1. Introduction
Macroevolutionary analyses aiming at exploring form–function relationships investigate how phenotypes are organized to perform relevant ecological functions by focusing on species-specific, broad ecological characteristics like i.e., trophic levels, dietary resource types and foraging behaviors (e.g., Pigot et al. 2020, Xu et al. 2023). However, the understanding of these form–function relationship evolution requires identifying what are the selection pressures acting on individuals, and it is therefore necessary to downscale these broad ecological characteristics into their constituent phenotypic traits. The paradigm of Arnold (Arnold 1983, Emerson & Arnold 1989) provides a useful framework to infer how the natural selection acting on phenotypic traits can modulate individual fitness. According to this paradigm, pertinent phenotypic traits (e.g., morphological, physiological, behavioral) are shaped by natural selection to optimize individual performance (Arnold 1983, Emerson & Arnold 1989), i.e., any quantitative measure of how well an organism performs an ecologically relevant task that is vital for its fitness (Irschik & Higham 2016). The ecological functions fulfilled by the individual will therefore result from two processes: the integration of its phenotypic traits, and the optimization of its performance, with evolutionary feed-backs between them. Despite the availability of this theoretical framework, experimental studies of performances that explicitly address form, i.e., the phenotypic integration of functional morphology (body design and mechanics) and of behavior are still rare (Green et al. 2021), probably because separating the “why” question (behaviors) of the “how” question (the design and mechanics of the body), and vice versa, is very complex as these two questions are so closely intertwined (Higham 2007, Bels et al. 2021, Green et al. 2021, Higham et al. 2021). We define here behavior as the internally coordinated responses (actions or inactions) of whole living organisms to internal and/or external stimuli, excluding responses more easily understood as developmental changes (Levitis et al. 2009). We consider behavior as a phenotypic trait at the same level than morphology or physiology according to Emerson & Arnold (1989) and Green et al. (2021), and unlike Garland & Losos (1994) or Bels et al. (2021) who use behavior as a way to capture how an organism’s performance (usually measured as a maximum value in the lab) is realized in natural conditions (similar to the “realized ecological niche”, Pianka 1984) (Green et al. 2021).
Predator-prey interactions might be excellent model systems in studies of behavior and functional morphology because both predators and prey can evolve extreme adaptations due to the coevolutionary “arms race” between them (Higham et al. 2017). The extreme specialization for capturing prey or escaping predators could then be used to test the relative roles of functional morphology and behavior in driving performances in nature. Here we use a couple of predator-prey species under completely natural situation to investigate how the body design of a predator and its behavioral register are integrated to shape its performance in prey acquisition. Gulls, i.e., bird species of the family Laridae, are characterized by the uniformity of their body plan organization (Burger & Gochfeld 1996). In this family of about 50 species (Burger & Gochfeld 1996), the proportions of appendage size relative to body size remain fairly constant, with the exception of rather slight variations in length and thickness of the beak. This constancy in morphology contrasts with what happens in some other families belonging to the same order of Charadriiforme than the Laridae, and particularly in the shorebirds (Scolopacidae and Charadriidae), in which the size and shape of the legs, toes, wings and especially the beak show huge interspecific variation. Despite this homogeneity of morphology, gull species generally show great inter- and intra-specific versatility in their diet (e.g., Cramp & Simmons 1983, Götmark 1984, Burger & Gochfeld 1996). This translates for example in a very high adaptability to the use of foods of human origin, including the use of specialized behaviors such as kleptoparasitism to steal meals from the hands of inattentive tourists in their seaside holyday resorts (Raghav & Boogert 2022). However, certain species of Laridae exhibit a strong dietary specialization.
This is the case of the slender-billed gull (Chroicocephalus genei) that feeds mainly on small invertebrates and fish (Isenmann 1976, Burger et al. 2020), and especially on brine shrimps (Artemia sp.) on salt pans during its breeding season (Britton & Johnson 1987, Oro 2002). We have here an exceptional, quasi-experimental situation to study if and how the effectiveness of a performance depends rather on body design or behavior. Indeed, brine shrimps are the only predictable prey on which gulls can feed in this very particular environment. Artemia sp. adults measure a maximum of 10 mm long and weight a maximum of 10 mg (Mason 1963). These ostracods are extremely abundant in salt pans, reaching for instance at adult summer peak density a biomass of 1600 kg/ha in the Mediterranean salinas of Salin de Giraud (Britton & Johnson 1987).
