Submitted:
01 July 2025
Posted:
02 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Definition of the Dependent Variable: Complete Tooth Loss
2.3. Description of Independent Variable: Cardiovascular Diseases
2.4. The Potential Confounding Variable
2.5. Statistical Methods
3. Results
4. Discussion
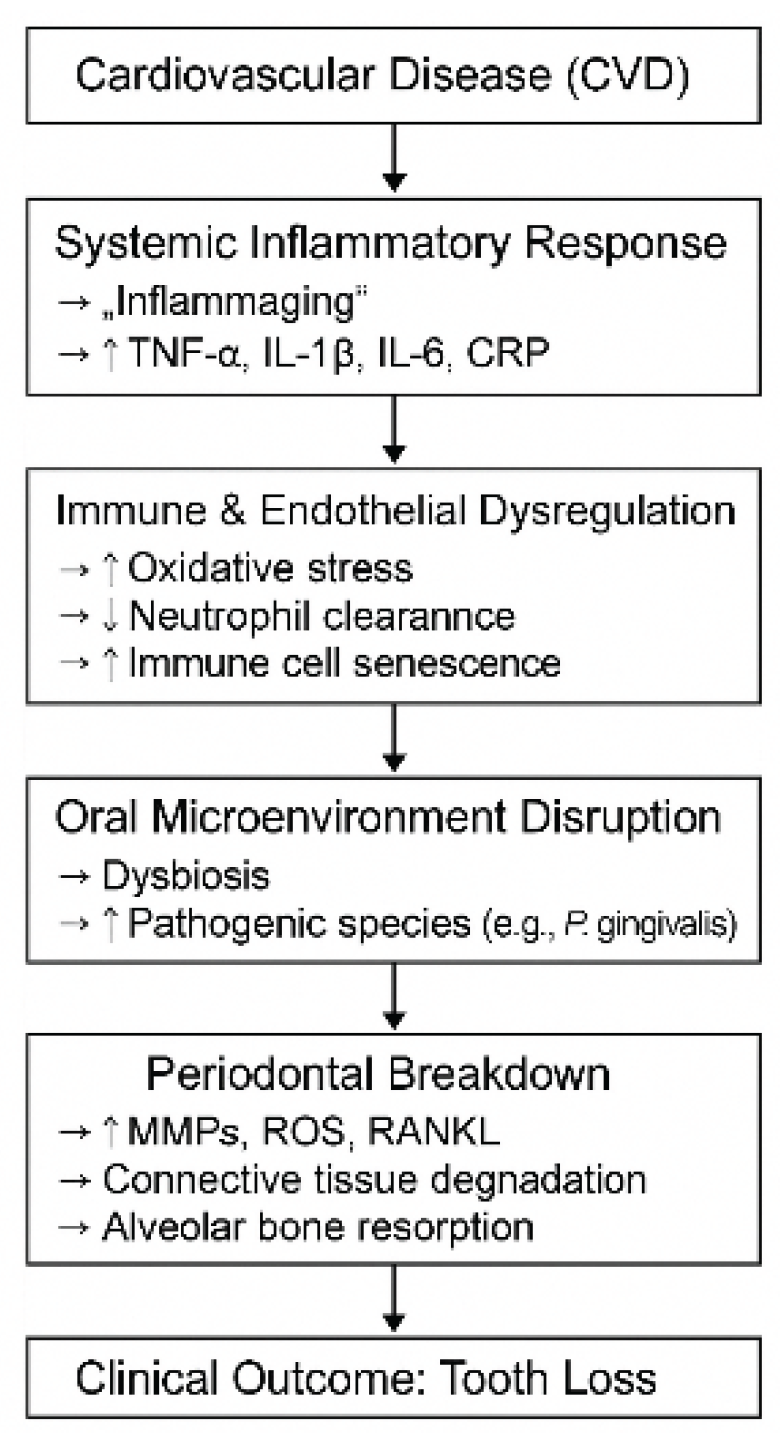
4.1. Limitation of Research
4.2. Future Perspectives
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marcenes, W.; Kassebaum, N.J.; Bernabé, E.; Flaxman, A.; Naghavi, M.; Lopez, A.; Murray, C.J.L. Global Burden of Oral Conditions in 1990–2010: A Systematic Analysis. J. Dent. Res.2013, 92, 592–597. [Google Scholar] [CrossRef] [Green Version]. [CrossRef]
- Kassebaum, N.J.; Smith, A.G.C.; Bernabé, E.; Fleming, T.D.; Reynolds, A.E.; Vos, T.; Murray, C.J.L.; Marcenes, W. Global, Regional, and National Prevalence, Incidence, and Disability-Adjusted Life Years for Oral Conditions for 195 Countries, 1990–2015: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors. J. Dent. Res. 2017, 96, 380–387. [Google Scholar] [CrossRef]. [CrossRef]
- Abodunrin, O.R.; Olagunju, M.T.; Alade, O.T.; Foláyan, M.O. Relationships between Oral Health and the Sustainable Development Goals: A Scoping Review. BioMed 2023, 3, 460-470. [CrossRef]
- Zhang, S.-Z.; Xie, L.; Shang, Z.-J. Burden of Oral Cancer on the 10 Most Populous Countries from 1990 to 2019: Estimates from the Global Burden of Disease Study 2019. Int. J. Environ. Res. Public Health 2022, 19, 875. [CrossRef]
- Al-Rafee, M. A. (2020). The epidemiology of edentulism and the associated factors: A literature review. Journal of Family Medicine and Primary Care, 9(4), 1841–1843. [CrossRef]. [CrossRef]
- Emami, E., de Souza, R. F., Kabawat, M., & Feine, J. S. (2013). The impact of edentulism on oral and general health. International Journal of Dentistry, 2013, Article 498305. [CrossRef]. [CrossRef]
- McGarry, T. J., Nimmo, A., Skiba, J. F., Ahlstrom, R. H., Smith, C. R., & Koumjian, J. H. (1999). Classification system for complete edentulism. Journal of Prosthodontics, 8(1), 27–39. [CrossRef]. [CrossRef]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global Burden of Severe Tooth Loss: A Systematic Review and Meta-Analysis. J. Dent. Res. 2014, 93, 7. [Google Scholar] [CrossRef] [Green Version]. [CrossRef]
- Holmstrup, P.; Damgaard, C.; Olsen, I.; Björn, K.; Allan, F.; Claus, H.; Nielsen, P.; Riis, H. Comorbidity of Periodontal Disease: Two Sides of the Same Coin? An Introduction for the Clinician. J. Oral Microbiol. 2017, 9, 1332710. [Google Scholar] [CrossRef]. [CrossRef]
- Romandini, M.; Baima, G.; Antonoglou, G.; Bueno, J.; Figuero, E.; Sanz, M. Periodontitis, Edentulism, and Risk of Mortality: A Systematic Review with Meta-Analyses. J. Dent. Res.2021, 100, 37–49. [Google Scholar] [CrossRef] [PubMed]. [CrossRef]
