Submitted:
25 June 2025
Posted:
26 June 2025
You are already at the latest version
Abstract
Keywords:
Introduction
The microcirculation and endothelial dysfunction
A note on systems biology explanations of cause and effect
The microcirculation from the point of view of ‘blood stasis’ in Traditional Chinese Medicine
Laser speckle (contrast) imaging (LSI/ LSCI)
Laser Doppler Imaging (LDI)
Comparison of the two techniques
Discussion
Comparison of technological advantages and innovative breakthroughs
Unity and specificity of cross-disease mechanisms
Opportunities and challenges for further clinical translation
Future research directions and technological innovation
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guven, G., Hilty, M. P. and Ince, C. (2020) Microcirculation: Physiology, Pathophysiology, and Clinical Application. Blood Purif. 49, 143-150. [CrossRef]
- Lai, C. and Teboul, J. L. (2023) Hemodynamic monitoring: current practice and new perspectives. In The sepsis codex (Sa, M. B., Hidalgo, J. and Perez-Fernandez, J., eds.). pp. 75-87, Elsevier, Amsterdam.
- Munoz, C. J., Lucas, A., Williams, A. T. and Cabrales, P. (2020) A Review on Microvascular Hemodynamics: The Control of Blood Flow Distribution and Tissue Oxygenation. Crit Care Clin. 36, 293-305. [CrossRef]
- Orellana Jimenez, C. E. A. (2023) Sepsis and Microcirculation. In The sepsis codex (Sa, M. B., Hidalgo, J. and Perez-Fernandez, J., eds.). pp. 29-34, Elsevier, Amsterdam.
- Slovinski, A. P., Hajjar, L. A. and Ince, C. (2019) Microcirculation in Cardiovascular Diseases. J Cardiothorac Vasc Anesth. 33, 3458-3468. [CrossRef]
- Yu, D. Y., Cringle, S. J., Yu, P. K., Balaratnasingam, C., Mehnert, A., Sarunic, M. V., An, D. and Su, E. N. (2019) Retinal capillary perfusion: Spatial and temporal heterogeneity. Prog Retin Eye Res. 70, 23-54. [CrossRef]
- Alberts, B., Johnson, A., Lewis, J., Morgan, D., Raff, M., Roberts, K. and Walter, P. (2016) Molecular biology of the cell, 6th Ed. Garland Science, New York.
- Foote, C. A., Soares, R. N., Ramirez-Perez, F. I., Ghiarone, T., Aroor, A., Manrique-Acevedo, C., Padilla, J. and Martinez-Lemus, L. (2022) Endothelial Glycocalyx. Compr Physiol. 12, 3781-3811. [CrossRef]
- Kesimer, M., Ehre, C., Burns, K. A., Davis, C. W., Sheehan, J. K. and Pickles, R. J. (2013) Molecular organization of the mucins and glycocalyx underlying mucus transport over mucosal surfaces of the airways. Mucosal Immunol. 6, 379-392. [CrossRef]
- Ait-Oufella, H., Maury, E., Lehoux, S., Guidet, B. and Offenstadt, G. (2010) The endothelium: physiological functions and role in microcirculatory failure during severe sepsis. Intensive Care Med. 36, 1286-1298. [CrossRef]
- Cusack, R., Leone, M., Rodriguez, A. H. and Martin-Loeches, I. (2022) Endothelial Damage and the Microcirculation in Critical Illness. Biomedicines. 10, 3150. [CrossRef]
- Crabb, J. W. (2014) The proteomics of drusen. Cold Spring Harb Perspect Med. 4, a017194. [CrossRef]
- Dentchev, T., Milam, A. H., Lee, V. M., Trojanowski, J. Q. and Dunaief, J. L. (2003) Amyloid-beta is found in drusen from some age-related macular degeneration retinas, but not in drusen from normal retinas. Mol Vis. 9, 184-190.
- Wang, J., Ohno-Matsui, K., Yoshida, T., Kojima, A., Shimada, N., Nakahama, K., Safranova, O., Iwata, N., Saido, T. C., Mochizuki, M. and Morita, I. (2008) Altered function of factor I caused by amyloid beta: implication for pathogenesis of age-related macular degeneration from Drusen. J Immunol. 181, 712-720. [CrossRef]
- Isas, J. M., Luibl, V., Johnson, L. V., Kayed, R., Wetzel, R., Glabe, C. G., Langen, R. and Chen, J. (2010) Soluble and mature amyloid fibrils in drusen deposits. Invest Ophthalmol Vis Sci. 51, 1304-1310. [CrossRef]
- Luibl, V., Isas, J. M., Kayed, R., Glabe, C. G., Langen, R. and Chen, J. (2006) Drusen deposits associated with aging and age-related macular degeneration contain nonfibrillar amyloid oligomers. J Clin Invest. 116, 378-385. [CrossRef]
- Shoda, C., Kitagawa, Y., Shimada, H., Yuzawa, M., Tateno, A. and Okubo, Y. (2018) Relationship of Area of Soft Drusen in Retina with Cerebral Amyloid-beta Accumulation and Blood Amyloid-beta Level in the Elderly. J Alzheimers Dis. 62, 239-245. [CrossRef]
- Anderson, D. H., Talaga, K. C., Rivest, A. J., Barron, E., Hageman, G. S. and Johnson, L. V. (2004) Characterization of beta amyloid assemblies in drusen: the deposits associated with aging and age-related macular degeneration. Exp Eye Res. 78, 243-256. [CrossRef]
- Mullins, R. F., Russell, S. R., Anderson, D. H. and Hageman, G. S. (2000) Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 14, 835-846.
- Friedman, E. (1997) A hemodynamic model of the pathogenesis of age-related macular degeneration. Am J Ophthalmol. 124, 677-682. [CrossRef]
- Friedman, E. (2008) The pathogenesis of age-related macular degeneration. Am J Ophthalmol. 146, 348-349. [CrossRef]
- Kubicka-Trząska, A. (2007) Macular microcirculation blood flow in patients with age related macular degeneration treated with photodynamic therapy and transpupillary thermotherapy. Klin Oczna. 109, 138-141.
- Lipecz, A., Miller, L., Kovacs, I., Czakó, C., Csipo, T., Baffi, J., Csiszar, A., Tarantini, S., Ungvari, Z., Yabluchanskiy, A. and Conley, S. (2019) Microvascular contributions to age-related macular degeneration (AMD): from mechanisms of choriocapillaris aging to novel interventions. Geroscience. 41, 813-845. [CrossRef]
- Lylyk, I., Bleise, C., Lylyk, P. N., Perez, N., Lundquist, J., Scrivano, E., Francone, A. A., Charles, M., Zompa, T. and Lylyk, P. (2022) Ophthalmic artery angioplasty for age-related macular degeneration. J Neurointerv Surg. 14, 968-972. [CrossRef]
- Stefánsson, E., Geirsdóttir, A. and Sigurdsson, H. (2011) Metabolic physiology in age related macular degeneration. Prog Retin Eye Res. 30, 72-80. [CrossRef]
- Alameddine, R. S., Hamieh, L. and Shamseddine, A. (2014) From sprouting angiogenesis to erythrocytes generation by cancer stem cells: evolving concepts in tumor microcirculation. Biomed Res Int. 2014, 986768. [CrossRef]
- Fukumura, D., Duda, D. G., Munn, L. L. and Jain, R. K. (2010) Tumor microvasculature and microenvironment: novel insights through intravital imaging in pre-clinical models. Microcirculation. 17, 206-225. [CrossRef]
- Gao, W. (2018) Quantitative depth-resolved microcirculation imaging with optical coherence tomography angiography (Part I): Blood flow velocity imaging. Microcirculation. 25, e12375. [CrossRef]
- Li, H. M. (2016) Microcirculation of liver cancer, microenvironment of liver regeneration, and the strategy of Chinese medicine. Chin J Integr Med. 22, 163-167. [CrossRef]
- Mayr, N. A., Hawighorst, H., Yuh, W. T., Essig, M., Magnotta, V. A. and Knopp, M. V. (1999) MR microcirculation assessment in cervical cancer: correlations with histomorphological tumor markers and clinical outcome. J Magn Reson Imaging. 10, 267-276. [CrossRef]
- Puleri, D. F., Balogh, P. and Randles, A. (2021) Computational models of cancer cell transport through the microcirculation. Biomech Model Mechanobiol. 20, 1209-1230. [CrossRef]
- Wei, F., Su, Y., Quan, Y., Li, X., Zou, Q., Zhang, L., Li, S., Jiang, M., Lin, G., Liang, P., He, J. and Xie, K. (2023) Anticoagulants Enhance Molecular and Cellular Immunotherapy of Cancer by Improving Tumor Microcirculation Structure and Function and Redistributing Tumor Infiltrates. Clin Cancer Res. 29, 2525-2539. [CrossRef]
- Bacelova, M., Nikolova, J., Alakidi, A., Petkova, V., Mihaylova, V., Dimov, I., Tafradzhiyska-Hadjiolova, R., Dzhiganska, T., Mileva-Popova, R. and Bivolarska, A. (2025) Microcirculation and cardiovascular risk: Diagnostic value and clinical relevance. Pharmacia. 72, 1-8. [CrossRef]
- Ciaramella, L., Di Serafino, L., Mitrano, L., De Rosa, M. L., Carbone, C., Rea, F. S., Monaco, S., Scalamogna, M., Cirillo, P. and Esposito, G. (2023) Invasive Assessment of Coronary Microcirculation: A State-of-the-Art Review. Diagnostics (Basel). 14, 86. [CrossRef]
- Kalia, N. (2023) A historical review of experimental imaging of the beating heart coronary microcirculation in vivo. J Anat. 242, 3-16. [CrossRef]
- Lazaridis, A., Triantafyllou, A., Mastrogiannis, K., Malliora, A., Doumas, M. and Gkaliagkousi, E. (2023) Assessing skin microcirculation in patients at cardiovascular risk by using laser speckle contrast imaging. A narrative review. Clin Physiol Funct Imaging. 43, 211-222. [CrossRef]
- Tibiriçá, E., Lorenzo, A. and de Oliveira, G. M. M. (2018) Microcirculation and Cardiovascular Diseases. Arq Bras Cardiol. 111, 120-121. [CrossRef]
- Ullrich-Daub, H., Daub, S., Olschewski, M., Münzel, T. and Gori, T. (2023) Diseases of the Coronary Microcirculation: Diagnosis and Treatment. Dtsch Arztebl Int. 120, 739-746. [CrossRef]
- Widmer, R. J., Samuels, B., Samady, H., Price, M. J., Jeremias, A., Anderson, R. D., Jaffer, F. A., Escaned, J., Davies, J., Prasad, M., Grines, C. and Lerman, A. (2019) The functional assessment of patients with non-obstructive coronary artery disease: expert review from an international microcirculation working group. EuroIntervention. 14, 1694-1702. [CrossRef]
- Xu, S., Ilyas, I., Little, P. J., Li, H., Kamato, D., Zheng, X., Luo, S., Li, Z., Liu, P., Han, J., Harding, I. C., Ebong, E. E., Cameron, S. J., Stewart, A. G. and Weng, J. (2021) Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol Rev. 73, 924-967. [CrossRef]
- Pries, A. R. (2014) Microcirculation in hypertension and cardiovascular disease. Eur Heart J Suppl. 16, A28-A29. [CrossRef]
- Pries, A. R., Kuebler, W. M. and Habazettl, H. (2018) Coronary Microcirculation in Ischemic Heart Disease. Curr Pharm Des. 24, 2893-2899. [CrossRef]
- Souza, A. C. d. A. H., Troschel, A. S., Marquardt, J. P., Hadžić, I., Foldyna, B., Moura, F. A., Hainer, J., Divakaran, S., Blankstein, R., Dorbala, S., Di Carli, M. F., Aerts, H. J. W. L., Lu, M. T., Fintelmann, F. J. and Taqueti, V. R. (2025) Skeletal muscle adiposity, coronary microvascular dysfunction, and adverse cardiovascular outcomes. Eur Heart J. 46, 1112-1123. [CrossRef]
- Taqueti, V. R. and Di Carli, M. F. (2018) Coronary Microvascular Disease Pathogenic Mechanisms and Therapeutic Options: JACC State-of-the-Art Review. J Am Coll Cardiol. 72, 2625-2641. [CrossRef]
- Taqueti, V. R., Solomon, S. D., Shah, A. M., Desai, A. S., Groarke, J. D., Osborne, M. T., Hainer, J., Bibbo, C. F., Dorbala, S., Blankstein, R. and Di Carli, M. F. (2018) Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J. 39, 840-849. [CrossRef]
- Obert, P., Walther, G., Dutheil, F., Lesourd, B., Chapier, R., Courteix, D. and Vinet, A. (2018) Regional myocardial function abnormalities are associated with macro- and microcirculation dysfunction in the metabolic syndrome: the RESOLVE study. Heart Vessels. 33, 688-694. [CrossRef]
- Uchida, Y., Ichimiya, S., Ishii, H., Kanashiro, M., Watanabe, J., Yoshikawa, D., Takeshita, K., Sakai, S., Amano, T., Matsubara, T. and Murohara, T. (2012) Impact of metabolic syndrome on various aspects of microcirculation and major adverse cardiac events in patients with ST-segment elevation myocardial infarction. Circ J. 76, 1972-1979. [CrossRef]
- Roskal-Wałek, J., Golębiewska, J., Mackiewicz, J., Wałek, P., Bociek, A., Biskup, M., Odrobina, D. and Jaroszyński, A. (2023) The Haemodialysis Session Effect on the Choroidal Thickness and Retinal and Choroidal Microcirculation-A Literature Review. J Clin Med. 12, 7729. [CrossRef]
- Chudzik, M., Cender, A., Mordaka, R., Zielinski, J., Katarzynska, J., Marcinek, A. and Gebicki, J. (2022) Chronic Fatigue Associated with Post-COVID Syndrome versus Transient Fatigue Caused by High-Intensity Exercise: Are They Comparable in Terms of Vascular Effects? Vasc Health Risk Manag. 18, 711-719. [CrossRef]
- Haunhorst, S., Dudziak, D., Scheibenbogen, C., Seifert, M., Sotzny, F., Finke, C., Behrends, U., Aden, K., Schreiber, S., Brockmann, D., Burggraf, P., Bloch, W., Ellert, C., Ramoji, A., Popp, J., Reuken, P., Walter, M., Stallmach, A. and Puta, C. (2024) Towards an understanding of physical activity-induced post-exertional malaise: Insights into microvascular alterations and immunometabolic interactions in post-COVID condition and myalgic encephalomyelitis/chronic fatigue syndrome. Infection. 53, 1-13. [CrossRef]
- Khan, F., Spence, V., Kennedy, G. and Belch, J. J. (2003) Prolonged acetylcholine-induced vasodilatation in the peripheral microcirculation of patients with chronic fatigue syndrome. Clin Physiol Funct Imaging. 23, 282-285. [CrossRef]
- Ryabkova, V. A., Gavrilova, N. Y., Fedotkina, T. V., Churilov, L. P. and Shoenfeld, Y. (2022) Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and Post-COVID Syndrome: A Common Neuroimmune Ground? Diagnostics (Basel). 13, 66. [CrossRef]
- Spence, V. A., Khan, F., Kennedy, G., Abbot, N. C. and Belch, J. J. (2004) Acetylcholine mediated vasodilatation in the microcirculation of patients with chronic fatigue syndrome. Prostaglandins Leukot Essent Fatty Acids. 70, 403-407. [CrossRef]
- Wirth, K. J. and Löhn, M. (2024) Microvascular Capillary and Precapillary Cardiovascular Disturbances Strongly Interact to Severely Affect Tissue Perfusion and Mitochondrial Function in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Evolving from the Post COVID-19 Syndrome. Medicina (Kaunas). 60, 194. [CrossRef]
- Wollina, U., Abdel-Naser, M. B. and Mani, R. (2006) A review of the microcirculation in skin in patients with chronic venous insufficiency: the problem and the evidence available for therapeutic options. Int J Low Extrem Wounds. 5, 169-180. [CrossRef]
- Abrard, S., Coquet, T., Riou, J., Rineau, E., Hersant, J., Vincent, A., Cordoval, J., Jacquet-Lagreze, M., Allaouchiche, B., Lukaszewicz, A. C. and Henni, S. (2024) Detection and Quantification of Microcirculatory Dysfunction in Severe Covid-19 Not Requiring Mechanical Ventilation: A Three-Arm Cohort Study. Shock. 62, 673-681. [CrossRef]
- Belcaro, G., Cornelli, U., Cesarone, M. R., Scipione, C., Scipione, V., Hu, S., Feragalli, B., Corsi, M., Cox, D., Cotellese, R., Hosoi, M. and Burki, C. (2022) Preventive effects of Pycnogenol(R) on cardiovascular risk factors (including endothelial function) and microcirculation in subjects recovering from coronavirus disease 2019 (COVID-19). Minerva Med. 113, 300-308. [CrossRef]
- Colantuoni, A., Martini, R., Caprari, P., Ballestri, M., Capecchi, P. L., Gnasso, A., Lo Presti, R., Marcoccia, A., Rossi, M. and Caimi, G. (2020) COVID-19 Sepsis and Microcirculation Dysfunction. Front Physiol. 11, 747. [CrossRef]
- Glazkov, A. A., Ulbashev, D. S., Borshchev, G. G., Pulin, A. A., Glazkova, P. A. and Kulikov, D. A. (2023) Skin microcirculation reactivity to local thermal hyperaemia in patients with COVID-19 - A pilot observational study. Clin Hemorheol Microcirc. 83, 19-29. [CrossRef]
- Koutsiaris, A. G. (2024) A Blood Supply Pathophysiological Microcirculatory Mechanism for Long COVID. Life (Basel). 14, 1076. [CrossRef]
- Mesquida, J., Caballer, A., Cortese, L., Vila, C., Karadeniz, U., Pagliazzi, M., Zanoletti, M., Pacheco, A. P., Castro, P., García-de-Acilu, M., Mesquita, R. C., Busch, D. R., Durduran, T. and Consortium, H.-. (2021) Peripheral microcirculatory alterations are associated with the severity of acute respiratory distress syndrome in COVID-19 patients admitted to intermediate respiratory and intensive care units. Crit Care. 25, 381. [CrossRef]
- Netiazhenko, V. Z., Mostovyi, S. I., Safonova, O. M. and Mikhaliev, K. O. (2023) Microcirculatory Alterations in Stable Coronary Artery Disease Patients with Concomitant Covid-19. Wiad Lek. 76, 2224-2238. [CrossRef]
- Sabioni, L., De Lorenzo, A., Castro-Faria-Neto, H. C., Estato, V. and Tibirica, E. (2023) Long-term assessment of systemic microcirculatory function and plasma cytokines after coronavirus disease 2019 (COVID-19). Braz J Infect Dis. 27, 102719. [CrossRef]
- Schlick, S., Lucio, M., Wallukat, G., Bartsch, A., Skornia, A., Hoffmanns, J., Szewczykowski, C., Schroder, T., Raith, F., Rogge, L., Heltmann, F., Moritz, M., Beitlich, L., Schottenhamml, J., Herrmann, M., Harrer, T., Ganslmayer, M., Kruse, F. E., Lammer, R., Mardin, C. and Hohberger, B. (2022) Post-COVID-19 Syndrome: Retinal Microcirculation as a Potential Marker for Chronic Fatigue. Int J Mol Sci. 23, 13683. [CrossRef]
- Szewczykowski, C., Mardin, C., Lucio, M., Wallukat, G., Hoffmanns, J., Schröder, T., Raith, F., Rogge, L., Heltmann, F., Moritz, M., Beitlich, L., Schottenhamml, J., Herrmann, M., Harrer, T., Ganslmayer, M., Kruse, F. E., Kräter, M., Guck, J., Lammer, R., Zenkel, M., Giessl, A. and Hohberger, B. (2022) Long COVID: Association of Functional Autoantibodies against G-Protein-Coupled Receptors with an Impaired Retinal Microcirculation. Int J Mol Sci. 23, 7209. [CrossRef]
- Xiang, M., Wu, X., Jing, H., Liu, L., Wang, C., Wang, Y., Novakovic, V. A. and Shi, J. (2022) The impact of platelets on pulmonary microcirculation throughout COVID-19 and its persistent activating factors. Front Immunol. 13, 955654. [CrossRef]
- Zharkikh, E. V., Loktionova, Y. I., Fedorovich, A. A., Gorshkov, A. Y. and Dunaev, A. V. (2023) Assessment of Blood Microcirculation Changes after COVID-19 Using Wearable Laser Doppler Flowmetry. Diagnostics (Basel). 13, 920. [CrossRef]
- Lip, S., Tran, T. Q. B., Hanna, R., Nichol, S., Guzik, T. J., Delles, C., McClure, J., McCallum, L., Touyz, R. M., Berry, C. and Padmanabhan, S. (2025) Long-term effects of SARS-CoV-2 infection on blood vessels and blood pressure - LOCHINVAR. J Hypertens. 43, 1057-1065. [CrossRef]
- Chang, C. H., Tsai, R. K., Wu, W. C., Kuo, S. L. and Yu, H. S. (1997) Use of dynamic capillaroscopy for studying cutaneous microcirculation in patients with diabetes mellitus. Microvasc Res. 53, 121-127. [CrossRef]
- Hansen, T. W. and Ripa, R. S. (2025) Advances in Imaging Techniques for Assessing Myocardial Microcirculation in People with Diabetes : An Overview of Current Techniques, Emerging Techniques, and Clinical Applications. Diabetes Ther. 16, 785-797. [CrossRef]
- Iwase, T., Ueno, Y., Tomita, R. and Terasaki, H. (2023) Relationship Between Retinal Microcirculation and Renal Function in Patients with Diabetes and Chronic Kidney Disease by Laser Speckle Flowgraphy. Life (Basel). 13, 424. [CrossRef]
- Jung, C. H., Cho, Y. Y., Choi, D., Kim, B. Y., Kim, C. H. and Mok, J. O. (2020) Relationship of Sarcopenia with Microcirculation Measured by Skin Perfusion Pressure in Patients with Type 2 Diabetes. Endocrinol Metab (Seoul). 35, 578-586. [CrossRef]
- Koivukangas, V., Oikarinen, A., Salmela, P. I. and Lahti, A. (2000) Microcirculatory response of skin to benzoic acid and methyl nicotinate in patients with diabetes. Diabet Med. 17, 130-133. [CrossRef]
- Nyberg, M., Gliemann, L. and Hellsten, Y. (2015) Vascular function in health, hypertension, and diabetes: effect of physical activity on skeletal muscle microcirculation. Scand J Med Sci Sports. 25 Suppl 4, 60-73. [CrossRef]
- Sawada, S., Tsuchiya, S., Kodama, S., Kurosawa, S., Endo, A., Sugawara, H., Hosaka, S., Kawana, Y., Asai, Y., Yamamoto, J., Munakata, Y., Izumi, T., Takahashi, K., Kaneko, K., Imai, J., Ito, A., Yasuda, M., Kunikata, H., Nakazawa, T. and Katagiri, H. (2020) Vascular resistance of carotid and vertebral arteries is associated with retinal microcirculation measured by laser speckle flowgraphy in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 165, 108240. [CrossRef]
- Strain, W. D. and Paldanius, P. M. (2018) Diabetes, cardiovascular disease and the microcirculation. Cardiovasc Diabetol. 17, 57. [CrossRef]
- Tooke, J. E. (1989) Microcirculation and diabetes. Br Med Bull. 45, 206-223. [CrossRef]
- Zhong, M., Song, X., Zhang, X., Chen, J., Wang, L., Xia, J., Tang, X., Chen, Q. I. and Yang, B. (2020) Treatment of microcirculation dysfunction in type 2 diabetic mellitus with Shenqi compound prescription: A protocol of systematic review and meta-analysis of randomized clinical trials. Medicine (Baltimore). 99, e22347. [CrossRef]
- Riaz, A., Asghar, S., Shahid, S., Tanvir, H., Ejaz, M. H. and Akram, M. (2024) Prevalence of Metabolic Syndrome and Its Risk Factors Influence on Microvascular Complications in Patients With Type 1 and Type 2 Diabetes Mellitus. Cureus. 16, e55478. [CrossRef]
- Balasubramanian, G. V., Chockalingam, N. and Naemi, R. (2021) The Role of Cutaneous Microcirculatory Responses in Tissue Injury, Inflammation and Repair at the Foot in Diabetes. Front Bioeng Biotechnol. 9, 732753. [CrossRef]
- Lowry, D., Saeed, M., Narendran, P. and Tiwari, A. (2017) The Difference Between the Healing and the Nonhealing Diabetic Foot Ulcer: A Review of the Role of the Microcirculation. J Diabetes Sci Technol. 11, 914-923. [CrossRef]
- Li, Q., Liu, X., Jia, M., Sun, F., Li, Y., Zhang, H., Liu, X., He, H., Zhao, Z., Yan, Z. and Zhu, Z. (2022) Assessment of sublingual microcirculation for the screening of diabetic nephropathy. Diabetol Metab Syndr. 14, 90. [CrossRef]
- Al-Allaf, A. W., Khan, F., Moreland, J., Belch, J. J. and Pullar, T. (2001) Investigation of cutaneous microvascular activity and flare response in patients with fibromyalgia syndrome. Rheumatology (Oxford). 40, 1097-1101. [CrossRef]
- Bengtsson, A. and Bengtsson, M. (1988) Regional sympathetic blockade in primary fibromyalgia. Pain. 33, 161-167. [CrossRef]
- Casas-Barragán, A., Molina, F., Tapia-Haro, R. M., García-Ríos, M. C., Correa-Rodríguez, M. and Aguilar-Ferrándiz, M. E. (2021) Association of core body temperature and peripheral blood flow of the hands with pain intensity, pressure pain hypersensitivity, central sensitization, and fibromyalgia symptoms. Ther Adv Chronic Dis. 12, 2040622321997253. [CrossRef]
- Choi, D. H. and Kim, H. S. (2015) Quantitative analysis of nailfold capillary morphology in patients with fibromyalgia. Korean J Intern Med. 30, 531-537. [CrossRef]
- Frödin, T., Bengtsson, A. and Skogh, M. (1988) Nail fold capillaroscopy findings in patients with primary fibromyalgia. Clin Rheumatol. 7, 384-388. [CrossRef]
- Jeschonneck, M., Grohmann, G., Hein, G. and Sprott, H. (2000) Abnormal microcirculation and temperature in skin above tender points in patients with fibromyalgia. Rheumatology (Oxford). 39, 917-921. [CrossRef]
- Kasikcioglu, E., Dinler, M. and Berker, E. (2006) Reduced tolerance of exercise in fibromyalgia may be a consequence of impaired microcirculation initiated by deficient action of nitric oxide. Med Hypotheses. 66, 950-952. [CrossRef]
- Le Goff, P. (2006) Is fibromyalgia a muscle disorder? Joint Bone Spine. 73, 239-242. [CrossRef]
- Morf, S., Amann-Vesti, B., Forster, A., Franzeck, U. K., Koppensteiner, R., Uebelhart, D. and Sprott, H. (2005) Microcirculation abnormalities in patients with fibromyalgia - measured by capillary microscopy and laser fluxmetry. Arthritis Res Ther. 7, R209-216. [CrossRef]
