Submitted:
14 July 2025
Posted:
15 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
- Detailed characterization of the arrhythmogenic substrate
- Optimal planning of access strategies
- Real-time guidance during the procedure
- Assessment of ablation efficacy
2. Pre-Procedural Imaging Assessment
3. Intraprocedural Imaging: Focus on Intracardiac Echocardiography
3.1. Anatomical Delineation and Catheter Navigation
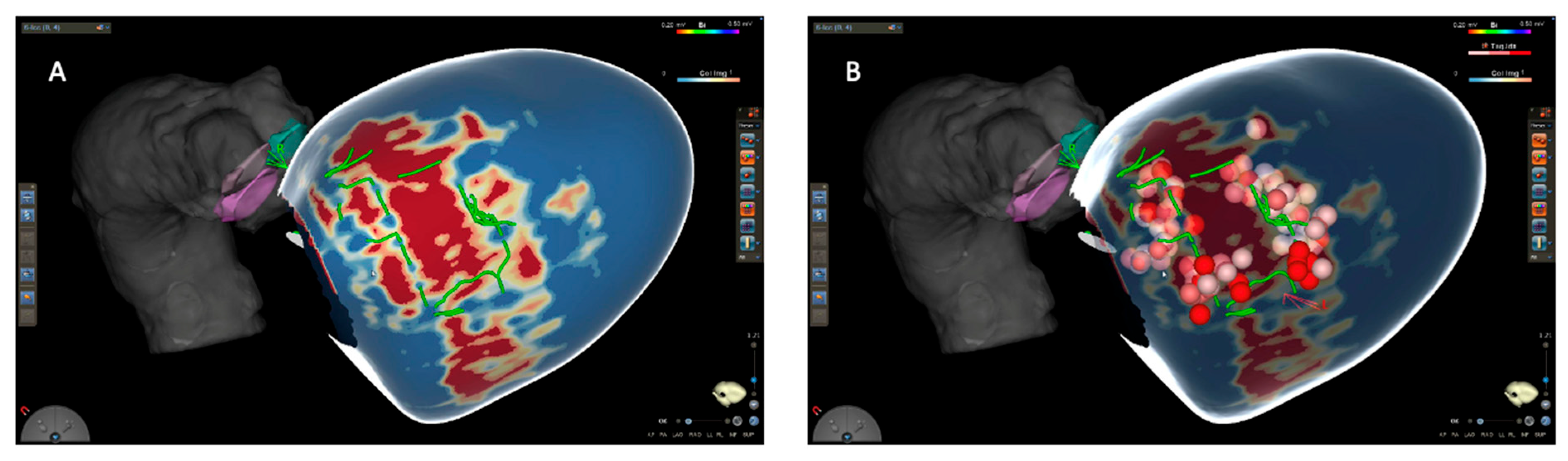
3.2. Substrate Characterization
3.3. Catheter-Tissue Contact Assessment
3.4. Complication Preventing and Monitoring
3.5. Reduction in Fluoroscopy Exposure
3.6. Procedural Outcomes and Clinical Impact
3.7. Future Perspectives
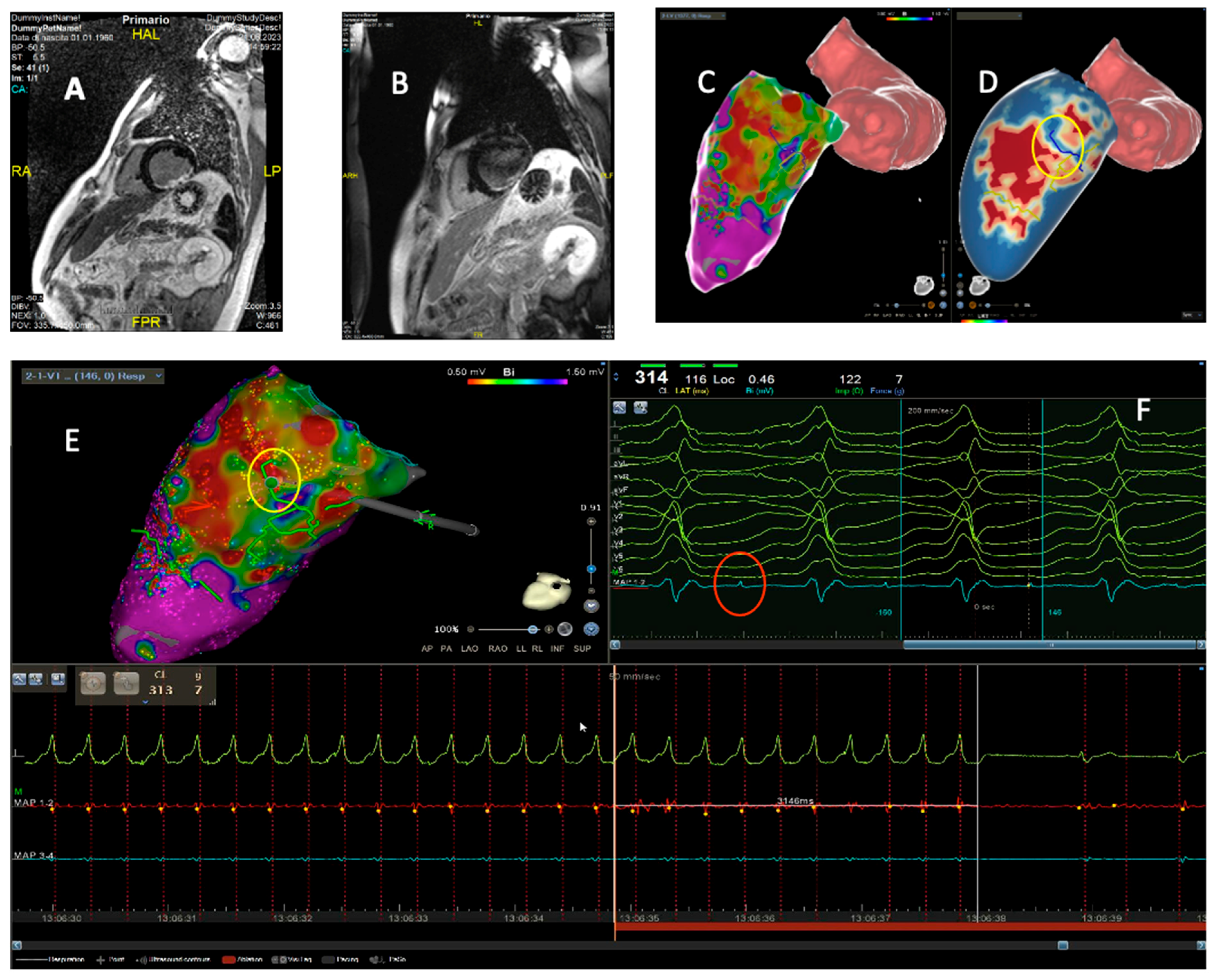
4. Intraprocedural Imaging: Focus on CT and CMR
5. Limitations
6. Conclusions
Conflicts of Interest
References
- Zeppenfeld, K.; Tfelt-Hansen, J.; De Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef]
- Della Bella, P.; Baratto, F.; Vergara, P.; Bertocchi, P.; Santamaria, M.; Notarstefano, P.; et al. Does Timing of Ventricular Tachycardia Ablation Affect Prognosis in Patients With an Implantable Cardioverter Defibrillator? Results From the Multicenter Randomized PARTITA Trial. Circulation. 2022, 145, 1829–1838. [Google Scholar] [CrossRef] [PubMed]
- Cronin, E.M.; Bogun, F.M.; Maury, P.; Peichl, P.; Chen, M.; Namboodiri, N.; et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. EP Eur. 2019, 21, 1143–1144. [Google Scholar]
- Arenal, Á.; Ávila, P.; Jiménez-Candil, J.; Tercedor, L.; Calvo, D.; Arribas, F.; et al. Substrate Ablation vs Antiarrhythmic Drug Therapy for Symptomatic Ventricular Tachycardia. J Am Coll Cardiol. 2022, 79, 1441–1453. [Google Scholar] [CrossRef] [PubMed]
- Tung, R.; Xue, Y.; Chen, M.; Jiang, C.; Shatz, D.Y.; Besser, S.A.; et al. First-Line Catheter Ablation of Monomorphic Ventricular Tachycardia in Cardiomyopathy Concurrent With Defibrillator Implantation: The PAUSE-SCD Randomized Trial. Circulation. 2022, 145, 1839–1849. [Google Scholar] [CrossRef]
- Sapp, J.L.; Tang, A.S.L.; Parkash, R.; Stevenson, W.G.; Healey, J.S.; Gula, L.J.; et al. Catheter Ablation or Antiarrhythmic Drugs for Ventricular Tachycardia. N Engl J Med. 2024, NEJMoa2409501. [Google Scholar] [CrossRef]
- Berruezo, A.; Penela, D.; Jáuregui, B.; Soto-Iglesias, D. The role of imaging in catheter ablation of ventricular arrhythmias. Pacing Clin Electrophysiol. 2021, 44, 1115–1125. [Google Scholar] [CrossRef]
- Ujeyl, A.; Inada, K.; Hillmann, K.; Wohlmuth, P.; Kato, M.; Tedrow, U.; et al. Right Heart Function Prediction of Outcome in Heart Failure Patients After Catheter Ablation for Recurrent Ventricular Tachycardia. JACC Heart Fail. 2013, 1, 281–289. [Google Scholar] [CrossRef]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef]
