Submitted:
13 July 2025
Posted:
15 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Importance of Understanding Pharmacological Approaches
2. Antivirals Against Mpox: Repurposed Drugs
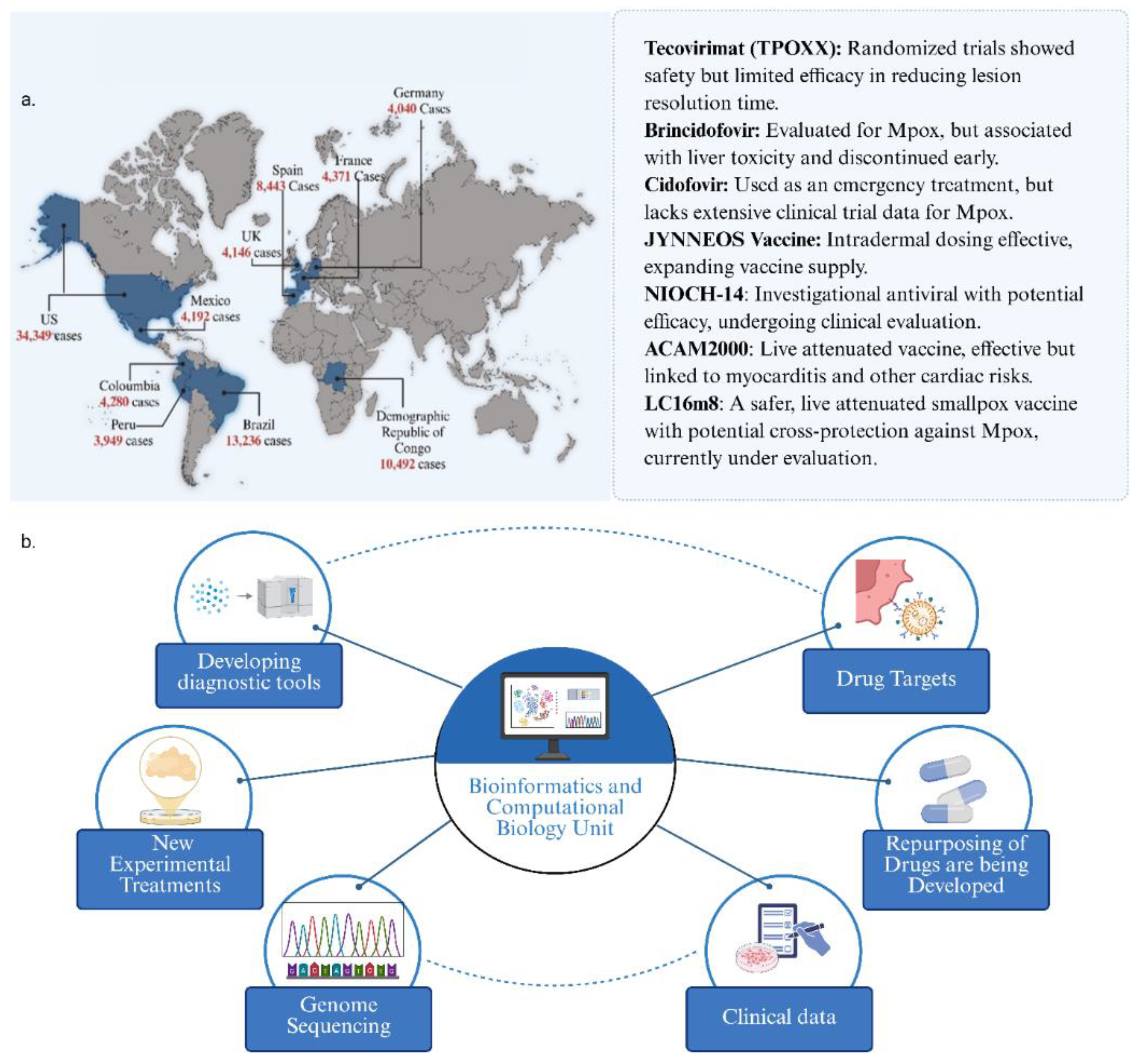
2.1. Tecovirimat (TPOXX)
2.2. Cidofovir and Brincidofovir
2.3. NIOCH-14
2.4. Vaccinia Immune Globulin
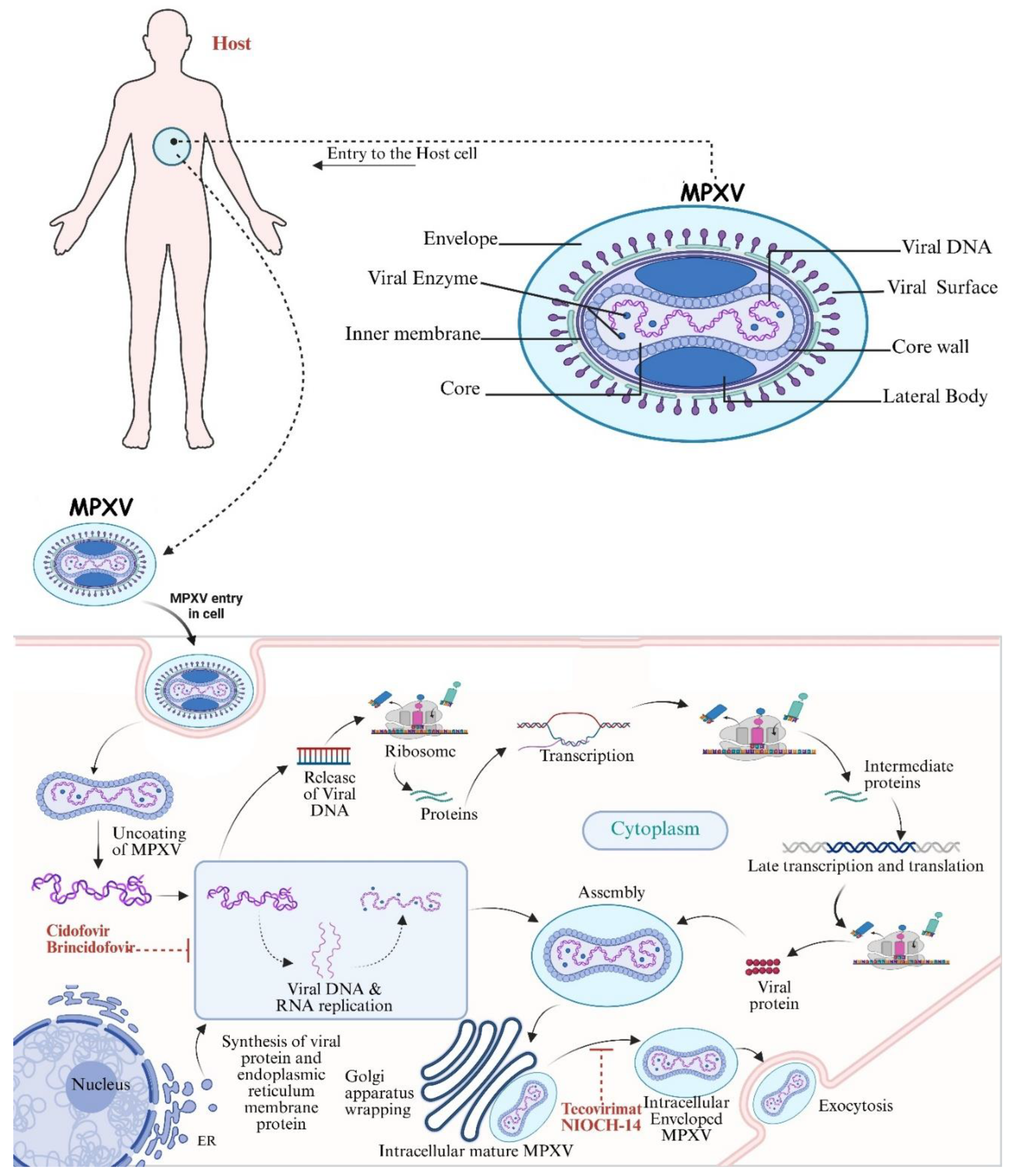
3. Mechanisms of Action
3.1. Viral Pathogenesis and Host Interactions
3.2. Targeting Viral Replication
3.3. Modulation of Host Immune Responses
3.4. Challenges in Understanding Mechanistic Pathways
4. Challenges in Drug Development
4.1. Barriers to Effective Therapeutics Development
4.2. Regulatory Considerations and Clinical Trial Design
4.3. Need for Comprehensive Pharmacovigilance
5. Next-Generation Therapeutic Approaches
5.1. Computational and Bioinformatics Approaches
| Drug Name | Drug Bank ID | Protein Interaction | Mode of Action | Refs |
|---|---|---|---|---|
| Eluxadoline | DB09011 | Mu-opioid receptor | Serves as both an agonist and an antagonist of mu-opioid receptors | [125] |
| Dihydroergotamine | DB00630 | 5-HT1B and 5-HT1D serotonin receptors | Vasoconstrictor, primarily used for migraine treatment | [126] |
| Tobramycin | DB00583 | 30S ribosomal subunit | Inhibits bacterial protein synthesis | [125] |
| Nebivolol | DB01298 | Beta-adrenergic receptors | Selective beta-1 blocker with vasodilating properties | [125] |
| Pimozide | DB00501 | Dopamine D2 receptor | Antipsychotic that blocks dopamine receptors | [126,127] |
| Triptorelin | DB00112 | Gonadotropin-releasing hormone receptor | GnRH analog that inhibits gonadotropin release | [126] |
| Carfilzomib | DB08892 | Proteasome | Proteasome inhibitor used in cancer therapy | [126] |
| Tolvaptan | DB06155 | Vasopressin V2 receptor | Vasopressin receptor antagonist used for treating hyponatremia | [126,127] |
| Cobicistat | DB09019 | Cytochrome P450 3A4 | CYP3A inhibitor that increases the effectiveness of certain HIV medications | [126] |
| Conivaptan | DB04874 | Vasopressin V1A and V2 receptors | Dual vasopressin receptor antagonist used for hyponatremia treatment | [126,127] |
| Tenapanor | DB11607 | Sodium/hydrogen exchanger NHE3 | Inhibits NHE3 to reduce sodium absorption in the intestine | [126] |
| Fludarabine | DB00336 | DNA polymerase | Antimetabolite that interferes with DNA synthesis in cancer cells | [28,127,128] |
| Tigecycline | DB02280 | Bacterial ribosomal subunit | Tigecycline, a glycylcycline, binds to the 30S ribosomal subunit and prevents amino acyl tRNA molecules from entering the ribosome’s A site, therefore inhibiting protein translation in bacteria | [127] |
| Eravacycline | DB12329 | DNA-dependent RNA polymerase | Broad-spectrum antibiotic; inhibits bacterial protein synthesis. | [127] |
5.2. Pipeline Pharmaceuticals and Vaccines Against Mpox
| Vaccine Description | Animal Model | Key Findings | Refs |
|---|---|---|---|
| Canine distemper virus (CDV) and Vaccinia immune globulin (VIG) | EiJ mice (aged 6–12 weeks) | Immunological responses and protective effectiveness | [140] |
| Dryvax vs non-vaccinated Control | Prairie dogs | Viral pathophysiology and vaccine-induced immunity | [141] |
| Sham-vaccinated and Elstree-RIVM vaccine | Cynomolgus macaques (Macaca fascicularis) | Evaluated the safety of vaccines and protective immunity; safe and immunogenic | [142] |
| Attenuated virus NYCBH-AESL (E3L Gene Deleted) | Cynomolgus monkeys | Examined the immune system’s reaction to genetic changes | [143] |
| Modified vaccinia Ankara (MVA) | Cynomolgus monkeys | Demonstrated safety and immunogenicity of MVA-based vaccines | [144] |
| Recombinant MVA | Rhesus macaques (Macaca mulatta) | Explored enhanced immunogenicity through genetic modifications | [145] |
| MVA or Dryvax | Cynomolgus monkeys | Immune responses to conventional and modified vaccinations were compared | [146] |
| Dryvax and TPOXX | BALB/c mice | Evaluated the combined effect of vaccination and antiviral treatment which was shown to be effective | [147] |
| JYNNEOS or ACAM2000 | Cynomolgus monkeys | Compared efficacy and safety profiles of both vaccines, with safety profiles | [148] |
| LC16m8 and Lister (Elstree Strain) | Cynomolgus monkeys | Immunogenic and safe | [149] |
| Dryvax, ACAM2000, or JYNNEOS | Prairie dogs | Vaccine induced protection | [150] |
| JYNNEOS or ACAM2000 | Prairie dogs | Vaccine efficacy against orthopoxvirus infections. | [151] |
| Live smallpox vaccine | BALB/c mice | Immune responses and durability of immunity | [152] |
| ACAM2000 and TPOXX | Cynomolgus monkeys | Synergistic effects of vaccination and antiviral therapy | ([153] |
| Dryvax | Cynomolgus monkeys | Safety and long-term immunity of vaccines | [154,155] |
| Brincidofovir | Black-tailed prairie dogs | Examined the effectiveness of antiviral drug in treating poxvirus infections which showed good responses | [44] |
| InterventionTrial No. | Country | Year (Phase), Status | Number of Subjects | Primary Endpoints | Key Findings |
|---|---|---|---|---|---|
| TPOXX NCT05597735 |
