1. Introduction
Equine sarcoids (
Figure 1), the most prevalent cutaneous neoplasms in horses, represent a significant challenge in veterinary oncology, with a global prevalence of 1–2% that escalates to 25% in bovine papillomavirus (BPV)-endemic regions [
1,
2]. These fibroblastic tumors are etiologically linked to BPV-1 and BPV-2 genotypes, which drive oncogenesis through viral DNA integration and inactivation of tumor suppressor pathways (p53 and pRb). Beyond their clinical aggressiveness and high recurrence rates, sarcoids impose substantial economic burdens, particularly in athletic and elite horses, due to treatment costs and reduced performance [
3].
Conventional therapies as surgical excision, cryotherapy, and topical chemotherapy remain plagued by recurrence rates of 30–50% and complications such as keloid formation or systemic toxicity [
4,
5,
6]. This therapeutic stagnation stems from sarcoids’ molecular heterogeneity, ranging from localized nodular forms to invasive, multicentric lesions resistant to standardized protocols [
7]. Emerging immunotherapies, however, offer promise by targeting the tumor microenvironment (TME) to mitigate recurrence a paradigm shift supported by recent advances in comparative oncolog [
8].
Bacillus Calmette-Guérin (BCG), a live-attenuated
Mycobacterium bovis strain, has been a cornerstone of human bladder cancer immunotherapy since the 1970s. Its efficacy hinges on activating innate immune cells (macrophages, NK cells) via Toll-like receptor (TLR) signaling and stimulating adaptive responses through CD8+ T-cell recruitment. In equine medicine, intralesional BCG has demonstrated tumor regression in 40–70% of cases since the 1980s, though clinical adoption is hindered by protocol heterogeneity (e.g., strain selection: BCG Tokyo vs. Pasteur) and adverse inflammatory reactions [
9,
10,
11]. Controversy persists regarding optimal dosing and treatment intervals, with studies like Compston et al. [
13] underscoring the lack of consensus, while Hollis [
2] emphasizes gaps in predictive biomarker identification.
This review synthesizes fourdecades of preclinical and clinical evidence (1986–2025) to critically evaluate BCG’s role in sarcoid management. We address three unresolved questions: how does BCG overcome BPV-induced immune evasion mechanisms? Can protocol standardization improve reproducibility across diverse clinical settings? What translational insights do sarcoids offer for HPV-associated human cancers? By integrating findings from studies, including randomized trials and translational research, we highlight BCG’s potential as a cost-effective immunotherapy and its alignment with One Health principles, which bridge veterinary and human oncology. Our analysis further explores combinatorial strategies (e.g., BCG + checkpoint inhibitors) and omics-driven approaches to optimize therapeutic outcomes, advocating for multidisciplinary collaboration to address current limitations.
2. Materials and Methods
This study is a narrative literature review following the framework proposed by Grant and Booth [
13], focusing on veterinary medicine and comparative oncology to synthesize multidisciplinary evidence on Bacillus Calmette-Guérin (BCG) immunotherapy for equine sarcoids. A systematic search was conducted across databases (PubMed, CAB Abstracts, Web of Science, Scopus, SciELO, Google Scholar, Capes Journals, ScienceDirect, and Veterinary Information Network) using terms like BCG immunotherapy, equine sarcoid, bovine papillomavirus, comparative oncology, and immune response, refined with Boolean operators. Inclusion criteria prioritized peer-reviewed articles and new data from the authors of this review, never published (1986–2025) addressing BCG use in equine sarcoids, including preclinical (in vitro, animal models) and clinical studies (trials, case series) focused on immuno-oncological mechanisms or translational applications, while excluding non-systematic reviews, studies without quantitative data, or those unrelated to equines/conventional therapies (e.g., surgery, cryotherapy). Study quality was assessed based on design hierarchy (RCTs > case series > experimental studies), methodological rigor (sample size, controls), and relevance to BCG’s efficacy/safety. From an initial pool of 28 studies, duplicates were removed, retaining 55 publications, reflecting limited high-level evidence. The synthesis bridged veterinary and human oncology through insights into viral immunosuppression (BPV in horses vs. HPV in humans) and aligned with the One Health framework, emphasizing interdisciplinary collaboration to address shared oncological challenges.
3. Immunological Mechanisms of BCG in Horses
The immunotherapy with Bacillus Calmette-Guérin (BCG) in equine sarcoids is rooted in its ability to modulate innate and adaptive immune responses. BCG activates macrophages and natural killer (NK) cells via binding to Toll-like receptors (TLR2 and TLR4), triggering the secretion of pro-inflammatory cytokines such as TNF-α and IL-12. These cytokines promote direct cytotoxicity against tumor cells and recruit dendritic cells to the sarcoid microenvironment. Additionally, nitric oxide production by activated macrophages induces apoptosis of neoplastic cells, while polarization toward the M1 (pro-inflammatory) phenotype inhibits tumor progression [
10,
11,
14,
15].
This innate immune activation is complemented by adaptive responses, marked by a significant increase in tumor-infiltrating CD8+ T lymphocytes. These cells, characterized by granzyme B and IFN-γ expression, recognize bovine papillomavirus (BPV) viral antigens presented via MHC-I, promoting the destruction of infected cells. BCG enhances this mechanism by stimulating antigen presentation, creating immunological memory that reduces recurrence risk. However, efficacy depends on the integrity of the MHC-I pathway, which is often compromised by BPV-induced immunosuppression [
10,
11].
Persistent challenges arise from interspecies differences. Equine TLR receptors, for example, exhibit distinct affinities for BCG components (e.g., MPB64 protein) compared to humans, potentially limiting immune response magnitude. Chronic viral immunosuppression—mediated by TLR9 downregulation and interferon pathway inhibition—creates a tumor microenvironment favoring immune evasion. These factors underscore the need for translational studies to adapt BCG protocols to equine physiology, integrating human and veterinary immunology insights. For instance, combining BCG with adjuvants that restore TLR signaling (e.g., imiquimod) or block viral immunosuppression (e.g., CRISPR-Cas9 targeting of BPV E5 oncogenes) could enhance therapeutic outcomes [
14,
15].
Understanding BCG’s immunological mechanisms in horses not only clarifies its antitumor action but also identifies targets to overcome therapeutic resistance. Such advances are critical for transforming BCG from an empirical therapy into an evidence-based strategy, particularly in regions like the Amazon Biome, where environmental factors modulate immune responses. Furthermore, comparative oncology insights—linking BPV-induced immunosuppression in horses to HPV mechanisms in humans—highlight opportunities for synergistic therapies (e.g., BCG + checkpoint inhibitors) under the One Health framework [
10,
11,
16,
17,
18,
19].
