Submitted:
18 July 2025
Posted:
21 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Sampling Sites
2.2. Dinophysis Acuminata Winter 2020 Bloom Analysis at Torquay Canal, Rehoboth Beach, DE
2.2.1. Field Collection
2.2.2. Laboratory Sample Processing2.2.2. a. Microscopy
2.2.2. b. Chlorophyll-a
2.2.3. Transect DNA Processing, Preservation, and Analysis
2.2.4. Dissolved Chemical Nutrient Analysis
2.2.5. Statistical Analysis
2.3. Benthic Marine Sediment Monitoring for Dinophysis acuminata Using Molecular Methods
2.3.1. Field Sample and Data Collection
2.3.2. Dissolved Chemical Nutrient Analysis
2.3.3. Molecular Methods to Determine D. acuminata Presence in Sediment Samples
2.3.4. Statistical Analysis
3. Results
3.1. Dinophysis Acuminata Winter 2020 Bloom Analysis at Torquay Canal, Rehoboth Beach, DE
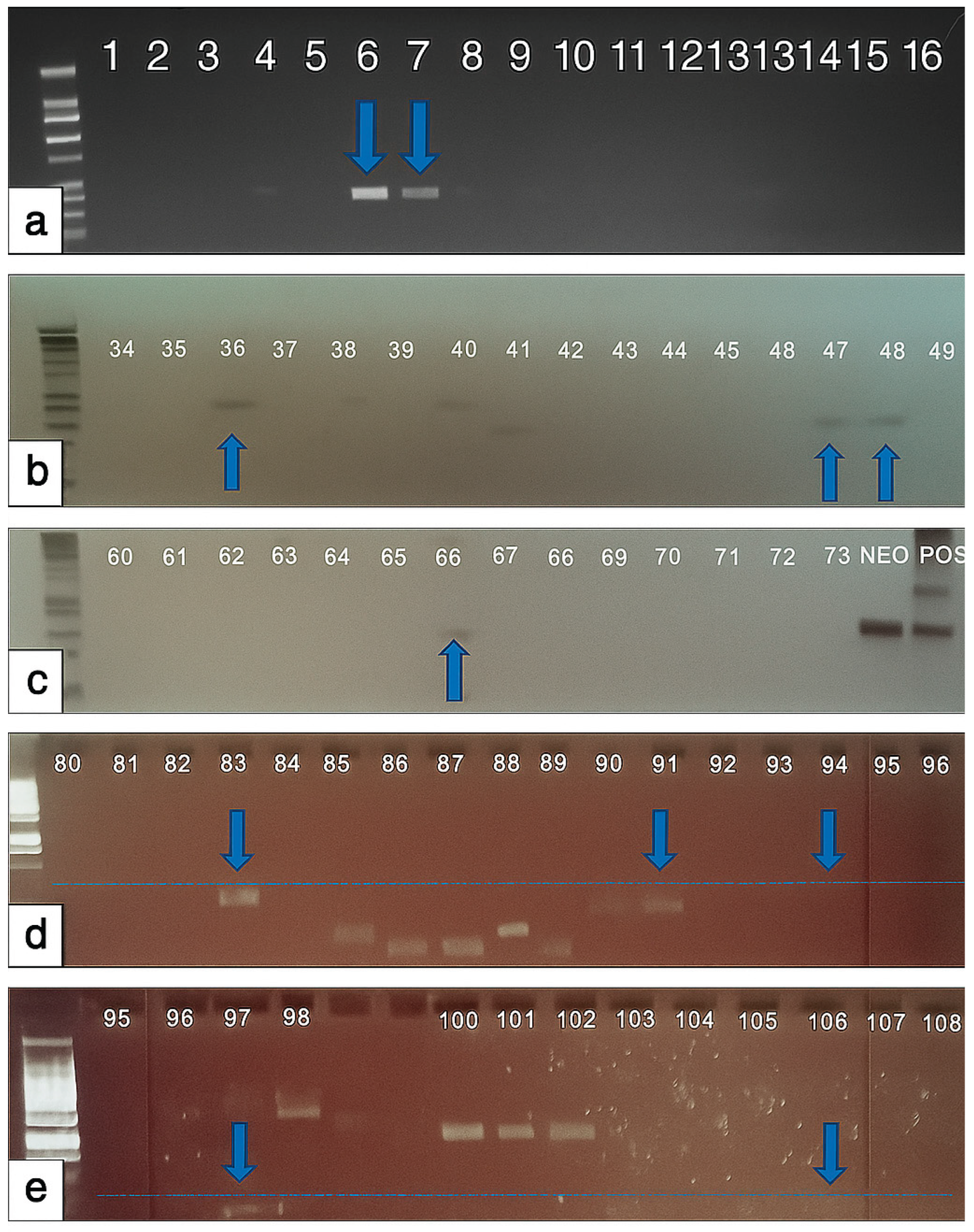
3.2. Benthic Marine Sediment Monitoring for Dinophysis acuminata Using Molecular Methods
4. Discussion
4.1. Project Limitations and Future Recommendations
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benedetti, F., Jalabert, L., Sourisseau, M., Becker, B., Cailliau, C., Desnos, C., Elineau, A., Irisson, J.O., Lombard, F., Picheral, M., Stemmann, L. & Pouline P. 2019.The seasonal and interannual fluctuations of plankton abundance and community structure in a North Atlantic Marine Protected Area. Front. Mar. Sci. 6:214. [CrossRef]
- Kimambo, O.N., Gumbo, J.R., & Chikoore, H. 2019. The occurrence of cyanobacteria blooms in freshwater ecosystems and their link with hydro-meterological and environmental variations in Tanzania. Heliyon 5(3): e01312. [CrossRef]
- Barton, A., Pershing, A.J., Litchman, E., Record, N., Edwards, K.F., Finkel, Z., Kiørboe, T. & Ward, B. 2013. The biogeography of marine plankton traits. Ecology Letters 16(4):522- 534. [CrossRef]
- Gallegos, C.L. & Bergstrom, P.W. 2005. Effects of a Prorocentrum minimum bloom on light availability for and potential impacts on submersed aquatic vegetation in upper Chesapeake Bay. Harmful Algae 4 (3):553-574. [CrossRef]
