Submitted:
04 July 2025
Posted:
07 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Framework and Target Population
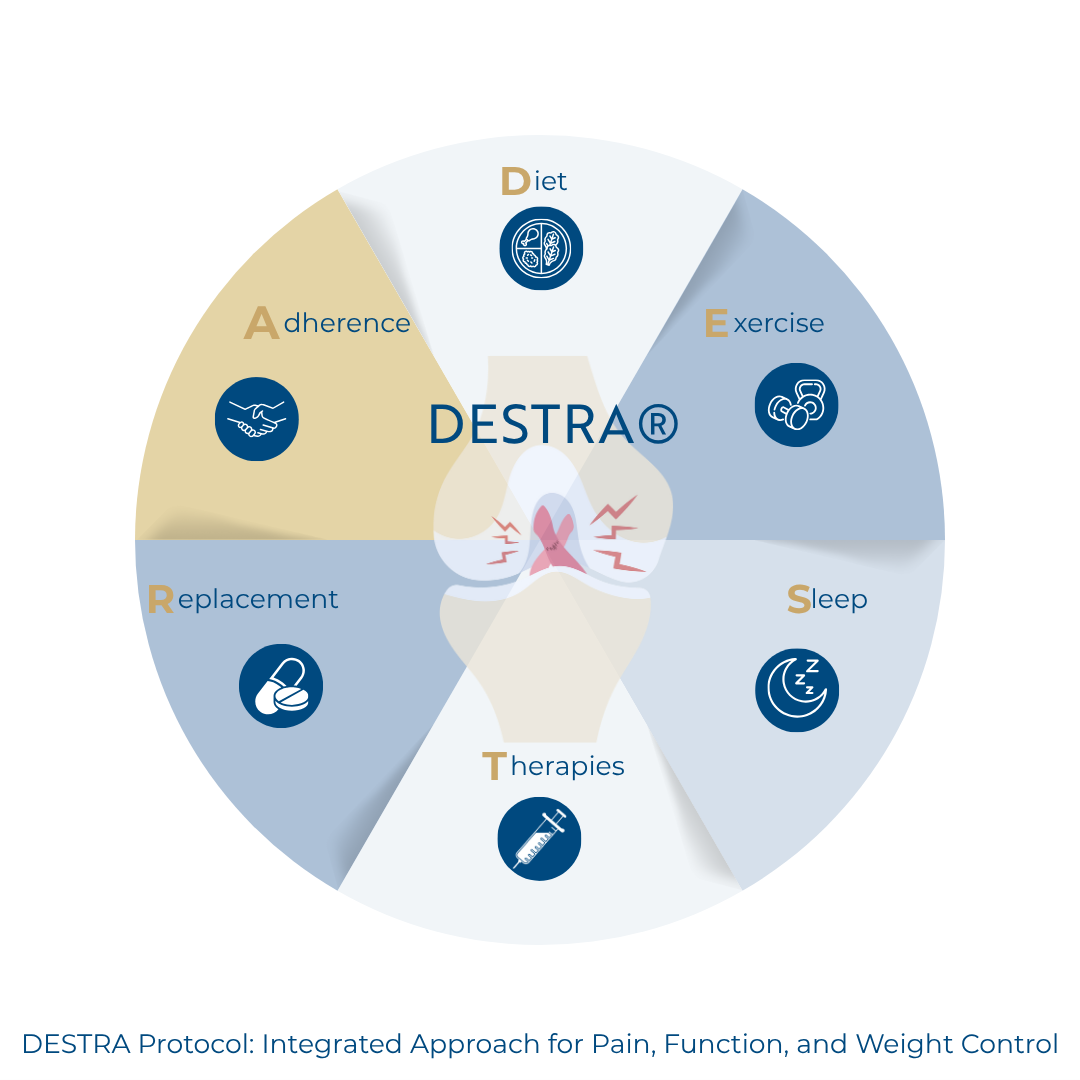
2.2. DESTRA Protocol Components
- Individualized anti-inflammatory dietary plans prioritizing foods rich in omega-3 fatty acids, antioxidants, fiber, and phytonutrients.
- Reduction of ultra-processed foods, added sugars, saturated fats, and pro-inflammatory dietary patterns.
- Incorporation of Mediterranean or plant-forward strategies tailored to patient preferences and cultural context.
- Nutritional counseling to ensure adherence and manage expectations.
- The goal is to reduce systemic inflammation, improve gut microbiota balance, and support weight loss, indirectly modulating pain pathways.