- Nigro, A. (2025) Microvascular and cerebrovascular alterations in Raynaud's phenomenon and fibromyalgia. Angiogenesis. 28, 22. [CrossRef]
- Rubio-Zarapuz, A., Parraca, J. A., Tornero-Aguilera, J. F. and Clemente-Suárez, V. J. (2025) Unveiling the link: exploring muscle oxygen saturation in fibromyalgia and its implications for symptomatology and therapeutic strategies. Med Gas Res. 15, 58-72. [CrossRef]
- Shang, Y., Gurley, K., Symons, B., Long, D., Srikuea, R., Crofford, L. J., Peterson, C. A. and Yu, G. (2012) Noninvasive optical characterization of muscle blood flow, oxygenation, and metabolism in women with fibromyalgia. Arthritis Res Ther. 14, R236. [CrossRef]
- Feuer, D. S., Handberg, E. M., Mehrad, B., Wei, J., Bairey Merz, C. N., Pepine, C. J. and Keeley, E. C. (2022) Microvascular Dysfunction as a Systemic Disease: A Review of the Evidence. Am J Med. 135, 1059-1068. [CrossRef]
- Gutterman, D. D., Chabowski, D. S., Kadlec, A. O., Durand, M. J., Freed, J. K., Ait-Aissa, K. and Beyer, A. M. (2016) The Human Microcirculation: Regulation of Flow and Beyond. Circ Res. 118, 157-172. [CrossRef]
- Morris, G., Puri, B. K., Olive, L., Carvalho, A., Berk, M., Walder, K., Gustad, L. T. and Maes, M. (2020) Endothelial dysfunction in neuroprogressive disorders-causes and suggested treatments. BMC Med. 18, 305. [CrossRef]
- Paříková, A. (2022) Rheopheresis and Its Use in the Treatment of Diseases with Impaired Microcirculation. A Review. Cesk Slov Oftalmol. 79, 3-5. [CrossRef]
- Rajendran, P., Rengarajan, T., Thangavel, J., Nishigaki, Y., Sakthisekaran, D., Sethi, G. and Nishigaki, I. (2013) The vascular endothelium and human diseases. Int J Biol Sci. 9, 1057-1069. [CrossRef]
- Ray, A., Maharana, K. C., Meenakshi, S. and Singh, S. (2023) Endothelial dysfunction and its relation in different disorders: Recent update. Health Sci Rev. 7, 100084. [CrossRef]
- Hilty, M. P., Akin, S., Boerma, C., Donati, A., Erdem, O., Giaccaglia, P., Guerci, P., Milstein, D. M., Montomoli, J., Toraman, F., Uz, Z., Veenstra, G. and Ince, C. (2020) Automated Algorithm Analysis of Sublingual Microcirculation in an International Multicentral Database Identifies Alterations Associated With Disease and Mechanism of Resuscitation. Crit Care Med. 48, e864-e875. [CrossRef]
- Hilty, M. P. and Ince, C. (2020) Automated quantification of tissue red blood cell perfusion as a new resuscitation target. Curr Opin Crit Care. 26, 273-280. [CrossRef]
- Bekkers, A., Borren, N., Ederveen, V., Fokkinga, E., Andrade De Jesus, D., Sanchez Brea, L., Klein, S., van Walsum, T., Barbosa-Breda, J. and Stalmans, I. (2020) Microvascular damage assessed by optical coherence tomography angiography for glaucoma diagnosis: a systematic review of the most discriminative regions. Acta Ophthalmol. 98, 537-558. [CrossRef]
- Aizawa, N., Kunikata, H., Shiga, Y., Yokoyama, Y., Omodaka, K. and Nakazawa, T. (2014) Correlation between structure/function and optic disc microcirculation in myopic glaucoma, measured with laser speckle flowgraphy. BMC Ophthalmol. 14, 113. [CrossRef]
- Aizawa, N., Kunikata, H., Yokoyama, Y. and Nakazawa, T. (2014) Correlation between optic disc microcirculation in glaucoma measured with laser speckle flowgraphy and fluorescein angiography, and the correlation with mean deviation. Clin Exp Ophthalmol. 42, 293-294. [CrossRef]
- Aizawa, N., Kunikata, H. and Nakazawa, T. (2019) Diagnostic power of laser speckle flowgraphy-measured optic disc microcirculation for open-angle glaucoma: Analysis of 314 eyes. Clin Exp Ophthalmol. 47, 680-683. [CrossRef]
- Bojikian, K. D., Nobrega, P., Wen, J. C., Zhang, Q., Mudumbai, R. C., Johnstone, M. A., Wang, R. K. and Chen, P. P. (2019) Macular Vascular Microcirculation in Eyes With Open-angle Glaucoma Using Different Visual Field Severity Classification Systems. J Glaucoma. 28, 790-796. [CrossRef]
- Hohberger, B., Lucio, M., Schlick, S., Wollborn, A., Hosari, S. and Mardin, C. (2021) OCT-angiography: Regional reduced macula microcirculation in ocular hypertensive and pre-perimetric glaucoma patients. PLoS One. 16, e0246469. [CrossRef]
- Hou, W., Feng, J., Chen, J., Li, X., Yang, G. and Sun, X. (2023) Analysis of the Optic Nerve Head Microcirculation Using Optical Coherence Tomography Angiography and the Upstream Macrocirculation Using Color Doppler Imaging in Low-Tension and High-Tension Glaucoma Patients. Ophthalmic Res. 66, 579-589. [CrossRef]
- Lin, P. W. and Chiu, L. W. (2024) Evaluation of Optic Nerve Head Microcirculation in Open-Angle Glaucoma Patients with Unilateral Visual Field Defect. Ophthalmic Res. 67, 257-265. [CrossRef]
- Taylor, L., Bojikian, K. D., Jung, H., Chu, Z., Zhou, X., Zhang, Q., Mudumbai, R. C., Waang, R. K. and Chen, P. P. (2020) Peripapillary and Macular Microcirculation in Glaucoma Patients of African and European Descent Using Optical Coherence Tomography Angiography. J Glaucoma. 29, 885-889. [CrossRef]
- Wang, T., Ling, Q., Shen, B. and Jia, X. (2025) The strong correlation between visual function improvement and retinal microcirculation enhancement in glaucoma. Front Med (Lausanne). 12, 1537741. [CrossRef]
- Yokoyama, Y., Aizawa, N., Chiba, N., Omodaka, K., Nakamura, M., Otomo, T., Yokokura, S., Fuse, N. and Nakazawa, T. (2011) Significant correlations between optic nerve head microcirculation and visual field defects and nerve fiber layer loss in glaucoma patients with myopic glaucomatous disk. Clin Ophthalmol. 5, 1721-1727. [CrossRef]
- Mourad, J. J., des Guetz, G., Debbabi, H. and Levy, B. I. (2008) Blood pressure rise following angiogenesis inhibition by bevacizumab. A crucial role for microcirculation. Ann Oncol. 19, 927-934. [CrossRef]
- Sane, D. C., Anton, L. and Brosnihan, K. B. (2004) Angiogenic growth factors and hypertension. Angiogenesis. 7, 193-201. [CrossRef]
- Duprez, D., De Buyzere, M., De Backer, T., Vercammen, J., Brusselmans, F. and Clement, D. L. (1992) Impaired microcirculation in mild-to-moderate essential arterial hypertension. J Hypertens. 10, 251-254. [CrossRef]
- Feihl, F., Liaudet, L., Waeber, B. and Levy, B. I. (2006) Hypertension: a disease of the microcirculation? Hypertension. 48, 1012-1017. [CrossRef]
- Agabiti-Rosei, C., Saxton, S. N., De Ciuceis, C., Lorenza Muiesan, M., Rizzoni, D., Agabiti Rosei, E. and Heagerty, A. M. (2024) Influence of Perivascular Adipose Tissue on Microcirculation: A Link Between Hypertension and Obesity. Hypertension. 81, 24-33. [CrossRef]
- De Ciuceis, C., Rizzoni, D. and Palatini, P. (2023) Microcirculation and Physical Exercise In Hypertension. Hypertension. 80, 730-739. [CrossRef]
- Feihl, F., Liaudet, L. and Waeber, B. (2009) The macrocirculation and microcirculation of hypertension. Curr Hypertens Rep. 11, 182-189. [CrossRef]
- Flores, J., Pena, C. and Nugent, K. (2025) Skin microcirculation and hypertension: is there a connection? J Bras Nefrol. 47, e202440192. [CrossRef]
- Gracia-Sancho, J., Marrone, G. and Fernandez-Iglesias, A. (2019) Hepatic microcirculation and mechanisms of portal hypertension. Nat Rev Gastroenterol Hepatol. 16, 221-234. [CrossRef]
- Hu, Y., Hu, A. and Song, S. (2024) Photoplethysmography for Assessing Microcirculation in Hypertensive Patients After Taking Antihypertensive Drugs: A Review. J Multidiscip Healthc. 17, 263-274. [CrossRef]
- Laurent, S., Agabiti-Rosei, C., Bruno, R. M. and Rizzoni, D. (2022) Microcirculation and Macrocirculation in Hypertension: A Dangerous Cross-Link? Hypertension. 79, 479-490. [CrossRef]
- Tsioufis, C., Dimitriadis, K., Katsiki, N. and Tousoulis, D. (2015) Microcirculation in Hypertension: An Update on Clinical Significance and Therapy. Curr Vasc Pharmacol. 13, 413-417. [CrossRef]
- Vicaut, E. (1992) Hypertension and the microcirculation: a brief overview of experimental studies. J Hypertens Suppl. 10, S59-68. [CrossRef]
- Vicaut, E. (2003) Hypertension and the microcirculation. Arch Mal Coeur Vaiss. 96, 893-903.
- Cesarone, M. R., Laurora, G. and Belcaro, G. V. (1992) Microcirculation in Systemic Hypertension. Angiology. 43, 899-903. [CrossRef]
- Durante, A., Mazzapicchi, A. and Baiardo Redaelli, M. (2024) Systemic and Cardiac Microvascular Dysfunction in Hypertension. Int J Mol Sci. 25, 13294. [CrossRef]
- Fedorovich, A. A., Loktionova, Y. I., Zharkikh, E. V., Gorshkov, A. Y., Korolev, A. I., Dadaeva, V. A., Drapkina, O. M. and Zherebtsov, E. A. (2022) Skin microcirculation in middle-aged men with newly diagnosed arterial hypertension according to remote laser Doppler flowmetry data. Microvasc Res. 144, 104419. [CrossRef]
- Jan, M. Y., Hsiu, H., Hsu, T. L., Wang, Y. Y. and Wang, W. K. (2000) The importance of pulsatile microcirculation in relation to hypertension. IEEE Eng Med Biol Mag. 19, 106-111. [CrossRef]
- Junqueira, C. L. C., Magalhaes, M. E. C., Brandao, A. A., Ferreira, E., Cyrino, F., Maranhao, P. A., Souza, M., Bottino, D. A. and Bouskela, E. (2018) Microcirculation and biomarkers in patients with resistant or mild-to-moderate hypertension: a cross-sectional study. Hypertens Res. 41, 515-523. [CrossRef]
- Korolev, A. I., Fedorovich, A. A., Gorshkov, A. Y., Dadaeva, V. A., Omelyanenko, K. V., Chashchin, M. G. and Drapkina, O. M. (2023) Structural and functional state of various parts of skin microcirculation at an early stage of hypertension in working-age men. Microvasc Res. 145, 104440. [CrossRef]
- Lewandowska, K., Marzyńska, D., Rzesoś, P., Partyka, A., Dydowicz, F., Lewandowski, M., Pawlak-Chomicka, R., Tykarski, A. and Uruski, P. (2023) Methods for the assessment of microcirculation in patients with hypertension. Arterial Hypertens. 27, 1-12. [CrossRef]
- Rizzoni, D., Agabiti-Rosei, C., Boari, G. E. M., Muiesan, M. L. and De Ciuceis, C. (2023) Microcirculation in Hypertension: A Therapeutic Target to Prevent Cardiovascular Disease? J Clin Med. 12, 4892. [CrossRef]
- Zweifach, B. W. (1983) The microcirculation in experimental hypertension. State-of-the-art review. Hypertension. 5, I10-16. [CrossRef]
- Bernardino, V. R., Rodrigues, A. C. and Panarra, A. (2019) Raynaud's phenomenon and inflammatory bowel disease: The possible role of microcirculation. Eur J Intern Med. 62, e16. [CrossRef]
- Caliskan, Z., Keles, N., Gokturk, H. S., Ozdil, K., Aksu, F., Ozturk, O., Kahraman, R., Kostek, O., Tekin, A. S., Ozgur, G. T. and Caliskan, M. (2016) Is activation in inflammatory bowel diseases associated with further impairment of coronary microcirculation? Int J Cardiol. 223, 176-181. [CrossRef]
- Danese, S. (2007) Inflammation and the mucosal microcirculation in inflammatory bowel disease: the ebb and flow. Curr Opin Gastroenterol. 23, 384-389. [CrossRef]
- Foitzik, T., Kruschewski, M., Kroesen, A. and Buhr, H. J. (1999) Does microcirculation play a role in the pathogenesis of inflammatory bowel diseases? Answers from intravital microscopic studies in animal models. Int J Colorectal Dis. 14, 29-34. [CrossRef]
- Khalil, P. N., Weiler, V., Nelson, P. J., Khalil, M. N., Moosmann, S., Mutschler, W. E., Siebeck, M. and Huss, R. (2007) Nonmyeloablative stem cell therapy enhances microcirculation and tissue regeneration in murine inflammatory bowel disease. Gastroenterology. 132, 944-954. [CrossRef]
- Laroux, F. S. and Grisham, M. B. (2001) Immunological basis of inflammatory bowel disease: role of the microcirculation. Microcirculation. 8, 283-301. [CrossRef]
- Frisbee, J. C., Goodwill, A. G., Frisbee, S. J., Butcher, J. T., Wu, F. and Chantler, P. D. (2016) Microvascular perfusion heterogeneity contributes to peripheral vascular disease in metabolic syndrome. J Physiol. 594, 2233-2243. [CrossRef]
- Jang, K. W., Hur, J., Lee, D. W. and Kim, S. R. (2024) Metabolic Syndrome, Kidney-Related Adiposity, and Kidney Microcirculation: Unraveling the Damage. Biomedicines. 12, 2706. [CrossRef]
- Krentz, A. J., Clough, G. and Byrne, C. D. (2009) Vascular disease in the metabolic syndrome: do we need to target the microcirculation to treat large vessel disease? J Vasc Res. 46, 515-526. [CrossRef]
- Marini, E., Mariani, P. G., Ministrini, S., Pippi, R., Aiello, C., Reginato, E., Siepi, D., Innocente, S., Lombardini, R., Paltriccia, R., Kararoudi, M. N., Lupattelli, G., De Feo, P. and Pasqualini, L. (2019) Combined aerobic and resistance training improves microcirculation in metabolic syndrome. J Sports Med Phys Fitness. 59, 1571-1576. [CrossRef]
- Nikolova, D. and Kamenov, Z. (2025) New Markers for the Assessment of Microvascular Complications in Patients with Metabolic Syndrome. Metabolites. 15, 184. [CrossRef]
- Serné, E. H., de Jongh, R. T., Eringa, E. C., Ijzerman, R. G., de Boer, M. P. and Stehouwer, C. D. (2006) Microvascular dysfunction: causative role in the association between hypertension, insulin resistance and the metabolic syndrome? Essays Biochem. 42, 163-176. [CrossRef]
- Serné, E. H., de Jongh, R. T., Eringa, E. C., IJzerman, R. G. and Stehouwer, C. D. (2007) Microvascular dysfunction: a potential pathophysiological role in the metabolic syndrome. Hypertension. 50, 204-211. [CrossRef]
- Shiba, T., Takahashi, M., Matsumoto, T. and Hori, Y. (2017) Relationship between Metabolic Syndrome and Ocular Microcirculation Shown by Laser Speckle Flowgraphy in a Hospital Setting Devoted to Sleep Apnea Syndrome Diagnostics. J Diabetes Res. 2017, 3141678. [CrossRef]
- Wiernsperger, N., Nivoit, P., De Aguiar, L. G. and Bouskela, E. (2007) Microcirculation and the metabolic syndrome. Microcirculation. 14, 403-438. [CrossRef]
- Brożyna-Tkaczyk, K., Myśliński, W., Dybala, A. and Paprzycki, P. (2025) Assessment of microcirculation among patients with obstructive sleep apnea after CPAP treatment. Ann Agric Environ Med. 32, 98-103. [CrossRef]
- Christou, E. E., Kostikas, K., Asproudis, C., Zafeiropoulos, P., Stefaniotou, M. and Asproudis, I. (2022) Retinal microcirculation characteristics in obstructive sleep apnea/hypopnea syndrome evaluated by OCT-angiography: a literature review. Int Ophthalmol. 42, 3977-3991. [CrossRef]
- Patt, B. T., Jarjoura, D., Haddad, D. N., Sen, C. K., Roy, S., Flavahan, N. A. and Khayat, R. N. (2010) Endothelial dysfunction in the microcirculation of patients with obstructive sleep apnea. Am J Respir Crit Care Med. 182, 1540-1545. [CrossRef]
- Lin, P. W., Chiu, L. W., Lin, C. W., Chang, C. T. and Lin, H. C. (2025) Alterations on Microcirculation of Optic Nerve Head Before and After OSA Surgery. Nat Sci Sleep. 17, 1249-1258. [CrossRef]
- Christou, E. E., Asproudis, I., Asproudis, C., Giannakis, A., Stefaniotou, M. and Konitsiotis, S. (2022) Macular microcirculation characteristics in Parkinson's disease evaluated by OCT-Angiography: a literature review. Semin Ophthalmol. 37, 399-407. [CrossRef]
- Kell, D. B., Kell, L., Kenny, L. C., Merriel, A., Moore, J. B. and Pretorius, E. (2025) The roles of placental senescence, autophagy and senotherapeutics in the development and prevention of pre-eclampsia: a focus on ergothioneine. Preprints, https://www.preprints.org/manuscript/202504.201261/v202501. [CrossRef]
- Kell, D. B. and Kenny, L. C. (2016) A dormant microbial component in the development of pre-eclampsia. Front Med Obs Gynecol. 3, 60. [CrossRef]
- Kenny, L. C. and Kell, D. B. (2018) Immunological tolerance, pregnancy and pre-eclampsia: the roles of semen microbes and the father. Front Med Obs Gynecol. 4, 239. [CrossRef]
- Granger, J. P., Alexander, B. T., Llinas, M. T., Bennett, W. A. and Khalil, R. A. (2001) Pathophysiology of hypertension during preeclampsia linking placental ischemia with endothelial dysfunction. Hypertension. 38, 718-722.
- Lamarca, B. (2012) Endothelial dysfunction. An important mediator in the pathophysiology of hypertension during pre-eclampsia. Minerva Ginecol. 64, 309-320.
- McElwain, C. J., Tuboly, E., McCarthy, F. P. and McCarthy, C. M. (2020) Mechanisms of Endothelial Dysfunction in Pre-eclampsia and Gestational Diabetes Mellitus: Windows Into Future Cardiometabolic Health? Front Endocrinol (Lausanne). 11, 655. [CrossRef]
- Possomato-Vieira, J. S. and Khalil, R. A. (2016) Mechanisms of Endothelial Dysfunction in Hypertensive Pregnancy and Preeclampsia. Adv Pharmacol. 77, 361-431. [CrossRef]
- Csiki, Z., Garai, I., Varga, J., Szucs, G., Galajda, Z., Andras, C., Zeher, M. and Galuska, L. (2005) Microcirculation of the fingers in Raynaud's syndrome: (99m)Tc-DTPA imaging. Nuklearmedizin. 44, 29-32. [CrossRef]
- Gregorczyk-Maga, I., Frołow, M., Kaczmarczyk, P. and Maga, P. (2019) Microcirculation disorders of the oral cavity in patients with primary Raynaud phenomenon. Pol Arch Intern Med. 129, 36-42. [CrossRef]
- Latuskiewicz-Potemska, J., Chmura-Skirlinska, A., Gurbiel, R. J. and Smolewska, E. (2016) Nailfold capillaroscopy assessment of microcirculation abnormalities and endothelial dysfunction in children with primary or secondary Raynaud syndrome. Clin Rheumatol. 35, 1993-2001. [CrossRef]
- Mosdósi, B., Bölcskei, K. and Helyes, Z. (2018) Impairment of microcirculation and vascular responsiveness in adolescents with primary Raynaud phenomenon. Pediatr Rheumatol Online J. 16, 20. [CrossRef]
- Radić, M., Snow, M., Frech, T. M., Saketkoo, L. A., Cutolo, M. and Smith, V. (2020) Consensus-based evaluation of dermatoscopy versus nailfold videocapillaroscopy in Raynaud's phenomenon linking USA and Europe: a European League against Rheumatism study group on microcirculation in rheumatic diseases project. Clin Exp Rheumatol. 38 Suppl 125, 132-136.
- Ingegnoli, F., Ughi, N., Dinsdale, G., Orenti, A., Boracchi, P., Allanore, Y., Foeldvari, I., Sulli, A., Cutolo, M., Smith, V., Herrick, A. L. and EULAR Study Group on Microcirculation in Rheumatic Diseases. (2017) An international SUrvey on non-iNvaSive tecHniques to assess the mIcrocirculation in patients with RayNaud's phEnomenon (SUNSHINE survey). Rheumatol Int. 37, 1879-1890. [CrossRef]
- Szabo, N., Csiki, Z., Szanto, A., Danko, K., Szodoray, P. and Zeher, M. (2008) Functional and morphological evaluation of hand microcirculation with nailfold capillaroscopy and laser Doppler imaging in Raynaud's and Sjogren's syndrome and poly/dermatomyositis. Scand J Rheumatol. 37, 23-29. [CrossRef]
- Bourcier, S., Joffre, J., Dubee, V., Preda, G., Baudel, J. L., Bige, N., Leblanc, G., Levy, B. I., Guidet, B., Maury, E. and Ait-Oufella, H. (2017) Marked regional endothelial dysfunction in mottled skin area in patients with severe infections. Crit Care. 21, 155. [CrossRef]
- Lundy, D. J. and Trzeciak, S. (2009) Microcirculatory dysfunction in sepsis. Crit Care Clin. 25, 721-731, viii. [CrossRef]
- Lundy, D. J. and Trzeciak, S. (2011) Microcirculatory dysfunction in sepsis. Crit Care Nurs Clin North Am. 23, 67-77. [CrossRef]
- Joffre, J., Hellman, J., Ince, C. and Ait-Oufella, H. (2020) Endothelial Responses in Sepsis. Am J Respir Crit Care Med. 202, 361-370. [CrossRef]
- Alexandre, A. R., Leitão, A. T. and Póvoa, P. (2025) Optical coherence tomography angiography as a novel tool to assess microcirculatory dysfunction in septic shock. Intensive Care Med. 51, 632-634. [CrossRef]
- Courtie, E., Gilani, A., Veenith, T. and Blanch, R. J. (2022) Optical coherence tomography angiography as a surrogate marker for end-organ resuscitation in sepsis: A review. Front Med (Lausanne). 9, 1023062. [CrossRef]
- Bakker, J. and Ince, C. (2020) Monitoring coherence between the macro and microcirculation in septic shock. Curr Opin Crit Care. 26, 267-272. [CrossRef]
- De Backer, D., Creteur, J., Preiser, J. C., Dubois, M. J. and Vincent, J. L. (2002) Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med. 166, 98-104. [CrossRef]
- De Backer, D., Orbegozo Cortes, D., Donadello, K. and Vincent, J. L. (2014) Pathophysiology of microcirculatory dysfunction and the pathogenesis of septic shock. Virulence. 5, 73-79. [CrossRef]
- De Backer, D., Ricottilli, F. and Ospina-Tascón, G. A. (2021) Septic shock: a microcirculation disease. Curr Opin Anaesthesiol. 34, 85-91. [CrossRef]
- González, R., Urbano, J. and López-Herce, J. (2024) Resuscitating the macro- vs. microcirculation in septic shock. Curr Opin Pediatr. 36, 274-281. [CrossRef]
- Gruartmoner, G., Mesquida, J. and Ince, C. (2017) Microcirculatory monitoring in septic patients: Where do we stand? Med Intensiva. 41, 44-52. [CrossRef]
- Hinshaw, L. B. (1996) Sepsis/septic shock: participation of the microcirculation: an abbreviated review. Crit Care Med. 24, 1072-1078. [CrossRef]
- Lipińska-Gediga, M. (2016) Sepsis and septic shock-is a microcirculation a main player? Anaesthesiol Intensive Ther. 48, 261-265. [CrossRef]
- Massey, M. J., Hou, P. C., Filbin, M., Wang, H., Ngo, L., Huang, D. T., Aird, W. C., Novack, V., Trzeciak, S., Yealy, D. M., Kellum, J. A., Angus, D. C., Shapiro, N. I. and Pro, C. i. (2018) Microcirculatory perfusion disturbances in septic shock: results from the ProCESS trial. Crit Care. 22, 308. [CrossRef]
- Obonyo, N. G., Fanning, J. P., Ng, A. S., Pimenta, L. P., Shekar, K., Platts, D. G., Maitland, K. and Fraser, J. F. (2016) Effects of volume resuscitation on the microcirculation in animal models of lipopolysaccharide sepsis: a systematic review. Intensive Care Med Exp. 4, 38. [CrossRef]
- Potter, E. K., Hodgson, L., Creagh-Brown, B. and Forni, L. G. (2019) Manipulating the Microcirculation in Sepsis - the Impact of Vasoactive Medications on Microcirculatory Blood Flow: A Systematic Review. Shock. 52, 5-12. [CrossRef]
- Saugel, B., Trepte, C. J., Heckel, K., Wagner, J. Y. and Reuter, D. A. (2015) Hemodynamic management of septic shock: is it time for "individualized goal-directed hemodynamic therapy" and for specifically targeting the microcirculation? Shock. 43, 522-529. [CrossRef]
- Shapiro, N. I. and Angus, D. C. (2014) A review of therapeutic attempts to recruit the microcirculation in patients with sepsis. Minerva Anestesiol. 80, 225-235.
- Siegemund, M., Hollinger, A., Gebhard, E. C., Scheuzger, J. D. and Bolliger, D. (2019) The value of volume substitution in patients with septic and haemorrhagic shock with respect to the microcirculation. Swiss Med Wkly. 149, w20007. [CrossRef]
- Spronk, P. E., Zandstra, D. F. and Ince, C. (2004) Bench-to-bedside review: sepsis is a disease of the microcirculation. Crit Care. 8, 462-468. [CrossRef]
- Tang, A., Shi, Y., Dong, Q., Wang, S., Ge, Y., Wang, C., Gong, Z., Zhang, W. and Chen, W. (2024) Prognostic Value of Sublingual Microcirculation in Sepsis: A Systematic Review and Meta-analysis. J Intensive Care Med. 39, 1221-1230. [CrossRef]
- Wang, H., Ding, H., Wang, Z. Y. and Zhang, K. (2024) Research progress on microcirculatory disorders in septic shock: A narrative review. Medicine (Baltimore). 103, e37273. [CrossRef]
- Elbers, P. W. G. and Ince, C. (2007) The Microcirculation Is a Vulnerable Organ in Sepsis. In Mechanisms of Sepsis-Induced Organ Dysfunction and Recovery. Update in Intensive Care and Emergency Medicine (Abraham, E. and Singer, M., eds.). pp. 249-262, Springer Berlin Heidelberg, Berlin, Heidelberg.
