Submitted:
23 July 2025
Posted:
24 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction

2. Pharmaceuticals vs Nutraceuticals and Dietary Supplements
2.1. What Are the Differences Between the Requirements for the Quality of Pharmaceuticals and Dietary Supplements of Botanical Origin?
2.2. Regulatory Classification, Purpose of Use, Dose and Potency, Labeling and Claims
3. Progress, Trends, Pitfalls, and Challenges in the Adaptogens Research

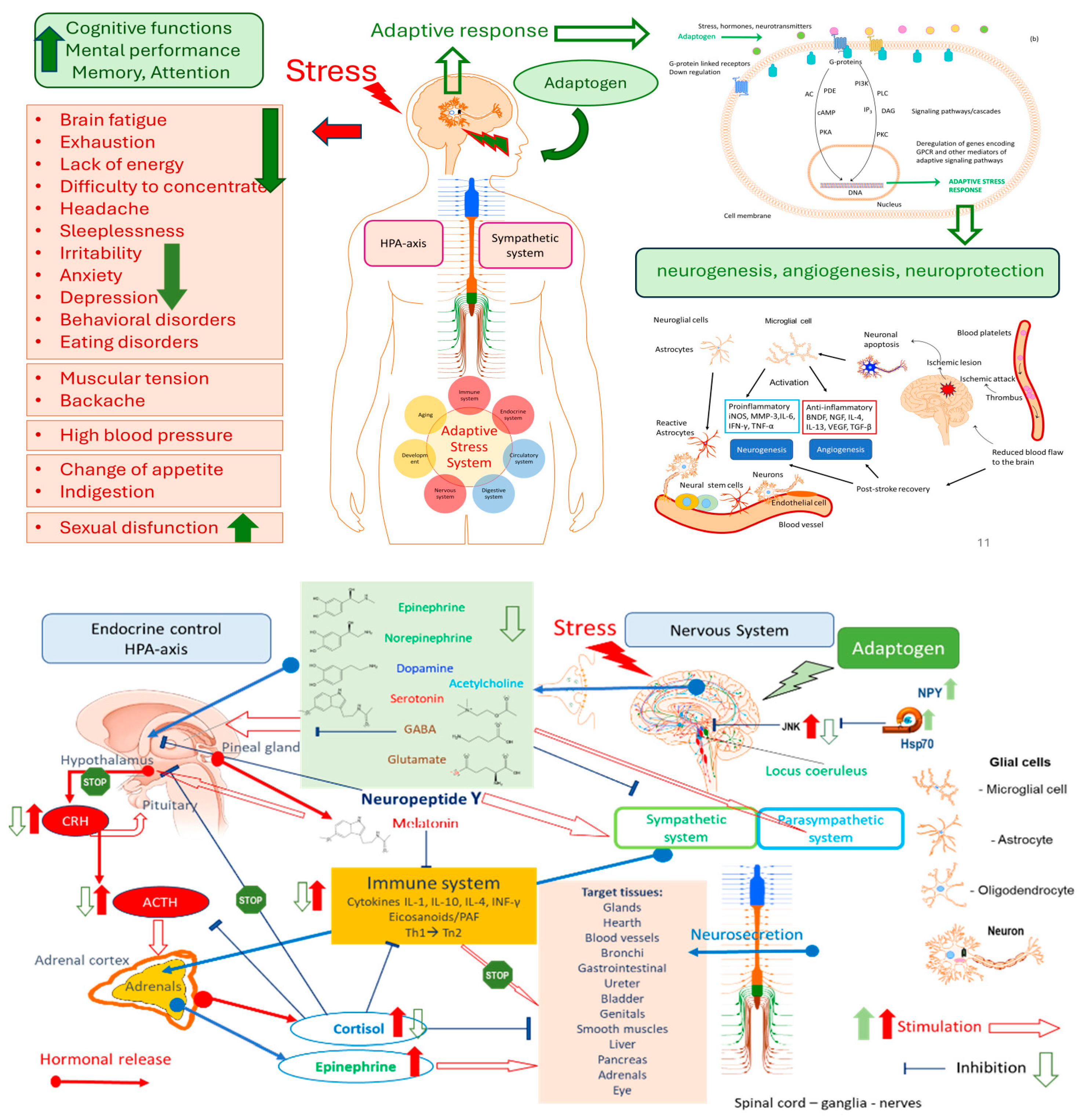
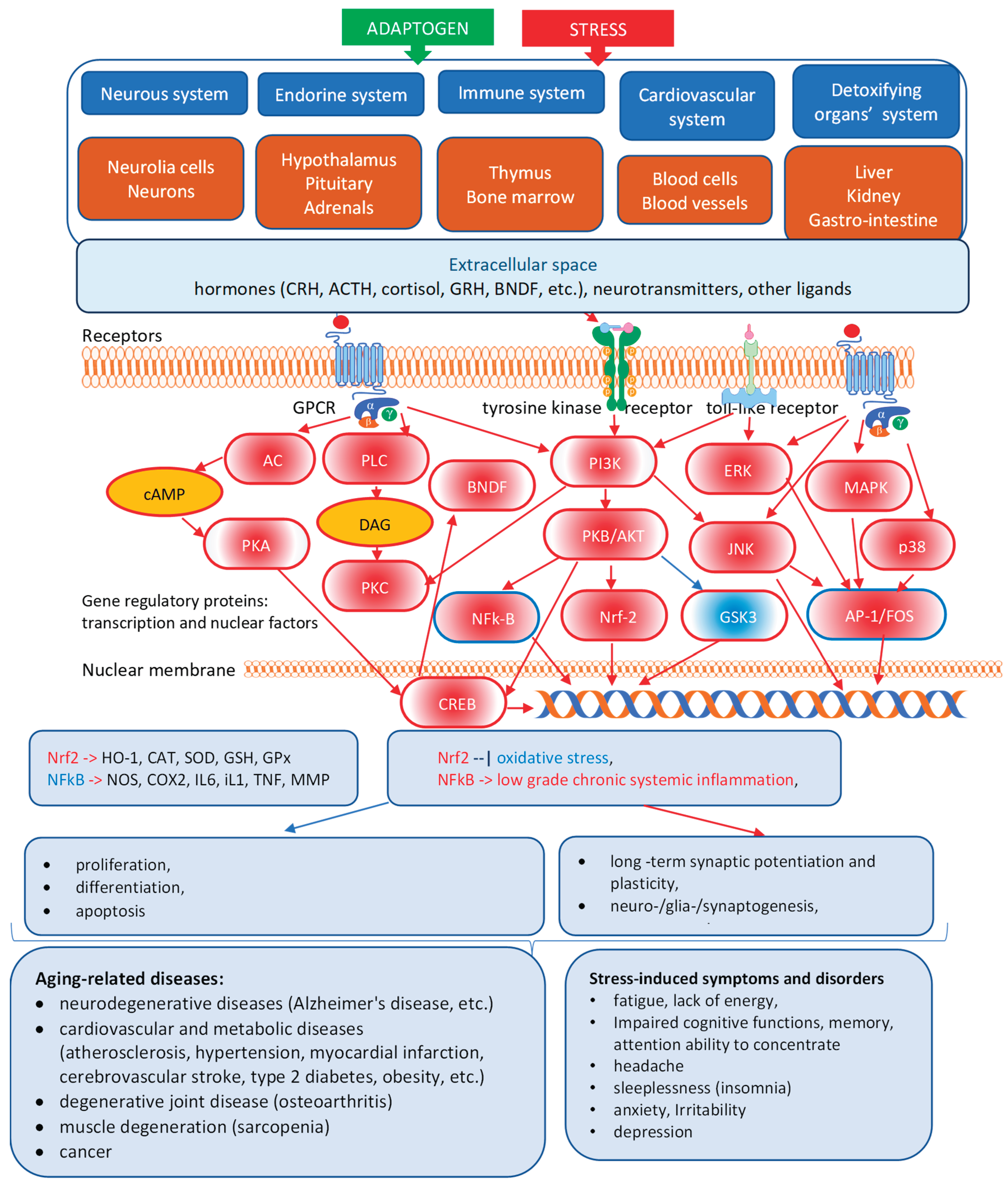
3.1. Adaptogens Are Stress Protectors
3.2. Adaptogens Are Stimulants
3.3. What Is Necessary and Sufficient to Be Classified as an Adaptogenic Plant?
3.4. Progress and Trends in Adaptogens Research
- elucidation of the mechanisms of action of medicinal plants,
- understanding the synergistic therapeutic actions of complex bioactive components in medicinal plants,
- providing a rationale for traditional Chinese medicine, enhancing the quality,
- of TCM drug research, and the speed and efficiency of developing new TCM products,
- discovering and developing new botanical hybrid combinations,
3.5. Pitfalls in Adaptogen Research: Inadequate Assignment of Some Plants to Adaptogens or Insufficient Scientific Data
- Active compounds of Eurycoma are 4300 dalton glycopeptides consisting of 36 amino acids.
- According to the authors Talbott et al., 2013 [37] , the mechanism of action is related to "the bioactive complex 4300 dalton glycopeptides ("eurypeptides" with 36 amino acids) has been shown to activate the CYP17 enzyme (17 alphahydroxylase and 17,20 lyase) to enhance the metabolism of pregnenolone and progesterone to yield more DHEA (dehydroepiandrosterone) and androstenedione, respectively [40]. This glycoprotein water-soluble extract of Eurycoma longifolia has been shown to deliver anti-aging and antistress benefits subsequent to its testosterone balancing effects".