- Collier, P.; Phelan, D.; Klein, A. A Test in Context: Myocardial Strain Measured by Speckle-Tracking Echocardiography. J Am Coll Cardiol. 2017, 69, 1043–1056. [Google Scholar] [CrossRef]
- Trivedi, S.J.; Campbell, T.; Stefani, L.D.; Thomas, L.; Kumar, S. Strain by speckle tracking echocardiography correlates with electroanatomic scar location and burden in ischaemic cardiomyopathy. Eur Heart J - Cardiovasc Imaging. 2021, 22, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Joyce, E.; Ninaber, M.K.; Katsanos, S.; Debonnaire, P.; Kamperidis, V.; Bax, J.J.; et al. Subclinical left ventricular dysfunction by echocardiographic speckle-tracking strain analysis relates to outcome in sarcoidosis. Eur J Heart Fail. 2015, 17, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Taha, K.; Kirkels, F.P.; Teske, A.J.; Asselbergs, F.W.; van Tintelen, J.P.; Doevendans, P.A.; et al. Echocardiographic Deformation Imaging for Early Detection of Genetic Cardiomyopathies: JACC Review Topic of the Week. J Am Coll Cardiol. 2022, 79, 594–608. [Google Scholar] [CrossRef] [PubMed]
- Kirkels, F.P.; Lie, Ø.H.; Cramer, M.J.; Chivulescu, M.; Rootwelt-Norberg, C.; Asselbergs, F.W.; et al. Right Ventricular Functional Abnormalities in Arrhythmogenic Cardiomyopathy: Association With Life-Threatening Ventricular Arrhythmias. JACC Cardiovasc Imaging. 2021, 14, 900–910. [Google Scholar] [CrossRef]
- Berte, B.; Sacher, F.; Venlet, J.; Andreu, D.; Mahida, S.; Aldhoon, B.; et al. VT Recurrence After Ablation: Incomplete Ablation or Disease Progression? A Multicentric European Study. J Cardiovasc Electrophysiol. 2016, 27, 80–87. [Google Scholar] [CrossRef]
- Shen, L.; Liu, S.; Zhang, Z.; Xiong, Y.; Lai, Z.; Hu, F.; et al. Catheter ablation of ventricular tachycardia in patients with arrhythmogenic right ventricular cardiomyopathy and biventricular involvement. Europace 2024, 26, euae059. [Google Scholar] [CrossRef]
- John, L.A.; John, I.I.; Tedford, R.J.; Gregoski, M.J.; Gold, M.R.; Field, M.E.; et al. Substrate Imaging Before Catheter Ablation of Ventricular Tachycardia. JACC Clin Electrophysiol. 2023, 9, 1684–1693. [Google Scholar] [CrossRef]
- Chery, G.; Khoshknab, M.; Nazarian, S. Imaging to Facilitate Ventricular Tachycardia Ablation. JACC Clin Electrophysiol. 2024, 10, 2277–2292. [Google Scholar] [CrossRef]
- Fernández-Armenta, J.; Berruezo, A.; Andreu, D.; Camara, O.; Silva, E.; Serra, L.; et al. Three-Dimensional Architecture of Scar and Conducting Channels Based on High Resolution ce-CMR: Insights for Ventricular Tachycardia Ablation. Circ Arrhythm Electrophysiol. 2013, 6, 528–537. [Google Scholar] [CrossRef]
- Estner, H.L.; Zviman, M.M.; Herzka, D.; Miller, F.; Castro, V.; Nazarian, S.; et al. The critical isthmus sites of ischemic ventricular tachycardia are in zones of tissue heterogeneity, visualized by magnetic resonance imaging. Heart Rhythm. 2011, 8, 1942–1949. [Google Scholar] [CrossRef]