- Gomez, H., Ince, C., De Backer, D., Pickkers, P., Payen, D., Hotchkiss, J. and Kellum, J. A. (2014) A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 41, 3-11. [CrossRef]
- Ince, C. and Mik, E. G. (2016) Microcirculatory and mitochondrial hypoxia in sepsis, shock, and resuscitation. J Appl Physiol (1985). 120, 226-235. [CrossRef]
- Legrand, M., Klijn, E., Payen, D. and Ince, C. (2010) The response of the host microcirculation to bacterial sepsis: does the pathogen matter? J Mol Med (Berl). 88, 127-133. [CrossRef]
- Lima, A., van Rooij, T., Ergin, B., Sorelli, M., Ince, Y., Specht, P. A. C., Mik, E. G., Bocchi, L., Kooiman, K., de Jong, N. and Ince, C. (2018) Dynamic Contrast-Enhanced Ultrasound Identifies Microcirculatory Alterations in Sepsis-Induced Acute Kidney Injury. Crit Care Med. 46, 1284-1292. [CrossRef]
- Sakr, Y., Dubois, M. J., De Backer, D., Creteur, J. and Vincent, J. L. (2004) Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med. 32, 1825-1831. [CrossRef]
- Top, A. P. C., Ince, C., de Meij, N., van Dijk, M. and Tibboel, D. (2011) Persistent low microcirculatory vessel density in nonsurvivors of sepsis in pediatric intensive care. Crit Care Med. 39, 8-13. [CrossRef]
- Birkhoff, W., de Vries, J., Dent, G., Verma, A., Kerkhoffs, J. L., van Meurs, A. H. F., de Kam, M., Moerland, M. and Burggraaf, J. (2018) Retinal microcirculation imaging in sickle cell disease patients. Microvasc Res. 116, 1-5. [CrossRef]
- Catella, J., Turpin, E., Connes, P., Nader, E., Carin, R., Martin, M., Rezigue, H., Nougier, C., Dargaud, Y., Josset-Lamaugarny, A., Dugrain, J., Marano, M., Leuci, A., Boisson, C., Renoux, C., Joly, P., Poutrel, S., Hot, A., Guillot, N. and Fromy, B. (2024) Impaired microvascular function in patients with sickle cell anemia and leg ulcers improved with healing. Br J Haematol. 205, 2459-2469. [CrossRef]
- Clarke, K., Mannath, A., Anastasi, M., Nasr, M., Pan, S., Balaskas, K., Dinah, C., Sarunic, M. V. and Asaria, R. (2025) Optical coherence tomography angiography as a tool for diagnosis and monitoring of sickle cell related eye disease: a systematic review and meta-analysis. Eye (Lond), online. [CrossRef]
- Grego, L., Pignatto, S., Alfier, F., Arigliani, M., Rizzetto, F., Rassu, N., Samassa, F., Prosperi, R., Barbieri, F., Dall'Amico, R., Cogo, P. and Lanzetta, P. (2020) Optical coherence tomography (OCT) and OCT angiography allow early identification of sickle cell maculopathy in children and correlate it with systemic risk factors. Graefes Arch Clin Exp Ophthalmol. 258, 2551-2561. [CrossRef]
- Mgboji, G. E., Cain, D. and Scott, A. W. (2022) Conjunctival optical coherence tomography angiography imaging in sickle cell maculopathy. Am J Ophthalmol Case Rep. 26, 101428. [CrossRef]
- Möckesch, B., Charlot, K., Jumet, S., Romana, M., Divialle-Doumdo, L., Hardy-Dessources, M. D., Petras, M., Tressieres, B., Tarer, V., Hue, O., Etienne-Julan, M., Connes, P. and Antoine-Jonville, S. (2017) Micro- and macrovascular function in children with sickle cell anaemia and sickle cell haemoglobin C disease. Blood Cells Mol Dis. 64, 23-29. [CrossRef]
- Raffa, E. H., Raffa, L., Almadani, S., Murad, W. and Alshanti, H. (2024) Optical Coherence Tomography Angiography of Macular Microangiopathy in Children With Sickle Cell Disease. J Pediatr Hematol Oncol. 46, 349-355. [CrossRef]
- Sapozhnikov, M., Rehman, M., Johnson, C., Daich, J., Salciccioli, L., Gillette, P. and Lazar, J. M. (2019) Characterization of microvascular disease in patients with sickle cell disease using nailfold capillaroscopy. Microvasc Res. 125, 103877. [CrossRef]
- Arsava, E. M., Arat, A., Topcuoglu, M. A., Peker, A., Yemisci, M. and Dalkara, T. (2018) Angiographic Microcirculatory Obstructions Distal to Occlusion Signify Poor Outcome after Endovascular Treatment for Acute Ischemic Stroke. Transl Stroke Res. 9, 44-50. [CrossRef]
- Charidimou, A., Kakar, P., Fox, Z. and Werring, D. J. (2013) Cerebral microbleeds and recurrent stroke risk: systematic review and meta-analysis of prospective ischemic stroke and transient ischemic attack cohorts. Stroke. 44, 995-1001. [CrossRef]
- Dalkara, T. and Arsava, E. M. (2012) Can restoring incomplete microcirculatory reperfusion improve stroke outcome after thrombolysis? J Cereb Blood Flow Metab. 32, 2091-2099. [CrossRef]
- Deng, G., Chu, Y. H., Xiao, J., Shang, K., Zhou, L. Q., Qin, C. and Tian, D. S. (2023) Risk Factors, Pathophysiologic Mechanisms, and Potential Treatment Strategies of Futile Recanalization after Endovascular Therapy in Acute Ischemic Stroke. Aging Dis. 14, 2096-2112. [CrossRef]
- Ginsberg, M. D. (2011) Visualizing the cortical microcirculation in patients with stroke. Crit Care Med. 39, 1228-1230. [CrossRef]
- Hu, J., Nan, D., Lu, Y., Niu, Z., Ren, Y., Qu, X., Huang, Y. and Jin, H. (2023) Microcirculation No-Reflow Phenomenon after Acute Ischemic Stroke. Eur Neurol. 86, 85-94. [CrossRef]
- Ishikawa, M., Cooper, D., Russell, J., Salter, J. W., Zhang, J. H., Nanda, A. and Granger, D. N. (2003) Molecular determinants of the prothrombogenic and inflammatory phenotype assumed by the postischemic cerebral microcirculation. Stroke. 34, 1777-1782. [CrossRef]
- Jin, H., Chen, Y., Ding, C., Lin, Y., Chen, Y., Jiang, D. and Su, L. (2018) Microcirculatory Disorders and Protective Role of Xuebijing in Severe Heat Stroke. Sci Rep. 8, 4553. [CrossRef]
- Liu, J., Ding, N., Yu, Y., Liu, L., Yuan, X., Lv, H., Zhao, Y. and Ma, Z. (2019) Whole-brain microcirculation detection after ischemic stroke based on swept-source optical coherence tomography. J Biophotonics. 12, e201900122. [CrossRef]
- Swanepoel, A. C. and Pretorius, E. (2012) Scanning electron microscopy analysis of erythrocytes in thromboembolic ischemic stroke. Int J Lab Hematol. 34, 185-191. [CrossRef]
- Xu, Q., Liu, J., Guo, X., Tang, Y., Zhou, G., Liu, Y., Huang, Q., Geng, Y., Liu, Z. and Su, L. (2015) Xuebijing injection reduces organ injuries and improves survival by attenuating inflammatory responses and endothelial injury in heatstroke mice. BMC Complement Altern Med. 15, 4. [CrossRef]
- Xu, K., Deng, B., Jia, T., Ren, M., Chen, H., Zhang, J., Guo, J., Li, Y. and Wang, J. (2024) A review of the Bovis Calculus's intervention mechanism and clinical application in ischemic stroke. Front Pharmacol. 15, 1510779. [CrossRef]
- Zhang, X., Pei, J., Xue, L., Zhao, Z., Xu, R., Zhang, C., Zhang, C., Fu, L., Zhang, X. and Cui, L. (2024) An-Gong-Niu-Huang-Wan (AGNHW) regulates cerebral blood flow by improving hypoperfusion, cerebrovascular reactivity and microcirculation disturbances after stroke. Chin Med. 19, 73. [CrossRef]
- McMahon, C. J., Hopkins, S., Vail, A., King, A. T., Smith, D., Illingworth, K. J., Clark, S., Rothwell, N. J. and Tyrrell, P. J. (2013) Inflammation as a predictor for delayed cerebral ischemia after aneurysmal subarachnoid haemorrhage. J Neurointerv Surg. 5, 512-517. [CrossRef]
- Clarke, J. V., Suggs, J. M., Diwan, D., Lee, J. V., Lipsey, K., Vellimana, A. K. and Zipfel, G. J. (2020) Microvascular platelet aggregation and thrombosis after subarachnoid hemorrhage: A review and synthesis. J Cereb Blood Flow Metab. 40, 1565-1575. [CrossRef]
- Dóczi, T. P. (2001) Impact of cerebral microcirculatory changes on cerebral blood flow during cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 32, 817. [CrossRef]
- Li, S., Wu, L., Li, N. and Zhao, X. (2025) Early Microcirculatory Dysfunction on Perfusion CT Is Related to Prognosis After Aneurysmal Subarachnoid Hemorrhage. Transl Stroke Res. [CrossRef]
- Naraoka, M., Shimamura, N. and Ohkuma, H. (2024) Cilostazol Alleviates Delayed Cerebral Ischemia After Subarachnoid Hemorrhage by Attenuating Microcirculatory Dysfunction. Transl Stroke Res. [CrossRef]
- Ohkuma, H., Manabe, H., Tanaka, M. and Suzuki, S. (2000) Impact of cerebral microcirculatory changes on cerebral blood flow during cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 31, 1621-1627. [CrossRef]
- Østergaard, L., Aamand, R., Karabegovic, S., Tietze, A., Blicher, J. U., Mikkelsen, I. K., Iversen, N. K., Secher, N., Engedal, T. S., Anzabi, M., Jimenez, E. G., Cai, C., Koch, K. U., Naess-Schmidt, E. T., Obel, A., Juul, N., Rasmussen, M. and Sørensen, J. C. (2013) The role of the microcirculation in delayed cerebral ischemia and chronic degenerative changes after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 33, 1825-1837. [CrossRef]
- Sabri, M., Ai, J., Lakovic, K. and Macdonald, R. L. (2013) Mechanisms of microthrombosis and microcirculatory constriction after experimental subarachnoid hemorrhage. Acta Neurochir Suppl. 115, 185-192. [CrossRef]
- Song, J. N., Chen, H., Zhang, M., Zhao, Y. L. and Ma, X. D. (2013) Dynamic change in cerebral microcirculation and focal cerebral metabolism in experimental subarachnoid hemorrhage in rabbits. Metab Brain Dis. 28, 33-43. [CrossRef]
- Terpolilli, N. A., Brem, C., Buhler, D. and Plesnila, N. (2015) Are We Barking Up the Wrong Vessels? Cerebral Microcirculation After Subarachnoid Hemorrhage. Stroke. 46, 3014-3019. [CrossRef]
- Tso, M. K. and Macdonald, R. L. (2014) Subarachnoid hemorrhage: a review of experimental studies on the microcirculation and the neurovascular unit. Transl Stroke Res. 5, 174-189. [CrossRef]
- Zhou, J., Guo, P., Guo, Z., Sun, X., Chen, Y. and Feng, H. (2022) Fluid metabolic pathways after subarachnoid hemorrhage. J Neurochem. 160, 13-33. [CrossRef]
- Blaise, S., Boulon, C., Mangin, M., Senet, P., Lazareth, I., Imbert, B., Lapebie, F. X., Lacroix, P., Seinturier, C., Constans, J. and Carpentier, P. H. (2025) Digital ulcers in systemic sclerosis are strongly associated with digital arterial disease: a finger-by-finger analysis of finger brachial pressure index measurements. Rheumatology (Oxford). 64, 1975-1980. [CrossRef]
- Correa, M. J., Andrade, L. E. and Kayser, C. (2010) Comparison of laser Doppler imaging, fingertip lacticemy test, and nailfold capillaroscopy for assessment of digital microcirculation in systemic sclerosis. Arthritis Res Ther. 12, R157. [CrossRef]
- Cutolo, M. and Smith, V. (2021) Detection of microvascular changes in systemic sclerosis and other rheumatic diseases. Nat Rev Rheumatol. 17, 665-677. [CrossRef]
- Della Rossa, A., Cazzato, M., d'Ascanio, A., Tavoni, A., Bencivelli, W., Pepe, P., Mosca, M., Baldini, C., Rossi, M. and Bombardieri, S. (2013) Alteration of microcirculation is a hallmark of very early systemic sclerosis patients: a laser speckle contrast analysis. Clin Exp Rheumatol. 31, 109-114.
- Mandujano, A. and Golubov, M. (2022) Animal Models of Systemic Sclerosis: Using Nailfold Capillaroscopy as a Potential Tool to Evaluate Microcirculation and Microangiopathy: A Narrative Review. Life (Basel). 12, 703. [CrossRef]
- Mostmans, Y., Cutolo, M., Giddelo, C., Decuman, S., Melsens, K., Declercq, H., Vandecasteele, E., De Keyser, F., Distler, O., Gutermuth, J. and Smith, V. (2017) The role of endothelial cells in the vasculopathy of systemic sclerosis: A systematic review. Autoimmun Rev. 16, 774-786. [CrossRef]
- Paolino, S., Gotelli, E., Goegan, F., Casabella, A., Ferrari, G., Patane, M., Albertelli, M., Gatto, F., Pizzorni, C., Cattelan, F., Sulli, A., Smith, V. and Cutolo, M. (2021) Body composition and bone status in relation to microvascular damage in systemic sclerosis patients. J Endocrinol Invest. 44, 255-264. [CrossRef]
- Ruaro, B., Sulli, A., Pizzorni, C., Paolino, S., Smith, V. and Cutolo, M. (2016) Correlations between skin blood perfusion values and nailfold capillaroscopy scores in systemic sclerosis patients. Microvasc Res. 105, 119-124. [CrossRef]
- Vanhaecke, A., Debusschere, C., Cutolo, M., Smith, V. and EULAR Study Group on Microcirculation in Rheumatic Diseases. (2022) Predictive value of laser speckle contrast analysis in systemic sclerosis. A systematic review and pilot study. Eur J Clin Invest. 52, e13672. [CrossRef]
- Yu, S., Hu, S. C., Yu, H. S., Chin, Y. Y., Cheng, Y. C. and Lee, C. H. (2019) Early sign of microangiopathy in systemic sclerosis: The significance of cold stress test in dynamic laser Doppler flowmetry. Clin Hemorheol Microcirc. 71, 373-378. [CrossRef]
- Bragin, D. E., Bragina, O. A., Trofimov, A. O., Huang, P. L. and Atochin, D. N. (2022) Involvement of Endothelial Nitric Oxide Synthase in Cerebral Microcirculation and Oxygenation in Traumatic Brain Injury. Adv Exp Med Biol. 1395, 3-7. [CrossRef]
- Rafols, J. A., Kreipke, C. W. and Petrov, T. (2007) Alterations in cerebral cortex microvessels and the microcirculation in a rat model of traumatic brain injury: a correlative EM and laser Doppler flowmetry study. Neurol Res. 29, 339-347. [CrossRef]
- Trofimov, A., Dubrovin, A., Martynov, D., Agarkova, D., Trofimova, K., Zorkova, A. and Bragin, D. E. (2021) Microcirculatory Biomarkers of Secondary Cerebral Ischemia in Traumatic Brain Injury. Acta Neurochir Suppl. 131, 3-5. [CrossRef]
- Villalba, N., Sackheim, A. M., Nunez, I. A., Hill-Eubanks, D. C., Nelson, M. T., Wellman, G. C. and Freeman, K. (2017) Traumatic Brain Injury Causes Endothelial Dysfunction in the Systemic Microcirculation through Arginase-1-Dependent Uncoupling of Endothelial Nitric Oxide Synthase. J Neurotrauma. 34, 192-203. [CrossRef]
- Domizi, R., Damiani, E., Scorcella, C., Carsetti, A., Castagnani, R., Vannicola, S., Bolognini, S., Gabbanelli, V., Pantanetti, S. and Donati, A. (2019) Association between sublingual microcirculation, tissue perfusion and organ failure in major trauma: A subgroup analysis of a prospective observational study. PLoS One. 14, e0213085. [CrossRef]
- Kell, D. B. and Pretorius, E. (2018) No effects without causes. The Iron Dysregulation and Dormant Microbes hypothesis for chronic, inflammatory diseases. Biol Rev. 93, 1518-1557. [CrossRef]
- Abrashev, H., Abrasheva, D., Nikolov, N., Ananiev, J. and Georgieva, E. (2025) A Systematic Review of Endothelial Dysfunction in Chronic Venous Disease-Inflammation, Oxidative Stress, and Shear Stress. Int J Mol Sci. 26, 3660. [CrossRef]
- Angjelova, A., Jovanova, E., Polizzi, A., Lagana, L., Santonocito, S., Ragusa, R. and Isola, G. (2024) Impact of Periodontitis on Endothelial Risk Dysfunction and Oxidative Stress Improvement in Patients with Cardiovascular Disease. J Clin Med. 13. [CrossRef]
- Dou, B., Zhu, Y., Sun, M., Wang, L., Tang, Y., Tian, S. and Wang, F. (2024) Mechanisms of Flavonoids and Their Derivatives in Endothelial Dysfunction Induced by Oxidative Stress in Diabetes. Molecules. 29, 3265. [CrossRef]
- Faro, D. C., Di Pino, F. L. and Monte, I. P. (2024) Inflammation, Oxidative Stress, and Endothelial Dysfunction in the Pathogenesis of Vascular Damage: Unraveling Novel Cardiovascular Risk Factors in Fabry Disease. Int J Mol Sci. 25, 8273. [CrossRef]
- Fodor, A., Tiperciuc, B., Login, C., Orasan, O. H., Lazar, A. L., Buchman, C., Hanghicel, P., Sitar-Taut, A., Suharoschi, R., Vulturar, R. and Cozma, A. (2021) Endothelial Dysfunction, Inflammation, and Oxidative Stress in COVID-19-Mechanisms and Therapeutic Targets. Oxid Med Cell Longev. 2021, 8671713. [CrossRef]
- Higashi, Y. (2022) Roles of Oxidative Stress and Inflammation in Vascular Endothelial Dysfunction-Related Disease. Antioxidants (Basel). 11, 1958. [CrossRef]
- Incalza, M. A., D'Oria, R., Natalicchio, A., Perrini, S., Laviola, L. and Giorgino, F. (2018) Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol. 100, 1-19. [CrossRef]
- Janaszak-Jasiecka, A., Siekierzycka, A., Płoska, A., Dobrucki, I. T. and Kalinowski, L. (2021) Endothelial Dysfunction Driven by Hypoxia-The Influence of Oxygen Deficiency on NO Bioavailability. Biomolecules. 11, 982. [CrossRef]
- Joffre, J. and Hellman, J. (2021) Oxidative Stress and Endothelial Dysfunction in Sepsis and Acute Inflammation. Antioxid Redox Signal. 35, 1291-1307. [CrossRef]
- Matsuoka, H. (2001) Endothelial dysfunction associated with oxidative stress in human. Diabetes Res Clin Pract. 54 Suppl 2, S65-72. [CrossRef]
- Nguyen, T. T. U., Yeom, J. H. and Kim, W. (2021) Beneficial Effects of Vitamin E Supplementation on Endothelial Dysfunction, Inflammation, and Oxidative Stress Biomarkers in Patients Receiving Hemodialysis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int J Mol Sci. 22, 11923. [CrossRef]
- Schulz, E., Gori, T. and Munzel, T. (2011) Oxidative stress and endothelial dysfunction in hypertension. Hypertens Res. 34, 665-673. [CrossRef]
- Sena, C. M., Leandro, A., Azul, L., Seica, R. and Perry, G. (2018) Vascular Oxidative Stress: Impact and Therapeutic Approaches. Front Physiol. 9, 1668. [CrossRef]
- Shaito, A., Aramouni, K., Assaf, R., Parenti, A., Orekhov, A., Yazbi, A. E., Pintus, G. and Eid, A. H. (2022) Oxidative Stress-Induced Endothelial Dysfunction in Cardiovascular Diseases. Front Biosci (Landmark Ed). 27, 105. [CrossRef]
- Shi, X., Li, P., Liu, H. and Prokosch, V. (2022) Oxidative Stress, Vascular Endothelium, and the Pathology of Neurodegeneration in Retina. Antioxidants (Basel). 11, 543. [CrossRef]
- Silva, B. R., Pernomian, L. and Bendhack, L. M. (2012) Contribution of oxidative stress to endothelial dysfunction in hypertension. Front Physiol. 3, 441. [CrossRef]
- Prajapat, S. K., Maharana, K. C. and Singh, S. (2024) Mitochondrial dysfunction in the pathogenesis of endothelial dysfunction. Mol Cell Biochem. 479, 1999-2016. [CrossRef]
- Pober, J. S. and Sessa, W. C. (2007) Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 7, 803-815. [CrossRef]
- Bloom, S. I., Islam, M. T., Lesniewski, L. A. and Donato, A. J. (2023) Mechanisms and consequences of endothelial cell senescence. Nat Rev Cardiol. 20, 38-51. [CrossRef]
- González, I. and Maldonado-Agurto, R. (2025) The role of cellular senescence in endothelial dysfunction and vascular remodelling in arteriovenous fistula maturation. J Physiol. [CrossRef]
- Graves, S. I. and Baker, D. J. (2020) Implicating endothelial cell senescence to dysfunction in the ageing and diseased brain. Basic Clin Pharmacol Toxicol. 127, 102-110. [CrossRef]
- Honda, S., Ikeda, K., Urata, R., Yamazaki, E., Emoto, N. and Matoba, S. (2021) Cellular senescence promotes endothelial activation through epigenetic alteration, and consequently accelerates atherosclerosis. Sci Rep. 11, 14608. [CrossRef]
- Jia, G., Aroor, A. R., Jia, C. and Sowers, J. R. (2019) Endothelial cell senescence in aging-related vascular dysfunction. Biochim Biophys Acta Mol Basis Dis. 1865, 1802-1809. [CrossRef]
- Kasal, D. A., Sena, V., Huguenin, G. V. B., De Lorenzo, A. and Tibirica, E. (2025) Microvascular endothelial dysfunction in vascular senescence and disease. Front Cardiovasc Med. 12, 1505516. [CrossRef]
- Kim, S. Y. and Cheon, J. (2024) Senescence-associated microvascular endothelial dysfunction: A focus on the blood-brain and blood-retinal barriers. Ageing Res Rev. 100, 102446. [CrossRef]
- Nunes, M., Kell, L., Slaghekke, A., Wüst, R., Fielding, B., Kell, D. B. and Pretorius, E. (2025) Virus-induced endothelial senescence as a cause and driving factor for ME/CFS and Long COVID: mediated by a dysfunctional immune system. Preprints, 202505.201875.v202501. [CrossRef]
- Csik, B., Nyúl-Tóth, Á., Gulej, R., Patai, R., Kiss, T., Delfavero, J., Nagaraja, R. Y., Balasubramanian, P., Shanmugarama, S., Ungvari, A., Chandragiri, S. S., Kordestan, K. V., Nagykaldi, M., Mukli, P., Yabluchanskiy, A., Negri, S., Tarantini, S., Conley, S., Oh, T. G., Ungvari, Z. and Csiszar, A. (2025) Senescent Endothelial Cells in Cerebral Microcirculation Are Key Drivers of Age-Related Blood-Brain Barrier Disruption, Microvascular Rarefaction, and Neurovascular Coupling Impairment in Mice. Aging Cell, e70048. [CrossRef]
- Duangchan, T., Kotepui, M., Sukati, S., Rattanapan, Y. and Wangdi, K. (2023) A Systematic Review and Meta-Analysis of the Proportion Estimates of Disseminated Intravascular Coagulation (DIC) in Malaria. Trop Med Infect Dis. 8, 289. [CrossRef]
- Gando, S., Levi, M. and Toh, C. H. (2016) Disseminated intravascular coagulation. Nat Rev Dis Primers. 2, 16037. [CrossRef]
- Gando, S., Levi, M. and Toh, C. H. (2024) Trauma-induced innate immune activation and disseminated intravascular coagulation. J Thromb Haemost. 22, 337-351. [CrossRef]
- Gong, F., Zheng, X., Zhao, S., Liu, H., Chen, E., Xie, R., Li, R. and Chen, Y. (2025) Disseminated intravascular coagulation: cause, molecular mechanism, diagnosis, and therapy. MedComm. 6, e70058. [CrossRef]
- Iba, T., Levi, M., Thachil, J. and Levy, J. H. (2022) Disseminated Intravascular Coagulation: The Past, Present, and Future Considerations. Semin Thromb Hemost. 48, 978-987. [CrossRef]
- Kiya, G. T., Abebe, G., Mekonnen, Z., Tadasa, E., Milkias, G. and Asefa, E. T. (2025) A comparison of disseminated intravascular coagulation scoring systems and their performance to predict mortality in sepsis patients: A systematic review and meta-analysis. PLoS One. 20, e0315797. [CrossRef]
- Levi, M. and van der Poll, T. (2014) A short contemporary history of disseminated intravascular coagulation. Semin Thromb Hemost. 40, 874-880. [CrossRef]
- Levi, M. and Thachil, J. (2020) Coronavirus Disease 2019 Coagulopathy: Disseminated Intravascular Coagulation and Thrombotic Microangiopathy-Either, Neither, or Both. Semin Thromb Hemost. [CrossRef]
- Li, W., Sheng, S. and Zhu, F. (2025) Efficacy and safety of antithrombin or recombinant human thrombomodulin in the treatment of disseminated intravascular coagulation: A systematic review and meta-analysis. Thromb Res. 249, 109302. [CrossRef]
- Okamoto, K., Tamura, T. and Sawatsubashi, Y. (2016) Sepsis and disseminated intravascular coagulation. J Intensive Care. 4, 23. [CrossRef]
- Toh, C. H. and Dennis, M. (2003) Disseminated intravascular coagulation: old disease, new hope. BMJ. 327, 974-977. [CrossRef]
- Umemura, Y., Scarlatescu, E., Nwagha, T. U., Levy, J. H., Othman, M., Moore, H., O'Reilly, D., Helms, J. and Iba, T. (2025) Mortality, diagnosis, and etiology of disseminated intravascular coagulation-a systematic review and meta-analysis: communication from the ISTH SSC subcommittee on disseminated intravascular coagulation. J Thromb Haemost. [CrossRef]
- Unar, A., Bertolino, L., Patauner, F., Gallo, R. and Durante-Mangoni, E. (2023) Decoding Sepsis-Induced Disseminated Intravascular Coagulation: A Comprehensive Review of Existing and Emerging Therapies. J Clin Med. 12, 6128. [CrossRef]
- Kell, D. B. and Pretorius, E. (2018) To what extent are the terminal stages of sepsis, septic shock, SIRS, and multiple organ dysfunction syndrome actually driven by a toxic prion/amyloid form of fibrin? Semin Thromb Hemost. 44, 224-238. [CrossRef]
- Niu, C. Y., Zhao, Z. G., Zhang, Y. P., Hou, Y. L., Li, J. J., Jiang, H. and Zhang, J. (2013) Exogenous normal lymph alleviates microcirculation disturbances and abnormal hemorheological properties in rats with disseminated intravascular coagulation. Braz J Med Biol Res. 46, 138-147. [CrossRef]
- Popescu, N. I., Lupu, C. and Lupu, F. (2022) Disseminated intravascular coagulation and its immune mechanisms. Blood. 139, 1973-1986. [CrossRef]
- ten Cate, H., Schoenmakers, S. H. H. F., Franco, R., Timmerman, J. J., Groot, A. P., Spek, C. A. and Reitsma, P. H. (2001) Microvascular coagulopathy and disseminated intravascular coagulation. Crit Care Med. 29, S95-97; discussion S97-98. [CrossRef]
- Vignolo-Scalone, W. H., Vignolo-Puglia, W. H. and Kitchens, C. S. (1984) Microvascular alterations in thrombin-induced experimental disseminated intravascular coagulation in the dog. Angiology. 35, 261-268. [CrossRef]
- Trevino-Peinado, C., Zubieta, J. L. and Fernandez, M. M. (2015) Subcortical Microbleeds in Disseminated Intravascular Coagulation Mimicking Amyloid Angiopathy. J Neuroimaging. 25, 660-661. [CrossRef]
- Schofield, J., Abrams, S. T., Jenkins, R., Lane, S., Wang, G. and Toh, C. H. (2024) Microclots, as defined by amyloid-fibrinogen aggregates, predict risks of disseminated intravascular coagulation and mortality. Blood Adv. 8, 2499-2508. [CrossRef]
- Adingupu, D. D., Thorn, C. E., Casanova, F., Elyas, S., Gooding, K., Gilchrist, M., Aizawa, K., Gates, P. E., Shore, A. C. and Strain, D. W. (2015) Blood Oxygen Saturation After Ischemia is Altered With Abnormal Microvascular Reperfusion. Microcirculation. 22, 294-305. [CrossRef]
- Casanova, F., Adingupu, D. D., Adams, F., Gooding, K. M., Looker, H. C., Aizawa, K., Dove, F., Elyas, S., Belch, J. J. F., Gates, P. E., Littleford, R. C., Gilchrist, M., Colhoun, H. M., Shore, A. C., Khan, F. and Strain, W. D. (2017) The impact of cardiovascular co-morbidities and duration of diabetes on the association between microvascular function and glycaemic control. Cardiovasc Diabetol. 16, 114. [CrossRef]
- Elyas, S., Adingupu, D., Aizawa, K., Casanova, F., Gooding, K., Fulford, J., Mawson, D., Gates, P. E., Shore, A. C. and Strain, D. (2021) Cerebral small vessel disease, systemic vascular characteristics and potential therapeutic targets. Aging (Albany NY). 13, 22030-22039. [CrossRef]
- Levy, B. I., Schiffrin, E. L., Mourad, J. J., Agostini, D., Vicaut, E., Safar, M. E. and Struijker-Boudier, H. A. (2008) Impaired tissue perfusion: a pathology common to hypertension, obesity, and diabetes mellitus. Circulation. 118, 968-976. [CrossRef]
