Submitted:
21 July 2025
Posted:
22 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Design and Participants
3. Results
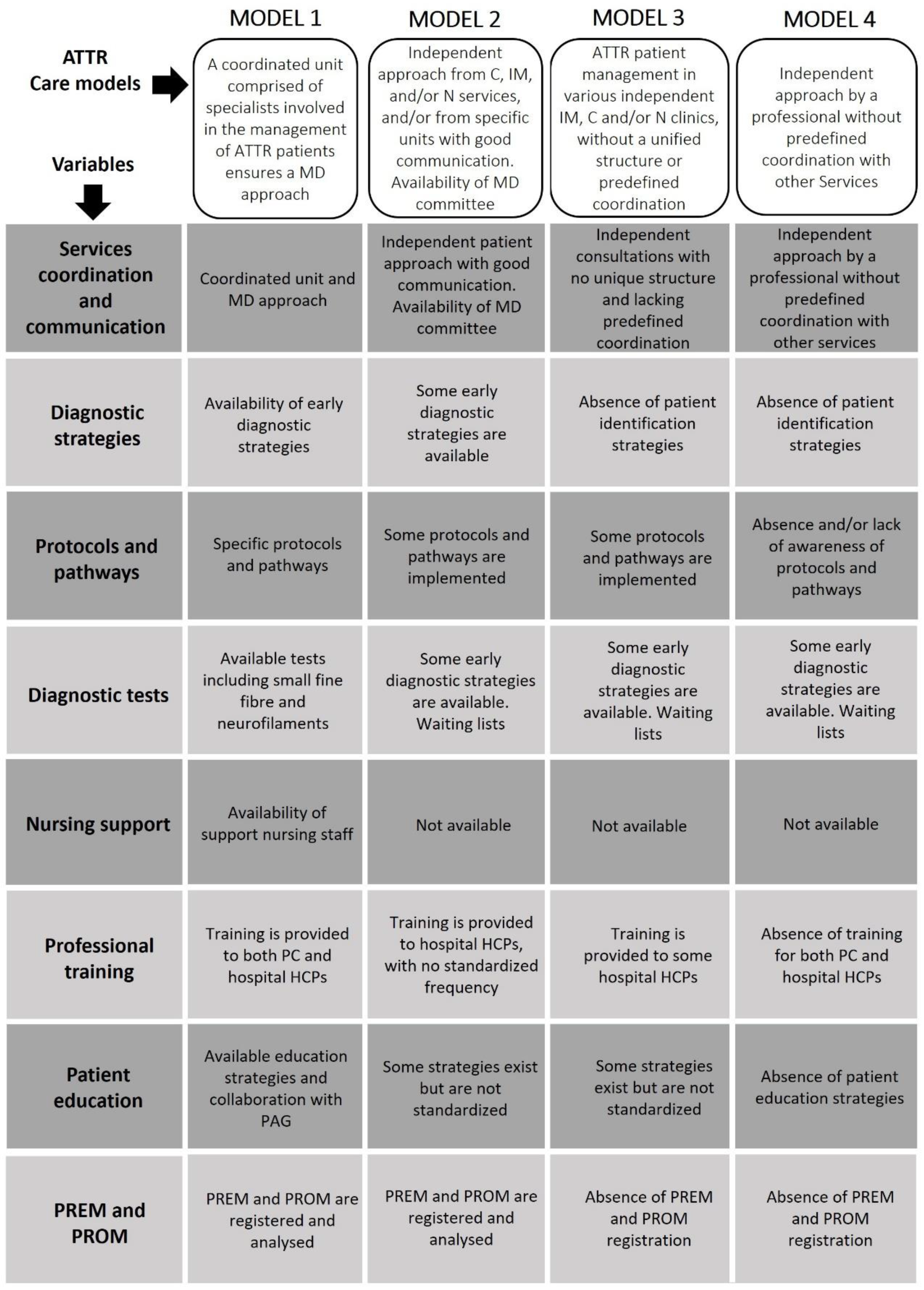
3.1. Characterization of ATTR Care Models
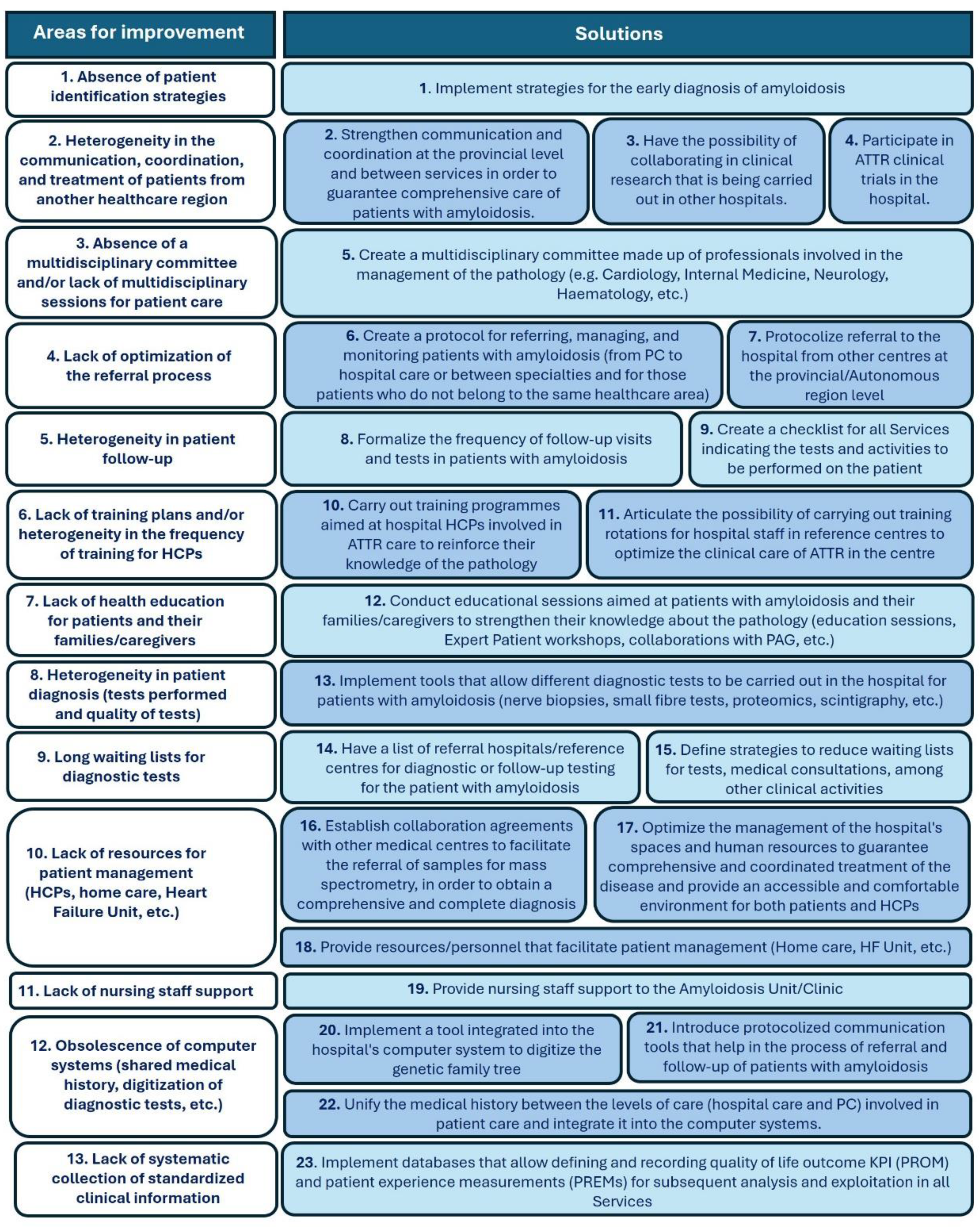
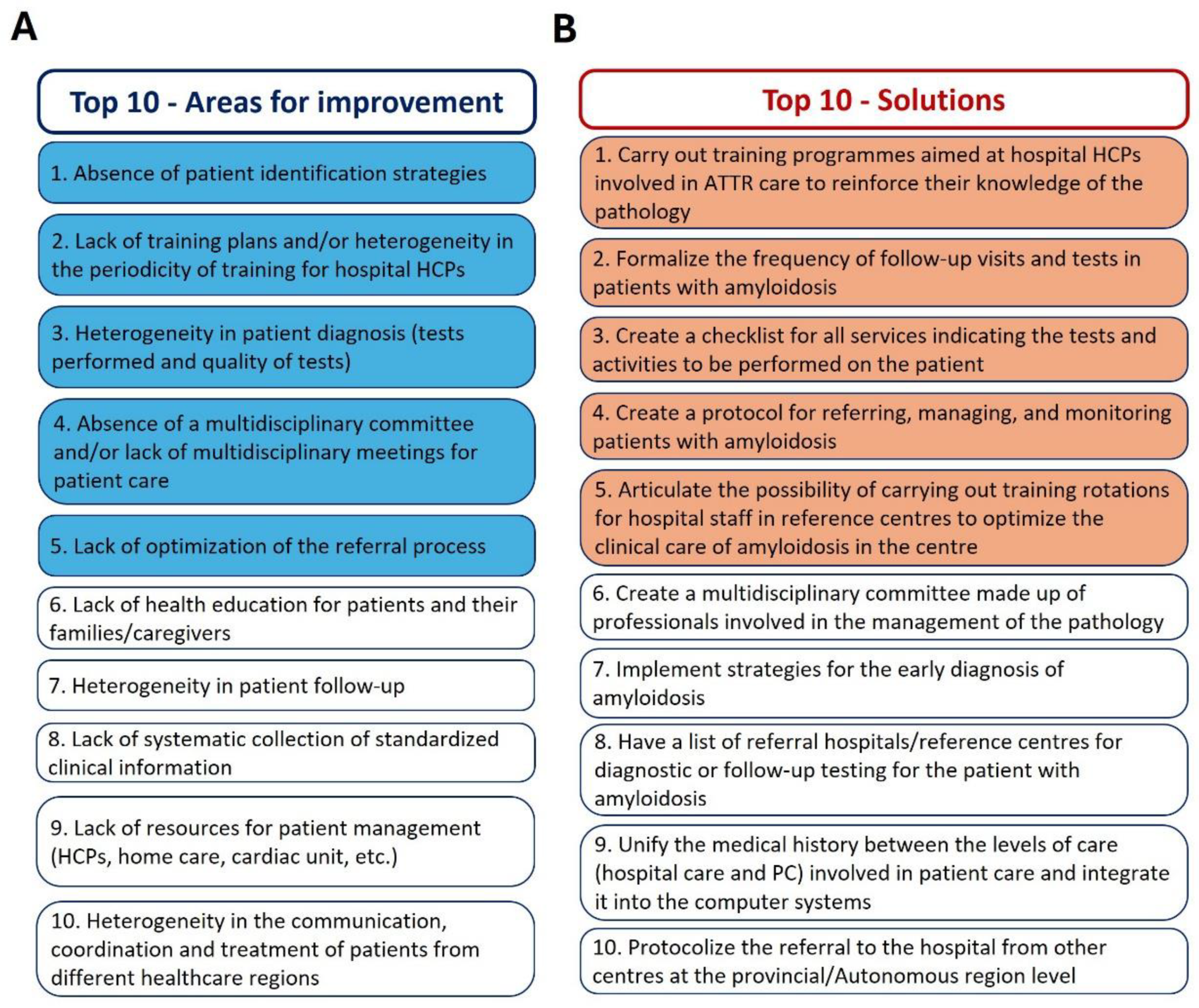
3.2. Indicators for the Evaluation of ATTR Care Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Appendix A. CARABELA ATTR Scientific Committee
References
- Yun, S.; González-Costello, J.; Formiga, F. Amiloidosis por transtiretina: lo que ahora vemos es solo la punta del iceberg. Revista Española de Geriatría y Gerontología 2020, 55, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Nativi-Nicolau, J.N.; Karam, C.; Khella, S.; Maurer, M.S. Screening for ATTR amyloidosis in the clinic: overlapping disorders, misdiagnosis, and multiorgan awareness. Heart Fail Rev 2022, 27, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Planté-Bordeneuve, V.; Ferreira, A.; Lalu, T.; Zaros, C.; Lacroix, C.; Adams, D.; Said, G. Diagnostic pitfalls in sporadic transthyretin familial amyloid polyneuropathy (TTR-FAP). Neurology 2007, 69, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Ando, Y.; Beirão, J.M.; Coelho, T.; Gertz, M.A.; Gillmore, J.D.; Hawkins, P.N.; Lousada, I.; Suhr, O.B.; Merlini, G. Expert consensus recommendations to improve diagnosis of ATTR amyloidosis with polyneuropathy. J Neurol 2021, 268, 2109–2122. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, P.N.; Ando, Y.; Dispenzeri, A.; Gonzalez-Duarte, A.; Adams, D.; Suhr, O.B. Evolving landscape in the management of transthyretin amyloidosis. Ann Med 2015, 47, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Rozenbaum, M.H.; Large, S.; Bhambri, R.; Stewart, M.; Whelan, J.; van Doornewaard, A.; Dasgupta, N.; Masri, A.; Nativi-Nicolau, J. Impact of Delayed Diagnosis and Misdiagnosis for Patients with Transthyretin Amyloid Cardiomyopathy (ATTR-CM): A Targeted Literature Review. Cardiol Ther 2021, 10, 141–159. [Google Scholar] [CrossRef] [PubMed]
