Submitted:
17 July 2025
Posted:
18 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
- Homogeneous: The catalyst and reactants are in the same phase, typically liquid. Gas-phase homogeneous catalysis is rare but exemplified by the oxidation of SO₂ to SO₃ using nitrogen oxides [16].
- Biocatalysis: Catalysis mediated by whole microorganisms or isolated enzymes, typically in the liquid phase. While classical biocatalytic processes include alcohols [23,24] and citric acid production [25,26,27,28], more recent developments involve engineered enzymes for specialty chemical [29,30,31,32,33,34] and pharmaceutical synthesis, including enantioselective transformations and active pharmaceutical ingredient (API) production [35,36,37,38].
2. Properties of Catalysts
- (i)
- Chemical composition and crystallographic structure.
- (ii)
- Texture and physical-chemical properties.
- (iii)
- Temperature and chemical stability.
- (iv)
- Mechanical stability.
- (v)
- Mass, heat and electrical transport properties.
- (vi)
- Catalytic performance.
2.1. Chemical Composition and Crystallographic Structure
- Chemical analysis and determination of the molecular structure, typically achieved through classical spectroscopic and analytical techniques.
- Chemical analysis of both the support material and the active catalyst with deposited metal, often involving decomposition in acidic media followed by atomic absorption (AAS) or emission spectrophotometry – Inductively Coupled Plasma Optical Emission Spectroscopy and Mass Spectroscopy (ICP OES, ICP MS).
- Nuclear Magnetic Resonance (NMR) performed in both solution and solid state, to elucidate the structural features of the support, including surface functional groups.
- X-ray reflection spectroscopy (XRF) to characterize surface composition.
- X-ray Powder Diffraction (XRPD), used for phase identification and crystallographic analysis; also applicable for estimating average crystallite size via the Scherrer equation.
- Electron Diffraction X-ray Analysis (EDX), often coupled with electron microscopy, to determine surface composition and the spatial distribution of metal species.
- Wavelength-dispersive X-ray spectroscopy, (WDS, WDX), offering enhanced sensitivity over EDX for surface elemental analysis.
- Fourier Transform Infrared Spectroscopy (FTIR), utilized for the identification of surface functional groups and chemical bonding environments.
- Raman spectroscopy for characterizing molecular and surface species.
- X-ray photoelectron spectroscopy (XPS), critical for determining the valence state of metal particles on the surface.
2.2. Texture and Physical-Chemical Properties
- Particle size distribution measurements in the range of 10 nm to 5 mm.
- Optical microscopy for determining particle size, shape, and surface texture (resolution: 250 nm; high-quality laser scanning confocal microscopy (LSCM): resolution down to 0.5 nm).
- Raman spectroscopy for identifying surface phases and irregularities, e.g., quantification of graphite and disordered carbon content on activated carbon [114].
- Mercury porosimetry (applicable only to mechanically stable materials) for pore size distribution ranging from 7.5 nm at 200 MPa to 15 μm at atmospheric pressure.
- Transmission electron microscopy (TEM), including High-Resolution TEM (HR-TEM) and Scanning TEM (STEM), for determining the particle size distribution of metal crystallites, atomic arrangement, and crystallographic phases (resolution: 0.1 nm).
- Chemisorption of H2 or CO for evaluating the specific surface area of metallic crystallites or agglomerates (applicable when internal volume is accessible — see [85]).
- Titration with basic components, e.g. NaOH solution to determine acidity.
- Adsorption measurements of basic components (e.g., NH3, organic amines) to evaluate acidity, FTIR spectroscopy is used to identify Lewis and Brønsted acid sites.
- Titration with acidic components, e.g. HCl to determine alkalinity.
- Adsorption measurements of acidic components, e.g. CO2 to determine alkalinity.
- Contact angle (CA), water is used as the reference hydrophilic liquid. A CA value less than 90° indicates a hydrophilic surface, while a value greater than 90° indicates a hydrophobic surface. This is a static measurement method. Powder samples must be compacted into larger particles with a “flat surface” (e.g., tablets) to enable accurate measurements.
- Washburn dynamic method (WDM), A liquid with defined polarity rises through a porous medium in a cylindrical setup; both the rate of wetting and the maximum height achieved are recorded [118]. The referenced study also provides useful correlations between CA and WDM data.
- Adsorption of adsorptives with a different polarity: This method evaluates surface polarity based on the adsorption behavior of molecules with varying polarities. Surface roughness significantly affects the results [119].
- Zeta Potential (ζ-Potential); values close to zero suggest low surface polarity and are typically associated with hydrophobic surfaces. However, establishing a quantitative correlation between zeta potential and hydrophilicity is complex and not straightforward [120].
2.3. Temperature and Chemical Stability
- Thermogravimetric analysis (TGA) – Measures the extent of decomposition of the carrier or catalyst as temperature increases. TGA can be performed in an oxidative atmosphere (Temperature Programmed Oxidation – TPO) or a reductive atmosphere (Temperature Programmed Reduction – TPR).
- Differential scanning calorimetry (DSC) – Measures decomposition and thermal effects, such as the release of water from the crystalline lattice or pyrolysis effects in organic polymer carriers. DSC is often followed by analysis of degradation products (e.g., using GC or GC-MS).
- (Micro)Pyrolysis combined with pyrolysis product analysis – Similar to DSC with follow-up analysis but carried out under different conditions to study pyrolysis products in more details.
- Hydrolytic, Acidic, Basic, Aminolytic, Alcoholytic and Other Stability Tests – These tests are particularly important for evaluating the stability of polymer-based catalysts.
2.4. Mechanical Stability
- Crush Strength.
- Young’s Modulus of Elasticity.
- Breakage by Collision.
- Breakage by Stress in a Fixed Bed.
- Breakage in Contiguous Equipment.
2.5. Mass, Heat and Electrical Transport Properties
- Diffusivity in pores – Includes bulk diffusion, Knudsen diffusion, wall effects, and the impact of tortuosity.
- Thermal conductivity – Critical for heat management in exothermic or endothermic reactions.
- Hydrodynamic Resistance – Resistance of the catalyst bed or layer to the flow of gas/vapor or gas–vapor–liquid mixtures.
- Electrical Conductivity – Particularly important for catalyst supports, as it strongly influences metal–support interactions.
- Character of a semiconductors surface – Especially relevant for metal oxides (e.g., ZnO, NiO, Fe₂O₃, V₂O₅, Cr₂O₃). These materials can exhibit n-type behavior (donating electrons to chemisorbed molecules) or p-type behavior (withdrawing electrons) [8,18]. This distinction is important for quantifying Van der Waals interactions and their influence on HOMO and LUMO energy levels, see e.g. [122]. Measurements of isoelectric points provide an initial estimation of surface electronic properties [123].
2.6. Catalytic Performance
- Katal, kat – SI coherent derived unit for catalytic activity, defined as the amount of reactant (in moles) converted per second: (molreactant·s-1).
- Molar Catalytic Activity (kat/mol) – Catalytic activity per mole of catalyst: (molreactant·s⁻¹·molcatalyst⁻¹).
- Mass Catalytic Activity (kat/mcatalyst) – Catalytic activity per unit mass of catalyst:(molreactant·s⁻¹·kgcatalyst⁻¹).
- Turnover Frequency (TOF) [109] – Also expressed as (molreactant s-1 molcatalyst-1), TOF is functionally identical to kat/mol but predates it and remains widely used, especially in heterogeneous catalysis. TOF refers to the number of reactant molecules converted per active site per second. It is crucial to specify how the number of active sites is determined - e.g., by acidity, accessible metal atoms via chemisorption, etc. Additionally, temperature, pressure, and reactant concentrations must be reported to fully specify TOF.
- Turnover Number (TON) – Defined as the number of moles of reactant converted per mole of catalyst during its lifetime :(molreactant molcatalyst-1). TON is useful for describing catalyst durability in non-regenerated systems (e.g., in continuous reactors over time on stream). However, it is generally discouraged, especially when estimated from batch reactors, as it can be misleading [109].
- Catalyst productivity (CatProd) – Expressed in the same units as TON (molreactant molcatalyst-1), this parameter describes the total productivity of a catalyst under practical conditions. While not an official IUPAC term, CatProd is useful in process design and techno-economic evaluations. It requires well-defined experiments, typically continued to the point at which the catalyst is no longer economically viable or is fully consumed, as in some polymerization catalysts [124]. Like TOF, full specification includes temperature, pressure, and reactant concentrations.
- Selectivity to a desired product (Sdes_pr) – The number of moles of the desired product relative to the theoretical maximum according to stoichiometry, at a given conversion of a key reactant.
- Catalyst lifetime (CLT) – The total time the catalyst remains active in the reactor (typically in days or years), from its introduction until deactivation to an unacceptable level.
- Catalyst life cycle (CLC) – The full period covering catalyst use, deactivation, regeneration, and eventual replacement. It is also expressed in days or years.
- 0.
- Transport of reactants from the bulk phase to the vicinity of the catalyst(Often neglected due to intensive mixing).
- 1.
- Transport of reactants through the external surface layer surrounding the catalyst.
- 2.
- Diffusion of reactants into the catalyst pores toward active sites,
- 3.
- Chemisorption of at least one reactant onto the active sites,
- 4.
- Surface catalytic reaction.
- 5.
- Desorption of products from the catalyst surface.
- 6.
- Diffusion of products out of the catalyst pores.
- 7.
- Transport of products through the external surface layer.
- 8.
- Transport of products into the bulk phase (Often neglected due to intensive mixing).
- Apparent Activation Energy (Ea) as an indicator:
- Effect of Particle Size:
2.6.1. Kinetic Regime
- Statistical, experimental, usually in the form of power-law equations
-
Mechanistic, considering steps of chemical transformation. Commonly, three basic types of chemical reactions are considered:
- a.
- Reactants are chemisorbed on catalytic centers (Langmuir-Houghen-Hinshelwood -Watson models - LHHW)
- b.
- One reactant is chemisorbed and another one comes as not-chemisorbed (Elley- Rideal models - ER)
- c.
- One of reactants (e.g. oxygen) is exchanging positions in the crystal framework of the catalysts (Mars van Krevelen models - MarKre)
2.6.2. Mass and Heat Transport
3. Heterogeneous Catalysts
- Unsupported catalysts – Catalytic centers are relatively uniformly distributed throughout the entire catalyst volume. All components are typically mixed before the final formulation of the catalyst.
- Supported catalysts – Catalytic centers are located on the surface of a support, including within micropores. This includes surface functionalization of a pre-formed support and/or the deposition or generation of metal nanoparticles on the support.
3.1. Unsupported Catalysts
3.2. Supported Catalysts
- Preparation of relatively large catalyst particles (up to 20 mm), which helps minimize resistance to fluid flow (gas and/or liquid).
- Increase in mechanical strength compared to unsupported catalysts.
- Reduced amounts of catalytically active components, particularly expensive metals and their compounds.
- Favorable interactions with the support, including reactant–support, product–support, solvent–support, catalytic moiety–support, e.g., metal (nano)particles, which influence chemisorption of both reactants and products.
- Stabilization of catalytic moieties on the surface of support (e.g. minimization of sintration by embedding particles in pores of a support, or by interactions with functional groups of the support).
- Enhanced control over reaction temperature and contact time by adjusting the amount and distribution of catalytic moieties (e.g., Pd on alumina for selective hydrogenation of acetylene in ethylene production).
- Inorganic (e.g., elemental carbon or metals, oxides, carbonates)
- Organic (various types of polymers)
- Hybrid (inorganic-organic composites)
- Natural polymers (e.g. celluloses, lignocellulose, chitin, starch)
- Synthetic polymers, primarily those containing heteroatoms (O, N, P, S, etc.)
- Covalent Organic Frameworks (COFs) and Metal-Organic Frameworks (MOFs)
- Interactions with Atoms and Surface Defects of the Support Framework: This type is especially relevant for inorganic semiconductor-type supports, and has been recognized since the early systematic studies of catalysis (pre-1960) [8,10]. The electronic nature of atoms in the support—whether electron-donating or electron-withdrawing—significantly influences catalytic performance. One way to evaluate these effects is by comparing electronegativities (χ). For example: effect of gold (χ=2.54) as electron attracting atom with respect to Fe (χ=1.83) exhibited positive effect on lowering the activation energy (EA) in oxidation of CO over the Fe2O3/Al2O3 catalyst, and manganese (χ=1.55) as electron repelling with respect to Fe increased EA [155]. A peculiar position belongs to cerium (χ=1.12) mainly as CeO2. Due to low electronegativity a strong electron repelling effect helps in reactions where activation starts with addition of electron to a reactant, e.g. in oxidations, the best known examples are convertors for oxidation abatement of flue gases [18]. Low electronegativities are also exhibited by lantanoids (χ=1.1 - 1.3), however industrial applications of these metals and their compounds as promoters are limited due to their relatively high prices. Furthermore, metal particles supported on oxides or other substrates can exhibit Strong Metal–Support Interactions (SMSI), a phenomenon studied extensively since the 1970s [156]. Notable support materials involved in SMSI include ZnO, TiO₂, CeO₂, MgO, and nitrides, especially in combination with transition non-noble or noble metals. These interactions are now well characterized using advanced analytical techniques (see Figure 5).
- Interactions with Functional Groups: Functional groups on the support—especially those containing lone-pair donating atoms like nitrogen (e.g., –NH₂, –NH–CO– in polyamides, polyurethanes, polyureas, and porphyrins) or phosphorus—can significantly influence the behavior of supported catalysts [85]. In the case of metal particles, these can form Covalent Metal–Support Interactions (CMSI), enhancing stability and tuning reactivity.
-
Chemical modification of a support:
- a.
- Oxidation – to introduce functional groups such as carboxyl, keto, aldehyde, and hydroxyl
- b.
- Amination – addition of amine, substituted amine, or tetraalkylamine groups
- c.
- Sulfonation – formation of –SO₃H groups
- d.
- Covalent binding of organocatalysts – e.g., nitrogen- or phosphorus-based moieties, metal-organic catalytic complexes
- e.
- Immobilization of enzymes or enzyme-like catalytic moieties
-
Introduction of metal (nano)particles:
- f.
- Fixation of pre-synthesized nanoparticles
- g.
-
Adsorption of metal precursors followed by decomposition or reduction, through techniques such as:
- Wetting processes
- Adsorption, ion-exchange procedure
- Chemical vapor deposition
- Electrochemical deposition, especially effective for single-atom catalysts (SACs)
3.3. Composite Catalysts
3.4. Promoters
-
Promoters Permanently Incorporated into the Catalyst Structure.These modifiers are embedded into the catalyst and influence performance through various mechanisms:
- Structural Promoters: Prevent undesirable structural changes, such as the sintering of metal nanoparticles, thereby preserving active surface area.
- Electronic Promoters: Modify electron density at active sites. For instance, alkali metals increase the electron density on iron catalyst surfaces, enhancing nitrogen adsorption and activation in ammonia synthesis [6]
- Surface modifiers: Influence surface chemistry and physical properties. Examples include: Sulfur-induced poisoning of certain sites on Fischer–Tropsch catalysts to improve olefin selectivity [167], conversion of hydrophilic to hydrophobic surfaces via tethering of polytetrafluoroethylene oligomers [168], ionic liquids [169], or anchoring of stereospecific ligands (heterogenized) to improve enantioselectivity. For example, over 90% selectivity to (R)-1-phenyl-1-propanol was achieved using PS-supported hydroxyamides in the enantioselective ethylation of benzaldehyde [170].
- Promoters Introduced into the Reaction Medium: These act dynamically, affecting surface interactions and reaction pathways. They may compete for adsorption sites, modify the reaction mechanism, or create transient intermediates. For instance: Chlorine-containing species formed in situ during ethylene epoxidation facilitate oxygen activation over silver catalysts [166], water vapor used to suppress carbon deposition in the dehydrogenation of ethylbenzene to styrene over iron catalysts [171]. Numerous additional cases highlight the use of in situ promoters to alter surface behavior and enhance catalyst performance [18].
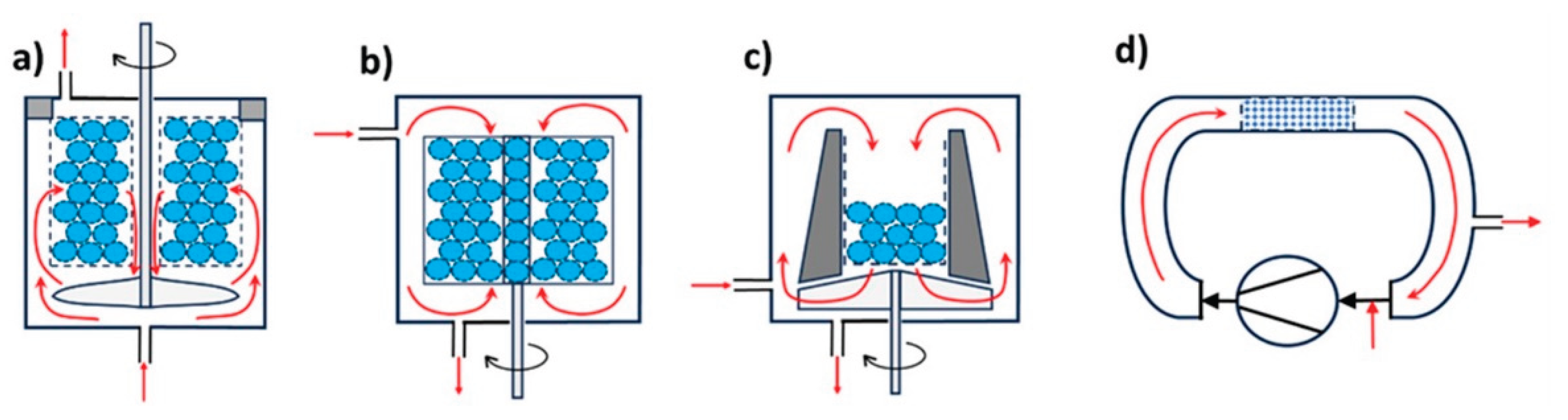
3.5. Structured Catalysts
-
Structures formed as a result of standard preparation processes
-
- a.
-
Fibers and meshes:
- b.
-
Skeletal catalysts:
- i.
-
Corrugated supports prepared from inorganic or organic sheets:
- ii.
-
Extruded and 3D printed:
- Inorganic: Skeletons are typically made of silica, spinels (e.g., MgAl₂O₄), or cordierite ((Mg,Fe)₂[Si₅Al₄O₁₈]·nH₂O), using extrusion. Additives like aluminum hydroxide or zinc salts improve plasticity and structural stability [136]. After calcination, monoliths can withstand up to 1200 °C. Porosity is improved via a washcoat layer (e.g., Al₂O₃) with anchored catalytic nanoparticles [185]. For more complex architectures, 3D printing is employed, enabling vertical and horizontal channel formation [191]. Additive Manufacturing (AM 3D) methods can include zeolites, MOFs, COFs, or burnable organic additives to increase porosity during calcination.
- c.
- Hierarchial catalysts (HiCat). Figure 2b refers to the multi-level porous architecture (micro-, meso-, and macropores) that facilitates enhanced diffusion and accessibility to active sites. These catalysts are particularly relevant for zeolites [161,177,197,198,199]. Conventional zeolites are microporous (0.4–0.8 nm). Post-treatment (e.g., desilication with alkaline hydroxides) introduces mesoporosity, improving performance by reducing transport limitations. Hierarchical structuring can also be achieved using bulky templates (alcohols, amines), polymer nanoparticles, or carbon black, followed by calcination. In this context the term hierarchy factor (HF) is important; HF = (Vmicrop/Vp)x(Smesop/SBET) with V = volume, S = surface. Typical HiCats of zeolite type exhibit HFs from 0.15 to 0.2. HiCats zeolite type extended potential for their application [53,199,200]. Besides zeolites, some other HiCats were prepared and tested [176,201,202,203]. OMOPs, including MOFs and COFs StrCats with regular texture are also considered as HiCats [204,205,206,207,208,209].
- Encapsuled catalysts (EnCat). Encapsulation of catalytic active moieties (enzymes, metal organic complexes, metal nanoparticles) is an effective method for protecting catalysts from deactivation. The active species are enclosed within a capsule with permeable walls that allow the passage of reactants and products. Encapsulation prevents the formation of agglomerates (e.g., sintering in the case of inorganic materials), while diffusion through the surrounding layer can regulate selectivity. Various techniques for preparing EnCats, including the encapsulation of magnetic particles for easy catalyst separation, are summarized in [210]. EnCats are also used industrially, for example, palladium encapsulated in polyureas [211,212]. EnCats based on polymeric organic films are typically suited for mild reaction conditions, with operational temperatures not exceeding 150 °C [210,213,214,215]. When enzymes are encapsulated, the working temperature is even lower (around 75 °C), although this is still higher than the operational limit for free enzymes [216]. Catalytic species can also be encapsulated within porous inorganic materials, such as zeolites, which allow much higher working temperatures—up to 400 °C [217,218]. In this context, it is worth comparing encapsulated catalysts with metal catalysts anchored to supports containing strong chelating groups (e.g., nitrogen-based moieties). Recent studies have demonstrated that palladium supported on crosslinked polyureas exhibits higher stability than encapsulated variants [160]. This finding highlights the importance of considering both approaches during catalyst design, with attention to both reaction rate and catalyst lifetime.
3.6. Electrocatalysts
- Faradaic efficiency:
- Energetic efficiency
3.7. Photocatalysts
3.8. Deactivation
- Poisoning by impurities, e.g., reaction of sulfur compounds with metals in hydrogenation processes.
- Formation of side products that adhere to the surface and block reactants from accessing catalytic sites (e.g., tar formation on cracking catalysts).
- Detachment of anchored functional groups from the surface of heterogenized catalysts (acidic groups, basic groups, metal-organic groups, etc.).
- Transformation of catalytic centers into soluble or vaporizable compounds and subsequent leaching into the reaction environment (common in reductions involving oxidizing reactants such as nitro- and nitroso-compounds).
- Growth of metal (nano)particles into larger ones (sintering), resulting in loss of activity due to decreased specific surface area.
- Abrasion or breakage of catalyst particles, mainly occurring in fluid and suspension reactor systems, although catalysts in fixed-bed reactors also experience this slowly over time.
- Use catalysts that are chemically stable and operate within appropriate temperature ranges; for example, observe the limited working temperature of OMOP catalysts.
- Operate at the lowest possible temperature that still ensures good catalyst performance.
- Utilize supported catalysts, preferably structured catalysts (StrCats) when possible.
- Choose suitable chelating groups for supported catalysts.
- Minimize the presence of chemical compounds that can attack or poison the catalyst.
- Ensure proper flow conditions around the catalyst surface—adequate for efficient transport of reactants and products but not so intense as to cause catalyst abrasion; this also applies to mixing intensity.
4. Catalytic Technologies
4.1. Gas Phase Reactors (G-S)
4.2. Liquid Phase Reactors (L-S)
4.3. Gas Liquid Phase Reactors (G-L-S)
- G-L:
- L-S (Eq. 10)
- Internal diffusion (Eq. 5)
| Property | Trickle bed | Mechan. stirred | Bubble column | Ebullated | Loop |
| Dcat (mm) | 2-30 | 0.01 - 1 | 0.01 – 0.2 | 0.01 – 0.2 | 0.01 – 0.1 |
| V (m3) | 3-20 | 10-4 -1000 | 1-20 | 1-20 | 0.05 - 5 |
| Pmax (MPa) | 5 | 15 | 5 | 3 | 3 |
| Tmax (°C) | 600 | 500 | 400 | 400 | 300 |
| Mass_transport | Average | Very good | Average | Average | Excellent |
| Heat_transport | Bad | Very good | Average | Good | Excellent |
| CAPEX | Low | High | Low | High | Very high |
| OPEX | Low | High | High | Very high | Very high |
| Examples | Hydrotreatment | All processes | Hydrogenation | Hydrotreatment | Hydrogenation, Oxidation |
4.5. Special Chemical Catalytic Reactors
4.6. Electrocatalytic Processes and Reactors
- Electron transfer
- Reorganization of intramolecular bonds
- Reorganization of the solvation shell
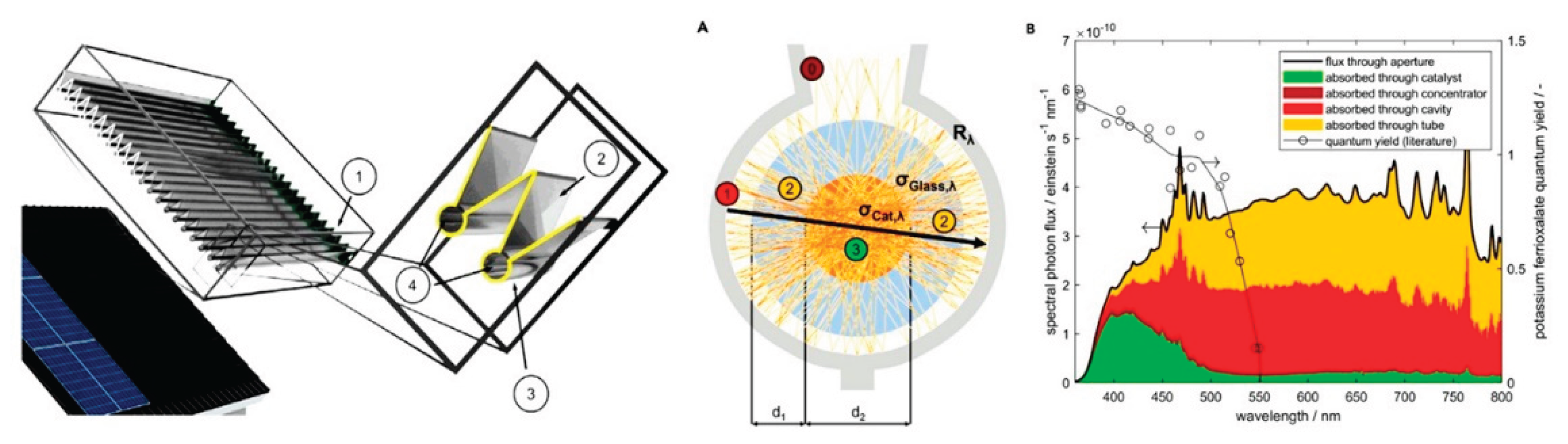
4.7. Heterogeneous Photocatalytic Processes and Reactors
-
Light source:
- a.
- Natural or Artificial
- b.
-
Light concentrators (mirrors, lens):
- i.
- Without Light Concentrators
- ii.
- With Light Concentrators
- c.
-
Wavelength:
- i.
- Visible (400-780 nm)
- ii.
- UV (190-400 nm)
- d.
-
Type:
- i.
- Conventional Lamps
- ii.
- Light Emitting diodes (LED)
-
Catalyst:
- a.
- TiO2 or not TiO2 based
- b.
- Size:
- i.
- Micron
- ii.
- Nanometer
-
Catalyst movement
- a.
- Immobilized (On the wall of the reactor, Bed, Fibers, Membrane, or Disk)
- b.
- Suspended
-
Reactor:
- a.
- Batch or Continuous
- b.
- Hydrodynamic behavior:
- i.
- Continuously Stirred (CSTR)
- ii.
- Plug Flow (PFR)
-
C1 chemistry:
-
Waste treatment:
-
Special chemicals:
- ○
- ○
- ○
4.7. Microreactors (MRs)
- Silicon/ceramic materials (well-characterized, high precision but expensive fabrication, high temperature resistance up to 1300 K)
- Glass (allows visualization of reaction and flow, electroosmotic flow (EOF) possible, withstands high operating pressures up to 50 bar)
- Polymers (low cost, various fabrication techniques, tunable properties, disposable microreactors possible, low chemical and temperature resistance—max 500 K)
- Metals (durable, well-established fabrication techniques, sensitivity to reaction environment, suitable for higher temperature—up to 1100 K—and pressure—up to 50 bar—regimes).
- High intensity of mixing; enhanced mass and heat transport
- Increased chemical reaction rates
- Efficient application of external energy sources (e.g., light, microwaves, ultrasound)
- Potential integration with membrane technologies (especially “tube-in-tube” reactors)
- Precise temperature control
- High operational pressure tolerance
- High selectivity
- High volumetric productivity
- Fouling and clogging
- High pressure drop
- Requirement for high-purity reactants
- Catalyst deactivation (due to abrasion or leaching)
- Not suitable for large-scale production (despite high volume-specific productivity)
5. Design of Catalytic Technologies
- Basic Research (BaR, also known as fundamental or pure research)
- Applied Research (ApR)
- Technological Research (TeR)
-
Large-Scale Technologies (Bulk Chemical Production)
- a.
- Inorganic (NH3, HNO3, H2SO4, etc.)
- b.
- Organic (components for fuels, monomers, polymers, etc.)
- c.
- Biotechnologies (fermentation, enzymatic hydrolysis, etc.)
-
Chemical specialties:
- a.
- Inorganic (components for production of catalysts, pure chemicals for electronics, special technical products – glasses, etc.)
- b.
- Organic and organic-inorganic mixed (additives to polymers, antidegradants, explosives, dyes, biologically active substances)
- c.
- Biotechnologies (production of API and their precursors, e.g. penicillin, etc.)
- Waste Treatment (liquid, gas, soil)
- A relatively inexpensive catalyst with average activity and selectivity, combined with a simple separation system
- A more expensive, highly active and selective catalyst, which may be sensitive to impurities, temperature, and pressure fluctuations. This choice would necessitate high-purity inputs and sophisticated process control systems, increasing costs.
- TeR
- Feasibility Study – Includes process principles, process flow diagrams (PFDs), raw material and energy requirements, waste treatment strategies, a preliminary layout, economic evaluation (CAPEX and OPEX), safety and environmental assessments, and comparison with Best Available Technology (BAT).
- Basic Design (BD) – Covers process principles, PFDs, material and energy balances, equipment selection and integration, control systems, Piping and Instrumentation Diagrams (P&IDs), utility requirements (cooling, water supply, energy), analytics, packaging and transportation of products, waste treatment, preliminary economic and ecological assessments, HAZOP (Hazard and Operability Study), and identification of unresolved issues.
- Legislation – Involves Environmental Impact Assessment (EIA), REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals), IPPC (Integrated Pollution Prevention and Control), and other applicable regulatory requirements, including those related to hazardous substances and construction permits.
- Detailed Design (DD) – Provides a more detailed version of all BD elements.
- Realization – Physical construction and implementation of the technology.
- Testing – Includes commissioning and validation of the process.
- Update of DD Documentation – Reflects changes based on real-world implementation.
- Routine operation
- Optimization
- Retrofit or Decommissioning of Technology
- New technology development (novel catalyst, reactor, separation system).
- (ii) Retrofit (existing or new product, with a new catalyst and adaptation of existing equipment). Retrofits are more cost-effective and quicker, but come with limitations—material, volume, mechanical strength (e.g., maximum pressure and temperature), and constraints related to heating/cooling, mixing, and separation units [273,517,518].
