Submitted:
08 July 2025
Posted:
11 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Reactants
2.2. Synthesis of MCM-41-Based Solids
2.3. Characterization of MCM-41/N and MCM-41/NN
2.4. Cr(VI) Removal Tests
3. Results and Discussion
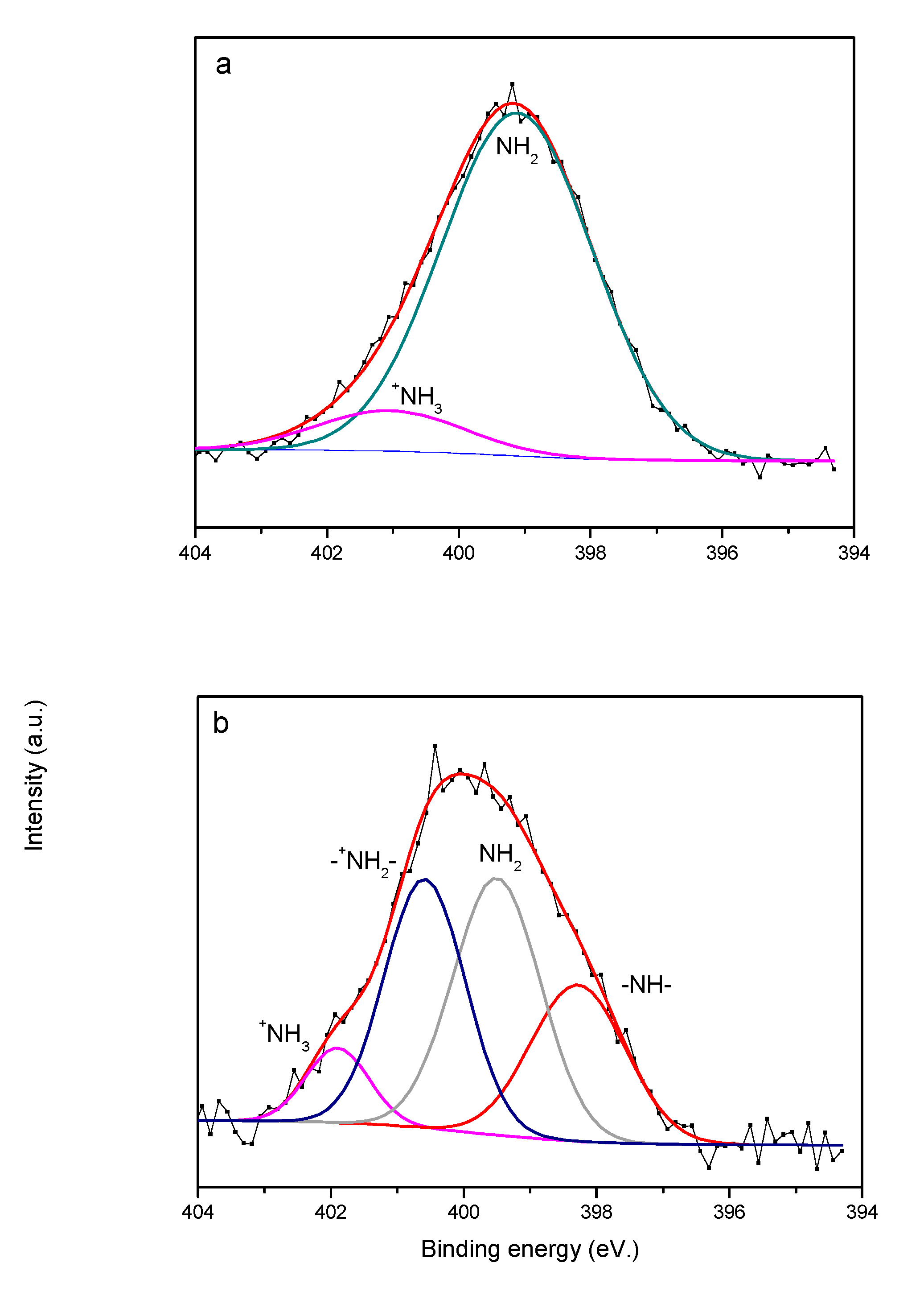
3.1. Characterization of MCM-41-N and MCM-41-NN
3.2. Performance of MCM-41-N and MCM-41-NN in Cr(VI) Removal
3.3. Characterization of Used Samples
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
References
- Chai, C.; Liu, B.; Lv, P.; Bai, Y.; Wang, J.; Su, W. , Song X. , Yu G.; Xu G.; Microwave synthesis of amino-functionalized MCM-41 from coal gasification fine slag for efficient bidirectional adsorption of anionic and cationic dyes, Chemosphere 2024, 351, 141229. [Google Scholar] [PubMed]
- Costa J. A., S. , de Jesus R. A., Santos D. O., Mano J. F., Romão L. P. C., Paranhos C. M., Recent progresses in the adsorption of organic, inorganic, and gas compounds by MCM-41-based mesoporous materials. Micro. Meso. Mater. 2020, 291, 109698. [Google Scholar] [CrossRef]
- Freitas Cavalcante, J.C. , da Silva A. M.; Batista Caldas P. M., de Sousa Barbosa B.V.; da Silva Júnior H.B.; Nicácio Alves J.J., Characterization and optimization of biodiesel production from corn oil using heterogeneous MoO3/MCM-41 catalysts, Catalysis Today 2025, 446, 115119. [Google Scholar]
- Kuppireddy, S. , Varghese A. M.; Araj H., Hart P.; Ramantani T.; Bampos G.; Karanikolos G. N.; A combined experimental and simulations assessment of CO2 capture and CO2/H2 separation performance of aminosilane-grafted MCM-41 and pore-expanded MCM-41, Micro. Meso. Mater. 2024, 377, 113220. [Google Scholar]
- Vera-Baquero, F.L.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Sierra, I. Exploring Adsorption Performance of Functionalized Mesoporous Silicas with a Different Pore Structure as Strong Cation-Exchange Sorbents for Solid-Phase Extraction of Atropine and Scopolamine. Appl. Sci. 2025, 15, 646. [Google Scholar] [CrossRef]
- Mallik A., K. , Moktadir Md. A., Rahman Md.A., Shahruzzaman Md., M. M. Rahman, Progress in surface-modified silicas for Cr(VI) adsorption: A review, J. Hazardous Materials 2022, 423, 127041. [Google Scholar]
- Tandon, R.K. , Crisp P. T., Ellis J., Effect of pH on Chromium (VI) species in solution, Talanta 1984, 31, 227. [Google Scholar]
- Zhao, B. , Wang Y. , Luo X., Luo J., Li G., Deng L., Cao Y., Interfacial adsorption behavior of amine-functionalized MCM-41 for Mo(VI) capture from aqueous solution, Environ. Res. 2025, 269, 120821. [Google Scholar]
- Liao, P. , Li B. , Xie L., Bai X., Qiao H., Li Q., Yang B., Liu C., Immobilization of Cr (VI) on engineered silicate nanoparticles: Microscopic mechanisms and site energy distribution, J. Hazardous Mater. 2020, 383, 121145. [Google Scholar]
