1. Introduction
Climate change represents a defining challenge of our era, with profound implications for human health and the healthcare sector. The Intergovernmental Panel on Climate Change (IPCC), in its 2023 Sixth Assessment Synthesis Report, warned of “a rapidly closing window of opportunity to secure a livable and sustainable future for all” [
1]. The World Health Organization (WHO) has echoed these concerns, describing climate change as a fundamental threat to global health, capable of reversing decades of progress in development and widening health inequities [
2].
Healthcare systems are both vulnerable to climate change and significant contributors to it. The global healthcare sector accounts for approximately 4.6% of net greenhouse gas (GHG) emissions, more than double that of the aviation industry [
3,
4]. These emissions arise not only from direct activities, such as fuel combustion and facility operations, but also from indirect sources across the energy supply chain and procurement systems. These are commonly categorized as Scope 1 (direct emissions), Scope 2 (indirect emissions from purchased electricity), and Scope 3 (all other indirect emissions, including those from supply chains and service delivery [
5]. In high-income countries, healthcare contributes 3-8.5% of national emissions, including 7.6% in the United States (US) and 5.4% in the United Kingdom (UK) [
3].
Cancer care, encompassing surgery, systemic anti-cancer therapy, radiation therapy, and clinical research, has a particularly high environmental burden. Overtreatment, waste from single-use plastics and cytotoxics, and energy-intensive treatment modalities contribute significantly to healthcare’s carbon footprint [
6,
7,
8]. Clinical trials, a cornerstone of oncology innovation, are increasingly recognized as carbon-intensive due to extensive travel, resource consumption, and expanding data storage needs. Notably, trials often collect large volumes of redundant data, with one analysis finding that just 5% of collected data informs the primary endpoint, and only 20% is used in the final publication [
9], highlighting opportunities for carbon reduction through streamlined trial design. One estimate places the carbon footprint of global clinical research at 100 million tons of carbon dioxide equivalent (CO₂e) annually [
10], with cancer trials accounting for over 100,000 active studies worldwide.
Despite this, few tools exist to quantify or mitigate the carbon footprint of clinical trials. While some efforts, such as the Sustainable Clinical Trials Group (SCTG) guidelines [
11] and more recent initiatives by the Low Carbon Clinical Trials Working Group [
12], have proposed frameworks and calculators, widespread adoption remains limited. Integration of sustainability into trial design is hampered by infrastructure gaps, limited institutional awareness, and entrenched design practices [
13]. A recent survey by Hoffmann
et al. [
13] revealed that over 60% of academic and industry stakeholders were unaware of sustainability measures in trial design.
Ireland’s 2025 National Climate Change Risk Assessment recognizes the healthcare sector as both vulnerable to and responsible for climate risk, calling for cross-sectoral action, including research and innovation [
14]. Ireland is uniquely positioned to lead in sustainable cancer research within Europe. As the European Union (EU)’s largest net pharmaceutical exporter [
15] and host to nine of the world’s ten largest pharmaceutical companies [
16], the country has a well-established research infrastructure. Cancer Trials Ireland (CTI), the national coordinating body for oncology clinical trials, is well-positioned to take a leadership role in this domain. CTI oversees research across seven Health Research Board (HRB)-funded university-hospital clusters and also has strong industry collaboration, with 47% of CTI trials being industry-sponsored. However, a study by Myo Oo
et al. has previously found that climate advocacy among cancer clinical trial organizations remains limited [
17], highlighting the need for greater institutional engagement in sustainability efforts.
This study explores the intersection of cancer clinical trials and environmental sustainability in Ireland. It assesses awareness of the carbon footprint of clinical trials, identifies perceived barriers and enablers to sustainable practices, and examines opportunities to embed sustainability into trial design and conduct. Drawing on the principles of eco-design, originally developed in engineering to minimize environmental impact across a product’s life cycle, this study considers how trials can be reimagined to reduce emissions. In this context, eco-design involves integrating sustainability at every stage of a trial’s lifecycle, from protocol development to data management and close-out, without compromising scientific integrity or patient safety.
2. Materials and Methods
We conducted a cross-sectional survey incorporating both quantitative and qualitative elements to explore sustainability practices in cancer clinical trials in Ireland. A pragmatic approach was adopted to generate practical, real-world insights rather than test a specific hypothesis. Data analysis was guided by abductive reasoning, allowing themes to emerge inductively from participant responses while remaining grounded in existing frameworks and evidence.
