1. Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD) is one of the most common forms of chronic liver diseases worldwide. As the rates of obesity have rose over the last few decades, the risk for developing MASLD continues to increase. Previously, steatotic liver disease was defined as Non-alcoholic fatty liver disease (NAFLD) but with recent advances in research showing a correlation between metabolic syndrome and NAFLD, the nomenclature has been updated to reflect this association [
1].
As the incidence of obesity, metabolic syndrome, and type 2 diabetes mellitus have rose in the United States, so has the incidence of MASLD. In 2020, it was estimated that 33.7% of the United States adult population had MASLD and it is projected to increase to 36.8% by 2030 [
2]. The increasing prevalence of the disease will lead to higher health-care utilization, poor patient outcomes and an overall decrease in quality of life.
Thus, it is crucial to highlight the importance of screening and educating patients at risk of developing MASLD. In order to improve patient outcomes, a multi-disciplinary approach will be key. The purpose of this review is to highlight the current treatment landscape of Metabolic-associated Steatotic fatty liver disease (MASLD) and discuss areas of future research.
2. Materials and Methods
A comprehensive literature search was conducted on 6th May 2025, using PubMed (
https://www.ncbi.nlm.nih.gov/) and Google Scholar databases. Search terms included: “new treatments for MASLD,” “Clinical trials for fatty liver disease,” “treatment out-comes,” “current clinical trials for MASLD treatment” and “epidemiology of MASLD.” The search was limited to articles published in the last 10 years (2015–2025), written in Eng-lish, and involving human subjects. ChatGPT 2.0 was utilized to make
Table 1.
A total of 36 articles were initially retrieved. After title and abstract screening, 25 full-text articles were assessed for eligibility. Ultimately, 15 studies were included in this review based on the following criteria. Studies that focused on epidemiology, management, cur-rent clinical trials and treatments were evaluated. Studies providing updated clinical guidelines or recent public health data were also included. Non-English language publica-tions and publications older than 10 years were excluded.
3. Results
3.1. Background
Metabolic dysfunction-associated-steatotic liver disease (MASLD) incidence is rapidly increasing [
3]. Currently, MASLD is increasing in parallel to the increasing rates of obesity and type 2 diabetes (Type 2 DM) and it is currently the most common form of chronic liver disease amongst adults [
3]. According to recent data, the global prevalence of fatty liver disease has increased from 25.3% in 1990-2006 to 38% in 2016-2019 [
3]. As disease burden rises, it will become even more important to incorporate preventative strategies, in addition to continually evaluating patients for advanced therapies.
MASLD is an overarching term used to characterize a condition where there is evidence of hepatic steatosis (> 5% hepatic steatosis) on imaging or histology (macro-vesicular steatosis) with the absence of secondary readily identifiable causes of steatosis [
4,
5]. Those secondary causes include the absence of significant alcohol consumption, starvation, medications or hereditary disorders that may contribute to the development of liver disorders. The definition of significant alcohol use includes < 20 g/day for women and < 30 g/day for men [
5]. Steatotic (fatty) liver disease is notably a comprehensive term that is further classified by MASLD (Metabolic dysfunction-associated steatotic liver disease) which is a fatty liver with at least one metabolic risk factor such as dyslipidemia or obesity [
1,
5,
6]. This category was formerly known as NAFLD (non-alcoholic fatty liver disease). MASLD with MASH (metabolic dysfunction-associated steatohepatitis) is defined by histologic evidence of inflammation, hepatocellular injury such as ballooning of hepatocytes with or without fibrosis, which was previously known as non-alcoholic steatohepatitis (NASH) [
1,
5,
6]. It is important to note the differences between the two as the nomenclatures for each disease has been updated.
Patients at risk of developing of MASLD go through several phases of progression. The progression of disease starts with simple steatosis, steatohepatitis, fibrosis and ultimately cirrhosis [
4]. Although the disease course is benign, the more advanced forms of disease can have many long-term implications in affected patients.
It is key to note that several factors increase the risk for developing MASLD. Since the correlation between cardiac risk factors and MASLD was established, people with type 2 DM, hyperlipidemia, central obesity and hypertension are at the highest risk for developing progressive liver disease [
5]. In addition, having several of these metabolic abnormalities con-currently confers an even greater risk for histological progression of MASLD [
5].
As the burden of fatty liver disease rises worldwide, it is crucial for clinicians to recognize and implement preventative and lifestyle measures to stop progression. The cornerstone of treatment includes a healthy diet, minimizing alcohol intake and exercise for the vast majority of patients [
5]. Even if weight loss is not needed, an improved diet composition and increased exercise can promote cardiovascular health in addition to improved control of metabolic co-morbidities [
5]. There are specific drugs that have shown a benefit in treating patients with MASLD. First, we will discuss drugs that were originally targeted for Type 2 DM such as glucagon-like peptide 1 receptor agonist, Pioglitazone, and sodium-glucose transport 2 inhibitors which are now being considered to treat fatty liver disease. We will also discuss specific drugs such as Resmetiron, fibroblast growth-factor 21 analogs and Lanifibranor as they are currently undergoing phase 3 clinical trials, but showing great promise in treating patients suffering from MASLD.
