1. Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs), which are widely used in veterinary medicine, play a significant role, particularly in managing pain, inflammation, and fever. They are often preferred in conditions such as postoperative pain, musculoskeletal diseases (e.g., osteoarthritis and laminitis), visceral pain (e.g., colic), inflammatory diseases (e.g., pneumonia and mastitis), and fever reduction [
1]. NSAIDs mainly suppress prostaglandin synthesis by inhibiting cyclooxygenase (COX) enzymes, thereby reducing pain, fever, and inflammation [
2]. While COX-1 is involved in normal physiological functions, COX-2 is induced during inflammation. Consequently, COX-2 selective NSAIDs (e.g., meloxicam, firocoxib) have fewer gastrointestinal side effects [
1,
2]. The most common adverse effects linked with NSAID use include gastrointestinal ulceration, renal toxicity, hepatotoxicity, hematologic disorders, and irritation at injection sites [
2]. Although NSAIDs are highly effective and essential drugs widely used in veterinary medicine, they should be administered with caution and awareness. Meloxicam belongs to the enolic acid group of NSAIDs and exhibits analgesic, anti-inflammatory, and antipyretic effects [
3]. It preferentially inhibits COX-2 over COX-1, but it is not entirely COX-2 selective; thus, at higher doses, its selectivity for the COX-2 isoenzyme diminishes [
4]. Meloxicam is extensively metabolised in the liver and undergoes significant enterohepatic recirculation [
5]. Its metabolites have not been found to possess any pharmacological activity [
6]. Meloxicam is an approved NSAID for cattle, horses, cats, and dogs, while it is used off-label in sheep and goats. Recent pharmacological research increasingly highlights gender-based differences in drug absorption, distribution, metabolism, and excretion. Incorporating gender as a crucial variable in dosage adjustment is important. Moyer et al. [
7] discussed how gender chromosomes and endogenous steroid hormones modulate drug transporters, metabolic enzymes, and receptors, thereby influencing pharmacokinetics and adverse drug responses. Moreover, Bosch et al. [
8] stated that female sex hormones, particularly estrogen, significantly impact drug metabolism mediated by CYP and UGT enzyme activity. These factors are especially relevant for orally administered drugs due to the influence of first-pass hepatic metabolism affected by estrogenic activity. Data on gender-dependent pharmacokinetics of drugs used in ruminants remains limited. It is scientifically and clinically valuable to explore how the pharmacokinetics of meloxicam, a commonly used NSAID in veterinary medicine, are affected by gender in food-producing species like goats. While existing literature addresses gender differences in humans and laboratory animals, no prior studies have focused on meloxicam in goats. Therefore, our research aimed to assess whether and how the pharmacokinetic parameters of meloxicam differ between female and male Saanen goats following intravenous (0.5 mg/kg) and oral (1.0 mg/kg) administration.
4. Discussion
It is imperative to comprehend how gender influences drug disposition to optimize therapeutic strategies, ensure animal welfare, and refine dosage recommendations, particularly in food-producing species such as goats. In both genders, pharmacokinetic variability can have both clinical and regulatory implications. The present study was conducted to evaluate the pharmacokinetics of meloxicam, a commonly used NSAID in veterinary medicine, in male and female Saanen goats following both IV and PO administration.