4. Clinical Efficacy and Limitations of BCG
The clinical efficacy of Bacillus Calmette-Guérin (BCG) immunotherapy in equine sarcoids is marked by promising yet heterogeneous outcomes. Studies report tumor regression rates of 40–70% with intralesional protocols, but recurrence occurs in 15–30% of cases within 12 months, reflecting inconsistent therapeutic responses. This variability is closely tied to prognostic factors such as anatomic location and histological subtype. For example, limb sarcoids show higher success rates due to reduced vascularization and ease of localized application, while periorbital lesions, with their anatomical complexity, respond less favorably. Additionally, nodular subtypes (with limited stromal infiltration) regress more effectively than fibroblastic subtypes, which exhibit greater biological aggressiveness [
10,
11,
14,
15].
The heterogeneity also stems from a lack of standardized protocols. Doses vary widely (0.1–10 mg per lesion), and while the Pasteur strain is often preferred over Tokyo for its higher immunogenicity, there is no consensus on the optimal BCG strain. Equally critical is the absence of guidelines for adjuvant use. Combinations with cryotherapy or immunotherapies, though empirically reported, lack robust validation, leaving gaps in understanding potential synergies or risks of secondary immunosuppression. For instance, cryotherapy may disrupt the tumor microenvironment, inadvertently reducing BCG’s immune activation [
10,
11,
20,
21].
Adverse effects remain a significant barrier. Severe local inflammatory reactions (e.g., edema, necrosis) occur in 15% of cases, often requiring corticosteroid intervention and treatment discontinuation. Pre-sensitized equines in endemic regions may develop systemic complications like anaphylaxis or granulomas, limiting repeated application. These challenges underscore the need for personalized protocols that balance tumor burden with individual immune status. For example, equines in the Amazon Biome face additional environmental stressors (e.g., heat, humidity) that exacerbate immunosuppression, necessitating region-specific adaptations [
14,
15,
22,
23].
Despite its potential, BCG’s clinical adoption requires advances in: a). Protocol standardization - multicenter randomized trials to optimize doses, strains, and adjuvant combinations (e.g., BCG + TLR agonists like imiquimod); b). Predictive biomarkers - omics technologies (e.g., transcriptomics) could identify resistance pathways (TGF-β, PD-L1) or stratify patients by recurrence risk; c). Innovative formulations - nanoparticles or thermostable BCG formulations to overcome logistical barriers in remote areas; d). Combination therapies - CRISPR-Cas9 editing to silence BPV oncogenes (e.g., E5) and enhance BCG efficacy. While BCG offers a cost-effective alternative to conventional therapies like cisplatin, its transformation into a reliable, scalable option hinges on bridging translational gaps between veterinary and human oncology under the One Health framework [
16,
17,
18,
19,
20,
21,
22,
23].
5. Opportunities in Comparative Oncology
Equine sarcoids, associated with bovine papillomavirus (BPV), serves as a valuable model for studying viral immunosuppression in cancer, offering direct parallels to HPV-linked human neoplasms [
24,
25]. Both viruses share immune evasion mechanisms, such as TLR9 downregulation, which impair viral DNA detection and interferon-dependent responses, facilitating tumor progression. These similarities enable equine research to not only advance veterinary therapies but also validate translational strategies for humans, such as TLR agonists to restore immune surveillance [
26].
BCG’s ability to activate macrophages and dendritic cells creates synergy with immune checkpoint inhibitors (e.g., anti-PD-1), which reverse T-cell exhaustion by blocking PD-1/PD-L1 interactions [
24,
25,
26]. Preliminary studies in equine models show enhanced tumor clearance in PD-L1-high sarcoids. DNA vaccines [
27] targeting BPV antigens (e.g., E5 oncoprotein) could further complement BCG by inducing virus-specific immunity, reducing recurrence risks an approach already explored in human cervical cancer.
Genomic signatures (e.g., PD-L1 expression, NOTCH1 mutations) and cytokine profiles (e.g., elevated IFN-γ post-treatment correlating with regression) may stratify patients likely to respond to BCG or combination therapies. Transcriptomic analysis of sarcoids pre- and post-BCG could identify resistance pathways (TGF-β, PD-L1) and guide CRISPR-Cas9 editing to silence BPV oncogenes (e.g., E5), enhancing therapeutic precision [
28,
29].
The zoonotic potential of BPV (rare human infections in veterinarians) underscores the need for integrated surveillance and therapeutic strategies that benefit both species. BCG’s cost-effectiveness compared to cisplatin aligns with One Health principles, offering scalable solutions for low-resource regions like the Amazon Biome, where environmental stressors exacerbate immunosuppression [
16,
18,
19]. Future directions are required: a). Nanoparticle formulations - thermostable BCG encapsulated in TLR agonist-loaded nanoparticles could improve targeted delivery and reduce systemic inflammation [
26]; b). Collaborative networks - partnerships between academia and industry are critical to develop BCG protocols adaptable to diverse settings, including remote areas lacking cold-chain infrastructure [
20,
21]; c). Cross-species trials - leveraging BPV-HPV parallels, clinical trials could test BCG combinations (e.g., with checkpoint inhibitors) in both equine and human patients under a unified framework [
22,
23]. By bridging veterinary and human oncology, comparative oncology accelerates therapeutic innovation, creating a virtuous cycle where discoveries in one species inform the other [
24]. This approach is pivotal to overcoming shared challenges like viral immunosuppression and tumor heterogeneity [
25].
6. Integration with the One Health Approach
The One Health framework, linking human, animal, and environmental health, is critical for optimizing BCG immunotherapy in equine sarcoids. Bovine papillomavirus (BPV), the causative agent of sarcoids, poses an emerging zoonotic risk, with documented human infections (e.g., veterinarians exposed to lesions or contaminated fomites). These cases highlight the need for integrated surveillance and strategies to reduce viral spread in shared environments, aligning animal welfare with public health priorities [
1,
2,
30,
31].
BCG is a cost-effective alternative to conventional therapies like cisplatin, offering simplified protocols and 20–40% lower recurrence rates, reducing financial strain in low-resource regions. In environments like the Amazon Biome, where heat and humidity threaten drug stability, thermostable BCG formulations and decentralized training for rural veterinarians are essential for accessibility [
9,
30,
31].
Standardized protocols (e.g., 0.5 mg BCG per lesion) and hands-on training via digital simulations improve adoption in endemic areas [
9]. Innovations like BCG-loaded nanoparticles with TLR agonists (e.g., imiquimod) enhance targeted delivery while minimizing systemic inflammation [
32]. Partnerships between academia, industry, and rural communities are key to developing cold-chain-free distribution networks in tropical regions [
33].