- Reinés, J.B.; Vera, T.R.; Martín, M.U.; Serra, H.A.; Campins, M.M.C.; Millán, J.M.D.; Lezaun, C.G.; Cruz, M.R. Epidemiology of transthyretin-associated familial amyloid polyneuropathy in the Majorcan area: Son Llàtzer Hospital descriptive study. Orphanet Journal of Rare Diseases 2014, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Álvarez Rubio, J.; Manovel Sánchez, A.J.; González-Costello, J.; García-Pavía, P.; Limeres Freire, J.; García-Pinilla, J.M.; Zorio Grima, E.; García-Álvarez, A.; Valverde Gómez, M.; Espinosa Castro, M.Á.; et al. Characterization of hereditary transthyretin cardiac amyloidosis in Spain. Revista Española de Cardiología (English Edition) 2022, 75, 488–495. [Google Scholar] [CrossRef] [PubMed]
- González-Moreno, J.; Losada-López, I.; Cisneros-Barroso, E.; Garcia-Pavia, P.; González-Costello, J.; Muñoz-Beamud, F.; Campistol, J.M.; Fernandez-Torron, R.; Chapman, D.; Amass, L. A Descriptive Analysis of ATTR Amyloidosis in Spain from the Transthyretin Amyloidosis Outcomes Survey. Neurol Ther 2021, 10, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Zampino, S.; Sheikh, F.H.; Vaishnav, J.; Judge, D.; Pan, B.; Daniel, A.; Brown, E.; Ebenezer, G.; Polydefkis, M. Phenotypes Associated With the Val122Ile, Leu58His, and Late-Onset Val30Met Variants in Patients With Hereditary Transthyretin Amyloidosis. Neurology 2023, 100, e2036–e2044. [Google Scholar] [CrossRef] [PubMed]