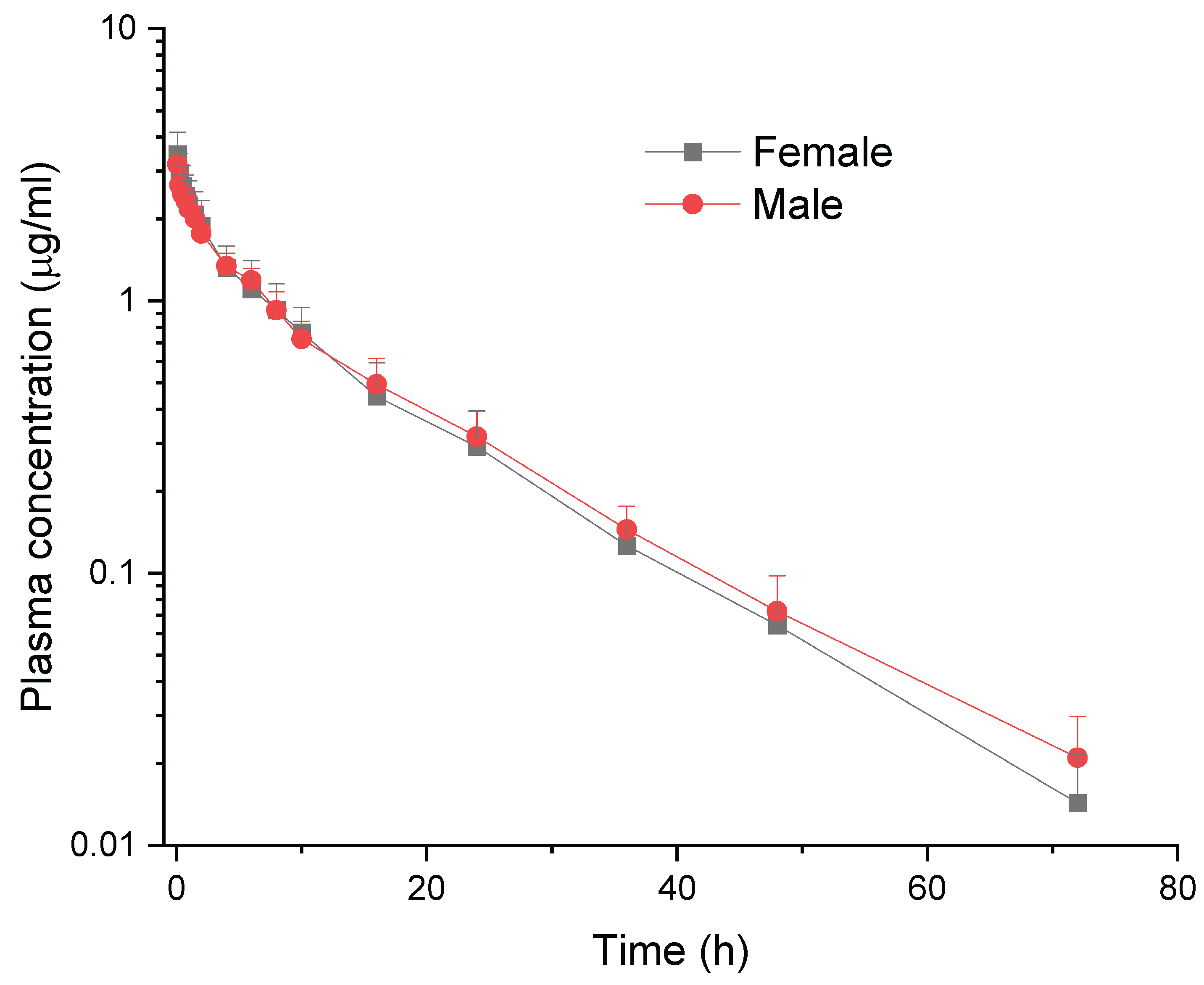
Following IV administration of meloxicam at 0.5 mg/kg, a statistically significant difference was observed in the T
1/2λz between female and male goats. The T
1/2λz was notably longer in males (11.72 ± 0.74 h) than in females (10.09 ± 0.97 h), suggesting that meloxicam is cleared more slowly in male goats. This prolonged elimination could reflect gender-based differences in hepatic metabolism, protein binding, or renal excretion rates. Despite the absence of statistically significant differences in other pharmacokinetic parameters, such as C
0, AUC
0–∞, Cl, and volume of distribution (Vss), between the genders, the consistently higher MRT
0–∞ and AUMC values observed in males provide further evidence that supports the hypothesis of a slower drug disposition in males. These findings indicate that, despite similar initial plasma concentrations and exposure levels, meloxicam remains in the system for a prolonged persistence in male goats. It is imperative to consider the implications of this prolonged systemic persistence on both therapeutic efficacy and withdrawal time considerations. A recent study investigated the pharmacokinetic differences of meloxicam between male and female sheep following IV administration. The results showed that the total clearance (Cl
T) and Vdss were significantly higher in male sheep, while the half-life (T
1/2λz) was significantly shorter compared to female sheep [
12]. In our study, meloxicam was administered at a dose of 0.5 mg/kg IV to Saanen goats, whereas Corum et al. [