- Singh, A.; Gupta, A.; Peres, M.A.; Watt, R.G.; Tsakos, G.; Mathur, M.R. Association Between Tooth Loss and Hypertension Among a Primarily Rural Middle Aged and Older Indian Adult Population. J. Public Health Dent. 2016, 76, 198–205. [Google Scholar] [CrossRef]. [CrossRef]
- Taboza, Z.A.; Costa, K.L.; Silveira, V.R.; Furlaneto, F.A.; Montenegro, R., Jr.; Russell, S.; Dasanayake, A.; Rego, R.O. Periodontitis, Edentulismo and Glycemic Control in Patients with Type 2 Diabetes: A Cross-Sectional Study. BMJ Open Diabetes Res. Care 2018, 6, 000453. [Google Scholar] [CrossRef] [Green Version].
- Muñoz-Torres, F.J.; Mukamal, K.J.; Pai, J.K.; Willett, W.; Joshipura, K.J. Relationship between Tooth Loss and Peripheral Arterial Disease among Women. J. Clin. Periodontol. 2017, 44, 989–995. [Google Scholar] [CrossRef] [PubMed]. [CrossRef]
- Cheng, F.; Zhang, M.; Wang, Q.; Xu, H.; Dong, X.; Gao, Z.; Chen, J.; Wei, Y.; Qin, F. Tooth Loss and Risk of Cardiovascular Disease and Stroke: A Dose-Response Meta Analysis of Prospective Cohort Studies. PLoS ONE 2018, 13, e0194563. [Google Scholar] [CrossRef] [Green Version]. [CrossRef]
- Lee, H.J.; Choi, E.K.; Park, J.B.; Han, K.D.; Oh, S. Tooth Loss Predicts Myocardial Infarction, Heart Failure, Stroke, and Death. J. Dent. Res. 2019, 98, 164–170. [Google Scholar] [CrossRef] [PubMed]. [CrossRef]
- Goteiner, D.; Craig, R.G.; Ashmen, R.; Janal, M.N.; Eskin, B.; Lehrman, N. Endotoxin Levels are Associated with High-Density Lipoprotein, Triglycerides, and Troponin in Patients with Acute Coronary Syndrome and Angina: Possible Contributions from Periodontal Sources. J. Periodontol. 2008, 79, 2331–2339. [Google Scholar] [CrossRef] [Green Version]. [CrossRef]
- Nascimento, G.G.; Leite, F.R.; Conceição, D.A.; Ferrúa, C.P.; Singh, A.; Demarco, F.F. Is there a Relationship between Obesity and Tooth Loss and Edentulism? A Systematic Review and Meta-Analysis. Obes. Rev. 2016, 17, 587–598. [Google Scholar] [CrossRef] [PubMed]. [CrossRef]
- Choi, H.M.; Han, K.; Park, Y.G.; Park, J.B. Associations between the Number of Natural Teeth and Renal Dysfunction. Medicine 2016, 95, 4681. [Google Scholar] [CrossRef] [PubMed]. [CrossRef]
- Barros, S.P.; Suruki, R.; Loewy, Z.G.; Beck, J.D.; Offenbacher, S. A Cohort Study of the Impact of Tooth Loss and Periodontal Disease on Respiratory Events among COPD Subjects: Modulatory Role of Systemic Biomarkers of Inflammation. PLoS ONE 2013, 8, e68592. [Google Scholar] [CrossRef]. [CrossRef]
- Yoo, J.J.; Yoon, J.H.; Kang, M.J.; Kim, M.; Oh, N. The Effect of Missing Teeth on Dementia in Older People: A Nationwide Population-Based Cohort Study in South Korea. BMC Oral Health2019, 19, 61. [Google Scholar] [CrossRef] [Green Version]. [CrossRef]
- Cademartori, M.G.; Gastal, M.T.; Nascimento, G.G.; Demarco, F.F.; Corrêa, M.B. Is Depression Associated with Oral Health Outcomes in Adults and Elders? A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2018, 22, 2685–2702. [Google Scholar] [CrossRef]. [CrossRef]