- Gertz, M.A.; Benson, M.D.; Dyck, P.J.; Grogan, M.; Coelho, T.; Cruz, M.; Berk, J.L.; Plante-Bordeneuve, V.; Schmidt, H.H.J.; Merlini, G. Diagnosis, Prognosis, and Therapy of Transthyretin Amyloidosis. Journal of the American College of Cardiology 2015, 66, 2451–2466. [Google Scholar] [CrossRef] [PubMed]
- de Frutos, F.; Ochoa, J.P.; Gómez-González, C.; Reyes-Leiva, D.; Aróstegui, J.I.; Casasnovas, C.; Barriales-Villa, R.; Sevilla, T.; Gonzalez-Lopez, E.; Ramil, E.; et al. Phenotype and clinical outcomes of Glu89Lys hereditary transthyretin amyloidosis: a new endemic variant in Spain. Amyloid 2023, 30, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Hahn, V.S.; Yanek, L.R.; Vaishnav, J.; Ying, W.; Vaidya, D.; Lee, Y.Z.J.; Riley, S.J.; Subramanya, V.; Brown, E.E.; Hopkins, C.D.; et al. Endomyocardial Biopsy Characterization of Heart Failure With Preserved Ejection Fraction and Prevalence of Cardiac Amyloidosis. JACC Heart Fail 2020, 8, 712–724. [Google Scholar] [CrossRef] [PubMed]
- Connors, L.H.; Sam, F.; Skinner, M.; Salinaro, F.; Sun, F.; Ruberg, F.L.; Berk, J.L.; Seldin, D.C. Heart Failure Resulting From Age-Related Cardiac Amyloid Disease Associated With Wild-Type Transthyretin: A Prospective, Observational Cohort Study. Circulation 2016, 133, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Castaño, A.; Narotsky, D.L.; Hamid, N.; Khalique, O.K.; Morgenstern, R.; DeLuca, A.; Rubin, J.; Chiuzan, C.; Nazif, T.; Vahl, T.; et al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. European Heart Journal 2017, 38, 2879–2887. [Google Scholar] [CrossRef] [PubMed]
- González-López, E.; Gallego-Delgado, M.; Guzzo-Merello, G.; de Haro-del Moral, F.J.; Cobo-Marcos, M.; Robles, C.; Bornstein, B.; Salas, C.; Lara-Pezzi, E.; Alonso-Pulpon, L.; et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. European Heart Journal 2015, 36, 2585–2594. [Google Scholar] [CrossRef] [PubMed]
