1. Introduction
Clonazepam is a drug that belongs to the group of benzodiazepines. Its mechanism of action involves allosteric interactions between central benzodiazepine receptors and gamma-aminobutyric acid (GABA) receptors in the brain, enhancing the effects of GABA. In Spain it is indicated in most of the clinical forms of epileptic disease and seizures in infants, children and adults. In the last group it is used in status epilepticus too [
1,
2].
The INSST and the American Institute for Occupational Safety and Health of the United States (NIOSH) include clonazepam in their List of Hazardous Drugs in Healthcare Settings and classify it as a group 3 non-antineoplastic drug that primarily has adverse reproductive effects. These risks affect men and women of reproductive age, pregnant women or those who are breastfeeding, but do not pose a significant risk to other personnel [
3,
4]. The FDA classified clonazepam as a category “D” pregnancy risk drug prior to 2015 [
5]. According to NIOSH and INSST guidelines, special protection is not required for handling clonazepam except for personnel at reproductive risk (pregnancy, breastfeeding, or those actively trying to conceive). For this risk group, it is recommended to handle the drug with double gloves, gown, face mask and eye protection as a safeguard against the risk of splashes or inhalation.
In Spain, there are two presentations of clonazepam commercialized in solution: oral drops (2.5 mg/mL) and parenteral (1 mg/mL); both are used in our hospital. For the reasons stated above, the hospital’s Pharmacy and Therapeutic Commission, together with the Occupational Risk Prevention Service, proposed the possibility of dispensing the two clonazepam solutions in pre-filled syringes, prepared under aseptic conditions in the Pharmacy Service, because a pre-filled syringe is a ready-to-use system that decreases the hazards of drug manipulation and also saves nursing time.
After evaluating the request from a pharmaceutical point of view, the work would consist of repackaging these drugs (the oral and the parenteral solution) from their original packaging into a PP syringe. The oral solution is packaged in a topaz glass bottle and the parenteral in a topaz glass ampoule. The conservation conditions of two presentations are temperature equal o less than 30ºC, in its secondary packaging to protect from the light. In these conditions, both have a three-year shelf life if unopened [
1,
2]. The issues to be clarified would therefore be: how long are these drugs physicochemical stable, in PP syringes, at concentrations of 1 mg/mL and 2.5 mg/mL?
PP is considered one of the most inert plastics and with the lowest tendency to adsorb drugs; in practice, adsorption to PP is usually low, but it can be significant in drugs of low concentration, of protein or peptide nature, or of high unit value, such as certain radiopharmaceuticals or insulin. In addition, many studies have shown that the physicochemical stability of most drugs is adequate in PP syringes, with no appreciable degradation over periods ranging from several days to weeks, depending on the drug and the storage conditions (refrigeration, darkness). PP offers low permeability to water vapor and is relatively impermeable to oxygen, which helps protect the solution from degradation by oxidation or hydrolysis. However, its gas barrier is not as hermetic as that of glass, so in long-term storage situations the shelf life of very sensitive solutions could be affected. Pharmaceutical-grade PP syringes minimize the presence of particles and the migration of additives [
6,
7,
8]. Therefore, the stability of clonazepam solutions (2,5 mg/mL and 1 mg/mL) could be affected by PP under different conditions. The relationship between the concentration of a drug in solution and its stability is not generally directly proportional, but rather depends on multiple chemical, physical, and environmental factors surrounding the drug. The chemical stability of a drug in solution can be affected by concentration, but the relationship is not always linear or direct. In certain cases, higher concentrations can favor stability by reducing the proportion of water, but in other cases, they can increase degradation due to chemical or physical reactions that depend on the active ingredient concentration and on other factors such as pH, temperature, and the presence of ions or catalysts. Stability also depends on degradation kinetics. This determines how the drug concentration changes over time and how it degrades, regardless of whether its concentration is low or high. For example, in some cases the degradation rate is proportional to the concentration, and in others it is not. For these reasons the stability must be specifically studied for each formulation, as behavior can vary depending on the drug and its environment [
9,
10,
11,
12,
13,
14].