- Functional exercises supervised with attention to pain thresholds, joint protection, and neuromuscular control.
- Progressive loading respecting arthrogenic inhibition and central sensitization phenomena.
- Low-impact aerobic activities, aquatic therapy, and lower limb strength training.
- Strategies to prevent kinesiophobia and restore proprioception.
- Periodic reassessments to personalize progressions based on pain, functional capacity, and tolerance.
- Assessment of sleep patterns using validated tools.
- Sleep hygiene education with consistent sleep–wake times, reduced evening screen exposure, and improved bedroom conditions.
- Screening for sleep disorders requiring referral.
- Emphasis on sleep’s role in regulating pain perception, cortisol balance, and metabolic health.
- GLP-1 and GIP receptor analogs to support weight loss and decrease inflammation.
- Orthobiologics (Viscosupplementation with hyaluronic acid, BMA, PRF, iPRF, PRP) following clinical indications and performed with ultrasound guidance.
- Assessment of nutritional and vitamin status (vitamin D, B complex, iron, magnesium, zinc, among others).
- Laboratory testing according to evidence-based guidelines.
- Personalized supplementation plans to correct deficiencies that may contribute to fatigue, mood changes, and delayed tissue repair.
- Use of motivational interviewing and coaching techniques to encourage lifestyle changes.
- Educational materials and support groups for empowerment and engagement.
- Periodic follow-up consultations to track progress and overcome barriers.
- Application of gamification principles (e.g., goal setting, rewards, progress visualization) to promote sustained adherence.
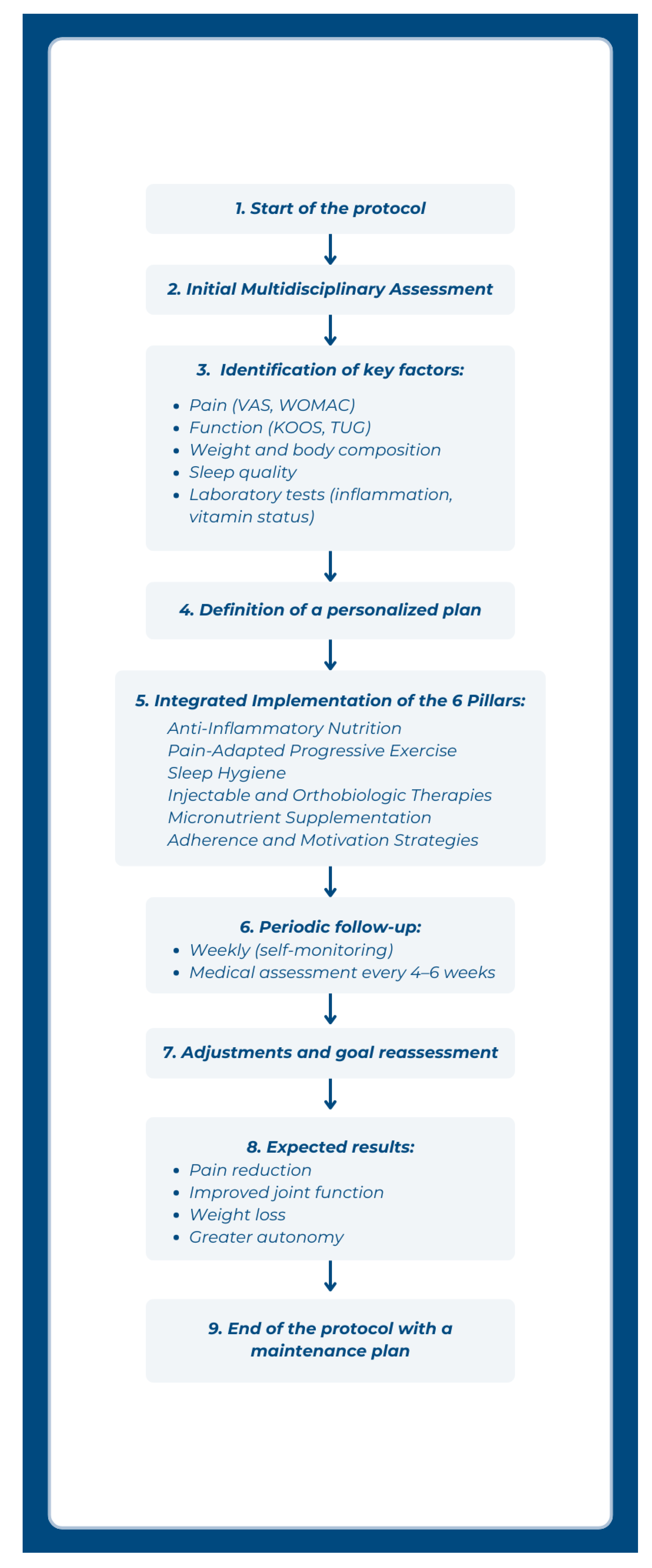
2.3. Implementation Roadmap
- pain scores (e.g., VAS, WOMAC)
- functional status (e.g., KOOS, timed up-and-go test)
- weight and body composition
- sleep quality metrics
- biochemical markers (inflammatory profile, vitamin levels)
3. Discussion
4. Conclusions
References
- Katz JN, Arant KR, Loeser RF. Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA. 2021;325(6):568–578. [CrossRef]
- Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–1759. [CrossRef]
- Bliddal H, Leeds AR, Christensen R. Osteoarthritis, obesity and weight loss: evidence, hypotheses and horizons — a scoping review. Obes Rev. 2014;15(7):578–586. [CrossRef]
- Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2019;27(11):1578–1589. [CrossRef]
- Kittelson AJ, George SZ. Physical therapy for knee osteoarthritis: why does it fail, and what might change that? J Orthop Sports Phys Ther. 2022;52(1):3–7. [CrossRef]
- McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis, update 2022. Osteoarthritis Cartilage. 2022;30(4):383–406. [CrossRef]