- González-Moreno, J.; Dispenzieri, A.; Grogan, M.; Coelho, T.; Tournev, I.; Waddington-Cruz, M.; Wixner, J.; Diemberger, I.; Garcia-Pavia, P.; Chapman, D.; et al. Clinical and Genotype Characteristics and Symptom Migration in Patients With Mixed Phenotype Transthyretin Amyloidosis from the Transthyretin Amyloidosis Outcomes Survey. Cardiol Ther 2024, 13, 117–135. [Google Scholar] [CrossRef] [PubMed]
- Gentile, L.; Coelho, T.; Dispenzieri, A.; Conceição, I.; Waddington-Cruz, M.; Kristen, A.; Wixner, J.; Diemberger, I.; Gonzalez-Moreno, J.; Cariou, E.; et al. A 15-year consolidated overview of data in over 6000 patients from the Transthyretin Amyloidosis Outcomes Survey (THAOS). Orphanet J Rare Dis 2023, 18, 350. [Google Scholar] [CrossRef] [PubMed]
- Swiecicki, P.L.; Zhen, D.B.; Mauermann, M.L.; Kyle, R.A.; Zeldenrust, S.R.; Grogan, M.; Dispenzieri, A.; Gertz, M.A. Hereditary ATTR amyloidosis: a single-institution experience with 266 patients. Amyloid 2015, 22, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Waddington-Cruz, M.; Wixner, J.; Amass, L.; Kiszko, J.; Chapman, D.; Ando, Y. Characteristics of Patients with Late- vs. Early-Onset Val30Met Transthyretin Amyloidosis from the Transthyretin Amyloidosis Outcomes Survey (THAOS). Neurol Ther 2021, 10, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Wixner, J.; Mundayat, R.; Karayal, O.N.; Anan, I.; Karling, P.; Suhr, O.B. THAOS: gastrointestinal manifestations of transthyretin amyloidosis - common complications of a rare disease. Orphanet J Rare Dis 2014, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Lovley, A.; Raymond, K.; Guthrie, S.D.; Pollock, M.; Sanchorawala, V.; White, M.K. Patient-reported burden of hereditary transthyretin amyloidosis on functioning and well-being. J Patient Rep Outcomes 