- Van Dolah, F.M. 2000. Marine algal toxins: origins, health effects, and their increased occurrence. Environmental Health Perspectives (108) Suppl 1: 133-41.
- Deeds, J.R., Wiles, K., Heideman, G.B., White, K.D. & Abraham, A. 2010. First US report of shellfish harvesting closures due to confirmed okadaic acid in Texas Gulf coast oysters. Toxicon 55: 1138-1146. [CrossRef]
- Garcia-Portela, M., Reguera, B., Sibat, M., Altenburger, A., Rodriguez, F., & Hess, P. 2018. Metabolomic Profiles of Dinophysis acuminata and Dinophysis acuta Using Non-Targeted High-Resolution Mass Spectrometry: Effect of Nutritional Status and Prey. Marine Drugs 16(143), 1–25. [CrossRef]
- Lloyd, J.K., Duchin, J.S., Borchert, J., Quintana, H.F. & Robertson, A. 2013. Diarrhetic Shellfish Poisoning, Washington, USA, 2011. Emerg. Infect. Dis. 19:. [CrossRef]
- Yasumoto, T., Oshima, Y., Sugawara, W., Fukuyo, Y., Oguri, H., Igarashi, T., Fujita, N. 1980. Identification of Dinophysis fortii as the causative organism of diarrhetic shellfish poisoning. Nippon Suisan Gakkaishi 46(11): 1405-1411. [CrossRef]
- Quilliam, M.A., Mojmir, J., Lawrence, J.F. 1993. Characterization of the oxidation products of paralytic shellfish poisoning toxins by liquid chromatography/mass spectrometry. Analytical Science Advances 7(6): e1290070616. [CrossRef]
- Hossen, V., Jourdan da Silva, N., Guillos-Becel, Y., Marchal, J. & Krys, S. 2011. Food poisoning outbreaks linked to mussels contaminated with okadaic acid and dinophystoxin-3 in France, June 2009 Eurosurveill 16(46):20020. [CrossRef]
- Reizopoulou, S., Strogyloudi, E., Giannakourou, A., Pagou, K., Hatzianestis, I., Pyrgaki, C. & Graneli, E. 2008. Okadaic acid accumulation in macrofilter feeders subjected to natural blooms of Dinophysis acuminata. Harm. Algae 7: 228-234. [CrossRef]
- Torgersen, T., Aasen, J., and Aune, T. 2005. Diarrhetic shellfish poisoning by okadaic acid esters from Brown crabs (Cancer pagurus) in Norway. Toxicon 46(5): 572-578. [CrossRef]
- Reguera, B., Riobo, P., Rodriguez, F., Diaz, P., Pizarro, G., Paz, B., Franco, J. M. &Blanco, J. 2014. Dinophysis Toxins: Causative Organisms, Distribution and Fate in Shellfish. Marine Drugs 12(1): 394-461.
