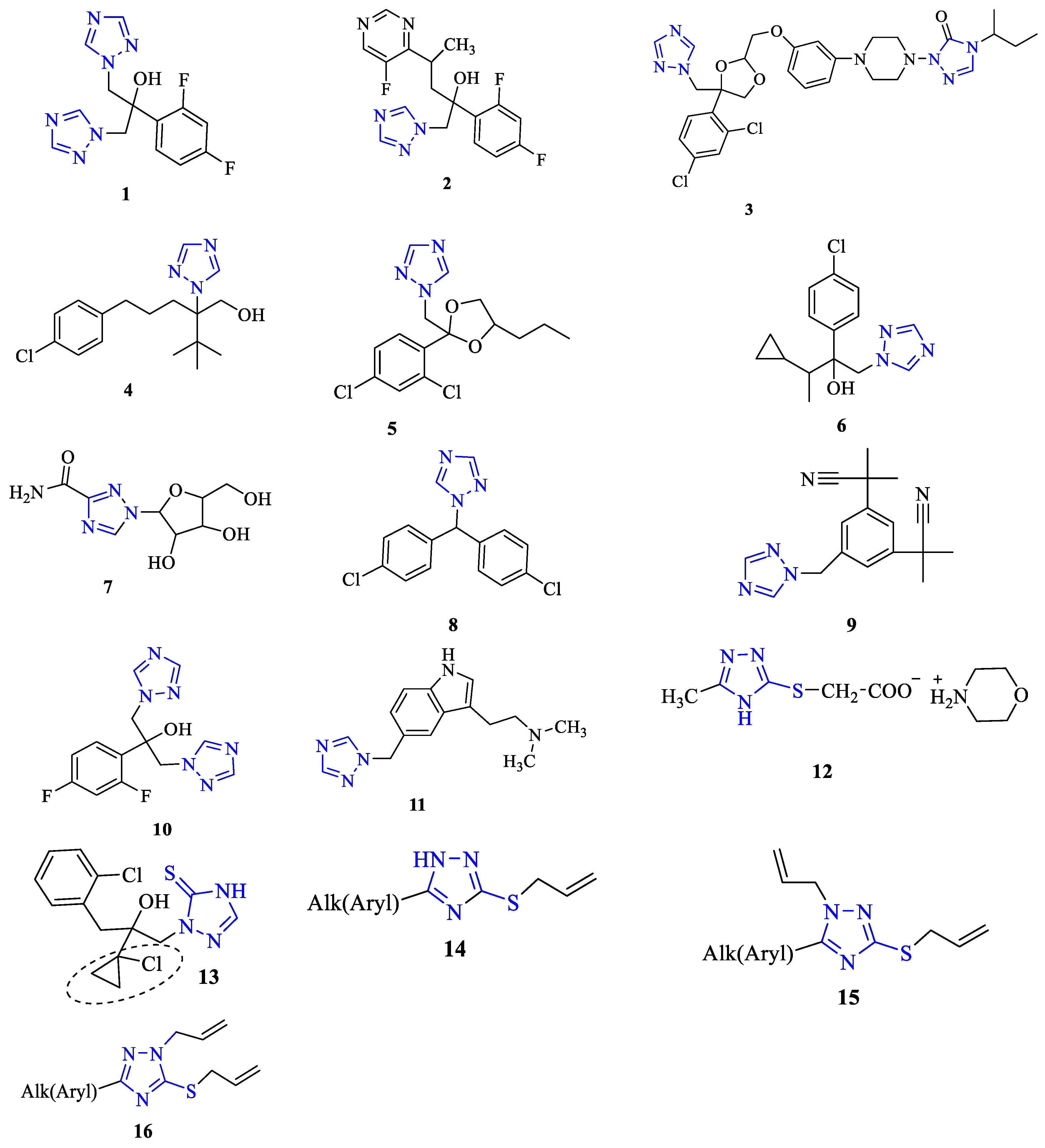
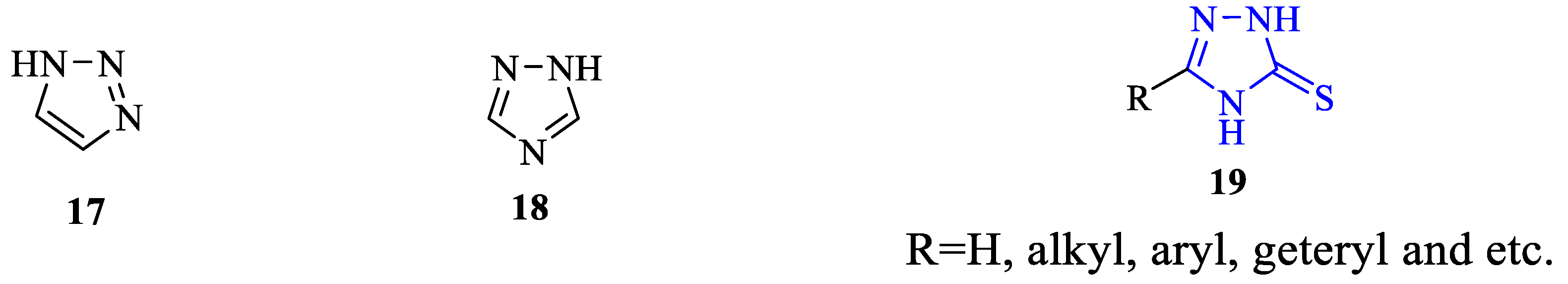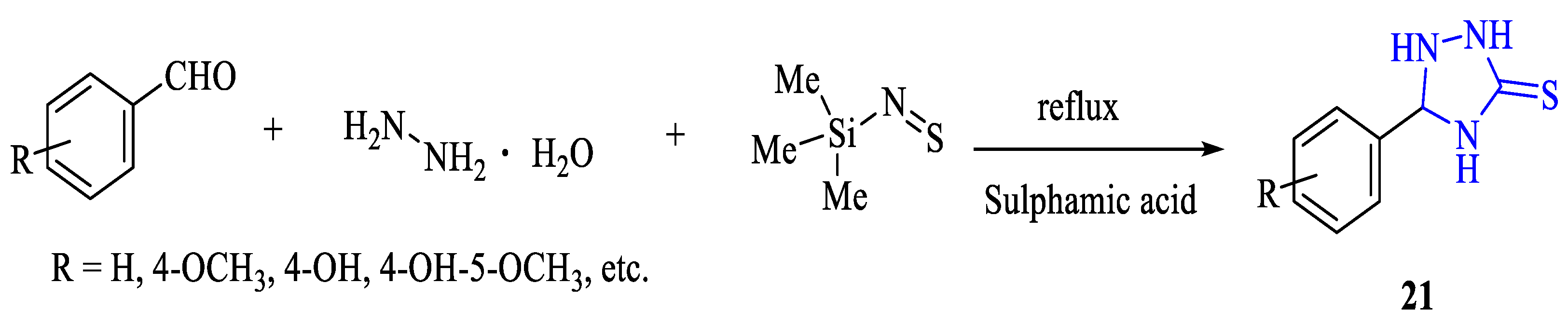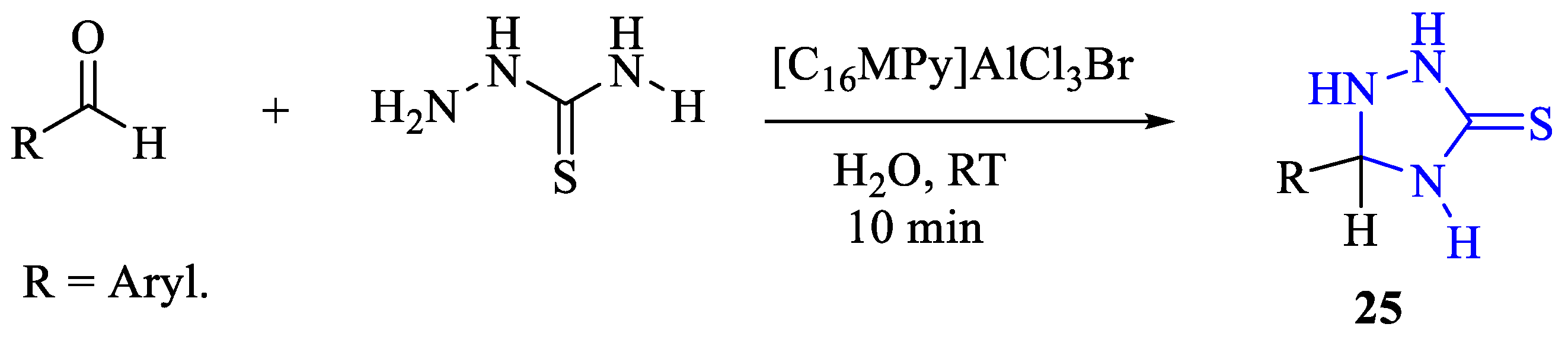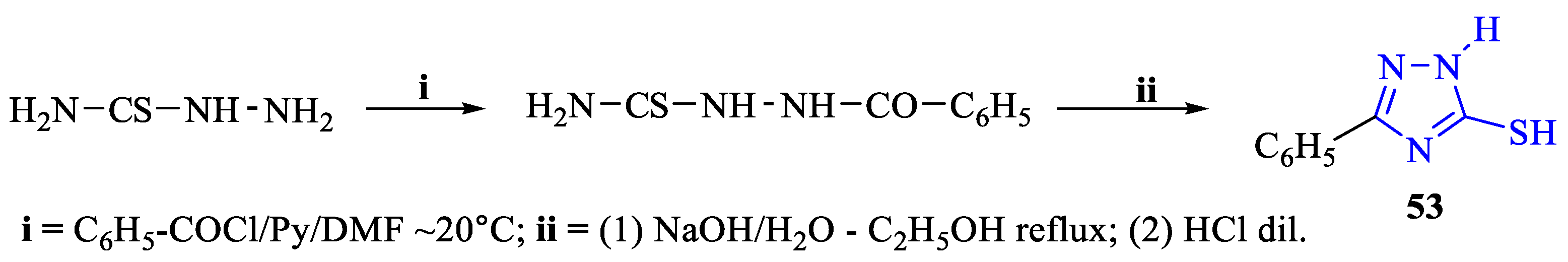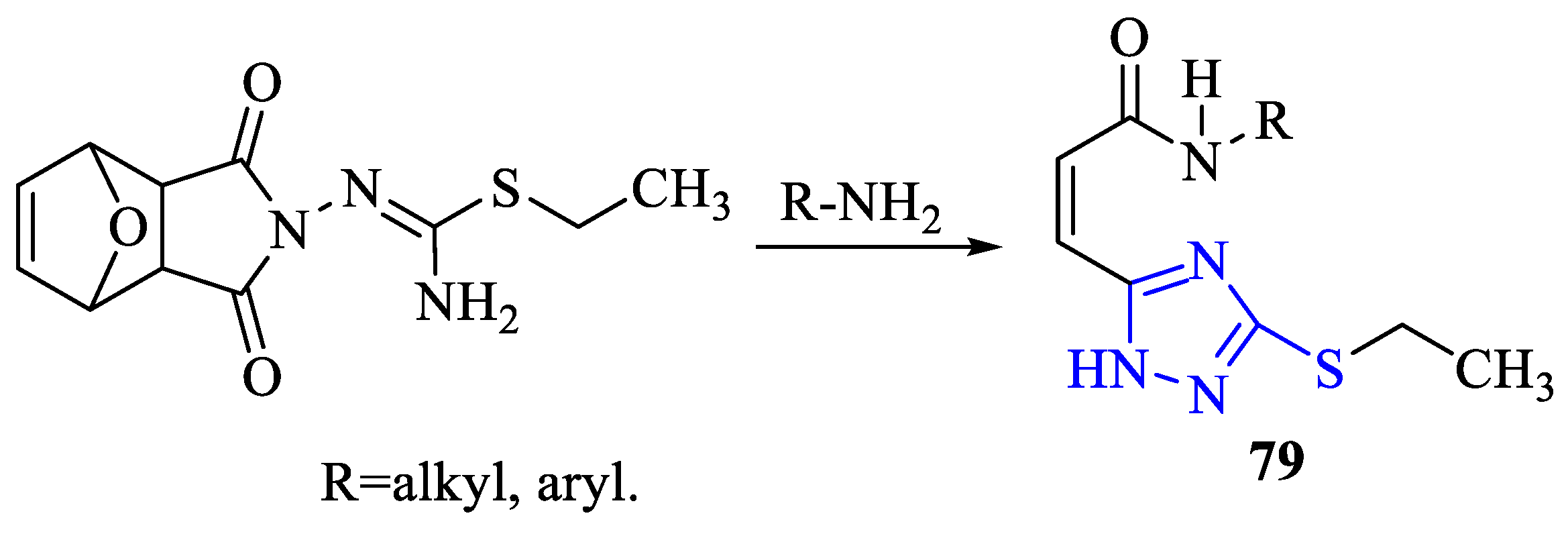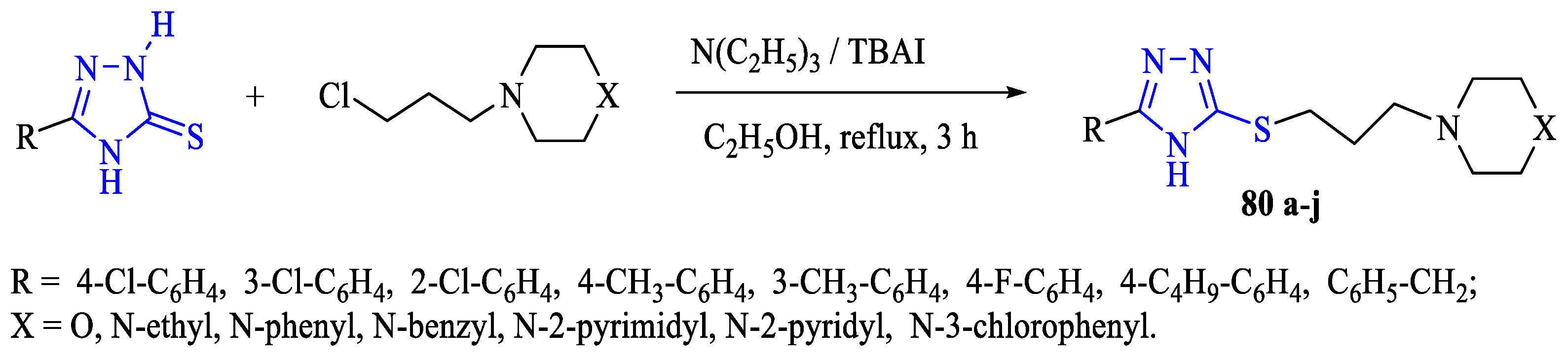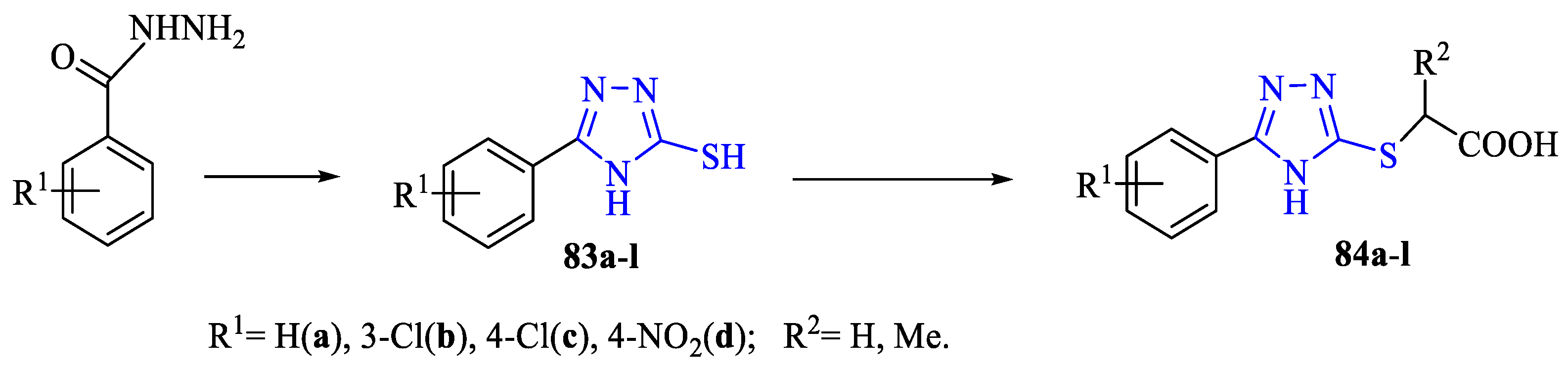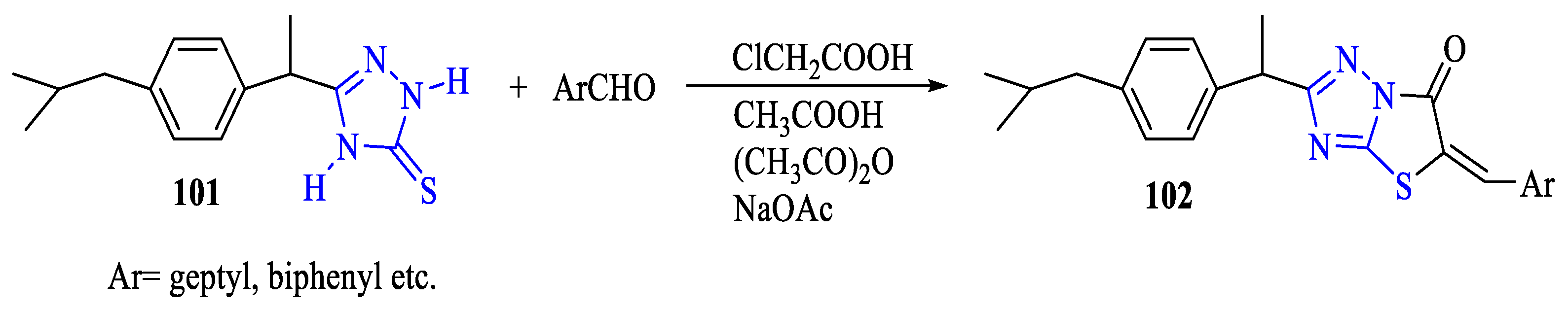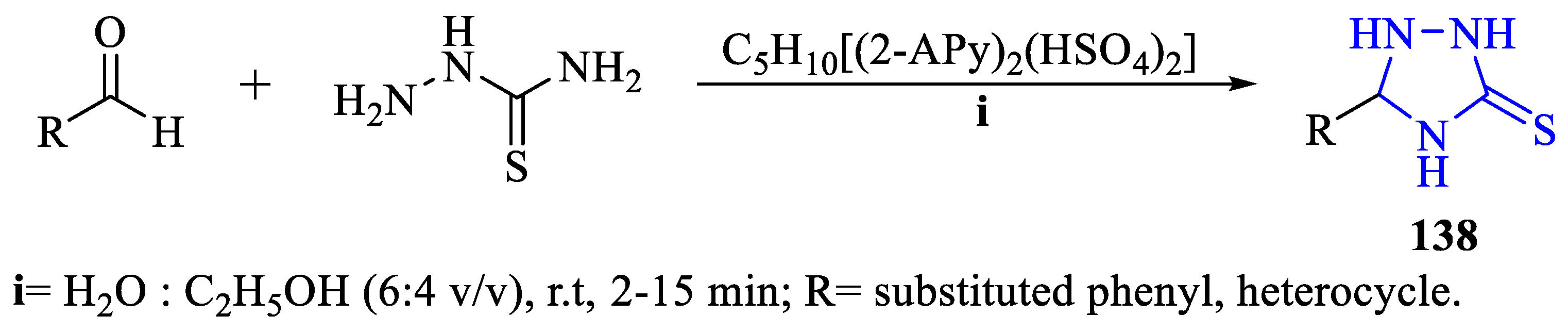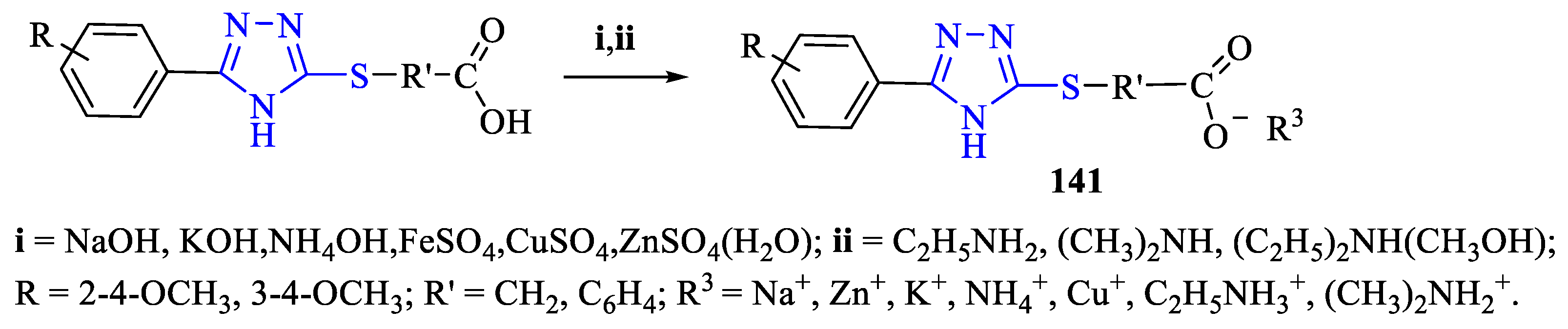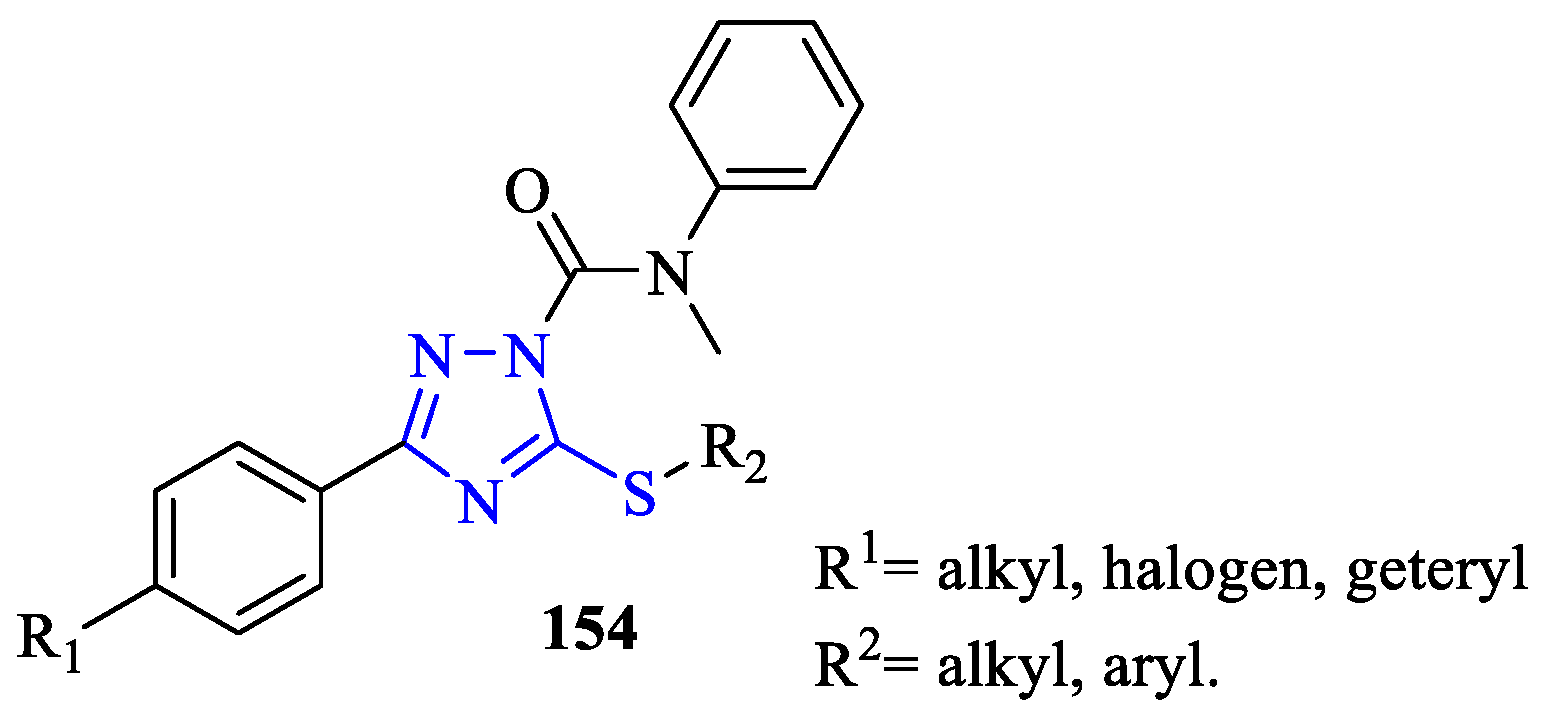3. Antibacterial and Antifungal Activity
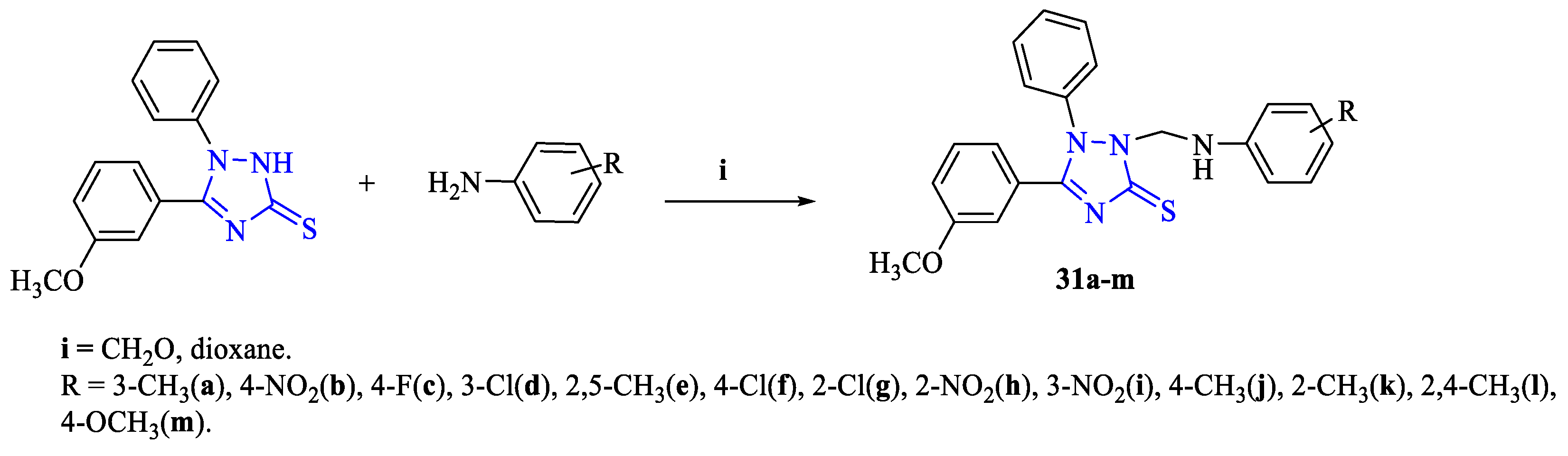
Godhani D.R. et al. [
58] synthesized a series of new Mannich bases by reacting 5-(3-methoxypenyl)-1-phenyl-1H-1,2,4-triazole-3(2H)-thione with substituted anilines (CH
2O, dioxane, yields 52-74%) (
Scheme 10):
All the obtained 2-((arylamino)methyl)-5-(3-methoxypenyl)-1-phenyl-1H-1,2,4-triazole-3(2H)-thiones 31a-m were tested in vitro for antibacterial activity against Gram-positive bacteria Staphylococcus aureus (MTCC-96), S. pyogenes (MTCC-442), Gram-negative bacteria Escherichia coli (MTCC-443), Pseudomonas aeruginosa (MTCC-1688) and fungi Candida albicans (MTCC 227), Aspergillus niger (MTCC 282) and A. clavatus (MTCC 1323). The same compounds were tested for anti-tuberculosis activity against Mycobacterium tuberculosis (H37Rv), where isoniazid was the standard. Of the tested substances, 31d,e,j,k showed good antibacterial activity, and compounds 31a,d,e,j had fairly good anti-tuberculosis activity. However, all compounds did not show fungicidal activity.
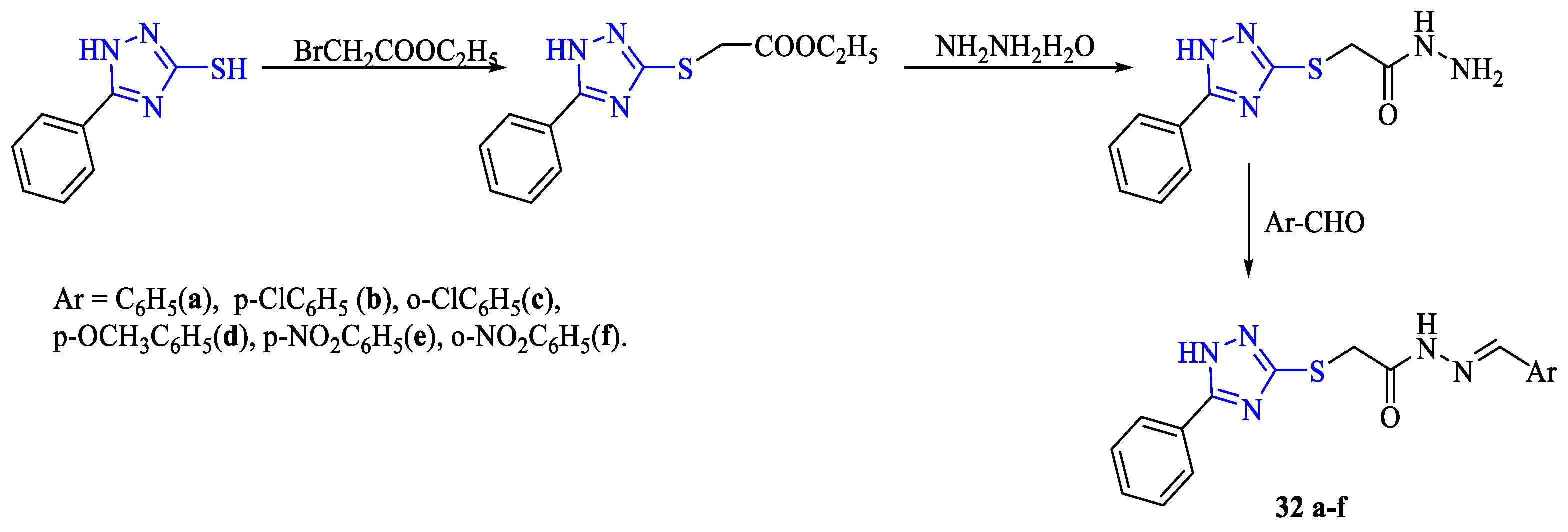
In order to search for new antimicrobial and antifungal agents, Dayama D.S. et al. [
59] obtained several new arylhydrazones of 5-phenyl-1-H-1,2,4-triazole-3-thione with good (66-73%) yields using multi-step reactions (
Scheme 11):
Synthesized derivatives 32a-f bearing various substituents in the aromatic ring were evaluated in vitro for antibacterial activity against S. aureus, E. coli, P. aeruginosa (standard ciprofloxacin). Fungicidal activity was studied against A. niger, C. albicans (standard fluconazole). Among the tested compounds, substances 32b and 32d showed the highest antibacterial activity (MIC 200 mg/ml) compared to other compounds 32a-f. The most effective antifungal compound was 32d (Ar = 4-OCH3C6H4) against C. albicans and A. niger.