- Insufficient description of the study medication (see above),
- Randomization (a method used to generate the random allocation sequence, including details of restriction)
- Implementation (who generated the allocation sequence, enrolled participants, assigned participants to their groups, etc.)
- Blinding (preparation had the same appearance, test, and odor as placebo; how care providers, those assessing outcomes, were blinded; how the success of blinding was evaluated)
- Allocation concealment (the mechanism used to implement the random allocation sequence, such as sequentially numbered containers, describing any steps taken to conceal the sequence until interventions were assigned; it is not clear whether the sequence was concealed until interventions were assigned),
- Procedure for treatment compliance (how measurements of compliance of individual patients with the treatment regimen under study were documented).
- Monitoring,
- Settings and locations where the data were collected,
- Quality assurance and quality control,
- Deviations from the protocol
- Selective reporting
- The trial was conducted per ICH guidelines for GCP.
- Voucher specimen (i.e., retention sample was retained and, if so, where it is kept or deposited).
- The role of the study sponsor/funder,
- Inappropriate statistical tools and statistical analysis (e.g., lack of between-groups comparison of changes from the baseline by two-way ANOVA, etc.).
- ⇒
- a purposive sampling in a randomized, double-blinded, placebo-controlled, parallel-group study.
- ⇒
- lost to follow-up and missing data points
- ⇒
- the lack of nutritional intake information, which can be a limitation for a comprehensive analysis of the potential influence of dietary factors on the observed outcomes.
