1. Introduction
Sleep plays an essential role in maintaining human health and well-being. Insufficient or poor-quality sleep has been linked to a variety of negative health outcomes, including cognitive impairment, mood disorders, weakened immune function, and an increased risk of chronic diseases such as hypertension, diabetes, and obesity. While the quantity of sleep is important, research has increasingly focused on sleep quality, including factors such as sleep architecture, which refers to the structure and distribution of different sleep stages throughout the night [
1,
2]. Optimizing sleep architecture, which includes periods of light sleep, deep sleep, and rapid eye movement (REM) sleep, is crucial for restorative sleep that promotes both physical and mental recovery [
3,
4].
High-quality sleep enhances mental and physical health and aids in fatigue recovery. Several studies have evaluated the effects of environmental factors (e.g., temperature, humidity, airflow, light, and noise) on sleep quality [
5,
6,
7,
8]. Thermal environments, in particular, affect skin temperature, which varies dynamically across sleep stages (e.g., N1–3 and REM stages) [
9]. Thermoneutrality, defined as the temperature range in which minimal metabolic heat production balances environmental heat loss, is critical for maintaining sleep quality. Extreme or sudden temperature changes outside this range are known to deteriorate sleep quality [
10,
11,
12,
13].
Maintaining thermoneutral conditions for the skin during sleep, achievable through appropriate ambient and mattress temperatures, is essential for ensuring optimal sleep quality [
13,
14]. However, while most studies focus on ambient temperature, mattress temperature—closer to the body—has been found to exert a greater influence on sleep quality [
9,
12,
14]. Stable and appropriate mattress temperatures contribute significantly to achieving high-quality sleep, and certain studies have identified optimal ranges for mattress temperature to support this [
15,
16,
17,
18].
Various studies have explored the effects of environmental temperature on sleep quality. Many have demonstrated that sleeping in an environment that is too hot or too cold can disrupt sleep continuity, increase wakefulness, and shorten periods of REM and deep sleep, which are key for cognitive restoration and physical recovery [
14,
19,
20]. Traditionally, thermal interventions have focused on controlling the ambient temperature of the room, but recent advancements in sleep technologies have enabled more localized temperature regulation, such as through heated blankets or temperature-adjustable mattresses. However, most current approaches utilize a constant temperature throughout the night, which may not reflect the body’s dynamic thermoregulatory needs during different sleep stages [
21,
22,
23].
This study aims to address this gap by investigating the effects of real-time mattress temperature adjustment (RTA) based on sleep stage transitions. Unlike constant temperature control (CTC), which maintains a fixed mattress temperature throughout the night, RTA dynamically adjusts the temperature in response to the sleep stage, thereby aligning the mattress temperature with the body’s changing thermoregulatory needs. Specifically, the temperature is lowered during REM sleep to reflect the body’s reduced ability to regulate temperature during this stage, while returning to a higher, more comfortable baseline during non-REM sleep. We hypothesize that this adaptive approach will result in improvements in sleep architecture, including increased total sleep time, sleep efficiency, and enhanced durations of REM and deep sleep, while reducing wakefulness after sleep onset (WASO).
2. Materials and Methods
2.1. Study Design and Participants
This study employed a prospective longitudinal cohort design to evaluate the effects of real-time temperature adjustment (RTA) on sleep quality compared to constant temperature control (CTC). Participants aged 19 to 60 years were recruited, ensuring equal representation across each decade of age. Eligibility criteria required participants to maintain consistent sleep-wake schedules for at least five days per week and to have no aversion to thermal mattress use. Exclusion criteria included individuals using medical devices or with prior surgeries that could affect polysomnography (PSG) results, as well as those requiring treatment for severe sleep disorders or mental health conditions. Recruitment was conducted via public advertisements, and all participants received detailed explanations of the study’s purpose, methods, and potential benefits before providing written informed consent. This study protocol was approved by the Institutional Review Board (IRB #P01-202401-01-042). Importantly, each participant underwent PSG on three separate occasions, allowing for robust within-subject comparisons under varying conditions.
2.2. Sleep Environment and Measurement Tools
The sleep environment was standardized to ensure consistency, with room temperature maintained at 18–20 °C and humidity controlled at 50–55%. The study was conducted during the winter months (December 2023 to February 2024) in Seoul, Republic of Korea, where the average outdoor temperature was approximately −2 °C, and relative humidity ranged from 55% to 74%. Sleep measurements were conducted using level 1 full-night PSG with the Nox A1 PSG system (Nox Medical, Reykjavik, Iceland), which complies with the standards of the American Academy of Sleep Medicine (AASM). PSG recordings included electroencephalography, electrooculography, chin and limb electromyography, electrocardiography, nasal pressure transducer, chest and abdomen respiratory inductance plethysmography, and pulse oximetry. Physiological data, including key sleep architecture metrics such as total sleep time, sleep efficiency, wake after sleep onset, and REM and deep sleep durations, were analyzed using the Noxturnal software (Nox Medical, Reykjavik, Iceland) and reviewed by a sleep expert.