- Martin, P. , Rafti M. , Marchetti S., Fellenz N., MCM-41-based composite with enhanced stability for Cr(VI) removal from aqueous media, Solid State Sci. 2020, 106, 106300. [Google Scholar]
- Fellenz, N. , Perez-Alonso F. J., Martin P.P., García-Fierro J.L., Bengoa J.F., Marchetti S. G., Rojas S., Chromium (VI) removal from water by means of adsorption-reduction at the surface of amino-functionalized MCM-41 sorbents, Micro. Meso. Mater. 2017, 239, 138. [Google Scholar]
- Fellenz, N. , Martin P. P., Marchetti S.G., Bengoa J.F., Aminopropyl-modified mesoporous silica nanospheres for the adsorption of Cr(VI) from water, J. Porous Mater. 2015, 22, 729. [Google Scholar]
- Ko, Y. , Choi K. , Lee S., Jung K., Hong S., Mizuseki H., Choi J., Lee W., Strong chromate-adsorbent based on pyrrolic nitrogen structure: An experimental and theoretical study on the adsorption mechanism, Water Research 2018, 145, 287. [Google Scholar]
- Zaitseva, N. , Zaitsev V. , Walcarius A., Chromium(VI) removal via reduction-sorption on bi-functional silica adsorbents, J. Hazardous Mater. 2013, 250, 454. [Google Scholar]
- Dong, X. , Shi L., Ma S. , Chen X., Cao S., Li W., Z. Zhao, C. Chen, H. Deng, Chitin/Chitosan Nanofibers Toward a Sustainable Future: From Hierarchical Structural Regulation to Functionalization Applications, Nano Letters 2024, 24, 12014. [Google Scholar]
- Malinkina, O. , Shmakov S. L., Shipovskaya A. B., Structure, the energy, sorption and biological properties of chiral salts of chitosan with l- and d-ascorbic acid, Int. J. of Biological Macromol. 2024, 257, 128731. [Google Scholar]
- Nandi, R. , Laskar S. and Saha B., Surfactant-promoted enhancement in bioremediation of hexavalent chromium to trivalent chromium by naturally occurring wall algae. Res. Chem. Intermed. 2017, 43, 1619. [Google Scholar] [CrossRef]
- Ukhurebor, K. , Aigbe U. O., Onyancha R.B., Nwankwo W., Osibote O.A., Paumo H. K., Ama O.M., Adetunji C.O., Siloko I.U., Effect of hexavalent chromium on the environment and removal techniques: a review, J. Environ. Manag. 2021, 280, 1–25. [Google Scholar]
- Karthik, C. , Barathi S. , Pugazhendhiv, Ramkumar V.S., Thi N., Arulselvi P.I., Characterization of multifarious plant growth promoting traits of rhizobacterial strain AR6 under Chromium(VI) stress, Microbiol. Res. 2017, 204, 65–71. [Google Scholar]
- Wang, Q. , Zuo W. , Tian Y., Kong L., Cai G., Zhang H., Li L., Zhang J., An ultralight and flexible nanofibrillated cellulose/chitosan aerogel for efficient chromium removal: Adsorption-reduction process and mechanism, Chemosphere 2023, 329, 138622. [Google Scholar]
- Grün, M. , Unger K.K., Matsumoto A., Tsutsumi K., Novel pathways for the preparation of mesoporous MCM-41 materials: control of porosity and morphology, Micro. Meso. Mater. 1999, 27 207.