Brazil | 2024 (3), recruiting | Not specified | Reduction of illness duration and contagiousness | The primary result is the duration required for the healing and re-epithelialization of all visible lesions (skin and mucosal). |
| TPOXX Oral Capsule NCT06156566 |
Belgium, France, Spain | 2024 (4), ongoing | 150 | Anal pain assessments using the health-related symptom Index, on days 7, 14, and 90 |
The health-related Symptom Index was utilized to evaluate anal pain on days 7, 14, and 90. From the 28th day of randomization until the first day, every lesion was fully healed and new skin grown |
| TPOXX Oral Capsule NCT05559099 |
The Democratic Republic of the Congo | 2022 (Randomized, placebo-controlled, double-blind study) completed | 597 | The time to lesion resolution, measured in days until all lesions are scabbed or a new layer of skin is developed |
Without posing any serious safety risks, the treatment greatly decreased viral shedding, accelerated lesion healing, and alleviated symptoms. |
| TPOXX (antiviral medication) NCT00728689 |
United States | 2008 (1), completed | 12 | A single dose of ST-246 Form I versus Form V was tested for pharmacokinetics and safety in healthy volunteers | The study found no serious adverse events with ST-246 Form I and Form V. Pharmacokinetic parameters were similar for both forms |
| MVA-BN (JYNNEOS) NCT06549530 |
The Democratic Republic of the Congo | 2024 (2), ongoing | 460 | Two weeks after the second MVA-BN injection, serum neutralizing antibodies against the vaccinia virus were measured using plaque reduction neutralization tests (PRNTs). | Test MVABN standard regimen neutralizing antibody response durability |
| JYNNEOS (vaccine for the prevention of smallpox and Mpox) NCT05512949 |
United States of America | 2022 (2), completed | 229 | The PRNT assay was used to analyse venous blood on Study Day 43 to determine if the intradermal regimen of 2x107 TCID50 MVA-BN and 1x107 TCID50 MVA-BN were non-inferior to the licensed subcutaneous regimen of 1x108 TCID50 MVA-BN | At six weeks, the dose-sparing intradermal Mpox immunization regimen induced antibody responses comparable to the conventional regimen. The vaccine was found to be safe |
| Bavarian Nordic smallpox vaccine NCT05745987 |
Nigeria, Uganda | 2024 (4), ongoing | 1560 | Degree of symptoms and RT-PCR-confirmed Mpox |
This research will ascertain whether the smallpox vaccine lessens the frequency of recurrent infections and the intensity of symptoms in those who have been exposed to Mpox. |
| MVA-BN vaccine NCT05734508 |
The Democratic Republic of the Congo | 2023 (4), completed | 500 | New drug healing time and symptom reduction |
The MVA-BN vaccine was safe, with no serious adverse events. Minor injection-site reactions were most common |
| NIOCH-14 (National Institute of Occupational Health and Safety) NCT05976100 |
Russian Federation | 2020 (1), completed | 90 | Monitoring various blood parameters (like erythrocyte, leukocyte, and platelet levels), biochemical markers (such as glucose and creatinine levels), and the occurrence of adverse events over time | NIOCH-14 was determined to be safe and well-tolerated in healthy volunteers; no significant adverse effects were noted |
| VAC∆6 vaccine NCT05846243 |
Russian Federation- Novosibirsk Region | 2021 (2 and 3), completed | 334 | The percentage of vaccinees with a titre of virus-neutralizing antibodies to vaccinia virus ≥ 1:40 | VAC∆6 vaccine was safe and well-tolerated, with no adverse and only mild reaction at the site of injection |
| LC16m8 live attenuated vaccine of vaccinia virus NCT06223919 |
Colombia | 2024 (3), active, not recruiting, Replicative live attenuated vaccinia virus vaccine: A randomized trial | 8686 | Based on each cohort’s Poisson distribution, the laboratory-confirmed Mpox incidence rate will be computed with a 95% CI. To assess efficacy, immunogenicity and, safety | The vaccine was well-tolerated, with no serious adverse events observed. |
| BNT166a (mRNA vaccines by BioNTech NCT05988203 |
United Kingdom | 2023 (2), Active, not recruiting, A Phase I/II randomized, partially observer-blind, dose-escalation trial of investigational RNA-based Mpox vaccine | 96 | The proportion of individuals who experienced at least one adverse event of special interest (AESI) between Dose 1 and day 201 after dose 1, inclusive. Overall, Safety and immunogenicity | No clinical results are published yet. Preclinical studies showed strong immune responses and full protection in animal models. |
6. Pharmacological Landscape: A Comparative Analysis
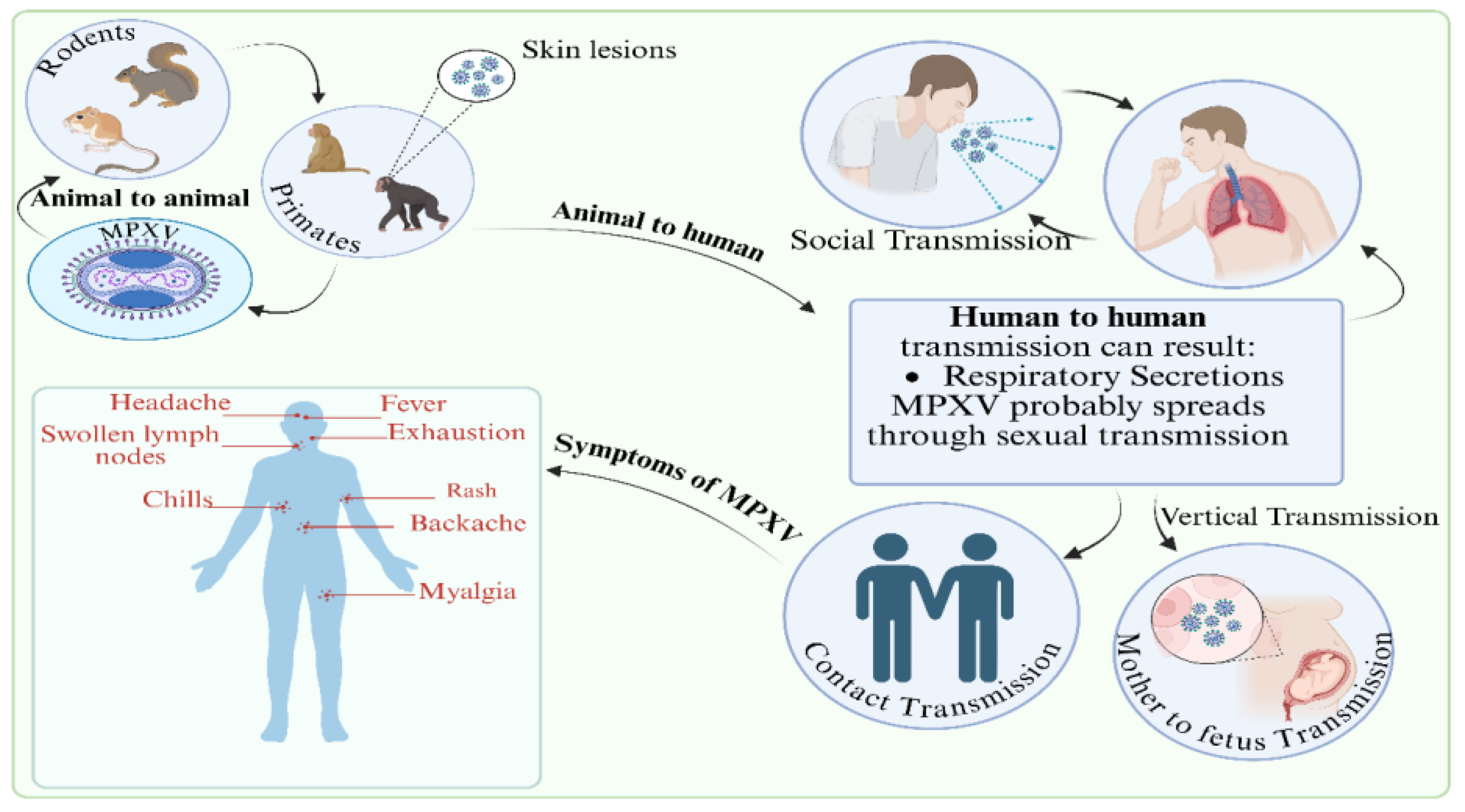
- Whilst Mpox is self-limiting and usually resolve without treatment. Those with symptoms can be managed with anti-histamines for rashes, pain relief medications for headache and muscle aches, topical creams (such as antiseptics, hydrocortisone) for skin lesions, and regular hydration. In rare instances complications such as pneumonia, secondary bacterial infections, eye infections and encephalitis may occur, and medical help is advised. In such instances antivirals may be used based on the specific circumstances. Vaccines are used to prevent further spread during outbreaks.
