Submitted:
04 July 2025
Posted:
07 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
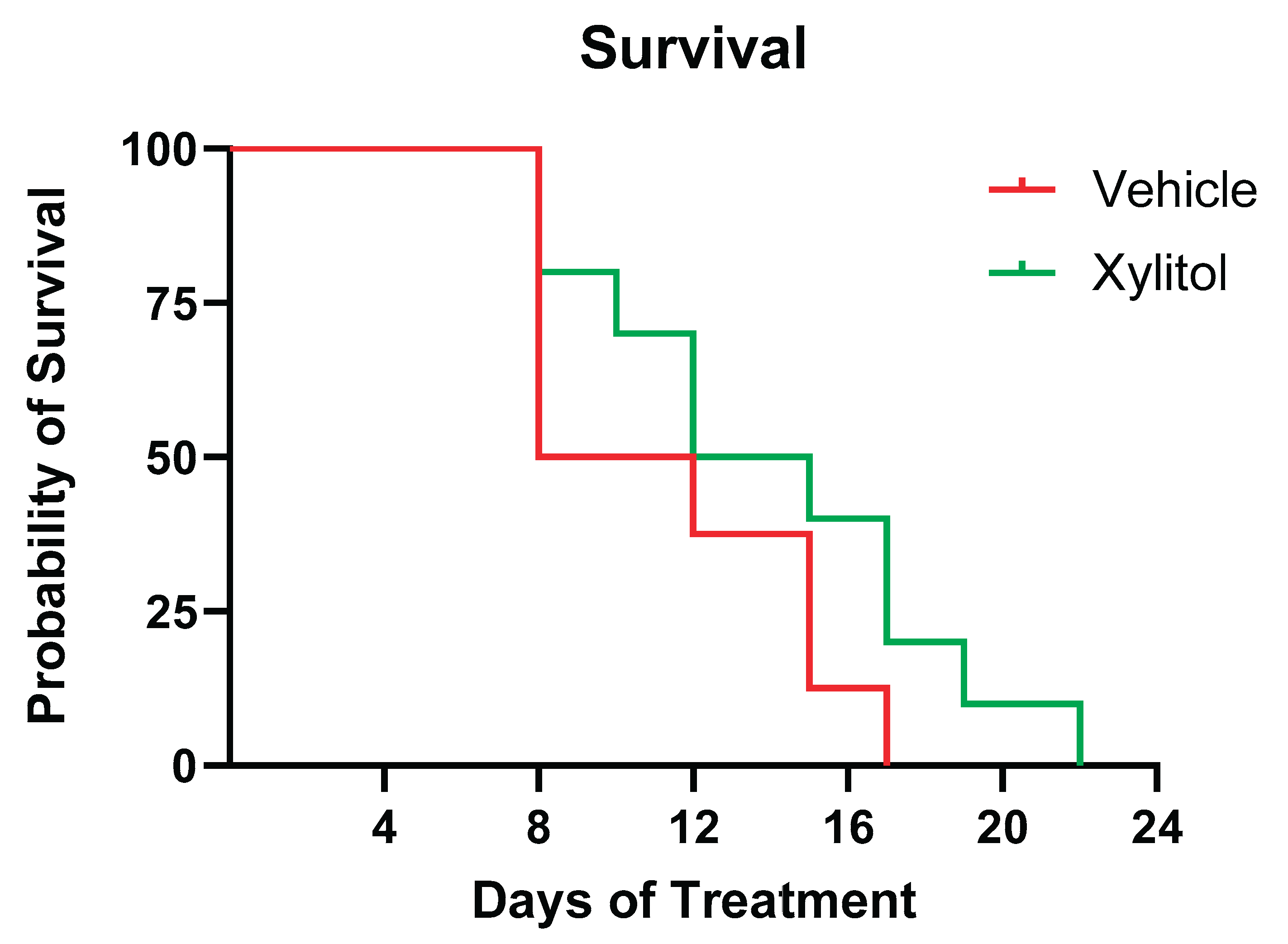
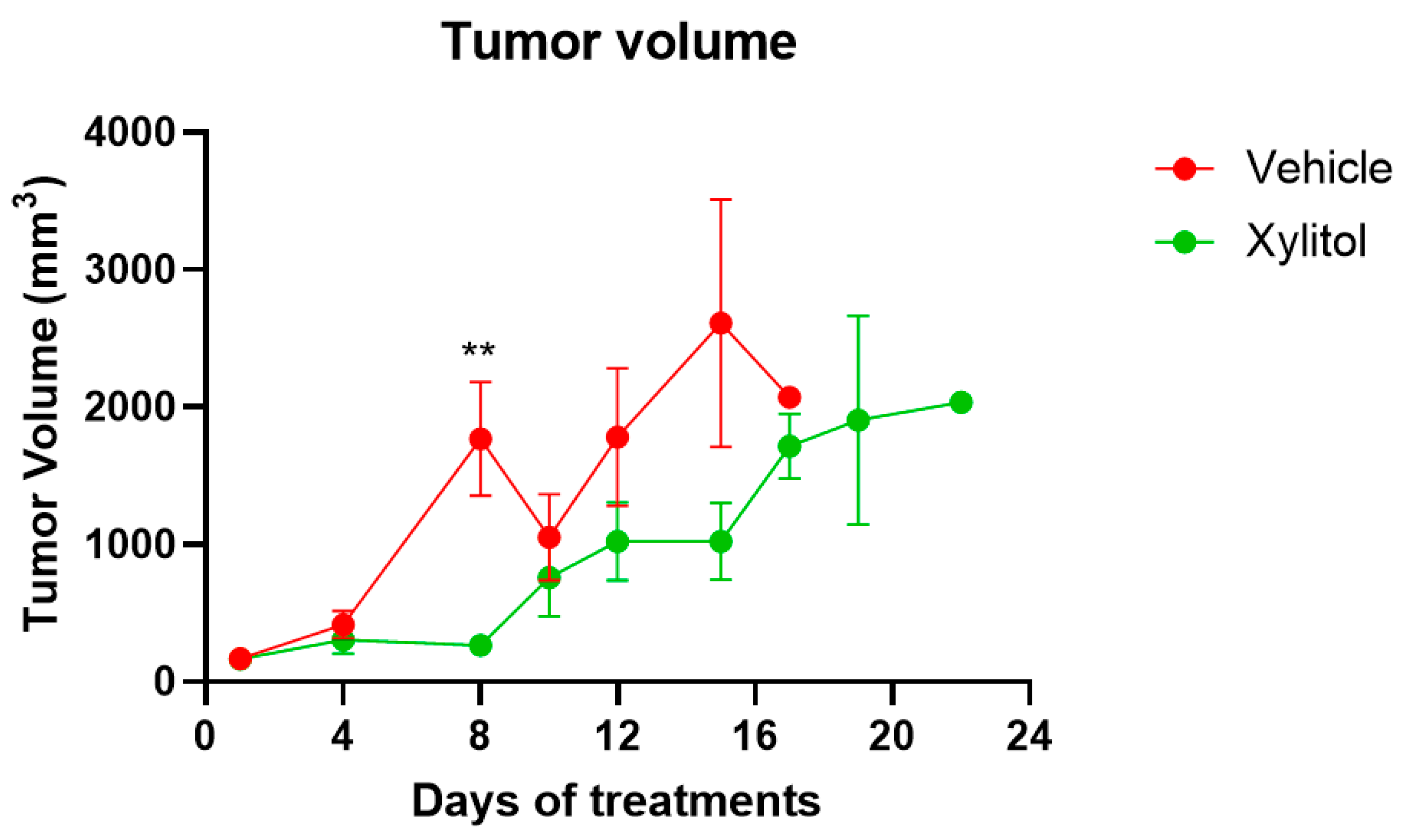
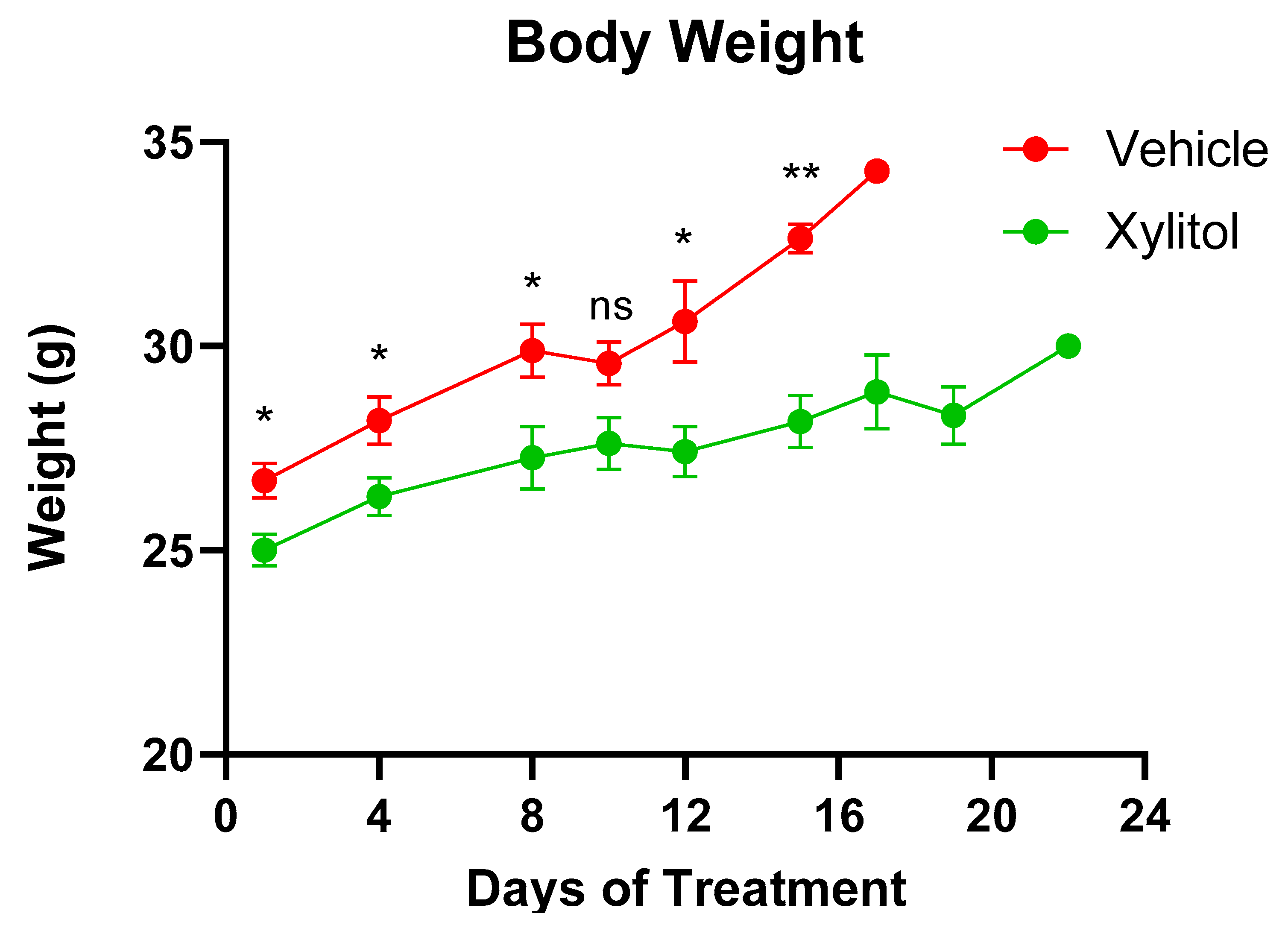
3. Results
4. Discussion
5. Conclusions
Ethics Approval and Consent to Participate
Authors Contributions
Competing Interests
Consent for Publication
Acknowledgments
Funding Details
Disclosure of Interest
Data Availability Statement
References
- Milgrom P, Ly KA, Rothen M. Xylitol and its vehicles for public health needs. Adv Dent Res. 2009;21:44–47. [CrossRef]
- Mooradian AD, Smith M, Tokuda M. The role of artificial and natural sweeteners in reducing the consumption of table sugar: a narrative review. Clin Nutr ESPEN. 2017;18:1–8. [CrossRef]
- Knuuttila MLE, Makinen KK. Effect of xylitol on the growth and metabolism of Streptococcus mutans. Caries Res. 1975;9:177–189. [CrossRef]
- Ly KA, Milgrom P, Rothen M. Xylitol, sweeteners, and dental caries. Pediatr Dent. 2006;28:154–163.
- Bradshaw DJ, Marsh PD. Effect of sugar alcohols on the composition and metabolism of a mixed culture of oral bacteria grown in a chemostat. Caries Res. 1994;28:251–256. [CrossRef]
- Soderling EM. Xylitol, mutans streptococci, and dental plaque. Adv Dent Res. 2009;21:74–78. [CrossRef]
- Scheinin A. Caries control through the use of sugar substitutes. Int Dent J. 1976;26:4–13.
