Submitted:
02 July 2025
Posted:
04 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Location
2.2. Animals
2.3. Methods
2.4. Statistical Analysis
3. Results
3.1. Prevalence of Sarcocystis Aucheniae in Alpaca Carcasses
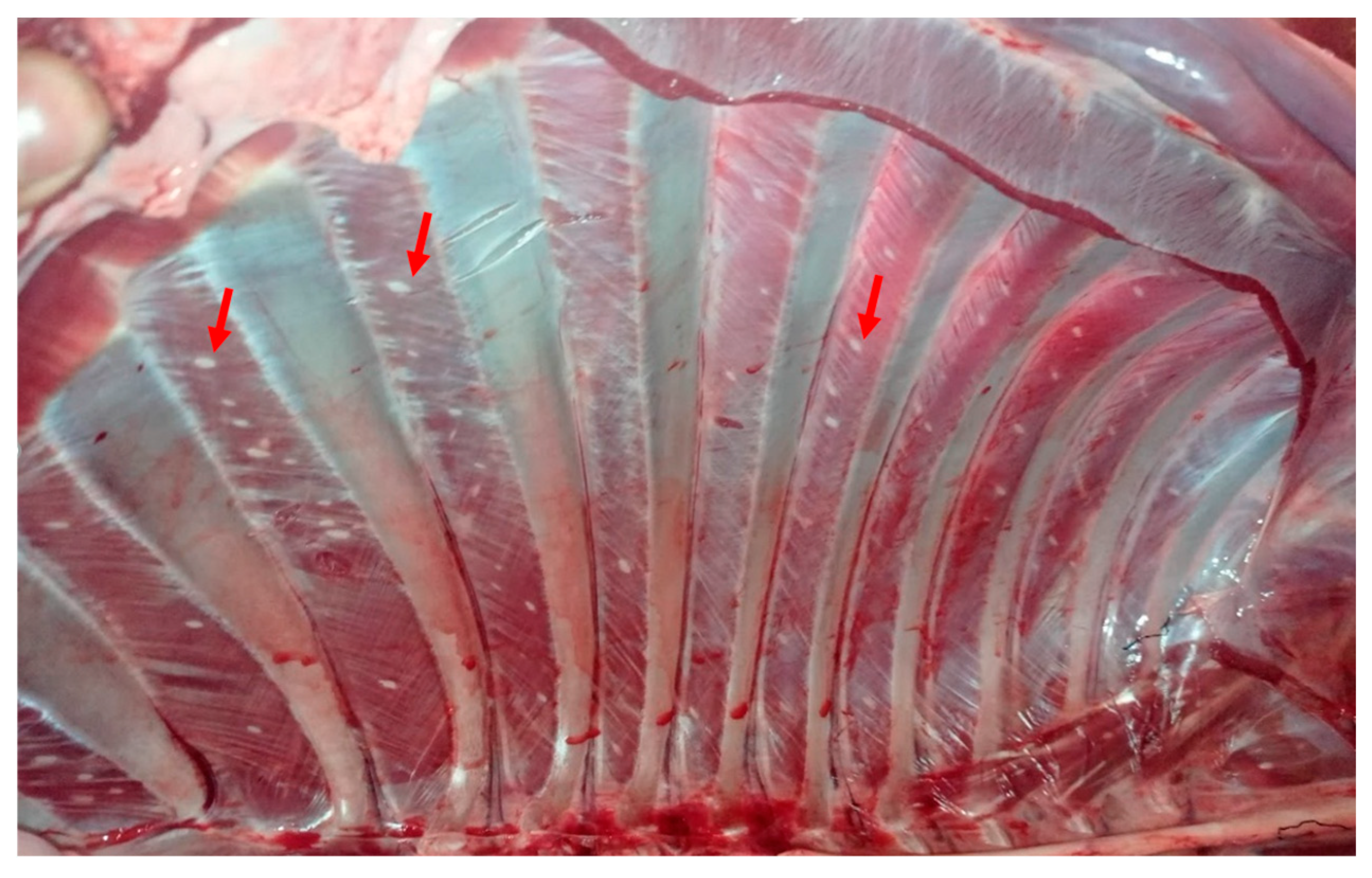
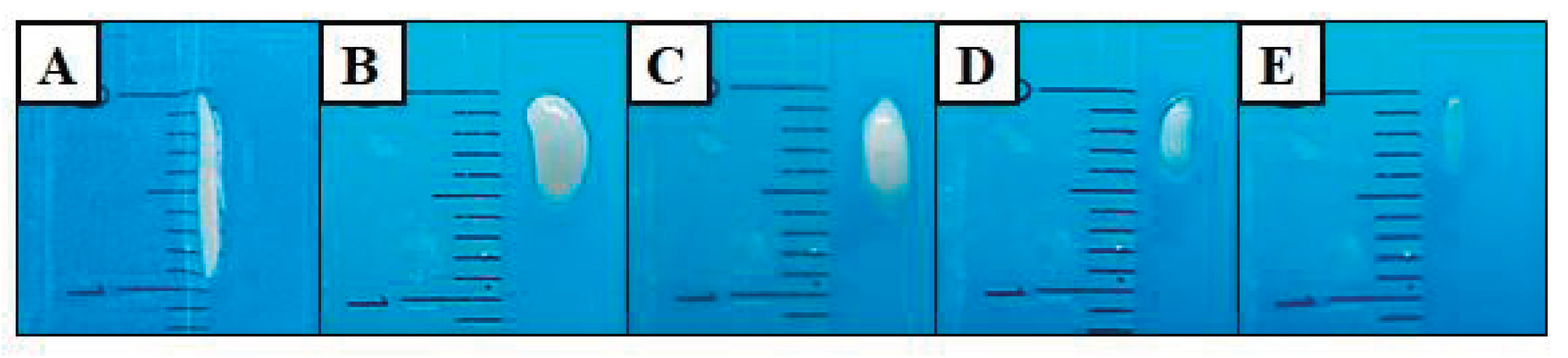
3.2. Sarcocystis Macrocyst Count in Alpaca Carcasses
3.3. Live Weight, Carcass Weight, and Carcass Yield of Alpacas
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| S. aucheniae | Sarcocystis aucheniae |
| S. lamacanis | Sarcocystis lamacanis |
| DL | Baby tooth |
| 2D | Two teeth |
| 4D | Four teeth |
| BLL | Full mouth |
| kg | kilogram |
| LI | Lower Limit |
| LS | Upper Limit |
References
- Quiroz, H. Parasitologia y Enfermedades Parasitarias de Animales Domesticos.; Limusa, Ed.; Mexico, 2000;
- Leguía, G. Infección Experimental En Primates No Humanos (Saimiri Boliviensis) y Voluntarios Humanos Con Micro y Macroquistes de Sarcocystis de Alpacas. Rev Acad Peru Cienc Vet 2003, 4, 11–15. [Google Scholar]
- Lucas, J.R. Sarcocystis Spp. En El Perú. Peruvian J. Parasitolgy 2012, 20, 64–73. [Google Scholar]
- Medrano, G.; Hung, A.; Rubio, N. Detección Molecular Temprana de Sarcocystis En El Animal Vivo y Su Estudio Filogenético Basado En El Análisis Del Gen SSU rRNA En Alpacas En Perú. Mosaico Cient 2006, 3, 5–9. [Google Scholar]
- Huanca, W. Los Desafíos En El Manejo Reproductivo de Los Camélidos Sudamericanos The Challenges in Reproductive Management of South American Camelids. Arch. Latinoam. Prod. Anim. 2013, 21, 233–236. [Google Scholar]
- Vilá, B.; Arzamendia, Y. South American Camelids: Their Values and Contributions to People. Sustain. Sci. 2022, 17, 707–724. [Google Scholar] [CrossRef]