- Fernandes L, Hagen KB, Bijlsma JW, et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis. 2013;72(7):1125–1135. [CrossRef]
- Whibley D, AlKandari N, Kristensen K, et al. Sleep and pain: a systematic review of the bidirectional relationship. Pain. 2019;160(8):1699–1704. [CrossRef]
- Koyanagi A, Garin N, Olaya B, et al. Chronic conditions and sleep problems among adults aged 50 years or over in nine countries: a multicountry study. PLoS One. 2014;9(12):e114742. [CrossRef]
- Gallo VS, Jean Y, Lohrmann D. Impact of weight loss interventions on knee osteoarthritis: a systematic review. Orthop Nurs. 2020;39(2):94–100. [CrossRef]
- Nicholas MK, Molloy AR, Tonkin LE, Beeston L. Manage chronic pain: do more, not less. Pain Rep. 2022;7(4):e1017. [CrossRef]
- Ramires LC, Santos GS, da Fonseca LF, et al. The association between gut microbiota and osteoarthritis: does the disease begin in the gut? Int J Mol Sci. 2022;23(3):1494. [CrossRef]
- Charles-Messance H, Mitchelson KAJ, De Marco Castro E, Sheedy FJ, Roche HM. Regulating metabolic inflammation by nutritional modulation. J Allergy Clin Immunol. 2020;146(4):706–720. [CrossRef]
- Lana JF, Lana JVB, Santos GS, et al. SDIMMMER: a proposed clinical approach to optimize cellular physiology in regenerative medicine. Life. 2024;14(10):1287. [CrossRef]
| Pillar | Description | Key Objectives |
|---|---|---|
| 1. Anti-Inflammatory Nutrition | Personalized meal plans emphasizing omega-3, antioxidants, fiber, and low glycemic load; avoidance of ultra-processed foods and pro-inflammatory items | Reduce systemic inflammation; support microbiota; promote weight loss |
| 2. Pain-Adapted Progressive Exercise | Structured functional exercise with progressive loading respecting pain thresholds; includes aerobic, strength, and proprioceptive training | Restore joint function; improve neuromuscular control; counteract kinesiophobia |
| 3. Sleep Hygiene | Assessment and education on sleep patterns; establishing consistent habits and environmental adjustments | Optimize hormonal balance; reduce pain sensitization; improve recovery |
| 4. Injectable and Orthobiologic Therapies | Viscosupplementation, GLP-1/GIP analogs, orthobiologics (BMA, PRF, iPRF, PRP) | Enhance pain control; promote joint homeostasis; stimulate regeneration |
| 5. Micronutrient Supplementation | Targeted correction of deficiencies (vitamin D, B complex, magnesium, iron, zinc) | Improve tissue repair; support mood and energy; modulate inflammation |
| 6. Behavioral and Adherence Strategies | Coaching, motivational interviewing, patient education, support groups, gamification | Sustain engagement; reinforce autonomy; improve treatment adherence |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





