1. Introduction
Vaccines stand as one of the most impactful achievements in the history of medicine. Their transformative power is exemplified by the global eradication of smallpox and the near elimination of poliomyelitis, successes achieved through the establishment of sterilizing immunity in vaccinated individuals. However, the term "vaccine" has become increasingly imprecise, as technological, and functional differences across “vaccine” platforms result in a wide spectrum of protective effects against disease, as well as significantly different impacts on the prevention of infection and transmission.
The COVID-19 pandemic brought this disparity into sharp focus. mRNA “vaccines,” initially promoted as transmission-blocking agents, elicited high titers of neutralizing antibodies directed, solely, at the SARS-CoV-2 spike protein. However, these products, like all single epitope approaches, ultimately proved unable to prevent infection or viral spread. The failure to communicate this clearly to the public contributed significantly to distrust and to fueling vaccine hesitancy. Surveys conducted in 2024 revealed that approximately 20% of United States (U.S.) adults declined COVID-19 booster doses, citing perceived overpromises and a misalignment between anticipated and realized outcomes [
1]. This skepticism arises from a widespread, and often implicit, assumption that the presence of neutralizing antibodies in vitro, and the belief that the moniker Vaccine guarantees robust protection from infection in vivo, an assumption that has been repeatedly disproven.
To address this growing crisis of confidence and to clarify the true efficacy of different immune-modulatory products, we propose a three-tier classification system. Tier assignments are derived from peer-reviewed efficacy data and clinical outcomes, ensuring an objective, data-driven approach rather than subjective preferences. This system aims to rebuild public trust by aligning expectations with actual outcomes, enhance understanding of true neutralizing immunity among both medical professionals and the public, and ultimately, contribute to improved global health outcomes. This paper will provide a detailed explanation of how each type of vaccine product functions, address safety advancements in live attenuated vaccines (LAVs), [the only technology that has consistently reached the highest threshold (Tier 1)] which mitigate historical risks and highlight the crucial role of our tiered system in countering distrust and misinformation.
2. The Need for a Tiered Classification System
The indiscriminate use of the term "vaccine" to encompass products that primarily reduce disease severity, rather than preventing infection and transmission, has demonstrably contributed to growing vaccine hesitancy. For example, Measles, Mumps, and Rubella (MMR) vaccine coverage in the U.S. fell to 92.7% in 2023–2024, below the 95% threshold required for herd immunity. This decline in coverage contributed to the resurgence of measles outbreaks, exemplified by the outbreak reported in Texas in 2025 [
2]. The current blanket application of the term "vaccine" obscures critical differences in efficacy, failing to distinguish between products that have the potential to eradicate pandemics and those that primarily mitigate symptoms. A transparent and rigorous classification system is essential for several reasons:
Clarifying Efficacy: The system will explicitly distinguish between vaccines that prevent infection and transmission (Tier 1) from those that primarily prevent disease (Tier 2) or products which may reduce disease severity (Tier 3). This clarifies the role of each product in broader public health strategies.
Restoring Trust: By aligning expectations with actual clinical outcomes, the classification system will reduce skepticism and rebuild public confidence in vaccination programs. Studies have shown that transparent and honest communication about vaccine capabilities can increase uptake by 15–20% [
3].
Enhancing Knowledge: The system will equip healthcare professionals with the knowledge needed to accurately explain the benefits and limitations of the different technologies and products. For example, it will allow them to articulate the difference between the Tier 3 status of the COVID mRNA vaccines and the Tier 1 status of the MMR vaccine. This enhanced understanding will empower healthcare providers to effectively address patient concerns and combat inaccurate information.
Guiding Policy: The system will provide a rational basis for public health policy decisions. For example, if a mandate is ever deemed appropriate, it would reserve the recommendation of mandatory vaccination policies for Tier 1 vaccines that demonstrate >90% effectiveness in preventing transmission while adhering to a strict safety standard, ensuring that such measures are ethically justified.
Informing Resource Allocation: By clearly defining which vaccines are truly sterilizing, public health agencies and funding bodies can prioritize the development and deployment of Tier 1 vaccines, maximizing their impact on global health security. The proposed tiered classification system is defined as follows:
Tier 1 (Green): Vaccines – These products prevent infection, transmission, and disease, and elicit true neutralizing immunity; the ability to prevent pathogen entry and replication in vivo. These vaccines are suitable for consideration for mandatory vaccination policies, especially during pandemic threats.
Tier 2 (Yellow): Therapeutic Vaccines – These products prevent disease, but do not prevent infection or transmission. They elicit neutralizing antibodies, but these antibodies lack true neutralizing immunity as defined above. These vaccines should be recommended, but not mandated, for individuals at risk of severe disease.
Tier 3 (Red): Immunomodulatory Therapeutics – These products may reduce disease severity but do not prevent infection, transmission, or disease, they are stated as capable of ameliorating disease severity. The neutralizing antibodies elicited by these products fail to confer true neutralizing immunity. These products should not be categorized as vaccines and should be regulated as therapeutics rather than as vaccines, with appropriate policy distinctions, labeling and patient education.
2.1. Refining the Classification System for Contextual Efficacy
To enhance the flexibility of the tiered classification system, we propose the inclusion of a modifier for vaccines with context-dependent efficacy, such as the Bacillus Calmette-Guérin (BCG) vaccine, which exhibits variable performance by region (e.g., 50–80% efficacy against disseminated tuberculosis in children, but limited protection against pulmonary tuberculosis in adults, particularly in tropical regions [
4]). This modifier, denoted as “C” (e.g., Tier 2-C), indicates that efficacy may vary based on factors such as geographic location, population immunity, or pathogen strain. For example, BCG would be classified as Tier 2-C to reflect its inconsistent transmission prevention across settings.
Additionally, the system accommodates vaccines with partial transmission prevention, such as the rotavirus vaccine, which reduces transmission by 60–80% [
5]. Vaccines achieving 50–90% transmission reduction are assigned to Tier 1 with a “P” modifier (e.g., Tier 1-P), indicating partial but significant transmission prevention. This avoids rigid categorizations while maintaining clarity. For instance, rotavirus is classified as Tier 1-P due to its 85–98% efficacy against severe gastroenteritis and substantial transmission reduction, distinguishing it from Tier 2 vaccines with negligible transmission impact [
5].