3.6. Dual-Use Dilemma and Inconsistencies in Botanical Risk Assessments in the Case of Withania Somnifera
- In India, Withania somnifera is officially included in the Indian Herbal Pharmacopeia [54]. The monograph outlines:
- o
- the plant names
- o
- geographical distribution
- o
- macroscopic and microscopic description of the roots,
- o
- chemical constituents - steroidal lactones including withanone, withaferin A, withanolides I, II, III, A, D, E, F, G, H, I, J, K, L, M, WS-L, P, and S, withasomnidienone, withanolide C, and alkaloids viz., cuscohygrine, anahygrine, tropine, pseudotropine, anaferine, isopellatierine, 3-tropyltigloate,
- o
- Assays/analytical methods including HPLC conditions fingerprints, identifying withaferin A in extracts and withanolide J in vitro culture,
- o
- quantitative standards (including total alkaloids (in total about0,2%),
- o
- adulteration,
- o
- pharmacology section,
- o
- reported activities including antistress, immunomodulatory, anticancer, antioxidant, anticonvulsive, anthelminthic, antiarthritic, chemopreventive, antibacterial, cardioprotective, antidepressant, antitoxic, hypoglycemic, diuretic, hypercholesterolemic, immunosuppressive, antiradical, and adaptogenic activities,
- o
- Therapeutic category: Adaptogen.
- In China, Ashwagandha is regulated under traditional Chinese medicine standards. Quality control measures are in place for the selection of raw materials, extraction processes, and product testing to ensure the safety and efficacy of the final product.
- In the U.S., Ashwagandha is permitted as a dietary supplement. The United States Pharmacopeia (USP) provides guidelines for its quality control, including High-Performance Liquid Chromatography (HPLC) methods to assess total withanolide content.
- Ashwagandha is allowed as a food supplement in the UK. The Medicines and Healthcare Products Regulatory Agency (MHRA) has approved clinical trials involving Ashwagandha, indicating its acceptance within certain regulatory frameworks.
- Ashwagandha is included in the Australian Register of Therapeutic Goods (ARTG), with over 320 listed medicines containing it, reflecting its acceptance in therapeutic products.
- In France, Ashwagandha root is classified under List B of medicinal plants, indicating that its potential adverse effects may outweigh its therapeutic benefits. This classification restricts its use in medicinal preparations.
- Ashwagandha is available as a dietary supplement in Germany. However, the Federal Institute for Risk Assessment (BfR) has recommended its inclusion in the EU's list of substances for which safety has not been conclusively established, suggesting caution in its use.
- Poland permits the use of Ashwagandha root but restricts the use of other parts of the plant. Additionally, the daily intake of withanolides is limited to less than 10 mg, reflecting a cautious approach to its consumption.
- In Sweden, the regulation of Ashwagandha is decentralized, allowing local authorities to make decisions regarding its use. This approach permits its availability under certain conditions.
- Within the Association of Southeast Asian Nations (ASEAN), Ashwagandha is not uniformly included in national pharmacopeias. However, efforts are underway to harmonize traditional medicine regulations across member states. The ASEAN Common Technical Document (ACTD) framework is being utilized to standardize quality, safety, and efficacy requirements for herbal products, such as Ashwagandha [2].
3.7. Key Issues Identified
- Blurring of Pharmacological and Nutritional Frameworks
- 2.
- Inappropriate Aggregation of Data from Different Plant Parts
- 3.
- Selective and Outdated Use of Scientific Literature
- 4.
- Absence of Peer Review and Transparency
- 5.
- Regulatory Disparity and Industry Impact
3.8. Critical Assessment of Common Technical Documentation Submitted by Drug Manufacturers to Drug Authorities
- The published clinical trials exhibit considerable deficiencies in their quality and show methodological problems.
- o
- insufficiently characterized herbal preparations,
- o
- open (label) studies,
- o
- small sample size,
- o
- missing ITT analysis, regardless of a detailed description of dropouts and reasons for exclusion in the analysis of an outcome measure,
- o
- healthy subjects,
- o
- The efficacy score has not been validated
- o
- The results from trials on clinical pharmacology are contradictory.
- o
- There is a lack of independent replications of the single different studies.
- extraction solvents and dry herb: dry native Extract Ratio (DER),
- The content of active markers, providing HPLC fingerprints to ensure consistent quality and reproducible pharmacological activity.
- The analytical methods were not validated for selectivity, accuracy, and precision.
- The authors declared that placebo capsules containing microcrystalline cellulose and silicon dioxide had the same appearance, odor, and taste as the R. rosea product, which is very unlikely due to their strong specific rose odor, test, and color, particularly when "participants were asked to self-determine their need for one additional capsule (i.e., a half dose), to be taken within four hours of the initial dose.
- The authors have not reported (or not assessed) the results of treatment compliance (counting of unused tablets), and that is a serious flaw.
- All outcome measures of the study were subjective based on self-assessment questionnaires of QOL in 48 nurses instead of the only doctor having the same unified "standard."
3.9. Other Challenges in Adaptogens Research
3.10. Proposed Solutions
- Establish Internationally Harmonized Guidelines for evaluating botanicals based on their intended use (e.g., pharmaceutical vs. dietary Supplement), incorporating traditional use data alongside modern scientific methods.
- Encourage Peer Review and Transparency in national assessments by mandating public disclosure of methodologies, data sources, and expert affiliations.
- Create a Tiered Evidence Framework that allows differentiated standards of proof for traditional botanical supplements versus pharmaceutical candidates.
- Promote International Scientific Dialogues among regulators, researchers, and industry to develop consensus positions and avoid unilateral bans that may lack scientific rigor.
4. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patwardhan, B.; Chaturvedi, S.; Tillu, G.; Deshpande, S.; Hegde, B. M. Danish ban on Ashwagandha: Truth, evidence, ethics, and regulations. Journal of Ayurveda and integrative medicine 2024, 15, 101028. [Google Scholar] [CrossRef] [PubMed]
- Ramadoss, M.S.K.; Koumaravelou, K. Regulatory Requirement Sufficiency for Registration of Traditional Herbal Medicine-Based Products in Association of Southeast Asian Nations Regions: A Model Study with Ashwagandha Tablets. https://www.ijpsonline.com/articles/regulatory-requirement-sufficiency-for-registration-of-traditionalherbal- medicine-based-products-in-association-of-southeast-asia-4579.html. Indian J Pharm Sci, 2022; 84, 501–512. [Google Scholar]
- Ministry of Ayush, Government of India, New Delhi. Safety of Ashwagandha. (Withania somnifera). Report of the Expert Committee constituted by Ministry of Ayush Govt. of India. Central Council for Research in Ayuredic Sciences, Ministry of Ayush, Government of India, New Delhi, 2020, 1-70. www.ccras.nic.
- Danish Technical University (DTU) Food Institute's risk assessments of Withania somnifera (Ashwagandha) root (DTU DOCX No. 19/1030299, 2020.
- Danish Technical University (DTU) Food Institute's risk assessments of Withania somnifera (Ashwagandha) root DTU DOCX 25/100205, 2025).
- EMA/HMPC/24186/2023. Final assessment report on Rhodiola rosea L., rhizoma et radix - Revision 1. Committee on Herbal Medicinal Products (HMPC). 21/05/2024; https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-rhodiola-rosea-l-rhizoma-et-radix-revision-1_en.
- EMA/HMPC/27745/2023. Final assessment report on Panax ginseng C.A.Mey., radix - Revision 1. Committee on Herbal Medicinal Products (HMPC). 12/07/2024. https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-panax-ginseng-camey-radix-revision-1_en.pdf. (accessed on 22 July 2025).
- EMA/HMPC/681519/2012. Final public statement on Withania somnifera (L.) Dunal, radix - First version. Herbal Medicinal Products (HMPC). 03/09/2013; https://www.ema.europa.eu/en/documents/public-statement/final-public-statement-withania-somnifera-l-dunal-radix-first-version_en.
- EMA/HMPC/680615/2013.Final assessment report on Eleutherococcus senticosus (Rupr. et Maxim.) Maxim., radix. Committee on Herbal Medicinal Products (HMPC). 06/10/2014 https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-eleutherococcus-senticosus-rupr-et-maxim-maxim-radix_en.
- EMA/HMPC/39455/2015. Final assessment report on Sideritis scardica Griseb.; Sideritis clandestina (Bory & Chaub.) Hayek; Sideritis raeseri Boiss. & Heldr.; Sideritis syriaca L., herba. Committee on Herbal Medicinal Products (HMPC). 28/10/2016; https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-sideritis-scardica-griseb-sideritis-clandestina-bory-chaub-hayek-sideritis-raeseri-boiss-heldr-sideritis-syriaca-l-herba_en.
- Ginseng Radix. In: WHO monographs on selected medicinal plants. World Health Organization, Geneva, Switzerland, 1999; 1:168-182.
- Radix Eleutherococci. In: WHO monographs on selected medicinal plants. World Health Organization, Geneva, Switzerland, 2002; 2: 83-96.
- Fructus Schisandrae. In: WHO monographs on selected medicinal plants. World Health Organization, Geneva, Switzerland, 2007; 3: 296-313.
- Radix Withaniae. In: WHO monographs on selected medicinal plants. World Health Organization, Geneva, Switzerland, 2009; 4: 373-391.
- National Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China, English ed.; National Pharmacopoeia Committee: Beijing, China, 2010. [Google Scholar]
- Panossian, A. Understanding adaptogenic activity: specificity of the pharmacological action of adaptogens and other phytochemicals. Annals of the New York Academy of Sciences, 1401. [Google Scholar]
- Panossian, A. G. , Efferth, T., Shikov, A. N., Pozharitskaya, O. N., Kuchta, K., Mukherjee, P. K., Banerjee, S., Heinrich, M., Wu, W., Guo, D. A., & Wagner, H. (2021). Evolution of the adaptogenic concept from traditional use to medical systems: Pharmacology of stress- and aging-related diseases. Medicinal research reviews, 41(1), 630–703. [CrossRef]
- Canguilhem, G. Essai sur quelques problèmes concernant le normal et le pathologique. In The Normal and the Pathological; Fawcett, C.R., Cohen, R.S., Translators, *!!! REPLACE !!!*, Eds.; Zone Books: New York, NY, USA, 1991; Available online: https://www.undergroundbooks.net/pages/books/11553/georges-canguilhem-michel-foucault-intro/the-normal-and-the-pathological (accessed on 10 February 2025).