- Loader, J., Khouri, C., Taylor, F., Stewart, S., Lorenzen, C., Cracowski, J. L., Walther, G. and Roustit, M. (2019) The continuums of impairment in vascular reactivity across the spectrum of cardiometabolic health: A systematic review and network meta-analysis. Obes Rev. 20, 906-920. [CrossRef]
- Roustit, M. and Cracowski, J. L. (2013) Assessment of endothelial and neurovascular function in human skin microcirculation. Trends Pharmacol Sci. 34, 373-384. [CrossRef]
- Strain, W. D., Chaturvedi, N., Hughes, A., Nihoyannopoulos, P., Bulpitt, C. J., Rajkumar, C. and Shore, A. C. (2010) Associations between cardiac target organ damage and microvascular dysfunction: the role of blood pressure. J Hypertens. 28, 952-958. [CrossRef]
- Thorn, C. E., Gates, P. E., Casanova, F., Ramalli, A., Tortoli, P., Palombo, C., Shore, A. C. and Aizawa, K. (2024) Interaction of macro- and microvascular function underlies brachial artery flow-mediated dilation in humans. Am J Physiol Heart Circ Physiol. 327, H268-H274. [CrossRef]
- Williams, J., Gilchrist, M., Strain, D., Fraser, D. and Shore, A. (2020) The systemic microcirculation in dialysis populations. Microcirculation. 27, e12613. [CrossRef]
- Strain, W. D., Adingupu, D. D. and Shore, A. C. (2012) Microcirculation on a large scale: techniques, tactics and relevance of studying the microcirculation in larger population samples. Microcirculation. 19, 37-46. [CrossRef]
- Maniewski, R. and Liebert, A. (2000) Manifestation of internal organs malfunction by laser Doppler study on microcirculation. Front Med Biol Eng. 10, 233-238. [CrossRef]
- Deegan, A. J. and Wang, R. K. (2019) Microvascular imaging of the skin. Phys Med Biol. 64, 07TR01. [CrossRef]
- Pretorius, E., Bester, J. and Kell, D. B. (2016) A bacterial component to Alzheimer-type dementia seen via a systems biology approach that links iron dysregulation and inflammagen shedding to disease J Alzheimers Dis. 53, 1237-1256. [CrossRef]
- Pretorius, E., Mbotwe, S., Bester, J., Robinson, C. J. and Kell, D. B. (2016) Acute induction of anomalous and amyloidogenic blood clotting by molecular amplification of highly substoichiometric levels of bacterial lipopolysaccharide. J R Soc Interface. 123, 20160539. [CrossRef]
- Kell, D. B. and Pretorius, E. (2017) Proteins behaving badly. Substoichiometric molecular control and amplification of the initiation and nature of amyloid fibril formation: lessons from and for blood clotting. Progr Biophys Mol Biol. 123, 16-41. [CrossRef]
- Grixti, J. M., Theron, C. W., Salcedo-Sora, J. E., Pretorius, E. and Kell, D. B. (2024) Automated microscopic measurement of fibrinaloid microclots and their degradation by nattokinase, the main natto protease. J Exp Clin Appl Chin Med. 5, 30-55. [CrossRef]
- Pretorius, E., Nunes, M., Pretorius, J. and Kell, D. B. (2024) Flow Clotometry: Measuring Amyloid Microclots in ME/CFS, Long COVID, and Healthy Samples with Imaging Flow Cytometry. Research Square, https://www.researchsquare.com/article/rs-4507472/v4507471. [CrossRef]
- Pretorius, E., Thierry, A., Sanchez, C., Ha, T., Pastor, B., Mirandola, A., Pisareva, E., Prevostel, C., Laubscher, G., Usher, T., Venter, C., Turner, S., Waters, M. and Kell, D. B. (2024) Circulating microclots are structurally associated with Neutrophil Extracellular Traps and their amounts are strongly elevated in long COVID patients. Res Square https://www.researchsquare.com/article/rs-4666650/v4666651. [CrossRef]
- Turner, S., Laubscher, G. J., Khan, M. A., Kell, D. B. and Pretorius, E. (2023) Accelerating discovery: A novel flow cytometric method for detecting fibrin(ogen) amyloid microclots using long COVID as a model Heliyon. 9, e19605. [CrossRef]
- Pretorius, E. and Kell, D. B. (2024) A Perspective on How Fibrinaloid Microclots and Platelet Pathology May be Applied in Clinical Investigations. Semin Thromb Hemost. 50, 537-551. [CrossRef]
- Bunch, C. M., Moore, E. E., Moore, H. B., Neal, M. D., Thomas, A. V., Zackariya, N., Zhao, J., Zackariya, S., Brenner, T. J., Berquist, M., Buckner, H., Wiarda, G., Fulkerson, D., Huff, W., Kwaan, H. C., Lankowicz, G., Laubscher, G. J., Lourens, P. J., Pretorius, E., Kotze, M. J., Moolla, M. S., Sithole, S., Maponga, T. G., Kell, D. B., Fox, M., Gillespie, L., Khan, R. Z., Mamczak, C. N., March, R., Macias, R., Bull, B. S. and Walsh, M. M. (2022) Immuno-thrombotic Complications of COVID-19: Implications for Timing of Surgery and Anticoagulation. Front Surg. 9, 889999. [CrossRef]
- Grobbelaar, L. M., Venter, C., Vlok, M., Ngoepe, M., Laubscher, G. J., Lourens, P. J., Steenkamp, J., Kell, D. B. and Pretorius, E. (2021) SARS-CoV-2 spike protein S1 induces fibrin(ogen) resistant to fibrinolysis: implications for microclot formation in COVID-19. Biosci Rep. 41, BSR20210611. [CrossRef]
- Grobbelaar, L. M., Kruger, A., Venter, C., Burger, E. M., Laubscher, G. J., Maponga, T. G., Kotze, M. J., Kwaan, H. C., Miller, J. B., Fulkerson, D., Huff, W., Chang, E., Wiarda, G., Bunch, C. M., Walsh, M. M., Raza, S., Zamlut, M., Moore, H. B., Moore, E. E., Neal, M. D., Kell, D. B. and Pretorius, E. (2022) Relative hypercoagulopathy of the SARS-CoV-2 Beta and Delta variants when compared to the less severe Omicron variants is related to TEG parameters, the extent of fibrin amyloid microclots, and the severity of clinical illness. Semin Thromb Haemost. 48, 858-868. [CrossRef]
- Grobler, C., Maphumulo, S. C., Grobbelaar, L. M., Bredenkamp`, J., Laubscher, J., Lourens, P. J., Steenkamp, J., Kell, D. B. and Pretorius, E. (2020) COVID-19: The Rollercoaster of Fibrin(ogen), D-dimer, von Willebrand Factor, P-selectin and Their Interactions with Endothelial Cells, Platelets and Erythrocytes. Int J Mol Sci. 21, 5168. [CrossRef]
- Laubscher, G. J., Lourens, P. J., Venter, C., Kell, D. B. and Pretorius, E. (2021) TEG®, Microclot and Platelet Mapping for Guiding Early Management of Severe COVID-19 Coagulopathy. J Clin Med. 10, 5381. [CrossRef]
- Pretorius, E., Venter, C., Laubscher, G. J., Lourens, P. J., Steenkamp, J. and Kell, D. B. (2020) Prevalence of readily detected amyloid blood clots in ‘unclotted’ Type 2 Diabetes Mellitus and COVID-19 plasma: A preliminary report. Cardiovasc Diabetol. 19, 193. [CrossRef]
- de Waal, G. M., Engelbrecht, L., Davis, T., de Villiers, W. J. S., Kell, D. B. and Pretorius, E. (2018) Correlative Light-Electron Microscopy detects lipopolysaccharide and its association with fibrin fibres in Parkinson's Disease, Alzheimer's Disease and Type 2 Diabetes Mellitus. Sci Rep. 8, 16798. [CrossRef]
- Grobler, C., van Tongeren, M., Gettemans, J., Kell, D. and Pretorius, E. (2023) Alzheimer-type dementia: a systems view provides a unifying explanation of its development. J Alz Dis. 91, 43-70. [CrossRef]
- Pretorius, E., Bester, J., Page, M. J. and Kell, D. B. (2018) The potential of LPS-binding protein to reverse amyloid formation in plasma fibrin of individuals with Alzheimer-type dementia. Frontiers Aging Neurosci. 10, 257. [CrossRef]
- Pretorius, L., Kell, D. B. and Pretorius, E. (2018) Iron Dysregulation and Dormant Microbes as Causative Agents for Impaired Blood Rheology and Pathological Clotting in Alzheimer's Type Dementia. Front Neurosci. 12, 851. [CrossRef]
- Pretorius, E., Oberholzer, H. M., van der Spuy, W. J., Swanepoel, A. C. and Soma, P. (2011) Qualitative scanning electron microscopy analysis of fibrin networks and platelet abnormalities in diabetes. Blood Coagul Fibrinol. 22, 463-467. [CrossRef]
- Pretorius, E., Bester, J., Vermeulen, N., Alummoottil, S., Soma, P., Buys, A. V. and Kell, D. B. (2015) Poorly controlled type 2 diabetes is accompanied by significant morphological and ultrastructural changes in both erythrocytes and in thrombin-generated fibrin: implications for diagnostics. Cardiovasc Diabetol. 134, 30. [CrossRef]
- Pretorius, E., Page, M. J., Engelbrecht, L., Ellis, G. C. and Kell, D. B. (2017) Substantial fibrin amyloidogenesis in type 2 diabetes assessed using amyloid-selective fluorescent stains. Cardiovasc Diabetol. 16, 141. [CrossRef]
- Kell, D. B., Laubscher, G. J. and Pretorius, E. (2022) A central role for amyloid fibrin microclots in long COVID/PASC: origins and therapeutic implications. Biochem J. 479, 537-559. [CrossRef]
- Kruger, A., Vlok, M., Turner, S., Venter, C., Laubscher, G. J., Kell, D. B. and Pretorius, E. (2022) Proteomics of fibrin amyloid microclots in Long COVID/ Post-Acute Sequelae of COVID-19 (PASC) shows many entrapped pro-inflammatory molecules that may also contribute to a failed fibrinolytic system. Cardiovasc Diabetol. 21, 190. [CrossRef]
- Pretorius, E., Vlok, M., Venter, C., Bezuidenhout, J. A., Laubscher, G. J., Steenkamp, J. and Kell, D. B. (2021) Persistent clotting protein pathology in Long COVID/ Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc Diabetol. 20, 172. [CrossRef]
- Pretorius, E., Venter, C., Laubscher, G. J., Kotze, M. J., Oladejo, S., Watson, L. R., Rajaratnam, K., Watson, B. W. and Kell, D. B. (2022) Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with Long COVID/ Post-Acute Sequelae of COVID-19 (PASC) Cardiovasc Diabetol. 21, 148. [CrossRef]
- Turner, S., Khan, M. A., Putrino, D., Woodcock, A., Kell, D. B. and Pretorius, E. (2023) Long COVID: pathophysiological factors and abnormal coagulation. Trends Endocrinol Metab. 34, 321-344. [CrossRef]
- Dalton, C. F., de Oliveira, M. I. R., Stafford, P., Peake, N., Kane, B., Higham, A., Singh, D., Jackson, N., Davies, H., Price, D., Duncan, R., Tattersall, N., Barnes, A. and Smith, D. P. (2024) Increased fibrinaloid microclot counts in platelet-poor plasma are associated with Long COVID. medRxiv, 2024.2004.2004.24305318. [CrossRef]
- Kell, D. B., Khan, M. A., Kane, B., Lip, G. Y. H. and Pretorius, E. (2024) Possible role of fibrinaloid microclots in Postural Orthostatic Tachycardia Syndrome (POTS): focus on Long COVID. J Personalised Medicine. 14, 170. [CrossRef]
- Kell, D. B., Khan, M. A. and Pretorius, E. (2024) Fibrinaloid microclots in Long COVID: assessing the actual evidence properly. Res Pract Thromb Haemost. 8, 102566. [CrossRef]
- Kruger, A., Joffe, D., Lloyd-Jones, G., Khan, M. A., Šalamon, Š., Laubscher, G. J., Putrino, D., Kell, D. B. and Pretorius, E. (2025) Vascular pathogenesis in acute and long covid: current insights and therapeutic outlook Semin Throm Hemost. 51, 256-271. [CrossRef]
- Dalton, C. J. and Lemmon, C. A. (2021) Fibronectin: Molecular Structure, Fibrillar Structure and Mechanochemical Signaling. Cells. 10, 2443. [CrossRef]
- de Villiers, S., Bester, J., Kell, D. B. and Pretorius, E. (2019) Erythrocyte health and the possible role of amyloidogenic blood clotting in the evolving haemodynamics of female migraine-with-aura pathophysiology: Results from a pilot study. Frontiers Neurol. 10, 1262. [CrossRef]
- Arron, H. E., Marsh, B. D., Kell, D. B., Khan, M. A., Jaeger, B. R. and Pretorius, E. (2024) Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: the biology of a neglected disease. Front Immunol. 15, 1386607. [CrossRef]
- Nunes, J. M., Kruger, A., Proal, A., Kell, D. B. and Pretorius, E. (2022) The occurrence of hyperactivated platelets and fibrinaloid microclots in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Pharmaceuticals (Basel). 15, 931. [CrossRef]
- Nunes, J. M., Kell, D. B. and Pretorius, E. (2023) Cardiovascular and haematological pathology in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): a role for Viruses. Blood Rev. 60, 101075. [CrossRef]
- Nunes, J. M., Kell, D. B. and Pretorius, E. (2024) Herpesvirus Infection of Endothelial Cells as a Systemic Pathological Axis in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Viruses. 16, 572. [CrossRef]
- Pretorius, E., Page, M. J., Mbotwe, S. and Kell, D. B. (2018) Lipopolysaccharide-binding protein (LBP) can reverse the amyloid state of fibrin seen or induced in Parkinson’s disease. PlosOne. 13, e0192121. [CrossRef]
- van Vuuren, M. J., Nell, T. A., Carr, J. A., Kell, D. B. and Pretorius, E. (2021) Iron dysregulation and inflammagens related to oral and gut health are central to the development of Parkinson’s disease. Biomolecules. 11, 30. [CrossRef]
- Bezuidenhout, J., Venter, C., Roberts, T., Tarr, G., Kell, D. and Pretorius, E. (2020) The Atypical Fibrin Fibre Network in Rheumatoid Arthritis and its Relation to Autoimmunity, Inflammation and Thrombosis. bioRxiv, 2020.2005.2028.121301v121301. [CrossRef]
- Pretorius, E., Oberholzer, H. M., van der Spuy, W. J., Swanepoel, A. C. and Soma, P. (2012) Scanning electron microscopy of fibrin networks in rheumatoid arthritis: a qualitative analysis. Rheumatol Int. 32, 1611-1615. [CrossRef]
- Pretorius, E., Akeredolu, O.-O., Soma, P. and Kell, D. B. (2017) Major involvement of bacterial components in rheumatoid arthritis and its accompanying oxidative stress, systemic inflammation and hypercoagulability. Exp Biol Med. 242, 355-373. [CrossRef]
- Kell, D. B., Pretorius, E. and Zhao, H. (2025) A direct relationship between ‘blood stasis’ and fibrinaloid microclots in chronic, inflammatory and vascular diseases, and some traditional natural products approaches to treatment. Pharmaceuticals. 18, 712. [CrossRef]
- Kell, D. B. and Pretorius, E. (2022) The potential role of ischaemia-reperfusion injury in chronic, relapsing diseases such as rheumatoid arthritis, long COVID and ME/CFS: evidence, mechanisms, and therapeutic implications. Biochem J. 479, 1653-1708. [CrossRef]
- Kell, D. B. and Pretorius, E. (2023) Are fibrinaloid microclots a cause of autoimmunity in Long Covid and other post-infection diseases? Biochem J. 480, 1217-1240. [CrossRef]
- Kell, D. B., Lip, G. Y. H. and Pretorius, E. (2024) Fibrinaloid Microclots and Atrial Fibrillation. Biomedicines. 12, 891. [CrossRef]
- Kell, D. B. and Pretorius, E. (2024) Potential roles of fibrinaloid microclots in fibromyalgia syndrome. OSF preprint, https://osf.io/9e2y5/. [CrossRef]
- Nunes, J. M., Vlok, M., Proal, A., Kell, D. B. and Pretorius, E. (2024) Data-independent LC-MS/MS analysis of ME/CFS plasma reveals a dysregulated coagulation system, endothelial dysfunction, downregulation of complement machinery. Cardiovasc. Diabetol. 23, 254. [CrossRef]
- Kell, D. B. and Pretorius, E. (2024) Proteomic evidence for amyloidogenic cross-seeding in fibrinaloid microclots. Int J Mol Sci. 25, 10809. [CrossRef]
- Kell, D. B. and Pretorius, E. (2025) The proteome content of blood clots observed under different conditions: successful role in predicting clot amyloid(ogenicity). Molecules. 30, 668. [CrossRef]
- Grixti, J. M., Chandran, A., Pretorius, J. H., Walker, M., Sekhar, A., Pretorius, E. and Kell, D. B. (2024) The clots removed from ischaemic stroke patients by mechanical thrombectomy are amyloid in nature. medRxiv, 10.1101/2024.1111.1101.24316555v24316551. [CrossRef]
- Grixti, J. M., Chandran, A., Pretorius, J. H., Walker, M., Sekhar, A., Pretorius, E. and Kell, D. B. (2025) Amyloid presence in acute ischemic stroke thrombi: observational evidence for fibrinolytic resistance. Stroke, online. [CrossRef]
- Biancalana, M. and Koide, S. (2010) Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim Biophys Acta. 1804, 1405-1412. [CrossRef]
- Biancardi, A., Biver, T. and Mennucci, B. (2017) Fluorescent dyes in the context of DNA-binding: The case of Thioflavin T. Int J Quant Chem. 117, e25349. [CrossRef]
- Gade Malmos, K., Blancas-Mejia, L. M., Weber, B., Buchner, J., Ramirez-Alvarado, M., Naiki, H. and Otzen, D. (2017) ThT 101: a primer on the use of thioflavin T to investigate amyloid formation. Amyloid. 24, 1-16. [CrossRef]
- Xue, C., Lin, T. Y., Chang, D. and Guo, Z. (2017) Thioflavin T as an amyloid dye: fibril quantification, optimal concentration and effect on aggregation. R Soc Open Sci. 4, 160696. [CrossRef]
- Kell, D. B. and Pretorius, E. (2025) On the utility of nailfold capillaroscopy in detecting the effects of fibrinaloid microclots in diseases involving blood stasis. Preprints, 202505.202356/v202501. [CrossRef]
- Ciaffi, J., Ajasllari, N., Mancarella, L., Brusi, V., Meliconi, R. and Ursini, F. (2020) Nailfold capillaroscopy in common non-rheumatic conditions: A systematic review and applications for clinical practice. Microvasc Res. 131, 104036. [CrossRef]
- Cutolo, M. and Smith, V. (2013) State of the art on nailfold capillaroscopy: a reliable diagnostic tool and putative biomarker in rheumatology? Rheumatology (Oxford). 52, 1933-1940. [CrossRef]
- Herrick, A. L., Berks, M. and Taylor, C. J. (2021) Quantitative nailfold capillaroscopy-update and possible next steps. Rheumatology (Oxford). 60, 2054-2065. [CrossRef]
- Kintrup, S., Listkiewicz, L., Arnemann, P. H. and Wagner, N. M. (2024) Nailfold videocapillaroscopy - a novel method for the assessment of hemodynamic incoherence on the ICU. Crit Care. 28, 400. [CrossRef]
- Komai, M., Takeno, D., Fujii, C., Nakano, J., Ohsaki, Y. and Shirakawa, H. (2024) Nailfold Capillaroscopy: A Comprehensive Review on Its Usefulness in Both Clinical Diagnosis and Improving Unhealthy Dietary Lifestyles. Nutrients. 16, 1914. [CrossRef]
- Lim, M. W. S., Setjiadi, D., Dobbin, S. J. H., Lang, N. N., Delles, C. and Connelly, P. J. (2023) Nailfold video-capillaroscopy in the study of cardiovascular disease: a systematic review. Blood Press Monit. 28, 24-32. [CrossRef]
- Mansueto, N., Rotondo, C., Corrado, A. and Cantatore, F. P. (2021) Nailfold capillaroscopy : a comprehensive review on common findings and clinical usefulness in non-rheumatic disease. J Med Invest. 68, 6-14. [CrossRef]
- Silva, I., Teixeira, A., Oliveira, J., Almeida, I., Almeida, R., Aguas, A. and Vasconcelos, C. (2015) Endothelial Dysfunction and Nailfold Videocapillaroscopy Pattern as Predictors of Digital Ulcers in Systemic Sclerosis: a Cohort Study and Review of the Literature. Clin Rev Allergy Immunol. 49, 240-252. [CrossRef]
- Balamurugan, S., Agrawal, A., Kato, Y. and Sano, H. (2011) Intra operative indocyanine green video-angiography in cerebrovascular surgery: An overview with review of literature. Asian J Neurosurg. 6, 88-93. [CrossRef]
- Breuking, E. A., de Fraiture, E. J., Krijgh, D. D., van Wessem, K., de Bruin, I. G. J. M., Hietbrink, F. and Ruiterkamp, J. (2025) Current applications of indocyanine green fluorescence angiography in trauma patients and its potential impact: a systematic review. BMJ Open. 15, e099755. [CrossRef]
- Iwamoto, M., Ueda, K. and Kawamura, J. (2022) A Narrative Review of the Usefulness of Indocyanine Green Fluorescence Angiography for Perfusion Assessment in Colorectal Surgery. Cancers (Basel). 14, 5623. [CrossRef]
- Le-Nguyen, A., O'Neill Trudeau, M., Dodin, P., Keezer, M. R., Faure, C. and Piché, N. (2021) The Use of Indocyanine Green Fluorescence Angiography in Pediatric Surgery: A Systematic Review and Narrative Analysis. Front Pediatr. 9, 736242. [CrossRef]
- Li, K., Zhang, Z., Nicoli, F., D'Ambrosia, C., Xi, W., Lazzeri, D., Feng, S., Su, W., Li, H., Ciudad, P., Tremp, M. and Zhang, Y. X. (2018) Application of Indocyanine Green in Flap Surgery: A Systematic Review. J Reconstr Microsurg. 34, 77-86. [CrossRef]
- Vega-Moreno, D. A., Janković, D., Azouz, H., Nakipuria, M. and Kato, Y. (2023) Dual Microscope Indocyanine Green Video Angiography and Endoscopic Review to Treat Intracranial Aneurysm: A Review of the Literature Regarding a Case. Asian J Neurosurg. 18, 701-707. [CrossRef]
- Aumann, S., Donner, S., Fischer, J. and Muller, F. (2019) Optical Coherence Tomography (OCT): Principle and Technical Realization. In High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics (Bille, J. F., ed.). pp. 59-85, Cham (CH).
- Greig, E. C., Duker, J. S. and Waheed, N. K. (2020) A practical guide to optical coherence tomography angiography interpretation. Int J Retina Vitreous. 6, 55. [CrossRef]
- Akil, H., Karst, S., Heisler, M., Etminan, M., Navajas, E. and Maberley, D. (2019) Application of optical coherence tomography angiography in diabetic retinopathy: a comprehensive review. Can J Ophthalmol. 54, 519-528. [CrossRef]
- de Carlo, T. E., Romano, A., Waheed, N. K. and Duker, J. S. (2015) A review of optical coherence tomography angiography (OCTA). Int J Retina Vitreous. 1, 5. [CrossRef]
- Ge, J. Y., Teo, Z. L. and Loo, J. L. (2024) Recent advances in the use of optical coherence tomography in neuro-ophthalmology: A review. Clin Exp Ophthalmol. 52, 220-233. [CrossRef]
- Koutsiaris, A. G., Batis, V., Liakopoulou, G., Tachmitzi, S. V., Detorakis, E. T. and Tsironi, E. E. (2023) Optical Coherence Tomography Angiography (OCTA) of the eye: A review on basic principles, advantages, disadvantages and device specifications. Clin Hemorheol Microcirc. 83, 247-271. [CrossRef]
- Langlo, C. S., Amin, A. and Park, S. S. (2025) Optical coherence tomography retinal imaging: narrative review of technological advancements and clinical applications. Ann Transl Med. 13, 17. [CrossRef]
- Lohrasbi, F., Karimi, E., Gouravani, M., Fooladi Sarabi, S., Mafi, A., Beikmarzehei, A., Sanjari Moghaddam, H., Parsaei, M., Tabatabaei, S. M. and Arevalo, J. F. (2025) Analysis of Retinal and Choroidal Microvasculature in Systemic Sclerosis Using Optical Coherence Tomography Angiography: A Systematic Review and Meta-Analysis. Ophthalmic Res. 68, 23-40. [CrossRef]
- Pradeep, K., Jeyakumar, V., Bhende, M., Shakeel, A. and Mahadevan, S. (2024) Artificial intelligence and hemodynamic studies in optical coherence tomography angiography for diabetic retinopathy evaluation: A review. Proc Inst Mech Eng H. 238, 3-21. [CrossRef]
- Wan, K. H. and Leung, C. K. S. (2017) Optical coherence tomography angiography in glaucoma: a mini-review. F1000Res. 6, 1686. [CrossRef]
- Yao, X., Alam, M. N., Le, D. and Toslak, D. (2020) Quantitative optical coherence tomography angiography: A review. Exp Biol Med (Maywood). 245, 301-312. [CrossRef]
- Ring, H. C., Themstrup, L., Banzhaf, C. A., Jemec, G. B. and Mogensen, M. (2016) Dynamic Optical Coherence Tomography Capillaroscopy: A New Imaging Tool in Autoimmune Connective Tissue Disease. JAMA Dermatol. 152, 1142–1146. [CrossRef]
- Themstrup, L., Welzel, J., Ciardo, S., Kaestle, R., Ulrich, M., Holmes, J., Whitehead, R., Sattler, E. C., Kindermann, N., Pellacani, G. and Jemec, G. B. (2016) Validation of Dynamic optical coherence tomography for non-invasive, in vivo microcirculation imaging of the skin. Microvasc Res. 107, 97-105. [CrossRef]
- Briers, D., Duncan, D. D., Hirst, E., Kirkpatrick, S. J., Larsson, M., Steenbergen, W., Stromberg, T. and Thompson, O. B. (2013) Laser speckle contrast imaging: theoretical and practical limitations. J Biomed Opt. 18, 066018. [CrossRef]
- Couturier, A., Bouvet, R., Cracowski, J. L. and Roustit, M. (2022) Reproducibility of high-resolution laser speckle contrast imaging to assess cutaneous microcirculation for wound healing monitoring in mice. Microvasc Res. 141, 104319. [CrossRef]
- Dunn, A. K. (2012) Laser speckle contrast imaging of cerebral blood flow. Ann Biomed Eng. 40, 367-377. [CrossRef]
- Hellmann, M., Kalinowski, L. and Cracowski, J. L. (2022) Laser speckle contrast imaging to assess microcirculation. Cardiol J. 29, 1028-1030. [CrossRef]
- Hren, R., Brezar, S. K., Marhl, U. and Sersa, G. (2024) Laser speckle contrast imaging of perfusion in oncological clinical applications: a literature review. Radiol Oncol. 58, 326-334. [CrossRef]
- Humeau-Heurtier, A., Guerreschi, E., Abraham, P. and Mahé, G. (2013) Relevance of laser Doppler and laser speckle techniques for assessing vascular function: state of the art and future trends. IEEE Trans Biomed Eng. 60, 659-666. [CrossRef]
- Kazmi, S. M., Richards, L. M., Schrandt, C. J., Davis, M. A. and Dunn, A. K. (2015) Expanding applications, accuracy, and interpretation of laser speckle contrast imaging of cerebral blood flow. J Cereb Blood Flow Metab. 35, 1076-1084. [CrossRef]
- Linkous, C., Pagan, A. D., Shope, C., Andrews, L., Snyder, A., Ye, T. and Valdebran, M. (2023) Applications of Laser Speckle Contrast Imaging Technology in Dermatology. JID Innov. 3, 100187. [CrossRef]
- Senarathna, J., Rege, A., Li, N. and Thakor, N. V. (2013) Laser Speckle Contrast Imaging: theory, instrumentation and applications. IEEE Rev Biomed Eng. 6, 99-110. [CrossRef]
- Vaz, P. G., Humeau-Heurtier, A., Figueiras, E., Correia, C. and Cardoso, J. (2016) Laser Speckle Imaging to Monitor Microvascular Blood Flow: A Review. IEEE Rev Biomed Eng. 9, 106-120. [CrossRef]
- Girkantaite, Z., Laucyte-Cibulskiene, A., Ryliskyte, L., Juceviciene, A. and Badariene, J. (2022) Laser Doppler flowmetry evaluation of skin microvascular endothelial function in patients with metabolic syndrome. Microvasc Res. 142, 104373. [CrossRef]
- Magnain, C., Castel, A., Boucneau, T., Simonutti, M., Ferezou, I., Rancillac, A., Vitalis, T., Sahel, J. A., Paques, M. and Atlan, M. (2014) Holographic laser Doppler imaging of microvascular blood flow. J Opt Soc Am A Opt Image Sci Vis. 31, 2723-2735. [CrossRef]
- Briers, J. D. (1996) Laser Doppler and time-varying speckle: a reconciliation. J Opt Sco Amer. 13, 345-350. [CrossRef]
- Tew, G. A., Klonizakis, M., Crank, H., Briers, J. D. and Hodges, G. J. (2011) Comparison of laser speckle contrast imaging with laser Doppler for assessing microvascular function. Microvasc Res. 82, 326-332. [CrossRef]
- Fell, D. A. (1996) Understanding the control of metabolism. Portland Press, London.