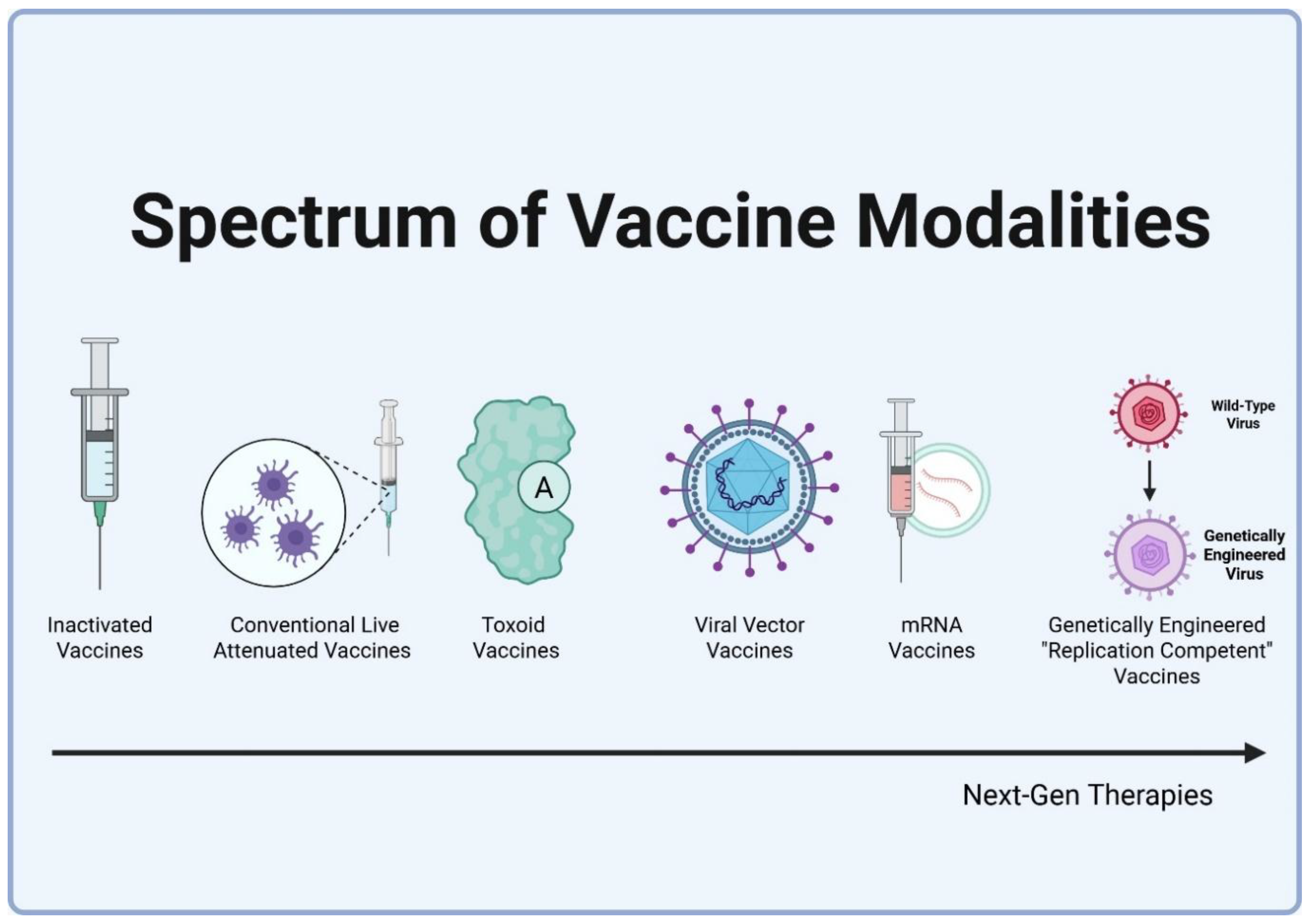
3. How Vaccines Work: A Functional Overview
The products currently classified as vaccines globally are based on five distinct technological platforms: live attenuated, killed/inactivated, subunit/conjugate/toxoid, mRNA, and viral vector. Additionally, next generation genetically engineered, replication competent vaccine candidates are currently in development (discussed in section 4.2.) These platforms differ significantly in antigenic breadth, the nature of the induced immune response (e.g., systemic vs. mucosal), and their ability to confer protection (disease prevention) and prevention (infection/transmission blockade). This inherent variability makes the application of a single, undifferentiated term "vaccine" wholly inadequate. For example, while most conjugate vaccines, such as DTaP or PCV13, are assigned to Tier 2 due to limited transmission prevention [
6,
7], the
Haemophilus influenzae type b (Hib) conjugate vaccine achieves Tier 1 status by preventing infection, transmission, and disease through true neutralizing immunity [
8], highlighting that efficacy not technology should be the main reason when classifying these products.
Table 1 summarizes these platforms, their mechanisms of action, and their corresponding tier assignments within the proposed classification system. This table highlights the wide range of outcomes associated with each technology.
Table 1.
Vaccine Technologies, Functions, and Tier Classification.
Table 1.
Vaccine Technologies, Functions, and Tier Classification.
| Technology |
Function |
Antigenic Breadth |
Examples |
Neutralizing Antibodies |
True Sterilizing Immunity |
Protection / Prevention Outcomes |
Tier |
| Live Attenuated |
Mimics natural infection; stimulates humoral (IgG, IgA), cellular (T-cell), and mucosal immunity |
Broad; multiple antigens |
MMR, OPV, Yellow Fever, Varicella* |
Yes, strong correlation |
Yes, blocks infection and transmission |
Prevents disease and halts community spread |
1 (Except BCG (2-C), Dengvaxia (2), Influenza Nasal (3)) |
| Inactivated |
Killed pathogens induce systemic antibodies; typically lacks mucosal immunity. Requires boosters/adjuvants. |
Moderate; whole pathogen |
IPV, Hepatitis A, Sinovac |
Yes, effective for disease |
No; viral shedding possible |
Prevents disease; limited impact on transmission |
2 or 3 (e.g., Sinovac) |
| Subunit / Conjugate / Toxoid |
Specific antigens induce targeted antibodies (often with adjuvants); limited mucosal immunity due to narrow antigen focus. Exception: Hib induces mucosal IgA and reduces carriage. |
Narrow; single/few antigens |
Hepatitis B, HPV, DTaP, PCV13, Hib |
Yes, effective for disease. Hib: strong correlation with transmission prevention |
No; does not block transmission. Hib: Yes; blocks infection and transmission |
Prevents disease; does not prevent spread. Hib: Prevents disease and halts community spread |
2 (Hib: 1) |
| mRNA |
Encodes antigen via mRNA; induces systemic antibodies and T-cell responses. Mucosal (IgA) response is minimal. |
Narrow; single antigen |
COVID-19 (Pfizer-BioNTech, Moderna) |
Yes, induces high titers |
No; minimal effect on transmission |
Reduces disease severity; limited transmission control |
3 |
| Viral Vector |
Delivers DNA encoding antigens via viral carrier; induces systemic immunity. Mucosal response generally lacking. |
Narrow; single antigen |
COVID-19 (Janssen, Sputnik V) |
Yes, moderate antibody titers |
No; does not block transmission |
Reduces disease severity; limited transmission control |
3 |
| Genetically Engineered Replication Competent |
Mimics natural infection; stimulates humoral (IgG, IgA), cellular (T-cell), and mucosal immunity |
Broad; multiple antigens |
None yet approved |
Yes, strong correlation |
Yes; blocks infection and transmission |
Prevents disease and halts community spread |
Expected Tier 1 |
Figure 1.
Spectrum of Vaccine Modalities and Antigenic Complexity.
Figure 1.
Spectrum of Vaccine Modalities and Antigenic Complexity.
3.1. Live Attenuated Vaccines (LAVs)
Function: Genetically engineered or traditionally weakened pathogens are capable of limited replication
in vivo, mimicking natural infection. This elicits a robust and multifaceted immune response, including IgG (systemic protection), IgA (mucosal protection at entry points like the nose/gut), and T-cells (CD4+/CD8+), targeting multiple antigens [
9]. The ability to stimulate mucosal immunity is a key differentiator for Tier 1 vaccines.
Examples and Outcomes:
MMR (Measles, Mumps, Rubella): Demonstrates 97% efficacy [95% CI: 95–99%] against measles, preventing transmission with <5% breakthrough cases being transmissible [
10].
OPV (Oral Polio Vaccine): Exhibits >95% efficacy [95% CI: 93–97%]. Mucosal IgA significantly reduces poliovirus shedding by 90%, providing robust community-level protection [
11].
Yellow Fever Vaccine: Shows >95% efficacy [95% CI: 92–98%], providing lifelong protection and preventing transmission by mosquitoes [
12].
Varicella (Chickenpox) Vaccine: Demonstrates a 90% efficacy and reduces household transmission [
13].
Rotavirus Vaccine: Provides 85–98% efficacy [95% CI: 82–99%] and reduces transmission by 60–80% [
5].
Cholera Vaccine (CVD 103-HgR): Offers 80–90% efficacy and effectively prevents transmission [
14].
Typhoid Vaccine (Ty21a): Demonstrates 50–80% efficacy and reduces fecal shedding by 70–85% [
15].
Tier: Generally assigned to Tier 1 (Green) due to the induction of neutralizing antibodies that reflect true neutralizing immunity, preventing both infection and transmission. However, exceptions exist based on specific clinical efficacy data.