Genomic surveillance in BPV hotspots can preempt interspecies transmission, while BCG-adjuvant combinations (e.g., Amazonian copaiba oil) may boost efficacy in heat-stressed equines [
34]. Open-access platforms sharing BCG response rates across biomes can accelerate translational insights. These initiatives exemplify One Health principles, promoting equity, sustainability, and zoonotic risk reduction [
16,
17,
18,
19].
7. Epidemiology and BCG Use in the Amazon Biome
Equine sarcoid distribution in Brazil is heterogeneous, influenced by environmental, genetic, and management factors. In the Amazon Biome, high humidity, hematophagous vectors (e.g., horseflies), and widespread bovine papillomavirus (BPV) exposure drive sarcoid incidence, particularly among working equines in rural area. Regional studies estimate that up to 20% of equines exhibit sarcoid-like lesions, often undiagnosed due to limited access to specialized veterinary care [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
14,
15,
28,
32,
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48].
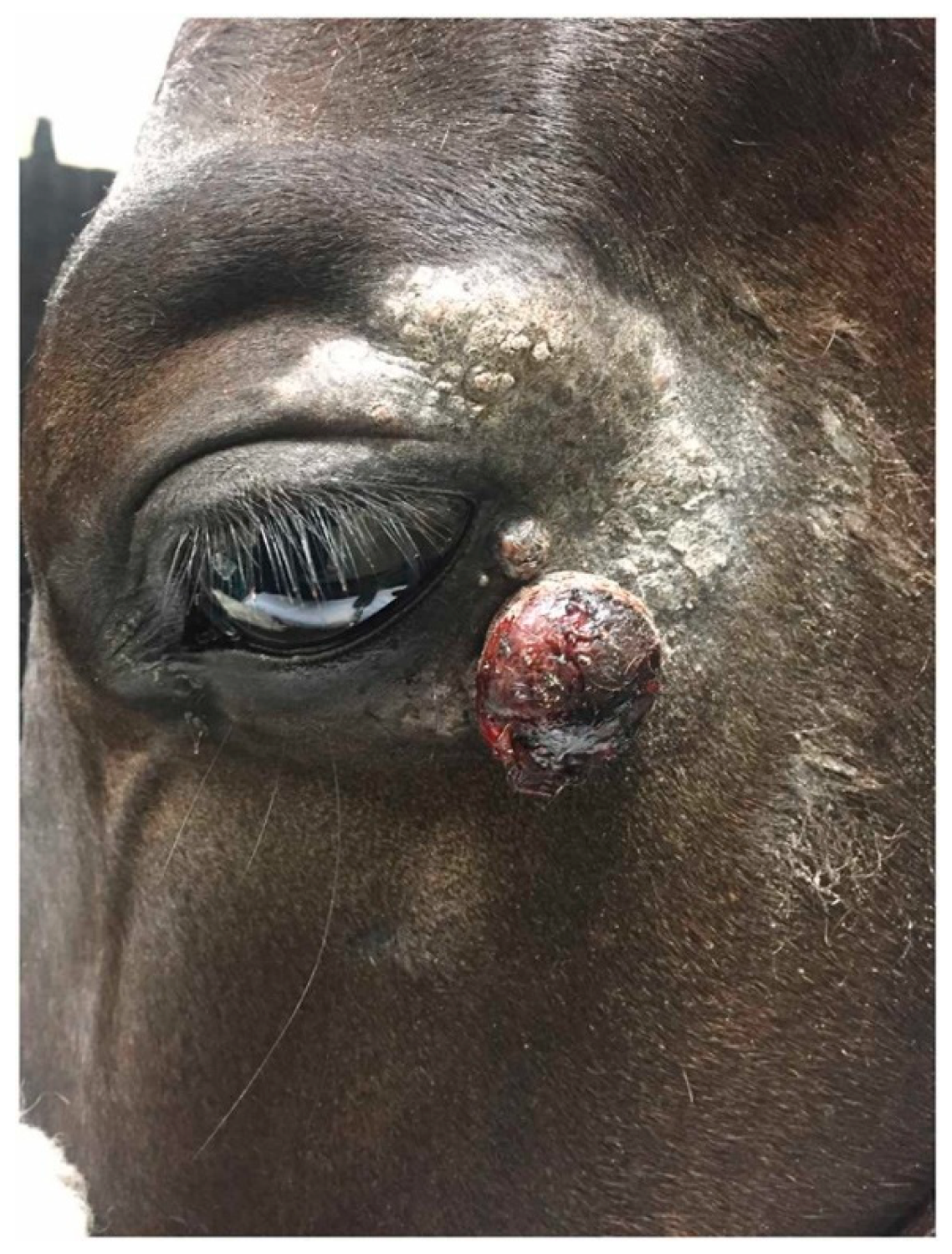
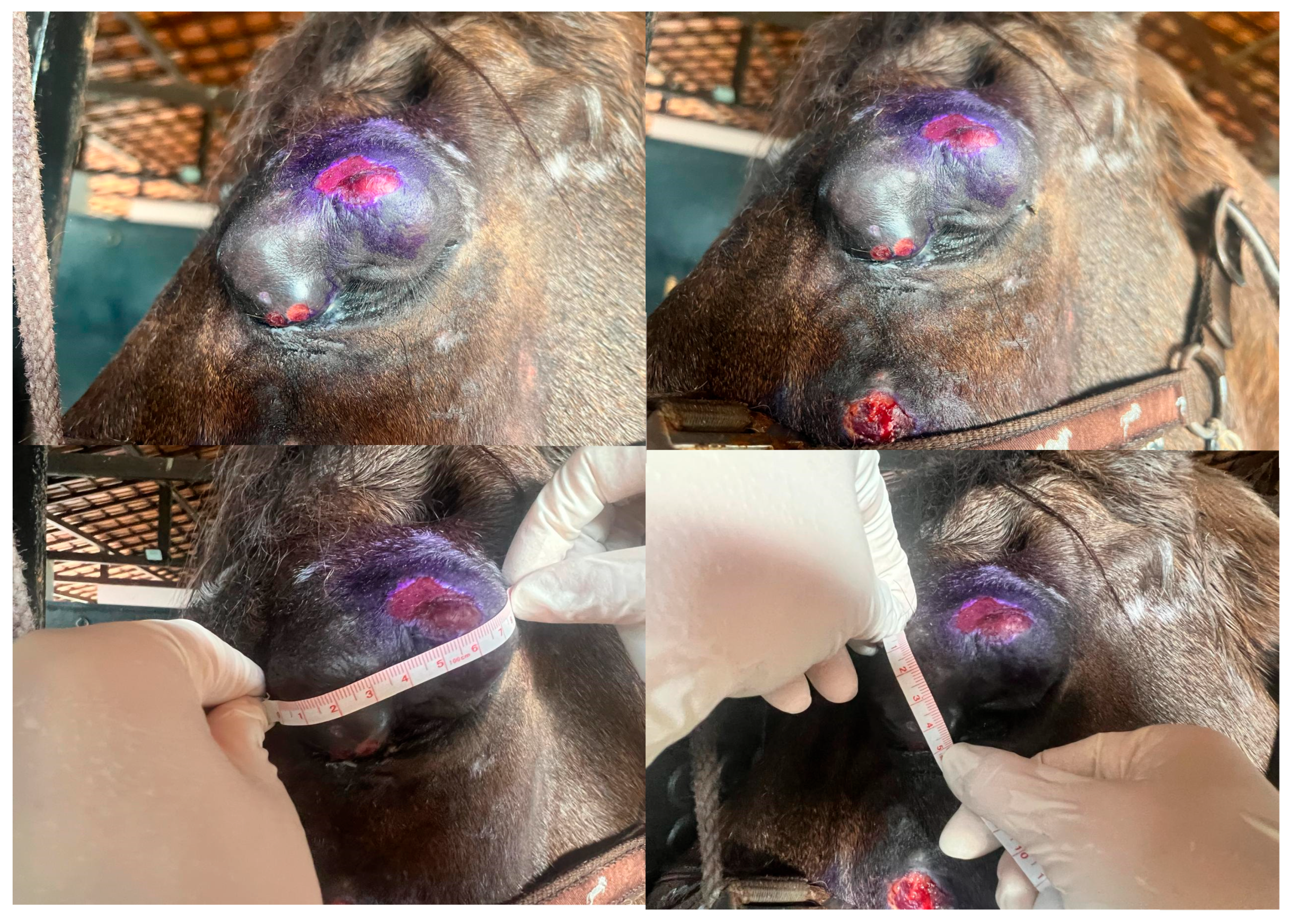
A 10-year-old Lusitano horse from the Amazon Biome was admitted to the Veterinary Hospital (HV) of the Institute of Veterinary Medicine (IMV) at the Federal University of Pará (UFPA) for evaluation of a nodular lesion affecting the upper eyelid. The mass measured approximately 9 cm in length, 5 cm in width, and 3 cm in depth, presenting a proliferative and ulcerated surface (