A structured, 21-item questionnaire was developed to explore: (1) knowledge of the carbon footprint associated with cancer clinical trials, (2) awareness of sustainability-enhancing innovations, and (3) perceived barriers and incentives to implementing such measures. Questions were informed by existing literature and tools, including the SCTG guidelines [
11], the National Institute for Health and Care Research (NIHR) sustainability assessment tool [
18], ‘My Green Lab’ (MGL) Certification [
19], and publications by Hoffmann
et al. and Griffiths
et al. [
12,
13]. The questionnaire included both closed and open-ended formats, with “don’t know” options where appropriate. The questionnaire was piloted with a consultant medical oncologist, resulting in minor refinements for clarity and scope.
Participants included members and stakeholders affiliated with CTI, representing clinical, academic, and industry sectors. The survey was distributed via email to 613 individuals using CTI’s membership database. Respondents were purposively sampled from the CTI database to ensure representation across research roles, cancer subspecialties, and geographical locations. Data collection was conducted through SurveyMonkey over a three-week period beginning 3rd April 2024.
Participation was voluntary and anonymous. Informed consent was obtained on the survey landing page, which detailed the study objectives, data protection measures, and rights to withdraw. No personal identifiers or IP addresses were collected. Data were securely stored and scheduled for deletion six months post-analysis. The study adhered to principlist and care-based ethical frameworks to ensure participant confidentiality and data integrity.
Qualitative data from open-text responses were analyzed using thematic analysis as described by Braun and Clarke [
20]. Descriptive statistics were used to summarize categorical data.
3. Results
A total of 126 individuals responded to the survey out of 613 invited members and stakeholders of CTI, yielding a response rate of 20.6%. Respondents represented a wide range of professional roles across the clinical, academic, and industry sectors (see
Table 1). The majority were hospital-based clinicians, research nurses, or clinical trial coordinators, with additional input from principal investigators, trial managers, and stakeholders from sponsor organizations and contract research organizations (CROs). Representation spanned all four provinces of Ireland, with the largest proportion based in Leinster (63%).
3.1. Awareness and Knowledge of Carbon Footprint Tools
Awareness of carbon footprint assessment tools specific to clinical trials was low among respondents. Of the 71 individuals who answered this question, 68% reported no awareness of established approaches such as the SCTG guidelines, the NIHR carbon footprint calculator, or MGL certification. Only 21% were aware of the SCTG guidelines, 20% were aware of MGL certification, and fewer than 6% had heard of the NIHR calculator. A small minority (4%) indicated involvement in other approaches, including paperless trials and use of electronic site files.
In Questions 7 and 8, respondents were asked about the extent to which they believed carbon footprint is considered during the design phase of industry-sponsored versus academic clinical trials. Among the 72 respondents to each question:
49% believed it was rarely considered in industry-sponsored trials, compared to 43% for academic trials.
29% believed it was occasionally considered for academic trials, while only 15% thought the same of industry trials.
Just 2.8% (industry) and 4.2% (academic) felt that sustainability was considered most of the time.
Notably, a large proportion selected “don’t know”; 33% for industry trials and 24% for academic trials.
The majority of responses were based on opinion rather than direct knowledge; 36% for industry trials and 39% for academic trials.
More respondents had direct experience related to academic trials (14%) than to industry-sponsored trials (4%).
These findings highlight a widespread lack of clarity and direct exposure to sustainability practices within trial design, particularly in the industry sector. Free-text comments reinforced this, with several noting that environmental sustainability is rarely discussed or incentivized during protocol development stages.
3.2. Perceived Contributors to Trial Emissions
In Question 9, respondents were asked to rank ten trial-related activities in order of their perceived impact on a trial’s carbon footprint, with 1 indicating the highest impact and 10 the lowest. A total of 70 respondents completed this ranking task. The activities were selected based on literature including Griffiths
et al. [
12] to align with categories used in the NIHR carbon footprint calculator.