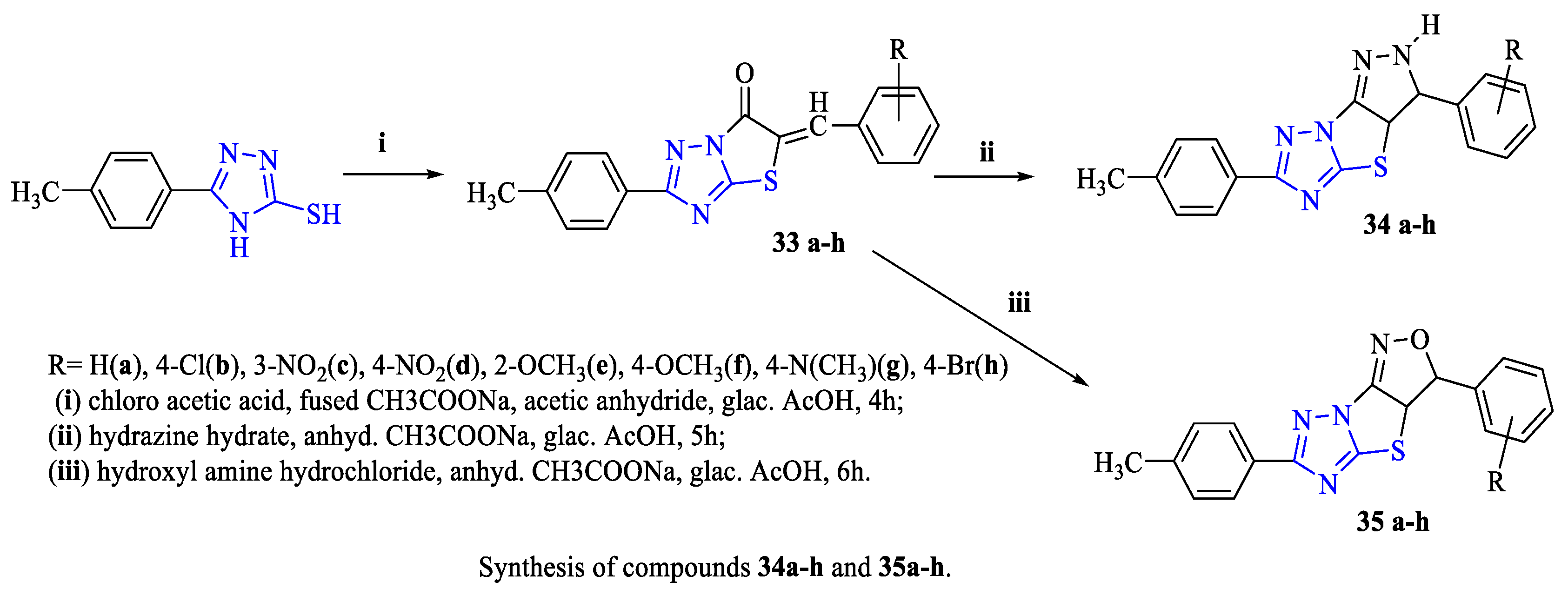
Seelam N. et al. [
60] synthesized derivatives combining 1,2,4-triazole, thiazole, pyrazole or isoxazole fragments in one molecule by reacting 5-mercapto-3-(p-tolyl)-1,2,4-triazole with chloroacetic acid, the corresponding aldehyde and acetic anhydride in the presence of anhydrous CH
3COONa in glacial AcOH to obtain chalcone derivatives of 2-(p-tolyl)thiazolo [3,2-b][1,2,4]triazol-6(5H)-one
33a-h (
Scheme 12):
The obtained compounds 33a-h were then converted by condensation reactions with hydrazine hydrate (anhyd. CH3COONa, glac. AcOH, 5h, yield 59-65%) and hydroxylamine hydrochloride (anhyd. CH3COONa, glac. AcOH, 6h, yield 66-71%) into the target compounds - 3-(substituted-phenyl)-6-(p-tolyl)-3,3a-dihydo-2H-pyrazolo [3/,4/:4,5]thiazolo [3,2-b][1,2,4]-triazole 34a-h and 3-(substituted-phenyl)-6-(p-tolyl)-3,3a-dihydo-isoxazolo [3/,4/:4,5]thiazolo [3,2-b] [1,2,4]-triazole 35a-h, respectively. These compounds were screened for their antimicrobial activity against various strains of bacteria and fungi (B. subtilis (MTCC-1133), B. thuringiensis (MTCC-4714), E. coli (MTCC-443), P. aeruginosa (MTCC-2297). The results showed that most of the tested compounds showed moderate antimicrobial activity comparable to the standards (streptomycin and chloramphenicol). Compounds 34b, 34d, 34h, 35d and 35h showed high activity at the level of the standard drug streptomycin (MIC 3.125 mg/ml) against Mycobacterium tuberculosis H37Rv. It should be noted that the compounds that showed good anti-tuberculosis activity have electron-donating (Cl, NO2, Br) substituents.
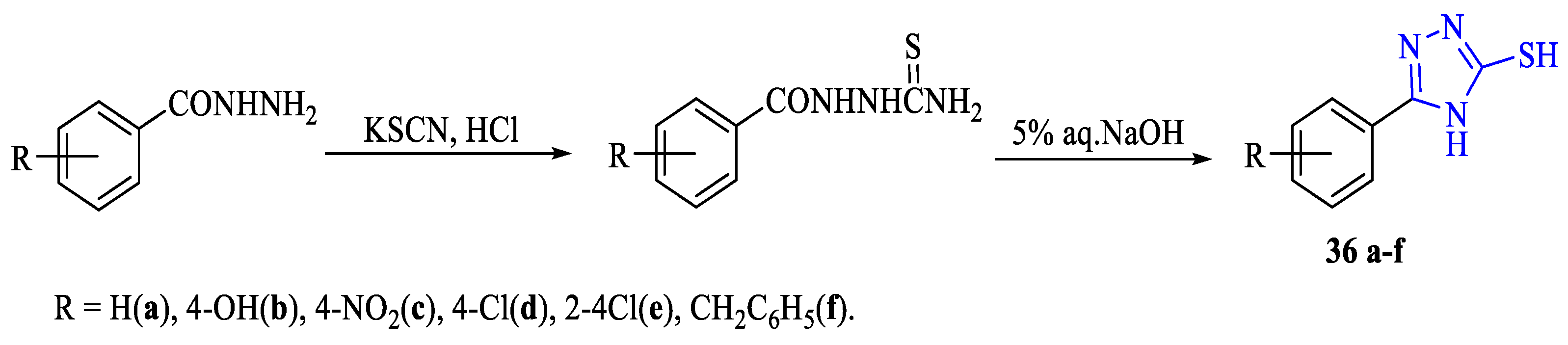
Agrawal R. et al. [
61] synthesized and characterized a series of 5-aryl-substituted-4H-1,2,4-triazole-3-thiols
36 having various aryl substituents from the corresponding thiosemicarbazides in medium (51-75%) yields (
Scheme 13):
All these compounds were tested in vitro for antibacterial activity against six different bacterial strains - E. coli (ATCC ® 10536), Staphylococcus aureus (ATCC ® 25923), Staphylococcus cohnii (MPCST 121), Proteus vulgaris (ATCC ® 6380), Klebsiella pneumoniae (ATCC ® 13883), Pseudomonas aeruginosa (ATCC ® 25619) and against two fungal strains Aspergillus niger (ATCC ® 16404) and Candida albicans (ATCC ® 14053). Gentamicin was used as a standard drug for antibacterial activity, and amphotericin B for antifungal activity. Most of the compounds showed significant activity against more than three different strains of microorganisms. In this case, the authors explained the role of substituents in the aromatic ring attached to 1,2,4-triazole in the manifestation of biological activity. For example, compound 36b has a hydroxyl group at the 4-position of the aromatic ring, which increases the hydrogen bond of the compound with the cell wall proteins of bacteria and fungi containing free sulfhydryl groups (-SH). This contributes to the significant activity of compound 36b. For compound 36c, the presence of a nitro group at the 4-position of the aromatic ring allows it to penetrate the bacterial and fungal cell wall very easily, which also leads to high activity. In contrast to these examples, the introduction of Cl leads to a decrease or complete loss of the antimicrobial and antifungal activity of compounds 36d and 36e. Compound 36f showed the highest activity against Candida albicans and Aspergillus niger with MIC of 0.1-0.2 mg/ml in both cases, which is comparable to the standard drug amphotericin B. The same compound 36f showed MIC of 0.1-0.15 mg/ml against E. coli, which is the lowest MIC among all tested compounds.
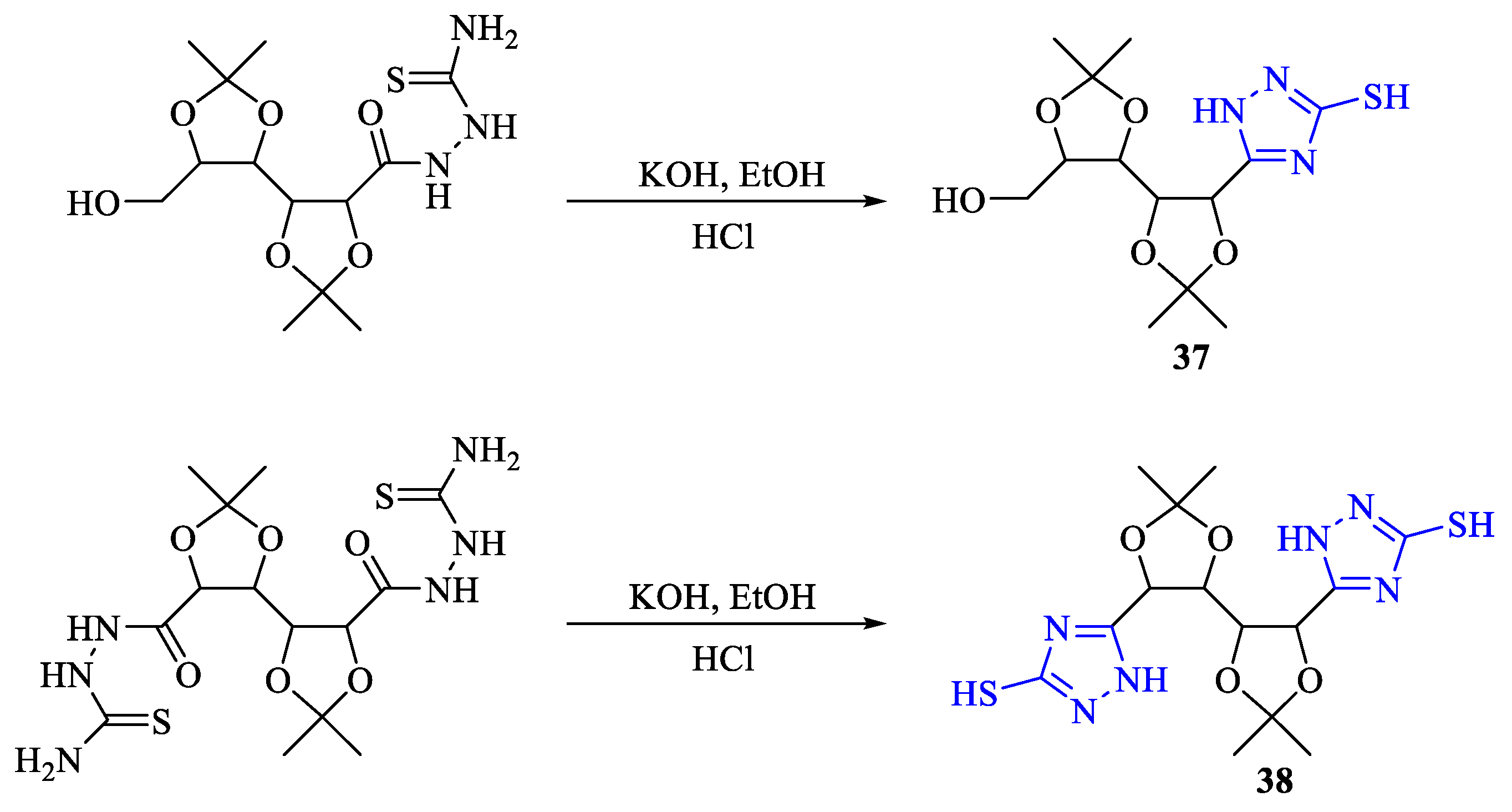
Using one of the main methods for the synthesis of 1,2,4-triazole-3-thiones, M. Belkadi et al. [
62] obtained from 2-{[5’-(hydroxymethyl)-2,2,2’,2’-tetramethyl-4,4’-bi-1,3-dioxol-5-yl]carbonyl}hydrazine-carbothioamide and 2,2,2’,2’-tetramethyl-4,4’-bi-1,3-dioxolane-5,5’-dihydrazine-carbothioamide by boiling in ethanol (KOH) in high yields (84-85%) the corresponding [5’-(5-mercapto-2H-1,2,4-triazole-3-yl)- 2,2,2’,2’-tetramethyl-4,4’-bi-(1,3-dioxolanyl)-5-yl]methanol
37 and 5,5’-(2,2,2’,2’-tetramethyl-4,4’-bi-1,3-dioxolane-5,5’-diyl) bis (1H-1,2,4-triazole-3-thiol)
38 (
Scheme 14):
The synthesized triazole 37 and bis triazole 38 were tested in vitro for antibacterial activity against S. aureus (ATCC 25923), E. coli (ATCC 25882), B. subtilis (ATCC 6633), P. aeruginosa (ATCC 27833) - the standard for comparison is ampicillin. Fungicidal activity was tested on the C. albicans (ATCC 64550) and C. krusei (ATCC 14243) - the standards are ketanazole and fluconazole. The results for antibacterial and fungicidal activity were negative.
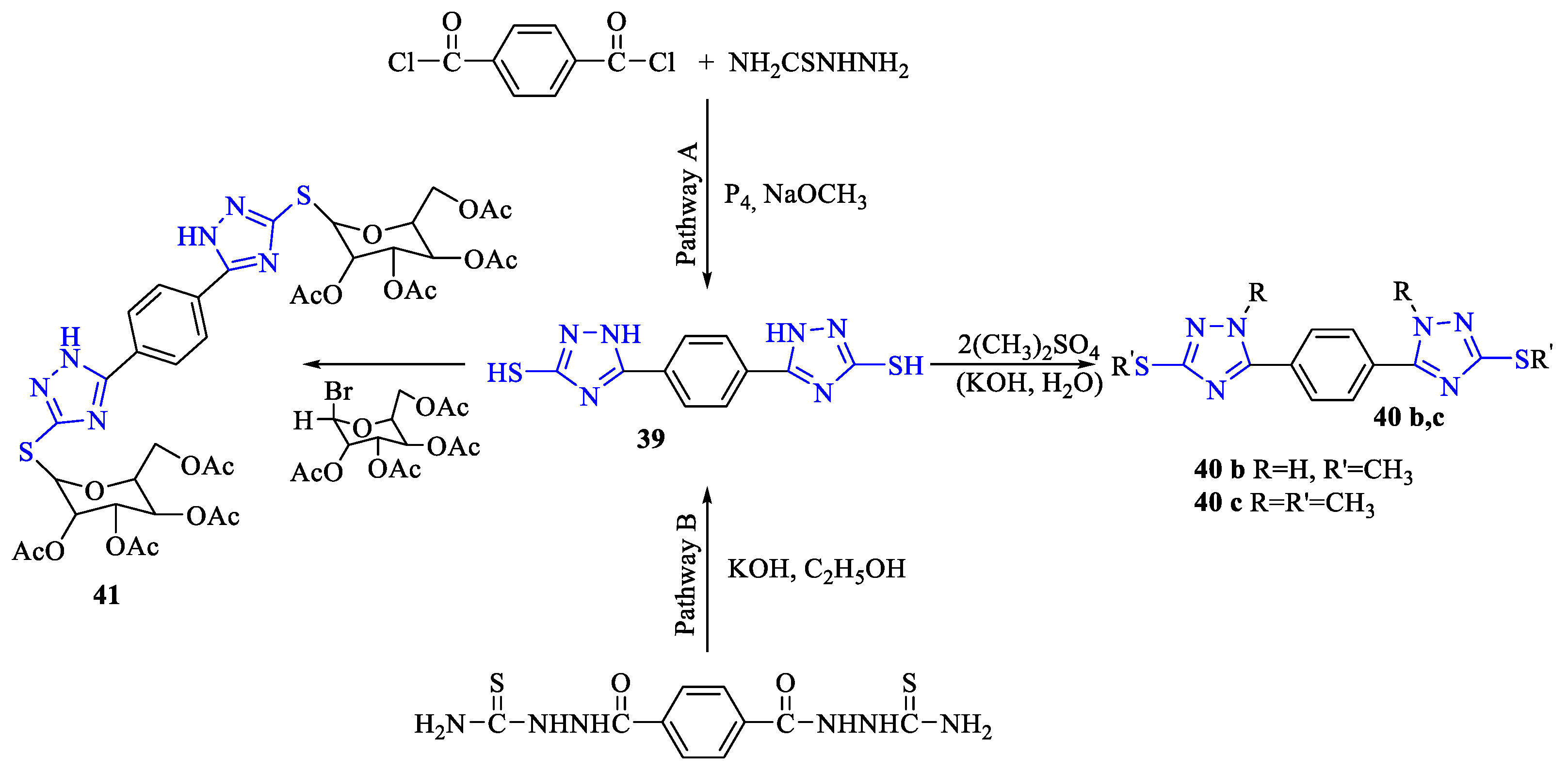
Using two different methods (method A: terephthalolyl dichloride, thiosemicarbazide, pyridine, stirring at room temperature; method B: 2,2’-(Benzene-1,4-diyldicarbonyl)dihydrazinecarbothioamide, ethanolic solution of KOH, refluxed) Datoussaid Y. et al. [
63] synthesized an interesting bis triazolethione - 5,5’-benzene-1,4-diylbis(1H-1,2,4-triazole-3-thiol)
39. However, the yields of
39 were significantly different, 72% for method A and 96% for method B. Further interaction of
39 with dimethyl sulfate in an aqueous KOH solution with an equimolar and two-fold excess of the alkylating agent yielded 5,5’-Benzene-1,4-diylbis [3-(methylsulfanyl)-1H-1,2,4-triazole]
39b and 5,5’-Benzene-1,4-diylbis [1-methyl-3-(methylsulfanyl)-1H-1,2,4-triazole]
39c, respectively, in the same (75%) yields (
Scheme 15):
1,4-Bis [5’-S-(2”,3”,4”,6”-tetra-O-acetate-1”-S-glucosidyl)-1’H-1’,2’,4’-triazo-3’-yl]pheneline 10 was also synthesized by reaction of 39 with tetraacetate bromoglucoside in chloroform using NaOH.
Synthesized compounds 39-41 were tested in vitro using Mueller Hinton agar medium against several gram-positive bacteria – S. aureus (ATCC 25923), E. faecalis (ATTC 29212) and three gram-negative bacteria – E. coli (ATCC 25924), P. aeruginosa (ATCC 10145), P. fluorescens (reference drugs antibiotic Cefotaxim and Gentamycin). Unsubstituted triazole 39 shows noticeable activity against P. aeruginosa at a minimum inhibitory concentration of 1.25 µg/ml. Methyl-substituted 40b showed similar action on P. aeruginosa and E. coli at the same concentration. The greatest effect on E. coli was observed with dimethyl-substituted triazole 40c (R=R’=CH3) at the lowest concentration (0.36 µg/ml).
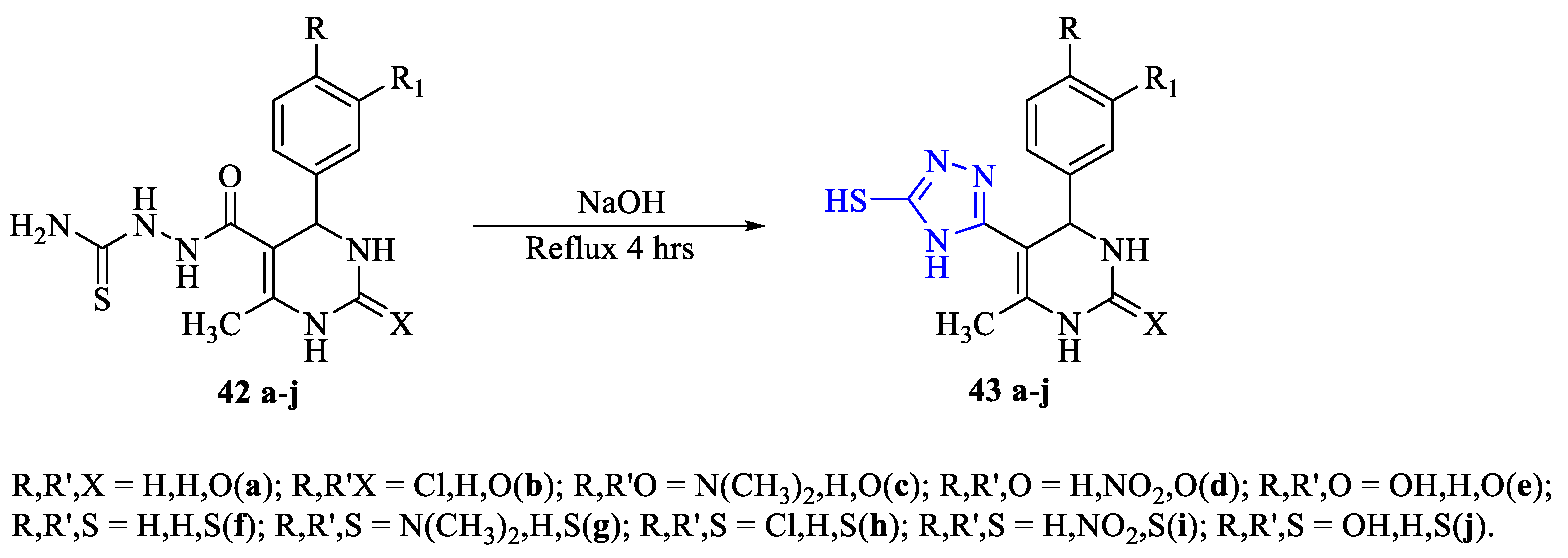
A series of pyrimidine derivatives were synthesized by Andrews B. et al. [
64] - 3,4-dihydro-5-(5-mercapto-4H-1,2,4-triazol-3-yl)-6-methyl-4-(R-phenyl)pyrimidin-2(1H)-one
43a-e and its thio analogue 3,4-dihydro-5-(5-mercapto-4H-1,2,4-triazol-3-yl)-6-methyl-4-(R-phenyl)pyrimidine-2(1H)-thione
43f-j were obtained by treatment of the corresponding carbothioamide compounds
42a-j in good yields, 76-90% (
Scheme 16):
Some (43b,e,g,i) of the synthesized compounds showed promising (12-23 mm) antibacterial activity against S. aureus, P. aeruginosa and E. coli.
In another work [
65] these authors present data on antifungal screening of the above-described compounds
43a-e,
43f-j against
C. albicans, Penicillium sps and
A. niger. Amphotericin-B was used as a standard drug. All the studied compounds showed moderate activity at a concentration of 10 mg/ml against all three strains. At the same time, relatively good activity was noted against
A. niger.
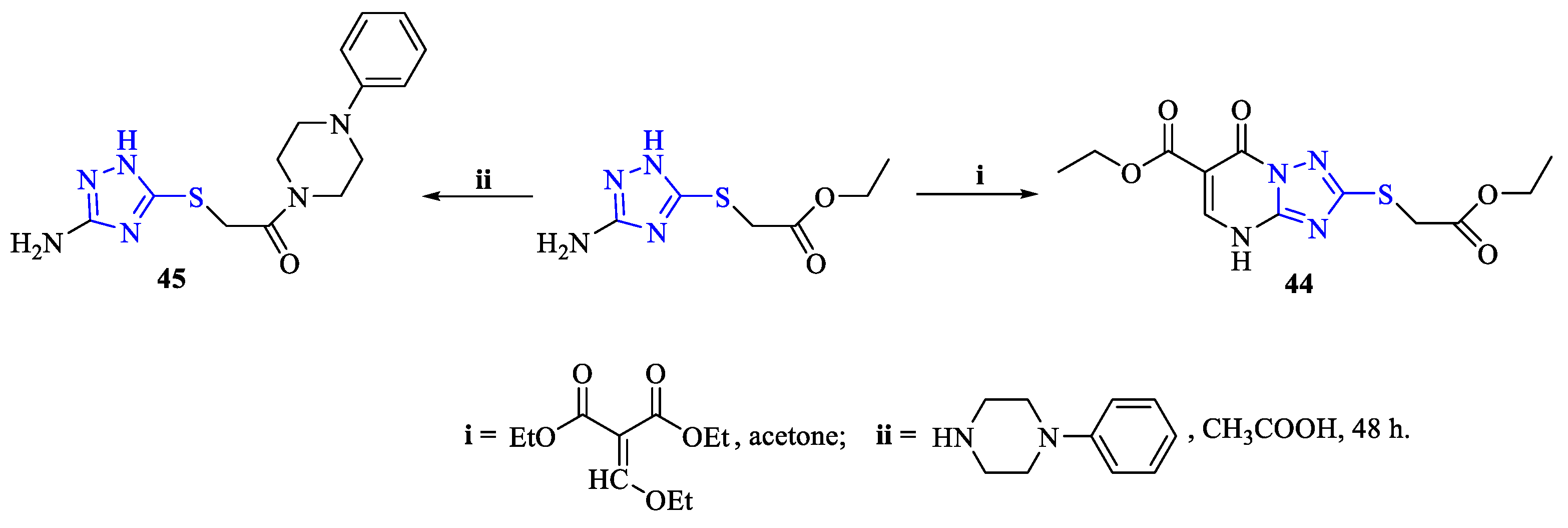
El-Feky Sh.M. et al. [
66] reacted ethyl 2-(3-amino-1H-[1,2,4]-triazol-5-ylthio)acetate with diethyl ethoxymethylenemalonate in acetone to obtain Ethyl 2-(2-ethoxy-2-oxoethylthio)-7-oxo-4,7-dihydro-[1,2,4]triazolo [1,5-a]pyrimidine-6-carboxylate
44 (83%). Prolonged stirring (48 h) of ethyl 2-(3-amino-1H-[1,2,4]-triazol-5-ylthio)acetate and phenylpiprazine in glacial acetic acid affords 2-(3-amino-1H-[1,2,4]-triazol-5-ylthio)-1-(4-phenylpiperazin-1-yl)ethanone
45 in an average yield of 66% (
Scheme 17):
The results of testing compounds 44 and 45 for both antifungal (C. albicans) and antibacterial (S. aureus, E. coli) activity showed that they exhibited no activity.
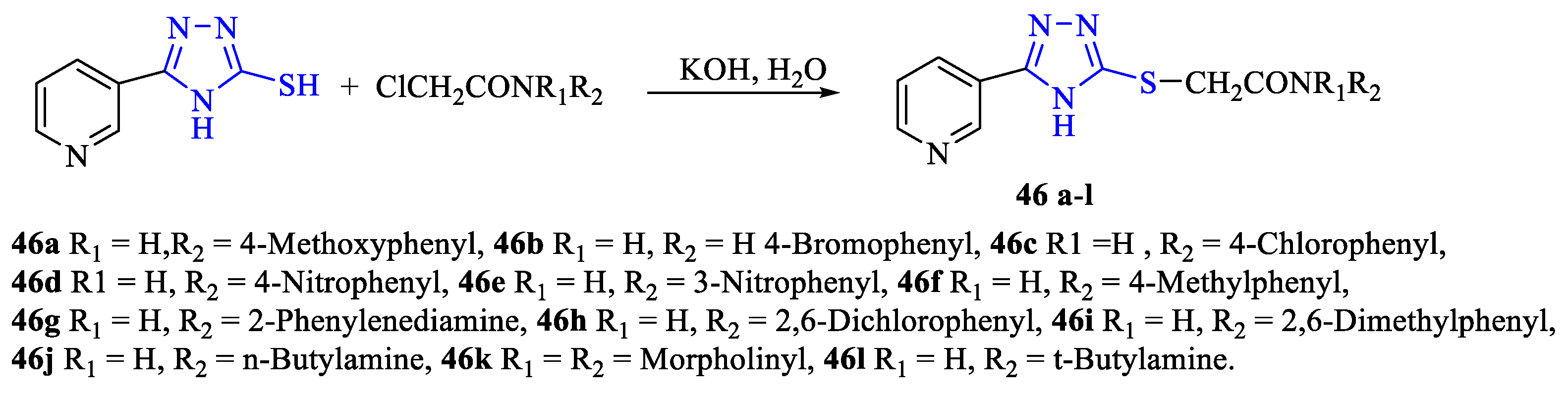
Reactions of 3-(3’-pyridyl)-1,2,4-triazole-5-thiol with the corresponding N-substituted-α-chloroacetanilides carried out by Mali R.K. et al. [
67] in an aqueous solution of potassium hydroxide gave the corresponding 5-(N-substituted carboxamidomethylthio)-3-(3’-pyridyl)-1,2,4-triazoles (
46a-l) in 60-86% yield (
Scheme 18):
All the newly synthesized compounds 46a-l were screened for antifungal activity against Candida albicans and Aspergillus niger at 50 and 100 mg/ml concentrations using fluconazole as a standard. Among all the tested compounds, 46a-46d, 46f and 46h showed the best activity against Candida albicans and Aspergillus niger at 100 mg/ml concentration, while 46a and 46d showed excellent antifungal activity against C. albicans and A. niger even at 50 mg/ml concentration. Substances 46c,d,e,h,i,r,l showed very good anti-tuberculosis activity at a dose of 50 mg/ml against Mycobacterium tuberculosis H37Rv (ATCC 27294) (97-100%, standard Rifampicin 98%).
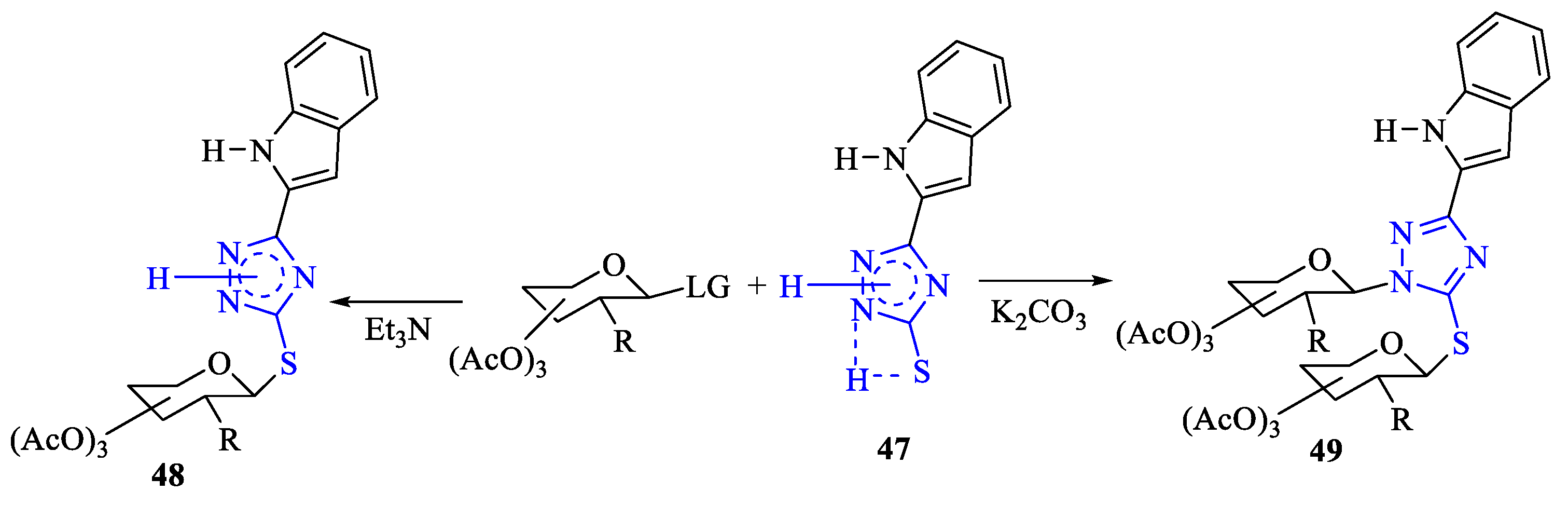
El Ashry E.S.H. et al. [
68] studied the glycosylation of 1,2-dihydro-5-(1H-indol-2-yl)-1,2,4-triazole-3-thione
47 in the presence of Et
3N and K
2CO
3 as acid scavengers with 2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide and 2-acetamido-3,4,6-tri-O-acetyl-2-deoxy-α-D-glucopyranosyl chloride. By using Et
3N, regioselective S-glycosides (S-)
48 were obtained, whereas using K
2CO
3, mixtures of two products (S- and S,N-1) having two glycoside fragments
49 were obtained (
Scheme 19):
The obtained compounds were screened for their antibacterial and antifungal activity, where some of them showed strong inhibitory activity compared to the reference drugs (chloramphenicol and Baneocin).