BCG (Bacillus Calmette-Guérin) Vaccine: Tier 2-C. Demonstrates 50–80% efficacy [95% CI: 40–85%] against disseminated TB in children but does not prevent pulmonary TB or transmission to others, with efficacy varying by region [
4].
Influenza Vaccine (Nasal): Tier 3. Exhibits 40–60% efficacy [95% CI: 35–65%] and does not prevent transmission. Its primary impact is reducing the severity and duration of illness [
16].
3.2. Inactivated Vaccines
Function: Inactivated vaccines utilize killed pathogens to stimulate the production of systemic IgG antibodies. However, they typically require booster doses and/or adjuvants to achieve adequate immunogenicity. Critically, they generally fail to elicit mucosal IgA responses, which allows for pathogen shedding at entry points [
9].
Examples and Outcomes:
IPV (Inactivated Polio Vaccine): Exhibits near-100% efficacy [95% CI: 98–100%] against paralytic polio; however, it allows for viral shedding in 20–30% of vaccinated individuals [
11].
Hepatitis A Vaccine: Demonstrates >94% efficacy [95% CI: 90–97%] but allows for shedding in 10–15% of vaccinated individuals [
17].
Cholera Vaccine (Dukoral): Provides 60–85% efficacy; some transmission may still occur [
14].
COVID-19 Vaccines (Sinovac, Covaxin): Demonstrate 50–70% efficacy [95% CI: 45–80%]; however, they offer only minimal prevention of infection and transmission [
18,
19].
Tier: Generally assigned to Tier 2 (Yellow). Inactivated COVID-19 vaccines (e.g., Sinovac) are assigned to Tier 3 (Red) due to their limited impact on transmission and their lack of true neutralizing immunity [
18].
3.3. Subunit, Conjugate, and Toxoid Vaccines
Function: These vaccines utilize specific antigens (proteins, polysaccharides, or inactivated toxins) to induce targeted IgG antibody responses, often in conjunction with adjuvants. The narrow focus of these vaccines typically limits the induction of mucosal immunity [
9].
Examples and Outcomes:
Hepatitis B Vaccine: Demonstrates >90% efficacy [95% CI: 85–95%]; however, viral shedding may occur in 5–10% of vaccinated individuals [
20].
HPV (Human Papillomavirus) Vaccine: Exhibits 97% efficacy [95% CI: 94–99%] in preventing precancerous lesions; however, it does not block transmission of the virus [
21].
PCV13 (Pneumococcal Conjugate Vaccine): Provides 80–90% efficacy [95% CI: 75–93%]; however, nasopharyngeal carriage of Streptococcus pneumoniae may persist in 15–20% of vaccinated individuals [
22].
DTaP (Diphtheria, Tetanus, and Pertussis) Vaccine: Demonstrates 84% short-term efficacy [95% CI: 80–88%]; however, it does not prevent transmission of pertussis [
6].
MenACWY (Meningococcal Conjugate Vaccine): Exhibits >85% efficacy [95% CI: 80–90%]; however, persistent colonization with
Neisseria meningitidis may occur [
23].
MenB (Meningococcal B Vaccine): Provides 70–90% efficacy [95% CI: 65–92%]; however, colonization data is limited [
24].
Shingles Vaccine: Demonstrates 90–97% efficacy [95% CI: 88–98%]; however, transmission data is limited [
25].
Malaria Vaccine (RTS, S): Exhibits 30–50% efficacy [95% CI: 24–55%]; however, transmission data is limited [
26].
Tier: Assigned to Tier 2 (Yellow), as neutralizing antibodies, often, prevent disease but do not prevent transmission [
23,
24,
25,
26].
An exception among conjugate vaccines is the
Haemophilus influenzae type b (Hib) vaccine, which achieves Tier 1 status due to its ability to prevent infection, transmission, and disease. Hib, a bacterium spread through respiratory droplets, historically caused approximately 20,000 cases of invasive disease annually in the U.S., including meningitis with 5 to 10 percent mortality. The Hib conjugate vaccine, utilizing polysaccharide antigens linked to protein carriers such as tetanus toxoid, elicits robust systemic IgG and mucosal IgA responses, reducing nasopharyngeal carriage from 3 to 5 percent to less than 1 percent in vaccinated populations. This carriage reduction interrupts transmission, yielding herd immunity that protects unvaccinated individuals. With 95 to 100 percent efficacy against invasive Hib disease, the vaccine has decreased incidence by over 90 percent globally, justifying its inclusion in mandatory pediatric immunization schedules. Unlike most conjugate vaccines, Hib’s non-adjuvanted formulation relies on conjugation for immunogenicity, minimizing reactogenicity while achieving true neutralizing immunity [
8].
3.4. mRNA “Vaccines”
Function: mRNA “vaccines” deliver genetic instructions encoding specific antigens (e.g., the SARS-CoV-2 spike protein) to host cells, which then produce the antigen, eliciting a systemic IgG and T-cell response. Critically, mRNA vaccines do not typically induce mucosal IgA responses [
9].
Examples and Outcomes:
COVID-19 “Vaccines” (Pfizer, Moderna): Demonstrate approximately 35% vaccine effectiveness (VE) [95% CI: 30–40%] against infection and 50% [95% CI: 45–55%] against hospitalization (data from 2024–2025). These “vaccines” do not significantly reduce transmission [
27].
Tier: Assigned to Tier 3 (Red), as the neutralizing antibodies elicited by these “vaccines” fail to confer true neutralizing immunity, and they have a limited impact on infection or transmission and have been touted for their ability to reduce disease severity [
27].
3.5. Viral Vector Vaccines
Function: Viral vector vaccines utilize non-replicating viral vectors (e.g., adenovirus) to deliver DNA encoding specific antigens to host cells. This induces systemic IgG and T-cell responses. Like mRNA vaccines, viral vector vaccines typically do not induce mucosal immunity [
9].
Examples and Outcomes:
COVID-19 “Vaccines” (Janssen, Sputnik V): Demonstrate 66–91% efficacy [95% CI: 60–94%] against symptomatic disease, but <50% efficacy against infection and minimal prevention of transmission [
28].
Tier: Assigned to Tier 3 (Red), as the neutralizing antibodies elicited by these “vaccines” lack true neutralizing immunity and offer limited protection against infection and transmission [
28].
3.6. Classification Nuances and Emerging Technologies
Live attenuated vaccines achieve Tier 1 status due to their broad antigen presentation and induction of mucosal immunity. However, exceptions like the BCG and Dengvaxia vaccines underscore that clinical efficacy is the primary driver of tier assignment [
4,
29]. Currently, no non-live vaccines are classified as Tier 1. However, future multivalent inactivated or mucosal mRNA vaccines may potentially achieve this status [
30]. Emerging platforms like DNA vaccines and nanoparticle vaccines are currently in development, but not yet approved for widespread use, confirming that the five technological platforms described above encompass all currently licensed vaccines [
31].
4. Safety Advances in Live Attenuated Vaccines (LAVs)
Historically, the LAV approach has been associated with safety concerns due to their reliance on the weakening of wild-type pathogens through serial passage or temperature control, which could potentially lead to vaccine-derived disease, viral shedding, or genetic recombination [
32]. Thanks to technological advances, Modern replication competent vaccine candidates are genetically engineered mutants designed to mitigate these risks, enhancing safety while preserving efficacy [
33].
4.1. Historical Safety Concerns
Vaccine-Derived Disease: Weakened pathogens could, in rare instances, cause mild to moderate disease, particularly in immunocompromised individuals. For example, the oral polio vaccine (OPV) was associated with vaccine-associated paralytic poliomyelitis (VAPP) in 2–4 per million doses due to rare reversion to virulence [
11].
Vaccine Shedding: traditional LAVs replicate in vivo, potentially shedding weakened viruses that could infect others. While often harmless (e.g., MMR shedding is rare and non-pathogenic), OPV shedding has been linked to outbreaks in under-vaccinated areas [
11].