- Brief description of technology
- Block flow diagram (BFD), i.e. definition of main technological units linked with streams, including recycles
- If possible, Comparison with best available technology (BAT)
- Raw-material and energy demands
- Catalyst and type of reactor (see above)
- Kinetics
- Lifetime, regeneration and disposal of deactivated catalyst
- Preliminary calculation of reactor volume
- Range of temperature and pressure for operation
- Recommendations for separation units
- Physical-chemical, explosive and hazardous/toxic properties of individual components and their mixture
- Characterization of wastes
- Preliminary economic, safety, ecological and technological risk analysis
6. Examples of Technologies
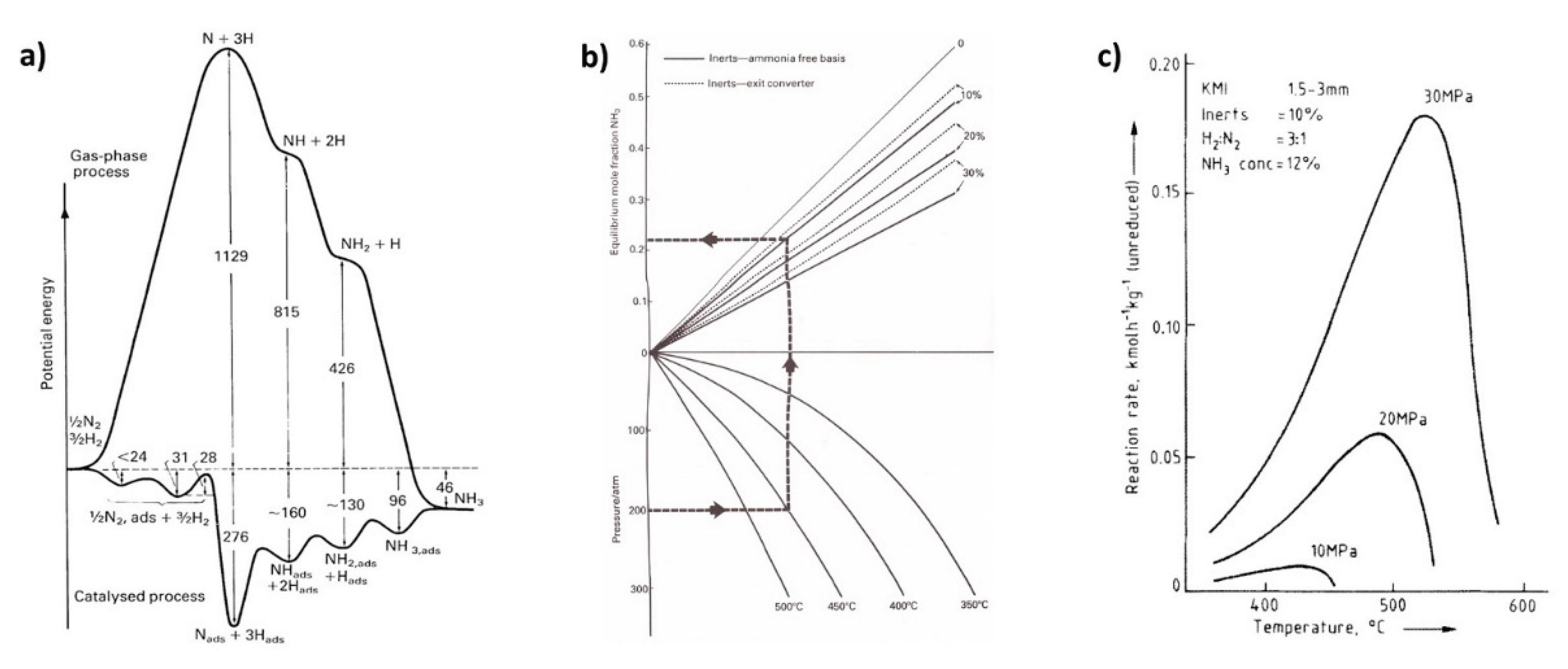
6.1. Ammonia
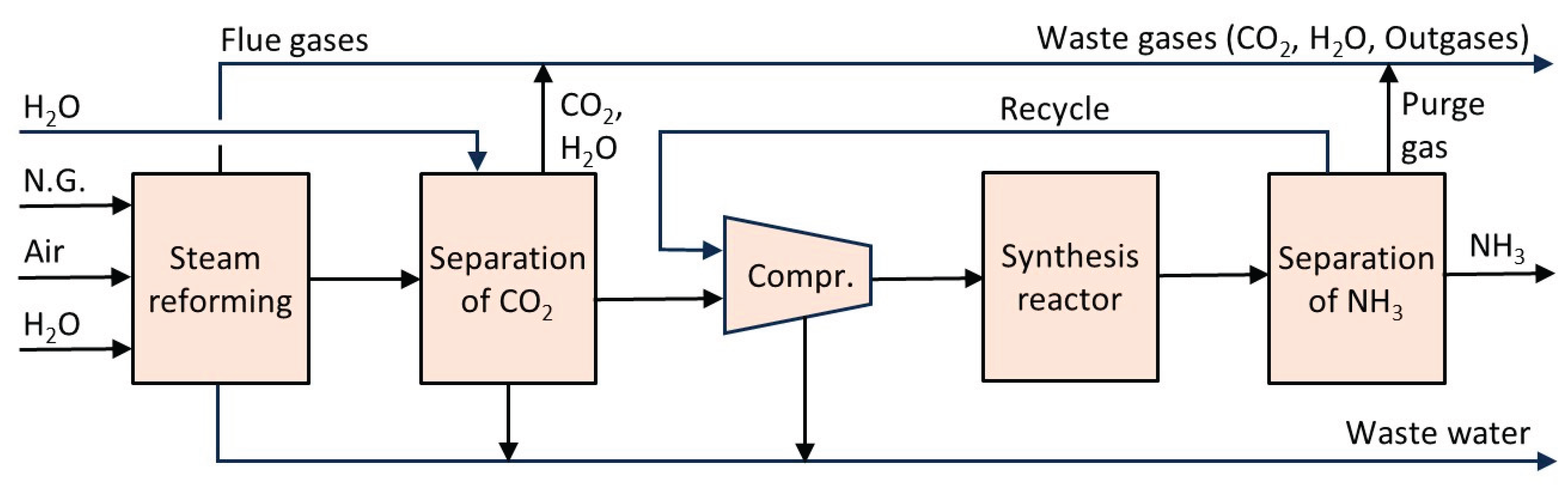
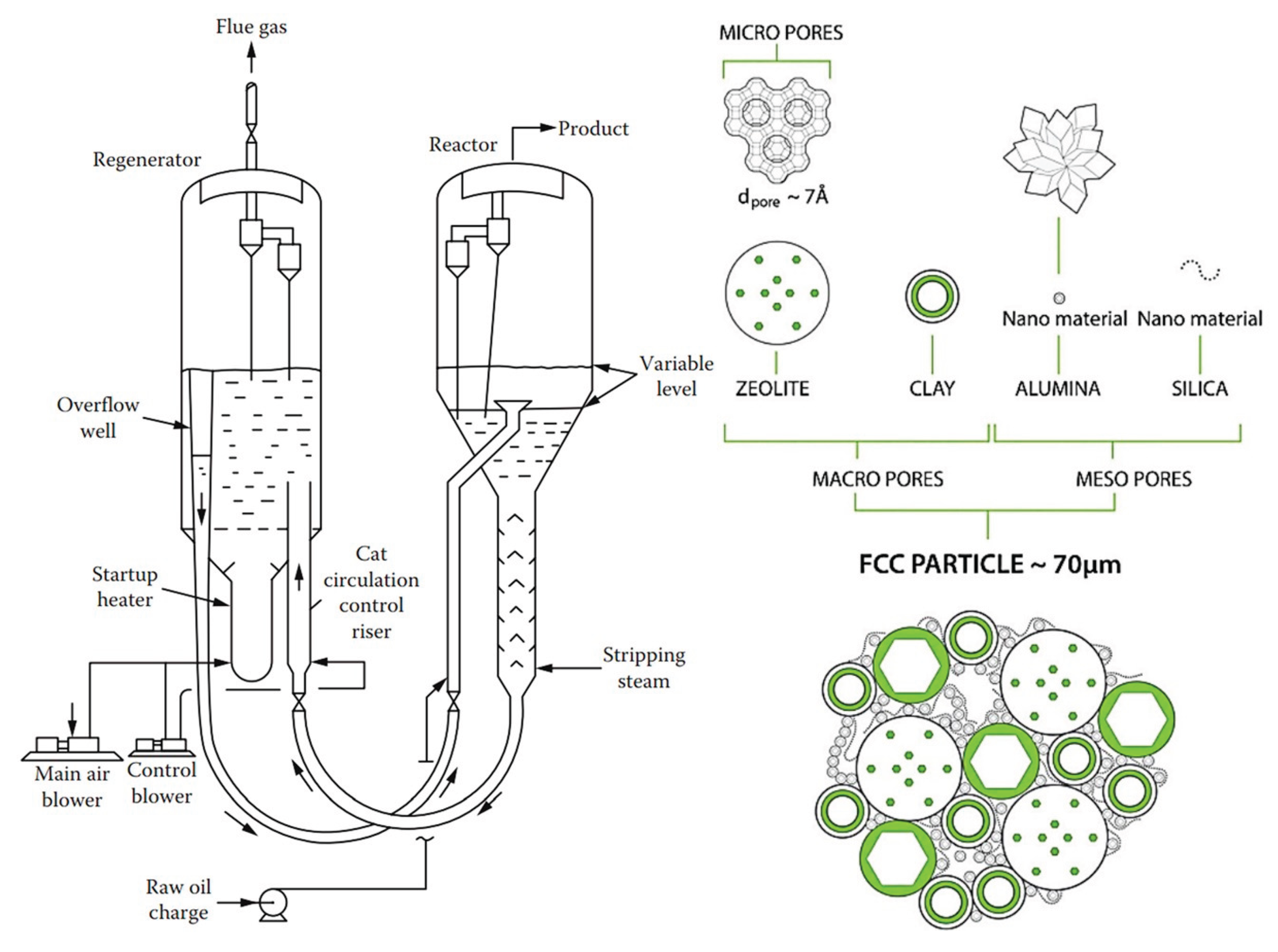
6.2. Fluid Catalytic Cracking (FCC)
6.3. Methanol
6.4. Alkyl Tert-Butyl Ethers (ATBE)
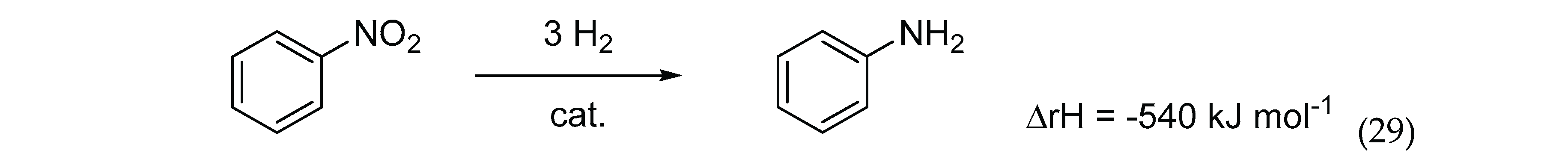
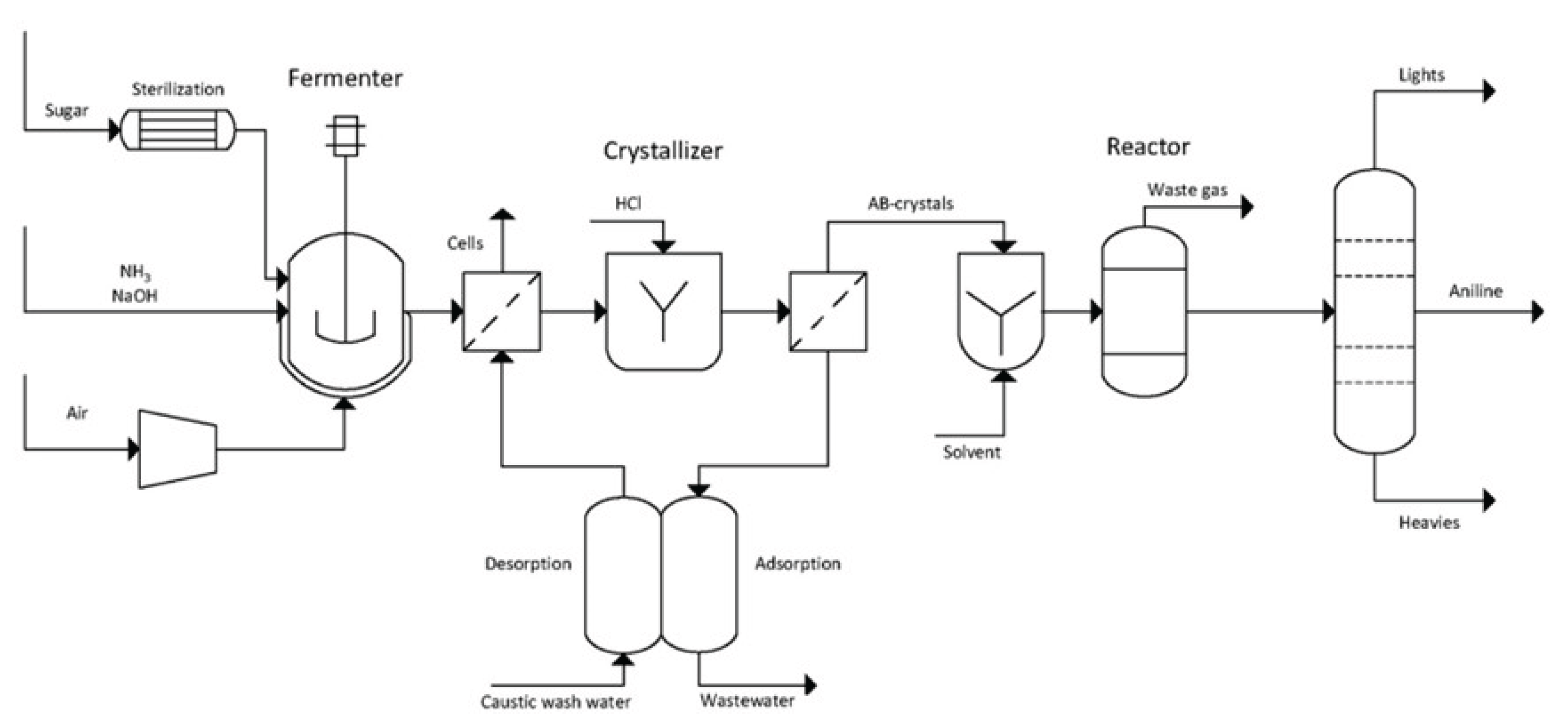
6.5. Aniline (AN)
- Fermentation of sugars in the presence of ammonia to form ammonium 2-aminobenzoate (NH₄–OAB)
- Thermal and/or catalytic decarboxylation NH4-OAB to aniline
- Extraction of the resulting aniline using a suitable solvent (e.g., dodecanol)
- Separation of aniline via rectification and solvent recycling
- Separation and recycling of ammonia
7. Conclusions
- Prioritize biotechnological methods (see examples in [588]).
- Utilize electrochemical processes when affordable electricity is available.
- Apply photocatalytic techniques for waste treatment and the production of chemical specialties.
- When opting for a heterogeneous catalyst, verify its availability from commercial suppliers.
- Catalyst development should follow thorough physicochemical analysis, including molecular modeling (using appropriate software), diffusion properties, thermal and chemical stability, toxicity, cost of preparation chemicals, ecological impact, regeneration potential, disposal methods, and reproducibility. Adhere strictly to principles of green and sustainable chemistry [22,105,108].
- Use commercial laboratory reactors or design custom ones for catalytic testing. Conduct sufficient number of experiments to evaluate activity, selectivity, temperature effects on performance, and catalyst lifetime.
- Develop a robust physicochemical model of the reactor suitable for scale-up, employing appropriate software tools.
- Favor continuous reactors over batch reactors whenever feasible.
- For low-scale production (up to 1 kg/h), consider the use of flow microreactors.
- In exothermic reactions, design for the highest possible operating temperature to efficiently utilize generated heat, while accounting for material stability and product selectivity.
- Address the trade-off between reaction rate and equilibrium composition by optimizing temperature regimes and feed locations. Consider autothermal reactors (e.g., those used in ammonia synthesis [6]).
- Compile data on the physicochemical hazards and toxicities of individual components and mixtures (e.g., explosion limits).
- If application tests are required (for new polymers/composites, biologically active compounds, polymer additives, fertilizers, etc.), propose test protocols and estimate the necessary sample quantities.
- Prepare detailed Block Flow Diagrams.
- Estimate raw material and energy consumption, along with waste generation.
- Assess economic feasibility.
- Identify and evaluate risks: economic, safety, ecological, and technological.
- Define open questions and uncertainties.
- Recommend pilot plant implementation when necessary to resolve open questions and minimize risks.
- Collaborate on feasibility studies and basic design phases.
- Participate in large-scale technology testing.
- Engage in the optimization of large-scale processes.
- Collect and maintain data for the future development of similar technologies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Jess, A.; Wasserscheid, P., Chemical Technology: From Principles to Products. 2nd ed.; John Wiley & Sons: SPi Global, Chennai, India, 2020; p 1-912.
- Murzin, D. Y., Chemical Product Technology. De Gruyter: 2018; p 1-280.
- Rothenberg, G., Catalysis: Concepts and Green Applications. John Wiley and Sons: 2008; p 1-279.
- Benvenuto, M. A., Industrial Biotechnology. 2nd ed.; De Gruyter, Incorporated: 2024. p 1-151.
- de Gonzalo, G.; Lavandera, I., Biocatalysis for Practitioners: Techniques, Reactions and Applications. Wiley: 2021; p 1-509.
- Twigg, M. V., Catalyst Handbook, Second Edition. Manson Publishing Ltd: Frome, England, 2016; p 1-608.
- Chorkendorff, I.; Niemantsverdriet, J. W., Concepts of Modern Catalysis and Kinetics, Third, completely revised and Enlarged Edition. WILEY-VCH Verlag GmbH & Co. KGaA,: Weinheim, Germany, 2017; p 1-526.
- Germain, J. E., Catalyse hétérogène. Dunod: Paris, 1959; p 1-230.
- Stratton, S. M.; Zhang, S.; Montemore, M. M., Addressing complexity in catalyst design: From volcanos and scaling to more sophisticated design strategies. Surf. Sci. Rep. 2023, 78, (3), 100597. [CrossRef]
- Berkman, S.; Morell, J. C.; Egloff, G., Catalysis, Inorganic and Organic. Reinhold Publishing Corporation: New Yourk, 1940; p 1-1130.
- García-Urdiales, E.; Lavandera, I.; Gotor, V., Concepts in Biocatalysis. In Enzyme Catalysis in Organic Synthesis, Third Edition, Wiley-VCH: 2012; Vol. 1, pp 43-66.
- Deutschmann, O.; Knozinger, H.; Kochloefl, K.; Turek, T., Heterogeneous Catalysis and Solid Catalysts. In Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA,: Weinheim, Germany, 2009. [CrossRef]
- Constable, F. H., On the present position of the Theory of Centres of Activity in Heterogeneous Catalysis. Math. Proc. Camb. Philos. Soc. 1928, 24, (2), 291-306. [CrossRef]
- Vogt, C.; Weckhuysen, B. M., The concept of active site in heterogeneous catalysis. Nat. Rev. Chem. 2022, 6, (2), 89-111. [CrossRef]
- Hanefeld, U. E.; Lefferts, L. E., Catalysis: An Integrated Textbook for Students. Wiley-VCH: SPi Global, Chennai, India, 2018.
- Lloyd, L., Handbook of Industrial Catalysts. Springer: New York, 2011; p 1-490.
- Beeckman, J. W. L., Catalyst Engineering Technology. Fundamentals and Applications. Wiley: Pondicherry, India, 2020; p 1-301.
- Ertl, G.; Knözinger, H.; Schüth, F.; J., W.; (Eds.), Handbook of Heterogeneous Catalysis, 2nd Ed. Wiley-VCH Weinheim, 2008; p 1-3865.
- Hattori, H.; Ono, Y., Solid Acid Catalysis, From Fundamentals to Applications. CRC, Pan Stanford Publishing: Boca Raton, 2015. p 1-530. [CrossRef]
- Palmisano, G.; Jitan, S. A.; Garlisi, C., Heterogeneous Catalysis: Fundamentals, Engineering and Characterizations (with accompanying presentation slides and instructor's manual). Elsevier: 2022; p 1-319.
- Schmal, M., Heterogeneous Catalysis and its Industrial Applications. Springer University of Sao Paulo, Brazil, 2016. p 1-373.
- Török, B.; Schäfer, C.; Kokel, A., Heterogeneous Catalysis in Sustainable Synthesis. 2021; p 1-664.
- Dhagat, S.; Jujjavarapu, S. E.; Sampath Kumar, N. S.; Mahapatra, C., Recent Advances in Bioprocess Engineering and Bioreactor Design. Springer Nature: 2024; p 1-298.
- Cardona Alzate, C. A.; Solarte Toro, J. C.; Peña, Á. G., Fermentation, thermochemical and catalytic processes in the transformation of biomass through efficient biorefineries. Catal. Today 2018, 302, 61-72. [CrossRef]
- Ciriminna, R.; Meneguzzo, F.; Delisi, R.; Pagliaro, M., Citric acid: Emerging applications of key biotechnology industrial product. Chem. Cent. J. 2017, 11, (1), 2222. [CrossRef]
- Książek, E., Citric Acid: Properties, Microbial Production, and Applications in Industries. Molecules 2024, 29, (1), 22. [CrossRef]
- Dhillon, G. S.; Brar, S. K.; Verma, M.; Tyagi, R. D., Recent Advances in Citric Acid Bio-production and Recovery. Food. Bioprocess Technol. 2011, 4, (4), 505-529. [CrossRef]
- Show, P. L.; Oladele, K. O.; Siew, Q. Y.; Aziz Zakry, F. A.; Lan, J. C. W.; Ling, T. C., Overview of citric acid production from Aspergillus niger. Front. Life Sci. 2015, 8, (3), 271-283. [CrossRef]
- Reetz, M. T., Biocatalysis in organic chemistry and biotechnology: Past, present, and future. J. Am. Chem. Soc. 2013, 135, (34), 12480-12496. [CrossRef]
- Boukid, F.; Ganeshan, S.; Wang, Y.; Tülbek, M. Ç.; Nickerson, M. T., Bioengineered Enzymes and Precision Fermentation in the Food Industry. Int. J. Mol. Sci. 2023, 24, (12), 10156. [CrossRef]
- Ingram, A. A.; Oike, K., Artificial Biocatalysis: Quo Vadis? ChemCatChem 2024, 16, (13), e202301759. [CrossRef]
- Rudroff, F.; Mihovilovic, M. D.; Gröger, H.; Snajdrova, R.; Iding, H.; Bornscheuer, U. T., Opportunities and challenges for combining chemo- and biocatalysis. Nat. Catal. 2018, 1, (1), 12-22. [CrossRef]
- de Kok, N. A. W.; Schmidt, S., Tapping into abiological reaction chemistries in biocatalysis. Chem. Catal. 2023, 3, (3), 100493. [CrossRef]
- Jain, S.; Ospina, F.; Hammer, S. C., A New Age of Biocatalysis Enabled by Generic Activation Modes. JACS Au 2024, 4, (6), 2068-2080. [CrossRef]
- Zhao, F.; Mattana, A.; Alam, R.; Montgomery, S. L.; Pandya, A.; Manetti, F.; Dominguez, B.; Castagnolo, D., Cooperative chemoenzymatic and biocatalytic cascades to access chiral sulfur compounds bearing C(sp3)–S stereocentres. Nat. Commun. 2024, 15, (1), 8332. [CrossRef]
- France, S. P.; Lewis, R. D.; Martinez, C. A., The Evolving Nature of Biocatalysis in Pharmaceutical Research and Development. JACS Au 2023, 3, (3), 715-735. [CrossRef]
- Zhang, N.; Domínguez de María, P.; Kara, S., Biocatalysis for the Synthesis of Active Pharmaceutical Ingredients in Deep Eutectic Solvents: State-of-the-Art and Prospects. Catalysts 2024, 14, (1), 84. [CrossRef]
- Santi, M.; Sancineto, L.; Nascimento, V.; Azeredo, J. B.; Orozco, E. V. M.; Andrade, L. H.; Gröger, H.; Santi, C., Flow biocatalysis: A challenging alternative for the synthesis of APIs and natural compounds. Int. J. Mol. Sci. 2021, 22, (3), 990. [CrossRef]
- Dalko, P. I.; Moisan, L., Enantioselective organocatalysis. Angew. Chem. Int. Ed. 2001, 40, (20), 3726-3748. [CrossRef]
- Cmelová, P.; Vargová, D.; Sebesta, R., Hybrid Peptide-Thiourea Catalyst for Asymmetric Michael Additions of Aldehydes to Heterocyclic Nitroalkenes. J. Org. Chem. 2021, 86, (1), 581-592. [CrossRef]
- Yang, X. F.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T., Single-atom catalysts: A new frontier in heterogeneous catalysis. Acc. Chem. Res. 2013, 46, (8), 1740-1748. [CrossRef]
- Yang, J.; Huang, Y.; Qi, H.; Zeng, C.; Jiang, Q.; Cui, Y.; Su, Y.; Du, X.; Pan, X.; Liu, X.; Li, W.; Qiao, B.; Wang, A.; Zhang, T., Modulating the strong metal-support interaction of single-atom catalysts via vicinal structure decoration. Nat. Commun. 2022, 13, (1), 4244. [CrossRef]
- He, L.; Guan, C.; Bulushev, D. A.; Xiang, Q., Regulation of Metal-Support Interaction in Single-Atom Catalysis. Small 2024. [CrossRef]
- Melchionna, M.; Fornasiero, P., On the Tracks to “Smart” Single-Atom Catalysts. J. Am. Chem. Soc. 2025, 147, (3), 2275-2290. [CrossRef]
- Allen J. Bard, A. J.; Larry R. Faulkner, L. R.; Henry S. White, H. S., Electrochemical Methods, Fundamentals and Applications, Third Edition. John Wiley & Sons, Ltd: New York, 2022; p 1-1104.
- Duca, M.; Koper, M. T. M., Fundamental Aspects of Electrocatalysis. In Surface and Interface Science, Volume 8: Interfacial Electrochemistry, wiley: 2018; Vol. 8, pp 773-890.
- Prajapati, A.; Hahn, C.; Weidinger, I. M.; Shi, Y. M.; Lee, Y. H. Y.; Alexandrova, A. N.; Thompson, D.; Bare, S. R.; Chen, S.; Yan, S.; Kornienko, N., Best practices for in-situ and operando techniques within electrocatalytic systems. Nat. Commun. 2025, 16, (1), 202593. [CrossRef]
- Liu, K. K.; Meng, Z.; Fang, Y.; Jiang, H. L., Conductive MOFs for electrocatalysis and electrochemical sensor. eScience 2023, 3, (6), 100133. [CrossRef]
- Palma, V.; Barba, D.; Cortese, M.; Martino, M.; Renda, S.; Meloni, E., Microwaves and heterogeneous catalysis: A review on selected catalytic processes. Catalysts 2020, 10, (2), 246. [CrossRef]
- Alazemi, A. M.; Dawood, K. M.; Al-Matar, H. M.; Tohamy, W. M., Efficient and Recyclable Solid-Supported Pd(II) Catalyst for Microwave-Assisted Suzuki Cross-Coupling in Aqueous Medium. ACS Omega 2022, 7, (33), 28831-28848. [CrossRef]
- Baruah, D.; Saikia, U. P.; Pahari, P.; Konwar, D., Cu-nanoparticles on cellulose/H2O-CH3CN/microwave: A green system for the selective oxidation of alcohols to aldehydes. Tetrahedron Lett. 2015, 56, (20), 2543-2547. [CrossRef]
- Bo, L.; Sun, S., Microwave-assisted catalytic oxidation of gaseous toluene with a Cu-Mn-Ce/cordierite honeycomb catalyst. Front. Chem. Sci. Eng. 2019, 13, (2), 385-392. [CrossRef]
- Xiang, H.; Zainal, S.; Jones, H.; Ou, X.; D'Agostino, C.; Esteban, J.; Parlett, C. M. A.; Fan, X., Hierarchical zeolite catalysed fructose dehydration to 5-hydroxymethylfurfural within a biphasic solvent system under microwave irradiation. RSC Sustain. 2023, 1, (6), 1530-1539. [CrossRef]
- Toukoniitty, B.; Mikkola, J. P.; Murzin, D. Y.; Salmi, T., Utilization of electromagnetic and acoustic irradiation in enhancing heterogeneous catalytic reactions. Appl. Catal. A: Gen. 2005, 279, (1-2), 1-22. [CrossRef]
- Suslick, K. S.; Skrabalak, S. E., Sonocatalysis. In Handbook of Heterogeneous Catalysis, Ertl, G.; Knozinger, H.; Weitkamp, J., Eds. Weinheim: Wiley-VCH, 2008; Vol. 4, pp 2006-2017.
- Kuna, E.; Behling, R.; Valange, S.; Chatel, G.; Colmenares, J. C., Sonocatalysis: A Potential Sustainable Pathway for the Valorization of Lignocellulosic Biomass and Derivatives. Top. Curr. Chem. 2017, 375, (2), 41. [CrossRef]
- Cintas, P.; Cravotto, G.; Gaudino, E. C.; Orio, L.; Boffa, L., Reticulated Pd(II)/Cu(I) cyclodextrin complexes as recyclable green catalyst for Sonogashira alkynylation. Catal. Sci. Technol. 2012, 2, (1), 85-87. [CrossRef]
- Albano, G.; Interlandi, S.; Evangelisti, C.; Aronica, L. A., Polyvinylpyridine-Supported Palladium Nanoparticles: A Valuable Catalyst for the Synthesis of Alkynyl Ketones via Acyl Sonogashira Reactions. Catal. Lett. 2020, 150, (3), 652-659. [CrossRef]
- Swiegers, G. F., Mechanical Catalysis: Methods of Enzymatic, Homogeneous, and Heterogeneous Catalysis. John Wiley and Sons: 2008; p 1-351.
- Čarný, T.; Kisszékelyi, P.; Markovič, M.; Gracza, T.; Koóš, P.; Šebesta, R., Mechanochemical Pd-Catalyzed Amino- and Oxycarbonylations using FeBr2(CO)4 as a CO Source. Org. Lett. 2023, 25, (48), 8617-8621. [CrossRef]
- Danielis, M.; Colussi, S.; Divins, N. J.; Soler, L.; Trovarelli, A.; Llorca, J., Mechanochemistry: A Green and Fast Method to Prepare a New Generation of Metal Supported Catalysts. Johns. Matthey Technol. Rev. 2024, 68, (2), 217-231. [CrossRef]
- Nezhad, S. M.; Pourmousavi, S. A.; Zare, E. N.; Heidari, G.; Makvandi, P., Magnetic Sulfonated Melamine-Formaldehyde Resin as an Efficient Catalyst for the Synthesis of Antioxidant and Antimicrobial Pyrazolone Derivatives. Catalysts 2022, 12, (6), 626. [CrossRef]
- Liu, D.; Huang, Y.; Hu, J.; Wang, B.; Lu, Y., Multiscale Catalysis Under Magnetic Fields: Methodologies, Advances, and Trends. ChemCatChem 2022, 14, (24), e202200889. [CrossRef]
- Khan, M. M., Principles and mechanisms of photocatalysis. In Photocatalytic Systems by Design: Materials, Mechanisms and Applications, Elsevier: 2021; pp 1-22.