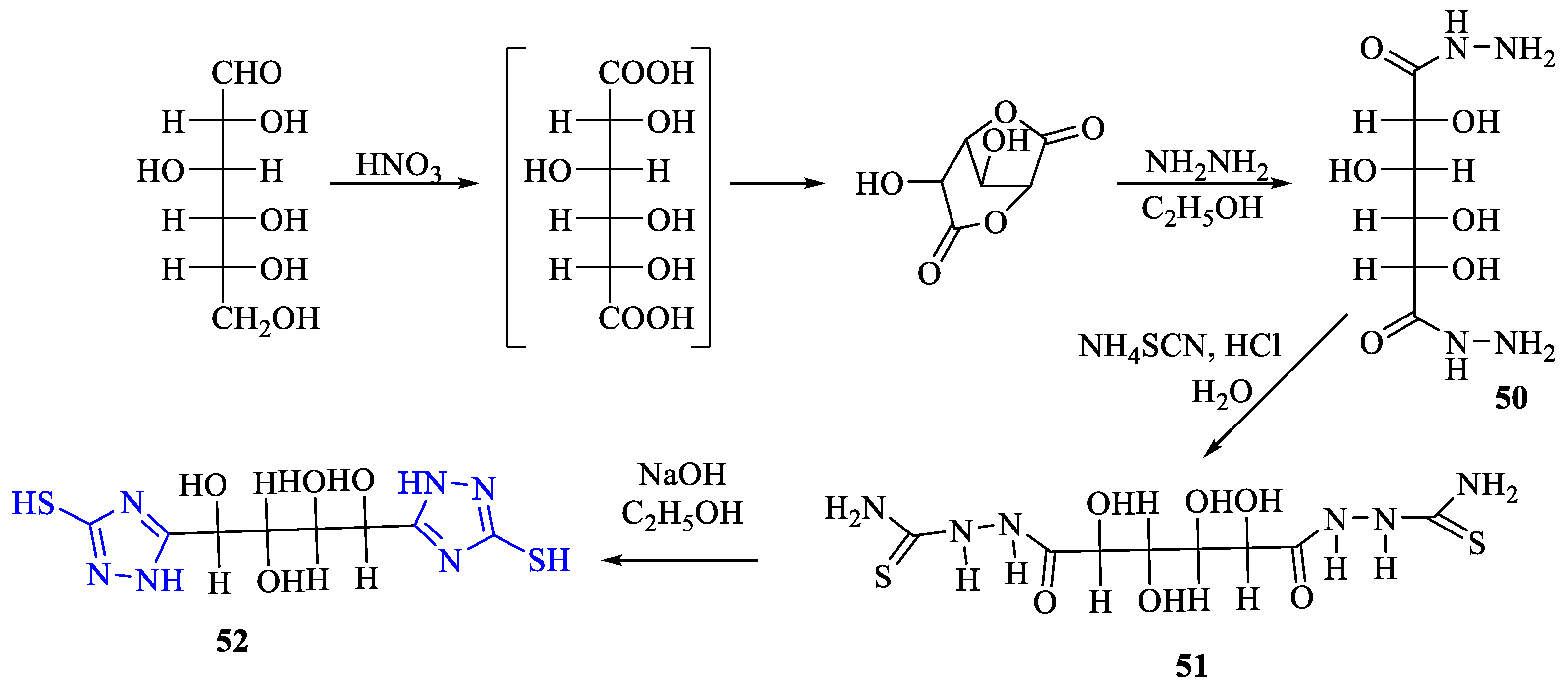
By a sequential synthesis in several stages, Amara S. et al. [
69] synthesized 2,3,4,5-tetrahydroxyhexanedihydrazide
50, from which 2,2’-(2,3,4,5-Tetrahydroxy-1,6-dioxohexane-1,6-diyl)dihydrazinecarbo-thioamide
51 was obtained. The authors then carried out cyclization reactions of
51 to obtain 1,4-bis(3-mercapto-1H-1,2,4-triazol-5-yl)butane-1,2,3,4-tetrol
52. Experiments carried out in an aqueous NaOH solution for 8 h at a temperature of 80°C proceed with a good yield (88%) of bis-triazole
52, while in ethanol this cyclization occurs spontaneously at room temperature with a yield of 84.4%. It should be noted that all experiments were carried out without protection of the hydroxyl groups of D-glucose (
Scheme 20):
The obtained 1,4-bis(3-mercapto-1H-1,2,4-triazol-5-yl)butane-1,2,3,4-tetrol 52 showed activity only against the gram-negative bacterium Klebsiella pneumoniae (ATCC 700603, inhibition zone diameter 14 mm, standard - amoxicillin + clavulanic acid 18 mm) at MIC 1.875 mg/ml. Compound 52 was not active against other Gram-positive bacteria tested in vitro: S. aureus (ATCC 25923), L. inovanii (ATCC 19119) and Gram-negative bacteria Salmonella sp., E. coli (ATCC 25922).
According to the traditional method, Ledeţi I. et al. [
70] obtained 1H-5-mercapto-3-phenyl-1,2,4-triazole
53 by benzoylation of thiosemicarbazide with benzoyl chloride followed by cyclization of 1-benzoylthiosemicarbazide with NaOH in an aqueous-alcoholic medium (
Scheme 21):
Synthesized 1H-5-mercapto-3-phenyl-1,2,4-triazole 53 was tested for antibacterial activity against three bacterial strains – S. aureus (ATCC 25923), E. coli (ATCC 25922) and P. aeruginosa (ATCC 27853) by disk diffusion. The test results showed that compound 53 was active only against gram-positive bacteria S. aureus and did not show activity against gram-negative bacteria E. coli and P. aeruginosa. The obtained results demonstrate the specific antimicrobial activity of 1H-5-mercapto-3-phenyl-1,2,4-triazole 53 against gram-positive bacterial infections at a concentration of 25 mg/ml. The authors consider the synthesis of new compounds based on this triazole as potential antibacterial agents to be promising.
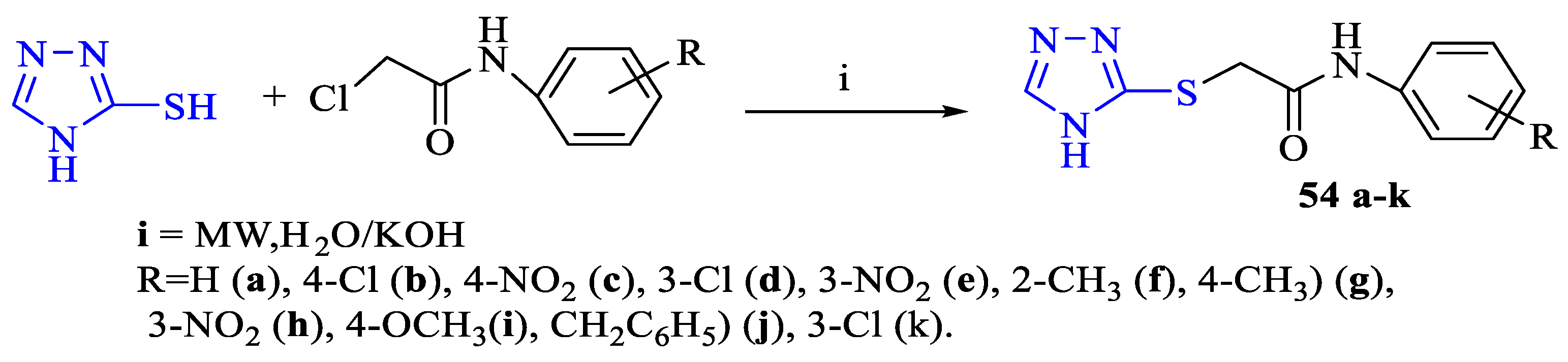
Using microwave irradiation methods, Manikrao A.M. et al. [
71] synthesized a series of 3-(N-substituted carboximidomethylthio)-(4H)-1,2,4-triazoles
54a-k by the reaction of 3-mercapto-(4H)-1,2,4-triazole and N-substituted chloroacetamides in aqueous KOH. This method was found to be rapid and economical, with microwave reactions proceeding smoothly within 2-6 min in yields of 62-85%
54a-k.
However, the conventional method of carrying out the reaction required continuous stirring at a temperature of 60-70 °C for 36 hours and the yields of the target products were significantly lower, 45-80% (
Scheme 22):
All compounds were tested in vitro for preliminary antibacterial (S. aureus, K. pneumoniae, E. coli, P. aeruginosa) and antifungal (A. flavus, A. fumigatus, Penicillium sp.) activity at two concentrations of 100 and 150 mg/mL. Streptomycin and griseofulvin were used as standards at the same concentrations (100 and 150 mg/mL), respectively. Among the tested compounds, 54b and 54d showed significant (inhibition zone diameter 11-16 mm, standard 13-22 mm) activity against E. coli, P. aeruginosa and K. pneumoniae, with moderate activity against S. aureus. Of the tested compounds, only 54c and 54e (8-12 mm, standard 11-17 mm) showed good activity against all tested fungi. The remaining compounds showed minimal or moderate activity.
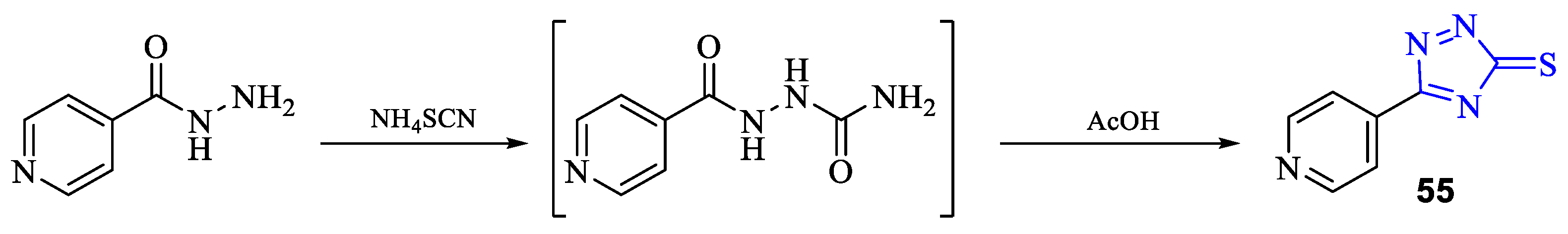
Farhan M.E. et al. [
72] obtained isonicotinic acid thiosemicarbazide by reacting isonicotinic acid hydrazide with ammonium thiocyanate, followed by N-cyclization of which in an acidic medium (AcOH) to synthesize 5-(Pyridin-4-yl)-3H-1,2,4-triazole-3-thione
55 (
Scheme 23):
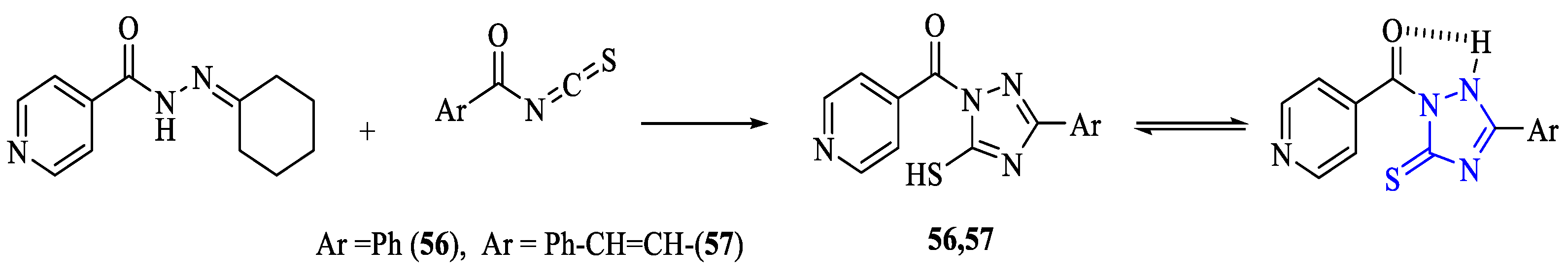
Other triazole derivatives containing a pyridine ring were also obtained. Thus, by boiling equal amounts of N’-cyclohexylidene benzohydrazide and benzoyl isothiocyanate in acetone for 1 hour, (3-phenyl-5-thioxo-4,5-dihydro-1H-1,2,4-triazol-1-yl) (pyridin-4-yl)methanone
56 was synthesized in 51% yield. Replacing benzoyl isothiocyanate with cinnamoyl isothiocyanate under similar conditions, pyridin-4-yl(3-styryl-5-thioxo-2,5-dihydro-1H-1,2,4-triazol-1-yl)methanone
57 was obtained in 51% yield (
Scheme 24):
Antimicrobial activity was tested at a concentration of 5 mg/ml against the bacterial and fungal strains E. coli (ATCC 25955), S. typhimurium (ATCC14028), S. aureus (RCMB010010), B. subtilis (NRRLB-543), A. flavus (RCMB002002) and C. albicans (ATCC 10231). The test results of compounds 56 and 57 showed moderate (12-14 mm, gentamicin standard 23-27 mm) activity only against S. aureus and S. typhimurium. 5-(Pyridin-4-yl)-3H-1,2,4-triazole-3-thione 55 was inactive against all test strains.
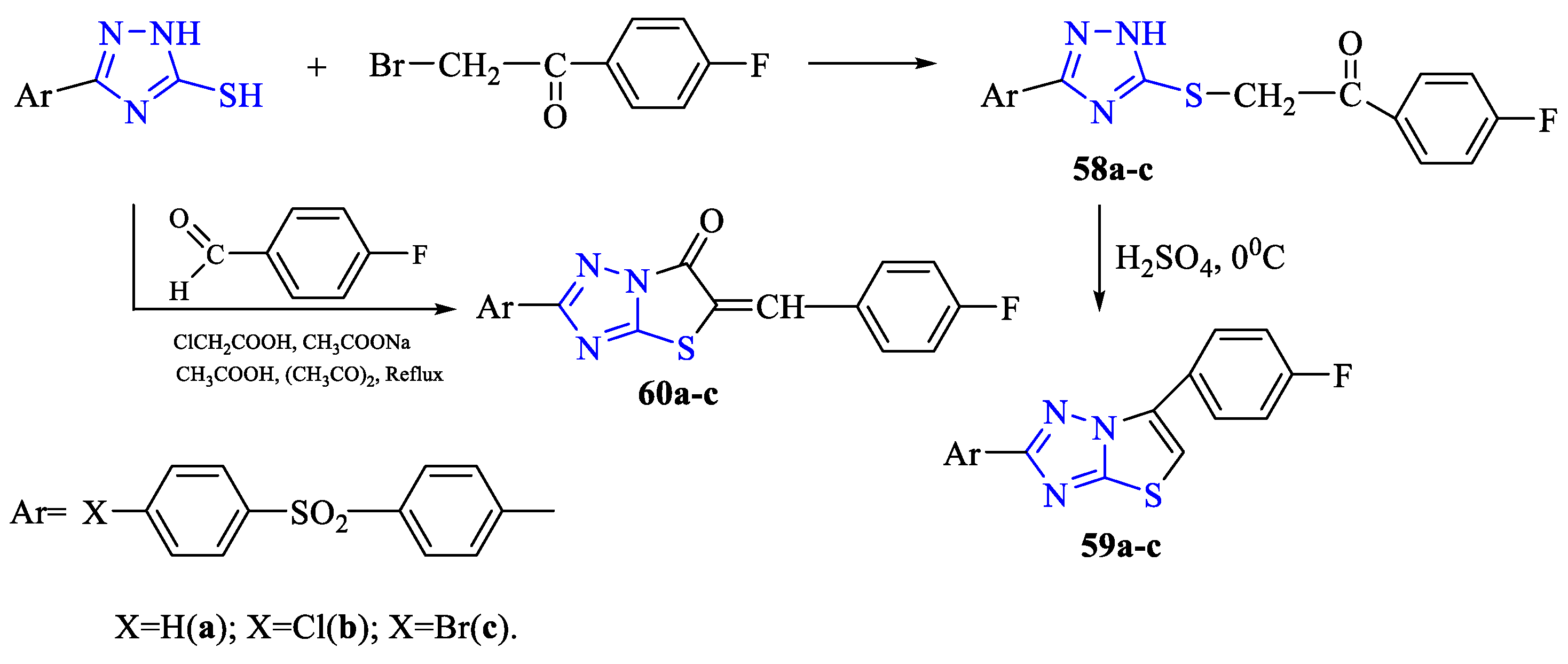
Barbuceanu S.F. et al. [
73] developed a series of syntheses of new heterocyclic fused systems
59a-
c,
60a-
c containing a thiazolo [3,2-b][1,2,4]triazole framework. The syntheses started with the preparation of some 4-(4-(4-x-phenylsulfonyl)phenyl)-4H-1,2,4-triazole-3-thiols (X = H, Cl, Br), and then their reaction with 2-bromo-4’-fluoroacetophenone in DMSO at room temperature gave 2-(5-(4-(4-x-phenylsulfonyl)phenyl)-2H-1,2,4-triazol-3-ylthio)-1-(4-fluorophenyl)ethanones
58a-
c. Cyclization of S-alkylated 1,2,4-triazoles
58a-
c in sulfuric acid at 0°C leads to the formation of 2-(4-(4-x-phenylsulfonyl)phenyl)-6-(4-fluorophenyl)thiazolo [3,2-b][1,2,4]triazoles
59a-
c in 88-93% yield. The synthesis of another series of new cyclic compounds 2-(4-(4-x-phenylsulfonyl)phenyl)-5-(4-fluorobenzylidene)-thiazolo [3,2-b][1,2,4]triazol-6(5H)-ones
60a-
c based on 1,2,4-triazole-3-thiols was carried out by their reaction with fluorobenzaldehyde and chloroacetic acid in a catalytic amount of anhydrous CH
3COONa under reflux in a mixture of acetic acid and acetic anhydride (
Scheme 25):
The antimicrobial activity of the studied compounds was tested against some reference bacterial: S. aureus (ATCC 29213), B. cereus (ATCC 13061), E. coli (ATCC 25922), E. cloacae (ATCC 49141), A. baumannii (ATCC 19606), P. aeruginosa (ATCC 27853) and fungal strains: C. albicans (ATCC 90028), C. parapsilosis (ATCC 22019), C. glabrata (ATCC 15126) and C. tropicalis (ATCC 13803). The screening results showed that the best activity was demonstrated by compounds 58b and 60b against A. baumannii (MIC=16 mg/ml). Both compounds have a chlorine atom in the para-position in the diphenylsulfone moiety. Compounds 58a, 59b, 60c also showed good activity against the same strain (MIC=32 mg/ml). The best promising results were obtained for compounds 58b, c and 60b against the Bacillus cereus strain with MIC=8 mg/ml, which should be used for further studies.
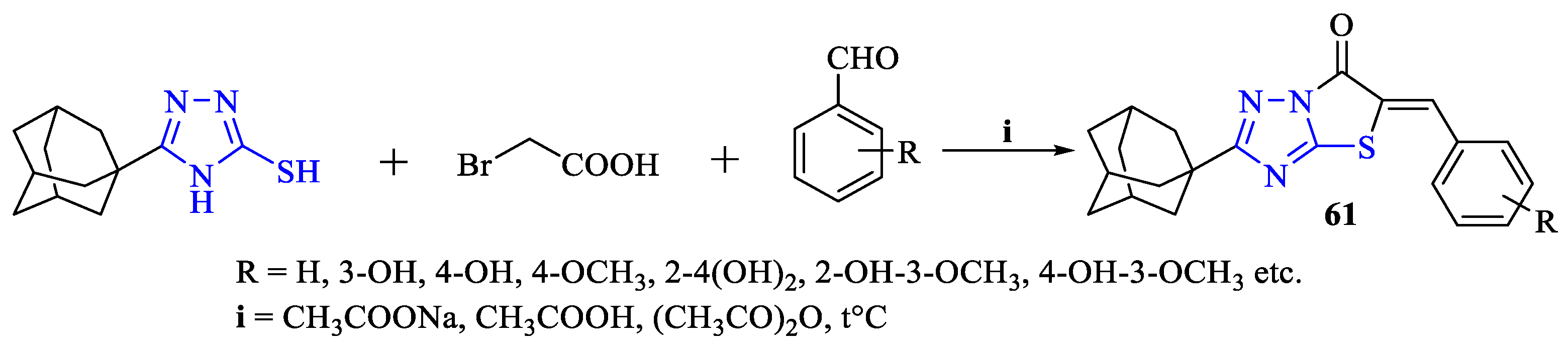
More than twenty substituted 5-benzylidene-2-adamantylthiazole [3,2-b][1,2,4]triazol-6(5H)ones
61 were synthesized in good yields (55-88%) by Tratrat C. et al. [
74] in a one-pot method by condensation of 5-adamantyl-4H-1,2,4-triazole-3-thiol with bromoacetic acid and the corresponding substituted benzaldehydes in the presence of sodium acetate and acetic anhydride (
Scheme 26):
The obtained compounds were evaluated in vitro for their antimicrobial properties against Gram-positive (B. cereus (clinical isolate), M. flavus (ATCC 10240), L. monocytogenes (NCTC 7973) and S. aureus (ATCC 6538), Gram-negative bacteria (E. coli (ATCC 35210), P. aeruginosa (ATCC 27853), S. typhimurium (ATCC 13311), E. cloacae (human isolate)) and fungal strains (A. niger (ATCC 6275), A. ochraceus (ATCC 12066), A. fumigatus (human isolate), A. versicolor (ATCC 11730), P. funiculosum (ATCC 36839), P. ochrochloron (ATCC 9112), T. viride (IAM 5061) and C. albicans (human isolate)). Almost all tested compounds showed antibacterial activity to varying degrees. In some cases, the activity was even higher than that of streptomycin against L. monocytogenes and E. coli. The antifungal effect of all compounds had MIC in the range of 3.67–34.6*10-2 μmol/ml and MFC in the range of 7.35–39.6*10-2 μmol/ml. Moreover, most compounds showed the best activity against A. ochraceus, A. versicolor and A. fumigatus, while the most resistant species was C. albicans.
Venkatachalam T. et al. [
75] designed and synthesized 2-substituted-1,5-diphenyl-1,2-dihydro-3H-1,2,4-triazole-3-thiones
63 as new inhibitors of
Mycobacterium tuberculosis (
M. tuberculosis H37Rv) (
Scheme 27):
The anti-tuberculosis activity of the synthesized compounds was studied in vitro on the M. tuberculosis H37Rv strain using the LRP method. At concentrations of 100 and 500 μg/ml, all tested substances show a high percentage of inhibition (89-98.6%).
The one-pot Mannich reaction of 5-methyl-1Hs-triazole-3-thiol with formaldehyde and primary aliphatic amines in ethanol at room temperature, carried out by the authors [
76], leads to the formation of cyclic products - 2-methyl-6-substituted-6,7-dihydro-5H-s-triazolo [5,1-b]-1,3,5-thiadiazines
64. In reactions of this triazole under similar conditions with primary aromatic amines, the authors obtained non-cyclized 3-methyl-1-((substituted-amino)methyl)-1H-s-triazole-5-thiols
65 Scheme 28):
As noted by the authors of the study, all synthesized compounds 64, 65 showed biological activity against E. coli, P. aeruginosa, B. subtilis, A. niger, A. flavus and A. fumigatus. It was also found that these compounds have the ability to remove Mg2+, Pb2+, Cd2+ and Ca2+ from an aqueous solution with the results of 70.27-93.92%, 72.29-92.40%, 70.95-92.00% and 53.92-89.00%, respectively.
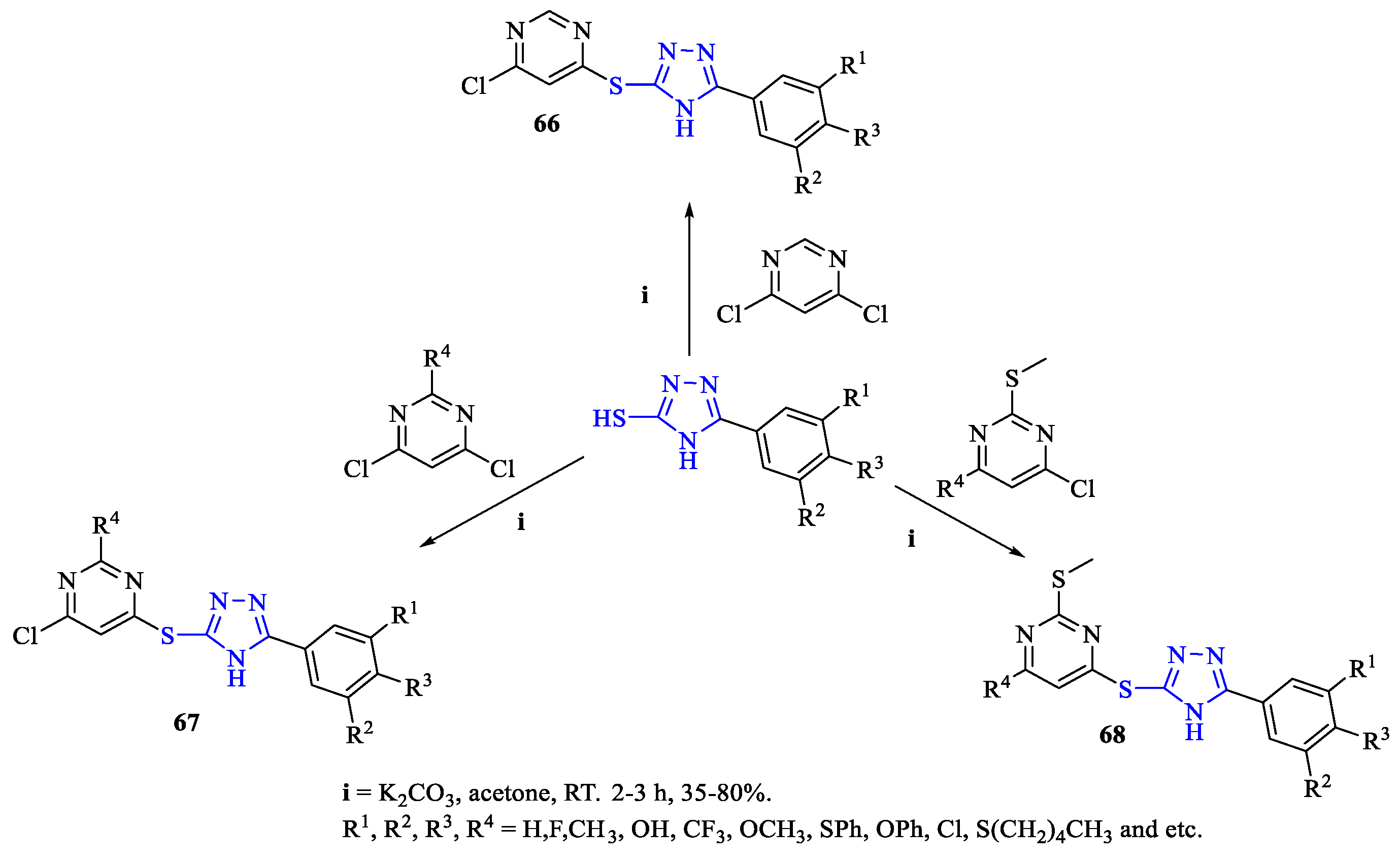
Cui J. et al. [
77] synthesized a series of triazole-pyrimidine compounds and evaluated them as novel Sec A inhibitors with IC
50 and MIC values in the low to submicromolar range (
Scheme 29):
Pyrimidine compounds 66-68 were prepared by reactions (K2CO3, acetone, room temperature, 2-3 h) of 5-(substituted-phenyl)-4H-1,2,4-triazole-3-thiols with substituted 4,6-dichloropyrimidines in 35-80% yields.
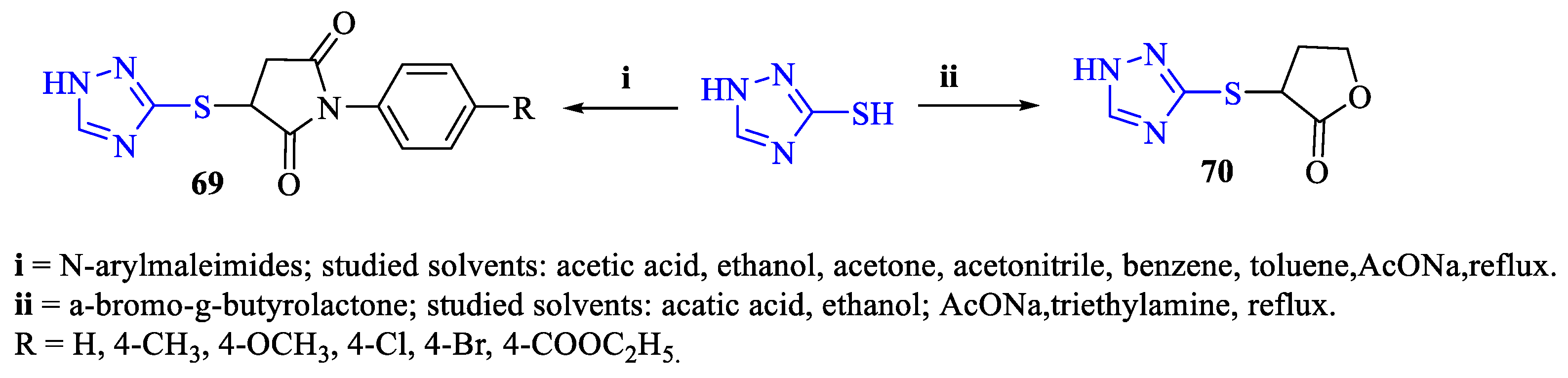
Holota S. et al. [
78] synthesized new triazole derivatives
69, 70 by reacting 1,2,4-triazole-3(5)-thiol with electrophilic reagents such as N-arylmaleimides and α-bromo-γ-butyrolactones by boiling in various solvents (acetic acid, ethanol, acetone, acetonitrile, benzene, toluene) in the presence of AcONa and triethylamine (
Scheme 30):
Preliminary screening of the antimicrobial activity of the synthesized 1-(R-phenyl)-3-(2H-[1,2,4]triazol-3-ylsulfanyl)-pyrrolidine-2,5-dione 69 and 3-((1H-1,2,4-triazol-3-yl)thio)dihydrofuran-2(3H)-one 70 against gram-positive (S. aureus, S. epidermidis) and gram-negative bacteria (E. coli), as well as yeast (C. albicans) showed that they have promising antimicrobial properties.
4. Cytotoxic Activity
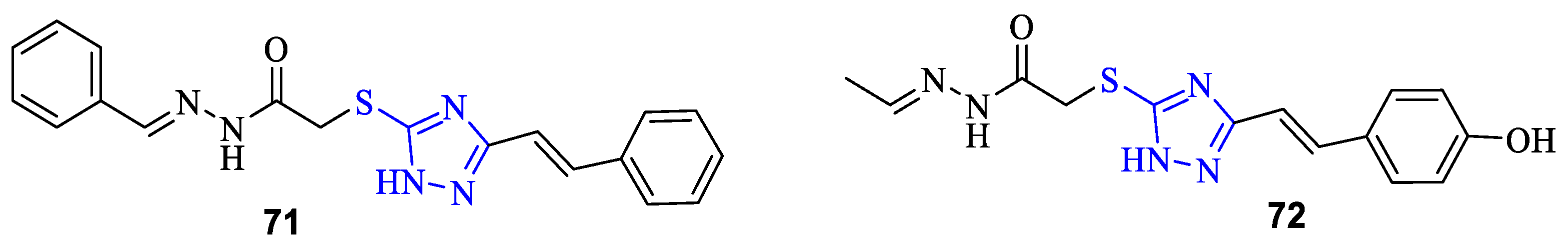
Mioc M. et al. [
79] generated a library of compounds containing 3-mercapto-1,2,4-triazole derivatives using a virtual docking screening method to predict molecules with potential antitumor properties active in colorectal cancer. After screening the library against two protein targets (VEGFR-2 and EGFR-1), two molecules
71 and
72 were selected that showed good binding properties (
Figure 3):
Based on the results of the studies, the authors hypothesized that compound
71 would be able to inhibit both VEGFR/EGFR proteins and would be very useful as a dual inhibitor. The authors reported obtaining and verifying the predicted activity for these two molecules
71 and
72 in their other work [
80].