Recombination: Attenuated viruses could recombine with wild-type viruses, potentially regaining virulence. OPV recombination has resulted in circulating vaccine-derived polioviruses (cVDPVs) in areas with low vaccination coverage [
34].
Varicella Vaccine Latency and Shingles Risk: A significant historical concern with LAVs is exemplified by the varicella (chickenpox) vaccine, which uses the Oka strain of varicella-zoster virus (VZV) attenuated through serial passage in cell culture. This crude method introduces random, uncontrolled mutations, allowing the Oka strain to establish latency in sensory nerve ganglia, like wild-type VZV [
35]. This latency can lead to reactivation as shingles (herpes zoster), a concern for a vaccine recommended for children. A 2019 study of 6.2 million children reported a shingles incidence of 0.8 per 1000 person-years in vaccinated children, compared to 3.6 per 1000 person-years in those with natural infection, a 78% risk reduction (relative risk: 0.22, 95% CI: 0.18–0.27) [
36]. This risk, though low, highlights the limitations of traditional attenuation methods, which lack the precision to eliminate latency, unlike modern genetically engineered replication competent candidates, like those discussed in sections 4.2 and 4.3.
4.2. Genetic Engineering Solutions
Advances in molecular virology have enabled the creation of highly stable replication competent mutants that minimize, if not eliminate, historical risks:
Codon De-optimization: Alters synonymous codons (codons that code for the same amino acid) to reduce mRNA stability and protein production. This strategy limits viral replication and prevents reversion to virulence. Codon de-optimization is currently being explored in the development of improved influenza and SARS-CoV-2 LAVs [
37].
Gene Deletions: Removes specific virulence genes, significantly enhancing safety. For example, near complete deletion of the ICP0 gene in HSV vaccines eliminates the risk of latency [
38]. This approach contrasts sharply with the varicella vaccine’s Oka strain, which relies on random mutations from serial passage, allowing latency and potential shingles reactivation [
35].
Attenuation Mutations: Introduces specific mutations in key viral genes (e.g., polymerase 3D in nOPV2) to stabilize the attenuated phenotype and prevent reversion to virulence [
34].
4.3. Examples of Genetically Engineered, Replication Competent Candidates
SARS-CoV-2 OTS-228: This live-attenuated SARS-CoV-2 vaccine incorporates OTS codons, Nsp1 mutations (K164A/H165A), ORF6–8 knockouts, and deletion of the spike protein polybasic cleavage site. Preclinical studies in Syrian hamsters demonstrated no evidence of transmission, no significant side effects, and sterilizing immunity against wild-type SARS-CoV-2. Furthermore, this candidate provides broad protection against Omicron variants (BA.2, BA.5, XBB.1.5), making it a promising Tier 1 vaccine candidate [
39].
Rational Vaccines’ HSV-2 candidate RVx201: This HSV-2 replication competent vaccine is a purposefully created mutant, engineered with targeted deletions in the ICP0 gene to be interferon-sensitive, enhancing immune clearance by preventing the virus from counteracting host interferon responses, and incapable of establishing latency [
38]. Unlike the varicella vaccine’s Oka strain, which relies on random mutations from serial passage and risks shingles reactivation [
35], RVx201 is not derived from weakening the wild-type viruses but is a novel genetically engineered construct designed without wild-type capabilities. A 2021 preclinical study in guinea pigs demonstrated no evidence of latency after 28 days, no HSV-2-associated disease, and a >90% reduction in viral shedding compared to wild-type infection, confirming its safety and efficacy as both a preventive and therapeutic vaccine [
38]. A 2021 study further validated RVx201’s robust mucosal and systemic immunity without reactivation, positioning it as a model for next-generation replication competent vaccines that address historical risks, like those of the varicella vaccine [
40].
Rational Vaccines VC2 RVX 202: was derived from herpes simplex virus type 1 (HSV-1), for oncolytic viral immunotherapy. This virus (VC2; RVx202) is based on the HSV-1 genome and has deletions in glycoprotein K and UL20 genes, rendering it replication-competent yet neuro-attenuated, thereby eliminating the risk of latency or neuro-invasion and establishment of latency. It has been shown in several animal studies, including mouse, guinea pig, and non-human primate studies, that the parental VC2 strain is a safe and immunogenic vaccine strain, and confers protection of mice and guinea pigs against lethal HSV genital and ocular infection [
41,
42].
ILIAD’s BPZE1 Pertussis Candidate: BPZE1 includes a dnt gene knockout, an ampG gene replacement and S1 subunit mutations (R9K, E129G), which eliminate dermonecrotic toxin, tracheal cytotoxin and pertussis toxin activity [
43] and ensure genetic stability after 20 passages without reversion [
44]. In addition to prevention against disease, BPZE1 reduces shedding and protects against virulent strains in mice (60) and non-human primates, with 99.9% reduction in nasal colonization after challenge [
45]. In humans, BPZE1 has also demonstrated nasopharyngeal protection against both attenuated [
46] and virulent pertussis strains, suggesting the potential to protect against transmission as well as disease and to induce broader immunity compared to current pertussis vaccines.
Codon De-optimized Influenza LAV: A 2024 study described the development of an influenza LAV with codon de-optimization, resulting in an 80% reduction in viral shedding in ferrets and preventing reversion to virulence. This candidate induced robust mucosal immunity, suggesting the potential for Tier 1 status [
47].
Codagenix’s CoviLiv: This replication-competent, live-attenuated SARS-CoV-2 vaccine uses codon deoptimization to limit replication, ensuring safety while eliciting robust mucosal and systemic immunity, potentially blocking transmission, which may make it a strong Tier 1 candidate [
48].
These Genetically engineered candidates are the next generation of replication competent vaccines. They demonstrate that modern genetic engineering approaches can effectively overcome historical safety concerns associated with LAVs, enabling the development of safe and effective Tier 1 vaccines [
37,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
49].
5. Neutralizing Antibodies vs. True Neutralizing Immunity
5.1. The Misconception
Equating the presence of sterilizing antibodies in vitro with true neutralizing immunity in vivo misleads stakeholders and undermines public trust. While sterilizing antibodies can bind to antigens in laboratory assays, true neutralizing immunity requires the presence of mucosal IgA, robust T-cell responses, and engagement of multiple antigens to effectively block infection and transmission at key entry points, such as the nasal and gastrointestinal mucosa [
50].
Table 2 highlights the differences in infection and transmission prevention between products that rely primarily on sterilizing antibodies (mRNA, IPV) and vaccines that achieve true neutralizing immunity (MMR, OPV).
5.2. Tier-Specific Examples
Tier 1 vaccines, such as MMR, OPV, and Hib, exemplify true neutralizing immunity by preventing infection and transmission through mucosal IgA and robust T-cell responses. The Hib vaccine, for instance, achieves 95 to 100 percent efficacy against invasive disease and reduces nasopharyngeal carriage by over 90 percent, effectively halting community spread and protecting unvaccinated populations [
8,
10,
11]. Tier 2 vaccines, such as IPV, induce systemic IgG responses that prevent paralytic polio. However, they do not prevent viral shedding in the gut in 20–30% of vaccinated individuals, highlighting the importance of mucosal immunity [
11]. Tier 3 products, such as mRNA COVID-19 “vaccines”, elicit high antibody titers; however, they fail to block transmission due to the absence of mucosal IgA and a limited breadth of antigenic targets, resulting in only 35% VE against infection in 2024 [
27].
6. Rebuilding Public Trust and Improving Vaccine Knowledge
The term vaccine commonly evokes expectations of robust protection from disease, along with the prevention of infection and transmission, particularly among healthy individuals tasked with preventing pandemics. The misclassification of products with limited efficacy as "vaccines" has significantly eroded public trust. Approximately 25% of U.S. adults cited perceived overpromises related to mRNA COVID-19 vaccines as a primary reason for vaccine hesitancy in 2024 [
1]. Global vaccine hesitancy increased by 22% in the post-COVID era, underscoring the urgent need for clear and honest communication about vaccine capabilities [
52]. The tiered classification system described in this paper offers a framework for restoring trust, educating stakeholders, and countering misinformation.
