1. Introduction
Hypertension remains one of the most prevalent and pressing global health challenges, contributing significantly to cardiovascular morbidity and mortality (Kumar et al., 2022). Despite the widespread availability of pharmacological treatments, their limitations—including hepatic metabolism, reduced bioavailability, and adverse effects—often result in poor adherence and suboptimal outcomes. These challenges underscore the urgent need for innovative therapeutic strategies that are both effective and well-tolerated.
Molecular hydrogen (H₂), a small, colorless, and odorless gas, has emerged as a potential therapeutic agent owing to its selective antioxidant, anti-inflammatory, and anti-apoptotic properties (Xie et al., 2023; Zhang et al., 2018; Saengsin et al., 2023). Studies have demonstrated its benefits in cardiovascular and metabolic disorders through mechanisms such as scavenging reactive oxygen species (ROS), modulating gene expression, and regulating cellular processes like autophagy and apoptosis (Barancik et al., 2020). H₂ can be administered via inhalation, hydrogen-rich water, or hydrogen-rich saline, but its therapeutic application is limited by delivery and stability challenges.
Nanobubble technology offers a promising solution by encapsulating therapeutic gases in nanoscale gas-filled bubbles, thereby enhancing their bioavailability, stability, and cellular uptake (Kumar et al., 2022). In the context of hypertension, nanotechnology-based delivery systems have shown potential to overcome barriers faced by conventional therapies. The combination of molecular hydrogen and nanobubbles, referred to as oxyhydrogen nanobubble (NB-HHO) therapy, represents a novel approach that may potentiate the therapeutic effects of H₂ while mitigating delivery limitations (Kumar et al., 2022; Xie et al., 2023). This case report describes the clinical response to NB-HHO therapy in a patient with Stage 1 hypertension, highlighting its potential as a safe and effective non-pharmacological intervention.
2. Case Presentation
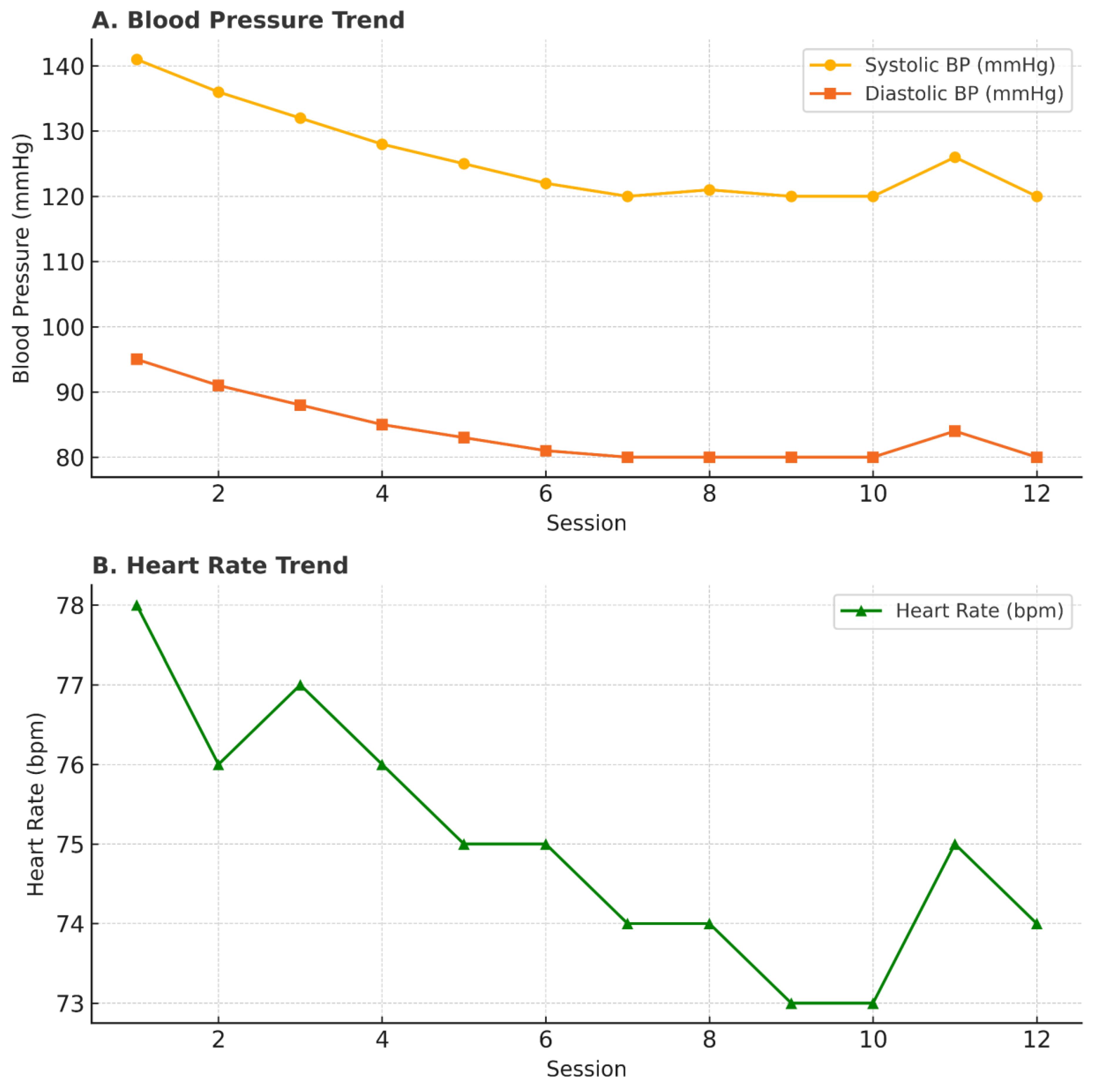