- Mamani-Linares, L.W.; Gallo, C.B. Meat Quality, Proximate Composition and Muscle Fatty Acid Profile of Young Llamas (Lama Glama) Supplemented with Hay or Concentrate during the Dry Season. Meat Sci. 2014, 96, 394–399. [Google Scholar] [CrossRef]
- Popova, T.; Tejeda, L.; Peñarrieta, J.M.; Smith, M.A.; Bush, R.D.; Hopkins, D.L. Meat of South American Camelids - Sensory Quality and Nutritional Composition. Meat Sci. 2021, 171, 108285. [Google Scholar] [CrossRef]
- Chávez, A.; Leyva V., V.; Panez L., S.; Ticona S., D.; García V, W.; Pezo C., D. Sarcocistiosis Y La Eficiencia Productiva De La Alpaca. Rev. Investig. Vet. Perú 2008, 19, 160–167. [Google Scholar] [CrossRef]
- Céspedes, C.; Vilca, M.; Ramos, D.; Sam, R.; Lucas, J. Saneamiento y Detoxificación de Carne de Alpaca (Vicugna Pacos) Con Sarcocistiosis Mediante Tratamientos Físicos y Químicos de Uso Doméstico. Rev Inv Vet Perú 2013, 24, 404–410. [Google Scholar]
- Beldomenico, P.M.; Uhart, M.; Bono, M.F.; Marull, C.; Baldi, R.; Peralta, J.L. Internal Parasites of Free-Ranging Guanacos from Patagonia. Vet. Parasitol. 2003, 118, 71–77. [Google Scholar] [CrossRef]
- Saeed, M.A.; Rashid, M.H.; Vaughan, J.; Jabbar, A. Sarcocystosis in South American Camelids: The State of Play Revisited. Parasit. Vectors 2018, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Servicio Nacional de Meteorología e Hidrología del Perú (SENAMHI) Climas del Perú: Mapa de Clasificación Climática - Servicio Nacional de Meteorología e Hidrología del Perú. Available online: https://www.gob.pe/institucion/senamhi/informes-publicaciones/2158106-climas-del-peru-mapa-de-clasificacion-climatica (accessed on 14 January 2024).