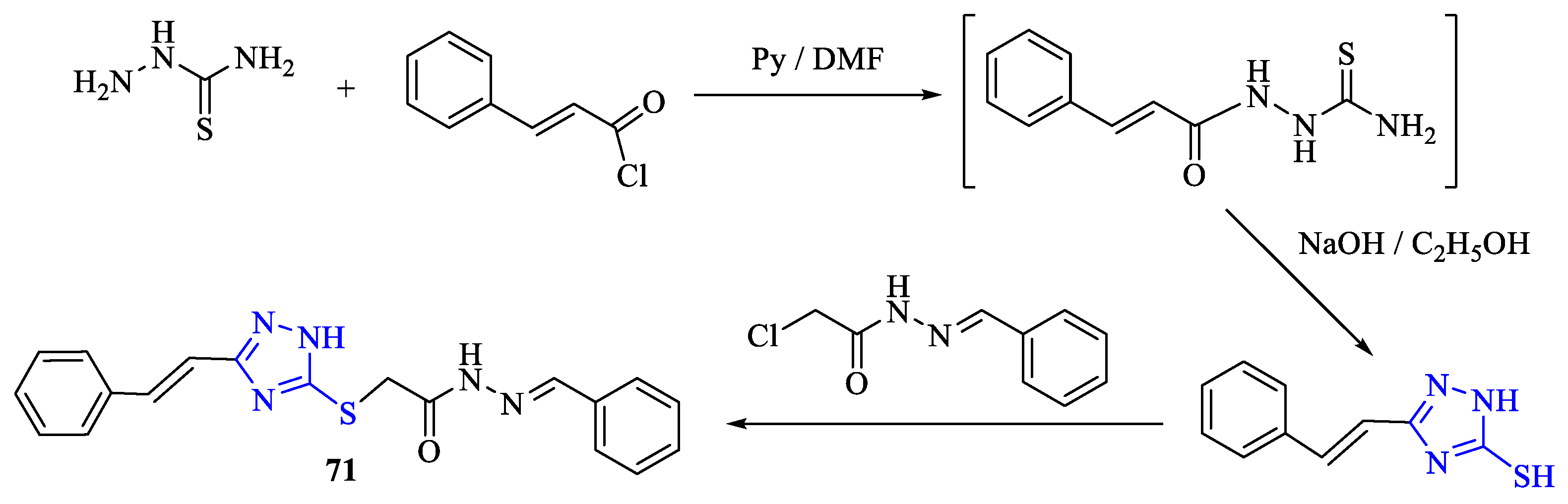
Synthesis of 1-H-3-styryl-5-benzylidenehydrazinocarbonylmethylsulfanyl-1,2,4-triazoles
71 was carried out in the following sequence: acylation of thiosemicarbazide with cinnamoyl chloride (pyridine, N,N-dimethylformamide) gave 1-cinnamoyl-thiosemicarbazide, then its cyclization (ethanol, NaOH, under reflux) led to 1H-3-styryl-5-mercapto-1,2,4-triazole. The target compound
71 was synthesized by alkylation of 1H-3-styryl-5-mercapto-1,2,4-triazole with N-(benzylideneamino)-2-chloroacetamide (
Scheme 31):
As mentioned above, 1-H-3-styryl-5-benzylidenehydrazino-carbonylmethylsulfanyl-1,2,4-triazole 71 was selected as a suitable ligand for the VEGFR-2 and EGFR1 receptors based on molecular docking. In vitro biological evaluation of 71 using the Alamar Blue assay revealed weak antiproliferative activity against the A375, A549 and B164A5 cell lines (human melanoma, lung carcinoma and murine melanoma, respectively), while stronger activity was reported against the MDA-MB-231 breast cancer cell line (triple-negative breast carcinoma).
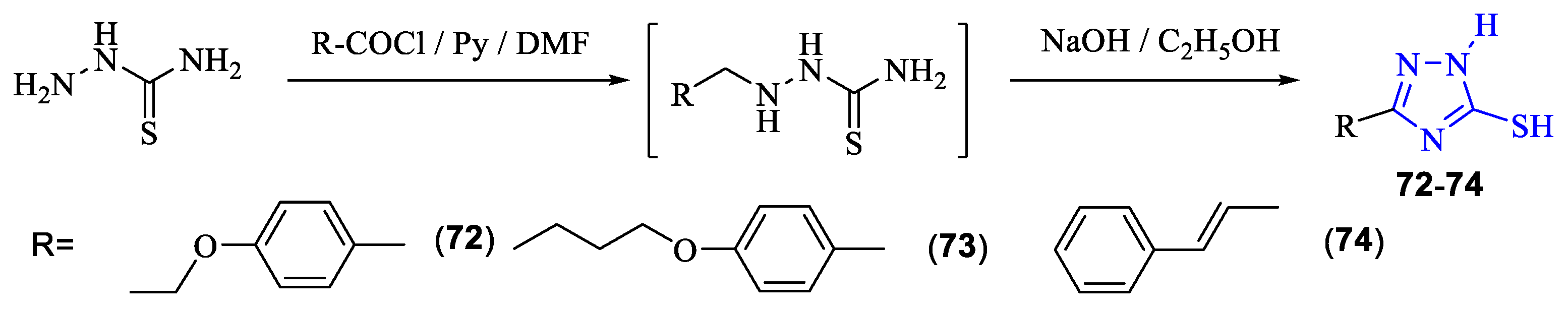
In another work by Mioc M. et al. [
81], the antiproliferative activity of several more 1H-3-R-5-mercapto-1,2,4-triazoles
72-
74, synthesized according to the scheme described in work [
80], was studied on the same cell lines (A375, B164A5, MDA-MB-231 and A549), as well as on a healthy cell line - human keratinocytes (HaCaT) (
Scheme 32):
The antiproliferative activity of 72-74 against A375 and B164A5 was moderate, while stronger activity was observed against the A549 and MDA-MB-231, acting in a dose-dependent manner. The authors note the low toxicity of compounds 72-74 against normal cell lines (HaCaT).
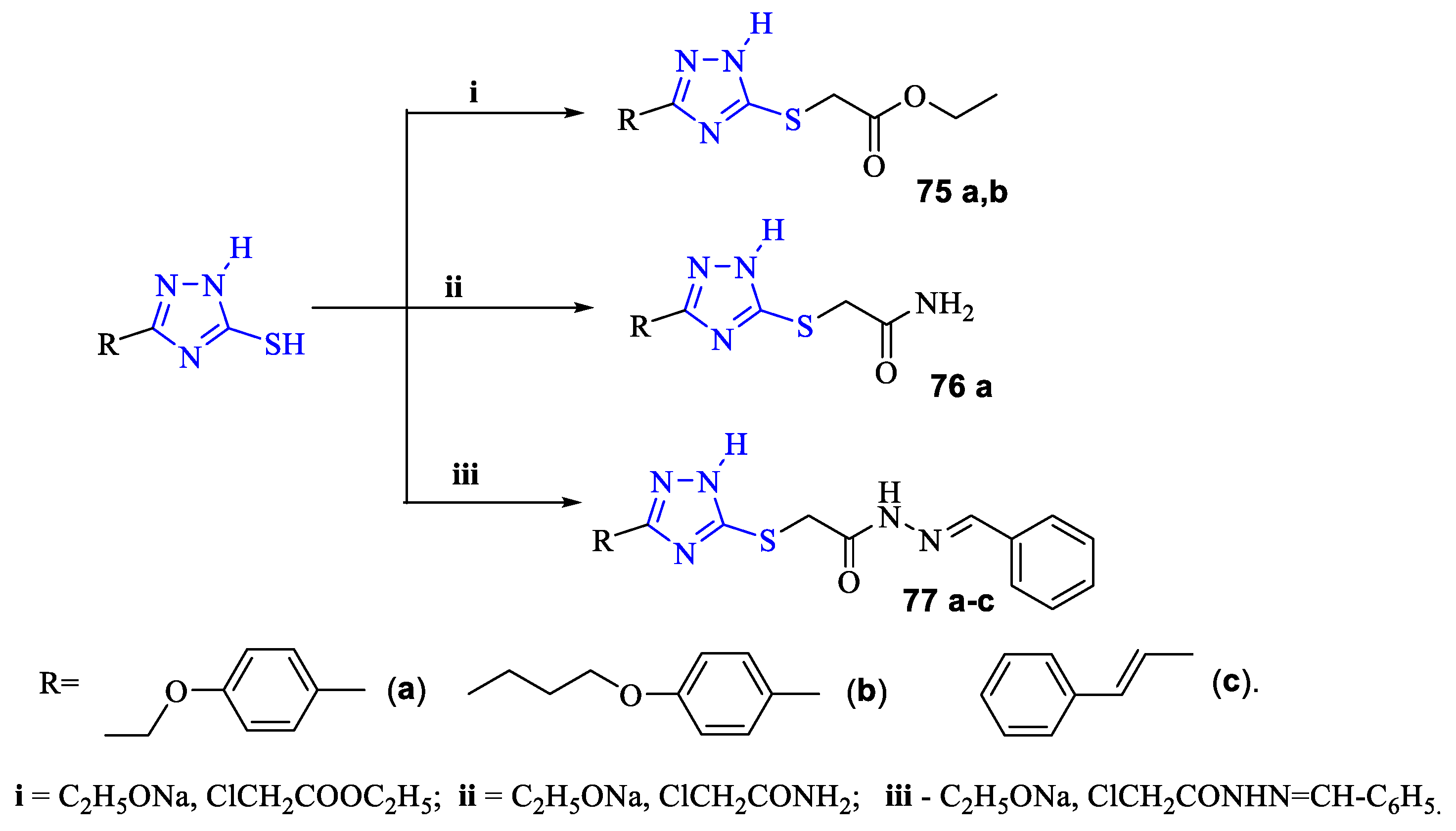
Continuing the studies of antiproliferative activity using the colorectal cancer cell line HT-29 as an example, Miok M. et al. [
82] synthesized several S-alkyl derivatives of 1H-3-R-5-mercapto-1,2,4-triazoles
75a,
75b,
76a,
77a-
c. These compounds were selected based on the results of virtual docking screening (
Scheme 33):
The test results showed that the obtained S-alkylated derivatives exhibited strong cytotoxic activity. It was found that S-substituted compounds containing -CO-NH-N=C-group 77a-c showed higher activity compared to other compounds. Also, the length of the alkyl substituent associated with the hydroxyl part in position 4’ of the aromatic ring affects the antiproliferative activity. In the case of a shorter alkyl chain 75a, 77a showed stronger cytotoxic activity than in comparison with compounds with a longer alkyl group 75b, 77b. Compound 77b, which was selected as a possible PDK1 inhibitor, exhibited the most significant cytotoxic activity against the HT-29 tumor cell line (IC50=87.95µM). Compounds 77a-c led to significant cell cycle arrest in both the sub G0/G1 and G0/G1 phases. These studies show prospects for the synthesis of new compounds containing 1,2,4-mercaptotriazole ring with antiproliferative activity in colorectal cancer.
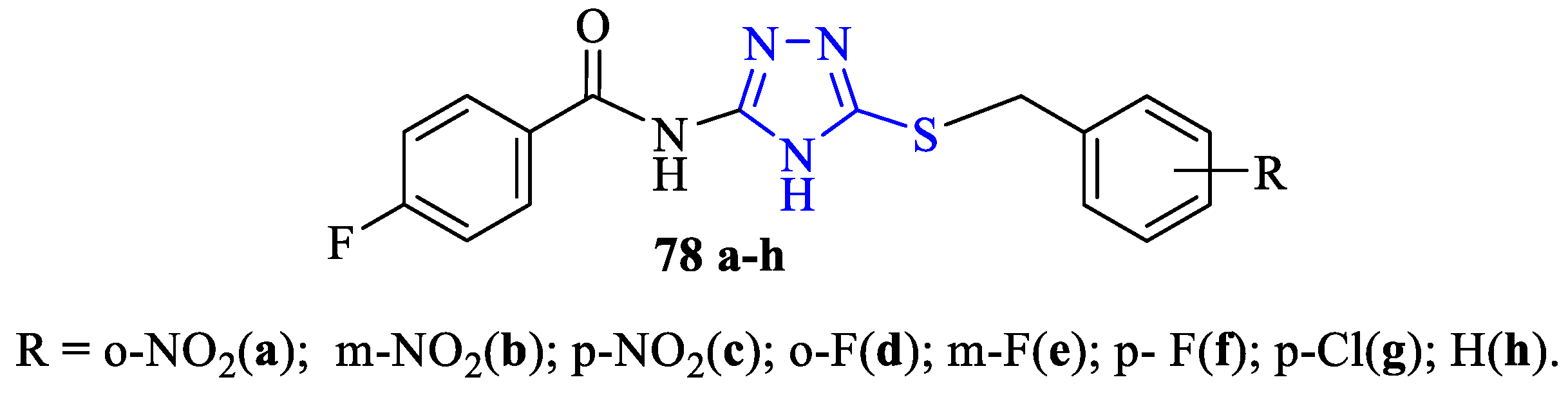
Aliabadi A. et al. [
83] synthesized and evaluated the cytotoxicity of a series of new 1,2,4-triazole derivatives - N-(5-R-benzylthio)-4H-1,2,4-triazol-3-yl)-4-fluorobenzamides
78a-
h (
Figure 4):
In vitro tests were performed on PC3 (prostate cancer), HT-29 (colon cancer) and SKNMC (neuroblastoma) cell lines using the MTT assay (reference drug imatinib). None of the tested compounds showed greater activity than imatinib on PC3 and SKNMC cell lines. However, on HT-29 cells, compound 78b (IC50 = 3.69 ± 0.9 µM) and 78e (IC50 = 15.31 ± 2.1 µM) showed higher activity than imatinib (18.1 ± 2.6 µM). Based on these results, the authors propose some of the obtained 1,2,4-triazole derivatives as potential antitumor agents, in particular against colorectal cancer.
New N-substituted amides of 3-(3-ethylthio-1,2,4-triazol-5-yl)propenoic acid
79 were prepared by Pachuta-Stec A. et al. [
84] by the condensation reaction of exo-S-ethyl-7-oxabicyclo-[2.2.1]-hept-5-ene-2,3-dicarbonylisothiosemicarbazide with primary amines (
Scheme 34):
Synthesized compounds 79 were tested for antitumor activity in vitro. A clearly expressed antiproliferative effect of the compounds in concentrations from 0.35 μM to 0.16 μM was established in relation to the breast carcinoma cell line. The lowest cytotoxicity was noted at concentrations of 0.16 mM and 0.03 mM in relation to the normal fibroblast cell line and breast carcinoma cells in vitro after 24- and 48-hour incubation.
By reaction of 5-substituted-[1,2,4]triazole-3-thiones and 1-(3-chloropropyl)-4-substituted cyclic amines in the presence of triethylamine and a catalytic amount of tetra-butyl ammonium iodide (TBAI) in ethanol, Murty M.S.R. et al. [
85] obtained 3-[3-[4-(substituted)-1-cyclic amine]propyl]thio-5-substituted [1,2,4]triazoles
80a-
j in good yields (63-75%) (
Scheme 35):
Triazole derivatives 80a-j were tested for cytotoxic activity against human cancer cell lines U937, THP-1, Colo 205, MCF 7 and HL-60. The results showed that they were more effective on U937 and HL-60 cells than on the other three cell lines. The highest activity among all tested compounds was shown by 5-(3-methylphenyl)-4H-1,2,4-triazol-3-yl 3-[4-(2-pyridyl)piperazino]propyl sulfide 80i and 5-(3-chlorophenyl)-4H-1,2,4-triazol-3-yl 3-[4-(2-pyrimidinyl)piperazino]propyl sulfide 80j against U937 and HL-60, respectively (IC50 = 52.33±3.12, 49.13±2.86 and 29.36±2.23, 18.51±1.16 μM, etoposide standard 10.43±2.0; 1.84±0.20 μM).
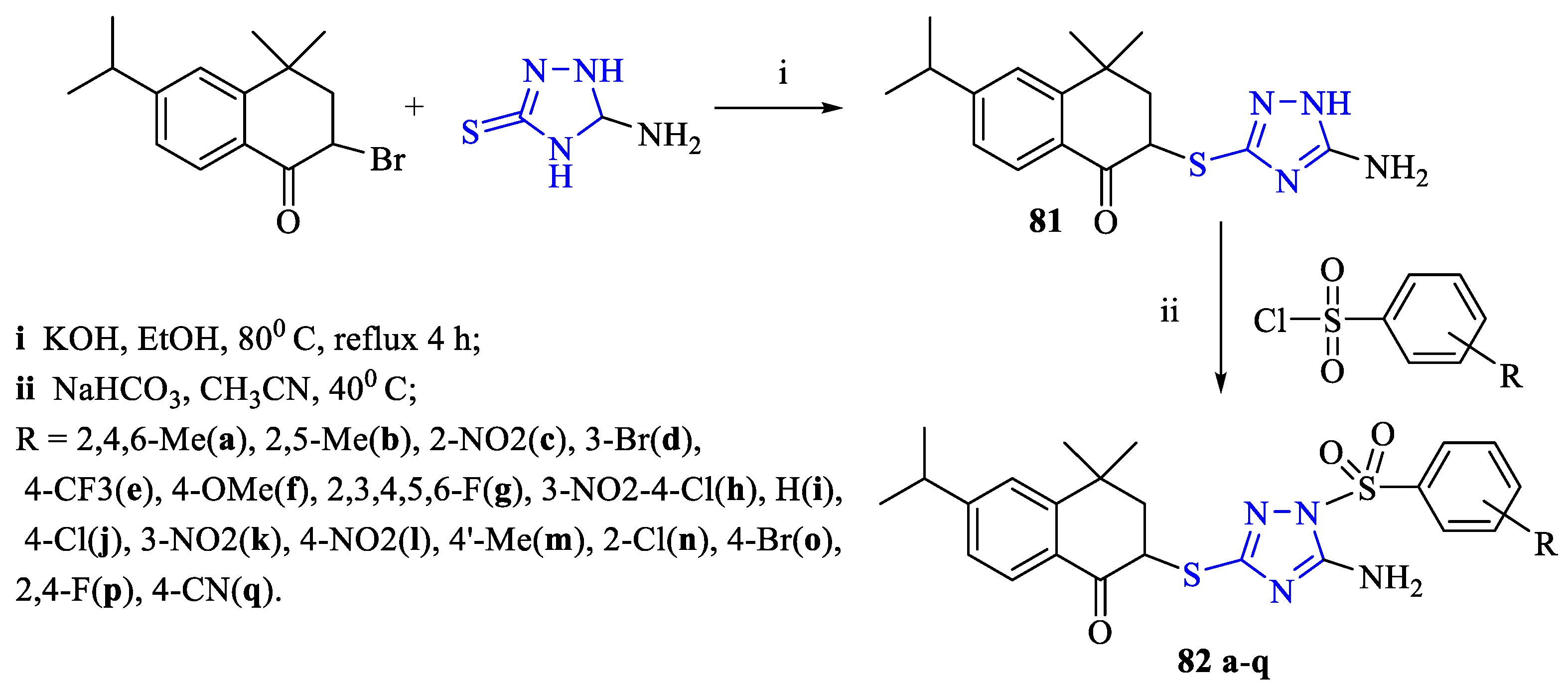
Zhu X.-P. et al. [
86] synthesized a large series of new 2-(5-amino-1-(substituted sulfonyl)-1H-1,2,4-triazol-3-ylthio)-6-isopropyl-4,4-dimethyl-3,4-dihydronaphthalen-1(2H)-ones
82a-q by the reaction of 2-(5-amino-1H-1,2,4-triazol-3-ylthio)-6-isopropyl-4,4-dimethyl-3,4-dihydronaphthalen-1(2H)-one
81 with a series of substituted sulfonyl chlorides (
81 – sulfonyl chloride ratio 1.2:1.5 mmol, NaHCO
3 – 0.13 g, stirring in acetonitrile for 24 h at 40 °C) (
Scheme 36):
The antiproliferative activity of 82a-q against five human cancer cell lines (T-24, MCF-7, HepG2, A549, and HT-29) was assessed by the MTT assay using the antitumor drug 5-fluorouracil (5-FU) as a control. The authors found that the compounds exhibited different antitumor activities against all five cancer cell lines. Thus, compounds 82g, 82h, and 82d demonstrated excellent and broad-spectrum antitumor activity against almost all cancer cell lines studied, whereas compounds 82b, 82c, and 82f demonstrated good activity against A549 and HT-29. It should be noted that the activity of these compounds was better or comparable to that of the control (5-FU). For example, compounds 82g have activity against MCF-7 with IC50 values of 4.42 ± 2.93 µM and 82h against A549 with IC50 values of 9.89 ± 1.77 µM, while the standard has >100 µM. The authors also found and discussed the influence of substituents on the activity exhibited. For example, compound 82h (R=3-NO2-4-Cl) exhibits clearly better antitumor activity than compound 82k (R=3-NO2) and 82j (R=4-Cl), etc. All this, according to the authors, indicated that the position, type and number of substituents significantly affect the antitumor activity.
A series of 5-aryl-1,2,4-triazole-3-thiones
83a-
l and their new derivatives 5-aryl-1,2,4-triazole-3-mercaptocarboxylic acids
84a-
l were synthesized (
Scheme 37):
The authors Shahzad S.A. et al. [
87] studied their inhibitory potential against the enzyme thymidine phosphorylase (TP), which is widely used in the search for compounds with anticancer activities. Of the synthesized compounds
83b,
c,
f,
l showed good inhibitory activity in terms of IC
50 values in the range from 61.98 ± 0.43 to 273.43 ± 0.96 µM, with indicators with IC
50 = 38.68 ± 4.42 µM of the standard 7-deazaxanthin. Based on these parameters, the authors tested 5-aryl-1,2,4-triazole-3-mercaptocarboxylic acids
84a-
l where some of them
84b-
84g showed good inhibitory potential in the range of 43.86±1.11 - 163.43±2.03 µM.
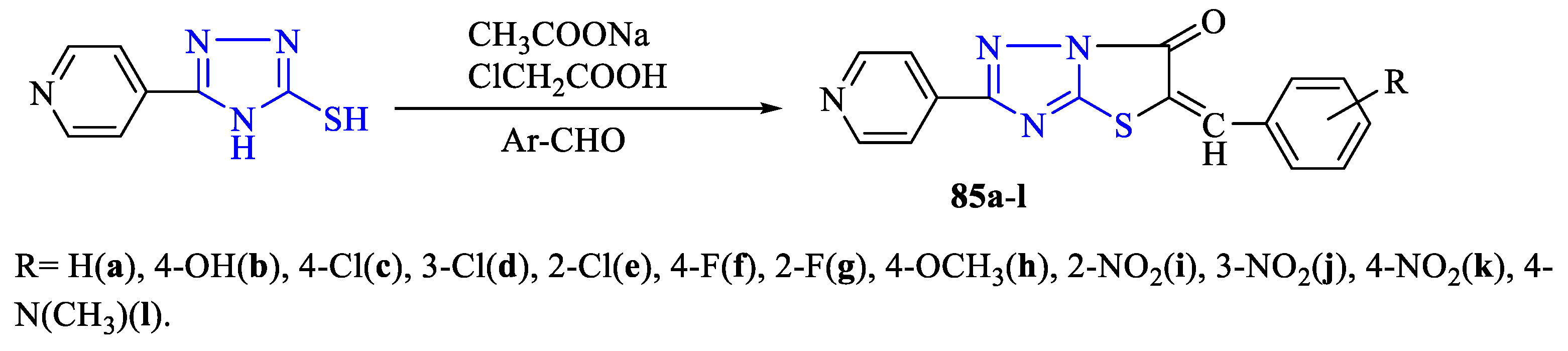
Using a multicomponent reaction, Mruthyunjaya J.H. et al. [
88] synthesized biheterocyclic 2-(pyridin-4-yl)thiazolo [3,2-b][1,2,4]triazol-6(5h)-ones
85a-l by refluxing 5-(pyridin-4-yl)-4H-1,2,4-triazole-3-thiol, monochloroacetic acid, the corresponding benzaldehyde, anhydrous sodium acetate, acetic anhydride, and glacial acetic acid in average yields of 50-73% in ethanol (
Scheme 38):
The cytotoxic activity of the synthesized compounds 85a-l was assessed using a standard MTT assay against two human tumor cell lines - HEK293, and HT-29. Compounds 85a, 85c, 85f, 85h exhibit high extracorporeal cytotoxic activity against the HT-29 cell line - IC50 values 8.25, 6.20, 8.40 and 5.74 μM, respectively. Against the HEK 293 cell line, 85c, 85f and 85h of the tested compounds showed pronounced activity with IC50 values of 6.40, 9.60 and 5.87 µM, respectively. The results of compounds 85a and 85e against the same HEK293 cell line were lower (14.9 and 18.4 µM). According to the authors, the presence of electron-donor groups such as OH, OCH3, N(CH3)2, etc. in the phenyl ring bound by the triazole ring contributes to the manifestation of significant indicators.
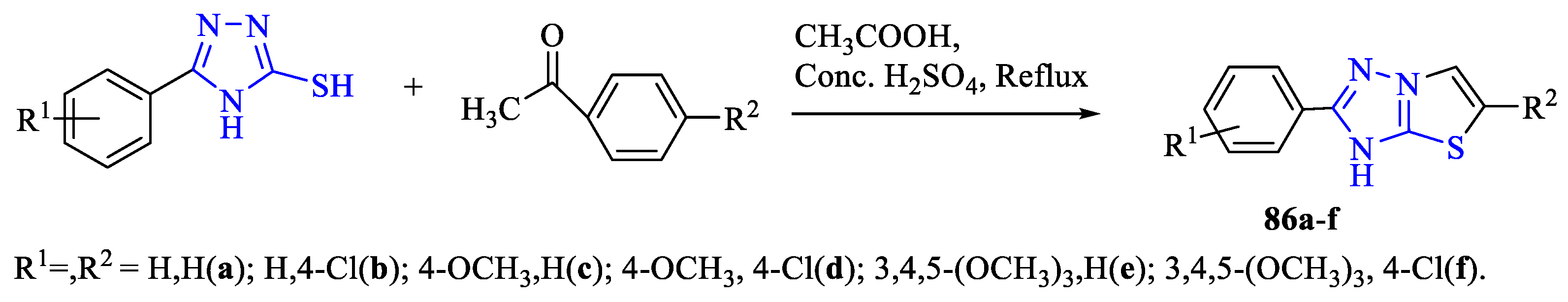
A series of new compounds containing the 1,2,4-triazole framework, 2,5-di(substituted phenyl)thiazolo [3,2-b][1,2,4]triazoles
86a–
f, were obtained. H.A.M. El-Sherif H.A.M. et al. [
89] synthesized compounds
86a–
f by refluxing the corresponding mercaptotriazoles (10 mmol) and substituted acetophenones (15 mmol) in acetic acid for 2-3 h in 63-77% yield (
Scheme 39):
Antiproliferative activity was assessed against the full NCI-60 human tumor cell line panel. Thiazolo [3,2-b][1,2,4]triazoles 86a-e showed variable antiproliferative activity against the same cell lines. Compound 86d was found to be active at five different doses in the NCI assay, showing GI50 values ranging from 0.30 to 6.99 μM.
Compounds 86a-e were also tested against four cell lines using the MTT assay, selecting compounds with the lowest IC50 against three known anticancer targets - EGFR, BRAF and tubulin. The results showed that compound 86d showed promising inhibitory activity against EGFR.
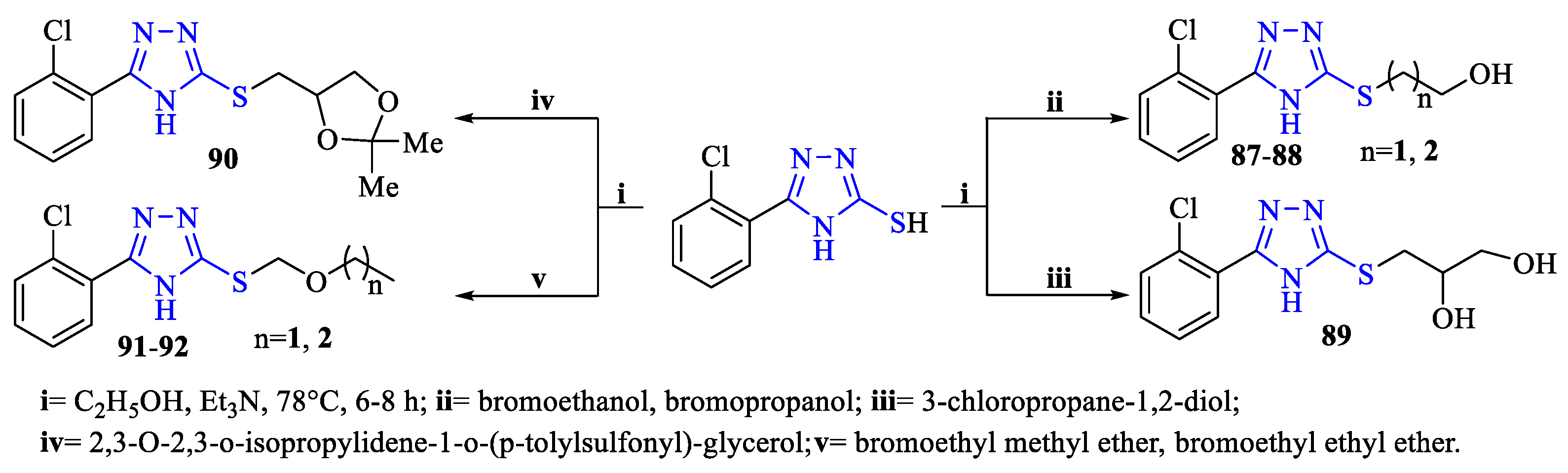
The authors Aouad M.R. et al. [
90] developed and synthesized a new series of regioselective analogues of 5-(2-chlorophenyl)-2,4-dihydro-1,2,4-triazole-3-thione with a yield of 85-91% (C
2H
5OH, TEA, 78°C, 6-8 h) (
Scheme 40):
Synthesized S-acyclonucleosides 87-92 were screened as cytotoxic agents against three cancer cell lines Hep G2, MCF-7, and HCT116. All tested derivatives showed significant cytotoxic activity with IC50 values ranging from 1.05 ± 0.02 µM to 86.62 ± 4.36 µM compared to the reference drug Staurosporine.
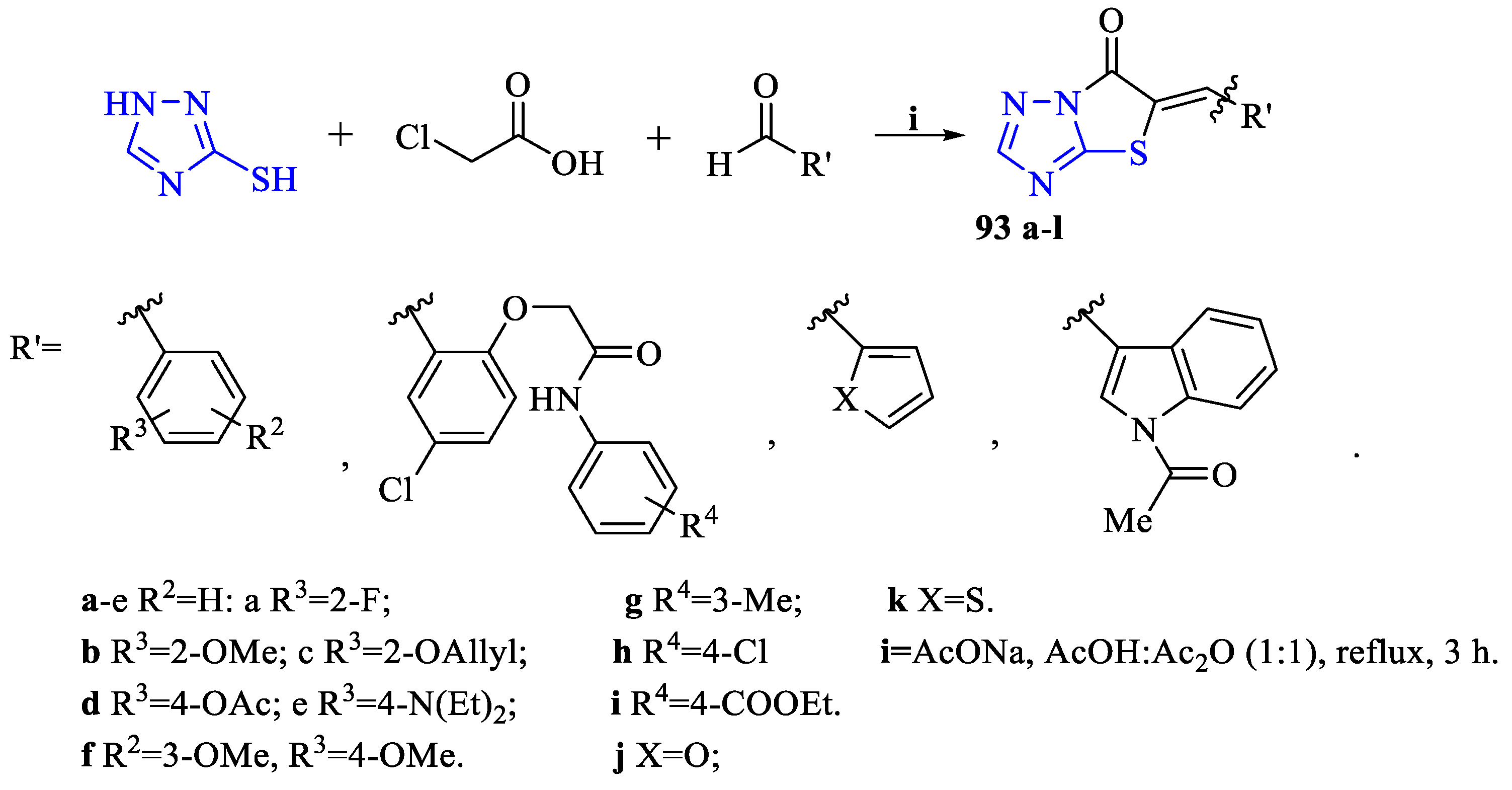
Holota S. et al. [
91] carried out a three-component one-pot reaction of 1,2,4-triazole-3-thiol with chloroacetic acid and aromatic/heteroaromatic aldehydes in a mixture of acetic acid and acetic anhydride (AcOH:Ac
2O) in the presence of AcONa and under gentle heating to give 5-aryl(heteryl)idene-thiazolo [3,2-b][1,2,4]triazole-6(5H)-ones
93a-
l (yield 51–68%) (
Scheme 41):
By selecting different substituents at the C-5 position, the authors aimed to investigate their effect on the pharmacological (anticancer) properties of the obtained thiazolo [3,2-b][1,2,4]triazol-6(5H)-ones 93a–l and to establish the structure-activity relationship. Of the synthesized compounds, 93h and 93i were the most active against cancer cell lines at 10 μM, without exerting toxic effects on normal somatic (HEK293) cells.