Trial-specific meetings and travel were perceived as the most significant contributors, receiving the highest weighted average score (7.31), and were ranked among the top four by 48 respondents. This was closely followed by trial supplies and equipment (score: 7.13; ranked in top four by 46 respondents). Other high-impact categories included collection and shipment of samples (6.57), emissions from Clinical Trial Research Units (CTRUs) (6.52), and trial set-up activities (6.41).
In contrast, trial close-out was consistently viewed as the least impactful, with 54% of respondents ranking it lowest. Additional lower-impact activities included patient assessments (ranked 8th by 29%) and laboratory-related activities (ranked 9th by 24%).
These findings suggest that travel, both for staff and trial activities, is viewed as the leading source of emissions, consistent with existing research. Notably, several respondents commented on their uncertainty in assessing the relative carbon impact of these activities, citing a lack of knowledge or tools. This highlights a broader issue of limited carbon literacy within the cancer clinical trials workforce.
3.3. Training, Confidence, and Willingness to Implement Sustainable Practices
In Question 10, respondents were asked whether they had received any education or training in their workplace on measuring or reducing the carbon footprint of clinical trials. Of the 71 individuals who answered this question, 97% reported that they had not received any formal training, highlighting a significant gap in structured education on environmental sustainability within the cancer trials sector. Only two respondents (3%) indicated they had received such training, one was a translational scientist currently working as a Principal Investigator, while the other did not specify their professional role.
Question 11 explored perceived competence in advising on or implementing carbon-reductive measures within trials. Of the 71 respondents, only 2.8% (n=2) felt very confident in their ability to do so. A further 17% believed they could contribute with some assistance, whereas the vast majority (82%) indicated that they would require significant assistance or guidance to take meaningful action in this area.
Despite this lack of confidence and training, responses to Question 12 revealed a strong underlying enthusiasm. When asked about their willingness to engage in sustainability initiatives, 41% of respondents stated they would be willing to participate in advising on or implementing sustainability measures. An additional 45% expressed interest but noted that their current role would not allow for such involvement.
3.4. Perceptions of Innovative Measures to Reduce Carbon Footprint
To address the second objective, assessing awareness of the potential impact of innovative measures to reduce the carbon footprint of cancer clinical trials, respondents were asked in Question 13 to select five measures they believed would have the greatest impact. Of the 71 who responded, only 10% indicated they lacked sufficient knowledge to make selections, suggesting a generally high level of engagement with the topic.
The most commonly selected innovation was reducing sample kit waste (59%), followed by full implementation of electronic patient records (55%), and virtual assessments for patient follow-up (54%). Other frequently selected measures included streamlining ethics and regulatory approvals and reducing patient site visits. Conversely, membership in Green Lab initiatives and establishment of a national biobank were selected least often, possibly reflecting lower familiarity or perceived feasibility among respondents.
In Question 14, respondents were invited to share additional practical suggestions for reducing a trial’s carbon footprint. Eighteen responses were submitted and analyzed thematically using Braun and Clarke’s method. Fifteen themes emerged, eight of which were unrelated to the list in Question 13, demonstrating the breadth of ideas among stakeholders. Locally focused suggestions included resource-sharing across trial units, evaluating the energy efficiency of storage practices, and consolidating site monitoring to a single Clinical Research Associate. Externally oriented proposals included linking sustainability performance to trial accreditation or funding, engaging regulators in environmental accountability, and minimizing the use of shipping materials (e.g., gel packs, dry ice) in industry-sponsored trials.
Question 15 invited respondents to describe any observed or known impacts resulting from the implementation of such innovations. Six participants provided input. While there was no clear thematic convergence, the examples were diverse and informative. Reported initiatives included raising freezer temperatures from -80°C to -70°C, using electronic investigator site files, collecting adverse event data on iPads, emailing patient information leaflets, and conducting fully paperless trials with electronic databases. One respondent referenced the development of a green charter and trial carbon calculator.
3.5. Barriers and Incentives to Sustainable Practice in Cancer Clinical Trials
To address the third research objective, identifying perceived barriers and incentives to adopting sustainable practices, respondents were asked a series of structured and open-ended questions.