- Food and Agriculture Organization (FAO) Proyecto de Cooperación Técnica En Apoyo a La Crianza y Aprovechamiento de Los Camélidos Sudamericanos En La Región Andina TCP/RLA/2914. Situación Actual de Los Camélidos Sudamericanos En Perú; Food and Agriculture Organizatio, 2007;
- Vázquez, J.; Cedeño, M.; Collazo, M.; Jiménez, M.; Quintero, L.; Barletta, J. Folleto de Protozoología y Técnicas Parasitológicas. Rev. Electrónica Las Cienc. Médicas En Cienfuegos 2012, 10, 151–162. [Google Scholar]
- Leguía, G.; Santiago, B. Infectividad de quistes de Sarcocystis sp. en alpacas (Vicugna pacos) en una ganadería de la sierra central del Perú. Biotempo 2019, 16, 209–213. [Google Scholar] [CrossRef]
- Palomino, R. Prevalencia de Sarcocistiosis En Alpacas (Vicugna Pacos) Beneficiadas En El Camal de Pilpichaca, Huancavelica Años 2010 al 2012. 2013.
- Decker-Franco, C. Avances En El Conocimiento de La Sarcocistiosis de Camélidos Sudamericanos y Desarrollo de Herramientas Para Su Control. Trabajo de tesis para optar al título de Doctora de la Universidad de Buenos Aires, Argentina, 2018.
- Velásquez, L.; Soncco, J.; Valderrama, A. Sarcocystis aucheniae en camélidos sudamericanos y factores de riesgo en la provincia de Lucanas. Salud Tecnol. Vet. 2019, 7, 8–13. [Google Scholar] [CrossRef]
- Santiago, B.; Leguía, G. Prevalencia de Sarcocystis En Alpacas (Lama Pacos) y En Perros Pastores de Una Ganadería de La Sierra Central Del Perú. Biotempo 2018, 15, 59–62. [Google Scholar] [CrossRef]
- Vizarreta, M.; Huaylla, R. Prevalencia de Sarcocystiosis Macroscópica y Microscópica En Carcasa de Alpacas y Llamas Beneficiados En El Camal Municipal de Espinar, Universidad Nacional de San Antonio Abad del Cusco: Cusco Perú, 2023.