- Makinen KK, Isotupa KP, Kivilompolo T, Makinen PL, Toivanen J, Soderling E. Comparison of erythritol and xylitol saliva stimulants in the control of dental plaque and mutans streptococci. Caries Res. 2001;35:129–135. [CrossRef]
- Soderling EM. Xylitol, mutans streptococci, and dental plaque. Adv Dent Res. 2009;21:74–78. [CrossRef]
- Burt BA. The use of sorbitol- and xylitol-sweetened chewing gum in caries control. J Am Dent Assoc. 2006;137:190–196. [CrossRef]
- Makinen KK, Isotupa KP, Kivilompolo T, Makinen PL, Murtomaa S, Petaja J, Toivanen J, Soderling E. The effect of polyol-combinant saliva stimulants on S. mutans levels in plaque and saliva of patients with mental retardation. Spec Care Dentist. 2002;22:187–193. [CrossRef]
- Makinen KK, Saag M, Isotupa KP, Olak J, Nommela R, Soderling E, Makinen PL. Similarity of the effects of erythritol and xylitol on some risk factors of dental caries. Caries Res. 2005;39:207–215. [CrossRef]
- Janakiram C, Deepan Kumar CV, Joseph J, Xylitol in preventing dental caries: a systematic review and meta-analyses. J Nat Sci Biol Med. 2017;8:16–21. [CrossRef]
- Kõljalg S, Smidt I, Chakrabarti A, Bosscher D, Mändar R. Exploration of singular and synergistic effect of xylitol and erythritol on causative agents of dental caries. Sci Rep. 2020;10:6297. [CrossRef]
- Mäkinen KK. Sugar alcohols and prevention of oral diseases–comments and rectifications. Oral Health Care. 2017;2:1–8. [CrossRef]
- Salli K, Lehtinen MJ, Tiihonen K, Ouwehand AC. Xylitol’s health benefits beyond dental health: a comprehensive review. Nutrients. 2019;11:1813. [CrossRef]
- Meng C, Bai C, Brown TD, Hood LE, Tian Q. Human gut microbiota and gastrointestinal cancer. Genomics Proteomics Bioinformatics. 2018;16:33–49. [CrossRef]
- Tsai YL, Lin TL, Chang CJ, Wu TR, Lai WF, Lu CC, Lai HC. Probiotics, prebiotics and amelioration of diseases. J Biomed Sci. 2019;26:3. [CrossRef]
- Yadav MK, Kumari I, Singh B, Sharma KK, Tiwari SK. Probiotics, prebiotics and synbiotics: safe options for next-generation therapeutics. Appl Microbiol Biotechnol. 2022;106:505–521. doi: 10.1007/s00253-021-11646-8.
- Liu X, Chen Y, Zhang S, Dong L. Gut microbiota-mediated immunomodulation in tumor. J Exp Clin Cancer Res. 2021;40:221. doi: 10.1186/s13046-021-01983-x.
- Abreu MT, Peek RM Jr. Gastrointestinal malignancy and the microbiome. Gastroenterology. 2014;146:1534–1546.e3. [CrossRef]
- Zhou CB, Zhou YL, Fang JY. Gut microbiota in cancer immune response and immunotherapy. Trends Cancer. 2021;7:647–660. [CrossRef]
- Ting NL, Lau HC, Yu J. Cancer pharmacomicrobiomics: targeting microbiota to optimise cancer therapy outcomes. Gut. 2022;71:1412–1425. [CrossRef]
- Park E, Park MH, Na HS, Chung J. Xylitol induces cell death in lung cancer A549 cells by autophagy. Biotechnol Lett. 2015;37:983–990. [CrossRef]
- Tomonobu N, Komalasari NLGY, Sumardika IW, Jiang F, Chen Y, Yamamoto KI, Kinoshita R, Murata H, Inoue Y, Sakaguchi M. Xylitol acts as an anticancer monosaccharide to induce selective cancer death via regulation of the glutathione level. Chem Biol Interact. 2020;324:109085. [CrossRef]
- Sahasakul Y, Angkhasirisap W, Lam-Ubol A, Aursalung A, Sano D, Takada K, Trachootham D. Partial substitution of glucose with xylitol prolongs survival and suppresses cell proliferation and glycolysis of mice bearing orthotopic xenograft of oral cancer. Nutrients. 2022;14;2023. [CrossRef]
- Mehnert H, Förster H, Dehmel KH. The effect of intravenous administration of xylitol solutions in normal persons and in patients with liver diseases and diabetes mellitus. In: Horecker BL, Lang K, Takagi Y, editors. International symposium on metabolism, physiology, and clinical use of pentoses and pentitols, Hakone, Japan, August 1967; 1967 Aug 27–29; Hakone, Japan. Berlin (Germany): Springer, 1969. p. 293–302.
- Sato J, Wang YM, van Eys J. Metabolism of xylitol and glucose in rats bearing hepatocellular. Cancer Res. 1981;41:3192–3199.
- Yi EY, Kim YJ. Xylitol inhibits in vitro and in vivo angiogenesis by suppressing the NF-κB and Akt signaling pathways. Int J Oncol. 2013;43:315–320. [CrossRef]
- Ylikahri RH, Leino T. Metabolic interactions of xylitol and ethanol in healthy males. Metabolism. 1979;28:25–29. [CrossRef]
- Hutcheson RM, Reynolds VH, Touster O. The reduction of L-xylulose to xylitol by guinea pig liver mitochondria. J Biol Chem. 1956;221:697–709.