Although there have been qualitative field observations of slender-billed gulls feeding on brine shrimps, it is unclear how this diet is sufficient to satisfy all their metabolic requirements. We will first evaluate to what extent the performance of gulls feeding on brine shrimps, resumed by their food intake rate, allows them to cope with their daily energy requirements. For this, we will use the food metabolic rate (FMR), which is the total sum of energy that a free-ranging animal metabolizes over a specified time (Dunn et al. 2018). By using the model estimating field metabolic rates (FMR) in seabirds of Dunn et al. (2018), we will demonstrate that the energy assimilated by this performance, i.e., the food intake rate, is more than sufficient to cover an adult gull FMR during three different periods of its breeding season. We will then look at how slender-billed gulls acquire these very particular preys and show that brine shrimp capture does not involve the use of specialized morphological structures, but rather involves a particular behavioral sequence that associates a mode of locomotion, a mode of capture and a mode of transport of the prey from the beak to the pharynx. The comparison of this sequence to the register of food acquisition behaviors used by other Charadriiformes (shorebirds: Baguette et al. 2024) reveals its convergence with a similar sequence of locomotion, capture and food transport behaviors that is used by a shorebird species also feeding on prey captured on saltwater surface. Altogether, our study supports (1) a causal chain in which a performance results from the interaction between morphological structures and behaviors according to Emerson & Arnold (1989) and Green et al. (2021), and (2) the idea that the performance peak of a realized phenotype (Higham et al. 2021) can be reached by using the best combination of behaviors either by convergence evolution, or by their conservation among those available in a phylogenetically determined register.
2. Materials and Methods
2.1. Data Collection
Slender billed gull food acquisition behaviors were video recorded during a one-week field session between April 1st and April 8th, 2024, in the salt pans of Salin de Giraud, Camargue, France. We worked at the They de St Ursule lagoon (43,359290° N, 4,78394° E), in which the salinity of water is between 140 and 270g/l (Britton & Johnson 1987). Data were collected as follows: observers avoiding bird disturbance were positioned at distances ranging from 5 m to 20 m, in or behind a car, and filmed using an Olympus OMD-EM1x with a M.Zuiko Digital ED 150-400 mm F4,5 lens, which provides magnification up to 40 times. Focal individuals (Altmann, 1974) were selected (among flocks when necessary) and filmed at speed of 60fps. Care was taken to avoid filming the same individual twice, which guarantees the independence of the data on food intakes between individuals. We ended with 11 video sequences that contain usable prey capture data by 21 focal individuals. Besides these video recordings, we observed and photographed slender-billed gulls feeding on Artemia at the same place in April 2021, July 2023 and September 2021, which allows us to generalize the conclusions of video recordings during the period of presence of these migratory birds on their breeding sites in Camargue.
2.2. Data Analysis
2.2.1. Intake Rate
In our study site and at this period of the year, adults and nauplii of Artemia sp. are the main available invertebrate prey for foraging birds (Britton & Johnson 1987), and the only one to be near the surface. Due to their positive phototaxis (e.g., Bradley & Forward 1984), brine shrimps move close to the water surface, where they are pecked by swimming gulls with the tip of their beak, without having to immerse their head. The movement of the gull associated with capture is clearly identifiable: while swimming, the bird tilts its head, pushes quicky the tip of its beak into the water, then pulls it out by raising its head. Beak movements associated with the transport of the prey towards the pharynx are clearly visible, as well as prey swallowing. Accordingly, we viewed each sequence for our 21 individuals, and counted the number of events starting with an immersion of the tip of the beak and ending with swallowing movements. Each of these events is considered a capture. We summed the number of these captures over the entire video sequence for a given individual. By dividing the number of captures by the duration of the video sequence, we inferred the individual's intake rate.
2.2.2. Field Metabolic Rate
To investigate the field metabolic rate of Larus genei feeding on Artemia sp. at the They de St Ursule lagoon, we averaged the intake rate over our 21 individuals. Then we computed the mean hourly energy intake of a gull by multiplying the mean hourly prey intake rate by the weight and energy content of an Artemia sp. (Equation 1).