- Piers, S.R.D.; Tao, Q.; De Riva Silva, M.; Siebelink, H.M.; Schalij, M.J.; Van Der Geest, R.J.; et al. CMR–Based Identification of Critical Isthmus Sites of Ischemic and Nonischemic Ventricular Tachycardia. JACC Cardiovasc Imaging. 2014, 7, 774–784. [Google Scholar] [CrossRef]
- Padmanabhan, D.; Kella, D.K.; Deshmukh, A.J.; Mulpuru, S.K.; Mehta, R.A.; Dalzell, C.M.; et al. Safety of thoracic magnetic resonance imaging for patients with pacemakers and defibrillators. Heart Rhythm. 2019, 16, 1645–1651. [Google Scholar] [CrossRef]
- Russo, R.J.; Costa, H.S.; Silva, P.D.; Anderson, J.L.; Arshad, A.; Biederman, R.W.W.; et al. Assessing the Risks Associated with MRI in Patients with a Pacemaker or Defibrillator. N Engl J Med. 2017, 376, 755–764. [Google Scholar] [CrossRef]
- Schwitter, J.; Gold, M.R.; Al Fagih, A.; Lee, S.; Peterson, M.; Ciuffo, A.; et al. Image Quality of Cardiac Magnetic Resonance Imaging in Patients With an Implantable Cardioverter Defibrillator System Designed for the Magnetic Resonance Imaging Environment. Circ Cardiovasc Imaging. 2016, 9, e004025. [Google Scholar] [CrossRef]
- Mesubi, O.; Ahmad, G.; Jeudy, J.; Jimenez, A.; Kuk, R.; Saliaris, A.; et al. Impact of ICD artifact burden on late gadolinium enhancement cardiac MR imaging in patients undergoing ventricular tachycardia ablation. Pacing Clin Electrophysiol PACE. 2014, 37, 1274–1283. [Google Scholar] [CrossRef]
- Sasaki, T.; Hansford, R.; Zviman, M.M.; Kolandaivelu, A.; Bluemke, D.A.; Berger, R.D.; et al. Quantitative Assessment of Artifacts on Cardiac Magnetic Resonance Imaging of Patients With Pacemakers and Implantable Cardioverter-Defibrillators. Circ Cardiovasc Imaging. 2011, 4, 662–670. [Google Scholar] [CrossRef]
- Rashid, S.; Rapacchi, S.; Vaseghi, M.; Tung, R.; Shivkumar, K.; Finn, J.P.; et al. Improved Late Gadolinium Enhancement MR Imaging for Patients with Implanted Cardiac Devices. Radiology [Internet]. /: 1 [cited 2025 Mar 2]; Available from: https, 2025. [Google Scholar]
- Roca-Luque, I.; Van Breukelen, A.; Alarcon, F.; Garre, P.; Tolosana, J.M.; Borras, R.; et al. Ventricular scar channel entrances identified by new wideband cardiac magnetic resonance sequence to guide ventricular tachycardia ablation in patients with cardiac defibrillators. EP Eur. 2020, 22, 598–606. [Google Scholar] [CrossRef]
- Bhuva, A.N.; Kellman, P.; Graham, A.; Ramlall, M.; Boubertakh, R.; Feuchter, P.; et al. Clinical impact of cardiovascular magnetic resonance with optimized myocardial scar detection in patients with cardiac implantable devices. Int J Cardiol. 2019, 279, 72–78. [Google Scholar] [CrossRef]
- Hilbert, S.; Weber, A.; Nehrke, K.; Börnert, P.; Schnackenburg, B.; Oebel, S.; et al. Artefact-free late gadolinium enhancement imaging in patients with implanted cardiac devices using a modified broadband sequence: current strategies and results from a real-world patient cohort. Eur Eur Pacing Arrhythm Card Electrophysiol J Work Groups Card Pacing Arrhythm Card Cell Electrophysiol Eur Soc Cardiol. 2018, 20, 801–807. [Google Scholar] [CrossRef]
