1. Introduction
A broad and frequently debilitating category of inherited and acquired illnesses are metabolic brain diseases (MBDs), also referred to as neurometabolic disorders. Disruptions in vital cellular functions, such as energy production, substrate metabolism, or detoxification pathways, are what essentially define these disorders [
1]. A variety of severe neurological symptoms, including cognitive decline, motor disturbances, and seizures, are often the result of such metabolic dysfunctions, which often lead to progressive neurodegeneration and significantly reduce patients' quality of life and lifespan [
1]. Clinicians face significant diagnostic challenges due to the inherent heterogeneity of these disorders and their variable age of onset, which can range from neonatal to adulthood [
1,
2].
The need for less invasive but highly informative methods has led to a significant evolution in the diagnostic landscape for diseases of the central nervous system (CNS). In the past, invasive techniques like lumbar punctures and brain biopsies have been necessary for the conclusive diagnosis of numerous CNS disorders. These techniques offer direct tissue access, but they come with risks, such as infection and bleeding, and they might not adequately represent the molecular diversity that defines a number of neurological conditions [
3]. Liquid biopsy has become a revolutionary substitute in this regard. By examining circulating biomarkers present in different body fluids, this minimally invasive method provides a flexible and approachable way to detect, track, and characterize diseases at the molecular level [
3]. Among the various analytes in liquid biopsy, cell-free DNA (cfDNA) has garnered considerable attention. Released from dying cells into the circulation, cfDNA provides a "real-time" molecular snapshot of ongoing pathological processes within the body [
4].
While the utility of cfDNA in liquid biopsy has been extensively explored in oncology, its potential in non-malignant neurological disorders, particularly MBDs, is now gaining significant momentum [
5,
6,
7,
8,
9]. A compelling aspect of cfDNA analysis lies in the interrogation of epigenetic modifications, most notably DNA methylation patterns. These epigenetic marks are stable, inherently tissue-specific, and critically reflect the gene regulatory changes that define cellular identity and function [
9,
10,
11,
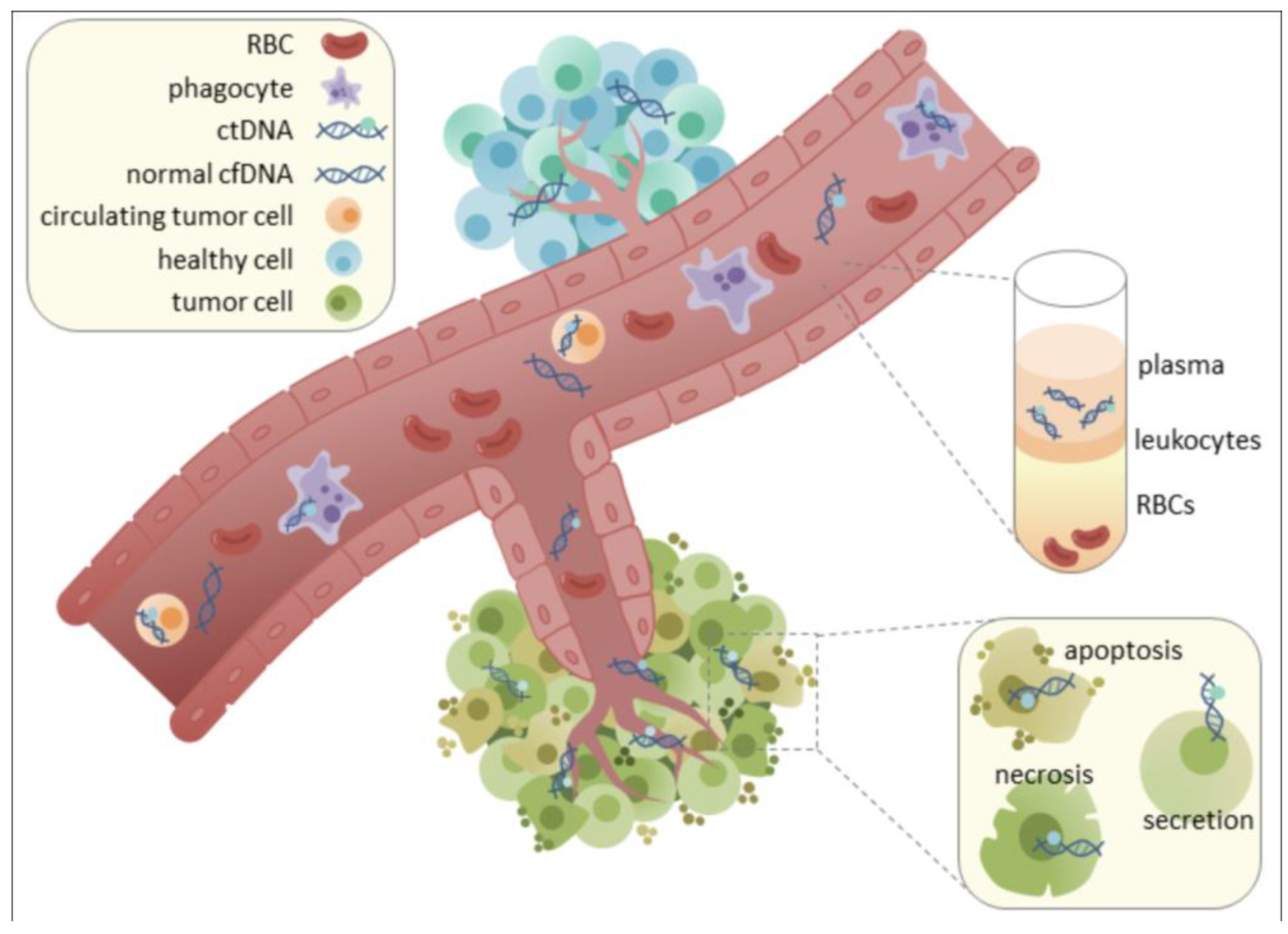
12]. Crucially, the unique epigenetic signatures carried by cfDNA fragments can reveal their tissue and even cell type of origin, enabling the precise mapping of CNS-specific damage or dysfunction (
Figure 1) [
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
26]. Cerebrospinal fluid (CSF), which directly bathes the brain and spinal cord, stands out as an ideal biofluid for capturing CNS-derived cfDNA. This direct anatomical proximity allows CSF to overcome the limitations imposed by the blood-brain barrier (BBB), which often restricts the passage of CNS-derived biomarkers into the peripheral bloodstream, thereby limiting the utility of plasma-based analyses for many brain pathologies [
3]. This review aims to critically examine the emerging role of epigenetic profiling of CSF-derived cfDNA, exploring its potential to revolutionize the diagnostic and prognostic landscape for MBDs by providing unprecedented molecular-level insights into CNS metabolic dysfunction.
2. Methodology
This narrative review was conducted to synthesize current knowledge regarding the diagnostic and prognostic utility of cerebrospinal fluid (CSF)-derived cell-free DNA (cfDNA) methylation profiling in metabolic brain diseases (MBDs). A comprehensive literature search was conducted across the PubMed, Scopus, and Web of Science databases from their inception through June 2025. Search terms included combinations of “cerebrospinal fluid,” “cfDNA,” “cell-free DNA,” “DNA methylation,” “epigenetics,” “liquid biopsy,” “metabolic brain disease,” “mitochondrial encephalopathy,” “lysosomal storage disease,” “GLUT1 deficiency,” and “diabetes-associated cognitive decline.” Only articles published in English were considered.
Eligible sources included peer-reviewed primary research articles, systematic reviews, epigenetic technology reports, and consensus statements addressing either traditional or emerging CSF biomarkers in neurologic and neurometabolic conditions. Studies were included if they described or evaluated CSF-based biomarkers in metabolic brain diseases, investigated cfDNA methylation or epigenetic alterations relevant to central nervous system pathology, or offered mechanistic or translational insights with potential clinical implications. Case reports lacking biomarker analysis, studies not involving CSF or cfDNA, and non-CNS-focused investigations were excluded. Abstract-only conference proceedings without accessible full-text data were also omitted.
In instances where multiple studies addressed similar topics, such as cfDNA profiling in MELAS or lysosomal storage disorders, priority was given to recent, high-impact, or methodologically rigorous publications. Technical platforms—including cfMeDIP-seq, enzymatic methyl-sequencing (EM-seq), and bisulfite-based approaches—were reviewed using both primary validation studies and, where necessary, technical documentation from established protocols.
The synthesis of findings was organized thematically around four key domains: (1) traditional CSF biomarkers used in metabolic brain disease diagnosis and monitoring; (2) the emerging methodologies for cfDNA methylation profiling, including technological innovations and limitations; (3) disease-specific applications and insights across select disorders such as MELAS, Niemann-Pick, and GLUT1 deficiency syndrome; and (4) barriers to clinical translation, including technical challenges, data interpretation, regulatory considerations, and ethical concerns. This thematic approach enabled a structured, integrative perspective on how cfDNA methylation profiling may complement or surpass conventional diagnostic modalities in the evaluation of metabolic brain disorders.
3. Metabolic Brain Diseases: Clinical Spectrum and Diagnostic Imperatives
The diagnosis of neurometabolic disorders presents a significant challenge due to their inherent heterogeneity and the often nonspecific nature of their clinical presentations. This section delineates the diverse manifestations and current diagnostic limitations of key metabolic brain diseases, underscoring the pressing need for advanced biomarker strategies.
3.1. Mitochondrial Encephalopathies (e.g., MELAS)
Mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) is a maternally inherited mitochondrial disorder primarily caused by mutations in mitochondrial DNA (mtDNA), with the m.3243A>G mutation in the
MT-TL1 gene being the most common genetic culprit (
Figure 2 and
Figure 3 and
Table 1) [
20,
23,
24]. These genetic alterations compromise the mitochondria's ability to synthesize proteins, utilize oxygen, and generate energy, leading to widespread cellular dysfunction, particularly in metabolically demanding tissues such as the brain and muscles [
20,
21,
22].
Clinically, MELAS typically manifests in childhood, often between 2 and 10 years of age, following a period of seemingly normal early psychomotor development [
20,
21,
22]. A hallmark feature of the syndrome is the occurrence of stroke-like episodes, which frequently appear before the age of 40. These episodes can present with varied neurological deficits, including aphasia, cortical vision loss, motor weakness (hemiparesis), severe headaches, seizures, and altered mental status, leading to a progressive accumulation of neurological impairment over time [
20,
21,
22]. Other common symptoms include encephalopathy (manifesting as seizures and/or dementia), recurrent migrainous headaches often accompanied by vomiting, generalized muscle weakness (myopathy), exercise intolerance, and chronic lactic acidosis detectable in both blood and CSF [
20,
21,
22]. Patients may also exhibit short stature and progressive sensorineural hearing loss [
20,
21,
22]. Less frequently observed manifestations include involuntary muscle spasms (myoclonus), impaired muscle coordination (ataxia), cardiac and renal complications, diabetes mellitus, and various psychiatric disturbances [
20,
21,
22].
Despite these characteristic features, current diagnostic approaches for MELAS face several limitations. While elevated lactate levels in blood and CSF are common, this finding is not specific to MELAS and can be observed in a range of other mitochondrial diseases, metabolic disorders, or systemic illnesses [
20,
21,
22]. Notably, a minority of individuals with MELAS may even present with normal serum lactic acid levels, further complicating diagnosis [
20,
21,
22]. The genetic diagnosis of MELAS is complicated by mitochondrial heteroplasmy, which refers to the co-existence of mutated and normal mtDNA within the same individual. The clinical expression of MELAS is highly dependent on the proportion and tissue distribution of the mutated mtDNA [
20,
21,
22]. This phenomenon means that the pathogenic mtDNA variant may be undetectable in readily accessible samples, such as leukocytes, often necessitating genetic testing from other tissues, including buccal mucosa, urinary sediment, or skeletal muscle [
20,
21,
22]. This variability in mutational load across tissues can lead to false-negative results if only blood samples are analyzed [
20,
21,
22]. Furthermore, while a muscle biopsy revealing ragged red fibers (RRFs) is suggestive of mitochondrial myopathy, it is not always a mandatory or definitive diagnostic criterion, as biochemical results from muscle tissue can sometimes appear normal despite the presence of MELAS (
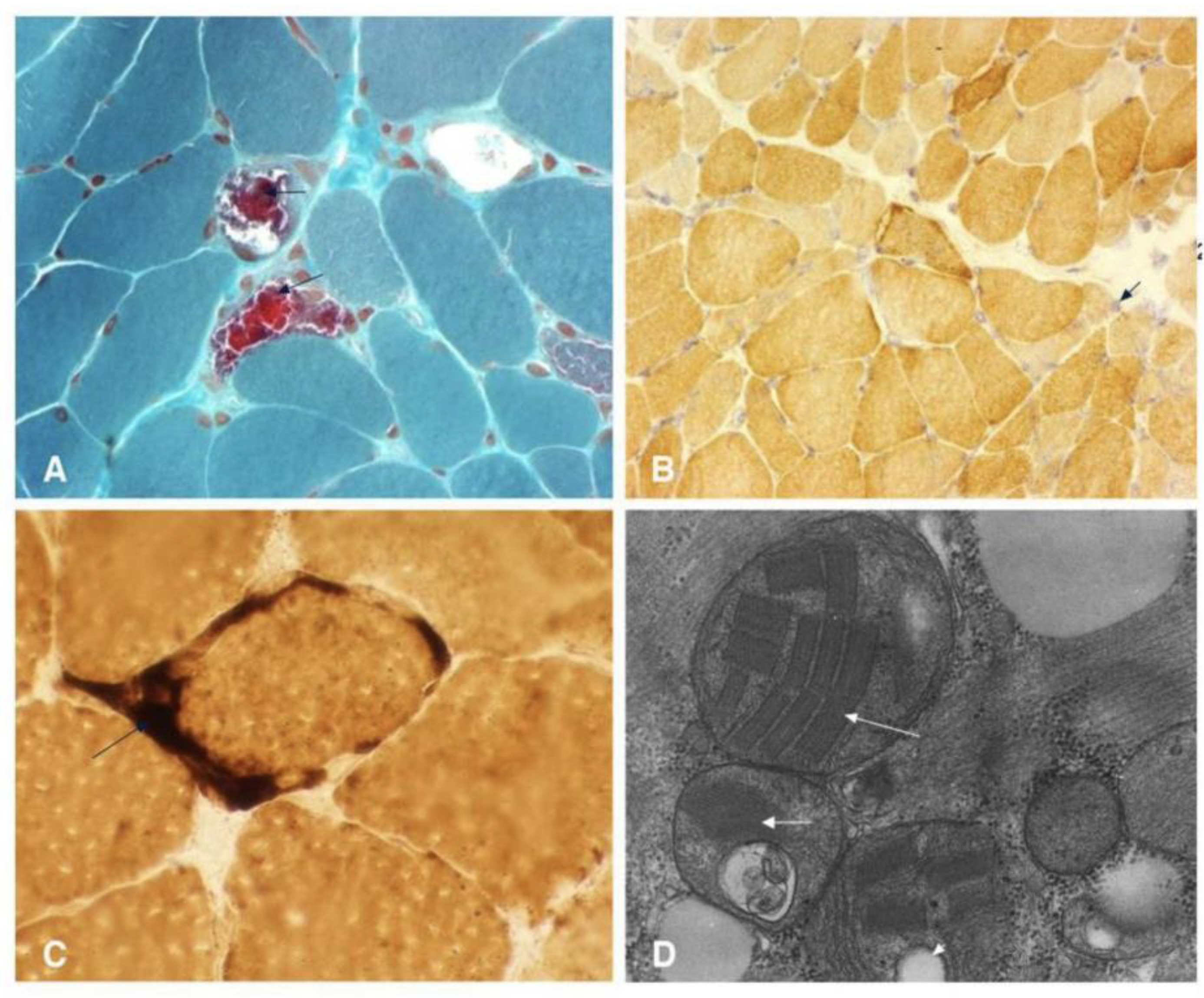
Figure 4 and
Table 1) [
20,
21,
22]. Neuroimaging, such as MRI, may reveal stroke-like lesions that do not conform to typical vascular territories; however, these findings are also not unique to MELAS and can be observed in other conditions [
20,
21,
22].
The non-specific and variable nature of MELAS symptoms, coupled with the complexities of heteroplasmy in traditional genetic testing, frequently leads to underdiagnosis or significant delays in establishing a definitive diagnosis. The current diagnostic reliance on invasive procedures like muscle biopsy or spinal tap, or on tests limited by tissue-specific mutational distribution, means that patients often endure a prolonged diagnostic odyssey. This "hidden" disease burden suggests that the true prevalence of MELAS may be higher than current estimates, which range from 1 in 500,000 to 16 in 100,000 in studies from Japan and Finland, respectively [
20,
21,
22]. This highlights a critical need for more sensitive, less invasive, and comprehensive diagnostic tools capable of overcoming the challenges posed by tissue-specific heteroplasmy and non-specific clinical presentations, for which CSF cfDNA epigenetic profiling offers a promising avenue.