- Ziegenbalg, D.; Pannwitz, A.; Rau, S.; Dietzek-Ivanšić, B.; Streb, C., Comparative Evaluation of Light-Driven Catalysis: A Framework for Standardized Reporting of Data**. Angew. Chem. Int. Ed. 2022, 61, (28), e202114106. [CrossRef]
- Pellejero, I.; Clemente, A.; Reinoso, S.; Cornejo, A.; Navajas, A.; Vesperinas, J. J.; Urbiztondo, M. A.; Gandía, L. M., Innovative catalyst integration on transparent silicone microreactors for photocatalytic applications. Catal. Today 2022, 383, 164-172. [CrossRef]
- Do, H. H.; Tran, T. K. C.; Ung, T. D. T.; Dao, N. T.; Nguyen, D. D.; Trinh, T. H.; Hoang, T. D.; Le, T. L.; Tran, T. T. H., Controllable fabrication of photocatalytic TiO2 brookite thin film by 3D-printing approach for dyes decomposition. J. Water Process Eng. 2021, 43, 102319. [CrossRef]
- Abhishek, B.; Jayarama, A.; Rao, A. S.; Nagarkar, S. S.; Dutta, A.; Duttagupta, S. P.; Prabhu, S. S.; Pinto, R., Challenges in photocatalytic hydrogen evolution: Importance of photocatalysts and photocatalytic reactors. Int. J. Hydrogen Energy 2024, 81, 1442-1466. [CrossRef]
- Li, Z.; Zhi, Y.; Shao, P.; Xia, H.; Li, G.; Feng, X.; Chen, X.; Shi, Z.; Liu, X., Covalent organic framework as an efficient, metal-free, heterogeneous photocatalyst for organic transformations under visible light. Appl. Catal. B: Environ. 2019, 245, 334-342. [CrossRef]
- Butova, V. V.; Burachevskaya, O. A.; Podshibyakin, V. A.; Shepelenko, E. N.; Tereshchenko, A. A.; Shapovalova, S. O.; Il’in, O. I.; Bren’, V. A.; Soldatov, A. V., Photoswitchable zirconium mof for light-driven hydrogen storage. Polymers 2021, 13, (22), 4052. [CrossRef]
- Yan, G.; Sun, X.; Zhang, K.; Zhang, Y.; Li, H.; Dou, Y.; Yuan, D.; Huang, H.; Jia, B.; Li, H.; Ma, T., Integrating Covalent Organic Framework with Transition Metal Phosphide for Noble-Metal-Free Visible-Light-Driven Photocatalytic H2 Evolution. Small 2022, 18, (25), 2201340. [CrossRef]
- Jia, T.; Zhao, Y.; Song, W.; Zhao, J., Metallaphotocatalytic Platforms Based on Covalent Organic Frameworks for Cross-Coupling Reactions. ChemCatChem 2024, 16, (7), e202301522. [CrossRef]
- Araujo, T. P.; Quiroz, J.; Barbosa, E. C. M.; Camargo, P. H. C., Understanding plasmonic catalysis with controlled nanomaterials based on catalytic and plasmonic metals. Curr. Opin. Colloid Interface Sci. 2019, 39, 110-122. [CrossRef]
- Zhang, Z.; Cao, X.; Geng, C.; Sun, Y.; He, Y.; Qiao, Z.; Zhong, C., Machine learning aided high-throughput prediction of ionic liquid@MOF composites for membrane-based CO2 capture. J. Membr. Sci. 2022, 650, 120399. [CrossRef]
- Martirez, J. M. P.; Bao, J. L.; Carter, E. A., First-Principles Insights into Plasmon-Induced Catalysis. Annu. Rev. Phys. Chem. 2020, 72, 99-119. [CrossRef]
- Rogolino, A.; Claes, N.; Cizaurre, J.; Marauri, A.; Jumbo-Nogales, A.; Lawera, Z.; Kruse, J.; Sanromán-Iglesias, M.; Zarketa, I.; Calvo, U.; Jimenez-Izal, E.; Rakovich, Y. P.; Bals, S.; Matxain, J. M.; Grzelczak, M., Metal-Polymer Heterojunction in Colloidal-Phase Plasmonic Catalysis. J. Phys. Chem. Lett. 2022, 13, (10), 2264-2272. [CrossRef]
- Bößl, F.; Tudela, I., Piezocatalysis: Can catalysts really dance? Curr. Opin. Green Sustain. Chem. 2021, 32, 100537. [CrossRef]
- Meng, N.; Liu, W.; Jiang, R.; Zhang, Y.; Dunn, S.; Wu, J.; Yan, H., Fundamentals, advances and perspectives of piezocatalysis: A marriage of solid-state physics and catalytic chemistry. Prog. Mater. Sci. 2023, 138, 101161. [CrossRef]
- He, J.; Dong, C.; Chen, X.; Cai, H.; Chen, X.; Jiang, X.; Zhang, Y.; Peng, A.; Badsha, M. A. H., Review of Piezocatalysis and Piezo-Assisted Photocatalysis in Environmental Engineering. Crystals 2023, 13, (9), 1382. [CrossRef]
- Fojt, J.; Erhart, P.; Schäfer, C., Controlling Plasmonic Catalysis via Strong Coupling with Electromagnetic Resonators. Nano Lett. 2024, 24, (38), 11913-11920. [CrossRef]
- Vensaus, P.; Liang, Y.; Ansermet, J. P.; Soler-Illia, G. J. A. A.; Lingenfelder, M., Enhancement of electrocatalysis through magnetic field effects on mass transport. Nat. Commun. 2024, 15, (1), 2867. [CrossRef]
- Song, X.; Shan, X.; Xue, H.; Li, X.; Liu, R.; Kong, J.; Zuo, Z.; Su, X.; Zhang, Q.; Yin, Y.; Cai, Y., Advances in Photothermal Catalysis: Mechanisms, Materials, and Environmental Applications. ACS Appl. Nano Mater. 2024, 7, (23), 26489-26514. [CrossRef]
- Jadhav, H.; Gogate, P.; Annapure, U., Intensification of synthesis of triglyceride of Decanoic acid in the presence of amberlyst 15 as catalyst based on the use of ultrasound and microwave irradiations. Chem. Eng. Process.: Process Intensif. 2021, 165, 108424. [CrossRef]
- Thomas, G. G.; Davies, C. W., lon Exchange Resins as Catalysts. Nature 1947, 159, 372-372,.
- Králik, M.; Koóš, P.; Markovič, M.; Lopatka, P., Organic and Metal–Organic Polymer-Based Catalysts—Enfant Terrible Companions or Good Assistants? Molecules 2024, 29, (19), 4623. [CrossRef]
- Sherrington, D. C., Polymer-supported metal complex oxidation catalysts. Pure Appl. Chem. 1988, 60, (3), 401-414. [CrossRef]
- Li, W.; Wang, F.; Shi, Y.; Yu, L., Polyaniline-supported tungsten-catalyzed oxidative deoximation reaction with high catalyst turnover number. Chin. Chem. Lett. 2023, 34, (1), 107505. [CrossRef]
- Macho, V.; Králik, M.; Jurecekova, E.; Hudec, J.; Jurecek, L., Dehydration of C4 alkanols conjugated with a positional and skeletal isomerisation of the formed C4 alkenes. Appl. Catal. A: Gen. 2001, 214, (2), 251-257. [CrossRef]
- Cui, X.; Li, W.; Ryabchuk, P.; Junge, K.; Beller, M., Bridging homogeneous and heterogeneous catalysis by heterogeneous single-metal-site catalysts. Nat. Catal. 2018, 1, (6), 385-397. [CrossRef]
- Astruc, D.; Lu, F.; Aranzaes, J. R., Nanoparticles as recyclable catalysts: The frontier between homogeneous and heterogeneous catalysis. Angew. Chem. Int. Ed. 2005, 44, (48), 7852-7872. [CrossRef]
- Mayer, A. B. R.; Mark, J. E., Transition metal nanoparticles protected by amphiphilic block copolymers as tailored catalyst systems. Colloid Polym. Sci. 1997, 275, (4), 333-340. [CrossRef]
- Králik, M.; Biffis, A., Catalysis by metal nanoparticles supported on functional organic polymers. J. Mol. Catal. A: Chem. 2001, 177, (1), 113-138. [CrossRef]
- Ding, S.; Motokura, K., Recent advances in immobilized noble metal catalysts in aqueous media for organic reactions. Curr. Opin. Green Sustain. Chem. 2023, 40, 100753. [CrossRef]
- Cozzi, F., Immobilization of organic catalysts: When, why, and how. Adv. Synth. Catal. 2006, 348, (12-13), 1367-1390. [CrossRef]
- Alács, B.; Zrinyi, A.; Hornyánszky, G.; Poppe, L.; Bell, E., Upgrading Epoxy Supports for Enzyme Immobilization by Affinity Function Doping—A Case Study with Phenylalanine Ammonia-Lyase from Petroselinum crispum. Catalysts 2024, 14, (1), 14. [CrossRef]
- Sheldon, R. A.; van Pelt, S., Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, (15), 6223-6235. [CrossRef]
- Lyu, X.; Gonzalez, R.; Horton, A.; Li, T., Immobilization of enzymes by polymeric materials. Catalysts 2021, 11, (10), 1211. [CrossRef]
- Moulijn, J.; Makkee, M.; Van Diepen, A. E., Chemical Process Technology, 2nd Ed. Wiley: New Delhi, India, 2013; p 1-566.
- Satterfield, C. N., Heterogeneous Catalysis in Industrial Practice. McGraw-Hill: New York, 1996; p 1-564.
- Cole-Hamilton, D. J., Homogeneous catalysis - New approaches to catalyst separation, recovery, and recycling. Science 2003, 299, (5613), 1702-1706. [CrossRef]
- Fassbach, T. A.; Ji, J. M.; Vorholt, A. J.; Leitner, W., Recycling of Homogeneous Catalysts─Basic Principles, Industrial Practice, and Guidelines for Experiments and Evaluation. ACS Catal. 2024, 14, (9), 7289-7298. [CrossRef]
- Salmi, T.; Murzin, D. Y.; Wärn, J.; Kangas, M.; Toukoniitty, E.; Nieminen, V., An integrated approach to modelling of chemical transformations in chemical reactors. Comput. Aided Chem. Eng. 2005, 20, (C), 1531-1536. [CrossRef]
- Murzin, D. Y.; Salmi, T., Catalytic Kinetics: Chemistry and Engineering: Second Edition. Elsevier Inc.: Amsterdam, Netherlands, 2016; p 1-740.
- Murzin, D. Y., Chemical Reaction Technology, 2nd ed. De Gruyter: Berlin, 2022; p 1-625.
- Anastas, P.; Eghbali, N., Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, (1), 301-312. [CrossRef]
- Amoneit, M.; Weckowska, D.; Spahr, S.; Wagner, O.; Adeli, M.; Mai, I.; Haag, R., Green chemistry and responsible research and innovation: Moving beyond the 12 principles. J. Clean. Prod. 2024, 484, 144011. [CrossRef]
- Kaur, A.; Goyal, A.; Kumari, S.; Garg, M.; Sharma, S.; Akram, M.; Laila, U.; Khalil, M. T.; Kaur, P.; Sahu, S. K. In Green chemistry and catalysis: An emerging sustainable approach in synthetic chemistry, BIO Web of Conferences, 2024; EDP Sciences.
- Kurul, F.; Doruk, B.; Topkaya, S. N., Principles of green chemistry: building a sustainable future. Discov. Chem. 2025, 2, 1-25. [CrossRef]
- Haber, J., Manual on catalyst characterization. Pure Appl. Chem. 1991, 63, (9), 1227-1246. [CrossRef]
- Dybkaer, R., Unit "katal" for catalytic activity (IUPAC Technical Report). Pure Appl. Chem. 2001, 73, (6), 927-931. [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A. V.; Olivier, J. P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K. S. W., Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, (9-10), 1051-1069. [CrossRef]
- Bond, J. Q.; Stangland, E. E.; Cybulskis, V. J., Best practices in the characterization of bulk catalyst properties. J. Catal. 2024, 433, 115487. [CrossRef]
- Che, M.; Védrine, J. C., Characterization of Solid Materials and Heterogeneous Catalysts: From Structure to Surface Reactivity, Volume 1&2. Wiley-VCH: 2012; Vol. 1-2, p 1-1224.
- Gašparovičová, D.; Králik, M.; Horváth, B.; Soták, T.; Hudec, P., Liquid phase oxidation of cyclopentanone over metal-free carbon catalysts. Chem. Pap. 2024, 78, (10), 5943-5960. [CrossRef]
- Králik, M., Adsorption, chemisorption, and catalysis. Chem. Pap. 2014, 68, (12), 1625-1638. [CrossRef]
- Biffis, A.; Corain, B.; Cvengros ̌ová, Z.; Hronec, M.; Jer ̌ábek, K.; Králik, M., Relationships between physico-chemical properties and catalytic activity of polymer-supported palladium catalysts. Part I. Experimental investigations. Appl. Catal. A: Gen. 1995, 124, (2), 355-365. [CrossRef]
- Králik, M.; Corain, B.; Zecca, M., Catalysis by metal nanoparticles supported on functionalized polymers. Chem. Pap. 2000, 54, (4), 254-264,.
- Galet, L.; Patry, S.; Dodds, J., Determination of the wettability of powders by the Washburn capillary rise method with bed preparation by a centrifugal packing technique. J. Colloid Interface Sci. 2010, 346, (2), 470-475. [CrossRef]
- Saidi, M.; Bihl, F.; Gimello, O.; Louis, B.; Roger, A. C.; Trens, P.; Salles, F., Evaluation of the Hydrophilic/Hydrophobic Balance of 13X Zeolite by Adsorption of Water, Methanol, and Cyclohexane as Pure Vapors or as Mixtures. Nanomaterials 2024, 14, (2), 213. [CrossRef]
- Lopes, M. A.; Monteiro, F. J.; Santos, J. D.; Serro, A. P.; Saramago, B., Hydrophobicity, surface tension, and zeta potential measurements of glass-reinforced hydroxyapatite composites. J. Biomed. Mater. Res. 1999, 45, (4), 370-375. [CrossRef]
- Jablonský, M.; Botková, M.; Šutý, S.; Šmatko, L.; Šima, J., Accelerated ageing of newsprint paper: Changes in swelling ability, WRV and electrokinetic properties of fibres. Fibres Text. East. Eur. 2014, 104, (2), 108-113,.
- Luna-Valenzuela, A.; Cabellos, J. L.; Alonso, J. A.; Posada-Amarillas, A., Effects of van der Waals interactions on the structure and stability of Cu8-xPdx (x = 0, 4, 8) cluster isomers. Mater. Today Commun. 2021, 26, 102024. [CrossRef]
- Parks, G. A., The Isoelectric Points of Solid Oxides, Solid Hydroxides, and Aqueous Hydroxo Complex Systems. Chem. Rev. 1965, 65, (2), 177-198. [CrossRef]
- Chang, M.; Liu, X.; Nelson, P. J.; Munzing, G. R.; Gegan, T. A.; Kissin, Y. V., Ziegler-Natta catalysts for propylene polymerization: Morphology and crystal structure of a fourth-generation catalyst. J. Catal. 2006, 239, (2), 347-353. [CrossRef]
- Ountaksinkul, K.; Sripinun, S.; Bumphenkiattikul, P.; Vongachariya, A.; Praserthdam, P.; Assabumrungrat, S., A Review of Packed Bed Reactor and Gradient-less Recycle Reactor for Determination of Intrinsic Reaction Kinetics. Eng. J. 2022, 26, (12), 17-42. [CrossRef]
- Carberry, J. J., Chemical and Catalytic Reaction Engineering. Courier Corporation, 2001: General Publishing Company, Ontario, Canada, 2001; p 1-642.
- GmbH, H. Laboratory systems. https://www.hte-company.com/en/laboratory-systems/ (Feb. 15, 2025),.
- Čejka, J.; Morris, R. E.; Nachtigall, P.; (Eds.), Zeolites in Catalysis: Properties and Applications. RSC: London, 2017; p 1-546.
- Hou, Q.; Zheng, B.; Bi, C.; Luan, J.; Zhao, Z.; Guo, H.; Wang, G.; Li, Z., Liquid-phase cascade acylation/dehydration over various zeolite catalysts to synthesize 2-methylanthraquinone through an efficient one-pot strategy. J. Catal. 2009, 268, (2), 376-383. [CrossRef]
- Murzin, D. Y., Rate Equations of Structure-Sensitive Catalytic Reactions with Arbitrary Kinetics. Chem. Eng. 2023, 7, (1), 12. [CrossRef]
- Lu, L.; Zou, S.; Fang, B., The Critical Impacts of Ligands on Heterogeneous Nanocatalysis: A Review. ACS Catal. 2021, 11, (10), 6020-6058. [CrossRef]
- Hao, L.; Zhao, Y.; Yu, B.; Zhang, H.; Xu, H.; Xu, J.; Liu, Z., Polyurea-supported metal nanocatalysts: Synthesis, characterization and application in selective hydrogenation of o-chloronitrobenzene. J. Colloid Interface Sci. 2014, 424, 44-48. [CrossRef]
- Mahdavi, H.; Sahraei, R., Synthesis and Application of Hyperbranched Polyester-Grafted Polyethylene (HBPE-g-PE) Containing Palladium Nanoparticles as Efficient Nanocatalyst. Catal. Lett. 2016, 146, (5), 977-990. [CrossRef]
- Murzin, D. Y., Kinetics of enantioselective heterogeneous nanocatalysis with limited cluster occupancy: Beyond the coverage concept. Chem. Eng. J. 2024, 497, 154570. [CrossRef]
- Stiles, A. B.; Koch, T. A., Catalyst Manufacture, Second Edition. CRC Press: 1995; p 1-312.
- Smith, A. J.; Trimm, D. L., The preparation of skeletal catalysts. Annu. Rev. Mater. Res. 2005, 35, 127-142. [CrossRef]
- Bendová, H.; Kamenická, B.; Weidlich, T.; Beneš, L.; Vlček, M.; Lacina, P.; Švec, P., Application of Raney Al-Ni Alloy for Simple Hydrodehalogenation of Diclofenac and Other Halogenated Biocidal Contaminants in Alkaline Aqueous Solution under Ambient Conditions. Materials 2022, 15, (11), 3939. [CrossRef]
- Salanov, A. N.; Serkova, A. N.; Chesnokova, N. M.; Isupova, L. A.; Parmon, V. N., Characterization of platinum alloy used in ammonia oxidation. Morphology and microstructure of Pt–Pd–Rh–Ru gauzes after the oxidation of NH3 with air at 1133 K. Mater. Chem. Phys. 2021, 273, 125138. [CrossRef]
- Wittstock, A.; Bäumer, M., Catalysis by unsupported skeletal gold catalysts. Acc. Chem. Res. 2014, 47, (3), 731-739. [CrossRef]
- Shigapov, A. N.; Graham, G. W.; McCabe, R. W.; Peck, M. P.; Plummer Jr, H. K., The preparation of high-surface-area cordierite monolith by acid treatment. Appl. Catal. A: Gen. 1999, 182, (1), 137-146. [CrossRef]
- Zhu, Y.; Lv, G.; Song, C.; Li, B.; Zhu, Y.; Liu, Y.; Zhang, W.; Wang, Y., Optimization of the washcoat slurry for hydrotalcite-based lnt catalyst. Catalysts 2019, 9, (8), 696. [CrossRef]
- Lam, E.; Luong, J. H. T., Carbon materials as catalyst supports and catalysts in the transformation of biomass to fuels and chemicals. ACS Catal. 2014, 4, (10), 3393-3410. [CrossRef]
- Ma, A. Z.; Muhler, M.; Grünert, W., Selective catalytic reduction of NO by ammonia over Raney-Ni supported Cu-ZSM-5. II. Interactions between support and supported Cu-ZSM-5. Appl. Catal. B: Environ. 2000, 27, (1), 37-47. [CrossRef]
- Niculescu, A. G.; Tudorache, D. I.; Bocioagă, M.; Mihaiescu, D. E.; Hadibarata, T.; Grumezescu, A. M., An Updated Overview of Silica Aerogel-Based Nanomaterials. Nanomaterials 2024, 14, (5), 469. [CrossRef]
- Dunn, B. C.; Cole, P.; Covington, D.; Webster, M. C.; Pugmire, R. J.; Ernst, R. D.; Eyring, E. M.; Shah, N.; Huffman, G. P., Silica aerogel supported catalysts for Fischer-Tropsch synthesis. Appl. Catal. A: Gen. 2005, 278, (2), 233-238. [CrossRef]
- Bang, J. H.; Jang, Y. N.; Kim, W.; Song, K. S.; Jeon, C. W.; Chae, S. C.; Lee, S. W.; Park, S. J.; Lee, M. G., Specific surface area and particle size of calcium carbonate precipitated by carbon dioxide microbubbles. Chem. Eng. J. 2012, 198-199, 254-260. [CrossRef]
- Wang, X.; Fang, Q.; Wang, J.; Gui, K.; Thomas, H. R., Effect of CaCO3 on catalytic activity of Fe-Ce/Ti catalysts for NH3-SCR reaction. RSC Adv. 2020, 10, (73), 44876-44883. [CrossRef]
- Diamond, S.; Kinter, E. B., Surface Areas Of Clay Minerals As Derived From Measurements Of Glycerol Retention Clays and Clay Minerals 1956, 334-347. [CrossRef]
- Trujillano, R.; González-García, I.; Morato, A.; Rives, V., Controlling the synthesis conditions for tuning the properties of hydrotalcite-like materials at the nano scale. Chem. Eng. 2018, 2, (3), 1-1531. [CrossRef]
- Fierro, J. L. G., Structure and composition of perovskite surface in relation to adsorption and catalytic properties. Catal. Today 1990, 8, (2), 153-174. [CrossRef]
- Shi, L.; Chang, D.; Ji, X.; Lu, W., Using Data Mining to Search for Perovskite Materials with Higher Specific Surface Area. J. Chem. Inf. Model. 2018, 58, (12), 2420-2427. [CrossRef]
- Elhage, A.; Wang, B.; Marina, N.; Marin, M. L.; Cruz, M.; Lanterna, A. E.; Scaiano, J. C., Glass wool: a novel support for heterogeneous catalysis. Chem. Sci. 2018, 9, (33), 6844-6852. [CrossRef]
- Hronec, M.; Cvengrošová, Z.; Králik, M.; Palma, G.; Corain, B., Hydrogenation of benzene to cyclohexene over polymer-supported ruthenium catalysts. J. Mol. Catal. A: Chem. 1996, 105, (1-2), 25-30. [CrossRef]
- Cui, H.; Li, P.; Chen, Y.; Ai, W.; Liu, Y.; Zou, J.; Jiao, M.; Shi, X. L., Practical Applications of Fiber-Supported Catalysts in Organic Transformations. ChemistrySelect 2024, 9, (43), e202402382. [CrossRef]
- Radwan, N. R. E.; El-Sharkawy, E. A.; Youssef, A. M., Influence of gold and manganese as promoters on surface and catalytic performance of Fe2O3/Al2O3 system. Appl. Catal. A: Gen. 2005, 281, (1-2), 93-106. [CrossRef]
- Luo, Z.; Zhao, G.; Pan, H.; Sun, W., Strong Metal–Support Interaction in Heterogeneous Catalysts. Adv. Energy Mater. 2022, 12, (37), 2201395. [CrossRef]
- Poelman, H.; Eufinger, K.; Depla, D.; Poelman, D.; De Gryse, R.; Sels, B. F.; Marin, G. B., Magnetron sputter deposition for catalyst synthesis. Appl. Catal. A: Gen. 2007, 325, (2), 213-219. [CrossRef]
- Rossnagel, S. M., Magnetron sputtering. J. Vac. Sci. Technol. A 2020, 38, (6), 060805. [CrossRef]
- Králik, M.; Hronec, M.; Lora, S.; Palma, G.; Zecca, M.; Biffis, A.; Corain, B., Microporous poly-N,N-dimethylacrylamide-p-styrylsulfonate-methylene bis(acrylamide): a promising support for metal catalysis. J. Mol. Catal. A: Chem. 1995, 97, (3), 145-155. [CrossRef]
- Markovič, M.; Lopatka, P.; Koóš, P.; Soták, T.; Ház, A.; Gracza, T.; Ley, S. V.; Králik, M., Palladium catalysts supported on biodegradable urea-based polymers in synthesis with CO – Part B. Catal. Today 2024, 440, 114831. [CrossRef]
- Cornils, B.; Herrmann, W. A.; Zanthoff, H.-W.; Wong, C.-H., Catalysis from A to Z: A Concise Encyclopedia. Wiley: Singapore, 2013.
- Hutchings, G. J., Promotion in heterogeneous catalysis: A topic requiring a new approach? Catal. Lett. 2001, 75, (1-2), 1-12. [CrossRef]
- Brosda, S.; Vayenas, C. G.; Wei, J., Rules of chemical promotion. Appl. Catal. B: Environ. 2006, 68, (3-4), 109-124. [CrossRef]
- Cao, A.; Bukas, V. J.; Shadravan, V.; Wang, Z.; Li, H.; Kibsgaard, J.; Chorkendorff, I.; Nørskov, J. K., A spin promotion effect in catalytic ammonia synthesis. Nat. Commun. 2022, 13, (1), 2382. [CrossRef]
- Rocha, T. C. R.; Hävecker, M.; Knop-Gericke, A.; Schlögl, R., Promoters in heterogeneous catalysis: The role of Cl on ethylene epoxidation over Ag. J. Catal. 2014, 312, 12-16. [CrossRef]
- Santos, V. P.; Plauck, A.; Gold, J.; Majumdar, P.; McAdon, M. H.; Calverley, T., The Complex Chlorination Effects on High Selectivity Industrial EO Catalysts: Dynamic Interplay between Catalyst Composition and Process Conditions. ACS Catal. 2024, 14, (14), 10839-10852. [CrossRef]
- McCue, A. J.; Anderson, J. A., Sulfur as a catalyst promoter or selectivity modifier in heterogeneous catalysis. Catal. Sci. Technol. 2014, 4, (2), 272-294. [CrossRef]
- Yamada, H.; Saito, K.; Goto, S.; Tagawa, T., Effect of Teflon powder deposition on the catalyst in a four-phase trickle-bed reactor. Ind. Eng. Chem. Res. 2005, 44, (16), 6403-6405. [CrossRef]
- Bartlewicz, O.; Dąbek, I.; Szymańska, A.; Maciejewski, H., Heterogeneous catalysis with the participation of ionic liquids. Catalysts 2020, 10, (11), 1227. [CrossRef]
- Sánchez-Carnerero, E. M.; Sandoval-Torrientes, R.; Urieta-Mora, J.; Moreno, F.; Maroto, B. L.; de la Moya, S., Speeding up heterogeneous catalysis with an improved highly reusable catalyst for the preparation of enantioenriched secondary alcohols. React. Funct. Polym. 2017, 113, 23-30. [CrossRef]
- Zha, K.; Zeng, T.; Zhu, M.; Wei, C.; Song, L.; Miao, C., Insights into promotional effects for ethylbenzene dehydrogenation to styrene with steam over Fe-K, Fe-K-Ce and Fe-K-Ce-Mo mixed oxide catalysts. Appl. Catal. A: Gen. 2023, 666, 119372. [CrossRef]
- Morbidelli, M.; Gavriilidis, A.; Varma, A., Catalyst Design: Optimal Distribution of Catalyst in Pellets, Reactors, and Membranes. Cambridge University Press: Cambridge, 2001; p 1-240.
- Basile, A. E.; Di Paola, L. E.; Hai, F. I. E.; Piemonte, V. E., Membrane Reactors for Energy Applications and Basic Chemical Production. Elsevier Inc.: 2015; p 1-673.
- Tan, L.; Tan, B., Hypercrosslinked porous polymer materials: Design, synthesis, and applications. Chem. Soc. Rev. 2017, 46, (11), 3322-3356. [CrossRef]
- Manaenkov, O.; Nikoshvili, L.; Bykov, A.; Kislitsa, O.; Grigoriev, M.; Sulman, M.; Matveeva, V.; Kiwi-Minsker, L., An Overview of Heterogeneous Catalysts Based on Hypercrosslinked Polystyrene for the Synthesis and Transformation of Platform Chemicals Derived from Biomass. Molecules 2023, 28, (24), 8126. [CrossRef]
- Sun, M.; Chen, C.; Chen, L.; Su, B., Hierarchically porous materials: Synthesis strategies and emerging applications. Front. Chem. Sci. Eng. 2016, 10, (3), 301-347. [CrossRef]
- Venezia, V.; Pota, G.; Silvestri, B.; Costantini, A.; Vitiello, G.; Luciani, G., Tailoring Structure: Current Design Strategies and Emerging Trends to Hierarchical Catalysts. Catalysts 2022, 12, (10), 1152. [CrossRef]
- Yang, M.; Mao, Y.; Wang, B.; Lin, L.; Wang, Y.; Zhang, L.; Jiang, Y.; Zhao, M.; Chen, H.; Zhang, Y., Heterometallic Mg@Fe-MIL-101/TpPa-1-COF grown on stainless steel mesh: Enhancing photo-degradation, fluorescent detection and toxicity assessment for tetracycline hydrochloride. Colloids Surf. A: Physicochem. Eng. Asp. 2021, 631, 127725. [CrossRef]
- Yang, L.; Li, J.; Liu, B., Recent Advances of Monolithic Metal Mesh-Based Catalysts for CO Oxidation. ChemCatChem 2024, 16, (21), e202401122. [CrossRef]
- Gheorghiu, C. C.; García-Bordejé, E.; Job, N.; Román-Martínez, M. C., Structured carbons as supports for hydrogenation hybrid catalysts prepared by the immobilization of a Rh diamine complex. Chem. Eng. J. 2016, 291, 47-54. [CrossRef]
- Jarrah, N. A.; Li, F.; Van Ommen, J. G.; Lefferts, L., Immobilization of a layer of carbon nanofibres (CNFs) on Ni foam: A new structured catalyst support. J. Mater. Chem. 2005, 15, (19), 1946-1953. [CrossRef]
- Lopatin, S.; Mikenin, P.; Elyshev, A.; Udovichenko, S.; Zagoruiko, A., Catalytic device on the base of glass-fiber catalyst for environmentally safe combustion of fuels and utilization of toxic wastes. Chem. Eng. J. 2019, 373, 406-412. [CrossRef]
- Colacot, T. J. In Palladium based FibreCat and SMOPEX® as supported homogenous catalyst systems for simple to challenging carbon-carbon coupling reactions, Top. Catal., 2008; p 91-98.
- Jiang, N.; Liu, H.; Zhao, G.; Li, H.; Yang, S.; Xu, X.; Zhuang, X.; Cheng, B., Aramid nanofibers supported metal-organic framework aerogel for protection of chemical warfare agent. J. Colloid Interface Sci. 2023, 640, 192-198. [CrossRef]
- Baharudin, L.; Watson, M. J., Monolithic substrate support catalyst design considerations for steam methane reforming operation. Rev. Chem. Eng. 2018, 34, (4), 481-501. [CrossRef]
- Pérez, N. C.; Miró, E. E.; Zamaro, J. M. In Cu, Ce/mordenite coatings on FeCrAl-alloy corrugated foils employed as catalytic microreactors for CO oxidation, Catal. Today, 2013; pp 183-191.