Nevertheless, currently the lack of stability studies of clonazepam in pre-filled PP syringes prevents the pharmacy services from preparing and storing it. Only, one study of clonazepam solution 0.5 mg/mL in water for injection, packaged in PP syringes has been published, with the result of 48 hours of stability, at 25º C, not protected from light [
15].
Therefore, this study investigates the physicochemical stability of clonazepam in pre-filled PP syringes in several different conditions.
2. Materials and Methods
2.1. Sample Preparation of Pre-Filled Syringes
Oral clonazepam 2.5 mg/mL solution syringes: Amber polypropylene 1 ml light protected oral syringes (Becton DickinsonTM, Madrid –Spain-) with a tip cap were pre-filled with 0.4 mL of clonazepam 2.5 mg/mL oral solution drops (Rivotril
®, Roche Farma, S.A., Madrid, -Spain-). Each syringe contained 1 mg of clonazepam (
Figure 1). Two groups of syringes were stored at either controlled room temperature (25ºC) exposed to ambient light, or under refrigerated conditions (2-8ºC) protected from light.
Parenteral clonazepam 1 mg/mL solution syringes: Luer lock polypropylene syringes (Nipro Europe Group Companies, Madrid –Spain-) for parental use were pre-filled with 1 mL of clonazepam 1 mg/mL parenteral solution (Rivotril
® powder 1 mg + 1 mL solvent, Roche Farma, S.A., Madrid, -Spain-), connected to a closed safety system (TexiumTM, Becton Dickinson España, S.A., Madrid, -Spain-) as shown in
Figure 2. Two groups of syringes were stored at either controlled room temperature (25ºC) protected and unprotected from light, or under refrigerated conditions (2-8ºC) protected from light.
2.2. Chemical Stability
The chemical stability of oral and parenteral clonazepam in pre-filled syringes was studied over 30 days of storage (day 0; days 1 to 4; days: 7, 9, 11, 14, 17, 21, 24, 28 and 30).
The chemical stability was studied on the selected days by withdrawing an aliquot of each syringe that was then diluted with the mobile phase to a concentration of 25 µg/mL. Three different batches of each preparation were analyzed in triplicate by HPLC within 10 minutes of dilution.
If the drug concentration remained between 90% and 110% of the initial concentration during the 30 days of storage, the preparation was considered stable [
9,
14,
16].
2.3. Chromatographic Method
Conditions: A Waters Breeze HPLC system (Waters Cromatography, S.A., Barcelona, -Spain-) and a XBridge 5 µm C18 (130 Å pore size, 4.6 x 150 mm) reversed-phase column (Waters Cromatography, S.A., Barcelona, -Spain-) were used. The chromatographic conditions were: isocratic mobile phase composed of ultrapure water/acetonitrile/methanol (40/30/30 v/v) at a flow rate of 1 mL/min, ultraviolet detector at 254 nm, 30°C column temperature, injection volume of 20 μL and run time of 5 minutes [
17,
18]. The HPLC reagents acetonitrile (HPLC-grade) and methanol (HPLC-grade) were purchased from Panreac Química S.L.U. (Barcelona, -Spain-). The reference drug clonazepam was obtained from Roche Farma, S.A. (Madrid, -Spain-).
Validation of the method: The HPLC method was validated in terms of linearity, precision and accuracy according to the ICH guidelines [
19].
Linearity: Linearity between the peak area and the clonazepam concentration was evaluated by performing six measurements in a concentration range of 0-45 μg/mL (0, 6, 15, 24, 30 and 45 μg/mL). A calibration curve and the corresponding linear regression analysis were performed, obtaining the results of the coefficient of determination (R
2), the slope (a) and the Y intercept (b) [
16].
Precision: Precision was verified by repeatability in intra and inter-day studies. The intra-day study consisted of analyzing five times, on the same day, the samples at 80, 100 and 120% of the target concentration (25 µg/mL). In the inter-day study the samples were analyzed two times during four different days, at 80, 100 and 120% of the target concentration. The mean, the standard deviation and the coefficient of variation were calculated, with less than 1% variation being accepted for intra-day repeatability, and less than 2% for inter-day repeatability [
16,
20].