- Individuals positive for Mpox are generally isolated to prevent spread and during outbreaks personal protective equipment such as masks, hygiene practices and social distancing are encouraged.
- TPOXX, approved by the FDA as a smallpox antiviral, has been shown to be effective against Mpox, reducing the mortality rate from 3.6% to 1.7% [157]; but was not effective against clade I Mpox lesions. It is currently used as a frontline treatment.
- CDV an antiviral that inhibits the synthesis of viral DNA, is used to treat MPXV, in particular those with severe diseases or who have not responded to TPOXX.
- BCV a derivative of cidofovir has been investigated to treat Mpox. Its mechanism of action is similar to cidofovir with better safety profile to cidofovir. Both CDV and BCV have been shown to considerably reduce the viral replication of Mpox clade IIa and IIb [51].
- The development of drug-resistant Mpox strains emphasizes the requirement for continuous research and surveillance to develop new antivirals or use of combination treatments [158].
- New experimental therapies are currently being developed and tested, including vaccinia immune globulin, antibodies targeting the vaccinia virus that may also cross-react with Mpox, and repurposed drugs identified through computational and biological testing for their potential effectiveness in treating Mpox.
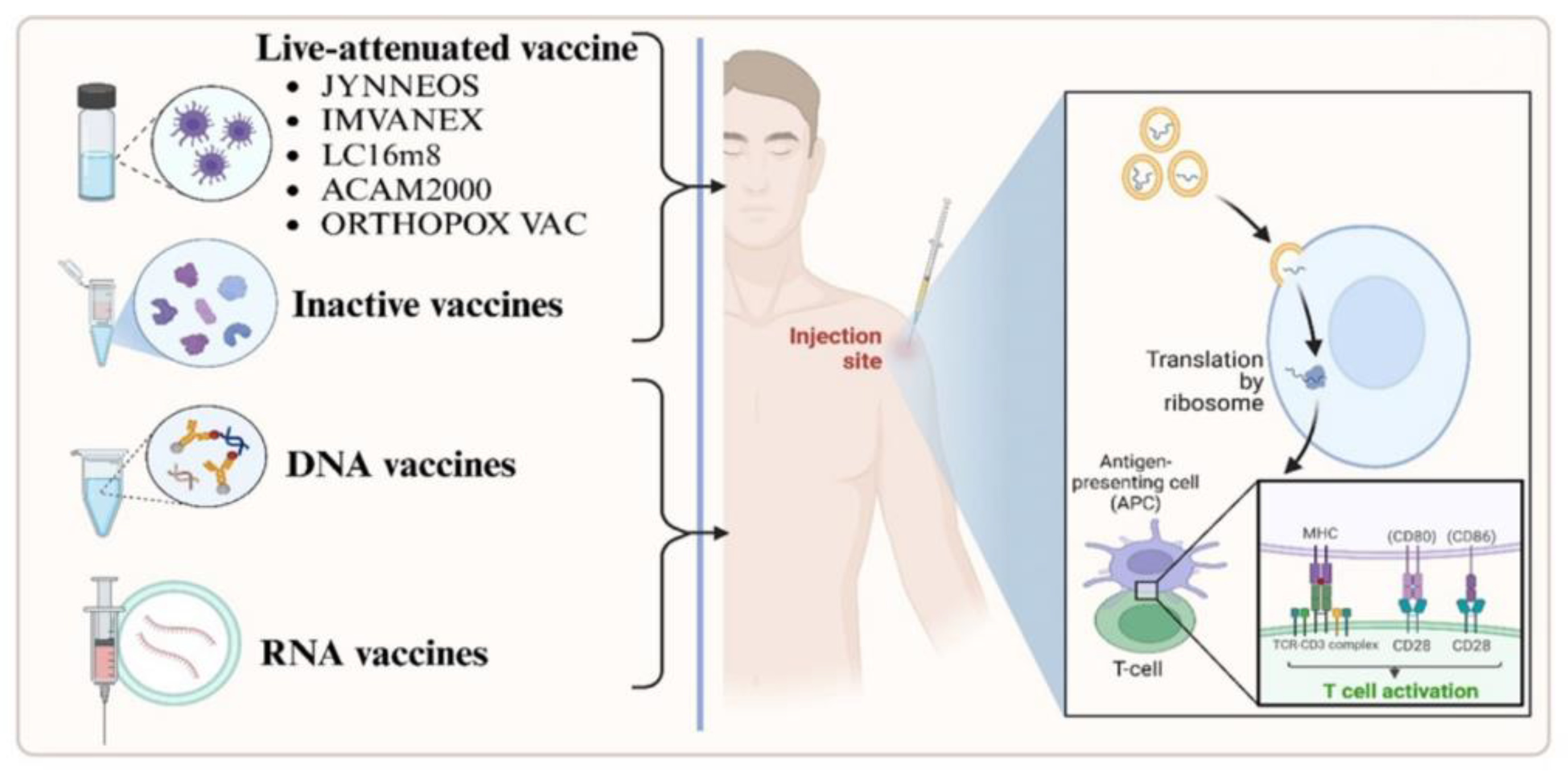
- JYNNEOS vaccine against smallpox and Mpox, a modified non-replicating vaccinia Ankara virus is safe, provides strong immune responses to both smallpox virus and Mpox, and is the preferred vaccine for use during Mpox outbreaks.
- ACAM2000 vaccine against smallpox, is a live vaccinia virus vaccine and is restricted in its use against Mpox, especially in those who are immunocompromised; it has shown some protection against MPXV infections.
- JYNNEOS and ACAM2000 are regarded as safer alternatives to the older smallpox vaccines, like Dryvax, which were used during the smallpox eradication effort. While effective, the older smallpox vaccines use live, replication-competent vaccinia virus vaccine, and their use is not preferred due to the risks associated with live virus vaccines.
- LC16m8 has received emergency use authorization, LC16m8 shields animals from deadly dosages of viruses like Mpox. Little information is available on LC16m8 usage during Mpox outbreaks. The virus in LC16m8 is weakened. Itching, redness, swollen lymph nodes, fever, and exhaustion are some of the side symptoms. The vaccine virus has the potential to spread to other bodily parts. To date, LC16m8 has been donated to over 90,000 individuals. These dosages did not result in any notable safety signals, even in 50,000 youngsters. Pregnant women, those with specific skin conditions, and immunocompromised individuals should not get LC16m8 [159].
7. Future Directions in Mpox Research
7.1. Integrating Mechanistic Insights into Drug Development
7.2. Collaborative Efforts in Global Health Contexts
7.3. Potential for Personalized Medical Approaches
8. Limitations
9. Conclusions
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
Generative AI Statement
Abbreviations
| MPXV | Monkeypox Virus |
| Mpox | Monkeypox |
| CDC | Centers for Disease Control and Prevention |
| WHO | World Health Organization |
| PCR | Polymerase Chain Reaction |
| DNA | Deoxyribonucleic Acid |
| RNA | Ribonucleic Acid |
| TPOXX | Tecovirimat |
| HIV | Human Immunodeficiency Virus |
| STIs | Sexually Transmitted Infections |
| FDA | Food and Drug Administration |
References
- Abdel-Rahman, S.M.; Bayici, B.Z.; Keske, Ş.; Kuşkucu, M.; Özsürekçi, Y.; Rimoin, A.W.; Rodriguez-Morales, A.J.; Ergönül, Ö. Mpox primer for clinicians: what makes the difference in 2024? Curr Opin Infect Dis 2025, 38, 143–149. [Google Scholar] [CrossRef] [PubMed]
- García-Iglesias, J.; Cabezas-Pino, A.; Membrillo de Novales, F.J.; Bautista Pérez, A.R.; Garrido Fuentes, J.; Villaamil Pérez, F.; Rodríguez-Morales, A.J.; Zamora Estay, D.; Guajardo Zuñiga, E.; Núñez Saavedra, L.J.; et al. Renaming mpox in Spanish, French, and Portuguese: using language to address stigma and racism. Lancet 2024, 404, 1301–1302. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Sridhar, S.B.; Shareef, J.; Talath, S.; Mohapatra, P.; Khatib, M.N.; Ballal, S.; Kaur, M.; Nathiya, D.; Sharma, S.; et al. The resurgence of monkeypox: Epidemiology, clinical features, and public health implications in the post-smallpox eradication era. New Microbes New Infect 2024, 62, 101487. [Google Scholar] [CrossRef] [PubMed]
- Lum, F.-M.; Torres-Ruesta, A.; Tay, M.Z.; Lin, R.T.; Lye, D.C.; Rénia, L.; Ng, L.F. Monkeypox: disease epidemiology, host immunity and clinical interventions. Nature Reviews Immunology 2022, 22, 597–613. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Chatterjee, P.; Nasipuri, M.; Basu, S.; Chakraborti, T. Computational drug repurposing for viral infectious diseases: a case study on monkeypox. Briefings in Functional Genomics 2024, 23, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Sah, R.; Mohanty, A.; Abdelaal, A.; Reda, A.; Rodriguez-Morales, A.J.; Henao-Martinez, A.F. First Monkeypox deaths outside Africa: no room for complacency. Ther Adv Infect Dis 2022, 9, 20499361221124027. [Google Scholar] [CrossRef] [PubMed]
- Sah, R.; Srivastava, S.; Mehta, R.; Kumar, S.; Sah, S.; Mohanty, A.; Feehan, J.; Al-Tawfiq, J.A.; Apostolopoulos, V. Global Mpox outbreak: Are we prepared for emerging strains? New Microbes New Infect 2024, 62, 101466. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA’s role in mpox preparedness and response, and information about mpox (formerly referred to as monkeypox). Availabe online: https://www.fda.gov/emergency-preparedness-and-response/mcm-issues/mpox (accessed on 12-01-2025).