2021, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.M.; Rosenthal, J.L.; Grodin, J.L.; Maurer, M.S.; Grogan, M.; Cheng, R.K. ATTR Amyloidosis: Current and Emerging Management Strategies: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol 2021, 3, 488–505. [Google Scholar] [CrossRef] [PubMed]
- Witteles, R.M.; Bokhari, S.; Damy, T.; Elliott, P.M.; Falk, R.H.; Fine, N.M.; Gospodinova, M.; Obici, L.; Rapezzi, C.; Garcia-Pavia, P. Screening for Transthyretin Amyloid Cardiomyopathy in Everyday Practice. JACC Heart Fail 2019, 7, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Damy, T.; Kristen, A.V.; Suhr, O.B.; Maurer, M.S.; Planté-Bordeneuve, V.; Yu, C.R.; Ong, M.L.; Coelho, T.; Rapezzi, C. Transthyretin cardiac amyloidosis in continental Western Europe: an insight through the Transthyretin Amyloidosis Outcomes Survey (THAOS). Eur Heart J 2022, 43, 391–400. [Google Scholar] [CrossRef] [PubMed]
- González-Duarte, A.; Conceição, I.; Amass, L.; Botteman, M.F.; Carter, J.A.; Stewart, M. Impact of Non-Cardiac Clinicopathologic Characteristics on Survival in Transthyretin Amyloid Polyneuropathy. Neurol Ther 2020, 9, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Gertz, M.; Adams, D.; Ando, Y.; Beirão, J.M.; Bokhari, S.; Coelho, T.; Comenzo, R.L.; Damy, T.; Dorbala, S.; Drachman, B.M.; et al. Avoiding misdiagnosis: expert consensus recommendations for the suspicion and diagnosis of transthyretin amyloidosis for the general practitioner. BMC Fam Pract 2020, 21, 198. [Google Scholar] [CrossRef] [PubMed]
- Benson, M.D.; Dasgupta, N.R.; Rao, R. Diagnosis and Screening of Patients with Hereditary Transthyretin Amyloidosis (hATTR): Current Strategies and Guidelines. Therapeutics and clinical risk management 2020, 16, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis: a position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2021, 42, 1554–1568. [Google Scholar] [CrossRef] [PubMed]