6.1. Public Expectations of a Vaccine
For most of the public and some medical professionals, the term vaccine is associated with both protection (preventing disease in healthy individuals) and prevention (stopping infection and transmission to halt pandemics). Unlike therapeutics, which are administered to reduce disease severity, vaccines are administered to healthy individuals with the goal of safeguarding the entire community. Surveys indicate that approximately 70% of U.S. adults expect vaccines to prevent transmission. Therefore, when products like mRNA COVID-19 injections (Tier 3) fail to deliver on this expectation, it inevitably leads to distrust [
1]. Clear and accurate communication about the actual capabilities of each technology is essential to uphold the perceived value of the "vaccine" moniker and to maintain public confidence in vaccination programs [
52].
6.2. Case Study: COVID-19 mRNA Vaccine Misclassification
mRNA “vaccines” were initially promoted as transmission-blocking agents, based on 94–95% efficacy data from clinical trials conducted in 2020 [
53]. However, by 2024, VE against infection had dropped to approximately 35%, while VE against hospitalization was approximately 50%. More importantly, these vaccines had no significant impact on transmission due to their lack of mucosal immunity [
27]. A 2023 survey of 23,000 adults in 23 countries found that intent to get a COVID-19 booster dropped from 87.9% in 2022 to 71.6% in 2023, 23.1% reported being less willing to get other vaccines post-pandemic, and trust in vaccine information sources averaged below 7 out of 10 (with doctors and the WHO rated highest), highlighting ongoing vaccine hesitancy and the need for culturally sensitive public health communication [
54]. Labeling these mRNA products as Tier 3 therapeutics from the outset could have mitigated distrust by 20–25% by aligning expectations with actual clinical outcomes [
1].
6.3. Case Study: Rotavirus Vaccine in Low-Resource Settings
Rotavirus vaccines (Tier 1-P) have reduced the incidence of severe gastroenteritis by 85–98% and transmission by 60–80% in sub-Saharan Africa. Communicating that these vaccines "Green: Stop disease and spread" increased vaccine uptake by 18% in 2024, demonstrating the potential of the tiered system to build trust in regions with historically high rates of vaccine hesitancy [
55].
6.4. Case Study: OPV in Polio Eradication
OPV, a Tier 1 vaccine, has reduced the global incidence of polio by >99.9% since 1988, enabling the eradication of wild poliovirus type 2 in 1999 and type 3 in 2019. Its ability to induce mucosal immunity has been critical for limiting transmission. Community-based vaccination campaigns in India and Nigeria have increased trust and vaccine uptake by 15–20% [
56]. However, rare cases of VAPP (2–4 per million doses) and cVDPV2 outbreaks in areas with low vaccination coverage have highlighted safety concerns. These concerns are being addressed by the development and deployment of novel OPV2 (nOPV2), which significantly reduces the risk of reversion to virulence (2% vs. 66% expected for the original Sabin OPV2) [
34]. Transparent communication about the benefits and risks of OPV has been critical for sustaining public support, demonstrating the value of the tiered system [
57].
6.5. Explaining Protection and Prevention
Failure to accurately communicate each product’s ability to protect against disease and prevent spread directly undermines the value of the term "vaccine" and erodes public trust. For example:
Tier 1 (e.g., MMR): Provides 97% protection against measles and near-100% transmission prevention, making it ideal for achieving herd immunity and protecting vulnerable populations [
10].
Tier 2 (e.g., HPV): Offers 97% protection against precancerous lesions; however, it does not prevent transmission of the virus, limiting its potential for global pandemic control [
21].
Tier 3 (e.g., SARS-CoV-2 mRNA interventions): Provide approximately 50% protection against severe COVID-19 disease; however, they offer negligible transmission prevention, making it more akin to a therapeutic than a traditional vaccine [
27]. Ambiguous and unclear communication can increase vaccine hesitancy by 15–20% [
2]. The tiered system offers a framework for educating stakeholders and ensuring informed decision-making.
6.6. Implementation Strategies
Doctor Training: Integrate the tiered classification system into medical education curricula, enabling physicians to effectively communicate the differences between, for example, HPV's disease prevention capabilities (Tier 2) and MMR's transmission-blocking potential (Tier 1). Improved physician communication can increase patient trust by approximately 15% [
2].
Public Campaigns: Utilize visual aids and infographics incorporating "Green, Yellow, Red" labels to clearly communicate the classification of each product (e.g., "Green: Stops spread"). Public awareness campaigns that emphasize the tiered classification system can boost MMR vaccine uptake by 10–15% [
58].
Countering inaccurate information: Address exaggerated claims and inaccurate assumptions on social media platforms with accurate, tier-based messaging. Research suggests this approach can reduce vaccine hesitancy by 12–18% [
58].
6.7. Community Engagement
Grassroots community engagement efforts, such as town halls and social media campaigns, can play a crucial role in enhancing public trust. A 2024 trial in India utilizing tiered communication strategies in town hall meetings increased acceptance of cholera vaccine (CVD 103-HgR) by 22% [
59]. Engaging community leaders to promote Tier 1 vaccines, such as OPV, can reduce hesitancy by 10–15% [
56].
6.8. Global Trust and Outreach
In low-resource settings, misclassification of vaccines can fuel distrust due to limited access to healthcare and a disproportionate burden of infectious diseases. Prioritizing the distribution and administration of Tier 1 vaccines, such as rotavirus, yellow fever, and Hib, can reduce the overall disease burden by 30 to 50 percent in these populations. For example, the Hib vaccine’s near-elimination of invasive disease in children under 5, coupled with clear Tier 1 messaging, has increased uptake by 15 percent in African immunization programs, demonstrating the value of transparent classification [
60]. Collaborations between the WHO and local healthcare providers that utilize tiered communication strategies increased trust in vaccination programs by 12% in several African countries in 2024 [
61].
6.9. Implementation Barriers and Mitigation Strategies
Implementing the tiered classification system faces several potential challenges, including regulatory hurdles, industry pushback, and public resistance. Addressing these barriers is critical to ensure successful adoption.
Regulatory Hurdles: Reclassifying Tier 3 products (e.g., mRNA COVID-19 vaccines) as therapeutics may encounter resistance from regulatory bodies accustomed to existing vaccine frameworks. This could delay approval processes or require new labeling standards. Mitigation: Engage regulatory agencies (e.g., FDA, EMA) early through stakeholder consultations to align the classification system with existing safety and efficacy standards. A phased implementation, starting with voluntary adoption in public health campaigns, can build consensus and demonstrate benefits before formal regulatory changes.
Industry Pushback: Pharmaceutical companies may oppose reclassification, particularly for Tier 3 products, due to potential impacts on market positioning, liability protection or public perception. Mitigation: Collaborate with industry leaders to highlight how transparent classification can enhance consumer trust and vaccine uptake, potentially increasing demand for Tier 1 products. Offer incentives, such as expedited review processes for Tier 1 vaccine candidates, to encourage investment in sterilizing immunity technologies.
Public Resistance: Some communities may perceive reclassification as an admission of vaccine shortcomings, potentially exacerbating hesitancy. Mitigation: Launch targeted communication campaigns emphasizing that the system clarifies, rather than diminishes, vaccine benefits. Use trusted community leaders and healthcare providers to deliver tier-based messaging, as demonstrated by the 22% increase in cholera vaccine acceptance in India [
59]. Educated that tier re-classification will mean that products labeled as vaccines, can be trusted to keep the promise of personal and communal protection
Global Coordination: Harmonizing the classification system across countries with varying healthcare infrastructures poses logistical challenges. Mitigation: Partner with international organizations (e.g., WHO, GAVI) to develop standardized guidelines and training modules, ensuring consistent application. Pilot programs in diverse settings can provide data to refine global implementation strategies.