- Wahid, M.; Mandal, R.K.; Sikander, M.; Khan, M.R.; Haque, S.; Nagda, N.; Ahmad, F.; Rodriguez-Morales, A.J. Safety and Efficacy of Repurposed Smallpox Vaccines Against Mpox: A Critical Review of ACAM2000, JYNNEOS, and LC16. J Epidemiol Glob Health 2025, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, A.; Reda, A.; Lashin, B.I.; Katamesh, B.E.; Brakat, A.M.; Al-Manaseer, B.M.; Kaur, S.; Asija, A.; Patel, N.K.; Basnyat, S., et al. Preventing the Next Pandemic: Is Live Vaccine Efficacious against Monkeypox, or Is There a Need for Killed Virus and mRNA Vaccines? Vaccines (Basel) 2022, 10. [CrossRef]
- Branda, F.; Romano, C.; Ciccozzi, M.; Giovanetti, M.; Scarpa, F.; Ciccozzi, A.; Maruotti, A. Mpox: An Overview of Pathogenesis, Diagnosis, and Public Health Implications. Journal of Clinical Medicine 2024, 13. [CrossRef]
- Plakkot, G.; Koka, S.; Koka, R.S.; Matus, C. Impact of the 2022 Mpox Outbreak on Future Public Health Initiatives in Ohio. Ohio Journal of Public Health 2023. [CrossRef]
- Farahat, R.A.; Sah, R.; El-Sakka, A.A.; Benmelouka, A.Y.; Kundu, M.; Labieb, F.; Shaheen, R.S.; Abdelaal, A.; Abdelazeem, B.; Bonilla-Aldana, D.K.; et al. Human monkeypox disease (MPX). Infez Med 2022, 30, 372–391. [Google Scholar] [CrossRef] [PubMed]
- Antinori, S.; Casalini, G.; Giacomelli, A.; Rodriguez-Morales, A.J. Update on Mpox: a brief narrative review. Infez Med 2023, 31, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Monkeypox (clade IIB). Monkeypox | SUNY Schenectady. Accessed March 29. Availabe online: https://sunysccc.edu/Current-Students/Monkeypox.html.
- About Mpox. Centers for Disease Control and Prevention. July 22, A.F., 2023. Availabe online: https://www.cdc.gov/poxvirus/mpox/about/index.html.
- Ortiz-Saavedra, B.; León-Figueroa, D.A.; Montes-Madariaga, E.S.; Ricardo-Martínez, A.; Alva, N.; Cabanillas-Ramirez, C.; Barboza, J.J.; Siddiq, A.; Coaguila Cusicanqui, L.A.; Bonilla-Aldana, D.K., et al. Antiviral Treatment against Monkeypox: A Scoping Review. Trop Med Infect Dis 2022, 7, doi:10.3390/tropicalmed7110369. [CrossRef]
- Patient’s Guide to mpox treatment with Tecovirimat (TPOXX). Centers for Disease Control and Prevention. Updated November 28, A.F., 2023. Availabe online: https://www.cdc.gov/poxvirus/mpox/if-sick/treatment.html#:~:text=There%20are%20no%20treatments%20specifically,used%20to%20treat% 20monkeypox%20effectively.
- De Clercq, E. Clinical potential of the acyclic nucleoside phosphonates cidofovir, adefovir, and tenofovir in treatment of DNA virus and retrovirus infections. Clinical microbiology reviews 2003, 16, 569–596. [Google Scholar] [CrossRef] [PubMed]
- Shamim, M.A.; Satapathy, P.; Padhi, B.K.; Veeramachaneni, S.D.; Akhtar, N.; Pradhan, A.; Agrawal, A.; Dwivedi, P.; Mohanty, A.; Pradhan, K.B.; et al. Pharmacological treatment and vaccines in monkeypox virus: a narrative review and bibliometric analysis. Front Pharmacol 2023, 14, 1149909. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Mu, L.; Wang, W. Monkeypox: epidemiology, pathogenesis, treatment and prevention. Signal Transduction and Targeted Therapy 2022, 7, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Ophinni, Y.; Frediansyah, A.; Sirinam, S.; Megawati, D.; Stoian, A.M.; Enitan, S.S.; Akele, R.Y.; Sah, R.; Pongpirul, K.; Abdeen, Z.; et al. Monkeypox: Immune response, vaccination and preventive efforts. Narra J 2022, 2, e90. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, E.A.; Sassine, J. Antivirals with activity against mpox: a clinically oriented review. Clinical infectious diseases 2023, 76, 155–164. [Google Scholar] [CrossRef] [PubMed]
- tecovirimat., F.a.D.A.H.o.p.i.-. Availabe online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214518s000lbl.pdf.
- monkeypox., C.f.D.C.a.P.a.I.c.g.f.t.t.o. Availabe online: https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html.
- Pauli, G.; Blümel, J.; Burger, R.; Drosten, C.; Gröner, A.; Gürtler, L.; Heiden, M.; Hildebrandt, M.; Jansen, B.; Montag-Lessing, T. Orthopox viruses: infections in humans. Transfusion Medicine and Hemotherapy 2010, 37, 351. [Google Scholar] [CrossRef] [PubMed]
- Rizk, J.G.; Lippi, G.; Henry, B.M.; Forthal, D.N.; Rizk, Y. Prevention and treatment of monkeypox. Drugs 2022, 82, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Hudu, S.A.; Alshrari, A.S.; Al Qtaitat, A.; Imran, M. VP37 protein inhibitors for Mpox treatment: highlights on recent advances, patent literature, and future directions. Biomedicines 2023, 11, 1106. [Google Scholar] [CrossRef] [PubMed]
- Duraffour, S.; Snoeck, R.; De Vos, R.; Van Den Oord, J.J.; Crance, J.-M.; Garin, D.; Hruby, D.E.; Jordan, R.; De Clercq, E.; Andrei, G. Activity of the anti-orthopoxvirus compound ST-246 against vaccinia, cowpox and camelpox viruses in cell monolayers and organotypic raft cultures. Antiviral therapy 2007, 12, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Shabil, M.; Khatib, M.N.; Ballal, S.; Bansal, P.; Tomar, B.S.; Ashraf, A.; Kumar, M.R.; Sinha, A.; Rawat, P.; Gaidhane, A.M.; et al. Effectiveness of Tecovirimat in Mpox Cases: A Systematic Review of Current Evidence. J Med Virol 2024, 96, e70122. [Google Scholar] [CrossRef] [PubMed]
- Pourkarim, F.; Entezari-Maleki, T. Clinical considerations on monkeypox antiviral medications: An overview. Pharmacology Research & Perspectives 2024, 12, e01164. [Google Scholar] [CrossRef]
- Congo, N.I.o.H.T.a.t.i.s.b.d.n.i.c.I.m.r.i.D.R.o.t. Availabe online: https://www.niaid.nih.gov/news-events/antiviral-tecovirimat-safe-did-not-improve-clade-i-mpox-resolution-democratic-republic.
- Witwit, H.; Cubitt, B.; Khafaji, R.; Castro, E.M.; Goicoechea, M.; Lorenzo, M.M.; Blasco, R.; Martinez-Sobrido, L.; de la Torre, J.C. Repurposing Drugs for Synergistic Combination Therapies to Counteract Monkeypox Virus Tecovirimat Resistance. Viruses 2025, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Cao, B.; Bei, Z.C.; Xu, L.; Zhang, D.; Zhao, L.; Song, Y.; Wang, H. Disulfide-incorporated lipid prodrugs of cidofovir: Synthesis, antiviral activity, and release mechanism. Eur J Med Chem 2023, 258, 115601. [Google Scholar] [CrossRef] [PubMed]
- Amaral, B.P.; Cargnelutti, J.F.; Mortari, A.P.G.; Merchioratto, I.; Feio, L.M.; Nogueira, C.W.; Weiblen, R.; Flores, E. Diphenyl diselenide and cidofovir present anti-viral activity against Bovine Alphaherpesvirus 2 in vitro and in a sheep model. Res Vet Sci 2021, 134, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Toro, J.R.; Wood, L.V.; Patel, N.K.; Turner, M.L. Topical cidofovir: a novel treatment for recalcitrant molluscum contagiosum in children infected with human immunodeficiency virus 1. Archives of Dermatology 2000, 136, 983–985. [Google Scholar] [CrossRef] [PubMed]
- Lea, A.P.; Bryson, H.M. Cidofovir. Drugs 1996, 52, 225–230, discussion 231. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.L.; Rodríguez, C.A.; Mucci, M.; Ingrosso, A.; Duncan, B.A.; Nickens, D.J. Pharmacokinetics and renal effects of cidofovir with a reduced dose of probenecid in HIV-infected patients with cytomegalovirus retinitis. The Journal of Clinical Pharmacology 2003, 43, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Cundy, K.C. Clinical pharmacokinetics of the antiviral nucleotide analogues cidofovir and adefovir. Clinical pharmacokinetics 1999, 36, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Tseng, A.L.; Mortimer, C.B.; Salit, I.E. Iritis associated with intravenous cidofovir. Ann Pharmacother 1999, 33, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Pourkarim, F.; Entezari-Maleki, T. Clinical considerations on monkeypox antiviral medications: An overview. Pharmacol Res Perspect 2024, 12, e01164. [Google Scholar] [CrossRef] [PubMed]
- Andrei, G.; Snoeck, R. Cidofovir activity against poxvirus infections. Viruses 2010, 2, 2803. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, N.; Shimizu, T.; Hirano, D.; Ishikane, M.; Kataoka, Y. Lack of clinical evidence of antiviral therapy for human monkeypox: A scoping review. Journal of Infection and Chemotherapy 2023, 29, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Hutson, C.L.; Kondas, A.V.; Mauldin, M.R.; Doty, J.B.; Grossi, I.M.; Morgan, C.N.; Ostergaard, S.D.; Hughes, C.M.; Nakazawa, Y.; Kling, C. Pharmacokinetics and efficacy of a potential smallpox therapeutic, brincidofovir, in a lethal monkeypox virus animal model. MSphere 2021, 6, e00927-20. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.W. Therapeutic strategies to address monkeypox. Expert Review of Anti-infective Therapy 2022, 20, 1249–1252. [Google Scholar] [CrossRef] [PubMed]
- FDA, U.S. FDA approves drug to treat smallpox. Availabe online: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-drug-treat-smallpox (accessed on 19).