Figure 2). Histopathological examination of a previously collected biopsy confirmed a mesenchymal neoplasm consistent with equine sarcoid. Molecular analysis by polymerase chain reaction (PCR) identified the presence of bovine papillomavirus type 1 (BPV-1), confirming the diagnosis. Due to the considerable size of the lesion and the risk of severe eyelid dysfunction following surgical excision which could potentially require enucleation alternative treatment strategies were considered. Given the patient’s geographic location, which limited access to advanced modalities such as electrochemotherapy requiring specialized anesthesia, intralesional immunotherapy with Bacillus Calmette-Guérin (BCG) vaccine (Pasteur strain) was chosen as the primary therapeutic approach. The protocol consisted of four intralesional injections of 0.2 mL BCG administered at 21-day intervals (
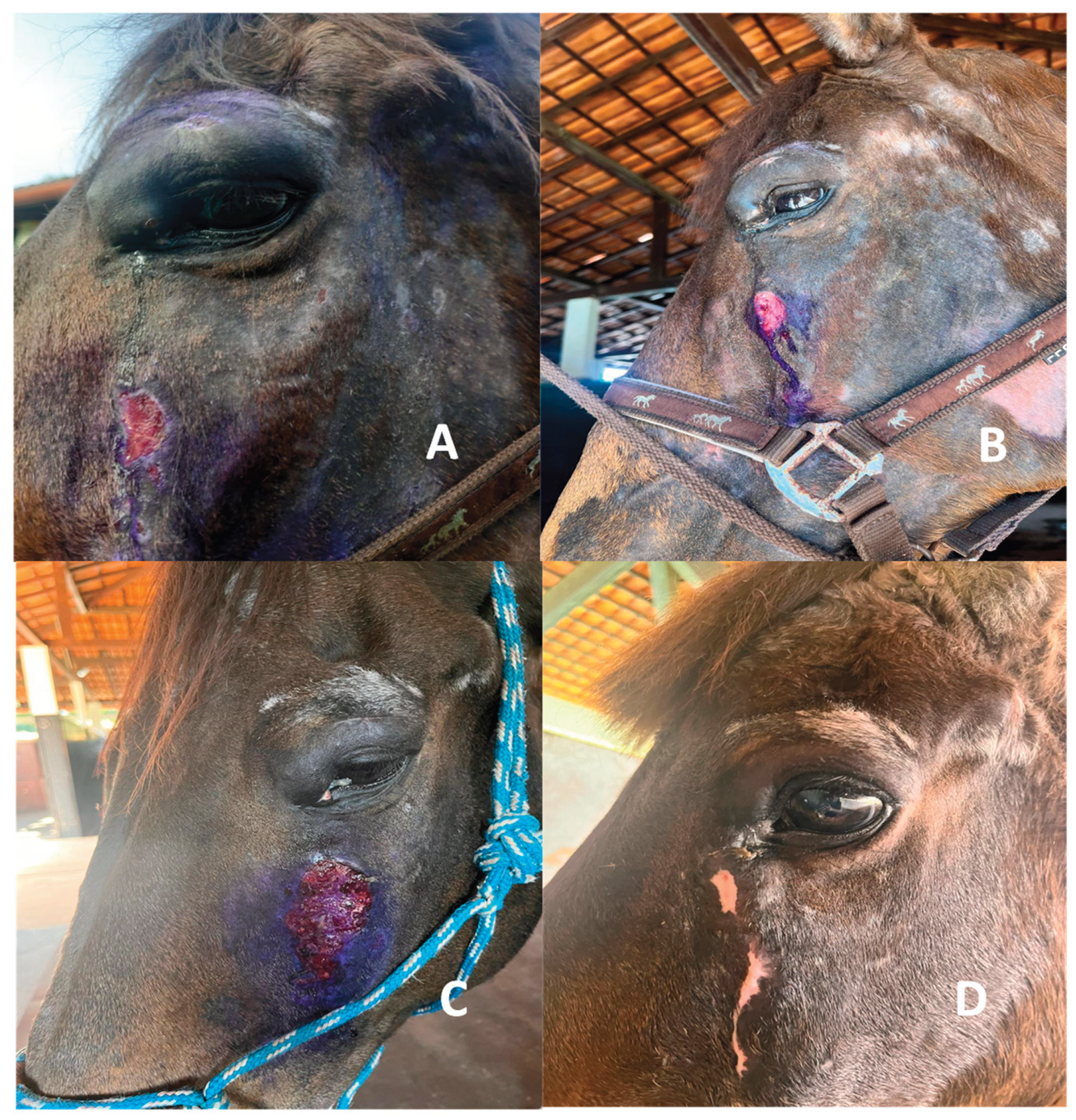
Figure 3). Significant clinical improvement was observed within the first weeks, with progressive reduction in lesion size. By the end of the treatment course, complete remission of the sarcoid was achieved, with full preservation of the ocular globe. Post-treatment ophthalmic evaluation showed no signs of corneal injury or tear film deficiency. The patient remained free of recurrence during a two-year follow-up period.
