Submitted:
01 June 2024
Posted:
05 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials & Methods
2.1. Filtering Metabolic Pathways and Genes of Interest
2.2. Building the “Ketosis” Module within the Kaleidoscope Lookup Tool
2.3. Querying Gene Lists and Generating Data Tables from Kaleidoscope Lookup
2.4. Transcriptomic Analysis among Neuropsychiatric Illness and Ketosis Datasets
2.5. Analysis on the Effect of Antipsychotics and Mood Stabilizers on Metabolic Gene Expression
3. Results
3.1. Final Pathways
3.2. Transcriptomic Results for Ketosis Datasets
3.3. Transcriptomic Results for Schizophrenia and Schizophrenia-Ketosis Comparison
3.4. Transcriptomic Results for Bipolar Disorder and Bipolar Disorder-Ketosis Comparison
3.5. Transcriptomic Results for Major Depressive-Disorder and Major Depressive Disorder-Ketosis Comparison
| Metabolic Pathway | Significantly Altered Genes in Chronic Antipsychotic-Treated vs. Vehicle-Treated Datasets (n=24) | Average LFC Values | Significantly Altered Genes in Chronic Mood Stabilizer-Treated vs. Vehicle-Treated Datasets (n=7) | Average LFC Values |
|---|---|---|---|---|
| Gluconeogenesis | Upregulated: PFKFB2, PCK2, G6PC1 | 0.39 | ||
| Downregulated: PFKFB2, PCK1, PCK2 | -0.23 | |||
| Glycolysis | Upregulated: PKLR, PGK1, PFKM, HK2, ALDOC, ENO2 | 0.36 | Upregulated: ENO3 | 0.37 |
| Downregulated: PFKP, GAPDH, ALDOB, GPI | -0.45 | Downregulated: PFKP, PKLR | -0.37 | |
| Lactate Shuttle (Neuron-Astrocyte) | Upregulated: SLC1A3, SLC1A2, SLC16A3, LDHD | 0.37 | Upregulated: GLUL, SLC1A3 | 0.37 |
| Downregulated: SLC2A3, SLC2A1, GLS, SLC1A6, GLUL | -0.31 | Downregulated: GLS2, GLS | -0.36 | |
| Tricarboxylic Acid (TCA) Cycle | Upregulated: SUCLG1, SDHB, IDH2, IDH1, ACO1 | 0.26 | Upregulated: SDHB, IDH2, ACO1, SDHAF4 | 0.35 |
| Downregulated: SDHAF4, PDHA2, IDH3A, ALDH5A1, ACLY | -0.47 | Downregulated: SUCLA2, ALDH5A1, DBT | -0.22 | |
| Electron Transport Chain (ETC) | Upregulated: UQCRH, SDHB, NDUFV2, NDUFC1, NDUFB5, COX7C, COX6B2, COX5B, COQ4, COQ10A, SDHAF2 | 0.24 | Upregulated: COQ7, COX7A2, COX6C, SDHB, SDHAF4 | 0.32 |
| Downregulated: SDHAF4, COX8A, COX7A1, COX6C, COX5A, COQ10B, MT-ND3 | -0.23 | Downregulated: NDUFS1, SDAHF2 | -0.26 | |
| Fatty Acid Synthesis | Upregulated: OXSM, HADH, ELOVL1, DECR1 | 0.32 | Upregulated: FASN | 0.33 |
| Downregulated: PPT2, MCAT, FASN, ELOVL6 | -0.44 | |||
| Fatty Acid Oxidation | Upregulated: HSD17B10, HADH, ECI1, ECHS1, ACAA2 | 0.27 | Upregulated: HADHB, ECHS1, CPT1C, SLC27A2, ECI1, HADHA | 0.28 |
| Ketogenesis | Upregulated: HMGCS2, BDH1, ACAT1, ACAA2 | 0.34 | ||
| Downregulated: HMGCS2, BDH2, HMGCS1 | -0.31 | |||
| Glycogenesis | Upregulated: HK2, GSK3A | 0.21 | Upregulated: PGM1, PGM2, GSK3B | 0.20 |
| Downregulated: PGM1, PGM2, GSK3B, GBE1, ADPGK | -0.39 | Downregulated: GCKR | -0.25 | |
| Glycogenolysis | Upregulated: G6PC1 | 0.20 | Upregulated: PGM1, PGM2 | 0.21 |
| Downregulated: PGM1, PGM2, AGL | -0.54 | |||
| Urea Cycle | Upregulated: ASS1, ASL, AGMAT | 0.44 | Upregulated: CAD | 0.24 |
| Downregulated: ARG2 | -0.18 | Downregulated: ASS1 | -0.65 | |
| Pentose Phosphate/ Glutathione Pathways | Upregulated: TKT, TALDO1, SOD1, RPIA, HK2, GSS, GCLC | 0.26 | ||
| Downregulated: RPE, GPI | -0.29 | Downregulated: GSS, RPIA | -0.37 |
3.5. Transcriptomic Results for Medication Analysis
4. Discussion
5. Conclusion
Supplementary Materials
References
- Whiteford HA, Ferrari AJ, Degenhardt L, Feigin V, Vos T. The global burden of mental, neurological and substance use disorders: an analysis from the Global Burden of Disease Study 2010. PLoS One. 2015;10(2):e0116820. Epub 20150206. [CrossRef] [PubMed] [PubMed Central]
- Wu Y, Wang L, Tao M, Cao H, Yuan H, Ye M, et al. Changing trends in the global burden of mental disorders from 1990 to 2019 and predicted levels in 25 years. Epidemiol Psychiatr Sci. 2023;32:e63. Epub 20231107. [CrossRef] [PubMed] [PubMed Central]
- Alshaya DS. Genetic and epigenetic factors associated with depression: An updated overview. Saudi J Biol Sci. 2022;29(8):103311. Epub 20220520. [CrossRef] [PubMed] [PubMed Central]
- Andlauer TFM, Mühleisen TW, Hoffstaedter F, Teumer A, Wittfeld K, Teuber A, et al. Genetic factors influencing a neurobiological substrate for psychiatric disorders. Transl Psychiatry. 2021;11(1):192. Epub 20210329. [CrossRef] [PubMed] [PubMed Central]
- Kolar D, Kleteckova L, Brozka H, Vales K. Mini-review: Brain energy metabolism and its role in animal models of depression, bipolar disorder, schizophrenia and autism. Neurosci Lett. 2021;760:136003. Epub 20210614. [CrossRef] [PubMed]