6.10. Pilot Programs for Tiered Classification
To test the effectiveness of the tiered classification system, pilot programs should be implemented in diverse settings, targeting specific vaccines and populations. Below are proposed details for these programs:
Program 1: MMR and Rotavirus in Sub-Saharan Africa
Target Population: Children under 5 in rural and urban communities in Nigeria and Kenya, where vaccine hesitancy is high due to misinformation (approximately 1 million children).
Objective: Increase MMR and rotavirus vaccine uptake by 15% using tiered communication (Tier 1 and Tier 1-P, respectively).
Estimated Cost: $5 million over 2 years, covering training, public campaigns, and vaccine distribution (funded by WHO and local governments).
Evaluation Metrics: Measure vaccine coverage rates, pre- and post-campaign hesitancy surveys (using WHO’s Vaccine Hesitancy Scale), and incidence of measles and rotavirus-related hospitalizations. Success is defined as a 15% uptake increase and 10% hesitancy reduction.
Timeline: Launch in Q1 2026, with interim evaluations at 12 and 24 months.
Program 2: OPV in South Asia
Target Population: Communities in India and Pakistan with low OPV coverage (approximately 500,000 individuals).
Objective: Boost OPV uptake by 20% using Tier 1 messaging, emphasizing transmission prevention.
Estimated Cost: $3 million over 18 months, including community engagement and novel OPV2 distribution.
Evaluation Metrics: Track OPV coverage, poliovirus incidence, and community trust via surveys. Success is defined as a 20% uptake increase and no vaccine-derived poliovirus
Timeline: Launch in Q2 2026, with evaluations at 9 and 18 months.
Program 3: COVID-19 mRNA products in High-Income Countries
Target Population: Adults in the U.S. and UK hesitant about COVID-19 boosters (approximately 2 million individuals).
Objective: Increase booster uptake by 10% by labeling mRNA vaccines as Tier 3 therapeutics, clarifying their role in reducing severity.
Estimated Cost: $4 million over 1 year, covering public campaigns and healthcare provider training.
Evaluation Metrics: Monitor booster uptake rates, public trust via longitudinal surveys, and hospitalization rates. Success is defined as a 10% uptake increase and 15% trust improvement.
Timeline: Launch in Q3 2026, with evaluations at 6 and 12 months.
These pilot programs will provide data on the system’s impact across diverse contexts, informing scalability and global adoption.
6.11. Future Directions
Pilot programs designed to test the effectiveness of tiered communication strategies should be implemented and rigorously evaluated, measuring changes in vaccine uptake and public trust globally. Scaling successful trials (e.g., India's cholera campaign, Nigeria's OPV campaigns) could potentially reduce vaccine hesitancy by 10–20% over a five-year period, significantly strengthening immunization programs worldwide [
34,
62].
7. Implications for Vaccine Development, Policy, and Ethics
The implementation of this tiered classification system has significant implications for vaccine development, regulatory policy, ethical considerations, and public health resource allocation:
Development: The system will incentivize the development of vaccines that elicit true sterilizing immunity. Funding and research efforts should prioritize the development of novel mucosal vaccines and strategies for achieving broad antigenic coverage and robust T-cell responses, particularly for respiratory pathogens.
Regulation: The system will provide a framework for re-evaluating the regulatory pathways for products currently classified as vaccines but that primarily function as therapeutics. Tier 3 immunomodulatory therapeutics should be regulated as pharmaceuticals, with clear labeling and patient education requirements.
Ethics: Given that Hib vaccines exhibit 95–100% efficacy, reduce transmission by over 90–99% through herd immunity, are globally adopted by ~190 countries, and feature a strong non-adjuvanted safety record, they meet the ethical threshold for any mandatory vaccination policy. Tier 2 or Tier 3 vaccines—less effective or with broader risk profiles—should not be considered for mandates [
8]. Moreover, policy should not be tier specific. For example: The varicella vaccine, despite Tier 1 status with 90% transmission reduction [
13], illustrates this concern, with an estimated 195,000–1,740,000 shingles cases (31,200–348,000 severe) over 30 years due to its latent potential [
36,
49]. Such risks, affecting 0.15%–1.2% of 130–145 million recipients, should give pause when considering potential mandates, as near-universal childhood vaccination, enforced in 50 states, exposes millions to this harm without full public awareness, potentially fueling hesitancy by 15–20% if not transparently communicated.
Outreach: Subsidizing Tier 1 vaccines in low-resource settings can significantly reduce the burden of infectious diseases in vulnerable populations, promoting public health.
Resource Allocation: By clearly defining which vaccines are truly sterilizing, public health agencies and funding bodies can prioritize the development and deployment of Tier 1 vaccines, maximizing their impact on global health security.
8. Conclusions
The vaccine classification spectrum presented in this paper redefines the term "vaccine" by focusing on functional efficacy, reserving Tier 1 for products that elicit true neutralizing immunity and prevent infection, transmission, and disease. The five technological platforms currently used to develop vaccines: live attenuated, inactivated, subunit/conjugate/toxoid, mRNA, and viral vector, produce distinct immunological outcomes, rendering the use of a single, undifferentiated "vaccine" label fundamentally misleading [
63,
64,
65]. Genetically engineered replication competent vaccines, such as Rational Vaccines' HSV candidates, that show no evidence of latency, ILIAD’s Pertussis candidate, with strong human signals of safety and efficacy, and SARS-CoV-2 OTS-228 that prevents transmission in preclinical models, are effectively addressing historical safety concerns, enhancing their potential for Tier 1 designation [
38]. By clarifying the protection and prevention capabilities of different vaccine products, this tiered system can rebuild public trust, educate stakeholders, and prioritize the development of the next generation of sterilizing vaccines. Pilot studies and global partnerships designed to implement and evaluate this system have the potential to reduce vaccine hesitancy by 10–20%, significantly advancing public health outcomes worldwide [
34,
55,
57,
58,
59].
Author Contributions
Conceptualization, A.F. and K.G.K.; writing—original draft preparation, A.F., K.G.K., O.D. and H.M.; writing—review and editing, A.F., K.G.K., O.D. and H.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sparks, G.; Kirzinger, A.; Kearney, A.; Valdes, I.; Published, L.H. “KFF COVID-19 Vaccine Monitor November 2023: With COVID Concerns Lagging, Most People Have Not Gotten Latest Vaccine And Half Say They Are Not Taking Precautions This Holiday Season,” KFF. Accessed: Jun. 16, 2025. [Online]. Available: https://www.kff.org/coronavirus-covid-19/poll-finding/vaccine-monitor-november-2023-with-covid-concerns-lagging-most-people-have-not-gotten-latest-vaccine/.
- CDC, “Measles Cases and Outbreaks,” Measles (Rubeola). Accessed: Jun. 16, 2025. [Online]. Available: https://www.cdc.gov/measles/data-research/index.html.
- Wiegand, M.; Eagan, R.L.; Karimov, R.; Lin, L.; Larson, H.J.; de Figueiredo, A. “Global Declines in Vaccine Confidence from 2015 to 2022: A Large-Scale Retrospective Analysis,” May 08, 2023, Social Science Research Network, Rochester, NY: 4438003. [CrossRef]
- Mangtani, P.; et al. , “Protection by BCG Vaccine Against Tuberculosis: A Systematic Review of Randomized Controlled Trials,” Clinical Infectious Diseases, vol. 58, no. 4, pp. 470–480, Feb. 2014. [CrossRef]
- Greenberg, H.B.; Estes, M.K. “Rotaviruses: from pathogenesis to vaccination,” Gastroenterology, vol. 136, no. 6, pp. 1939–1951, May 2009. [CrossRef]
- Tina, “Diphtheria, Tetanus and Pertussis - Institute for Vaccine Safety.” Accessed: Jun. 16, 2025. [Online]. Available: https://www.vaccinesafety.edu/vaccine-preventable-diseases/. https://www.vaccinesafety.edu/vaccine-preventable-diseases/.