3.2. Lysosomal Storage Diseases (e.g., Niemann-Pick, Gaucher)
Lysosomal storage diseases (LSDs) are a broad category of over 40 inherited metabolic disorders, each stemming from a deficiency in one of the lysosomal enzymes. This enzymatic defect leads to the progressive accumulation of undegraded substrates within lysosomes across various organs and tissues, ultimately causing cellular dysfunction and damage [
27,
28].
Niemann-Pick Disease (NPD) and Gaucher Disease (GD) are two of the most well-known LSDs. NPD mainly affects how the body uses and breaks down fats, including cholesterol and other lipids, inside cells, which causes them to build up toxically and eventually cause cell death [
28,
29,
30,
31]. NPD Types A and B are specifically brought on by mutations in the SMPD1 gene, which lead to a sphingomyelinase enzyme deficiency. On the other hand, NPD Type C results from intracellular cholesterol transport-related mutations in the NPC1 or NPC2 genes [
28,
29,
30,
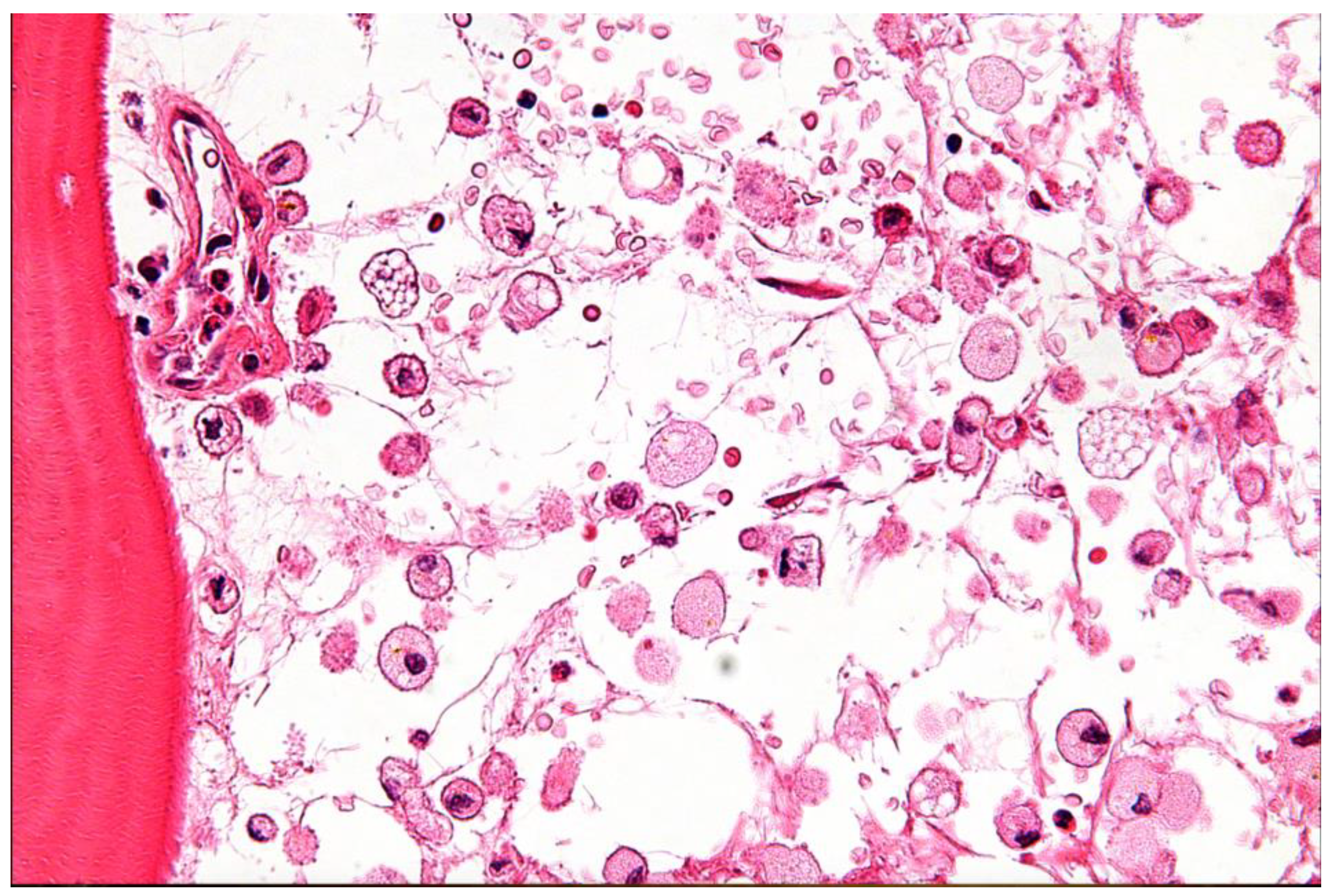
31]. Due to mutations in the GBA1 gene, Gaucher Disease (GD) is characterized by a lack of the lysosomal enzyme glucocerebrosidase. This deficiency leads to the accumulation of glucosylceramide within macrophages, forming characteristic "Gaucher cells" that infiltrate various organs (
Figure 5 and
Table 1) [
29,
30].
The clinical manifestations of LSDs are remarkably diverse and highly dependent on the specific enzyme deficiency and the patient's age of onset [
27,
28,
29,
30,
31,
32]. In early childhood, severe forms like NPD Type A often present with significant visceral involvement, including hepatosplenomegaly, prolonged jaundice, and pulmonary infiltrates, alongside neurological signs such as hypotonia, developmental delay, and failure to thrive [
27,
28,
29,
30,
31,
32]. As patients progress into later childhood or adolescence, conditions like NPD Type C and GD Type 3 are often dominated by progressive neurodegeneration, manifesting as ataxia, dysarthria, dysphagia, epileptic seizures, dystonia, and a progressive decline in cognitive function leading to dementia or intellectual disability [
27,
28,
29,
30,
31,
32]. Specific neurological signs like vertical supranuclear gaze palsy are highly characteristic of NPD Type C [
27,
28,
29,
30,
31,
32]. Other symptoms can include pain, organ enlargement, musculoskeletal abnormalities, muscle weakness, hearing loss, and corneal clouding [
27,
28,
29,
30,
31,
32]. In adults, particularly with NPD Type C or GD Type 1, presentations may be subtle, predominantly featuring early-onset dementia or psychiatric manifestations, though careful examination often reveals underlying neurological signs [
31]. It is noteworthy that non-neuronop[27–32athic GD Type 1 can increase susceptibility to Parkinson's disease later in life [
27,
28,
29,
30,
31,
32].
Despite the availability of biochemical tests and mutational analysis, which can generally confirm the diagnosis of LSDs [
27,
28,
29,
30,
31,
32], significant diagnostic limitations persist. A high index of suspicion is paramount for diagnosis due to the diverse and often nonspecific nature of early symptoms [
31]. For Niemann-Pick Type C (NP-C), specific challenges abound. Biomarkers such as oxysterols (cholestane-3β,5α,6β-triol and 7-ketocholesterol) are elevated in NP-C but are not exclusive to it, also appearing in NP-A, NP-B, and acid lipase deficiency, necessitating cautious interpretation [
27,
28,
29,
30,
31,
32]. Sample stability is another concern, as prolonged storage at room temperature can lead to cholesterol autoxidation, potentially yielding false-positive oxysterol results [
27,
28,
29,
30,
31,
32].
Genetic testing, while definitive, is complicated by the highly polymorphic nature of the
NPC1 gene, making the interpretation of novel mutations challenging. Routine sequencing methods may fail to detect large deletions/duplications or deep intronic changes, and variants of unknown significance (VUS) often require laborious functional assays for clarification [
27,
28,
29,
30,
31,
32]. The filipin staining test, once considered the gold standard, is no longer a first-line diagnostic tool and is now primarily reserved for assessing the functional significance of new genetic variants or confirming diagnoses when genetic testing is inconclusive [
27,
28,
29,
30,
31,
32] Neuroimaging findings, such as MRI or PET, are typically non-specific and may not be present in the early stages of the disease, thus their absence does not rule out NP-C [
31]. Clinically, the non-specific nature of symptoms like ataxia can lead to NP-C patients being "hidden" within broader patient cohorts, and challenges in psychiatric practice, such as incomplete medical histories, further impede timely diagnosis [
31]. While Gaucher disease diagnosis is confirmed by demonstrating glucocerebrosidase deficiency in leukocytes, the broad clinical spectrum, including the potential for later-onset neurological complications like Parkinsonism in Type 1, can still delay timely identification [
29,
30,
31,
32].
The profound heterogeneity of LSDs, encompassing a wide range of genetic causes and highly variable clinical presentations (including age of onset, organ involvement, and the balance between neurological and visceral symptoms), creates a complex diagnostic labyrinth. Even with established biochemical and genetic testing, the non-specific nature of early symptoms and the prevalence of variants of unknown significance can lead to substantial diagnostic delays and misdiagnoses, particularly for the neuronopathic forms. This complexity means that patients, especially those with atypical or adult-onset presentations, may experience prolonged diagnostic journeys, highlighting the inadequacy of current methods for early, definitive, and comprehensive diagnosis of CNS involvement. Epigenetic biomarkers, with their capacity to reflect tissue- and cell-specific changes and potentially capture subtle molecular alterations at an early stage, offer a promising approach to navigating this diagnostic complexity and identifying CNS involvement more promptly and accurately (
Figure 6) [
33].
3.3. Glucose Metabolism Disorders (e.g., GLUT1 Deficiency, Diabetes-Associated Cognitive Decline)
Disorders of glucose metabolism can profoundly impact brain function, ranging from rare genetic conditions to widespread acquired diseases. Two notable examples are Glucose Transporter Type 1 Deficiency Syndrome (GLUT1DS) and Diabetes-Associated Cognitive Decline (DCD).
GLUT1 Deficiency Syndrome (GLUT1DS): This is a rare genetic metabolic disorder caused by pathogenic variants in the
SLC2A1 gene, which encodes the GLUT1 protein [
34]. A deficiency in GLUT1 impairs the crucial transport of glucose across the blood-brain barrier (BBB) into the brain, resulting in chronic neuroglycopenia—a state of insufficient glucose supply to the brain, which relies primarily on glucose for energy [
34,
35,
36]. The clinical spectrum of GLUT1DS is highly variable [
34,
35,
36]. The most common manifestation is epilepsy, with seizures often commencing within the first six months of life. These can include generalized tonic-clonic, myoclonic, atypical absence, and atonic seizures [
34,
35,
36]. Beyond seizures, patients frequently exhibit deceleration of head growth during infancy, potentially leading to acquired microcephaly. Developmental delays and cognitive impairment, ranging from mild learning difficulties to severe intellectual disability, are also common [
34,
35,
36]. Movement disorders are prevalent, such as diminished muscle tone (hypotonia), poor balance and coordination (ataxia), slow and stiff limb movements (spasticity), and awkward postures (dystonia), including paroxysmal exercise-induced dyskinesia (PED) [
34,
35,
36]. Speech and language abnormalities, such as dysarthria and dysfluency, are also frequently observed [
34,
35,
36]. Interestingly, some individuals may present with non-classic forms of GLUT1DS, experiencing movement disorders and cognitive impairment without epilepsy, or may even be asymptomatic due to mosaicism, often identified when symptomatic family members undergo genetic testing [
34,
35,
36].
Diabetes-Associated Cognitive Decline (DCD): Cognitive impairment is a recognized and prevalent complication of both type 1 and type 2 diabetes [
37]. The pathophysiology of DCD is complex and multifactorial, involving direct effects of chronic hyperglycemia, insulin dysregulation and resistance, microvascular and macrovascular pathologies, oxidative stress, chronic low-grade inflammation, mitochondrial dysfunction, neurotransmitter dysregulation, and even epigenetic modifications [
37,
38,
39,
40]. Clinically, DCD manifests as impairments across multiple cognitive domains, including attention and concentration, memory (both short-term/working and long-term), executive functions (e.g., planning, decision-making, inhibition), and visuospatial abilities [
37,
38,
39,
40,
41,
42]. These deficits can range from mild cognitive impairment (MCI) to overt dementia [
42]. Diabetes significantly increases the risk of developing all types of dementia, including Alzheimer's disease and vascular dementia, with some studies suggesting a 65% higher risk of Alzheimer's in diabetics [
42,
43,
44].
Current diagnostic approaches for these disorders of glucose metabolism face notable limitations. For GLUT1DS, despite the classic presentation, the wide variability in symptoms and severity means that mild cases are often undiagnosed, contributing to a significant gap between estimated true prevalence (at least 1:24,000) and diagnosed cases [
34,
35,
36]. While reduced CSF glucose and lactate levels are key biochemical indicators, these levels can be near or within normal reference ranges, especially in milder presentations, making biochemical diagnosis challenging [
34,
35,
36,
37,
38,
39,
40,
41,
42]. Genetic testing for
SLC2A1 gene variants remains the gold standard for confirmation; however, variants in other genes (e.g.,
PURA,
HK1) can present with similar CSF biomarker profiles, complicating differential diagnosis. For instance,
HK1 variants result in low CSF glucose but
abnormally high CSF lactate, which distinguishes them from GLUT1DS, where lactate is low-normal or low [
40,
41,
42,
43,
44]. Functional assays, such as erythrocyte GLUT1 activity or protein quantification, are available but not universally accessible or definitive [
40,
41,
42,
43,
44]. Neuroimaging, including PET scans, can show reduced brain metabolism, but their accuracy and reliability in establishing a definitive diagnosis are not yet fully established [
40,
41,
42,
43,
44].
For DCD, the cognitive symptoms (e.g., memory, language, judgment issues) are often non-specific and can overlap considerably with normal age-related cognitive changes or other neurodegenerative conditions like Alzheimer's disease [
42,
43,
44]. Given that Type 2 Diabetes is known to hasten brain aging and cognitive decline, much like early Alzheimer's pathology, this mimicry is especially difficult [
40]. Additionally, changes in cerebral glucose metabolism, a major contributing factor to DCD, can occur decades before overt cognitive dysfunction manifests, making early detection challenging with available clinical tools [
39]. It is difficult to identify precise, actionable diagnostic markers or intervention targets due to the intricate and multifactorial pathophysiology of DCD, which involves a network of interrelated mechanisms (hyperglycemia, insulin resistance, vascular damage, and neuroinflammation) [
37].
The "silent progression" pattern shared by GLUT1DS and DCD is typified by early, subtle symptoms that are frequently missed or misdiagnosed. DCD specifically highlights how CNS pathology can be fueled by systemic metabolic dysfunction, like diabetes, through intricately linked mechanisms that closely resemble those of other neurodegenerative diseases. The crucial need for biomarkers that can identify disease processes before overt clinical symptoms appear or that can precisely distinguish these conditions from other neurodegenerative disorders or common age-related changes is highlighted by this clinical mimicry and the frequently subclinical onset. Present-day biomarkers are frequently non-specific or only show up when the disease is advanced. Epigenetic profiling of CSF cfDNA could offer a unique advantage by capturing early, subtle molecular shifts related to brain cell dysfunction or metabolic reprogramming, potentially enabling earlier and more accurate differentiation of these conditions, and distinguishing them from other neurodegenerative mimics (
Table 1) [
37,
38,
39,
40,
41,
42,
43,
44].
3.4. Overarching Challenges in the Diagnosis of Neurometabolic Disorders
The collective experience with MELAS, LSDs, and glucose metabolism disorders reveals several overarching challenges in the diagnosis of neurometabolic conditions. These diseases are individually rare but collectively numerous and highly heterogeneous, presenting with complex clinical pictures that can vary dramatically between pediatric and adult presentations [
1,
2]. This phenotypic complexity often leads to significant diagnostic delays and underdiagnosis, as symptoms frequently mimic more common neurological disorders [
1,
2]. Many symptoms, such as developmental delay, cognitive impairment, seizures, and movement disorders, are nonspecific and overlap across various MBDs and other neurological conditions, making differential diagnosis difficult [
1,
2]. While biochemical and genetic tests are often definitive for many MBDs, some still necessitate invasive procedures like muscle biopsies or spinal taps, which carry inherent risks and patient discomfort [
22]. Furthermore, traditional diagnostic measures often lack the sensitivity required for early disease detection, frequently revealing pathology only in late phases when potential therapeutic windows may have already closed [
15]. These collective limitations underscore the urgent need for novel, non-invasive, and highly sensitive diagnostic tools that can provide early, specific, and comprehensive molecular insights into CNS metabolic dysfunction (
Table 1).