- Saito, S.; Ohi, T.; Murakami, T.; Komiyama, T.; Miyoshi, Y.; Endo, K.; Satoh, M.; Asayama, K.; Inoue, R.; Kikuya, M.; et al. Association Between Tooth Loss and Cognitive Impairment in Community-Dwelling Older Japanese Adults: A 4-Year Prospective Cohort Study from the Ohasama Study. BMC Oral Health 2018, 18, 142. [Google Scholar] [CrossRef] [PubMed]. [CrossRef]
- Yang, B.; Petrick, J.L.; Abnet, C.C.; Graubard, B.I.; Murphy, G.; Weinstein, S.J.; Männistö, S.; Albanes, D.; McGlynn, K.A. Tooth Loss and Liver Cancer Incidence in a Finnish Cohort. Cancer Causes Control 2017, 28, 899–904. [Google Scholar] [CrossRef] [PubMed]. [CrossRef]
- Maisonneuve, P.; Amar, S.; Lowenfels, A.B. Periodontal Disease, Edentulism, and Pancreatic Cancer: A Meta-Analysis. Ann. Oncol. 2017, 28, 985–995. [Google Scholar] [CrossRef]. [CrossRef]
- Meyer, M.S.; Joshipura, K.; Giovannucci, E.; Michaud, D.S. A Review of the Relationship between Tooth Loss, Periodontal Disease, and Cancer. Cancer Causes Control 2008, 19, 895–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]. [CrossRef]
- Michaud, D.S.; Fu, Z.; Shi, J.; Chung, M. Periodontal Disease, Tooth Loss, and Cancer Risk. Epidemiol. Rev. 2017, 39, 49–58. [Google Scholar] [CrossRef] [Green Version]. [CrossRef]
- Islas-Granillo, H.; Borges-Yañez, S.A.; Navarrete-Hernández, J.J.; Veras-Hernández, M.A.; Casanova-Rosado, J.F.; Minaya-Sánchez, M.; Casanova-Rosado, A.J.; Fernández-Barrera, M.Á.; Medina-Solís, C.E. Indicators of Oral Health in Older Adults with and Without the Presence of Multimorbidity: A Cross-Sectional Study. Clin. Inter. Aging. 2019, 14, 219–224. [Google Scholar] [CrossRef] [Green Version]. [CrossRef]
- Islas-Granillo, H.; Medina-Solís, C.E.; Márquez-Corona, M.L.; de la Rosa-Santillana, R.; Fernández-Barrera, M.A.; Villalobos-Rodelo, J.J.; Hernández-Martínez, C.T.; Navarrete-Hernández, J.J.; Mendoza-Rodríguez, M. Prevalence of Multimorbidity in Institutionalized Subjects Aged 60 and Over in a Developing Country. Clin. Inter. Aging 2018, 13, 1129–1133. [Google Scholar] [CrossRef] [Green Version]. [CrossRef]
- Kailembo, A.; Preet, R.; Stewart Williams, J. Common Risk Factors and Edentulism in Adults, Aged 50 years and over, in China, Ghana, India and South Africa: Results from the WHO Study on Global AGEing and Adult Health (SAGE). BMC Oral Health 2016, 17, 29. [Google Scholar] [CrossRef] [Green Version]. [CrossRef]
- Hunter E, De Moura Brito L, Piyasena P, et al. Impact of edentulism on community-dwelling adults in low-income, middle-income and high-income countries: a systematic review BMJ Open 2024;14:e085479. [CrossRef]
- American Heart Association. (2017). Cardiovascular disease: A costly burden for America—Projections through 2035. [CrossRef].