- Do, D.H.; Eyvazian, V.; Bayoneta, A.J.; Hu, P.; Finn, J.P.; Bradfield, J.S.; et al. Cardiac magnetic resonance imaging using wideband sequences in patients with nonconditional cardiac implanted electronic devices. Heart Rhythm. 2018, 15, 218–225. [Google Scholar] [CrossRef]
- Patel, H.N.; Wang, S.; Rao, S.; Singh, A.; Landeras, L.; Besser, S.A.; et al. Impact of wideband cardiac magnetic resonance on diagnosis, decision-making and outcomes in patients with implantable cardioverter defibrillators. Eur Heart J - Cardiovasc Imaging. 2023, 24, 181–189. [Google Scholar] [CrossRef]
- Zghaib, T.; Ghasabeh, M.A.; Assis, F.R.; Chrispin, J.; Keramati, A.; Misra, S.; et al. Regional Strain by Cardiac Magnetic Resonance Imaging Improves Detection of Right Ventricular Scar Compared With Late Gadolinium Enhancement on a Multimodality Scar Evaluation in Patients With Arrhythmogenic Right Ventricular Cardiomyopathy. Circ Cardiovasc Imaging. 2018, 11, e007546. [Google Scholar] [CrossRef]
- Santangeli, P.; Muser, D.; Zado, E.S.; Magnani, S.; Khetpal, S.; Hutchinson, M.D.; et al. Acute Hemodynamic Decompensation During Catheter Ablation of Scar-Related Ventricular Tachycardia: Incidence, Predictors, and Impact on Mortality. Circ Arrhythm Electrophysiol. 2015, 8, 68–75. [Google Scholar] [CrossRef]
- Pontone, G.; Rossi, A.; Guglielmo, M.; Dweck, M.R.; Gaemperli, O.; Nieman, K.; et al. Clinical applications of cardiac computed tomography: a consensus paper of the European Association of Cardiovascular Imaging—part I. Eur Heart J - Cardiovasc Imaging. 2022, 23, 299–314. [Google Scholar] [CrossRef]
- Pontone, G.; Rossi, A.; Guglielmo, M.; Dweck, M.R.; Gaemperli, O.; Nieman, K.; et al. Clinical applications of cardiac computed tomography: a consensus paper of the European Association of Cardiovascular Imaging—part II. Eur Heart J - Cardiovasc Imaging. 2022, 23, e136–61. [Google Scholar] [CrossRef]
- Sasaki, T.; Calkins, H.; Miller, C.F.; Zviman, M.M.; Zipunnikov, V.; Arai, T.; et al. New insight into scar-related ventricular tachycardia circuits in ischemic cardiomyopathy: Fat deposition after myocardial infarction on computed tomography--A pilot study. Heart Rhythm. 2015, 12, 1508–1518. [Google Scholar] [CrossRef]
- Tian, J.; Jeudy, J.; Smith, M.F.; Jimenez, A.; Yin, X.; Bruce, P.A.; et al. Three-Dimensional Contrast-Enhanced Multidetector CT for Anatomic, Dynamic, and Perfusion Characterization of Abnormal Myocardium To Guide Ventricular Tachycardia Ablations. Circ Arrhythm Electrophysiol. 2010, 3, 496–504. [Google Scholar] [CrossRef]
- Esposito, A.; Palmisano, A.; Antunes, S.; Maccabelli, G.; Colantoni, C.; Rancoita, P.M.V.; et al. Cardiac CT With Delayed Enhancement in the Characterization of Ventricular Tachycardia Structural Substrate. JACC Cardiovasc Imaging. 2016, 9, 822–832. [Google Scholar] [CrossRef]