- Sullivan CR, O'Donovan SM, McCullumsmith RE, Ramsey A. Defects in Bioenergetic Coupling in Schizophrenia. Biol Psychiatry. 2018;83(9):739-50. Epub 20171024. [CrossRef] [PubMed] [PubMed Central]
- Zuccoli GS, Saia-Cereda VM, Nascimento JM, Martins-de-Souza D. The Energy Metabolism Dysfunction in Psychiatric Disorders Postmortem Brains: Focus on Proteomic Evidence. Front Neurosci. 2017;11:493. Epub 20170907. [CrossRef] [PubMed] [PubMed Central]
- Danan A, Westman EC, Saslow LR, Ede G. The Ketogenic Diet for Refractory Mental Illness: A Retrospective Analysis of 31 Inpatients. Front Psychiatry. 2022;13:951376. Epub 20220706. [CrossRef] [PubMed] [PubMed Central]
- Dietch DM, Kerr-Gaffney J, Hockey M, Marx W, Ruusunen A, Young AH, et al. Efficacy of low carbohydrate and ketogenic diets in treating mood and anxiety disorders: systematic review and implications for clinical practice. BJPsych Open. 2023;9(3):e70. Epub 20230417. [CrossRef] [PubMed] [PubMed Central]
- Tillery EE, Ellis KD, Threatt TB, Reyes HA, Plummer CS, Barney LR. The use of the ketogenic diet in the treatment of psychiatric disorders. Ment Health Clin. 2021;11(3):211-9. Epub 20210512. [CrossRef] [PubMed] [PubMed Central]
- Swerdlow RH. Bioenergetic medicine. Br J Pharmacol. 2014;171(8):1854-69. [CrossRef] [PubMed] [PubMed Central]
- douglas.ruderfer@vanderbilt.edu BDaSWGotPGCEa, Consortium BDaSWGotPG. Genomic Dissection of Bipolar Disorder and Schizophrenia, Including 28 Subphenotypes. Cell. 2018;173(7):1705-15.e16. [CrossRef] [PubMed] [PubMed Central]
- Gao X, Qin Y, Jiao S, Hao J, Zhao J, Wang J, et al. Genetic evidence for the causal relations between metabolic syndrome and psychiatric disorders: a Mendelian randomization study. Transl Psychiatry. 2024;14(1):46. Epub 20240120. [CrossRef] [PubMed] [PubMed Central]
- Ben-Shachar D, Karry R. Neuroanatomical pattern of mitochondrial complex I pathology varies between schizophrenia, bipolar disorder and major depression. PLoS One. 2008;3(11):e3676. Epub 20081107. [CrossRef] [PubMed] [PubMed Central]
- Halim ND, Lipska BK, Hyde TM, Deep-Soboslay A, Saylor EM, Herman MM, et al. Increased lactate levels and reduced pH in postmortem brains of schizophrenics: medication confounds. J Neurosci Methods. 2008;169(1):208-13. Epub 20071129. [CrossRef] [PubMed] [PubMed Central]
- Prabakaran S, Swatton JE, Ryan MM, Huffaker SJ, Huang JT, Griffin JL, et al. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol Psychiatry. 2004;9(7):684-97, 43. [CrossRef] [PubMed]
- Roberts RC, Barksdale KA, Roche JK, Lahti AC. Decreased synaptic and mitochondrial density in the postmortem anterior cingulate cortex in schizophrenia. Schizophrenia research. 2015;168(1-2):543-53. [CrossRef] [PubMed] [PubMed Central]
- Sun X, Wang JF, Tseng M, Young LT. Downregulation in components of the mitochondrial electron transport chain in the postmortem frontal cortex of subjects with bipolar disorder. J Psychiatry Neurosci. 2006;31(3):189-96. [PubMed] [PubMed Central]
- das Neves Duarte JM, Kulak A, Gholam-Razaee MM, Cuenod M, Gruetter R, Do KQ. N-acetylcysteine normalizes neurochemical changes in the glutathione-deficient schizophrenia mouse model during development. Biological psychiatry. 2012;71(11):1006-14. [CrossRef] [PubMed]
- Smolensky IV, Zajac-Bakri K, Gass P, Inta D. Ketogenic diet for mood disorders from animal models to clinical application. J Neural Transm (Vienna). 2023;130(9):1195-205. Epub 20230321. [CrossRef] [PubMed] [PubMed Central]
- Le Foll C, Levin BE. Fatty acid-induced astrocyte ketone production and the control of food intake. Am J Physiol Regul Integr Comp Physiol. 2016;310(11):R1186-92. Epub 20160427. [CrossRef] [PubMed] [PubMed Central]
- Longo R, Peri C, Cricrì D, Coppi L, Caruso D, Mitro N, et al. Ketogenic Diet: A New Light Shining on Old but Gold Biochemistry. Nutrients. 2019;11(10). Epub 20191017. [CrossRef] [PubMed] [PubMed Central]
- LaManna JC, Salem N, Puchowicz M, Erokwu B, Koppaka S, Flask C, et al. Ketones suppress brain glucose consumption. Adv Exp Med Biol. 2009;645:301-6. [CrossRef] [PubMed] [PubMed Central]
- Mattson MP, Moehl K, Ghena N, Schmaedick M, Cheng A. Intermittent metabolic switching, neuroplasticity and brain health. Nat Rev Neurosci. 2018;19(2):63-80. Epub 20180111. [CrossRef] [PubMed] [PubMed Central]