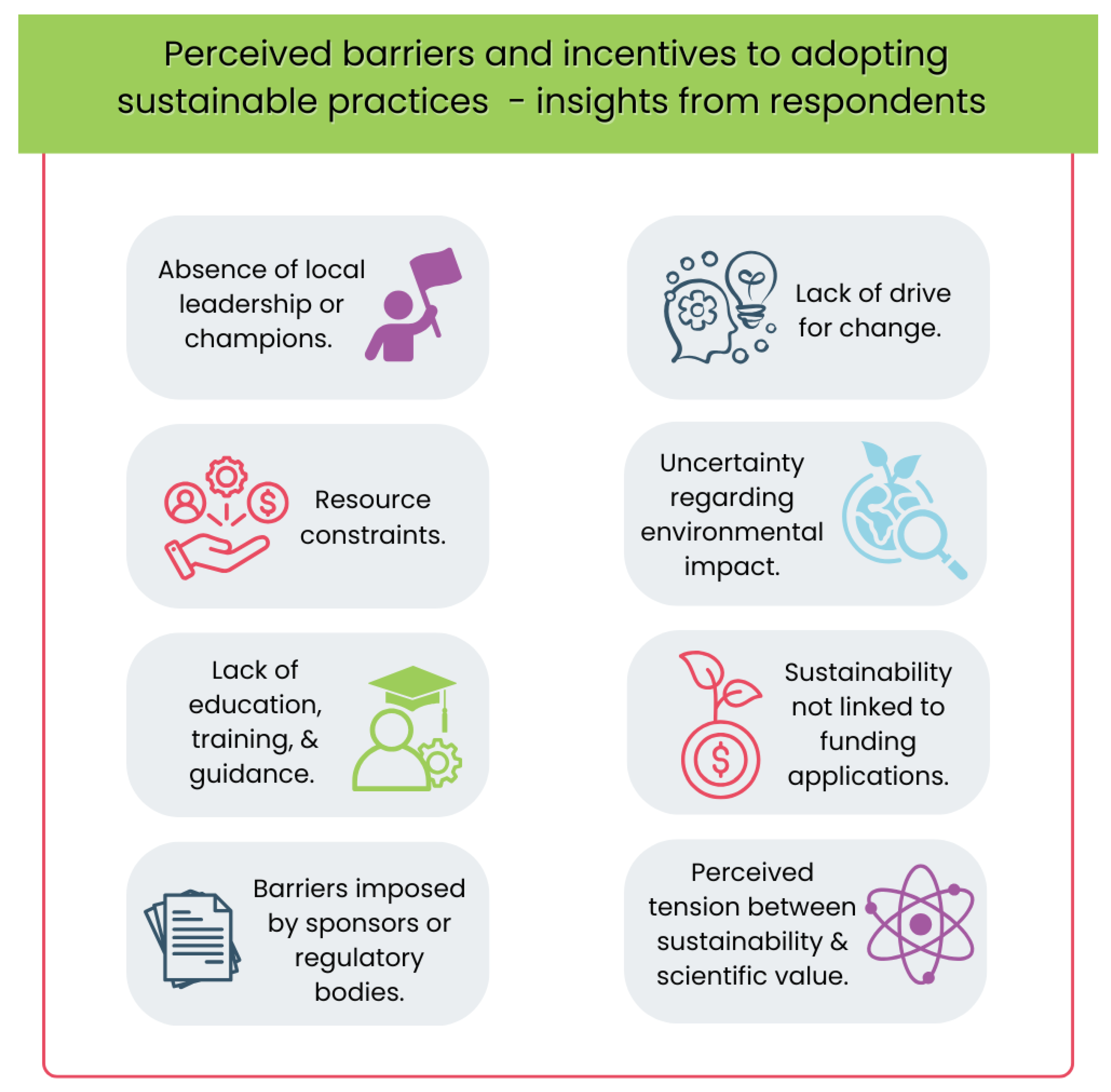
In Question 16, 64 respondents selected their three main perceived barriers from a list of nine options (see
Figure 1). The most commonly cited barrier was the lack of education, training, and guidance (66%), followed by resource constraints (39%) and lack of drive for change (33%). Other barriers included perceived tension between sustainability and scientific value, uncertainty regarding environmental impact, and absence of local leadership or champions. Additional comments revealed that some respondents viewed the primary barriers as external to their organization, particularly those imposed by sponsors or regulatory bodies. For example, one participant noted: “Many people just don’t think this is an important issue” (16:CM08), while another criticized the redundancy of reprinting lengthy patient information leaflets for reconsent (16:CM05).