- Komatsu, Y.; Cochet, H.; Jadidi, A.; Sacher, F.; Shah, A.; Derval, N.; et al. Regional Myocardial Wall Thinning at Multidetector Computed Tomography Correlates to Arrhythmogenic Substrate in Postinfarction Ventricular Tachycardia: Assessment of Structural and Electrical Substrate. Circ Arrhythm Electrophysiol. 2013, 6, 342–350. [Google Scholar] [CrossRef]
- Baroldi, G.; Silver, M.D.; De Maria, R.; Parodi, O.; Pellegrini, A. Lipomatous metaplasia in left ventricular scar. Can J Cardiol. 1997, 13, 65–71. [Google Scholar]
- Su, L.; Siegel, J.E.; Fishbein, M.C. Adipose tissue in myocardial infarction. Cardiovasc Pathol Off J Soc Cardiovasc Pathol. 2004, 13, 98–102. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Kitagawa, K.; Chino, S.; Ishida, M.; Matsuoka, K.; Tanigawa, T.; et al. Adipose tissue detected by multislice computed tomography in patients after myocardial infarction. JACC Cardiovasc Imaging. 2009, 2, 548–555. [Google Scholar] [CrossRef]
- Schmitt, M.; Samani, N.; McCann, G. Images in cardiovascular medicine. Lipomatous metaplasia in ischemic cardiomyopathy: a common but unappreciated entity. Circulation. 2007, 116, e5–e6. [Google Scholar]
- Xu, L.; Khoshknab, M.; Berger, R.D.; Chrispin, J.; Dixit, S.; Santangeli, P.; et al. Lipomatous Metaplasia Enables Ventricular Tachycardia by Reducing Current Loss Within the Protected Corridor. JACC Clin Electrophysiol. 2022, 8, 1274–1285. [Google Scholar] [CrossRef]
- Xu, L.; Zahid, S.; Khoshknab, M.; Moss, J.; Berger, R.D.; Chrispin, J.; et al. Lipomatous Metaplasia Facilitates Slow Conduction in Critical Ventricular Tachycardia Corridors Within Postinfarct Myocardium. JACC Clin Electrophysiol. 2023, 9, 1235–1245. [Google Scholar] [CrossRef]
- Tung, R.; Bauer, B.; Schelbert, H.; Lynch, J.P.; Auerbach, M.; Gupta, P.; et al. Incidence of abnormal positron emission tomography in patients with unexplained cardiomyopathy and ventricular arrhythmias: The potential role of occult inflammation in arrhythmogenesis. Heart Rhythm. 2015, 12, 2488–2498. [Google Scholar] [CrossRef]
- Sharma, R.; Kouranos, V.; Cooper, L.T.; Metra, M.; Ristic, A.; Heidecker, B.; et al. Management of cardiac sarcoidosis: A clinical consensus statement of the Heart Failure Association, the European Association of Cardiovascular Imaging, the ESC Working Group on Myocardial & Pericardial Diseases, and the European Heart Rhythm Association of the ESC. Eur Heart J. 2024, 45, 2697–2726. [Google Scholar]
- Tuominen, H.; Haarala, A.; Tikkakoski, A.; Kähönen, M.; Nikus, K.; Sipilä, K. FDG-PET in possible cardiac sarcoidosis: Right ventricular uptake and high total cardiac metabolic activity predict cardiovascular events. J Nucl Cardiol. 2021, 28, 199–205. [Google Scholar] [CrossRef]