- Barbero, B. P.; Costa-Almeida, L.; Sanz, O.; Morales, M. R.; Cadus, L. E.; Montes, M., Washcoating of metallic monoliths with a MnCu catalyst for catalytic combustion of volatile organic compounds. Chem. Eng. J. 2008, 139, (2), 430-435. [CrossRef]
- Koga, H.; Kitaoka, T.; Isogai, A., Chemically-modified cellulose paper as a microstructured catalytic reactor. Molecules 2015, 20, (1), 1495-1508. [CrossRef]
- Yan, W. Z.; Liu, J.; Zheng, X. J.; Zhang, J.; Tang, K. Y., Wood-derived high-performance cellulose structural materials. E-Polym. 2023, 23, (1), 20230010. [CrossRef]
- Krupšová, S.; Almáši, M., Cellulose–Amine Porous Materials: The Effect of Activation Method on Structure, Textural Properties, CO2 Capture, and Recyclability. Molecules 2024, 29, (5), 1158. [CrossRef]
- Soliman, A.; Alamoodi, N.; Karanikolos, G. N.; Doumanidis, C. C.; Polychronopoulou, K., A review on new 3-d printed materials’ geometries for catalysis and adsorption: Paradigms from reforming reactions and CO2 capture. Nanomaterials 2020, 10, (11), 2198. [CrossRef]
- D’Accolti, L.; De Cataldo, A.; Montagna, F.; Esposito Corcione, C.; Maffezzoli, A., The Role of 3D Printing in the Development of a Catalytic System for the Heterogeneous Fenton Process. Polymers 2023, 15, (3), 580. [CrossRef]
- Ma, T.; Zhang, Y.; Ruan, K.; Guo, H.; He, M.; Shi, X.; Guo, Y.; Kong, J.; Gu, J., Advances in 3D printing for polymer composites: A review. InfoMat 2024, 6, (6), e12568. [CrossRef]
- Vaes, D.; Van Puyvelde, P., Semi-crystalline feedstock for filament-based 3D printing of polymers. Prog. Polym. Sci. 2021, 118, 101411. [CrossRef]
- Bagheri, A.; Jin, J., Photopolymerization in 3D Printing. ACS Appl. Polymer Mat. 2019, 1, (4), 593-611. [CrossRef]
- He, T.; Yip, W. S.; Yan, E. H.; Tang, J.; Rehan, M.; Teng, L.; Wong, C. H.; Sun, L.; Zhang, B.; Guo, F.; Zhang, S.; To, S., 3D printing for ultra-precision machining: current status, opportunities, and future perspectives. Front. Mech. Eng. 2024, 19, (4), 23. [CrossRef]
- Oshimura, H.; Tanaka, S.; Nagata, S.; Matsuura, S.; Hashimoto, T.; Ishihara, A., Preparation of Novel Hierarchical Catalysts by Simultaneous Generation of β-Zeolite and Mesoporous Silica for Catalytic Cracking. ChemPlusChem 2024, 89, (12), e202400447. [CrossRef]
- Hartmann, M.; Thommes, M.; Schwieger, W., Hierarchically-Ordered Zeolites: A Critical Assessment. Adv. Mater. Interfaces 2021, 8, (4), 2001841. [CrossRef]
- Chen, L. H.; Sun, M. H.; Wang, Z.; Yang, W.; Xie, Z.; Su, B. L., Hierarchically structured zeolites: From design to application. Chem. Rev. 2020, 120, (20), 11194-11294. [CrossRef]
- Rutkowska, M.; Chmielarz, L., Application of Mesoporous/Hierarchical Zeolites as Catalysts for the Conversion of Nitrogen Pollutants: A Review. Catalysts 2024, 14, (5), 290. [CrossRef]
- Yuan, Z.; Liu, L.; Ru, W.; Zhou, D.; Kuang, Y.; Feng, J.; Liu, B.; Sun, X., 3D printed hierarchical spinel monolithic catalysts for highly efficient semi-hydrogenation of acetylene. Nano Res. 2022, 15, (7), 6010-6018. [CrossRef]
- Tian, Z.; Deng, X.; He, P.; Wang, G. H., Atomic Pt anchored on hierarchically porous monolithic carbon nanowires as high-performance catalyst for liquid hydrogenation. Nano Res. 2023, 16, (4), 5880-5886. [CrossRef]
- Huo, C.; Tian, X.; Nan, Y.; Qiu, Z.; Zhong, Q.; Huang, X.; Li, D., 3D printing of hierarchically porous monolithic TS-1 catalyst for one-pot synthesis of ethylene glycol. Chem. Eng. J. 2022, 450, 138259. [CrossRef]
- Luo, R.; Zhu, M.; Shen, X.; Li, S., Polymer catalyst with self-assembled hierarchical access for sortable catalysis. J. Catal. 2015, 331, 49-56. [CrossRef]
- Tan, L.; Tan, B., Functionalized hierarchical porous polymeric monoliths as versatile platforms to support uniform and ultrafine metal nanoparticles for heterogeneous catalysis. Chem. Eng. J. 2020, 390, 124485. [CrossRef]
- Sun, Y.; Shi, F.; Wang, B.; Shi, N.; Ding, Z.; Xie, L.; Jiang, J.; Han, M., Large-Scale Synthesis of Hierarchical Porous MOF Particles via a Gelation Process for High Areal Capacitance Supercapacitors. Nanomaterials 2023, 13, (10), 1691. [CrossRef]
- Kim, J. J.; Lim, C. R.; Reddy, B. M.; Park, S. E., Hierarchical porous organic polymer as an efficient metal-free catalyst for acetalization of carbonyl compounds with alcohols. Mol. Cat. 2018, 451, 43-50. [CrossRef]
- Yuan, S.; Zou, L.; Qin, J. S.; Li, J.; Huang, L.; Feng, L.; Wang, X.; Bosch, M.; Alsalme, A.; Cagin, T.; Zhou, H. C., Construction of hierarchically porous metal-organic frameworks through linker labilization. Nat. Commun. 2017, 8, 15356. [CrossRef]
- Tripathy, S. P.; Subudhi, S.; Parida, K., Inter-MOF hybrid (IMOFH): A concise analysis on emerging core–shell based hierarchical and multifunctional nanoporous materials. Coord. Chem. Rev. 2021, 434, 213786. [CrossRef]
- Li, M.; Yang, Y.; Yu, D.; Li, W.; Ning, X.; Wan, R.; Zhu, H.; Mao, J., Recent advances on the construction of encapsulated catalyst for catalytic applications. Nano Res. 2023, 16, (2), 3451-3474. [CrossRef]
- Reaxa EnCat catalysts. http://reaxa.com/ (July 13, 2024),.
- Ley, S. V.; Ramarao, C.; Gordon, R. S.; Holmes, A. B.; Morrison, A. J.; McConvey, I. F.; Shirley, I. M.; Smith, S. C.; Smith, M. D., Polyurea-encapsulated palladium(II) acetate: A robust and recyclable catalyst for use in conventional and supercritical media. Chem. Commun. 2002, 2, (10), 1134-1135. [CrossRef]
- Gao, C.; Lyu, F.; Yin, Y., Encapsulated Metal Nanoparticles for Catalysis. Chem. Rev. 2021, 121, (2), 834-881. [CrossRef]
- Sebati, W.; Ray, S. S., Advances in nanostructured metal-encapsulated porous organic-polymer composites for catalyzed organic chemical synthesis. Catalysts 2018, 8, (11), 492. [CrossRef]
- Begum, R.; Farooqi, Z. H.; Xiao, J.; Ahmed, E.; Sharif, A.; Irfan, A., Crosslinked polymer encapsulated palladium nanoparticles for catalytic reduction and Suzuki reactions in aqueous medium. J. Mol. Liq. 2021, 338, 116780. [CrossRef]
- Naseem, K.; Arif, M.; Ahmad Haral, A.; Tahir, M. H.; Khurshid, A.; Ahmed, K.; Majeed, H.; Haider, S.; Khan, S. U. D.; Nazar, M. F.; Aziz, A., Enzymes encapsulated smart polymer micro assemblies and their tuned multi-functionalities: a critical review. Int. J. Polym. Mater. Polym. Biomater. 2024, 73, (9), 785-816. [CrossRef]
- Yan, P.; Li, K., Readily scalable and controllable micro-structured catalyst with encapsulated Pd@Silicate-1 towards sustainable catalytic emission control. Chem. Eng. Sci. 2024, 293, 120037. [CrossRef]
- Kruse, N.; Machoke, A. G.; Schwieger, W.; Güttel, R., Nanostructured encapsulated catalysts for combination of fischer-tropsch synthesis and hydroprocessing. ChemCatChem 2015, 7, (6), 1018-1022. [CrossRef]
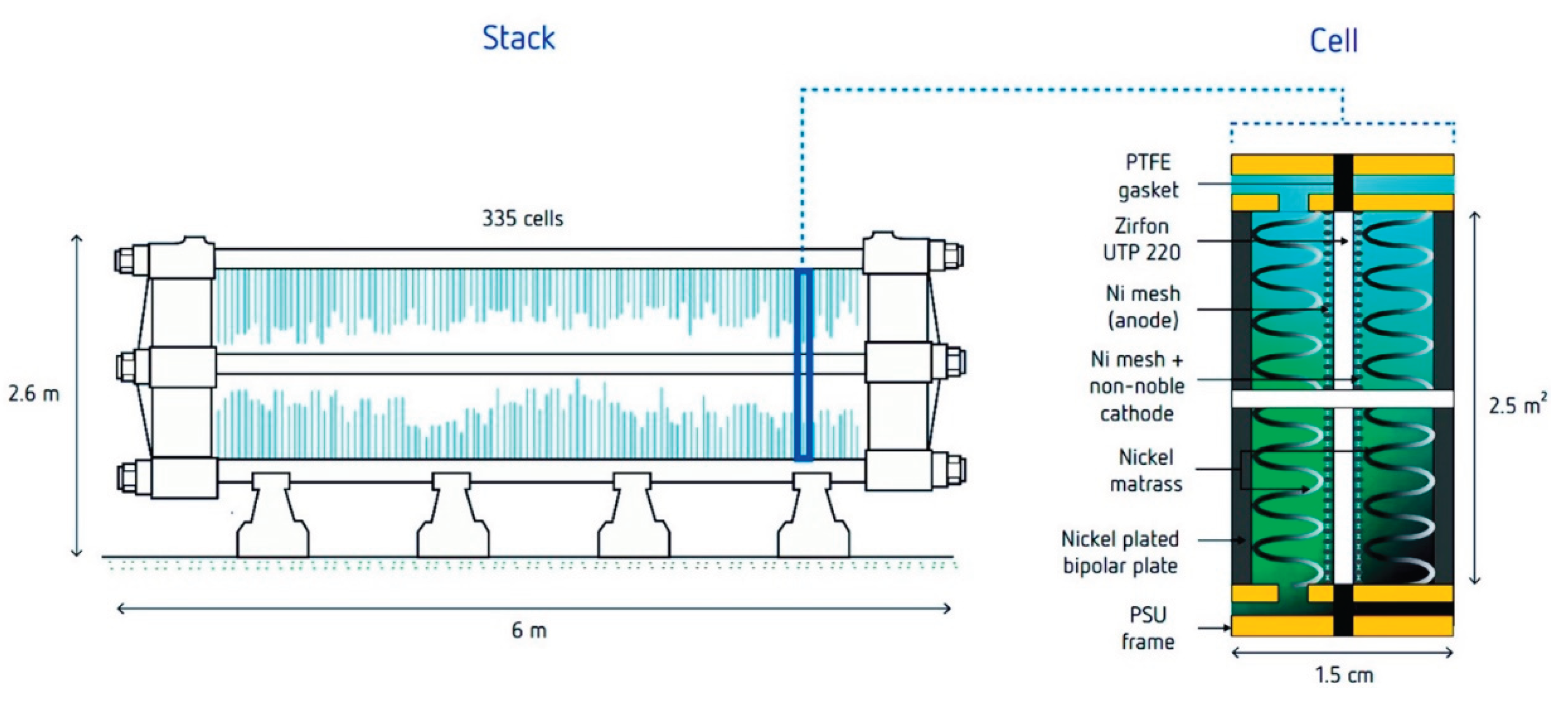
- Chatenet, M.; Pollet, B. G.; Dekel, D. R.; Dionigi, F.; Deseure, J.; Millet, P.; Braatz, R. D.; Bazant, M. Z.; Eikerling, M.; Staffell, I.; Balcombe, P.; Shao-Horn, Y.; Schäfer, H., Water electrolysis: from textbook knowledge to the latest scientific strategies and industrial developments. Chem. Soc. Rev. 2022, 51, (11), 4583-4762. [CrossRef]
- Franco, A.; Giovannini, C., Recent and Future Advances in Water Electrolysis for Green Hydrogen Generation: Critical Analysis and Perspectives. Sustainability 2023, 15, (24), 16917. [CrossRef]
- Godula-Jopek, A., Hydrogen Production: By Electrolysis. wiley: 2015; p 1-402.
- Shiva Kumar, S.; Himabindu, V., Hydrogen production by PEM water electrolysis – A review. Mater. Sci. Energy. Technol. 2019, 2, (3), 442-454. [CrossRef]
- Devi, Y.; Huang, P. J.; Chen, W. T.; Jhang, R. H.; Chen, C. H., Roll-to-Roll Production of Electrocatalysts Achieving High-Current Alkaline Water Splitting. ACS Appl. Mater. Interfaces 2023, 15, (7), 9231-9239. [CrossRef]
- Li, Y.; Shao, G.; Zheng, X.; Jia, Y.; Xia, Y.; Dou, Y.; Huang, M.; Gu, C.; Shi, J.; Zheng, J.; Dou, S., Cutting-edge advances in pressurized electrocatalytic reactors. eScience 2025, 5, (3), 100369. [CrossRef]
- Trasatti, S., Work function, electronegativity, and electrochemical behaviour of metals. III. Electrolytic hydrogen evolution in acid solutions. J Electroanal Chem 1972, 39, (1), 163-184. [CrossRef]
- Kempler, P. A.; Nielander, A. C., Reliable reporting of Faradaic efficiencies for electrocatalysis research. Nat. Commun. 2023, 14, (1), 1158. [CrossRef]
- Lamy, C.; Millet, P., A critical review on the definitions used to calculate the energy efficiency coefficients of water electrolysis cells working under near ambient temperature conditions. J. Power Sources 2020, 447, 227350. [CrossRef]
- Zhang, H.; Lin, G.; Chen, J., Evaluation and calculation on the efficiency of a water electrolysis system for hydrogen production. Int. J. Hydrogen Energy 2010, 35, (20), 10851-10858. [CrossRef]
- Malkow, T.; Pilenga, A., EU harmonised testing procedure: Determination of water electrolyser energy performance. Publications Office of the European Union Luxembourg, 2023; p 1-73.
- Li, W.; Liu, Y.; Azam, A.; Liu, Y.; Yang, J.; Wang, D.; Sorrell, C. C.; Zhao, C.; Li, S., Unlocking Efficiency: Minimizing Energy Loss in Electrocatalysts for Water Splitting. Adv. Mater. 2024, 36, (42), 2404658. [CrossRef]
- She, Z. W.; Kibsgaard, J.; Dickens, C. F.; Chorkendorff, I.; Nørskov, J. K.; Jaramillo, T. F., Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, (6321), eaad4998. [CrossRef]
- Jang, I.; S. A. Carneiro, J.; Crawford, J. O.; Cho, Y. J.; Parvin, S.; Gonzalez-Casamachin, D. A.; Baltrusaitis, J.; Lively, R. P.; Nikolla, E., Electrocatalysis in Solid Oxide Fuel Cells and Electrolyzers. Chem. Rev. 2024, 124, (13), 8233-8306. [CrossRef]
- Seo, J. M.; Noh, H. J.; Jeon, J. P.; Kim, H.; Han, G. F.; Kwak, S. K.; Jeong, H. Y.; Wang, L.; Li, F.; Baek, J. B., Conductive and Ultrastable Covalent Organic Framework/Carbon Hybrid as an Ideal Electrocatalytic Platform. J. Am. Chem. Soc. 2022, 144, (43), 19973-19980. [CrossRef]
- Ouimet, R. J.; Glenn, J. R.; De Porcellinis, D.; Motz, A. R.; Carmo, M.; Ayers, K. E., The Role of Electrocatalysts in the Development of Gigawatt-Scale PEM Electrolyzers. ACS Catal. 2022, 12, (10), 6159-6171. [CrossRef]
- Lai, F.; Shang, H.; Jiao, Y.; Chen, X.; Zhang, T.; Liu, X., Recent progress and perspective on electrocatalysis in neutral media: Mechanisms, materials, and advanced characterizations. Interdiscip. Mater. 2024, 3, (4), 492-529. [CrossRef]
- Kale, H. B.; Kute, A. D.; Gaikwad, R. P.; Fornasiero, P.; Zbořil, R.; Gawande, M. B., Synthesis and energy applications of copper-based single-atom electrocatalysts. Coord. Chem. Rev. 2024, 502, 215602. [CrossRef]
- Zhou, J.; Ming, F.; Liang, H., Application of functional coatings in water electrolyzers and fuel cells. Nanoscale 2025, 17, (14), 8289-8300. [CrossRef]
- Linnemann, J.; Kanokkanchana, K.; Tschulik, K., Design Strategies for Electrocatalysts from an Electrochemist's Perspective. ACS Catal. 2021, 11, (9), 5318-5346. [CrossRef]
- Chen, Z.; Ma, T.; Wei, W.; Wong, W. Y.; Zhao, C.; Ni, B. J., Work Function-Guided Electrocatalyst Design. Adv. Mater. 2024, 36, (29), 2401568. [CrossRef]
- Morab, S.; Sundaram, M. M.; Pivrikas, A., Review on Charge Carrier Transport in Inorganic and Organic Semiconductors. Coat. 2023, 13, (9), 1657. [CrossRef]
- Májek, M., Modern Organic Photochemistry and Electrochemistry. Comenius University in Bratislava: Bratislava, 2025; p 1-99.
- Parrino, F.; Bellardita, M.; García-López, E. I.; Marcì, G.; Loddo, V.; Palmisano, L., Heterogeneous Photocatalysis for Selective Formation of High-Value-Added Molecules: Some Chemical and Engineering Aspects. ACS Catal. 2018, 8, (12), 11191-11225. [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A. A., Heterojunction Photocatalysts. Adv. Mater. 2017, 29, (20), 1601694. [CrossRef]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J., S-Scheme Heterojunction Photocatalyst. Chem 2020, 6, (7), 1543-1559. [CrossRef]
- Zhao, Y.; Linghu, X.; Shu, Y.; Zhang, J.; Chen, Z.; Wu, Y.; Shan, D.; Wang, B., Classification and catalytic mechanisms of heterojunction photocatalysts and the application of titanium dioxide (TiO2)-based heterojunctions in environmental remediation. J. Environ. Chem. Eng. 2022, 10, (3), 108077. [CrossRef]
- Li, Z.; Zhang, J.; Wu, R.; Qiu, P.; Yao, Y.; Liao, X.; Jiang, Y.; Shi, J.; Chen, Y.; Lu, S., An S-scheme α-Fe2O3/g-C3N4 heterojunction nanostructure with superior visible-light photocatalytic activity for the aza-Henry reaction. J. Mater. Chem. C 2022, 10, (45), 17075-17083. [CrossRef]
- Marcolongo, D. M. S.; Nocito, F.; Ditaranto, N.; Comparelli, R.; Aresta, M.; Dibenedetto, A., Opto-Electronic Characterization of Photocatalysts Based on p,n-Junction Ternary and Quaternary Mixed Oxides Semiconductors (Cu2O-In2O3 and Cu2O-In2O3-TiO2). Catalysts 2022, 12, (2), 153. [CrossRef]
- Schumacher, L.; Marschall, R., Recent Advances in Semiconductor Heterojunctions and Z-Schemes for Photocatalytic Hydrogen Generation. Top. Curr. Chem. 2022, 380, (6), 53. [CrossRef]
- Li, C.; Ding, G.; Liu, X.; Huo, P.; Yan, Y.; Yan, Y.; Liao, G., Photocatalysis over NH2-UiO-66/CoFe2O4/CdIn2S4 double p-n junction: Significantly promoting photocatalytic performance by double internal electric fields. Chem. Eng. J. 2022, 435, 134740. [CrossRef]
- Sasikumar, K.; Rajamanikandan, R.; Ju, H., Construction of Z-Scheme ZIF67/NiMoO4 Heterojunction for Enhanced Photocatalytic Degradation of Antibiotic Pollutants. Materials 2024, 17, (24), 6225. [CrossRef]
- Xie, J.; Fu, C.; Quesne, M. G.; Guo, J.; Wang, C.; Xiong, L.; Windle, C. D.; Gadipelli, S.; Guo, Z. X.; Huang, W.; Catlow, C. R. A.; Tang, J., Methane oxidation to ethanol by a molecular junction photocatalyst. Nature 2025, 639, (8054), 368-374. [CrossRef]
- Li, J.; Li, Z.; Sun, Q.; Wang, Y.; Li, Y.; Peng, Y. K.; Li, Y.; Zhang, C.; Liu, B.; Zhao, Y., Recent Advances in the Large-Scale Production of Photo/Electrocatalysts for Energy Conversion and beyond. Adv. Energy Mater. 2024, 14, (45), 2402441. [CrossRef]
- Behul, M.; Marton, M.; Chvála, A.; Michniak, P.; Pifko, M.; Honig, H.; Scheler, T.; Kurniawan, M.; Vojs, M., Nanoscale Engineering of Si/BDD/TiO2 heterostructure interfaces to enhance photoelectrochemical performance. Appl. Surf. Sci. Adv. 2025, 27, 100757. [CrossRef]
- Sun, S.; He, L.; Yang, M.; Cui, J.; Liang, S., Facet Junction Engineering for Photocatalysis: A Comprehensive Review on Elementary Knowledge, Facet-Synergistic Mechanisms, Functional Modifications, and Future Perspectives. Adv. Funct. Mater. 2022, 32, (1), 2106982. [CrossRef]
- Zhang, Z., Plasmonic Photocatalysis, Principles and Applications. Springer: Singapore, 2022; p 1-91. [CrossRef]
- Xu, M.; Wang, H.; Yan, C.; Wang, H.; Li, B.; Song, X.; Liu, X.; Zhang, J.; Yang, Y.; Zhou, W.; Huo, P., Enhancing selective photoreduction of CO2 via bimetallic site directed electric fields in synergy with plasmonic near-field effects. Appl. Catal. B: Environ. 2025, 366, 124983. [CrossRef]
- Cai, J.; Peng, Y.; Jiang, Y.; Li, L.; Wang, H.; Li, K., Application of Fe-MOFs in Photodegradation and Removal of Air and Water Pollutants: A Review. Molecules 2023, 28, (20), 7121. [CrossRef]
- An, X.; Kays, J. C.; Lightcap, I. V.; Ouyang, T.; Dennis, A. M.; Reinhard, B. M., Wavelength-Dependent Bifunctional Plasmonic Photocatalysis in Au/Chalcopyrite Hybrid Nanostructures. ACS Nano 2022, 16, (4), 6813-6824. [CrossRef]
- De Barros, H. R.; García, I.; Kuttner, C.; Zeballos, N.; Camargo, P. H. C.; De Torresi, S. I. C.; López-Gallego, F.; Liz-Marzán, L. M., Mechanistic Insights into the Light-Driven Catalysis of an Immobilized Lipase on Plasmonic Nanomaterials. ACS Catal. 2021, 11, (1), 414-423. [CrossRef]
- Huang, H. J.; Wu, J. C. S.; Chiang, H. P.; Chau, Y. F. C.; Lin, Y. S.; Wang, Y. H.; Chen, P. J., Review of experimental setups for plasmonic photocatalytic reactions. Catalysts 2020, 10, (1), 46. [CrossRef]
- Pei, L.; Li, T.; Yuan, Y.; Yang, T.; Zhong, J.; Ji, Z.; Yan, S.; Zou, Z., Schottky junction effect enhanced plasmonic photocatalysis by TaON@Ni NP heterostructures. Chem. Commun. 2019, 55, (78), 11754-11757. [CrossRef]
- Zhang, H.; Wang, T.; Wang, J.; Liu, H.; Dao, T. D.; Li, M.; Liu, G.; Meng, X.; Chang, K.; Shi, L.; Nagao, T.; Ye, J., Surface-Plasmon-Enhanced Photodriven CO2 Reduction Catalyzed by Metal-Organic-Framework-Derived Iron Nanoparticles Encapsulated by Ultrathin Carbon Layers. Adv. Mater. 2016, 28, (19), 3703-3710. [CrossRef]
- Braslavsky, S. E.; Braun, A. M.; Cassano, A. E.; Emeline, A. V.; Litter, M. I.; Palmisano, L.; Parmon, V. N.; Serpone, N., Glossary of terms used in photocatalysis and radiation catalysis (IUPAC recommendations 2011). Pure Appl. Chem. 2011, 83, (4), 931-1014. [CrossRef]
- Schneider, J. E.; Bahnemann, D. E.; Ye, J. E.; Puma, G.-L. E.; Dionysiou, D.-D. E., Photocatalysis: Fundamentals and Perspectives. Royal Soc Chemistry: Cambridge, 2016; p 1-436.
- De Lasa, H.; Serrano, B.; Salaices, M., Photocatalytic reaction engineering. Springer US: 2005; p 1-187.
- Cao, S.; Piao, L., Considerations for a More Accurate Evaluation Method for Photocatalytic Water Splitting. Angew. Chem. Int. Ed. 2020, 59, (42), 18312-18320. [CrossRef]
- Serrano, B.; Ortíz, A.; Moreira, J.; De Lasa, H. I., Photocatalytic thermodynamic efficiency factors. Practical limits in photocatalytic reactors. Ind. Eng. Chem. Res. 2010, 49, (15), 6824-6833. [CrossRef]
- Lugo-Vega, C. S.; Serrano-Rosales, B.; de Lasa, H., Energy efficiency limits in Photo-CREC-Air photocatalytic reactors. Chem. Eng. Sci. 2016, 156, 77-88. [CrossRef]
- Sayago-Carro, R.; Jiménez-Chavarriga, L. J.; Fernández-García, E.; Kubacka, A.; Fernández-García, M., Efficiency in photocatalytic production of hydrogen: energetic and sustainability implications. Energy Adv. 2024, 3, (11), 2738-2757. [CrossRef]
- Forzatti, P.; Lietti, L., Catalyst deactivation. Catal. Today 1999, 52, (2-3), 165-181. [CrossRef]
- Argyle, M. D.; Bartholomew, C. H., Heterogeneous catalyst deactivation and regeneration: A review. Catalysts 2015, 5, (1), 145-269. [CrossRef]
- Walas, S. M., Chemical Process Equipment: Selection and Design. Butterworth-Heinemann: Newton. USA, 1990; p 1-755.
- Smith, R., Chemical Process Design and Integration, 2nd Edition. Wiley: Noida, India, 2016; p 1-928.
- Semmel, M.; Steiner, L.; Bontrup, M.; Sauer, J.; Salem, O., Catalyst screening and reaction kinetics of liquid phase DME synthesis under reactive distillation conditions. Chem. Eng. J. 2023, 455, 140525. [CrossRef]
- Kiss, A. A.; Muthia, R.; Pazmiño-Mayorga, I.; Harmsen, J.; Jobson, M.; Gao, X., Conceptual methods for synthesis of reactive distillation processes: recent developments and perspectives. J. Chem. Technol. Biotechnol. 2024, 99, (6), 1263-1290. [CrossRef]
- Kudaibergenov, S. E.; Dzhardimalieva, G. I., Flow-through catalytic reactors based on metal nanoparticles immobilized within porous polymeric gels and surfaces/hollows of polymeric membranes. Polymers 2020, 12, (3), 572. [CrossRef]
- Jun, B. M.; Al-Hamadani, Y. A. J.; Son, A.; Park, C. M.; Jang, M.; Jang, A.; Kim, N. C.; Yoon, Y., Applications of metal-organic framework based membranes in water purification: A review. Sep. Purif. Technol. 2020, 247, 116947. [CrossRef]
- Amin, A., Review of autothermal reactors: Catalysis, reactor design, and processes. Int. J. Hydrogen Energy 2024, 65, 271-291. [CrossRef]
- Paul, E. L.; Atiemo-Obeng, V. A.; Kresta, S. M., HANDBOOK OF INDUSTRIAL MIXING: SCIENCE AND PRACTICE. wiley: 2004; p 1-1433.
- Hill, J. C., Homogeneous Turbulent Mixing with Chemical-Reaction. Annu. Rev. Fluid Mech. 1976, 8, 135-161. [CrossRef]
- Bourne, J. R., Mixing and the selectivity of chemical reactions. Org. Process Res. Dev. 2003, 7, (4), 471-508. [CrossRef]
- Danckwerts, P. V., The definition and measurement of some characteristics of mixtures. Appl. Sci. Res. 1952, 3, (4), 279-296. [CrossRef]
- Gobert, S. R. L.; Kuhn, S.; Braeken, L.; Thomassen, L. C. J., Characterization of Milli- and Microflow Reactors: Mixing Efficiency and Residence Time Distribution. Org. Process Res. Dev. 2017, 21, (4), 531-542. [CrossRef]
- Silverstein, J.; Shinnar, R., Design of Fixed Bed Catalytic Microreactors. Ind. Eng. Chem. Process. Des. Dev. 1975, 14, (2), 127-137. [CrossRef]
- COMSOL COMSOL Multiphysics®. https://www.comsol.com/.
- Bartholomew, C. H.; Farrauto, R. J., Fundamentals of Industrial Catalytic Processes: Second Edition. John Wiley and Sons: 2010; p 1-966.
- Lachman, I. M.; Williams, J. L., Extruded monolithic catalyst supports. Catal. Today 1992, 14, (2), 317-329. [CrossRef]
- Erfani, N.; Symons, D.; Fee, C.; James Watson, M., A novel method to design monolithic catalysts for non-isothermal packed-bed reactors using topology optimisation. Chem. Eng. Sci. 2023, 267, 118347. [CrossRef]
- Boger, T.; Heibel, A. K.; Sorensen, C. M., Monolithic catalysts for the chemical industry. Ind. Eng. Chem. Res. 2004, 43, (16), 4602-4611. [CrossRef]
- Yates, J. G.; Paola Lettieri, P., Fluidized-Bed Reactors: Processes and Operating Conditions. Springer: Switzerland, 2016; p 1-214.
- Shirzad, M.; Karimi, M.; Silva, J. A. C.; Rodrigues, A. E., Moving Bed Reactors: Challenges and Progress of Experimental and Theoretical Studies in a Century of Research. Ind. Eng. Chem. Res. 2019, 58, (22), 9179-9198. [CrossRef]
- Haynes, T. N.; Caram, H. S., The simulated moving bed chemical reactor. Chem. Eng. Sci. 1994, 49, (24), 5465-5472. [CrossRef]
- Magyarová, Z.; Králik, M.; Soták, T., Utilization of zeolite catalysts in biomass exploitation: a minireview. Monatsh. Chem. 2023, 154, (8), 815-835. [CrossRef]
- Kouser, S.; Hezam, A.; Khadri, M. J. N.; Khanum, S. A., A review on zeolite imidazole frameworks: synthesis, properties, and applications. J. Porous Mater. 2022, 29, (3), 663-681. [CrossRef]
- Sklenár, M.; Danielik, V.; Hudec, P., Alkylation of diphenylamine with alkenes catalyzed by acidic catalysts based on bentonite. Pet. Coal 2016, 58, (6), 597-604,.