In Question 17, 23 participants described personal experiences with these barriers. Thematic analysis revealed recurring issues such as time constraints, resistance to change, staff shortages, and the perception that sustainability is not prioritized institutionally. Respondents also flagged rigid paper-based documentation, inflexible sample kit protocols, and ongoing requirements for wet signatures as impediments to greener practice. One response encapsulated the existential challenge of climate action within the clinical research community: “I think people feel overwhelmed by climate change… our work is about our health but our focus is on treating illness” (17:CM07). Others identified regulators as crucial enablers of change, with one respondent remarking: “I’ve seen how simple changes from regulators can enable much more efficient processes. Hence I believe regulators are key” (17:CM19). A call for CTI to lead institutional change also emerged (17:CM03).
In Question 18, 15 participants offered suggestions for overcoming these barriers. Frequently cited solutions included protected time, training, guidance, and additional resources. One respondent proposed enabling multi-study programming on a single machine as an example of pragmatic change for industry trials (18:CM06), while another recommended: “National guidance to allow/promote direct to electronic database data entry… needs advocacy” (18:CM02). Incremental improvement was also encouraged: “Don’t try to change everything at once” (18:CM12).
In Question 19, 65 respondents selected three key facilitators to incentivize sustainability. Financial support to introduce electronic trial master files ranked highest (52%), followed by guidance and training (49%), and mandatory inclusion of sustainability measures in grant applications (45%). The least favored facilitator was patient/public awareness (12%). Free-text suggestions reinforced the importance of regulatory flexibility, inclusion of sustainability costs in budgets, and linking funding to sustainability compliance, with one respondent stating: “I think you have to make funding contingent on sustainability – that’s the only way you will get the buy-in needed” (19:CM01).
Questions 20 and 21 gathered open feedback. Ten respondents commented on how to accelerate sustainable practice, highlighting 12 themes. Key suggestions included making sustainability mandatory in funding applications, introducing trial set-up carbon checklists, and offering practical guidance for time-poor clinicians (20:CM05). One respondent suggested sustainability become a mandatory module for all CTI members, while another recommended a ‘green trial’ social media campaign to raise awareness (21:CM08).
The need for education was a consistent theme: “Education is key to driving change” (21:CM05). Others stressed the role of IT infrastructure and predicted future EU legislation would likely mandate action: “We need to educate trial leads as to just how important this will be within 5 years” (21:CM07).
Overall, while barriers such as limited training, competing priorities, and perceived regulatory hurdles persist, respondents identified clear facilitators to support change. These include national guidance, continuous professional development (CPD)-accredited training, toolkits, and the appointment of green champions. The findings suggest that the Irish cancer clinical trials community is open to sustainable innovation, but meaningful progress will require system-wide alignment, structural incentives, and cultural transformation.
4. Discussion
The study drew participation from a multidisciplinary cohort of cancer research professionals in Ireland, with consultant medical oncologists, consultant radiation oncologists, consultant hematologists, and translational scientists collectively accounting for 63% of respondents. Surgeons constituted only 4% of the sample, which is noteworthy given the substantial role of surgery in cancer care and its associated environmental impact. Over half of participants (n=57) currently hold or have previously held senior roles in clinical trial research, placing them in positions of influence regarding trial design, process innovation, and leadership. All major clinical trial roles were represented, including members of ethics boards, patient advocacy groups, and individuals from academia, private healthcare, and cancer charities, reflecting a comprehensive cross-section of the cancer trials ecosystem.
The findings revealed a significant gap in awareness and training regarding sustainable clinical trial practices. Although guidance and tools such as Hoffmann
et al.’s carbon reduction checklist [
13] and the SCTG Guidelines [
11] exist, the majority of respondents were unfamiliar with these resources. Only 21% were aware of the SCTG Guidelines and 20% of the MGL Certification program [
19]. Awareness of the more recent NIHR guidelines [
18] was particularly low (<6%). This limited engagement with existing tools underscores a broader issue; while clinicians and research professionals are increasingly recognized as key agents in climate action [
21], many lack the institutional support and training needed to fulfil this role within the context of clinical trials. Dablander
et al. found that climate scientists report significantly higher levels of climate-related advocacy and personal behavior change compared to non-climate researchers [
22], suggesting that domain-specific knowledge may strongly influence sustainability engagement.