12] employed a 1.0 mg/kg IV dose in Romanov sheep. Despite this difference in dosing, both studies reveal that gender significantly influences meloxicam disposition, particularly in terms of T
1/2λz. In male goats, the T
1/2λz (11.72 ± 0.74 h) was longer than that observed in male sheep (9.47 ± 0.25 h), while female goats had a T
1/2λz (10.09 ± 0.97 h) slightly shorter than female sheep (11.96 ± 0.33 h). The fact that the T
1/2λz is longer in male goats than in male sheep is a surprising result because studies have shown that goats metabolize and eliminate compounds faster than sheep [
13,
14,
15,
16]. Although male sheep exhibited faster clearance (8.72 ± 1.34 mL/h/kg) and larger volume of distribution (Vdss: 100.95 ± 14.73 mL/kg) than females, such pronounced differences were not observed in goats. In our study, both Cl and Vd values were similar across genders, suggesting that species-related physiological or metabolic factors may play a more prominent role in drug disposition than gender alone in goats. Another notable distinction concerns the extent of systemic exposure. In sheep, female animals showed significantly higher AUC
0–∞ values (224.68 ± 28.22 μg·h/mL) compared to males (117.25 ± 20.38 μg·h/mL), indicating a marked gender-based difference in meloxicam exposure. In contrast, in goats, AUC values were comparable between genders (females: 25.57 ± 6.76 vs. males: 26.43 ± 4.30 μg·h/mL), further underscoring the interspecies variability in meloxicam pharmacokinetics. These differences could be attributed not only to species-specific hepatic enzyme activity (such as CYP2C9-mediated metabolism) but also to variations in plasma protein binding, tissue distribution, and renal excretion patterns between goats and sheep. Both studies highlight the importance of gender in determining the pharmacokinetics of meloxicam; however, the results again demonstrate that these effects are species-dependent.