- Conceição, I.; Coelho, T.; Rapezzi, C.; Parman, Y.; Obici, L.; Galán, L.; Rousseau, A. Assessment of patients with hereditary transthyretin amyloidosis – understanding the impact of management and disease progression. Amyloid 2019, 26, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Silva-Hernández, L.; Horga Hernández, A.; Valls Carbó, A.; Guerrero Sola, A.; Montalvo-Moraleda, M.T.; Galán Dávila, L. Red flags in patients with hereditary transthyretin amyloidosis at diagnosis in a non-endemic area of Spain. Neurologia (Engl Ed) 2023, 38, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Tschöpe, C.; Elsanhoury, A. Treatment of Transthyretin Amyloid Cardiomyopathy: The Current Options, the Future, and the Challenges. Journal of Clinical Medicine 2022, 11, 2148. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.S.; Schwartz, J.H.; Gundapaneni, B.; Elliott, P.M.; Merlini, G.; Waddington-Cruz, M.; Kristen, A.V.; Grogan, M.; Witteles, R.; Damy, T.; et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N Engl J Med 2018, 379, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Benson, M.D.; Waddington-Cruz, M.; Berk, J.L.; Polydefkis, M.; Dyck, P.J.; Wang, A.K.; Planté-Bordeneuve, V.; Barroso, F.A.; Merlini, G.; Obici, L.; et al. Inotersen Treatment for Patients with Hereditary Transthyretin Amyloidosis. N Engl J Med 2018, 379, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Gonzalez-Duarte, A.; O'Riordan, W.D.; Yang, C.C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N Engl J Med 2018, 379, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Graban, M.; Toussaint, J. Lean Hospitals: Improving Quality, Patient Safety, and Employee Engagement; 2018.