- Sklenár, M.; Danielik, V.; Hudec, P., Discontinuous alkylation of diphenylamine with nonene for maximum catalyst utilization. Chem. Pap. 2017, 71, (8), 1453-1461. [CrossRef]
- Hájek, M.; Tomášová, A.; Kocík, J.; Podzemná, V., Statistical evaluation of the mutual relations of properties of Mg/Fe hydrotalcites and mixed oxides as transesterification catalysts. Appl. Clay Sci. 2018, 154, 28-35. [CrossRef]
- Mališová, M.; Horňáček, M.; Mikulec, J.; Hudec, P.; Hájek, M.; Peller, A.; Jorík, V.; Frolich, K.; Hadvinová, M.; Hájeková, E., Transesterification of Camelina sativa Oil Catalyzed by Mg/Al Mixed Oxides with Added Divalent Metals. ACS Omega 2020, 5, (49), 32040-32050. [CrossRef]
- Mück, J.; Kocík, J.; Frolich, K.; Šimek, J.; Michálková, M.; Hájek, M., Transition Metal-Promoted Mg-Fe Mixed Oxides for Conversion of Ethanol to Valuable Products. ACS Omega 2023, 8, (22), 19374-19384. [CrossRef]
- Rahmani, E.; Rahmani, M., Al-Based MIL-53 Metal Organic Framework (MOF) as the New Catalyst for Friedel-Crafts Alkylation of Benzene. Ind. Eng. Chem. Res. 2018, 57, (1), 169-178. [CrossRef]
- Xiang, B. L.; Fu, L.; Li, Y.; Liu, Y., Preparation of Fe(II)/MOF-5 Catalyst for Highly Selective Catalytic Hydroxylation of Phenol by Equivalent Loading at Room Temperature. J. Chem. 2019, 2019, 8950630. [CrossRef]
- Greifenstein, R.; Ballweg, T.; Hashem, T.; Gottwald, E.; Achauer, D.; Kirschhöfer, F.; Nusser, M.; Brenner-Weiß, G.; Sedghamiz, E.; Wenzel, W.; Mittmann, E.; Rabe, K. S.; Niemeyer, C. M.; Franzreb, M.; Wöll, C., MOF-Hosted Enzymes for Continuous Flow Catalysis in Aqueous and Organic Solvents. Angew. Chem. Int. Ed. 2022, 61, (18), e202117144. [CrossRef]
- Wu, J.; Jiang, X.; Wheatley, A., Characterizing activated sludge process effluent by particle size distribution, respirometry and modelling. Desalination 2009, 249, (3), 969-975. [CrossRef]
- Kuśnierz, M., Scale of Small Particle Population in Activated Sludge Flocs. Water Air Soil Pollut. 2018, 229, (10), 327. [CrossRef]
- Shabestary, K.; Klamt, S.; Link, H.; Mahadevan, R.; Steuer, R.; Hudson, E. P., Design of microbial catalysts for two-stage processes. Nat. Rev. Bioeng. 2024, 2, (12), 1039-1055. [CrossRef]
- Hamawand, I., Energy Consumption in Water/Wastewater Treatment Industry—Optimisation Potentials. Energies 2023, 16, (5), 2433. [CrossRef]
- Yeoh, G. H. E.; Joshi, J. B. E., Handbook of Multiphase Flow Science and Technology. Springer Nature: 2023; p 1-1634.
- Zhang, H.; Song, N.; Yu, T.; Qu, C., Process Intensification in Gas–Liquid Mass Transfer by Modification of Reactor Design: A Review. Energy Technol. 2023, 11, (7), 2201495. [CrossRef]
- Gates, B. C., Catalytic Chemistry. Wiley: New York, 1992; p 1-480.
- Speight, J. G., Handbook of Petroleum Refining. 1st ed.; CRC Press: Boca Raton, 2016; p 1-789.
- Pjontek, D.; McKnight, C. A.; Wiens, J.; Macchi, A., Ebullated bed fluid dynamics relevant to industrial hydroprocessing. Chem. Eng. Sci. 2015, 126, 730-744. [CrossRef]
- Martinez, J.; Sanchez, J. L.; Ancheyta, J.; Ruiz, R. S., A review of process aspects and modeling of ebullated bed reactors for hydrocracking of heavy oils. Catal. Rev. - Sci. Eng. 2010, 52, (1), 60-105. [CrossRef]
- Chaudhari, R. V.; Shah, Y. T.; foster, N. R., Novel Gas-Liquid-Solid Reactors. Catalysis Reviews 1986, 28, (4), 431-518. [CrossRef]
- Dautzenberg, F. M.; Mukherjee, M., Process intensification using multifunctional reactors. Chem. Eng. Sci. 2001, 56, (2), 251-267. [CrossRef]
- AG, B. C. The BUSS-Loop® Reactor Explained. https://www.buss-ct.com/gas-liquid-reactions/buss-loop-reactor/ (April 2, 2025),.
- Li, W.; Sun, Z.; Ye, X.; He, Y., Ultra-thin PPS mesh-based PSF-ZrO2 composite diaphragm for efficient electrolysis of water for hydrogen production. Int. J. Hydrogen Energy 2024, 81, 918-926. [CrossRef]
- Wang, S.; Lu, A.; Zhong, C. J., Hydrogen production from water electrolysis: role of catalysts. Nano Converg. 2021, 8, (1), 4. [CrossRef]
- Zhao, P.; Wang, J.; Sun, L.; Li, Y.; Xia, H.; He, W., Optimal electrode configuration and system design of compactly-assembled industrial alkaline water electrolyzer. Energy Convers. Manage. 2024, 299, 117875. [CrossRef]
- Rizwan, M.; Alstad, V.; Jäschke, J., Design considerations for industrial water electrolyzer plants. Int. J. Hydrogen Energy 2021, 46, (75), 37120-37136. [CrossRef]
- Şahin, M. E., An Overview of Different Water Electrolyzer Types for Hydrogen Production. Energies 2024, 17, (19), 4944. [CrossRef]
- van ’t Noordende, H.; Ripson, P., A One-GigaWatt Green-Hydrogen Plant. Advanced Design and Total Installed-Capital Costs. . ISPT: Amersfoort, The Netherlands, 2023; p 1-45.
- Zeng, Y.; Zhao, M.; Zeng, H.; Jiang, Q.; Ming, F.; Xi, K.; Wang, Z.; Liang, H., Recent progress in advanced catalysts for electrocatalytic hydrogenation of organics in aqueous conditions. eScience 2023, 3, (5), 100156. [CrossRef]
- Kundu, B. K.; Sun, Y., Electricity-driven organic hydrogenation using water as the hydrogen source. Chem. Sci. 2024, 15, (40), 16424-16435. [CrossRef]
- Lee, K. M.; Lee, H. J.; Seo, J.; Lee, T.; Yoon, J.; Kim, C.; Lee, C., Electrochemical Oxidation Processes for the Treatment of Organic Pollutants in Water: Performance Evaluation Using Different Figures of Merit. ACS. ES&T Eng. 2022, 2, (10), 1797-1824. [CrossRef]
- Mitsudo, K.; Osaki, A.; Inoue, H.; Sato, E.; Shida, N.; Atobe, M.; Suga, S., Electrocatalytic hydrogenation of cyanoarenes, nitroarenes, quinolines, and pyridines under mild conditions with a proton-exchange membrane reactor. Beilstein J. Org. Chem. 2024, 20, 1560-1571. [CrossRef]
- Zhou, P.; Li, W.; Lan, J.; Zhu, T., Electroredox carbene organocatalysis with iodide as promoter. Nat. Commun. 2022, 13, (1), 3827. [CrossRef]
- Jensen, M. T.; Rønne, M. H.; Ravn, A. K.; Juhl, R. W.; Nielsen, D. U.; Hu, X. M.; Pedersen, S. U.; Daasbjerg, K.; Skrydstrup, T., Scalable carbon dioxide electroreduction coupled to carbonylation chemistry. Nat. Commun. 2017, 8, (1), 489. [CrossRef]
- Kusama, S.; Saito, T.; Hashiba, H.; Sakai, A.; Yotsuhashi, S., Crystalline Copper(II) Phthalocyanine Catalysts for Electrochemical Reduction of Carbon Dioxide in Aqueous Media. ACS Catal. 2017, 7, (12), 8382-8385. [CrossRef]
- Zhu, H. J.; Lu, M.; Wang, Y. R.; Yao, S. J.; Zhang, M.; Kan, Y. H.; Liu, J.; Chen, Y.; Li, S. L.; Lan, Y. Q., Efficient electron transmission in covalent organic framework nanosheets for highly active electrocatalytic carbon dioxide reduction. Nat. Commun. 2020, 11, (1), 497. [CrossRef]
- Park, S.; Wijaya, D. T.; Na, J.; Lee, C. W., Towards the large-scale electrochemical reduction of carbon dioxide. Catalysts 2021, 11, (2), 253. [CrossRef]
- Perry, S. C., Electrochemical carbon dioxide reduction. De Gruyter: 2021; p 1-152.
- Chen, T. W.; Chen, S. M.; Anushya, G.; Kannan, R.; G. Al-Sehemi, A.; Alargarsamy, S.; Gajendran, P.; Ramachandran, R., Development of Different Kinds of Electrocatalyst for the Electrochemical Reduction of Carbon Dioxide Reactions: An Overview. Molecules 2023, 28, (20), 7016. [CrossRef]
- Peng, L.; Zhang, Y.; He, R.; Xu, N.; Qiao, J., Research Advances in Electrocatalysts, Electrolytes, Reactors and Membranes for the Electrocatalytic Carbon Dioxide Reduction Reaction. Wuli Huaxue Xuebao (Acta Phys. -Chim. Sin.) 2023, 39, (12), 2302037. [CrossRef]
- Chen, Z.; Li, N.; Zhang, Q., Covalent Organic Frameworks as Promising Platforms for Efficient Electrochemical Reduction of Carbon Dioxide: A Review. Small Struct. 2024, 5, (5), 2300495. [CrossRef]
- Varhade, S.; Guruji, A.; Singh, C.; Cicero, G.; García-Melchor, M.; Helsen, J.; Pant, D., Electrochemical CO2 Reduction: Commercial Innovations and Prospects. ChemElectroChem 2025, 12, (2), e202400512. [CrossRef]
- Pourebrahimi, S.; Pirooz, M.; Ahmadi, S.; Kazemeini, M.; Vafajoo, L., Nanoengineering of metal-based electrocatalysts for carbon dioxide (CO2) reduction: A critical review. Mater. Today Phys. 2023, 38, 101250. [CrossRef]
- Ma, S.; Sadakiyo, M.; Luo, R.; Heima, M.; Yamauchi, M.; Kenis, P. J. A., One-step electrosynthesis of ethylene and ethanol from CO2 in an alkaline electrolyzer. J. Power Sources 2016, 301, 219-228. [CrossRef]
- Gao, D.; Li, W.; Wang, H.; Wang, G.; Cai, R., Heterogeneous Catalysis for CO2 Conversion into Chemicals and Fuels. Trans. Tianjin Univ. 2022, 28, (4), 245-264. [CrossRef]
- Díaz-Sainz, G.; Alvarez-Guerra, M.; Irabien, A., Continuous electroreduction of CO2 towards formate in gas-phase operation at high current densities with an anion exchange membrane. J. CO2 Util. 2022, 56, 101822. [CrossRef]
- Si, D.; Teng, X.; Xiong, B.; Chen, L.; Shi, J., Electrocatalytic functional group conversion-based carbon resource upgrading. Chem. Sci. 2024, 15, (17), 6269-6284. [CrossRef]
- Novaes, L. F. T.; Liu, J.; Shen, Y.; Lu, L.; Meinhardt, J. M.; Lin, S., Electrocatalysis as an enabling technology for organic synthesis. Chem. Soc. Rev. 2021, 50, (14), 7941-8002. [CrossRef]
- Gao, Y.; Ge, L.; Xu, H.; Davey, K.; Zheng, Y.; Qiao, S. Z., Electrocatalytic Refinery of Biomass-Based 5-Hydroxymethylfurfural to Fine Chemicals. ACS Catal. 2023, 13, (17), 11204-11231. [CrossRef]
- Tang, C.; Wei, C.; Fang, Y.; Liu, B.; Song, X.; Bian, Z.; Yin, X.; Wang, H.; Liu, Z.; Wang, G.; Xiao, X.; Duan, X., Electrocatalytic hydrogenation of acetonitrile to ethylamine in acid. Nat. Commun. 2024, 15, (1), 3233. [CrossRef]
- Zhu, Y.; Wu, D.; Tang, J.; Braaten, D.; Liu, B.; Peng, Z., Advances in electrocatalytic dehydrogenation of ethylamine to acetonitrile. Chem. Commun. 2024, 60, (68), 9007-9021. [CrossRef]
- Sen, R.; Das, S.; Nath, A.; Maharana, P.; Kar, P.; Verpoort, F.; Liang, P.; Roy, S., Electrocatalytic Water Oxidation: An Overview With an Example of Translation From Lab to Market. Front. Chem. 2022, 10, 861604. [CrossRef]
- Guan, M. H.; Wu, T.; Lu, A. H., Tandem Synthesis of Valuable Chemicals via Electrocatalysis. ChemCatChem 2023, 15, (24), e202301311. [CrossRef]
- Dong, L.-Y.; Guan, M.-H.; Ren, Y.-Q.; Hao, G.-P.; Lu, A.-H., Mesoporous Fe-Doped Carbon Electrocatalysts with Highly Exposed Active Sites for Efficient Synthesis of Hydrogen Peroxide and Tandem Epoxidation of Propene. Renewables 2023, 1, (1), 562–571. [CrossRef]
- Guan, M. H.; Dong, L. Y.; Wu, T.; Li, W. C.; Hao, G. P.; Lu, A. H., Boosting Selective Oxidation of Ethylene to Ethylene Glycol Assisted by In situ Generated H2O2 from O2 Electroreduction. Angew. Chem. Int. Ed. 2023, 62, (19), e202302466. [CrossRef]
- Lazaridis, T.; Stühmeier, B. M.; Gasteiger, H. A.; El-Sayed, H. A., Capabilities and limitations of rotating disk electrodes versus membrane electrode assemblies in the investigation of electrocatalysts. Nat. Catal. 2022, 5, (5), 363-373. [CrossRef]
- Dong, Q.; Li, T.; Yao, Y.; Wang, X.; He, S.; Li, J.; Luo, J.; Zhang, H.; Pei, Y.; Zheng, C.; Hong, M.; Qiao, H.; Gao, J.; Wang, D.; Yang, B.; Hu, L., A General Method for Regenerating Catalytic Electrodes. Joule 2020, 4, (11), 2374-2386. [CrossRef]
- Bisztyga-Szklarz, M.; Mech, K.; Marzec, M.; Kalendarev, R.; Szaciłowski, K., In situ regeneration of copper-coated gas diffusion electrodes for electroreduction of CO2 to ethylene. Materials 2021, 14, (12), 3171. [CrossRef]
- Sundar, K. P.; Kanmani, S., Progression of Photocatalytic reactors and it's comparison: A Review. Chem. Eng. Res. Des. 2020, 154, 135-150. [CrossRef]
- Kant, P.; Liang, S.; Rubin, M.; Ozin, G. A.; Dittmeyer, R., Low-cost photoreactors for highly photon/energy-efficient solar-driven synthesis. Joule 2023, 7, (6), 1347-1362. [CrossRef]
- Meng, T.; Su, X.; Sun, P.; Sun, W.; Santoro, D.; Yao, H.; Wang, H., UV-based advanced oxidation processes in photoreactors with reflective sleeves. J. Clean. Prod. 2023, 416, 137945. [CrossRef]
- Cabrera-Reina, A.; Salazar-González, R.; Marugán, J.; Sánchez Pérez, J. A.; Miralles-Cuevas, S., Comprehensive evaluation of a novel pilot-scale UVA-LED photoreactor for water treatment applications: Characterization, catalytic efficiency, energy performance and economic viability. J. Water Process Eng. 2024, 63, 105483. [CrossRef]
- photoreactors, P. Photoreactors – lab, pilot and industrial scale. https://photoreactors.com/?gad_source=1&gad_campaignid=15624145416&gbraid=0AAAAAD_YhgWeP7PoKLoGHPP3eqNmPBfwl&gclid=CjwKCAjw24vBBhABEiwANFG7y-M6wOmo0NjkS8wT4TZtLg5hSD7y07UG9zGvpD4CcVEKKNkrVnzHsBoCFkcQAvD_BwE (May 20, 2025),.
- Meinhardová, V.; Dubnová, L.; Drobná, H.; Matějová, L.; Kočí, K.; Čapek, L., Role of lamp type in conventional batch and micro-photoreactor for photocatalytic hydrogen production. Front. Chem. 2023, 11, 1271410. [CrossRef]
- Markushyna, Y.; Savateev, A., Light as a Tool in Organic Photocatalysis: Multi-Photon Excitation and Chromoselective Reactions. Eur. J. Org. Chem. 2022, 2022, (24), e202200026. [CrossRef]
- Veréb, G.; Gyulavári, T.; Virág, O.; Alapi, T.; Hernadi, K.; Pap, Z., Wavelength Dependence of the Photocatalytic Performance of Pure and Doped TiO2 Photocatalysts—A Reflection on the Importance of UV Excitability. Catalysts 2022, 12, (12), 1492. [CrossRef]
- Menzel, J. P.; Noble, B. B.; Blinco, J. P.; Barner-Kowollik, C., Predicting wavelength-dependent photochemical reactivity and selectivity. Nat. Commun. 2021, 12, (1), 1691. [CrossRef]
- Watts, R. J.; Kong, S.; Lee, W., Sedimentation and reuse of titanium dioxide: Application to suspended-photocatalyst reactors. J. Environ. Eng. 1995, 121, (10), 730-735. [CrossRef]
- Romero, R. L.; Alfano, O. M.; Cassano, A. E., Cylindrical Photocatalytic Reactors. Radiation Absorption and Scattering Effects Produced by Suspended Fine Particles in an Annular Space. Ind. Eng. Chem. Res. 1997, 36, (8), 3094-3109. [CrossRef]
- Negishi, N.; Sano, T., Photocatalytic Solar Tower Reactor for the Elimination of a Low Concentration of VOCs. Molecules 2014, 19, (10), 16624-16639. [CrossRef]
- Manassero, A.; Satuf, M. L.; Alfano, O. M., Photocatalytic degradation of an emerging pollutant by TiO2-coated glass rings: a kinetic study. Environ. Sci. Pollut. Res. 2017, 24, (7), 6031-6039. [CrossRef]
- Šuligoj, A.; Cerc Korošec, R.; Žerjav, G.; Novak Tušar, N.; Lavrenčič Štangar, U., Solar-Driven Photocatalytic Films: Synthesis Approaches, Factors Affecting Environmental Activity, and Characterization Features. Top. Curr. Chem. 2022, 380, (6), 51. [CrossRef]
- Chen, C.; Fei, L.; Wang, B.; Xu, J.; Li, B.; Shen, L.; Lin, H., MOF-Based Photocatalytic Membrane for Water Purification: A Review. Small 2024, 20, (1), 2305066. [CrossRef]
- Sriramoju, J. B.; Paramesh, C. C.; Halligudra, G.; Rangappa, D.; Shivaramu, P. D., Magnetic photocatalytic systems. In Photocatalytic Systems by Design: Materials, Mechanisms and Applications, Elsevier: 2021; p 503-536.
- Qian, Z.; Zhang, R.; Xiao, Y.; Huang, H.; Sun, Y.; Chen, Y.; Ma, T.; Sun, X., Trace to the Source: Self-Tuning of MOF Photocatalysts. Adv. Energy Mater. 2023, 13, (23), 2300086. [CrossRef]
- He, S.; Yin, B.; Niu, H.; Cai, Y., Targeted synthesis of visible-light-driven covalent organic framework photocatalyst via molecular design and precise construction. Appl. Catal. B: Environ. 2018, 239, 147-153. [CrossRef]
- Ruidas, S.; Chowdhury, A.; Ghosh, A.; Ghosh, A.; Mondal, S.; Wonanke, A. D. D.; Addicoat, M.; Das, A. K.; Modak, A.; Bhaumik, A., Covalent Organic Framework as a Metal-Free Photocatalyst for Dye Degradation and Radioactive Iodine Adsorption. Langmuir 2023, 39, (11), 4071-4081. [CrossRef]
- Li, S.; Luo, P.; Wu, H.; Wei, C.; Hu, Y.; Qiu, G., Strategies for Improving the Performance and Application of MOFs Photocatalysts. ChemCatChem 2019, 11, (13), 2978-2993. [CrossRef]
- Wang, S.; Sun, Q.; Chen, W.; Tang, Y.; Aguila, B.; Pan, Y.; Zheng, A.; Yang, Z.; Wojtas, L.; Ma, S.; Xiao, F. S., Programming Covalent Organic Frameworks for Photocatalysis: Investigation of Chemical and Structural Variations. Matter 2020, 2, (2), 416-427. [CrossRef]
- Guo, M.; Zhang, M.; Liu, R.; Zhang, X.; Li, G., State-of-the-Art Advancements in Photocatalytic Hydrogenation: Reaction Mechanism and Recent Progress in Metal-Organic Framework (MOF)-Based Catalysts. Adv. Sci. 2022, 9, (1), 2103361. [CrossRef]
- Mondal, I.; Ghosh, D.; Pal, U.; Mistry, S., Linker's Functionality: A Critical Exploration of Metal-Organic Frameworks and Their Derived Materials in Photocatalysis. ChemCatChem 2025, 17, (9), e202500022. [CrossRef]
- Binjhade, R.; Mondal, R.; Mondal, S., Continuous photocatalytic reactor: Critical review on the design and performance. J. Environ. Chem. Eng. 2022, 10, (3), 107746. [CrossRef]
- Du, R.; Chen, Y.; Ding, Z.; Fan, C.; Wang, G.; Zhang, J.; Tang, Z., Scale-up of slurry Taylor flow microreactor for heterogeneous photocatalytic synthesis of azo-products. React. Chem. Eng. 2024, 9, (8), 2249-2261. [CrossRef]
- Crawford, R.; Baumann, M., Continuous Flow Technology Enabling Photochemistry. Adv. Synth. Catal. 2025. [CrossRef]
- Thomson, C. G.; Lee, A. L.; Vilela, F., Heterogeneous photocatalysis in flow chemical reactors. Beilstein J. Org. Chem. 2020, 16, 1495-1549. [CrossRef]
- Shi, M.; Meng, X., Photothermal catalysis: From principles to applications. Int. J. Hydrogen Energy 2023, 48, (89), 34659-34676. [CrossRef]
- Gupta, A.; Likozar, B.; Jana, R.; Chanu, W. C.; Singh, M. K., A review of hydrogen production processes by photocatalytic water splitting – From atomistic catalysis design to optimal reactor engineering. Int. J. Hydrogen Energy 2022, 47, (78), 33282-33307. [CrossRef]
- Alvarado-Ávila, M. I.; De Luca, S.; Edlund, U.; Ye, F.; Dutta, J., Cellulose as sacrificial agents for enhanced photoactivated hydrogen production. Sustain. Energy Fuels 2023, 7, (8), 1981-1991. [CrossRef]
- Guan, X.; Shen, S.; Mao, S. S., Harvesting the two-electron process for solar water splitting. Cell Rep. Phys. Sci. 2023, 4, (1), 101211. [CrossRef]
- Zhou, P.; Navid, I. A.; Ma, Y.; Xiao, Y.; Wang, P.; Ye, Z.; Zhou, B.; Sun, K.; Mi, Z., Solar-to-hydrogen efficiency of more than 9% in photocatalytic water splitting. Nature 2023, 613, (7942), 66-70. [CrossRef]
- Li, X.; Zhang, C.; Geng, J.; Zong, S.; Wang, P., Photo(electro)catalytic Water Splitting for Hydrogen Production: Mechanism, Design, Optimization, and Economy. Molecules 2025, 30, (3), 630. [CrossRef]
- Mani, P.; Shenoy, S.; Sagayaraj, P. J. J.; Agamendran, N.; Son, S.; Bernaurdshaw, N.; Kim, H. I.; Sekar, K., Scaling up of photocatalytic systems for large-scale hydrogen generation. Appl. Phys. Rev. 2025, 12, (1), 011303. [CrossRef]
- Meng, X. Y.; Wang, T.; Li, J. J.; Ching, C. C.; Zhou, Y. N.; Pan, Y. X., Reactor for Photocatalytic Hydrogen Production from Water. ChemSusChem 2025. [CrossRef]
- Hao, Q. G.; Zhu, Y. H.; Li, Y.; Li, Z. H.; Yuan, H.; Ouyang, S. X., Rational design of a carbon nitride photocatalyst with in-plane electron delocalization for photocatalytic hydrogen evolution. Ind. Chem. Mater. 2025, 3, (2), 203-212. [CrossRef]
- Sasikumar, K.; Ju, H., Recent Advances in Vanadate-Based Materials for Photocatalytic Hydrogen Production. Molecules 2025, 30, (4), 789. [CrossRef]
- Sportelli, G.; Marchi, M.; Fornasiero, P.; Filippini, G.; Franco, F.; Melchionna, M., Photoelectrocatalysis for Hydrogen Evolution Ventures into the World of Organic Synthesis. Glob. Chall. 2024. [CrossRef]
- Tasleem, S.; Tahir, M., Investigating the performance of liquid and gas phase photoreactors for dynamic H2 production over bimetallic TiO2 and Ni2P dispersed MAX Ti3AlC2 monolithic nanocomposite under UV and visible light. J. Environ. Chem. Eng. 2021, 9, (4), 105351. [CrossRef]
- Wang, F. D.; Yang, L. J.; Wang, X. X.; Rong, Y.; Yang, L. B.; Zhang, C. X.; Yan, F. Y.; Wang, Q. L., Pyrazine-Functionalized Donor–Acceptor Covalent Organic Frameworks for Enhanced Photocatalytic H2 Evolution with High Proton Transport. Small 2023, 19, (23), 2207421. [CrossRef]
- Cui, C.; Zhao, X.; Su, X.; Xi, N.; Wang, X.; Yu, X.; Zhang, X. L.; Liu, H.; Sang, Y., Porphyrin-based Donor–Acceptor Covalent Organic Polymer/ZnIn2S4 Z-Scheme Heterostructure for Efficient Photocatalytic Hydrogen Evolution. Adv. Funct. Mater. 2022, 32, (47), 2208962. [CrossRef]
- Brito, J. F. D.; Bessegato, G. G.; Perini, J. A. L.; Torquato, L. D. D. M.; Zanoni, M. V. B., Advances in photoelectroreduction of CO2 to hydrocarbons fuels: Contributions of functional materials. J. CO2 Util. 2022, 55, 101810. [CrossRef]
- Yamada, T.; Nishiyama, H.; Akatsuka, H.; Nishimae, S.; Ishii, Y.; Hisatomi, T.; Domen, K., Production of Methane by Sunlight-Driven Photocatalytic Water Splitting and Carbon Dioxide Methanation as a Means of Artificial Photosynthesis. ACS Eng. Au 2023, 3, (5), 352-363. [CrossRef]
- Adeola, A. O.; Ighalo, J. O.; Kyesmen, P. I.; Nomngongo, P. N., Metal-Organic Polyhedra (MOPs) as emerging class of metal-organic frameworks for CO2 photocatalytic conversions: Current trends and future outlook. J. CO2 Util. 2024, 80, 102664. [CrossRef]
- Guene Lougou, B.; Geng, B. X.; Pan, R. M.; Wang, W.; Yan, T. T.; Li, F. H.; Zhang, H.; Djandja, O. S.; Shuai, Y.; Tabatabaei, M.; Sabi Takou, D., Solar-driven photothermal catalytic CO2 conversion: a review. Rare Met. 2024, 43, (7), 2913-2939. [CrossRef]
- Liu, H.; Sun, R.; Yang, Y.; Zhang, C.; Zhao, G.; Zhang, K.; Liang, L.; Huang, X., Review on Microreactors for Photo-Electrocatalysis Artificial Photosynthesis Regeneration of Coenzymes. Micromachines 2024, 15, (6), 789. [CrossRef]
- Saadh, M. J.; Mustafa, M. A.; Altalbawy, F. M. A.; Kaur, M.; Sharma, R.; Juraev, N.; Ghazy, H.; Abdulwahid, A. S.; Muften, N. F.; Saud, H. R.; Hulail, H. M.; Alam, M. M.; Abosaoda, M. K., Incorporation anthracene and Cu to NH2-Zr-UiO-67 metal-organic framework: Introducing the simultaneous selectivity and efficiency in photocatalytic CO2 reduction to ethanol. J. Mol. Struct. 2024, 1318, 139329. [CrossRef]
- Tian, J.; Xu, W.; Gao, J.; Zhu, T.; Xu, G.; Zhong, Z.; Su, F., Electrocatalytic and Photocatalytic CO2 Methanation: From Reaction Fundamentals to Catalyst Developments. ChemCatChem 2024, 16, (9), e202301406. [CrossRef]
- Yeo, C. I.; Tan, Y. S.; Awan, H. T. A.; Hanan, A.; Wong, W. P.; Walvekar, R.; Goh, B. H.; Khalid, M., A review on the advancements in covalent organic frameworks for photocatalytic reduction of carbon dioxide. Coord. Chem. Rev. 2024, 521, 216167. [CrossRef]
- Lei, K.; Wang, D.; Ye, L.; Kou, M.; Deng, Y.; Ma, Z.; Wang, L.; Kong, Y., A Metal-Free Donor–Acceptor Covalent Organic Framework Photocatalyst for Visible-Light-Driven Reduction of CO2 with H2O. ChemSusChem 2020, 13, (7), 1725-1729. [CrossRef]
- Wu, X.; Zhang, S.; Ning, S.; Yang, C.; Li, L.; Tang, L.; Wang, J.; Liu, R.; Yin, X.; Zhu, Y.; Chen, S.; Ye, J., Recent advances and developments in solar-driven photothermal catalytic CO2 reduction into multicarbon (C2+) products. Chem. Sci. 2025, 16, (11), 4568-4594. [CrossRef]
- Zhang, X.; Yang, Y.; Hu, Y.; Chen, Y.; Shen, J.; Tu, Y., Enhanced Photothermal Catalytic CO2 Reduction to High Selective C2H4 by Cooperative Interaction of Oxygen Vacancy and WTe2 Semimetal Cocatalyst In Situ Grown on WO3 Hollow Spheres. ACS Appl. Ener. Mat. 2024, 7, (24), 11859-11872. [CrossRef]
- Jiang, Y.; Li, S.; Fan, X.; Tang, Z., Recent advances on aerobic photocatalytic methane conversion under mild conditions. Nano Res. 2023, 16, (11), 12558-12571. [CrossRef]
- Xie, J.; Jiang, Y. H.; Li, S. Y.; Xu, P.; Zheng, Q.; Fan, X. Y.; Peng, H. L.; Tang, Z. Y., Stable Photocatalytic Coupling of Methane to Ethane with Water Vapor Using TiO2 Supported Ultralow Loading AuPd Nanoparticles. Acta Phys.-Chim. Sin. 2023, 39, (10), 2306037. [CrossRef]
- Bo, C.; Zhang, L.; Liu, X.; Chang, H.; Sun, Y.; Zhang, X.; Tan, T.; Piao, L., Highly selective photocatalytic oxidation of methane to methyl hydroperoxide. Nano Res. 2024, 17, (4), 2473-2480. [CrossRef]
- Wang, P.; Shi, R.; Zhao, J.; Zhang, T., Photodriven Methane Conversion on Transition Metal Oxide Catalyst: Recent Progress and Prospects. Adv. Sci. 2024, 11, (8), 2305471. [CrossRef]
- Ali, N. S.; Kalash, K. R.; Ahmed, A. N.; Albayati, T. M., Performance of a solar photocatalysis reactor as pretreatment for wastewater via UV, UV/TiO2, and UV/H2O2 to control membrane fouling. Sci. Rep. 2022, 12, (1), 16782. [CrossRef]
- Bazargan, A. E., Photocatalytic Water and Wastewater Treatment. IWA Publishing: Lodon, UK, 2022; p 1-207.