- Kruk, M.; et al. , Determination of Pore Size and Pore Wall Structure of MCM-41 by Using Nitrogen Adsorption, Transmission Electron Microscopy, and X-ray Diffraction, J. of Physical Chemistry B 2000, 104, 292. [Google Scholar] [CrossRef]
- Kaur, P. , Chopra H. K., SBA-15 supported benzoxazolium-based ionic liquids: Synthesis, characterization, and application in the adsorptive desulfurization, Fuel Processing Tech. 2022, 238, 107480. [Google Scholar]
- Calvo, A. , Angelome P. C., Sanchez V.M., Scherlis D.A., Williams F.J., Soler-Illia G.J.A.A., Mesoporous aminopropyl-functionalized hybrid thin films with modulable surface and environment-responsive behavior, Chem. Mater. 2008, 20, 4661–4668. [Google Scholar]
- Jabbari-Gargari, A.; et al. , Carboxylic acid decorated silica aerogel nanostructure as drug delivery carrier, Micro. Meso. Mater. 2021, 323, 111220. [Google Scholar] [CrossRef]
- Zhmud, B.V. , Sonnefeld J. , Aminopolysiloxane gels : production and properties, J. Non-Cryst. Sol. 1996, 195, 16–27. [Google Scholar]
- Walcarius, A. , Etienne M. , Lebeau B., Rate of Access to the Binding Sites in Organically Modified Silicates. 2. Ordered Mesoporous Silicas Grafted with Amine or Thiol Groups, Chem. Mater. 2003, 15, 2161–2173. [Google Scholar]
- Fang, L.; et al. , Insights into the proton-enhanced mechanism of hexavalent chromium removal by amine polymers in strong acid wastewater: Reduction of hexavalent chromium and sequestration of trivalent chromium, J. Coll. Interf. Science 2023, 650, 515–525. [Google Scholar] [CrossRef]
- Ko, Y.G. , Shin S. S., Choi U.S., Primary, secondary, and tertiary amines for CO2 capture: Designing for mesoporous CO2 adsorbents, J. Coll. Interf. Science 2011, 361, 594–602. [Google Scholar]
- Yismaw S., S. G. Ebbinghaus, M. Wenzel, D. Poppitz, R. Gläser, J. Matysik, F. Bauer, D. Enke, Selective functionalization of the outer surface of MCM-48-type mesoporous silica nanoparticles at room temperature; J. Nanopart. Res. 2020, 22, 279. [Google Scholar]
- Dogan F., K. D. Hammond, G. A. Tompsett, H. Huo, W. Curtis Conner Jr., SM. Auerbach, C. P. Grey, Searching for Microporous, Strongly Basic Catalysts: Experimental and Calculated 29Si NMR Spectra of Heavily Nitrogen-Doped Y Zeolites, J. Am. Chem. Soc. 2009, 131, 11062–11079. [Google Scholar]
- Zúñiga, E. , Belmar L., Toledo L, Torres C, Rivas B.L., Sánchez S.A., Urbano B.F., Rhodamine-loaded surface modified mesoporous silica particles embedded into a thermoresponsive composite hydrogel for prolonged release. Eur Polym J. 2017, 5, 358–367. [Google Scholar] [CrossRef]
- Miyajima, T. , Abry S. , Zhou W., Albela B., Laurent B., Y. Oumi, T. Sano and Yoshitake H., Estimation of spacing between 3-bromopropyl functions grafted on mesoporous silica surfaces by a substitution reaction using diamine probe molecules, J. Mater. Chem. 2007, 17, 3901–3909. [Google Scholar]
- Buttersack, C. , General Cluster Sorption Isotherm, Micro. Meso. Materials 2021, 316, 110909. [Google Scholar] [CrossRef]
- Popova M., A. Szegedi, K. Yoncheva, S. Konstantinov, G.P. Petrova, H.A. Aleksandrov, G.N. Vayssilov, P. Shestakova, New method for preparation of delivery systems of poorly soluble drugs on the basis of functionalized mesoporous MCM-41 nanoparticles, Micro. Meso. Materials 2014, 198, 247–255. [Google Scholar]
- Sun, S. ; H. Lyu, L. Gai, P. Sun a, B. Shen, J. Tang, Biochar-anchored low-cost natural iron-based composites for durable hexavalent chromium removal, Chemical Engineering J. 2023, 476, 146604. [Google Scholar]
- Verma R., P. K. Maji, S. Sarkar, Removal of hexavalent chromium from impaired water: Polyethylenimine-based sorbents− A review, J. Env. Chem. Eng. 2023, 11, 109598. [Google Scholar]
- Losev V., N.; Didukh-Shadrina S., L. , Trofimchuk A. K., Effective separation of chromium species in technological solutions using amino-immobilized silica prior to their determination, J. Hazardous Mater. 2021, 407, 124383. [Google Scholar]
- Pattnaik, S. , Dash D. , Mohapatra S., Pattnaik M., Marandi A. K., Das S., Samantaray D. P., Improvement of rice plant productivity by native Cr(VI) reducing and plant growth promoting soil bacteria Enterobacter cloacae, Chemosphere 2020, 240, 124895. [Google Scholar]
- Wu, J. , Yan M. , Lv S., Yin W., Bu H., Liu L., Li P., Deng H., Zheng X., Preparation of highly dispersive and antioxidative nano zero-valent iron for the removal of hexavalent chromium, Chemosphere 2021, 262, 127733. [Google Scholar]
- Yang, X.; Zhou, Y.; Hu, J.; Zheng, Q.; Zhao, Y.; Lv, G. , Liao L.; Clay minerals and clay-based materials for heavy metals pollution control, Science of The Total Environment 2024, 954, 176193. 954.
- Abbou, B. , Lebkiri I. , Ouaddari H., Evaluation of Illitic-Kaolinite clay as an adsorbent for Cr3+ removal from synthetic aqueous solutions: Isotherm, kinetic, and thermodynamic analyses, Chem. Physics Impact 2024, 8, 100527. [Google Scholar]
- Moulder J., F. , Stickle W. F., Sobol P. E., Bomben K. D. Handbook of X-ray Photoelectron Spectroscopy. Edited by J. Chastain (1992), ISBN: 0-9627026-2-5.