PreyMeanEnergyContent Brine shrimp adult maximum wet weight is 10 mg (Mason 1963), with a water content of 87% (Caudell & Conover 2006), giving a dry weight of 1,3 mg. Energy content is 22 kj/g of dry weight (Caudell & Conover 2006). We used this mean hourly energy intake to investigate the time needed by slender-billed gull individuals to acquire the energy required to achieve their daily field metabolic rate (FMR). We computed the FMR of slender-billed gulls at the They de St Ursule lagoon by using the model of Dunn et al. (2018), which provides FMR for any seabird populations. We used the ‘Seabird FMR calculator’ (Dunn et al. 2018), which is a dedicated app located at
https://ruthedunn.shinyapps.io/seabird_fmr_calculator/. We inputted into the model the mean mass of slender-billed gulls (285 g, Burger et al. 2020) and the latitude of the lagoon (43°).
2.3. Behavioral Data
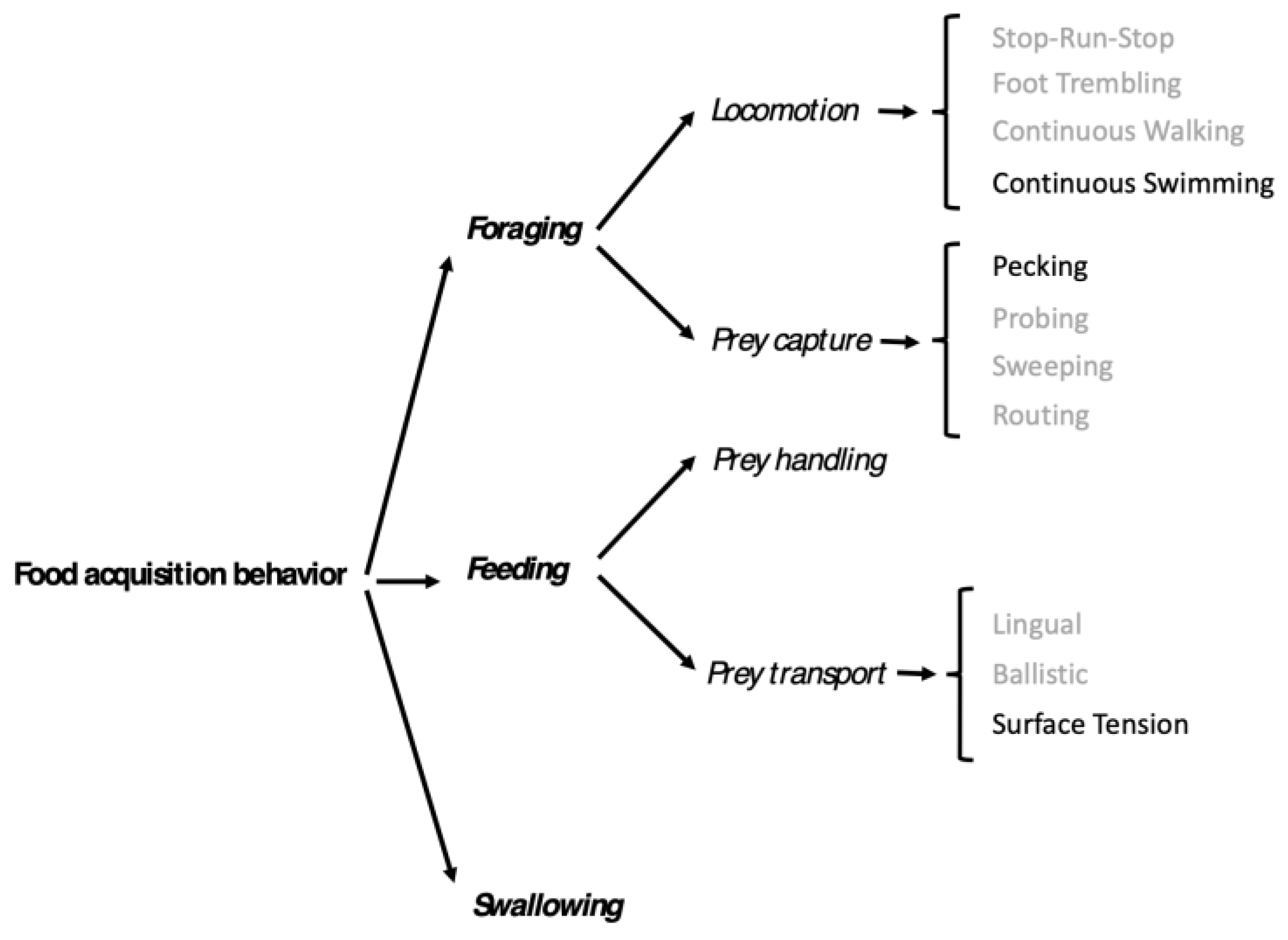
We used the approach we designed on shorebirds (Baguette et al. 2024) to perform a functional and integrative analysis of behaviors associated with food acquisition. Food acquisition is divided in three successive stages: foraging, feeding, and swallowing. The foraging stage concerns the different behaviors associated to food detection and capture, i.e. locomotion behaviors (how do birds move in suitable foraging habitats) and capture behaviors (how do birds locate and catch the prey. The feeding stage encompasses the behaviors used to handle the prey and to transport the prey into the pharynx. In the swallowing stage, the prey enters the digestive tractus. We viewed the video sequences of our 21 focal individuals, and detailed the behaviors associated with these three stages of food acquisition. We compared them to our behavioral register established on another group of Charadriiformes, the shorebirds.
3. Results
3.1. Hourly Energy Intake Rate
The number of captures, the duration of the observation and the resulting intake rate (captures/minute) for the 21 focal individuals are shown on
Table 1. The mean intake rate is 58.2 ± 17.7 captures/minute (min. 27.3, max. 921.9, which means a hourly prey intake rate of 3492 brine shrimps/hour. This food intake rate corresponds to our observations made in other years and at other times of the year.
We solved equation 1:
3492 x 0,0013 x 22 = 99,87 kJ/hr, which provided us with an estimate of the hourly energy intake rate by Chroicocephalus genei feeding on adult brine shrimps (Artemia sp.) in the salt pan of Salin de Giraud.
3.2. Catching Time Needed to Cover the Energetic Requirement of FMR
We used first the model of Dunn et al. (2018) to estimate the FMR of slender-billed gulls at three different phases of their breeding period (
Table 2). Classically, the FMR grows according to the development of the chicks, it is minimum during the incubation phase, it increases when the pulli are fed in the nest, and it is maximum when juveniles are in the crèche (
Table 2).
Next, we computed the time that an individual gull would need catching brine shrimps to cover these three different FMR (
Table 2). The increase of food demand by chicken over the reproduction period results in a 74% increase of the daily time a gull would spend fishing brine shrimps.
3.3. Food Acquisition Behaviors
Locomotion behavior associated with the foraging stage is continuously swimming according to the repertoire of Baguette et al. (2024). Gulls move continuously by vigorously moving their legs in the water and change direction frequently and abruptly (Figure 1). As such changes of direction are most often followed by the capture of a shrimp, we assume that hat they are triggered by prey visual contact. Capture behavior is pecking: the gull in motion picks the shrimp without stopping its continuous swimming. (Figure 1). The prey handling behavior that initiates the feeding stage is absent: upon capture, transport begins to bring the shrimp from the tip of the beak to the pharynx (Figure 1).
Figure 1.
Food acquisition behavior by slender-billed gull (Chroicocephalus genei) feeding on a brine shrimp. Capture begins at 0.42 sec and ends at 0.62 sec. Transport begins at 0.65 sec.
Figure 1.
Food acquisition behavior by slender-billed gull (Chroicocephalus genei) feeding on a brine shrimp. Capture begins at 0.42 sec and ends at 0.62 sec. Transport begins at 0.65 sec.
Figure 1.
(ctd). Food acquisition behavior by slender-billed gull (Chroicocephalus genei) feeding on a brine shrimp. Transport ends at 0.95 sec. Note the spitting of water droplets used for transport.
Figure 1.
(ctd). Food acquisition behavior by slender-billed gull (Chroicocephalus genei) feeding on a brine shrimp. Transport ends at 0.95 sec. Note the spitting of water droplets used for transport.