A 41-year-old male presented with a 10-year history of untreated hypertension, reporting persistent morning neck stiffness as his primary symptom. His baseline blood pressure (BP) was 141/95 mmHg, and heart rate was within normal limits. He declined pharmacological therapy due to concerns about long-term side effects and opted for an alternative approach. The patient underwent 12 sessions of NB-HHO therapy at our clinic over a period of four weeks. Each session involved intravenous infusion of NB-HHO in escalating doses (5–20 mL) combined with supplemental oxygen inhalation. No changes were made to his diet or physical activity levels during this period. BP showed a progressive decline over the first five sessions and stabilized at an average of 120/80 mmHg by session 10 (
Table 1).
A slight increase was noted in session 11 (attributed to work-related stress) but normalized by session 12 (
Figure 1). Heart rate remained stable throughout, and no adverse events were reported. The patient continued without conventional antihypertensive medications during and after therapy.
3. Discussion
This case highlights the potential of Hydrogen-Oxygen Nanobubble (NB-HHO) therapy as a non-pharmacological intervention for Stage 1 hypertension. The patient experienced progressive normalization of blood pressure (BP) over 12 sessions, without the need for conventional antihypertensives or any reported adverse effects. This favorable response underscores the therapeutic promise of molecular hydrogen (H₂) and nanobubble technology in managing cardiovascular risk.
Hypertension is a well-established contributor to global cardiovascular morbidity and mortality, responsible for over 50% of cases of stroke and heart disease (Zhou et al., 2021). Conventional therapies, while effective, often suffer from poor adherence due to adverse effects, polypharmacy, and the long-term commitment required. This necessitates exploring alternative strategies that are both efficacious and well-tolerated. Molecular hydrogen has emerged as a compelling candidate in this regard, offering selective antioxidant properties and minimal toxicity (Ohta, 2015).