Accuracy: Accuracy was determined by recovery studies in triplicate at 20, 25 and 30 μg/mL concentrations of clonazepam. Recovery was expressed as a percentage, and the mean value was compared with the theoretical value (100%), using Student’s t test [
16,
20,
21].
Limit of detection and limit of quantification: To determine the limit of detection (LOD) and the limit of quantification (LOQ) of clonazepam, the independent term “b” and the slope “a” were used in the equations LOD = 3 b/a and LOQ = 10 b/a [
16,
22].
2.4. Physical Stability
Physical stability was studied checking visual aspects, determining pH and observing crystal formation. 1 mL samples of each preparation were obtained on the selected evaluation days and were checked for visual aspects such as particle formation, crystals, turbidity, precipitation or color changes during storage. The pH was measured with a calibrated SevenMultiTM pH meter (Mettler Toledo, Cornellà de Llobregat, -Spain-). A visual inspection booth with both a black/white background [
12] and bright-field, and a SediMAX2TM phase contrast microscope (77 Elektronika, Budapest, -Hungary-), were used to determine the presence of particles and crystals.
3. Results
3.1. Validation of the Analytical Method
The method demonstrated excellent linearity, with a R
2 greater than 0.9996. The regression equation was calculated as y = 127436x + 8625. The results were highly satisfactory regarding the intra-day and inter-day repeatability of the three clonazepam quality control solutions. Accuracy ranged from 98.34% to 101.62%, while precision, expressed as relative standard deviation (RSD%), fell within the range of 0.094% to 0.682% for intra-day precision and 0.232% to 0.713% for inter-day precision. These RSD% values comfortably meet the ICH (International Conference on Harmonisation) standards, which stipulate a maximum RSD of 1% and 2% respectively. Likewise, the accuracy was between 98% and 102%. The LOD and the LOQ were calculated as 0.20 µg/mL and 0.68 µ/mL respectively (
Table 1 and
Table 2).
3.2. Stability Study
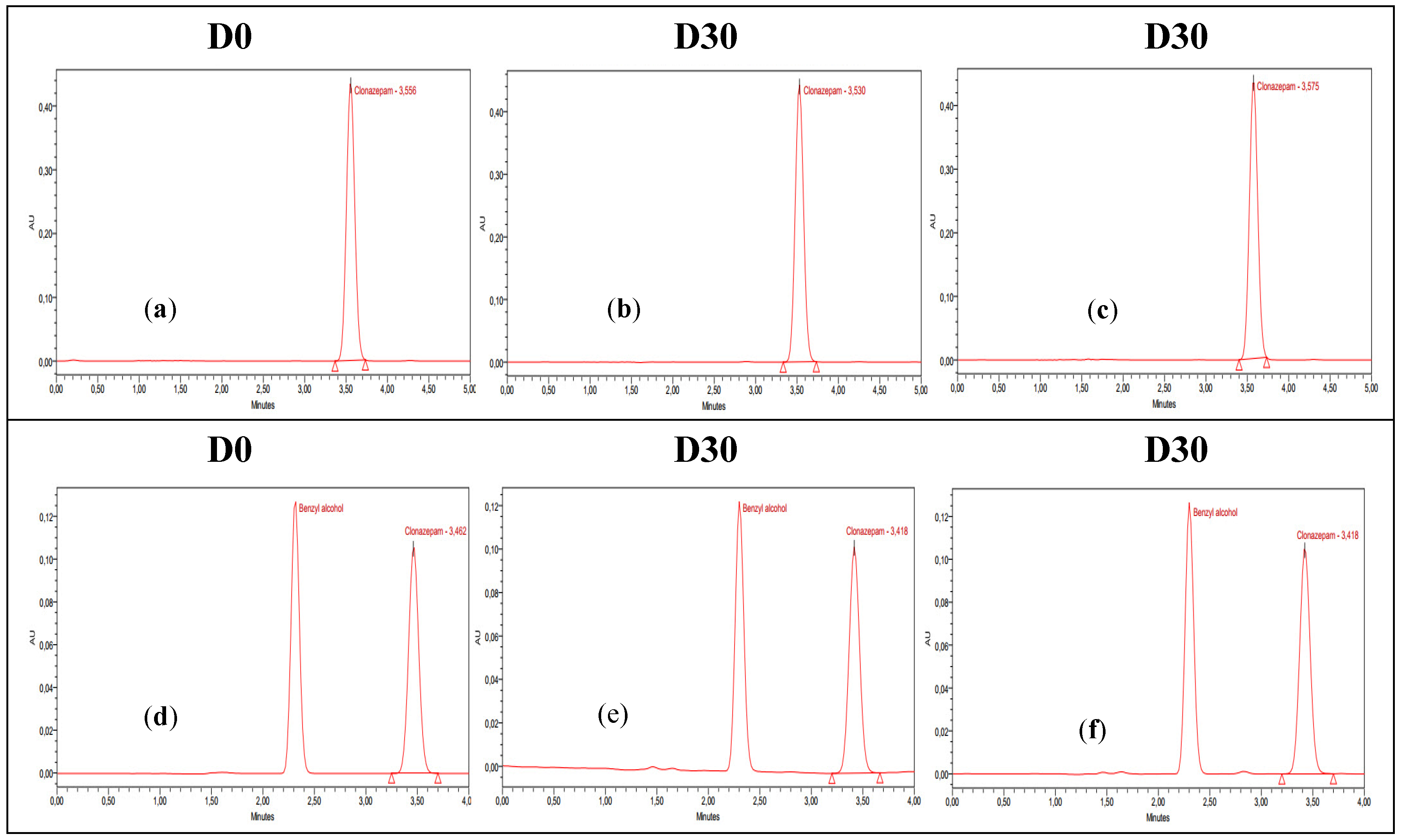
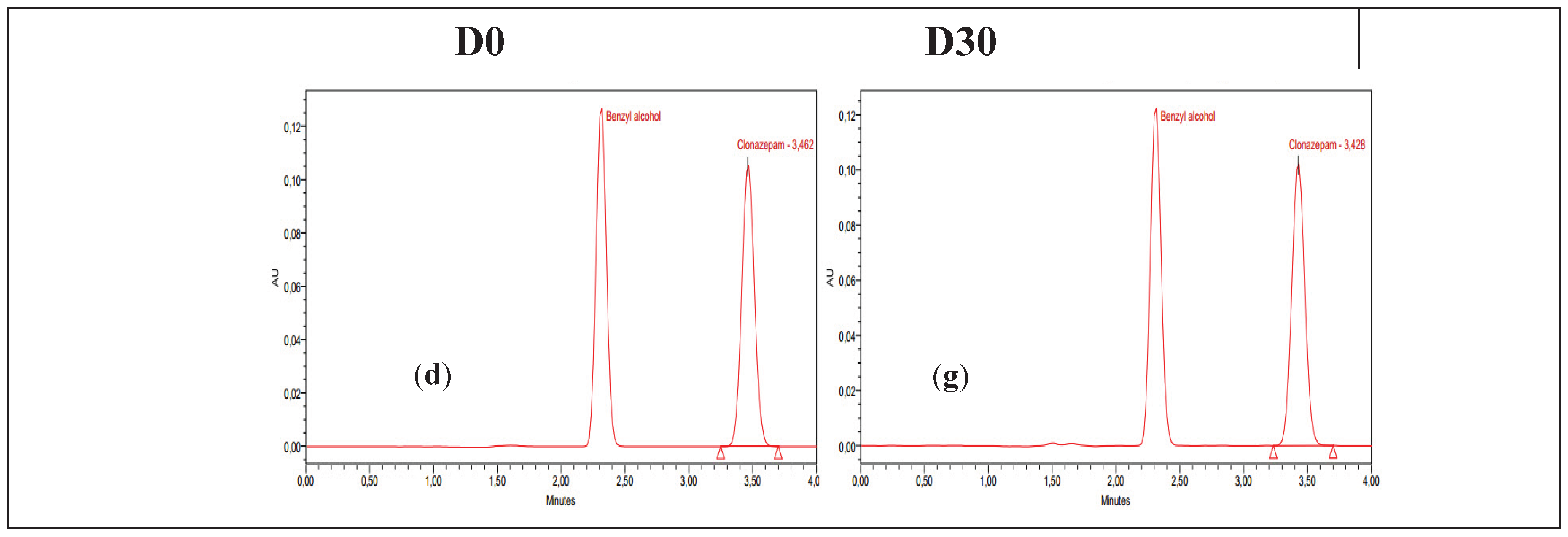
The stability study was carried out by measuring the concentration of clonazepam in pre-filled syringes on each day of the analysis, as described previously. The mean concentrations were calculated and expressed as recovery percentage with respect to the measurement on the first day (D0 = 100%). The chromatograms of D0 and D30 (day 30 of the study) for each preparation under the specified storage conditions are presented in
Figure 1. The clonazepam 1 mg/mL parenteral solution contains benzyl alcohol as a preservative agent. This compound exhibits absorption at 254 nm, which accounts for the appearance of a corresponding peak in the chromatograms.