This lack of familiarity extended to competence in using carbon footprint calculators, with most respondents expressing limited knowledge or experience. These findings mirror previous research by Carlberg and Jansson [
23], Huang
et al. [
24], Elia
et al. [
25], and Rosa
et al. [
26], which similarly identified the lack of education and training as a key barrier to adoption of sustainable practices. Encouragingly, despite these deficits, the majority of respondents expressed willingness to engage with sustainability initiatives, suggesting a readiness to act if appropriately supported. To address this gap, there is a strong rationale for a coordinated education and awareness campaign, potentially through a CPD-accredited training program, highlighting available tools, including the forthcoming the industry-wide Low Carbon Clinical Trials (iLCCT) online platform [
27]. CTI, as the national coordinating body, is well-positioned to lead the development of sustainability-focused training modules for clinical trial staff. Similar findings were reported by O’Reilly
et al. among Breast International Group members, where respondents expressed strong support for sustainability integration but cited practical and institutional barriers [
28].
Most respondents indicated that the carbon footprint of clinical trials is rarely considered during the design phase, a finding that aligns with the work of Hoffmann
et al. [
13]. While some respondents suggested that such considerations may be more common in academic-led studies, a significant proportion expressed uncertainty. Hoffmann
et al. also found that GHG emissions are generally not assessed during Institutional Review Board (IRB) submissions, suggesting that even in academic contexts, environmental impact is not routinely incorporated into trial design. These findings collectively highlight a broader gap in the integration of sustainability principles into the early planning stages of cancer clinical trials.
When evaluating knowledge of emissions from specific trial-related activities, there were notable discrepancies between respondent perceptions and empirical data from Griffiths
et al. [
12] and Mackillop
et al. [
29]. For instance, respondents ranked trial set-up and sample shipment as major contributors to emissions, whereas Griffiths
et al.’s empirical calculations placed these activities lower [
12]. While some variation in rankings may reflect trial-specific characteristics (e.g. internal flights or multiple radiotherapy visits), the findings underscore a need for greater clarity and standardization in calculating and communicating the environmental impacts of different trial activities.
The majority of respondents viewed the reduction of sample kit waste as the most impactful innovative strategy, aligning with previous calls by Ioannidis
et al. [
30] to reduce unnecessary waste in research. Transitioning to electronic patient records and virtual assessments were also highly rated, supported by existing evidence of their efficacy in reducing emissions associated with travel and physical documentation [
31,
32,
33]. However, refinement of database design, a measure with substantial potential to reduce data waste [
9,
34], was rarely prioritized by respondents. This suggests a possible underestimation of data inefficiencies and further illustrates the knowledge gap around less visible sources of carbon emissions. Other innovations, such as adjusting freezer storage temperatures, were also recognized, echoing the work of MGL and the Laboratory Efficiency Assessment Framework (LEAF) in reducing laboratory emissions [
35]. While resource constraints and lack of organizational drive were cited as common barriers, these did not appear to significantly diminish willingness to engage with sustainability initiatives, which remained high (86%).
Many respondents highlighted the pivotal role of regulators in driving sustainability through clearer guidance and incentive structures. Suggestions included the removal of paper record requirements, minimization of kit sizes, increased permission for multi-use equipment, and reductions in packaging, all areas where study sponsors and industry stakeholders could exert influence. Furthermore, respondents strongly supported the integration of sustainability into funding criteria. Almost half agreed that mandatory inclusion of carbon footprint considerations in grant applications would incentivize more sustainable practices. These findings support calls by Adshead
et al. [
36] for funders to require researchers to justify carbon usage and support the broader view that systemic levers, rather than voluntary change alone, are needed to drive behavioral shifts within the research community.
A key strength of this study is its national scope and the inclusion of diverse professional roles, from clinicians to CRO staff. The mixed-methods approach enabled both quantitative insight and rich contextual data. However, the modest response rate (20.6%) introduces possible response bias; individuals more interested in sustainability may have been more likely to respond. Underrepresentation of surgeons and some trial support roles, despite their relevance to emissions-heavy activities, limits generalizability. The study also focused on self-reported perceptions rather than direct emissions data. Future research should include carbon footprint measurement across trial phases.