- Heinrich, R. and Rapoport, T. A. (1974) A linear steady-state treatment of enzymatic chains. General properties, control and effector strength. Eur. J. Biochem. 42, 89-95. [CrossRef]
- Heinrich, R. and Schuster, S. (1996) The regulation of cellular systems. Chapman & Hall, New York.
- Kacser, H. and Burns, J. A. (1973) The control of flux. In Rate Control of Biological Processes. Symposium of the Society for Experimental Biology Vol 27 (Davies, D. D., ed.). pp. 65-104, Cambridge University Press, Cambridge.
- Kell, D. B. and Westerhoff, H. V. (1986) Metabolic control theory: its role in microbiology and biotechnology. FEMS Microbiol. Rev. 39, 305-320. [CrossRef]
- Minas, G. and Rand, D. A. (2019) Parameter sensitivity analysis for biochemical reaction networks. Math Biosci Eng. 16, 3965-3987. [CrossRef]
- Rand, D. A. (2008) Mapping global sensitivity of cellular network dynamics: sensitivity heat maps and a global summation law. J R Soc Interface. 5 Suppl 1, S59-69. [CrossRef]
- Cornish-Bowden, A. (1991) Failure of channelling to maintain low concentrations of metabolic intermediates. Eur J Biochem. 195, 103-108. [CrossRef]
- Cornish-Bowden, A. and Cardenas, M. L. (1993) Channelling can affect concentrations of metabolic intermediates at constant net flux: artefact or reality? Eur J Biochem. 213, 87-92. [CrossRef]
- Mendes, P., Kell, D. B. and Westerhoff, H. V. (1992) Channelling can decrease pool size. Eur J Biochem. 204, 257-266. [CrossRef]
- Mendes, P. and Kell, D. B. (1993) On the role of enzyme kinetic parameters in determining the effectiveness with which channelling can decrease the size of a metabolite pool. Acta Biotheoret. 41, 63-73. [CrossRef]
- Mendes, P., Kell, D. B. and Welch, G. R. (1995) Metabolic channeling in organized enzyme systems: experiments and models. In Enzymology in vivo (Brindle, K. M., ed.). pp. 1-19, JAI Press, London.
- Pethig, R. and Kell, D. B. (1987) The passive electrical properties of biological systems: their significance in physiology, biophysics and biotechnology. Phys. Med. Biol. 32, 933-970. [CrossRef]
- Sun, C., Liu, Y., Huang, W., Chen, Y., Deng, Y., Yuan, J., Deng, L., Xu, N., Shang, X., Wang, C., Yang, Z., Huang, L. and Qiu, Q. (2024) Uric acid, high density lipoprotein cholesterol levels and their ratio are related to microbial enterotypes and serum metabolites in females with a blood stasis constitution. Lipids Health Dis. 23, 90. [CrossRef]
- Qu, C., Pu, Z. J., Zhou, G. S., Wang, J., Zhu, Z. H., Yue, S. J., Li, J. P., Shang, L. L., Tang, Y. P., Shi, X. Q., Liu, P., Guo, J. M., Sun, J., Tang, Z. S., Zhao, J., Zhao, B. C. and Duan, J. A. (2017) Comparative analysis of main bio-active components in the herb pair Danshen-Honghua and its single herbs by ultra-high performance liquid chromatography coupled to triple quadrupole tandem mass spectrometry. J Sep Sci. 40, 3392-3401. [CrossRef]
- Zhang, Q., Liang, Z., Wang, X., Zhang, S. and Yang, Z. (2024) Exploring the potential mechanisms of Danshen against COVID-19 via network pharmacology analysis and molecular docking. Sci Rep. 14, 12780. [CrossRef]
- Wei, B., Sun, C., Wan, H., Shou, Q., Han, B., Sheng, M., Li, L. and Kai, G. (2023) Bioactive components and molecular mechanisms of Salvia miltiorrhiza Bunge in promoting blood circulation to remove blood stasis. J Ethnopharmacol. 317, 116697. [CrossRef]
- Lan, T., Yu, D., Zhao, Q., Qu, C. and Wu, Q. (2025) Ethnomedicine, phytochemistry, pharmacology, pharmacokinetics, and clinical application of Salvia miltiorrhiza Bunge (Lamiaceae): A comprehensive review. J Ethnopharmacol. 350, 120032. [CrossRef]
- Li, Y., Ren, T. T., Liu, S. S., Zhang, L., Yi, H., Li, C., Chen, L. M., Gao, H. M., Yan, L. H., Liu, X. Q. and Wang, Z. M. (2024) Fingerprint analysis of dang-gui-Si-Ni decoction and its anticoagulant activity in vivo-in vitro. J Ethnopharmacol. 325, 117890. [CrossRef]
- Ma, X. H., Chen, Y., Huang, X. Y., Jiang, J. R., Liu, X., An, D. Q. and He, R. R. (2024) Characteristics and efficacy of traditional Chinese medicine in the therapeutic strategy of chronic coronary syndrome: A systematic review and meta-analysis. Phytomedicine. 129, 155579. [CrossRef]
- Draijer, M., Hondebrink, E., van Leeuwen, T. and Steenbergen, W. (2009) Review of laser speckle contrast techniques for visualizing tissue perfusion. Lasers Med Sci. 24, 639-651. [CrossRef]
- Qureshi, M. M., Allam, N., Im, J., Kwon, H. S., Chung, E. and Vitkin, I. A. (2024) Advances in laser speckle imaging: From qualitative to quantitative hemodynamic assessment. J Biophotonics. 17, e202300126. [CrossRef]
- Hudetz, A. G. (1997) Blood flow in the cerebral capillary network: a review emphasizing observations with intravital microscopy. Microcirculation. 4, 233-252. [CrossRef]
- Yuan, S., Devor, A., Boas, D. A. and Dunn, A. K. (2005) Determination of optimal exposure time for imaging of blood flow changes with laser speckle contrast imaging. Appl Opt. 44, 1823-1830. [CrossRef]
- Berggren, J. V., Stridh, M. and Malmsjo, M. (2022) Perfusion Monitoring During Oculoplastic Reconstructive Surgery: A Comprehensive Review. Ophthalmic Plast Reconstr Surg. 38, 522-534. [CrossRef]
- Aizawa, N., Kunikata, H., Nitta, F., Shiga, Y., Omodaka, K., Tsuda, S. and Nakazawa, T. (2016) Age- and Sex-Dependency of Laser Speckle Flowgraphy Measurements of Optic Nerve Vessel Microcirculation. PLoS One. 11, e0148812. [CrossRef]
- Csipo, T., Lipecz, A., Mukli, P., Péterfi, A., Szarvas, Z., Ungvari, A., Alaoui, L. E., Sándor, M., Kállai, A., Fekete, M., Fülöp, G. Á., Tarantini, S., Csiszar, A., Benyó, Z., Sótonyi, P., Tabak, A. G., Merkely, B., Yabluchanskiy, A. and Ungvari, Z. (2025) Advancing prediction of age-related vascular cognitive impairment based on peripheral and retinal vascular health in a pilot study: a novel comprehensive assessment developed for a prospective workplace-based cohort (The Semmelweis Study). Geroscience. 47, 1329-1344. [CrossRef]
- Khalil, A., Humeau-Heurtier, A., Gascoin, L., Abraham, P. and Mahe, G. (2016) Aging effect on microcirculation: A multiscale entropy approach on laser speckle contrast images. Med Phys. 43, 4008. [CrossRef]
- Luft, N., Wozniak, P. A., Aschinger, G. C., Fondi, K., Bata, A. M., Werkmeister, R. M., Schmidl, D., Witkowska, K. J., Bolz, M., Garhofer, G. and Schmetterer, L. (2016) Ocular Blood Flow Measurements in Healthy White Subjects Using Laser Speckle Flowgraphy. PLoS One. 11, e0168190. [CrossRef]
- Broadhurst, D. I. and Kell, D. B. (2006) Statistical strategies for avoiding false discoveries in metabolomics and related experiments. Metabolomics. 2, 171-196. [CrossRef]
- Gille-Johnson, P., Kessler, I. and Tehrani, S. (2022) Impaired Microvascular Function in Patients With Critical COVID-19. Crit Care Explor. 4, e0643. [CrossRef]
- Tehrani, S. and Gille-Johnson, P. (2021) Microvascular Dysfunction in Patients with Critical Covid-19, a Pilot Study. Shock. 56, 964-968. [CrossRef]
- Orbegozo Cortés, D., Rahmania, L., Irazabal, M., Santacruz, C., Fontana, V., De Backer, D., Creteur, J. and Vincent, J. L. (2016) Microvascular reactivity is altered early in patients with acute respiratory distress syndrome. Respir Res. 17, 59. [CrossRef]
- Linton, E. F., Ahmad, N. U., Filister, R., Wang, J. K., Sohn, E. H. and Kardon, R. H. (2025) Laser Speckle Flowgraphy Reveals Widespread Reductions in Ocular Blood Flow in nonexudative Age-Related Macular Degeneration. Am J Ophthalmol. 273, 92-106. [CrossRef]
- Takizawa, H., Yasuda, M., Hoshi, K., Okabe, T., Kunikata, H. and Nakazawa, T. (2024) Changes in ocular blood flow in patients with neovascular age-related macular degeneration after intravitreal injection of ranibizumab biosimilar and brolucizumab. Int Ophthalmol. 44, 181. [CrossRef]
- Clemons, G. A., Silva, A. C. E., Acosta, C. H., Udo, M. S. B., Tesic, V., Rodgers, K. M., Wu, C. Y., Citadin, C. T., Lee, R. H., Neumann, J. T., Allani, S., Prentice, H., Zhang, Q. and Lin, H. W. (2024) Protein arginine methyltransferase 4 modulates nitric oxide synthase uncoupling and cerebral blood flow in Alzheimer's disease. J Cell Physiol. 239, e30858. [CrossRef]
- Crouzet, C., Phan, T., Wilson, R. H., Shin, T. J. and Choi, B. (2023) Intrinsic, widefield optical imaging of hemodynamics in rodent models of Alzheimer's disease and neurological injury. Neurophotonics. 10, 020601. [CrossRef]
- Jiang, Y., Lin, Y., Tan, Y., Shen, X., Liao, M., Wang, H., Lu, N., Han, F., Xu, N., Tang, C., Song, J. and Tao, R. (2023) Electroacupuncture ameliorates cerebrovascular impairment in Alzheimer's disease mice via melatonin signaling. CNS Neurosci Ther. 29, 917-931. [CrossRef]
- Korte, N., Barkaway, A., Wells, J., Freitas, F., Sethi, H., Andrews, S. P., Skidmore, J., Stevens, B. and Attwell, D. (2024) Inhibiting Ca(2+) channels in Alzheimer's disease model mice relaxes pericytes, improves cerebral blood flow and reduces immune cell stalling and hypoxia. Nat Neurosci. 27, 2086-2100. [CrossRef]
- Tarantini, S., Fulop, G. A., Kiss, T., Farkas, E., Zolei-Szenasi, D., Galvan, V., Toth, P., Csiszar, A., Ungvari, Z. and Yabluchanskiy, A. (2017) Demonstration of impaired neurovascular coupling responses in TG2576 mouse model of Alzheimer's disease using functional laser speckle contrast imaging. Geroscience. 39, 465-473. [CrossRef]
- Udo, M. S. B., Zaccarelli-Magalhães, J., Clemons, G. A., Citadin, C. T., Langman, J., Smith, D. J., Matuguma, L. H., Tesic, V. and Lin, H. W. (2025) Blockade of A(2A)R improved brain perfusion and cognitive function in a mouse model of Alzheimer's disease. Geroscience. [CrossRef]
- Ye, C., Liang, Y., Chen, Y., Xiong, Y., She, Y., Zhong, X., Chen, H. and Huang, M. (2021) Berberine Improves Cognitive Impairment by Simultaneously Impacting Cerebral Blood Flow and beta-Amyloid Accumulation in an APP/tau/PS1 Mouse Model of Alzheimer's Disease. Cells. 10, 1161. [CrossRef]
- Yu, T., Wei, Z., Wang, J., Song, C., Huang, W., Zhang, P., Shi, J., Zhang, R., Jiang, M., Wang, D., Zhang, Y., Chen, H. and Wang, H. (2025) Ginkgo biloba Extract GBE50 ameliorates cerebrovascular dysfunction and cognitive impairment in a mouse model of Alzheimer's disease. Phytomedicine. 141, 156646. [CrossRef]
- Zhao, P., Cheng, P., Wang, J., Zhu, G. and Wang, X. (2024) Shenqi Yizhi prescription prevents AbetaO-induced memory impairment in mice by regulating the contractility of brain pericytes. Phytomedicine. 129, 155639. [CrossRef]
- Dolgyras, P., Lazaridis, A., Anyfanti, P., Gavriilaki, E., Koletsos, N., Triantafyllou, A., Nikolaidou, B., Galanapoulou, V., Douma, S. and Gkaliagkousi, E. (2023) Microcirculation dynamics in systemic vasculitis: evidence of impaired microvascular response regardless of cardiovascular risk factors. Rheumatology (Oxford). 62, 2510-2516. [CrossRef]
- Tkaczyk, E. (2017) Innovations and Developments in Dermatologic Non-invasive Optical Imaging and Potential Clinical Applications. Acta Derm Venereol. Suppl 218, 5-13. [CrossRef]
- Yan, J., Kang, Y., Xu, S., Ong, L. L., Zhuo, S., Bunte, R. M., Chen, N., Asada, H. H., So, P. T., Wanless, I. R. and Yu, H. (2014) In vivo label-free quantification of liver microcirculation using dual-modality microscopy. J Biomed Opt. 19, 116006. [CrossRef]
- Burmeister, D. M., Ponticorvo, A., Yang, B., Becerra, S. C., Choi, B., Durkin, A. J. and Christy, R. J. (2015) Utility of spatial frequency domain imaging (SFDI) and laser speckle imaging (LSI) to non-invasively diagnose burn depth in a porcine model. Burns. 41, 1242-1252. [CrossRef]
- Crouzet, C., Nguyen, J. Q., Ponticorvo, A., Bernal, N. P., Durkin, A. J. and Choi, B. (2015) Acute discrimination between superficial-partial and deep-partial thickness burns in a preclinical model with laser speckle imaging. Burns. 41, 1058-1063. [CrossRef]
- De Mey, K., De Decker, I., Gush, R., Hoeksema, H., Verbelen, J., De Coninck, P., Blondeel, P., Monstrey, S. and Claes, K. E. Y. (2025) Validity of laser speckle contrast imaging for predicting wound healing potential in burns: A critical examination. Burns. 51, 107449. [CrossRef]
- Dijkstra, A., Guven, G., van Baar, M. E., Trommel, N., Hofland, H. W. C., Kuijper, T. M., Ince, C. and Van der Vlies, C. H. (2023) Laser speckle contrast imaging, an alternative to laser doppler imaging in clinical practice of burn wound care derivation of a color code. Burns. 49, 1907-1915. [CrossRef]
- Mirdell, R., Farnebo, S., Sjoberg, F. and Tesselaar, E. (2019) Interobserver reliability of laser speckle contrast imaging in the assessment of burns. Burns. 45, 1325-1335. [CrossRef]
- Mirdell, R., Farnebo, S., Sjoberg, F. and Tesselaar, E. (2020) Using blood flow pulsatility to improve the accuracy of laser speckle contrast imaging in the assessment of burns. Burns. 46, 1398-1406. [CrossRef]
- Ponticorvo, A., Burmeister, D. M., Yang, B., Choi, B., Christy, R. J. and Durkin, A. J. (2014) Quantitative assessment of graded burn wounds in a porcine model using spatial frequency domain imaging (SFDI) and laser speckle imaging (LSI). Biomed Opt Express. 5, 3467-3481. [CrossRef]
- Ponticorvo, A., Burmeister, D. M., Rowland, R., Baldado, M., Kennedy, G. T., Saager, R., Bernal, N., Choi, B. and Durkin, A. J. (2017) Quantitative long-term measurements of burns in a rat model using Spatial Frequency Domain Imaging (SFDI) and Laser Speckle Imaging (LSI). Lasers Surg Med. 49, 293-304. [CrossRef]
- Ponticorvo, A., Rowland, R., Baldado, M., Burmeister, D. M., Christy, R. J., Bernal, N. P. and Durkin, A. J. (2019) Evaluating clinical observation versus Spatial Frequency Domain Imaging (SFDI), Laser Speckle Imaging (LSI) and thermal imaging for the assessment of burn depth. Burns. 45, 450-460. [CrossRef]
- Qin, J., Reif, R., Zhi, Z., Dziennis, S. and Wang, R. (2012) Hemodynamic and morphological vasculature response to a burn monitored using a combined dual-wavelength laser speckle and optical microangiography imaging system. Biomed Opt Express. 3, 455-466. [CrossRef]
- Ragol, S., Remer, I., Shoham, Y., Hazan, S., Willenz, U., Sinelnikov, I., Dronov, V., Rosenberg, L. and Bilenca, A. (2015) Static laser speckle contrast analysis for noninvasive burn diagnosis using a camera-phone imager. J Biomed Opt. 20, 86009. [CrossRef]
- Ragol, S., Remer, I., Shoham, Y., Hazan, S., Willenz, U., Sinelnikov, I., Dronov, V., Rosenberg, L. and Bilenca, A. (2016) In vivo burn diagnosis by camera-phone diffuse reflectance laser speckle detection. Biomed Opt Express. 7, 225-237. [CrossRef]
- Sadhwani, A., Schomacker, K. T., Tearney, G. J. and Nishioka, N. S. (1996) Determination of Teflon thickness with laser speckle. I. Potential for burn depth diagnosis. Appl Opt. 35, 5727-5735. [CrossRef]
- Stewart, C. J., Frank, R., Forrester, K. R., Tulip, J., Lindsay, R. and Bray, R. C. (2005) A comparison of two laser-based methods for determination of burn scar perfusion: laser Doppler versus laser speckle imaging. Burns. 31, 744-752. [CrossRef]
- Zheng, K. J., Middelkoop, E., Stoop, M., van Zuijlen, P. P. M. and Pijpe, A. (2022) Validity of laser speckle contrast imaging for the prediction of burn wound healing potential. Burns. 48, 319-327. [CrossRef]
- Hajjarian, Z. and Nadkarni, S. K. (2021) Technological perspectives on laser speckle micro-rheology for cancer mechanobiology research. J Biomed Opt. 26, 090601. [CrossRef]
- Omarjee, L., Larralde, A., Jaquinandi, V., Stivalet, O. and Mahe, G. (2018) Performance of noninvasive laser Doppler flowmetry and laser speckle contrast imaging methods in diagnosis of Buerger disease: A case report. Medicine (Baltimore). 97, e12979. [CrossRef]
- Meyer, J., Gorbach, A. M., Liu, W. M., Medic, N., Young, M., Nelson, C., Arceo, S., Desai, A., Metcalfe, D. D. and Komarow, H. D. (2013) Mast cell dependent vascular changes associated with an acute response to cold immersion in primary contact urticaria. PLoS One. 8, e56773. [CrossRef]
- Molnár, B., Molnár, E., Fazekas, R., Gánti, B., Mikecs, B. and Vág, J. (2019) Assessment of Palatal Mucosal Wound Healing Following Connective-Tissue Harvesting by Laser Speckle Contrast Imaging: An Observational Case Series Study. Int J Periodontics Restorative Dent. 39, e64-e70. [CrossRef]
- Ruaro, B., Bruni, C., Wade, B., Baratella, E., Confalonieri, P., Antonaglia, C., Geri, P., Biolo, M., Confalonieri, M. and Salton, F. (2021) Laser Speckle Contrast Analysis: Functional Evaluation of Microvascular Damage in Connective Tissue Diseases. Is There Evidence of Correlations With Organ Involvement, Such as Pulmonary Damage? Front Physiol. 12, 710298. [CrossRef]
- Sulli, A., Hysa, E., Cere, A., Lalli, F., Pinelli, A., Sammori, S., Gotelli, E., Pizzorni, C., Malfait, F., Castori, M., Smith, V. and Cutolo, M. (2024) Microvascular status and skin thickness in adults with hypermobile Ehlers-Danlos syndrome: a pilot investigation. Clin Exp Rheumatol. 42, 682-688. [CrossRef]
- Yilmaz, O. K., Haeberle, S., Zhang, M., Fritzler, M. J., Enk, A. H. and Hadaschik, E. N. (2019) Scurfy Mice Develop Features of Connective Tissue Disease Overlap Syndrome and Mixed Connective Tissue Disease in the Absence of Regulatory T Cells. Front Immunol. 10, 881. [CrossRef]
- Ziółkowska, K., Słabón, A., Glik, J., Maj, M., Olszak, M., Mikuś-Zagórska, K., Strzelec, P., Czerny, K., Maciejowski, R., Gierek, M. and Łabuś, W. (2025) The Successful Treatment of a Patient with Ehlers-Danlos Syndrome (EDS) After an Extensive Burn Injury: A Case Report. Medicina (Kaunas). 61, 554. [CrossRef]
- Dal Canto, E., van Deursen, L., Hoek, A. G., Elders, P. J. M., den Ruijter, H. M., van der Velden, J., van Empel, V., Serne, E. H., Eringa, E. C. and Beulens, J. W. J. (2023) Microvascular endothelial dysfunction in skin is associated with higher risk of heart failure with preserved ejection fraction in women with type 2 diabetes: the Hoorn Diabetes Care System Cohort. Cardiovasc Diabetol. 22, 234. [CrossRef]
- El-Awaisi, J., Kavanagh, D. P. J. and Kalia, N. (2024) Monitoring coronary blood flow by laser speckle contrast imaging after myocardial ischaemia reperfusion injury in adult and aged mice. Front Cardiovasc Med. 11, 1358472. [CrossRef]
- Liu, H. L., Yuan, Y., Han, L., Bi, Y., Yu, W. Y. and Yu, Y. (2024) Wide dynamic range measurement of blood flow in vivo using laser speckle contrast imaging. J Biomed Opt. 29, 016009. [CrossRef]
- Pang, W., Yuan, C., Zhong, T., Huang, X., Pan, Y., Qu, J., Nie, L., Zhou, Y. and Lai, P. (2024) Diagnostic and therapeutic optical imaging in cardiovascular diseases. iScience. 27, 111216. [CrossRef]
- Woudstra, J., Mourmans, S. G. J., Vink, C. E. M., Marques, K. M. J., de Jong, E. A. M., Haddad, R. Y. R., van de Hoef, T. P., Chamuleau, S. A. J., Damman, P., Beijk, M. A. M., van Empel, V. P. M., Serne, E. H., Appelman, Y., Eringa, E. C. and consortium, I. (2024) Relationship between peripheral and intracoronary blood flow in patients with angina and nonobstructive coronary arteries. Am J Physiol Heart Circ Physiol. 327, H1086-H1097. [CrossRef]
- Betteridge, Z., Gunawardena, H., North, J., Slinn, J. and McHugh, N. (2007) Identification of a novel autoantibody directed against small ubiquitin-like modifier activating enzyme in dermatomyositis. Arthritis Rheum. 56, 3132-3137. [CrossRef]
- Abendroth, D., Schmand, J., Landgraf, R., Illner, W. D. and Land, W. (1991) Diabetic microangiopathy in type 1 (insulin-dependent) diabetic patients after successful pancreatic and kidney or solitary kidney transplantation. Diabetologia. 34 Suppl 1, S131-134. [CrossRef]
- Matheus, A. S. d. M., Clemente, E. L., de Lourdes Guimaraes Rodrigues, M., Valença, D. C. T. and Gomes, M. B. (2017) Assessment of microvascular endothelial function in type 1 diabetes using laser speckle contrast imaging. J Diabetes Complications. 31, 753-757. [CrossRef]
- Matheus, A. S. M., da Matta, M. d. F. B., Clemente, E. L. S., Rodrigues, M. L. G., Valença, D. C. T. and Gomes, M. B. (2018) Sensibility and specificity of laser speckle contrast imaging according to Endo-PAT index in type 1 diabetes. Microvasc Res. 117, 10-15. [CrossRef]
- Yu, H., Yang, C., Wang, G., Lv, J., Li, X., Qi, W. and Wang, X. (2025) Qizhi Kebitong Formula Ameliorates Sciatic Nerve Injury in Streptozocin-induced Diabetic Mice through PERK/ATF4/CHOP Endoplasmic Reticulum Stress Signaling Pathway. Curr Pharm Des. [CrossRef]
- Lamprou, S., Koletsos, N., Zografou, I., Lazaridis, A., Mintziori, G., Trakatelli, C. M., Kotsis, V., Gkaliagkousi, E., Doumas, M. and Triantafyllou, A. (2024) Skin Microvascular Dysfunction in Type 2 Diabetes Mellitus Using Laser Speckle Contrast Analysis and Association with Carotid Intima-Media Thickness. J Clin Med. 13, 4957. [CrossRef]
- Shiba, C., Shiba, T., Takahashi, M., Matsumoto, T. and Hori, Y. (2016) Relationship between glycosylated hemoglobin A1c and ocular circulation by laser speckle flowgraphy in patients with/without diabetes mellitus. Graefes Arch Clin Exp Ophthalmol. 254, 1801-1809. [CrossRef]
- Su, Z. Z. S., Ang, B. C. H., Balne, P. K., Tun, S. B. B., Htoon, H. M., Schmetterer, L., Barathi, V. A. and Agrawal, R. (2024) Effect of anti-VEGF on retinal blood flow in diabetic mice using laser speckle flowgraphy. Acta Ophthalmol. 102, e926-e934. [CrossRef]