- Roberts MN, Wallace MA, Tomilov AA, Zhou Z, Marcotte GR, Tran D, et al. A Ketogenic Diet Extends Longevity and Healthspan in Adult Mice. Cell Metab. 2017;26(3):539-46.e5. [CrossRef] [PubMed] [PubMed Central]
- Newman JC, Verdin E. β-Hydroxybutyrate: A Signaling Metabolite. Annu Rev Nutr. 2017;37:51-76. [CrossRef] [PubMed] [PubMed Central]
- Kraeuter AK, Loxton H, Lima BC, Rudd D, Sarnyai Z. Ketogenic diet reverses behavioral abnormalities in an acute NMDA receptor hypofunction model of schizophrenia. Schizophrenia research. 2015;169(1-3):491-3. Epub 2015/11/09. [CrossRef] [PubMed]
- Kraeuter AK, Archambault N, van den Buuse M, Sarnyai Z. Ketogenic diet and olanzapine treatment alone and in combination reduce a pharmacologically-induced prepulse inhibition deficit in female mice. Schizophrenia research. 2019;212:221-4. Epub 2019/08/14. [CrossRef] [PubMed]
- Kraeuter AK, Mashavave T, Suvarna A, van den Buuse M, Sarnyai Z. Effects of beta-hydroxybutyrate administration on MK-801-induced schizophrenia-like behaviour in mice. Psychopharmacology (Berl). 2020;237(5):1397-405. Epub 20200128. [CrossRef] [PubMed]
- Ramsey AJ. NR1 knockdown mice as a representative model of the glutamate hypothesis of schizophrenia. Prog Brain Res. 2009;179:51-8. Epub 2009/01/01. [CrossRef] [PubMed]
- Paula Farias Waltrick A, Henrique Bernardo de Lima Silva A, Cristina de Carvalho M, Aparecida Comotti de Oliveira B, Naliwaiko K, Maria da Cunha J, et al. Preventive treatment with fish oil facilitates the antidepressant-like effect of antidepressant drugs in type-1 diabetes mellitus rats: Implication of serotonergic system. Neurosci Lett. 2022;772:136477. Epub 20220125. [CrossRef] [PubMed]
- Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, et al. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006;60(2):223-35. [CrossRef] [PubMed]
- Phelps JR, Siemers SV, El-Mallakh RS. The ketogenic diet for type II bipolar disorder. Neurocase. 2013;19(5):423-6. Epub 20121003. [CrossRef] [PubMed]
- Kraft BD, Westman EC. Schizophrenia, gluten, and low-carbohydrate, ketogenic diets: a case report and review of the literature. Nutr Metab (Lond). 2009;6:10. Epub 20090226. [CrossRef] [PubMed] [PubMed Central]
- Palmer CM. Ketogenic diet in the treatment of schizoaffective disorder: Two case studies. Schizophr Res. 2017;189:208-9. Epub 20170203. [CrossRef] [PubMed]
- Palmer CM, Gilbert-Jaramillo J, Westman EC. The ketogenic diet and remission of psychotic symptoms in schizophrenia: Two case studies. Schizophrenia research. 2019;208:439-40. Epub 2019/04/10. [CrossRef] [PubMed]
- Kovács Z, D'Agostino DP, Diamond D, Kindy MS, Rogers C, Ari C. Therapeutic Potential of Exogenous Ketone Supplement Induced Ketosis in the Treatment of Psychiatric Disorders: Review of Current Literature. Front Psychiatry. 2019;10:363. Epub 20190523. [CrossRef] [PubMed] [PubMed Central]
- Adeva-Andany MM, Pérez-Felpete N, Fernández-Fernández C, Donapetry-García C, Pazos-García C. Liver glucose metabolism in humans. Biosci Rep. 2016;36(6). Epub 20161129. [CrossRef] [PubMed] [PubMed Central]
- Judge A, Dodd MS. Metabolism. Essays Biochem. 2020;64(4):607-47. [CrossRef] [PubMed] [PubMed Central]
- Nguyen P, Leray V, Diez M, Serisier S, Le Bloc'h J, Siliart B, et al. Liver lipid metabolism. J Anim Physiol Anim Nutr (Berl). 2008;92(3):272-83. [CrossRef] [PubMed]
- Harris MA, Clark J, Ireland A, Lomax J, Ashburner M, Foulger R, et al. The Gene Ontology (GO) database and informatics resource. Nucleic Acids Res. 2004;32(Database issue):D258-61. [CrossRef] [PubMed] [PubMed Central]
- Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47(D1):D607-D13. [CrossRef] [PubMed] [PubMed Central]
- Alganem K, Imami AS, Sahay S, Eby H, Arvay TO, Abel M, et al. Kaleidoscope: A Bioinformatics Web Application for In Silico Exploration of Omics Data. Journal of Bioinformatics and Systems Biology. 2023;6(4):327-38. Epub 28 November, 2023. [CrossRef]
- Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, et al. NCBI GEO: archive for functional genomics data sets--update. Nucleic Acids Res. 2013;41(Database issue):D991-5. Epub 20121127. [CrossRef] [PubMed] [PubMed Central]
- Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30(1):207-10. [CrossRef] [PubMed] [PubMed Central]
- Community G. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2022 update. Nucleic Acids Res. 2022;50(W1):W345-W51. [CrossRef] [PubMed] [PubMed Central]
- Wingett SW, Andrews S. FastQ Screen: A tool for multi-genome mapping and quality control. F1000Res. 2018;7:1338. Epub 20180824. [CrossRef] [PubMed] [PubMed Central]