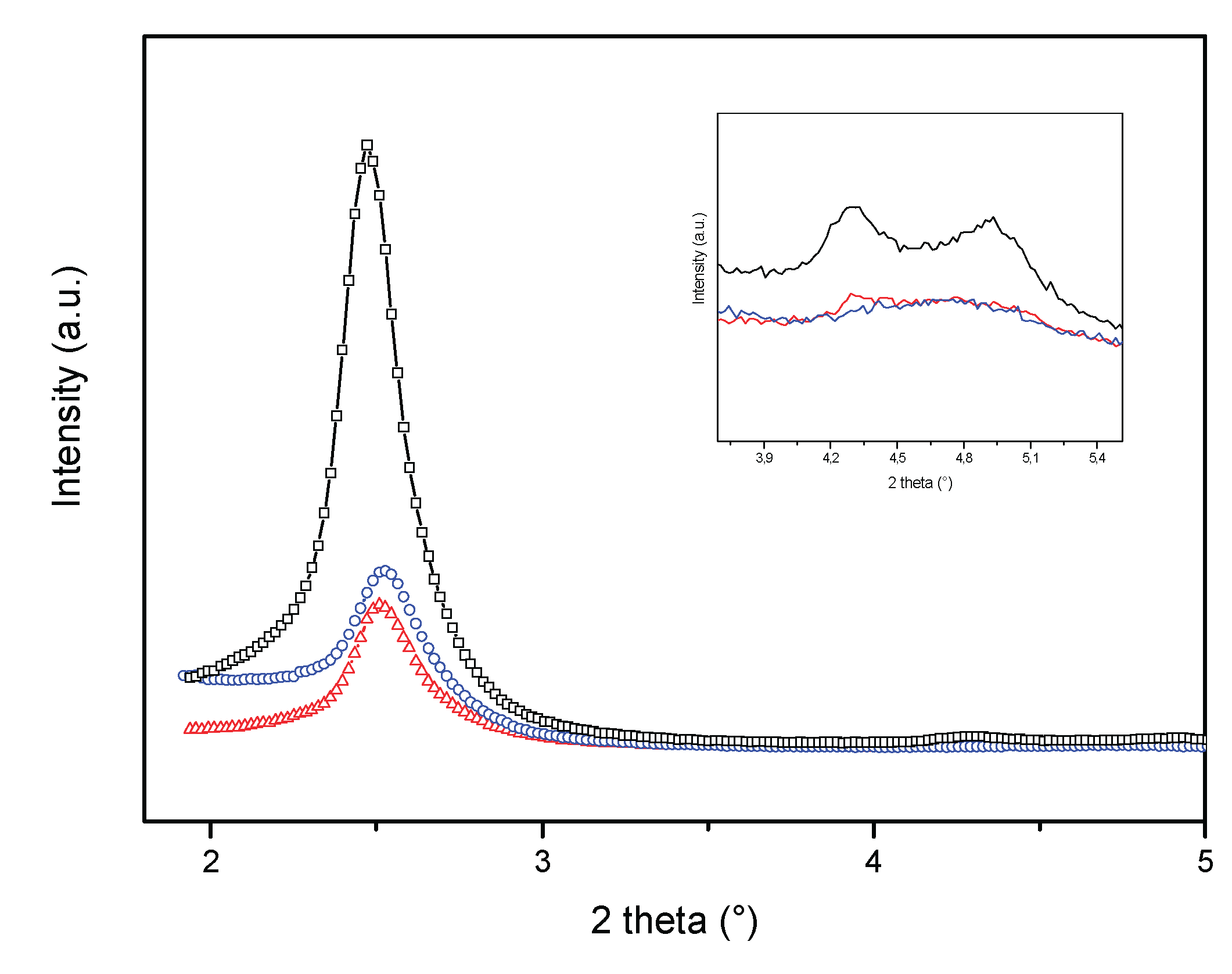
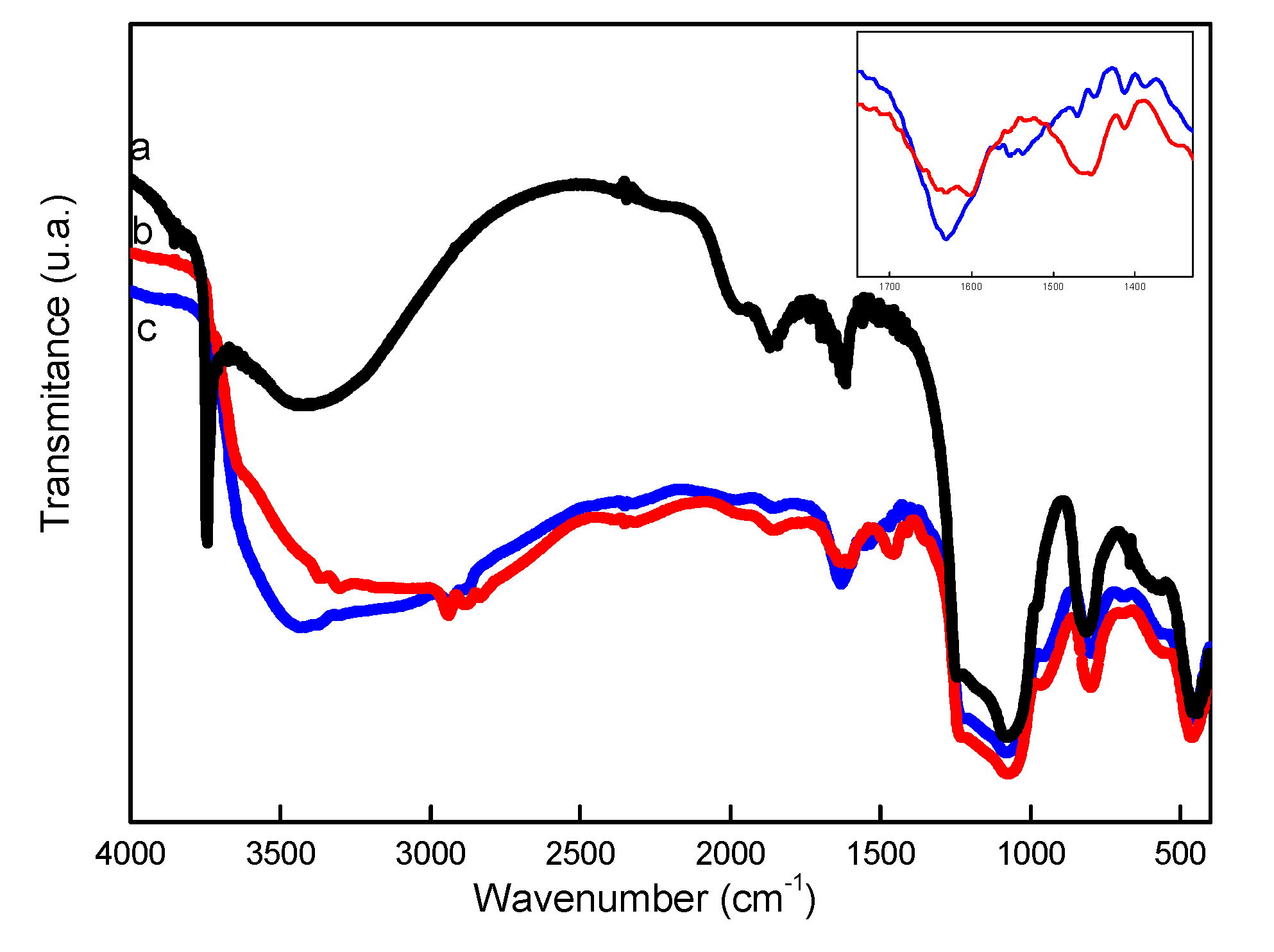
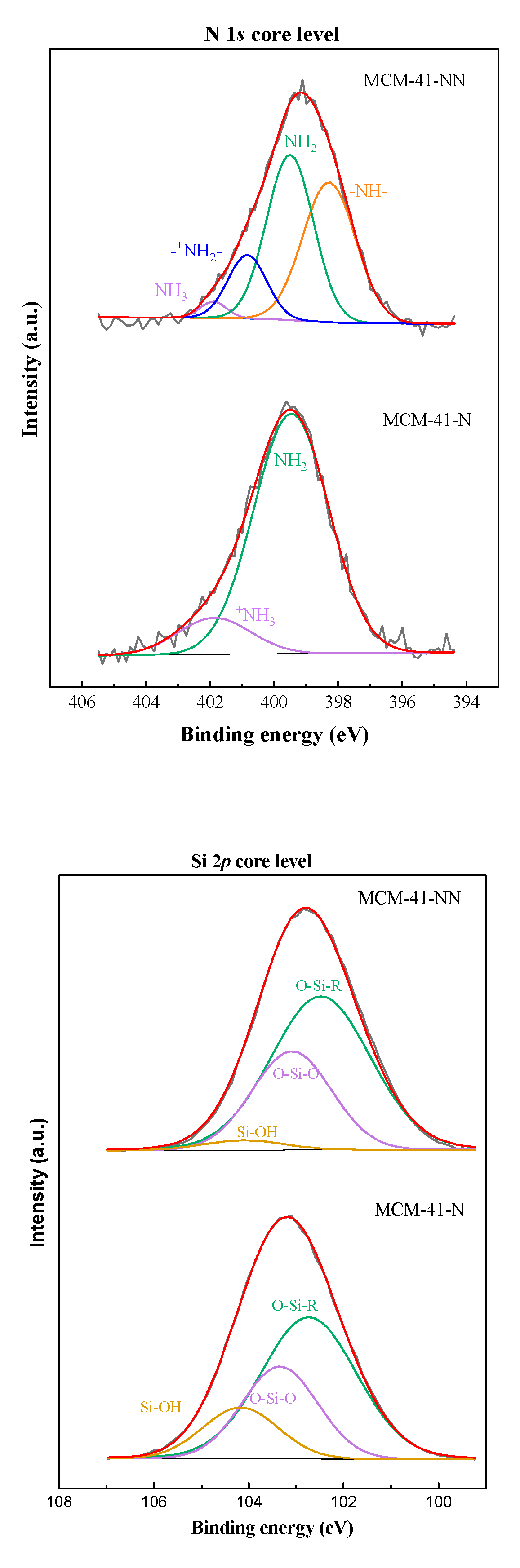
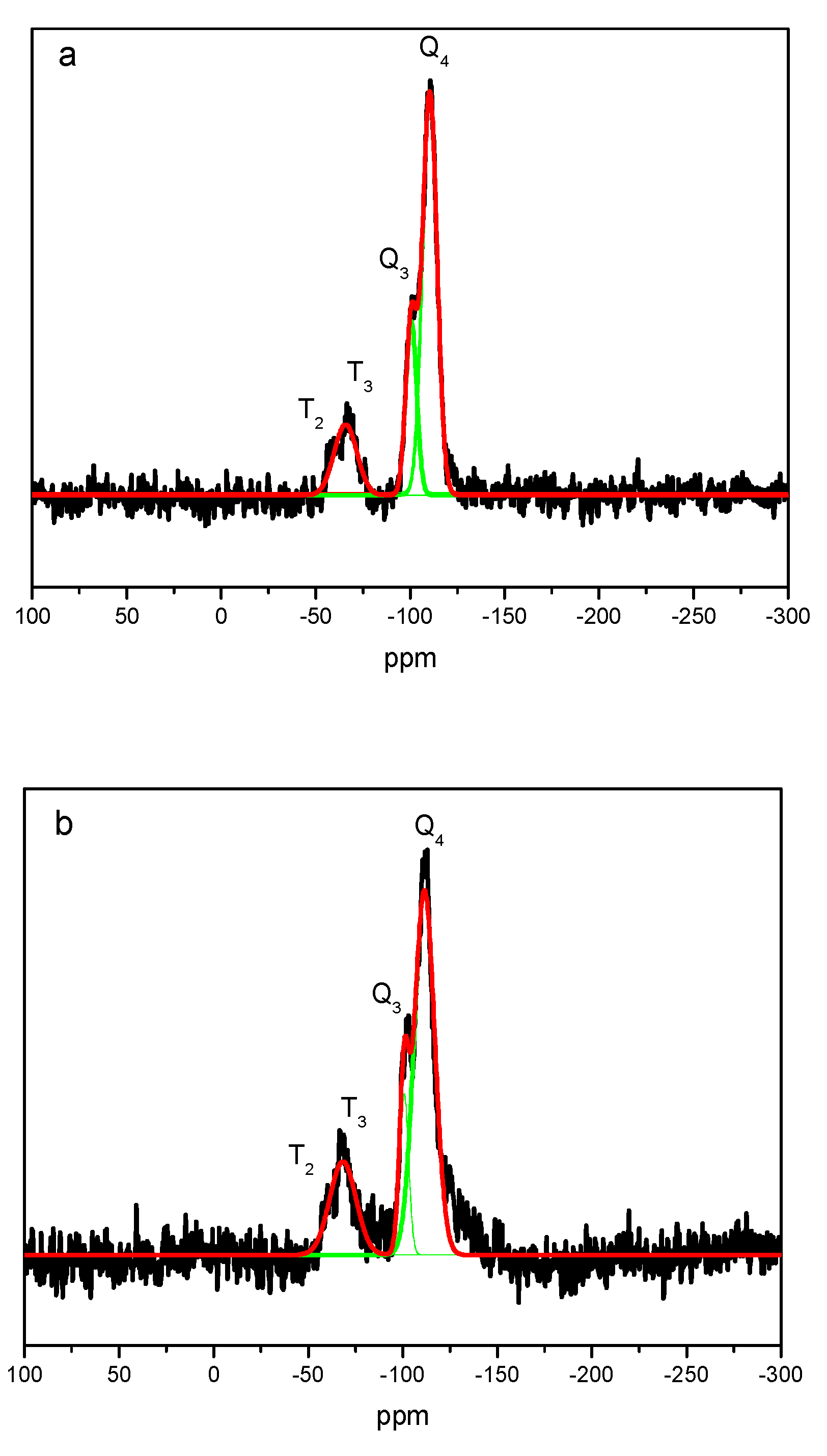