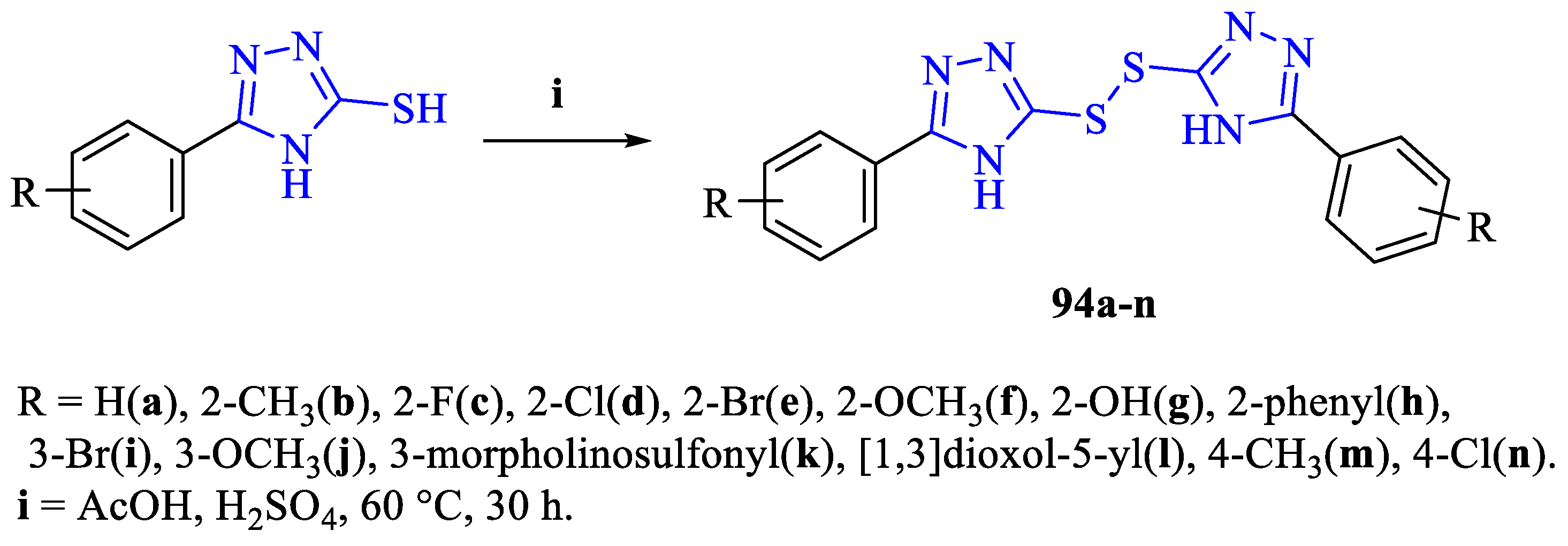
Zhou W. et al. [
92] synthesized 5-(R-phenyl)-4H-1,2,4-triazole-3-thiols with various substituents on the phenyl ring. Then, by heating these triazolethiols with catalytic amounts of concentrated sulfuric acid in acetic acid (AcOH), the corresponding dimer products, 1,2-bis(5-(R-phenyl)-4H-1,2,4-triazol-3-yl)disulfanes
94a-
n were obtained in good yields (67-92%) (
Scheme 42):
The conducted studies of the synthesized bis-products 94a-n showed that some of them (94h) suppressed neddylation of cullin 3 and prevented migration and invasion of two squamous cell carcinoma cell lines with increased expression of DCN1 (KYSE70 and H2170). Based on these results, the authors suggest that 94h may be a promising new compound for the development of anticancer drugs.
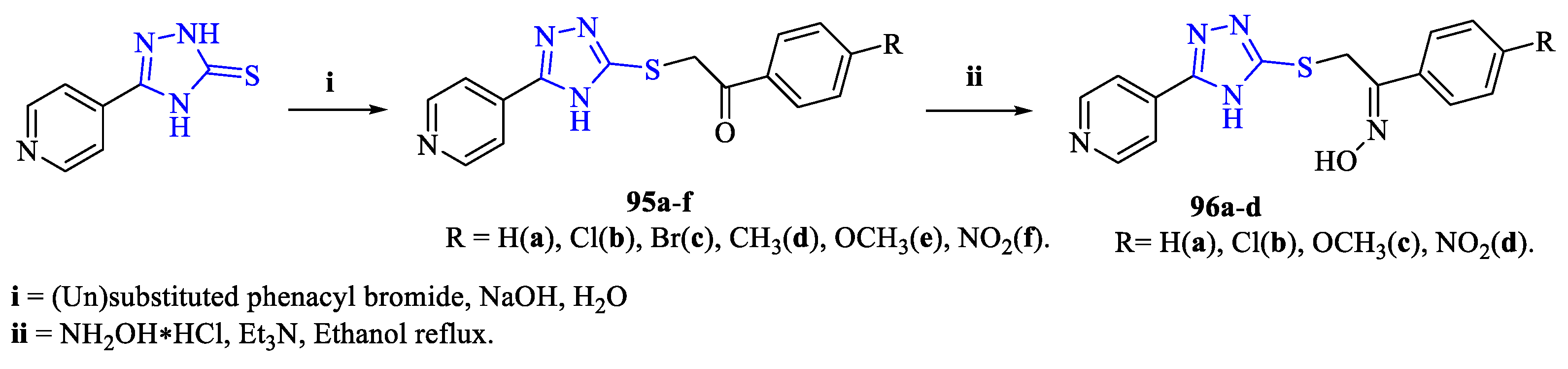
In the reaction of 3-(pyridyl-4-yl)-1H-1,2,4-triazole-5(4H)-thione with substituted phenacyl bromides in aqueous NaOH solution at room temperature, El-Wahab H.A.A.A. et al. [
93] obtained 1-(4-substituted phenyl)-2-((5-(pyridine-4-yl)-4H-1,2,4-triazole-3-yl)thio)ethan-1-one
95a-
f, which were converted by the reaction of NH
2OH⋅HCl (Et
3N, C
2H
5OH, reflux) into the corresponding oxime compounds - 1-(4-substituted phenyl)-2-((4-substituted5-(pyridin-4-yl)-4H-1,2,4-triazol-3-yl)thio)ethenone oxime
96a-
d (
Scheme 43):
All synthesized compounds were tested in vitro for their ability to inhibit the growth of human cancer cell lines NCI-60. The most active compounds 95e and 96b from this series were further tested for inhibition of the EGFR, where they showed IC50 values of 0.14 and 0.18 μM, respectively, compared to Gefitinib as a reference with an IC50 value of 0.06 μM.
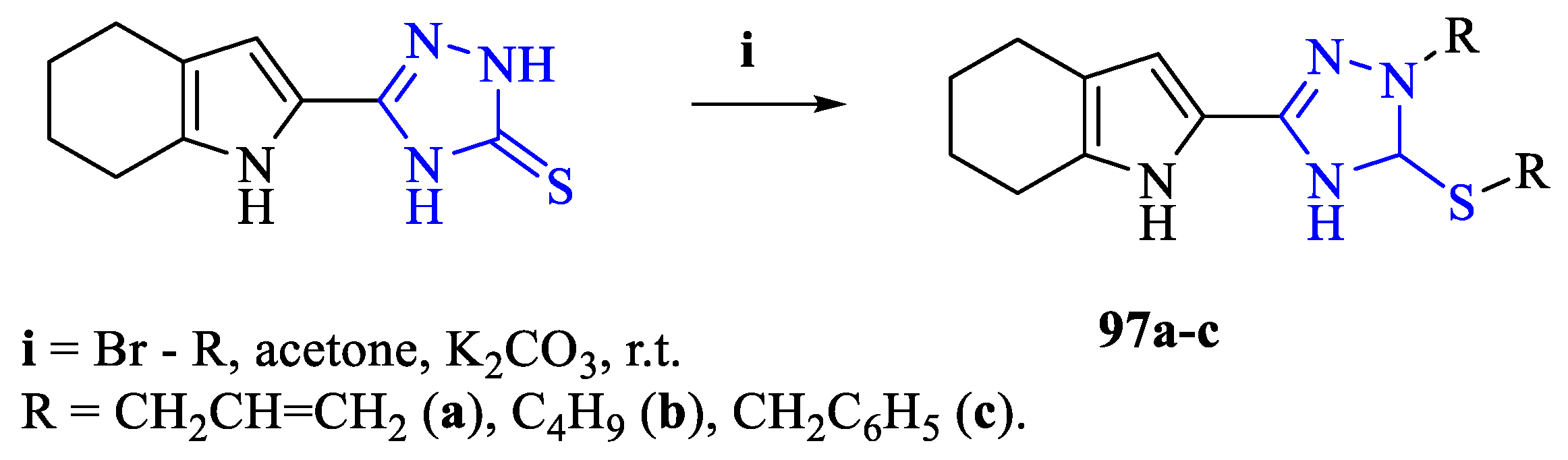
Boraei A.T.A. et al. [
94] synthesized bis S-, 2-N-alkyl isomers
97a-
c (alkyl=allyl, butyl, benzyl) of 1,2-dihydro-5-(1H-indol-2-yl)-1,2,4-triazole-3-thione (
Scheme 44):
The resulting bis products 97a-c were tested for antiproliferative activity on HepG2 and MCF-7 cancer cell lines. The results showed that the benzyl radical containing compound 97c was the most active with IC50 of 3.58 mg/ml and 4.53 mg/ml, respectively (standard drug doxorubicin - IC50 4.0 mg/ml).
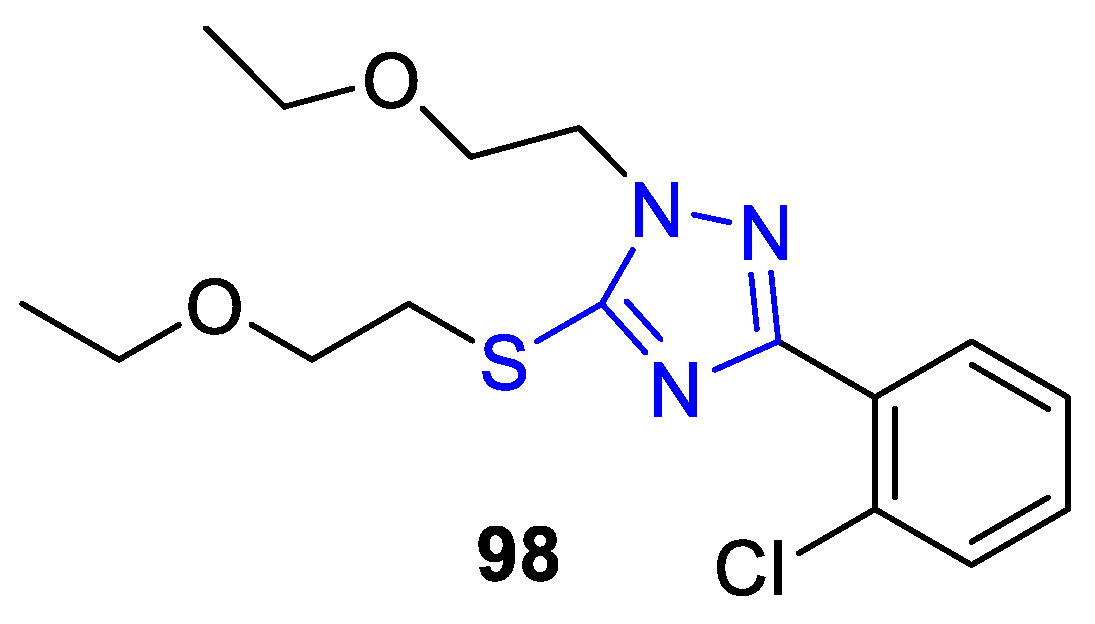
Also, the synthesis of the bis product, S,N-bis(acyclonucleoside) derivative of 5-(2-chlorophenyl)-2,4-dihydro-1,2,4-triazole-3-thione
98, was reported by Aouad M.R. et al. [
90] (
Figure 5):
Cytotoxic screening of S,N-bis(acyclonucleoside) derivative 98 on three different cancer cells - HepG2, MCF-7 and HCT116 showed significant anticancer activity (IC50 1.38, 5.16 and 3.38 μM, respectively).
5. Anti-Inflammatory and Analgesic Activities
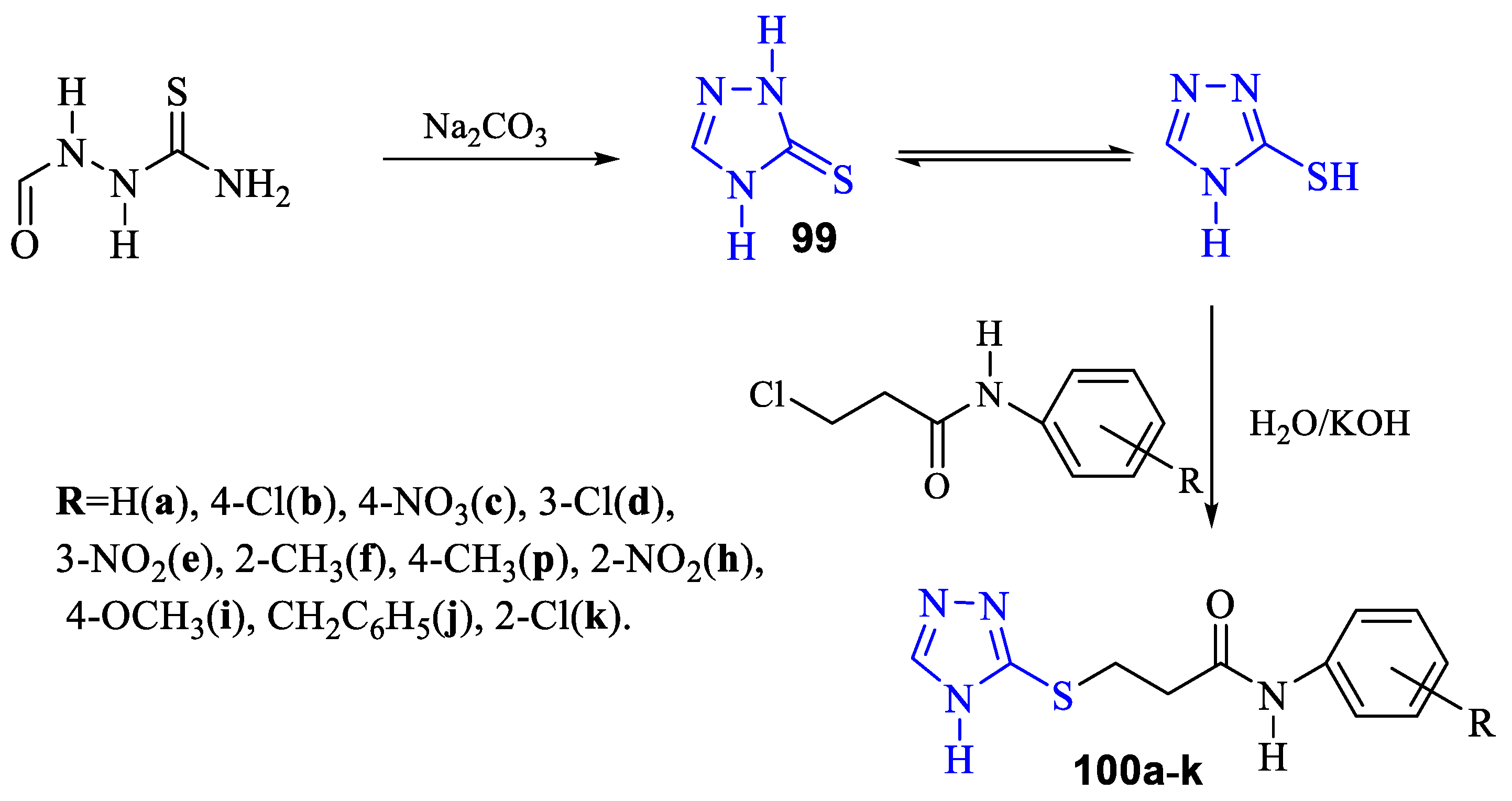
Manikrao A.M. et al. [
95] synthesized 5-unsubstituted 3-mercapto-(4H)-1,2,4-triazole
99 by cyclization of 1-formylthiosemicarbazide in sodium carbonate solution in 63% yield. Further reaction of 3-mercapto-(4H)-1,2,4-triazole with various N-substituted β-chloropropionamides in aqueous KOH solution afforded 3-(N-substituted carboxamidoethylthio)-(4H)-1,2,4-triazoles in moderate yields (24-45%)
100a-
k (
Scheme 45):
The synthesized triazole derivatives
100a-
k exhibited good anti-inflammatory activity, but showed low analgesic activity. Of the tested substances, N-phenyl carboxamidoethylthio-(4H)-1,2,4-triazole
100a showed equipotent anti-inflammatory and analgesic activity compared to standard drugs (Diclofenac Sodium and Tramadol, respectively). In another study by these authors [
96], virtual screening by molecular docking of six major tautomeric forms of compound
100a was investigated. It was found that hydroxy groups formed by tautomerism significantly improve the interaction of drug receptors.
By reacting equimolar amounts of (±)-3-[1-(4-(2-methylpropyl)phenyl)ethyl]-1,2,4-triazole-5-thione
101, the corresponding aromatic aldehydes, chloroacetic acid and sodium acetate in a mixture of acetic acid and acetic anhydride, Uzgören-Baran A. et al. [
97] obtained a series of 6-substituted thiazolo [3,2-b]-1,2,4-triazol-5(6H)-ones
102 containing an ibuprofen residue (
Scheme 46):
All compounds were evaluated for their anti-inflammatory and analgesic activity in vivo in mice. Several of them were found to exhibit analgesic/anti-inflammatory activity without gastrointestinal side effects.
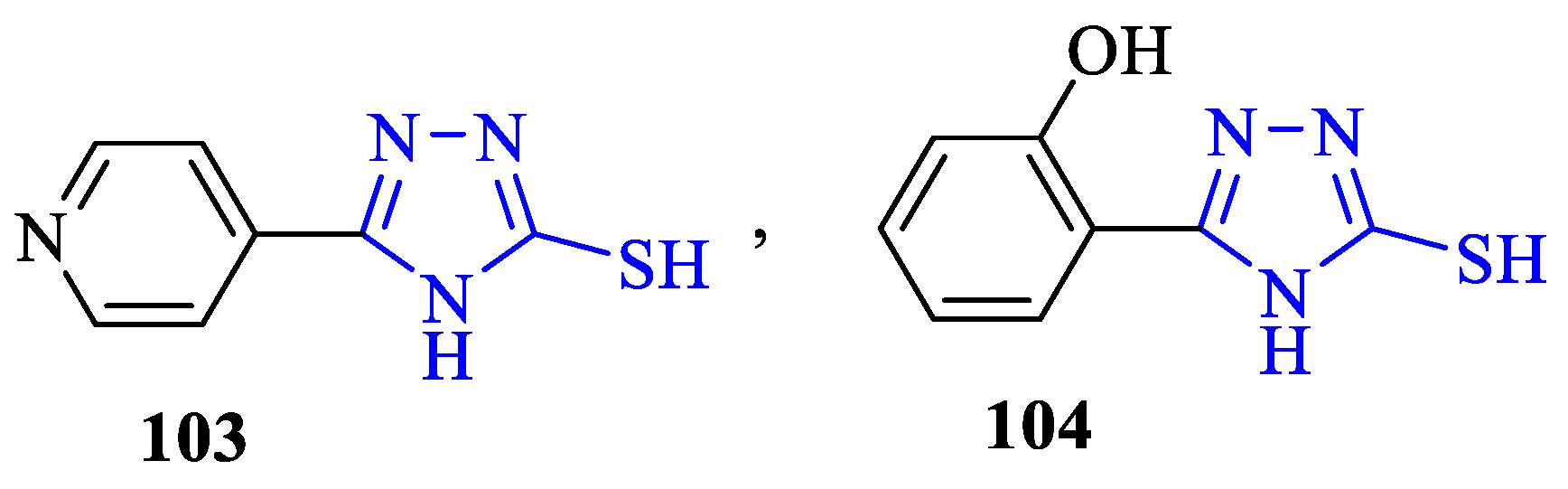
The authors Cetin A. et al. [
98] investigated the total antioxidant and metal chelating activities of 5-(pyridin-4-yl)-2,4-dihydro-1,2,4-triazole-3-thione
103 and 5-(2-hydroxyphenyl)-2,4-dihydro-1,2,4-triazole-3-thione
104. The activities were assessed using various antioxidant assays such as ABTS (2,2’-azino bis(3-ethylbenzothiazoline-6-sulfonate)) and DPPH (1,1-diphenyl-2-picrylhydrazyl) (
Figure 6):
As the authors note, the results were better than expected. Thus, the compound 5-(2-hydroxyphenyl)-2,4-dihydro-1,2,4-triazole-3-thione 104 had a high total antioxidant activity (TAA) with a value of 232.12±6.89 mmol/ml. It also showed fairly good activity with ABTS and DPPH with the values of IC50=4.59±4.19 and IC50=7.12±2.32 mg/ml (Trolox standard 5.76±0.54, BHA ND 38.04±0.98), respectively. The activity of 5-(pyridin-4-yl)-2,4-dihydro-1,2,4-triazole-3-thione 103 was more modest and amounted to 182.88±4.43 mmol/ml, 7.06±5.65 and 78.27±1.27 mg/ml, respectively, according to the TAA, ABTS and DPPH methods. The authors consider the obtained results to be promising for the development of antioxidant drugs.
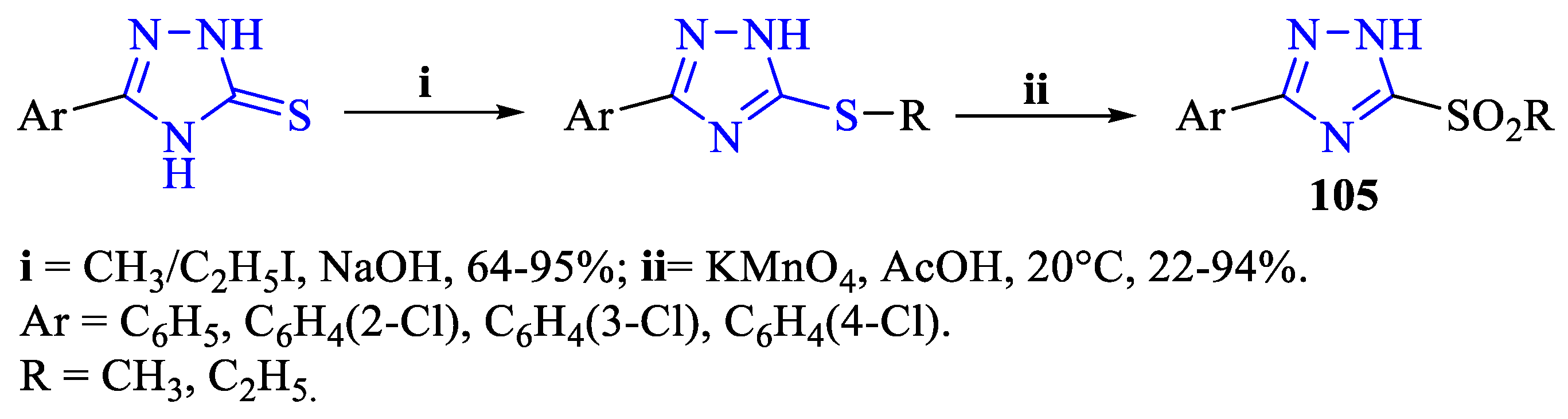
In order to study the analgesic and anti-inflammatory properties, Turkish researchers Tozkoparan B. et al. [
99] synthesized a series of sulfone derivatives from the corresponding 5-aryl-3-alkylthio-1,2,4-triazoles
105 (
Scheme 47):
In addition, studies were conducted in mice to assess ulcerogenic risk and acute toxicity. Compounds with 2-chlorophenyl and 4-chlorophenyl substituents showed significant activity, 37.9% and 40.2%, respectively, at a dose of 50 mg/kg. However, unlike the reference compounds acetylsalicylic acid and indomethacin, they did not cause gastric damage in experimental animals at similar doses. It was also found that alkyl sulfone derivatives are more active than the corresponding alkylthio analogues.
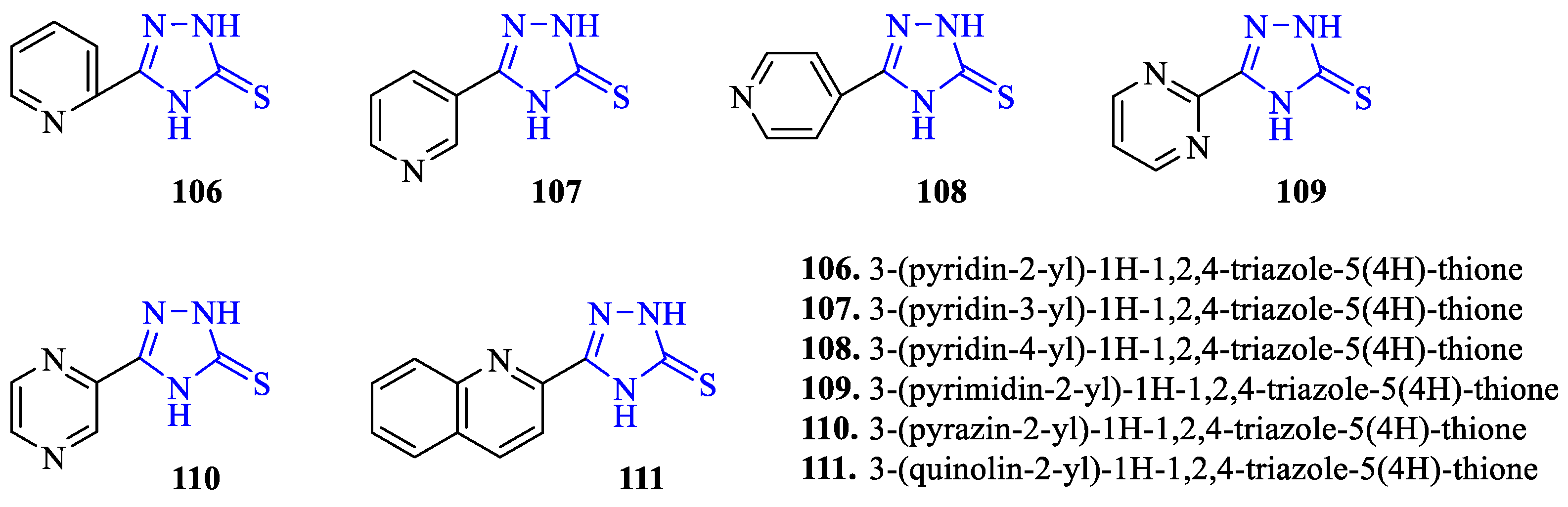
Muneer C.P. et al. [
100] studied the antioxidant activity of several 3-heteryl-1H-1,2,4-triazole-5(4H)-thiones
106-
111 (heteryl = 2,3,4-pyridine, pyrazine, pyrimidine, and quinoline) by spectrophotometrically measuring the change in absorption of DPPH (1,1-diphenyl-2-picrylhydrazyl) at 525 nm in DMSO (
Figure 7):
Of the tested triazolethiones, the highest activity (IC50 48.5 and 42.6 mg/ml) was demonstrated by 3-(pyridin-3-yl)-1H-1,2,4-triazole-5(4H)-thione 107 and 3-(pyridin-4-yl)-1H-1,2,4-triazole-5(4H)-thione 108 with an IC50 value of 49 mg/ml of the standard (ascorbic acid).
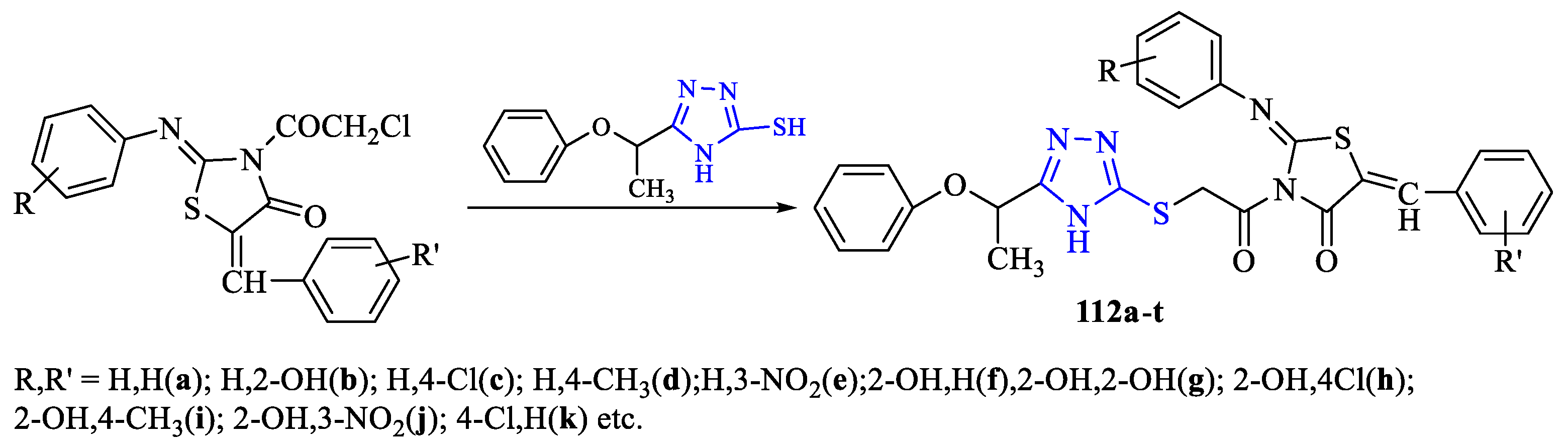
To study the anticonvulsant activity, Shiradkar M.R. et al. [
101] synthesized a series of new 2-[(substituted phenyl)imino]-5-(Z)-1-arylmethylidene-3-(2-[5-(1-phenoxyethyl)-4H-1,2,4-triazol-3-yl]sulfanylacetyl)-1,3-thiazolan-4-ones
112a-
t by reacting 3-(2-chloroacetyl)-2-arylimino-5-(Z)-1-arylmethylidene-1,3-thiazolan-4-one with 5-(1-phenoxyethyl)-4H-1,2,4-triazole-3-thiol in dry benzene (K
2CO
3, TEA) with good yields of the target product, 48-82%(
Scheme 48):
The anticonvulsant activity of all synthesized compounds was evaluated in two animal seizure models - maximal electroshock (MES) and subcutaneous pentylenetetrazole (scPTZ). Compounds 112i, 112g showed excellent anticonvulsant activity in both animal seizure models. The compounds were also evaluated for neurotoxicity.