H₂’s therapeutic effects are attributed to its ability to scavenge cytotoxic reactive oxygen species (ROS), particularly hydroxyl radicals (•OH) and peroxynitrite (ONOO⁻), without interfering with physiological ROS required for cellular signaling (Ohta, 2015). It also upregulates endogenous antioxidant defenses such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), contributing to redox homeostasis (Ichihara et al., 2021). Moreover, H₂ modulates inflammatory pathways by inhibiting NF-κB signaling and activating Nrf2 transcription, enhancing cellular resilience to oxidative stress (Chen et al., 2024). In the vascular context, H₂ improves endothelial function by increasing nitric oxide (NO) bioavailability and reducing systemic vascular resistance. These mechanisms may explain the gradual BP reduction and stabilization observed in this case. The adjunctive use of O₂ inhalation likely augmented mitochondrial bioenergetics and improved aerobic metabolism, indirectly contributing to autonomic balance and vascular tone regulation.
The delivery of H₂ via nanobubbles represents a technological advance, addressing challenges of poor solubility and rapid diffusion that have limited its clinical translation. Nanobubbles enhance gas stability and facilitate targeted delivery at the microvascular level (Ichihara et al., 2021). This synergy between molecular hydrogen and nanotechnology potentially amplifies therapeutic effects and improves patient compliance by reducing treatment frequency and invasiveness.
Although limited to a single case, the absence of adverse events and the sustained BP normalization suggest that NB-HHO therapy may be a viable adjunct or alternative in early-stage hypertension, particularly for patients reluctant to initiate pharmacotherapy. These findings align with preclinical and clinical studies demonstrating the cardiovascular benefits of molecular hydrogen (Ichihara et al., 2021; Ohta, 2015; Chen et al., 2024). However, the limitations must be acknowledged. This is an observational report without a control group, and biomarkers of oxidative stress and endothelial function were not assessed. Future studies should include randomized controlled trials with larger cohorts, standardized dosing regimens, and mechanistic endpoints to validate efficacy and elucidate underlying pathways.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Barancik, M., Kura, B., LeBaron, T.W., Bolli, R., Buday, J. and Slezak, J., 2020. Molecular and cellular mechanisms associated with effects of molecular hydrogen in cardiovascular and central nervous systems. Antioxidants, 9(12), p.1281. [CrossRef]
- Chen, J.Y., Yang, Y.J., Meng, X.Y., Lin, R.H., Tian, X.Y., Zhang, Y., Lai, W.F., Yang, C., Ma, X.Q. and Huang, M.Q., 2024. Oxysophoridine inhibits oxidative stress and inflammation in hepatic fibrosis via regulating Nrf2 and NF-κB pathways. Phytomedicine, 132, p.155585. [CrossRef]
- Ichihara, M., Sobue, S., Ito, M., Ito, M., Hirayama, M. and Ohno, K., 2015. Beneficial biological effects and the underlying mechanisms of molecular hydrogen-comprehensive review of 321 original articles. Medical gas research, 5, pp.1-21. [CrossRef]
- Kumar, G., Virmani, T., Pathak, K. and Alhalmi, A., 2022. A revolutionary blueprint for mitigation of hypertension via nanoemulsion. BioMed Research International, 2022(1), p.4109874. [CrossRef]
- Ohta, S., 2015. Molecular hydrogen as a novel antioxidant: overview of the advantages of hydrogen for medical applications. Methods in enzymology, 555, pp.289-317. [CrossRef]
- Saengsin, K., Sittiwangkul, R., Chattipakorn, S.C. and Chattipakorn, N., 2023. Hydrogen therapy as a potential therapeutic intervention in heart disease: From the past evidence to future application. Cellular and Molecular Life Sciences, 80(6), p.174.
- Xie, F., Song, Y., Yi, Y., Jiang, X., Ma, S., Ma, C., Li, J., Zhanghuang, Z., Liu, M., Zhao, P. and Ma, X., 2023. Therapeutic potential of molecular hydrogen in metabolic diseases from bench to bedside. Pharmaceuticals, 16(4), p.541. [CrossRef]
- Zhang, Y., Tan, S., Xu, J. and Wang, T., 2018. Hydrogen therapy in cardiovascular and metabolic diseases: from bench to bedside. Cellular Physiology and Biochemistry, 47(1), pp.1-10. [CrossRef]
- Zhou, B., Perel, P., Mensah, G.A. and Ezzati, M., 2021. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nature Reviews Cardiology, 18(11), pp.785–802. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).