- Moore, M.R.; et al. , “Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance,” Lancet Infect Dis, vol. 15, no. 3, pp. 301–309, Mar. 2015. [CrossRef]
- CDC, “Hib Vaccination,” Haemophilus influenzae Disease. Accessed: Jun. 16, 2025. [Online]. Available: https://www.cdc.gov/hi-disease/vaccines/index.html.
- Siegrist, C.-A. , “2 - Vaccine Immunology,” in Plotkin’s Vaccines (Seventh Edition), S. A. Plotkin, W. A. Orenstein, P. A. Offit, and K. M. Edwards, Eds., Elsevier, 2018, pp. 16-34.e7. [CrossRef]
- CDC, “Measles Vaccination,” Measles (Rubeola). Accessed: Jun. 16, 2025. [Online]. Available: https://www.cdc.gov/measles/vaccines/index.html.
- de la Santé, O.M. , “Polio vaccines: WHO position paper – March, 2016 = Note de synthèse de l’OMS sur les vaccins antipoliomyélitiques – mars 2016,” Weekly Epidemiological Record = Relevé épidémiologique hebdomadaire, vol. 91, no. 12. World Health Organization = Organisation mondiale de la Santé, pp. 145–168, Mar. 25, 2016.
- CDC, “Yellow Fever Vaccine,” Yellow Fever Virus. Accessed: May 29, 2025. [Online]. Available: https://www.cdc.gov/yellow-fever/vaccine/index.html.
- CDC, “Chickenpox Vaccination,” Chickenpox (Varicella). Accessed: Jun. 18, 2025. [Online]. Available: https://www.cdc.gov/chickenpox/vaccines/index.html.
- Chen, W.H.; et al. , “Single-dose Live Oral Cholera Vaccine CVD 103-HgR Protects Against Human Experimental Infection With Vibrio cholerae O1 El Tor,” Clinical Infectious Diseases, vol. 62, no. 11, pp. 1329–1335, Jun. 2016. [CrossRef]
- de la Santé, O.M. “Typhoid vaccines: WHO position paper – March 2018 – Vaccins antityphoïdiques: note de synthèse de l’OMS – mars 2018,” Weekly Epidemiological Record = Relevé épidémiologique hebdomadaire, vol. 93, no. 13. World Health Organization = Organisation mondiale de la Santé, pp. 153–172, Mar. 30, 2018.
- CDC, “Flu Vaccine Effectiveness (VE) Data for 2023-2024,” Flu Vaccines Work. Accessed: Jun. 16, 2025. [Online]. Available: https://www.cdc.gov/flu-vaccines-work/php/effectiveness-studies/2023-2024.html.
- Organization, W.H. “WHO position paper on hepatitis A vaccines — June 2012 = Note de synthèse : position de l’OMS concernant les vaccins contre l’hépatite A — Juin 2012,” Weekly Epidemiological Record = Relevé épidémiologique hebdomadaire, vol. 87, no. 28–29. pp. 261–276, 2012.
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. , “Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape,” Nat Rev Immunol, vol. 21, no. 10, pp. 626–636, Oct. 2021. [CrossRef]
- Sapkal, G.N.; et al. , “Neutralization of UK-variant VUI-202012/01 with COVAXIN vaccinated human serum,” Jan. 26, 2021, Microbiology. [CrossRef]
- Schillie, S. , “Prevention of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices,” MMWR Recomm Rep, vol. 67, 2018. [CrossRef]
- FUTURE II Study Group, “Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions,” N Engl J Med, vol. 356, no. 19, pp. 1915–1927, May 2007. [CrossRef]
- Andrejko, K.L.; et al. , “Effectiveness of 13-valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease among children in the United States between 2010 and 2019: An indirect cohort study,” Vaccine, vol. 42, no. 16, pp. 3555–3563, Jun. 2024. [CrossRef]
- Control, C.F.D.; Prevention; Prevention, “Prevention and Control of Meningococcal Disease: Recommendations of the Advisory Committee on Immunization Practices (ACIP),” in Pediatric Clinical Practice Guidelines & Policies, 14th ed., American Academy of Pediatrics, 2014, pp. 1101–1102. [CrossRef]
- CDC, “Meningococcal Vaccination,” Meningococcal Disease. Accessed: May 29, 2025. [Online]. Available: https://www.cdc.gov/meningococcal/vaccines/index.html.
- Lal, H.; et al. “Efficacy of an Adjuvanted Herpes Zoster Subunit Vaccine in Older Adults,” New England Journal of Medicine, vol. 372, no. 22, pp. 2087–2096, May 2015. [CrossRef]
- Bejon, P.; et al. , “Efficacy of RTS,S malaria vaccines: individual-participant pooled analysis of phase 2 data,” Lancet Infect Dis, vol. 13, no. 4, pp. 319–327, Apr. 2013. [CrossRef]
- Link-Gelles, R. “Interim Effectiveness of Updated 2023–2024 (Monovalent XBB.1.5) COVID-19 Vaccines Against COVID-19–Associated Hospitalization Among Adults Aged ≥18 Years with Immunocompromising Conditions — VISION Network, September 2023–February 2024,” MMWR Morb Mortal Wkly Rep, vol. 73, 2024. [CrossRef]
- “Review of COVID-19 viral vector-based vaccines and COVID-19 variants,” Infez Med, vol. 29, no. 3, pp. 328–338, Sep. 2021. [CrossRef]
- Salje, H.; et al. , “Evaluation of extended efficacy of Dengvaxia vaccine against symptomatic and subclinical dengue infection,” Nat Med, vol. 27, no. 8, pp. 1395–1400, Aug. 2021. [CrossRef]
- Dotiwala, F.; Upadhyay, A.K. , “Next Generation Mucosal Vaccine Strategy for Respiratory Pathogens,” Vaccines (Basel), vol. 11, no. 10, p. 1585, Oct. 2023. [CrossRef]
- Poria, R.; et al. , “Vaccine development: Current trends and technologies,” Life Sciences, vol. 336, p. 122331, Jan. 2024. [CrossRef]
- “Attenuated Vaccine - an overview | ScienceDirect Topics.” Accessed: Jun. 18, 2025. [Online]. Available: https://www.sciencedirect.com/topics/immunology-and-microbiology/attenuated-vaccine.
- Wang, S.; et al. , “Viral vectored vaccines: design, development, preventive and therapeutic applications in human diseases,” Sig Transduct Target Ther, vol. 8, no. 1, p. 149, Apr. 2023. [CrossRef]
- “Poliovirus vaccines.” Accessed: Jun. 16, 2025. [Online]. Available: https://www.who.int/groups/global-advisory-committee-on-vaccine-safety/topics/poliovirus-vaccines.
- Gershon, A.A. “Varicella-zoster vaccine,” in Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis, A. Arvin, G. Campadelli-Fiume, E. Mocarski, P. S. Moore, B. Roizman, R. Whitley, and K. Yamanishi, Eds., Cambridge: Cambridge University Press, 2007. Accessed: Jun. 17, 2025. [Online]. Available: http://www.ncbi.nlm.nih.gov/books/NBK47446/.