- Escalada, J.; Carretero Gomez, J.; Anguita, M.; de Sequera, P.; García-Río, F.; Dávila, I.; Soto Bonel, J.F.; García, J.P.; Rodríguez Ledo, P.; Barrena, E.; et al. Enhancing the management of chronic diseases in clinical practice: The CARABELA methodology. J Healthc Qual Res 2024. [Google Scholar] [CrossRef] [PubMed]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies: Developed by the task force on the management of cardiomyopathies of the European Society of Cardiology (ESC). European Heart Journal 2023, 44, 3503–3626. [Google Scholar] [CrossRef] [PubMed]
- Dorbala, S.; Ando, Y.; Bokhari, S.; Dispenzieri, A.; Falk, R.H.; Ferrari, V.A.; Fontana, M.; Gheysens, O.; Gillmore, J.D.; Glaudemans, A.; et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: Part 1 of 2-evidence base and standardized methods of imaging. J Nucl Cardiol 2019, 26, 2065–2123. [Google Scholar] [CrossRef] [PubMed]
- Kittleson, M.M.; Maurer, M.S.; Ambardekar, A.V.; Bullock-Palmer, R.P.; Chang, P.P.; Eisen, H.J.; Nair, A.P.; Nativi-Nicolau, J.; Ruberg, F.L. Cardiac Amyloidosis: Evolving Diagnosis and Management: A Scientific Statement From the American Heart Association. Circulation 2020, 142. [Google Scholar] [CrossRef] [PubMed]
- Moody, W.E.; Turvey-Haigh, L.; Knight, D.; Coats, C.J.; Cooper, R.M.; Schofield, R.; Robinson, S.; Harkness, A.; Oxborough, D.L.; Gillmore, J.D.; et al. British Society of Echocardiography guideline for the transthoracic echocardiographic assessment of cardiac amyloidosis. Echo Res Pract 2023, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Casado, J.; Pérez-Silvestre, J.; Salamanca, P.; Llàcer, P.; Quirós, R.; Ruiz-Hueso, R.; Méndez, M.; Manzano, L.; Formiga, F. Sospecha clínica, diagnóstico y seguimiento de la amiloidosis cardíaca: documento de actualización y resumen ejecutivo. Revista Clínica Española 2024, 224, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.S.; Moody, W.E. The importance of pathways to facilitate early diagnosis and treatment of patients with cardiac amyloidosis. Ther Adv Cardiovasc Dis 2023, 17, 17539447231216318. [Google Scholar] [CrossRef] [PubMed]
- Brailovsky, Y.; Rajapreyar, I.; Alvarez, R. TTR Amyloidosis: Current State of Affairs and Promise for the Future. JACC Case Rep 2023, 10, 101759. [Google Scholar] [CrossRef] [PubMed]
- Obici, L.; Callaghan, R.; Ablett, J.; Bibiloni, C.; Bueser, T.; Conceição, I.; Dongiglio, F.; Farrugia, A.; Knebel, F.; Lane, T.; et al. Consensus recommendations on holistic care in hereditary ATTR amyloidosis: an international Delphi survey of patient advocates and multidisciplinary healthcare professionals. BMJ Open 2023, 13, e073130. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Okumura, T.; Murohara, T.; Katsuno, M. Multidisciplinary Approaches for Transthyretin Amyloidosis. Cardiol Ther 2021, 10, 289–311. [Google Scholar] [CrossRef] [PubMed]
- Losada, I.; González-Moreno, J.; Rodriguez, A.; Uson, M.; Ripoll-Vera, T.; Ferrer-Nadal, A.; Rigo, E.; Andreu, H.; Figuerola, A.; Montalà, J.C.; et al. Multidisciplinary approach in the management of hATTR. Eur J Clin Invest 2020, 50, e13296. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Kittleson, M.M.; Wechalekar, A.D.; Alvarez-Cardona, J.; Mitchell, J.D.; Scarlatelli Macedo, A.V.; Dutra, J.P.P.; Campbell, C.M.; Liu, J.E.; Landau, H.J.; et al. Moving towards establishing centres of excellence in cardiac amyloidosis: an International Cardio-Oncology Society statement. Heart 2024. [Google Scholar] [CrossRef] [PubMed]
- Bumma, N.; Kahwash, R.; Parikh, S.V.; Isfort, M.; Freimer, M.; Vallakati, A.; Redder, E.; Campbell, C.M.; Sharma, N.; Efebera, Y.; et al. Multidisciplinary amyloidosis care in the era of personalized medicine. Front Neurol 2022, 13, 935936. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Reilly, M.M.; Coats, C.J.; Cooper, R.; Cox, H.; Coyne, M.R.E.; Green, A.J.; McGowan, R.; Moody, W.E.; Hawkins, P.N. Clinical and Genetic Evaluation of People with or at Risk of Hereditary ATTR Amyloidosis: An Expert Opinion and Consensus on Best Practice in Ireland and the UK. Adv Ther 2022, 39, 2292–2301. [Google Scholar] [CrossRef] [PubMed]