- Carhuapoma-Delacruz, V.; Jurado-Escobar, M.; Valencia-Mamani, N.; Castrejón Valdez, M.; Guillermo Sánchez-Araujo, V.; Virgilio Capcha-Huamaní, A.; Oscar Sánchez-Ramos, B.; Esparza, M. Infección por Sarcocystis aucheniae (Apicomplexa: Sarcocystidae) en tejido muscular de alpacas y llamas en Huancavelica, Perú. Rev. Cient. Fac. Vet. 2024, 34, 1–9. [Google Scholar] [CrossRef]
- Hidalgo-Pozo, J.; Yánez Ortiz, I.; Salazar Silva, R.; Mena Miño, L. Probabilidad de riesgo asociado a la presencia de macroquistes de Sarcocystis spp. en canales de camélidos sudamericanos domésticos en Ecuador. Ecuad. ES Calid. - Rev. Científica Ecuat. 2021, 8. [Google Scholar] [CrossRef]
- Rooney, A.L.; Limon, G.; Vides, H.; Cortez, A.; Guitian, J. Sarcocystis Spp. En Llamas ( Lama Glama ) En El Sur de Bolivia: Un Estudio Transversal de La Prevalencia, Factores de Riesgo y Pérdida de Ingresos Causados Por La Degradación de Las Canales. Prev. Vet. Med. 2014, 116, 296–304. [Google Scholar] [CrossRef]
- Decker-Franco, C.; Romero, S.; Ferrari, A.; Schnittger, L.; Florin-Christensen, M. Detection of Sarcocystis Aucheniae in Blood of Llama Using a Duplex Semi-Nested PCR Assay and Its Association with Cyst Infestation. Heliyon 2018, 4, e00928. [Google Scholar] [CrossRef]
- Chirinos, J. Frecuencia de Micro Quistes (Sarcocystis Lamacanis) Entre Las Fibras Musculares Cardíacas En Alpaca (Vicugna Pacos), Beneficiadas En Los Camales de Nuñoa y Ayaviri, Departamento de Puno, 2016. 2017.
- Romero, S.; Carletti, T.; Decker Franco, C.; Moré, G.; Schnittger, L.; Florin-Christensen, M. La Seropositividad a La Infección Por Sarcocystis En Llamas Se Correlaciona Con Las Prácticas de Cría. Vet. Parasitol. Reg. Stud. Rep. 2017, 10, 65–70. [Google Scholar] [CrossRef]
- Castro C., E.; Sam T., R.; López U., T.; González Z., A.; Silva I., M. Evaluación de La Edad Como Factor de Riesgo de Seropositividad a Sarcocystis Sp. En Alpacas. Rev. Investig. Vet. Perú 2004, 15, 83–86. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, J.; Hu, J.; Song, J.; Deng, S.; Zhu, N.; Yang, Y.; Tao, J. Morphological and Molecular Characterization, and Demonstration of a Definitive Host, for Sarcocystis Masoni from an Alpaca (Vicugna Pacos) in China. Biology 2022, 11, 1016. [Google Scholar] [CrossRef] [PubMed]
- López-Torres Torres, B.; Espinoza, J.L.; Rodríguez Sánchez, J.A.; Barrios-Arpi, M.; Arroyo, G.; Rodríguez, A.; Revuelta Rueda, L. Correlaciones entre la infección por microquistes de S. lamacanis y CK-MB, Ast y LDH alpacas. Rev. Complut. Cienc. Vet. 2015, 9, 40–51. [Google Scholar] [CrossRef]
- Jiang, N.; Xin, S.; Zhu, N.; Yang, L.; Huang, W.; Hu, J.; Zhu, X.; Yang, Y. First Report of Sarcocystis Masoni in a Captive Alpaca (Vicugna Pacos) From China. Front. Vet. Sci. 2021, 8, 759252. [Google Scholar] [CrossRef]