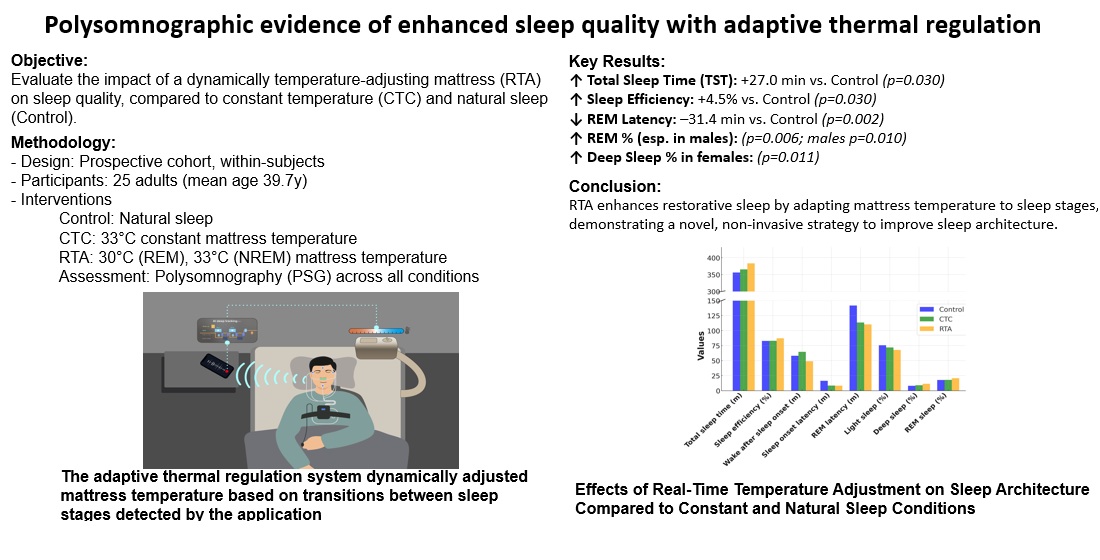
The intervention used a thermal mattress (EMW720, KyungDong Navien, Seoul, Republic of Korea), with temperature regulated through an AI-based application. This application recorded participants’ breathing sounds during sleep and used these data to predict sleep stages in real time. The adaptive thermal regulation system dynamically adjusted mattress temperature based on transitions between sleep stages detected by the application.
2.3. Study Procedure for Thermal Mattress Temperature Control
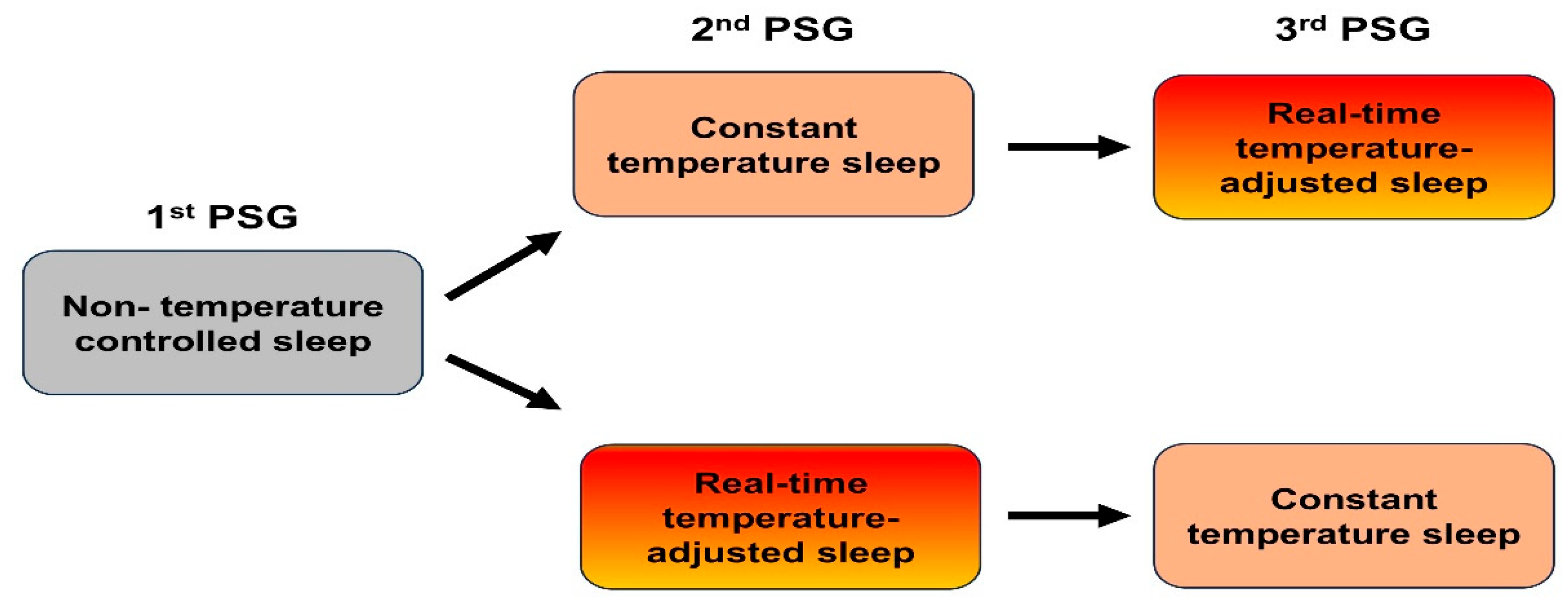
Participants were randomly assigned to one of two groups to minimize potential order effects. Group 1 first underwent a baseline natural sleep condition without mattress intervention, followed by the CTC condition, and then the RTA condition. Group 2 followed the reverse order, starting with natural sleep, then RTA, and concluding with CTC. Each condition was separated by a five-day interval to minimize residual effects and allow participants to return to their baseline state (
Figure 1).