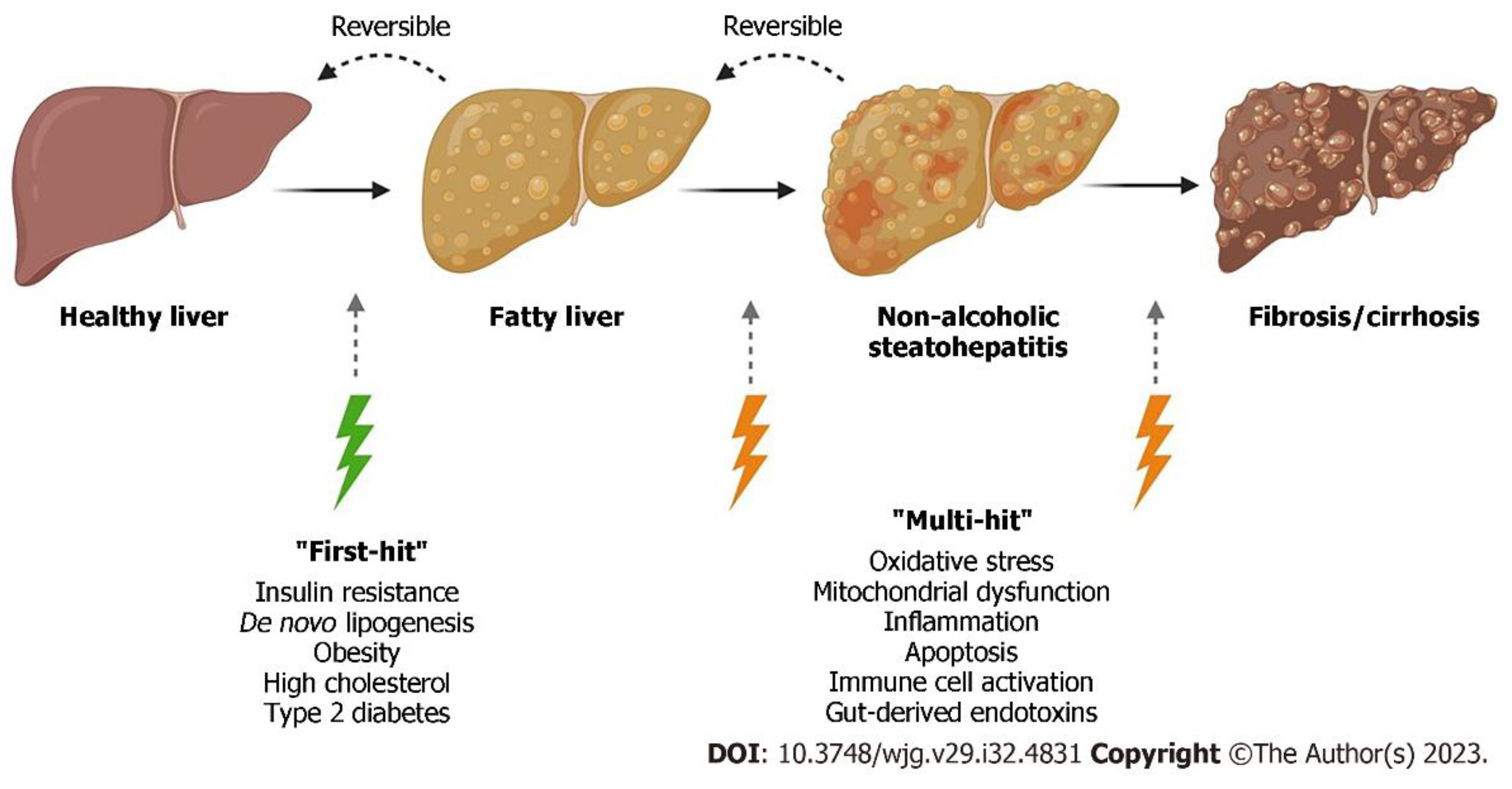
Figure 1.
Shows the progression of normal liver to liver fibrosis. In the “multiple-hit” theory of progression, the first cause or “first-hit” in NAFLD is insulin resistance, obesity, type 2 diabetes, and metabolic syndrome. As the first hit occurs, free fatty acids are stored in the liver as triglycerides, resulting in simple steatosis. Disease progresses when multiple factors, or “multi-hits”, such as oxidative stress, inflammatory mediators, apoptosis, and mitochondrial dysfunction cause liver damage
. *Note that fatty liver was previously referred to as NAFLD (Non-alcoholic fatty liver disease) which is now MASLD. NASH (non-alcoholic steatohepatitis) is MASH. Adapted from Petagine, et al. Reproduced under the Creative Commons Attribution License. [
37].
Figure 1.
Shows the progression of normal liver to liver fibrosis. In the “multiple-hit” theory of progression, the first cause or “first-hit” in NAFLD is insulin resistance, obesity, type 2 diabetes, and metabolic syndrome. As the first hit occurs, free fatty acids are stored in the liver as triglycerides, resulting in simple steatosis. Disease progresses when multiple factors, or “multi-hits”, such as oxidative stress, inflammatory mediators, apoptosis, and mitochondrial dysfunction cause liver damage
. *Note that fatty liver was previously referred to as NAFLD (Non-alcoholic fatty liver disease) which is now MASLD. NASH (non-alcoholic steatohepatitis) is MASH. Adapted from Petagine, et al. Reproduced under the Creative Commons Attribution License. [
37].
3.2. Benefits of Lifestyle Modifications
The majority of patients who are initially diagnosed with MASLD are treated with conservative management alone. As noted, a strong association between cardiac risk factors and the development of MASLD has been established. Thus, a set of general guidelines should be followed when recommending initial treatment. Management should start with weight loss as the primary therapy for MASLD [
6]. It is recommended that patients who are overweight or have obesity lose about 5-7% of body weight which is about 1-2 lbs. per week. Patients with suspected or biopsy-proven MASH (metabolic associated steatohepatitis), the weight loss goal is higher to 7-10% of body weight [
6]. It is shown that weight loss > 7% improves histological disease activity [
7]. Thus, it remains crucial for patients to make lifestyle changes to achieve this goal.