- Tessier, R.; Marteau, L.; Vivien, M.; Guyomarch, B.; Thollet, A.; Fellah, I.; et al. 18F-Fluorodeoxyglucose Positron Emission Tomography for the Detection of Myocardial Inflammation in Arrhythmogenic Left Ventricular Cardiomyopathy. Circ Cardiovasc Imaging. 2022, 15, e014065. [Google Scholar] [CrossRef]
- Miller, B.; Vunnam, R.; Mesubi, O.; Smith, M.F.; Chen, W.; Mahat, J.B.; et al. Metabolic heterogeneous zone assessed by18 FDG-PET is predictive of postablation mortality in patients with ventricular tachycardia. J Cardiovasc Electrophysiol. 2021, 32, 2238–2245. [Google Scholar] [CrossRef]
- Kanawati, J.; De Silva, K.; Bhaskaran, A.; Turnbull, S.; Zhou, J.; Kotake, Y.; et al. Intracardiac echocardiography techniques to identify ventricular arrhythmia substrate. Heart Rhythm O2. 2022, 3, 602–612. [Google Scholar] [CrossRef]
- Muser, D.; Lavalle, C.; Guarracini, F.; Sassone, B.; Conte, E.; Magnani, S.; et al. Role of cardiac imaging in patients undergoing catheter ablation of ventricular tachycardia. J Cardiovasc Med. 2021, 22, 727–737. [Google Scholar] [CrossRef]
- Enriquez, A.; Saenz, L.C.; Rosso, R.; Silvestry, F.E.; Callans, D.; Marchlinski, F.E.; et al. Use of Intracardiac Echocardiography in Interventional Cardiology: Working With the Anatomy Rather Than Fighting It. Circulation. 2018, 137, 2278–2294. [Google Scholar] [CrossRef]
- Marrouche, N.F.; Martin, D.O.; Wazni, O.; Gillinov, A.M.; Klein, A.; Bhargava, M.; et al. Phased-Array Intracardiac Echocardiography Monitoring During Pulmonary Vein Isolation in Patients With Atrial Fibrillation: Impact on Outcome and Complications. Circulation. 2003, 107, 2710–2716. [Google Scholar] [CrossRef]
- Madhavan, M.; Asirvatham, S.J. The Fourth Dimension: Endocavitary Ventricular Tachycardia. Circ Arrhythm Electrophysiol. 2010, 3, 302–304. [Google Scholar] [CrossRef]
- De Sensi, F.; Addonisio, L.; Cresti, A.; Limbruno, U. Anatomical reconstruction of right ventricular structures with intracardiac echocardiography during ablation of premature contractions from moderator band. Indian Pacing Electrophysiol J. 2024, 24, 155–157. [Google Scholar] [CrossRef]
- Sadek, M.M.; Benhayon, D.; Sureddi, R.; Chik, W.; Santangeli, P.; Supple, G.E.; et al. Idiopathic ventricular arrhythmias originating from the moderator band: Electrocardiographic characteristics and treatment by catheter ablation. Heart Rhythm. 2015, 12, 67–75. [Google Scholar] [CrossRef]
- Kautzner, J.; Peichl, P. Papillary Muscle Ventricular Tachycardia or Ectopy: Diagnostics, Catheter Ablation and the Role of Intracardiac Echocardiography. Arrhythmia Electrophysiol Rev. 2019, 8, 65–69. [Google Scholar] [CrossRef]
- Proietti, R.; Rivera, S.; Dussault, C.; Essebag, V.; Bernier, M.L.; Ayala-Paredes, F.; et al. Intracardiac echo-facilitated 3D electroanatomical mapping of ventricular arrhythmias from the papillary muscles: assessing the ‘fourth dimension’ during ablation.