- Marty, F.M.; Winston, D.J.; Chemaly, R.F.; Mullane, K.M.; Shore, T.B.; Papanicolaou, G.A.; Chittick, G.; Brundage, T.M.; Wilson, C.; Morrison, M.E.; et al. A Randomized, Double-Blind, Placebo-Controlled Phase 3 Trial of Oral Brincidofovir for Cytomegalovirus Prophylaxis in Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant 2019, 25, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Adler, H.; Gould, S.; Hine, P.; Snell, L.B.; Wong, W.; Houlihan, C.F.; Osborne, J.C.; Rampling, T.; Beadsworth, M.B.; Duncan, C.J. Clinical features and management of human monkeypox: a retrospective observational study in the UK. The Lancet Infectious Diseases 2022, 22, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Shamim, M.A.; Padhi, B.K.; Satapathy, P.; Veeramachaneni, S.D.; Chatterjee, C.; Tripathy, S.; Akhtar, N.; Pradhan, A.; Dwivedi, P.; Mohanty, A.; et al. The use of antivirals in the treatment of human monkeypox outbreaks: a systematic review. Int J Infect Dis 2023, 127, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Quenelle, D.C.; Lampert, B.; Collins, D.J.; Rice, T.L.; Painter, G.R.; Kern, E.R. Efficacy of CMX001 against herpes simplex virus infections in mice and correlations with drug distribution studies. The Journal of infectious diseases 2010, 202, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Prévost, J.; Sloan, A.; Deschambault, Y.; Tailor, N.; Tierney, K.; Azaransky, K.; Kammanadiminti, S.; Barker, D.; Kodihalli, S.; Safronetz, D. Treatment efficacy of cidofovir and brincidofovir against clade II Monkeypox virus isolates. Antiviral Research 2024, 231, 105995. [Google Scholar] [CrossRef] [PubMed]
- Shishkina, L.N.; Mazurkov, O.Y.; Bormotov, N.I.; Skarnovich, M.O.; Serova, O.A.; Mazurkova, N.A.; Skarnovich, M.A.; Chernonosov, A.A.; Selivanov, B.A.; Tikhonov, A.Y., et al. Safety and Pharmacokinetics of the Substance of the Anti-Smallpox Drug NIOCH-14 after Oral Administration to Laboratory Animals. Viruses 2023, 15. [CrossRef]
- Mazurkov, O.Y.; Kabanov, A.S.; Shishkina, L.N.; Sergeev, A.A.; Skarnovich, M.O.; Bormotov, N.I.; Skarnovich, M.A.; Ovchinnikova, A.S.; Titova, K.A.; Galahova, D.O. New effective chemically synthesized anti-smallpox compound NIOCH-14. Journal of General Virology 2016, 97, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.S.; Sergeev, A.A.; Shishkina, L.N.; Bulychev, L.E.; Skarnovich, M.O.; Sergeev, A.A.; Bormotov, N.I.; P’Iankov O, V.; Serova, O.A.; Bodnev, S.A.; et al. [A comparative study of the antiviral activity of chemical compounds concerning the orthopoxviruses experiments in vivo]. Vopr Virusol 2013, 58, 39–43. [Google Scholar] [PubMed]
- Шишкина, Л.; Бoгрянцева, М.; Бoрмoтoв, Н.; Усoва, С.; Скарнoвич, М.; Мазуркoв, О.; Башкина, Е.; Куцерубoва, Н.; Удальева, С.; Сергеев, А. Безoпаснoсть нoвoгo рoссийскoгo прoтивooспеннoгo препарата НИОХ-14: oткрытoе рандoмизирoваннoе клиническoе исследoвание I фазы. Безoпаснoсть и риск фармакoтерапии 2025.
- Weinstein, R.A.; Nalca, A.; Rimoin, A.W.; Bavari, S.; Whitehouse, C.A. Reemergence of monkeypox: prevalence, diagnostics, and countermeasures. Clinical infectious diseases 2005, 41, 1765–1771. [Google Scholar] [CrossRef] [PubMed]
- Wittek, R. Vaccinia immune globulin: current policies, preparedness, and product safety and efficacy. International journal of infectious diseases 2006, 10, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A.; Arunagiri, T.; Mani, S.; Kumaran, V.R.; Kannaiah, K.P.; Chanduluru, H.K. From pox to protection: understanding Monkeypox pathophysiology and immune resilience. Tropical Medicine and Health 2025, 53, 33. [Google Scholar] [CrossRef] [PubMed]
- Gilchuk, I.; Gilchuk, P.; Sapparapu, G.; Lampley, R.; Singh, V.; Kose, N.; Blum, D.L.; Hughes, L.J.; Satheshkumar, P.S.; Townsend, M.B. Cross-neutralizing and protective human antibody specificities to poxvirus infections. Cell 2016, 167, 684–694.e689. [Google Scholar] [CrossRef] [PubMed]
- Gilchuk, I.; Gilchuk, P.; Sapparapu, G.; Lampley, R.; Singh, V.; Kose, N.; Blum, D.L.; Hughes, L.J.; Satheshkumar, P.S.; Townsend, M.B.; et al. Cross-Neutralizing and Protective Human Antibody Specificities to Poxvirus Infections. Cell 2016, 167, 684–694.e689. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Wu, L.; Sun, J.; Liu, D.; Han, P.; Gao, Y.; Zhang, Y.; Xu, Y.; Qu, X.; Wang, H.; et al. Two noncompeting human neutralizing antibodies targeting MPXV B6 show protective effects against orthopoxvirus infections. Nat Commun 2024, 15, 4660. [Google Scholar] [CrossRef] [PubMed]
- Noy-Porat, T.; Tamir, H.; Alcalay, R.; Rosenfeld, R.; Epstein, E.; Cherry, L.; Achdout, H.; Erez, N.; Politi, B.; Yahalom-Ronen, Y.; et al. Generation of recombinant mAbs to vaccinia virus displaying high affinity and potent neutralization. Microbiol Spectr 2023, 11, e0159823. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.E.; Frattaroli, P.; Vu, C.A.; Paniagua, L.; Mintz, J.; Bravo-Gonzalez, A.; Zamudio, P.; Barco, A.; Rampersad, A.; Lichtenberger, P., et al. Successful Outcome after Treatment with Cidofovir, Vaccinia, and Extended Course of Tecovirimat in a Newly-Diagnosed HIV Patient with Severe Mpox: A Case Report. Vaccines (Basel) 2023, 11. [CrossRef]
- Zone., O.V. Availabe online: https://viralzone.expasy.org/149?outline=all_by_species (accessed on 07/04/2025).
- Lansiaux, E.; Jain, N.; Laivacuma, S.; Reinis, A. The virology of human monkeypox virus (hMPXV): A brief overview. Virus research 2022, 322, 198932. [Google Scholar] [CrossRef] [PubMed]
- Harapan, H.; Ophinni, Y.; Megawati, D.; Frediansyah, A.; Mamada, S.S.; Salampe, M.; Bin Emran, T.; Winardi, W.; Fathima, R.; Sirinam, S. Monkeypox: a comprehensive review. Viruses 2022, 14, 2155. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Wang, C.; Chuai, X.; Chiu, S. Monkeypox virus: a re-emergent threat to humans. Virologica Sinica 2022, 37, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Moss, B. Membrane fusion during poxvirus entry. In Proceedings of Seminars in cell & developmental biology; pp. 89-96.
- Challberg, M.D.; Englund, P.T. Purification and properties of the deoxyribonucleic acid polymerase induced by vaccinia virus. Journal of Biological Chemistry 1979, 254, 7812–7819. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Leng, P.; Zhou, H. Global transmission of monkeypox virus—a potential threat under the COVID-19 pandemic. Frontiers in Immunology 2023, 14, 1174223. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Kumar, S.; Jain, S.; Mohanty, A.; Thapa, N.; Poudel, P.; Bhusal, K.; Al-Qaim, Z.H.; Barboza, J.J.; Padhi, B.K. The Global Monkeypox (Mpox) outbreak: a comprehensive review. Vaccines 2023, 11, 1093. [Google Scholar] [CrossRef] [PubMed]
- Haller, S.L.; Peng, C.; McFadden, G.; Rothenburg, S. Poxviruses and the evolution of host range and virulence. Infection, Genetics and Evolution 2014, 21, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Dsouza, L.; Pant, A.; Offei, S.; Priyamvada, L.; Pope, B.; Satheshkumar, P.S.; Wang, Z.; Yang, Z. Antiviral activities of two nucleos (t) ide analogs against vaccinia and mpox viruses in primary human fibroblasts. bioRxiv 2023. [CrossRef]
- Thakur, M.; Das, P.; Sobti, R.C.; Kaur, T. Human monkeypox: epidemiology, transmission, pathogenesis, immunology, diagnosis and therapeutics. Molecular and Cellular Biochemistry 2023, 478, 2097–2110. [Google Scholar] [CrossRef] [PubMed]
- Organization, W.H. WHO advisory committee on variola virus research: report of the twenty-third meeting, virtual meeting, 3-4 November 2021; World Health Organization: 2022.