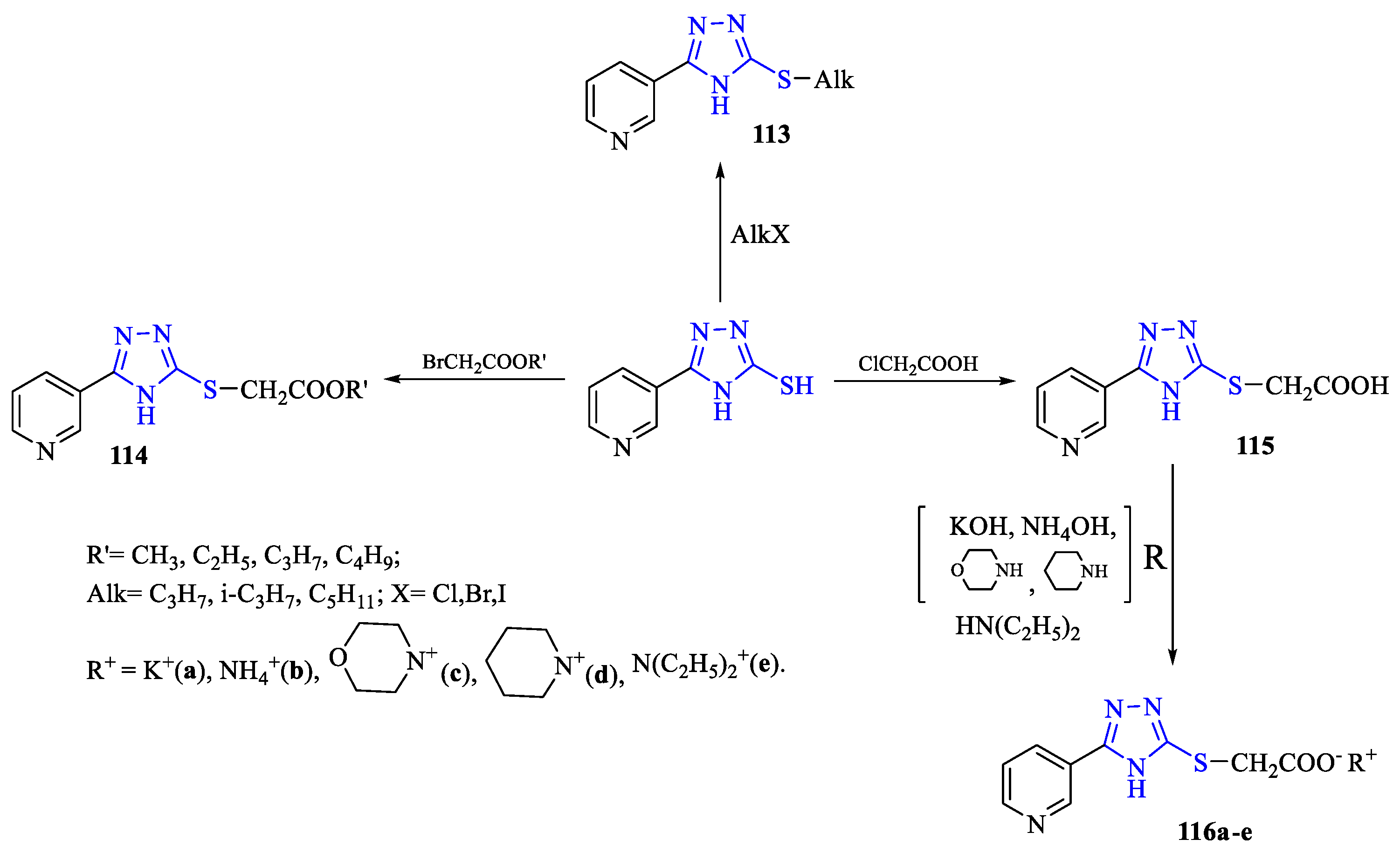
A targeted synthesis of various S-derivatives of 5-(pyridin-3-yl)-2H-1,2,4-triazole-3-thione
113-
116 were carried out with the aim of studying various pharmacological activities (antimicrobial, diuretic, anti-inflammatory, etc.)[
102] (
Scheme 49):
After numerous experiments, the patterns of the structure-action relationship were established. Thus, of the synthesized compounds, 3-[5-(alkylthio)-4R1-1,2,4-triazol-3-yl]pyridines 113 do not exhibit anti-inflammatory activity, whereas the transition to 2-[5-(pyridin-3-yl)-4R1-1,2,4-triazol-3-ylthio]acetic acids 114 and their salts 116a-e is accompanied by the appearance of high anti-inflammatory activity. For example, morpholinium 2-[5-(pyridin-3-yl)-1,2,4-triazol-3-ylthio]acetate 116c exhibits anti-inflammatory activity and low acute toxicity, and also has a pronounced anti-edematous effect in cerebral edema caused by broadband vibration.
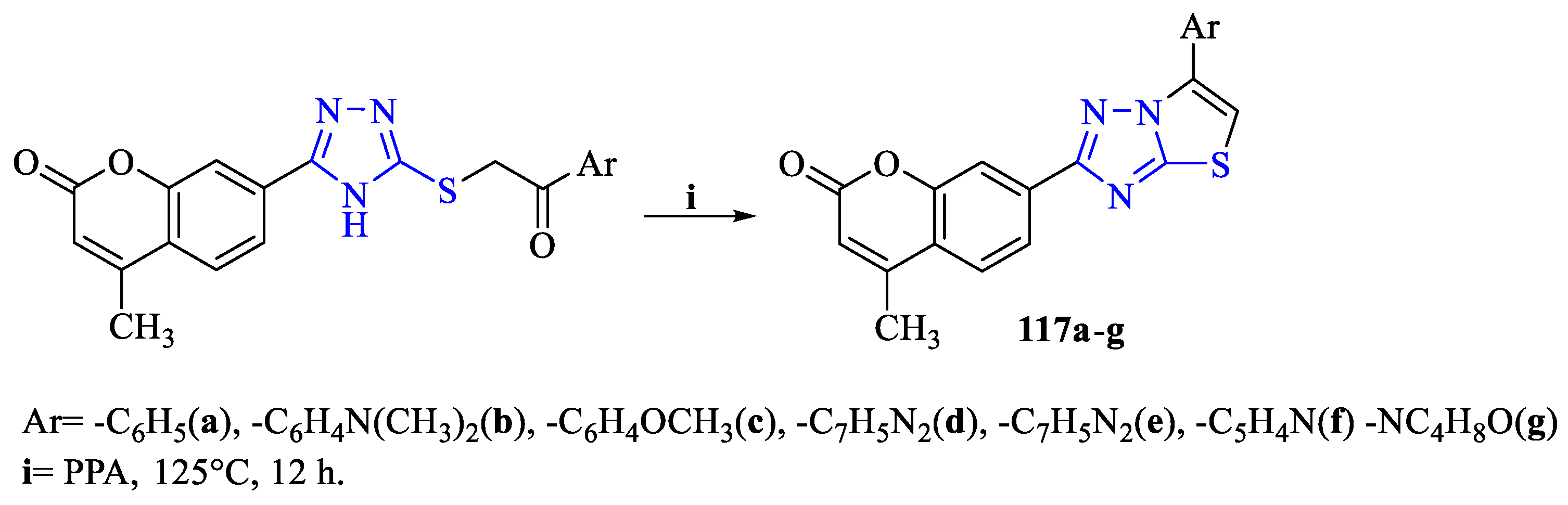
By cyclization in polyphosphoric acid at 125°C, Naseer M.A. et al. [
103] obtained a series of new chromene derivatives - 4-methyl-7-((6-substituted-thiazolo [3,2-b][1,2,4]triazol-2-yl)methoxy)-2H-chromen-2-one
117a-
g (
Scheme 50):
Some of the synthesized compounds 117c, 117f and 117g showed very good anti-inflammatory activity (90.83%, 85.81% and 88.40% respectively) with low gastrointestinal toxicity compared with the standard drug ibuprofen. Meanwhile, other compounds 117a, 117b, 117d and 117f from this group showed the highest analgesic activity with 52.54%, 54.02%, 56.76% and 52.45% respectively. Among them, compound 117d had a higher rate than the standard drug ibuprofen.
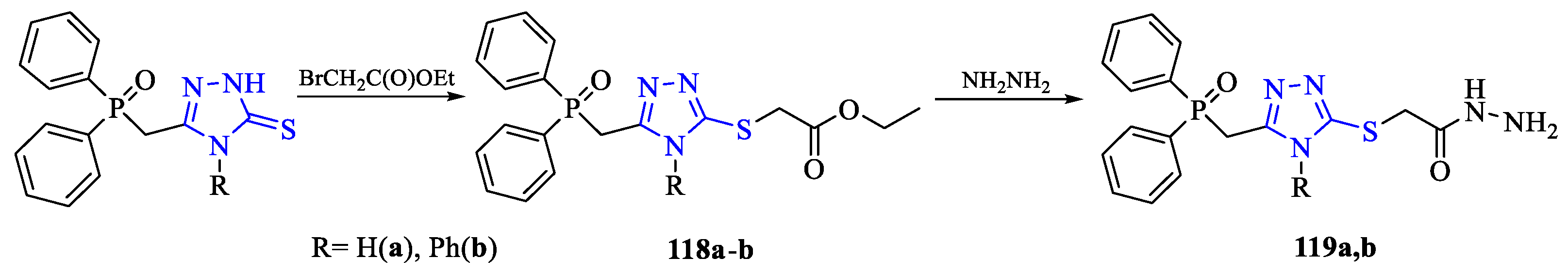
By alkylation at the exocyclic atom (S) of 5-[(Diphenylphosphoryl)methyl]-2,4-dihydro-3H1,2,4-triazole-3-thione with ethyl 2-bromoacetate, Krutov I.A. et al. [
104] obtained ethyl{5-[(diphenylphosphoryl)methyl]-4H-1,2,4-triazole-3-yl}sulphanylacetate
118 (K
2CO
3, acetone, yield 75%). Then, ester
118 was converted by hydrazinolysis (NH
2NH
2, ethanol) to the corresponding hydrazide 2-{5-[(Diphenylphosphoryl)methyl]-4H-1,2,4-triazole-3-yl}sulphanylacethydrazide
119 in high yield, 90% (
Scheme 51):
The study of pharmacological activity showed that the compounds exhibited neurotropic activity (behavioral tests at doses of 1/50 and 1/100 LD50) with low toxicity (abdominal injection to mice, LD50 value from 300 to 800 mg/kg). These results indicate the need to continue further work on the synthesis of new compounds as potential drugs with psychotropic properties.
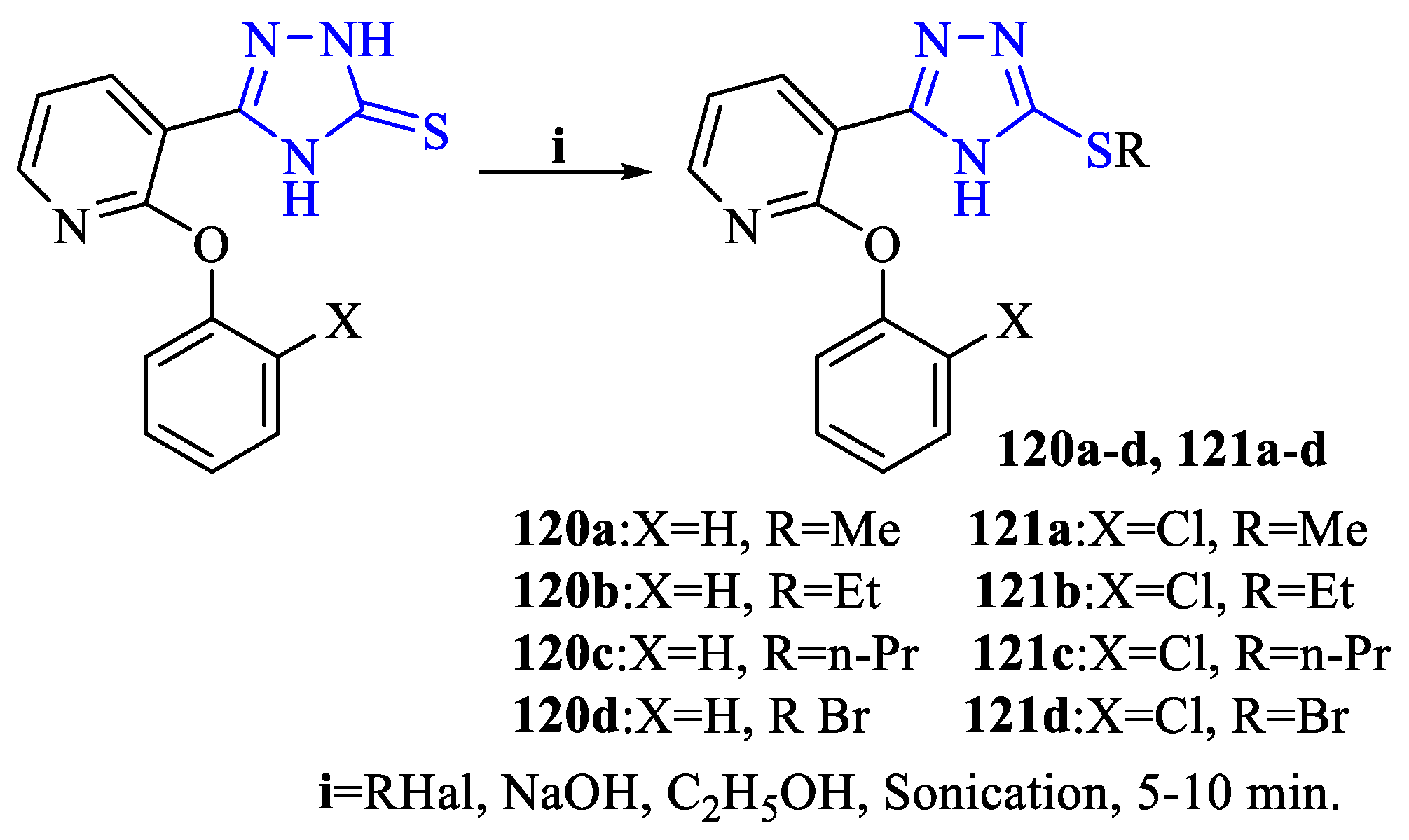
By ultrasonic treatment of 5-(2-phenoxypyridin-3-yl)-2,4H-1,2,4-triazole-3-thione and 5-(2-(2-chlorophenoxy)pyridin-3-yl)-2,4H-1,2,4-triazole-3-thione with alkyl halides in an aqueous-ethanol solution of sodium hydroxide, Navidpour L. et al. [
105] obtained the corresponding 3-alkylthio-5-(2-phenoxy-3-pyridyl)-4H-1,2,4-triazoles
120a-
d and 5-(2-(2-chlorophenoxy)-3-pyridyl)-3-methylthio-4H-1,2,4-triazoles
121a-
d (
Scheme 52):
Their anticonvulsant activity was assessed, with some of them (120b, 120c, 121b) having significantly higher (IC50 0.05, 0.06 and 0.04 μM, respectively) IC50 values at 2.4 μM than the reference drug diazepam.
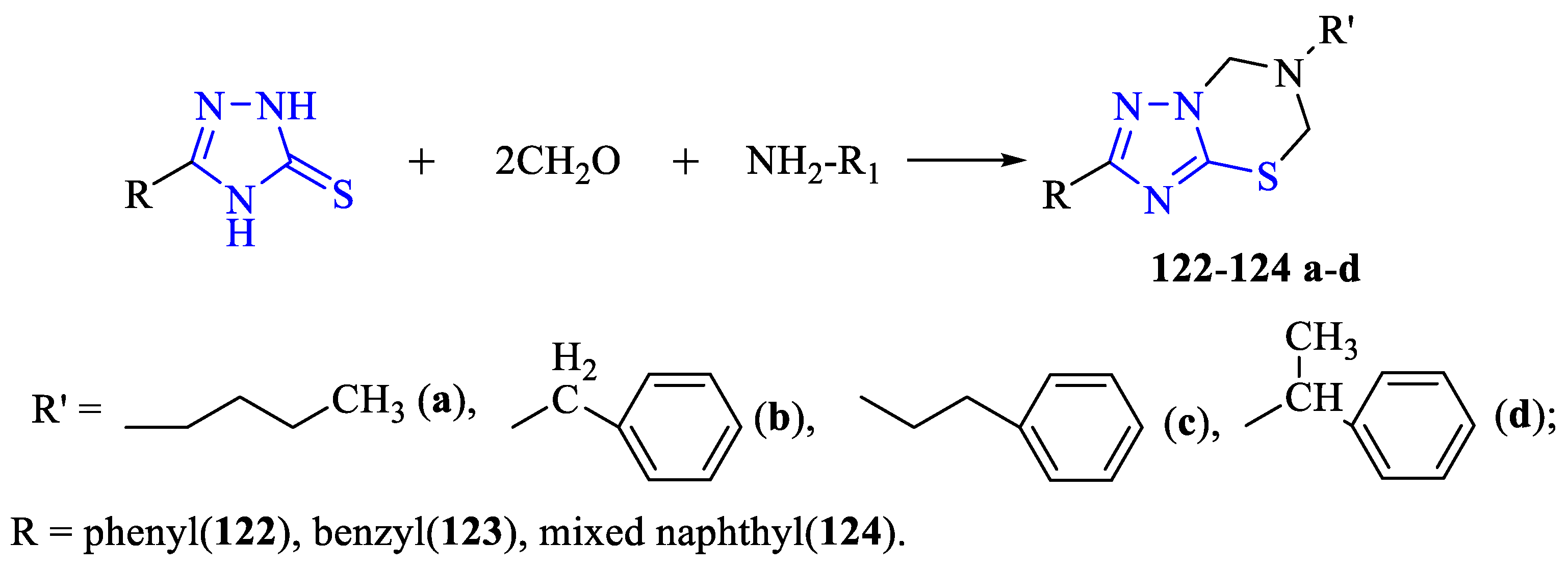
Mannich reactions were carried out by Sert-Ozgur S. et al. [
106] with 3-aryl-, 3-arylalkyl-1,2,4-triazole-5-thiones and various primary amines such as butyl-, benzyl-, 2-phenethyl- and phenethylamines using 2 mol of formaldehyde in ethanol (
Scheme 53):
The target 2,6-disubstituted-6,7-dihydro-5H-1,2,4-triazolo [3,2-b]-1,3,5-thiadiazines 122–124a–d were obtained in moderate to good yields (50–85%) and evaluated for anti-inflammatory and analgesic activity. Several fused compounds demonstrated analgesic activity comparable to reference drugs (naproxen, indomethacin). Compounds containing a benzyl group at the second position 123a–c showed strong anti-inflammatory activity.
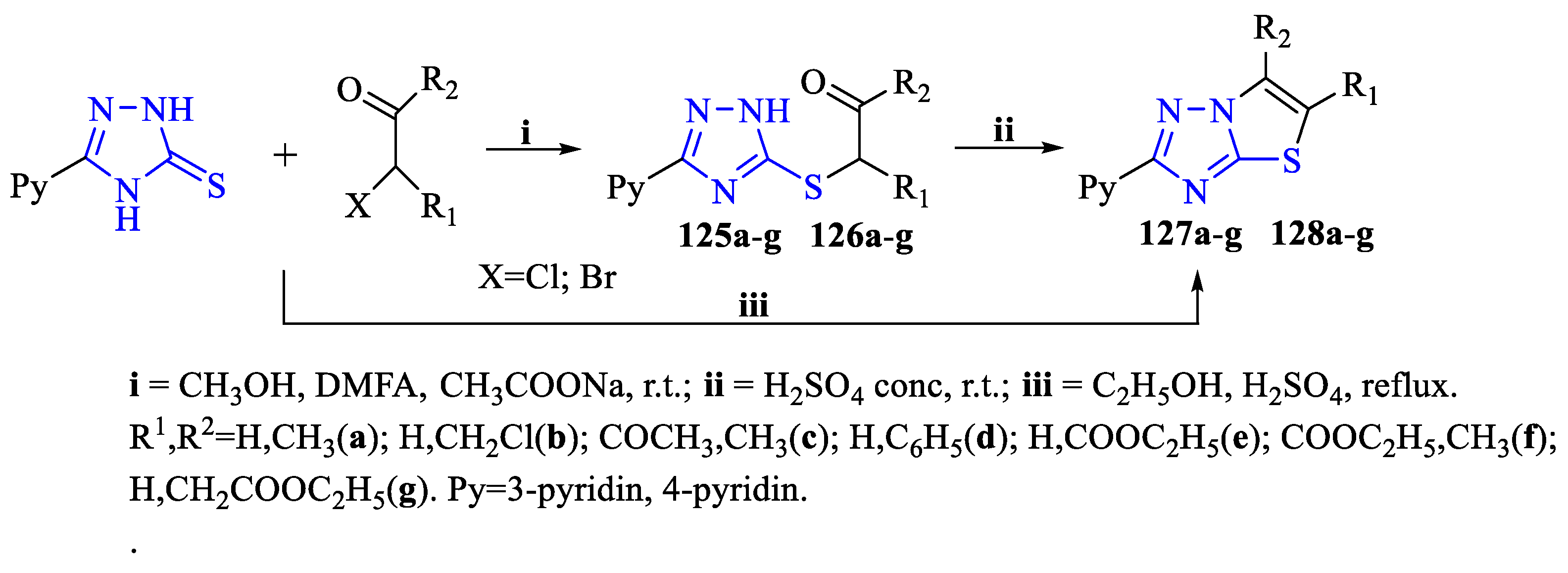
By condensation reaction of 5-pyridin-3/4-yl-1,2,4-triazole-3-thiols and various α-halocarbonyl compounds at room temperature and under basic conditions, Thoma A.et al. [
107] synthesized pyridin-3/4-yl S-alkylated 1,2,4-triazole compounds
125a-
g,
126a-
g. Further cyclization of these compounds under acidic conditions (H
2SO
4) leads to the formation of pyridin-3/4-yl-thiazolo [3,2-b][1,2,4]triazoles
127a-
g and
128a-
g. Carrying out this reaction at reflux and under acidic conditions also leads to the production of pyridin-3/4-yl-thiazolo [3,2-b][1,2,4]triazoles in one step without isolation of intermediate alkyl derivatives (
Scheme 54):
Anti-inflammatory screening of the obtained compounds showed that cyclic compounds with 4-pyridyl 128c,d,f possess good anti-inflammatory activity, while compounds with 3-pyridyl 127d,f show moderate activity. It should be noted that S-alkylated derivatives of pyridin-3/4-yl-1,2,4-triazoles 125d,f,g and 126c,d,f,g showed rapid but short-term anti-inflammatory activity.
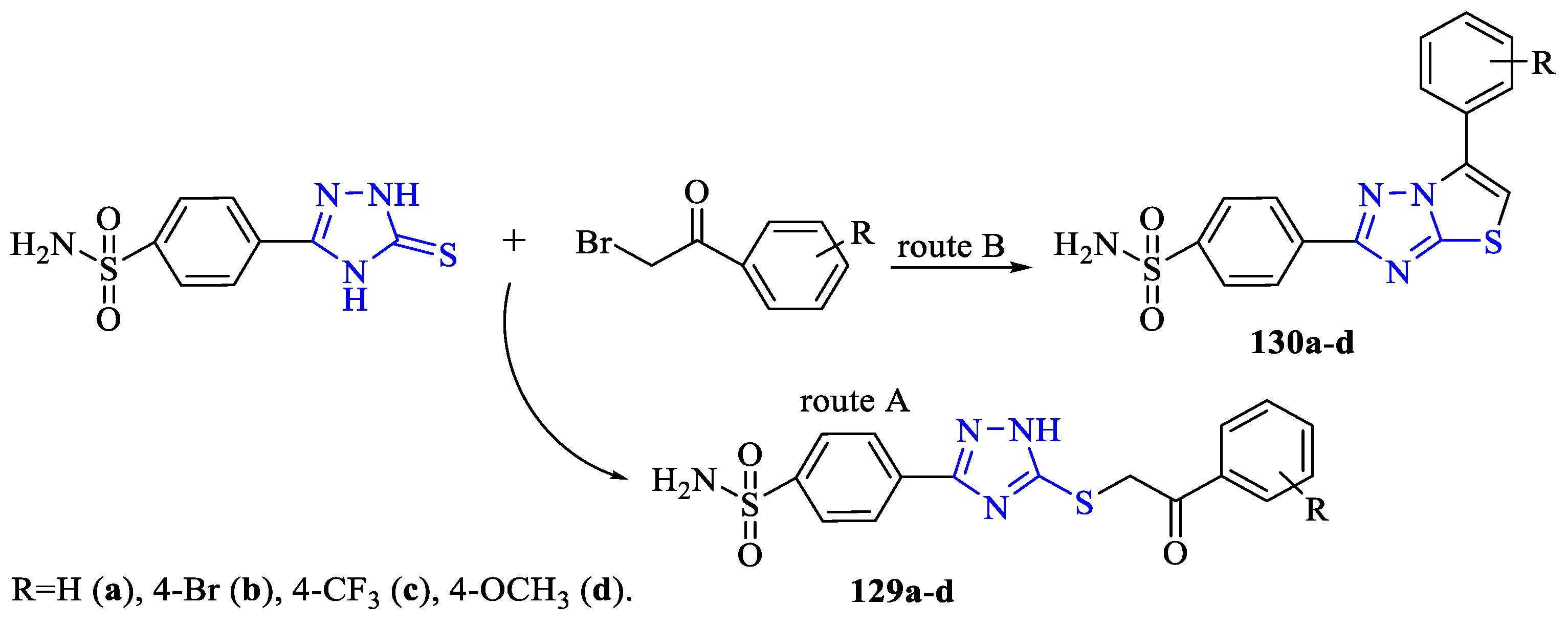
Cristina A. et al. [
108] synthesized several bicyclic 4-(6-(R-phenyl)thiazolo [3,2-b][1,2,4]triazol-2-yl)benzenesulfonamides
130 a-
d using two methods (route B). In the first procedure (route B), a mixture of 4-(5-thioxo-4,5-dihydro-1H-1,2,4-triazol-3-yl)benzenesulfonamide and the corresponding phenacyl bromide in absolute ethanol was refluxed for 2-3 h, and after cooling, concentrated H
2SO
4 was added to the mixture. According to the second route (route A), cyclization was carried out by keeping the previously obtained corresponding 4-(5-((2-aryl-2-oxoethyl)thio)-1H-1,2,4-triazol-3-yl)benzenesulfonamide
129a-d in concentrated sulfuric acid for 1-12 hours (
Scheme 55):
All synthesized compounds were evaluated in vivo for their anti-inflammatory (in a rat model of acute inflammation induced by λ-carrageenan) and antinociceptive activities. Compounds 129b, 129c and 130d showed significant anti-inflammatory activity compared to the control group, but their values were lower than those of the reference drug - diclofenac. Also, compounds 129 a,b,c and 130a,d showed a significant increase in the nociceptive threshold (model of inflammatory hyperalgesia).
6. Pesticidal Activity
As can be seen from the data presented in the previous sections, there is a lot of information on various pharmacological activities (antimicrobial, antioxidant, antitumor, etc.) of compounds containing the heterocycle 2,4-dihydro-1,2,4-triazole-3-thione. Our analysis of the literature shows a small number of works devoted to the pesticidal activity of the object under consideration.
Currently, several commercial preparations containing the 1,2,4-triazole group in the form of free or condensed substituents are used in practice. These preparations include the herbicides Penoxsulam (trade name Granite
® manufacturer Dow Agro Sciences, 2004) active ingredient 2-(2,2-difluoroethoxy)-N-(5,8-dimethoxy [1,2,4]triazolo [1,5-c]pyrimidin-2-yl)-6-(trifluoromethyl)-benzenesulfonamide, Pyroxsulam (Simplicity
® Dow Agro Sciences 2008) active ingredient N-(5,7-dimethoxy [1,2,4]triazolo [1,5-a]pyrimidin-2-yl)-2-methoxy-4-(trifluoromethyl)-3-pyridinesulfonamide, Thienecarbazone-methyl (Adengo
® Bayer Crop Science 2008) active ingredient Methyl ester 4-[[[(4,5-dihydro-3-methoxy-4-methyl-5-oxo-1H-1,2,4-triazol-1-yl)carbonyl]amino]sulfonyl]-5-methyl-3-thiophenecarboxylic acid [
29].
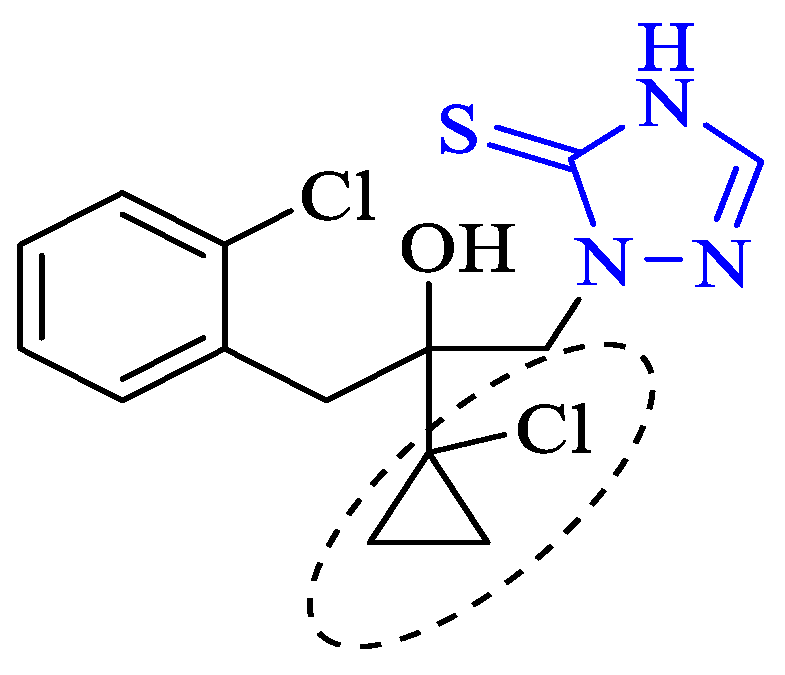
Another commercial product developed by Bayer Crop Science in 2004 is the fungicide Prothioconazole (Proline
®), the active ingredient of which is 2-[2-(1-chlorocyclopropyl)-3-(2-chlorophenyl)-2-hydroxypropyl]-1,2-dihydro-3H-1,2,4-triazole-3-thione (
Figure 8):
Prothioconazole, in addition to the 1,2,4-triazole-5-thione ring system, contains an o-chlorobenzyl substituent together with an innovative chlorinated cyclopropyl moiety, which, as new lipophilic moieties, exhibit high fungicidal activity. The commercial product prothioconazole is a mixture of two active enantiomers, which allows it to exhibit a broad spectrum of fungicidal activity, high bioavailability and long-term efficacy. It shows very good results in the control of agricultural pathogens in cereals and legumes, including stem and base diseases, the all-important leaf spot diseases, as well as rusts of cereals (
Puccinia spp.), powdery mildew (
Blumeria graminis) and white mold (
Sclerotinia sclerotorium) of rapeseed [
109,
110,
111,
112]. In addition, prothioconazole exhibits plant growth promoting activity (PGR), which is a useful tool for managing plant development [
113].