- Ahuja V, Macho M, Ewe D, Singh M, Saha S, Saurav K. Biological and pharmacological potential of xylitol: a molecular insight of unique metabolism. Foods. 2020;9:1592. [CrossRef]
- K.K. Makinen, E. Soderling , A quantitative study of mannitol, sorbitol, xylitol, and xylose in wild berries and commercial fruits. Journal of Food Science, 45 (1980), pp. 367-374.
- Islam MS. Effects of xylitol as a sugar substitute on diabetes-related parameters in nondiabetic rats. J Med Food. 2011;14:505–511. [CrossRef]
- Park E, Na HS, Kim SM, Wallet S, Cha S, Chung J. Xylitol, an anticaries agent, exhibits potent inhibition of inflammatory responses in human THP-1-derived macrophages infected with Porphyromonas gingivalis. J Periodontol. 2014;85:e212–e223. [CrossRef]
- Trachootham D, Chingsuwanrote P, Yoosadiang P, Mekkriangkrai D, Ratchawong T, Buraphacheep N, Kijanukul S, Saekhow S, Pongpitchayadej O, Vongvachvasin K, et al. Partial substitution of glucose with xylitol suppressed the glycolysis and selectively inhibited the proliferation of oral cancer cells. Nutr Cancer. 2017;69:862–872. [CrossRef]
- Qusa MH, Siddique AB, Nazzal S, El Sayed KA. Novel olive oil phenolic (−)-oleocanthal (+)-xylitol-based solid dispersion formulations with potent oral anti-breast cancer activities. Int J Pharm. 2019;569:118596. [CrossRef]
- Ireson CR, Alavijeh MS, Palmer AM, Fowler ER, Jones HJ. The role of mouse tumour models in the discovery and development of anticancer drugs. Br J Cancer. 2019;121:101–108. [CrossRef]
- Cogels MM, Rouas R, Ghanem GE, Martinive P, Awada A, van Gestel D, Krayem M. Humanized mice as a valuable pre-clinical model for cancer immunotherapy research. Front Oncol. 2021;11:784947. [CrossRef]
- Wang, Y., Qi, H., Liu, Y., Duan, C., Liu, X., Xia, T., Chen, D., Piao, H. L., & Liu, H. X. (2021). The double-edged roles of ROS in cancer prevention and therapy. Theranostics, 11(10), 4839–4857. [CrossRef]
- Hegde, S. S., Chandler, J., Vetting, M. W., Yu, M., & Blanchard, J. S. (2007). Mechanistic and structural analysis of human spermidine/spermine N1-acetyltransferase. Biochemistry, 46(24), 7187-7195. [CrossRef]
- Ni, Y. Q., & Liu, Y. S. (2021). New Insights into the Roles and Mechanisms of Spermidine in Aging and Age-Related Diseases. Aging and disease, 12(8), 1948–1963. [CrossRef]
- Holbert, C. E., Cullen, M. T., Casero, R. A., Jr, & Stewart, T. M. (2022). Polyamines in cancer: integrating organismal metabolism and antitumour immunity. Nature reviews. Cancer, 22(8), 467–480. [CrossRef]
- Lian, J., Liang, Y., Zhang, H., Lan, M., Ye, Z., Lin, B., Qiu, X., & Zeng, J. (2022). The role of polyamine metabolism in remodeling immune responses and blocking therapy within the tumor immune microenvironment. Frontiers in immunology, 13, 912279. [CrossRef]
- Timson D. J. (2019). Fructose 1,6-bisphosphatase: getting the message across. Bioscience reports, 39(3), BSR20190124. [CrossRef]
- Cho, E. S., Cha, Y. H., Kim, H. S., Kim, N. H., & Yook, J. I. (2018). The Pentose Phosphate Pathway as a Potential Target for Cancer Therapy. Biomolecules & therapeutics, 26(1), 29–38. [CrossRef]
- Bartrons, R., Simon-Molas, H., Rodríguez-García, A., Castaño, E., Navarro-Sabaté, À., Manzano, A., & Martinez-Outschoorn, U. E. (2018). Fructose 2,6-Bisphosphate in Cancer Cell Metabolism. Frontiers in oncology, 8, 331. [CrossRef]
- Prasher, P., Sharma, M., Singh, S. K., Gulati, M., Chellappan, D. K., Rajput, R., Gupta, G., Ydyrys, A., Kulbayeva, M., Abdull Razis, A. F., Modu, B., Sharifi-Rad, J., & Dua, K. (2023). Spermidine as a promising anticancer agent: Recent advances and newer insights on its molecular mechanisms. Frontiers in chemistry, 11, 1164477. [CrossRef]