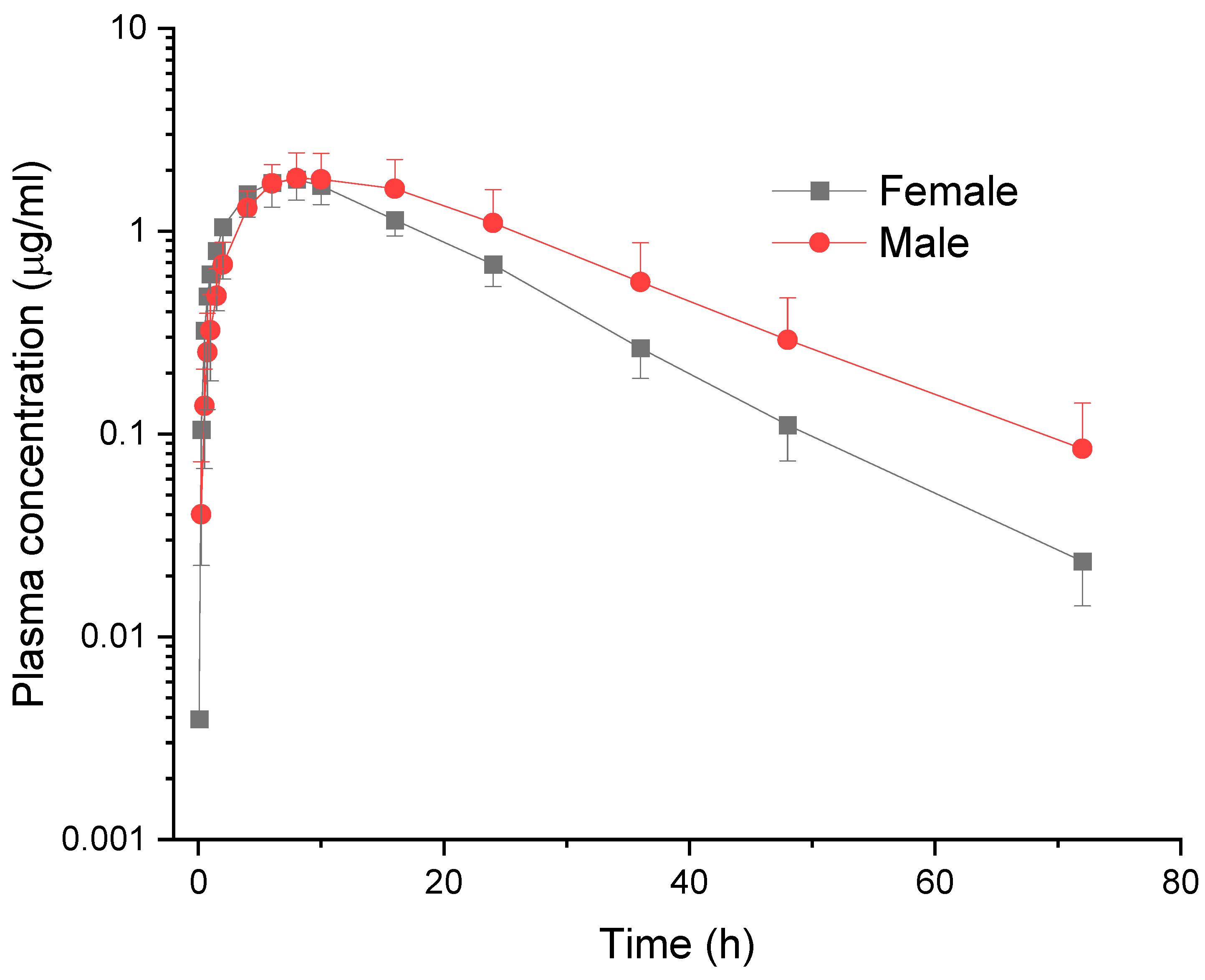
In the present study, gender-related pharmacokinetic differences were also observed in Saanen goats following a single PO administration of meloxicam at a dose of 1.0 mg/kg. Male goats exhibited a significantly longer T
1/2λz (13.10 ± 2.01 h) compared to females (9.87 ± 0.85 h), indicating slower clearance of the drug. This was further supported by the longer MRT
0–∞ observed in males (22.18 ± 3.47 h vs. 17.12 ± 1.73 h in females), suggesting extended systemic persistence of meloxicam in male animals. In addition, although C
max was similar between genders, total drug exposure represented by AUC
0–∞ was higher in males (55.36 ± 22.38 μg·h/mL) than in females (39.59 ± 7.45 μg·h/mL), although not statistically significant, but with a high degree of inter-individual variability. These findings suggest that meloxicam is more systemically available in males than females following PO administration in goats. Meloxicam is subject to complete metabolic conversion to four pharmacologically inactive metabolites, primarily through the cytochrome P450 2C pathway [
17]. Thus, the observed differences in elimination and exposure in male and female animals may be attributable to gender-based variations in hepatic enzyme activity, enterohepatic recirculation, gastrointestinal transit times, or hormonal influences affecting drug metabolism and excretion.