BCG has emerged as a cost-effective intralesional therapy in Brazil, including the Amazon, due to its adaptability to resource-limited settings. However, implementation faces unique challenges: logistical constraints (e.g., cold-chain maintenance in remote areas), workforce gaps (few veterinarians trained in immunotherapy), and reliance on traditional herbal remedies that delay conventional treatment. Despite these barriers, BCG’s simplified protocols (e.g., 0.1–0.5 mg per lesion) and lower recurrence rates compared to conventional therapies highlight its potential for scalable adoption [
15,
34,
37,
38,
42,
49].
Effective BCG adoption requires multifocal strategies. Farmer education programs on early lesion identification and training agricultural technicians to administer BCG could reduce disease progression. Research is urgently needed to validate climate-adapted protocols, such as thermostable BCG formulations and combinations with plant-derived adjuvants (e.g., copaiba oil, which has immunomodulatory properties). These initiatives align with One Health principles, mitigating zoonotic BPV risks (linked to human infections in veterinarians) while enhancing climate-resilient animal health systems [
15,
34,
37,
38,
42,
49].
Integrating HV case studies (e.g., lesion regression rates, adverse effects) would refine region-specific protocols and address knowledge gaps. Partnerships with local universities and NGOs are critical to decentralize BCG training and improve cold-chain infrastructure. Additionally, genomic surveillance in BPV hotspots and open-access platforms for clinical data sharing could accelerate translational insights for both veterinary and human oncology. By bridging gaps between ecosystems and species, BCG immunotherapy exemplifies the One Health ethos, offering equitable and sustainable solutions for zoonotic risk reduction [
50,
51,
52,
53].
8. Cost-Benefit Comparison of BCG vs. Conventional Therapies
Conventional treatments for equine sarcoids (
Table 1), surgical excision, electrochemotherapy, cryotherapy, and topical cisplatin show success rates of 50–80% but suffer from high recurrence (20–40%), particularly in aggressive subtypes like fibroblastic sarcoids Surgery, while rapid, requires specialized infrastructure and postoperative care, increasing costs in remote regions. Topical cisplatin, effective in 70–85% of cases, demands 4–6 applications and strict biosafety protocols due to systemic toxicity, making it impractical for small-scale farms or nonspecialized practitioners. While cisplatin-based electrochemotherapy demonstrates satisfactory outcomes in equine tumor management, the requirement for general anesthesia substantially increases both procedural costs and risks. Potential anesthetic complications and recovery-related adverse events represent significant limitations for equine patients. Surgical excision followed by topical Acyclovir dressings has also been reported with favorable success rates in equine sarcoid treatment. However, this approach similarly requires a preliminary surgical procedure [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12]. BCG immunotherapy offers moderate efficacy (60–70% tumor regression) but superior economic benefits. Its cost per dose is up to 10 times lower than cisplatin, and it requires only 1–3 intralesional sessions, reducing logistical expenses (transport, materials) BCG does not require advanced equipment or chemical protection, simplifying use in rural areas. Studies emphasize its cost-effectiveness in endemic outbreaks, where durable immunity reduces recurrence compared to cytotoxic like cisplatin [
14,
15,
28,
32]
BCG’s efficacy varies with tumor location and subtype. Lesions in critical areas (e.g., eyelids) or verrucous subtypes respond poorly, often requiring adjunct therapies (e.g., cryotherapy), which increase costs. Adverse reactions (edema, abscesses) occur in 30% of cases, necessitating additional veterinary care. Despite these challenges, large-scale cost-benefit analyses confirm BCG’s economic advantage over cisplatin, especially in herds with multiple affected animals, where reduced cumulative recurrence offsets side-effect management costs. Cisplatin requires specialized training for safe handling, while BCG can be administered by veterinarians with basic training using simplified protocols (e.g., 0.1–0.5 mg per lesion). This accessibility aligns BCG with One Health principles, promoting equitable solutions in low-resource settings [
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48].
9. Future Directions, Gaps and Novel Insights
BCG immunotherapy for equine sarcoids represents a promising yet underexplored frontier in veterinary oncology. Despite regression rates of 50–70%, individual response variability remains a critical challenge. Key gaps include incomplete understanding of BCG’s mechanisms in equines, such as how macrophage and dendritic cell activation counteracts BPV-induced immunosuppression in specific tumor microenvironments Additionally, the lack of standardized dosing (0.1–1 mg/lesion) and application frequency (weekly vs. biweekly) hinders cross-study comparisons, underscoring the need for multicenter randomized trials, particularly in complex biomes like the Amazon, where environmental factors modulate immune responses [
3,
10,
11,
14,
15].
Emerging omics technologies offer transformative insights. Transcriptomic analysis of sarcoids pre- and post-BCG could identify resistance pathways (e.g., TGF-β signaling or PD-L1 expression) and predict candidates for combinatorial therapies. CRISPR-Cas9 editing to silence BPV viral genes (e.g., E5), which mediate immune evasion, may enhance BCG efficacy. Dynamic biomarkers, such as exosomal pro-inflammatory microRNAs in blood, could enable noninvasive real-time monitoring of therapeutic responses [
29].
Innovative delivery systems like BCG-loaded nanoparticles combined with TLR agonists (e.g., imiquimod) may improve drug targeting to tumor microenvironments, reducing systemic effects and enhancing compliance in cases of multiple lesions. Adjuvant strategies, such as immunomodulatory probiotics to amplify Th1 responses, could synergize with BCG, leveraging insights from human oncology. These approaches align with One Health principles, bridging veterinary and human cancer research to address shared challenges like viral immunosuppression [
20,
21,
54,
55,
56].