- Ewels P, Magnusson M, Lundin S, Käller M. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics. 2016;32(19):3047-8. Epub 20160616. [CrossRef] [PubMed] [PubMed Central]
- Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12(4):357-60. Epub 20150309. [CrossRef] [PubMed] [PubMed Central]
- Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30(7):923-30. Epub 20131113. [CrossRef] [PubMed]
- Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139-40. Epub 20091111. [CrossRef] [PubMed] [PubMed Central]
- Kaidanovich-Beilin O, Cha DS, McIntyre RS. Crosstalk between metabolic and neuropsychiatric disorders. F1000 Biol Rep. 2012;4:14. Epub 20120702. [CrossRef] [PubMed] [PubMed Central]
- Campbell IH, Campbell H. The metabolic overdrive hypothesis: hyperglycolysis and glutaminolysis in bipolar mania. Mol Psychiatry. 2024. Epub 20240125. [CrossRef] [PubMed]
- Gu X, Ke S, Wang Q, Zhuang T, Xia C, Xu Y, et al. Energy metabolism in major depressive disorder: Recent advances from omics technologies and imaging. Biomed Pharmacother. 2021;141:111869. Epub 20210702. [CrossRef] [PubMed]
- Regenold WT, Pratt M, Nekkalapu S, Shapiro PS, Kristian T, Fiskum G. Mitochondrial detachment of hexokinase 1 in mood and psychotic disorders: implications for brain energy metabolism and neurotrophic signaling. J Psychiatr Res. 2012;46(1):95-104. Epub 20111022. [CrossRef] [PubMed]
- Shan D, Mount D, Moore S, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal partitioning of hexokinase 1 suggests disruption of a glutamate transport protein complex in schizophrenia. Schizophr Res. 2014;154(1-3):1-13. Epub 20140221. [CrossRef] [PubMed] [PubMed Central]
- Stork C, Renshaw PF. Mitochondrial dysfunction in bipolar disorder: evidence from magnetic resonance spectroscopy research. Mol Psychiatry. 2005;10(10):900-19. [CrossRef] [PubMed]
- Sullivan CR, Mielnik CA, O'Donovan SM, Funk AJ, Bentea E, DePasquale EA, et al. Connectivity Analyses of Bioenergetic Changes in Schizophrenia: Identification of Novel Treatments. Mol Neurobiol. 2019;56(6):4492-517. Epub 20181018. [CrossRef] [PubMed] [PubMed Central]
- Sullivan CR, Koene RH, Hasselfeld K, O'Donovan SM, Ramsey A, McCullumsmith RE. Neuron-specific deficits of bioenergetic processes in the dorsolateral prefrontal cortex in schizophrenia. Mol Psychiatry. 2019;24(9):1319-28. Epub 20180301. [CrossRef] [PubMed] [PubMed Central]
- Bubber P, Hartounian V, Gibson GE, Blass JP. Abnormalities in the tricarboxylic acid (TCA) cycle in the brains of schizophrenia patients. Eur Neuropsychopharmacol. 2011;21(3):254-60. Epub 20101130. [CrossRef] [PubMed] [PubMed Central]
- Büttiker P, Weissenberger S, Esch T, Anders M, Raboch J, Ptacek R, et al. Dysfunctional mitochondrial processes contribute to energy perturbations in the brain and neuropsychiatric symptoms. Front Pharmacol. 2022;13:1095923. Epub 20230105. [CrossRef] [PubMed] [PubMed Central]
- Middleton FA, Mirnics K, Pierri JN, Lewis DA, Levitt P. Gene expression profiling reveals alterations of specific metabolic pathways in schizophrenia. J Neurosci. 2002;22(7):2718-29. [CrossRef] [PubMed] [PubMed Central]
- Henkel ND, Wu X, O'Donovan SM, Devine EA, Jiron JM, Rowland LM, et al. Schizophrenia: a disorder of broken brain bioenergetics. Mol Psychiatry. 2022;27(5):2393-404. Epub 20220309. [CrossRef] [PubMed]
- Kuang H, Duong A, Jeong H, Zachos K, Andreazza AC. Lactate in bipolar disorder: A systematic review and meta-analysis. Psychiatry Clin Neurosci. 2018;72(8):546-55. Epub 20180607. [CrossRef] [PubMed]
- Roman C, Egert L, Di Benedetto B. Astrocytic-neuronal crosstalk gets jammed: Alternative perspectives on the onset of neuropsychiatric disorders. Eur J Neurosci. 2021;54(5):5717-29. Epub 20200727. [CrossRef] [PubMed]
- Özaslan MS, Balcı N, Demir Y, Gürbüz M, Küfrevioğlu Ö. Inhibition effects of some antidepressant drugs on pentose phosphate pathway enzymes. Environ Toxicol Pharmacol. 2019;72:103244. Epub 20190816. [CrossRef] [PubMed]
- Sarandol A, Sarandol E, Eker SS, Erdinc S, Vatansever E, Kirli S. Major depressive disorder is accompanied with oxidative stress: short-term antidepressant treatment does not alter oxidative-antioxidative systems. Hum Psychopharmacol. 2007;22(2):67-73. [CrossRef] [PubMed]
- Parnell LD, McCaffrey KS, Brooks AW, Smith CE, Lai CQ, Christensen JJ, et al. Rate-Limiting Enzymes in Cardiometabolic Health and Aging in Humans. Lifestyle Genom. 2023;16(1):124-38. Epub 20230720. [CrossRef] [PubMed]