- Dutt, M.; Kumar, A.; Rout, M.; Dehury, B.; Martinez, G.; Ndishimye, P.; Kelvin, A.A.; Kelvin, D.J. Drug repurposing for Mpox: Discovery of small molecules as potential inhibitors against DNA-dependent RNA polymerase using molecular modeling approach. Journal of Cellular Biochemistry 2023, 124, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Alkhalil, A.; Strand, S.; Mucker, E.; Huggins, J.W.; Jahrling, P.B.; Ibrahim, S.M. Inhibition of monkeypox virus replication by RNA interference. Virol J 2009, 6, 188. [Google Scholar] [CrossRef] [PubMed]
- Hishiki, T.; Morita, T.; Akazawa, D.; Ohashi, H.; Park, E.S.; Kataoka, M.; Mifune, J.; Shionoya, K.; Tsuchimoto, K.; Ojima, S.; et al. Identification of IMP Dehydrogenase as a Potential Target for Anti-Mpox Virus Agents. Microbiol Spectr 2023, 11, e0056623. [Google Scholar] [CrossRef] [PubMed]
- Alkhalil, A.; Strand, S.; Mucker, E.; Huggins, J.W.; Jahrling, P.B.; Ibrahim, S.M. Inhibition of Monkeypox virus replication by RNA interference. Virology journal 2009, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Pevear, D.C.; Davies, M.H.; Collett, M.S.; Bailey, T.; Rippen, S.; Barone, L.; Burns, C.; Rhodes, G.; Tohan, S. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus challenge. Journal of virology 2005, 79, 13139–13149. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.K.; Olson, V.A.; Karem, K.L.; Jordan, R.; Hruby, D.E.; Damon, I.K. In vitro efficacy of ST246 against smallpox and monkeypox. Antimicrobial agents and chemotherapy 2009, 53, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Chiem, K.; Nogales, A.; Lorenzo, M.; Morales Vasquez, D.; Xiang, Y.; Gupta, Y.K.; Blasco, R.; de la Torre, J.C.; Martínez-Sobrido, L. Identification of In Vitro Inhibitors of Monkeypox Replication. Microbiology Spectrum 2023, 11, e0474522. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.; Berryman, H.; Surani, Y.M.; Bewley, K.; Wand, M.E.; Sutton, J.M.; Tree, J.A. The antiviral activity of licensed therapeutics against Mpox clade Ib, in vitro; alternative options for the treatment of Mpox. bioRxiv 2025, 2025.2001. 2011.632516.
- Tiwari, H.; Sharma, S.; Dixit, A.; Kumar, R.; Fatima, K.; Hazarika, Z.; Ilyas, A.; Upadhyay, S.; Borkotoky, S. Unlocking the Secret Vault of Promising Drug Targets from Mpox Proteome-A Computational Approach. 2024.
- Akazawa, D.; Ohashi, H.; Hishiki, T.; Morita, T.; Iwanami, S.; Kim, K.S.; Jeong, Y.D.; Park, E.S.; Kataoka, M.; Shionoya, K.; et al. Potential Anti-Mpox Virus Activity of Atovaquone, Mefloquine, and Molnupiravir, and Their Potential Use as Treatments. J Infect Dis 2023, 228, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Suraweera, C.D.; Hinds, M.G.; Kvansakul, M. Poxviral strategies to overcome host cell apoptosis. Pathogens 2020, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Boys, I.N.; Johnson, A.G.; Quinlan, M.R.; Kranzusch, P.J.; Elde, N.C. Structural homology screens reveal host-derived poxvirus protein families impacting inflammasome activity. Cell reports 2023, 42. [CrossRef]
- Martínez-Fernández, D.E.; Fernández-Quezada, D.; Casillas-Muñoz, F.A.G.; Carrillo-Ballesteros, F.J.; Ortega-Prieto, A.M.; Jimenez-Guardeño, J.M.; Regla-Nava, J.A. Human Monkeypox: a comprehensive overview of epidemiology, pathogenesis, diagnosis, treatment, and prevention strategies. Pathogens 2023, 12, 947. [Google Scholar] [CrossRef] [PubMed]
- Albarnaz, J.D.; Kite, J.; Oliveira, M.; Li, H.; Di, Y.; Christensen, M.H.; Paulo, J.A.; Antrobus, R.; Gygi, S.P.; Schmidt, F.I. Quantitative proteomics defines mechanisms of antiviral defence and cell death during modified vaccinia Ankara infection. Nature Communications 2023, 14, 8134. [Google Scholar] [CrossRef] [PubMed]
- An, T.-Q.; Li, J.-N.; Su, C.-M.; Yoo, D. Molecular and cellular mechanisms for PRRSV pathogenesis and host response to infection. Virus research 2020, 286, 197980. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Liu, Y.; Dou, D.; Su, B. The unique immune evasion mechanisms of the mpox virus and their implication for developing new vaccines and immunotherapies. Virologica Sinica 2024. [CrossRef]
- Zandi, M.; Shafaati, M.; Hosseini, F. Mechanisms of immune evasion of monkeypox virus. Frontiers in microbiology 2023, 14, 1106247. [Google Scholar] [CrossRef] [PubMed]
- Hammarlund, E.; Dasgupta, A.; Pinilla, C.; Norori, P.; Früh, K.; Slifka, M.K. Monkeypox virus evades antiviral CD4+ and CD8+ T cell responses by suppressing cognate T cell activation. Proceedings of the National Academy of Sciences 2008, 105, 14567–14572. [Google Scholar] [CrossRef] [PubMed]
- Otieno, J.R.; Ruis, C.; Onoja, A.B.; Kuppalli, K.; Hoxha, A.; Nitsche, A.; Brinkmann, A.; Michel, J.; Mbala-Kingebeni, P.; Mukadi-Bamuleka, D. Global genomic surveillance of monkeypox virus. Nature Medicine 2024, 1-1. [CrossRef]
- Alakunle, E.; Kolawole, D.; Diaz-Canova, D.; Alele, F.; Adegboye, O.; Moens, U.; Okeke, M.I. A comprehensive review of monkeypox virus and mpox characteristics. Frontiers in Cellular and Infection Microbiology 2024, 14, 1360586. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Xing, H.; Wang, C.; Tang, M.; Wu, C.; Ye, F.; Yin, L.; Yang, Y.; Tan, W.; Shen, L. Mpox (formerly monkeypox): pathogenesis, prevention, and treatment. Signal Transduction and Targeted Therapy 2023, 8, 458. [Google Scholar] [CrossRef] [PubMed]
- Caetano-Pinto, P.; Nordell, P.; Nieskens, T.; Haughan, K.; Fenner, K.S.; Stahl, S.H. Amplifying the impact of kidney microphysiological systems: predicting renal clearance using mechanistic modelling based on reconstructed drug secretion. ALTEX-Alternatives to animal experimentation 2023, 40, 408–424. [Google Scholar] [CrossRef] [PubMed]
- Velioglu, A.; Erdogan, E.; Tigen, E.; Ozgen, Z.; Tuglular, S. Topical cidofovir-related acute kidney injury in a kidney transplant recipient. Clinical Transplantation 2022, 36, e14824. [Google Scholar] [CrossRef] [PubMed]
- Sankar, S.; Balakrishnan, P.; Yong, Y.K.; Raju, S.; Velu, V.; Shankar, E.M.; Larsson, M. Mpox Virus as a Global Public Health Emergency: A Scoping Review. Can J Infect Dis Med Microbiol 2025, 2025, 6683501. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Xu, A.; Guan, L.; Tang, Y.; Chai, G.; Feng, J.; Wu, Y.; Li, M.; Zhang, C.; Liu, X. A review of Mpox: Biological characteristics, epidemiology, clinical features, diagnosis, treatment, and prevention strategies. In Proceedings of Exploration; p. 20230112.
- ICMRA. Summary report of the International Coalition of Medicines Regulatory Authorities (ICMRA) workshop on mpox, October 2, 2024. Availabe online: https://www.icmra.info/drupal/news/mpox_news_announcement_25oct2024/mpox_ws_report_2oct2024.
- WHO. Clinical management of mpox (monkeypox). 2024.
- Islam, M.A.; Hemo, M.K.; Chopra, H.; Amin, M.R.; Bhattacharya, P.; Dhama, K. Old enemy with a New Face: Re-emerging Monkeypox Disease--An Update. Journal of Pure & Applied Microbiology 2022, 16. [CrossRef]
- Group, P.W. Tecovirimat for clade I MPXV infection in the Democratic Republic of Congo. New England Journal of Medicine 2025, 392, 1484–1496. [Google Scholar] [CrossRef] [PubMed]
- CTV. Study to Assess the Safety and Immunogenicity of TPOXX® When Administered Orally for 28 Days With JYNNEOS. Availabe online: https://ctv.veeva.com/study/study-to-assess-the-safety-and-pharmacokinetics-of-tpoxx-r-when-administered-orally-for-28-days.
- Witwit, H.; Cubitt, B.; Khafaji, R.; Castro, E.M.; Goicoechea, M.; Lorenzo, M.M.; Blasco, R.; Martinez-Sobrido, L.; de la Torre, J.C. Repurposing Drugs for Synergistic Combination Therapies to Counteract Monkeypox Virus Tecovirimat Resistance. Viruses 2025, 17, 92. [Google Scholar] [CrossRef] [PubMed]
- Ghazy, R.M.; Elrewany, E.; Gebreal, A.; ElMakhzangy, R.; Fadl, N.; Elbanna, E.H.; Tolba, M.M.; Hammad, E.M.; Youssef, N.; Abosheaishaa, H. Systematic review on the efficacy, effectiveness, safety, and immunogenicity of monkeypox vaccine. Vaccines 2023, 11, 1708. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Guruparan, D.; Karuppanan, K.; Kumar, K.S. Comprehensive Insights into Monkeypox (mpox): Recent Advances in Epidemiology, Diagnostic Approaches and Therapeutic Strategies. Pathogens 2024, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Vivancos, R.; Anderson, C.; Blomquist, P.; Balasegaram, S.; Bell, A.; Bishop, L.; Brown, C.S.; Chow, Y.; Edeghere, O.; Florence, I. Community transmission of monkeypox in the United Kingdom, April to May 2022. Eurosurveillance 2022, 27, 2200422. [Google Scholar] [CrossRef] [PubMed]
- Tambo, E.; Al-Nazawi, A.M. Combating the global spread of poverty-related Monkeypox outbreaks and beyond. Infectious Diseases of Poverty 2022, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, M.; Wu, P.; Wu, S.; Lee, T.-Y.; Bai, C. Application of computational biology and artificial intelligence in drug design. International journal of molecular sciences 2022, 23, 13568. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Singla, R.K. Bioinformatics Tools for Pharmaceutical Drug Product Development. Indo Global Journal of Pharmaceutical Sciences 2022, 12, 281–294. [Google Scholar] [CrossRef]
- Parvathaneni, V.; Kulkarni, N.S.; Muth, A.; Gupta, V. Drug repurposing: a promising tool to accelerate the drug discovery process. Drug discovery today 2019, 24, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Odhar, H.A. Computational Repurposing of FDA Approved Drugs Against Monkeypox Virus Cysteine Proteinase: A Molecular Docking and Dynamics Simulation Study. 2022.