Unlocking BCG’s full potential requires overcoming practical barriers. Collaborative networks among universities, field clinics, and pharmaceutical industries could accelerate the development of thermostable lyophilized BCG formulations, eliminating cold-chain dependence in tropical regions. Concurrently, continuing education programs must train veterinarians in immunotherapy techniques, including simulations for complex cases (e.g., periocular sarcoids) and adverse reaction management. Such efforts democratize access to advanced therapies while fostering climate-resilient animal health systems [
3,
4,
9].
9. Conclusion
BCG immunotherapy consolidates as a transformative yet underutilized strategy for equine sarcoids, achieving 40–70% tumor regression through dual activation of innate immunity (TLR-mediated macrophage polarization, TNF-α release) and adaptive responses (BPV-specific CD8+ T cells), while addressing critical One Health challenges in resource-limited settings like the Amazon Biome. Despite persistent gaps—including protocol heterogeneity (0.1–1 mg/lesion dosing variability), strain-dependent efficacy (Pasteur vs. Tokyo), and severe inflammation in 15–30% of cases—BCG outperforms conventional therapies (cryotherapy, cisplatin) in cost-effectiveness (10x cheaper per dose) and scalability, particularly for endemic outbreaks. Emerging omics technologies, such as CRISPR-Cas9 editing of BPV’s immune-evasion genes (e.g., E5) and transcriptomic profiling of TGF-β/PD-L1 pathways, now enable precision protocols to overcome viral immunosuppression, while thermostable nanoparticle formulations promise equitable access across tropical regions. Crucially, sarcoids serve as a translational bridge to HPV-associated cancers, with synergistic strategies like BCG + anti-PD-1 inhibitors demonstrating cross-species potential. To unlock this promise, multicenter randomized trials must standardize protocols (dose, frequency) and validate dynamic biomarkers (e.g., exosomal miRNAs), supported by collaborative networks linking academia, pharmaceutical innovators, and field clinics to development independent BCG formulations and train veterinarians in adverse reaction management. Thus, BCG transcends species boundaries, epitomizing a sustainable, One Health-aligned solution where veterinary advances catalyze breakthroughs in human immuno-oncology.
Author Contributions
Conceptualization, R.M.C.T. and F.M.S..; methodology, B.J.S.d.S., E.L.A.d.C., A.J.M.P., R.T., R.M.C.T. and F.M.S.; formal analysis, B.J.S.d.S., E.L.A.d.C., A.J.M.P., R.T., R.M.C.T. and F.M.S.; investigation, B.J.S.d.S., E.L.A.d.C., A.J.M.P., R.T., R.M.C.T. and F.M.S.; data curation, B.J.S.d.S., E.L.A.d.C., A.J.M.P., R.T., R.M.C.T. and F.M.S.; writing—original draft preparation, B.J.S.d.S., E.L.A.d.C. and A.J.M.P., writing—review and editing, R.T., R.M.C.T. and F.M.S.; supervision, R.M.C.T. and F.M.S.; project administration, R.M.C.T. and F.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol number Nº 6261300323 (ID 002208), approval date: 27 April 2023 was approved by the National Council for Control of Animal Experimentation (CONCEA) and was approved by the Ethics Committee on Animal Use of the Federal University of Para (CEUA/UFPA).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors are grateful to CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), FAPESPA (Fundação Amazônia de Amparo a Estudos e Pesquisas do Estado do Pará), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Goodrich, L.; Gerber, H.; Marti, E.; Antczak, D.F. Equine Sarcoids. Vet. Clin. N. Am. Equine Pract. 1998, 14, 607–623. [CrossRef]
- Hollis, A.R. Management of Equine Sarcoids. Vet. J. 2023, 291, 105926. [CrossRef]
- Ogłuszka, M.; Starzyński, R.R.; Pierzchała, M.; Otrocka-Domagała, I.; Raś, A. Equine Sarcoids—Causes, Molecular Changes, and Clinicopathologic Features: A Review. Vet. Pathol. 2021, 58, 472–482. [CrossRef]
- Taylor, S.; Haldorson, G. A Review of Equine Sarcoid. Equine Vet. Educ. 2013, 25, 210–216. [CrossRef]
- Tamzali, Y.; Borde, L.; Rols, M.P.; Golzio, M.; Lyazrhi, F.; Teissie, J. Successful Treatment of Equine Sarcoids with Cisplatin Electrochemotherapy: A Retrospective Study of 48 Cases. Equine Vet. J. 2012, 44, 214–220. [CrossRef]
- Byam-Cook, K.L.; Henson, F.M.; Slater, J.D. Treatment of Periocular and Non-Ocular Sarcoids in 18 Horses by Interstitial Brachytherapy with Iridium-192. Vet. Rec. 2006, 159, 337–341. [CrossRef]
- Altamura, G.; Strazzullo, M.; Corteggio, A.; Francioso, R.; Roperto, F.; D’Esposito, M.; Roperto, S. O(6)-Methylguanine-DNA Methyltransferase in Equine Sarcoids: Molecular and Epigenetic Analysis. BMC Vet. Res. 2012, 8, 218. [CrossRef]
- Jindra, C.; Hainisch, E.K.; Brandt, S. Immunotherapy of Equine Sarcoids—From Early Approaches to Innovative Vaccines. Vaccines 2023, 11, 769. [CrossRef]
- Offer, K.S.; Dixon, C.E.; Sutton, D.G.M. Treatment of Equine Sarcoids: A Systematic Review. Equine Vet. J. 2024, 56, 12–25. [CrossRef]
- Vanselow, B.A.; Abetz, I.; Jackson, A.R. BCG Emulsion Immunotherapy of Equine Sarcoid. Equine Vet. J. 1988, 20, 444–447. [CrossRef]
- Martens, A.; De Moor, A.; Vlaminck, L.; Pille, F.; Steenhaut, M. Evaluation of Excision, Cryosurgery and Local BCG Vaccination for the Treatment of Equine Sarcoids. Vet. Rec. 2001, 149, 665–669. [CrossRef]
- Compston, P.C.; Turner, T.; Wylie, C.E.; Payne, R.J. Laser Surgery as a Treatment for Histologically Confirmed Sarcoids in the Horse. Equine Vet. J. 2016, 48, 451–456. [CrossRef]
- Grant, M.J.; Booth, A. A Typology of Reviews: An Analysis of 14 Review Types and Associated Methodologies. Health Inf. Libr. J. 2009, 26, 91–108. [CrossRef]
- Klein, W.R.; Bras, G.E.; Misdorp, W.; Steerenberg, P.A.; de Jong, W.H.; Tiesjema, R.H.; Kersjes, A.W.; Ruitenberg, E.J. Equine Sarcoid: BCG Immunotherapy Compared to Cryosurgery in a Prospective Randomised Clinical Trial. Cancer Immunol. Immunother. 1986, 21, 133–140. [CrossRef]
- Carvalho, M.C.P.; Almeida, G.B.; Vieira, R.R.; Oliveira, A.F.; Silva, F.L. Imunomodulação com vacina BCG para tratamento de sarcoide em um equino. In Proceedings of the XI Simpósio Internacional do Cavalo Atleta, Belo Horizonte, MG, Brazil, 2023. Available online: https://www.even3.com.br/anais/xi-simcav-304492/623917-IMUNOMODULACAO-COM-VACINA-BCG-PARA-TRATAMENTO-DE--SARCOIDE-EM-UM-EQUINO (accessed on 9 April 2025).