An important characteristic to achieve these targeted weight loss goals is incorporating regular exercise. The American College of Sports Medicine (ACSM) recommends at least 150 min/weekly of moderate or 75 min/weekly of vigorous-intensity physical activity for all patients with MASLD [
8]. In return, this not only assists with weight loss but also increases insulin sensitivity, decreases free fatty acids and de novo lipogenesis [
8]. All of which play role in the pathophysiology of developing MASLD.
For patients who do not meet their weight loss goals after 6 months, other options such as Bariatric surgery can be discussed. In a recent observational study, a cohort of 431 participants were biopsy screened for histological MASLD [
9]. Out of the 288 that qualified for the study, 156 participants underwent Rou-en-Y gastric bypass or sleeve gastrectomy, while the others were assigned to life-style modifications only [
9]. When compared to life-style modifications alone, patients who underwent a Bariatric procedure had a 3.6 times higher likelihood of MASH resolution [
9]. Additionally, patients who underwent Bariatric surgery also had a lower risk of developing new-onset heart failure, cerebrovascular events and coronary artery interventions [
10].
3.3. Diabetic Drugs Targeting MASH
Besides weight loss and exercise, medical therapy including anti-diabetic drugs are rapidly gaining ground. These medications are becoming popular as they help control certain metabolic factors such as diabetes and lipid metabolism [
11]. These factors play a large role in developing metabolic syndrome and are directly implicated in MASLD development.
3.3.1. Glucagon-like Peptide-1 Receptor Agonists (GLP – 1 RA)
Glucagon-like peptide-1 receptor agonists (GLP – 1 RA’s) have become increasingly popular as a treatment for diabetes and weight loss. GLP -1 RA is an incretin hormone that stimulates the release of insulin from pancreatic beta-cells in response to carbohydrates that are absorbed from the gut [
12]. It also controls glucose homeostasis by delaying gastric emptying which in return helps control post-prandial hyperglycemia. Furthermore, GLP-1 RA’s affect specific portions of the hypothalamus where hunger is controlled and as a result, patients benefit from weight loss due to reduced caloric intake [
12]. By exerting such effects, patients can control their metabolic risk factors and reduce their chances of developing MASLD.
There has been ongoing research on the positive effects of GLP-1s and steatohepatitis. Semaglutide is part of an ongoing phase 3 clinical (ESSENCE) trial where its benefits on reducing biopsy proven steatohepatitis and liver fibrosis stage 2 or 3 is being studied [
13]. The primary end point of the study is the resolution of steatohepatitis and reduction of liver fibrosis without worsening steatohepatitis. Resolution of steatohepatitis was seen in 62.9% of the 534 patients in the Semaglutide group versus 34.4% of the 266 patients in the placebo group [
13]. In addition, a reduction in liver fibrosis without worsening of steatohepatitis was noted in 36.8% of patients in the Semaglutide group and in 22.4% of those in the placebo group. In patients who took Semaglutide, the reduction of steatohepatitis and moderate to advanced fibrosis proved to be beneficial when compared to placebo [
13].
Clinical trials that have been completed on other GLP-1 RA’s have also demonstrated significant clinical benefit. Tirzepatide, which is a dual GLP-1 and glucose-independent insulinotropic polypeptide (GIP), has shown even greater efficacy in weight loss when compared to Semaglutide. The SURMOUNT – 5 study, did a direct head-to-head comparison of Semaglutide vs Tirezepatide. In the study, the primary end point was to measure the greatest percent change in weight from week 1 to week 72 [
14]. The study only included individuals with obesity but without diabetes. The study enrolled 751 participants which underwent randomization. The end-point of the study revealed that among participants who took both medications, Tirzepatide was superior to Semaglutide when it came to reduction in body weight and waist circumference [
14]. Although the study did not reveal it’s direct effects on patients with MASLD, it does show favorable outcomes for patients with at least one metabolic risk factor leading to fatty liver disease.
The benefits of GLP-1/glucagon receptor co-agonists have also undergone extensive research. Cotadutide is a dual GLP-1 and glucagon receptor agonist that has shown benefit in patients suffering from MASLD. In a phase 2b study (NCT03235050), Cotadutide was assessed for HbA1c reduction and body weight improvement by week 14 [
15]. Liver bio-markers and liver fibrosis scores were also assessed. Participants in a randomized double-blind study were either assigned to a placebo group or cotadutide group or an open label liraglutide group [
15]. It was shown that cotadutide significantly decreased A1c levels and body weight at weeks 14 and 54. An improvement in AST and ALT levels, fibrosis – 4 index were observed in the cotadutide 300 ug vs placebo group, but not with liraglutide. It is important to note the benefits of weight loss, HbA1c and liver biomarkers was observed in a dose-dependent fashion with cotadutide [
15].
Survodutide is a glucagon/GLP-1 receptor dual agonist that is also showing great promise. In 2024, the US FDA gave the drug a breakthrough designation status to study the benefits of this therapy for patients living with MASH (metabolic associated steato-hepatitis) and fibrosis and MASH with compensated liver cirrhosis [
16]. In its phase 2 study, Survodutide proved to be superior when compared to placebo with respect to improvement in MASH without worsening of fibrosis when compared to placebo, which warranted further investigation in phase 3 trials [
17]. The phase III clinical trial was launched into two separate studies: the LIVERAGE trial designated for patients with MASH with moderate to advanced fibrosis and the LIVERAGE – Cirrhosis trial for those with MASH with compensated liver cirrhosis [
16].