- World Health Organization. (2021, June 11). Cardiovascular diseases (CVDs). [CrossRef].
- American Heart Association. (2021). AHA names top heart disease and stroke research advances of 2021. [CrossRef].
- Preisser JS, Moss K, Finlayson TL, Jones JA, Weintraub JA. Prediction Model Development and Validation of 12-Year Incident Edentulism of Older Adults in the United States. JDR Clinical & Translational Research. 2022;8(4):384-393. [CrossRef]
- Antonoglou, G. N., Romandini, M., Meurman, J. H., Surakka, M., Janket, S. J., & Sanz, M. (2023). Periodontitis and edentulism as risk indicators for mortality: Results from a prospective cohort study with 20 years of follow-up. Journal of periodontal research, 58(1), 12–21. [CrossRef]
- Janket, S.-J.; Kunhipurayil, H.H.; Tamimi, F.; Surakka, M.; Li, H.; Van Dyke, T.E.; Meurman, J.H. Edentulism or Poor Oral Hygiene: Which Is the Stronger Predictor for All-Cause Mortality? J. Clin. Med. 2025, 14, 371. [CrossRef]
- Juxiang Peng, Jukun Song, Jing Han, Zhu Chen, Xinhai Yin, Jianguo Zhu, Jinlin Song; The relationship between tooth loss and mortality from all causes, cardiovascular diseases, and coronary heart disease in the general population: systematic review and dose–response meta-analysis of prospective cohort studies. Biosci Rep 31 January 2019; 39 (1): BSR20181773. [CrossRef]
- Carra MC, Rangé H, Caligiuri G, Bouchard P. Periodontitis and atherosclerotic cardiovascular disease: A critical appraisal. Periodontol 2000. 2023; 00:1-34. [CrossRef]
- National Center for Health Statistics. About the National Health and Nutrition Examination Survey. Centers for Disease Control and Prevention. Available online: (accessed on 11 Dec 2024). https://www.cdc.gov/nchs/nhanes/about/.
- American Association of Oral and Maxillofacial Surgeons. Edentulism. MyOMS. Available online: [https://myoms.org/what-we-%20do/dental-implant-surgery/edentulism/ (accessed on 11 Dec 2024).
- Casanova-Rosado, A.J.; Casanova-Rosado, J.F.; Minaya-Sánchez, M.; Robles-Minaya, J.L.; Casanova-Sarmiento, J.A.; Márquez-Corona, M.d.L.; Pontigo-Loyola, A.P.; Isla-Granillo, H.; Mora-Acosta, M.; Márquez-Rodríguez, S.; et al. Association of Edentulism with Various Chronic Diseases in Mexican Elders 60+ Years: Results of a Population-Based Survey. Healthcare 2021, 9, 404. [CrossRef]. [CrossRef]
- Szerszeń, M.; Górski, B.; Kowalski, J. Clinical Condition of the Oral Cavity in the Adult Polish Population below 70 Years of Age after Myocardial Infarction—A Case–Control Study. Int. J. Environ. Res. Public Health2022, 19, 7265. [CrossRef]. [CrossRef]
- D’Orto, B.; Tetè, G.; Nagni, M.; Visconti, R.F.; Polizzi, E.; Gherlone, E.F. Full Arch Implant-Prosthetic Rehabilitation in Patients with Cardiovascular Diseases: A 7-Year Follow-Up Prospective Single Cohort Study. J. Clin. Med. 2024, 13, 924. [CrossRef]. [CrossRef]
- Ebersole, J.L.; Graves, C.L.; Gonzalez, O.A.; Dawson, D., 3rd; Morford, L.A.; Huja, P.E.; Hartsfield, J.K., Jr.; Huja, S.S.; Pandruvada, S.; Wallet, S.M. Aging, Inflammation, Immunity and Periodontal Disease. Periodontology 2000, 2016, 54–75. [Google Scholar] [CrossRef] [PubMed]. [CrossRef]
- D’Aiuto, F.; Graziani, F.; Tetè, S.; Gabriele, M.; Tonetti, M.S. Periodontitis: From Local Infection to Systemic Diseases. Int. J. Immunopathol. Pharmacol. 2005, 18, 1–11. [Google Scholar].