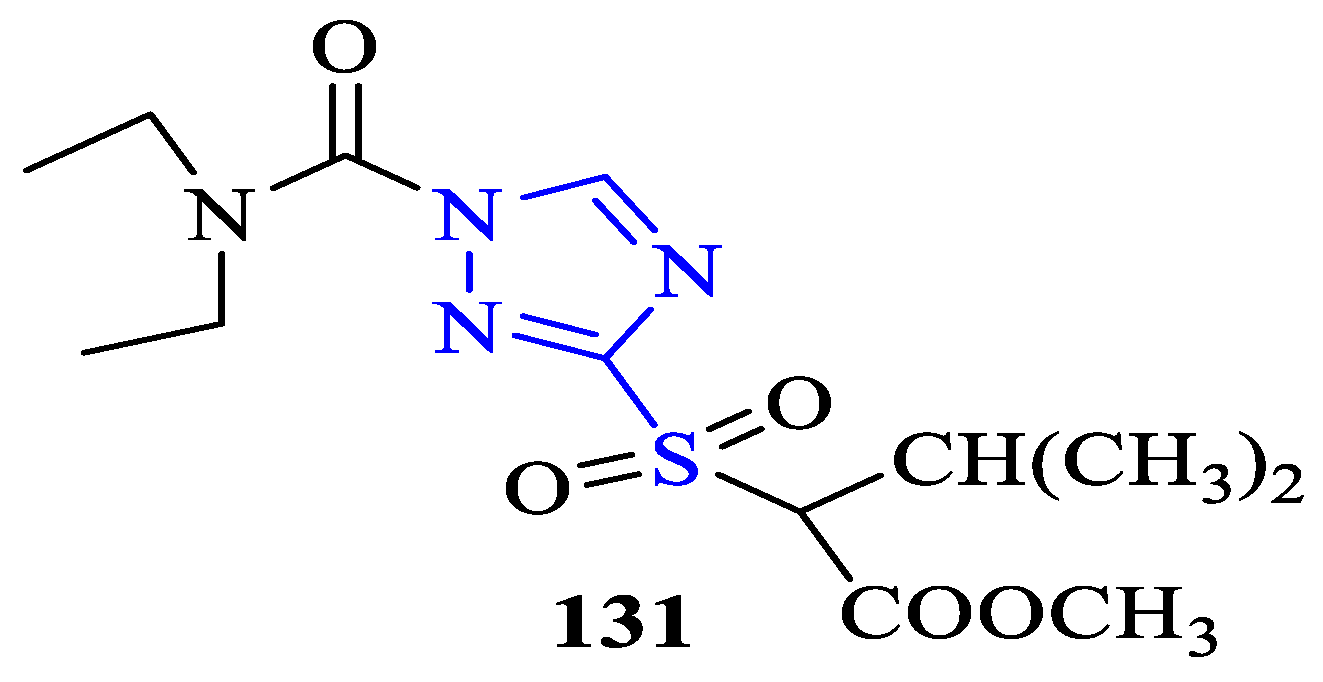
Yano T. et al. [
114] synthesized a series of 2-(1-N,N-dialkylcarbamoyl-1,2,4-triazol-3-ylsulfonyl)alkanoates
131 and tested them for herbicidal activity against the weeds
Monochoria vaginalis,
Echinochloa oryzicola, broadleaf weeds, and
Scirpus juncoides (
Figure 9):
The herbicidal efficacy varied depending on the substituents at the α-position of the alkoxycarbonyl group and the nitrogen atom of the carbamoyl fragment. It was found that of the tested compounds, 1-N,N-dialkylcarbamoyl-1,2,4-triazoles having a branched alkyl group at the α-position of the alkoxycarbonyl group exhibited the highest herbicidal activity. Based on the data obtained, isopropyl 2-(1-N,N-diethylcarbamoyl-1,2,4-triazol-3-ylsulfonyl)-4-methylpentanoate was selected as a promising herbicide for further studies on transplanted rice.
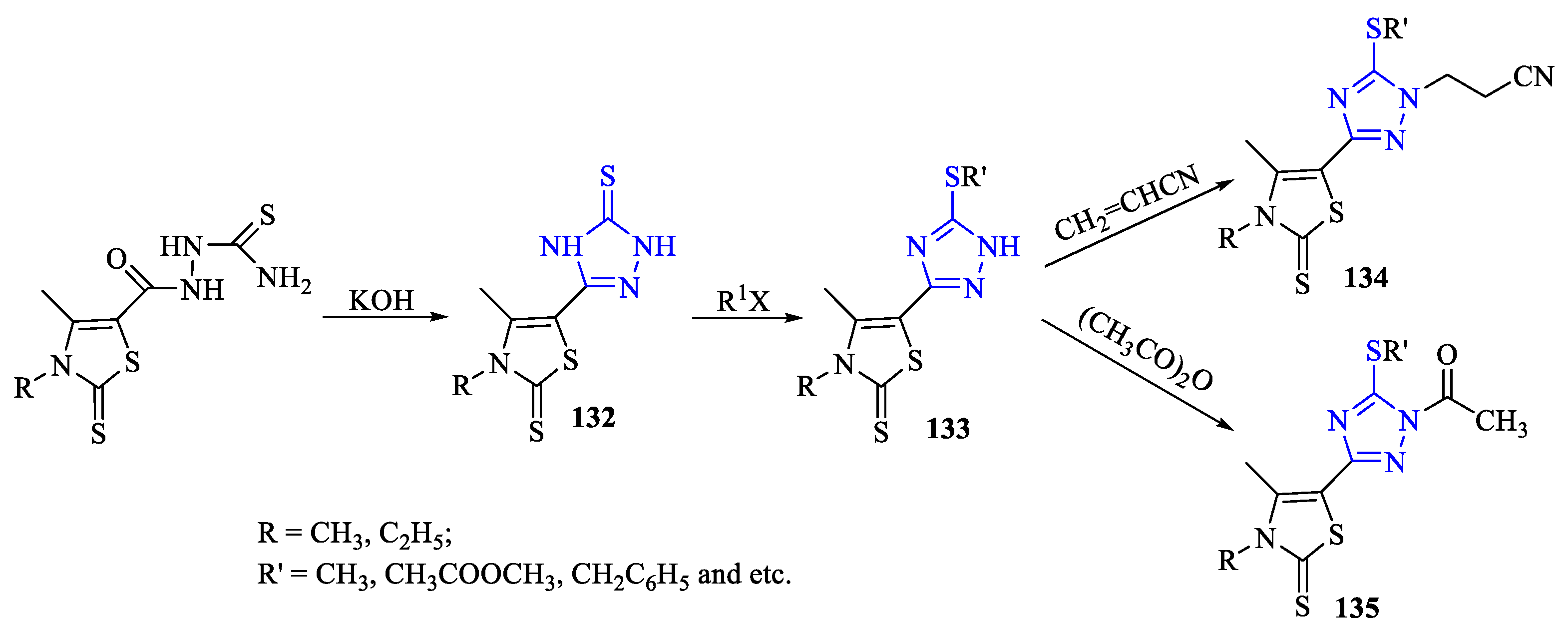
By cyclization of (2-thioxo-3-methyl(ethyl)-4-methyl-3H-thiazol-5-yl)-(thiosemicarbazide-1-yl)-methanones with an excess of aqueous potassium hydroxide solution upon heating, Knyazyan A.M. and co-authors [
115] obtained 5-(2-thioxo-3-methyl(ethyl)-4-methyl-3H-thiazol-5-yl)-2,4-dihydro-[1,2,4]-triazole-3-thiones
132, in the molecules of which the thiazole and 1,2,4-triazole rings are directly linked to each other (
Scheme 56):
The resulting bis-heterocycles 132 are alkylated (CH3I, ClCH2COOCH3, ClCH2C6H5, etc.) primarily at the exocyclic sulfur atom of the triazole ring to form the corresponding 5-sulfanyl derivatives 133. The compounds (R=R1=CH3) then react selectively with electrophilic reagents (acrylonitrile, phenyl isocyanate, and acetic anhydride) to form derivatives primarily at the nitrogen atom 134, 135 in the second position of the 1,2,4-triazole ring. The authors believed that the synthesis of compounds with a combination of two heterocycles and various substituents would be of interest as substances potentially possessing new physiological properties. Biological screening showed that the synthesized compounds exhibit a valuable combination of growth-stimulating and fungicidal action. Some substances demonstrated growth-stimulating activity in the experiment of 80-100% compared to the widely used preparation heteroauxin. At the same time, the compounds in concentrations of 0.1 and 0.01% completely suppress the growth of loose smut of wheat, and in the minimum concentration of 0.001% - from 60 to 90%. These data indicate the prospects for further studies of a new series of synthesized compounds in terms of searching for preparations with a combination of two important properties.
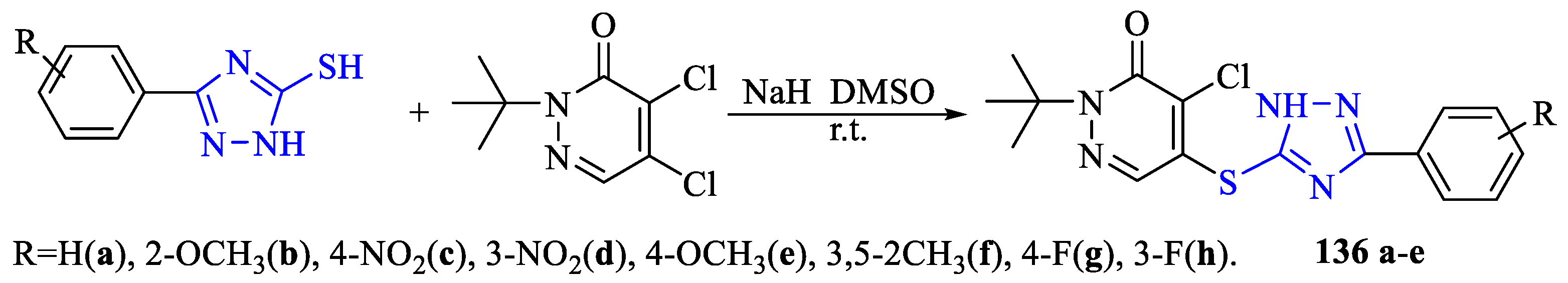
Eight new compounds 2-t-Butyl-4-chloro-5[(3-(R-phenyl)-1H-1,2,4-triazol-5yl)thio]pyridazin-3(2H)-one
136a-h were synthesized by Chai B. et al. [
116]. The reaction was carried out by stirring a mixture of equimolar amounts of 5-(R-phenyl)-1,2,4-triazole-3-thiones, 2-t-butyl-4,5-dichloro-pyridazinone and NaH in DMSO at room temperature. S-derivatives
136a-
h were obtained in 54-72% yield (
Scheme 57):
The activity of all synthesized compounds was tested by leaf dip method. Compounds 136d,e,g showed insecticidal activity against Aphis rumicis Linnaeus - 45%, 38% and 30% respectively at concentration of 500 mg.
7. Other Types of Biological Activity
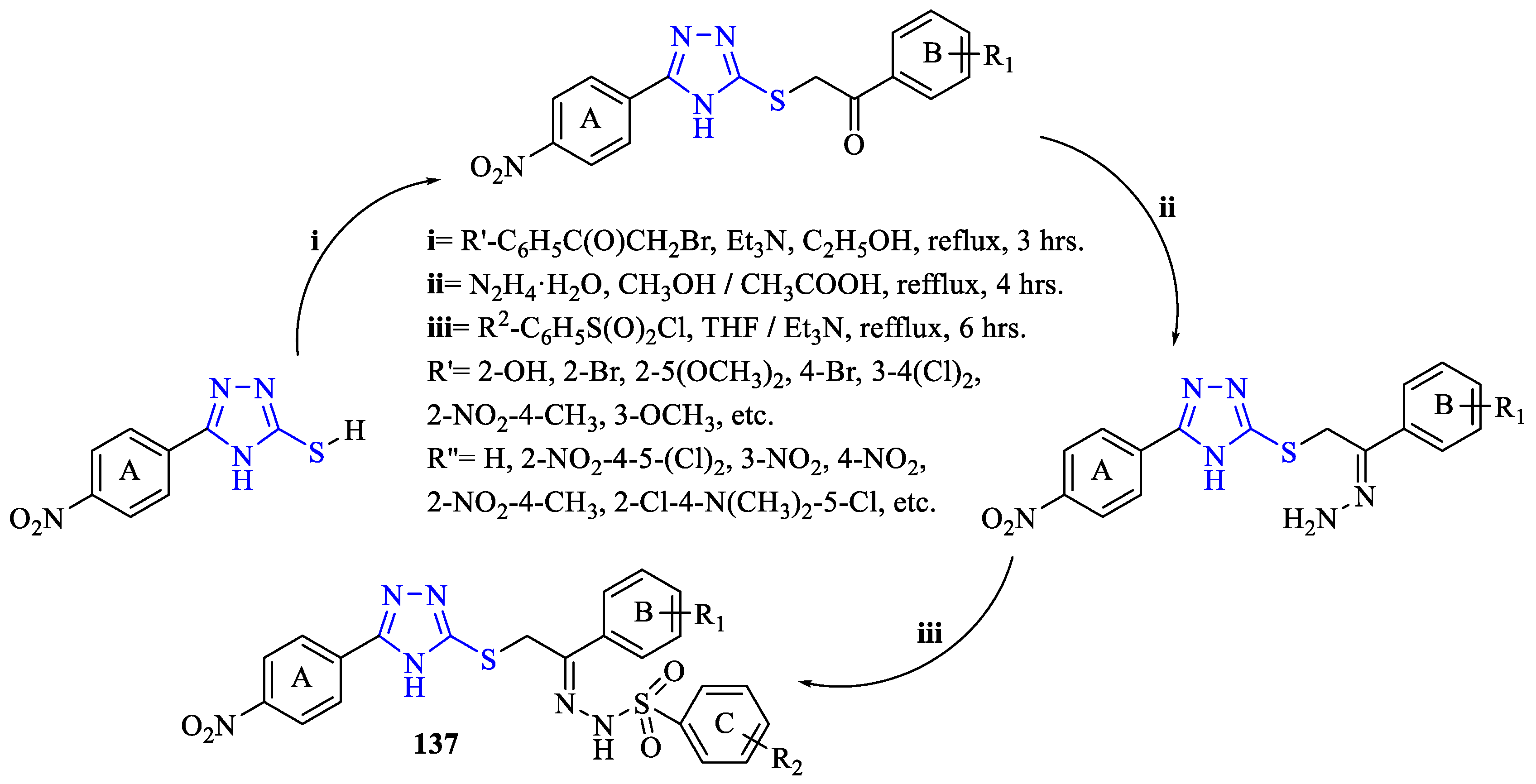
Othman M.S. et al. [
117] synthesized in several stages 1,2,4-triazole-containing derivatives of sulfhydrazide
137 having different (electron-withdrawing or electron-donating) properties on the phenyl rings (
Scheme 58):
Most of the synthesized compounds showed good or excellent inhibitory activity against acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) enzymes with IC50 values ranging from 0.30±0.050 to 15.21±0.50 μM (against AChE) and from 0.70±0.050 to 18.27±0.60 μM (against BuChE). The values of the reference drug Donepezil were IC50=2.16±0.12 and 4.5±0.11 μM, respectively. The highest result (IC50= 0.30±0.050 and 0.70±0.050 μM for AChE and BChE, respectively) was obtained for a compound containing chlorine atoms in the 3rd and 4th positions of ring B and a nitro group in the 3rd position of ring C. The authors identified a structure-activity relationship that mainly depends on the nature, position, and number of substitutions in the phenyl rings of the compounds studied.
Mahajan P.G. et al. [
118] designed and synthesized a new ionic liquid C
5H
10[(2-APy)
2(HSO
4)
2] and applied it to the synthesis of a series of 5-substituted-1,2,4-triazolidine-3-thiones. A short reaction of the corresponding aldehydes with thiosemicarbazide in the presence of this catalyst in a water/ethanol mixture (60:40 v/v) at room temperature afforded the target 1,2,4-triazolidine-3-thione derivatives
138 (
Scheme 59):
The synthesized triazolthiones 138 were tested for acetylcholinesterase (AChE) inhibitory activity and showed varying degrees of IC50 values in the range of 0.0269±0.002 - 1199.9167±3.8888 μM compared to standard neostigmine methyl sulfate. Compounds containing hydroxyl and disubstituted halogen groups in their structures were more potent AChE inhibitors. It was also found that the synthesized 1,2,4-triazolidine-3-thiones 138 exhibited significant free radical scavenging activity compared to standard vitamin C.
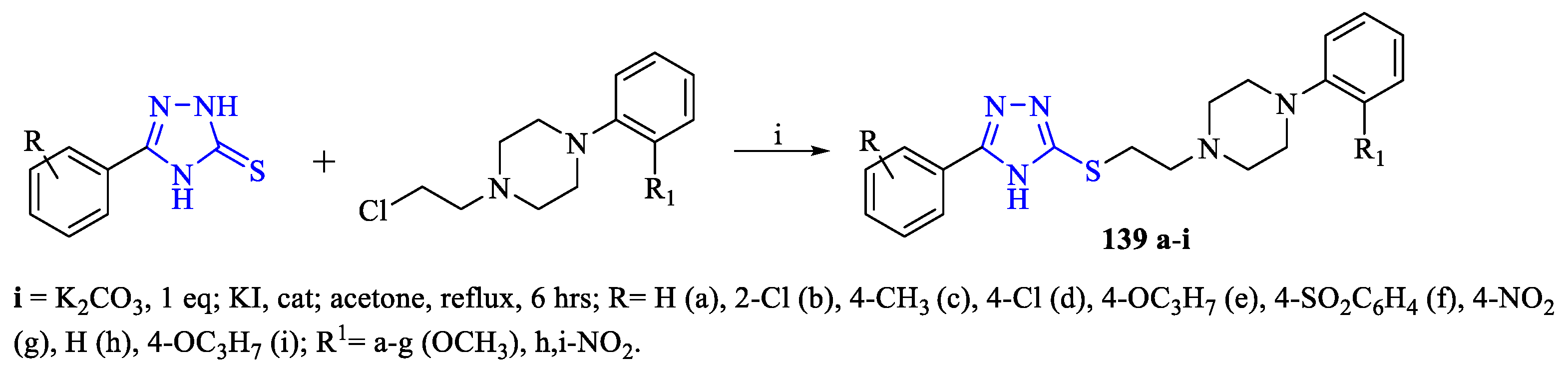
To obtain new selective ligands for the serotonin 5-HT1A receptor, Salerno L. et al. [
119] obtained 3-[[2-[4-(2-methoxy or 2-nitrophenyl)1-piperazinyl]ethyl]thio]-5-(R1-phenyl)[1,2,4]triazoles
139a-
i by reaction in acetone (heating with stirring) of the corresponding 5-aryl-2,4-dihydro-3H [1,2,4]triazole-3-thiones with 1-(2-chloroethyl)-4-(2-R1-phenyl)piperazines in the presence of K
2CO
3 and KI (
Scheme 60):
Most of the compounds 139a-i showed good Ki (nM) values in the nanomolar range and selectivity for the 5-HT1A receptor.
Samelyuk Yu.G. et al. [
120] synthesized new salts, derivatives of 2-(5-(4-methoxyphenyl(3,4,5-trimethoxyphenyl))-1,2,4-triazol-3-ylthio)-acetic acids
140 and studied their actoprotective activity (
Figure 10):
Among the synthesized substances, compounds with pronounced actoprotective activity were found. The authors studied the relationship between the structure of the obtained salts and their actoprotective action. It was found that the introduction of 3,4,5-trimethoxyphenyl radical into the molecule of 2-(5-R-1,2,4-triazol-3-ylthio)-acetate leads to a decrease in activity, in contrast to 2-(5-(4-methoxyphenyl)-1,2,4-triazol-3-ylthio)-acetate. The most pronounced actoprotective activity (42.57% (P<0.05)) of the studied compounds is possessed by ammonium 2-(5-(4-methoxyphenyl)-1,2,4-triazol-3-ylthio)-acetate, the activity of which exceeds the action of the known reference drug riboxin by 16.92%.
Dovbnia D. et al. [
121] developed methods for the synthesis of {5-[(2,4-,3,4-dimethoxyphenyl)-3H-1,2,4-triazol-3-yl]thio}(acetic, propanoic, benzoic) acids and, on their basis, obtained salts with organic and inorganic bases (
Scheme 61):
The hypoglycemic activity of the obtained salts 141 was studied, among which zinc (II) 2-{5-[(3,4-dimethoxyphenyl)-3H-1,2,4-triazol-3-yl]thio}acetate showed greater effectiveness in the ability to reduce blood glucose levels by 27.3% (approximately 1.3 times) compared to the reference drug metformin.
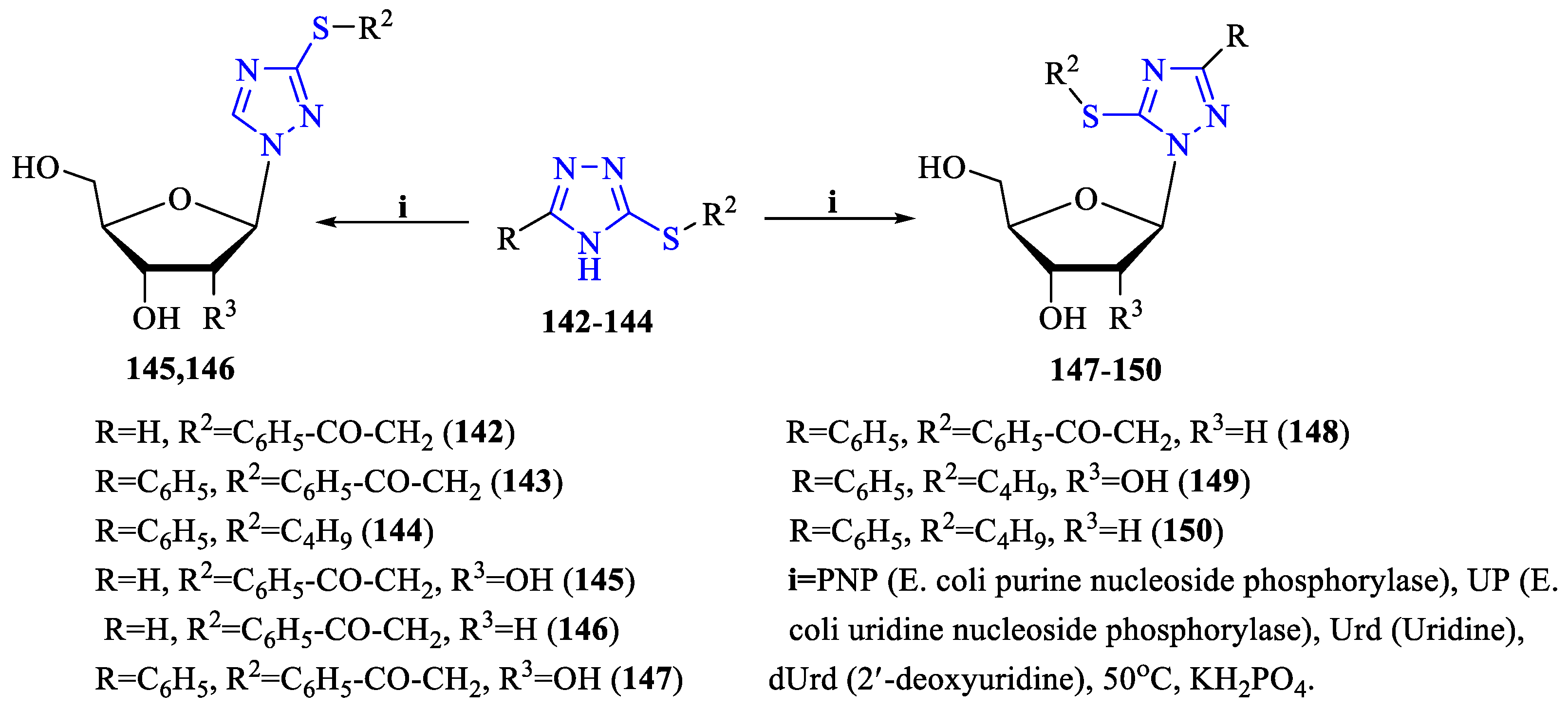
In order to synthesize new antiviral compounds, Fateev I.V. et al. [
122] obtained several derivatives of ribose and deoxyribose derivatives of 1,2,4-triazole-3-thione by enzymatic transglycosylation using recombinant nucleoside phosphorylases (
Scheme 62):
The highest antiviral activity against the wild-type HSV-1/L2(TK+) and the acyclovir-resistant strain (HSV-1/L2/RACV) was observed for the nucleosides 3-phenacylthio-1-(β-D-ribofuranosyl)-1,2,4-triazole 145 and 5-butylthio-1-(2-deoxy-β-D-ribofuranosyl)-3-phenyl-1,2,4-triazole 149, whose selectivity index significantly exceed those of the antiviral drug ribavirin.
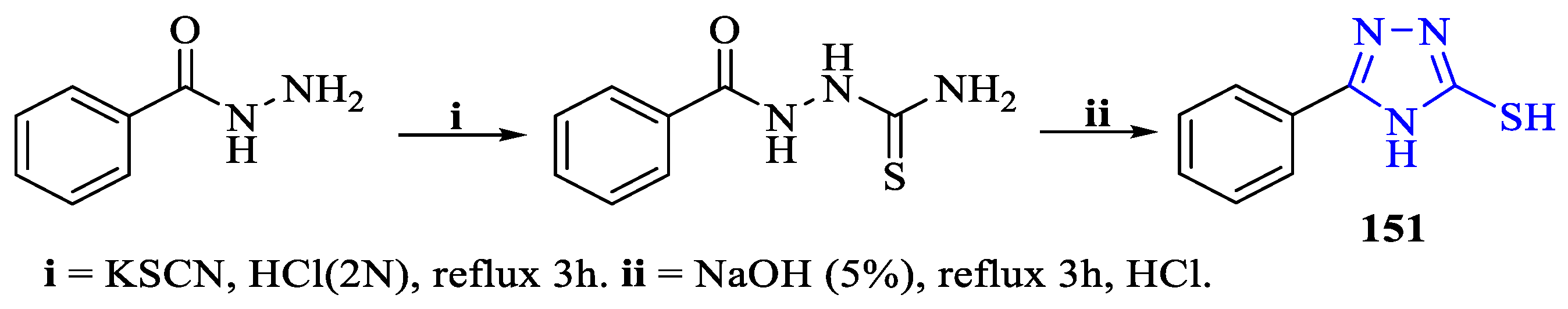
5-Phenyl-1,2,4-triazole-3-thiol
151 was synthesized by Hadjadj H. et al. [
123] via the preparation of benzoylthiosemicarbazide by the reaction of benzhydrazide with potassium thiocyanate (KSCN) and subsequent cyclization in alkaline (NaOH) solution (
Scheme 63):
A neurobehavioral study of compound 151 was conducted on Wistar rats. In this case, animals exposed to 5-phenyl-1,2,4-triazole-3-thiol 151 showed an increase in body weight and brain weight. Overall, the results of the studies showed that exposure to triazolethiol 151 can cause neurotoxic effects that impair spatial learning and memory performance, as well as induce a depressive state in animals.
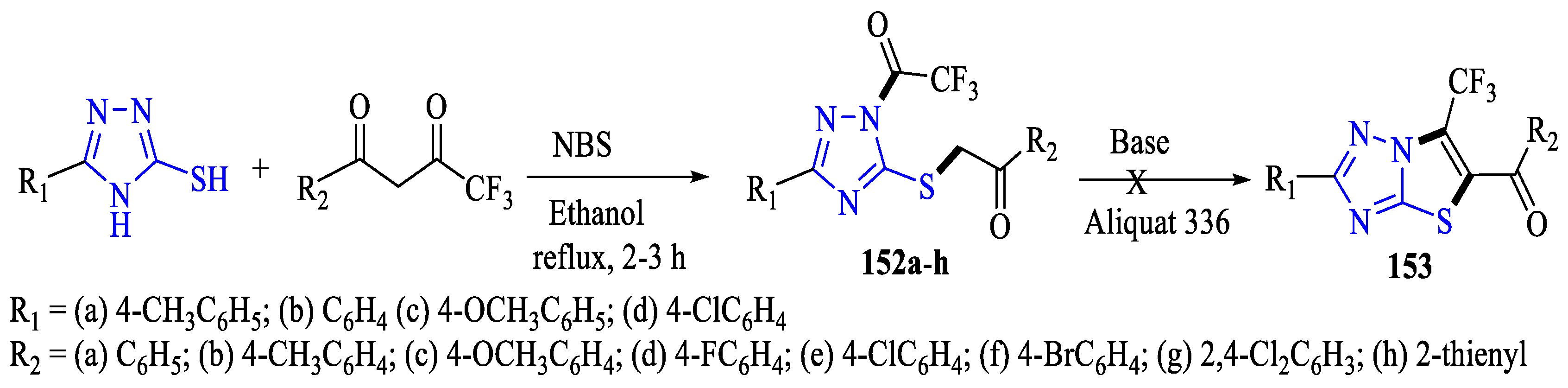
By performing a one-pot cascade reaction of 5-aryl-3-mercapto [1,2,4]triazoles with trifluoromethyl-b-dictetones in the presence of NBS (C
2H
5OH, reflux, 2-3 h), Aggarwal R. et al. [
124] obtained 1-trifluoroacetyl-3-aryl-5-(2-oxo-2-arylethylthio)-1,2,4-triazoles
152a-h. Attempts to cyclize compounds
152a-
h using Aliquat 336 and various bases (KOH, K
2CO
3, C
2H
5ONa, DABCO, and trimethylamine) as a catalyst to obtain cyclized thiazolo [3,2-b][1,2,4]triazoles
153 did not give the expected result (
Scheme 64):
The synthesized substances were tested for their ability to bind to the d(CGCGAATTCGCG)2 DNA duplex using molecular modeling tools and, according to the authors, the most promising compound is the compound (R1=4-OCH3C6H5, R2= 4-CH3C6H5) with a strong (Kb=1×105M−1) binding capacity of double-stranded DNA.
Ebdrup S. et al. [
125] synthesized new compounds based on 1,2,4-triazole with the general structure
154 exhibiting selective inhibition of hormone-sensitive lipase (HSL) (
Figure 11):
The selected methylphenylcarbamoyltriazoles, while inhibiting HSL, do not inhibit other hydrolases such as hepatolipase, lipoprotein lipase, pancreatic lipase and butyrylcholinesterase, indicating their antidiabetic activity.
Figure 1.
Drugs based on 1,2,4-triazole (1-11) and 1,2,4-triazole-3-thione (12-16).
Figure 1.
Drugs based on 1,2,4-triazole (1-11) and 1,2,4-triazole-3-thione (12-16).
Figure 2.
Structures of 1,2,3- (17), 1,2,4-(18) and 2,4-dihydro-1,2,4-triazole-3-thiones (19).
Figure 2.
Structures of 1,2,3- (17), 1,2,4-(18) and 2,4-dihydro-1,2,4-triazole-3-thiones (19).
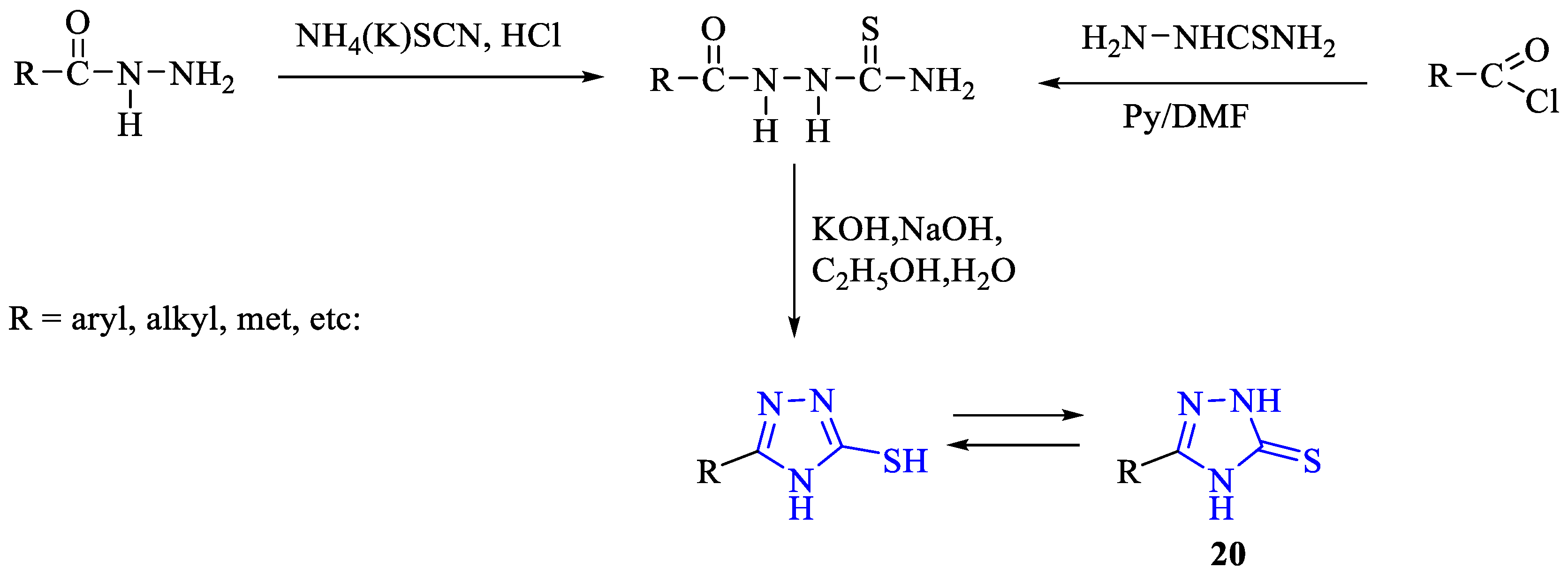
Scheme 1.
Synthesis of compounds 20.
Scheme 1.
Synthesis of compounds 20.