Table 1: This table serves as a crucial summary, consolidating complex information about the diverse clinical presentations and, more importantly, the shared and specific diagnostic limitations across different categories of metabolic brain diseases. By presenting this information concisely, it visually reinforces the argument for the necessity of novel, more precise diagnostic tools. The juxtaposition of "Key Clinical Manifestations" with "Key Diagnostic Limitations" directly highlights the diagnostic gaps that epigenetic profiling of CSF cfDNA aims to address, such as non-specificity of existing biomarkers, challenges in genetic testing due to biological complexities (e.g., heteroplasmy), and the late manifestation of definitive symptoms. This structured overview provides a clear foundation for understanding why the proposed liquid biopsy approach is not merely an incremental improvement but a potentially transformative solution to long-standing clinical challenges.
4. Cerebrospinal Fluid as a Source for Cell-Free DNA Biomarkers in CNS Pathologies
The utility of liquid biopsy hinges on the accessibility and informative content of circulating biomarkers. Cell-free DNA (cfDNA), comprising fragmented nucleic acids released from cells, primarily through apoptosis, necrosis, or active secretion, is a highly promising analyte [
9]. In healthy individuals, cfDNA levels in circulation are typically low (e.g., <10 ng/mL in plasma) and predominantly originate from hematopoietic cells [
12]. However, in disease states such as cancer, myocardial infarction, stroke, or diabetes, cfDNA concentrations can significantly increase, sometimes by orders of magnitude, reflecting ongoing cellular damage or turnover [
10]. The short half-life of cfDNA, estimated between 4 minutes and 12 hours, allows it to provide a dynamic, "real-time" snapshot of physiological and pathological processes [
11].
4.1. Unique Characteristics and Functions of Cerebrospinal Fluid
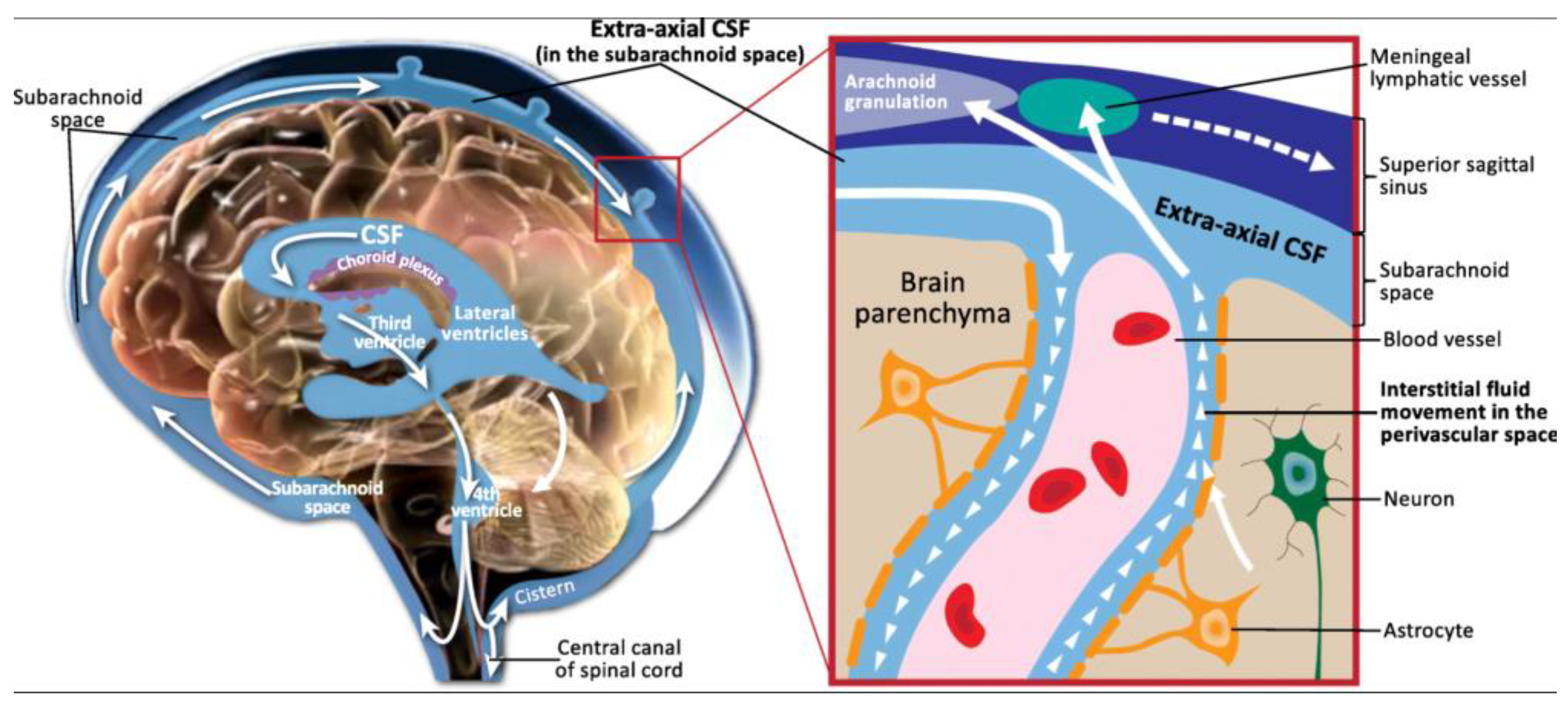
Cerebrospinal fluid (CSF) is a clear, colorless liquid that plays a vital role in the protection and physiological maintenance of the central nervous system (CNS) (
Figure 7) [
21,
47]. Primarily produced by the choroid plexus within the ventricular system, with additional contributions from the interstitial space of the brain and the subarachnoid space, CSF circulates throughout the brain and spinal cord [
45,
46]. Its production is a two-stage process involving passive filtration of plasma through capillary endothelium, driven by osmotic pressure, followed by active transport of plasma ultrafiltrate via choroid plexus epithelial cells, mediated by various ion transporters and aquaporins [
46]. This active transport, regulated by enzymes like carbonic anhydrase and specific ion pumps (e.g., Na+/K+ ATPase), ensures precise control over CSF composition [
46].
The functions of CSF are multifaceted. It provides crucial mechanical support, acting as a shock absorber that reduces the effective weight of the brain by 10-15 times, thereby protecting it from sudden impacts [
46,
47]. Beyond physical protection, CSF is integral to CNS homeostasis, facilitating the transport of essential nutrients, neurotransmitters, and hormones to brain cells while simultaneously eliminating metabolic waste products and toxic substances [
46,
47]. The integrity of the blood-cerebrospinal fluid barrier (BCB), formed by tight junctions between choroid plexus epithelial cells, is critical for maintaining CSF homeostasis and preventing uncontrolled substance movement [
46,
47]. Furthermore, CSF serves as an invaluable diagnostic medium, as changes in its composition (e.g., protein levels, cell counts, and the presence of specific biomarkers) are indicative of various CNS diseases, including infections, autoimmune disorders, and neurodegenerative conditions [
46,
47]. Its direct contact with brain tissue also makes it a conduit for administering drugs that might otherwise be impeded by the blood-brain barrier (BBB) [
46,
47].
4.2. Advantages of CSF-Derived cfDNA over Plasma cfDNA for CNS Pathologies
While plasma cfDNA has proven utility in many systemic diseases, its application for CNS pathologies, particularly brain-specific conditions, is often limited by the blood-brain barrier (BBB) [
3]. The BBB is a highly selective physiological barrier that restricts the passage of many molecules, including tumor-derived biomarkers like cfDNA, from the brain into the peripheral bloodstream [
3]. Consequently, in primary CNS tumors like gliomas, circulating tumor DNA (ctDNA) levels in plasma are typically very low, often below the detection threshold, making blood-based liquid biopsies challenging for these conditions [
3].
In stark contrast, CSF offers distinct advantages as a source for CNS-derived cfDNA. Being in direct anatomical contact with the brain and spinal cord, CSF directly reflects the molecular changes occurring within the CNS microenvironment. Studies have shown that the concentration of cfDNA in CSF can be significantly higher than in plasma. For instance, one study reported a mean cfDNA concentration of 26.82 ng/mL in CSF compared to 13.46 ng/mL in plasma [
19]. More importantly, the concentration of
tumor-specific cfDNA (ctDNA) in CSF can be several orders of magnitude higher than in plasma or urine in CNS malignancies like gliomas (
Figure 8) [
3,
48]. This elevated concentration directly translates to higher sensitivity for the detection of CNS-specific pathologies.
Beyond sheer quantity, the characteristics of cfDNA in CSF also differ from those in plasma. CSF samples exhibit a larger proportion of longer cfDNA fragments (e.g., >200bp) compared to plasma (37.8% in CSF vs. 22.8% in plasma) [
19]. Furthermore, distinct 6-bp end motif patterns allow for the clear separation of cfDNA from CSF and plasma, indicating unique fragmentation processes or cellular origins within each biofluid [
19]. CSF is also considered a "less complicated solution system" chemically than plasma, and it contains lower contamination from non-tumor cells, such as peripheral blood cells, which can confound analyses in plasma [
3]. This reduced background noise allows for more accurate and reliable detection of CNS-derived signals.
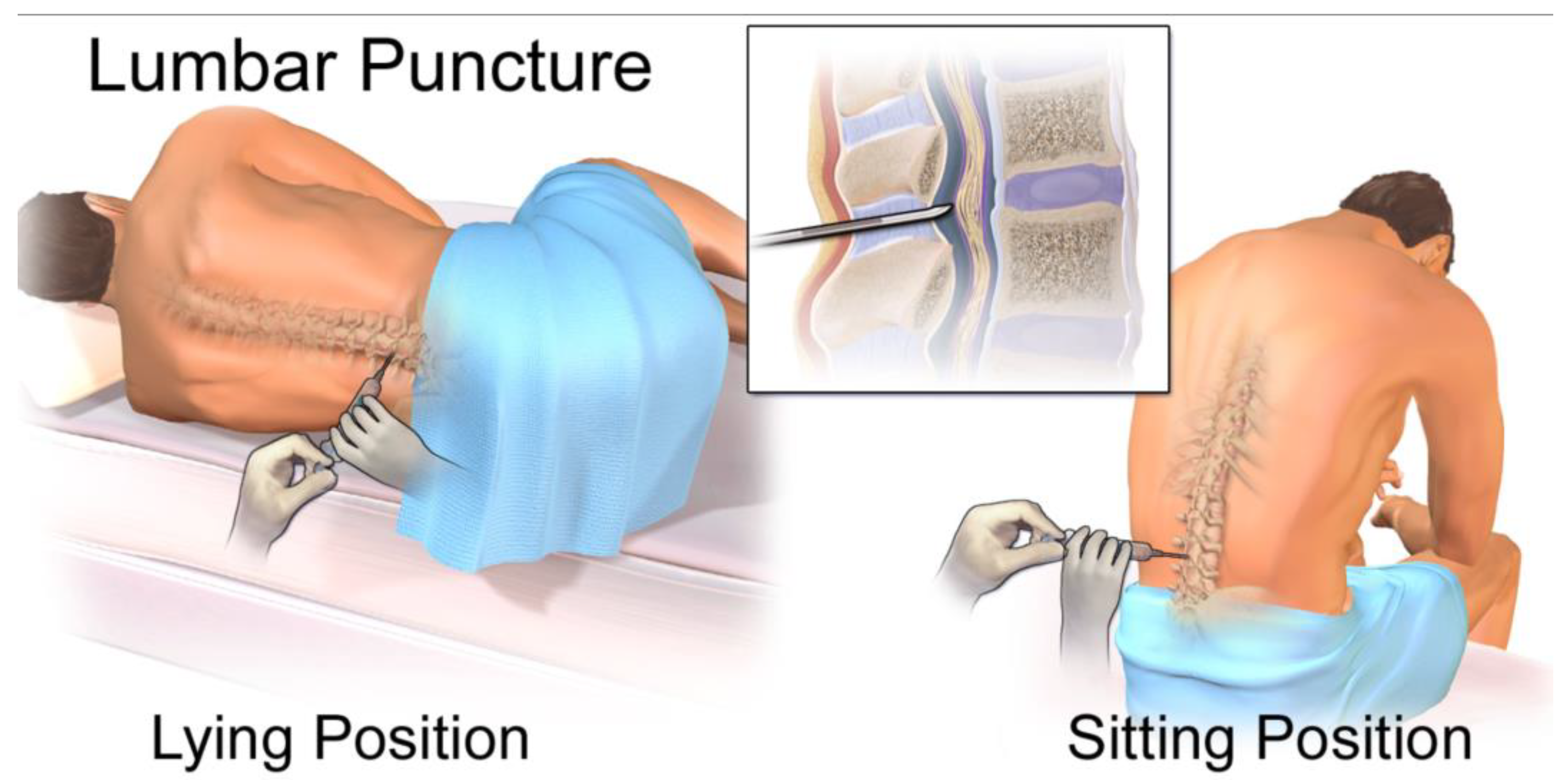
While lumbar puncture for CSF collection is more invasive than a blood draw, it is a routine clinical procedure that can be performed at the bedside, making longitudinal monitoring feasible for evaluating disease status and treatment response (Figure 9) [
3,
49]. This direct access to CNS-specific cfDNA, coupled with its higher concentration and lower background contamination, positions CSF as a superior biofluid for liquid biopsy applications in brain diseases, particularly when the blood-brain barrier limits the utility of peripheral blood samples. The distinct fragmentation patterns and end motifs observed in CSF cfDNA also suggest additional layers of information that can be leveraged for the development of advanced biomarkers, providing a richer molecular profile of CNS health and disease.
Figure 10.
Diagram of a lumbar puncture. This file is licensed under the Creative Commons Attribution 3.0 Unported license with permission from Wikimedia Commons [
49].
Figure 10.
Diagram of a lumbar puncture. This file is licensed under the Creative Commons Attribution 3.0 Unported license with permission from Wikimedia Commons [
49].
5. Epigenetic Profiling of cfDNA: Fundamental Mechanisms and Methodologies
Epigenetics refers to heritable changes in gene expression that occur without alterations to the underlying DNA sequence [
13]. These modifications are crucial for normal development, cell differentiation, and the maintenance of tissue-specific gene expression and cellular identity [
13]. Aberrant epigenetic changes are increasingly recognized as fundamental drivers of various human diseases, including cancer and neurodegenerative disorders [
13]. Unlike genetic mutations, epigenetic modifications are inherently dynamic and reversible, making them attractive targets for therapeutic intervention and powerful biomarkers for disease detection and monitoring [
13].