- Koukaras, K. & Nikolaidis, G. 2004. Dinophysis blooms in Greek coastal waters (Thermaikos Gulf, NW Aegean Sea). J. Plankton Rs 26 (4):445-457. [CrossRef]
- Hattenrath-Lehmann, T.K., Marcoval, M.A., Berry, D.L., Fire, S., Wang, Z., Morton, S.L., & Gobler, C.J. 2013. The emergence of Dinophysis acuminata blooms and DSP toxins in shellfish in New York waters. Harmful Algae, 26: 33-44. [CrossRef]
- Pease, S. K. D., Brosnahan, M. L., Sanderson, M. P., & Smith, J. L. (2022). Effects of Two Toxin-Producing Harmful Algae, Alexandrium catenella and Dinophysis acuminata (Dinophyceae), on Activity and Mortality of Larval Shellfish. Toxins, 14(5), 335. [CrossRef]
- Wolny, J.L., Egerton, T.A., Handy, S.M., Stutts, W.L., Smith, J.L., Whereat, E.B., Bachvaroff, T.R., Henrichs, D.W., Campbell, L., Deeds, J.R. 2020. Characterization of Dinophysis spp. (Dinophyceae, Dinophysiales) from the mid-Atlantic region of the United States. Journal of Phycol. 56(2):. [CrossRef]
- Maso, M., and Garces, E. 2006. Harmful microalgae blooms (HAB); problematic and conditions that induce them. Marine Pollution Bulletin, 53(10-12), 620-630. [CrossRef]
- Paerl, H.W., Hall, N.S, Peierls, B.L. & Rossignol, K.L. 2014. Evolving paradigms and challenges in estuarine and coastal eutrophication dynamics in a culturally and climatically stressed world. Estuaries and Coasts 37(2):243–258. [CrossRef]
- Whyte, C., Swan, S., Davidson, K. 2014. Changing wind patterns linked to unusually high Dinophysis blooms around the Shetland Islands, Scotland. Harmful Algae 39: 365-373. [CrossRef]
- Hauser, C. A., & Bason, C. W. (2020). The Economic Value of the Delaware Inland Bays. DE.
- Title of Site. Available online: URL (accessed on Day Month Year).
- Title of Site. Available online: URL (accessed on Day Month Year).
- Welschmeyer N. A. 1994. Fluorometric analysis of chlorophyll a in the presence of chlorophyll b and pheopigments. Limnol Oceanogr 39(8): 1985-1992. [CrossRef]
- Coyne, K. J., Hutchins, D. A., Hare, C. E. & Cary, S. C. 2001. Assessing temporal and spatial variability in Pfiesteria piscicida distributions using molecular probing techniques. Aquatic Microbial Ecology, 24: 275-285. [CrossRef]
- Dempster, E.L., Pryor, K.V., Francis, D., Young, J.E., & Rogers, H.J. 1999. Rapid DNA extraction from ferns for PCR-based analyses. Biotechniques 27:66–68. [CrossRef]
- Coyne, K.J., Handy, S.M., Demir, E., Whereat, E.B., Hutchins, D.A., Portune, K.J., Doblin, M.A., & Cary, S.C. 2005. Improved quantitative real-time PCR assays for enumeration of harmful algal species in field samples using exogenous DNA reference standard. Limnology and Oceanography, 3(9): 381-391. [CrossRef]
- Galluzzi, L., Bertozzini, E., Penna, A., Perini, F., Pigalarga, A., Graneli, E. and Magnani, M. (2008), Detection and quantification of Prymnesium parvum (Haptophyceae) by real-time PCR. Letters in Applied Microbiology, 46: 261-266. [CrossRef]
- Rosales, D., Ellett, A., Jacobs, J., Ozbay, G., Parveen, S., Pitula, J. 2022. Investigating the Relationship between Nitrate, Total Dissolved Nitrogen, and Phosphate with Abundance of Pathogenic Vibrios and Harmful Algal Blooms in Rehoboth Bay, Delaware. Applied and Environmental Microbiology 88(16):1-14. [CrossRef]
- Altschul, S.F., Gish, W., Miller, W., Myers, E.W., Lipman, D.J. 1990. Basic local alignment search tool. J Mol Biol. 215(3):403-10. [CrossRef]
- Moore, D. S., Notz, W. I, & Flinger, M. A. (2013). The basic practice of statistics (6th ed.): Chapter 4. New York, NY: W. H. Freeman and Company.
- Piehler, Michael and Lindsay Dubbs, directors. Taking a Sediment Core. Youtube.com/Taking a Sediment Core, University of North Carolina Coastal Studies Institute, 15 Aug. 2015, www.youtube.com/watch?v=0DixyJZCvVQ.
- Portune, K.J., Coyne, K.J., Hutchins, D.A., Handy, S.M., Cary, C.S. 2009. Quantitative real-time PCR for detecting germination of Heterosigma akashiwo and Chattonella subsalsa cysts from Delaware’s Inland Bays, USA. Aquatic Microbial Ecology 55: 229-239. [CrossRef]
- Hart, M.C., Green, D. H., Bresnan, E., & Bolch, C.J. 2007. Large subunit ribosomal RNA gene variation and sequence heterogeneity of Dinophysis (Dinophyceae) species from Scottish coastal waters. Harmful Algae 6: 271-287. [CrossRef]
- Scholin, C.A., Herzog, M., Sogin, M., & Anderson, D.M., 1994. Identification of group-specific and train-specific genetic-markers for globally distributed Alexandrium (Dinophyceae). II. Sequence analysis of a fragment of the LSU rRNA gene. J. Phycol. 30, 999–1011.