In the baseline condition, participants slept without any mattress intervention. In the CTC condition, the mattress temperature was maintained at a constant 33 °C throughout the night, reflecting the commonly preferred heating mat temperature in South Korea[
24]. In the RTA condition, the mattress temperature was dynamically adjusted: during REM sleep, the temperature decreased by 3 °C to 30 °C to accommodate reduced thermoregulation, while during non-REM sleep, it reverted to 33 °C. Thirty minutes before the scheduled wake time, the temperature was increased by 3 °C to 36 °C to facilitate arousal. (
Figure 2).
2.4. Statistical Analysis
Data from the three PSG sessions were analyzed to compare the effects of CTC and RTA conditions on sleep architecture. Repeated-measures ANOVA was conducted to evaluate differences across the conditions, followed by post-hoc tests using the Friedman correction to identify specific variations. Statistical significance was defined as p < 0.05. All analyses were performed using standard statistical software. The inclusion of three separate PSG sessions for each participant ensured a robust evaluation of the adaptive thermal regulation’s impact on sleep quality.
3. Results
3.1. General and Baseline Polysomnographic Characteristics
A total of 30 participants were initially recruited for the study. However, 5 participants were excluded from the final analysis due to their inability to complete all three polysomnographic (PSG) sessions. Therefore, the data from 25 participants, consisting of 13 males and 12 females, were analyzed. The mean age of the participants was 39.7 years, with males averaging 42.4 years and females averaging 36.9 years (p = 0.336). The mean body mass index (BMI) was 25.2 Kg/m2.
Baseline polysomnographic (PSG) results revealed significant gender differences in sleep parameters. The average total sleep time (TST) was 356.2 min, with females exhibiting a no significantly longer TST (372.6 min) compared to males (341.1 min) (p = 0.157). Sleep efficiency averaged 82.8%, with females achieving a no significantly higher efficiency (86.6%) than males (79.3%) (p = 0.176). Wake after sleep onset (WASO) was longer in males (68.5 min) compared to females (45.3 min), with a group average of 58.2 min, demonstrating a statistically no significant difference (p = 0.233). Sleep onset latency was also no significantly longer in males (21.0 min) compared to females (11.8 min) (p = 0.910), with a total group average of 16.3 min.