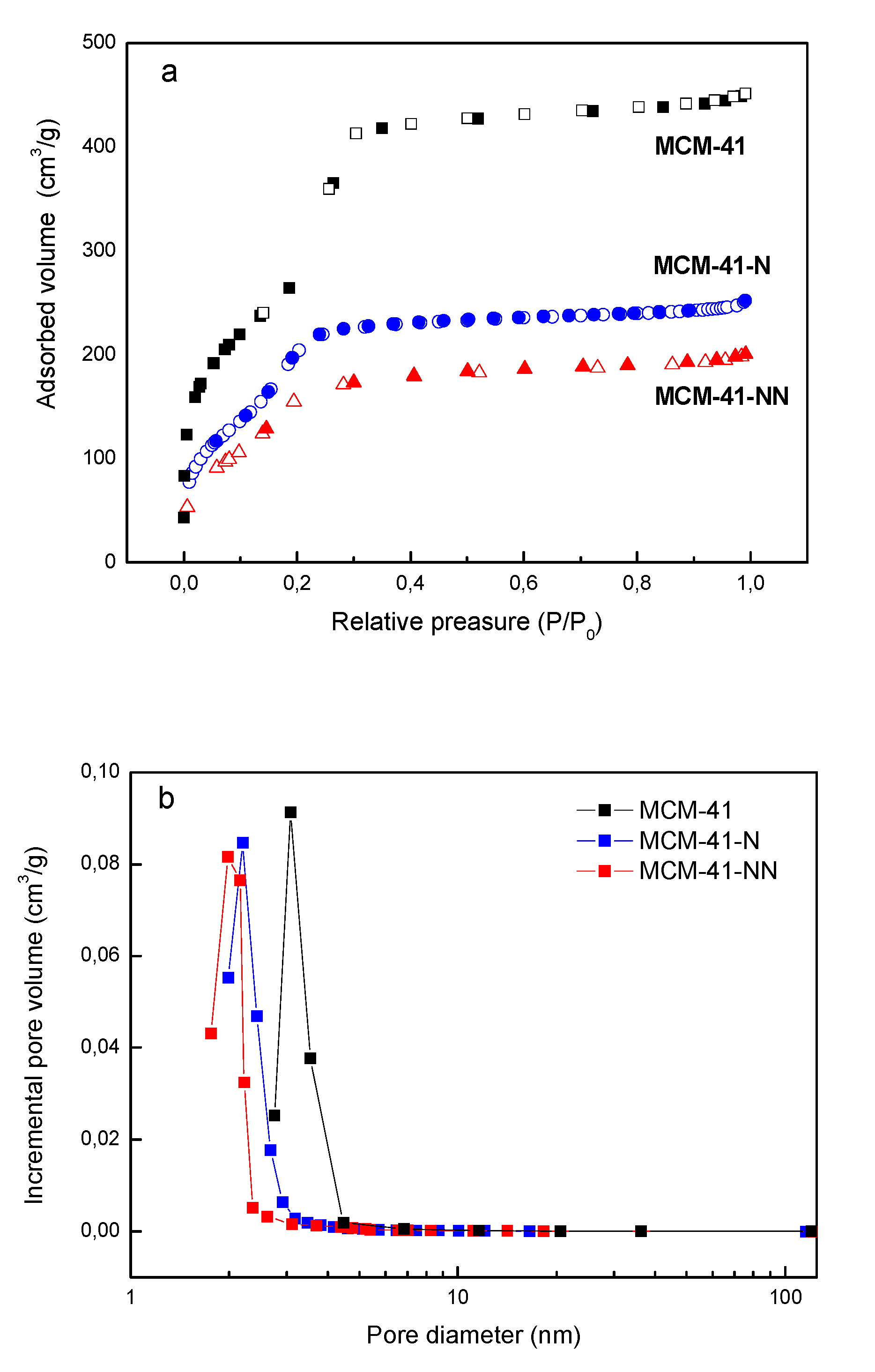
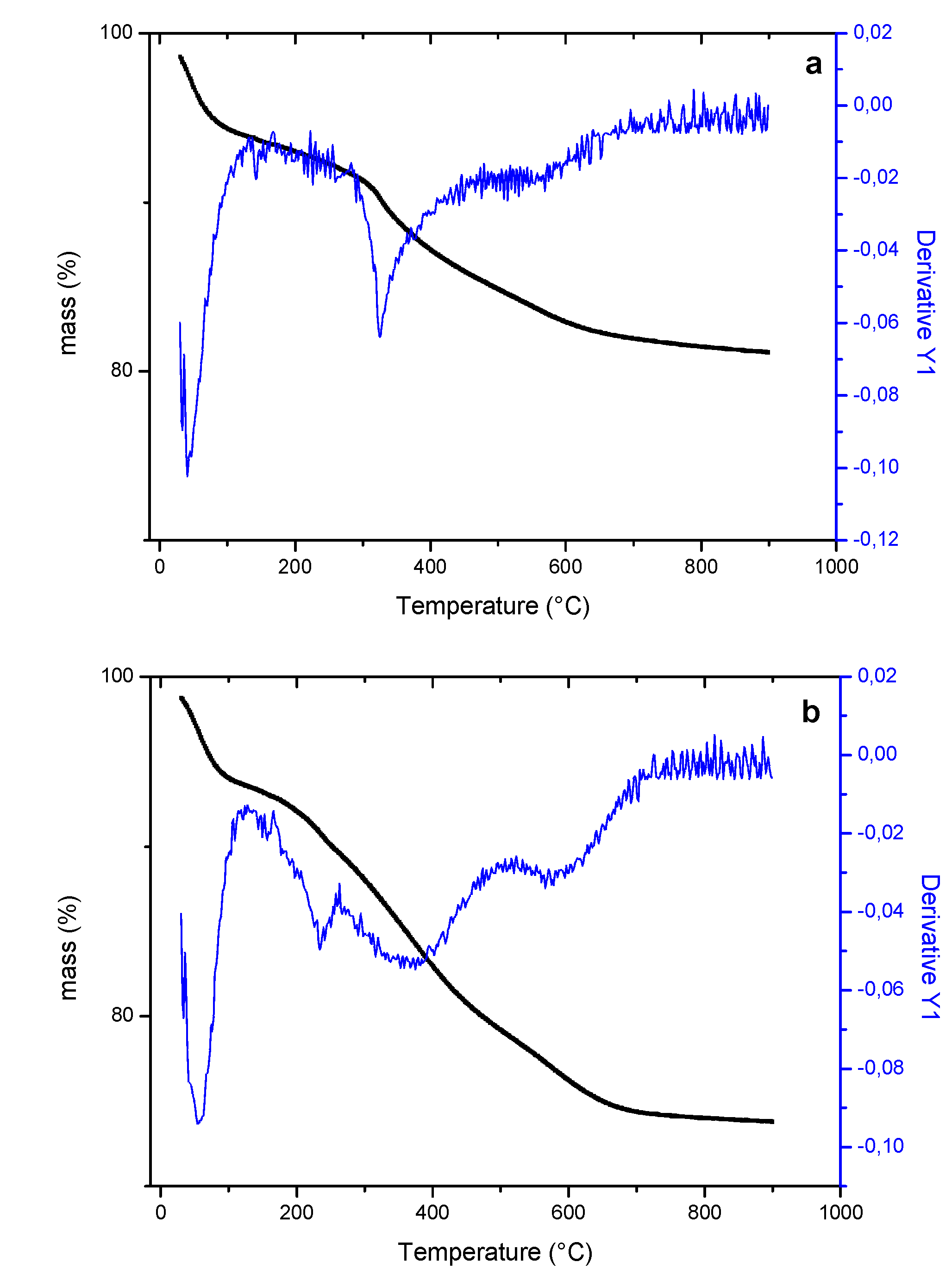
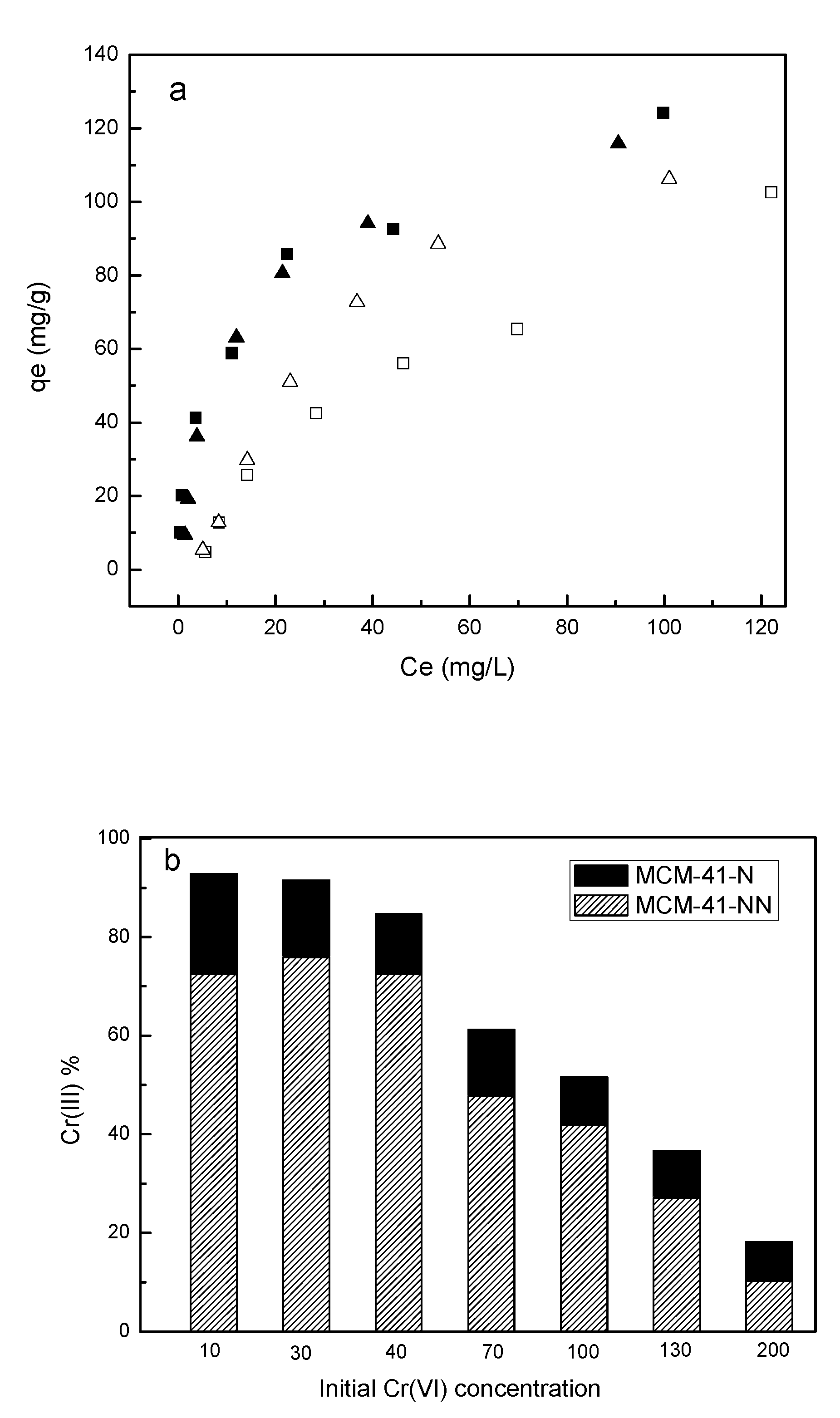
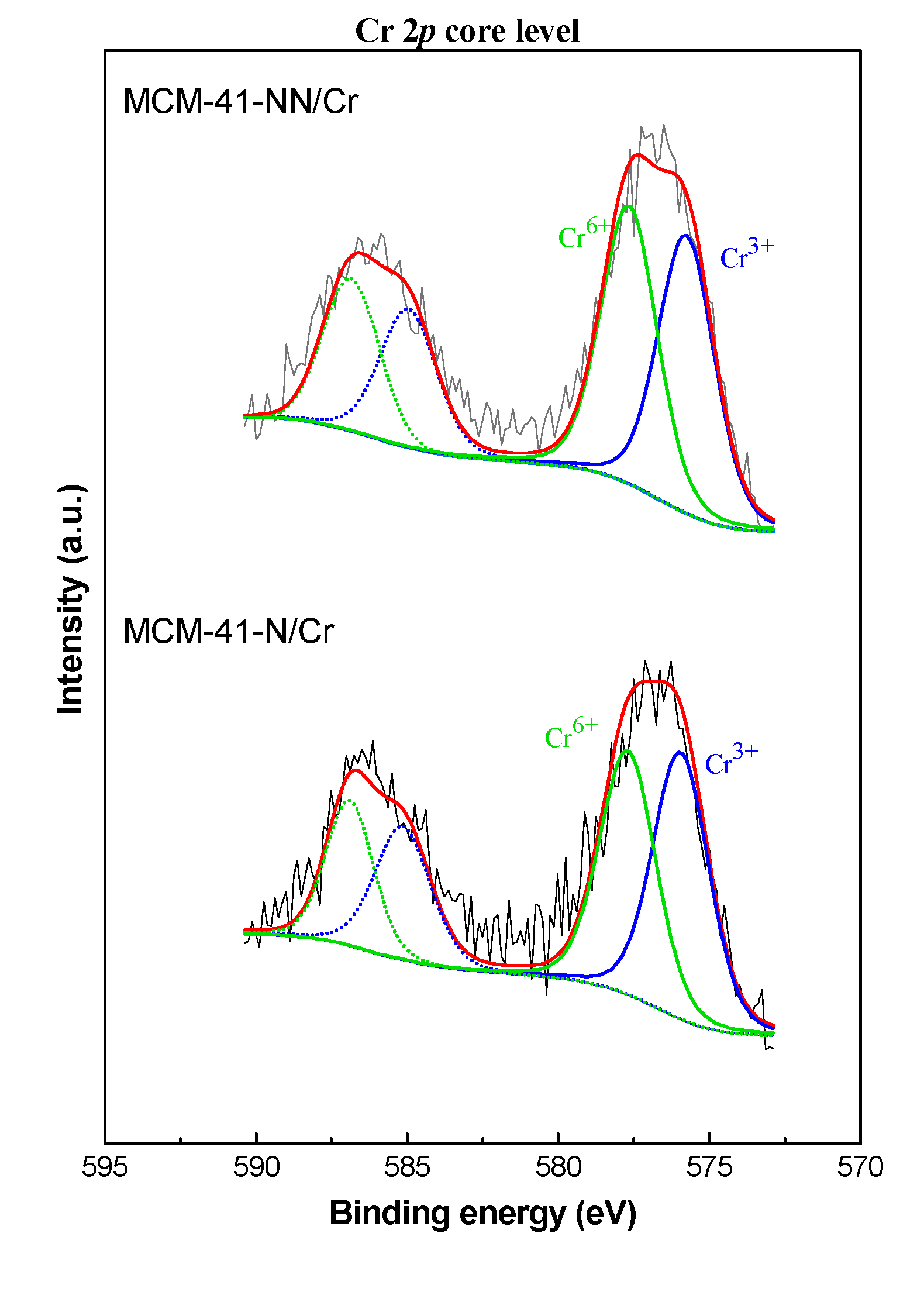
| Sample/ Parameter |
Sg (m2·g-1)a |
Vp (cm3·g-1)a |
Dp (nm)b |
Ca | Functional group loading (mmol·g-1)c |
d (mmol·nm-2) |
|---|---|---|---|---|---|---|
| MCM 41 | 991 | 0.7 | 3.1 | 95.7 | - | - |
| MCM 41-N | 715 | 0.4 | 2.2 | 41.0 | 1.3 | 11.0 |
| MCM 41-NN | 605 | 0.3 | 2.0 | 39.5 | 1.5 | 14.9 |
| Sample/Parameter | qm Cr(VI)a (mg·g-1) |
qm Cr(tot)b (mg·g-1) |
Cr(III)/Cr(VI)c |
|---|---|---|---|
| MCM-41-N | 129.9 | 107.1 | 1.1 |
| MCM-41-NN | 133.3 | 122.1 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