- United States Environmental Protection Agency. 2016. Estimated nitrate concentrations in groundwater used for drinking. Retrieved December 2020, from https://www.epa.gov/nutrient-policy-data/estimated-nitrate-concentrations-groundwater-used-drinking.
- United States Environmental Protection Agency. Ground Water and Drinking Water. February 14, 2020. National Primary Drinking Water Regulations. Retrieved December 6, 2020, from https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations.
- Ozbay, G. 2016. Delaware Inland Bays Eastern Oyster (Crassostrea virginica) Quality for Consumption and Application of Non-Thermal, High Hydrostatic Pressure (HHP) to Extend Oyster Shelf Life. Master of Science Thesis in Food Science & Biotechnology. Delaware State University, Human Ecology, Dover, DE USA. 173p.
- Bravo, I., Fraga, S., Figueroa, R.I., Pazos, Y., Massanet, A. & Ramilo, I. 2010. Bloom dynamics and life cycle strategies of two toxic dinoflagellates in a coasting upwelling system (NW Iberian Penninsula). Deep-Sea Research II, 57:222-234.
- Kremp, A. & Heiskanen, A.S. 1999. Sexuality and cyst formation of the spring bloom dinoflagellate Scrippsiella hangoei in the coastal northern Baltic Sea. Mar. Biol 134:771–777. [CrossRef]
- Warns, A., Hense, I. & Kremp, A. 2012. Modelling the life cycle of dinoflagellates; a case study with Biecheleria baltica. Journal of Plankton Research 35(2):379-392. [CrossRef]
- Hattenrath-Lehmann, TK., Smith, JL., Wallace, RB., Merlo, L., Koch, F., Mittelsdorf, H., Goleski, JA., Anderson, DM., Gobler, CJ. 2015b. The effects of elevated CO2 on the growth and toxicity of field populations and cultures of the saxitoxin-producing dinoflagellates, Alexandrium fundyense. Limnol Oceanogr 60(1):198-214. [CrossRef]
- Hattenrath-Lehmann, TK., Marcoval, M.A., Mittlesdorf H., Goleski, J.A., Wang, Z., Haynes, B. 2015a. Nitrogenous Nutrients Promote the growth and toxicity of Dinophysis acuminata during estuarine bloom events. PLoS ONE 10 (4):e0124148.
- Tong, M., Smith, J., Kulis, D., Anderson, D. 2015. Role of dissolved nitrate and orthophosphate in isolates of Mesodinium rubrum and toxin-producing Dinophysis acuminata. Aquatic Microbial Ecology 75:169-185. [CrossRef]
- Setälä, O., Autio, R., Kuosa, H., Rintala, J., & Ylöstalo, P. (2005). Survival and photosynthetic activity of different dinophysis acuminata populations in the northern Baltic Sea. Harmful Algae, 4(2), 337–350. [CrossRef]
- Tong, M., Zhou, Q., David, K. M., Jiang, T., Qi, Y., & Donald, A. M. 2010. Culture techniques and growth characteristics of Dinophysis acuminata and its prey. Chinese Journal of Oceanology and Limnology 28(6):1230-1239. [CrossRef]
- Bravo, I., & Figueroa, R. I. 2014. Towards an ecological understanding of dinoflagellate cyst functions. Microorganisms, 2, 11-32. [CrossRef]
- Berland, B.R., Maestrini, S.Y. & Grzebyk, D. 1995. Observation on possible life cycle stages of the dinoflagellates Dinophysis cf. acuminata, Dinophysis acuta and Dinophysis pavillardi. Aquatic Microbial Ecology. 9: 183-189. [CrossRef]
- Escalera, L. & Reguera, B. 2008. Planozygote Division and Other Observations on the Sexual Cycle of Several Species of Dinophysis (Dinophyceae, Dinophysiales). Journal of Phycology. 44. 1425 - 1436. [CrossRef]
- Collins, R.A., Wangensteen, O.S., O’Gorman, E.J., Mariani, S., Sims, D.W., & Genner, M.J. 2018. Persistence of environmental DNA in marine systems. Commun Biol 1, 185. [CrossRef]
- Coyne, K.J., Hare, C.E., Popels, L.C., Hutchins, D.A. & Cary, S.C. 2006. Distributions of Pfisteria piscida cyst populations in sediments of the Delaware Inland Bays. Harmful Algae, 5 (4):363-373.