- Rai, M.; Singh, A.V.; Paudel, N.; Kanase, A.; Falletta, E.; Kerkar, P.; Heyda, J.; Barghash, R.F.; Singh, S.P.; Soos, M. Herbal concoction unveiled: a computational analysis of phytochemicals’ pharmacokinetic and toxicological profiles using novel approach methodologies (NAMs). Current Research in Toxicology 2023, 5, 100118. [Google Scholar] [CrossRef] [PubMed]
- Karplus, M.; McCammon, J.A. Molecular dynamics simulations of biomolecules. Nature structural biology 2002, 9, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Shoichet, B.K.; McGovern, S.L.; Wei, B.; Irwin, J.J. Lead discovery using molecular docking. Current opinion in chemical biology 2002, 6, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Flores-Holguín, N.; Frau, J.; Glossman-Mitnik, D. Computational Study of the Chemical Reactivity and Bioactivity Rates of Marine Peptides Hemiasterlin and Its A and B Derivatives Used in the Cancer Treatment through Conceptual Density Functional Theory. Computational Molecular Bioscience 2019, 9, 95–107. [Google Scholar] [CrossRef]
- Kharwar, R.; Bhatt, M.; Patel, K.; Patel, S.; Daxini, N. A computational approach to identify natural putative inhibitors to combat monkeypox. In Nanotechnology and In Silico Tools, Elsevier: 2024; pp. 285-308.
- Aiman, S.; Alhamhoom, Y.; Ali, F.; Rahman, N.; Rastrelli, L.; Khan, A.; Farooq, Q.u.A.; Ahmed, A.; Khan, A.; Li, C. Multi-epitope chimeric vaccine design against emerging Monkeypox virus via reverse vaccinology techniques-a bioinformatics and immunoinformatics approach. Frontiers in immunology 2022, 13, 985450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L. Multi-epitope vaccines: a promising strategy against tumors and viral infections. Cellular & molecular immunology 2018, 15, 182–184. [Google Scholar] [CrossRef]
- Kleftogiannis, D.; Kalnis, P.; Bajic, V.B. Progress and challenges in bioinformatics approaches for enhancer identification. Briefings in Bioinformatics 2015, 17, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Pritam, M.; Banerjee, M.; Singh, A.K.; Singh, S.P. Genome based screening of epitope ensemble vaccine candidates against dreadful visceral leishmaniasis using immunoinformatics approach. Microbial pathogenesis 2019, 136, 103704. [Google Scholar] [CrossRef] [PubMed]
- Nnaemeka, E.J.; Oladokun, P.; Abubakar, I.; Afolabi, S. Leveraging AI and Deep Learning in Predictive Genomics for MPOX Virus Research using MATLAB.
- Sahu, A.; Gaur, M.; Mahanandia, N.C.; Subudhi, E.; Swain, R.P.; Subudhi, B.B. Identification of core therapeutic targets for Monkeypox virus and repurposing potential of drugs against them: An in silico approach. Computers in Biology and Medicine 2023, 161, 106971. [Google Scholar] [CrossRef] [PubMed]
- Arasu, M.V.; Vijayaragavan, P.; Purushothaman, S.; Rathi, M.; Al-Dhabi, N.A.; Gopalakrishnan, V.; Choi, K.C.; Ilavenil, S. Molecular docking of monkeypox (mpox) virus proteinase with FDA approved lead molecules. Journal of Infection and Public Health 2023, 16, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Alandijany, T.A.; El-Daly, M.M.; Tolah, A.M.; Bajrai, L.H.; Khateb, A.M.; Kumar, G.S.; Dubey, A.; Dwivedi, V.D.; Azhar, E.I. A multi-targeted computational drug discovery approach for repurposing tetracyclines against monkeypox virus. Scientific Reports 2023, 13, 14570. [Google Scholar] [CrossRef] [PubMed]
- Ashley, C.N.; Broni, E.; Wood, C.M.; Okuneye, T.; Ojukwu, M.-P.T.; Dong, Q.; Gallagher, C.; Miller III, W.A. Identifying potential monkeypox virus inhibitors: an in silico study targeting the A42R protein. Frontiers in Cellular and Infection Microbiology 2024, 14, 1351737. [Google Scholar] [CrossRef] [PubMed]
- CDC. Mpox in the United States and Around the World: Current Situation. Availabe online: https://www.cdc.gov/mpox/situation-summary/index.html (accessed on 20-03-2025).
- FDA, U. Key Facts About Vaccines to Prevent Mpox Disease. Availabe online: https://www.fda.gov/vaccines-blood-biologics/vaccines/key-facts-about-vaccines-prevent-mpox-disease (accessed on 20-03-2025).
- WHO. Overview of Mpox (monkeypox) vaccines and safety surveillance. Availabe online: https://www.who.int/news-room/questions-and-answers/item/mpox-vaccines.
- WHO. Mpox: Vaccines. Availabe online: https://www.who.int/news-room/questions-and-answers/item/mpox-vaccines (accessed on 2025).
- Garcia-Atutxa, I.; Mondragon-Teran, P.; Huerta-Saquero, A.; Villanueva-Flores, F. Advancements in monkeypox vaccines development: a critical review of emerging technologies. Front Immunol 2024, 15, 1456060. [Google Scholar] [CrossRef] [PubMed]
- Berhanu, A.; King, D.S.; Mosier, S.; Jordan, R.; Jones, K.F.; Hruby, D.E.; Grosenbach, D.W. Impact of ST-246® on ACAM2000™ smallpox vaccine reactogenicity, immunogenicity, and protective efficacy in immunodeficient mice. Vaccine 2010, 29, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.; Crump, R.; Foster, S.; Hartzler, H.; Hembrador, E.; Lanier, E.R.; Painter, G.; Schriewer, J.; Trost, L.C.; Buller, R.M. Co-administration of the broad-spectrum antiviral, brincidofovir (CMX001), with smallpox vaccine does not compromise vaccine protection in mice challenged with ectromelia virus. Antiviral research 2014, 111, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Atutxa, I.; Mondragon-Teran, P.; Huerta-Saquero, A.; Villanueva-Flores, F. Advancements in monkeypox vaccines development: a critical review of emerging technologies. Frontiers in Immunology 2024, 15, 1456060. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.B.; Mehta, A.; Zahid, M.J.; Candelario, K.; Shrestha, S.; Pokharel, P. Concerns over cardiovascular manifestations associated with monkeypox immunization: a literature review. Annals of Medicine and Surgery 2023, 85, 2797–2801. [Google Scholar] [CrossRef] [PubMed]
- CDC. Tecovirimat (TPOXX) for Treatment of Mpox. 2025.
- ARENA, C.T. Mpox: Three ongoing vaccine trials to watch. September 19, 2024.
- Parker, S.; D’Angelo, J.; Buller, R.M.; Smee, D.F.; Lantto, J.; Nielsen, H.; Jensen, A.; Prichard, M.; George, S.L. A human recombinant analogue to plasma-derived vaccinia immunoglobulin prophylactically and therapeutically protects against lethal orthopoxvirus challenge. Antiviral Research 2021, 195, 105179. [Google Scholar] [CrossRef] [PubMed]
- Keckler, M.S.; Salzer, J.S.; Patel, N.; Townsend, M.B.; Nakazawa, Y.J.; Doty, J.B.; Gallardo-Romero, N.F.; Satheshkumar, P.S.; Carroll, D.S.; Karem, K.L. IMVAMUNE® and ACAM2000® provide different protection against disease when administered postexposure in an intranasal monkeypox challenge prairie dog model. Vaccines 2020, 8, 396. [Google Scholar] [CrossRef] [PubMed]
- Stittelaar, K.J.; van Amerongen, G.; Kondova, I.; Kuiken, T.; van Lavieren, R.F.; Pistoor, F.H.; Niesters, H.G.; van Doornum, G.; van der Zeijst, B.A.; Mateo, L. Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. Journal of virology 2005, 79, 7845–7851. [Google Scholar] [CrossRef] [PubMed]
- Denzler, K.L.; Babas, T.; Rippeon, A.; Huynh, T.; Fukushima, N.; Rhodes, L.; Silvera, P.M.; Jacobs, B.L. Attenuated NYCBH vaccinia virus deleted for the E3L gene confers partial protection against lethal monkeypox virus disease in cynomolgus macaques. Vaccine 2011, 29, 9684–9690. [Google Scholar] [CrossRef] [PubMed]
- Earl, P.L.; Americo, J.L.; Wyatt, L.S.; Eller, L.A.; Whitbeck, J.C.; Cohen, G.H.; Eisenberg, R.J.; Hartmann, C.J.; Jackson, D.L.; Kulesh, D.A. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature 2004, 428, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Earl, P.L.; Americo, J.L.; Wyatt, L.S.; Eller, L.A.; Montefiori, D.C.; Byrum, R.A.; Piatak, M.; Lifson, J.D.; Amara, R.R.; Robinson, H.L., et al. Recombinant modified vaccinia virus Ankara provides durable protection against disease caused by an immunodeficiency virus as well as long-term immunity to an orthopoxvirus in a non-human primate. Virology 2007, 366 1, 84-97. [CrossRef]
- Earl, P.L.; Americo, J.L.; Wyatt, L.S.; Espenshade, O.; Bassler, J.; Gong, K.; Lin, S.; Peters, E.; Rhodes, L.; Spano, Y.E.; et al. Rapid protection in a monkeypox model by a single injection of a replication-deficient vaccinia virus. Proceedings of the National Academy of Sciences 2008, 105, 10889–10894. [Google Scholar] [CrossRef] [PubMed]