- Chasiotis D, Sahlin K, Hultman E. Regulation of glycogenolysis in human muscle at rest and during exercise. J Appl Physiol Respir Environ Exerc Physiol. 1982;53(3):708-15. [CrossRef] [PubMed]
- Roach PJ, Depaoli-Roach AA, Hurley TD, Tagliabracci VS. Glycogen and its metabolism: some new developments and old themes. Biochem J. 2012;441(3):763-87. [CrossRef] [PubMed] [PubMed Central]
- Miller TB, Larner J. Mechanism of control of hepatic glycogenesis by insulin. J Biol Chem. 1973;248(10):3483-8. [PubMed]
- Eyre JA, Stuart AG, Forsyth RJ, Heaviside D, Bartlett K. Glucose export from the brain in man: evidence for a role for astrocytic glycogen as a reservoir of glucose for neural metabolism. Brain Res. 1994;635(1-2):349-52. [CrossRef] [PubMed]
- Forsyth R, Fray A, Boutelle M, Fillenz M, Middleditch C, Burchell A. A role for astrocytes in glucose delivery to neurons? Dev Neurosci. 1996;18(5-6):360-70. [CrossRef] [PubMed]
- StatPearls. 2024.
- Churchward MA, Tchir DR, Todd KG. Microglial Function during Glucose Deprivation: Inflammatory and Neuropsychiatric Implications. Mol Neurobiol. 2018;55(2):1477-87. Epub 20170207. [CrossRef] [PubMed] [PubMed Central]
- Morris G, Berk M. The many roads to mitochondrial dysfunction in neuroimmune and neuropsychiatric disorders. BMC Med. 2015;13:68. Epub 20150401. [CrossRef] [PubMed] [PubMed Central]
- Pei L, Wallace DC. Mitochondrial Etiology of Neuropsychiatric Disorders. Biol Psychiatry. 2018;83(9):722-30. Epub 20171120. [CrossRef] [PubMed] [PubMed Central]
- Eggleston LV, Krebs HA. Regulation of the pentose phosphate cycle. Biochem J. 1974;138(3):425-35. [CrossRef] [PubMed] [PubMed Central]
- Stincone A, Prigione A, Cramer T, Wamelink MM, Campbell K, Cheung E, et al. The return of metabolism: biochemistry and physiology of the pentose phosphate pathway. Biol Rev Camb Philos Soc. 2015;90(3):927-63. Epub 20140922. [CrossRef] [PubMed] [PubMed Central]
- Ermakov EA, Dmitrieva EM, Parshukova DA, Kazantseva DV, Vasilieva AR, Smirnova LP. Oxidative Stress-Related Mechanisms in Schizophrenia Pathogenesis and New Treatment Perspectives. Oxid Med Cell Longev. 2021;2021:8881770. Epub 20210123. [CrossRef] [PubMed] [PubMed Central]
- Riganti C, Gazzano E, Polimeni M, Aldieri E, Ghigo D. The pentose phosphate pathway: an antioxidant defense and a crossroad in tumor cell fate. Free Radic Biol Med. 2012;53(3):421-36. Epub 20120511. [CrossRef] [PubMed]
- Dienel GA. Brain lactate metabolism: the discoveries and the controversies. J Cereb Blood Flow Metab. 2012;32(7):1107-38. Epub 20111221. [CrossRef] [PubMed] [PubMed Central]
- Hagihara H, Shoji H, Hattori S, Sala G, Takamiya Y, Tanaka M, et al. Large-scale animal model study uncovers altered brain pH and lactate levels as a transdiagnostic endophenotype of neuropsychiatric disorders involving cognitive impairment. Elife. 2024;12. Epub 20240326. [CrossRef] [PubMed] [PubMed Central]
- Jensen-Urstad AP, Semenkovich CF. Fatty acid synthase and liver triglyceride metabolism: housekeeper or messenger? Biochim Biophys Acta. 2012;1821(5):747-53. Epub 20111008. [CrossRef] [PubMed] [PubMed Central]
- Bartlett K, Eaton S. Mitochondrial beta-oxidation. Eur J Biochem. 2004;271(3):462-9. [CrossRef] [PubMed]
- Bhathena SJ. Relationship between fatty acids and the endocrine and neuroendocrine system. Nutr Neurosci. 2006;9(1-2):1-10. [CrossRef] [PubMed]
- Perica MM, Delas I. Essential fatty acids and psychiatric disorders. Nutr Clin Pract. 2011;26(4):409-25. [CrossRef] [PubMed]
- Ross BM, Seguin J, Sieswerda LE. Omega-3 fatty acids as treatments for mental illness: which disorder and which fatty acid? Lipids Health Dis. 2007;6:21. Epub 20070918. [CrossRef] [PubMed] [PubMed Central]
- Shi L, Tu BP. Acetyl-CoA and the regulation of metabolism: mechanisms and consequences. Curr Opin Cell Biol. 2015;33:125-31. Epub 20150220. [CrossRef] [PubMed] [PubMed Central]
- Krebs HA. Rate control of the tricarboxylic acid cycle. Adv Enzyme Regul. 1970;8:335-53. [CrossRef] [PubMed]
- Morella IM, Brambilla R, Morè L. Emerging roles of brain metabolism in cognitive impairment and neuropsychiatric disorders. Neurosci Biobehav Rev. 2022;142:104892. Epub 20220928. [CrossRef] [PubMed]
- Clay HB, Sillivan S, Konradi C. Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. Int J Dev Neurosci. 2011;29(3):311-24. Epub 20100915. [CrossRef] [PubMed] [PubMed Central]