- Fisher, M.A.; Taylor, G.W.; West, B.T.; McCarthy, E.T. Bidirectional Relationship between Chronic Kidney and Periodontal Disease: A Study Using Structural Equation Modeling. Kidney Int. 2011, 79, 347–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]. [CrossRef]
- Gacon, I.; Wieczorek, A. Coexistence of Lack of Clinical Manifestation of Oral Mycosis and Systemic Diseases in Edentulous Patients Using Removable Prosthetic Restorations. Int. J. Environ. Res. Public Health 2020, 17, 6348. [Google Scholar] [CrossRef] [PubMed]. [CrossRef]
- Ebersole, J.L.; Graves, C.L.; Gonzalez, O.A.; Dawson, D., 3rd; Morford, L.A.; Huja, P.E.; Hartsfield, J.K., Jr.; Huja, S.S.; Pandruvada, S.; Wallet, S.M. Aging, Inflammation, Immunity and Periodontal Disease. Periodontology 2000, 2016, 54–75. [Google Scholar] [CrossRef] [PubMed]. [CrossRef]
- D’Aiuto, F.; Graziani, F.; Tetè, S.; Gabriele, M.; Tonetti, M.S. Periodontitis: From Local Infection to Systemic Diseases. Int. J. Immunopathol. Pharmacol. 2005, 18, 1–11. [Google Scholar].
- Fisher, M.A.; Taylor, G.W.; West, B.T.; McCarthy, E.T. Bidirectional Relationship between Chronic Kidney and Periodontal Disease: A Study Using Structural Equation Modeling. Kidney Int. 2011, 79, 347–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]. [CrossRef]
- Tinti, F.; Lai, S.; Noce, A.; Rotondi, S.; Marrone, G.; Mazzaferro, S.; Di Daniele, N.; Mitterhofer, A.P. Chronic Kidney Disease as a Systemic Inflammatory Syndrome: Update on Mechanisms Involved and Potential Treatment. Life 2021, 11, 419. [CrossRef]
- Gacon, I.; Wieczorek, A. Coexistence of Lack of Clinical Manifestation of Oral Mycosis and Systemic Diseases in Edentulous Patients Using Removable Prosthetic Restorations. Int. J. Environ. Res. Public Health 2020, 17, 6348. [Google Scholar] [CrossRef] [PubMed]. [CrossRef]
- Bagatini, M.D.; Cardoso, A.M.; Reschke, C.R.; Carvalho, F.B. Immune System and Chronic Diseases 2018. J. Immunol. Res. 2018, 2018, 8653572. [Google Scholar] [CrossRef] [Green Version]. [CrossRef]
- You, Z.; Cushman, M.; Jenny, N.S.; Howard, G. Regards, Tooth Loss, Systemic Inflammation, and Prevalent Stroke among Participants in the Reasons for Geographic and Racial Difference in Stroke (REGARDS) Study. Atherosclerosis 2009, 203, 615–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]. [CrossRef]
- Sadek, K.M.; El Moshy, S.; Radwan, I.A.; Rady, D.; Abbass, M.M.S.; El-Rashidy, A.A.; Dörfer, C.E.; Fawzy El-Sayed, K.M. Molecular Basis beyond Interrelated Bone Resorption/Regeneration in Periodontal Diseases: A Concise Review. Int. J. Mol. Sci. 2023, 24, 4599. [CrossRef]
- Gutterman, D. D., Chabowski, D. S., Kadlec, A. O., Durand, M. J., Freed, J. K., Ait-Aissa, K., & Beyer, A. M. (2016). The human microcirculation: Regulation of flow and beyond. Circulation Research, 118(1), 157–172. [CrossRef]. [CrossRef]
- Cecoro, G., Annunziata, M., Iuorio, M. T., Nastri, L., & Guida, L. (2020). Periodontitis, Low-Grade Inflammation and Systemic Health: A Scoping Review. Medicina, 56(6), 272. [CrossRef]. [CrossRef]
- Hunter E, De Moura Brito L, Piyasena P, et al. Impact of edentulism on community-dwelling adults in low-income, middle-income and high-income countries: a systematic review BMJ Open 2024;14:e085479. [CrossRef]
- American Heart Association. (2017). Cardiovascular disease: A costly burden for America—Projections through 2035. [CrossRef].
- World Health Organization. (2021, June 11). Cardiovascular diseases (CVDs). [CrossRef].
- American Heart Association. (2021). AHA names top heart disease and stroke research advances of 2021. [CrossRef].