5. Conclusions
This study provides the first national insight into sustainability awareness and attitudes in Irish cancer clinical trials. While formal training and awareness of carbon footprinting tools remain low, there is clear enthusiasm among professionals to adopt sustainable practices. Barriers such as unclear regulatory expectations, lack of institutional support, and perceived misalignment with trial compliance norms suggest the need for coordinated, system-wide interventions.
Embedding sustainability into ethics reviews, grant assessments, and trial governance frameworks could position Ireland as a global leader in environmentally responsible oncology research. CTI is uniquely placed to lead this shift by developing national toolkits, offering CPD-accredited training, and supporting the workforce in implementing low-carbon trial designs. Future efforts should prioritize emissions quantification, address behavioral resistance, and engage underrepresented groups to broaden impact. This work aligns with Ireland’s Climate Action Plan and the EU Green Deal, reinforcing the need for sustainable transformation across all aspects of healthcare, including research.
Through deliberate, strategic action, Ireland has an opportunity to set a global benchmark, proving that clinical research excellence and environmental stewardship are mutually reinforcing goals.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, A.C.L. and S.O’R.; methodology, A.C.L.; validation, A.C.L.; formal analysis, A.C.L.; investigation, A.C.L. and S.O’R.; data curation, A.C.L. and S.O’R.; writing—original draft preparation, C.R.F. and A.C.L.; writing—review and editing, C.R.F., A.C.L. and S.O’R.; visualization, C.H.; supervision, S.O’R; project administration, A.C.L.; funding acquisition, A.C.L. All authors have read and agreed to the published version of the manuscript.”.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. Ethics approval was not required for this study.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2023: Synthesis Report. Summary for Policymakers. 2023.
- World Health Organization (WHO). Making the Case for Global Health in Climate Action 2021 [Available from: https://who.foundation/latest-updates/making-the-case-for-global-health-in-climate-action/.
- Health Care Without Harm. Health Care’s climate footprint: How the health sector contributes to the global climate crisis and opportunities for action. 2019.
- Romanello M, Napoli CD, Green C, Kennard H, Lampard P, Scamman D, et al. The 2023 report of the Lancet Countdown on health and climate change: the imperative for a health-centred response in a world facing irreversible harms. Lancet. 2023;402(10419):2346-94.
- World Resources Institute, World Business Council for Sustainable Development. Greenhouse Gas Protocol 2022 [Available from: https://ghgprotocol.org.
- Pak LM, Morrow M. Addressing the problem of overtreatment in breast cancer. Expert Rev Anticancer Ther. 2022;22(5):535-48.
- Zikhathile T, Atagana H, Bwapwa J, Sawtell D. A Review of the Impact That Healthcare Risk Waste Treatment Technologies Have on the Environment. Int J Environ Res Public Health. 2022;19(19).
- Chuter R, Stanford-Edwards C, Cummings J, Taylor C, Lowe G, Holden E, et al. Towards estimating the carbon footprint of external beam radiotherapy. Phys Med. 2023;112:102652.
- Crowley E, Treweek S, Banister K, Breeman S, Constable L, Cotton S, et al. Using systematic data categorisation to quantify the types of data collected in clinical trials: the DataCat project. Trials. 2020;21(535).
- Academy of Medical Sciences. Enabling greener biomedical research 2023 [Available from: https://acmedsci.ac.uk/file-download/61695123.
- Sustainable Trials Study Group. Towards sustainable clinical trials. BMJ. 2007;334(7595):671-3.
- Griffiths J, Fox L, Williamson PR, Low Carbon Clinical Trials G. Quantifying the carbon footprint of clinical trials: guidance development and case studies. BMJ Open. 2024;14(1):e075755.
- Hoffmann JM, Bauer A, Grossmann R. The carbon footprint of clinical trials: a global survey on the status quo and current regulatory guidance. BMJ Glob Health. 2023;8(9).
- Environmental Protection Agency. National Climate Change Risk Assessment: Summary for Policymakers. 2025.
- Irish Pharmaceutical Healthcare Association (IPHA). Pharmaceutical Preparations Manufacturing in Ireland - Industry Market Research Report 2025 [Available from: https://www.globenewswire.com/news-release/2024/11/11/2978464/28124/en/Ireland-Pharmaceutical-Preparations-Manufacturing-Market-Research-Report-2024-2029-A-Backlog-of-Healthcare-Appointments-has-Aided-Demand-for-Pharmaceutical-Products.html?utm_source=chatgpt.com.