- Panossian, A.; Lemerond, T.; Efferth, T. Adaptogens in Long-Lasting Brain Fatigue: An Insight from Systems Biology and Network Pharmacology. Pharmaceuticals 2025, 18, 261. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Nörr, H.; Winterhoff, H. Plant adaptogens. Phytomedicine 1994, 1, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A. , Wikman, G., & Wagner, H. (1999). Plant adaptogens. III. Earlier and more recent aspects and concepts on their mode of action. Phytomedicine : international journal of phytotherapy and phytopharmacology, 6(4), 287–300. [CrossRef]
- Panossian, A.G. Adaptogens: Tonic herbs for fatigue and stress. Altern. Compliment. Ther. 2003, 9, 327–332. [Google Scholar] [CrossRef]
- Panossian, A.; Wagner, H. Stimulating effect of adaptogens: an overview with particular reference to their efficacy following single dose administration. Phytother Res. 2005, 19, 819–838. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Wikman, G. Pharmacology of Schisandra chinensis Bail.: An overview of Russian research and uses in medicine. J. Ethnopharmacol. 2008, 118, 183–212. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Wikman, G.; Sarris, J. Rosenroot (Rhodiola rosea): Traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine 2010, 17, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Wikman, G.; Kaur, P.; Asea, A. Adaptogens exert a stress-protective effect by modulation of expression of molecular chaperones. Phytomedicine 2009, 16, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Wikman, G.; Kaur, P.; Asea, A. Adaptogens stimulate neuropeptide y and hsp72 expression and release in neuroglia cells. Front. Neurosci. 2012, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E. M. , Gomez, D., Caccamise, A., McPhail, D., & Hearing, M. (2019). Chronic unpredictable stress promotes cell-specific plasticity in prefrontal cortex D1 and D2 pyramidal neurons. Neurobiology of stress, 10, 100152. [CrossRef]
- Panossian, A. , Seo, E. J., & Efferth, T. (2018). Novel molecular mechanisms for the adaptogenic effects of herbal extracts on isolated brain cells using systems biology. Phytomedicine : international journal of phytotherapy and phytopharmacology, 50, 257–284. [CrossRef]
- Panossian, A. , & Efferth, T. (2022). Network Pharmacology of Adaptogens in the Assessment of Their Pleiotropic Therapeutic Activity. Pharmaceuticals (Basel, Switzerland), 15(9), 1051. [CrossRef]
- Panossian, A. , Lemerond, T., & Efferth, T. (2024). State-of-the-Art Review on Botanical Hybrid Preparations in Phytomedicine and Phytotherapy Research: Background and Perspectives. Pharmaceuticals (Basel, Switzerland), 17(4), 483. [CrossRef]
- Panossian, A. (2025). Trends and Pitfalls in the Progress of Network Pharmacology Research on Natural Products. Pharmaceuticals (Basel, Switzerland), 18(4), 538. [CrossRef]
- Panossian, A. (2023). Challenges in phytotherapy research. Frontiers in pharmacology, 14, 1199516. [CrossRef]
- Tambi, M. I. (2006). Eurycoma longifolia jack: a potent adaptogen in the form of water-soluble extract with the effects of maintaining men's health. Asian J Androl, 8(Suppl 1):49–50.
- George A, Liske E, Chen CK, and Ismail SB. (2013). J Sports Med Doping Stud, 2: Water Extract-Physta Does not Change Normal Ratios of Testosterone to Epitestosterone in Healthy Males, J Sports Med Doping Stud, 3:2.
- George, A. , & Henkel, R. (2014). Phytoandrogenic properties of Eurycoma longifolia as natural alternative to testosterone replacement therapy. Andrologia 46(7), 708–721. [CrossRef] [PubMed]
- Talbott, S. M. , Talbott, J. A., George, A., & Pugh, M. (2013). Effect of Tongkat Ali on stress hormones and psychological mood state in moderately stressed subjects. Journal of the International Society of Sports Nutrition 10(1), 28. [CrossRef] [PubMed]
- Thu, H. E. , Mohamed, I. N., Hussain, Z., Jayusman, P. A., & Shuid, A. N. (2017). Eurycoma Longifolia as a potential adoptogen of male sexual health: a systematic review on clinical studies. Chinese journal of natural medicines. [CrossRef]
- 2Chan, K (2021). Effects of Eurycoma longifolia supplementation: An evaluation of cell growth, exercise performance, and well-being in adult males. Doctoral thesis, Liverpool John Moores University. https://researchonline.ljmu.ac.uk/id/eprint/15104/ ; https://researchonline.ljmu.ac.uk/id/eprint/15104/1/2021kaiquinphd.
- Chinnappan, S.M. , George, A., Pandey, P., Narke, G., Choudhary, Y.K. Food & Nutrition Research, 5: aqueous root extract–Physta® on testosterone levels and quality of life in ageing male subjects: a randomised, double-blind, placebo-controlled multicentre studyFood & Nutrition Research 2021, 65, 2021. [Google Scholar] [CrossRef]
- Bhat, R. , & Karim, A. A. (2010). Tongkat Ali (Eurycoma longifolia Jack): a review on its ethnobotany and pharmacological importance. Fitoterapia, 81(7), 669–679. [CrossRef]
- Rehman, S. U. , Choe, K., & Yoo, H. H. (2016). Review on a Traditional Herbal Medicine, Eurycoma longifolia Jack (Tongkat Ali): Its Traditional Uses, Chemistry, Evidence-Based Pharmacology and Toxicology. Molecules (Basel, Switzerland). [CrossRef]
- Low, B. S. , Das, P. K., & Chan, K. L. (2013). Standardized quassinoid-rich Eurycoma longifolia extract improved spermatogenesis and fertility in male rats via the hypothalamic-pituitary-gonadal axis. Journal of ethnopharmacology, 145(3), 706–714. [CrossRef]
- Leisegang, K. , Finelli, R., Sikka, S. C., & Panner Selvam, M. K. (2022). Eurycoma longifolia (Jack) Improves Serum Total Testosterone in Men: A Systematic Review and Meta-Analysis of Clinical Trials. Medicina (Kaunas, Lithuania). [CrossRef]
- Henkel, R. R. , Wang, R., Bassett, S. H., Chen, T., Liu, N., Zhu, Y., & Tambi, M. I. (2014). Tongkat Ali as a potential herbal supplement for physically active male and female seniors--a pilot study. Phytotherapy research : PTR. [CrossRef]
- Khanijo, T. , & Jiraungkoorskul, W. (2016). Review Ergogenic Effect of Long Jack, Eurycoma Longifolia. Pharmacognosy reviews 10(20), 139–142. [CrossRef] [PubMed]
- George, A. , Udani, J., Abidin, N. Z., & Yusof, A. (2018). Efficacy and safety of Eurycoma longifolia(Physta®) water extract plus multivitamins on quality of life, mood and stress: a randomized placebo-controlled and parallel study. Food & nutrition research, 1374. [Google Scholar] [CrossRef]
- Insanu M, Pratiwi S N E, Fidrianny I. A review of the phytochemical compounds and pharmacological activities of Eurycoma longifolia. International Journal of Research in Pharmaceutical Sciences. (IJRPS), 1949.