- Hunter E, De Moura Brito L, Piyasena P, et al. Impact of edentulism on community-dwelling adults in low-income, middle-income and high-income countries: a systematic review BMJ Open 2024;14:e085479. [CrossRef]
- Gutterman, D. D., Chabowski, D. S., Kadlec, A. O., Durand, M. J., Freed, J. K., Ait-Aissa, K., & Beyer, A. M. (2016). The human microcirculation: Regulation of flow and beyond. Circulation Research, 118(1), 157–172. [CrossRef]. [CrossRef]
- Celik, D.; Kantarci, A. Vascular Changes and Hypoxia in Periodontal Disease as a Link to Systemic Complications. Pathogens 2021, 10, 1280. [CrossRef]. [CrossRef]
- Theodorakis, N.; Nikolaou, M. From Cardiovascular-Kidney-Metabolic Syndrome to Cardiovascular-Renal-Hepatic-Metabolic Syndrome: Proposing an Expanded Framework. Biomolecules 2025, 15, 213. [CrossRef]
- Tinti, F.; Lai, S.; Noce, A.; Rotondi, S.; Marrone, G.; Mazzaferro, S.; Di Daniele, N.; Mitterhofer, A.P. Chronic Kidney Disease as a Systemic Inflammatory Syndrome: Update on Mechanisms Involved and Potential Treatment. Life 2021, 11, 419. [CrossRef]
- Gacon, I.; Wieczorek, A. Coexistence of Lack of Clinical Manifestation of Oral Mycosis and Systemic Diseases in Edentulous Patients Using Removable Prosthetic Restorations. Int. J. Environ. Res. Public Health 2020, 17, 6348. [Google Scholar] [CrossRef] [PubMed]. [CrossRef]
- Desai, J.P.; Nair, R.U. Oral Health Factors Related to Rapid Oral Health Deterioration among Older Adults: A Narrative Review. J. Clin. Med.2023,12,3202. [CrossRef]
- Marchini, L.; Ettinger, R.L. The Prevention, Diagnosis, and Treatment of Rapid Oral Health Deterioration (ROHD) among Older Adults. J. Clin. Med. 2023, 12, 2559. [CrossRef]
- Pawinska, M.; Kondrat, A.; Jamiolkowski, J.; Paszynska, E. Dental Status and Oral Health Behaviors of Selected 45–74-Year-Old Men from Northeastern Poland. Int. J. Environ. Res. Public Health 2023, 20, 6005. [CrossRef]
- Maria Febbraio, Christopher Bryant Roy, Liran Levin, Is There a Causal Link Between Periodontitis and Cardiovascular Disease? A Concise Review of Recent Findings, International Dental Journal, Volume 72, Issue 1, 2022, Pages 37-51, ISSN 0020-6539, [CrossRef]. [CrossRef]
| Independent variable | With Dentition (n) |
% | Complete Tooth Loss (n) |
% | Total (n) |
||
|---|---|---|---|---|---|---|---|
| Heart Disease* | No heart disease | 8,583 | 84.5 | 1,378 | 6.42 | 9,961 | |
| Have heart disease | 941 | 7.33 | 385 | 1.76 | 1,326 | ||
| Heart Attack (Myocardial Infarction MI) | Yes | 356 | 2.78 | 165 | 0.78 | 521 | |
| No | 9,155 | 89.06 | 1,593 | 7.42 | 10,748 | ||
| Coronary Heart Disease (CHF) | Yes | 349 | 3.08 | 160 | 0.74 | 509 | |
| No | 9,147 | 88.78 | 1,590 | 7.39 | 10,737 | ||
| Congestive Heart Failure (CHF) | Yes | 271 | 1.8 | 144 | 0.58 | 415 | |
| No | 9,229 | 90.02 | 1,616 | 7.61 | 10,845 | ||
| Stroke | Yes | 346 | 2.37 | 136 | 0.64 | 482 | |
| No | 9,167 | 89.46 | 1,624 | 7.52 | 10,791 | ||
| Gender | Male | 6,963 | 42.21 | 2,486 | 6.65 | 9,449 | 0.53 |
| Female | 7,329 | 44.36 | 2,447 | 6.78 | 9,776 | ||
| Race/Ethnicity | Hispanic | 4,065 | 15.14 | 1,351 | 2.72 | 5,416 | |