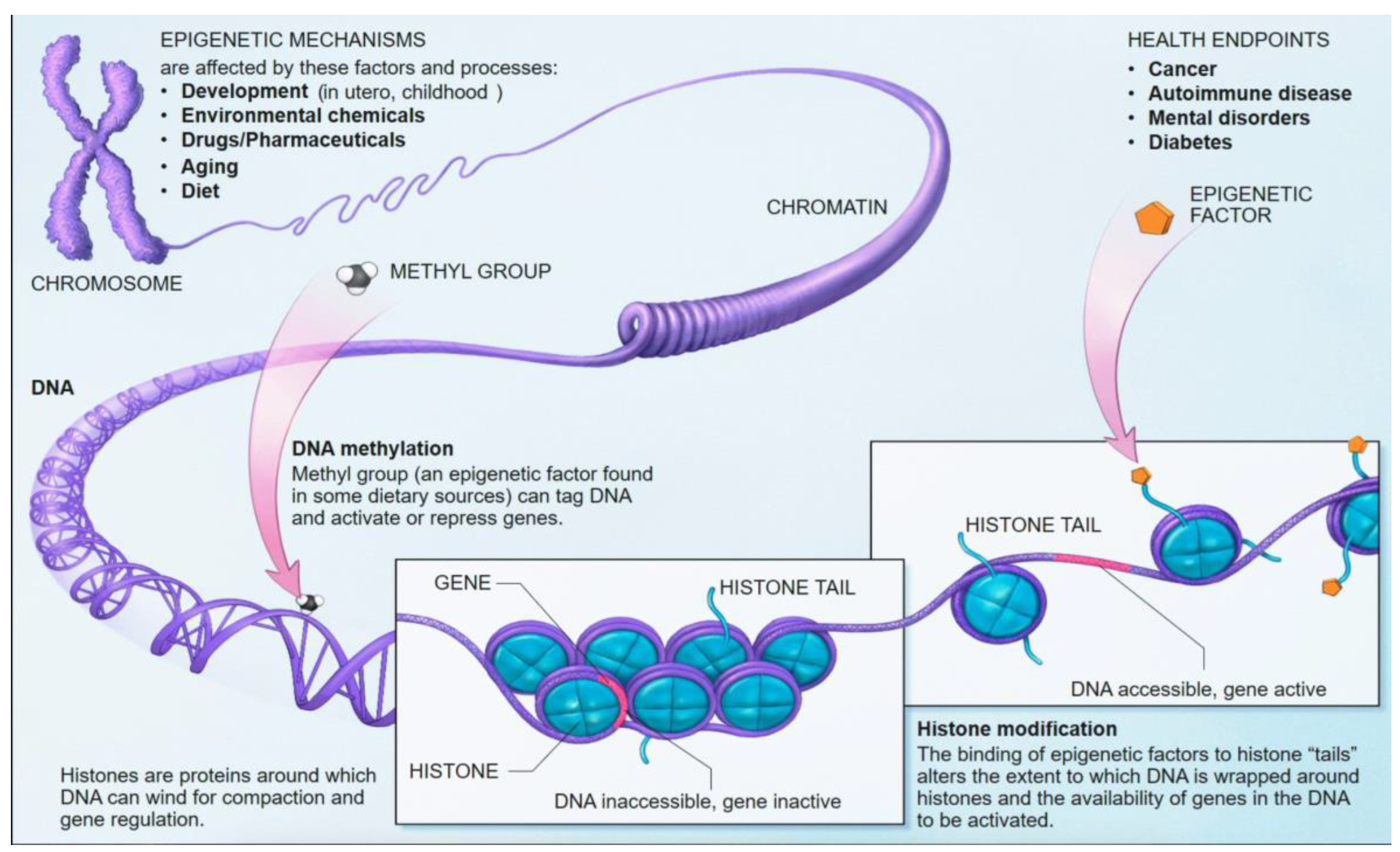
5.1. DNA Methylation: Mechanisms and Role in Gene Regulation
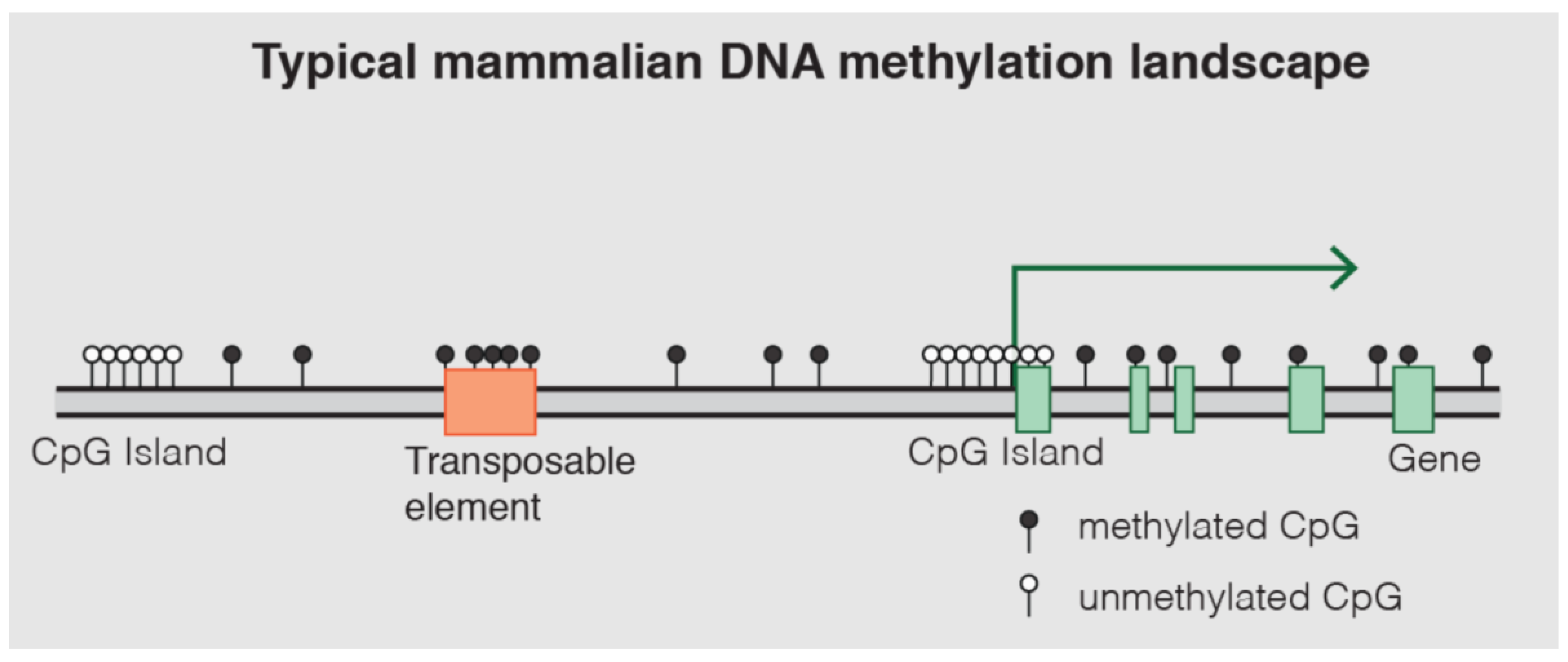
DNA methylation is one of the most well-characterized epigenetic mechanisms in mammals [
14]. It involves the covalent addition of a methyl group (CH3) to the fifth carbon position of a cytosine nucleotide, predominantly occurring within cytosine-guanine (CpG) dinucleotides [
13,
14,
15]. These CpG sites are often clustered in regions known as CpG islands, which are frequently located in gene promoter regions [
14]. Approximately 70% of gene promoter regions lie within CpG islands (
Figure 11) [
14,
50].
The process of DNA methylation is dynamically regulated by a balance of "writer" and "eraser" enzymes [
51,
52]. DNA methyltransferases (DNMTs) are the "writers" responsible for establishing and maintaining methylation patterns. DNMT3A and DNMT3B are
de novo methyltransferases that establish new methylation marks on unmethylated CpG sites. At the same time, DNMT1 acts as a "maintenance" methyltransferase, ensuring that methylation patterns are faithfully copied to newly synthesized DNA strands during replication [
52]. Conversely, Ten-Eleven Translocation (TET) enzymes act as "erasers," initiating DNA demethylation by oxidizing 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC) and further oxidized forms (5fC, 5caC), facilitating both passive (replication-dependent) and active (replication-independent) demethylation pathways [
52]. The interplay between DNMTs and TETs sculpts the intricate DNA methylation landscape, which is crucial for the flow of epigenetic information across cell generations [
53].
DNA methylation plays a critical role in gene regulation and chromatin structure. When methylation occurs within gene promoter regions, it typically leads to gene silencing. This occurs through two primary mechanisms: methylated cytosines can recruit gene suppressor proteins, and methylation can also induce the formation of heterochromatin. This condensed chromatin state physically prevents the binding of transcription factors and transcriptional machinery to the DNA [
11]. In contrast, methylation within gene bodies can be associated with transcript splicing alterations [
11]. Aberrant DNA methylation patterns are a hallmark of many diseases. For instance, in cancer, there is often global hypomethylation of the genome, accompanied by focal hypermethylation of tumor suppressor genes, both of which contribute to tumorigenesis [
13,
14]. Beyond disease, DNA methylation is essential for fundamental biological processes such as tissue-specific gene regulation, genomic imprinting, and X-chromosome inactivation [
11]. The unique methylation patterns across different cell types, despite having an identical underlying DNA sequence, form a fundamental aspect of tissue identity and cellular function [
13]. This inherent tissue specificity allows DNA methylation patterns to be used to determine the tissue of origin of cfDNA, which is a powerful principle for identifying the source of pathological processes [
11].
5.2. Methodologies for Methylome Interrogation in cfDNA
Analyzing DNA methylation in cfDNA, particularly from low-input samples such as CSF, requires highly sensitive and specific methodologies. Several advanced techniques have been developed for this purpose.
5.2.1. cfMeDIP-seq
Cell-free methylated DNA immunoprecipitation and high-throughput sequencing (cfMeDIP-seq) is a method specifically designed for identifying DNA methylation patterns in low-input cfDNA samples [
54,
55,
56,
57,
58,
59,
60,
61,
62]. The technical principle involves isolating cfDNA from the biofluid, followed by shearing the DNA (e.g., via sonication). Antibodies specifically targeting methylated DNA fragments are then used to immunoprecipitate and isolate these methylated regions [
62]. The enriched methylated DNA fragments are subsequently identified and quantified using next-generation sequencing (NGS). cfMeDIP-seq offers several advantages [
60]. It is not constrained by the need to identify specific tumor mutations; instead, it examines cancer-associated DNA methylation signatures across the entire genome in a tumor-agnostic fashion [
60]. Studies have demonstrated its high efficiency and specificity, with cfMeDIP-seq enriching for highly methylated regions, capturing ≥97% of methylated spike-in control fragments with ≤3% non-specific binding [
61]. It shows a preference for fragments with higher G+C content and more CpGs, which are often key regulatory regions [
61]. This method has been successfully applied to detect various cancers non-invasively, even with minimal sample input, and has shown high sensitivity and specificity in identifying hepatocellular carcinoma (HCC) from plasma cfDNA [
60,
61]. Its ability to assess peripheral blood plasma from cancer patients without prior knowledge of tumor origin or specific mutations makes it ideal for liquid biopsy applications [
60,
61].
5.2.2. Targeted Bisulfite Sequencing
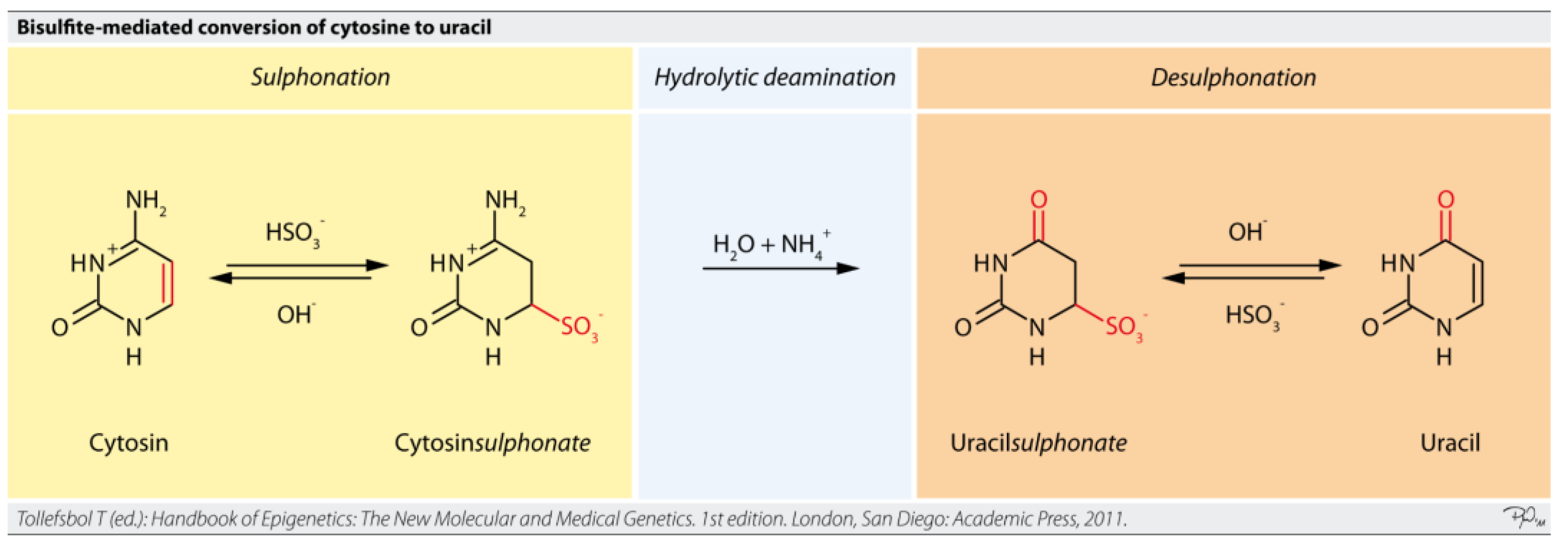
Targeted bisulfite sequencing is an approach that focuses on analyzing DNA methylation in specific regions of interest within the genome, rather than the entire genome (
Figure 12) [
63,
69]. This method combines the fundamental principle of bisulfite conversion with targeted enrichment strategies. Sodium bisulfite treatment converts unmethylated cytosine residues to uracil, while methylated cytosines remain unchanged [
57]. During subsequent PCR amplification, uracil is read as thymine, allowing for the differentiation of methylated from unmethylated cytosines by comparing the sequenced reads to a reference genome [
63].
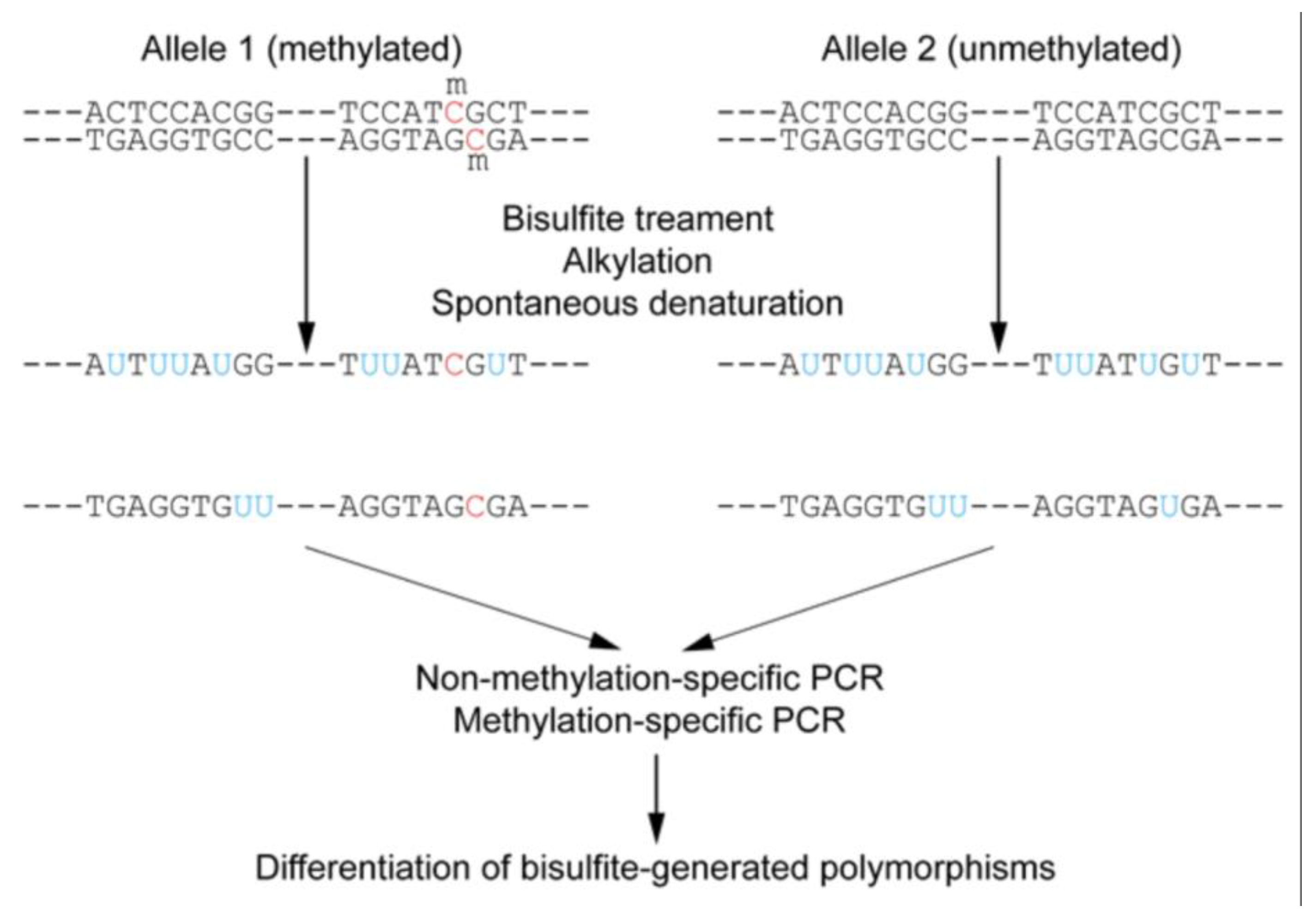
Targeted bisulfite sequencing can be performed in various ways. PCR-based enrichment involves designing specific primers to amplify only the target regions of interest (
Figure 13) [
63,
70]. Another variation, Reduced Representation Bisulfite Sequencing (RRBS), enriches for CpG-rich regions by using restriction enzymes (e.g., MspI) to digest genomic DNA, reducing the amount of sequencing required and lowering costs compared to whole-genome bisulfite sequencing (WGBS) [
63,
64,
65,
66]. However, RRBS may not cover all CpG regions and is unable to distinguish between 5mC and 5hmC [
66]. Oxidative bisulfite sequencing (oxBS-Seq) addresses this by enabling the absolute quantification of both 5mC and 5hmC at single-base resolution [
64].