Transport behavior is clearly achieved by surface tension: close-up images indicate the presence of prey in a water droplet that is inserted between the upper and lower mandibles (
Figure 2).
In addition, the swallowing movements associated with the swallowing stage are accompanied by the release of drops of water from the tip of the beak. The food acquisition behavior of slender-billed gulls is summarized at
Figure 3.
4. Discussion
Here we investigate how the body design and the food acquisition behaviors of a predator (the slender-billed gull Chroicocephalus genei) do interact to optimize its food intake rate on its prey (the brine shrimp Artemia sp.), and to what extent this performance is sufficient to cover its daily energetic requirements. In a first step, we quantified the energetic requirement of gulls during their breeding period, and assessed whether daily brine shrimp intake rate could cover these energy costs. Our calculation showed that the prey catching time needed by an individual to cope with energetic demand of the different phases of the breeding period seems rather coherent, and thus that a diet composed of brine shrimps only could be enough to meet these needs. However, here we must mention that it is very difficult to obtain reliable data on the physiology of Artemia sp. living in natural conditions (Cuellar 1990). There are so many contradictory studies on laboratory strains that we have favored parameters measured on wild individuals (Mason 1963).
Notwithstanding these difficulties in parameter estimate, our measurement of the daily energy acquisition of the gulls observed at our study site shows that it is sufficient to cover the energy requirements required during the breeding period, as predicted by the FMR modelling approach of Dunn et al. (2018). Even if the feeding period of the young requires a significant increase in catching time by the parents, their rate of intake of brine shrimp allows them a sufficient energy supply to meet these needs. Brine shrimps show a huge seasonal variation in the salt pans of Salin de Giraud: it is only present in the form of dormant eggs (cysts) on the bottom of salinas during winter, while adults reach a density of 16 000 individuals/m2 in open water at their summer peak density (Britton & Johnson 1987). Added to this enormous density is positive phototaxis, which causes Artemia to be attracted to the surface to feed on photosynthetic algae and cyanobacteria (e.g., Bradley & Forward 1984), and a lack of adaptation to resist predation (Britton & Johnson 1987). Even if this energy-rich prey is extremely abundant and easy to capture, the fact remains that to cover their FMR during the breeding season, slender-billed gulls must capture them at a sustained rate. Our measurements provided impressive food intake rates, with a mean of 58 captures/minute, peaking at 92 captures/minute.
We mention here that we use in our calculation the maximum weight of adult brine shrimps available in the literature, which is the one that will yield the maximum energy return to the feeding gull. It is obviously impossible to measure the size of each shrimp caught by a feeding gull. Besides, the size distribution within shrimp populations is expected to be highly variable, because Artemia is a multivoltine species with several overlapping generations in a year (Britton & Jonson 1987), which means that both nauplii and adult of different sizes would be simultaneously available for predation. However, we wish to highlight two points to support our conclusions: (1) we believe that slender-billed gulls will maximize their food intake rate by preferentially hunting large shrimp, and (2) even if the shrimps were half the size used in our calculation, the hunting periods necessary for a bird to cover its FMR remain entirely achievable over the course of a day.
We can now turn to the central question of our paper, i.e., the interaction between the body design and the food acquisition behaviors in allowing the gull to achieve this performance. Beginning by morphology, we might expect that slender-billed gulls have a slender beak than other gulls. However, these gulls are not comparable with other gulls with a white head: slender-billed gulls are now considered as members of the
Chroicocephalus genus, most members of which are masked gulls (Burger et al. 2020). The comparison of beak length-depth rations between
C. genei and other
Chroicocephalus species (
Table 3) show that the slender-billed gull does not fully deserve its vernacular name: even it has the slender beak among its congeneric species, it is not by much.