- Keafer, B.A., Buesseler, K.O. & Anderson, D.M. 1992. Burial of living dinoflagellate cysts in estuarine and nearshore sediments. Mar. Micropaleontol. 20, 147–161. [CrossRef]
- McQuoid, M.R., Godhe, A. & Nordberg, K. 2002. Viability of phytoplankton resting stages in the sediments of a coastal Swedish fjord. Eur. J. Phycol. 37: 191–201. [CrossRef]
- Rozan, T.F., Taillefert, M., Trouwborst, R.E., Glazer, B.T., Ma, S., Herszage, J., Valdes, L.M., Price, K.S. & Luther, G.W. 2002. Iron– sulfur–phosphorus cycling in the sediments of a shallow coastal bay: implications for sediment nutrient release and benthic macroalgal blooms. Limnol. Oceanogr. 47, 1346–1354. [CrossRef]
- Taillefert, M., Rozan, T.F., Glazer, B.T., Herszage, J., Trouwborst, R.E. & Luther, G.W. 2002. Seasonal variations of soluble organicFe(III) in sediment porewaters as revealed by voltammetric microelectrodes. In: Taillefert, M., Rozan, T.F. (Eds.), Analyses of Trace Elemental Biogeochemistry. American Chemical Society, pp. 247–264.