3.2. Comparative Analysis of Sleep Architecture Across Control, CTC, and RTA Conditions
This study evaluated sleep parameters under three conditions: natural sleep (Control), constant temperature control (CTC), and real-time temperature adjustment (RTA). The results are summarized in
Table 1.
3.2.1. Total Sleep Time and Sleep Efficiency
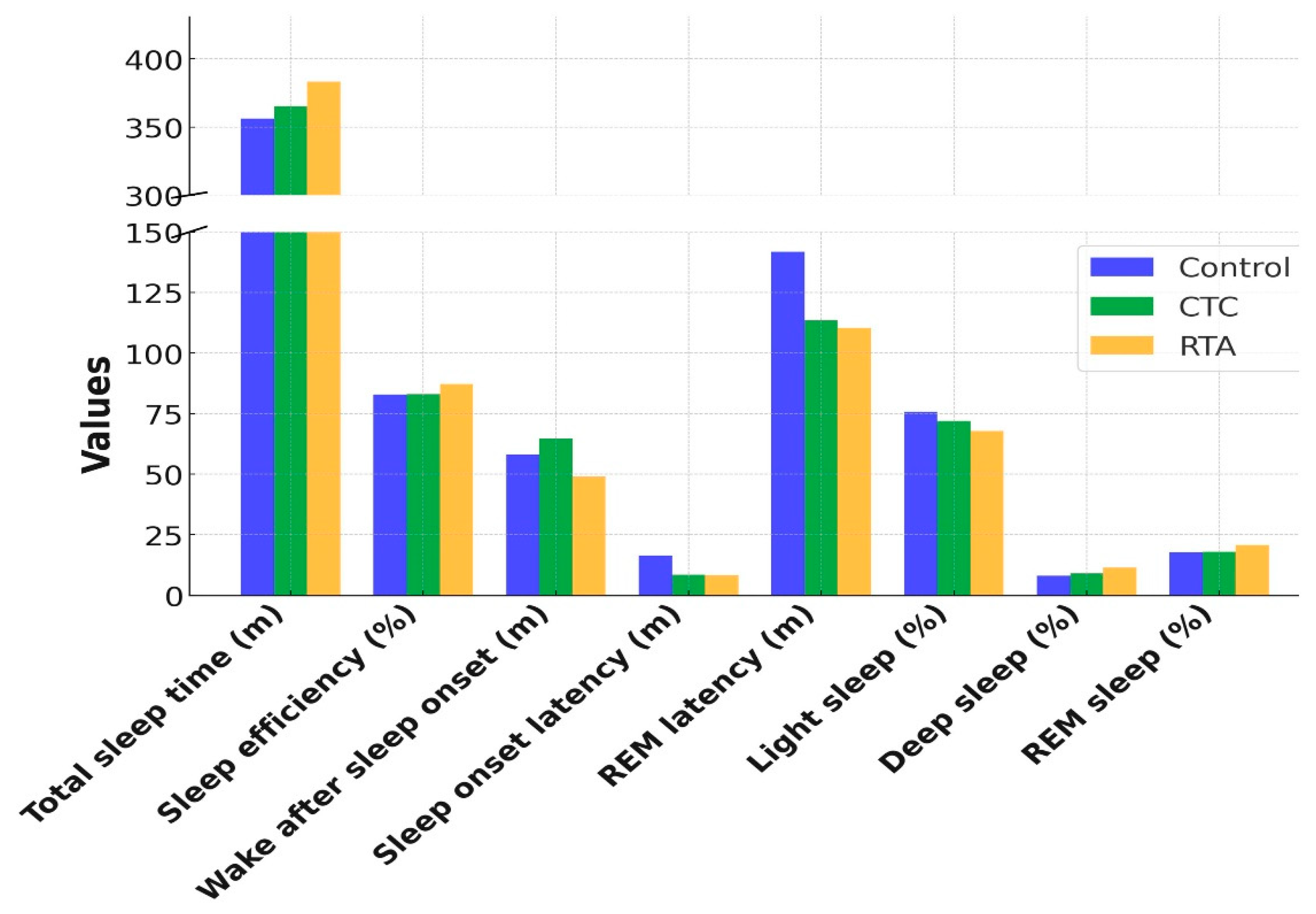
Significant improvements in both total sleep time (TST) and sleep efficiency were observed with the RTA condition. Total sleep time increased from 356.2 min under Control to 383.2 min under RTA (p = 0.030). Post-hoc analysis revealed a statistically significant difference between Control and RTA (p = 0.025), while the difference between CTC and RTA (p = 0.054) approached significance. Similarly, sleep efficiency improved significantly from 82.8% under Control to 87.3% under RTA (p = 0.030). Post-hoc comparisons indicated significant differences between Control and RTA (p = 0.004) as well as CTC and RTA (p = 0.010).
3.2.2. Wake After Sleep Onset, Sleep Onset Latency and Rem Latency
Wake after sleep onset (WASO) decreased under the RTA condition (49.0 min) compared to Control (58.2 min) and CTC (64.6 min), with a trend toward significance (p = 0.067). Post-hoc analysis showed a significant reduction in WASO between CTC and RTA (p = 0.041).
Sleep onset latency showed no significant differences across the conditions (p = 0.383), with RTA maintaining a similar latency to CTC (8.3 vs. 8.4 min, respectively). REM latency significantly decreased under RTA (110.4 min) compared to Control (141.8 min) and CTC (113.5 min; p = 0.002). Post-hoc analysis revealed significant reductions in REM latency between Control and RTA (p = 0.020).
3.2.3. Sleep Stages
For light sleep, a significant reduction was observed across conditions (
p = 0.002) with post-hoc analysis showing a significant difference between Control and RTA (
p = 0.011). No significant changes were observed in deep sleep duration (
p = 0.482). REM sleep percentage showed an increasing trend from Control (17.7%) to RTA (20.8%;
p = 0.006), though post-hoc comparisons did not reveal significant differences among individual conditions (
Figure 3).