The diet composition of other members of the
Chroicocephalus genus is rather generalist (
Table 3), and the slender-billed gull seems rather special by the fact that it does not feed much on offal. It shares this particularity with Bonaparte's gull (
C. philadelphia) and the gray-headed gull (
C. cirrocephalus).
The stages of the slender-billed gull's food acquisition behavior when feeding on brine shrimps are quite distinct and do not show variation. As the density of shrimp is very high, the sequence of gull locomotion and prey capture, handling, and transport occurs very quickly. Visual detection of prey is evident in the sudden and rapid changes of direction of the swimming bird. This fluidity in the sequence of these successive stages undoubtedly explains the very high food intake rate we report here. Overall, the food acquisition behavior of the slender-billed gull is completely similar to that which we described in shorebirds, the grey phalarope (Phalaropus fulicarius) and the red-necked phalarope (P. lobatus) feeding on floating invertebrates: locomotion by continuous swimming, capture by pecking on or near the sea surface, without immersion of the head, and transport of the prey from the tip of the beak to the pharynx by surface tension (Baguette et al. 2024, Baguette & Hernandez--Possémé pers. obs.). It is salient to note that this similarity in food acquisition behavior with phalaropes has also been explicitly noted in another member of the genus Chroicocephalus, the Bonaparte's gull (C. philadelphia) (Burger & Gochfeld 2020).
The very high food intake rate achieved by slender-billed gulls does not seem to be due to the evolution of a specialized morphological structure, as its beak length-depth ratio quite is comparable to that of its congeneric species. Rather, it seems to be due to the optimal use of an existing structure by highly effective behavioral sequences in this environmental context of high density of so small preys that they require a very quick acquisition. Putting this result in the context of the causal chain that arises from the paradigm of Arnold (Arnold 1983, Emerson & Arnold 1989) indicates that a performance - in this case the food intake rate, which is quantifiable and has a direct effect on the fitness of the individual - is achieved by the interaction between a series of stereotyped behaviors and the morphology of the beak of the bird. We also show that the integration of these two phenotypic traits (the morphology and mechanics of the beak on the one hand, the behavioral sequences on the other hand) optimizes the performance of food intake rate so as to be able to cover the FMR of the gull even at its peak during the maximum demand of offspring.
The similarity in food acquisition behaviors between the slender-billed gull and the two species of phalaropes we report here is surprising given the difference in beak morphology between these two species. The phalaropes have a beak length-depth ratio of 9 and 9,5 (24,4 mm/2,7 mm and 31,4 mm/3,3 mm, for the red necked-phalarope and the grey phalarope, respectively, data from Avonet [Tobias et al. 2022]), to compare to the equivalent value of 5,5 for the gull, which means that the beaks of the phalaropes are much slimmer. However, phalaropes and slender-billed gulls use exactly the same sequence of locomotion, capture and transport behaviors to locate, capture and transport preys on or near the sea surface. Despite their different evolutionary trajectories – according to the dated phylogeny of the Charadriiforme order [Cerny & Natale 2023], these taxa would have diverged ca. 65 Myr ago) – that produced quite different body designs, we therefore show that they use the same behavioral sequences to ensure food intake. A direct comparison of the intake rates between the two taxa would be meaningless given the huge differences in the environmental settings where these birds were observed. However, this similarity of behavioral sequences associated to food intake reinforces the idea that a performance in a given context can be optimized by the selection of the best combination of behaviors. Besides, this similarity raises the question the origin of these behaviors, either from their conservation in a phylogenetically determined register, or from their convergent evolution. The reconstruction of ancestral behavioral repertoires (Hernandez et al. 2021) paves the way for answering this question.
Author Contributions
Conceptualization, M.B.; methodology, M.B.; validation, M.d.S., L.H., V.B., N.S.; formal analysis, M.d.S.S., L.H., V.B., N.S., M.B.; investigation, M.d.S.S., L.H., M.B.; resources, M.d.S.S., L.H.; data curation, M.B.; writing—original draft preparation, M.d.S.S., L.H., MB.; writing—review and editing, V.B., N.S.; supervision, M.B..; project administration, M.B.; funding acquisition, M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Agence National de la Recherche, grant number ANR-10-LABX-41, France, to M.B. and by the ISYEB UMR 7205 via internal grants to M.B.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Acknowledgments
In this section, you can acknowledge any support given which is not covered by the author contribution or funding sections.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Arnold, S.J. 1983. Morphology, performance and fitness. American Zoologist 232, 347–361.