- Coyne, K.J. & Cary, S.C. 2005. Molecular approaches to the investigation of viable dinoflagellate cysts in natural sediments from estuarine environments. Eukaryotic Microbiology, 52 (2): 90-94. [CrossRef]
- Jephson, T. & Carlsson, P. 2009. Species and stratification dependent diel vertical migration behaviour of three dinoflagellate species in a laboratory study. Journal of Plankton Research 31(11): 1353-1362. [CrossRef]
- Lassus, P., Proniewski, F., Pigeon, C., Veret, L., Le Dean, L., Bardouil, M., & Truquet, P. 1990. The diurnal vertical migrations of Dinophysis acuminata in an outdoor tank at Antifer (Normandy, France). Aquatic Living Resources 3(2):143-145. [CrossRef]
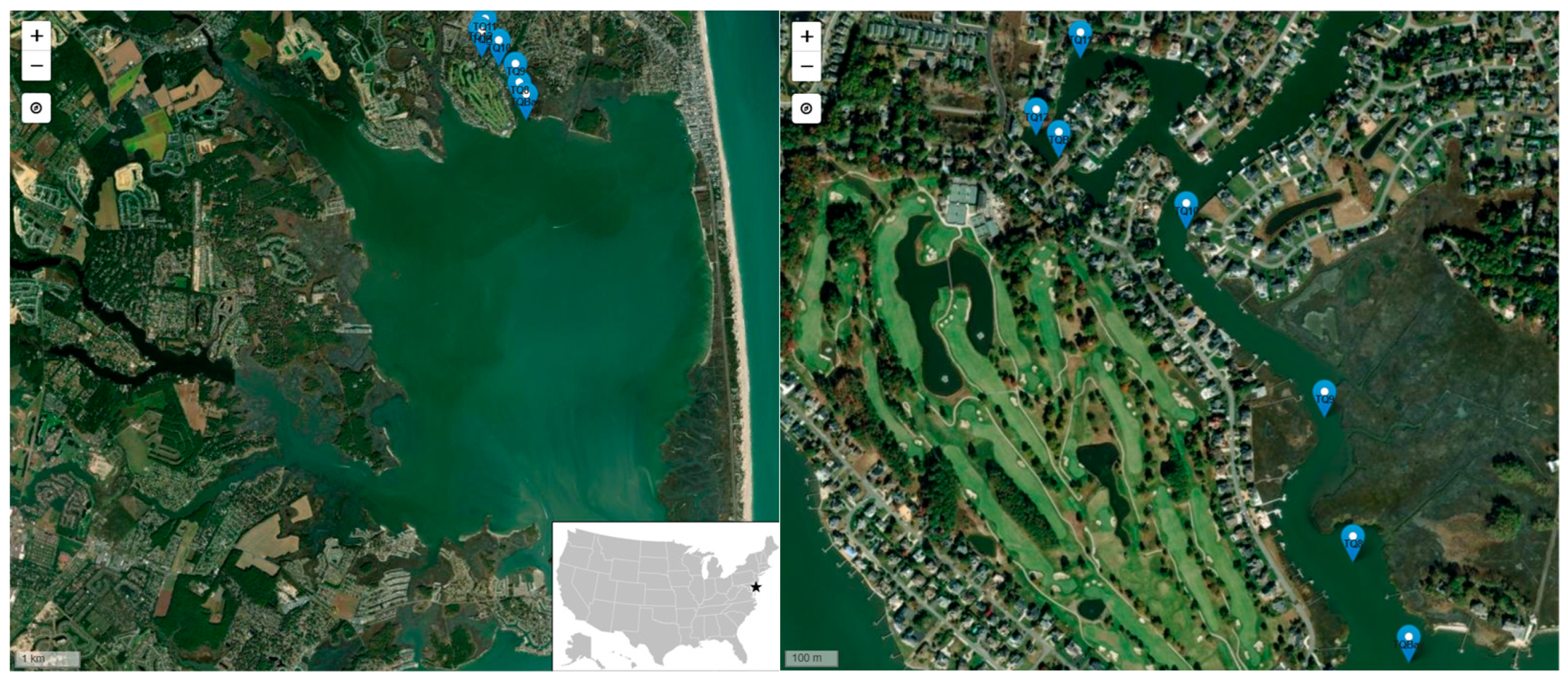
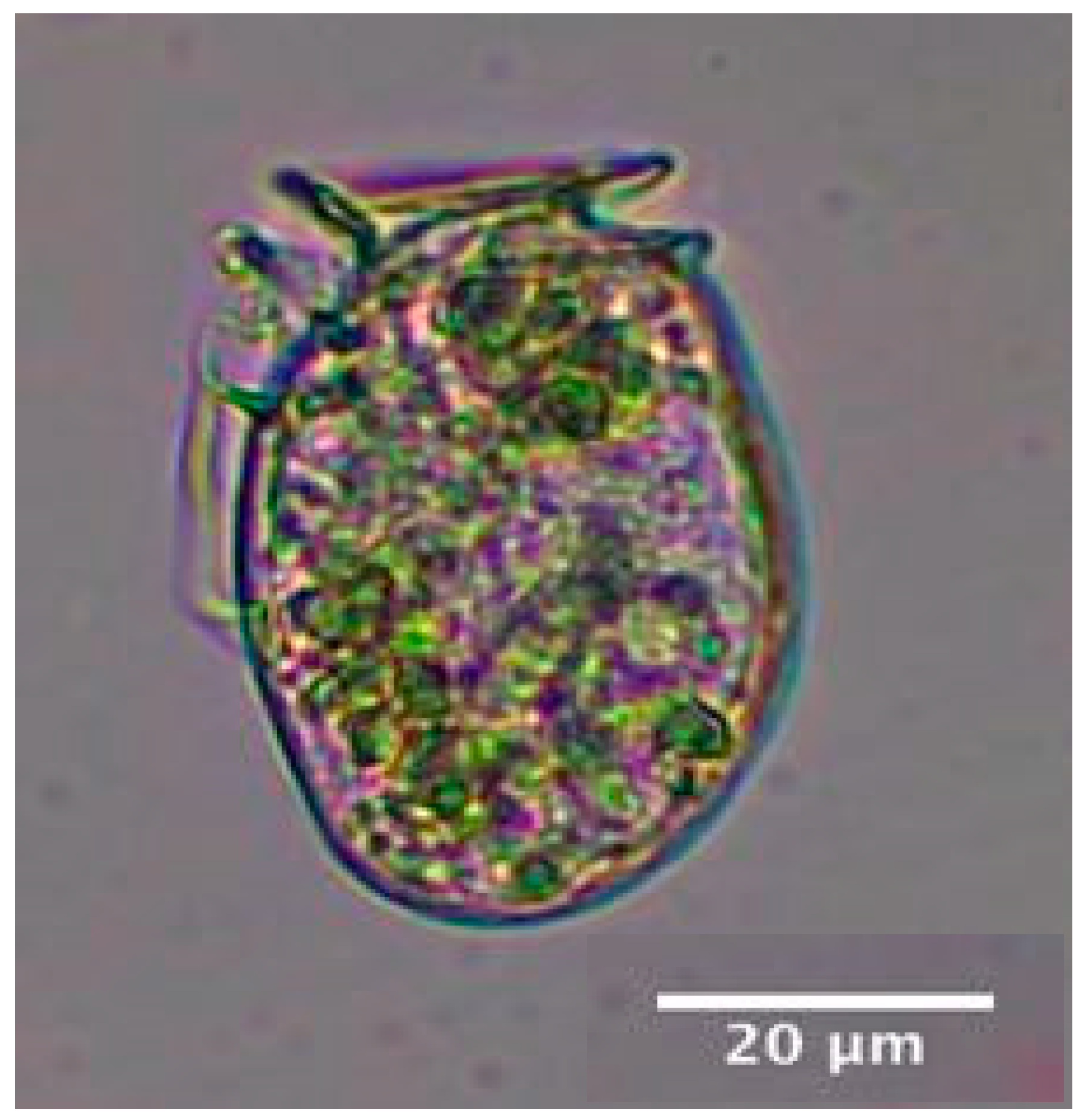