- Grosenbach, D.W.; Jordan, R.; King, D.S.; Berhanu, A.; Warren, T.K.; Kirkwood-Watts, D.L.; Tyavanagimatt, S.R.; Tan, Y.; Wilson, R.L.; Jones, K.F.; et al. Immune responses to the smallpox vaccine given in combination with ST-246, a small-molecule inhibitor of poxvirus dissemination. Vaccine 2007, 26, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Hatch, G.J.; Graham, V.A.; Bewley, K.R.; Tree, J.; Dennis, M.J.; Taylor, I.; Funnell, S.G.P.; Bate, S.R.; Steeds, K.; Tipton, T.; et al. Assessment of the Protective Effect of Imvamune and Acam2000 Vaccines against Aerosolized Monkeypox Virus in Cynomolgus Macaques. Journal of Virology 2013, 87, 7805–7815. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, I.; Ami, Y.; Suzaki, Y.; Nagata, N.; Fukushi, S.; Ogata, M.; Morikawa, S.; Hasegawa, H.; Mizuguchi, M.; Kurane, I., et al. A Single Vaccination of Nonhuman Primates with Highly Attenuated Smallpox Vaccine, LC16m8, Provides Long-term Protection against Monkeypox. Japanese journal of infectious diseases 2017, 70 4, 408-415. [CrossRef]
- Keckler, M.S.; Carroll, D.S.; Gallardo-Romero, N.F.; Lash, R.R.; Salzer, J.S.; Weiss, S.; Patel, N.R.; Clemmons, C.J.; Smith, S.K.; Hutson, C.L.; et al. Establishment of the Black-Tailed Prairie Dog (Cynomys ludovicianus) as a Novel Animal Model for Comparing Smallpox Vaccines Administered Preexposure in both High- and Low-Dose Monkeypox Virus Challenges. Journal of Virology 2011, 85, 7683–7698. [Google Scholar] [CrossRef] [PubMed]
- Keckler, M.S.; Salzer, J.S.; Patel, N.R.; Townsend, M.B.; Nakazawa, Y.J.; Doty, J.B.; Gallardo-Romero, N.F.; Satheshkumar, P.S.; Carroll, D.S.; Karem, K.L.; et al. IMVAMUNE® and ACAM2000® Provide Different Protection against Disease When Administered Postexposure in an Intranasal Monkeypox Challenge Prairie Dog Model. Vaccines 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Phelps, A.L.; Gates, A.J.; Eastaugh, L.S.; Hillier, M.; Ulaeto, D. Comparative Efficacy of Intramuscular and Scarification Routes of Administration of Live Smallpox Vaccine in a Murine Challenge Model. Vaccine 2017, 35, 3889–3896. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.T.; Berhanu, A.; Bigger, C.B.; Prigge, J.T.; Silvera, P.M.; Grosenbach, D.W.; Hruby, D.E. Co-administration of tecovirimat and ACAM2000™ in non-human primates: Effect of tecovirimat treatment on ACAM2000 immunogenicity and efficacy versus lethal monkeypox virus challenge. Vaccine 2019, 38, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Huang, D.; Wei, H.; Wang, R.C.; Chen, C.Y.; Shen, L.; Zhang, W.; Jin, J.; Chen, Z.W. Expansion, Reexpansion, and Recall-Like Expansion of Vγ2Vδ2 T Cells in Smallpox Vaccination and Monkeypox Virus Infection. Journal of Virology 2009, 83, 11959–11965. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Atutxa, I.; Mondragón-Terán, P.; Huerta-Saquero, A.; Villanueva-Flores, F. Advancements in monkeypox vaccines development: a critical review of emerging technologies. Frontiers in Immunology 2024, 15. [CrossRef]
- Trials.gov, C. Mpox \(Monkeypox\), Phase: Early Phase 1, 1, 2, 3, 4, Interventional, Observational studies Availabe online: https://clinicaltrials.gov/search?cond=Mpox%20%5C(Monkeypox%5C)&aggFilters=phase:0%201%202%203%204,studyType:obs%20int (accessed on 28-01-2025).
- NIH. The antiviral tecovirimat is safe but did not improve clade I mpox resolution in Democratic Republic of the Congo. Availabe online: https://www.nih.gov/news-events/news-releases/antiviral-tecovirimat-safe-did-not-improve-clade-i-mpox-resolution-democratic-republic-congo (accessed on 20-03-2025).
- Li, P.; Al-Tawfiq, J.A.; Memish, Z.A.; Pan, Q. Preventing drug resistance: combination treatment for mpox. The Lancet 2023, 402, 1750–1751. [Google Scholar] [CrossRef] [PubMed]
- Grabenstein, J.D.; Hacker, A. Vaccines against mpox: MVA-BN and LC16m8. Expert Rev Vaccines 2024, 23, 796–811. [Google Scholar] [CrossRef] [PubMed]
- Cambaza, E.M. A Review of the Molecular Understanding of the Mpox Virus (MPXV): Genomics, Immune Evasion, and Therapeutic Targets. Zoonotic Diseases 2025, 5, 3. [Google Scholar] [CrossRef]
- Lu, J.; Xing, H.; Wang, C.; Tang, M.; Wu, C.; Ye, F.; Yin, L.; Yang, Y.; Tan, W.; Shen, L. Mpox (formerly monkeypox): Pathogenesis, prevention and treatment. Signal Transduction and Targeted Therapy 2023, 8, 458. [Google Scholar] [CrossRef] [PubMed]
- Azari, P.P.; Rukerd, M.R.Z.; Charostad, J.; Bashash, D.; Farsiu, N.; Behzadi, S.; Khoshnazar, S.M.; Heydari, S.; Nakhaie, M. Monkeypox (Mpox) vs. Innate immune responses: Insights into evasion mechanisms and potential therapeutic strategies. Cytokine 2024, 183, 156751. [Google Scholar] [CrossRef] [PubMed]
- WHO. MPOX. Availabe online: https://www.who.int/news-room/fact-sheets/detail/mpox (accessed on 25-01-2025).
- Srivastava, S.; Laxmi, *!!! REPLACE !!!*; Sharma, K.; Sridhar, S.B.; Talath, S.; Shareef, J.; Mehta, R.; Satapathy, P.; Sah, R. Clade Ib: a new emerging threat in the Mpox outbreak. Frontiers in Pharmacology 2024, 15, 1504154. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.; Halappa, N.R.; Fnu, K.; Reddy, S.M.; Han, G.; Yogesh, R.; Fareed, M.U.; Defne, Ş.; Danyal, B.; Palash, R. Unraveling Monkeypox: An Emerging Threat in Global Health. Cureus 2023, 15. [CrossRef]
- Kumar, D.; Malviya, R.; Srivastava, S.; Sridhar, S.B.; Talath, S.; Shareef, J.; Prajapati, B.G. Personalized Immunization against Mpox Clades I and Ib: strategies to combat the emerging epidemic. Infectious Medicine 2025, 100166. [CrossRef]
- Jeyakumar, T.; Younus, S.; Zhang, M.; Clare, M.; Charow, R.; Karsan, I.; Dhalla, A.; Al-Mouaswas, D.; Scandiffio, J.; Aling, J. Preparing for an artificial intelligence–enabled future: patient perspectives on engagement and health care professional training for adopting artificial intelligence technologies in health care settings. JMIR AI 2023, 2, e40973. [Google Scholar] [CrossRef] [PubMed]
- Najjar, R. Redefining radiology: a review of artificial intelligence integration in medical imaging. Diagnostics 2023, 13, 2760. [Google Scholar] [CrossRef] [PubMed]
- Alowais, S.A.; Alghamdi, S.S.; Alsuhebany, N.; Alqahtani, T.; Alshaya, A.I.; Almohareb, S.N.; Aldairem, A.; Alrashed, M.; Bin Saleh, K.; Badreldin, H.A. Revolutionizing healthcare: the role of artificial intelligence in clinical practice. BMC medical education 2023, 23, 689. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.B.; Wei, W.Q.; Weeraratne, D.; Frisse, M.E.; Misulis, K.; Rhee, K.; Zhao, J.; Snowdon, J.L. Precision medicine, AI, and the future of personalized health care. Clinical and translational science 2021, 14, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Bohr, A.; Memarzadeh, K. Artificial intelligence in healthcare; Academic Press: 2020.
- Thieme, A.H.; Zheng, Y.; Machiraju, G.; Sadee, C.; Mittermaier, M.; Gertler, M.; Salinas, J.L.; Srinivasan, K.; Gyawali, P.; Carrillo-Perez, F. A deep-learning algorithm to classify skin lesions from mpox virus infection. Nature medicine 2023, 29, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, E.; Bayat, Z.; Farrokhi, M.; Karimian, S.; Zahedpasha, R.; Sabzehie, H.; Ramezani Poor, S.; Jafari Khouzani, P.; Aminpour, S.; Karami, M.; et al. Monkeypox: A Comprehensive Review of Virology, Epidemiology, Transmission, Diagnosis, Prevention, Treatment, and Artificial Intelligence Applications. Arch Acad Emerg Med 2024, 12, e70. [Google Scholar] [CrossRef] [PubMed]
- Nyame, J.; Punniyakotti, S.; Khera, K.; Pal, R.S.; Varadarajan, N.; Sharma, P. Challenges in the treatment and prevention of monkeypox infection; A comprehensive review. Acta Trop 2023, 245, 106960. [Google Scholar] [CrossRef] [PubMed]
| Vaccine Name | Description | Dosing Regimen | Approved Countries | Reported Side Effects | Refs |
|---|---|---|---|---|---|
| ACAM2000 | Live, replicating vaccinia virus (NYCBH strain) | Single percutaneous dose (2.5 μL) | USA | Myocarditis, pericarditis, encephalitis, progressive vaccinia; contraindicated in immunocompromised individuals and those with certain skin conditions | [130,131] |
| JYNNEOS (also known as Imvanex® or Imvamune®) | Modified Vaccinia Ankara (MVA), non-replicating strain | Two subcutaneous doses (0.5 mL each), 4 weeks apart | USA, Canada, European Union | Mild to moderate local reactions (pain, redness, swelling); fewer systemic side effects; considered safe for immunocompromised individuals | [130,132] |
| LC16m8 (also known as LC16-KMB®) | Live attenuated, minimally replicating vaccinia virus | Single percutaneous dose (2 μL) | Japan | Generally well-tolerated; specific side effects not extensively reported; ongoing studies to assess safety profile | [131] |
| Orthopox Vaccine | Recombinant MVA-based vaccine (live vaccinia VACdelta6-based culture vaccine) | Dosing regimen not specified | Russian Federation (approved in November 2022) | Limited available data on side effects; further studies are required to establish safety and efficacy | [131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