A thorough evaluation of meloxicam pharmacokinetics after IV and PO administration in both female and male Saanen goats shows significant differences in drug disposition based on the route of administration and gender. While T1/2λz values remained generally consistent across genders and administration routes—suggesting similar elimination kinetics once meloxicam enters the systemic circulation—statistically significant differences emerged in drug exposure and MRT, especially following PO administration. In both genders, PO administration of meloxicam at 1.0 mg/kg resulted in significantly higher systemic exposure compared to 0.5 mg/kg IV administration. This was evident in the AUC0–∞ values, which increased from 25.57 ± 6.76 to 39.59 ± 7.45 μg·h/mL in females and from 26.43 ± 4.30 to 55.36 ± 22.38 μg·h/mL in males. Correspondingly, AUMC0–∞ and MRT0–∞ values were significantly elevated after PO dosing in both genders, indicating prolonged systemic presence of meloxicam via the oral route. Notably, the increase in MRT0–∞ from IV to PO was more pronounced in males (14.27 h vs. 22.18 h) than in females (12.77 h vs. 17.12 h), suggesting that male goats may exhibit a slower drug turnover and more sustained drug retention after PO administration. Interestingly, the calculated absolute bioavailability (F) of oral meloxicam was 77.43% in females and 104.73% in males. While the high F value in males may partly reflect nonlinear pharmacokinetics at the higher dose or enterohepatic recirculation, it also highlights inter-individual variability and possible differences in gastrointestinal absorption or hepatic metabolism between genders. The consistent Tmax values (7.33 ± 1.03 h in females vs. 8.33 ± 1.51 h in males) suggest a delayed yet efficient absorption profile for oral meloxicam across both genders, with slightly slower absorption kinetics in males. From a clinical standpoint, these findings underscore that although both genders achieve therapeutic plasma levels of meloxicam after PO administration, male goats may maintain these levels for a longer duration due to prolonged MRT and higher AUC. While T1/2λz remained unaffected by the administration route, the overall pharmacokinetic behavior was modulated by both the administration route and gender, reinforcing the need to account for these variables in designing dosage regimens, determining dosing intervals, and establishing withdrawal periods for food-producing animals.
The observed gender-related pharmacokinetic differences in meloxicam disposition in goats may be partially attributed to hormonal modulation of drug metabolism, as supported by previous studies using model compounds such as antipyrine. In their comparative work, Witkamp et al. [
18] demonstrated that the metabolism and clearance of antipyrine varied significantly not only between species (goat, cattle, rabbit, rat) but also between genders within a species. In goats, although gender differences in antipyrine plasma clearance were inconsistent across years, females tended to eliminate the drug more rapidly than males in some instances. Importantly, metabolite profiling revealed that the production of certain oxidative metabolites (e.g., 3-hydroxymethyl antipyrine and norantipyrine) was consistently lower in males, suggesting a potential gender-linked difference in cytochrome P450 isoenzyme activity. Extending these findings, Witkamp et al. [
19] showed that exogenous administration of gonadal hormones in dwarf goats selectively affected the formation of specific antipyrine metabolites. Testosterone treatment in female goats and castrated males significantly suppressed the production of 3-hydroxymethyl antipyrine, nor antipyrine, and 4,4’-dihydroxy antipyrine, while estradiol administration in intact males reduced the clearance of 4-hydroxy antipyrine. These results support the hypothesis that gonadal steroids can modulate specific metabolic pathways, most likely by altering the expression or activity of hepatic P450 enzymes involved in phase I oxidation reactions. These data are consistent with our findings, where male goats exhibited longer elimination half-lives and higher systemic exposure to meloxicam, especially after PO administration. Although the elimination rate of meloxicam was not drastically different between genders after IV administration, the prolonged MRT and elevated AUC values in males suggest that endogenous hormonal differences may influence drug clearance capacity. In particular, testosterone-mediated suppression of hepatic oxidative metabolism -as observed with antipyrine- may be extrapolated to meloxicam, a drug extensively metabolized by the liver in ruminants.