- Beura, R.; Sooraj, K. P.; Singh, P.; Ranjan, M.; Mohapatra, S., Sunlight driven photocatalytic degradation of organic pollutants by solvothermally synthesized rGO-BiVO4 nanohybrids. Chem. Phys. Impact 2024, 8, 100595. [CrossRef]
- Chaudhary, M.; Kumar, C.; Raghav, S.; Panwar, M.; Pandey, S.; Painuli, R., Sunlight-driven photocatalytic degradation of industrial dyes using Withania somnifera decorated MnO2 nanoparticles. Discov. Nano 2024, 19, (1), 206. [CrossRef]
- Davari, N.; Falletta, E.; Bianchi, C. L.; Yargeau, V.; Rodriguez-Seco, C.; Boffito, D. C., Comparing the photocatalytic activity of suspended and floating Ag-decorated TiO2 for dye removal. Tetrahedron Green Chem. 2025, 5, 100059. [CrossRef]
- Díaz-Angulo, J.; Cotillas, S.; Gomes, A. I.; Miranda, S. M.; Mueses, M.; Machuca-Martínez, F.; Rodrigo, M. A.; Boaventura, R. A. R.; Vilar, V. J. P., A tube-in-tube membrane microreactor for tertiary treatment of urban wastewaters by photo-Fenton at neutral pH: A proof of concept. Chemosphere 2021, 263, 128049. [CrossRef]
- Gao, Z.; Pan, C.; Choi, C. H.; Chang, C. H., Continuous-flow photocatalytic microfluidic-reactor for the treatment of aqueous contaminants, simplicity, and complexity: A mini-review. Symmetry 2021, 13, (8), 1325. [CrossRef]
- Ge, X. H.; Wei, N.; Hu, X.; Xie, Q.; Wang, X.; Li, L.; Qiu, T., The integrated microfluidic photocatalytic planar reactor under continuous operation. Front. Chem. Eng. 2024, 6, 1375071. [CrossRef]
- Gogoi, R.; Jena, S. K.; Singh, A.; Sharma, K.; Khanna, K.; Chowdhury, S.; Kumar, R.; Siril, P. F., Mechanically pulverized covalent organic framework as a metal-free photocatalyst for fenton-like degradation of organic pollutants and hexavalent chromium reduction. J. Environ. Chem. Eng. 2024, 12, (2), 112006. [CrossRef]
- Gupta, B.; Gupta, A. K.; Bhatnagar, A., Treatment of pharmaceutical wastewater using photocatalytic reactor and hybrid system integrated with biofilm based process: Mechanistic insights and degradation pathways. J. Environ. Chem. Eng. 2023, 11, (1), 109141. [CrossRef]
- Khositanon, C.; Deepracha, S.; Assabumrungrat, S.; Ogawa, M.; Weeranoppanant, N., Simple Fabrication of a Continuous-Flow Photocatalytic Reactor Using Dopamine-Assisted Immobilization onto a Fluoropolymer Tubing. Ind. Eng. Chem. Res. 2022, 61, (3), 1322-1331. [CrossRef]
- Kumar Jaiswal, V.; Dutta Gupta, A.; Verma, V.; Sharan Singh, R., Degradation of p-cresol in the presence of UV light driven in an integrated system containing photocatalytic and packed bed biofilm reactor. Bioresour. Technol. 2023, 387, 129706. [CrossRef]
- Lv, H.; Zhao, X.; Niu, H.; He, S.; Tang, Z.; Wu, F.; Giesy, J. P., Ball milling synthesis of covalent organic framework as a highly active photocatalyst for degradation of organic contaminants. J. Hazard. Mater. 2019, 369, 494-502. [CrossRef]
- Manassero, A.; Satuf, M. L.; Alfano, O. M., Photocatalytic reactors with suspended and immobilized TiO2: Comparative efficiency evaluation. Chem. Eng. J. 2017, 326, 29-36. [CrossRef]
- Mei, J.; Gao, X.; Zou, J.; Pang, F., Research on Photocatalytic Wastewater Treatment Reactors: Design, Optimization, and Evaluation Criteria. Catalysts 2023, 13, (6), 974. [CrossRef]
- Parmanbek, N.; Sütekin, S. D.; Barsbay, M.; Aimanova, N. A.; Mashentseva, A. A.; Alimkhanova, A. N.; Zhumabayev, A. M.; Yanevich, A.; Almanov, A. A.; Zdorovets, M. V., Environmentally friendly loading of palladium nanoparticles on nanoporous PET track-etched membranes grafted by poly(1-vinyl-2-pyrrolidone) via RAFT polymerization for the photocatalytic degradation of metronidazole. RSC Adv. 2023, 13, (27), 18700-18714. [CrossRef]
- Chu, C.; Chen, Z.; Yao, D.; Liu, X.; Cai, M.; Mao, S., Large-Scale Continuous and In Situ Photosynthesis of Hydrogen Peroxide by Sulfur-Functionalized Polymer Catalyst for Water Treatment. Angew. Chem. Int. Ed. 2024, 63, (10), e202317214. [CrossRef]
- Meng, J.; Huang, Y.; Wang, X.; Liao, Y.; Zhang, H.; Dai, W., Photocatalytic Production of Hydrogen Peroxide from Covalent-Organic-Framework-Based Materials: A Mini-Review. Catalysts 2024, 14, (7), 429. [CrossRef]
- Yong, Z.; Ma, T., Solar-to-H2O2 Catalyzed by Covalent Organic Frameworks. Angew. Chem. Int. Ed. 2023, 62, (49), e202308980. [CrossRef]
- Imamura, K.; Ikeuchi, K.; Sakamoto, Y.; Aono, Y.; Oto, T.; Onda, A., Photocatalytic hydrogenation of nitrobenzene to aniline over titanium(IV) oxide using various saccharides instead of hydrogen gas. RSC Adv. 2021, 11, (51), 32300-32304. [CrossRef]
- López, J.; Chávez, A. M.; Rey, A.; Álvarez, P. M., Insights into the stability and activity of mil-53(Fe) in solar photocatalytic oxidation processes in water. Catalysts 2021, 11, (4), 448. [CrossRef]
- Wang, Y.; Torres, J. A.; Shviro, M.; Carmo, M.; He, T.; Ribeiro, C., Photocatalytic materials applications for sustainable agriculture. Prog. Mater. Sci. 2022, 130, 100965. [CrossRef]
- Basak, A.; Karak, S.; Banerjee, R., Covalent Organic Frameworks as Porous Pigments for Photocatalytic Metal-Free C-H Borylation. J. Am. Chem. Soc. 2023, 145, (13), 7592-7599. [CrossRef]
- Oudi, S.; Oveisi, A. R.; Daliran, S.; Khajeh, M.; Dhakshinamoorthy, A.; García, H., A Porphyrin-Based Covalent Organic Framework as Metal-Free Visible-LED-Light Photocatalyst for One-Pot Tandem Benzyl Alcohol Oxidation/Knoevenagel Condensation. Nanomaterials 2023, 13, (3), 558. [CrossRef]
- Feng, S.; Su, R., Synthetic Chemistry in Flow: From Photolysis & Homogeneous Photocatalysis to Heterogeneous Photocatalysis. ChemSusChem 2024, 17, (16), e202400064. [CrossRef]
- Ghosh, I.; König, B., Chromoselective Photocatalysis: Controlled Bond Activation through Light-Color Regulation of Redox Potentials. Angew. Chem. Int. Ed. 2016, 55, (27), 7676-7679. [CrossRef]
- Martínez-Gualda, A. M.; Cano, R.; Marzo, L.; Pérez-Ruiz, R.; Luis-Barrera, J.; Mas-Ballesté, R.; Fraile, A.; de la Peña O’Shea, V. A.; Alemán, J., Chromoselective access to Z- or E- allylated amines and heterocycles by a photocatalytic allylation reaction. Nat. Commun. 2019, 10, (1), 26342634. [CrossRef]
- Markushyna, Y.; Schüßlbauer, C. M.; Ullrich, T.; Guldi, D. M.; Antonietti, M.; Savateev, A., Chromoselective Synthesis of Sulfonyl Chlorides and Sulfonamides with Potassium Poly(heptazine imide) Photocatalyst. Angew. Chem. Int. Ed. 2021, 60, (37), 20543-20550. [CrossRef]
- Schmermund, L.; Reischauer, S.; Bierbaumer, S.; Winkler, C. K.; Diaz-Rodriguez, A.; Edwards, L. J.; Kara, S.; Mielke, T.; Cartwright, J.; Grogan, G.; Pieber, B.; Kroutil, W., Chromoselective Photocatalysis Enables Stereocomplementary Biocatalytic Pathways**. Angew. Chem. Int. Ed. 2021, 60, (13), 6965-6969. [CrossRef]
- Zou, Y.; Abednatanzi, S.; Gohari Derakhshandeh, P.; Mazzanti, S.; Schüßlbauer, C. M.; Cruz, D.; Van Der Voort, P.; Shi, J. W.; Antonietti, M.; Guldi, D. M.; Savateev, A., Red edge effect and chromoselective photocatalysis with amorphous covalent triazine-based frameworks. Nat. Commun. 2022, 13, (1), 2171. [CrossRef]
- Galushchinskiy, A.; Pulignani, C.; Szalad, H.; Reisner, E.; Albero, J.; Tarakina, N. V.; Pelicano, C. M.; García, H.; Savateev, O.; Antonietti, M., Heterostructured PHI-PTI/Li+Cl- Carbon Nitrides for Multiple Photocatalytic Applications. Sol. RRL 2023, 7, (14), 2300077. [CrossRef]
- Hossain, M. M.; Shaikh, A. C.; Kaur, R.; Gianetti, T. L., Red Light-Blue Light Chromoselective C(sp2)-X Bond Activation by Organic Helicenium-Based Photocatalysis. J. Am. Chem. Soc. 2024, 146, (12), 7922-7930. [CrossRef]
- Ehrfeld, W.; Hessel, V.; Löwe, H., Microreactors, New Technology for Modern Chemistry. WILEY-VCH Verlag GmbH: Weinheim, Germany, 2000; p 1-293.
- Mills, P. L.; Quiram, D. J.; Ryley, J. F., Microreactor technology and process miniaturization for catalytic reactions-A perspective on recent developments and emerging technologies. Chem. Eng. Sci. 2007, 62, (24), 6992-7010. [CrossRef]
- Saldana, M.; Gallegos, S.; Gálvez, E.; Castillo, J.; Salinas-Rodríguez, E.; Cerecedo-Sáenz, E.; Hernández-Ávila, J.; Navarra, A.; Toro, N., The Reynolds Number: A Journey from Its Origin to Modern Applications. Fluids 2024, 9, (12), 299. [CrossRef]
- Houzelot, J. L.; Villermaux, J., Mass transfer in annular cylindrical reactors in laminar flow. Chem. Eng. Sci. 1977, 32, (12), 1465-1470. [CrossRef]
- Wißdorf, W.; Müller, D.; Brachthäuser, Y.; Langner, M.; Derpmann, V.; Klopotowski, S.; Polaczek, C.; Kersten, H.; Brockmann, K.; Benter, T., Gas Flow Dynamics in Inlet Capillaries: Evidence for non Laminar Conditions. J. Am. Soc. Mass Spectrom. 2016, 27, (9), 1550-1563. [CrossRef]
- Diehm, J.; Franzreb, M., Modeling the extra column volume of a micro simulated moving bed chromatography system: Introducing the equivalent radial flow rate distribution. J. Chromatogr. A 2025, 1740, 465543. [CrossRef]
- Antony, R.; Giri Nandagopal, M. S.; Sreekumar, N.; Rangabhashiyam, S.; Selvaraju, N., Liquid-liquid slug flow in a microchannel reactor and its mass transfer properties - A review. Bull. Chem. React. Eng. Catal. 2014, 9, (3), 207-223. [CrossRef]
- Zong, J.; Yue, J., Gas–Liquid Slug Flow Studies in Microreactors: Effect of Nanoparticle Addition on Flow Pattern and Pressure Drop. Front. Chem. Eng. 2021, 3, 788241. [CrossRef]
- Zong, J.; Yue, J., Continuous Solid Particle Flow in Microreactors for Efficient Chemical Conversion. Ind. Eng. Chem. Res. 2022, 61, (19), 6269-6291. [CrossRef]
- Chen, Y.; Zhang, Y.; Zou, H.; Li, M.; Wang, G.; Peng, M.; Zhang, J.; Tang, Z., Tuning the gas-liquid-solid segmented flow for enhanced heterogeneous photosynthesis of Azo- compounds. Chem. Eng. J. 2021, 423, 130226. [CrossRef]
- Radhi, H. H.; Sharifzadeh, E.; Azimi, N., Intensification of Liquid-Liquid Extraction in a Microreactor Equipped with Low and High-Frequency Ultrasound Waves: A Numerical Study. Iran. J. Chem. Chem. Eng.-Int. Engl. Ed. 2024, 43, (8), 3021-3032,.
- Wojnicki, M.; Yang, X.; Zabinski, P.; Mutschke, G., Enhanced Mixing in Microflow Systems Using Magnetic Fields—Experimental and Numerical Analyses. Micromachines 2025, 16, (4), 422. [CrossRef]
- Ma, T.; Zhao, S.; Tang, W.; Zhong, W.; Liu, Y.; Feng, Y.; Fang, Z.; Qin, H.; Xu, H.; Li, Y.; Zhao, Y.; Meng, F.; Yi, L.; He, W.; Guo, K., Microreactors with multivariate external force field used for the chemical process intensification. Chem. Eng. J. 2024, 493, 152508. [CrossRef]
- Tanimu, A.; Jaenicke, S.; Alhooshani, K., Heterogeneous catalysis in continuous flow microreactors: A review of methods and applications. Chem. Eng. J. 2017, 327, 792-821. [CrossRef]
- Suryawanshi, P. L.; Gumfekar, S. P.; Bhanvase, B. A.; Sonawane, S. H.; Pimplapure, M. S., A review on microreactors: Reactor fabrication, design, and cutting-edge applications. Chem. Eng. Sci. 2018, 189, 431-448. [CrossRef]
- Bojang, A. A.; Wu, H. S., Design, fundamental principles of fabrication and applications of microreactors. Processes 2020, 8, (8), 891. [CrossRef]
- Bugay, C. A.; Caballas, M. C.; Mercado, S. B.; Rubio, J. F.; Serote, P. K.; Villarte, P. N.; Rubi, R. V. C., A Review of Microreactors for Process Intensification. Eng. Proc. 2024, 67, (1), 21. [CrossRef]
- Abiev, R. S.; Makusheva, I. V., Energy Dissipation Rate and Micromixing in a Two-Step Micro-Reactor with Intensively Swirled Flows. Micromachines 2022, 13, (11), 1859. [CrossRef]
- Templis, C. C.; Papayannakos, N. G., Mass and heat transfer coefficients in automotive exhaust catalytic converter channels. Catalysts 2019, 9, (6), 507. [CrossRef]
- Yue, J., Multiphase flow processing in microreactors combined with heterogeneous catalysis for efficient and sustainable chemical synthesis. Catal. Today 2018, 308, 3-19. [CrossRef]
- Abbas, I.; Romeggio, F.; Pilarczyk, K.; Kuhn, S.; Damsgaard, C. D.; Kibsgaard, J.; Lievens, P.; Grandjean, D.; Janssens, E., High pressure microreactor for minute amounts of catalyst on planar supports: A case study of CO2 hydrogenation over Pd0.25Zn0.75Ox nanoclusters. Chem. Eng. J. 2025, 503, 158127. [CrossRef]
- Togashi, S.; Miyamoto, T.; Asano, Y.; Endo, Y., Yield improvement of chemical reactions by using a microreactor and development of a pilot plant using the numbering-up of microreactors. J. Chem. Eng. Jpn. 2009, 42, (7), 512-519. [CrossRef]
- Frost, C. G.; Mutton, L., Heterogeneous catalytic synthesis using microreactor technology. Green Chem. 2010, 12, (10), 1687-1703. [CrossRef]
- Liu, X.; Ünal, B.; Jensen, K. F., Heterogeneous catalysis with continuous flow microreactors. Catal. Sci. Technol. 2012, 2, (10), 2134-2138. [CrossRef]
- Feng, H.; Zhang, Y.; Liu, J.; Liu, D., Towards Heterogeneous Catalysis: A Review on Recent Advances of Depositing Nanocatalysts in Continuous–Flow Microreactors. Molecules 2022, 27, (22), 8052. [CrossRef]
- Zhang, L.; Yue, J., Packed Bed Microreactors for Sustainable Chemistry and Process Development. Chem. 2025, 7, (2), 29. [CrossRef]
- Markovič, M.; Lopatka, P.; Gracza, T.; Koóš, P., Recent Applications of Continuous Flow in Homogeneous Palladium Catalysis. Synthesis 2020, 52, (23), 3511-3529. [CrossRef]
- Fanelli, F.; Parisi, G.; Degennaro, L.; Luisi, R., Contribution of microreactor technology and flow chemistry to the development of green and sustainable synthesis. Beilstein J. Org. Chem. 2017, 13, 520-542. [CrossRef]
- Petrusová, Z.; Slouka, Z.; Vobecká, L.; Polezhaev, P.; Hasal, P.; Přibyl, M.; Izák, P., Microreaction and membrane technologies for continuous single-enantiomer production: A review. Catal. Rev. - Sci. Eng. 2023, 65, (3), 773-821. [CrossRef]
- Hone, C. A.; Kappe, C. O., Membrane Microreactors for the On-Demand Generation, Separation, and Reaction of Gases. Chem. Eur. J. 2020, 26, (58), 13108-13117. [CrossRef]
- Koybasi, H. H.; Avci, A. K., Modeling of a membrane integrated catalytic microreactor for efficient DME production from syngas with CO2. Catal. Today 2022, 383, 133-145. [CrossRef]
- Wirth, T., Microreactors in Organic Chemistry and Catalysis: Second Edition. Wiley-VCH: 2013. p 1-465. [CrossRef]
- Alza, E.; Mannhold, R.; Buschmann, H.; Holenz, J., Flow and Microreactor Technology in Medicinal Chemistry. wiley: 2022; p 1-344.
- Yang, L.; Jensen, K. F., Mass transport and reactions in the tube-in-tube reactor. Org. Process Res. Dev. 2013, 17, (6), 927-933. [CrossRef]
- Gross, U.; Koos, P.; O'Brien, M.; Polyzos, A.; Ley, S. V., A general continuous flow method for palladium catalysed carbonylation reactions using single and multiple tube-in-tube gas-liquid microreactors. Eur. J. Org. Chem. 2014, 2014, (29), 6418-6430. [CrossRef]
- Neyt, N. C.; Riley, D. L., Application of reactor engineering concepts in continuous flow chemistry: A review. React. Chem. Eng. 2021, 6, (8), 1295-1326. [CrossRef]
- Lomel, S.; Falk, L.; Commenge, J. M.; Houzelot, J. L.; Ramdani, K., The microreactor: A systematic and efficient tool for the transition from batch to continuous process? Chem. Eng. Res. Des. 2006, 84, (5 A), 363-369. [CrossRef]
- Elvira, K. S.; I Solvas, X. C.; Wootton, R. C. R.; Demello, A. J., The past, present and potential for microfluidic reactor technology in chemical synthesis. Nat. Chem. 2013, 5, (11), 905-915. [CrossRef]
- Xing, R.; Huang, R.; Su, R.; Kong, J.; Dickey, M. D.; Qi, W., 3D-Printing of Hierarchical Porous Copper-Based Metal-Organic-Framework Structures for Efficient Fixed-Bed Catalysts. Chem. Bio. Eng. 2024, 1, (3), 264-273. [CrossRef]
- Rossetti, I., Continuous flow (micro-)reactors for heterogeneously catalyzed reactions: Main design and modelling issues. Catal. Today 2018, 308, 20-31. [CrossRef]
- Chen, X.; Fu, M.; He, J.; Lu, J.; Wang, C.; Jiang, D.; Li, Y.; Yu, C., Immobilization strategies for heterogeneous catalysts in micro flow chemistry: Key techniques for efficient and sustainable chemical transformations. Coord. Chem. Rev. 2025, 538, 216723. [CrossRef]
- An, H.; Li, A.; Sasmito, A. P.; Kurnia, J. C.; Jangam, S. V.; Mujumdar, A. S., Computational fluid dynamics (CFD) analysis of micro-reactor performance: Effect of various configurations. Chem. Eng. Sci. 2012, 75, 85-95. [CrossRef]
- Yang, Y.; Qian, J.; Fang, G.; Guan, Z.; Han, H.; Liu, Q.; Liu, H.; Wang, Y.; Li, W., Research progress on the process analysis technology for microreactors. Microchem. J. 2025, 212, 113373. [CrossRef]
- Schoenitz, M.; Grundemann, L.; Augustin, W.; Scholl, S., Fouling in microstructured devices: A review. Chem. Commun. 2015, 51, (39), 8213-8228. [CrossRef]
- Wang, H.; Wang, X.; Ren, Y.; Zhang, L., Macrostructural Design Approach of the Monolithic Catalyst and Its Application in Nitrobenzene Hydrogenation. Ind. Eng. Chem. Res. 2024. [CrossRef]
- Liu, M.; Zhu, X.; Liao, Q.; Chen, R.; Ye, D.; Chen, G., Stacked Catalytic Membrane Microreactor for Nitrobenzene Hydrogenation. Ind. Eng. Chem. Res. 2020, 59, (20), 9469-9477. [CrossRef]
- Yan, W. W.; Tsuru, T., Overview: Insight into NH3 permselective membranes and their application to a membrane reactor for efficient ammonia production. Sep. Purif. Technol. 2025, 370, 133275. [CrossRef]
- Fang, H.; Xiao, Q.; Wu, F.; Floreancig, P. E.; Weber, S. G., Rapid catalyst screening by a continuous-flow microreactor interfaced with ultra-high-pressure liquid chromatography. J. Org. Chem. 2010, 75, (16), 5619-5626. [CrossRef]
- Nieuwelink, A. E.; Vollenbroek, J. C.; Tiggelaar, R. M.; Bomer, J. G.; van den Berg, A.; Odijk, M.; Weckhuysen, B. M., High-throughput activity screening and sorting of single catalyst particles with a droplet microreactor using dielectrophoresis. Nat. Catal. 2021, 4, (12), 1070-1079. [CrossRef]
- Hone, C. A.; Lopatka, P.; Munday, R.; O'Kearney-McMullan, A.; Kappe, C. O., Continuous-flow Synthesis of Aryl Aldehydes by Pd-catalyzed Formylation of Aryl Bromides Using Carbon Monoxide and Hydrogen. ChemSusChem 2019, 12, (1), 326-337. [CrossRef]
- Greco, R.; Goessler, W.; Cantillo, D.; Kappe, C. O., Benchmarking immobilized di- and triarylphosphine palladium catalysts for continuous-flow cross-coupling reactions: Efficiency, durability, and metal leaching studies. ACS Catal. 2015, 5, (2), 1303-1312. [CrossRef]
- Cantillo, D.; Kappe, C. O., Immobilized transition metals as catalysts for cross-couplings in continuous flow - A critical assessment of the reaction mechanism and metal leaching. ChemCatChem 2015, 6, (12), 3286-3305. [CrossRef]
- Hübner, S.; De Vries, J. G.; Farina, V., Why Does Industry Not Use Immobilized Transition Metal Complexes as Catalysts? Adv. Synth. Catal. 2016, 358, (1), 3-25. [CrossRef]
- Tongtummachat, T.; Jaree, A.; Akkarawatkhoosith, N., Green synthesis of 5-hydroxymethylfurfural through non-catalytic conversion of glucose in a microreactor. Energy Convers. Manage. X 2021, 12, 100141. [CrossRef]
- Cohen, K.; Blanchard, J.; Rodriguez, P.; Kelly, K.; Dorman, J. A.; Dooley, K. M., Non-Catalytic direct partial oxidation of methane to methanol in a Wall-Coated microreactor. Chem. Eng. J. 2024, 482, 149049. [CrossRef]
- El Zanati, E. M.; Elnahas, G. M., Design and scale-up of continuous flow micro-reactor. J. Eng. Sci. Technol. 2018, 13, (8), 2481-2494,.
- Lopatka, P.; Markovič, M.; Koóš, P.; Ley, S. V.; Gracza, T., Continuous Pd-Catalyzed Carbonylative Cyclization Using Iron Pentacarbonyl as a CO Source. J. Org. Chem. 2019, 84, (22), 14394-14406. [CrossRef]
- Lopatka, P.; Gavenda, M.; Markovič, M.; Koóš, P.; Gracza, T., Flow Pd(II)-catalysed carbonylative cyclisation in the total synthesis of Jaspine B. Catalysts 2021, 11, (12), 1513. [CrossRef]
- Ahn, G. N.; Yu, T.; Lee, H. J.; Gyak, K. W.; Kang, J. H.; You, D.; Kim, D. P., A numbering-up metal microreactor for the high-Throughput production of a commercial drug by copper catalysis. Lab Chip 2019, 19, (20), 3535-3542. [CrossRef]
- Ciemięga, A.; Maresz, K.; Mrowiec-Białoń, J., Bifunctionalised acid-base hierarchically structured monolithic microreactor for continuous-flow tandem catalytic process of cyanocinnamate synthesis. Sci. Rep. 2024, 14, (1), 25332. [CrossRef]
- Poletti, L.; De Risi, C.; Ragno, D.; Di Carmine, G.; Tassoni, R.; Massi, A.; Dambruoso, P., Organocatalytic Packed-Bed Reactors for the Enantioselective Flow Synthesis of Quaternary Isotetronic Acids by Direct Aldol Reactions of Pyruvates. Molecules 2025, 30, (2), 296. [CrossRef]
- Rao Pala, L. P.; Peela, N. R., Green Hydrogen Production in an Optofluidic Planar Microreactor via Photocatalytic Water Splitting under Visible/Simulated Sunlight Irradiation. Energy & Fuels 2021, 35, (23), 19737-19747. [CrossRef]
- Zhang, H.; Liu, Y.; Liu, N.; Kang, S., Understanding the interface properties of photocatalytic reactors for rational engineering applications. Chem. Eng. J. 2023, 472, 145057. [CrossRef]
- Lu, J. M.; Wang, H. F.; Guo, Q. H.; Wang, J. W.; Li, T. T.; Chen, K. X.; Zhang, M. T.; Chen, J. B.; Shi, Q. N.; Huang, Y.; Shi, S. W.; Chen, G. Y.; Pan, J. Z.; Lu, Z.; Fang, Q., Roboticized AI-assisted microfluidic photocatalytic synthesis and screening up to 10,000 reactions per day. Nat. Commun. 2024, 15, (1), 8826. [CrossRef]
- Towler, G.; Sinnott, R., Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design. Elsevier: 2021; p 1-1027.
- Data, R. A. Top 10 Industrial Catalyst Companies in the World. https://www.reportsanddata.com/blog/top-industrial-catalyst-companies-in-the-world (June 6, 2025),.
- Shi, H.; Zhou, T., Computational design of heterogeneous catalysts and gas separation materials for advanced chemical processing. Front. Chem. Sci. Eng. 2021, 15, (1), 49-59. [CrossRef]
- Aris, R.; Bell, A. T.; Boudart, M.; Chen, N. Y.; C., G. B.; Haag, W. O.; Somorjai, G. A.; J., W.; Hegedus, L. S. E., Catalyst Design: Progress and Perspectives Wiley-Interscience: New York, US, 1987; p 1-288.
- Hagen, J., Industrial Catalysis: A Practical Approach: Second Edition. John Wiley and Sons: 2006; p 1-507.
- Schmidle, C. J.; Mansfield, R. C., Catalysis by anion exchange resins. Ind. Eng. Chem. 1952, 44, (6), 1388-1390. [CrossRef]
- Lyu, Y.; Scrimin, P., Mimicking Enzymes: The Quest for Powerful Catalysts from Simple Molecules to Nanozymes. ACS Catal. 2021, 11, (18), 11501-11509. [CrossRef]
- Illanes, A., Enzyme biocatalysis: Principles and applications. Springer Netherlands: 2008; p 1-391.
- Winkler, C. K.; Schrittwieser, J. H.; Kroutil, W., Power of Biocatalysis for Organic Synthesis. ACS Cent. Sci. 2021, 7, (1), 55-71. [CrossRef]
- Albayati, S. H.; Nezhad, N. G.; Taki, A. G.; Rahman, R. N. Z. R. A., Efficient and easible biocatalysts: Strategies for enzyme improvement. A review. Int. J. Biol. Macromol. 2024, 276, 133978. [CrossRef]
- Reisenbauer, J. C.; Sicinski, K. M.; Arnold, F. H., Catalyzing the future: recent advances in chemical synthesis using enzymes. Curr. Opin. Chem. Biol. 2024, 83, 102536. [CrossRef]
- Man, T.; Xu, C.; Liu, X. Y.; Li, D.; Tsung, C. K.; Pei, H.; Wan, Y.; Li, L., Hierarchically encapsulating enzymes with multi-shelled metal-organic frameworks for tandem biocatalytic reactions. Nat. Commun. 2022, 13, (1), 305. [CrossRef]
- Wang, Y.; Tong, H.; Ni, S.; Huo, K.; Liu, W.; Zan, X.; Yuan, X.; Wang, S., Combining Hard Shell with Soft Core to Enhance Enzyme Activity and Resist External Disturbances. Adv. Sci. 2025, 12, (10), e2411196. [CrossRef]
- Králik, M. In Research and development of chemical technologies, 39th International Conference of Slovak Society of Chemical Engineering, Tatranské Matliare, Slovakia, 2012, 2012; Markoš, J., Ed. SSCHE Bratislava: Tatranské Matliare, Slovakia, 2012; p 451–456.
- Králik, M. In Liquid phase hydrogenations over dispersed metal catalysts, 12 thPannonian Symposium on Catalysis, Třešť, Czech Republic, 2014; Klusoň, P., Ed. Institute of Chemical Technology, Prague: Třešť, Czech Republic, p K1.
- group, J. M., Licensed processes A portfolio of DAVY licensed processes and technologies. group, J. M., Ed. Johnson Matthey group: Billingham, UK, 2021; pp 1-16.
- Setoyama, T., Design of heterogeneous catalysts and process technologies reflecting on the relevant reaction mechanism as a methodology of research at chemical company. Res. Chem. Intermed. 2021, 47, (1), 211-223. [CrossRef]
- Erfani, N.; Baharudin, L.; Watson, M., Recent advances and intensifications in Haber-Bosch ammonia synthesis process. Chem. Eng. Process.: Process Intensif. 2024, 204, 109962. [CrossRef]
- Sebesta, R. W., Concepts of Programming Languages, 11th Ed. Pearson: 2015; p 1-800.
- Brunovska, A.; Burianek, J.; Ilavsky, J.; Valtyni, J., Modelling of catalyst pellet deactivation: an irreversible chemisorption model. Adsorpt. Sci. Technol. 1986, 3, 201-215. [CrossRef]
- Ilavský, J.; Brunovská, A.; Valtýni, J.; Buriánek, J., Modeling of catalytic reactors with catalyst deactivation. I. Pseudo-homogeneous model of a plug-flow reactor. Chem. Pap. 1983, 37, (4), 433–451.
- Markoš, J.; Brunovská, A.; Ilavský, J., Modelling of fixed bed catalytic reactors catalyst poisoning. Collect. Czech. Chem. Commun. 1985, 50, 2228-2244. [CrossRef]
- Barto, M.; Brunovská, A.; Ilavský, J., Modelling of catalytic reactors with catalyst deactivation III. Pseudohomogeneous model of reactor with axial dispersion. Chem. Pap. 1986, 40, (4), 551-563,.