- Baguette M., Le Floch G., Hannier L., Kirchhoff F., Schtickzelle N., Stevens V.M., Bels V. 2024. Repertoire of food acquisition behaviors in Western Palearctic shorebirds (Aves, Charadriiformes). Frontiers in Ethology 3, 1351994. [CrossRef]
- Bels V. L., Pallandre J.-P., Pelle E., Kirchhoff F. 2022. Studies of the behavioral sequences: The neuroethological morphology concept crossing ethology and functional morphology. Animals 12, 1336. [CrossRef]
- Bradley, D.J., Forward, R.B.Jr. 1984. Phototaxis of adult brine shrimp, Artemia salina. Canadian Journal of Zoology 56, 708-711.
- Britton R.H., Johnson A.R. 1987. An ecological account of a Mediterranean salina: The Salin de Giraud, Camargue (S. France). Biological Conservation 42, 185-230.
- Burger J., Gochfeld M. 1996. Family Laridae (Gulls). In Handbook of the Birds of the World. Vol. 3. Hoatzin to Auks, ed. J. del Hoyo, A. Elliott, J. Sargatal. Lynx Edicions, Barcelona, pp. 572-599.
- Burger, J., Gochfeld M. 2020. Bonaparte's Gull (Chroicocephalus philadelphia), version 1.0. In Birds of the World (A. F. Poole and F. B. Gill, Editors). Cornell Lab of Ornithology, Ithaca, NY, USA. https://doi-org.mnhn.idm.oclc.org/10.2173/bow.bongul.01.
- Burger J., Gochfeld M., Garcia E.F.J. (2020). Slender-billed Gull (Chroicocephalus genei), version 1.0. In Birds of the World (J. del Hoyo, A. Elliott, J. Sargatal, D. A. Christie, and E. de Juana, Editors). Cornell Lab of Ornithology, Ithaca, NY, USA. https://doi-org.mnhn.idm.oclc.org/10.2173/bow.slbgul1.01.
- Caudell J.N., Conover M.R. 2006. Energy content and digestibility of brine shrimp (Artemia franciscana) and other prey items of Eared Grebes (Podiceps nigricollis) on the Great Salt Lake. Biological Conservation 130, 251-254.
- Cramp, S., Simmons, K. E. L. (1983). Handbook of the birds of Europe, the middle east and North Africa. Vol. 3. Waders to gulls. Oxford University Press, Oxford.
- Cuellar O. 1990. Ecology of brine shrimp from Great Salt Lake, Utah, U.S.A. (Branchiopoda, Anostraca). Crustaceana 59, 25-34.
- Dunn R.E., White C.R., Green J.A. 2018 A model to estimate seabird field metabolic rates. Biology Letters 14, 20180190. [CrossRef]
- Emerson S. B., Arnold, S.I. 1989. Intra- and interspecific relationships between morphology performance, and fitness. In Complex Organismal Functions: Integration and Evolution in Vertebrates, ed. D. B. Wake, G. Roth. John Wlley and Sons, Chichester, pp. 295-314.
- Garland T.J., Losos J.B. 1994. Ecological morphology of locomotor performance in squamate reptiles. In: Wainwright PC, Reilly SM, editors. Ecological morphology: integrative organismal biology. The University of Chicago Press, Chicago & London, pp. 240-302.
- Green P.A., McHenry M.J., Rico-Guevara A. 2021. Mechanoethology: the physical mechanisms of behavior. Integrative and Comparative Biology 612, 613–623.
- Hernández D.G., Rivera C., Cande J., Zhou B., Stern D., Berman G.J. 2021. A framework for studying behavioral evolution by reconstructing ancestral repertoires. eLife 10, e61806.
- Higham T.E. 2007. The integration of locomotion and prey capture in vertebrates: morphology, behavior, and performance. Integrative and Comparative Biology 47, 82-95.
- Higham, T.E., Clark R.W., Collins C.E., Whitford M.D., Freymille G.A. 2017. Rattlesnakes are extremely fast and variable when striking at kangaroo rats in nature: three-dimensional high-speed kinematics at night. Scientific Reports 7, 40412. [CrossRef]
- Higham T.E., Ferry L.A., Schmitz L., Irschick D.J. Starko S., Anderson P.S., Bergmann P.J., Jamniczky H.A., Monteiro L.R., Navon D., Messier J., Carrington E., Farina S.C., Feilich K.L., Hernandez P., Johnson M.A., Kawano S.M., Law C.J., Longo S.J., Martin C.H., Martone P.T., Rico-Guevara A., Santana S.E., Niklas K.J. 2021. Linking ecomechanical models and functional traits to understand phenotypic diversity. Trends in Ecology and Evolution 369, 860–873.