Farkouh et al. [
20] stated that there are differences in basic pharmacokinetic parameters such as bioavailability, volume of distribution and clearance between both genders in commonly prescribed drugs, and emphasized that these differences may lead to gender-specific changes in the effect of some drugs. A previous study showed that there were notable gender-related differences in the plasma disposition of ivermectin between male and female goats after pour-on administration [
21]. While C
max, T
max, and AUC values did not differ significantly between the genders, the half-life (T
1/2λz) and mean residence time (MRT) were considerably longer in male goats compared to female goats. Moyer et al. [
7] highlighted that gender chromosomes and steroid hormones modulate various pharmacokinetic processes, including hepatic enzyme expression (e.g., cytochrome P450 isoforms), transporter activity, and receptor signaling. These molecular differences can alter both the rate and extent of drug metabolism, particularly for drugs with high hepatic extraction or those undergoing first-pass metabolism, such as meloxicam. Importantly, the influence of gender on pharmacokinetics becomes more pronounced with orally administered drugs, due to potential differences in gastrointestinal physiology (e.g., pH, motility), enzyme activity, and portal blood flow, all of which may explain the extended MRT and increased AUC observed in male goats in this study.
Bosch et al. [
8] reviewed the modulatory role of estrogens and other female sex hormones on drug metabolism and pharmacokinetics. They reported that estrogens can induce or inhibit specific metabolic pathways, especially those involving CYP and UGT enzymes, leading to faster clearance in females in some contexts. This may help explain the more rapid elimination of meloxicam in female goats, particularly after oral dosing. Furthermore, these hormonal effects can vary dynamically with reproductive status and age factors that may contribute to inter-individual variability, even within the same gender. In our study, PO administration (1.0 mg/kg) resulted in disproportionately greater systemic exposure and longer MRT in males than in females, despite the same dosing and formulation. While part of this difference may stem from dose-dependent kinetics or enterohepatic recirculation, the consistency of the gender effect across multiple parameters supports a biologically rooted difference in metabolism. Interestingly, the bioavailability of meloxicam was calculated as 77.43% in females and 104.73% in males, suggesting more complete and sustained absorption in males, which may further support a hormonal influence on drug disposition. Gleiter and Gundert-Remy [
22] reported that CYP3A4 activity is higher in females, whereas phase II conjugation reactions (e.g., glucuronidation) are more dominant in males. These enzymatic differences are consistent with the slower meloxicam clearance and longer MRT observed in male goats in our study. Scandlyn et al. [
23] examined the gender-specific CYP isoform distribution in humans and showed that CYP1A2 and CYP2E1 were more active in men, while the CYP3A family was more dominant in women.
Schwartz [
24] emphasized that these differences are gender-based not only at the enzyme level but also in physiological parameters such as body composition, plasma volume, fat/muscle ratio, renal function and gastrointestinal motility; this revealed that pharmacokinetic processes are affected by gender. It has been suggested that these physiological and biochemical variables may lead to significant pharmacokinetic variations, especially in orally administered drugs [
25]. Soldin and Mattison [
26] also stated that male-female differences in pharmacokinetic and pharmacodynamic processes are at a level that can affect clinical outcomes, and these differences should be taken into account, especially in hepatic metabolism, renal elimination and tissue distribution of the drug. Spoletini et al. [
27] explained how hormonal balances that change throughout life affect drug effects and metabolic pathways; they showed that estrogen can accelerate the hepatic clearance of drugs by increasing CYP3A4 expression. In the study conducted by Martin et al. [
28], differences in pain biomarkers and plasma meloxicam levels were observed following meloxicam administration in male and female calves, demonstrating that pharmacokinetic differences were directly reflected in pharmacodynamic results. These data suggest that gender-specific dosing strategies may affect not only clinical efficacy but also drug residual duration and food safety.
Taken together, these findings underscore the need to incorporate gender as a biological variable in pharmacokinetic studies, particularly for veterinary drugs administered orally in food-producing animals. The integration of literature findings with our data suggests that sex hormones can affect the activity of hepatic enzymes involved in meloxicam metabolism, which may lead to clinically relevant differences in drug exposure and clearance. These findings could have significant implications for adjusting dosage regimens and withdrawal periods to ensure both therapeutic efficacy and food safety.