- Králik, M.; Ilavský, J.; Pašek, J.; Lehocký, P., Mathematical dynamic model of the continuous bubble column slurry reactor. Chem. Eng. Proc. 1990, 28, (2), 127-132. [CrossRef]
- Markov, P. V.; Mashkovsky, I. S.; Bragina, G. O.; Wärnå, J.; Gerasimov, E. Y.; Bukhtiyarov, V. I.; Stakheev, A. Y.; Murzin, D. Y., Particle size effect in liquid-phase hydrogenation of phenylacetylene over Pd catalysts: Experimental data and theoretical analysis. Chem. Eng. J. 2019, 358, 520-530. [CrossRef]
- Murzin, D. Y.; Wärnå, J.; Haario, H.; Salmi, T., Parameter estimation in kinetic models of complex heterogeneous catalytic reactions using Bayesian statistics. React. Kinet. Mech. Catal. 2021, 133, (1). [CrossRef]
- Suárez-Méndez, A.; Mendoza-Cruz, R.; Martínez-Klimov, M. E.; Wärnå, J.; Albuquerque, J. S.; Murzin, D. Y.; Klimova, T. E., NiPt supported on zeolite Y and SBA-15 composites as efficient catalysts for the hydrodeoxygenation of anisole under mild conditions. Fuel 2025, 400, 135793. [CrossRef]
- Murzin, D. Y., Chemical reactors, Treatment of data and mathematical modelling. Králik, M., Ed. Abo-Turku, Bratislava, 2025.
- Wikipedia List of chemical process simulators. https://en.wikipedia.org/wiki/List_of_chemical_process_simulators (June 6, 2025),.
- Haydary, J., Chemical Process Design and Simulation. Aspen Plus and Aspen HYSYS Applications. John Wiley & Sons, Inc.: New Delhi, India, 2018; p 1-418.
- de la Hera, G.; Ruiz-Gutiérrez, G.; Viguri, J. R.; Galán, B., Flexible Green Ammonia Production Plants: Small-Scale Simulations Based on Energy Aspects. Environ. 2024, 11, (4), 71. [CrossRef]
- Beale, M. H.; T., H. M.; Demuth, H. B., Deep Learning Toolbox™ Reference, MATLAB. The MathWorks, Inc.: Natick, Massachusetts, U.S., 2023; p 1-2706.
- Anderson, E.; Bai, Z.; Bischof, C.; Blackford, S.; Demmel, J.; Dongarra, J.; Croz, J.-D.; Greenbaum, A.; Hammarling, S.; McKenney, A.; Sorensen, D., gPROMS Introductory User Guide. Process Systems Enterprise Ltd.: London, UK, 2004; p 254.
- Qayyum, H.; Cheema, I. I.; Abdullah, M.; Amin, M.; Khan, I. A.; Lee, E. J.; Lee, K. H., One-dimensional modeling of heterogeneous catalytic chemical looping steam methane reforming in an adiabatic packed bed reactor. Front. Chem. 2023, 11, 1295455. [CrossRef]
- Hachhach, M.; Russo, V.; Simakova, I.; Eränen, K.; Murzin, D. Y.; Salmi, T., Packed bed reactor technology in sugar oxidation. Chem. Eng. Sci. 2025, 311, 121580. [CrossRef]
- Nyemba, W. R., Computer Aided Design: Engineering Design and Modeling Using AutoCAD. CRC Press: 2022; p 1-292.
- Schindel; Polyakova; Harding; Weinhold; Stenger; Grunewald; Bramsiepe, General approach for technology and Process Equipment Assembly (PEA) selection in process design. Chem. Eng. Process.: Process Intensif. 2021, 159, 108223. [CrossRef]
- Ismaeel, H. K.; Albayati, T. M.; Dhahad, H. A.; Al-Sudani, F. T.; Salih, I. K.; Saady, N. M. C.; Zendehboudi, S., Strategies for biodiesel production with the role of reactor technologies: A comprehensive review. Chem. Eng. Process.: Process Intensif. 2024, 200, 109767. [CrossRef]
- Perathoner, S.; Centi, G., Science and Technology Roadamap on Catalysis for Europe, a Path to Create a Sustainable Future. ERIC aisbl: Brussel, BG, 2016.
- Appl, M., Ammonia: Principles and Industrial Practice. wiley: 1999; p 1-301.
- Humphreys, J.; Lan, R.; Tao, S., Development and Recent Progress on Ammonia Synthesis Catalysts for Haber–Bosch Process. Adv. Energy Sustain. Res. 2021, 2, (1), 2000043. [CrossRef]
- Fuller, J.; An, Q.; Fortunelli, A.; Goddard, W. A., Reaction Mechanisms, Kinetics, and Improved Catalysts for Ammonia Synthesis from Hierarchical High Throughput Catalyst Design. Acc. Chem. Res. 2022, 55, (8), 1124-1134. [CrossRef]
- Nadiri, S.; Attari Moghaddam, A.; Folke, J.; Ruland, H.; Shu, B.; Fernandes, R.; Schlögl, R.; Krewer, U., Ammonia Synthesis Rate Over a Wide Operating Range: From Experiments to Validated Kinetic Models. ChemCatChem 2024, 16, (23), e202400890. [CrossRef]
- Rouwenhorst, K. H. R.; Travis, A. S.; Lefferts, L., 1921–2021: A Century of Renewable Ammonia Synthesis. Sustain. Chem. 2022, 3, (2), 149-171. [CrossRef]
- Varadwaj, P. R.; Marques, H. M.; Grabowski, I., Ammonia Synthesis over Transition Metal Catalysts: Reaction Mechanisms, Rate-Determining Steps, and Challenges. Int. J. Mol. Sci. 2025, 26, (10), 4670. [CrossRef]
- Saygin, D.; Blanco, H.; Boshell, F.; Cordonnier, J.; Rouwenhorst, K.; Lathwal, P.; Gielen, D., Ammonia Production from Clean Hydrogen and the Implications for Global Natural Gas Demand. Sustainability 2023, 15, (2), 1623. [CrossRef]
- Hansen, J. B., High efficient Ammonia Synthesis Systems. Haldor Topsøe A/S: Lyngby, Denmark, 2019; p 1-23.
- Wang, B.; Li, T.; Gong, F.; Othman, M. H. D.; Xiao, R., Ammonia as a green energy carrier: Electrochemical synthesis and direct ammonia fuel cell - a comprehensive review. Fuel Process. Technol. 2022, 235, 107380. [CrossRef]
- Arabczyk, W.; Pelka, R.; Jasińska, I.; Lendzion-Bieluń, Z., Thermodynamics of Iron Ammonia Synthesis Catalyst Sintering. Crystals 2024, 14, (2), 188. [CrossRef]
- Stull, D. R.; E.F., W. J.; Sinke, G. C., The chemical thermodynamics of organic compounds. John Wiley and Sons: New York, 1969.
- Cuevas-Castillo, G. A.; Michailos, S.; Hughes, K.; Ingham, D.; Pourkashanian, M., A comprehensive process modelling, techno-economic and life cycle assessment of a power to ammonia process. Sustain. Energy Technol. Assess 2025, 76, 104278. [CrossRef]
- Bian, X.; Zhao, Y.; Waterhouse, G. I. N.; Miao, Y.; Zhou, C.; Wu, L. Z.; Zhang, T., Quantifying the Contribution of Hot Electrons in Photothermal Catalysis: A Case Study of Ammonia Synthesis over Carbon-supported Ru Catalyst. Angew. Chem. Int. Ed. 2023, 62, (25), e202304452. [CrossRef]
- Vogt, E. T. C.; Weckhuysen, B. M., Fluid catalytic cracking: recent developments on the grand old lady of zeolite catalysis. Chem. Soc. Rev. 2015, 44, (20), 7342-7370. [CrossRef]
- Tasneem, N.; Ahmed, S.; Hossain, M. M., Enhancement of Olefins from Fluid Catalytic Cracking Processes: A Review on Effects of Catalysts and Kinetics. Arab. J. Sci. Eng. 2025, 50, (6), 3649-3670107513. [CrossRef]
- Cordero-Lanzac, T.; Bilbao, J., Deactivation kinetic models for the fluid catalytic cracking (FCC). A review. Chem. Eng. J. 2025, 514, 162856. [CrossRef]
- Ferreira, J. M. M.; Sousa-Aguiar, E. F.; Aranda, D. A. G., FCC Catalyst Accessibility—A Review. Catalysts 2023, 13, (4), 784. [CrossRef]
- Fals, J.; Garcia-Valencia, J. F.; Puello-Polo, E.; Tuler, F.; Márquez, E., Hierarchical Y Zeolite-Based Catalysts for VGO Cracking: Impact of Carbonaceous Species on Catalyst Acidity and Specific Surface Area. Molecules 2024, 29, (13), 3085. [CrossRef]
- Tedeeva, M. A.; Kustov, A. L.; Batkin, A. M.; Garifullina, C.; Zalyatdinov, A. A.; Yang, D.; Dai, Y.; Yang, Y.; Kustov, L. M., Catalytic systems for hydrogenation of CO2 to methanol. Mol. Cat. 2024, 566, 114403. [CrossRef]
- Huang, F.; Wang, S.; Zhang, L., Design and recent advances of novel MoS2-based catalysts for methanol from carbon dioxide hydrogenation. Sep. Purif. Technol. 2025, 365, 132681. [CrossRef]
- Bozzano, G.; Manenti, F., Efficient methanol synthesis: Perspectives, technologies and optimization strategies. Prog. Energy Combust. Sci. 2016, 56, 71-105. [CrossRef]
- Sheldon, D., Methanol production - A technical history. Johns. Matthey Technol. Rev. 2017, 61, (3), 172-182. [CrossRef]
- Atsbha, T. A.; Yoon, T.; Seongho, P.; Lee, C. J., A review on the catalytic conversion of CO2 using H2 for synthesis of CO, methanol, and hydrocarbons. J. CO2 Util. 2021, 44, 101413. [CrossRef]
- Tezel, E.; Sannes, D. K.; Svelle, S.; Szilágyi, P. I.; Olsbye, U., Direct CO2 to methanol reduction on Zr6-MOF based composite catalysts: a critical review. Mater. Adv. 2023, 4, (22), 5479-5495. [CrossRef]
- Sahoo, K. K.; Katari, J. K.; Das, D., Recent advances in methanol production from methanotrophs. World J. Microbiol. Biotechnol. 2023, 39, (12), 360. [CrossRef]
- Qu, T.; Wei, S.; Xiong, Z.; Zhang, J.; Zhao, Y., Progress and prospect of CO2 photocatalytic reduction to methanol. Fuel Process. Technol. 2023, 251, 107933. [CrossRef]
- Wawrzyniak, A.; Koper, M. T. M., Electrocatalytic CO2 Reduction to Methanol on Pt(111) Modified with a Pd Monolayer. ACS Catal. 2025, 15, (3), 1514-1521. [CrossRef]
- Gielen, D.; Dolan, G., Innovation Outlook : Renewable Methanol. International Renewable Energy Agency Abu Dhabi, , 2021; p 1-124.