- Enterprise Ireland. Pharma: Innovating to enhance patient outcomes and deliver a sustainable future 2025 [Available from: https://www.enterprise-ireland.com/en/global/pharma?utm_source=chatgpt.com.
- Oo NM, Weadick CS, Murphy L, O'Reilly S. Climate change advocacy and cancer clinical trial organisations. BJC Rep. 2024;2(1):49.
- National Institute for Health and Care Research. NIHR Carbon Reduction Guidelines 2019 [Available from: https://www.nihr.ac.uk/about-us/what-we-do/key-initiatives/climate-health-sustainability/carbon-reduction-guidelines.
- My Green Lab. My Green Lab Certification 2024 [Available from: https://www.mygreenlab.org/green-lab-certification.html.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77-101.
- Braithwaite J, Pichumani A, Crowley P. Tackling climate change: the pivotal role of clinicians. BMJ. 2023;382:e076963.
- Dablander F, Sachisthal MSM, Haslbeck JMB. Climate actions by climate and non-climate researchers. npj clim action. 2024;3(105).
- Carlberg C, Jansonn C. ‘Barriers to Eco-Innovation – A Qualitative Study on Large Manufacturing Companies’, MSc Thesis submission 2019 [Available from: https://www.diva-portal.org/smash/get/diva2:1333528/FULLTEXT01.pdf.
- Huang Y-F, Chen AP-S, Do M-H, Chung J-C. Assessing the Barriers of Green Innovation Implementation: Evidence from the Vietnamese Manufacturing Sector. Sustainability. 2022;14(8).
- Elia MR, Toygar I, Tomlins E, Bagcivan G, Parsa S, Ginex PK. Climate change, climate disasters and oncology care: a descriptive global survey of oncology healthcare professionals. Support Care Cancer. 2024;32(11):764.
- Rosa C, Marsch LA, Winstanley EL, Brunner M, Campbell ANC. Using digital technologies in clinical trials: Current and future applications. Contemp Clin Trials. 2021;100:106219.
- Sustainable Healthcare Coalition. Clinical Trials: Carbon footprint assessment guidance [Available from: https://shcoalition.org/clinical-trials-carbon-footprint-assessment-guidance-guidance-document/.
- O'Reilly S, Griffiths J, Fox L, Weadick CS, My Oo N, Murphy L, et al. Climate change impacts and sustainability integration among breast international group members. Breast. 2025;81:104469.
- Mackillop N, Shah J, Collins M, Costelloe T, Ohman D. Carbon footprint of industry-sponsored late-stage clinical trials. BMJ Open. 2023;13(8):e072491.
- Ioannidis JP, Greenland S, Hlatky MA, Khoury MJ, Macleod MR, Moher D, et al. Increasing value and reducing waste in research design, conduct, and analysis. Lancet. 2014;383(9912):166-75.
- Subaiya S, Hogg E, Roberts I. Reducing the environmental impact of trials: a comparison of the carbon footprint of the CRASH-1 and CRASH-2 clinical trials. Trials. 2011;12:31.
- Coombs NJ, Coombs JM, Vaidya UJ, Singer J, Bulsara M, Tobias JS, et al. Environmental and social benefits of the targeted intraoperative radiotherapy for breast cancer: data from UK TARGIT-A trial centres and two UK NHS hospitals offering TARGIT IORT. BMJ Open. 2016;6(5):e010703.
- National Health Service. Delivering a “Net Zero” National Health Service 2020 [Available from: B1728-delivering-a-net-zero-nhs-july-2022.pdf (england.nhs.uk).
- Tufts-Center for the Study of Drug Development. Rising protocol design complexity is driving rapid growth in clinical trial data volume. Impact Report Jan/Feb. 2021;23(1).
- Kelly FJ. How can we reduce biomedical research's carbon footprint? PLoS Biol. 2023;21(11):e3002363.
- Adshead F, Al-Shahi Salman R, Aumonier S, Collins M, Hood K, McNamara C, et al. A strategy to reduce the carbon footprint of clinical trials. Lancet. 2021;398(10297):281-2.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).