- Schmidt, D. R., Patel, R., Kirsch, D. G., Lewis, C. A., Vander Heiden, M. G., & Locasale, J. W. (2021). Metabolomics in cancer research and emerging applications in clinical oncology. CA: a cancer journal for clinicians, 71(4), 333–358. [CrossRef]
- Oldenburg, O., Qin, Q., Sharma, A. R., Cohen, M. V., Downey, J. M., & Benoit, J. N. (2002). Acetylcholine leads to free radical production dependent on K(ATP) channels, G(i) proteins, phosphatidylinositol 3-kinase and tyrosine kinase. Cardiovascular research, 55(3), 544–552. [CrossRef]
- Liu, P., Huang, F., Lin, P. et al. Histidine metabolism drives liver cancer progression via immune microenvironment modulation through metabolic reprogramming. J Transl Med 23, 262 (2025). [CrossRef]
- Zanoni, M., Pegoraro, A., Adinolfi, E., & De Marchi, E. (2022). Emerging roles of purinergic signaling in anti-cancer therapy resistance. Frontiers in cell and developmental biology, 10, 1006384. [CrossRef]
- Cox, M. A., Bassi, C., Saunders, M. E., Nechanitzky, R., Morgado-Palacin, I., Zheng, C., & Mak, T. W. (2020). Beyond neurotransmission: acetylcholine in immunity and inflammation. Journal of internal medicine, 287(2), 120–133. [CrossRef]
- Purves D, Augustine GJ, Fitzpatrick D, et al., editors. Neuroscience. 2nd edition. Sunderland (MA): Sinauer Associates; 2001. G-Proteins and Their Molecular Targets. Available from: https://www.ncbi.nlm.nih.gov/books/NBK10832/.
- Snezhkina, A. V., Kudryavtseva, A. V., Kardymon, O. L., Savvateeva, M. V., Melnikova, N. V., Krasnov, G. S., & Dmitriev, A. A. (2019). ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxidative medicine and cellular longevity, 2019, 6175804. [CrossRef]
- Yagüe-Capilla, M., & Rudd, S. G. (2024). Understanding the interplay between dNTP metabolism and genome stability in cancer. Disease models & mechanisms, 17(8), dmm050775. [CrossRef]
- He, H., Huang, J., Wu, S., Jiang, S., Liang, L., Liu, Y., Liu, W., Xie, L., Tao, Y., Jiang, Y., & Cong, L. (2021). The roles of GTPase-activating proteins in regulated cell death and tumor immunity. Journal of hematology & oncology, 14(1), 171. [CrossRef]
- Novak I. (2003). ATP as a signaling molecule: the exocrine focus. News in physiological sciences : an international journal of physiology produced jointly by the International Union of Physiological Sciences and the American Physiological Society, 18, 12–17. [CrossRef]
- Yegutkin, G. G., & Boison, D. (2022). ATP and Adenosine Metabolism in Cancer: Exploitation for Therapeutic Gain. Pharmacological reviews, 74(3), 797–822. [CrossRef]
- Savio, L. E. B., Leite-Aguiar, R., Alves, V. S., Coutinho-Silva, R., & Wyse, A. T. S. (2021). Purinergic signaling in the modulation of redox biology. Redox biology, 47, 102137. [CrossRef]
- Scolaro, T., Manco, M., Pecqueux, M., Amorim, R., Trotta, R., Van Acker, H. H., Van Haele, M., Shirgaonkar, N., Naulaerts, S., Daniluk, J., Prenen, F., Varamo, C., Ponti, D., Doglioni, G., Ferreira Campos, A. M., Fernandez Garcia, J., Radenkovic, S., Rouhi, P., Beatovic, A., Wang, L., … Mazzone, M. (2024). Nucleotide metabolism in cancer cells fuels a UDP-driven macrophage cross-talk, promoting immunosuppression and immunotherapy resistance. Nature cancer, 5(8), 1206–1226. [CrossRef]
- Juan, C. A., Pérez de la Lastra, J. M., Plou, F. J., & Pérez-Lebeña, E. (2021). The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. International journal of molecular sciences, 22(9), 4642. [CrossRef]
- Jang, J. H., Kim, D. H., & Chun, K. S. (2025). Tumor microenvironment regulation by reactive oxygen species-mediated inflammasome activation. Archives of pharmacal research, 48(2), 115–131. [CrossRef]