- Tahrani, A. A., Ali, A., Raymond, N. T., Begum, S., Dubb, K., Mughal, S., Jose, B., Piya, M. K., Barnett, A. H. and Stevens, M. J. (2012) Obstructive sleep apnea and diabetic neuropathy: a novel association in patients with type 2 diabetes. Am J Respir Crit Care Med. 186, 434-441. [CrossRef]
- Unal-Cevik, I., Orhan, D., Acar-Ozen, N. P. and Mamak-Ekinci, E. B. (2021) Small Fiber Functionality in Patients with Diabetic Neuropathic Pain. Pain Med. 22, 2068-2078. [CrossRef]
- Zharkikh, E., Dremin, V., Zherebtsov, E., Dunaev, A. and Meglinski, I. (2020) Biophotonics methods for functional monitoring of complications of diabetes mellitus. J Biophotonics. 13, e202000203. [CrossRef]
- Mennes, O. A., van Netten, J. J., van Baal, J. G. and Steenbergen, W. (2019) Assessment of microcirculation in the diabetic foot with laser speckle contrast imaging. Physiol Meas. 40, 065002. [CrossRef]
- Mennes, O. A., Selles, M., van Netten, J. J., van Baal, J. G., Steenbergen, W. and Slart, R. (2020) Semi-Automatic Tracking of Laser Speckle Contrast Images of Microcirculation in Diabetic Foot Ulcers. Diagnostics (Basel). 10, 1054. [CrossRef]
- Mennes, O. A., van Netten, J. J., van Baal, J. G., Slart, R. and Steenbergen, W. (2021) The Association between Foot and Ulcer Microcirculation Measured with Laser Speckle Contrast Imaging and Healing of Diabetic Foot Ulcers. J Clin Med. 10, 3844. [CrossRef]
- Sato, T., Mito, K. and Ishii, H. (2020) Relationship between impaired parasympathetic vasodilation and hyposalivation in parotid glands associated with type 2 diabetes mellitus. Am J Physiol Regul Integr Comp Physiol. 318, R940-R949. [CrossRef]
- Yen, S., Wang, Y. and Liao, L. D. (2024) Exploring the translational impact of type 1 diabetes on cerebral neurovascular function through ECoG-LSCI. APL Bioeng. 8, 036108. [CrossRef]
- Hanaguri, J., Yokota, H., Watanabe, M., Yamagami, S., Kushiyama, A., Kuo, L. and Nagaoka, T. (2021) Retinal blood flow dysregulation precedes neural retinal dysfunction in type 2 diabetic mice. Sci Rep. 11, 18401. [CrossRef]
- Nitta, F., Kunikata, H., Aizawa, N., Omodaka, K., Shiga, Y., Yasuda, M. and Nakazawa, T. (2014) The effect of intravitreal bevacizumab on ocular blood flow in diabetic retinopathy and branch retinal vein occlusion as measured by laser speckle flowgraphy. Clin Ophthalmol. 8, 1119-1127. [CrossRef]
- Patel, D. D. and Lipinski, D. M. (2020) Validating a low-cost laser speckle contrast imaging system as a quantitative tool for assessing retinal vascular function. Sci Rep. 10, 7177. [CrossRef]
- Barsotti, S., d'Ascanio, A., Valentina, V., Chiara, S., Silvia, B., Laura, A., Mosca, M. and Della Rossa, A. (2020) Is there a role for laser speckle contrast analysis (LASCA) in predicting the outcome of digital ulcers in patients with systemic sclerosis? Clin Rheumatol. 39, 69-75. [CrossRef]
- Di Battista, M., Della Rossa, A., Da Rio, M., De Mattia, G., Morganti, R. and Mosca, M. (2023) Preliminary Clinical and Laser Speckle Contrast Analysis Data on Selexipag Efficacy for the Treatment of Digital Vasculopathy in Systemic Sclerosis. J Rheumatol. 50, 1029-1031. [CrossRef]
- Di Battista, M., Della Rossa, A. and Mosca, M. (2024) Long-Term Data on Efficacy and Safety of Selexipag for Digital Systemic Sclerosis Vasculopathy. J Rheumatol. 51, 899-903. [CrossRef]
- Gigante, A., Villa, A. and Rosato, E. (2021) Laser speckle contrast analysis predicts major vascular complications and mortality of patients with systemic sclerosis. Rheumatology (Oxford). 60, 1850-1857. [CrossRef]
- Marjanovic, E., Moore, T. L., Manning, J. B., Dinsdale, G., Wilkinson, S., Dickinson, M. R., Herrick, A. L. and Murray, A. K. (2020) Systemic sclerosis-related digital calcinosis; a pilot study of cutaneous oxygenation and perfusion. Rheumatology (Oxford). 59, 3573-3575. [CrossRef]
- Pauling, J. D., Hackett, N., Guida, A. and Merkel, P. A. (2020) Performance of laser-derived imaging for assessing digital perfusion in clinical trials of systemic sclerosis-related digital vasculopathy: A systematic literature review. Semin Arthritis Rheum. 50, 1114-1130. [CrossRef]
- Ruaro, B., Paolino, S., Pizzorni, C., Cutolo, M. and Sulli, A. (2017) Assessment of treatment effects on digital ulcer and blood perfusion by laser speckle contrast analysis in a patient affected by systemic sclerosis. Reumatismo. 69, 134-136. [CrossRef]
- Barcelos, A., Tibirica, E. and Lamas, C. (2018) Evaluation of microvascular endothelial function and capillary density in patients with infective endocarditis using laser speckle contrast imaging and video-capillaroscopy. Microvasc Res. 118, 61-68. [CrossRef]
- Puissant, C., Abraham, P., Durand, S., Humeau-Heurtier, A., Faure, S., Leftheriotis, G., Rousseau, P. and Mahe, G. (2013) Reproducibility of non-invasive assessment of skin endothelial function using laser Doppler flowmetry and laser speckle contrast imaging. PLoS One. 8, e61320. [CrossRef]
- Souza, E. G., De Lorenzo, A., Huguenin, G., Oliveira, G. M. and Tibirica, E. (2014) Impairment of systemic microvascular endothelial and smooth muscle function in individuals with early-onset coronary artery disease: studies with laser speckle contrast imaging. Coron Artery Dis. 25, 23-28. [CrossRef]
- Verri, V., Brandao, A. and Tibirica, E. (2015) The evaluation of penile microvascular endothelial function using laser speckle contrast imaging in healthy volunteers. Microvasc Res. 99, 96-101. [CrossRef]
- Hellmann, M., Imbert, B. and Cracowski, J. L. (2019) Microvascular imaging of primary erythromelalgia. Pol Arch Intern Med. 129, 632-633. [CrossRef]
- Appelman, B., Charlton, B. T., Goulding, R. P., Kerkhoff, T. J., Breedveld, E. A., Noort, W., Offringa, C., Bloemers, F. W., van Weeghel, M., Schomakers, B. V., Coelho, P., Posthuma, J. J., Aronica, E., Joost Wiersinga, W., van Vugt, M. and Wüst, R. C. I. (2024) Muscle abnormalities worsen after post-exertional malaise in long COVID. Nat Commun. 15, 17. [CrossRef]
- Basak, K., Manjunatha, M. and Dutta, P. K. (2012) Review of laser speckle-based analysis in medical imaging. Med Biol Eng Comput. 50, 547-558. [CrossRef]
- Cutolo, M., Vanhaecke, A., Ruaro, B., Deschepper, E., Ickinger, C., Melsens, K., Piette, Y., Trombetta, A. C., De Keyser, F., Smith, V. and EULAR Study Group on Microcirculation in Rheumatic Diseases. (2018) Is laser speckle contrast analysis (LASCA) the new kid on the block in systemic sclerosis? A systematic literature review and pilot study to evaluate reliability of LASCA to measure peripheral blood perfusion in scleroderma patients. Autoimmun Rev. 17, 775-780. [CrossRef]
- Nadort, A., Kalkman, K., van Leeuwen, T. G. and Faber, D. J. (2016) Quantitative blood flow velocity imaging using laser speckle flowmetry. Sci Rep. 6, 25258. [CrossRef]
- Vennemann, P., Lindken, R. and Westerweel, J. (2007) In vivo whole-field blood velocity measurement techniques. Exp Fluids. 42, 495-511. [CrossRef]
- Fan, N., Wang, P., Tang, L. and Liu, X. (2015) Ocular Blood Flow and Normal Tension Glaucoma. Biomed Res Int. 2015, 308505. [CrossRef]
- Vinnett, A., Kandukuri, J., Le, C., Cho, K. A., Sinha, A., Asanad, S., Thompson, G., Chen, V., Rege, A. and Saeedi, O. J. (2022) Dynamic Alterations in Blood Flow in Glaucoma Measured with Laser Speckle Contrast Imaging. Ophthalmol Glaucoma. 5, 250-261. [CrossRef]
- Gu, C., Li, A. and Yu, L. (2021) Diagnostic performance of laser speckle flowgraphy in glaucoma: a systematic review and meta-analysis. Int Ophthalmol. 41, 3877-3888. [CrossRef]
- Kiyota, N., Kunikata, H., Shiga, Y., Omodaka, K. and Nakazawa, T. (2018) Ocular microcirculation measurement with laser speckle flowgraphy and optical coherence tomography angiography in glaucoma. Acta Ophthalmol. 96, e485-e492. [CrossRef]
- Fulop, G. A., Ahire, C., Csipo, T., Tarantini, S., Kiss, T., Balasubramanian, P., Yabluchanskiy, A., Farkas, E., Toth, A., Nyúl-Tóth, Á., Toth, P., Csiszar, A. and Ungvari, Z. (2019) Cerebral venous congestion promotes blood-brain barrier disruption and neuroinflammation, impairing cognitive function in mice. Geroscience. 41, 575-589. [CrossRef]
- Gkaliagkousi, E., Lazaridis, A., Anyfanti, P., Stavropoulos, K., Imprialos, K., Triantafyllou, A., Mastrogiannis, K., Douma, S. and Doumas, M. (2022) Assessment of skin microcirculation in primary aldosteronism: impaired microvascular responses compared to essential hypertensives and normotensives. J Hum Hypertens. 36, 1066-1071. [CrossRef]
- Lorenzo, A., Santos, E. M. D., Bello Moreira, A. S., Huguenin, G. V. B. and Tibirica, E. (2021) Dietary supplementation with whey protein improves systemic microvascular function in heart failure patients: a pilot study. Braz J Med Biol Res. 54, e10577. [CrossRef]
- Marino, P., de Oliveira Lopes, G., Pereira Borges, J., Carolina Terra Cola, M., Arkader Kopiler, D. and Tibirica, E. (2018) Evaluation of systemic microvascular reactivity in adults with congenital heart disease. Congenit Heart Dis. 13, 978-987. [CrossRef]
- Mourmans, S. G. J., Achten, A., Hermans, R., Scheepers, M. J. E., D'Alessandro, E., Swennen, G., Woudstra, J., Appelman, Y., Goor, H. V., Schalkwijk, C., Knackstedt, C., Weerts, J., Eringa, E. C. and van Empel, V. P. M. (2025) The effect of empagliflozin on peripheral microvascular dysfunction in patients with heart failure with preserved ejection fraction. Cardiovasc Diabetol. 24, 182. [CrossRef]
- Calcinaghi, N., Wyss, M. T., Jolivet, R., Singh, A., Keller, A. L., Winnik, S., Fritschy, J. M., Buck, A., Matter, C. M. and Weber, B. (2013) Multimodal imaging in rats reveals impaired neurovascular coupling in sustained hypertension. Stroke. 44, 1957-1964. [CrossRef]
- Crahim, V., Verri, V., De Lorenzo, A. and Tibirica, E. (2024) Reduced systemic microvascular function in patients with resistant hypertension and microalbuminuria: an observational study. J Hum Hypertens. 38, 806-813. [CrossRef]
- Lazaridis, A., Triantafyllou, A., Dipla, K., Dolgyras, P., Koletsos, N., Anyfanti, P., Aslanidis, S., Douma, S. and Gkaliagkousi, E. (2022) Skin microvascular function, as assessed with laser speckle contrast imaging, is impaired in untreated essential and masked hypertension. Hypertens Res. 45, 445-454. [CrossRef]
- Liu, L., He, W., Liu, S., Li, Y., Wang, P., Yan, F., Yang, W., Yang, Y. and Guo, J. (2025) The flavonoid of Dracocephalum heterophyllum Benth. ameliorates cerebral small vessel disease by inhibiting the autophagy via Angs-Tie2 signaling pathway. Front Pharmacol. 16, 1500307. [CrossRef]
- Mottard, N., Berkowitz, D. E. and Santhanam, L. (2020) Assessing Renal Microvascular Reactivity by Laser Speckle-Contrast Imaging in Angiotensin-II-Treated Mice. Int J Nephrol Renovasc Dis. 13, 45-51. [CrossRef]
- Pappelis, K., Choritz, L. and Jansonius, N. M. (2020) Microcirculatory model predicts blood flow and autoregulation range in the human retina: in vivo investigation with laser speckle flowgraphy. Am J Physiol Heart Circ Physiol. 319, H1253-H1273. [CrossRef]
- Tamplin, M. R., Broadhurst, K. A., Vitale, A. H., Hashimoto, R., Kardon, R. H. and Grumbach, I. M. (2021) Longitudinal Testing of Retinal Blood Flow in a Mouse Model of Hypertension by Laser Speckle Flowgraphy. Transl Vis Sci Technol. 10, 16. [CrossRef]
- Zou, G., Yu, R., Zhao, D., Duan, Z., Guo, S., Wang, T., Ma, L., Yuan, Z. and Yu, C. (2024) Celastrol ameliorates energy metabolism dysfunction of hypertensive rats by dilating vessels to improve hemodynamics. J Nat Med. 78, 191-207. [CrossRef]
- Pai, V., Bileck, A., Hommer, N., Janku, P., Lindner, T., Kauer, V., Rumpf, B., Haslacher, H., Hagn, G., Meier-Menches, S. M., Schmetterer, L., Schmidl, D., Gerner, C. and Garhöfer, G. (2024) Impaired retinal oxygen metabolism and perfusion are accompanied by plasma protein and lipid alterations in recovered COVID-19 patients. Sci Rep. 14, 8395. [CrossRef]
- Frodlund, M., Wetterö, J., Dahle, C., Dahlström, Ö., Skogh, T., Rönnelid, J. and Sjöwall, C. (2020) Longitudinal anti-nuclear antibody (ANA) seroconversion in systemic lupus erythematosus: a prospective study of Swedish cases with recent-onset disease. Clin Exp Immunol. 199, 245-254. [CrossRef]
- Koletsos, N., Gkaliagkousi, E., Lazaridis, A., Triantafyllou, A., Anyfanti, P., Dolgyras, P., Dipla, K., Galanopoulou, V., Aslanidis, S. and Douma, S. (2021) Skin microvascular dysfunction in systemic lupus erythematosus patients with and without cardiovascular risk factors. Rheumatology (Oxford). 60, 2834-2841. [CrossRef]
- Koletsos, N., Lazaridis, A., Triantafyllou, A., Anyfanti, P., Lamprou, S., Stoimeni, A., Papadopoulos, N. G., Koravou, E. E. and Gkaliagkousi, E. (2024) Accumulation of Microvascular Target Organ Damage in Systemic Lupus Erythematosus Patients Is Associated with Increased Cardiovascular Risk. J Clin Med. 13, 2140. [CrossRef]
- Ruaro, B., Sulli, A., Casabella, A., Pizzorni, C., Paolino, S., Smith, V. and Cutolo, M. (2021) Peripheral blood perfusion in patients with systemic lupus erythematosus and in primary Raynaud's phenomenon. Eur J Rheumatol. 8, 7-11. [CrossRef]
- Dönmez-Demir, B., Yemisci, M., Kılıç, K., Gürsoy-Özdemir, Y., Söylemezoğlu, F., Moskowitz, M. and Dalkara, T. (2018) Microembolism of single cortical arterioles can induce spreading depression and ischemic injury; a potential trigger for migraine and related MRI lesions. Brain Res. 1679, 84-90. [CrossRef]
- Eikermann-Haerter, K., Lee, J. H., Yuzawa, I., Liu, C. H., Zhou, Z., Shin, H. K., Zheng, Y., Qin, T., Kurth, T., Waeber, C., Ferrari, M. D., van den Maagdenberg, A. M., Moskowitz, M. A. and Ayata, C. (2012) Migraine mutations increase stroke vulnerability by facilitating ischemic depolarizations. Circulation. 125, 335-345. [CrossRef]
- Eikermann-Haerter, K., Arbel-Ornath, M., Yalcin, N., Yu, E. S., Kuchibhotla, K. V., Yuzawa, I., Hudry, E., Willard, C. R., Climov, M., Keles, F., Belcher, A. M., Sengul, B., Negro, A., Rosen, I. A., Arreguin, A., Ferrari, M. D., van den Maagdenberg, A. M. J. M., Bacskai, B. J. and Ayata, C. (2015) Abnormal synaptic Ca(2+) homeostasis and morphology in cortical neurons of familial hemiplegic migraine type 1 mutant mice. Ann Neurol. 78, 193-210. [CrossRef]
- Staehr, C., Rajanathan, R., Postnov, D. D., Hangaard, L., Bouzinova, E. V., Lykke-Hartmann, K., Bach, F. W., Sandow, S. L., Aalkjaer, C. and Matchkov, V. V. (2020) Abnormal neurovascular coupling as a cause of excess cerebral vasodilation in familial migraine. Cardiovasc Res. 116, 2009-2020. [CrossRef]
- Unal-Cevik, I. and Yilmaz, E. (2020) Quantification of Trigeminovascular Hypersensitivity Using Laser Speckle Contrast Analysis in a Patient With Chronic Migraine. Pain Pract. 20, 204-210. [CrossRef]
- Xing, Z., Peng, F., Chen, Y., Wan, F., Peng, C. and Li, D. (2024) Metabolomic profiling integrated with molecular exploring delineates the action of Ligusticum chuanxiong hort. on migraine. Phytomedicine. 134, 155977. [CrossRef]
- Seifart, U. (2023) Post-COVID-More than chronic fatigue? Herz. 48, 229-233. [CrossRef]
- Lin, P. W., Chiu, L. W., Chang, C. T. and Lin, H. C. (2024) Impaired blood flow of optic nerve head in patients with severe obstructive sleep apnea/hypopnea syndrome. J Sleep Res, e14422. [CrossRef]
- Hijazi, M. M., Buchmann, S. J., Sedghi, A., Illigens, B. M., Reichmann, H., Schackert, G. and Siepmann, T. (2020) Assessment of cutaneous axon-reflex responses to evaluate functional integrity of autonomic small nerve fibers. Neurol Sci. 41, 1685-1696. [CrossRef]
- Noda, T., Noda, K., Hirooka, K., Kase, S. and Ishida, S. (2022) Subretinal fluid accumulation in a patient with polycythemia vera after receiving a prostaglandin I2 analogue treatment. Am J Ophthalmol Case Rep. 27, 101568. [CrossRef]
- Buch, J., Karagaiah, P., Raviprakash, P., Patil, A., Kroumpouzos, G., Kassir, M. and Goldust, M. (2021) Noninvasive diagnostic techniques of port wine stain. J Cosmet Dermatol. 20, 2006-2014. [CrossRef]
- Barr, L. C., Pudwell, J. and Smith, G. N. (2021) Postpartum microvascular functional alterations following severe preeclampsia. Am J Physiol Heart Circ Physiol. 320, H1393-H1402. [CrossRef]
- Cumsille, P., Lara, E., Verdugo-Hernandez, P., Acurio, J. and Escudero, C. (2022) A robust quantitative approach for laser speckle contrast imaging perfusion analysis revealed anomalies in the brain blood flow in offspring mice of preeclampsia. Microvasc Res. 144, 104418. [CrossRef]
- Sato, T., Sugawara, J., Aizawa, N., Iwama, N., Takahashi, F., Nakamura-Kurakata, M., Saito, M., Sugiyama, T., Kunikata, H., Nakazawa, T. and Yaegashi, N. (2017) Longitudinal changes of ocular blood flow using laser speckle flowgraphy during normal pregnancy. PLoS One. 12, e0173127. [CrossRef]
- Chizari, A., Schaap, M. J., Knop, T., Boink, Y. E., Seyger, M. M. B. and Steenbergen, W. (2021) Handheld versus mounted laser speckle contrast perfusion imaging demonstrated in psoriasis lesions. Sci Rep. 11, 16646. [CrossRef]
- Chizari, A., Schaap, M. J., Knop, T., Seyger, M. M. B. and Steenbergen, W. (2025) Mitigation of Motion Artifacts in Handheld Laser Speckle Contrast Imaging Illustrated on Psoriasis Lesions. IEEE Trans Biomed Eng. 72, 70-78. [CrossRef]
- Margouta, A., Anyfanti, P., Lazaridis, A., Nikolaidou, B., Mastrogiannis, K., Malliora, A., Patsatsi, A., Triantafyllou, A., Douma, S., Doumas, M. and Gkaliagkousi, E. (2022) Blunted Microvascular Reactivity in Psoriasis Patients in the Absence of Cardiovascular Disease, as Assessed by Laser Speckle Contrast Imaging. Life (Basel). 12, 1796. [CrossRef]
- Schaap, M. J., Chizari, A., Knop, T., Groenewoud, H. M. M., van Erp, P. E. J., de Jong, E. M. G. J., Steenbergen, W. and Seyger, M. M. B. (2022) Perfusion measured by laser speckle contrast imaging as a predictor for expansion of psoriasis lesions. Skin Res Technol. 28, 104-110. [CrossRef]
- Trombetta, A. C., Pizzorni, C., Ruaro, B., Paolino, S., Sulli, A., Smith, V. and Cutolo, M. (2016) Effects of Longterm Treatment with Bosentan and Iloprost on Nailfold Absolute Capillary Number, Fingertip Blood Perfusion, and Clinical Status in Systemic Sclerosis. J Rheumatol. 43, 2033-2041. [CrossRef]
- Aleksiev, T., Ivanova, Z., Dobrev, H. and Atanasov, N. (2021) Application of a novel finger temperature device in the assessment of subjects with Raynaud's phenomenon. Skin Res Technol. 27, 1110-1115. [CrossRef]
- Herrick, A. L., Oogarah, P. K., Freemont, A. J., Marcuson, R., Haeney, M. and Jayson, M. I. (1994) Vasculitis in patients with systemic sclerosis and severe digital ischaemia requiring amputation. Ann Rheum Dis. 53, 323-326. [CrossRef]
- Huntgeburth, M., Kiessling, J., Weimann, G., Wilberg, V., Saleh, S., Hunzelmann, N. and Rosenkranz, S. (2018) Riociguat for the Treatment of Raynaud's Phenomenon: A Single-Dose, Double-Blind, Randomized, Placebo-Controlled Cross-Over Pilot Study (DIGIT). Clin Drug Investig. 38, 1061-1069. [CrossRef]
- van Roon, A. M., Kuijpers, M., van de Zande, S. C., Abdulle, A. E., van Roon, A. M., Bos, R., Bouma, W., Klinkenberg, T. J., Bootsma, H., DeJongste, M. J. L., Mariani, M. A., Smit, A. J. and Mulder, D. J. (2020) Treatment of resistant Raynaud's phenomenon with single-port thoracoscopic sympathicotomy: a novel minimally invasive endoscopic technique. Rheumatology (Oxford). 59, 1021-1025. [CrossRef]
- Rosato, E., Borghese, F., Pisarri, S. and Salsano, F. (2009) Laser Doppler perfusion imaging is useful in the study of Raynaud's phenomenon and improves the capillaroscopic diagnosis. J Rheumatol. 36, 2257-2263. [CrossRef]
- Rosato, E., Molinaro, I., Rossi, C., Pisarri, S. and Salsano, F. (2011) The combination of laser Doppler perfusion imaging and photoplethysmography is useful in the characterization of scleroderma and primary Raynaud's phenomenon. Scand J Rheumatol. 40, 292-298. [CrossRef]
- Anyfanti, P., Gavriilaki, E., Nikolaidou, B., Triantafyllou, A., Dolgyras, P., Zarifis, H., Lazaridis, A., Koletsos, N., Douma, S. and Gkaliagkousi, E. (2022) Impaired skin microcirculation dynamics in patients with rheumatoid arthritis: association with arterial stiffness and coronary microvascular perfusion. J. Hypertens. 40(Supple 1), e51-e52. [CrossRef]
- Anyfanti, P., Gavriilaki, E., Dolgyras, P., Nikolaidou, B., Dimitriadou, A., Lazaridis, A., Mastrogiannis, K., Koletsos, N., Triantafyllou, A., Dimitroulas, T., Aslanidis, S., Doumas, M., Douma, S. and Gkaliagkousi, E. (2023) Skin microcirculation dynamics are impaired in patients with rheumatoid arthritis and no cardiovascular comorbidities. Clin Exp Rheumatol. 41, 1507-1515. [CrossRef]
- Dunn, J. F., Forrester, K. R., Martin, L., Tulip, J. and Bray, R. C. (2011) A transmissive laser speckle imaging technique for measuring deep tissue blood flow: an example application in finger joints. Lasers Surg Med. 43, 21-28. [CrossRef]
- Vanden Bulcke, M., Vanhaecke, A., Deschepper, E., Cutolo, M., Jacques, P. and Smith, V. (2022) Laser speckle contrast analysis in rheumatoid arthritis: a pilot study. Clin Exp Rheumatol. 40, 129-134. [CrossRef]
- Paolino, S., Goegan, F., Cimmino, M. A., Casabella, A., Pizzorni, C., Patane, M., Schenone, C., Tomatis, V., Sulli, A., Gotelli, E., Smith, V. and Cutolo, M. (2020) Advanced microvascular damage associated with occurence of sarcopenia in systemic sclerosis patients: results from a retrospective cohort study. Clin Exp Rheumatol. 38 Suppl 125, 65-72.