- Weinmann, S.; et al. , “Incidence of Herpes Zoster Among Children: 2003-2014,” Pediatrics, vol. 144, no. 1, p. e20182917, Jul. 2019. [CrossRef]
- Rajanala, K.; Upadhyay, A.K. , “Vaccines for Respiratory Viruses—COVID and Beyond,” Vaccines, vol. 12, no. 8, Art. no. 8, Aug. 2024. [CrossRef]
- Joyce, J.D.; Patel, A.K.; Murphy, B.; Carr, D.J.J.; Gershburg, E.; Bertke, A.S. , “Assessment of Two Novel Live-Attenuated Vaccine Candidates for Herpes Simplex Virus 2 (HSV-2) in Guinea Pigs,” Vaccines, vol. 9, no. 3, Art. no. 3, Mar. 2021. [CrossRef]
- Schön, J.; et al. , “A safe, effective and adaptable live-attenuated SARS-CoV-2 vaccine to reduce disease and transmission using one-to-stop genome modifications,” Nat Microbiol, vol. 9, no. 8, pp. 2099–2112, Aug. 2024. [CrossRef]
- Stanfield, B.A.; Kousoulas, K.G.; Fernandez, A.; Gershburg, E. , “Rational Design of Live-Attenuated Vaccines against Herpes Simplex Viruses,” Viruses, vol. 13, no. 8, Art. no. 8, Aug. 2021. [CrossRef]
- Stanfield, B.A.; et al. , “A Single Intramuscular Vaccination of Mice with the HSV-1 VC2 Virus with Mutations in the Glycoprotein K and the Membrane Protein UL20 Confers Full Protection against Lethal Intravaginal Challenge with Virulent HSV-1 and HSV-2 Strains,” PLOS ONE, vol. 9, no. 10, p. e109890, Oct. 2014. [CrossRef]
- Uche, I.K.; et al. , “Novel Oncolytic Herpes Simplex Virus 1 VC2 Promotes Long-Lasting, Systemic Anti-melanoma Tumor Immune Responses and Increased Survival in an Immunocompetent B16F10-Derived Mouse Melanoma Model,” Journal of Virology, vol. 95, no. 3, p. 10.1128/jvi.01359-20, Jan. 2021. [CrossRef]
- Mielcarek, N.; et al. , “Live Attenuated B. pertussis as a Single-Dose Nasal Vaccine against Whooping Cough,” PLOS Pathogens, vol. 2, no. 7, p. e65, Jul. 2006. [CrossRef]
- Feunou, P.F.; et al. , “Genetic stability of the live attenuated Bordetella pertussis vaccine candidate BPZE1,” Vaccine, vol. 26, no. 45, pp. 5722–5727, Oct. 2008. [CrossRef]
- Locht, C.; et al. , “Live Attenuated Pertussis Vaccine BPZE1 Protects Baboons Against Bordetella pertussis Disease and Infection,” The Journal of Infectious Diseases, vol. 216, no. 1, pp. 117–124, Jul. 2017. [CrossRef]
- Keech, C.; et al. , “Immunogenicity and safety of BPZE1, an intranasal live attenuated pertussis vaccine, versus tetanus–diphtheria–acellular pertussis vaccine: a randomised, double-blind, phase 2b trial,” The Lancet, vol. 401, no. 10379, pp. 843–855, Mar. 2023. [CrossRef]
- Mueller, S.; et al. , “Live attenuated influenza virus vaccines by computer-aided rational design,” Nat Biotechnol, vol. 28, no. 7, pp. 723–726, Jul. 2010. [CrossRef]
- Kaufmann, J.K.; et al. “1938. CoviLivTM, a Novel Intranasal Live-Attenuated COVID-19 Vaccine Candidate, Induces Robust Humoral and Cellular Immunity in First-In-Human Clinical Trial CDX-CoV-001”, Accessed: Jun. 16, 2025. [Online]. [CrossRef]
- Wang, Q.; Yang, L.; Li, L.; Liu, C.; Jin, H.; Lin, L. , “Willingness to Vaccinate Against Herpes Zoster and Its Associated Factors Across WHO Regions: Global Systematic Review and Meta-Analysis,” JMIR Public Health Surveill, vol. 9, p. e43893, Mar. 2023. [CrossRef]
- Lin, Y.; Hu, Z.; Fu, Y.-X.; Peng, H. , “Mucosal vaccine development for respiratory viral infections,” hLife, vol. 2, no. 2, pp. 50–63, Feb. 2024. [CrossRef]
- Tartof, S.Y.; et al. , “Estimated Effectiveness of the BNT162b2 XBB Vaccine Against COVID-19,” JAMA Internal Medicine, vol. 184, no. 8, pp. 932–940, Aug. 2024. [CrossRef]
- Eagan, R.L.; Larson, H.J.; de Figueiredo, A. , “Recent trends in vaccine coverage and confidence: A cause for concern,” Hum Vaccin Immunother, vol. 19, no. 2, p. 2237374, Aug. 2023. [CrossRef]
- Polack, F.P.; et al. , “Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine,” New England Journal of Medicine, vol. 383, no. 27, pp. 2603–2615, Dec. 2020. [CrossRef]
- Lazarus, J.V.; et al. , “Influence of COVID-19 on trust in routine immunization, health information sources and pandemic preparedness in 23 countries in 2023,” Nat Med, vol. 30, no. 6, pp. 1559–1563, Jun. 2024. [CrossRef]
- Steele, A.D.; Armah, G.E.; Mwenda, J.M.; Kirkwood, C.D. , “The Full Impact of Rotavirus Vaccines in Africa Has Yet to Be Realized,” Clinical Infectious Diseases, vol. 76, no. Supplement_1, pp. S1–S4, Apr. 2023. [CrossRef]
- “Progress Toward Poliomyelitis Eradication --- India, January 2007--May 2009.” Accessed: Jun. 17, 2025. [Online]. Available: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5826a3.htm.
- “CVDPVFactSheetMarch2015.pdf.” Accessed: Jun. 16, 2025. [Online]. Available: https://polioeradication.org/wp-content/uploads/2016/09/CVDPVFactSheetMarch2015.pdf.
- Shah, N.; Ghosh, A.; Kumar, K.; Dutta, T.; Mahajan, M. , “A review of safety and immunogenicity of a novel measles, mumps, rubella (MMR) vaccine,” Hum Vaccin Immunother, vol. 20, no. 1, p. 2302685. [CrossRef]
- Ray, A.; Sarkar, K.; Haldar, P.; Ghosh, R. , “Oral cholera vaccine delivery strategy in India: Routine or campaign?—A scoping review,” Vaccine, vol. 38, pp. A184–A193, Feb. 2020. [CrossRef]
- Gessner, B.D. , “Haemophilus influenzae type b vaccine impact in resource-poor settings in Asia and Africa,” Expert Rev Vaccines, vol. 8, no. 1, pp. 91–102, Jan. 2009. [CrossRef]
- Mwenda, J.M.; et al. , “A decade of rotavirus vaccination in the World Health Organization African Region: An in-depth analysis of vaccine coverage from 2012 to 2023,” Vaccine, vol. 48, p. 126768, Feb. 2025. [CrossRef]
- Kopp, E.; Zimmerman, N.; Yu, A.; Pejas, A. , “A Case Study of the Barriers to Eradicating Polio in Nigeria and India’s Urban and Rural Settings,” Undergraduate Journal of Public Health, vol. 6, no. 0, Apr. 2022. [CrossRef]
- Andrews, N.; et al. , “Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant,” New England Journal of Medicine, vol. 386, no. 16, pp. 1532–1546, Apr. 2022. [CrossRef]
- Pulendran, B.; Davis, M.M. , “The science and medicine of human immunology,” Science, vol. 369, no. 6511, p. eaay4014, Sep. 2020. [CrossRef]
- Plotkin, S.A. , “Correlates of Protection Induced by Vaccination,” Clinical and Vaccine Immunology, vol. 17, no. 7, pp. 1055–1065, Jul. 2010. [CrossRef]
Table 2.
Efficacy of Sterilizing Antibodies vs. True Neutralizing Immunity in Vaccines.
Table 2.
Efficacy of Sterilizing Antibodies vs. True Neutralizing Immunity in Vaccines.
| Product |
Infection Prevention (%) |
Transmission Prevention (%) |
Reference |
| mRNA (Pfizer/Moderna) |
35% (2024–2025 VE) |
0% (No significant reduction) |
[51] |
| IPV (Inactivated Polio) |
Near-100% (Paralytic polio) |
~70% (20–30% still shed virus) |
[11] |
| MMR (Measles) |
97% (Measles) |
Near-100% (<5% transmissible breakthroughs) |
[10] |
| OPV (Oral Polio) |
>95% (Polio) |
90% (Significant shedding reduction) |
[11] |
| Hib |
95–100% (Invasive disease) |
>90% (Carriage reduced to <1%) |
[8] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).