- White HM. The Role of TCA Cycle Anaplerosis in Ketosis and Fatty Liver in Periparturient Dairy Cows. Animals (Basel). 2015;5(3):793-802. Epub 20150818. [CrossRef] [PubMed] [PubMed Central]
- Bostock EC, Kirkby KC, Taylor BV. The Current Status of the Ketogenic Diet in Psychiatry. Front Psychiatry. 2017;8:43. Epub 20170320. [CrossRef] [PubMed] [PubMed Central]
- Brietzke E, Mansur RB, Subramaniapillai M, Balanzá-Martínez V, Vinberg M, González-Pinto A, et al. Ketogenic diet as a metabolic therapy for mood disorders: Evidence and developments. Neurosci Biobehav Rev. 2018;94:11-6. Epub 20180731. [CrossRef] [PubMed]
- Sethi S, Ford JM. The Role of Ketogenic Metabolic Therapy on the Brain in Serious Mental Illness: A Review. J Psychiatr Brain Sci. 2022;7(5). Epub 20221031. [CrossRef] [PubMed] [PubMed Central]
- Liu Y, Fiskum G, Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem. 2002;80(5):780-7. [CrossRef] [PubMed]
- Ramzan R, Kadenbach B, Vogt S. Multiple Mechanisms Regulate Eukaryotic Cytochrome C Oxidase. Cells. 2021;10(3). Epub 20210228. [CrossRef] [PubMed] [PubMed Central]
- Giménez-Palomo A, Dodd S, Anmella G, Carvalho AF, Scaini G, Quevedo J, et al. The Role of Mitochondria in Mood Disorders: From Physiology to Pathophysiology and to Treatment. Front Psychiatry. 2021;12:546801. Epub 20210706. [CrossRef] [PubMed] [PubMed Central]
- Mailloux RJ. Teaching the fundamentals of electron transfer reactions in mitochondria and the production and detection of reactive oxygen species. Redox Biol. 2015;4:381-98. Epub 20150207. [CrossRef] [PubMed] [PubMed Central]
- Pinto A, Bonucci A, Maggi E, Corsi M, Businaro R. Anti-Oxidant and Anti-Inflammatory Activity of Ketogenic Diet: New Perspectives for Neuroprotection in Alzheimer's Disease. Antioxidants (Basel). 2018;7(5). Epub 20180428. [CrossRef] [PubMed] [PubMed Central]
- Yoshimi N, Futamura T, Kakumoto K, Salehi AM, Sellgren CM, Holmén-Larsson J, et al. Blood metabolomics analysis identifies abnormalities in the citric acid cycle, urea cycle, and amino acid metabolism in bipolar disorder. BBA Clin. 2016;5:151-8. Epub 20160403. [CrossRef] [PubMed] [PubMed Central]
- Practice guideline for the treatment of patients with schizophrenia. American Psychiatric Association. Am J Psychiatry. 1997;154(4 Suppl):1-63. [CrossRef] [PubMed]
- Association AP. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(4 Suppl):1-50. [PubMed]
- Kokras N, Dalla C. Sex differences in animal models of psychiatric disorders. Br J Pharmacol. 2014;171(20):4595-619. Epub 20140701. [CrossRef] [PubMed] [PubMed Central]
- Labonté B, Engmann O, Purushothaman I, Menard C, Wang J, Tan C, et al. Sex-specific transcriptional signatures in human depression. Nat Med. 2017;23(9):1102-11. Epub 20170821. [CrossRef] [PubMed] [PubMed Central]
- Consortium C-DGotPG. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381(9875):1371-9. Epub 20130228. [CrossRef] [PubMed] [PubMed Central]
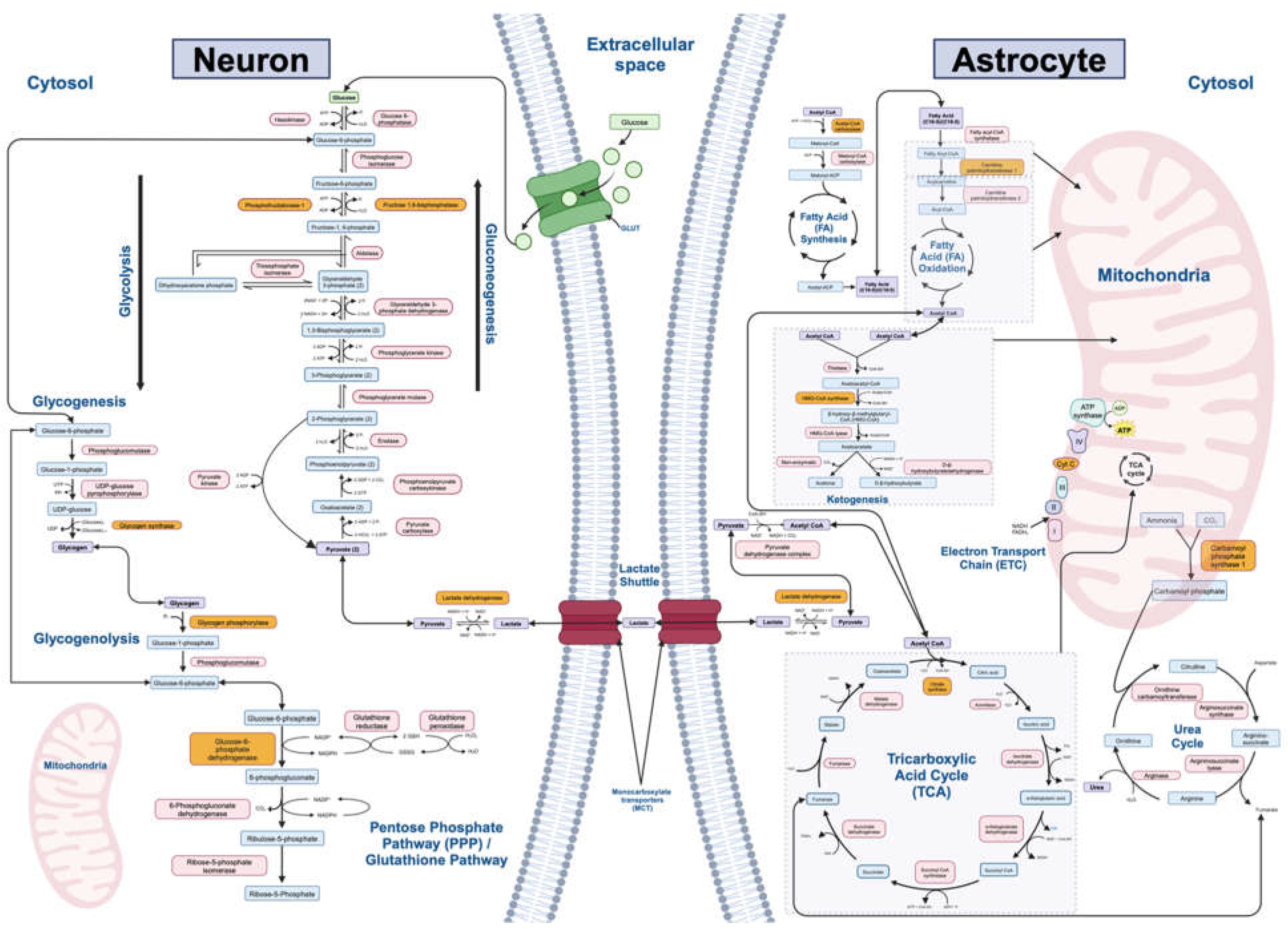
| Metabolic Pathway (n=12) | Rate-Limiting Enzyme | Final Gene List (Output from Kaleidoscope STRING) | Number of Genes (n=242) |
|---|---|---|---|
| Gluconeogenesis | Fructose-1,6-bisphosphate | PC, PCK1, PCK2, FBP1, FBP2, G6PC1, G6PC2, G6PC3, PFKFB2 | 9 |
| Glycolysis | Phosphofructokinase | HK1, HK2, HK3, GCK, GPI, PFKL, PFKM, PFKP, ALDOA, ALDOB, ALDOC, TPI1, GAPDH, GAPDHS, PGK1, PGK2, BPGM, PGAM1, PGAM2, PGAM4, ENO1, ENO2, ENO3, ENO4, PKLR, PKM | 27 |
| Lactate Shuttle (Neuron-Astrocyte) | Lactate dehydrogenase | SLC2A3, LDHA, LDHC, LDHD, SLC16A1, SLC16A3, GLS, GLS2, GLUL, SLC2A1, SLC2A10, SLC2A11, SLC2A12, SLC2A14, SLC1A1, SLC1A2, SLC1A3, SLC1A6 | 18 |
| Tricarboxylic Acid (TCA) Cycle | Citrate Synthase | CS, ACLY, ACO1, ACO2, IREB2, IDH1, IDH2, IDH3A, IDH3B, IDH3G, OGDH, OGDHL, SUCLA2, SUCLG2, SUCLG1, SDHA, SDHB, SDHC, SDHD, SDHAF1, SDHAF4, ALDH5A1, FH, MDH1B, MDH2, PDHA1, PDHA2, DBT, DLAT, DLD | 30 |
| Electron Transport Chain (ETC) | Cytochrome C oxidase | NDUFA8, NDUFS4, NDUFV3, NDUFA11, NDUFS5, NDUFC1, NDUFC2, NDUFS1, NDUFV2, NDUFV1, NDUFA12, NDUFB5, MT-ND1, MT-ND2, MT-ND3, MT-ND4, MT-ND5, MT-ND6, MT-ND4L, SDHA, SDHB, SDHC, SDHD, SDHAF1, SDHAF2, SDHAF3, SDHAF4, UQCRB, UQCRQ, UQCRC1, UQCRC2, UQCR10, MT-CYB, CYC1, UQCRFS1, UQCRH, UQCR10, UQCR11, COX4I1, COX4I2, COX5A, COX5B, COX6A1, COX6A2, COX6B1, COX6B2, COX6C, COX7A1, COX7A2, COX7B, COX7B2, COX7C, COX8A, COX8C, MT-CO1, MT-CO2, MT-CO3, COQ4, COQ7, COQ10A, COQ10B, CYCS, ATP5F1A, ATP5F1B, ATP5F1C, ATP5F1D, ATP5F1E, MT-ATP6, MT-ATP8 | 69 |