Efinopegdutide and pemvidutide are other combined glucagon/GLP-1 medications that have been studied recently. In a phase II randomized trial, Efinopegdutide 10 mg was noted to have statistically significant decrease in liver fat content in patients with MASH when compared to semaglutide 1 mg once weekly for 24 weeks [
18]. Additionally, a greater reduction in other metabolic risk factors such as HDL, LDL, TGL levels and weight reduction was noted in the efinopegdutide group when compared to semaglutide [
18]. Additionally, pemvidutide (1.2 mg, 1.8 mg, 2.4 mg once weekly) was compared to placebo, the 1.2 mg and 1.8 mg demonstrated significant reduction in liver fat content when compared to placebo [
18].
In summary, GLP-1 receptor agonists and their dual agonist counterparts represent a promising class of therapies for patients with MASLD and MASH. By targeting multiple metabolic pathways—ranging from glycemic control and appetite suppression to direct hepatic effects—these agents have demonstrated significant benefits in reducing liver fat, improving liver enzyme profiles, and even reversing steatohepatitis and fibrosis in clinical trials. As ongoing phase 3 studies continue to evaluate long-term efficacy and safety, these therapies may soon become integral components of a multidisciplinary approach to managing MASLD.
3.3.2. Pioglitazone
Pioglitazone is a thiazolidinedione which increases insulin sensitivity and lipid metabolism by acting on peroxisome proliferator-activated receptor-gamma (PPARγ) receptors. It reduces insulin resistance by improving lipid storage/redistribution and glucose utilization [
19]. Pioglitazone was one of the first anti-diabetic drugs to show promise when it came to treating patients with MASLD [
19]. In a randomized clinical trial (RCT) involving 55 people with pre-diabetes/Type 2 DM and biopsy proven MASH, it showed a significant difference when compared to placebo in reducing liver fat content and improvement in histologic findings [
20,
21]. In a meta-analysis of eight RCT’s involving 516 people with biopsy proven MASH, pioglitazone was noted to have improved advanced fibrosis of any stage and MASH resolution in patients. Despite showing great benefits, pioglitazone has been implicated with adverse events such as heart failure, weight gain and increased fracture risk which has made the drug less favorable [
19]. As a result, pioglitazone is not routinely recommended for use in patients with MASLD.
3.3.3. Sodium-Glucose Co-Transporter-2 Inhibitors (SGLT-2 Inhibitors)
Sodium-glucose co-transporter- 2 inhibitors (SGLT-2 inhibitors) have shown promise in treating patients with MASLD. The drug has already been proven to reduce the risk of chronic kidney disease (CKD) progression, reduce heart failure exacerbations and improve diabetic outcomes. These agents work by inhibiting the reabsorption of glucose in the proximal renal tubules to facilitate urinary glucose excretion [
22]. This glycemic control, combined with weight loss has made this drug class an important area of study [
22]. In addition, SGLT-2 inhibitors can decrease plasma triglyceride levels and increase high-density lipoprotein which can improve dyslipidemia and be helpful in alleviating hepatic steatosis.
A recent meta-analysis reviewed 18 eligible RCT’s involving 1330 participants and based on those results, SGLT-2 inhibitors were shown to slightly improve hepatic steatosis and fibrosis when compared to controls with low to moderate certainty of evidence [
23]. This study included: empagliflozin, dapagliflozin, tofogliflozin, luseogliflozin, licogliflozin and ipragliflozin. Despite showing promise, SGLT-2 inhibitors need more randomized controlled trials and a larger sample size before they can be considered as a standard of treatment for MASLD patients.
3.3.4. Vitamin E
Vitamin E, a potent anti-oxidant, has also been considered as a potential therapeutic option for treating non-diabetic patients with MASLD. It manages to prevent liver injury by reducing oxidative stress. In the setting of metabolic syndrome, the increased delivery of fatty acids to the liver results in amplified oxidative stress through fatty acid oxidation and oxidative phosphorylation [
24]. This produces an environment high in reactive oxygen species which can cause hepatocyte injury and hepatic damage. Vitamin E manages to reduce oxidative stress via various pathways which directly aids in improving hepatic steatosis, lobular inflammation and hepatocyte ballooning [
24].
In a recent double-blind, randomized trial by Song et. al, Vitamin E was compared to placebo. The study included 124 non-diabetic patients with biopsy proven MASH. When compared with placebo, the patients who took Vitamin E 300 mg had significant improvement in steatosis, lobular inflammation and liver fibrosis [
25]. In addition, a systematic review of 11 studies concluded that patients who took Vitamin E 800 IU daily noticed improvement in liver enzymes (ALT and AST), hepatic steatosis, and inflammation [
26]. Although it showed promising results in those areas, it did not have significant impact on liver fibrosis. Despite initial success, the studies that have been conducted for Vitamin E have been limited in size which calls for future research prior to being considered as routine therapy for MASLD (
Table 1).