While bisulfite conversion is considered the gold standard for DNA methylation analysis, it has limitations, particularly for ultra-low-input cfDNA. The bisulfite treatment process can cause DNA fragmentation and degradation (up to 30-50% degradation), requiring sufficient DNA input for optimal recovery and potentially leading to lower sequencing quality [
4,
64,
65,
66]. Bisulfite-treated DNA is also AT-rich, which can lead to non-specific PCR amplification [
64,
65,
66]. These challenges are particularly pronounced with highly fragmented, low-input cfDNA, where WGBS remains too costly for routine application due to its non-targeted nature and high sequencing budget requirements for robust quantification [
4]. RRBS also struggles with highly fragmented or degraded DNA [
63,
66,
67,
68].
5.2.3. Enzymatic Methyl-Sequencing (EM-seq) and EPIC Arrays
Newer enzymatic methods, such as Enzymatic Methyl-sequencing (EM-seq), offer significant advantages over traditional bisulfite conversion. EM-seq uses a milder enzymatic conversion process involving TET2 and APOBEC3A enzymes to convert non-methylated cytosines to uracil, while protecting 5mC and 5hmC from deamination [
4]. This enzymatic approach significantly reduces DNA damage and loss (degradation rate <5%), leading to higher quality and yield of sequencing libraries, especially from limited or degraded DNA samples, such as cfDNA [
4]. EM-seq has demonstrated high sensitivity, accurately detecting DNA methylation at single-base resolution with as little as 100 pg of DNA, making it highly suitable for cfDNA analysis in liquid biopsies [
68].
DNA methylation arrays, such as the Illumina Infinium MethylationEPIC v2.0 kit, provide a cost-effective and high-throughput alternative for profiling methylation across hundreds of thousands of CpG sites [
71]. These microarrays quantitatively interrogate CpGs at single-nucleotide resolution, providing accurate and precise methylation measurements independent of read depth [
71]. They cover extensive regions, including CpG islands, genes, and enhancers, and are compatible with various sample types, including formalin-fixed paraffin-embedded (FFPE) tissues, which is valuable for retrospective studies [
71]. While arrays offer broad coverage at a lower cost per sample, they provide only partial genomic coverage compared to sequencing methods, potentially missing key methylation sites not included on the array [
57].
The choice of methodology for cfDNA methylation analysis depends on the specific research question, available sample input, and desired resolution. While WGBS provides the most comprehensive data, its cost and computational demands are substantial [
57]. Targeted approaches like cfMeDIP-seq and targeted bisulfite sequencing offer cost-effectiveness and higher depth in specific regions. Newer enzymatic methods like EM-seq address the critical challenge of DNA degradation in low-input samples, improving data quality and yield. The ongoing advancements in these methodologies are crucial for overcoming the technical hurdles associated with ultra-low-input cfDNA, paving the way for more robust and widespread clinical applications of epigenetic liquid biopsies [
72,
73].
6. Translational Applications: Mapping Brain Cell-Type-Specific Injury, Inflammatory Signaling, and Metabolic Reprogramming
The ability to profile epigenetic signatures in CSF-derived cfDNA opens unprecedented avenues for understanding the molecular underpinnings of metabolic brain diseases. This approach moves beyond traditional bulk tissue analysis, offering the potential to resolve disease processes at the level of specific brain cell types, track inflammatory responses, and identify metabolic reprogramming events.
6.1. Brain Cell-Type-Specific Injury
A profound strength of cfDNA methylation profiling lies in its capacity to identify the tissue and even cell type of origin of circulating DNA fragments.
11 Each cell type in the body possesses a unique DNA methylation pattern, which is intrinsically linked to its gene expression profile and cellular identity [
11]. These methylation patterns are remarkably stable under physiological and pathological conditions, allowing them to serve as reliable markers of cell death in specific organs [
15]. When cells in a particular brain region or of a specific cell type undergo damage or death, they release their DNA into the CSF, carrying these unique methylation signatures [
9].
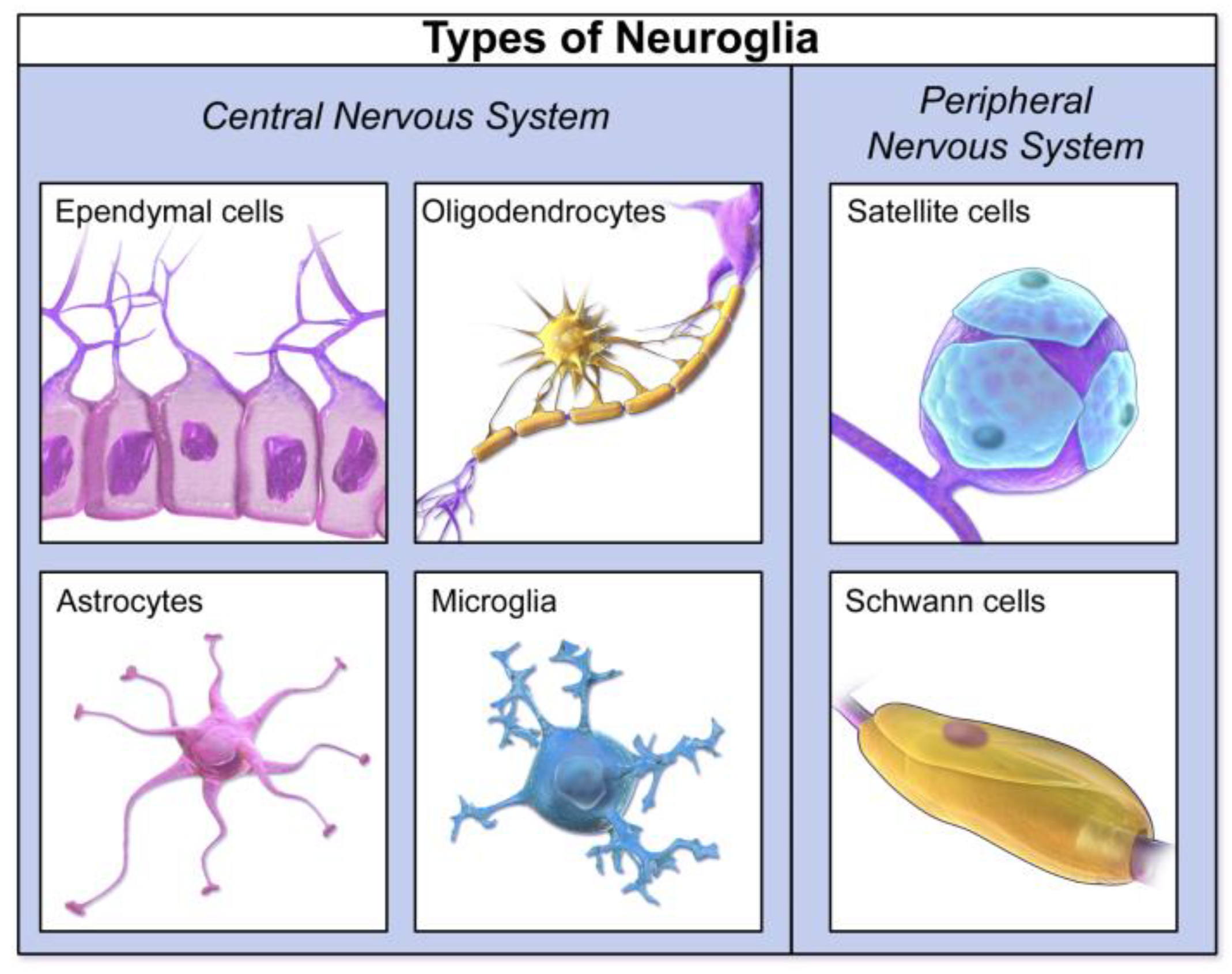
Sophisticated deconvolution algorithms have been developed to analyze the mixed cfDNA methylation profiles in CSF and estimate the proportional contributions from different brain cell types, such as neurons, astrocytes, oligodendrocytes, and microglia [
11]. For instance, studies have shown that cfDNA methylation changes can reflect cell-type-specific gene dysregulation in the brains of patients with neurodegenerative diseases, such as Amyotrophic Lateral Sclerosis (ALS), particularly in excitatory neurons and astrocytes [
75,
76,
77]. In Alzheimer's disease (AD), specific DNA methylation signatures initially identified in cortical neurons and brain tissue have been validated in CSF-derived cfDNA, demonstrating the potential to diagnose AD even before clinical manifestation [
75,
78,
79,
80,
81]. This capability to detect cfDNA originating from minority cell populations, such as oligodendroglial lineage cells in multiple sclerosis, highlights the exquisite sensitivity of this approach (
Figure 14) [
11,
74,
78,
79,
80,
81].
The ability to pinpoint the specific brain cell types undergoing injury or death offers a granular understanding of disease progression that is unattainable with bulk tissue analysis or traditional neuroimaging. This is particularly relevant for metabolic brain diseases, where different cell populations (e.g., neurons, glia) may be differentially affected by metabolic defects. For example, in GLUT1DS, understanding which specific brain cells are most impacted by glucose deficiency could refine therapeutic strategies. Similarly, in neurodegenerative conditions like frontotemporal dementia (FTD), cfDNA methylation changes in CSF have been observed to be dependent on the clinical stage and can even show subtype-specific patterns, providing clues about the genomic location of these changes and their tissue origin [
79]. This level of detail allows for a more precise mapping of localized neurodegeneration and provides valuable insights into the underlying pathological mechanisms.
6.2. Inflammatory Signaling
Neuroinflammation is a common pathological feature across various neurological disorders, including metabolic brain diseases, and contributes significantly to neuronal damage and cognitive decline [
41]. Epigenetic mechanisms, particularly DNA methylation, play a crucial role in regulating inflammatory gene expression and signaling pathways within the CNS [
80].
In Niemann-Pick disease type C1 (NPC1), for instance, neuroinflammation is a prominent part of the pathological cascade [
82]. Studies in NPC1 mouse models and human post-mortem brain tissues have identified significant changes in the expression of inflammation-associated genes, such as complement 3 (C3) [
82]. Analysis of CSF from NPC1 patients has revealed altered levels of various inflammatory markers, including increased interleukin 3 (IL-3), chemokine (C-X-C motif) ligand 5 (CXCL5), interleukin 16 (IL-16), and chemokine ligand 3 (CCL3), alongside decreased levels of anti-inflammatory cytokines like IL-4, IL-10, and IL-13 [
82]. These findings suggest that CSF-derived cfDNA methylation patterns could reflect these inflammatory shifts, providing a molecular readout of neuroinflammation.
Similarly, in neuronopathic Gaucher disease (nGD), neuroinflammation is a central pathological feature driven by the accumulation of glycosphingolipids and the activation of microglia, NK cells, astrocytes, and neurons [
83]. Targeted genetic rescue experiments have demonstrated that ameliorating the buildup of glucosylceramide (GlcCer) and glucosylsphingosine (GlcSph), particularly in microglia and macrophages, can reverse neuroinflammation and improve survival [
83,
84,
85,
86]. This highlights the critical role of specific cell types in driving the inflammatory response. In diabetes-associated cognitive decline (DCD), hyperglycemia itself triggers a neuroinflammatory state by promoting the formation of advanced glycation end products (AGEs) and inducing oxidative stress, leading to the upregulation of inflammatory cytokines (e.g., TNF-α, IL-1, IL-2, IL-6) and damage to the blood-brain barrier [
41]. Sustained glial activation and inflammation can make the brain more susceptible to injury and neurodegeneration [
41].
Epigenetic profiling of CSF cfDNA offers a unique opportunity to monitor these inflammatory signaling pathways. Changes in DNA methylation patterns on cfDNA fragments originating from activated microglia or astrocytes, or neurons undergoing inflammatory stress, could serve as sensitive biomarkers of neuroinflammation. Given that cfDNA levels themselves can be influenced by inflammation and can trigger immune-inflammatory reactions, analyzing the epigenetic landscape of CSF cfDNA could provide a comprehensive picture of the inflammatory status within the CNS, aiding in disease monitoring and evaluating the efficacy of anti-inflammatory therapies (
Table 2) [
12].
6.3. Metabolic Reprogramming
Metabolic reprogramming, the alteration of cellular metabolic pathways, is a fundamental process in many diseases, including metabolic brain disorders. Epigenetic modifications are intricately intertwined with cellular metabolism, as metabolites can serve as substrates or cofactors for epigenetic modifying enzymes, and conversely, epigenetics can regulate the expression of metabolic genes [
51].
In GLUT1 Deficiency Syndrome, the primary metabolic defect is an insufficient transport of glucose into the brain.
33 Beyond the direct energy deficit, this chronic neuroglycopenia can cause broader disruptions at the cellular level. Next-generation metabolic screening of CSF from GLUT1DS patients has identified novel biomarkers, including decreased levels of gluconic and galactonic acid, as well as xylose-α1-3-glucose and xylose-α1-3-xylose-α1-3-glucose [
34,
35,
36]. The latter two, potentially originating from O-glycosylated proteins, suggest that insufficient glucose may affect protein glycosylation, impacting various cellular processes beyond just energy production [
34,
35,
36]. These findings imply that brain glucose deficiency leads to metabolic reprogramming, which could be reflected in cfDNA methylation patterns (
Table 2).
In diabetes-associated cognitive decline (DCD), impaired glucose homeostasis and insulin signaling play a central role in brain dysfunction [
40]. Chronic hyperglycemia and insulin resistance induce oxidative stress, neuroinflammation, and impaired synaptic plasticity, all of which contribute to cognitive impairment [
37]. The brain's reliance on glucose catabolism for energy production and neurotransmission means that metabolic modifications, particularly those affecting glucose oxidation pathways and the glutamine-glutamate/GABA cycling between astrocytes and neurons, are crucial for functional deterioration [
40]. Epigenetic modifications are known to be influenced by various extrinsic factors, including nutrition and metabolic state [
51]. For instance, maternal obesity has been linked to altered offspring brain DNA methylation profiles and behavior, detectable through cell-free fetal DNA (cffDNA) methylation in maternal blood, correlating with metabolic and immune markers [
84,
85,
86]. This demonstrates a clear link between systemic metabolic state, epigenetic changes, and neurodevelopment (
Table 2).
CSF cfDNA methylation profiling can provide insights into these metabolic reprogramming events within the brain. By identifying specific methylation patterns associated with altered glucose utilization, mitochondrial dysfunction, or shifts in metabolic pathways (e.g., one-carbon metabolism, glycolysis/OXPHOS switch) in specific brain cell types, this approach can uncover the molecular signatures of metabolic stress and adaptation [
84,
85,
86,
87,
88,
89,
90,
91,
92,
93,
94,
95,
96,
97]. This offers a powerful means to understand how metabolic defects translate into neurological dysfunction and to monitor the efficacy of interventions aimed at restoring metabolic balance in the brain (
Table 2).
7. Diagnostic and Prognostic Utility of CSF cfDNA Methylation Biomarkers
The inherent properties of cfDNA, combined with the specificity of epigenetic signatures, position CSF cfDNA methylation profiling as a powerful tool with significant diagnostic and prognostic potential for metabolic brain diseases. This approach offers advantages over traditional CSF markers and neuroimaging, particularly in early detection and dynamic monitoring (
Table 2).
7.1. Comparison with Traditional CSF Markers and Neuroimaging
Traditional CSF markers for neurological diseases typically include protein levels, cell counts, glucose, and lactate, which provide general indicators of infection, inflammation, or metabolic disturbances [
78,
85,
86,
87,
88,
89,
90,
91,
92]. For specific MBDs, markers like elevated CSF lactate in MELAS are indicative but lack specificity [
20,
21,
22]. In GLUT1DS, low CSF glucose and lactate are characteristic, but these levels can be borderline or near normal in mild cases, limiting their diagnostic accuracy [
34,
35,
36]. Neuroimaging techniques, such as MRI and PET, provide structural and functional insights into the brain. While MRI can reveal stroke-like lesions in MELAS or atrophy in advanced LSDs, these findings are often nonspecific or absent in early disease stages [
34,
35,
36]. PET scans can show reduced brain metabolism in GLUT1DS or DCD, but their reliability for definitive diagnosis is not fully established (
Table 1 and
Table 2) [
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36].
CSF cfDNA methylation biomarkers offer several distinct advantages. Firstly, they provide molecular-level information directly from the CNS, reflecting cell death and gene regulatory changes that are often more specific to disease pathology than general metabolic markers or structural imaging [
9]. The ability to identify the tissue and cell type of origin of cfDNA fragments allows for precise mapping of CNS damage, which is a significant leap beyond bulk CSF protein measurements or broad imaging findings [
11]. For instance, while neurofilament light chain (NfL) is a valuable general biomarker of neurodegeneration, it lacks the targeted insights that cfDNA methylation can provide regarding specific cell populations [
75] (
Table 1 and
Table 2).