- Wang, L., Kuang, Z., Zhang, D., Gao, Y., Ying, M., & Wang, T. (2021). Reactive oxygen species in immune cells: A new antitumor target. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 133, 110978. [CrossRef]
- Cao, Y., Yi, Y., Han, C., & Shi, B. (2024). NF-κB signaling pathway in tumor microenvironment. Frontiers in immunology, 15, 1476030. [CrossRef]
- Montero, J., Dutta, C., van Bodegom, D. et al. p53 regulates a non-apoptotic death induced by ROS. Cell Death Differ 20, 1465–1474 (2013). [CrossRef]
- An, X., Yu, W., Liu, J. et al. Oxidative cell death in cancer: mechanisms and therapeutic opportunities. Cell Death Dis 15, 556 (2024). [CrossRef]
- Aggarwal, V., Tuli, H. S., Varol, A., Thakral, F., Yerer, M. B., Sak, K., Varol, M., Jain, A., Khan, M. A., & Sethi, G. (2019). Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules, 9(11), 735. [CrossRef]
- Nakamura, H., & Takada, K. (2021). Reactive oxygen species in cancer: Current findings and future directions. Cancer science, 112(10), 3945–3952. [CrossRef]
- Rigoulet, M., Bouchez, C. L., Paumard, P., Ransac, S., Cuvellier, S., Duvezin-Caubet, S., Mazat, J. P., & Devin, A. (2020). Cell energy metabolism: An update. Biochimica et biophysica acta. Bioenergetics, 1861(11), 148276. [CrossRef]
- Fiorillo, M., Ózsvári, B., Sotgia, F., & Lisanti, M. P. (2021). High ATP Production Fuels Cancer Drug Resistance and Metastasis: Implications for Mitochondrial ATP Depletion Therapy. Frontiers in oncology, 11, 740720. [CrossRef]
- Miriam Yagüe-Capilla, Sean G. Rudd; Understanding the interplay between dNTP metabolism and genome stability in cancer. Dis Model Mech 1 August 2024; 17 (8): dmm050775. [CrossRef]
- Bordier, V., Teysseire, F., Senner, F., Schlotterbeck, G., Drewe, J., Beglinger, C., Wölnerhanssen, B. K., & Meyer-Gerspach, A. C. (2022). Absorption and Metabolism of the Natural Sweeteners Erythritol and Xylitol in Humans: A Dose-Ranging Study. International journal of molecular sciences, 23(17), 9867. [CrossRef]
- Wölnerhanssen, B. K., Meyer-Gerspach, A. C., Beglinger, C., & Islam, M. S. (2020). Metabolic effects of the natural sweeteners xylitol and erythritol: A comprehensive review. Critical reviews in food science and nutrition, 60(12), 1986–1998. [CrossRef]
- Uebanso, T., Kano, S., Yoshimoto, A., Naito, C., Shimohata, T., Mawatari, K., & Takahashi, A. (2017). Effects of Consuming Xylitol on Gut Microbiota and Lipid Metabolism in Mice. Nutrients, 9(7), 756. [CrossRef]
- Ahuja, V., Macho, M., Ewe, D., Singh, M., Saha, S., & Saurav, K. (2020). Biological and Pharmacological Potential of Xylitol: A Molecular Insight of Unique Metabolism. Foods (Basel, Switzerland), 9(11), 1592. [CrossRef]
- Amo, K., Arai, H., Uebanso, T., Fukaya, M., Koganei, M., Sasaki, H., Yamamoto, H., Taketani, Y., & Takeda, E. (2011). Effects of xylitol on metabolic parameters and visceral fat accumulation. Journal of clinical biochemistry and nutrition, 49(1), 1–7. [CrossRef]
- Ylikahri R. (1979). Metabolic and nutritional aspects of xylitol. Advances in food research, 25, 159–180. [CrossRef]
- Kabashima, T., Kawaguchi, T., Wadzinski, B. E., & Uyeda, K. (2003). Xylulose 5-phosphate mediates glucose-induced lipogenesis by xylulose 5-phosphate-activated protein phosphatase in rat liver. Proceedings of the National Academy of Sciences of the United States of America, 100(9), 5107–5112. [CrossRef]
- Jakob, A., Williamson, J. R., & Asakura, T. (1971). Xylitol metabolism in perfused rat liver. Interactions with gluconeogenesis and ketogenesis. The Journal of biological chemistry, 246(24), 7623–7631.