- Verkuijl, C.; Smit, J.; Green, J.M.H.; Nordquist, R.E.; Sebo, J.; Hayek, M.N.; Hötzel, M.J. Climate change, public health, and animal welfare: towards a One Health approach to reducing animal agriculture’s climate footprint. Front. Anim. Sci. 2024, 5, 1281450. [CrossRef]
- Danasekaran, R. One Health: A Holistic Approach to Tackling Global Health Issues. Indian J. Community Med. 2024, 49, 260–263. [CrossRef]
- Ahmed, M.M.; Okesanya, O.J.; Othman, Z.K.; Ibrahim, A.M.; Adigun, O.A.; Ukoaka, B.M.; Abdi, M.I.; Lucero-Prisno, D.E. Holistic Approaches to Zoonoses: Integrating Public Health, Policy, and One Health in a Dynamic Global Context. Zoonotic Dis. 2025, 5, 5. [CrossRef]
- Horefti, E. The Importance of the One Health Concept in Combating Zoonoses. Pathogens 2023, 12, 977. [CrossRef]
- Singh, S.; Saavedra-Avila, N.A.; Tiwari, S.; Porcelli, S.A. A Century of BCG Vaccination: Immune Mechanisms, Animal Models, Non-Traditional Routes and Implications for COVID-19. Front. Immunol. 2022, 13, 959656. [CrossRef]
- Lidagoster, S.; Ben-David, R.; De Leon, B.; Sfakianos, J.P. BCG and Alternative Therapies to BCG Therapy for Non-Muscle-Invasive Bladder Cancer. Curr. Oncol. 2024, 31, 1063–1078. [CrossRef]
- Pereira, M.; Paixão, E.; Trajman, A.; de Souza, R.A.; da Natividade, M.S.; Pescarini, J.M.; Pereira, S.M.; Barreto, F.R.; Ximenes, R.; Dalcomo, M.; Ichihara, M.Y.; Nunes, C.; Barral-Netto, M.; Barreto, M.L. The Need for Fast-Track, High-Quality and Low-Cost Studies about the Role of the BCG Vaccine in the Fight against COVID-19. Respir. Res. 2020, 21, 178. [CrossRef]
- Hannouneh, Z.A.; Hijazi, A.; Alsaleem, A.A.; Hami, S.; Kheyrbek, N.; Tanous, F.; Khaddour, K.; Abbas, A.; Alshehabi, Z. Novel immuno-therapeutic options for BCG-unresponsive high-risk non-muscle-invasive bladder cancer. Cancer Med. 2023, 12, 21944–21968. [CrossRef]
- Sultan, F.; Ganaie, B.A. Comparative Oncology: Integrating Human and Veterinary Medicine. Open Vet. J. 2018, 8, 25–34. [CrossRef]
- Youssef, E.; Palmer, D.; Fletcher, B.; Vaughn, R. Exosomes in Precision Oncology and Beyond: From Bench to Bedside in Diagnostics and Therapeutics. Cancers 2025, 17, 940. [CrossRef]
- Kader, M.; Smith, A.P.; Guiducci, C.; Wonderlich, E.R.; Normolle, D.; Watkins, S.C.; Barrat, F.J.; Barratt-Boyes, S.M. Blocking TLR7- and TLR9-Mediated IFN-α Production by Plasmacytoid Dendritic Cells Does Not Diminish Immune Activation in Early SIV Infection. PLoS Pathog. 2013, 9, e1003530. [CrossRef]
- Pandya, A.; Shah, Y.; Kothari, N.; Postwala, H.; Shah, A.; Parekh, P.; Chorawala, M.R. The Future of Cancer Immunotherapy: DNA Vaccines Leading the Way. Med. Oncol. 2023, 40, 200. [CrossRef]
- Semik, E.; Gurgul, A.; Ząbek, T.; Ropka-Molik, K.; Koch, C.; Mählmann, K.; Bugno-Poniewierska, M. Transcriptome analysis of equine sarcoids. Vet. Comp. Oncol. 2017, 15, 1370–1381. [CrossRef]
- Feng, X.; Li, Z.; Liu, Y.; Chen, D.; Zhou, Z. CRISPR/Cas9 technology for advancements in cancer immunotherapy: from uncovering regulatory mechanisms to therapeutic applications. Exp. Hematol. Oncol. 2024, 13, 102. [CrossRef]
- Coman, M.A.; Marcu, A.; Chereches, R.M.; Leppälä, J.; Van Den Broucke, S. Educational Interventions to Improve Safety and Health Literacy Among Agricultural Workers: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 1114. [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; Saadh, M.J.; Amajd, A.; Abo-Zaid, M.A.; Castillo-Acobo, R.Y.; Ismail, A.H.; Amin, A.H.; Akhavan-Sigari, R. Progressing nanotechnology to improve targeted cancer treatment: overcoming hurdles in its clinical implementation. Mol. Cancer 2023, 22, 169. [CrossRef]
- Nogueira, S.A.; Torres, S.M.; Malone, E.D.; Diaz, S.F.; Jessen, C.; Gilbert, S. Efficacy of imiquimod 5% cream in the treatment of equine sarcoids: A pilot study. Vet. Dermatol. 2006, 17, 259–265. [CrossRef]
- Pagliusi, S.; Che, Y.; Dong, S. The art of partnerships for vaccines. Vaccine 2019, 37, 5909–5919. [CrossRef]
- Lorga, A.D.; Amaro, F.; Gomes, A.R.C.; Cocco, M.; Gularte, A.; Silva, Y.; Silva, J. Application of Euphorbia tirucalli Sap in Sarcoid Treatment in Horses – Case Report. Arq. Bras. Med. Vet. Zootec. 2022, 74, 509–513. [CrossRef]
- Knottenbelt, D.C.; Kelly, D.F. The Diagnosis and Treatment of Periorbital Sarcoid in the Horse: 445 Cases from 1974 to 1999. Vet. Ophthalmol. 2000, 3, 169–191. [CrossRef]
- Knottenbelt, D.C. The Equine Sarcoid: Why Are There So Many Treatment Options? Vet. Clin. North Am. Equine Pract. 2019, 35, 243–262. [CrossRef]