4. Novel Therapeutics for MASLD
4.1. Resmetirom
The therapeutic landscape for MASLD continues to evolve, with Resmetirom emerging as one of the newest FDA-approved agents for patients with moderate to advanced fibrosis. Approved in 2024, Resmetiron exerts its effects by targeting THR-β (thyroid hormone receptor – beta) selective receptor on the liver [
27]. THR-β is responsible for regulating metabolic pathways in the liver and it is frequently impaired in MASLD patients [
27]. As a result, this affects lipid metabolism, fatty acid oxidation and energy production which can potentially worsen MASLD and liver fibrosis. The lipotoxicity that occurs in fatty liver disease induces intrahepatic hypothyroidism resulting in reduced conversion of pro-hormone T4 to active T3. Instead, there is an increased conversion of T4 to the inactive metabolite reverse T3 (rT3). By targeting the thyroid hormone receptor, Resmetirom proved to be efficacious in MASLD resolution and in improving fibrosis by at least one stage without any significant worsening in MASLD activity score [
27].
This pivotal data was revealed from the MAESTRO-NASH trial where 1759 participants underwent a double blind, placebo-controlled trial. After 52 weeks of treatment, both 100 mg and 80 mg of Resmetirom demonstrated significant improvement in the treatment arm when compared to placebo. The primary end-points of the study were MASLD resolution (including a reduction in disease activity score ≥2 points) with no worsening fibrosis and an improvement in fibrosis by at least one stage without evidence of worsening in the disease activity score [
27]. Despite its promising efficacy, the cost and accessibility of Resmetirom may limit widespread adoption. While the manufacturer offers financial assistance programs, broader use will likely depend on commercial insurance coverage.
4.2. Lanifibranor
Lanifibranor has emerged as one of the most promising investigational therapies for managing metabolic associated steatohepatitis (MASH). Although not yet FDA-approved, the drug gained early momentum in 2020 when it received Breakthrough Therapy Designation from the U.S. Food and Drug Administration [
28]. This designation is reserved for treatments that demonstrate substantial potential in early clinical trials and is intended to expedite drug development and review [
28]. Lanifibranor is a pan-PPAR (peroxisome proliferator- activated receptor) agonist that modulates key metabolic, inflammatory, and fibrogenic pathways in the pathogenesis of MASH [
29]. Initial findings in the phase 2b, double-blind, placebo controlled NATIVE trial showed that Lanifibranor (1200 mg and 800 mg) had a significant improvement in steatosis, activity, fibrosis (SAF) scores when compared to placebo. The SAF score (0-4) - a histologic scoring system where higher values reflect more active disease, is a score that incorporate scores for ballooning and inflammation without worsening of fibrosis [
29].
The NATIVE trial enrolled a total of 247 patients who underwent randomization, 103 had type 2 diabetes mellitus and 188 (76%) had significant (moderate) or advanced fibrosis. Compared to placebo, the percentage of patients who had a decrease of at least 2 points in the SAF score without worsening fibrosis was much higher in the 1200 mg Lanifibranor group than those who took 800 mg of Lanifibranor [
29]. Although the drug’s effects were modest with the lower dose, the results still favored the treatment group over the placebo. In addition, the Lanifibranor group had decreased liver enzyme levels and improvement in the majority of lipid, inflammatory and fibrosis markers [
29].
These encouraging findings paved the way for advancement to a Phase 3 trial (NATiV3), which recently completed enrollment [
30]. Final results are anticipated in the first half of 2026 and will be pivotal in determining whether Lanifibranor gains regulatory approval. Out of the current treatment landscape, Lanifibranor shows huge promise as an emerging therapy specifically for MASH [
30]. If approved, it could significantly expand the therapeutic arsenal available for patients with MASLD.
4.3. Fibroblast Growth Factor (FGF) Analogs
Fibroblast growth factor (FGF)-based agents represent an emerging class of therapeutics showing promise in the treatment of MASLD and MASH. Fibroblast growth factors and their receptors play an important role in maintaining metabolic homeostasis in the liver. Dysregulation in these pathways have been implicated in contributing to hepatic lipid accumulation and chronic inflammation leading to MASLD and MASH, respectively [
31]. In the liver, fibroblast growth factors (FGF19, FGF21, FGF23), have been shown to regulate hepatic lipid metabolism, fasting response and bile acid homeostasis [
31]. Therapeutics focusing on FGF21 have been of particular interest as up-regulation in FGF21 has been linked to progression of fatty liver disease. In obese children, elevated expression of FGF21 has been confirmed as a risk factor for steatosis [
31].
As a result, Pegbelfermin, which is a recombinant PEGylated analog of human FGF21 showed great initial results when treating patients with MASH and stage 2 fibrosis. The medication exerted its effects by increasing adiponectin and decrease in serum pro-C3, which yielded in decreased liver fat, transaminases and liver stiffness as assessed by MR elastography [
31]. Despite showing early promise, the results of the phase 2b FALCON trials did not yield favorable outcomes when comparing to placebo and as a result, further trials of the drug were abated [
32].
Efruxifermin, another FGF21 analog, has shown promising results in recent phase 2 trials. Although initial studies did not demonstrate significant improvement in fibrosis scores compared to placebo [
33], efruxifermin showed favorable trends in steatosis reduction, liver enzyme normalization, and non-invasive fibrosis markers. Consequently, it has progressed to a robust phase 3 clinical development program (SYNCHRONY). This includes trials focused on both fibrosis regression in F2–F3 patients and treatment of compensated cirrhosis (F4) [
34]. The SYNCHRONY program is actively enrolling patients, with results anticipated by 2032 [
35].