Secondly, cfDNA has a short half-life (minutes to hours), making it a dynamic biomarker that can provide a "real-time" assessment of disease activity and treatment response [
11]. This contrasts with some traditional protein biomarkers or neuroimaging findings that may represent late-stage pathology or accumulate slowly [
15]. For example, in Alzheimer's disease, while amyloid-beta changes are noted early, pTau levels, crucial for differential diagnosis, often increase only in advanced stages [
78]. Epigenetic cfDNA analysis can potentially detect early, subtle molecular shifts before overt clinical symptoms or significant structural changes are visible, thereby expanding the therapeutic window (
Table 1 and
Table 2) [
9].
Table 2.
Comparative Overview – Imaging vs CSF Biomarkers [
105,
107,
108,
109,
110,
111,
112,
113,
114,
115,
116].
Table 2.
Comparative Overview – Imaging vs CSF Biomarkers [
105,
107,
108,
109,
110,
111,
112,
113,
114,
115,
116].
| Modality |
Measured Target |
Quantitative/Qualitative Output |
Diagnostic Accuracy |
Limitations |
| CSF Lactate [105,107,108,109,110 |
Anaerobic CNS metabolism |
4.4 mmol/L in MELAS vs ~1.6 in controls; cut-off >2.2 mmol/L; AUC 0.994 |
High sensitivity (94–100%) & specificity (100%) in pediatric series |
Requires lumbar puncture; affected by seizures/infection |
| ^1H-MRS (MELAS) [111 |
Brain lactate peak (lactate/Cr) |
0.40 ±0.05 (frontal), 0.32 ±0.03 (occipital) vs zero controls; correlated with CSF lactate (r=0.85) |
Qualitative, binary presence detection |
Not quantitative; low dynamic range |
| ^31P-MRS (Muscle MELAS) [111 |
Pi/PCr ratio |
Elevated post-exercise; lowered post-recovery |
Reflects peripheral mitochondrial dysfunction |
Indirect CNS measure; muscle-specific |
| MRI (MELAS) [111 |
Stroke-like lesions |
FLAIR/T2 hyperintensities, inverted lactate double peak, NAA/Cr low (~0.79 vs 1.8–2.2) |
Visual identification of lesions |
Structural, post-lesion; no early detection |
| PET/CSF in Alzheimer’s |
Aβ + tau PET vs CSF Aβ42/t-tau |
PET AUC 0.92–0.93; CSF Aβ42/t-tau AUC 0.93–0.94; Sens 97%, Spec 83% |
Equally accurate; CSF slightly better |
Both require LP or PET; cost |
| GLUT1 Deficiency Syndrome |
CSF glucose and CSF/serum ratio |
CSF glucose: 34–44% of blood; CSF/serum ratio ~0.34–0.44; no standardized lactate cut-off but often low-normal. |
FDG-PET: Lenticular/thalami SUV ratio cut-off ≥1.54 discriminates GLUT1-DS vs. epilepsy controls with 100% sensitivity & 98% specificity. Widespread hypometabolism in thalamus, cerebellum, and temporal cortex by SPM |
CSF glucose remains a more direct, earlier marker; PET shows high accuracy but requires high-cost imaging |
7.2. Diagnostic and Prognostic Applications in Neurometabolic Disorders
The application of CSF cfDNA methylation profiling holds substantial promise for improving the diagnosis and prognosis of metabolic brain diseases (Table 3).
Early Detection and Differential Diagnosis: The capacity of cfDNA methylation to identify specific tissue and cell-type signatures means it can potentially detect early cellular damage or dysfunction in the brain before widespread clinical symptoms emerge [
16]. This is particularly critical for MBDs, where early intervention can significantly impact disease progression and patient outcomes [
2]. Moreover, by providing highly specific molecular fingerprints, cfDNA methylation patterns can aid in differentiating between various MBDs that share common, non-specific clinical presentations, or distinguish them from other neurological disorders they mimic [
11]. For example, in CNS tumors, methylation-based classification of CSF cfDNA has shown high accuracy in discriminating major malignant brain tumor types, approaching the accuracy of standard-of-care tissue biopsies [
89]. While MBDs are not tumors, this demonstrates the principle of specific classification.
Monitoring Disease Progression and Treatment Response: The dynamic nature of cfDNA, with its short half-life, allows for repeated sampling and real-time monitoring of disease activity [
9]. Changes in the levels or methylation patterns of cfDNA originating from specific brain cell types could indicate progression of neurodegeneration, shifts in inflammatory states, or the effectiveness of therapeutic interventions [
9]. This is valuable for assessing the impact of therapies aimed at correcting metabolic defects or mitigating neuroinflammation. For instance, in glioma, longitudinal CSF cfDNA monitoring has shown changes in tumor-associated variant allele frequencies in response to chemoradiation, even through pseudoprogression [
91]. This principle can be extended to MBDs to track the molecular response to treatments.
Prognostic Insights: Elevated cfDNA concentrations, particularly of circulating tumor DNA (ctDNA) in oncology, often correlate with overall tumor burden and advanced disease stage, indicating poorer clinical outcomes [
4]. While direct correlations for MBDs are still emerging, it is plausible that specific cfDNA methylation signatures or quantitative changes could provide prognostic information, predicting disease severity, progression rates, or response to specific therapies. This could enable more precise risk stratification and personalized treatment planning for patients with MBDs (
Table 2) [
9].
The integration of CSF cfDNA methylation analysis into clinical evaluation holds the potential to transform the diagnostic paradigm for metabolic brain disorders. By offering a minimally invasive window into the molecular pathology of the CNS, it can facilitate earlier, more accurate diagnoses, enable dynamic disease monitoring, and provide critical prognostic insights, ultimately paving the way for more targeted and effective patient management strategies.
Table 3.
Proposed CSF cfDNA Epigenetic Biomarkers in Metabolic Brain Diseases. Epigenome-wide profiling in CSF shows distinct CNS-derived methylation patterns in genes linked to neuronal development and energy metabolism.
Table 3.
Proposed CSF cfDNA Epigenetic Biomarkers in Metabolic Brain Diseases. Epigenome-wide profiling in CSF shows distinct CNS-derived methylation patterns in genes linked to neuronal development and energy metabolism.
| Disease |
Proposed cfDNA Epigenetic Features |
Potential Advantages |
| MELAS [98,102,104,106] |
Neuron/glia-specific methylation shifts affecting mitochondrial genes |
Early detection of cell-type-specific injury; more precise than lactate |
| Lysosomal Disorders (e.g., Niemann-Pick, Gaucher) [99] |
Methylation alterations in microglia/neuron cfDNA tied to inflammation and lipid pathways |
Better detection of neuroinflammation vs. unstable small molecules |
| GLUT1DS [35,85,100,101,102] |
cfDNA methylation changes in glucose transport/metabolism genes (e.g., SLC2A1 targets) |
Detects CNS metabolic stress even if glucose is near-normal; complements new metabolic markers |
| Neurodegenerative-Metabolic (e.g., PD, AD, MS) [97,103,105] |
cfDNA profiles reflecting insulin signaling, oxidative stress, and synaptic integrity |
Enables differentiation from pure neurodegeneration |
8. Challenges and Future Directions in Epigenetic Liquid Biopsy for Neurometabolic Disorders
Despite the immense promise of epigenetic profiling of CSF-derived cfDNA for metabolic brain diseases, several technical, interpretive, and clinical challenges must be addressed to facilitate its widespread clinical translation.
8.1. Technical Challenges
The analysis of ultra-low-input cfDNA, particularly from CSF, presents significant technical hurdles.
Low DNA Concentration: While CSF cfDNA concentrations can be higher than plasma for CNS pathologies, the absolute quantities remain low compared to traditional tissue biopsies [
5]. This low input can compromise the sensitivity and reliability of methylation assays, increasing the risk of false-positive results [
4,
63]. Methods like cfMeDIP-seq and enzymatic methyl-sequencing (EM-seq) are designed for low-input samples, but consistent high-quality data remains a challenge [
60].
DNA Integrity and Degradation: Bisulfite conversion, a cornerstone of many methylation analysis methods, can cause significant DNA fragmentation and degradation, impacting sequencing quality and accuracy [
4]. While EM-seq offers a milder, less degradative alternative, optimizing library preparation for highly fragmented cfDNA is crucial [
63].
Contamination and Background Noise: Although CSF generally has lower non-tumor cfDNA contamination than plasma, contamination from cellular DNA during sample collection or processing can still occur [
3,
5]. This can introduce high molecular weight DNA contamination, affecting the accuracy of methylation classification [
5]. Careful sample handling protocols, including immediate centrifugation and proper storage, are essential to minimize this [
11].
Standardization of Assays: A major barrier to clinical adoption is the lack of standardized protocols for cfDNA collection, processing, and analysis [
11]. Variability in laboratory procedures, from DNA extraction kits (e.g., MagMAX) to library preparation (e.g., SureSelect XT HS2) and sequencing platforms (e.g., Illumina NovaSeq), can lead to inconsistent results across studies and laboratories [
5]. Establishing robust, reproducible, and universally accepted protocols is critical for validating cfDNA methylation biomarkers for routine clinical use [
73].
8.2. Interpretive Challenges
The complexity of cfDNA methylation data necessitates advanced bioinformatics and careful interpretation.
Data Complexity and Bioinformatics: Genome-wide methylation profiling generates vast, high-dimensional datasets that require significant computational resources and specialized bioinformatics expertise for processing, analysis, and interpretation [
4]. Developing user-friendly software tools and pipelines that can accurately deconvolve tissue-of-origin signals from complex cfDNA mixtures and identify disease-specific methylation patterns is an ongoing challenge [
88].
Cell-Type Deconvolution Accuracy: While deconvolution algorithms (e.g., CelFiE, CelFEER, cfDecon) are advancing, accurately estimating cell-type proportions from cfDNA methylation data, especially for rare cell types or in the presence of unknown cell types, remains challenging [
88]. The accuracy of these methods relies on robust reference methylome datasets for various brain cell types, which are still being developed [
17].
Dynamic Nature of Epigenetic Signatures: Epigenetic signatures can change throughout disease progression or in response to treatment [
11]. While this dynamism offers opportunities for monitoring, it also adds complexity to interpretation, as a single methylation profile may not capture the full disease trajectory. This necessitates longitudinal studies and the development of dynamic predictive models.
Overfitting and Generalizability: Methylation classifiers, especially for rare diseases or subtypes, can suffer from overfitting if training datasets are not comprehensive or representative [
57]. This can limit their generalizability to new, unseen patient populations, potentially leading to misclassification [
57].
8.3. Clinical Integration and Regulatory Challenges
Translating epigenetic liquid biopsy from research to routine clinical practice involves significant regulatory, ethical, and economic considerations.
Invasiveness of CSF Collection: While less invasive than brain biopsy, lumbar puncture for CSF collection is still an invasive procedure that can cause discomfort and carries risks such as post-dural puncture headache [
5]. This limits its applicability for widespread screening or very frequent monitoring, particularly in pediatric populations where ethical standards are stricter [
6].
Regulatory Frameworks: The regulatory landscape for novel liquid biopsy biomarkers, especially for non-oncological CNS conditions, is still evolving [
6]. Clear guidelines for assay validation, clinical utility, and reimbursement are necessary to accelerate adoption. The FDA's emphasis on clear scientific rationale, risk minimization, and comprehensive informed consent for biopsies in clinical trials underscores the stringent requirements for new diagnostic tools [
6].
Cost-Effectiveness: The advanced technologies required for cfDNA methylation analysis, such as next-generation sequencing, can be expensive, with costs primarily driven by consumables [
4]. While liquid biopsies can offer downstream economic benefits by avoiding more invasive procedures or guiding more efficient therapy allocation, the initial cost remains a significant barrier to widespread clinical use, particularly for rare diseases.
11 Developing more cost-effective molecular profiling methods and non-proprietary assay panels is crucial [
11].
Ethical Considerations: Beyond the invasiveness of CSF collection, data privacy concerns and ethical issues related to genetic and epigenetic information derived from liquid biopsies need careful consideration, especially as these technologies become more accessible [
95].
8.4. Future Directions
Despite the challenges, the field of epigenetic liquid biopsy for neurometabolic disorders is rapidly advancing, with several promising future directions.
Integration with Artificial Intelligence (AI) and Machine Learning: AI and machine learning algorithms are pivotal for processing complex cfDNA methylation data, improving diagnostic accuracy, and identifying novel methylation signatures [
7]. AI can enhance biomarker discovery, optimize classification models, and aid in the interpretation of complex genomic and epigenomic landscapes [
87].
Multi-Omics Approaches: Combining cfDNA methylation profiling with other omics data, such as proteomics (e.g., neurofilament light chain, tau, α-synuclein), transcriptomics (cfRNA), and metabolomics, can provide a more comprehensive and holistic understanding of disease mechanisms and progression [
86]. This integrated approach can capture multi-layered genomic and epigenomic information, enhancing diagnostic and prognostic precision [
75].
Longitudinal Studies and Biomarker Validation: Large-scale, prospective longitudinal studies are essential to validate the diagnostic and prognostic utility of CSF cfDNA methylation biomarkers across diverse patient populations and disease stages [
18]. Such studies will help establish robust reference ranges, track dynamic changes over time, and correlate molecular findings with clinical outcomes and treatment responses.
Development of Less Invasive CSF Collection Methods: Research into less invasive or alternative methods for accessing CNS-derived biomarkers, potentially through advanced blood-based approaches that can overcome BBB limitations or novel micro-invasive techniques, could broaden the applicability of these powerful molecular tools.
Therapeutic Monitoring and Precision Medicine: Given the reversible nature of epigenetic modifications, cfDNA methylation biomarkers hold significant potential for monitoring the efficacy of epigenetic therapies or other targeted interventions in real-time [
11]. This aligns with the principles of precision medicine, allowing for tailored therapeutic strategies based on an individual's unique molecular profile and disease response [
4].
9. Conclusion
Epigenetic profiling of cell-free DNA in cerebrospinal fluid represents a burgeoning frontier in the diagnosis and management of metabolic brain diseases. These disorders, characterized by their clinical heterogeneity, nonspecific early symptoms, and the limitations of traditional diagnostic methods, present a significant unmet clinical need. The ability of CSF-derived cfDNA to provide a direct, molecular-level window into CNS pathology, circumventing the blood-brain barrier and offering insights into specific brain cell-type injury, inflammatory signaling, and metabolic reprogramming, positions it as a highly promising source of biomarkers.
Current methodologies, including cfMeDIP-seq and targeted bisulfite sequencing, are continually evolving to address the challenges of ultra-low-input cfDNA, with newer enzymatic approaches, such as EM-seq, demonstrating improved efficiency and reduced DNA degradation. The inherent tissue-specificity of DNA methylation patterns allows for precise deconvolution of cfDNA origin, offering an unparalleled resolution for understanding localized brain dysfunction. This molecular granularity provides a significant advantage over conventional CSF markers and neuroimaging, enabling potentially earlier and more accurate diagnosis, dynamic monitoring of disease progression, and assessment of therapeutic response.
While technical hurdles related to DNA concentration, assay standardization, and complex bioinformatics remain, ongoing advancements in AI-driven analysis and multi-omics integration are poised to overcome these limitations. The ethical and logistical considerations associated with CSF collection, alongside the cost-effectiveness of these advanced assays, require careful consideration for broader clinical implementation. Nevertheless, the transformative potential of epigenetic cfDNA analysis in CSF for providing non-invasive, molecular-level insights into CNS metabolic dysfunction is undeniable. Continued research and collaborative efforts are essential to translate this promising technology into routine clinical practice, ultimately improving diagnostic timelines, guiding personalized therapies, and enhancing outcomes for patients afflicted with metabolic brain diseases.
References
- Souravh Bais, Renu Kumari, Nirmal Dongre, Aakash Singh Panwar. Insights into neurometabolic diseases[J]. AIMS Molecular Science, 2024, 11(2): 116-139. [CrossRef]
- Fernández-Eulate G, Carreau C, Benoist J, et al. Diagnostic approach in adult-onset neurometabolic diseases. Journal of Neurology, Neurosurgery & Psychiatry 2022;93:413-421.
- Otsuji R, Fujioka Y, Hata N, Kuga D, Hatae R, Sangatsuda Y, Nakamizo A, Mizoguchi M, Yoshimoto K. Liquid Biopsy for Glioma Using Cell-Free DNA in Cerebrospinal Fluid. Cancers. 2024; 16(5):1009. [CrossRef]
- CD Genomics. Cell-free DNA as a Biomarker in Precision Oncology. Cd-genomics.com. Published 2023. Accessed June 30, 2025. https://www.cd-genomics.com/resource-data-precision-oncology-cfdna.html.