- Brum, J.S.; Souza, T.M.; Barros, C.S.L. Aspectos epidemiológicos e distribuição anatômica das diferentes formas clínicas do sarcoide equino no Rio Grande do Sul: 40 casos. Pesqui. Vet. Bras. 2010, 30, 839–843. [CrossRef]
- Anjos, B.L.; Silva, M.S.; Diefenbach, A.; Brito, M.F.; Seppa, G.S.; Brum, M.C.S. Sarcoide equino associado ao papilomavírus bovino BR-UEL-4. Ciênc. Rural 2010, 40, 1456–1459. [CrossRef]
- Alcântara, B.K.; Alfieri, A.A.; Headley, S.A.; Rodrigues, W.B.; Otonel, R.A.A.; Lunardi, M.; Alfieri, A.F. Caracterização molecular de DNA de Delta papillomavirus bovino (BPV1, 2 e 13) em sarcoides equinos. Pesqui. Vet. Bras. 2015, 35, 431–436. [CrossRef]
- Munday, J.S.; Orbell, G.; Fairley, R.A.; Hardcastle, M.; Vaatstra, B. Evidence from a Series of 104 Equine Sarcoids Suggests That Most Sarcoids in New Zealand Are Caused by Bovine Papillomavirus Type 2, although Both BPV1 and BPV2 DNA Are Detectable in around 10% of Sarcoids. Animals 2021, 11, 3093. [CrossRef]
- Karalus, W.; Subharat, S.; Orbell, G.; Vaatstra, B.; Munday, J.S. Equine Sarcoids: A Clinicopathologic Study of 49 Cases, with Mitotic Count and Clinical Type Predictive of Recurrence. Vet. Pathol. 2024, 61, 357–365. [CrossRef]
- Bogaert, L.; Martens, A.; Van Poucke, M.; Ducatelle, R.; De Cock, H.; Dewulf, J.; De Baere, C.; Peelman, L.; Gasthuys, F. High prevalence of bovine papillomaviral DNA in the normal skin of equine sarcoid-affected and healthy horses. Vet. Microbiol. 2008, 129, 58–68. [CrossRef]
- McConaghy, F.F.; Davis, R.E.; Reppas, G.P.; Rawlinson, R.J.; McClintock, S.A.; Hutchins, D.R.; Hodgson, D.R. Management of equine sarcoids: 1975–93. N. Z. Vet. J. 1994, 42, 180–184. [CrossRef]
- Stewart, A.A.; Rush, B.; Davis, E. The efficacy of intratumoural 5-fluorouracil for the treatment of equine sarcoids. Aust. Vet. J. 2006, 84, 101–106. [CrossRef]
- Stadler, S.; Kainzbauer, C.; Haralambus, R.; Brehm, W.; Hainisch, E.; Brandt, S. Successful treatment of equine sarcoids by topical aciclovir application. Vet. Rec. 2011, 168, 187. [CrossRef]
- Walker, M.; Adams, W.; Hoskinson, J.; Held, J.P.; Blackford, J.; Geiser, D.; Henton, J. Iridium-192 brachytherapy for equine sarcoid, one and two year remission rates. Vet. Radiol. 1991, 32, 206–208. [CrossRef]
- Spoormakers, T.J.; Klein, W.R.; Jacobs, J.J.; Van Den Ingh, T.S.; Koten, J.W.; Den Otter, W. Comparison of the efficacy of local treatment of equine sarcoids with IL-2 or cisplatin/IL-2. Cancer Immunol. Immunother. 2003, 52, 179–184. [CrossRef]
- Berruex, F.; Gerber, V.; Wohlfender, F.D.; Burger, D.; Koch, C. Clinical course of sarcoids in 61 Franches-Montagnes horses over a 5–7 year period. Vet. Q. 2016, 36, 189–196. [CrossRef]
- Assis-Brasil, N.D.; Marcolongo-Pereira, C.; Stigger, A.L.; Fiss, L.; Santos, B.L.; Coelho, A.C.B.; Sallis, E.S.V.; Fernandes, C.G.; Schild, A.L. Equine Dermatopathies in Southern Brazil: A Study of 710 Cases. Ciênc. Rural 2015, 45, 519–524. [CrossRef]
- Baust, J.G.; Gage, A.A. The Molecular Basis of Cryosurgery. BJU Int. 2005, 95, 1187–1191. [CrossRef]
- Schaffer, P.A.; Wobeser, B.; Martin, L.E.; Dennis, M.M.; Duncan, C.G. Cutaneous Neoplastic Lesions of Equids in the Central United States and Canada: 3,351 Biopsy Specimens from 3,272 Equids (2000–2010). J. Am. Vet. Med. Assoc. 2013, 242, 99–104. [CrossRef]
- Ireland, J.L.; Wylie, C.E.; Collins, S.N.; Verheyen, K.L.; Newton, J.R. Preventive Health Care and Owner-Reported Disease Prevalence of Horses and Ponies in Great Britain. Res. Vet. Sci. 2013, 95, 418–424. [CrossRef]
- Aminin, D.; Wang, Y.M. Macrophages as a “Weapon” in Anticancer Cellular Immunotherapy. Kaohsiung J. Med. Sci. 2021, 37, 749–758. [CrossRef]
- Perez-Penco, M.; Byrdal, M.; Lara de la Torre, L.; et al. The Antitumor Activity of TGFβ-Specific T Cells Is Dependent on IL-6 Signaling. Cell Mol. Immunol. 2025, 22, 111–126. [CrossRef]
- Collinet, A.; Grimm, P.; Jacotot, E.; Julliand, V. Biomarkers for monitoring the equine large intestinal inflammatory response to stress-induced dysbiosis and probiotic supplementation. J. Anim. Sci. 2022, 100, skac268. [CrossRef]
- Kober, A.K.M.H.; Riaz Rajoka, M.S.; Mehwish, H.M.; Villena, J.; Kitazawa, H. Immunomodulation Potential of Probiotics: A Novel Strategy for Improving Livestock Health, Immunity, and Productivity. Microorganisms 2022, 10, 388. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).