- Khalid, M.; Dharaskar, S. A.; Sillanpää, M.; Siddiqui, H., Emerging Carbon Capture Technologies: Towards a Sustainable Future. Elsevier: 2022; p 1-479.
- Yee, K. F.; Mohamed, A. R.; Tan, S. H., A review on the evolution of ethyl tert-butyl ether (ETBE) and its future prospects. Renew. Sustain. Energy Rev. 2013, 22, 604-620. [CrossRef]
- Mahdi, H. I.; Muraza, O., Conversion of Isobutylene to Octane-Booster Compounds after Methyl tert-Butyl Ether Phaseout: The Role of Heterogeneous Catalysis. Ind. Eng. Chem. Res. 2016, 55, (43), 11193-11210. [CrossRef]
- Králik, M.; Turáková, M.; Mačák, I.; Lehocký, P., Aniline-catalysis and chemical engineering. In the 41st International Conference of Slovak Society of Chemical Engineering, Markoš, J., Ed. SSCHI, ICEE, STU in Bratislava: Tatranské Matliare, Slovakia, 2014; Vol. 1, pp 723–733.
- Graham, D. P.; Spiegler, L. Hydrogenation of Nitro Compounds to Amines and Catalyst therefore. 1958.
- Gelder, E. A.; Jackson, S. D.; Lok, C. M., The hydrogenation of nitrobenzene to aniline: A new mechanism. Chem. Commun. 2005, (4), 522-524. [CrossRef]
- Turáková, M.; Králik, M.; Lehocký, P.; Pikna, Ľ.; Smrčová, M.; Remeteiová, D.; Hudák, A., Influence of preparation method and palladium content on Pd/C catalysts activity in the liquid phase hydrogenation of nitrobenzene to aniline. Appl. Catal. A: Gen. 2014, 476, 103-112. [CrossRef]
- Turáková, M.; Salmi, T.; Eränen, K.; Wärnå, J.; Murzin, D. Y.; Králik, M., Liquid phase hydrogenation of nitrobenzene. Appl. Catal. A: Gen. 2015, 499, 66-76. [CrossRef]
- Bombay, C., Aniline Plant. Chematur Bombay: Bombay, 2012; p 1-13.
- Tang, W.; Liu, Y.; Jin, Y.; Wang, Y.; Shi, W.; Ma, P.; Niu, J.; Wang, J., Photocatalytic Reduction of Nitrobenzene to Aniline by an Intriguing {Ru(C6H6)}-Based Heteropolytungstate. Inorg. Chem. 2024, 63, (14), 6260-6267. [CrossRef]
- Islam, I. U.; Zhang, Y.; Dong, B.; Iqbal, A.; Abbas, S.; Zai, J.; Ahmad Shah, S. S.; Qian, X., Highly Selective Electroreduction of Nitrobenzene to Aniline by Co-Doped 1T-MoS2. ACS Appl. Mater. Interfaces 2024, 16, (19), 25090-25100. [CrossRef]
- Driessen, R. T.; Kamphuis, P.; Mathijssen, L.; Zhang, R.; van der Ham, L. G. J.; van den Berg, H.; Zeeuw, A. J., Industrial Process Design for the Production of Aniline by Direct Amination. Chem. Eng. Technol. 2017, 40, (5), 838-846. [CrossRef]
- Bugosen, S.; Mantilla, I. D.; Tarazona-Vasquez, F., Techno-economic analysis of aniline production via amination of phenol. Heliyon 2020, 6, (12), e05778. [CrossRef]
- Liu, X.; Chen, W.; Zou, J.; Ye, L.; Yuan, Y., Liquid-Phase Amination of Phenol to Aniline over the Pd/MgO Catalyst without External Hydrogen Addition. ACS Sustain. Chem. Eng. 2022, 10, (21), 6988-6998. [CrossRef]
- Gernot, J.; Magnus, J.; Moussa, A. S. Production of aniline via anthranilate. US 10,173 ,969 B2, Jan . 8 , 2019, 2019.
- Winter, B.; Meys, R.; Bardow, A., Towards aromatics from biomass: Prospective Life Cycle Assessment of bio-based aniline. J. Clean. Prod. 2021, 290, 125818. [CrossRef]
- Covestro World’s First Pilot Plant for Bio-Based Aniline. https://www.covestro.com/press/worlds-first-pilot-plant-for-bio-based-aniline/ (June, 15, 2025 ),.
- Koch, W., Pathway design for industrial fermentation. wiley: 2024; p 1-496.
- McCusker, L. B.; Olson, D. H.; Baerlocher, C., Atlas of Zeolite Framework Types. Elsevier: 2007.
- Lai, N. S.; Tew, Y. S.; Zhong, X.; Yin, J.; Li, J.; Yan, B.; Wang, X., Artificial Intelligence (AI) Workflow for Catalyst Design and Optimization. Ind. Eng. Chem. Res. 2023, 62, (43), 17835-17848. [CrossRef]
- McCullough, K. E.; King, D. S.; Chheda, S. P.; Ferrandon, M. S.; Goetjen, T. A.; Syed, Z. H.; Graham, T. R.; Washton, N. M.; Farha, O. K.; Gagliardi, L.; Delferro, M., High-Throughput Experimentation, Theoretical Modeling, and Human Intuition: Lessons Learned in Metal-Organic-Framework-Supported Catalyst Design. ACS Cent. Sci. 2022, 9, (2), 266-276. [CrossRef]
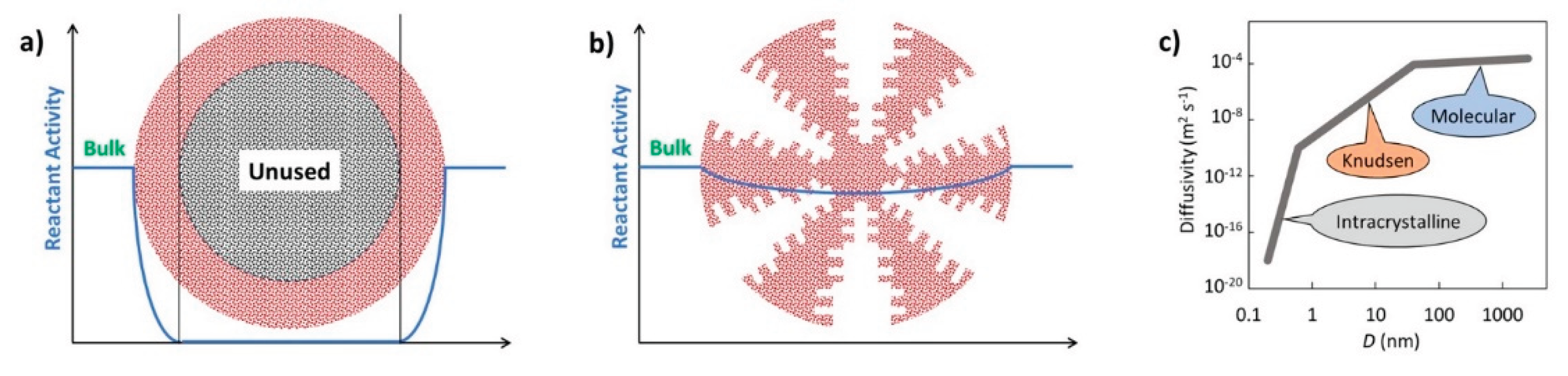
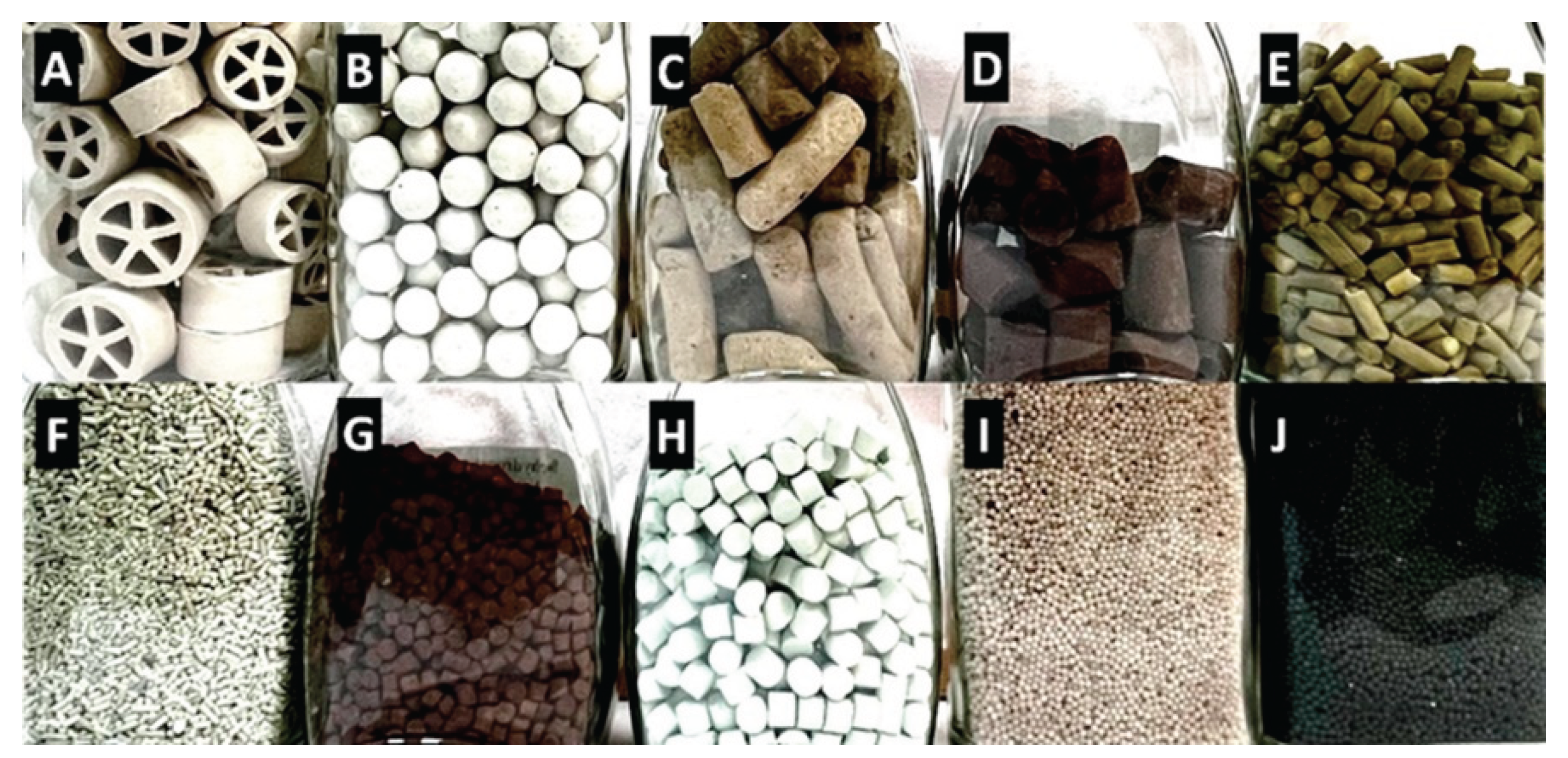
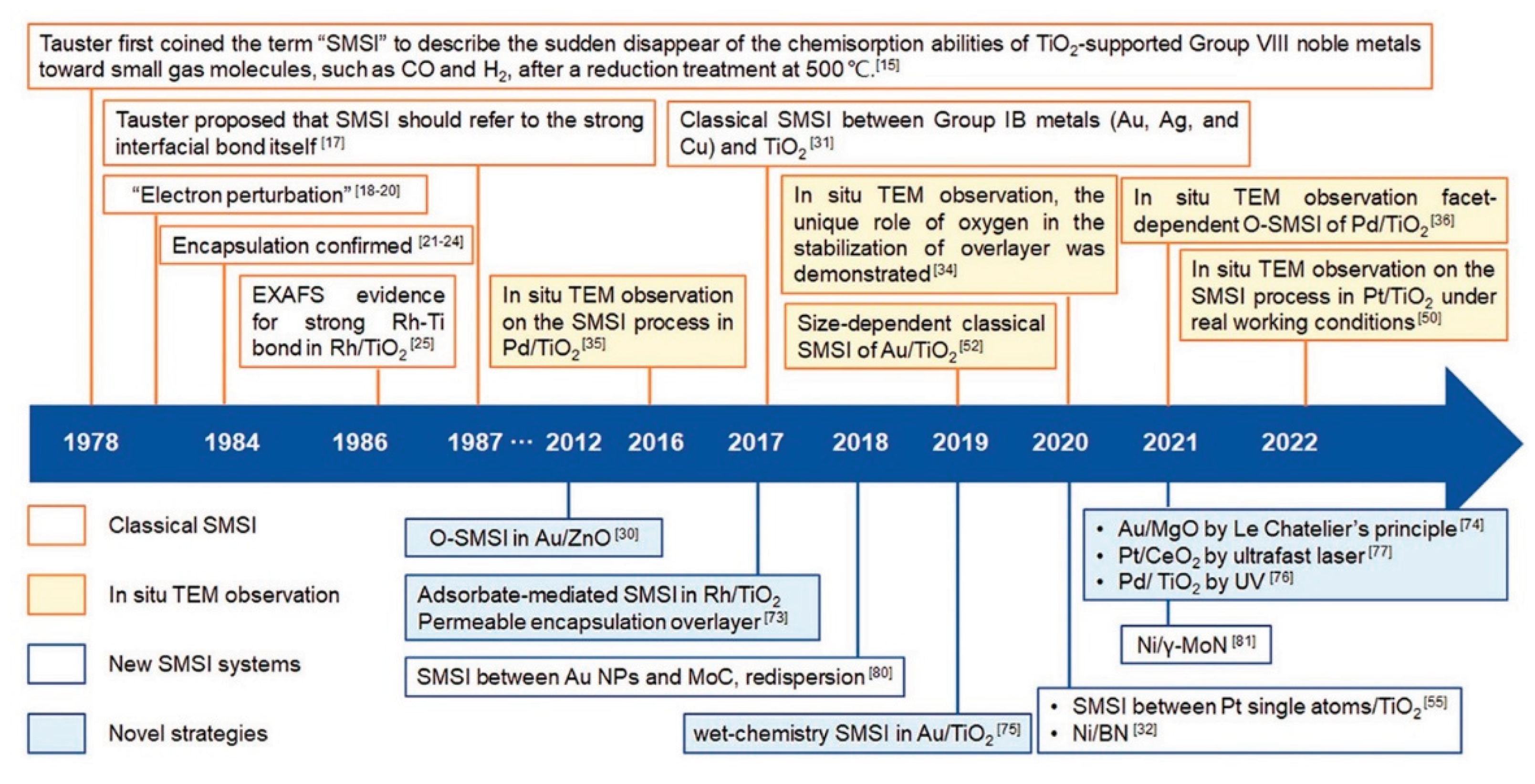

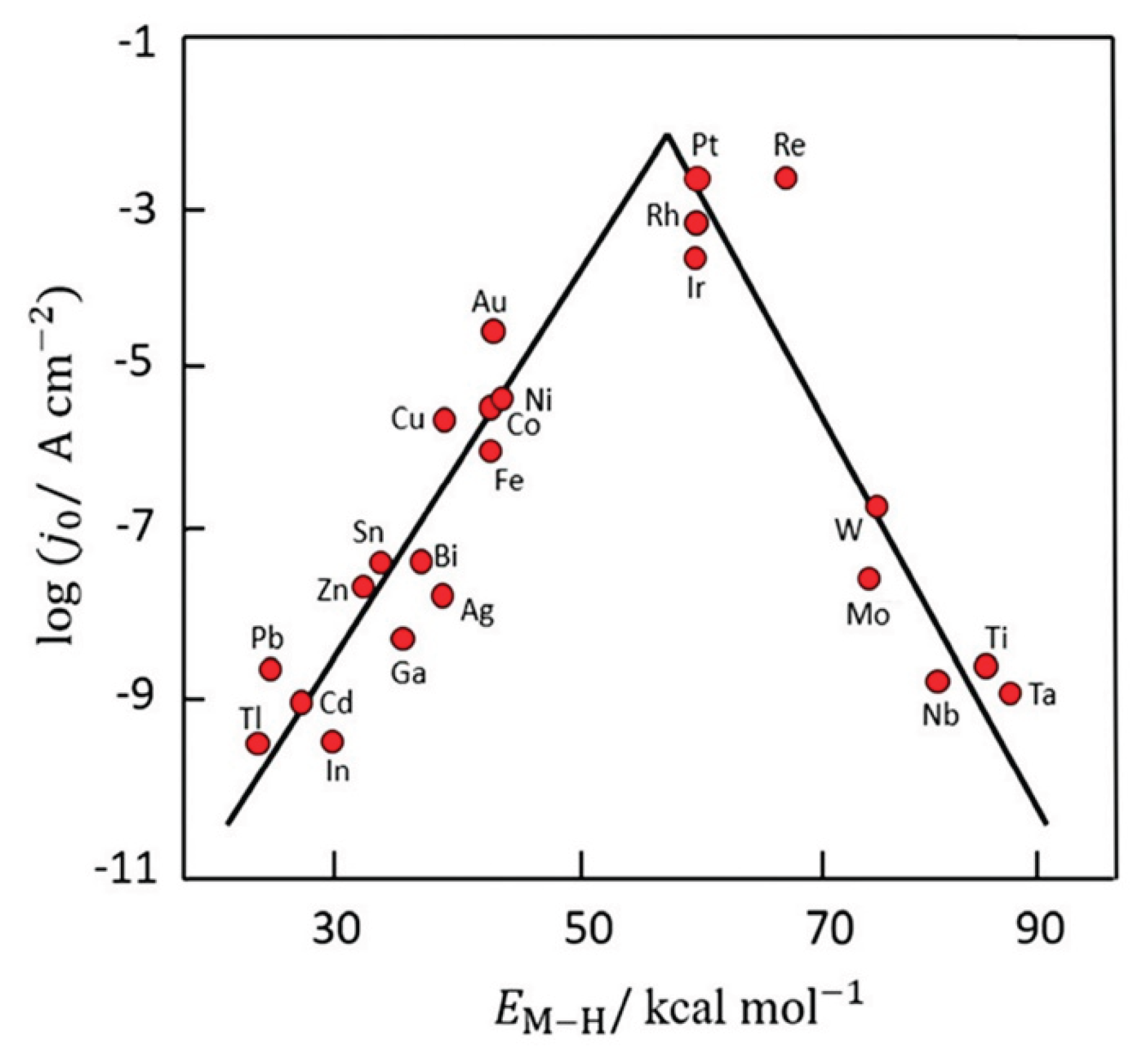
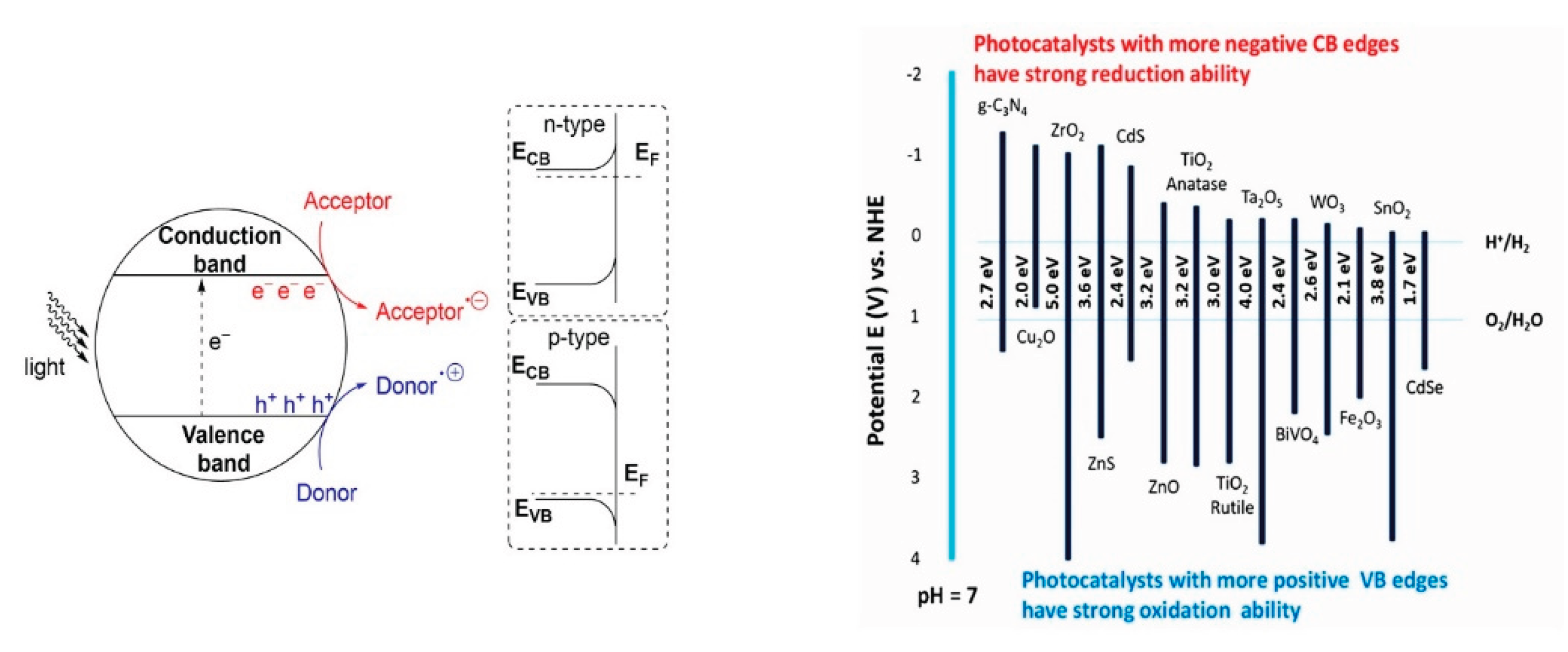
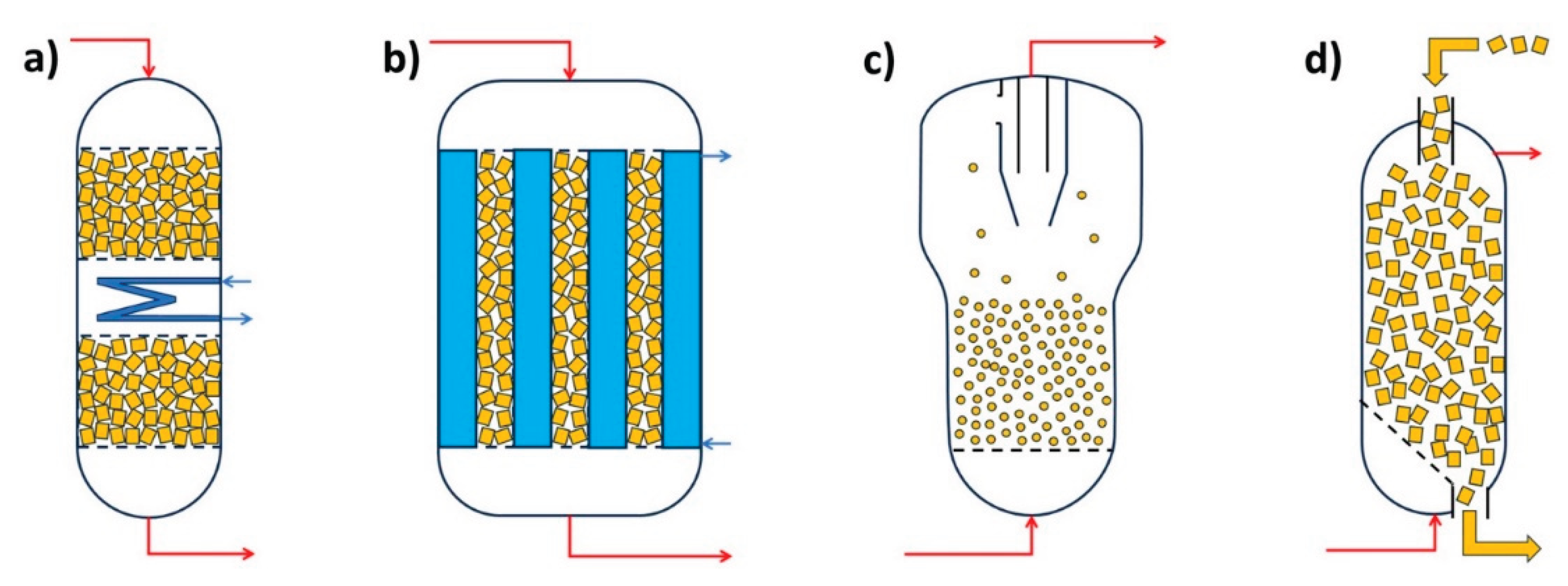
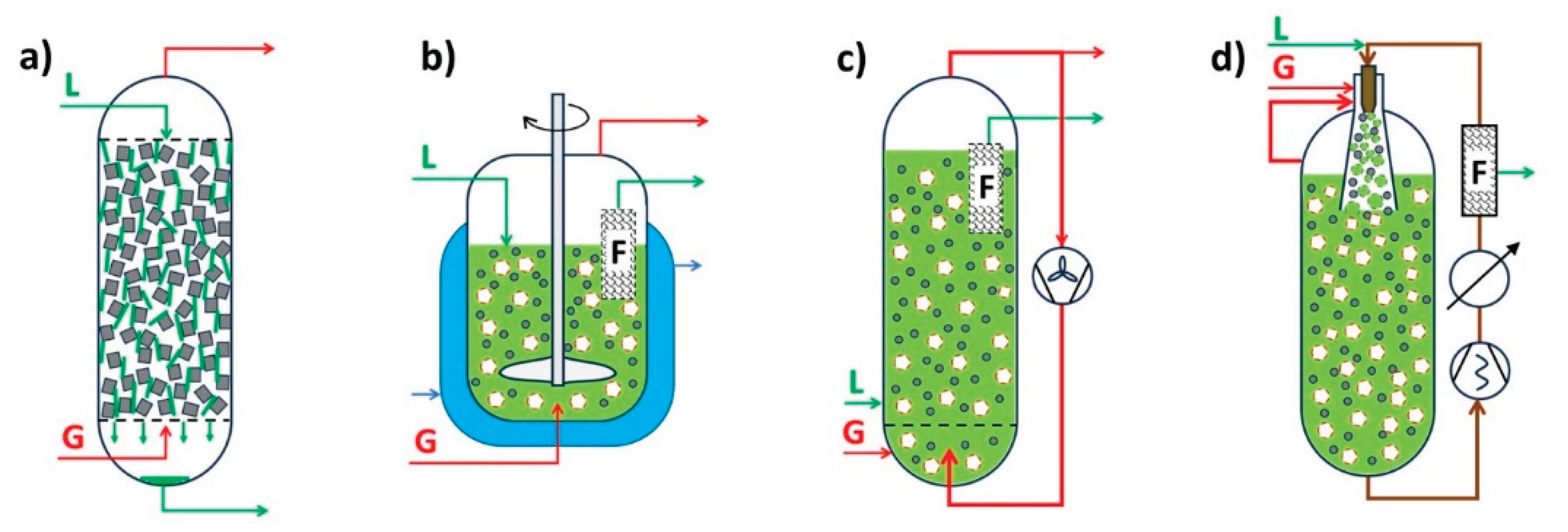
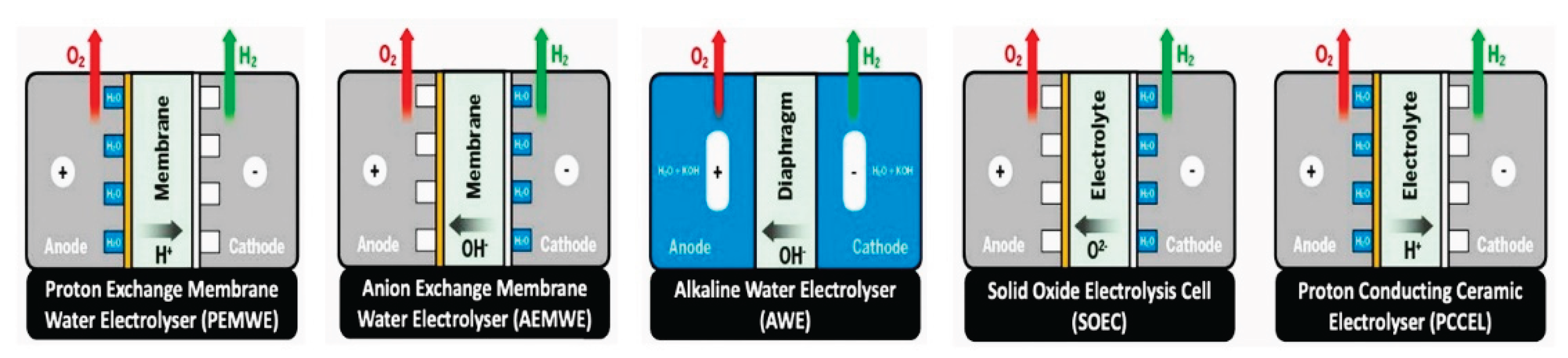
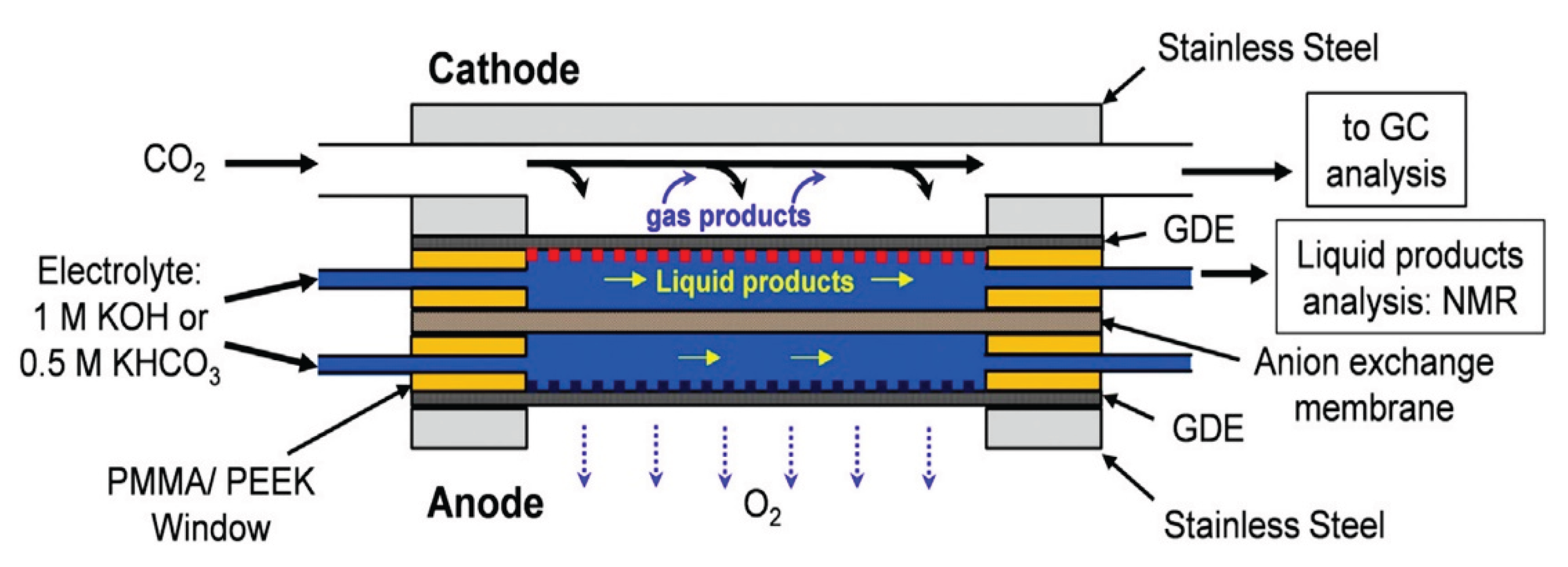
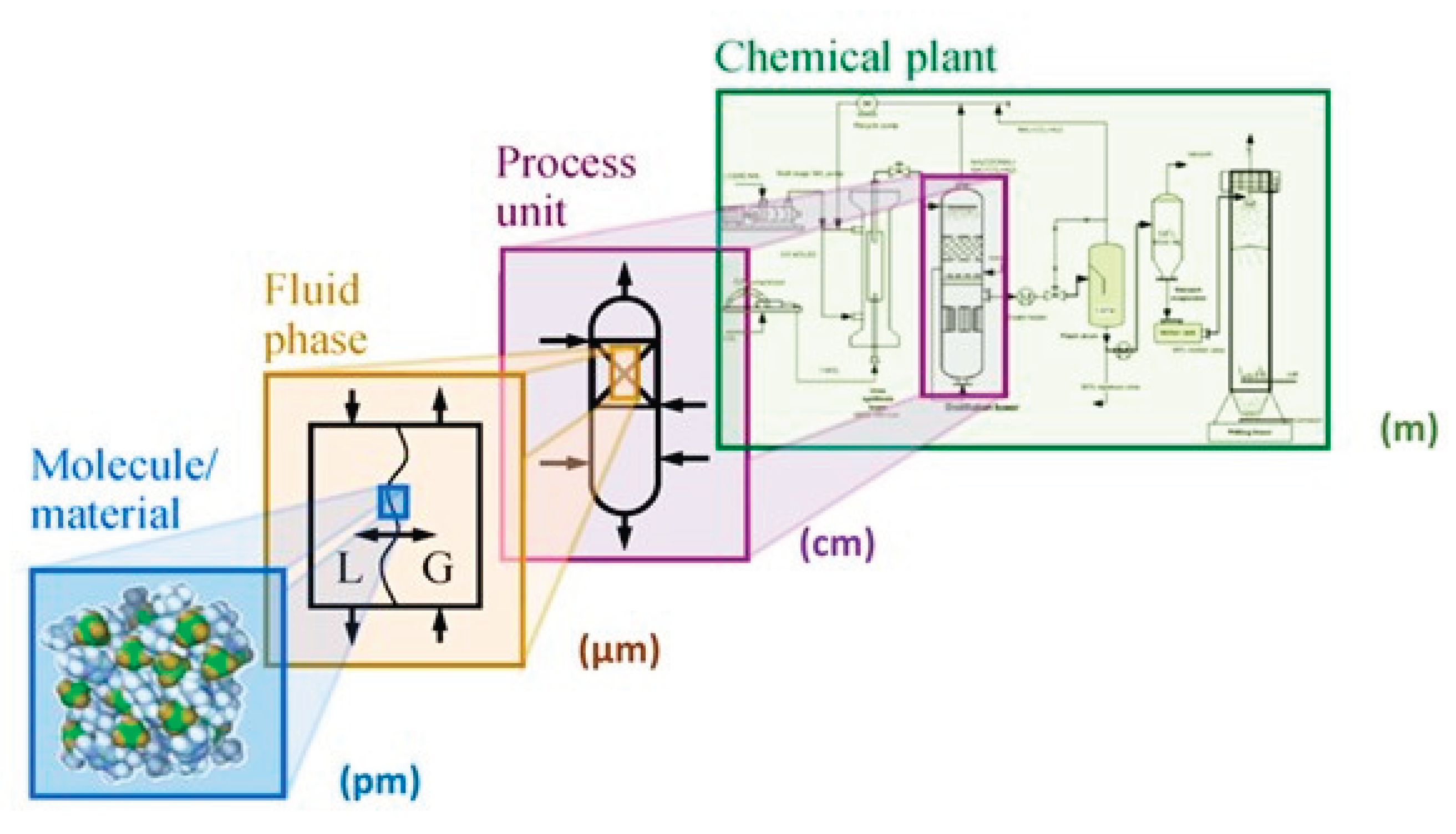
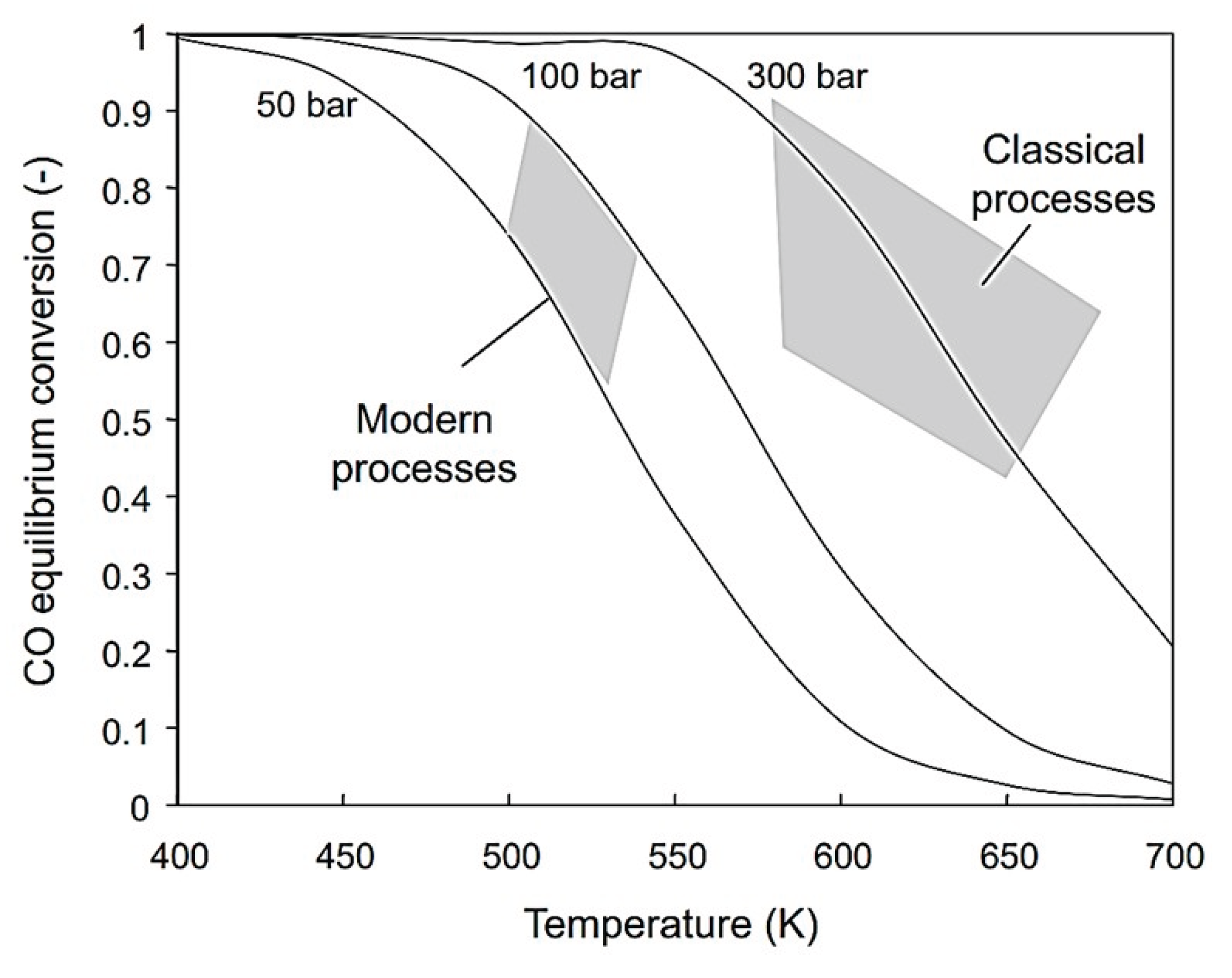
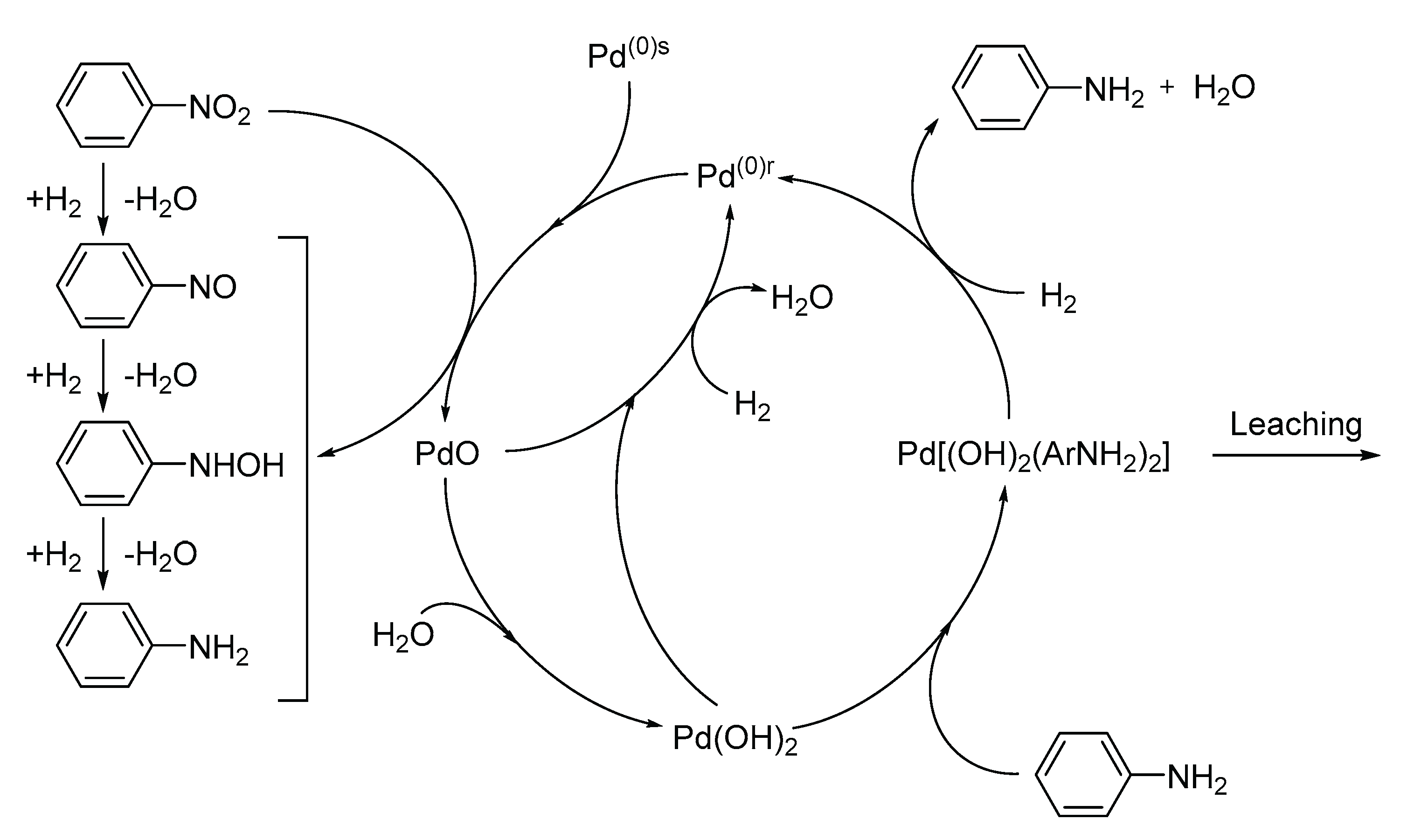
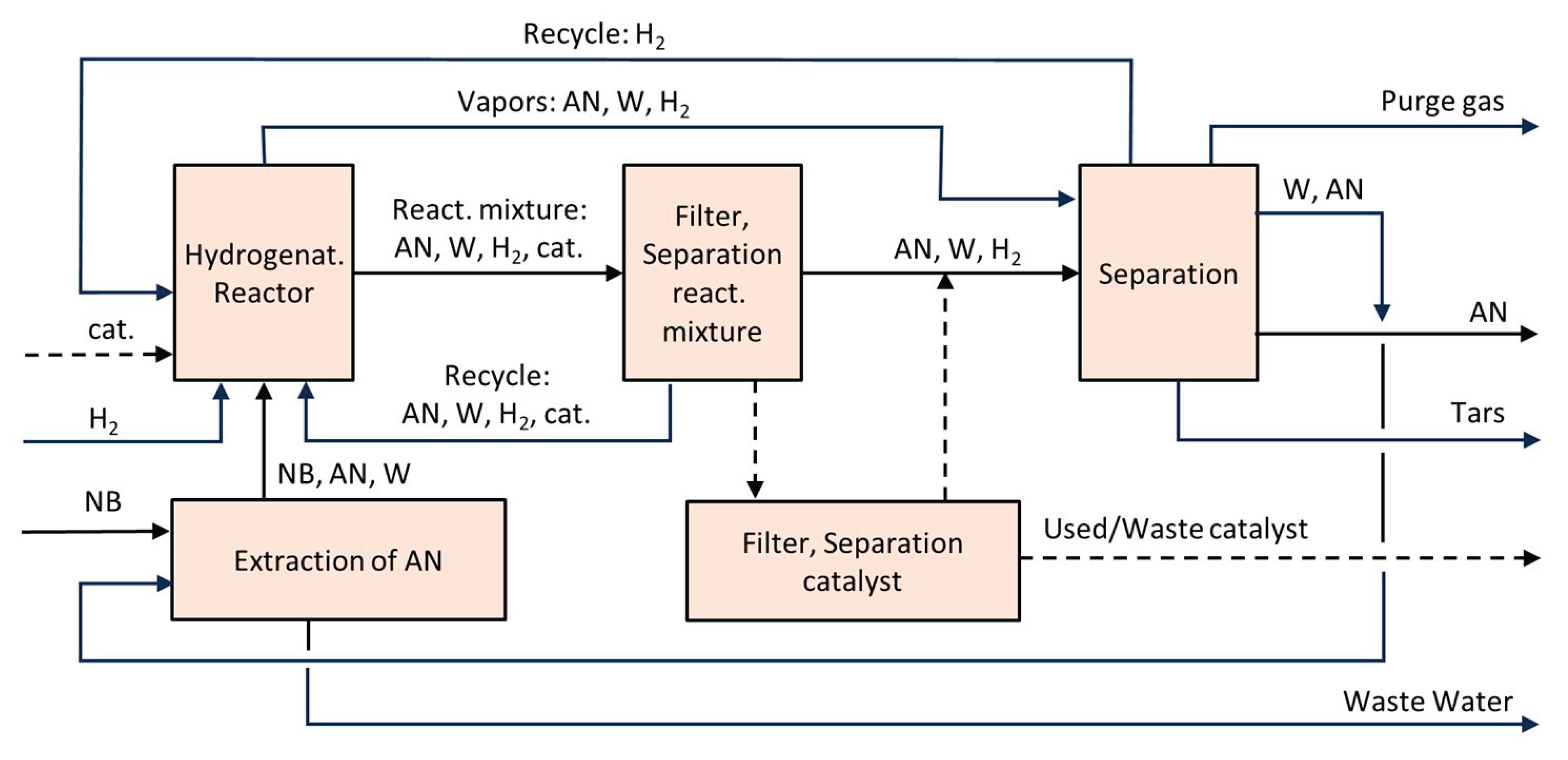
| Catalyst | Process | CLT1), deactivation |
| Fe/Al2O3/CaO/K Hollow extrudates |
NH3 synth. (N2 + 3H2 -> 2NH3), 400 – 550 °C, 150 – 500 bar |
5-10 years, slow sintering |
| Pt-Pd-Ru-Rh alloy gauze | Oxidation of NH3 to NOx, production of HNO3, 800-940 °C, 1-10 bar | 1-6 months, detoriation/ evaporation of atoms from wires |
| V2O5/K2SO4/SiO2 extrudates |
sulfuric acid (2 SO2 + O2 -> 2 SO3) 400-600 °C, 1 bar |
5-10 years, slow physical detoriation (dust), pressure drop |
| Cu/ZnO/Al2O3 pellets |
CH3OH synth. (CO + 2H2 -> CH3OH) 200-300 °C, 50 -200 bar |
2-8 years, slow sintering |
| Co/Mo sulf./Al2O3 extrudates | Petrochem. Desulf. (R2S + 2H2 -> 2RH + H2S) 300-400 °C, 20-40 bar |
2-8 years, slow coking, pressure drop |
| Ag granules, 1-3 mm |
Oxidat. (CH3OH + 1/2O2 -> HCHO + H2O) 500-600 °C, 1 bar |
0.3-1 year, coking, loss of selectivity |
| Raney Ni skeletalparticles | Hydrogenation, e.g. vegetable oils, 100 – 200 °C, 3-30 bar |
1-10 days, deactivation by sticking of side products on the surface |
| Zeolites/SiO2-Al2O3 microspheroids |
Fluid catalytic cracking (FCC), 350-550 °C, 1-5 bar |
1-10 s, permanent loss of activity, reactivation by burning coke |
| Support | S1) (m2 g-1) |
Vp2) (cm3 g-1) |
Other properties | Top.max3) (°C) |
|---|---|---|---|---|
| Graphite [142] | 10 - 100 | 0.01−0.1 | Mesopor. Struct., high el. 4) and heat conduct. 5) | 500 |
| Activated carbon [142] | 200 – 3000 | 0.6−2 | Micropor. Struct., low el. and heat conduct. | 400 |
| Carbon nanotubes, [142] | 50 - 500 | 2-2.5 | Micropor. Struct., high el. and heat conduct. | 300 |
| Graphene sheets [142] | 1500 - 3000 | 2-3.5 | Micropor. Struct., low el. and heat conduct. | 300 |
| Nickel (Raney) [143] | 5-30 | 0.01-0.05 | Low micropor struct. high el. and heat conduct. | 300 |
| γ-Al2O3 [18] | 50 - 300 | 0.4-0.8 | Meso-micropor. Struct., mild el. and heat conduct. | 800 |
| α-Al2O3 [18] | 0.3 - 5 | 0.01-0.05 | Low meso-micropor. Struct., mild el. Conduct., good heat conduct. | 1100 |
| SiO2 – gels [144,145] | 100-800 | 0.2-0.6 | Micropor. Struct., mild el. and heat conduct. | 400 |
| CaCO3 – precipitated [146,147] | 5- 40 | 0.01-0.05 | Micropor. Struct., mild el. and heat conduct. | 400 |
| Clays [148] | 250 - 800 | 0.1-0.3 | Micropore., mild el. and heat conduct. Used mainly as an acid catalyst (H+ form), after calcination (> 400 °C) mixed oxides are formed – basic catalysts | 200 |
| Hydrotalcites [149] | 100 - 300 | 0.1-0.3 | Micropore., mild el. and heat conduct. Used mainly as a base catalyst (OH+ form), after calcination (> 400 °C) mixed oxides are formed – basic catalysts | 200 |
| Zeolites [128] | 300 - 800 | 0.5- 2 | Regular micropore structure 0.5- 2 nm, mild el. and heat conduct, shape selectivity, in H+ form very efficient acid catalysts, e.g. in FCC | 900 |
| Cordierite, 2MgO·2Al2O3·5SiO2 Skeleton [140,141], |
1 - 8 | 0.01-0.05 | mild el. and heat conduct. (semiconductor), high mechanical strength and toughness | 900 |
| TiO2 [18] | 20 - 400 | 0.1- 0.6 | mild el. and heat conduct. (semiconductor), photocatalytic act. |
400 |
| Perovskites – “ABO3”, [150,151] CaTiO3 as a representative | 5 - 40 | 0.05 – 0.6 | mild el. and heat conduct. (semiconductor), photocatalytic act. |
300 |
| Glasses [18,152] | 0.01 – 0.1 | 1 – 40 | low el. and heat conduct. Surface treatment is needed | 400 |
| Property | Fixed bed | Multitube | Fluidized | Moving bed |
| Dcat (mm) | 2-30 | 1-5 | 0.1-0.5 | 2-30 |
| V (m3) | 3-20 | 1-10 | 3-20 | 3-20 |
| Pmax (MPa) | 15 | 5 | 3 | 5 |
| Tmax (°C) | 1000 | 1000 | 800 | 600 |
| Mass_transport | Average | Good | Very good | Average |
| Heat_transport | Bad | Very good | Very good | Bad |
| CAPEX | Low | Very high | Average | Average |
| OPEX | Low | Very high | Very High | High |
| Examples | Oxidation, hydrogenation, alkylation, hydrocracking | Oxidation | Polymerization, cracking | Hydrotreatment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).