| White | 4,474 | 52.46 | 1,742 | 7.41 | 6,216 | ||
| Black | 3,221 | 10.22 | 1,023 | 1.67 | 4,244 | ||
| Asian | 1,747 | 4.83 | 463 | 0.75 | 2,210 | ||
| Others | 785 | 3.92 | 354 | 0.87 | 1,139 | ||
| Age in years at screening | Less than 6 | 165 | 0.5 | 2,815 | 6.91 | 2,980 | |
| 11-Jun | 2,234 | 7.52 | 213 | 0.28 | 2,447 | ||
| 18-Dec | 2,111 | 9.29 | 121 | 0.15 | 2,232 | ||
| 19-44 | 4,345 | 32.54 | 455 | 1.38 | 4,800 | ||
| 45-59 | 2,376 | 18.66 | 339 | 1.56 | 2,715 | ||
| Above 60 | 3,061 | 18.07 | 990 | 3.14 | 4,051 | ||
| Education level | 0-11 | 1,931 | 10.8 | 550 | 2.13 | 2,481 | |
| HS/Ged | 2,118 | 21.46 | 443 | 2.48 | 2,561 | ||
| >HS | 5,466 | 59.58 | 762 | 3.55 | 6,228 | ||
| Ratio of family | <100%FPL | 2,889 | 69.58 | 1,263 | 30.42 | 4,152 | |
| 100%-99%FPL | 3,564 | 74.46 | 1,222 | 25.54 | 4,786 | <0.0001 | |
| 200%-399%FPL | 3,382 | 77.18 | 1,000 | 22.82 | 4,382 | ||
| 400%+FPL | 2,886 | 79.68 | 736 | 20.32 | 3,622 | ||
| BMI | Underweight | 1,877 | 7.61 | 1,288 | 4.39 | 3,165 | |
| normal | 4,268 | 26.16 | 453 | 1.97 | 4,721 | ||
| Overweight | 3,648 | 24.58 | 409 | 2.09 | 4,057 | ||
| Obese | 4,355 | 30.68 | 463 | 2.52 | 4,818 | ||
| Diabetes | Yes | 1,365 | 7.38 | 384 | 1.24 | 1,749 | |
| No | 12,647 | 78.54 | 3,737 | 10.99 | 16,384 | ||
| borderline | 273 | 1.67 | 58 | 0.17 | 331 | ||
| Covariate | Odds Ratio | 95% Confidence Interval | P-value |
|---|---|---|---|
| Heart attack (Myocardial Infarction) | 1.78 | 1.27- 2.49 | 0.001 |
| Sex | 1.04 | 0.86- 1.26 | 0.648 |
| Age (45-59) | 2.23 | 1.61- 3.09 | <0.001 |
| Age (Above 60) | 4.31 | 2.88-6.46 | <0.001 |
| Education (HS/GED) | 0.64 | 0.50-0.82 | 0.001 |
| Education (>HS) | 0.40 | 0.31- 0.50 | <0.001 |
| Poverty (100%-199% FPL) | 0.92 | 0.69- 1.23 | 0.592 |
| Poverty (200%-399% FPL) | 0.54 | 0.37- 0.78 | 0.002 |
| Poverty (400%+ FPL) | 0.38 | 0.25- 0.57 | <0.001 |
| Race (Non-Hispanic White) | 1.54 | 1.05-2.25 | 0.027 |
| Rece (Non-Hispanic Black) | 1.62 | 1.13-2.33 | 0.010 |
| Race (Non-Hispanic Asian) | 1.99 | 1.25- 3.18 | 0.005 |
| Race (Other) | 2.50 | 1.53-4.09 | 0.001 |
| Diabetes (Yes) | 1.13 | 0.85-1.49 | 0.367 |
| Diabetes (Borderline) | 1.12 | 0.68- 1.83 | 0.631 |
| BMI (Normal) | 0.71 | 0.23- 2.18 | 0.545 |
| BMI (Overweight) | 0.84 | 0.26- 2.66 | 0.760 |
| BMI (Obese) | 0.74 | 0.24- 2.25 | 0.596 |
| Variable | Odds Ratio | 95% Confidence Interval | P-value |
|---|---|---|---|
| Heart Disease* | 1.70 | 1.35- 2.14 | <0.001 |
| Sex | 1.04 | 0.86- 1.25 | 0.664 |
| Age (45-59) | 2.19 | 1.59- 3.02 | <0.001 |
| Age (Above 60) | 4.06 | 2.72- 6.07 | <0.001 |
| Education (HS/GED) | 0.65 | 0.50- 0.83 | 0.001 |
| Education (>HS) | 0.40 | 0.32- 0.50 | <0.001 |
| Poverty (100%-199% FPL) | 0.92 | 0.69- 1.22 | 0.566 |
| Poverty (200%-399% FPL) | 0.54 | 0.37- 0.78 | 0.002 |
| Poverty (400%+ FPL) | 0.38 | 0.26- 0.58 | <0.001 |
| Race (White) | 1.49 | 1.02- 2.19 | 0.039 |
| Race (Black) | 1.57 | 1.09- 2.26 | 0.017 |
| Race (Asian) | 1.99 | 1.24-3.19 | 0.006 |
| Race (Other) | 2.45 | 1.48- 4.06 | 0.001 |
| Diabetes (Yes) | 1.08 | 0.82- 1.42 | 0.558 |
| Diabetes (Borderline) | 1.06 | 0.65- 1.74 | 0.785 |