The results show that the concentrations remained stable for 30 days in all of the storage conditions used for the oral clonazepam 2.5 mg/mL solution syringes (
Table 3) and the parenteral clonazepam 1 mg/mL solution syringes (
Table 4).
No significant variation was observed with regards to visual aspects (color changes, turbidity) and pH throughout the study (
Table 3 and
Table 4).
4. Discussion
Since the publication of the document on hazardous drugs and preventive measures for their preparation and administration, Hospital Pharmacy Services have been involved in implementing measures to adapt practices to the recommendations given by the INSST and European Agency for Safety and Health at Work in its guidance document for the safe management of hazardous medicinal products at work [
23]. Among the adaptation options is the possibility of direct delivery of the drug from the Pharmacy Service in a standardized dose and container, ready for administration. If stability studies of the drug in standardized containers are available, it is possible to prepare and store the drug in the Pharmacy Service, depending on consumption, to avoid the need for shift or daily preparation.
There are currently only two studies published that evaluate the stability of oral clonazepam solutions. One of them assessed the stability of clonazepam 0.2 mg/mL oral solution stored under refrigeration (2-8ºC) and at room temperature, using clonazepam in powder form to prepare the solution and packed on amber-colored glass bottles, concluding that the solution was stable for 90 days [
18]. The other analyzed the stability of clonazepam 0.1 mg/mL oral solution prepared from commercial tablets, placed in polyethylene terephthalate bottles, both stored under refrigeration (2-8ºC) and at room temperature protected from light, observing that the solution remained stable for 60 days under both storage conditions [
17]. To date, only one study has investigated the stability of clonazepam in polypropylene syringes, although at a lower concentration (0.5 mg/mL). Unlike the previous cases, this study has evaluated the stability of commercialized clonazepam drugs in the presentations of 2.5 mg/mL oral drops and 1 mg/mL injectable solution, repackaged in pre-filled PP syringes. Until this study was carried out, stability of these commercials solutions were known in glass containers, but not in polypropylene containers. Both concentrations are significantly higher than the concentrations mentioned in the studies beforehand, a condition that does not seem to affect the stability observed in the current work.
The assessment of the microbiological stability of commercial clonazepam solutions repackaged in polypropylene syringes was not within the objectives of this study. Nevertheless, according to the manufacturer’s product information, the oral solution at a concentration of 2.5 mg/mL has a shelf-life of 120 days after opening. Regarding the 1 mg/mL solution intended for parenteral administration, it should be noted that it contains benzyl alcohol as a preservative. Therefore, since repackaging process was carried out under aseptic conditions in a laminar airflow cabinet, the stability of both commercial solutions was not considered to be compromised.