- Qi, H., Zhang, X., Zhang, Z., Gao, Y., Tian, D., Zhao, G., Xie, Z., Zeng, J., Zhang, L., Zeng, N. and Yang, R. (2025) The extract of chrysanthemum flos mitigates post-stroke sarcopenia by inhibiting PANoptosis and restoring muscle homeostasis. Phytomedicine. 142, 156784. [CrossRef]
- Qi, H., Gao, Y., Zhang, Z., Zhang, X., Tian, D., Jiang, Y., Zhang, L., Zeng, N. and Yang, R. (2025) HouShiHeiSan attenuates sarcopenia in middle cerebral artery occlusion (MCAO) rats. J Ethnopharmacol. 337, 118917. [CrossRef]
- De Backer, D., Donadello, K., Sakr, Y., Ospina-Tascon, G., Salgado, D., Scolletta, S. and Vincent, J. L. (2013) Microcirculatory alterations in patients with severe sepsis: impact of time of assessment and relationship with outcome. Crit Care Med. 41, 791-799. [CrossRef]
- Hsieh, M. C., Hu, J. J., Lin, Y. R., Li, S. Y., Hsieh, P. Y., Shing Ching, C. T. and Liao, L. D. (2024) Improving the early diagnosis and clinical outcomes of shock patients via laser speckle contrast imaging assessment of peripheral hemodynamics. iScience. 27, 111307. [CrossRef]
- Ruan, Z., Li, R., Dong, W., Cui, Z., Yang, H. and Ren, R. (2022) Laser speckle contrast imaging to monitor microcirculation: An effective method to predict outcome in patients with sepsis and septic shock. Front Bioeng Biotechnol. 10, 1067739. [CrossRef]
- Sand, C. A., Starr, A., Wilder, C. D., Rudyk, O., Spina, D., Thiemermann, C., Treacher, D. F. and Nandi, M. (2015) Quantification of microcirculatory blood flow: a sensitive and clinically relevant prognostic marker in murine models of sepsis. J Appl Physiol (1985). 118, 344-354. [CrossRef]
- Panda, A., Revadi, G., Sharma, J. P., Pakhare, A., Singhai, A., Joshi, R. and Saigal, S. (2022) On Admission, Microcirculation Abnormality is an Independent Predictor of Sepsis and Sepsis-related Mortality: A Hospital-based Study. Indian J Crit Care Med. 26, 294-301. [CrossRef]
- Bian, E. J., Chen, C. W., Cheng, C. M., Kuan, C. Y. and Sun, Y. Y. (2023) Impaired post-stroke collateral circulation in sickle cell anemia mice. Front Neurol. 14, 1215876. [CrossRef]
- Minniti, C. P., Gorbach, A. M., Xu, D., Hon, Y. Y., Delaney, K. M., Seidel, M., Malik, N., Peters-Lawrence, M., Cantilena, C., Nichols, J. S., Mendelsohn, L., Conrey, A., Grimes, G. and Kato, G. J. (2014) Topical sodium nitrite for chronic leg ulcers in patients with sickle cell anaemia: a phase 1 dose-finding safety and tolerability trial. Lancet Haematol. 1, e95-e103. [CrossRef]
- Minniti, C. P., Delaney, K. M., Gorbach, A. M., Xu, D., Lee, C. C., Malik, N., Koroulakis, A., Antalek, M., Maivelett, J., Peters-Lawrence, M., Novelli, E. M., Lanzkron, S. M., Axelrod, K. C. and Kato, G. J. (2014) Vasculopathy, inflammation, and blood flow in leg ulcers of patients with sickle cell anemia. Am J Hematol. 89, 1-6. [CrossRef]
- Armitage, G. A., Todd, K. G., Shuaib, A. and Winship, I. R. (2010) Laser speckle contrast imaging of collateral blood flow during acute ischemic stroke. J Cereb Blood Flow Metab. 30, 1432-1436. [CrossRef]
- Chen, Y., Wang, L., Zhou, Y., Wang, Y., Qin, W., Wang, M., Liu, B., Tian, Q., Xu, H., Shen, H. and Zheng, C. (2025) Exendin-4 improves cerebral ischemia by relaxing microvessels, rapidly increasing cerebral blood flow after reperfusion. Basic Res Cardiol. 120, 423-441. [CrossRef]
- Hecht, N., Muller, M. M., Sandow, N., Pinczolits, A., Vajkoczy, P. and Woitzik, J. (2016) Infarct prediction by intraoperative laser speckle imaging in patients with malignant hemispheric stroke. J Cereb Blood Flow Metab. 36, 1022-1032. [CrossRef]
- Hu, J., Li, Y., Quan, X., Han, Y., Chen, J., Yuan, M., Chen, Y., Zhou, M., Yu, E., Zhou, J., Wang, D., Wang, R. and Zhao, Y. (2025) Shengui Sansheng San alleviates the worsening of blood-brain barrier integrity resulted from delayed tPA administration through VIP/VIPR1 pathway. Chin Med. 20, 38. [CrossRef]
- Huang, Y. X., Mahler, S., Abedi, A., Tyszka, J. M., Lo, Y. T., Lyden, P. D., Russin, J., Liu, C. and Yang, C. (2024) Correlating stroke risk with non-invasive cerebrovascular perfusion dynamics using a portable speckle contrast optical spectroscopy laser device. Biomed Opt Express. 15, 6083-6097. [CrossRef]
- Li, Y., Zhu, S., Yuan, L., Lu, H., Li, H. and Tong, S. (2013) Predicting the ischemic infarct volume at the first minute after occlusion in rodent stroke model by laser speckle imaging of cerebral blood flow. J Biomed Opt. 18, 76024. [CrossRef]
- Liu, Q., Chen, S., Soetikno, B., Liu, W., Tong, S. and Zhang, H. F. (2018) Monitoring Acute Stroke in Mouse Model Using Laser Speckle Imaging-Guided Visible-Light Optical Coherence Tomography. IEEE Trans Biomed Eng. 65, 2136-2142. [CrossRef]
- Lu, H., Li, Y., Yuan, L., Li, H., Lu, X. and Tong, S. (2014) Induction and imaging of photothrombotic stroke in conscious and freely moving rats. J Biomed Opt. 19, 96013. [CrossRef]
- Roberts, L., Coutts, G., Dickie, B. R., Smith, C. J., South, K. and Allan, S. M. (2025) Comparison of the Novel Thrombolytic Constitutively Active ADAMTS13 With Clinical Thrombolytics in a Murine Stroke Model. Stroke. 56, 1589-1595. [CrossRef]
- Taha, B. A., Kadhim, A. C., Addie, A. J., Al-Jubouri, Q., Azzahrani, A. S., Haider, A. J., Alkawaz, A. N. and Arsad, N. (2025) Optical Spectroscopy of Cerebral Blood Flow for Tissue Interrogation in Ischemic Stroke Diagnosis. ACS Chem Neurosci. 16, 895-907. [CrossRef]
- Yosovich, A., Agdarov, S., Beiderman, Y., Beiderman, Y. and Zalevsky, Z. (2025) Speckle pattern analysis with deep learning for low-cost stroke detection: a phantom-based feasibility study. J Biomed Opt. 30, 056003. [CrossRef]
- Zhu, L., Huang, R., Feng, J. R., Zhang, M., Huang, X. J., Chen, Z., Wang, W. and Chen, Y. (2025) Shexiang Tongxin Dropping Pills attenuate ischemic microvascular dysfunction via suppressing P66Shc-mediated mitochondrial respiration deficits. J Ethnopharmacol. 346, 119664. [CrossRef]
- Neulen, A., Pantel, T., Kosterhon, M., Kramer, A., Kunath, S., Petermeyer, M., Moosmann, B., Lotz, J., Kantelhardt, S. R., Ringel, F. and Thal, S. C. (2019) Neutrophils mediate early cerebral cortical hypoperfusion in a murine model of subarachnoid haemorrhage. Sci Rep. 9, 8460. [CrossRef]
- Cutolo, C. A., Cere, A., Toma, P., Cannavacciuolo, T., Toma, C., Balito, S., Gerli, V., Smith, V., Sulli, A., Paolino, S., Gotelli, E., Traverso, C. E., Nicolò, M., Cutolo, M. and Hysa, E. (2024) Peripheral and ocular microvascular alterations in systemic sclerosis: observations from capillaroscopic assessments, perfusion peripheral analysis, and optical coherence tomography angiography. Rheumatol Int. 44, 107-118. [CrossRef]
- Gonzalez, J. M. and Valenzuela, A. (2024) Vascular, Soft Tissue, and Musculoskeletal Imaging in Systemic Sclerosis. Rheum Dis Clin North Am. 50, 661-681. [CrossRef]
- Lambrecht, V., Cutolo, M., De Keyser, F., Decuman, S., Ruaro, B., Sulli, A., Deschepper, E. and Smith, V. (2016) Reliability of the quantitative assessment of peripheral blood perfusion by laser speckle contrast analysis in a systemic sclerosis cohort. Ann Rheum Dis. 75, 1263-1264. [CrossRef]
- Ruaro, B., Sulli, A., Alessandri, E., Pizzorni, C., Ferrari, G. and Cutolo, M. (2014) Laser speckle contrast analysis: a new method to evaluate peripheral blood perfusion in systemic sclerosis patients. Ann Rheum Dis. 73, 1181-1185. [CrossRef]
- Sulli, A., Ruaro, B. and Cutolo, M. (2014) Evaluation of blood perfusion by laser speckle contrast analysis in different areas of hands and face in patients with systemic sclerosis. Ann Rheum Dis. 73, 2059-2061. [CrossRef]
- Wilkinson, J. D., Leggett, S. A., Marjanovic, E. J., Moore, T. L., Allen, J., Anderson, M. E., Britton, J., Buch, M. H., Del Galdo, F., Denton, C. P., Dinsdale, G., Griffiths, B., Hall, F., Howell, K., MacDonald, A., McHugh, N. J., Manning, J. B., Pauling, J. D., Roberts, C., Shipley, J. A., Herrick, A. L. and Murray, A. K. (2018) A Multicenter Study of the Validity and Reliability of Responses to Hand Cold Challenge as Measured by Laser Speckle Contrast Imaging and Thermography: Outcome Measures for Systemic Sclerosis-Related Raynaud's Phenomenon. Arthritis Rheumatol. 70, 903-911. [CrossRef]
- Willems, S., Smith, V., Wallaert, S., Gotelli, E., Du Four, T., Wyckstandt, K., Cere, A. and Cutolo, M. (2023) Description of Peripheral Blood Perfusion by Laser Speckle Contrast Analysis (LASCA) in 'Early' versus 'Clinically Overt' Systemic Sclerosis in Routine Clinics. Diagnostics (Basel). 13, 1566. [CrossRef]
- Rosato, E., Rossi, C., Molinaro, I., Giovannetti, A., Pisarri, S. and Salsano, F. (2011) Laser Doppler perfusion imaging in systemic sclerosis impaired response to cold stimulation involves digits and hand dorsum. Rheumatology (Oxford). 50, 1654-1658. [CrossRef]
- Langri, D. S. and Sunar, U. (2023) Non-Invasive Continuous Optical Monitoring of Cerebral Blood Flow after Traumatic Brain Injury in Mice Using Fiber Camera-Based Speckle Contrast Optical Spectroscopy. Brain Sci. 13, 1365. [CrossRef]
- Liu, H., He, J., Zhang, Z., Liu, L., Huo, G., Sun, X. and Cheng, C. (2018) Evolution of cerebral perfusion in the peri-contusional cortex in mice revealed by in vivo laser speckle imaging after traumatic brain injury. Brain Res. 1700, 118-125. [CrossRef]
- Kofman, I. and Abookasis, D. (2015) Dual-wavelength laser speckle imaging for monitoring brain metabolic and hemodynamic response to closed head traumatic brain injury in mice. J Biomed Opt. 20, 106009. [CrossRef]
- Wang, G., Zhang, Y. P., Gao, Z., Shields, L. B. E., Li, F., Chu, T., Lv, H., Moriarty, T., Xu, X. M., Yang, X., Shields, C. B. and Cai, J. (2018) Pathophysiological and behavioral deficits in developing mice following rotational acceleration-deceleration traumatic brain injury. Dis Model Mech. 11, dmm030387. [CrossRef]
- Bari, F., Tóth-Szüki, V., Domoki, F. and Kálmán, J. (2005) Flow motion pattern differences in the forehead and forearm skin: Age-dependent alterations are not specific for Alzheimer's disease. Microvasc Res. 70, 121-128. [CrossRef]
- Lobanov, A. A., Irina, A. G., Andronov, S. V., Gleb, N. B., Andrey, I. P., Anatoliy, D. F., Elena, P. I., Maccarone, M. C. and Stefano, M. (2022) Can aquatic exercises contribute to the improvement of the gait stereotype function in patients with Long COVID outcomes? Eur J Transl Myol. 32. [CrossRef]
- Raia, L., Urbina, T., Gabarre, P., Bonny, V., Hariri, G., Ehrminger, S., Bigé, N., Baudel, J. L., Guidet, B., Maury, E., Joffre, J. and Ait-Oufella, H. (2022) Impaired skin microvascular endothelial reactivity in critically ill COVID-19 patients. Ann Intensive Care. 12, 51. [CrossRef]
- Sabioni, L. R., Tibirica, E., Lamas, C. C., Amorim, G. D. and De Lorenzo, A. (2020) Systemic microvascular dysfunction in COVID-19. Am J Cardiovasc Dis. 10, 386-391.
- Sabioni, L., De Lorenzo, A., Lamas, C., Muccillo, F., Castro-Faria-Neto, H. C., Estato, V. and Tibirica, E. (2021) Systemic microvascular endothelial dysfunction and disease severity in COVID-19 patients: Evaluation by laser Doppler perfusion monitoring and cytokine/chemokine analysis. Microvasc Res. 134, 104119. [CrossRef]
- Mazela, J., Merritt, A., Terry, M. H., Gregory, T. J. and Blood, A. B. (2012) Comparison of poractant alfa and lyophilized lucinactant in a preterm lamb model of acute respiratory distress. Pediatr Res. 72, 32-37. [CrossRef]
- Troiani, S., Cardona, A., Milioni, M., Monacelli, D., Verrotti, A., Gehring, M. and Ambrosio, G. (2017) Evidence of impaired microvascular dilatation in preterms with acute respiratory distress syndrome. Int J Cardiol. 241, 83-86. [CrossRef]
- Berisha, F., Feke, G. T., Trempe, C. L., McMeel, J. W. and Schepens, C. L. (2007) Retinal abnormalities in early Alzheimer's disease. Invest Ophthalmol Vis Sci. 48, 2285-2289. [CrossRef]
- Connelly, P. J., Adams, F., Tayar, Z. I. and Khan, F. (2019) Peripheral vascular responses to acetylcholine as a predictive tool for response to cholinesterase inhibitors in Alzheimer's disease. BMC Neurol. 19, 88. [CrossRef]
- Dai, S. J., Zhang, J. Y., Bao, Y. T., Zhou, X. J., Lin, L. N., Fu, Y. B., Zhang, Y. J., Li, C. Y. and Yang, Y. X. (2018) Intracerebroventricular injection of Abeta(1-42) combined with two-vessel occlusion accelerate Alzheimer's disease development in rats. Pathol Res Pract. 214, 1583-1595. [CrossRef]
- Feke, G. T., Hyman, B. T., Stern, R. A. and Pasquale, L. R. (2015) Retinal blood flow in mild cognitive impairment and Alzheimer's disease. Alzheimers Dement (Amst). 1, 144-151. [CrossRef]
- Frost, S., Kanagasingam, Y., Sohrabi, H., Vignarajan, J., Bourgeat, P., Salvado, O., Villemagne, V., Rowe, C. C., Macaulay, S. L., Szoeke, C., Ellis, K. A., Ames, D., Masters, C. L., Rainey-Smith, S., Martins, R. N. and Group, A. R. (2013) Retinal vascular biomarkers for early detection and monitoring of Alzheimer's disease. Transl Psychiatry. 3, e233. [CrossRef]
- Khalil, Z., Poliviou, H., Maynard, C. J., Beyreuther, K., Masters, C. L. and Li, Q. X. (2002) Mechanisms of peripheral microvascular dysfunction in transgenic mice overexpressing the Alzheimer's disease amyloid Abeta protein. J Alzheimers Dis. 4, 467-478. [CrossRef]
- Li, Z., Zhao, G., Qian, S., Yang, Z., Chen, X., Chen, J., Cai, C., Liang, X. and Guo, J. (2012) Cerebrovascular protection of beta-asarone in Alzheimer's disease rats: a behavioral, cerebral blood flow, biochemical and genic study. J Ethnopharmacol. 144, 305-312. [CrossRef]
- Lin, A. J., Liu, G., Castello, N. A., Yeh, J. J., Rahimian, R., Lee, G., Tsay, V., Durkin, A. J., Choi, B., LaFerla, F. M., Chen, Z., Green, K. N. and Tromberg, B. J. (2014) Optical imaging in an Alzheimer's mouse model reveals amyloid-beta-dependent vascular impairment. Neurophotonics. 1, 011005. [CrossRef]
- Liu, D., Ahmet, I., Griess, B., Tweedie, D., Greig, N. H. and Mattson, M. P. (2021) Age-related impairment of cerebral blood flow response to K(ATP) channel opener in Alzheimer's disease mice with presenilin-1 mutation. J Cereb Blood Flow Metab. 41, 1579-1591. [CrossRef]
- Liu, D., Hsueh, S. C., Tweedie, D., Price, N., Glotfelty, E., Lecca, D., Telljohann, R., deCabo, R., Hoffer, B. J. and Greig, N. H. (2024) Chronic inflammation with microglia senescence at basal forebrain: impact on cholinergic deficit in Alzheimer's brain haemodynamics. Brain Commun. 6, fcae204. [CrossRef]
- Sarkaki, A., Farbood, Y., Badavi, M., Ghadiri, A., Ghasemi Dehcheshmeh, M., Mansouri, E. and Navabi, S. P. (2018) The protective effect of betulinic acid on microvascular responsivity and protein expression in alzheimer disease induced by cerebral micro-injection of beta-amyloid and streptozotocin. Microcirculation. 25, e12503. [CrossRef]
- Ye, X., Shao, S., Wang, Y. and Su, W. (2023) Ginsenoside Rg2 alleviates neurovascular damage in 3xTg-AD mice with Alzheimer's disease through the MAPK-ERK pathway. J Chem Neuroanat. 133, 102346. [CrossRef]
- Yu, S., Hsu, C. Y., Chuang, H. Y., Yang, C. C., Lai, C. L. and Yu, H. S. (2021) Abnormalities in Cutaneous Microcirculation in Patients with Alzheimer's Disease, Mild Cognitive Impairment, and Chronic Insomnia Disorder. J Clin Med. 10, 5718. [CrossRef]
- Filer, A. D., Gardner-Medwin, J. M., Thambyrajah, J., Raza, K., Carruthers, D. M., Stevens, R. J., Liu, L., Lowe, S. E., Townend, J. N. and Bacon, P. A. (2003) Diffuse endothelial dysfunction is common to ANCA associated systemic vasculitis and polyarteritis nodosa. Ann Rheum Dis. 62, 162-167. [CrossRef]
- Fluhr, J. W., Zuberbier, T. and Darlenski, R. (2018) Noninvasive measures in atopic dermatitis. Curr Opin Allergy Clin Immunol. 18, 417-424. [CrossRef]
- Stücker, M., Heese, A., Hoffmann, K., El Gammal, C. and Altmeyer, P. (1998) Quantification of vascular dysregulation in atopic dermatitis using laser Doppler perfusion imaging. Skin Res Technol. 4, 9-13. [CrossRef]
- Ferraz, J. G., McKnight, W., Sharkey, K. A. and Wallace, J. L. (1995) Impaired vasodilatory responses in the gastric microcirculation of anesthetized rats with secondary biliary cirrhosis. Gastroenterology. 108, 1183-1191. [CrossRef]
- Stevens, S., Allen, J., Murray, A., Jones, D. and Newton, J. (2009) Microvascular optical assessment confirms the presence of peripheral autonomic dysfunction in primary biliary cirrhosis. Liver Int. 29, 1467-1472. [CrossRef]
- Asif, M., Chin, A. G. M., Lagziel, T., Klifto, K. M., Modica, A. D., Duraes, E., Caffrey, J. and Hultman, C. S. (2020) The Added Benefit of Combining Laser Doppler Imaging With Clinical Evaluation in Determining the Need for Excision of Indeterminate-Depth Burn Wounds. Cureus. 12, e8774. [CrossRef]
- Claes, K. E. Y., De Decker, I., Vyncke, T., Verbelen, J., Dhooghe, N., Monstrey, S. and Hoeksema, H. (2023) Enzymatic Debridement With Nexobrid((R))Reduces Surgery in Laser Doppler Imaging-Confirmed Deep Burns. Ann Burns Fire Disasters. 36, 347-354.
- Jan, S. N., Khan, F. A., Bashir, M. M., Nasir, M., Ansari, H. H., Shami, H. B., Nazir, U., Hanif, A. and Sohail, M. (2018) Comparison of Laser Doppler Imaging (LDI) and clinical assessment in differentiating between superficial and deep partial thickness burn wounds. Burns. 44, 405-413. [CrossRef]
- Jaskille, A. D., Ramella-Roman, J. C., Shupp, J. W., Jordan, M. H. and Jeng, J. C. (2010) Critical review of burn depth assessment techniques: part II. Review of laser doppler technology. J Burn Care Res. 31, 151-157. [CrossRef]
- Khatib, M., Jabir, S., Fitzgerald O'Connor, E. and Philp, B. (2014) A systematic review of the evolution of laser Doppler techniques in burn depth assessment. Plast Surg Int. 2014, 621792. [CrossRef]
- Owoso, T., Kankam, H. K. N., Abdulsalam, A. and Lewis, D. (2023) The Use of Laser Doppler Imaging in Nitric Acid Burns: A Case Report and Literature Review. J Burn Care Res. 44, 1440-1444. [CrossRef]
- Rozo, A., Miskovic, V., Rose, T., Keersebilck, E., Iorio, C. and Varon, C. (2022) U-Net based Mapping from Digital Images to Laser Doppler Imaging for Burn Assessment. Annu Int Conf IEEE Eng Med Biol Soc. 2022, 459-462. [CrossRef]
- Ryabkov, M. G., Peretyagin, P. V., Shestakova, S. A., Ptushko, S. S., Koshmanev, M. S., Bederina, Y. L., Potapov, A. L., Sirotkina, M. A., Gladkova, N. D. and Kiseleva, E. B. (2024) Diagnosis of Skin Burn-Induced Colon Circulatory Disorders Using Optical Coherence Tomography Angiography and Laser Doppler Flowmetry (Experimental Study). Sovrem Tekhnologii Med. 16, 47-55. [CrossRef]
- Shahid, S., Duarte, M. C., Zhang, J., Markeson, D. and Barnes, D. (2023) Laser doppler imaging - the role of poor burn perfusion in predicting healing time and guiding operative management. Burns. 49, 129-136. [CrossRef]
- Sharma, V. P., O'Boyle, C. P. and Jeffery, S. L. (2011) Man or machine? The clinimetric properties of laser Doppler imaging in burn depth assessment. J Burn Care Res. 32, 143-149. [CrossRef]
- Shin, J. Y. and Yi, H. S. (2016) Diagnostic accuracy of laser Doppler imaging in burn depth assessment: Systematic review and meta-analysis. Burns. 42, 1369-1376. [CrossRef]
- Stewart, T. L., Ball, B., Schembri, P. J., Hori, K., Ding, J., Shankowsky, H. A., Tredget, E. E. and Wound Healing Research Group. (2012) The use of laser Doppler imaging as a predictor of burn depth and hypertrophic scar postburn injury. J Burn Care Res. 33, 764-771. [CrossRef]
- Wang, R., Zhao, J., Zhang, Z., Cao, C., Zhang, Y. and Mao, Y. (2020) Diagnostic Accuracy of Laser Doppler Imaging for the Assessment of Burn Depth: A Meta-analysis and Systematic Review. J Burn Care Res. 41, 619-625. [CrossRef]
- Enejder, A. M., af Klinteberg, C., Wang, I., Andersson-Engels, S., Bendsoe, N., Svanberg, S. and Svanberg, K. (2000) Blood perfusion studies on basal cell carcinomas in conjunction with photodynamic therapy and cryotherapy employing laser-Doppler perfusion imaging. Acta Derm Venereol. 80, 19-23. [CrossRef]
- Kondziołka, J., Wilczyński, S. and Michalecki, L. (2022) Potential Use of Novel Image and Signal Processing Methods to Develop a Quantitative Assessment of the Severity of Acute Radiation Dermatitis in Breast Cancer Radiotherapy. Clin Cosmet Investig Dermatol. 15, 725-733. [CrossRef]
- Wårdell, K., Richter, J. and Zsigmond, P. (2024) Cerebral Microcirculation: Progress and Outlook of Laser Doppler Flowmetry in Neurosurgery and Neurointensive Care. Microcirculation. 31, e12884. [CrossRef]
- Dalla Vecchia, L., Palombo, C., Ciardetti, M., Porta, A., Milani, O., Kozàkovà, M., Lucini, D. and Pagani, M. (2004) Contrasting effects of acute and chronic cigarette smoking on skin microcirculation in young healthy subjects. J Hypertens. 22, 129-135. [CrossRef]
- Low, B. H., Lin, Y. D., Huang, B. W., Chia, T., Bau, J. G. and Huang, H. Y. (2020) Impaired Microvascular Response to Muscle Stretching in Chronic Smokers With Type 2 Diabetes. Front Bioeng Biotechnol. 8, 602. [CrossRef]
- Omae, T., Nagaoka, T. and Yoshida, A. (2016) Effects of Habitual Cigarette Smoking on Retinal Circulation in Patients With Type 2 Diabetes. Invest Ophthalmol Vis Sci. 57, 1345-1351. [CrossRef]
- Rose, K., Flanagan, J. G., Patel, S. R., Cheng, R. and Hudson, C. (2014) Retinal blood flow and vascular reactivity in chronic smokers. Invest Ophthalmol Vis Sci. 55, 4266-4276. [CrossRef]
- Tehan, P. E., Mills, J., Leask, S., Oldmeadow, C., Peterson, B., Sebastian, M. and Chuter, V. (2024) Toe-brachial index and toe systolic blood pressure for the diagnosis of peripheral arterial disease. Cochrane Database Syst Rev. 10, CD013783. [CrossRef]
- Uehara, K., Sone, R. and Yamazaki, F. (2010) Cigarette smoking following a prolonged mental task exaggerates vasoconstriction in glabrous skin in habitual smokers. J UOEH. 32, 303-316. [CrossRef]
- Booi, D. I., Debats, I. B., Boeckx, W. D. and van der Hulst, R. R. (2008) A study of perfusion of the distal free-TRAM flap using laser Doppler flowmetry. J Plast Reconstr Aesthet Surg. 61, 282-288. [CrossRef]
- Eustachio, R. R., Ferreira, R., Brondino, C. M. N., Damante, C. A., De Rezende, M. L. R., Sant'ana, A. C. P., Greghi, S. L. A. and Zangrando, M. S. R. (2018) Clinical parameters, histological analysis, and laser Doppler flowmetry of different subepithelial connective tissue grafts. J Indian Soc Periodontol. 22, 348-352. [CrossRef]
- Forrester, K., Doschak, M. and Bray, R. (1997) In vivo comparison of scanning technique and wavelength in laser Doppler perfusion imaging: measurement in knee ligaments of adult rabbits. Med Biol Eng Comput. 35, 581-586. [CrossRef]
- Hellekes, D. and Hettich, R. (1995) Countinuous Laser-Doppler free-flap monitoring via the telephone line. Preliminary experiences. Int J Clin Monit Comput. 12, 241-244. [CrossRef]
- Kouadio, A. A., Jordana, F., Koffi, N. J., Le Bars, P. and Soueidan, A. (2018) The use of laser Doppler flowmetry to evaluate oral soft tissue blood flow in humans: A review. Arch Oral Biol. 86, 58-71. [CrossRef]
- Saemann, L., Grosskopf, A., Hoorn, F., Veres, G., Guo, Y., Korkmaz-Icöz, S., Karck, M., Simm, A., Wenzel, F. and Szabó, G. (2021) Relationship of Laser-Doppler-Flow and coronary perfusion and a concise update on the importance of coronary microcirculation in donor heart machine perfusion. Clin Hemorheol Microcirc. 79, 121-128. [CrossRef]
- Saemann, L., Kohl, M., Veres, G., Korkmaz-Icöz, S., Grosskopf, A., Karck, M., Simm, A., Wenzel, F. and Szabó, G. (2022) Prediction Model for Contractile Function of Circulatory Death Donor Hearts Based on Microvascular Flow Shifts During Ex Situ Hypothermic Cardioplegic Machine Perfusion. J Am Heart Assoc. 11, e027146. [CrossRef]
- Łoś, A., Walczak, I., Bieńkowski, M., Kutryb-Zając, B. and Hellmann, M. (2024) New insight into the aortic microcirculation in coronary disease: Intraoperative laser Doppler flow measurement and vasa vasorum imaging. Kardiol Pol. 82, 1008-1009. [CrossRef]
- Agarwal, S. C., Allen, J., Murray, A. and Purcell, I. F. (2012) Laser Doppler assessment of dermal circulatory changes in people with coronary artery disease. Microvasc Res. 84, 55-59. [CrossRef]
- Dawn, A., Thevarajah, S., Cayce, K. A., Carroll, C. L., Duque, M. L., Chan, Y. H., Jorizzo, J. L. and Yosipovitch, G. (2007) Cutaneous blood flow in dermatomyositis and its association with disease severity. Skin Res Technol. 13, 285-292. [CrossRef]
- Gunawardena, H., Harris, N. D., Carmichael, C. and McHugh, N. J. (2007) Microvascular responses following digital thermal hyperaemia and iontophoresis measured by laser Doppler imaging in idiopathic inflammatory myopathy. Rheumatology (Oxford). 46, 1483-1486. [CrossRef]
- Au, M. and Rattigan, S. (2012) Barriers to the management of Diabetes Mellitus - is there a future role for Laser Doppler Flowmetry? Australas Med J. 5, 627-632. [CrossRef]
- Fuchs, D., Dupon, P. P., Schaap, L. A. and Draijer, R. (2017) The association between diabetes and dermal microvascular dysfunction non-invasively assessed by laser Doppler with local thermal hyperemia: a systematic review with meta-analysis. Cardiovasc Diabetol. 16, 11. [CrossRef]
- Jan, Y. K., Kelhofer, N., Tu, T., Mansuri, O., Onyemere, K., Dave, S. and Pappu, S. (2024) Diagnosis, Pathophysiology and Management of Microvascular Dysfunction in Diabetes Mellitus. Diagnostics (Basel). 14, 2830. [CrossRef]
- Clough, G. F., Kuliga, K. Z. and Chipperfield, A. J. (2017) Flow motion dynamics of microvascular blood flow and oxygenation: Evidence of adaptive changes in obesity and type 2 diabetes mellitus/insulin resistance. Microcirculation. 24. [CrossRef]
- Hu, H. F., Hsiu, H., Sung, C. J. and Lee, C. H. (2017) Combining laser-Doppler flowmetry measurements with spectral analysis to study different microcirculatory effects in human prediabetic and diabetic subjects. Lasers Med Sci. 32, 327-334. [CrossRef]
- López-Galán, E., Montoya-Pedrón, A., Barrio-Deler, R., Sánchez-Hechavarría, M. E., Muñoz-Bustos, M. E. and Muñoz-Bustos, G. A. (2023) Reactive Hyperemia and Cardiovascular Autonomic Neuropathy in Type 2 Diabetic Patients: A Systematic Review of Randomized and Nonrandomized Clinical Trials. Medicina (Kaunas). 59. [CrossRef]
- Zhao, X., Schalkwijk, C., Kroon, A., Schram, M. T., Stehouwer, C. and Houben, A. (2024) Different Measures of Hyperglycemia Are Negatively Associated With Skin Microvascular Flowmotion: The Maastricht Study. Microcirculation. 31, e12882. [CrossRef]
- Zharkikh, E., Loktionova, Y. and Dunaev, A. (2024) Microcirculatory Dysfunction in Patients With Diabetes Mellitus Detected by a Distributed System of Wearable Laser Doppler Flowmetry Analysers. J Biophotonics. 17, e202400297. [CrossRef]
- Cleofort, V., Attal, R., Sayegh, J., Yannoutsos, A., Lazareth, I., Emmerich, J. and Priollet, P. (2023) Evaluation of the ankle brachial index and toe brachial index for peripheral arterial disease diagnosis in patients over 70 years with lower limb ulcers. J Med Vasc. 48, 11-17. [CrossRef]
- Cobb, J. and Claremont, D. (2001) An in-shoe laser Doppler sensor for assessing plantar blood flow in the diabetic foot. Med Eng Phys. 23, 417-425. [CrossRef]
- Godavarty, A., Leiva, K., Amadi, N., Klonoff, D. C. and Armstrong, D. G. (2023) Diabetic Foot Ulcer Imaging: An Overview and Future Directions. J Diabetes Sci Technol. 17, 1662-1675. [CrossRef]
- Held, M., Bender, D., Krauss, S., Wenger, A., Daigeler, A. and Rothenberger, J. (2019) Quantitative Analysis of Heel Skin Microcirculation Using Laser Doppler Flowmetry and Tissue Spectrophotometry. Adv Skin Wound Care. 32, 88-92. [CrossRef]
- Nemcsik, J., Cseprekál, O., Egresits, J., Kielstein, J., Kümpers, P., Lukasz, A., Tabák, A., Marton, A., Németh, Z. K., Járai, Z., Godina, G., Sallai, L., Farkas, K., Kiss, I. and Tislér, A. (2017) The role of laser Doppler flowmetry tests, serum angiopoietin-2, asymmetric and symmetric dimethylarginine to predict outcome in chronic kidney disease. J Hypertens. 35, 1109-1118. [CrossRef]
- Green, A. Q., Krishnan, S., Finucane, F. M. and Rayman, G. (2010) Altered C-fiber function as an indicator of early peripheral neuropathy in individuals with impaired glucose tolerance. Diabetes Care. 33, 174-176. [CrossRef]
- Park, H. S., Yun, H. M., Jung, I. M. and Lee, T. (2016) Role of Laser Doppler for the Evaluation of Pedal Microcirculatory Function in Diabetic Neuropathy Patients. Microcirculation. 23, 44-52. [CrossRef]
- Aso, Y., Inukai, T. and Takemura, Y. (1997) Evaluation of microangiopathy of the skin in patients with non-insulin-dependent diabetes mellitus by laser Doppler flowmetry; microvasodilatory responses to beraprost sodium. Diabetes Res Clin Pract. 36, 19-26. [CrossRef]
- Pemp, B. and Schmetterer, L. (2008) Ocular blood flow in diabetes and age-related macular degeneration. Can J Ophthalmol. 43, 295-301. [CrossRef]
- Blaise, S., Boulon, C., Mangin, M., Senet, P., Lazareth, I., Imbert, B., Lapebie, F. X., Lacroix, P., Constans, J. and Carpentier, P. (2022) Finger Systolic Blood Pressure Index Measurement: A Useful Tool for the Evaluation of Arterial Disease in Patients With Systemic Sclerosis. Arthritis Care Res (Hoboken). 74, 828-832. [CrossRef]
- Herrick, A. L. (2021) Raynaud's phenomenon and digital ulcers: advances in evaluation and management. Curr Opin Rheumatol. 33, 453-462. [CrossRef]
- Herrick, A. L., Hughes, M. and Murray, A. (2025) Recent advances in non-invasive imaging of systemic sclerosis-related digital ulcers. J Scleroderma Relat Disord, 23971983251339703. [CrossRef]
- Busila, I., Onofriescu, M., Gramaticu, A., Hogas, S., Covic, A. and Florea, L. (2015) Endothelial Dysfunction Assessed by Laser Doppler Post-Occlusive Hyperemia in Chronic Kidney Disease Patients. Rev Med Chir Soc Med Nat Iaşi. 119, 1001-1009.