In addition to FGF21-based agents, aldafermin (NGM282), an analog of FGF19, has demonstrated therapeutic potential for MASLD. This medication exerts its effects on FGF19 which suppresses bile acid synthesis via CYP7A1, thereby reducing bile acid-mediated hepatotoxicity. It also improves metabolic parameters, including insulin sensitivity and liver fat content [
36]. In a recent retrospective trial, 491 patients were included where Aldafermin showed a dose-dependent reduction in liver fat content, alanine aminotransferase levels (ALT), aspartate aminotransferase levels (AST) and enhanced liver fibrosis scores, in the 1 mg and 3 mg subgroups [
36]. However, the treatments impact on improving histologic fibrosis lacked statistical significance. As a result, larger and longer trials are needed to effectively establish the robustness of this drug therapy.
5. Discussion
As the incidence of MASLD rises worldwide, patients are at an increased risk of developing sequalae of progressive liver disease including liver cirrhosis, Hepatocellular carcinoma (HCC), and liver related mortality. The key to prevention will be to reduce the risk of developing metabolic syndrome, which culminates from a cluster of conditions including: type 2 DM, hyperlipidemia, central obesity and hypertension. These risk factors are well-established contributors to the development and progression of MASLD, making metabolic control a vital component of any therapeutic approach.
As various therapies still remain under investigation, there have been promising drug developments that will make treating MASLD less challenging. Resmetirom, GLP-1 receptor agonists, Lanifibranor, and Efruxifermin are some of the few that have shown favorable clinical trial results. Notably, Resmetirom has emerged as the therapy of choice as it is the first and only FDA approved medication available to treat MASLD. However, given the complex and multifactorial pathophysiology of MASLD, it is unlikely that a single therapy will serve as a universal solution.
Thus, it will be important to closely follow phase 3 trial results of multiple medications that remain in the pipeline. For years, the treatment options for MASLD were limited and largely supportive. Today, as targeted therapies continue to emerge, we are witnessing a shift toward disease-modifying interventions that not only improve liver histology and fibrosis but also address the broader cardiometabolic risks that accompany MASLD. This evolving treatment landscape offers new hope for patients and clinicians alike in the fight against the global liver disease epidemic.
Author Contributions
Conceptualization, P.P.; methodology, P.P.; software, P.P.; validation, P.P.; formal analysis, P.P.; investigation, P.P.; resources, P.P.; data curation, P.P.; writing—original draft preparation, P.P.; writing—review and editing, P.P.; visualization, P.P.; supervision, P.P.; project administration, P.P. All authors have read and agreed to the published version of the manuscript.”
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
During the preparation of this manuscript/study, the author(s) used [ChatGPT 2.0] for the purposes of editing and creating text tables. The author(s) have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986.
- Le P, Tatar M, Dasarathy S, et al. Estimated Burden of Metabolic Dysfunction–Associated Steatotic Liver Disease in US Adults, 2020 to 2050. JAMA Netw Open. 2025;8(1):e2454707. [CrossRef]
- Younossi, Z. M., Koenig, A. B., Abdelatif, D., Fazel, Y., Henry, L., & Wymer, M. (2023). Changing epidemiology, global trends and implications for outcomes of NAFLD. Journal of Hepatology, 79(6), 1179–1191. [CrossRef]
- Kudaravalli P, John S. Nonalcoholic Fatty Liver. [Updated 2023 Apr 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; Accessed 21 May 2025. Available from: https://www.ncbi.nlm.nih.gov/books/NBK541033/.
- Rinella, Mary E.1; Neuschwander-Tetri, Brent A.2; Siddiqui, Mohammad Shadab3; Abdelmalek, Manal F.4; Caldwell, Stephen5; Barb, Diana6; Kleiner, David E.7; Loomba, Rohit8. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 77(5):p 1797-1835, May 2023. |. [CrossRef]
- Chalasani, N., Younossi, Z., Lavine, J. E., Diehl, A. M., Brunt, E. et al. (2024). Management of metabolic dysfunction-associated steatotic liver disease (nonalcoholic fatty liver disease) in adults. UpToDate. Retrieved May 26, 2025.
- Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. 2012 Apr;55(4):885-904. Epub 2012 Jan 27. PMID: 22278337. [CrossRef]
- Stine JG, Long MT, Corey KE, Sallis RE, Allen AM, Armstrong MJ, Conroy DE, et al. American College of Sports Medicine (ACSM) International Multidisciplinary Roundtable report on physical activity and nonalcoholic fatty liver disease. Hepatol Commun. 2023 Mar 30;7(4):e0108. PMID: 36995998; PMCID: PMC10069861. [CrossRef]
- Verrastro, O, Panunzi, S, Gissey-Castragneto, L, Gaetano DE, A, Lembo, E, Capristo, E, et al. Bariatric-metabolic surgery vs lifestyle intervention plus best medical care in non-alcoholic steatohepatitis (BRAVES): a multi-centre, open-label, randomized trial. The Lancet. 2023 April 20; 401(10390) 1786-1797. [CrossRef]
- Krishnan A, Hadi Y, Alqahtani SA, Woreta TA, Fang W, Abunnaja S, Szoka N, Tabone LE, Thakkar S, Singh S. Cardiovascular Outcomes and Mortality After Bariatric Surgery in Patients With Nonalcoholic Fatty Liver Disease and Obesity. JAMA Netw Open. 2023 Apr 3;6(4):e237188. PMID: 37027156; PMCID: PMC10082402. [CrossRef]
- Koullias E, Papavdi M, Koskinas J, et al. (January 01, 2025) Targeting Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): Available and Future Pharmaceutical Options. Cureus 17(1): e76716. [CrossRef]
- Boer GA et al (2023): Obesity pharmacotherapy: incretin action in the central nervous system. Trends Pharmacol Sci 44(1):50-63. PMID: 36462999. [CrossRef]
- Sanyal AJ, Newsome PN, Kliers I, Østergaard LH, Long MT, Kjær MS, Cali AMG, et al. ESSENCE Study Group. Phase 3 Trial of Semaglutide in Metabolic Dysfunction-Associated Steatohepatitis. N Engl J Med. 2025 Jun 5;392(21):2089-2099. Epub 2025 Apr 30. PMID: 40305708. [CrossRef]
- Aronne LJ, Horn DB, le Roux CW, Ho W, Falcon BL, Gomez Valderas E, Das S, et al. SURMOUNT-5 Trial Investigators. Tirzepatide as Compared with Semaglutide for the Treatment of Obesity. N Engl J Med. 2025 May 11. Epub ahead of print. PMID: 40353578. [CrossRef]
- Nahra R, Wang T, Gadde KM, Oscarsson J, Stumvoll M, Jermutus L, Hirshberg B, Ambery P. Effects of Cotadutide on Metabolic and Hepatic Parameters in Adults With Overweight or Obesity and Type 2 Diabetes: A 54-Week Randomized Phase 2b Study. Diabetes Care. 2021 Jun;44(6):1433-1442. Epub 2021 May 20. Erratum in: Diabetes Care. 2022 Dec 1;45(12):3112. doi: 10.2337/dc22-er12. PMID: 34016612; PMCID: PMC8247525. [CrossRef]
- Boehringer Ingelheim. Boehringer receives U.S. FDA Breakthrough Therapy designation and initiates two phase III trials in MASH for survodutide. Available at: https://www.boehringer-ingelheim.com/human-health/metabolic-diseases/survodutide-us-fda-breakthrough-therapy-phase-3-trials-mash. Accessed June 2025.