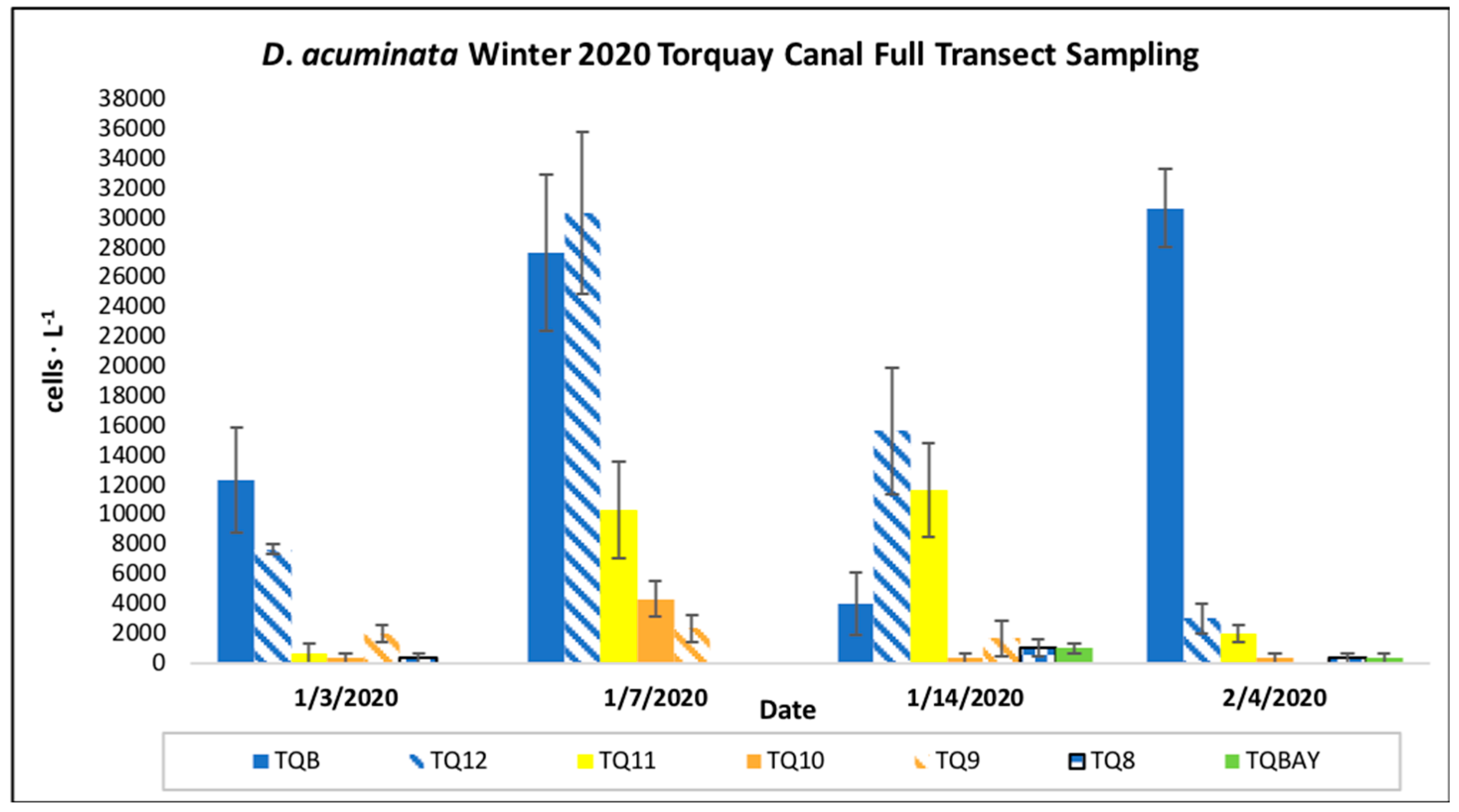
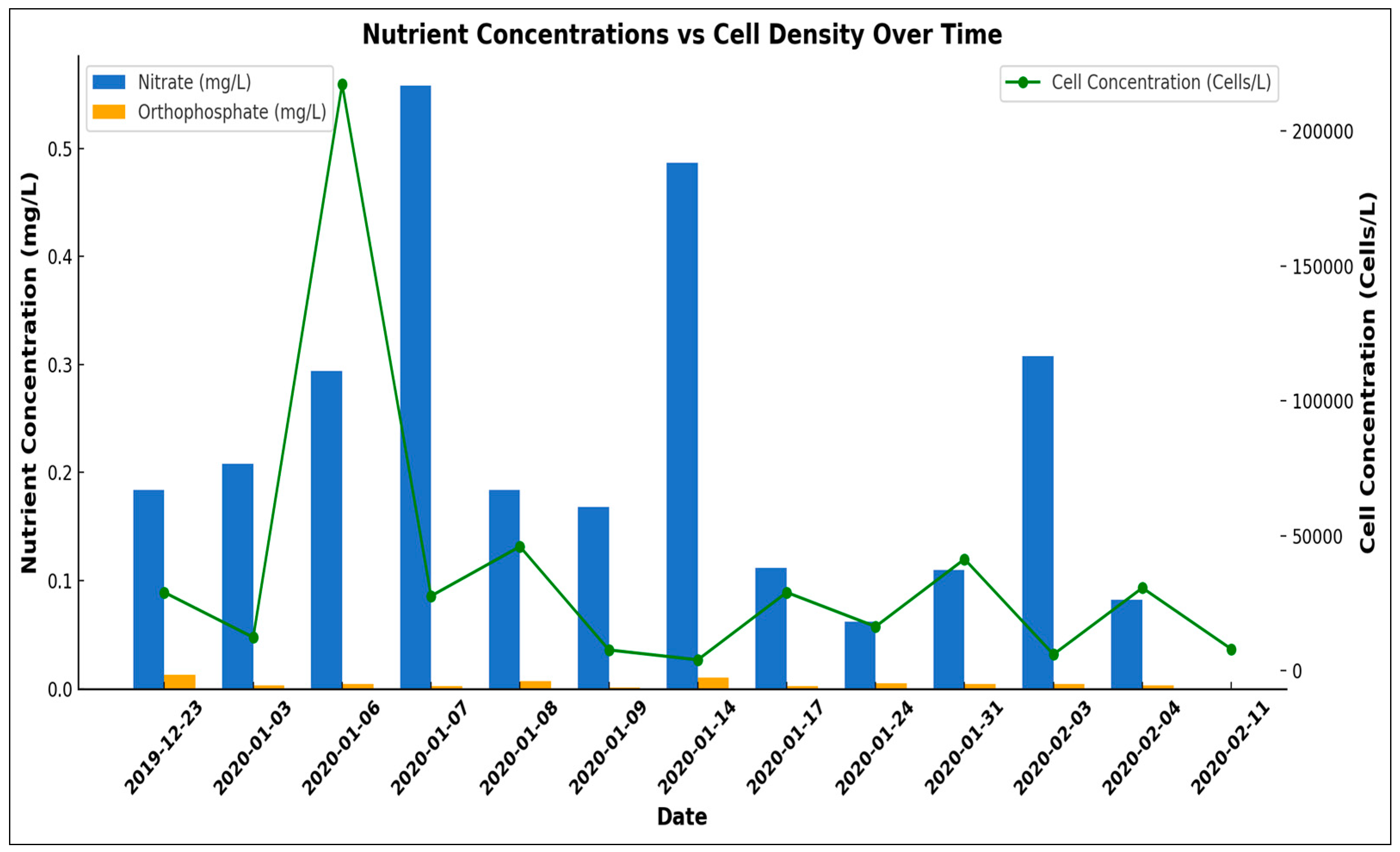
| Site Name | Description | Coordinates |
|---|---|---|
| TQB | Transect starting point | 38.699079, -75.112431 |
| TQBay | Transect ending point | 38.690418, -75.104775 |
| Torquay Canal (TQ) | Dead-end residential canal | TQ8: 38.692135, -75.105985 TQ9: 38.694625, -75.106608 TQ10: 38.697852, -75.109639 TQ11: 38.700784, -75.111952 TQ12: 38.699462, -75.112918 |
| Camp Arrowhead (CA) | Pilot artificial oyster reef | A: 38.656779, -75.130685 B: 38.656037, -75.130158 C: 38.655379, -75.129155 |
| James Farm (JF) | Ecological preserve | A: 38.574917, -75.079540 B: 38.575082, -75.079304 C: 38.575078, -75.079439 |
| Pearson Correlation |
Water Temp (°C) | Chl-a ∙ (µg L-1) | Conductivity (mS/cm) | DO (mg∙ L-1) |
NOx (mg N ∙ L-1) |
OP (mg P ∙ L-1) |
pH |
|---|---|---|---|---|---|---|---|