- Bunch, T.J.; Weiss, J.P.; Crandall, B.G.; Day, J.D.; Dimarco, J.P.; Ferguson, J.D.; et al. Image Integration Using Intracardiac Ultrasound and 3D Reconstruction for Scar Mapping and Ablation of Ventricular Tachycardia. J Cardiovasc Electrophysiol. 2010, 21, 678–684. [Google Scholar] [CrossRef]
- Hussein, A.; Jimenez, A.; Ahmad, G.; Mesubi, O.; Klein, T.; Gurm, G.; et al. Assessment of Ventricular Tachycardia Scar Substrate by Intracardiac Echocardiography. Pacing Clin Electrophysiol. 2014, 37, 412–421. [Google Scholar] [CrossRef]
- Qian, P.C.; Tedrow, U.B. Intracardiac Echocardiography to Guide Catheter Ablation of Ventricular Arrhythmias in Ischemic Cardiomyopathy. Card Electrophysiol Clin. 2021, 13, 285–292. [Google Scholar] [CrossRef]
- Bala, R.; Ren, J.F.; Hutchinson, M.D.; Desjardins, B.; Tschabrunn, C.; Gerstenfeld, E.P.; et al. Assessing Epicardial Substrate Using Intracardiac Echocardiography During VT Ablation. Circ Arrhythm Electrophysiol. 2011, 4, 667–673. [Google Scholar] [CrossRef]
- Barrett, C.; Tzou, W.S. Utility of Intracardiac Echocardiography for Guiding Ablation of Ventricular Tachycardia in Nonischemic Cardiomyopathy. Card Electrophysiol Clin. 2021, 13, 337–343. [Google Scholar] [CrossRef]
- Lamberti, F.; Di Clemente, F.; Remoli, R.; Bellini, C.; De Santis, A.; Mercurio, M.; et al. Catheter ablation of idiopathic ventricular tachycardia without the use of fluoroscopy. Int J Cardiol.
- Kautzner, J.; Haskova, J.; Lehar, F. Intracardiac Echocardiography to Guide Non-fluoroscopic Electrophysiology Procedures. Card Electrophysiol Clin. 2021, 13, 399–408. [Google Scholar] [CrossRef]
- Rivera, S.; Vecchio, N.; Ricapito, P.; Ayala-Paredes, F. Non-fluoroscopic catheter ablation of arrhythmias with origin at the summit of the left ventricle. J Interv Card Electrophysiol. 2019, 56, 279–290. [Google Scholar] [CrossRef]
- Hasegawa, K.; Yoneda, Z.T.; Martines-Parachini, J.R.; Powers, E.M.; Davogustto, G.E.; Hu, T.Y.; et al. Can Intracardiac Echocardiography Reduce Steam Pops During Half-Normal Saline Irrigated Radiofrequency Ablation? Circ Arrhythm Electrophysiol [Internet]. 2024 Jun [cited 2025 Mar 17];17(6). Available from: https://www.ahajournals.org/doi/10.1161/CIRCEP.123. 0126. [Google Scholar]
- Peichl, P.; Wichterle, D.; Čihák, R.; Aldhoon, B.; Kautzner, J. Catheter Ablation of Ventricular Tachycardia in the Presence of an Old Endocavitary Thrombus Guided by Intracardiac Echocardiography: ABLATION IN PRESENCE OF THROMBUS. Pacing Clin Electrophysiol. 2016, 39, 581–587. [Google Scholar] [CrossRef]
- Raczka, F.; Granier, M.; Cung, T.T.; Davy, J.M. Intracardiac thrombus: a good indication of ultrasound image integration system (CartosoundTM) for radiofrequency ablation. Europace. 2010, 12, 591–592. [Google Scholar] [CrossRef]
- Enriquez, A.; Sadek, M.; Hanson, M.; Yang, J.; Matos, C.D.; Neira, V.; et al. Feasibility, Efficacy, and Safety of Fluoroless Ablation of VT in Patients With Structural Heart Disease. JACC Clin Electrophysiol. 2024, 10, 1287–1300. [Google Scholar] [CrossRef]
- Field, M.E.; Gold, M.R.; Reynolds, M.R.; Goldstein, L.; Lee, S.H.Y.; Kalsekar, I.; et al. Real-world outcomes of ventricular tachycardia catheter ablation with versus without intracardiac echocardiography. J Cardiovasc Electrophysiol. 2020, 31, 417–422. [Google Scholar] [CrossRef]
- Kitamura, T.; Nakajima, M.; Kawamura, I.; Kaszynski, R.H.; Ohbe, H.; Sasabuchi, Y.; et al. Safety and effectiveness of intracardiac echocardiography in ventricular tachycardia ablation: a nationwide observational study. Heart Vessels. 2021, 36, 1009–1015. [Google Scholar] [CrossRef]
- Di Biase, L.; Zou, F.; Lin, A.N.; Grupposo, V.; Marazzato, J.; Tarantino, N.; et al. Feasibility of three-dimensional artificial intelligence algorithm integration with intracardiac echocardiography for left atrial imaging during atrial fibrillation catheter ablation. Europace. 2023, 25, euad211. [Google Scholar] [CrossRef]
- Akerström, F.; Drca, N.; Jensen-Urstad, M.; Braunschweig, F. Feasibility of a novel algorithm for automated reconstruction of the left atrial anatomy based on intracardiac echocardiography. Pacing Clin Electrophysiol. 2022, 45, 1288–1294. [Google Scholar] [CrossRef]