- Mohamed, A. Farag, Abiodun O. Ajayi, Mohammed Taleb, KaiWang, and Iriny M. Ayoub. A Multifaceted Review of Eurycoma longifolia Nutraceutical Bioactives: Production, Extraction, and Analysis in Drugs and Biofluids. ACS Omega. 2023, 8, 1838−1850. [Google Scholar]
- Lee EL and Barnes, J. Tongkat Ali/Long Jack. https://www.publish.csiro.au/hc/pdf/HC22143. Journal of Primary Health Care. [CrossRef]
- Muniandy, S. , Yahya, H. M., Shahar, S., Kamisan Atan, I., Mahdy, Z. A., Rajab, N. F., George, A., & Chinnappan, S. M. (2023). Effects of Eurycoma longifolia Jack standardised water extract (Physta) on well-being of perimenopausal and postmenopausal women: protocol for a randomised, double-blinded, placebo-controlled, parallel group study. BMJ open. [CrossRef]
- Niamh Michail. 'Incorrect and misleading': Industry defends ashwagandha root extract safety amid Danish assessment. , 2023, https://www.vitafoodsinsights.com/botanicals-herbs/-incorrect-andmisleading- industry-defends-ashwagandha-root-extract-safety-amid-danish-assessment. 25 May.
- Indian Express. (2024). Denmark's ban on Ashwagandha lacks scientific rigor. Retrieved from https://indianexpress.com/article/cities/pune/denmarks-ban-on-ashwagandha-ban-based-on-2020-reportthat- lacks-scientific-rigor-says-ayush-ministry-9435664/. 2020.
- The Indian Herbal Pharmacopeia. Revised new edition 2002. Indian Drug manufacturers' association, Mumbai, pp. 467-478.
- Amritha, N. , Bhooma, V., & Parani, M. (2020). Authentication of the market samples of Ashwagandha by DNA barcoding reveals that powders are significantly more adulterated than roots. Journal of ethnopharmacology 256, 112725. [CrossRef] [PubMed]
- Balkrishna, A. , Mehta, A., & Yadav, D. (2024). Botanical risk misclassifications: Withania somnifera case review. Journal of Restorative and Alternative Medicine, Advance online publication.
- Anupama, N. , Rathi, P. Journal of Ayurveda and Integrative Medicine 14(1), 100420.
- Kalaivani, P. , Siva, R., Gayathri, V., & Langade, D. (2023). Ninety-day repeated dose toxicity of Ashwagandha (Withania somnifera) root extract in Wistar rats. Toxicology reports. [CrossRef]
- Tandon, N. , & Yadav, S. S. (2020). Safety and clinical effectiveness of Withania Somnifera (Linn.) Dunal root in human ailments. Journal of ethnopharmacology 255, 112768. [CrossRef]
- Verma, N. , Gupta, S. K., Tiwari, S., & Mishra, A. K. (2021). Safety of Ashwagandha Root Extract: A Randomized, Placebo-Controlled, study in Healthy Volunteers. Complementary therapies in medicine. [CrossRef]
- Vaidya, V. G. Gothwad, A., Ganu, G., Girme, A., Modi, S. J., & Hingorani, L. (2024). Clinical safety and tolerability evaluation of Withania somnifera (L.) Dunal (Ashwagandha) root extract in healthy human volunteers. Journal of Ayurveda and integrative medicine 15(1), 100859. [CrossRef] [PubMed]
- Mandlik Ingawale, D. S. , & Namdeo, A. G. (2021). Pharmacological evaluation of Ashwagandha highlighting its healthcare claims, safety, and toxicity aspects. Journal of dietary supplements 18(2), 183–226. [CrossRef]
- Paul, S. , Chakraborty, S., Anand, U., Dey, S., Nandy, S., Ghorai, M., Saha, S. C., Patil, M. T., Kandimalla, R., Proćków, J., & Dey, A. (2021). Withania somnifera (L.) Dunal (Ashwagandha): A comprehensive review on ethnopharmacology, pharmacotherapeutics, biomedicinal and toxicological aspects. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 143, 112175. [CrossRef]
- Mukherjee, P. K. , Banerjee, S., Biswas, S., Das, B., Kar, A., & Katiyar, C. K. (2021). Withania somnifera (L.) Dunal - Modern perspectives of an ancient Rasayana from Ayurveda. Journal of ethnopharmacology. [CrossRef]
- Mikulska, P. , Malinowska, M., Ignacyk, M., Szustowski, P., Nowak, J., Pesta, K., Szeląg, M., Szklanny, D., Judasz, E., Kaczmarek, G., Ejiohuo, O. P., Paczkowska-Walendowska, M., Gościniak, A., & Cielecka-Piontek, J. (2023). Ashwagandha (Withania somnifera)-Current Research on the Health-Promoting Activities: A Narrative Review. Pharmaceutics. [CrossRef]
- Khalid, M. U. , Sultan, M. T., Baig, I., Abbas, A., Noman, A. M., Zinedine, A., Bartkiene, E., & Rocha, J. M. (2025). A comprehensive review on the bioactivity and pharmacological attributes of Withania somnifera. Natural product research, 1–15. Advance online publication. [CrossRef]
- HMPC. EMA/HMPC/24186/2023. Assessment Report on Rhodiola rosea L., Rhizoma et Radix. Draft–Revision 1; Committee on Herbal Medicinal Products (HMPC): Amsterdam, The Netherlands, 2023; pp. 1–50. [Google Scholar]
- Punja, S. , Shamseer, L., Olson, K., & Vohra, S. (2014). Rhodiola rosea for mental and physical fatigue in nursing students: a randomized controlled trial. PloS one, 9(9), e108416. [CrossRef]