- Ma, P., Sun, W., Sun, C., Tan, J., Dong, X., He, J., Ali, A., Chen, M., Zhang, L., Wu, L., & Wang, P. (2025). Using gut microbiota and non-targeted metabolomics techniques to study the effect of xylitol on alleviating DSS-induced inflammatory bowel disease in mice. BMC immunology, 26(1), 18. [CrossRef]
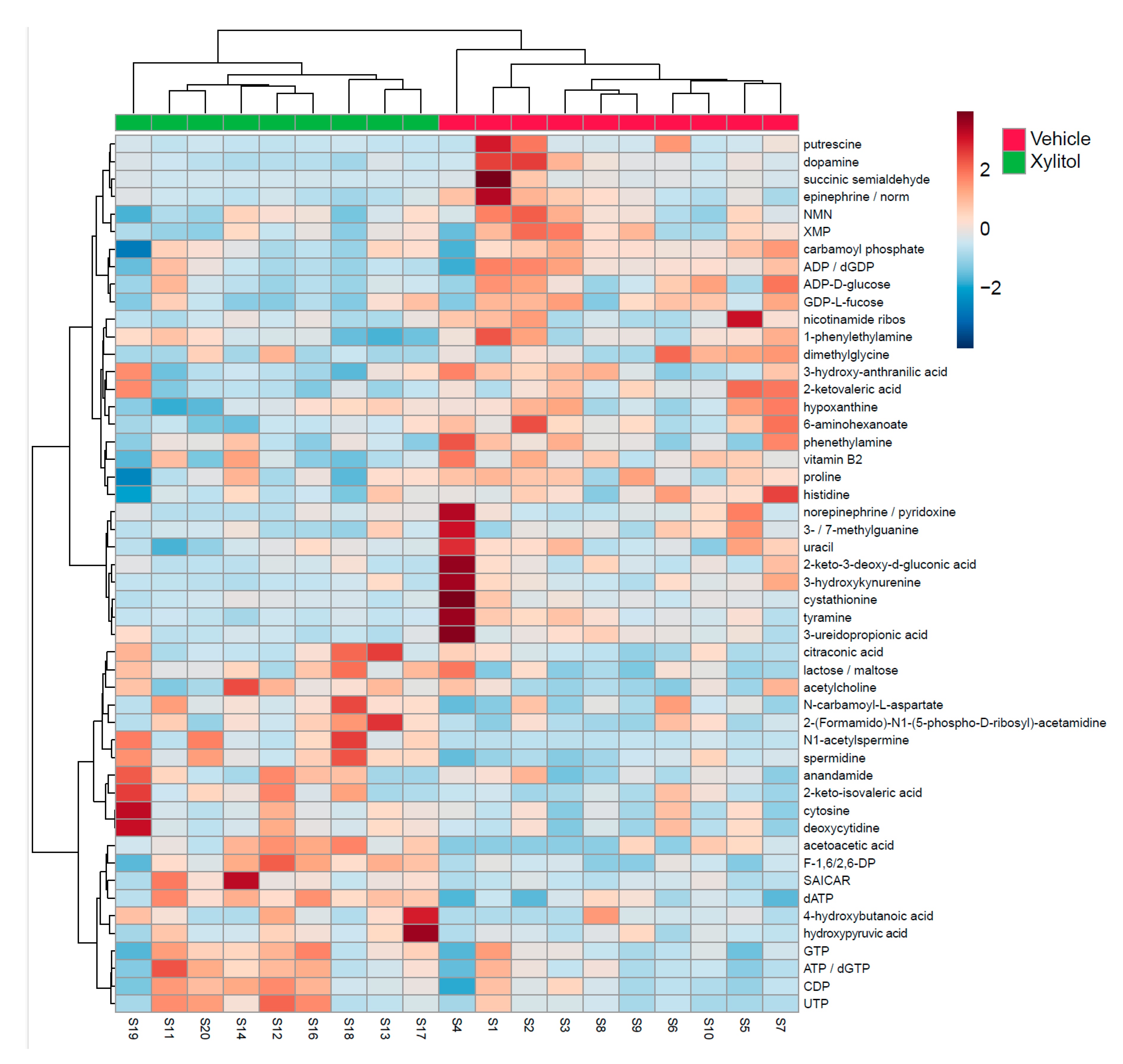
| Metabolite | t.stat | p.value |
| dATP | -4.1503 | 0.00067 |
| spermidine | -3.7167 | 0.001715 |
| F-1,6/2,6-DP | -3.2851 | 0.004368 |
| N1-acetylspermine | -3.1546 | 0.005786 |
| 6-aminohexanoate | 2.983 | 0.008352 |
| acetoacetic acid | -2.8922 | 0.010128 |
| dopamine | 2.5854 | 0.019254 |
| UTP | -2.4726 | 0.024271 |
| ADP / dGDP | 2.4571 | 0.025048 |
| SAICAR | -2.3512 | 0.031036 |
| tyramine | 2.3357 | 0.032018 |
| CDP | -2.3348 | 0.032075 |
| epinephrine / normetanephrine | 2.2182 | 0.040446 |
| vitamin B2 | 2.1843 | 0.043235 |
| 2-ketovaleric acid | 2.1751 | 0.044027 |
| ATP / dGTP | -2.1645 | 0.044953 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