| BMI (Normal) | 0.71 | 0.23- 2.18 | 0.542 |
| BMI (Overweight) | 0.83 | 0.26- 2.64 | 0.747 |
| BMI (Obese) | 0.73 | 0.24- 2.20 | 0.574 |
| Variable | Odds Ratio | 95% Confidence Interval | P-value |
|---|---|---|---|
| Coronary heart disease | 1.60 | 1.16- 2.20 | 0.005 |
| Sex | 1.03 | 0.85- 1.24 | 0.721 |
| Age (45-59) | 2.26 | 1.63- 3.12 | <0.001 |
| Age (Above 60) | 4.35 | 2.90- 6.52 | <0.001 |
| Education (HS/GED) | 0.66 | 0.51- 0.85 | 0.003 |
| Education (>HS) | 0.40 | 0.31- 0.51 | <0.001 |
| Poverty (100%-199% FPL) | 0.93 | 0.70- 1.23 | 0.617 |
| Poverty (200%-399% FPL) | 0.53 | 0.37- 0.78 | 0.002 |
| Poverty (400%+ FPL) | 0.37 | 0.25- 0.56 | <0.001 |
| Race (Non-Hispanic White) | 1.52 | 1.03- 2.24 | 0.035 |
| Race (Non-Hispanic Black) | 1.62 | 1.13- 2.34 | 0.010 |
| Race (Non-Hispanic Asian) | 1.99 | 1.25-3.17 | 0.005 |
| Race (Other) | 2.55 | 1.54- 4.21 | 0.001 |
| Diabetes (Yes) | 1.13 | 0.85- 1.52 | 0.365 |
| Diabetes (Borderline) | 1.08 | 0.65- 1.78 | 0.751 |
| BMI (Normal) | 0.71 | 0.23 2.17 | 0.537 |
| BMI (Overweight) | 0.82 | 0.25- 2.61 | 0.731 |
| BMI (Obese) | 0.74 | 0.24 2.25 | 0.593 |
| Variable | Odds Ratio | 95% Confidence Interval | P-value |
|---|---|---|---|
| Congestive HF | 1.85 | 1.29- 2.67 | 0.002 |
| Sex | 1.01 | 0.84- 1.23 | 0.840 |
| Age (45-59) | 2.26 | 1.64- 3.11 | <0.001 |
| Age (Above 60) | 4.42 | 2.92- 6.68 | <0.001 |
| Education (HS/GED) | 0.64 | 0.50- 0.83 | 0.001 |
| Education (>HS) | 0.39 | 0.31- 0.50 | <0.001 |
| Poverty (100%-199% FPL) | 0.92 | 0.69- 1.22 | 0.556 |
| Poverty (200%-399% FPL) | 0.53 | 0.37- 0.78 | 0.002 |
| Poverty (400%+ FPL) | 0.38 | 0.25- 0.57 | <0.001 |
| Race (Non-Hispanic White) | 1.54 | 1.04- 2.27 | 0.029 |
| Race (Non-Hispanic Black) | 1.59 | 1.10- 2.30 | 0.014 |
| Race (Non-Hispanic Asian) | 1.97 | 1.23- 3.15 | 0.006 |
| Race (Other) | 2.61 | 1.58- 4.32 | <0.001 |
| Diabetes (Yes) | 1.12 | 0.84- 1.50 | 0.404 |
| Diabetes (Borderline) | 1.06 | 0.64- 1.74 | 0.814 |
| BMI (Normal) | 0.71 | 0.23- 2.21 | 0.552 |
| BMI (Overweight) | 0.83 | 0.26- 2.67 | 0.751 |
| BMI (Obese) | 0.74 | 0.24- 2.22 | 0.581 |
| Variable | Odds Ratio | 95% Confidence Interval | P-value |
|---|---|---|---|
| Stroke | 1.64 | 1.17- 2.29 | 0.005 |
| Sex | 1.01 | 0.84- 1.23 | 0.849 |
| Age (45-59) | 2.26 | 1.64- 3.12 | <0.001 |
| Age (Above 60) | 4.44 | 2.95- 6.69 | <0.001 |
| Education (HS/GED) | 0.64 | 0.50- 0.83 | <0.001 |
| Education (>HS) | 0.40 | 0.31- 0.50 | <0.001 |
| Poverty (100%-199% FPL) | 0.92 | 0.69- 1.23 | 0.601 |
| Poverty (200%-399% FPL) | 0.54 | 0.37- 0.78 | 0.002 |
| Poverty (400%+ FPL) | 0.38 | 0.25- 0.57 | <0.001 |
| Race (Non-Hispanic White) | 1.55 | 1.05- 2.27 | 0.027 |
| Race (Non-Hispanic Black) | 1.60 | 1.11- 2.32 | 0.013 |
| Race (Non-Hispanic Asian) | 2.01 | 1.26- 3.20 | 0.005 |
| Race (Other) | 2.58 | 1.56- 4.29 | 0.001 |
| Diabetes (Yes) | 1.12 | 0.85- 1.49 | 0.390 |
| Diabetes (Borderline) | 1.06 | 0.65- 1.72 | 0.787 |
| BMI (Normal) | 0.68 | 0.22- 2.09 | 0.499 |
| BMI (Overweight) | 0.80 | 0.25- 2.53 | 0.698 |
| BMI (Obese) | 0.72 | 0.24- 2.17 | 0.557 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