- Fernandez-F, F.; Gutiérrez-A, R.; Pacheco-S, V.; Chirinos-T, J.; Lombardo, D.M.; Olivera, L.V.M.; Bernabe-Ortiz, J.C.; López-Casaperalta, P. Determinación de Microquistes de Sarcocystis Lamacanis En El Músculo Cardíaco de Alpacas ( Vicugna Pacos ) y Su Correlación Con La Troponina cTnI. Un Estudio Realizado En La Región Altoandina Del Sur Del Perú. Vet. Anim. Sci. 2022, 18, 100270. [Google Scholar] [CrossRef]
- Smith, M.A.; Bush, R.D.; Thomson, P.C.; Hopkins, D.L. Carcass Traits and Saleable Meat Yield of Alpacas (Vicugna Pacos) in Australia. Meat Sci. 2015, 107, 1–11. [Google Scholar] [CrossRef]
- Castellaro G., G.; García-Huidobro P de A, J.; Salinas, P. Alpaca Liveweight Variations and Fiber Production in Mediterranean Range of Chile. 51 1998. [Google Scholar] [CrossRef]
- Moré, G.; Regensburger, C.; Gos, M.L.; Pardini, L.; Verma, S.K.; Ctibor, J.; Serrano-Martínez, M.E.; Dubey, J.P.; Venturini, M.C. Sarcocystis Masoni, n. Sp. (Apicomplexa: Sarcocystidae), and Redescription of Sarcocystis Aucheniae from Llama (Lama Glama), Guanaco (Lama Guanicoe) and Alpaca (Vicugna Pacos). Parasitology 2016, 143, 617–626. [Google Scholar] [CrossRef]
- Bowler, B.; Grandez, R. Electrocardiographical Parameters in Alpacas Infected with Sarcocystis Lamacanis. Int J Appl Res Vet Med 2008, 6, 87–92. [Google Scholar]
| Sex | N | Positives | Prevalence | LI (95%) | LS (95%) |
| Female | 728 | 218 | 29.95a | 29.91 | 29.98 |
| Male | 633 | 192 | 30.33a | 30.30 | 30.37 |
| Total | 1361 | 410 | |||
| Class | |||||
| DL | 156 | 37 | 23.72a | 23.65 | 23.78 |
| 2D | 147 | 51 | 34.69a | 34.62 | 34.77 |
| 4D | 438 | 131 | 29.91a | 29.87 | 29.95 |
| BLL | 620 | 191 | 30.81a | 30.77 | 30.84 |
| Total | 1361 | 410 |
| Sex | Average | Min | Max |
| Female | 32.50a | 1 | 350 |
| Male | 26.18a | 1 | 154 |
| Probability | 0.085 |
| Category | Average | Min | Max |
| DL | 43a | 1 | 49 |
| 2D | 53ab | 1 | 139 |
| 4D | 134b | 1 | 188 |
| BLL | 192 b | 1 | 350 |
| Probability | 0.046 |
| Sarcocystosis | Live weight, kg | Carcass weight, kg | Carcass Yield, kg | |
| Negative | 45.09a | 23.22a | 51.32a | |
| Positive | 45.36a | 23.04a | 50.57a | |
| Probability | 0.793 | 0.763 | 0.584 | |
| Sarcocystosis | Age | |||
| Negative | DL | 37.54a | 18.45a | 49.12a |
| 2D | 44.54bc | 22.64bcd | 50.58a | |
| 4D | 49.95cd | 25.73d | 51.15a | |
| BLL | 48.32cd | 26.06d | 54.42a | |
| Positive | DL | 40.39ab | 19.58ab | 48.39a |
| 2D | 41.51ab | 20.93ab | 50.24a | |
| 4D | 46.08bc | 23.85cd | 51.66a | |
| BLL | 53.45d | 27.80d | 51.99a | |
| Probability | 0.001 | 0.026 | 0.088 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