5. Conclusions
In the present study, clonazepam 2.5 mg/mL oral solution in light-protected pre-filled PP syringes, both at room temperature and under refrigeration (2-8ºC), and clonazepam 1 mg/mL parenteral solution in pre-filled PP syringes at room temperature with and without light protection, and under refrigeration (2-8ºC) with light protection, are observed to be physically and chemically stable for at least 30 days. This has allowed for preparation of ready-to-use stock of this hazardous drug, minimizing drug manipulation by nursing staff and therefore reducing the risk of occupational exposure.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Author Contributions
Conceptualization, J.C.R.R.; methodology: J.C.R.R., I.T.H. and L.B.G.; validation, J.C.R.R., J.M.A.H. and A.C.V.; formal analysis, J.C.R.R., I.T.H. and L.B.G.; investigation: I.T.H. and L.B.G.; writing—original draft preparation, J.C.R.R., I.T.H. and L.B.G.; writing—review and editing, J.C.R.R., J.M.A.H. and A.C.V.; supervision, J.C.R.R., J.M.A.H. and A.C.V.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| D0 |
Day cero of the test |
| D30 |
Day thirty of test |
| GABA |
Gamma-aminobutyric acid |
| HPLC |
High-performance liquid chromatography |
| ICH |
International Conference on Harmonisation |
| INSST |
Spanish National Institute for Safety and Health at Work |
| LOD |
Limit of detection |
| LOQ |
Limit of quantification |
| NIOSH |
Health of the United States |
| PP |
Polypropylene |
| R2 |
Correlation coefficient |
| RSD% |
Relative standard deviation |
| SD |
Standard deviation |
| SEFH |
Spanish Society of Hospital Pharmacy |
References
- Spanish Medicines and Medical Devices Agency. Datasheet of Rivotril 1 mg/mL concentrate and solvent for injectable solution. [Internet]. Ficha Técnica Rivotril 1 mg/ ml concentrado y disolvente para solución inyectable. [cited 2024 Aug 12]. Available from: https://cima.aemps.es/cima/dochtml/ft/52332/FT_52332.html.
- Spanish Medicines and Medical Devices Agency. Datasheet of Rivotril 2,5 mg/mlL oral drops solution [Internet]. Ficha Técnica Rivotril 2,5 mg/ml gotas orales en solución. 2020 [cited 2024 Aug 12]. Available from: https://cima.aemps.es/cima/dochtml/ft/52333/FT_52333.html#5-propiedades-farmacol-gicas.
- National Institute for Occupational Safety and Health. Hazardous drugs. Preventive measures for their preparation and administration [Internet]. Medicamentos peligrosos. Medidas de prevención para su preparación y administración. 2016 [cited 2024 Aug 12]. Available from: https://www.insst.es/documentacion/catalogo-de-publicaciones/medicamentos-peligrosos.-medidas-de-prevencion-para-su-preparacion-y-administracion.
- Connor TH, MacKenzie, BA, DeBord DG, Trout DB, O’Callaghan JP, Ovesen JL, Whittaker C. Cincinnati, OH, National Institute for Occupational Safety and Health. NIOSH [2020]. NIOSH list of hazardous drugs in healthcare settings 2020 [Internet]. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; [cited 2024 Aug 5]. Available from: https://www.cdc.gov/niosh/docket/review/docket233c/pdfs/DRAFT-NIOSH-Hazardous-Drugs-List-2020.pdf.
- Lal, R. Drugs in Pregnancy and Lactation: Improved Benefit-Risk Information [Internet]. 2015 [cited 2024 Aug 27]. Available from: https://www.fda.gov/files/drugs/published/%22Drugs-in-Pregnancy-and-Lactation--Improved-Benefit-Risk-Information%22-January-22--2015-Issue.pdf.
- Tuárez-Párraga MA, Laz-Mero M, Córdova-Mosquera A, Panchana-Cedeño R, Gavilánes-López P, Solórzano Zambrano L, et al. Oxygen Permeability in Food Packaging made of Polypropylene by Injection Moldin. Revista Politécnica. 2024;53:27–36.
- Raimundo Piñero A, Selva Otaolarruvchi J. Interacción de fármacos y mezclas parenterales con productos sanitarios. Panorama Actual del Medicamento. 2020;11:587–92.
- Laboratorios Grifols, S,A,. Estabilidad y compatibilidad de nedicamentos [Internet]. Laboratorios Grifols, S,A,; Available from: https://gruposdetrabajo.sefh.es/gps/images/stories/descargas/Compatibilidad_medicamentos_diluyentes.pdf.