- Cornelli L, Van Paemel R, Ferro Dos Santos MR, Roelandt S, Willems L, Vandersteene J, Baert E, Mus LM, Van Roy N, De Wilde B, De Preter K. Diagnosis of pediatric central nervous system tumors using methylation profiling of cfDNA from cerebrospinal fluid. Clin Epigenetics. 2024 Jul 5;16(1):87. [CrossRef] [PubMed] [PubMed Central]
- BioIVT. BioIVT. Bioivt.com. Published April 11, 2025. Accessed June 30, 2025. https://bioivt.com/blogs/unpacking-the-fda-draft-guidance-on-biopsies-in-clinical-trials.
- Akabane M, Imaoka Y, Kawashima J, Pawlik TM. Advancing precision medicine in hepatocellular carcinoma: current challenges and future directions in liquid biopsy, immune microenvironment, single nucleotide polymorphisms, and conversion therapy. Hepat Oncol. 2025;12(1). [CrossRef]
- Armakolas A, Kotsari M, Koskinas J. Liquid Biopsies, Novel Approaches and Future Directions. Cancers. 2023; 15(5):1579. [CrossRef]
- Aydın Ş, Özdemir S, Adıgüzel A. The Potential of cfDNA as Biomarker: Opportunities and Challenges for Neurodegenerative Diseases. J Mol Neurosci. 2025 Mar 13;75(1):34. [CrossRef] [PubMed] [PubMed Central]
- Life in the Lab Stuff. Cell-Free DNA (cfDNA) vs. Circulating Tumor DNA (ctDNA) Explained. Life in the Lab. Published January 22, 2025. Accessed June 18, 2025. https://www.thermofisher.com/blog/life-in-the-lab/cfdna-vs-ctdna/.
- Gaitsch H, Franklin RJM, Reich DS. Cell-free DNA-based liquid biopsies in neurology. Brain. 2023 May 2;146(5):1758-1774. [CrossRef] [PubMed] [PubMed Central]
- Liu F, Su Y, Liu X, et al. Cell-free DNA: a metabolic byproduct with diagnostic and prognostic potential in rheumatic disorders. Frontiers in Pharmacology. 2025;16. [CrossRef]
- Pathak A, Tomar S, Pathak S. Epigenetics and Cancer: A Comprehensive Review. Asian Pacific Journal of Cancer Biology. 2023;8(1):75-89. [CrossRef]
- Al Aboud NM, Tupper C, Jialal I. Genetics, Epigenetic Mechanism. [Updated 2023 Aug 14]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532999/.
- R. Lehmann-Werman,D. Neiman,H. Zemmour,J. Moss,J. Magenheim,A. Vaknin-Dembinsky,S. Rubertsson,B. Nellgård,K. Blennow,H. Zetterberg,K. Spalding,M.J. Haller,C.H. Wasserfall,D.A. Schatz,C.J. Greenbaum,C. Dorrell,M. Grompe,A. Zick,A. Hubert,[...]& Y. Dor, Identification of tissue-specific cell death using methylation patterns of circulating DNA, Proc. Natl. Acad. Sci. U.S.A. 113 (13) E1826-E1834. (2016). [CrossRef]
- Jin Y, Allen EG, Jin P. Cell-free DNA methylation as a potential biomarker in brain disorders. Epigenomics. 2022 Apr;14(7):369-374. Epub 2022 Jan 17. [CrossRef] [PubMed] [PubMed Central]
- Cai M, Zhou J, McKennan C, Wang J. scMD facilitates cell type deconvolution using single-cell DNA methylation references. Commun Biol. 2024 Jan 2;7(1):1. [CrossRef] [PubMed] [PubMed Central]
- McEwen AE, Leary SES, Lockwood CM. Beyond the Blood: CSF-Derived cfDNA for Diagnosis and Characterization of CNS Tumors. Front Cell Dev Biol. 2020 Feb 18;8:45. [CrossRef] [PubMed] [PubMed Central]
- Jian Zhang et al. A characteristic comparison of cell-free DNA isolated from plasma and cerebrospinal fluid in patients with cancer.. JCO 41, e14008-e14008(2023). [CrossRef]
- Pia S, Lui F. Melas Syndrome. [Updated 2024 Jan 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532959/.
- MedlinePlus. Mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes: MedlinePlus Genetics. medlineplus.gov. Published December 1, 2013. Accessed June 30, 2025. https://medlineplus.gov/genetics/condition/mitochondrial-encephalomyopathy-lactic-acidosis-and-stroke-like-episodes/.
- El-Hattab AW, Almannai M, Scaglia F. MELAS. 2001 Feb 27 [Updated 2018 Nov 29]. In: Adam MP, Feldman J, Mirzaa GM, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2025. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1233/.
- File:Mitochondrial.jpg. Wikimedia Commons. https://commons.wikimedia.org/w/index.php?title=File:Mitochondrial.jpg&oldid=997298825. Published February 11, 2025. Accessed June 30, 2025.
- File:Mitochondrial DNA lg.jpg. Wikimedia Commons. https://commons.wikimedia.org/w/index.php?title=File:Mitochondrial_DNA_lg.jpg&oldid=946178322. Published October 18, 2024. Accessed June 30, 2025.
- File:Modified Gomori trichrome stain showing several ragged red fibers.jpg. Wikimedia Commons. https://commons.wikimedia.org/w/index.php?title=File:Modified_Gomori_trichrome_stain_showing_several_ragged_red_fibers.jpg&oldid=763868130. Published May 16, 2023. Accessed June 30, 2025.
- File:EPIC-seq workflow.png. Wikimedia Commons. https://commons.wikimedia.org/w/index.php?title=File:EPIC-seq_workflow.png&oldid=859065986. Published March 9, 2024. Accessed June 30, 2025.
- BMJ Best Practice. Common hereditary lysosomal storage diseases - Symptoms, diagnosis and treatment | BMJ Best Practice US. Bmj.com. Published May 30, 2025. Accessed June 30, 2025. https://bestpractice.bmj.com/topics/en-us/1021.
- Sun A. Lysosomal storage disease overview. Annals of Translational Medicine. 2017;6(24). [CrossRef]
- Stirnemann J, Belmatoug N, Camou F, Serratrice C, Froissart R, Caillaud C, Levade T, Astudillo L, Serratrice J, Brassier A, et al. A Review of Gaucher Disease Pathophysiology, Clinical Presentation and Treatments. International Journal of Molecular Sciences. 2017; 18(2):441. [CrossRef]
- Derralynn Hughes, Peter Mikosch, Nadia Belmatoug, Francesca Carubbi, TimothyM Cox, Ozlem Goker-Alpan, Andreas Kindmark, PramodK Mistry, Ludger Poll, Neal Weinreb, Patrick Deegan, Gaucher Disease in Bone: From Pathophysiology to Practice, Journal of Bone and Mineral Research, Volume 34, Issue 6, 1 June 2019, Pages 996–1013. [CrossRef]
- Patterson M. Niemann-Pick Disease Type C. 2000 Jan 26 [Updated 2020 Dec 10]. In: Adam MP, Feldman J, Mirzaa GM, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2025. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1296/.
- File:Gaucher disease - very high mag.jpg. Wikimedia Commons. https://commons.wikimedia.org/w/index.php?title=File:Gaucher_disease_-_very_high_mag.jpg&oldid=890184076. Published June 30, 2024. Accessed June 30, 2025.
- File:Epigenetic mechanisms.jpg. Wikimedia Commons. https://commons.wikimedia.org/w/index.php?title=File:Epigenetic_mechanisms.jpg&oldid=721049823. Published December 30, 2022. Accessed June 30, 2025.
- De Vivo D. Glucose Transporter Type 1 Deficiency Syndrome - NORD (National Organization for Rare Disorders). NORD (National Organization for Rare Disorders). Published July 30, 2024. https://rarediseases.org/rare-diseases/glucose-transporter-type-1-deficiency-syndrome/.
- Peters TMA, Merx J, Kooijman PC, et al. Novel CSF biomarkers of GLUT1 deficiency syndrome: implications beyond the brain’s energy deficit. medRxivorg. Published online April 17, 2022. [CrossRef]
- Steele G, Klepper J, Poduri A. GLUT1 Deficiency Syndrome (SLC2A1). Epilepsy Foundation. Published February 1, 2021. Accessed June 30, 2025. https://www.epilepsy.com/causes/metabolic/glut1.
- Yu X, He H, Wen J, Xu X, Ruan Z, Hu R, Wang F, Ju H. Diabetes-related cognitive impairment: Mechanisms, symptoms, and treatments. Open Med (Wars). 2025 Jan 13;20(1):20241091. [CrossRef] [PubMed] [PubMed Central]
- Kun Ma, Yang Xiao, Mechanisms of Cognitive Decline in Newly Diagnosed Diabetics: A Review of Pathophysiological Contributions and Intervention Strategies. MEDS Basic Medicine (2024) Vol. 2: 13-19. [CrossRef]
- Zhang S, Zhang Y, Wen Z, et al. Cognitive dysfunction in diabetes: abnormal glucose metabolic regulation in the brain. Frontiers in Endocrinology. 2023;14. [CrossRef]
- Duarte JM. Metabolic Alterations Associated to Brain Dysfunction in Diabetes. Aging Dis. 2015 Oct 1;6(5):304-21. [CrossRef] [PubMed] [PubMed Central]
- Abbatecola AM, Arosio B, Cerasuolo M, et al. Common neurodegenerative pathways in brain aging, cognitive decline, type 2 diabetes & metabolic syndrome. Journal of Gerontology and Geriatrics 2024;72:43-49.
- Mayo Clinic Staff. Mild cognitive impairment - Symptoms and causes. Mayo Clinic. Published October 24, 2024. Accessed June 30, 2025. https://www.mayoclinic.org/diseases-conditions/mild-cognitive-impairment/symptoms-causes/syc-20354578.
- Summers K. Study Unlocks How Diabetes Distorts Memory and Reward Processing. UNLV. Published March 25, 2025. Accessed June 30, 2025. https://www.unlv.edu/news/release/study-unlocks-how-diabetes-distorts-memory-and-reward-processing.
- Bhasin G, Flores E, Crew LA, et al. ACC reward location information is carried by hippocampal theta synchrony and suppressed in a Type 2 Diabetes model. Journal of Neuroscience. Published online March 24, 2025:e1546242025-e1546242025. [CrossRef]
- MedlinePlus. Cerebrospinal Fluid (CSF) Analysis: MedlinePlus Lab Test Information. Medlineplus.gov. Published March 13, 2025. Accessed June 30, 2025. https://medlineplus.gov/lab-tests/cerebrospinal-fluid-csf-analysis/.
- Czarniak N, Kamińska J, Matowicka-Karna J, Koper-Lenkiewicz OM. Cerebrospinal Fluid–Basic Concepts Review. Biomedicines. 2023; 11(5):1461. [CrossRef]
- File:CSF circulation.png. Wikimedia Commons. https://commons.wikimedia.org/w/index.php?title=File:CSF_circulation.png&oldid=859369762. Published March 10, 2024. Accessed June 30, 2025.
- File:CtDNA in circulation.png. Wikimedia Commons. https://commons.wikimedia.org/w/index.php?title=File:CtDNA_in_circulation.png&oldid=871699736. Published April 27, 2024. Accessed June 30, 2025.
- File:Blausen 0617 LumbarPuncture.png. Wikimedia Commons. https://commons.wikimedia.org/w/index.php?title=File:Blausen_0617_LumbarPuncture.png&oldid=825516397. Published November 27, 2023. Accessed June 30, 2025.
- File:DNAme landscape.png. Wikimedia Commons. https://commons.wikimedia.org/w/index.php?title=File:DNAme_landscape.png&oldid=873650162. Published May 4, 2024. Accessed June 30, 2025.
- Lossi L, Castagna C, Merighi A. An Overview of the Epigenetic Modifications in the Brain under Normal and Pathological Conditions. Int J Mol Sci. 2024 Mar 30;25(7):3881. [CrossRef] [PubMed] [PubMed Central]
- Younesian S, Yousefi AM, Momeny M, Ghaffari SH, Bashash D. The DNA Methylation in Neurological Diseases. Cells. 2022 Oct 31;11(21):3439. [CrossRef] [PubMed] [PubMed Central]
- Lio CWJ, Rao A. TET Enzymes and 5hmC in Adaptive and Innate Immune Systems. Frontiers in Immunology. 2019;10. [CrossRef]
- Williams K, Christensen J, Helin K. DNA methylation: TET proteins—guardians of CpG islands? EMBO reports. 2011;13(1):28-35. [CrossRef]
- Locke WJ, Guanzon D, Ma C, et al. DNA Methylation Cancer Biomarkers: Translation to the Clinic. Frontiers in Genetics. 2019;10. [CrossRef]
- Miller JL, Grant PA. The role of DNA methylation and histone modifications in transcriptional regulation in humans. Subcell Biochem. 2013;61:289-317em. 2013;61:289-317. [CrossRef] [PubMed] [PubMed Central]
- Hiemenz M, Chang J. DNA Methylation to the Rescue: An Update for Pathologists Navigating…. College of American Pathologists. Published 2025. Accessed June 30, 2025. https://www.cap.org/member-resources/articles/dna-methylation-to-the-rescue-an-update-for-pathologists-navigating-the-future-of-precision-tumor-diagnostics.
- Kakodkar P, Conway K, Santana-Santos L, et al. DNA methylation-based classification of the central nervous system tumors, achievements, and challenges. In: IntechOpen. Published 2025. [CrossRef]
- Wei Tang, Shixiang Wan, Zhen Yang, Andrew E Teschendorff, Quan Zou, Tumor origin detection with tissue-specific miRNA and DNA methylation markers, Bioinformatics, Volume 34, Issue 3, February 2018, Pages 398–406. [CrossRef]
- Chen K, Li Z, Kirsh BO, et al. Plasma cell-free DNA methylomes for hepatocellular carcinoma detection and monitoring after liver resection or transplantation. medRxivorg. Published online October 2, 2024. [CrossRef]
- Wilson SL, Shen SY, Harmon L, et al. Sensitive and reproducible cell-free methylome quantification with synthetic spike-in controls. bioRxiv (Cold Spring Harbor Laboratory). Published online November 19, 2021. [CrossRef]
- Biorad. Epigenetics and Chromatin Structure | Bio-Rad. www.bio-rad.com. Accessed June 30, 2025. https://www.bio-rad.com/en-us/applications-technologies/epigenetics-chromatin-structure?ID=LUSNMFHYP.
- CD Genomics. Innovation in WGBS: New Methods for DNA Methylation Analysis. Cd-genomics.com. Published 2023. Accessed June 30, 2025. https://www.cd-genomics.com/epigenetics/resource-new-wgbs-technology-pbat-wgbs.html.
- Abcam. Bisulfite sequencing in DNA methylation analysis | Abcam. Abcam.com. Published 2017. Accessed June 30, 2025. https://www.abcam.com/en-us/knowledge-center/dna-and-rna/bisulfite-sequencing.
- De Koker A, Raman L, Van Paemel R, et al. Development of circulating cell-free DNA reduced representation bisulfite sequencing for clinical methylomics diagnostics. bioRxiv. Published online April 9, 2025. [CrossRef]
- What are the limitations of reduced representation bisulfite sequencing (RRBS)? | AAT Bioquest. Aatbio.com. Published April 26, 2024. Accessed June 30, 2025. https://www.aatbiocom/resources/faqfrequently-251 asked-questions/what-are-the-limitations-of-reduced-representation-bisulfite-sequencing-rrbs.
- CD Genomics. DNA Methylation Array Workflow & Analysis: Process Optimization and Data Insights. Cd-genomics.com. Published 2021. Accessed June 30, 2025. https://www.cd-genomics.com/epigenetics/resource-dna-methylation-array-workflow-optimization-analysis.html.
- CD Genomics. Overview of EM-seq: A New Detection Approach for DNA Methylation - CD Genomics. Cd-genomics.com. Published 2021. Accessed June 30, 2025. https://www.cd-genomics.com/epigenetics/resource-dna-methylation-detection-em-seq.html.
- File:Bisulfite-reaction.png. Wikimedia Commons. https://commons.wikimedia.org/w/index.php?title=File:Bisulfite-reaction.png&oldid=824921164. Published November 25, 2023. Accessed June 30, 2025.
- File:Wiki Bisulfite sequencing Figure 1 small.png. Wikipedia. https://en.wikipedia.org/wiki/File:Wiki_Bisulfite_sequencing_Figure_1_small.png. Published March 22, 2012. Accessed June 30, 2025.