| Fatty Acid Synthesis | Acetyl-CoA carboxylase | ACACA, ACACB, MCAT, FASN, OXSM, DECR1, HADH, ELOVL1, ELOVL3, ELOVL6, OLAH, PPT1, PPT2 | 13 |
| Fatty Acid Oxidation | Carnitine Palmitoyltransferase I | SLC27A2, ACSBG2, ACSBG1, ECI1, ECHS1, HADHA, HSD17B10, HADH, HADHB, ACAA2, SCP2, VLCAD, SCAD, MCAD, LCAD, CPT1C, CPT2 | 17 |
| Ketogenesis | HMG-CoA synthase | ACAT1, ACAA1, ACAA2, HMGCS1, HMGCS2, HMGCL, HMGCLL1, BDH2, BDH1 | 9 |
| Glycogenesis | Glycogen synthase | ADPGK, GCKR, HK1, HK2, HK3, PGM1, PGM2, UGP2, GYS1, GYS2, GSK3A, GSK3B, GBE1 | 13 |
| Glycogenolysis | Glycogen phosphorylase | PYGL, PYGM, PYGB, AGL, PGM1, PGM2, G6PC1, G6PC2, G6PC3 | 9 |
| Urea Cycle | Carbamoyl phosphate synthetase I | OTC, OAT, CPS1, CAD, ASS1, ASL, ARG1, ARG2, AGMAT, NAGS, ACY1 | 11 |
| Pentose Phosphate/ Glutathione Pathways | Glucose-6-phosphate dehydrogenase | HK1, HK2, HK3, G6PD, PGD, TKT, TKTL1, TKTL2, TALDO1, GPI, GCLC, GSS, GSR, SOD1, RPE, RPIA, RGN | 17 |
| Metabolic Pathway | Significant Genes by Pathway in Schizophrenia (SCZ) Datasets (n=35) | SCZ Average LFC Values | Significant Genes by Pathway in Ketogenic Intervention (KI) Datasets (n=8) | KI Average LFC Values |
|---|---|---|---|---|
| Gluconeogenesis | Upregulated: PC, PCK1 | 1.08 | Upregulated: PC | 0.23 |
| Downregulated: G6PC3, PFKFB2 | -0.32 | Downregulated: PCK2 | -1.56 | |
| Glycolysis | Upregulated: ALDOA, ALDOB, ALDOC, GAPDHS, PGAM2, ENO1, ENO3, PKLR, PKM | 0.68 | ||
| Downregulated: GAPDH, PFKM, PFKP, PGK1, PGK2 | -0.58 | Downregulated: ENO1, GAPDH, PFKP | -0.56 | |
| Lactate Shuttle (Neuron-Astrocyte) | Upregulated: GLUL, LDHC, SLC16A3, SLC1A2, SLCIA3, SLC2A1 | 0.58 | Upregulated: SLC16A1 | 0.29 |
| Downregulated: GLS2, LDHA, SLC1A1, SLC2A11, SLC2A14 | -0.23 | |||
| Tricarboxylic Acid (TCA) Cycle | Upregulated: ACO1, ACO2, CS, DBT, IDH2, SDHA, SUCLG2 | 0.39 | ||
| Downregulated: DLD, IDH1, IDH3B, MDH2, SDHB, SDHD, SUCLA2 | -0.33 | Downregulated: CS, IDH3G, SDHA, SDHB, SUCLG2 | -0.67 | |
| Electron Transport Chain (ETC) | Upregulated: COX4I2, COX6B2, SDHA, UQCR10 | 0.56 | Upregulated: CYCS, COX4I2, SDHAF3 | 0.40 |
| Downregulated: ATP5F1A, ATP5F1B, ATP5F1C, ATP5F1E, COQ10B, COQ4, COQ7, COX5A, COX5B, COX6A1, COX6B1, COX6C, COX7A1, COX7A2, COX7B, COX7C, COX8A, CYC1, NDUFA12, NDUFA8, NDUFB5, NDUFC2, NDUFS4, NDUFS5, NDUFV1, NDUFV2, NDUFV3, SDHAF3, SDHB, SDHD, UQCR11, UQCRB, UQCRC1, UQCRFS1, UQCRQ | -0.30 | Downregulated: ATP5F1C, ATP5F1D, COX411, COX5B, COX6C, CYC1, SDHA, SDHB, NDUFV2, UQCRC1, UQCRFS1 | -0.62 | |
| Fatty Acid Synthesis | Upregulated: ACACB, DECR1, FASN, HADH | 0.50 | Upregulated: ACACB, HADH | 0.70 |
| Downregulated: ELOVL1, MCAT, OLAH, OXSM | -0.68 | Downregulated: DECR1, PPT1 | -0.69 | |
| Fatty Acid Oxidation | Upregulated: ACAA2, CPT1C, CPT2, HADHA, HSD17B10 | 0.56 | Upregulated: HADH | 0.41 |
| Downregulated: SCP2 | -0.48 | |||
| Ketogenesis | Upregulated: ACAA2, BDH1, BDH2, HMGCLL1 | 0.82 | ||
| Downregulated: ACAA1, HMGCS1 | -0.30 | |||
| Glycogenesis | Upregulated: HK2 | 0.33 | ||
| Downregulated: GBE1, GSK3A, GSK3B, GYS1 | -0.22 | Downregulated: PGM1 | -0.50 | |
| Glycogenolysis | Upregulated: PYGL | 0.54 | Upregulated: PYGL, PYGM | 0.56 |
| Downregulated: G6PC3 | -0.36 | Downregulated: PGM1 | -0.50 | |
| Urea Cycle | Upregulated: ACY1, AGMAT, CAD | 0.90 | ||
| Downregulated: ARG2, ASS1, CPS1, OAT | -0.32 | Downregulated: OAT | -0.56 | |
| Pentose Phosphate/ Glutathione Pathways | Upregulated: G6PD, GPI, HK2, RPE, TKT | 0.52 | ||
| Downregulated: GCLC, GSS, RPIA, SOD1, TALDO1 | -0.26 | Downregulated: G6PD, GSR, RPIA | -0.73 |
| Metabolic Pathway | Significant Genes by Pathway in Bipolar Disorder (BPD) Datasets (n=55) | BPD Average LFC Values | Significant Genes by Pathway in Ketogenic Intervention (KI) Datasets (n=8) | KI Average LFC Values |
|---|---|---|---|---|
| Gluconeogenesis | Upregulated: FBP2, PCK1 | 1.08 | Upregulated: PC | 0.23 |
| Downregulated: G6PC3 | -0.43 | Downregulated: PCK2 | -1.56 | |
| Glycolysis | Upregulated: BPGM, HK2, HK3, PGAM2 | 0.84 | ||
| Downregulated: ALDOA, ENO1, ENO2, GAPDH, GCK, GPI, PFKL, PFKM, PFKP, PGAM4, PGK1, PKLR | -0.58 | Downregulated: ENO1, GAPDH, PFKP | -0.56 | |
| Lactate Shuttle (Neuron-Astrocyte) | Upregulated: LDHC, SLC16A1, SLC2A1, SLC2A10, SLC2A12 | 1.06 | Upregulated: SLC16A1 | 0.29 |
| Downregulated: SLC1A2, SLC2A11, SLC2A3 | -0.84 | |||
| Tricarboxylic Acid (TCA) Cycle | Upregulated: ACO1, DBT, IDH1 | 0.38 | ||