- European Directorate for the Quality of Medicines & HealthCare. European Pharmacopoeia. 11th edn. Estrasburgo: European Directorate for the Quality of Medicines & HealthCare; 2022.
- Abraham J. Stability Testing of New Drug Sjustances and Products Q1A(R2). In: Tietje C, Brouder A, editors. International Conference On Harmonisation Of Technical Requirements For Registration Of Pharmaceuticals For Human Use [Internet]. Brill | Nijhoff; 2010 [cited 2025 Aug 16]. p. 1041–53. Available from: https://brill.com/view/book/edcoll/9789004181564/Bej.9789004163300.i-1081_085.xml.
- Troy DB, Beringer P. Remington: The Science and Practice of Pharmacy. Lippincott Williams & Wilkins; 2006.
- Spanish Agency of Medicines and Medical Devices. Visible particle contamination. Spanish Royal Pharmacopoeia online [Internet]. [cited 2024 Sept 10]. Available from: https://extranet.boe.es/farmacopea/doc.php?id=20920.
- Barrueco N, Escobar Rodríguez I, García Díaz B, Gil Alegre ME, López Lunar E, Ventura Valares MG. The stability of medicines in the clinical practice. From safety to efficiency. Farmacia Hospitalaria. 2013;37:175–7.
- United States Pharmacopeial Convention. United States Pharmacopeia and National Formulary (USP-NF). 2022nd edn. Rockville: US Pharmacopeia Convention, Inc; 2022.
- Guchelaar HJ, Hartog ME. De stabiliteit van clonazepaminjectievloeistof. [Internet]. Bibliographie - 2217 - Stabilis 4.0. [cited 2025 Aug 11]. Available from: https://www.stabilis.org/Bibliographie.php?IdBiblio=2217.
- Castro M, Gascón S, Pujol M, Sans J, Vicente L. Validation of Analytical Methods. Monograph. Commission on Good Manufacturing Practice and Quality Control Standards. Barcelona: Spanish Association of Industrial Pharmacists (AEFI); 1989.
- Allen LV, Erickson MA. Stability of acetazolamide, allopurinol, azathioprine, clonazepam, and flucytosine in extemporaneously compounded oral liquids. Am J Health Syst Pharm. 1996;53:1944–9. [CrossRef]
- Polonini HC, Loures S, Lima LC, Ferreira AO, Brandão MAF. Stability of Atenolol, Clonazepam, Dexamethasone, Diclofenac Sodium, Diltiazem, Enalapril Maleate, Ketoprofen, Lamotrigine, Penicillamine-D, and Thiamine in SyrSpend SF PH4 Oral Suspensions. Int J Pharm Compd. 2016;20:167–74.
- Abraham J. International Conference On Harmonisation Of Technical Requirements For Registration Of Pharmaceuticals For Human Use. In: Brouder A, Tietje C, editors. Handbook of Transnational Economic Governance Regimes [Internet]. Brill; 2009 [cited 2018 June 6]. p. 1041–54. Available from: http://booksandjournals.brillonline.com/content/books/10.1163/ej.9789004163300.i-1081.897.
- Sagar Baliram PM. A Validated Stability–Indicating HPLC Method estimation of Clonazepam In the bulk drug and Pharmaceutical Dosage Form. Pharmaceutica Analytica Acta [Internet]. 2015 [cited 2018 May 20];06. Available from: https://www.omicsonline.org/open-access/a-validated-stabilityindicating-hplc-method-estimation-of-clonazepam-in-the-bulk-drug-and-pharmaceutical-dosage-form-2153-2435.1000332.php?aid=40328.
- Statistical validation: Quantitative determination (General Explanations). Basle: Hoffman F. La Roche, 1987:1-9.
- N. Miller James, C. Miller Jane. Statistics and Chemometrics for Analytical Chemistry. 5a. Harlow: Pearson Education Limited; 2005.
- European Agency for Safety and Health at Work. Guidance for the safe management of hazardous medicinal products at work | Safety and health at work EU-OSHA [Internet]. European Commission; 2023 [cited 2024 Aug 28]. Available from: https://osha.europa.eu/en/publications/guidance-safe-management-hazardous-medicinal-products-work.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).