3.3. Effects of Adaptive Thermal Regulation: Male and Female Subgroup Analysis”
3.3.1. Males (n = 13)
Subgroup analysis results are shown in
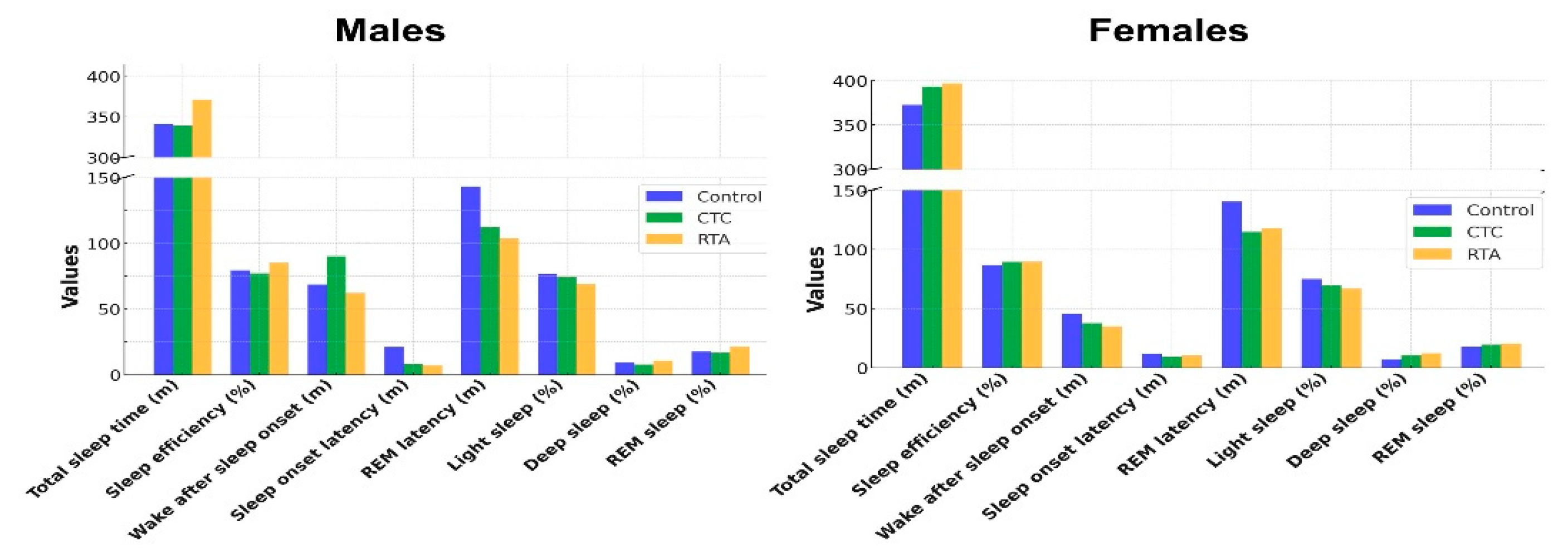
Table 2. In the male subgroup, significant improvements in sleep efficiency and REM sleep percentage were observed with real-time temperature adjustment (RTA). Sleep efficiency increased from 79.3% under Control and 77.2% under constant temperature control (CTC) to 85.1% under RTA (
p = 0.001), with post-hoc analysis revealing significant differences between Control and RTA (
p = 0.017) and CTC and RTA (
p = 0.001). REM sleep percentage also significantly increased from 17.5% under Control to 21.3% under RTA (
p = 0.010), though post-hoc analysis did not show significant differences between the conditions.
A reduction in REM latency was observed under RTA (103.6 min) compared to Control (143.3 min) and CTC (112.3 min; p = 0.001). Post-hoc analysis indicated a significant difference between Control and RTA (p = 0.018). Wake after sleep onset (WASO) and sleep onset latency showed trends toward improvement under RTA but did not reach statistical significance (p = 0.062 and p = 0.066, respectively).
Light sleep duration decreased under RTA (68.9%) compared to Control (76.5%) and CTC (74.1%), though this difference was not statistically significant (
p = 0.170). Deep sleep percentage showed no significant differences among the conditions (
p = 0.859) (
Figure 4).
3.3.2. Females (n = 12)
In the female subgroup, significant changes were observed in light sleep and deep sleep percentages. Light sleep decreased significantly under RTA (66.7%) compared to Control (75.1%) and CTC (69.8%; p = 0.001). Post-hoc analysis showed a significant difference between Control and RTA (p = 0.035). Deep sleep percentage increased from 7.1% under Control to 12.3% under RTA (p = 0.011), though post-hoc analysis did not reveal significant differences between the conditions.
Total sleep time (TST), sleep efficiency, WASO, sleep onset latency, and REM latency showed no significant differences across the conditions. REM sleep percentage exhibited a non-significant increase under RTA (20.3%) compared to Control (17.8%) and CTC (19.6%; p = 0.249) (Figure 5).
4. Discussion
The findings of this study provide compelling evidence that adaptive thermal regulation (RTA) significantly enhances sleep architecture compared to constant temperature control (CTC) and natural sleep. Improvements in total sleep time and sleep efficiency underscore the utility of dynamic temperature modulation in optimizing restorative sleep. By aligning mattress temperature with the body’s thermoregulatory needs during different sleep stages, RTA facilitates prolonged and uninterrupted sleep. These outcomes are consistent with prior studies that emphasize the role of environmental temperature in maintaining sleep continuity and reducing wakefulness after sleep onset [
25,
26]. RTA achieved a marked reduction in REM latency and an increase in REM sleep percentage, highlighting the critical role of temperature modulation during REM sleep in enhancing cognitive and emotional recovery processes [
27]. Furthermore, the significant increase in deep sleep percentage under RTA supports the hypothesis that adaptive thermal regulation enhances the body’s recovery by optimizing thermoregulatory efficiency during non-REM sleep. These findings advocate for the utility of RTA in improving overall sleep quality and promoting restorative functions.Most existing studies focus on identifying the optimal ranges for ambient and mattress temperatures, often relying on subjective measures such as surveys [
19,
28]. While these studies provide valuable insights, they largely overlook the dynamic temperature changes required during different sleep stages. This study differs from previous work by incorporating real-time temperature adjustments tailored to each sleep stage, accounting for physiological changes in body temperature [
9,
12,
14]. Prior studies have identified the optimal mattress temperature for general sleep comfort to range between 28 °C and 31 °C [
29], while some studies suggest higher limits, up to 35 °C, under specific conditions [
24,
30]. However, few studies have investigated the direct impact of mattress temperature adjustments on sleep stages. This study bridges this gap by assessing sleep quality under adaptive thermal conditions with advanced technologies, such as AI-based dynamic mattress temperature control.