| Coefficient (r) | -0.156 | 0.055 | -0.258 | -0.087 | -0.098 | 0.0567 | -0.404 |
| N | 109 | 35 | 35 | 37 | 37 | 37 | 37 |
| T statistic | 1.635 | 0.318 | 1.580 | 0.518 | 0.598 | 0.334 | 2.611 |
| DF | 107 | 33 | 35 | 35 | 35 | 35 | 35 |
| p-value | 0.105 | 0.753 | 0.123 | 0.608 | 0.566 | 0.738 | 0.0132 |
| Pearson Correlation | Date | Water Temp (°C) | Chl-a (µg L-1) | Conductivity (mS/cm) | DO (mg∙ L-1) |
NOx (mg N ∙ L-1) |
OP (mg P ∙ L-1) |
pH |
|---|---|---|---|---|---|---|---|---|
| Coefficient (r) | -0.253 | -0.152 | -0.698 | -0.087 | -0.264 | 0.047 | -0.058 | -0.3869 |
| N | 13 | 13 | 12 | 13 | 13 | 12 | 12 | 13 |
| T statistic | 0.868 | 0.510 | 0.221 | 0.290 | 0.909 | 0.150 | 0.182 | 1.391 |
| DF | 11 | 11 | 10 | 11 | 11 | 10 | 10 | 11 |
| p value | 0.404 | 0.620 | 0.829 | 0.777 | 0.383 | 0.884 | 0.859 | 0.192 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