- Illumina. Infinium MethylationEPIC Kit | Methylation profiling array for EWAS. Illumina.com. Published 2019. Accessed June 30, 2025. https://www.illumina.com/products/by-type/microarray-kits/infinium-methylation-epic.html.
- Sebastian A, Silvy M, Coiffard B, Reynaud-Gaubert M, Magdinier F, Chiaroni J, Picard C, Pedini P. A review of cell-free DNA and epigenetics for non-invasive diagnosis in solid organ transplantation. Front Transplant. 2024 Nov 15;3:1474920. [CrossRef] [PubMed] [PubMed Central]
- Tan WY, Nagabhyrava S, Ang-Olson O, Das P, Ladel L, Sailo B, He L, Sharma A, Ahuja N. Translation of Epigenetics in Cell-Free DNA Liquid Biopsy Technology and Precision Oncology. Current Issues in Molecular Biology. 2024; 46(7):6533-6565. [CrossRef]
- File:Blausen 0870 TypesofNeuroglia.png. Wikimedia Commons. https://commons.wikimedia.org/w/index.php?title=File:Blausen_0870_TypesofNeuroglia.png&oldid=859366013. Published March 10, 2024. Accessed July 1, 2025.
- Pollard CA, Saito ER, Burns JM, Hill JT, Jenkins TG. Considering Biomarkers of Neurodegeneration in Alzheimer’s Disease: The Potential of Circulating Cell-Free DNA in Precision Neurology. Journal of Personalized Medicine. 2024; 14(11):1104. [CrossRef]
- Southwood D, Singh S, Chatterton Z. Brain-derived cell-free DNA. Neural Regen Res. 2022 Oct;17(10):2213-2214. [CrossRef] [PubMed] [PubMed Central]
- Jin, Y., Conneely, K.N., Ma, W. et al. Whole-genome bisulfite sequencing of cell-free DNA unveils age-dependent and ALS-associated methylation alterations. Cell Biosci 15, 26 (2025). [CrossRef]
- Rados M, Klotz S, Regelsberger G, Stögmann E, Landegger LD, Fetahu IS. Distinct cerebrospinal fluid DNA methylation signatures linked to Alzheimer’s disease. bioRxiv. Published online April 7, 2025. [CrossRef]
- Giannini LAA, Boers RG, van der Ende EL, Poos JM, Jiskoot LC, Boers JB, van IJcken WFJ, Dopper EG, Pijnenburg YAL, Seelaar H, Meeter LH, van Rooij JGJ, Scheper W, Gribnau J, van Swieten JC. Distinctive cell-free DNA methylation characterizes presymptomatic genetic frontotemporal dementia. Ann Clin Transl Neurol. 2024 Mar;11(3):744-756. Epub 2024 Mar 13. [CrossRef] [PubMed] [PubMed Central]
- Ma Y, Wang W, Liu S, Qiao X, Xing Y, Zhou Q, Zhang Z. Epigenetic Regulation of Neuroinflammation in Alzheimer's Disease. Cells. 2023 Dec 29;13(1):79. [CrossRef] [PubMed] [PubMed Central]
- Bai I, Keyser C, Zhang Z, et al. Epigenetic regulation of autophagy in neuroinflammation and synaptic plasticity. Frontiers in immunology. 2024;15. [CrossRef]
- Cologna SM, Cluzeau CV, Yanjanin NM, Blank PS, Dail MK, Siebel S, Toth CL, Wassif CA, Lieberman AP, Porter FD. Human and mouse neuroinflammation markers in Niemann-Pick disease, type C1. J Inherit Metab Dis. 2014 Jan;37(1):83-92. Epub 2013 May 8. [CrossRef] [PubMed] [PubMed Central]
- Boddupalli CS, Nair S, Belinsky G, Gans J, Teeple E, Nguyen TH, Mehta S, Guo L, Kramer ML, Ruan J, Wang H, Davison M, Kumar D, Vidyadhara DJ, Zhang B, Klinger K, Mistry PK. Neuroinflammation in neuronopathic Gaucher disease: Role of microglia and NK cells, biomarkers, and response to substrate reduction therapy. Elife. 2022 Aug 16;11:e79830. [CrossRef] [PubMed] [PubMed Central]
- Fawal MA, Davy A. Impact of Metabolic Pathways and Epigenetics on Neural Stem Cells. Epigenet Insights. 2018 Dec 25;11:2516865718820946. [CrossRef] [PubMed] [PubMed Central]
- Peters TMA, Merx J, Kooijman PC, Noga M, de Boer S, van Gemert LA, Salden G, Engelke UFH, Lefeber DJ, van Outersterp RE, Berden G, Boltje TJ, Artuch R, Pías-Peleteiro L, García-Cazorla Á, Barić I, Thöny B, Oomens J, Martens J, Wevers RA, Verbeek MM, Coene KLM, Willemsen MAAP. Novel cerebrospinal fluid biomarkers of glucose transporter type 1 deficiency syndrome: Implications beyond the brain's energy deficit. J Inherit Metab Dis. 2023 Jan;46(1):66-75. Epub 2022 Oct 17. [CrossRef] [PubMed] [PubMed Central]
- Laufer BI, Hasegawa Y, Zhang Z, Hogrefe CE, Del Rosso LA, Haapanen L, Hwang H, Bauman MD, Van de Water J, Taha AY, Slupsky CM, Golub MS, Capitanio JP, VandeVoort CA, Walker CK, LaSalle JM. Multi-omic brain and behavioral correlates of cell-free fetal DNA methylation in macaque maternal obesity models. Nat Commun. 2022 Sep 21;13(1):5538. [CrossRef] [PubMed] [PubMed Central]
- Myrou A, Barmpagiannos K, Ioakimidou A, Savopoulos C. Molecular Biomarkers in Neurological Diseases: Advances in Diagnosis and Prognosis. International Journal of Molecular Sciences. 2025; 26(5):2231. [CrossRef]
- Wang Y, Li J, Li J, et al. cfDecon: Accurate and Interpretable methylation-based cell type deconvolution for cell-free DNA. bioRxiv (Cold Spring Harbor Laboratory). Published online February 15, 2025. [CrossRef]
- Zuccato JA, Patil V, Mansouri S, Voisin M, Chakravarthy A, Shen SY, Nassiri F, Mikolajewicz N, Trifoi M, Skakodub A, Zacharia B, Glantz M, De Carvalho DD, Mansouri A, Zadeh G. Cerebrospinal fluid methylome-based liquid biopsies for accurate malignant brain neoplasm classification. Neuro Oncol. 2023 Aug 3;25(8):1452-1460. [CrossRef] [PubMed] [PubMed Central]
- Cecile Riviere-Cazaux, Xiaoxi Dong, Wei Mo, Rahul Kumar, Chao Dai, Lucas P. Carlstrom, Amanda Munoz-Casabella, Keyvan Ghadimi, Cody L. Nesvick, Katherine M. Andersen, Matthew D. Hoplin, Nicholas Canaday, Ignacio Jusue-Torres, Noor Malik, Jian L. Campian, Michael W. Ruff, Joon H. Uhm, Jeanette E. Eckel Passow, Timothy J. Kaufmann, David M. Routman, Sani H. Kizilbash, Ugur Sener, Arthur E. Warrington, Robert B. Jenkins, Pan Du, Shidong Jia, Terry C. Burns; Longitudinal Glioma Monitoring via Cerebrospinal Fluid Cell-Free DNA. Clin Cancer Res 1 March 2025; 31 (5): 881–889. [CrossRef]
- Huang J, Wang L. Cell-Free DNA Methylation Profiling Analysis-Technologies and Bioinformatics. Cancers (Basel). 2019 Nov 6;11(11):1741. [CrossRef] [PubMed] [PubMed Central]
- Iser F, Hinz F, Hoffmann DC, Grassl N, Güngoör C, Meyer J, Dörner L, Hofmann L, Kelbch V, Göbel K, Mahmutoglu MA, Vollmuth P, Patel A, Nguyen D, Kaulen LD, Mildenberger I, Sahm K, Maaß K, Pajtler KW, Shankar GM, Weiler M, Wildemann B, Winkler F, von Deimling A, Platten M, Wick W, Sahm F, Kessler T. Cerebrospinal Fluid cfDNA Sequencing for Classification of Central Nervous System Glioma. Clin Cancer Res. 2024 Jul 15;30(14):2974-2985. [CrossRef] [PubMed] [PubMed Central]
- Ran Hu, Shuo Li, Mary L Stackpole, Qingjiao Li, Xianghong Jasmine Zhou, Wenyuan Li, cfTools: an R/Bioconductor package for deconvolving cell-free DNA via methylation analysis, Bioinformatics Advances, Volume 5, Issue 1, 2025, vbaf108. [CrossRef]
- Keukeleire P, Makrodimitris S, Reinders M. Cell type deconvolution of methylated cell-free DNA at the resolution of individual reads. NAR Genom Bioinform. 2023 Jun 2;5(2):lqad048. [CrossRef] [PubMed] [PubMed Central]
- Research and Markets. Liquid Biopsy Market Forecast to Reach US$13.44 Billion by 2030 at 12.19% CAGR | Next-Gen Sequencing and PCR Breakthroughs Fuel Innovation in Liquid Biopsy Diagnostics. GlobeNewswire News Room. Published April 9, 2025. Accessed June 30, 2025. https://www.globenewswire.com/news-release/2025/04/09/3058183/28124/en/Liquid-Biopsy-Market-Forecast-to-Reach-US-13-44-Billion-by-2030-at-12-19-CAGR-Next-Gen-Sequencing-and-PCR-Breakthroughs-Fuel-Innovation-in-Liquid-Biopsy-Diagnostics.html.
- Jinyue Yu, Craig Paterson, Phillippa Davies, Jennifer C Palmer, Julian P T Higgins, Kathreena M Kurian, Evaluating liquid biopsy biomarkers for early detection of brain metastasis: A systematic review, Neuro-Oncology Practice, 2025;, npaf032. [CrossRef]
- Liguori C, Stefani A, Fernandes M, Cerroni R, Mercuri NB, Pierantozzi M. Biomarkers of Cerebral Glucose Metabolism and Neurodegeneration in Parkinson's Disease: A Cerebrospinal Fluid-Based Study. J Parkinsons Dis. 2022;12(2):537-544. [CrossRef] [PubMed]
- Mattox AK, Yan H, Bettegowda C. The potential of cerebrospinal fluid-based liquid biopsy approaches in CNS tumors. Neuro Oncol. 2019 Dec 17;21(12):1509-1518. [CrossRef] [PubMed] [PubMed Central]
- Otto C, Kalantzis R, Kübler-Weller D, Kühn AA, Böld T, Regler A, Strathmeyer S, Wittmann J, Ruprecht K, Heelemann S. Comprehensive analysis of the cerebrospinal fluid and serum metabolome in neurological diseases. J Neuroinflammation. 2024 Sep 26;21(1):234. [CrossRef] [PubMed] [PubMed Central]
- Mastrangelo, M., Manti, F., Ricciardi, G. et al. The diagnostic and prognostic role of cerebrospinal fluid biomarkers in glucose transporter 1 deficiency: a systematic review. Eur J Pediatr 183, 3665–3678 (2024). [CrossRef]
- Leen WG, Wevers RA, Kamsteeg EJ, Scheffer H, Verbeek MM, Willemsen MA. Cerebrospinal fluid analysis in the workup of GLUT1 deficiency syndrome: a systematic review. JAMA Neurol. 2013 Nov;70(11):1440-4. [CrossRef] [PubMed]
- Cappuccio G, Pinelli M, Alagia M, Donti T, Day-Salvatore DL, Veggiotti P, De Giorgis V, Lunghi S, Vari MS, Striano P, Brunetti-Pierri N, Kennedy AD, Elsea SH. Biochemical phenotyping unravels novel metabolic abnormalities and potential biomarkers associated with treatment of GLUT1 deficiency with ketogenic diet. PLoS One. 2017 Sep 29;12(9):e0184022. [CrossRef] [PubMed] [PubMed Central]
- Bonomi CG, De Lucia V, Mascolo AP, Assogna M, Motta C, Scaricamazza E, Sallustio F, Mercuri NB, Koch G, Martorana A. Brain energy metabolism and neurodegeneration: hints from CSF lactate levels in dementias. Neurobiol Aging. 2021 Sep;105:333-339. Epub 2021 May 29. [CrossRef] [PubMed]
- Pan L, McClain L, Shaw P, Donnellan N, Chu T, Finegold D, Peters D. Non-invasive epigenomic molecular phenotyping of the human brain via liquid biopsy of cerebrospinal fluid and next generation sequencing. Eur J Neurosci. 2020 Dec;52(11):4536-4545. Epub 2020 Oct 22. [CrossRef] [PubMed]
- Albanese M, Zagaglia S, Landi D, Boffa L, Nicoletti CG, Marciani MG, Mandolesi G, Marfia GA, Buttari F, Mori F, Centonze D. Cerebrospinal fluid lactate is associated with multiple sclerosis disease progression. J Neuroinflammation. 2016 Feb 10;13:36. [CrossRef] [PubMed] [PubMed Central]
- Yang C, Pan RY, Guan F, Yuan Z. Lactate metabolism in neurodegenerative diseases. Neural Regen Res. 2024 Jan;19(1):69-74. [CrossRef] [PubMed] [PubMed Central]
- Magner M, Szentiványi K, Svandová I, Ješina P, Tesařová M, Honzík T, Zeman J. Elevated CSF-lactate is a reliable marker of mitochondrial disorders in children even after brief seizures. Eur J Paediatr Neurol. 2011 Mar;15(2):101-8. Epub 2010 Nov 12. [CrossRef] [PubMed]
- Abe K, Fujimura H, Nishikawa Y, Yorifuji S, Mezaki T, Hirono N, Nishitani N, Kameyama M. Marked reduction in CSF lactate and pyruvate levels after CoQ therapy in a patient with mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes (MELAS). Acta Neurol Scand. 1991 Jun;83(6):356-9. [CrossRef] [PubMed]
- Xu, S., Jiang, J., Chang, L. et al. Multisystem clinicopathologic and genetic analysis of MELAS. Orphanet J Rare Dis 19, 487 (2024). [CrossRef]
- Yamada K, Toribe Y, Yanagihara K, Mano T, Akagi M, Suzuki Y. Diagnostic accuracy of blood and CSF lactate in identifying children with mitochondrial diseases affecting the central nervous system. Brain Dev. 2012 Feb;34(2):92-74. Epub 2011 Aug 27. [CrossRef] [PubMed]
- Guerrero-Molina MP, Bernabeu-Sanz Á, Ramos-González A, Morales-Conejo M, Delmiro A, Domínguez-González C, Arenas J, Martín MA, González de la Aleja J. Magnetic resonance spectroscopy in MELAS syndrome: correlation with CSF and plasma metabolite levels and change after glutamine supplementation. Neuroradiology. 2024 Mar;66(3):389-398. Epub 2023 Dec 19. [CrossRef] [PubMed]
- Natsume J, Ishihara N, Azuma Y, Nakata T, Takeuchi T, Tanaka M, Sakaguchi Y, Okai Y, Ito Y, Yamamoto H, Ohno A, Kidokoro H, Hattori A, Nabatame S, Kato K. Lenticular nuclei to thalamic ratio on PET is useful for diagnosis of GLUT1 deficiency syndrome. Brain Dev. 2021 Jan;43(1):69-77. Epub 2020 Jul 30. [CrossRef] [PubMed]
- Marin-Valencia I, Kocabas A, Rodriguez-Navas C, Miloushev VZ, González-Rodríguez M, Lees H, Henry KE, Vaynshteyn J, Longo V, Deh K, Eskandari R, Mamakhanyan A, Berishaj M, Keshari KR. Imaging brain glucose metabolism in vivo reveals propionate as a major anaplerotic substrate in pyruvate dehydrogenase deficiency. Cell Metab. 2024 Jun 4;36(6):1394-1410.e12. [CrossRef] [PubMed] [PubMed Central]
- Meyer PT, Frings L, Gerta Rücker, Hellwig S. 18F-FDG PET in Parkinsonism: Differential Diagnosis and Cognitive Impairment in Parkinson’s disease. The Journal of Nuclear Medicine. 2017;58(12):1888-1898. [CrossRef]
- Probasco JC, Solnes L, Nalluri A, et al. Abnormal brain metabolism on FDG-PET/CT is a common early finding in autoimmune encephalitis. Neurology - Neuroimmunology Neuroinflammation. 2017;4(4):e352. [CrossRef]
- Akman CI, Provenzano F, Wang D, Engelstad K, Hinton V, Yu J, Tikofsky R, Ichese M, De Vivo DC. Topography of brain glucose hypometabolism and epileptic network in glucose transporter 1 deficiency. Epilepsy Res. 2015 Feb;110:206-15. Epub 2014 Dec 11. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).