- Blumenthal, C.J.; Hsue, W.; Chen, T.; Zhang, D.; Brem, E.; Garcia, F.C.; et al. Preclinical Experience Using 4D Intracardiac Echocardiography to Guide Cardiac Electrophysiology Procedures. J Cardiovasc Electrophysiol. 2025, 36, 480–486. [Google Scholar] [CrossRef]
- Mahida, S.; Sacher, F.; Dubois, R.; Sermesant, M.; Bogun, F.; Haïssaguerre, M.; et al. Cardiac Imaging in Patients With Ventricular Tachycardia. Circulation. 2017, 136, 2491–2507. [Google Scholar] [CrossRef]
- Wijnmaalen, A.P.; Van Der Geest, R.J.; Van Huls Van Taxis, C.F.B.; Siebelink, H.M.J.; Kroft, L.J.M.; Bax, J.J.; et al. Head-to-head comparison of contrast-enhanced magnetic resonance imaging and electroanatomical voltage mapping to assess post-infarct scar characteristics in patients with ventricular tachycardias: real-time image integration and reversed registration. Eur Heart J. 2011, 32, 104–114. [Google Scholar] [CrossRef]
- Tian, J.; Jeudy, J.; Smith, M.F.; Jimenez, A.; Yin, X.; Bruce, P.A.; et al. Three-dimensional contrast-enhanced multidetector CT for anatomic, dynamic, and perfusion characterization of abnormal myocardium to guide ventricular tachycardia ablations. Circ Arrhythm Electrophysiol. 2010, 3, 496–504. [Google Scholar] [CrossRef]
- Andreu, D.; Berruezo, A.; Ortiz-Pérez, J.T.; Silva, E.; Mont, L.; Borràs, R.; et al. Integration of 3D Electroanatomic Maps and Magnetic Resonance Scar Characterization Into the Navigation System to Guide Ventricular Tachycardia Ablation. Circ Arrhythm Electrophysiol. 2011, 4, 674–683. [Google Scholar] [CrossRef]
- Yamashita, S.; Sacher, F.; Mahida, S.; Berte, B.; Lim, H.S.; Komatsu, Y.; et al. Image Integration to Guide Catheter Ablation in Scar-Related Ventricular Tachycardia. J Cardiovasc Electrophysiol. 2016, 27, 699–708. [Google Scholar] [CrossRef]
- Takigawa, M.; Duchateau, J.; Sacher, F.; Martin, R.; Vlachos, K.; Kitamura, T.; et al. Are wall thickness channels defined by computed tomography predictive of isthmuses of postinfarction ventricular tachycardia? Heart Rhythm 2019, 16, 1661–1668. [Google Scholar] [CrossRef]
- Parollo, M.; Mazzocchetti, L.; Cori, A.D.; Segreti, L.; Lucia, R.D.; Grifoni, G.; et al. Lipomatous metaplasia as the most reliable computed tomography predictor for functional substrate localization in scar-related ventricular tachycardia. Heart Rhythm. 2023, 20, 1593–1594. [Google Scholar] [CrossRef]
- Di Cori, A.; Pistelli, L.; Parollo, M.; Zaurino, N.; Segreti, L.; Zucchelli, G. Approaching Ventricular Tachycardia Ablation in 2024: An Update on Mapping and Ablation Strategies, Timing, and Future Directions. J Clin Med. 2024, 13, 5017. [Google Scholar] [CrossRef]
- Sanchez-Somonte, P.; Garre, P.; Vázquez-Calvo, S.; Quinto, L.; Borràs, R.; Prat, S.; et al. Scar conducting channel characterization to predict arrhythmogenicity during ventricular tachycardia ablation. EP Eur. 2023, 25, 989–999. [Google Scholar] [CrossRef]
- Acosta, J.; Andreu, D.; Penela, D.; Cabrera, M.; Carlosena, A.; Korshunov, V.; et al. Elucidation of hidden slow conduction by double ventricular extrastimuli: a method for further arrhythmic substrate identification in ventricular tachycardia ablation procedures. EP Eur. 2018, 20, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Andreu, D.; Penela, D.; Acosta, J.; Fernández-Armenta, J.; Perea, R.J.; Soto-Iglesias, D.; et al. Cardiac magnetic resonance–aided scar dechanneling: Influence on acute and long-term outcomes. Heart Rhythm. 2017, 14, 1121–1128. [Google Scholar] [CrossRef]
- Soto-Iglesias, D.; Penela, D.; Jáuregui, B.; Acosta, J.; Fernández-Armenta, J.; Linhart, M.; et al. Cardiac Magnetic Resonance-Guided Ventricular Tachycardia Substrate Ablation. JACC Clin Electrophysiol. 2020, 6, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Lilli, A.; Parollo, M.; Mazzocchetti, L.; De Sensi, F.; Rossi, A.; Notarstefano, P.; et al. Ventricular tachycardia ablation guided or aided by scar characterization with cardiac magnetic resonance: rationale and design of VOYAGE study. BMC Cardiovasc Disord. 2022, 22, 169. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