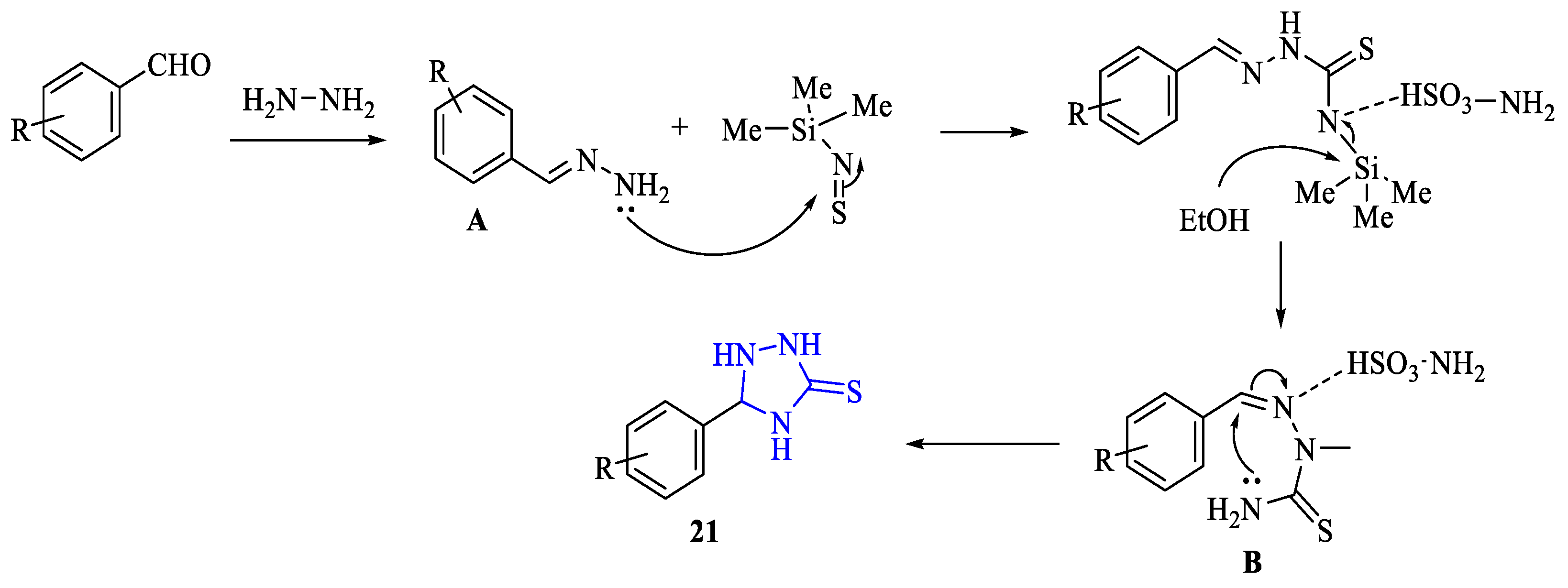
Scheme 2.
Synthesis pathway of compounds 21.
Scheme 2.
Synthesis pathway of compounds 21.
Scheme 3.
Synthesis reactions of compounds 21.
Scheme 3.
Synthesis reactions of compounds 21.
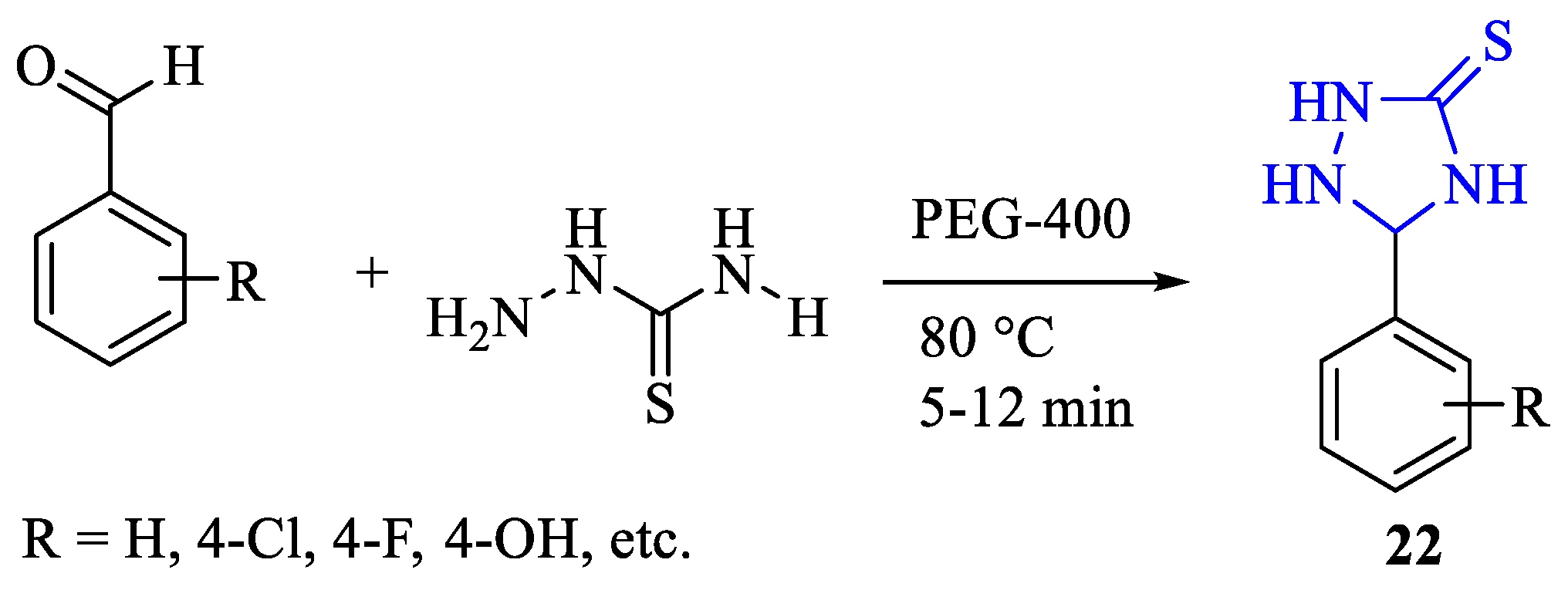
Scheme 4.
Synthesis of compounds 22.
Scheme 4.
Synthesis of compounds 22.
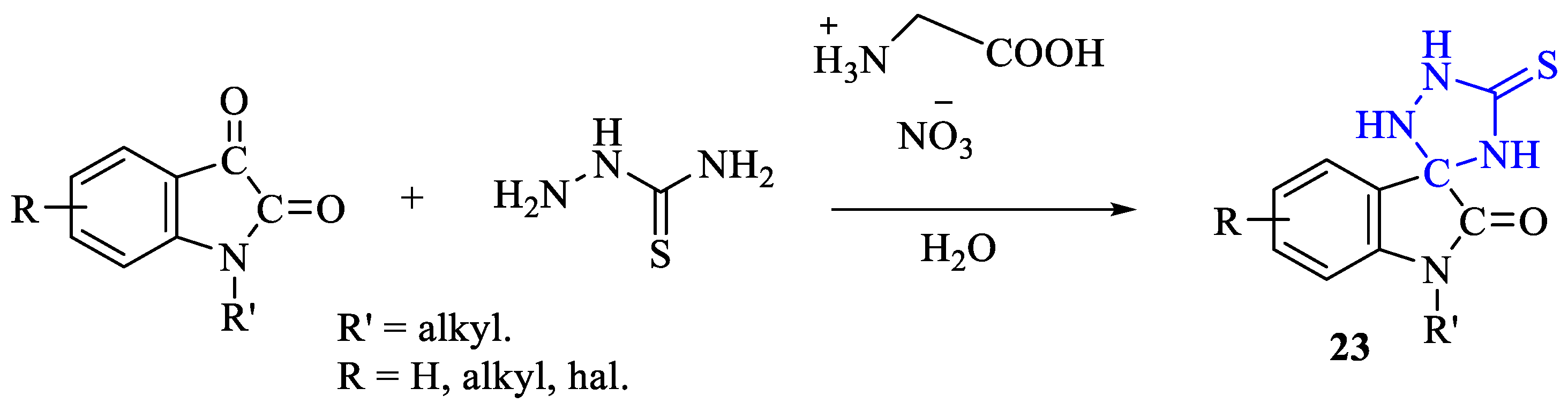
Scheme 5.
Synthesis pathway of compounds 23.
Scheme 5.
Synthesis pathway of compounds 23.
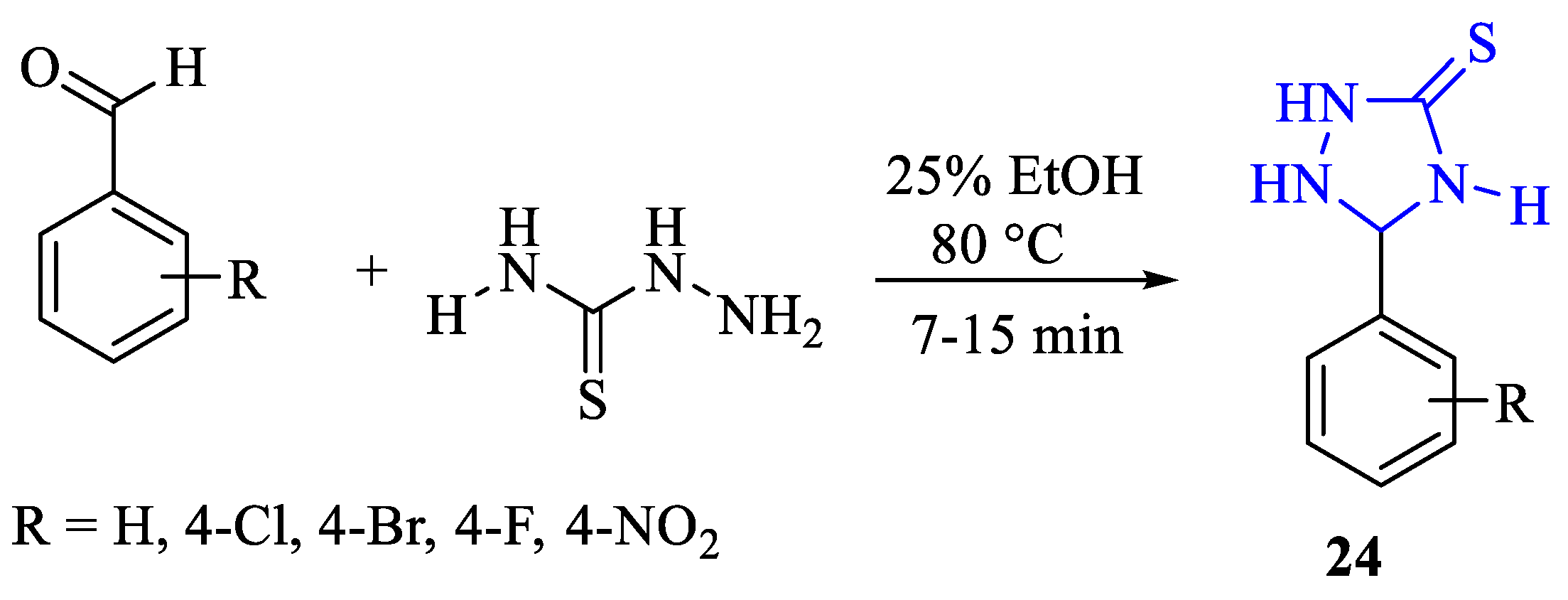
Scheme 6.
Synthesis of compounds 24.
Scheme 6.
Synthesis of compounds 24.
Scheme 7.
Synthesis of compounds 25.
Scheme 7.
Synthesis of compounds 25.
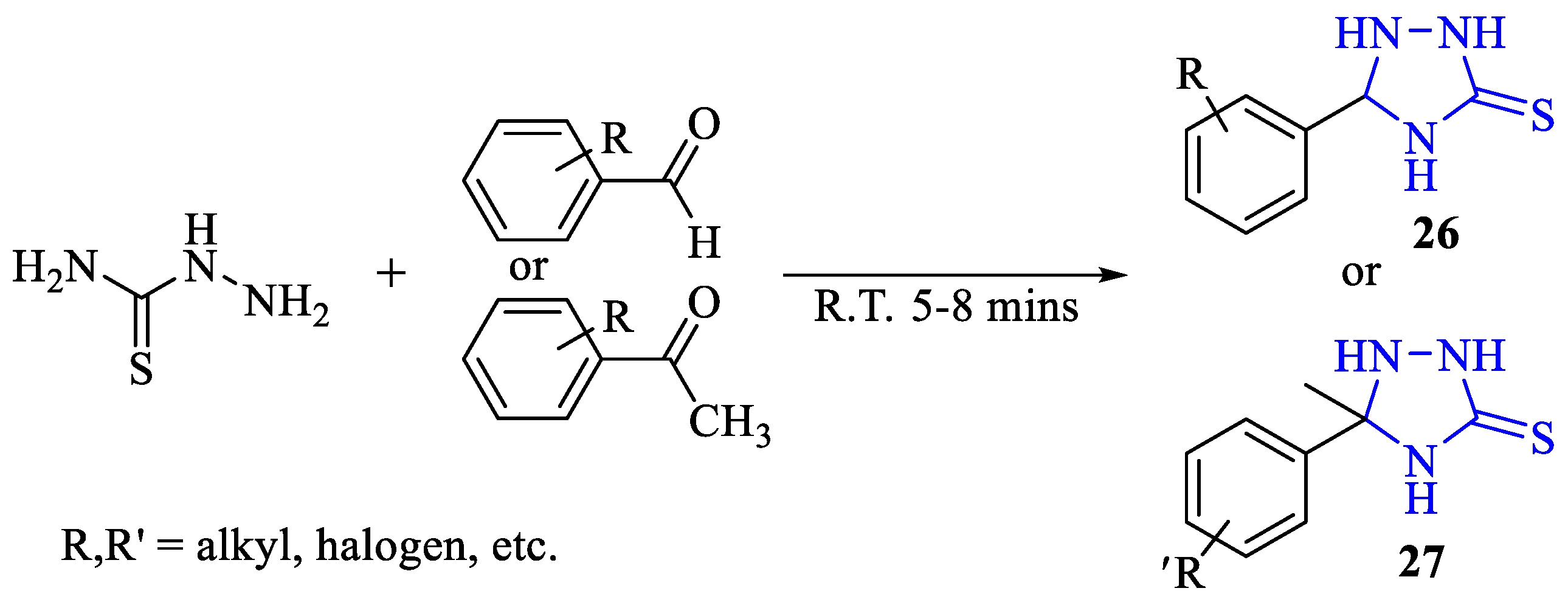
Scheme 8.
Synthesis pathway of compounds 26-27.
Scheme 8.
Synthesis pathway of compounds 26-27.
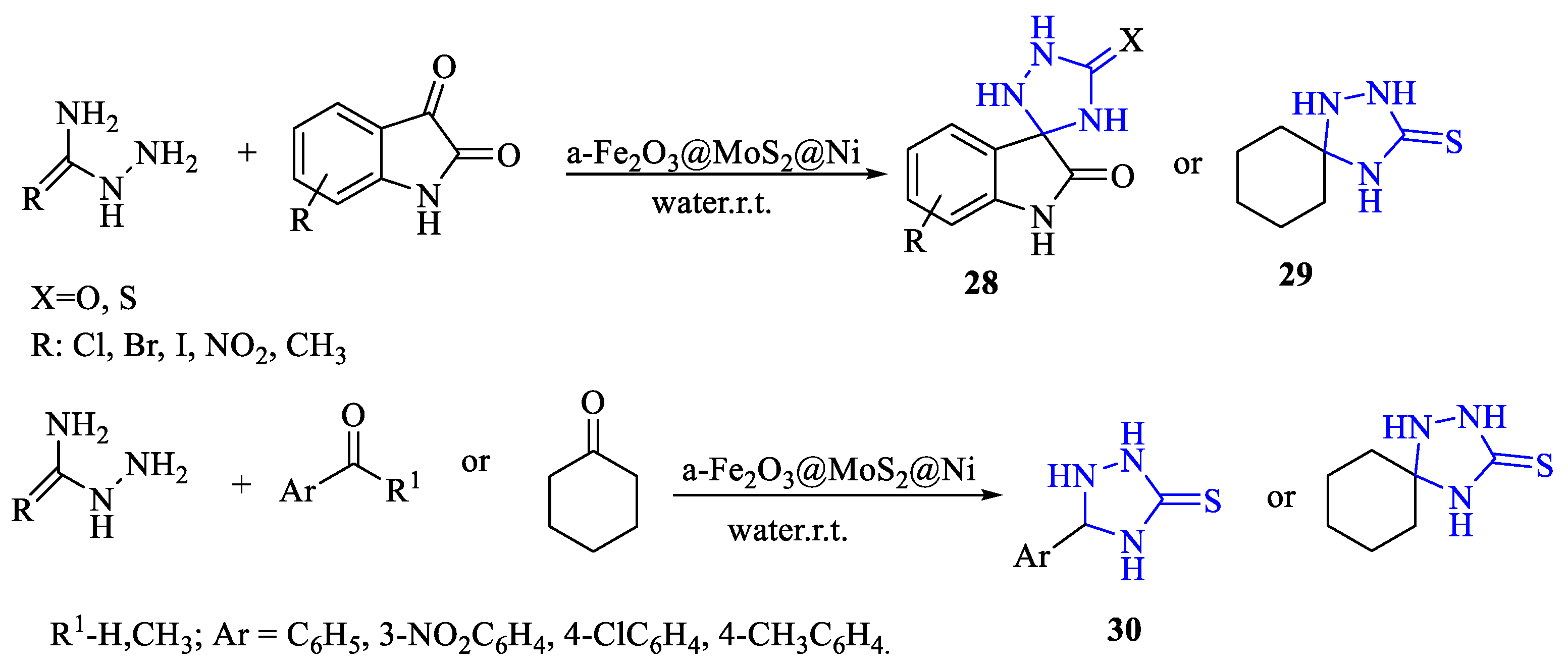
Scheme 9.
Synthesis pathway of compounds 28-30.
Scheme 9.
Synthesis pathway of compounds 28-30.
Scheme 10.
Synthesis of compounds 31.
Scheme 10.
Synthesis of compounds 31.
Scheme 11.
Synthesis pathway of compounds 32.
Scheme 11.
Synthesis pathway of compounds 32.
Scheme 12.
Synthesis pathway of compounds 33-35.
Scheme 12.
Synthesis pathway of compounds 33-35.
Scheme 13.
Synthesis of compounds 36 a-f.
Scheme 13.
Synthesis of compounds 36 a-f.
Scheme 14.
Synthesis of compounds 37 and 38.
Scheme 14.
Synthesis of compounds 37 and 38.
Scheme 15.
Synthesis pathway of compounds 39-41.
Scheme 15.
Synthesis pathway of compounds 39-41.
Scheme 16.
Synthesis of compounds 42, 43.
Scheme 16.
Synthesis of compounds 42, 43.
Scheme 17.
Synthesis pathway of compounds 44 and 45.
Scheme 17.
Synthesis pathway of compounds 44 and 45.
Scheme 18.
Synthesis of compounds 46a-l.
Scheme 18.
Synthesis of compounds 46a-l.
Scheme 19.
Synthesis pathway of compounds 48-49.
Scheme 19.
Synthesis pathway of compounds 48-49.
Scheme 20.
Synthesis pathway of compounds 50-52.
Scheme 20.
Synthesis pathway of compounds 50-52.
Scheme 21.
Synthesis pathway of compound 53.
Scheme 21.
Synthesis pathway of compound 53.
Scheme 22.
Synthesis pathway of compounds 54a-k.
Scheme 22.
Synthesis pathway of compounds 54a-k.
Scheme 23.
Synthesis of compound 55.
Scheme 23.
Synthesis of compound 55.
Scheme 24.
Synthesis pathway of compounds 56, 57.
Scheme 24.
Synthesis pathway of compounds 56, 57.
Scheme 25.
Synthesis pathway of compounds 58-60a-c.
Scheme 25.
Synthesis pathway of compounds 58-60a-c.
Scheme 26.
Synthesis pathway of compounds 61.
Scheme 26.
Synthesis pathway of compounds 61.
Scheme 27.
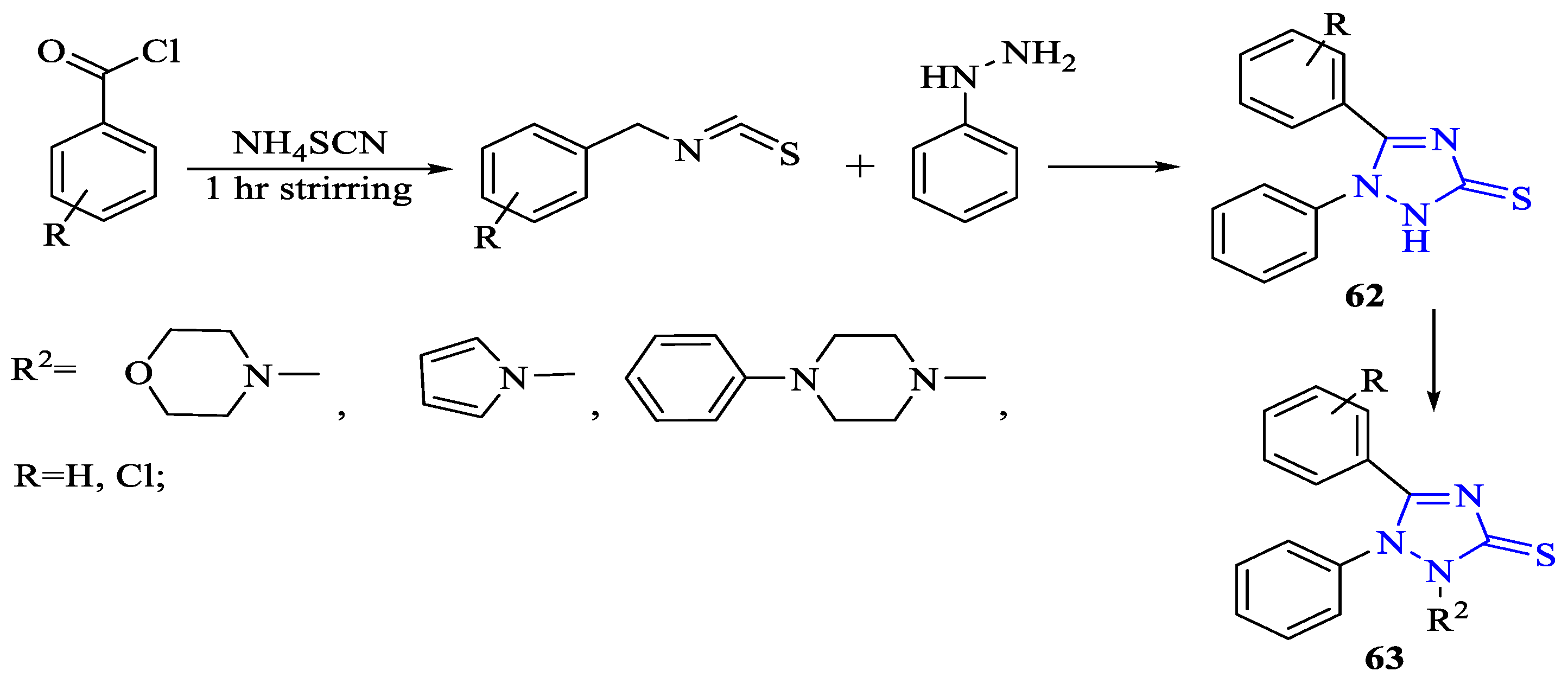
Synthesis pathway of compounds 62, 63.
Scheme 27.
Synthesis pathway of compounds 62, 63.
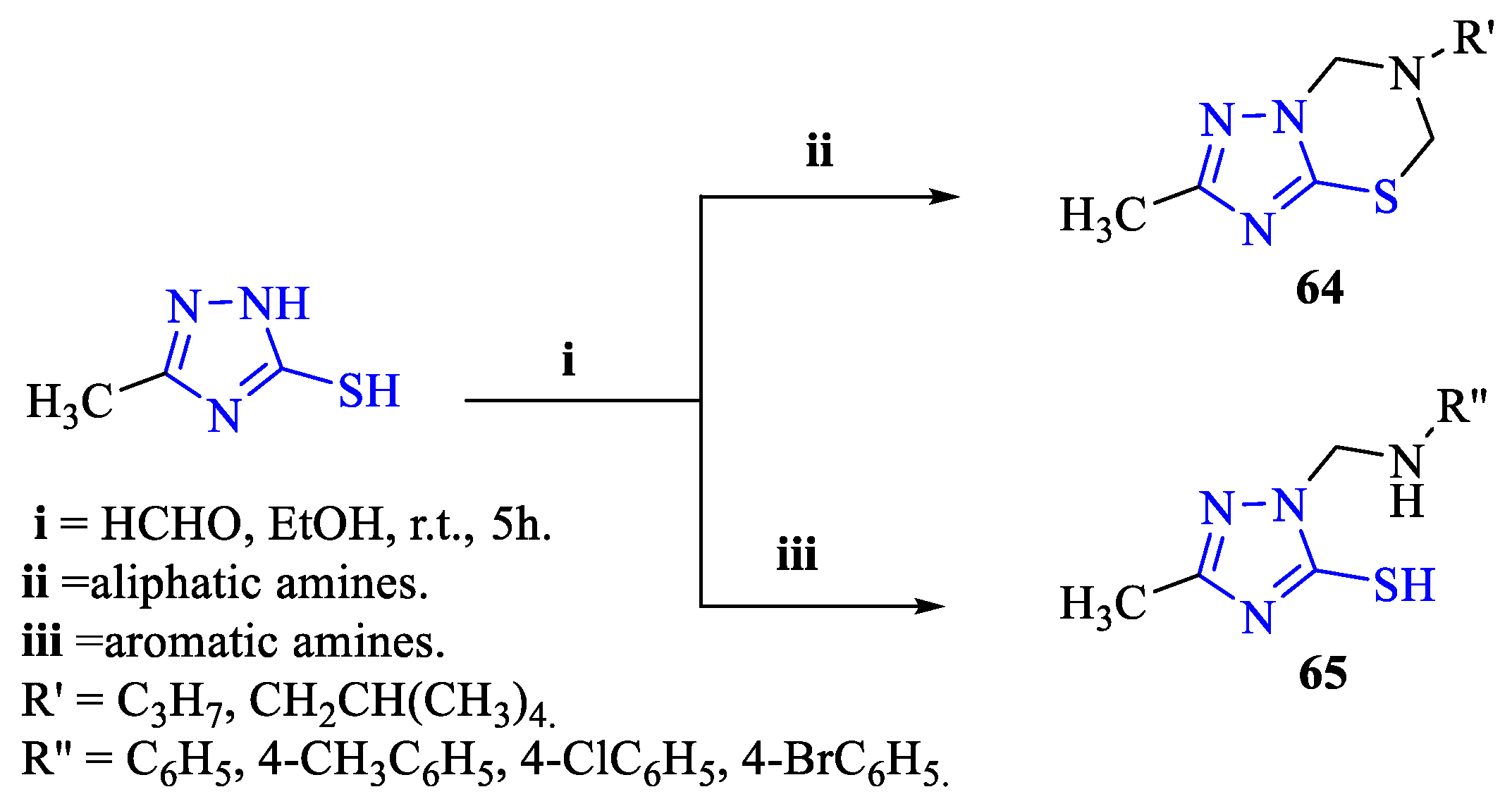
Scheme 28.
Synthesis pathway of compounds 64, 65.
Scheme 28.
Synthesis pathway of compounds 64, 65.
Scheme 29.
Synthesis pathway of compounds 66-68.
Scheme 29.
Synthesis pathway of compounds 66-68.
Scheme 30.
Synthesis pathway of compounds 69-70.
Scheme 30.
Synthesis pathway of compounds 69-70.
Figure 3.
Structure of compounds 71 and 72.
Figure 3.
Structure of compounds 71 and 72.
Scheme 31.
Synthesis pathway of compound 71.
Scheme 31.
Synthesis pathway of compound 71.
Scheme 32.
Synthesis of compounds 72-74.
Scheme 32.
Synthesis of compounds 72-74.
Scheme 33.
Synthesis pathway of compounds 75-77.
Scheme 33.
Synthesis pathway of compounds 75-77.
Figure 4.
Structure of compounds 78.
Figure 4.
Structure of compounds 78.
Scheme 34.
Synthesis of compounds 79.
Scheme 34.
Synthesis of compounds 79.
Scheme 35.
Synthesis of compounds 80.
Scheme 35.
Synthesis of compounds 80.
Scheme 36.
Synthesis pathway of compounds 81 and 82.
Scheme 36.
Synthesis pathway of compounds 81 and 82.
Scheme 37.
Synthesis pathway of compounds 83, 84.
Scheme 37.
Synthesis pathway of compounds 83, 84.
Scheme 38.
Synthesis of compounds 85.
Scheme 38.
Synthesis of compounds 85.
Scheme 39.
Synthesis of compounds 86.
Scheme 39.
Synthesis of compounds 86.
Scheme 40.
Synthesis pathway of compounds 87-92.
Scheme 40.
Synthesis pathway of compounds 87-92.
Scheme 41.
Synthesis pathway of compounds 93.
Scheme 41.
Synthesis pathway of compounds 93.
Scheme 42.
Synthesis of compounds 94.
Scheme 42.
Synthesis of compounds 94.
Scheme 43.
Synthesis pathway of compounds 95,96.
Scheme 43.
Synthesis pathway of compounds 95,96.
Scheme 44.
Synthesis of compounds 97.
Scheme 44.
Synthesis of compounds 97.
Figure 5.
Structure of compound 98.
Figure 5.
Structure of compound 98.
Scheme 45.
Synthesis pathway of compounds 99, 100.
Scheme 45.
Synthesis pathway of compounds 99, 100.
Scheme 46.
Synthesis of compounds 102.
Scheme 46.
Synthesis of compounds 102.
Figure 6.
Structure of compounds 103 and 104.
Figure 6.
Structure of compounds 103 and 104.
Scheme 47.
Synthesis pathway of compounds 105.
Scheme 47.
Synthesis pathway of compounds 105.
Figure 7.
Structure of compounds 106-111.
Figure 7.
Structure of compounds 106-111.
Scheme 48.
Synthesis of compounds 112.
Scheme 48.
Synthesis of compounds 112.
Scheme 49.
Synthesis pathway of compounds 113-116.
Scheme 49.
Synthesis pathway of compounds 113-116.
Scheme 50.
Synthesis of compounds 117.
Scheme 50.
Synthesis of compounds 117.
Scheme 51.
Synthesis pathway of compounds 118 and 119.
Scheme 51.
Synthesis pathway of compounds 118 and 119.
Scheme 52.
Synthesis of compounds 120, 121.
Scheme 52.
Synthesis of compounds 120, 121.
Scheme 53.
Synthesis of compounds 122-124.
Scheme 53.
Synthesis of compounds 122-124.
Scheme 54.
Synthesis pathway of compounds 125- 128.
Scheme 54.
Synthesis pathway of compounds 125- 128.
Scheme 55.
Synthesis pathway of compounds 129, 130.
Scheme 55.
Synthesis pathway of compounds 129, 130.
Figure 8.
Chemical structure of Prothioconazole.
Figure 8.
Chemical structure of Prothioconazole.
Figure 9.
Chemical structure of compounds 131.
Figure 9.
Chemical structure of compounds 131.
Scheme 56.
Synthesis pathway of compounds 132-135.
Scheme 56.
Synthesis pathway of compounds 132-135.
Scheme 57.
Synthesis of compounds 136.
Scheme 57.
Synthesis of compounds 136.
Scheme 58.
Synthesis pathway of compounds 137.
Scheme 58.
Synthesis pathway of compounds 137.
Scheme 59.
Synthesis of compounds 138.
Scheme 59.
Synthesis of compounds 138.
Scheme 60.
Synthesis of compounds 139.
Scheme 60.
Synthesis of compounds 139.
Figure 10.
Structure of compounds 140.
Figure 10.
Structure of compounds 140.
Scheme 61.
Synthesis of compounds 141.
Scheme 61.
Synthesis of compounds 141.
Scheme 62.
Synthesis pathway of compounds 142-150.
Scheme 62.
Synthesis pathway of compounds 142-150.
Scheme 63.
Synthesis pathway of compound 151.
Scheme 63.
Synthesis pathway of compound 151.
Scheme 64.
Synthesis pathway of compounds 152,153.
Scheme 64.
Synthesis pathway of compounds 152,153.
Figure 11.
Chemical structure of compounds 154.
Figure 11.
Chemical structure of compounds 154.