- Sanyal AJ, Bedossa P, Fraessdorf M, Neff GW, Lawitz E, Bugianesi E, Anstee QM, Hussain SA, Newsome PN, et al. 1404-0043 Trial Investigators. A Phase 2 Randomized Trial of Survodutide in MASH and Fibrosis. N Engl J Med. 2024 Jul 25;391(4):311-319. Epub 2024 Jun 7. PMID: 38847460. [CrossRef]
- Singh A, Sohal A, Batta A. GLP-1, GIP/GLP-1, and GCGR/GLP-1 receptor agonists: Novel therapeutic agents for metabolic dysfunction-associated steatohepatitis. World J Gastroenterol. 2024 Dec 28;30(48):5205-5211. PMID: 39735270; PMCID: PMC11612699. [CrossRef]
- Chan WK, Chuah KH, Rajaram RB, Lim LL, Ratnasingam J, Vethakkan SR. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J Obes Metab Syndr. 2023 Sep 30;32(3):197-213. Epub 2023 Sep 13. PMID: 37700494; PMCID: PMC10583766. [CrossRef]
-
Chopra S, Lai M. Management of metabolic dysfunction-associated steatotic liver disease (nonalcoholic fatty liver disease) in adults. UpToDate. Updated February 9, 2025. Available at: management-of-metabolic-dysfunction-associated-steatotic-liver-disease-nonalcoholic-fatty-liver-disease-in-adults.
- Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J, Berria R, Ma JZ, Dwivedi S, Havranek R, Fincke C, DeFronzo R, Bannayan GA, Schenker S, Cusi K. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006 Nov 30;355(22):2297-307. PMID: 17135584. [CrossRef]
- Michalopoulou E, Thymis J, Lampsas S, Pavlidis G, Katogiannis K, Vlachomitros D, Katsanaki E, et al. The Triad of Risk: Linking MASLD, Cardiovascular Disease and Type 2 Diabetes; From Pathophysiology to Treatment. J Clin Med. 2025 Jan 10;14(2):428. PMID: 39860434; PMCID: PMC11765821. [CrossRef]
- Ong Lopez AMC, Pajimna JAT. Efficacy of sodium glucose cotransporter 2 inhibitors on hepatic fibrosis and steatosis in non-alcoholic fatty liver disease: an updated systematic review and meta-analysis. Sci Rep. 2024 Jan 24;14(1):2122. PMID: 38267513; PMCID: PMC10808406. [CrossRef]
- Perumpail BJ, Li AA, John N, Sallam S, Shah ND, Kwong W, Cholankeril G, Kim D, Ahmed A. The Role of Vitamin E in the Treatment of NAFLD. Diseases. 2018 Sep 24;6(4):86. PMID: 30249972; PMCID: PMC6313719. [CrossRef]
- Song Y, Ni W, Zheng M, Sheng H, Wang J, Xie S, Yang Y, et al. Chinese NAFLD Clinical Research Network (CNAFLD CRN). Vitamin E (300 mg) in the treatment of MASH: A multi-center, randomized, double-blind, placebo-controlled study. Cell Rep Med. 2025 Feb 18;6(2):101939. PMID: 39970876; PMCID: PMC11866479. [CrossRef]
- Abera M, Suresh SB, Malireddi A, Boddeti S, Noor K, Ansar M, Malasevskaia I. Vitamin E and Non-alcoholic Fatty Liver Disease: Investigating the Evidence Through a Systematic Review. Cureus. 2024 Oct 29;16(10):e72596. PMID: 39610563; PMCID: PMC11602675. [CrossRef]
- Harrison SA, Taub R, Neff GW, Lucas KJ, Labriola D, Moussa SE, Alkhouri N, et al. Resmetirom for nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled phase 3 trial. Nat Med. 2023 Nov;29(11):2919-2928. Epub 2023 Oct 16. PMID: 37845512; PMCID: PMC10667098. [CrossRef]
- Inventiva. (2020, October 12). Inventiva receives FDA Breakthrough Therapy designation for lead drug candidate lanifibranor in NASH. Sofinnova Partners. https://sofinnovapartners.com/news/inventiva-receives-fda-breakthrough-therapy-designation-for-lead-drug-candidate-lanifibranor-in-nash?utm_source=chatgpt.com.