- Peral, C.; Formiga, F.; García, P.; Martín-Sánchez, J.; Navarro-Ruiz, A.; Tarilonte, P.; López, A.; Rubio-Rodriguez, D.; Rubio-Terrés, C. PCV24 Health and Economic IMPACT of the Correct Diagnosis of Transthyretin Cardiac Amyloidosis in Spain. Value in Health 2020, 23, S490–S491. [Google Scholar] [CrossRef]
- Stewart, M.; Shaffer, S.; Murphy, B.; Loftus, J.; Alvir, J.; Cicchetti, M.; Lenderking, W.R. Characterizing the High Disease Burden of Transthyretin Amyloidosis for Patients and Caregivers. Neurol Ther 2018, 7, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Giblin, G.T.; Cuddy, S.A.M. Multimodality Imaging in Cardiac Amyloidosis. Curr Cardiol Rep 2021, 23, 134. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Naharro, A.; Baksi, A.J.; Hawkins, P.N.; Fontana, M. Diagnostic imaging of cardiac amyloidosis. Nat Rev Cardiol 2020, 17, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Perez, R.; Vázquez-García, R.; Barge-Caballero, G.; Bouzas-Mosquera, A.; Soler-Fernandez, R.; Larrañaga-Moreira, J.M.; Crespo-Leiro, M.G.; Vazquez-Rodriguez, J.M. Diagnostic and prognostic value of cardiac imaging in amyloidosis. World J Cardiol 2020, 12, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Shouman, K.; Broski, S.M.; Muchtar, E.; Pendleton, C.A.; Johnson, G.B.; Tracy, J.; Engelstad, J.K.; Spinner, R.J.; Dyck, P.J.B. Novel imaging techniques using (18) F-florbetapir PET/MRI can guide fascicular nerve biopsy in amyloid multiple mononeuropathy. Muscle Nerve 2021, 63, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.S.; Elliott, P.; Comenzo, R.; Semigran, M.; Rapezzi, C. Addressing Common Questions Encountered in the Diagnosis and Management of Cardiac Amyloidosis. Circulation 2017, 135, 1357–1377. [Google Scholar] [CrossRef] [PubMed]
- Rintell, D.; Heath, D.; Braga Mendendez, F.; Cross, E.; Cross, T.; Knobel, V.; Gagnon, B.; Turtle, C.; Cohen, A.; Kalmykov, E.; et al. Patient and family experience with transthyretin amyloid cardiomyopathy (ATTR-CM) and polyneuropathy (ATTR-PN) amyloidosis: results of two focus groups. Orphanet J Rare Dis 2021, 16, 70. [Google Scholar] [CrossRef] [PubMed]
- Pack, A.P.; Zuleta, A.; Daugerdas, E.; Huang, W.; Batio, S.; Svoboda, S.; Zeitler, E.P.; Kumar, N.; Watt, S.; Fernandez-Arias, M.I.; et al. Developing, optimizing, and evaluating patient infographics for diagnosing cardiac amyloidosis. PEC Innovation 2023, 3, 100212. [Google Scholar] [CrossRef] [PubMed]
- FEDER. AMILO presenta en Palma su decálogo para mejorar la calidad de vida de los pacientes con amiloidosis. https://www.enfermedades-raras.org/movimiento-asociativo/actualidad-asociativa/amilo-presenta-en-palma-su-decalogo-para-mejorar-la-calidad-de-vida-de-los-pacientes-con-amiloidosis. 2023.
- Siu, A.; Sierra, I.; Zhang, M.; Dranow, L.; Hong, J.; Waldron, J.; Caballero, K.; Greene, T.; Kovacsovics, T.; Stehlik, J.; et al. Patient Reported Outcomes In Amyloidosis Cardiomyopathy. Journal of Cardiac Failure 2020, 26, S116–S117. [Google Scholar] [CrossRef]
- Aimo, A.; Rapezzi, C.; Perfetto, F.; Cappelli, F.; Palladini, G.; Obici, L.; Merlini, G.; Di Bella, G.; Serenelli, M.; Zampieri, M.; et al. Quality of life assessment in amyloid transthyretin (ATTR) amyloidosis. Eur J Clin Invest 2021, 51, e13598. [Google Scholar] [CrossRef] [PubMed]
- Aimo, A.; Teresi, L.; Castiglione, V.; Picerni, A.L.; Niccolai, M.; Severino, S.; Agazio, A.; Carnevale Baraglia, A.; Obici, L.; Palladini, G.; et al. Patient-reported outcome measures for transthyretin cardiac amyloidosis: the ITALY study. Amyloid 2024, 31, 52–61. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