| Downregulated: ACLY, ACO2, CS, DLAT, IDH2, IDH3B, IDH3G, MDH2, OGDHL, SDHAF4, SDHC, SUCLA2 | -0.51 | Downregulated: CS, IDH3G, SDHA, SDHB, SUCLG2 | -0.67 | |
| Electron Transport Chain (ETC) | Upregulated: COX4I2, COX6A2, COX6B2, COX8C, MT-ATP6, MT-CO1, MT-CO2, MT-CO3, MT-CYB, MT-ND1, MT-ND2, MT-ND3, MT-ND4, MT-ND4L, MT-ND5, MT-ND6, NDUFV2, UQCRC2, UQCRH | 0.65 | Upregulated: CYCS, COX4I2, SDHAF3 | 0.40 |
| Downregulated: ATP5F1A, ATP5F1B, ATP5F1D, ATP5F1E, COQ10A, COQ4, COQ7, COX5B, COX7B2, COX8A, CYC1, CYCS, NDUFA11, NDUFA12, NDUFB5, NDUFC2, NDUFS1, NDUFV1, SDHAF3, SDHAF4, SDHC, UQCR10, UQCR11, UQCRC1 | -0.57 | Downregulated: ATP5F1C, ATP5F1D, COX411, COX5B, COX6C, CYC1, SDHA, SDHB, NDUFV2, UQCRC1, UQCRFS1 | -0.62 | |
| Fatty Acid Synthesis | Upregulated: DECR1, ELOVL3, OLAH, OXSM | 0.90 | Upregulated: ACACB, HADH | 0.70 |
| Downregulated: ACACA, SLOVL6, FASN, MCAT, PPT1 | -0.51 | Downregulated: DECR1, PPT1 | -0.69 | |
| Fatty Acid Oxidation | Upregulated: CPT2, HADHB, SCP2 | 0.46 | Upregulated: HADH | 0.41 |
| Downregulated: ACSBG2, CPT1C, ECHS1 | -0.76 | |||
| Ketogenesis | Upregulated: HMGCL, HMGCS2 | 1.16 | ||
| Downregulated: ACAT1, HMGCLL1, HMGCS1 | -1.05 | |||
| Glycogenesis | Upregulated: HK2, HK3, UGP2 | 0.90 | ||
| Downregulated: PGM2 | -0.67 | Downregulated: PGM1 | -0.50 | |
| Glycogenolysis | Upregulated: PYGL | 0.49 | Upregulated: PYGL, PYGM | 0.56 |
| Downregulated: AGL, G6PC3, PGM2 | -0.58 | Downregulated: PGM1 | -0.50 | |
| Urea Cycle | Upregulated: ACY1, ARG1, OTC | 0.68 | ||
| Downregulated: ARG2, ASL, ASS1, CAD, OAT | -0.45 | Downregulated: OAT | -0.56 | |
| Pentose Phosphate/ Glutathione Pathways | Upregulated: GCLC, HK2, HK3, RPIA | 0.87 | ||
| Downregulated: G6PD, GPI, PGD, TKT | -0.61 | Downregulated: G6PD, GSR, RPIA | -0.73 |
| Metabolic Pathway | Significant Genes by Pathway in Major Depressive Disorder (MDD) Datasets (n=36) | MDD Average LFC Values | Significant Genes by Pathway in Ketogenic Intervention (KI) Datasets (n=8) | KI Average LFC Values |
|---|---|---|---|---|
| Gluconeogenesis | Upregulated: G6PC2 | 0.3 | Upregulated: PC | 0.23 |
| Downregulated: G6PC3, PCK1, PFKFB2 | -0.68 | Downregulated: PCK2 | -1.56 | |
| Glycolysis | Upregulated: ALDOA, ENO2, GCK, GPI, HK1, HK3, PFKP, PGAM1, PGAM4, PGK1, PKM, TPI1 | 0.59 | ||
| Downregulated: ENO4, PGK2 | -1.19 | Downregulated: ENO1, GAPDH, PFKP | -0.56 | |
| Lactate Shuttle (Neuron-Astrocyte) | Upregulated: GLS, LDHA, SLC16A1, SLC1A6 | 0.39 | Upregulated: SLC16A1 | 0.29 |
| Downregulated: GLUL, LDHD, SLC1A2, SLC1A3, SLC2A10, SLC2A11, SLC2A12, SLC2A3 | -0.53 | |||
| Tricarboxylic Acid (TCA) Cycle | Upregulated: ACO2, CS, DLD, FH, IDH3A, IDH3B, IDH3G, OGDH, PDHA1, SDHA, SDHB, SDHC, SDHD, SUCLA2, SUCLG1 | 0.45 | ||
| Downregulated: ACLY, ACO1, ALDH5A1, DBT, IDH1, IDH2, IREB2, SUCLG2 | -0.33 | Downregulated: CS, IDH3G, SDHA, SDHB, SUCLG2 | -0.67 | |
| Electron Transport Chain (ETC) | Upregulated: ATP5F1A, ATP5F1B, ATP5F1E, COQ10A, COQ10B, COQ7, COX5B, COX6B1, COX6C, COX7A1, COX7A2, COX7B, COX7C, CYC1, CYCS, MT-CO2, MT-CO3, NDUFA12, NDUFB5, NDUFC2, NDUFS4, NDUFS5, NDUFV1, SDHA, SDHAF3, SDHB, SDHC, SDHD, UQCR11, UQCRB, UQCRC1, UQCRC2, UQCRFS1, UQCRH, UQCRQ | 0.57 | Upregulated: CYCS, COX4I2, SDHAF3 | 0.40 |
| Downregulated: COX7B2, MT-ATP6, MT-ATP8, MT-ND2, MT-ND5 | -0.51 | Downregulated: ATP5F1C, ATP5F1D, COX411, COX5B, COX6C, CYC1, SDHA, SDHB, NDUFV2, UQCRC1, UQCRFS1 | -0.62 | |
| Fatty Acid Synthesis | Upregulated: ELOVL3, MCAT, OLAH | 0.24 | Upregulated: ACACB, HADH | 0.70 |
| Downregulated: DECR1, ELOVL1, FASN | -0.35 | Downregulated: DECR1, PPT1 | -0.69 | |
| Fatty Acid Oxidation | Upregulated: ACSBG2, HSD17B10, SLC27A2 | 0.29 | Upregulated: HADH | 0.41 |
| Downregulated: ACAA2, ACSBG1, CPT2, ECI1 | -0.39 | |||
| Ketogenesis | Upregulated: ACAT1, HMGCLL1 | 0.62 | ||
| Downregulated: ACAA1, ACAA2, BDH1, BDH2, HMGCS1 | -0.35 | |||
| Glycogenesis | Upregulated: HK1, HK3, UGP2 | 0.41 | ||
| Downregulated: ADPGK, GYS1, PGM2 | -0.33 | Downregulated: PGM1 | -0.50 | |
| Glycogenolysis | Upregulated: G6PC2, PYGL | 0.66 | Upregulated: PYGL, PYGM | 0.56 |
| Downregulated: AGL, G6PC3, PGM2, PYGB, PYGM | -0.44 | Downregulated: PGM1 | -0.50 | |
| Urea Cycle | Upregulated: ARG2, ASS1, OTC | 0.33 | ||
| Downregulated: ACY1, ASL, CPS1 | -0.62 | Downregulated: OAT | -0.56 | |
| Pentose Phosphate/ Glutathione Pathways | Upregulated: GCLC, GPI, GSR, HK1, HK3, PGD, RPIA, SOD1, TALDO1 | 0.34 | ||
| Downregulated: G6PD, GSR, RPIA | -0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