This study identified gender-specific differences in response to RTA, highlighting the physiological and possibly hormonal variations that influence sleep architecture [
31]. While males exhibited significant improvements in REM sleep percentage and efficiency, females benefited more from enhanced deep sleep duration. These differences may be attributed to distinct thermoregulatory profiles, as previous research suggests that hormonal fluctuations, such as those related to estrogen and progesterone levels, influence body temperature regulation and sleep patterns in females [
32]. The reduced light sleep percentage and increased deep sleep observed in females under RTA suggest that this intervention could be tailored to meet gender-specific thermoregulatory needs. Moreover, the subgroup analysis revealed that males showed a greater reduction in REM latency under RTA, potentially reflecting a heightened sensitivity to the optimized thermal environment during REM sleep. These findings advocate for future research to explore personalized thermal interventions based on individual physiological characteristics, including gender and age.
Enhanced sleep efficiency and reduced WASO is likely to improve sleep quality by minimizing sleep fragmentation and promoting more consolidated sleep episodes. These findings suggest that RTA not only improves nocturnal physiological processes but also supports better cognitive and emotional performance during the day. The reduced REM latency under RTA is particularly noteworthy, as shortened latency to REM sleep has been associated with better mood regulation and memory consolidation. By fostering a more efficient transition into restorative sleep stages, adaptive thermal regulation may have far-reaching implications for individuals with mood disorders or cognitive impairments linked to poor sleep quality[
33,
34].
The efficacy of RTA in enhancing sleep architecture can be attributed to its alignment with the body’s circadian and sleep-stage-specific thermoregulatory processes. During REM sleep, the body’s thermoregulatory capacity is significantly reduced, making it more vulnerable to external temperature fluctuations [
35,
36]. By lowering the mattress temperature during REM sleep, RTA compensates for this vulnerability, maintaining an optimal thermal environment that supports uninterrupted REM cycles. Similarly, the return to a higher baseline temperature during non-REM sleep aligns with the body’s increased thermoregulatory activity, promoting deep sleep and recovery. The pre-wake temperature increase observed in the RTA condition likely aids in facilitating arousal and reducing sleep inertia. This mechanism mirrors natural circadian temperature rhythms, where a rise in core body temperature occurs in the early morning hours to prepare the body for wakefulness [
37]. By synchronizing mattress temperature with these physiological changes, RTA enhances the natural sleep-wake cycle and promotes smoother transitions between sleep and wake states.
Despite the promising results, this study has several limitations that should be addressed in future research. First, the sample size of 25 participants, though sufficient for preliminary analysis, limits the generalizability of the findings. Larger-scale studies are needed to confirm the efficacy of RTA across diverse populations and age groups. Nevertheless, our study overcomes this limitation by employing a rigorous protocol involving polysomnographic assessments conducted on three separate occasions. This approach enhances the reliability of the findings and underscores the robustness of our methodology. Second, the short duration of the study precludes an evaluation of the long-term benefits and potential drawbacks of adaptive thermal regulation. Prolonged use of RTA and its impact on chronic sleep issues, health outcomes, and potential habituation effects remain unexplored. Nonetheless, the robust within-subject design of this study provides a strong foundation for understanding immediate impacts. Third, while the study employed robust PSG methods, the reliance on a single thermal mattress model and fixed temperature settings may not account for individual variations in thermal preferences and sensitivity. Future research should investigate customizable temperature algorithms to enhance user comfort and effectiveness. Despite this limitation, our use of AI-based real-time adjustments represents a significant advancement over static temperature controls. Finally, the study did not include participants with diagnosed sleep disorders, such as insomnia or obstructive sleep apnea, limiting the applicability of findings to clinical populations. Expanding the scope of RTA research to include these groups could provide valuable insights into its therapeutic potential. Nevertheless, our findings contribute valuable knowledge on the general population, serving as a critical reference for extending applications to clinical settings.
While the findings of this study highlight the benefits of adaptive thermal regulation, several avenues for future research remain. Longitudinal studies are needed to assess the sustained effects of RTA on sleep quality and its potential long-term health benefits. For instance, chronic improvements in sleep architecture could mitigate the risk of conditions such as hypertension, diabetes, and obesity, which are often associated with poor sleep quality. Additionally, exploring the application of RTA in populations with specific sleep disorders, such as insomnia, obstructive sleep apnea, or restless leg syndrome, could provide valuable insights into its therapeutic potential. Customizing thermal interventions for different demographic groups, including older adults and individuals with chronic illnesses, could further enhance the utility of RTA in promoting restorative sleep across diverse populations. Finally, advancements in wearable technology and AI-based sleep monitoring systems could enable the development of more sophisticated and user-friendly adaptive thermal regulation devices. By integrating real-time physiological data with personalized thermal adjustment algorithms, future innovations could provide tailored solutions that maximize sleep quality and overall well-being.