- Schröter, H.-B. , Neumann, D., Katritzky, A.R.; Swinbourne, F.J. Withasomnine. A pyrazole alkaloid from Withania somnifera Dun. Tetrahedron, 2895. [Google Scholar] [CrossRef]
- Rath, S. N. , Jena, L., Bhuyan, R., Mahanandia, N. C., & Patri, M. (2021). In silico discovery and evaluation of phytochemicals binding mechanism against human catechol-O-methyltransferase as a putative bioenhancer of L-DOPA therapy in Parkinson disease. Genomics & informatics, 19(1), e7. [CrossRef]
- Panossian A, Amaterdam J. 2017. Adaptogens in Psychiatric Practice. Chapter 8, Pp 155-181 in: Complementary and Integrative Treatments. Eds. PL. Gerbarg, PR. Muskin, RP. Brown, American Psychiatric Association Publishing. Arlington, 2018, 425 pages. https://www.appi.org/Complementary_and_Integrative_Treatments_in_Psychiatric_Practice.
- Akgul, Y. , Ferreira, D., Abourashed, E. A., & Khan, I. A. (2004). Lotaustralin from Rhodiola rosea roots. Fitoterapia, 75(6), 612–614. [CrossRef]
- Langeder, J. , Grienke, U., Döring, K., Jafari, M., Ehrhardt, C., Schmidtke, M., & Rollinger, J. M. (2021). High-performance Countercurrent Chromatography to Access Rhodiola rosea Influenza Virus Inhibiting Constituents. Planta medica, 87(10-11), 818–826. [CrossRef]
| Category | Pharmaceuticals | Dietary Supplements | ||
|---|---|---|---|---|
| RegulatoryOversight | Very strict | Lenient | ||
| Regulated by | Strictly regulated by drug authorities (e.g., FDA, EMA) | Loosely regulated; treated as a food category in many countries | ||
| Pre-market approval | Required (clinical trials, IND, NDA, etc.) | Not required; must follow labeling and safety guidelines. | ||
| Evidence of Safety and Efficacy | Required clinical + preclinical | Not required; General safety only | ||
| Efficacy | Must be proven through rigorous clinical trials | No requirement to prove efficacy before marketing | ||
| Safety | Extensive safety data required (nonclinical + clinical) | Only required to ensure general safety; no clinical trials mandated | ||
| Quality Standards | High | Moderate | ||
| Identity and purity | Must meet strict pharmacopoeial standards (e.g., USP, EP) | Less stringent, basic identity and purity testing is often enough | ||
| Standardization | Active ingredients must be quantified and consistent | Often contains a range of components; standardization is not always required | ||
| Contaminants (e.g., heavy metals, microbes) | Tightly controlled with established limits | Limits exist, but are less strictly enforced | ||
| Batch-to-batch consistency | Mandatory and validated | Expected but not strictly enforced | ||
| Manufacturing Requirements | Pharmaceutical grade, GMP | Food GMP | ||
| GMP Standards | Must follow pharmaceutical GMP (e.g., ICH Q7, EU GMP) | Must follow food-grade GMP (less stringent) | ||
| Process validation | Mandatory for all critical manufacturing steps | Not required for all processes | ||
| Change control & documentation. | Detailed documentation and validation are required. | Documentation is required, but it is generally simpler. | ||
| Consistencyof Botanical Source Specifics | Standardized | Variable | ||
| Botanical identity | Must be rigorously confirmed and controlled | Often confirmed, but methods may vary in rigor | ||
| Extraction process | Fully validated and standardized | May vary; often not standardized | ||
| Complex mixtures | Defined active constituents or fractions used | Often, a whole plant/extract with variable composition | ||
| Labeling Claims and Doses | Indications for use in diseases | Health supporting claims | ||
| Health claims | Medicine list "Dosage" based on age, weight, or condition.Can make therapeutic claims (e.g., "treats depression") | Supplements list "Suggested use" or "Serving size".Cannot make disease claims; only "structure/function" claims (e.g., "supports mood") | ||
| Labeling accuracy | Must match approved documentation | Must be truthful and not misleading, but with less scrutiny | ||
| Regulatory Classification |
|
|
||
| Purpose of Use | Medicines are designed for therapeutic effect and are often used for shorter-term or targeted purposes. | Supplements aim to provide nutritional support and are typically used on a long-term basis. | ||
| Aspect | FDA (U.S.) | EMA (EU) |
|---|---|---|
| Governing Bodies | - Pharmaceuticals: Center for Drug Evaluation and Research (CDER) - Supplements: Center for Food Safety and Applied Nutrition (CFSAN) | - Pharmaceuticals: European Medicines Agency (EMA) - Supplements: Regulated at member state level (e.g., Germany: BfArM, France: ANSM) |
| Applicable Legal Frameworks | - Drugs: FD&C Act, 21 CFR- Supplements: Dietary Supplement Health and Education Act (DSHEA, 1994) | - Drugs: Directive 2001/83/EC- Supplements: Food Supplements Directive (2002/46/EC), national laws |