- Irshick, D. J., and Higham, T. E. 2016. Animal Athletes: an ecological and evolutionary approach. Oxford University Press, Oxford, 255pp.
- Isenmann P. 1976b. Contribution à l'étude de la biologie de la reproduction et de l'étho-écologie du goéland railleur, Larus genei. Ardea 64, 48-61.
- Mason D.T. 1963. The growth response of Artemia salina (L.) to various feeding regimes. Crustaceana 5, 138-150.
- Oro, D. 2002. Breeding biology and population dynamics of Slender-billed Gulls at the Ebro Delta (Northwestern Mediterranean). Waterbirds. 25(1): 67–77.
- Pianka, E. R. 1984. Evolutionary Ecology. Harper Collins, New York. 486 pp.
- Pigot A.L., Sheard C., Miller E.T., Bregman T.P., Freeman B.G., Roll U., Seddon N., Trisos C.H., Weeks B.C., Tobias J.A. 2020. Macroevolutionary convergence connects morphological form to ecological function in birds. Nature Ecology and Evolution 4, 230-239. [CrossRef]
- Raghav S., Boogert N.J. 2022. Factors associated with herring gulls Larus argentatus stealing food from humans in coastal towns, Bird Study 69, 103-108. [CrossRef]
- Tobias J.A., Sheard C., Pigot A.L., Devenish A.J.M., Yang J., Sayol F., Neate-Clegg M.H.C., Alioravainen N., Weeks T.L., Barber R.A., Walkden P.A., MacGregor H.E.A., Jones S.E.I., Vincent C., Phillips A.G., Marples N.M. , Montano-Centellas F.A., Leandro-Silva V., Claramunt S., Darski B., Freeman B.J., Bregman T.P., Cooney C.R., Hughes E.C., Capp E.J.R., Varley Z.K., Friedman N.R., , Korntheuer H., Corrales-Vargas A., Trisos C.H., Weeks B.C., Hanz D.M., Topfer T., Bravo G.A., Remes V., Nowak L., CarneiroL.S., Moncada R.A.J., Matysiokova B., Baldassarre D.T., Martinez-Salinas A., Wolfe J.D., Chapman P.M., Daly B.G., Sorensen M.C., Neu A., Ford M.A., Mayhew R.J., Silveira L.F., Kelly D.J., Annorbah N.N.D., Pollock H.S., Grabowska-Zhang A.M., McEntee J.P., Gonzalez J.C.T., Meneses C.G., Munoz M.C., Powell L.L., Jamie G.A., Matthews T.J., Johnson O., Brito G.R.R., Zyskowski K., Crates R., Harvey M.G., Zevallos M.J., Hosner P.A., Bradfer-Lawrence T., Maley J.M., Stiles F.G., Lima H.S., Provost K.L., Chibesa M., Mashao M., Howard J.T., Mlamba E., Chua M.A.H., Li B., Gomez M.I., Garcia N.C., Packert M., Fuchs J., Ali J.R., Derryberry E.P., Carlson M.L., Urriza R.C., Brzeski K.E., Prawiradilaga D.M., Rayner M.J., Miller E.T., Bowie R.C.K., Lafontaine R.M., Scofield R.P., Lou Y., Somarathna L., Lepage D., Illif M., Neuschulz E.L., Templin M., Dehling D.M., Cooper J.C., Pauwels O.S.G., Analuddin K., Fjeldsa J., Seddon N., Sweet P.R., DeClerck F.A.J., Naka L.N., Brawn J.D., Aleixo A., Bohning-Gaese K., Rahbek C., Fritz S.A., Thomas G.H., Schleuning M. 2022. AVONET: morphological, ecological and geographical data for all birds. Ecology Letters 25, 581-597.
- Xu Y., Price M., Que P., Zhang K., Sheng S., He X., Wen Z., Wang B. 2023 Ecological predictors of interspecific variation in bird bill and leg lengths on a global scale. Proceedings of the Royal Society B 290: 20231387. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).