In conclusion, adaptive thermal regulation represents a promising intervention for optimizing sleep quality by dynamically aligning mattress temperature with the body’s thermoregulatory needs during different sleep stages. The significant improvements in sleep architecture observed in this study underscore the potential of RTA as a non-invasive and personalized approach to enhancing restorative sleep. By addressing individual and demographic variations in thermoregulatory responses, RTA could pave the way for innovative sleep solutions that promote better health and quality of life.
Author Contributions
For research articles with several authors, the following statements should be used “Conceptualization, Jeong-Whun Kim and Sungjin Heo; methodology, Sungjin Heo; software, Joonki Hong; validation, Dongheon Lee; formal analysis, Sungeun Moon; investigation, Sungjin Heo; resources, Jeong-Whun Kim; data curation, Dongheon Lee; writing—original draft preparation, Sungjin Heo; writing—review and editing, Sungjin Heo and Jeong-Whun Kim; visualization, Sungjin Heo; supervision, Jeong-Whun Kim; project administration, Jeong-Whun Kim; funding acquisition, Donghyuk Yang. All authors have read and agreed to the published version of the manuscript.”
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not Available
Acknowledgments
This research was supported by KyungDong Navien, Republic of Korea which provided funding and resources for the study. We sincerely appreciate their support in enabling this research on sleep regulation and thermal comfort. The findings and conclusions expressed in this paper are solely those of the authors and do not necessarily reflect the views of the funding organization.
Conflicts of Interest
This study was supported by KyungDong Navien; however, the sponsor had no role in the study design, data collection, analysis, interpretation, or manuscript preparation. The authors declare no other competing interests.
References
- Chattu, V.K.; Manzar, M.D.; Kumary, S.; Burman, D.; Spence, D.W.; Pandi-Perumal, S.R. The Global Problem of Insufficient Sleep and Its Serious Public Health Implications. Healthcare 2018, 7, 1. [CrossRef]
- Ramar, K.; Malhotra, R.K.; Carden, K.A.; Martin, J.L.; Abbasi-Feinberg, F.; Aurora, R.N.; Kapur, V.K.; Olson, E.J.; Rosen, C.L.; Rowley, J.A.; et al. Sleep is essential to health: An American Academy of Sleep Medicine position statement. J. Clin. Sleep. Med. 2021, 17, 2115–2119. [CrossRef]
- Desai, D.; Momin, A.; Hirpara, P.; Jha, H.; Thaker, R.; Patel, J. Exploring the Role of Circadian Rhythms in Sleep and Recovery: A Review Article. Cureus 2024, 16, e61568. [CrossRef]
- Zapalac, K.; Miller, M.; Champagne, F.A.; Schnyer, D.M.; Baird, B. The effects of physical activity on sleep architecture and mood in naturalistic environments. Sci. Rep. 2024, 14, 5637. [CrossRef]
- Henane, R.; Buguet, A.; Roussel, B.; Bittel, J. Variations in evaporation and body temperatures during sleep in man. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1977, 42, 50–55. [CrossRef]
- Buguet, A. Sleep under extreme environments: Effects of heat and cold exposure, altitude, hyperbaric pressure and microgravity in space. J. Neurol. Sci. 2007, 262, 145–152. [CrossRef]
- Haskell, E.H.; Palca, J.W.; Walker, J.M.; Berger, R.J.; Heller, H.C. Metabolism and thermoregulation during stages of sleep in humans exposed to heat and cold. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1981, 51, 948–954. [CrossRef]
- Okamoto-Mizuno, K.; Tsuzuki, K.; Mizuno, K. Effects of head cooling on human sleep stages and body temperature. Int. J. Biometeorol. 2003, 48, 98–102. [CrossRef]
- Troynikov, O.; Watson, C.G.; Nawaz, N. Sleep environments and sleep physiology: A review. J. Therm. Biol. 2018, 78, 192–203. [CrossRef]
- Caddick, Z.; Gregory, K.; Arsintescu, L.; Flynn-Evans, E. A review of the environmental parameters necessary for an optimal sleep environment. Build. Environ. 2018, 132, 11–20. [CrossRef]
- Lan, L.; Pan, L.; Lian, Z.; Huang, H.; Lin, Y. Experimental study on thermal comfort of sleeping people at different air temperatures. Build. Environ. 2014, 73, 24–31. [CrossRef]
- Bischof, W.; Madsen, T.L.; Clausen, J.; Madsen, P.L.; Wildschi∅dtz, G. Sleep and the temperature field of the bed. J. Therm. Biol. 1993, 18, 393–398. [CrossRef]
- Obradovich, N.; Migliorini, R.; Mednick, S.C.; Fowler, J.H. Nighttime temperature and human sleep loss in a changing climate. Sci. Adv. 2017, 3, e1601555. [CrossRef]
- Okamoto-Mizuno, K.; Mizuno, K. Effects of thermal environment on sleep and circadian rhythm. J. Physiol. Anthropol. 2012, 31, 14.