| Botanical Drugs | Defined as botanical drug products, subject to full NDA or IND path (e.g., Veregen®, Mytesi®) | Herbal medicinal products (HMPs), classified into: - Well-established use (WEU) - Traditional use (THMP) - Full marketing authorization |
| Supplements (Botanical) | Treated as foods, not drugs. No pre-market approval. No efficacy proof required. | Also treated as foods, but the EU is more restrictive on claims. Heavily influenced by EFSA assessments. |
| Quality Standards for Botanicals | Encourages use of USP monographs and FDA Botanical Drug Guidance (2004). Must define active constituents or marker compounds. | Uses European Pharmacopoeia (Ph. Eur.) monographs. Strict on identity, purity, and standardization. The Herbal Medicinal Products Committee (HMPC) oversees scientific guidelines. |
| Clinical Evidence (Botanical Drugs) | IND → NDA process: requires full clinical trials unless eligible for accelerated approval. | WEU: requires published literature and some clinical data. THMP: based on 30 years of traditional use (15 in the EU), with nonclinical safety evidence only |
| Labeling (Supplements) | Structure/function claims allowed: "supports immune health." Must carry a disclaimer: "This product is not intended to diagnose, treat, cure, or prevent any disease." | Health claims reviewed and authorized by EFSA; therapeutic claims prohibited on supplements. Stricter than the FDA. |
| GMP | - Drugs: 21 CFR Part 210/211- Supplements: 21 CFR Part 111 | - Drugs: EU GMP (Annexes)- Supplements: Food GMP (varies by country); less |
| Unique Points | Allows botanical drug development via standard drug approval paths. Dietary supplements are widely available with relatively light regulation, provided safety is ensured. Botanical Drug Development Guidance has been available since 2004. |
Provides a specific regulatory framework for traditional herbal medicinal products (THMPs) via simplified registration. More centralized regulation of herbal drugs via EMA's HMPC. Supplements are subject to tighter control over labeling and claims, often stricter than in the U.S. |
| Example | ||
| Echinacea supplement | Dietary Supplement, no pre-market approval | Food supplement; cannot claim therapeutic effects |
| Echinacea extract as a medicine | Must go through the full IND/NDA process | Can qualify as THMP or WEU based on evidence and monograph |
| Country | PharmacopoeiaMonographs | Rhodiola | Ginseng | Withania | Eleuthero-coccus | Schisandra | Eurycoma | Sideritis |
|---|---|---|---|---|---|---|---|---|
| Russia | State Pharmacopoeia | ✅ | ✅ | ✅ | ✅ | |||
| China | Pharmacopoeia of PRC | ✅ | ✅ | ✅ | ✅ | |||
| European Union | European Pharmacopoeia/EMA/HMPC Union herbal monograph | ✅ | ✅ | ✅ | ✅ | ✅ | ✅ | |
| United States | USP, USP Herbal Compendium monograph, AHP | ✅ | ✅ | ⚠️ | ⚠️ | ⚠️ | ||
| Germany | Commission E | ✅ | ✅ | ⚠️ | ✅ | ✅ | ||
| UK | ⚠️ | |||||||
| Mongolia | Mongolian Pharmacopoeia | ✅ | ✅ | ✅ | ||||
| India | Indian Herbal Pharmacopeia | ✅ | ✅ | |||||
| Pakistan | Unani/Ayurvedic Pharmacopoeia | ✅ | ||||||
| Bangladesh | Unani Pharmacopoeia | ✅ | ||||||
| Sri Lanka | Ayurvedic Pharmacopoeia | ✅ | ||||||
| South Korea | Korean Herbal Pharmacopoeia | ✅ | ✅ | ✅ | ||||
| Japan | Japanese Pharmacopoeia | ✅ | ⚠️ | ✅ | ||||
| Vietnam | Vietnamese Pharmacopoeia | ✅ | ✅ | |||||
| Lanka | ||||||||
| Australia | Australian Register of Therapeutic Goods (ARTG) | ✅ | ||||||
| Malaysia | Malaysian Pharmacopoeia | ✅ | ||||||
| Indonesia | Indonesia Pharmacopoeia | ✅ | ||||||
| South Africa | CAM regulatory framework | ⚠️ | ||||||
| WHO | WHO Monographs | ✅ | ✅ | ✅ |
| Feature | Traditional Pharmacology | Network Pharmacology |
|---|---|---|
| Philosophy | Reductionist – targets one gene/protein | Systems-oriented – considers multitarget interactions |
| Target Focus | Single molecule | Multiple targets, often in networks |
| Drug Design Goal | High specificity | Modulation of networks/pathways |
| View of Disease | Caused by the dysfunction of a single entity | Disease as a network perturbation |
| Data Used | Experimental pharmacokinetics/dynamics | Multi-omics, PPI networks, computational modeling |
| Mechanism Identification | Binding affinity and downstream effects | Topological influence on biological networks |
| Predictive Capacity | Limited to known targets | Broader scope; includes off-targets, repurposing, synergy predictions |
| Herbal/TCM Suitability | Not applicable | Especially suitable due to the multi-component nature |
| Validation | Strong experimental support | Requires computational and experimental integration |
| Limitations | Ignores complexity and off-target effects | Data noise, oversimplified networks, context-ignorant models |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