- Francque, S, Bedossa, P, Ratziu, V, Anstee, Q, Bugianesi, E, Sanyal, A, Loomba, R, et al. (2021). A randomized, controlled trial of the pan-PPAR agonist lanifibranor in NASH. New England Journal of Medicine. 385. 1547-1558. [CrossRef]
- Inventiva. (2025, April 1). Inventiva announces completion of enrollment in the Phase 3 NATiV3 clinical trial of lanifibranor in patients with MASH and advanced fibrosis. BioSpace. https://www.biospace.com/press-releases/inventiva-announces-completion-of-enrollment-in-the-phase-3-nativ3-clinical-trial-of-lanifibranor-in-patients-with-mash-and-advanced-fibrosis.
- Ocker M. Fibroblast growth factor signaling in non-alcoholic fatty liver disease and non-alcoholic steatohepatitis: Paving the way to hepatocellular carcinoma. World J Gastroenterol. 2020 Jan 21;26(3):279-290. PMID: 31988589; PMCID: PMC6969880. [CrossRef]
- Abdelmalek MF, Sanyal AJ, Nakajima A, Neuschwander-Tetri BA, Goodman ZD, Lawitz EJ, Harrison SA, et al. Pegbelfermin in Patients With Nonalcoholic Steatohepatitis and Compensated Cirrhosis (FALCON 2): A Randomized Phase 2b Study. Clin Gastroenterol Hepatol. 2024 Jan;22(1):113-123.e9. Epub 2023 Apr 23. PMID: 37088458. [CrossRef]
- Noureddin M, Rinella ME, Chalasani NP, Neff GW, Lucas KJ, Rodriguez ME, Rudraraju M, et al. Efruxifermin in Compensated Liver Cirrhosis Caused by MASH. N Engl J Med. 2025 May 9. Epub ahead of print. PMID: 40341827. [CrossRef]
- Akero Therapeutics. Clinical Trials – Efruxifermin for MASH. Akero Therapeutics website. Published 2025. Accessed June 22, 2025. https://akerotx.com/clinical-trials/.
- Akero Therapeutics. (2025). A phase 3, randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of efruxifermin in subjects with non-cirrhotic NASH/MASH and fibrosis (ClinicalTrials.gov Identifier: NCT06215716). Retrieved June 22, 2025, from ClinicalTrials.gov.
- Marey MM, Belal M, Awad AA, Rabea EM, Hassan MA, Abbas AW, Nashwan AJ. Efficacy and safety of aldafermin in non-alcoholic steatohepatitis: A systematic review and meta-analysis of randomized controlled trials. Clin Res Hepatol Gastroenterol. 2024 Jun;48(6):102357. Epub 2024 Apr 28. PMID: 38688423. [CrossRef]
- Petagine L, Zariwala MG, Patel VB. Non-alcoholic fatty liver disease: Immunological mechanisms and current treatments. World J Gastroenterol 2023; 29(32): 4831-4850 [PMID: 37701135]. [CrossRef]
Table 1.
Comparative effects of anti-diabetic drugs. [
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26]. Legend:
+++: Strong/Highly favorable effect,
++: Moderate/Favorable effect,
+: Mild/Modest benefit,
0: No significant effect,
−−: Negative impact.
Table 1.
Comparative effects of anti-diabetic drugs. [
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26]. Legend:
+++: Strong/Highly favorable effect,
++: Moderate/Favorable effect,
+: Mild/Modest benefit,
0: No significant effect,
−−: Negative impact.
| Drug Class |
Liver Fibrosis |
Hepatic Steatosis |
Body Weight |
Cardiorenal Effects |
MASH Resolution |
|
GLP-1 Receptor Agonists(e.g., Semaglutide, Tirzepatide, Cotadutide)
|
+++ (marked improvement shown in fibrosis markers and histology) |
+++ (significant reduction of liver fat content) |
+++ (consistent weight loss) |
+++ (robust cardiovascular and possible renal benefits) |
+++ (high rates of steatohepatitis resolution in trials) |
|
SGLT-2 Inhibitors(e.g., Empagliflozin, Dapagliflozin)
|
++ (modest improvement; evidence is low-to-moderate in certainty) |
++ (mild reduction in steatosis observed) |
++ (moderate weight loss) |
+++ (well-established cardiorenal protection) |
+ (limited/direct data on MASH resolution) |
|
DPP-IV Inhibitors(e.g., Sitagliptin, Saxagliptin)
|
0 (no significant effect demonstrated) |
0 (minimal to no impact) |
0 (weight-neutral) |
++ (generally safe with modest overall benefits) |
0 (no evidence of MASH resolution) |
| Pioglitazone |
++ (improvement in fibrosis demonstrated in MASH studies) |
++ (moderate reduction of steatosis) |
−− (commonly associated with weight gain) |
++ (some cardiorenal benefit overshadowed by risks [e.g., heart failure]) |
++ (moderate rates of MASH resolution noted in trials) |
| Metformin |
0 (no proven direct effect on fibrosis) |
+ (indirect, modest improvement via insulin sensitization) |
0–+ (weight-neutral to mild loss) |
0 (no listed cardiorenal benefits) |
0 (no clear evidence of MASH resolution) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).