- Muzet, A.; Libert, J.P.; Candas, V. Ambient temperature and human sleep. Experientia 1984, 40, 425–429. [CrossRef]
- Tsuzuki, K.; Okamoto-Mizuno, K.; Mizuno, K. Effects of humid heat exposure on sleep, thermoregulation, melatonin, and microclimate. J. Therm. Biol. 2004, 29, 31–36. [CrossRef]
- Raymann, R.J.; Swaab, D.F.; Van Someren, E.J. Cutaneous warming promotes sleep onset. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R1589–R1597. [CrossRef]
- Macpherson, R.K. Thermal stress and thermal comfort. Ergonomics 1973, 16, 611–622.
- Tsang, T.W.; Mui, K.W.; Wong, L.T. Investigation of thermal comfort in sleeping environment and its association with sleep quality. Build. Environ. 2021, 187, 107406. [CrossRef]
- Zheng, G.; Li, K.; Wang, Y. The Effects of High-Temperature Weather on Human Sleep Quality and Appetite. Int. J. Environ. Res. Public. Health 2019, 16, 270. [CrossRef]
- Aijazi, A.; Parkinson, T.; Zhang, H.; Schiavon, S. Passive and low-energy strategies to improve sleep thermal comfort and energy resilience during heat waves and cold snaps. Sci. Rep. 2024, 14, 12568. [CrossRef]
- Okamoto-Mizuno, K.; Tsuzuki, K.; Ohshiro, Y.; Mizuno, K. Effects of an electric blanket on sleep stages and body temperature in young men. Ergonomics 2005, 48, 749–757. [CrossRef]
- Lan, L.; Tsuzuki, K.; Liu, Y.F.; Lian, Z.W. Thermal environment and sleep quality: A review. Energy Build. 2017, 149, 101–113. [CrossRef]
- Harding, E.C.; Franks, N.P.; Wisden, W. The Temperature Dependence of Sleep. Front. Neurosci. 2019, 13, 336.
- Fan, X.; Shao, H.; Sakamoto, M.; Kuga, K.; Lan, L.; Wyon, D.P.; Ito, K.; Bivolarova, M.P.; Liao, C.; Wargocki, P. The effects of ventilation and temperature on sleep quality and next-day work performance: Pilot measurements in a climate chamber. Build. Environ. 2022, 209, 108666. [CrossRef]
- Johnson, D.A.; Jackson, C.L.; Guo, N.; Sofer, T.; Laden, F.; Redline, S. Perceived home sleep environment: Associations of household-level factors and in-bed behaviors with actigraphy-based sleep duration and continuity in the Jackson Heart Sleep Study. Sleep. 2021, 44, zsab163. [CrossRef]
- Goldstein, A.N.; Walker, M.P. The role of sleep in emotional brain function. Annu. Rev. Clin. Psychol. 2014, 10, 679–708. [CrossRef]
- Ngarambe, J.; Yun, G.Y.; Lee, K.; Hwang, Y. Effects of Changing Air Temperature at Different Sleep Stages on the Subjective Evaluation of Sleep Quality. Sustainability 2019, 11, 1417. [CrossRef]
- Joshi, S.S.; Lesser, T.J.; Olsen, J.W.; O’Hara, B.F. The importance of temperature and thermoregulation for optimal human sleep. Energy Build. 2016, 131, 153–157. [CrossRef]
- Rohles, F.H.; Munson, D.M. Sleep and the sleep environment temperature. J. Environ. Psychol. 1981, 1, 207–214. [CrossRef]
- Alosta, M.R.; Oweidat, I.; Alsadi, M.; Alsaraireh, M.M.; Oleimat, B.; Othman, E.H. Predictors and disturbances of sleep quality between men and women: Results from a cross-sectional study in Jordan. BMC Psychiatry 2024, 24, 200. [CrossRef]
- Dorsey, A.; de Lecea, L.; Jennings, K.J. Neurobiological and Hormonal Mechanisms Regulating Women’s Sleep. Front. Neurosci. 2020, 14, 625397. [CrossRef]
- Rasch, B.; Born, J. About sleep’s role in memory. Physiol. Rev. 2013, 93, 681–766.
- Riemann, D.; Krone, L.B.; Wulff, K.; Nissen, C. Sleep, insomnia, and depression. Neuropsychopharmacology 2020, 45, 74–89.
- Bigalke, J.A.; Cleveland, E.L.; Barkstrom, E.; Gonzalez, J.E.; Carter, J.R. Core body temperature changes before sleep are associated with nocturnal heart rate variability. J. Appl. Physiol. 2023, 135, 136–145. [CrossRef]
- Gonnissen, H.K.; Mazuy, C.; Rutters, F.; Martens, E.A.; Adam, T.C.; Westerterp-Plantenga, M.S. Sleep architecture when sleeping at an unusual circadian time and associations with insulin sensitivity. PLoS ONE 2013, 8, e72877. [CrossRef]
- Trotti, L.M. Waking up is the hardest thing I do all day: Sleep inertia and sleep drunkenness. Sleep. Med. Rev. 2017, 35, 76–84. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).