- Gomes, V., Gomes, M. B., Tibirica, E. and Lessa, M. A. (2014) Post-operative endothelial dysfunction assessment using laser Doppler perfusion measurement in cardiac surgery patients. Acta Anaesthesiol Scand. 58, 468-477. [CrossRef]
- Hogas, S. M., Voroneanu, L., Serban, D. N., Segall, L., Hogas, M. M., Serban, I. L. and Covic, A. (2010) Methods and potential biomarkers for the evaluation of endothelial dysfunction in chronic kidney disease: a critical approach. J Am Soc Hypertens. 4, 116-127. [CrossRef]
- Kruger, A., Stewart, J., Sahityani, R., O'Riordan, E., Thompson, C., Adler, S., Garrick, R., Vallance, P. and Goligorsky, M. S. (2006) Laser Doppler flowmetry detection of endothelial dysfunction in end-stage renal disease patients: correlation with cardiovascular risk. Kidney Int. 70, 157-164. [CrossRef]
- Škrha, J., Prázný, M., Haas, T., Kvasnička, J. and Kalvodová, B. (2001) Comparison of laser-Doppler flowmetry with biochemical indicators of endothelial dysfunction related to early microangiopathy in Type 1 diabetic patients. J Diabetes Complications. 15, 234-240. [CrossRef]
- Stiefel, P., Moreno-Luna, R., Vallejo-Vaz, A. J., Beltran, L. M., Costa, A., Gomez, L., Ordonez, A. and Villar, J. (2012) Which parameter is better to define endothelial dysfunction in a test of postocclusive hyperemia measured by laser-Doppler flowmetry? Coron Artery Dis. 23, 57-61. [CrossRef]
- Tao, J., Jin, Y. F., Yang, Z., Wang, L. C., Gao, X. R., Lui, L. and Ma, H. (2004) Reduced arterial elasticity is associated with endothelial dysfunction in persons of advancing age: comparative study of noninvasive pulse wave analysis and laser Doppler blood flow measurement. Am J Hypertens. 17, 654-659. [CrossRef]
- Esen, E. and Çetin, A. (2017) Microvascular functions in patients with fibromyalgia syndrome: effects of physical exercise. Turk J Phys Med Rehabil. 63, 215-223. [CrossRef]
- Schley, M., Legler, A., Skopp, G., Schmelz, M., Konrad, C. and Rukwied, R. (2006) Delta-9-THC based monotherapy in fibromyalgia patients on experimentally induced pain, axon reflex flare, and pain relief. Curr Med Res Opin. 22, 1269-1276. [CrossRef]
- Souza-Silva, E., Ascenso, R., Tonussi, C. R. and da Silva-Santos, J. E. (2021) Detection of blood flow perfusion and post - occlusive reactive hyperemia in the skeletal muscle of rats. Life Sci. 278, 119571. [CrossRef]
- Gorodkin, R., Herrick, A. L. and Murray, A. K. (2016) Microvascular Response in Patients with Complex Regional Pain Syndrome as Measured by Laser Doppler Imaging. Microcirculation. 23, 379-383. [CrossRef]
- Lenasi, H. (2011) Assessment of Human Skin Microcirculation and Its Endothelial Function Using Laser Doppler Flowmetry. In Medical imaging (Erondu, O. F., ed.). pp. 271–296, InTech, Rijeka.
- Schabauer, A. M. and Rooke, T. W. (1994) Cutaneous laser Doppler flowmetry: applications and findings. Mayo Clin Proc. 69, 564-574. [CrossRef]
- McIllhatton, A., Lanting, S. and Chuter, V. (2024) The Effect of Overweight/Obesity on Cutaneous Microvascular Reactivity as Measured by Laser-Doppler Fluxmetry: A Systematic Review. Biomedicines. 12, 2488. [CrossRef]
- Murray, A. K., Herrick, A. L. and King, T. A. (2004) Laser Doppler imaging: a developing technique for application in the rheumatic diseases. Rheumatology (Oxford). 43, 1210-1218. [CrossRef]
- Ben Simon, G. J., Moroz, I., Goldenfeld, M. and Melamed, S. (2003) Scanning laser Doppler flowmetry of nonperfused regions of the optic nerve head in patients with glaucoma. Ophthalmic Surg Lasers Imaging. 34, 245-250.
- Holló, G., van den Berg, T. J. T. P. and Greve, E. L. (1996) Scanning laser Doppler flowmetry in glaucoma. Int Ophthalmol. 20, 63-70. [CrossRef]
- Hosking, S. L., Embleton, S. J. and Cunliffe, I. A. (2001) Application of a local search strategy improves the detection of blood flow deficits in the neuroretinal rim of glaucoma patients using scanning laser Doppler flowmetry. Br J Ophthalmol. 85, 1298-1302. [CrossRef]
- Dubiel, M., Krolczyk, J., Gasowski, J. and Grodzicki, T. (2011) Skin microcirculation and echocardiographic and biochemical indices of left ventricular dysfunction in non-diabetic patients with heart failure. Cardiol J. 18, 270-276.
- Edvinsson, M. L., Uddman, E. and Andersson, S. E. (2011) Deteriorated function of cutaneous microcirculation in chronic congestive heart failure. J Geriatr Cardiol. 8, 82-87. [CrossRef]
- Edvinsson, M. L., Uddman, E., Edvinsson, L. and Andersson, S. E. (2014) Brain natriuretic peptide is a potent vasodilator in aged human microcirculation and shows a blunted response in heart failure patients. J Geriatr Cardiol. 11, 50-56. [CrossRef]
- Green, D. J., Maiorana, A. J., Siong, J. H., Burke, V., Erickson, M., Minson, C. T., Bilsborough, W. and O'Driscoll, G. (2006) Impaired skin blood flow response to environmental heating in chronic heart failure. Eur Heart J. 27, 338-343. [CrossRef]
- Hoit, B. D., Walsh, R. A., Shao, Y., Gabel, M. and Millard, R. (1993) Comparative assessment of regional left atrial perfusion by laser Doppler and radionuclide microsphere techniques. Cardiovasc Res. 27, 508-514. [CrossRef]
- Maréchaux, S., Samson, R., van Belle, E., Breyne, J., de Monte, J., Dedrie, C., Chebai, N., Menet, A., Banfi, C., Bouabdallaoui, N., Le Jemtel, T. H. and Ennezat, P. V. (2016) Vascular and Microvascular Endothelial Function in Heart Failure With Preserved Ejection Fraction. J Card Fail. 22, 3-11. [CrossRef]
- Marshall, R. A., Luchkanych, A. M. S., Morton, J. S., Boyes, N. G., Zhai, A., Marciniuk, D. D., Mei, Y., Allison, E. Y., Shoemaker, J. K., Al-Khazraji, B. K., Allen, M. D., Tomczak, C. R. and Olver, T. D. (2022) Cerebral haemodynamics during arrhythmia in health, ischaemic heart disease and heart failure with reduced ejection fraction, and in a preclinical swine model. J Physiol. 600, 2311-2325. [CrossRef]
- Paine, N. J., Hinderliter, A. L., Blumenthal, J. A., Adams, K. F., Jr., Sueta, C. A., Chang, P. P., O'Connor, C. M. and Sherwood, A. (2016) Reactive hyperemia is associated with adverse clinical outcomes in heart failure. Am Heart J. 178, 108-114. [CrossRef]
- Sansone, R., Stanske, B., Keymel, S., Schuler, D., Horn, P., Saeed, D., Boeken, U., Westenfeld, R., Lichtenberg, A., Kelm, M. and Heiss, C. (2015) Macrovascular and microvascular function after implantation of left ventricular assist devices in end-stage heart failure: Role of microparticles. J Heart Lung Transplant. 34, 921-932. [CrossRef]
- Shantsila, E., Wrigley, B., Shantsila, A., Tapp, L. D., Blann, A. D., Gill, P. S. and Lip, G. Y. H. (2011) Ethnic differences in macrovascular and microvascular function in systolic heart failure. Circ Heart Fail. 4, 754-762. [CrossRef]
- Tikhomirova, I., Petrochenko, E., Muravyov, A., Malysheva, Y., Petrochenko, A., Yakusevich, V. and Oslyakova, A. (2017) Microcirculation and blood rheology abnormalities in chronic heart failure. Clin Hemorheol Microcirc. 65, 383-391. [CrossRef]
- Muñoz-Hernández, R., Ampuero, J., Millán, R., Gil-Gómez, A., Rojas, Á., Macher, H. C., Gallego-Durán, R., Gato, S., Montero-Vallejo, R., Rico, M. C., Maya-Miles, D., Sánchez-Torrijos, Y., Soria, I. C., Stiefel, P. and Romero-Gómez, M. (2020) Hepatitis C Virus Clearance by Direct-Acting Antivirals Agents Improves Endothelial Dysfunction and Subclinical Atherosclerosis: HEPCAR Study. Clin Transl Gastroenterol. 11, e00203. [CrossRef]
- Nagaoka, T., Sato, E., Takahashi, A., Yokohama, S. and Yoshida, A. (2007) Retinal circulatory changes associated with interferon-induced retinopathy in patients with hepatitis C. Invest Ophthalmol Vis Sci. 48, 368-375. [CrossRef]
- Cankar, K., Potocnik, N. and Strucl, M. (2010) Alteration of skin laser-Doppler flux response to local cooling in gestational hypertension. Clin Auton Res. 20, 183-190. [CrossRef]
- Cesarone, M. R., Belcaro, G., Rulo, A., Griffin, M., Ricci, A., Ippolito, E., De Sanctis, M. T., Incandela, L., Bavera, P., Cacchio, M. and Bucci, M. (2001) Microcirculatory effects of total triterpenic fraction of Centella asiatica in chronic venous hypertension: measurement by laser Doppler, TcPO2-CO2, and leg volumetry. Angiology. 52 Suppl 2, S45-48.
- Farkas, K., Kolossvary, E., Járai, Z., Nemcsik, J. and Farsang, C. (2004) Non-invasive assessment of microvascular endothelial function by laser Doppler flowmetry in patients with essential hypertension. Atherosclerosis. 173, 97-102. [CrossRef]
- Yuan, X., Wu, Q., Shang, F., Li, B., Liu, M., Wang, B., Sheng, Y., Zhang, H. and Xiu, R. (2019) A comparison of the cutaneous microvascular properties of the Spontaneously Hypertensive and the Wistar-Kyoto rats by Spectral analysis of Laser Doppler. Clin Exp Hypertens. 41, 342-352. [CrossRef]
- Emmanuel, A. V. and Kamm, M. A. (1999) Laser Doppler measurement of rectal mucosal blood flow. Gut. 45, 64-69. [CrossRef]
- Guslandi, M., Sorghi, S., Polli, D. and Tittobello, A. (1998) Measurement of rectal blood flow by laser Doppler flowmetry in inflammatory bowel disease. Hepatogastroenterology. 45, 445-446.
- Anania, C., Norman, M., Heimburger, M., Gustafsson, T., Jogestrand, T., Hafström, I. and Frostegård, J. (2012) Microcirculation as determined by iontophoresis in SLE-patients and controls. Lupus. 21, 815-820. [CrossRef]
- Svensson, C., Eriksson, P., Bjarnegård, N., Jonasson, H., Strömberg, T., Sjöwall, C. and Zachrisson, H. (2021) Impaired Microcirculation and Vascular Hemodynamics in Relation to Macrocirculation in Patients With Systemic Lupus Erythematosus. Front Med (Lausanne). 8, 722758. [CrossRef]
- Yusof, M. Y. M., Britton, J., Edward, S., Hensor, E. M. A., Goodfield, M. J., Laws, P. M., Emery, P., Wittmann, M. and Vital, E. M. (2019) Validity and sensitivity to change of laser Doppler imaging as a novel objective outcome measure for cutaneous lupus erythematosus. Lupus. 28, 1320-1328. [CrossRef]
- Ibrahimi, K., Vermeersch, S., Frederiks, P., Geldhof, V., Draulans, C., Buntinx, L., Lesaffre, E., MaassenVanDenBrink, A. and de Hoon, J. (2017) The influence of migraine and female hormones on capsaicin-induced dermal blood flow. Cephalalgia. 37, 1164-1172. [CrossRef]
- Trzepizur, W., Gagnadoux, F., Abraham, P., Rousseau, P., Meslier, N., Saumet, J. L. and Racineux, J. L. (2009) Microvascular endothelial function in obstructive sleep apnea: Impact of continuous positive airway pressure and mandibular advancement. Sleep Med. 10, 746-752. [CrossRef]
- Yim-Yeh, S., Rahangdale, S., Nguyen, A. T., Jordan, A. S., Novack, V., Veves, A. and Malhotra, A. (2010) Obstructive sleep apnea and aging effects on macrovascular and microcirculatory function. Sleep. 33, 1177-1183. [CrossRef]
- Yim-Yeh, S., Rahangdale, S., Nguyen, A. T., Stevenson, K. E., Novack, V., Veves, A. and Malhotra, A. (2011) Vascular dysfunction in obstructive sleep apnea and type 2 diabetes mellitus. Obesity (Silver Spring). 19, 17-22. [CrossRef]
- Siepmann, T., Frenz, E., Penzlin, A. I., Goelz, S., Zago, W., Friehs, I., Kubasch, M. L., Wienecke, M., Löhle, M., Schrempf, W., Barlinn, K., Siegert, J., Storch, A., Reichmann, H. and Illigens, B. M. (2016) Pilomotor function is impaired in patients with Parkinson's disease: A study of the adrenergic axon-reflex response and autonomic functions. Parkinsonism Relat Disord. 31, 129-134. [CrossRef]
- Chen, Q. and Rosenson, R. S. (2018) Systematic Review of Methods Used for the Microvascular Assessment of Peripheral Arterial Disease. Cardiovasc Drugs Ther. 32, 301-310. [CrossRef]
- Oh, D. G., Hansen, L. and Taylor, W. R. (2025) Is Laser Doppler Perfusion Imaging Truly a "Gold Standard" for Preclinical Peripheral Artery Disease Research? JACC Basic Transl Sci. 10, 104-106. [CrossRef]
- Palzkill, V. R., Tan, J., Moparthy, D., Tice, A. L., Ferreira, L. F. and Ryan, T. E. (2025) A 6-Minute Limb Function Assessment for Therapeutic Testing in Experimental Peripheral Artery Disease Models. JACC Basic Transl Sci. 10, 88-103. [CrossRef]
- Sanip, Z., Pahimi, N., Bokti, N. A., Yusof, Z., Mohamed, M. S., WYH, W. I. and Rasool, A. H. (2023) Impaired peripheral microvascular reactivity in patients with nonobstructive coronary artery disease. Microcirculation. 30, e12807. [CrossRef]
- Valentini, J., Sigl, M., Dunckel, C., Krisam, J., Amendt, K. and Greten, H. J. (2024) Can acupuncture increase microcirculation in peripheral artery disease and diabetic foot syndrome? - a pilot study. Front Med (Lausanne). 11, 1371056. [CrossRef]
- de Mul, F. F. M., Blaauw, J., Aarnoudse, J. G., Smit, A. J. and Rakhorst, G. (2007) Diffusion model for iontophoresis measured by laser-Doppler perfusion flowmetry, applied to normal and preeclamptic pregnancies. J Biomed Opt. 12, 014032. [CrossRef]
- de Mul, F. F., Blaauw, J., Smit, R. J., Rakhorst, G. and Aarnoudse, J. G. (2009) Time development models for perfusion provocations studied with laser-Doppler flowmetry, applied to iontophoresis and PORH. Microcirculation. 16, 559-571. [CrossRef]
- Porto, L. B., Brandao, A. H. F., Leite, H. V. and Cabral, A. C. V. (2017) Longitudinal evaluation of uterine perfusion, endothelial function and central blood flow in early onset pre-eclampsia. Pregnancy Hypertens. 10, 161-164. [CrossRef]
- Pyevich, M., Alexander, L. M. and Stanhewicz, A. E. (2022) Women with a history of preeclampsia have preserved sensory nerve-mediated dilatation in the cutaneous microvasculature. Exp Physiol. 107, 175-182. [CrossRef]
- Ramsay, J. E., Stewart, F., Greer, I. A. and Sattar, N. (2003) Microvascular dysfunction: a link between pre-eclampsia and maternal coronary heart disease. BJOG. 110, 1029-1031.
- Alba, B. K., Greaney, J. L., Ferguson, S. B. and Alexander, L. M. (2018) Endothelial function is impaired in the cutaneous microcirculation of adults with psoriasis through reductions in nitric oxide-dependent vasodilation. Am J Physiol Heart Circ Physiol. 314, H343-H349. [CrossRef]
- Coates, L. C., Anderson, R. R., Fitzgerald, O., Gottlieb, A. B., Kelly, S. G., Lubrano, E., McGonagle, D. G., Olivieri, I., Ritchlin, C. T., Tan, A. L., De Vlam, K. and Helliwell, P. S. (2008) Clues to the pathogenesis of psoriasis and psoriatic arthritis from imaging: a literature review. J Rheumatol. 35, 1438-1442.
- Grajdeanu, I. A., Statescu, L., Vata, D., Popescu, I. A., Porumb-Andrese, E., Patrascu, A. I., Taranu, T., Crisan, M. and Solovastru, L. G. (2019) Imaging techniques in the diagnosis and monitoring of psoriasis. Exp Ther Med. 18, 4974-4980. [CrossRef]
- Hendriks, A. G. M., Steenbergen, W., Hondebrink, E., van Hespen, J. C. G., van de Kerkhof, P. C. M. and Seyger, M. M. B. (2014) Whole field laser Doppler imaging of the microcirculation in psoriasis and clinically unaffected skin. J Dermatolog Treat. 25, 18-21. [CrossRef]
- Hendriks, A. G. M., van de Kerkhof, P. C. M., de Jonge, C. S., Lucas, M., Steenbergen, W. and Seyger, M. M. B. (2015) Clearing of psoriasis documented by laser Doppler perfusion imaging contrasts remaining elevation of dermal expression levels of CD31. Skin Res Technol. 21, 340-345. [CrossRef]
- Lacarrubba, F., Pellacani, G., Gurgone, S., Verzi, A. E. and Micali, G. (2015) Advances in non-invasive techniques as aids to the diagnosis and monitoring of therapeutic response in plaque psoriasis: a review. Int J Dermatol. 54, 626-634. [CrossRef]
- Engelhart, M. and Kristensen, J. K. (1986) Raynaud's phenomenon: blood supply to fingers during indirect cooling, evaluated by laser Doppler flowmetry. Clin Physiol. 6, 481-488. [CrossRef]
- Markousis-Mavrogenis, G., Bournia, V. K., Sfikakis, P. P. and Mavrogeni, S. I. (2023) Raynaud phenomenon and microvasculopathy in systemic sclerosis: multi-modality imaging for diagnosis and evaluation. Curr Opin Rheumatol. 35, 324-333. [CrossRef]
- Wollersheim, H., Reyenga, J. and Thien, T. (1989) Postocclusive reactive hyperemia of fingertips, monitored by laser Doppler velocimetry in the diagnosis of Raynaud's phenomenon. Microvasc Res. 38, 286-295. [CrossRef]
- Kanetaka, T., Komiyama, T., Onozuka, A., Miyata, T. and Shigematsu, H. (2004) Laser Doppler skin perfusion pressure in the assessment of Raynaud's phenomenon. Eur J Vasc Endovasc Surg. 27, 414-416. [CrossRef]
- Maga, P., Henry, B. M., Kmiotek, E. K., Gregorczyk-Maga, I., Kaczmarczyk, P., Tomaszewski, K. A. and Nizankowski, R. (2016) Postocclusive Hyperemia Measured with Laser Doppler Flowmetry and Transcutaneous Oxygen Tension in the Diagnosis of Primary Raynaud's Phenomenon: A Prospective, Controlled Study. Biomed Res Int. 2016, 9645705. [CrossRef]
- Picart, C., Carpentier, P. H., Brasseur, S., Galliard, H. and Piau, J. M. (1998) Systemic sclerosis: blood rheometry and laser Doppler imaging of digital cutaneous microcirculation during local cold exposure. Clin Hemorheol Microcirc. 18, 47-58.
- Dimitroulas, T., Hodson, J., Sandoo, A., Smith, J. and Kitas, G. D. (2017) Endothelial injury in rheumatoid arthritis: a crosstalk between dimethylarginines and systemic inflammation. Arthritis Res Ther. 19, 32. [CrossRef]
- Fenton, S. A. M., Sandoo, A., Metsios, G. S., Duda, J. L., Kitas, G. D. and Veldhuijzen van Zanten, J. J. C. S. (2018) Sitting time is negatively related to microvascular endothelium-dependent function in rheumatoid arthritis. Microvasc Res. 117, 57-60. [CrossRef]
- Khan, F., Galarraga, B. and Belch, J. J. (2010) The role of endothelial function and its assessment in rheumatoid arthritis. Nat Rev Rheumatol. 6, 253-261. [CrossRef]
- Klimek, E., Sulicka, J., Gryglewska, B., Skalska, A., Kwaśny-Krochin, B., Korkosz, M. and Grodzicki, T. K. (2017) Alterations in skin microvascular function in patients with rheumatoid arthritis and ankylosing spondylitis. Clin Hemorheol Microcirc. 65, 77-91. [CrossRef]
- Meyer, M. F., Schmidt, O., Hellmich, B., Schatz, H., Klein, H. H. and Braun, J. (2007) Microvascular dysfunction in rheumatoid arthritis assessed by laser Doppler anemometry: relationship to soluble adhesion molecules and extraarticular manifestations. Rheumatol Int. 28, 145-152. [CrossRef]
- Sandoo, A., Carroll, D., Metsios, G. S., Kitas, G. D. and Veldhuijzen van Zanten, J. J. (2011) The association between microvascular and macrovascular endothelial function in patients with rheumatoid arthritis: a cross-sectional study. Arthritis Res Ther. 13, R99. [CrossRef]
- Sandoo, A., Hodson, J., Douglas, K. M., Smith, J. P. and Kitas, G. D. (2013) The association between functional and morphological assessments of endothelial function in patients with rheumatoid arthritis: a cross-sectional study. Arthritis Res Ther. 15, R107. [CrossRef]
- Ferrell, W. R., Balint, P. V., Egan, C. G., Lockhart, J. C. and Sturrock, R. D. (2001) Metacarpophalangeal joints in rheumatoid arthritis: laser Doppler imaging--initial experience. Radiology. 220, 257-262. [CrossRef]
- Ait-Oufella, H., Bourcier, S., Alves, M., Galbois, A., Baudel, J. L., Margetis, D., Bige, N., Offenstadt, G., Maury, E. and Guidet, B. (2013) Alteration of skin perfusion in mottling area during septic shock. Ann Intensive Care. 3, 31. [CrossRef]
- Contreras, R., Hernández, G., Valenzuela, E. D., González, C., Ulloa, R., Soto, D., Castro, R., Guzman, C., Oviedo, V., Alegria, L., Vidal, D., Morales, S., Ospina-Tascon, G. A., Bakker, J. and Kattan, E. (2023) Exploring the relationship between capillary refill time, skin blood flow and microcirculatory reactivity during early resuscitation of patients with septic shock: a pilot study. J Clin Monit Comput. 37, 839-845. [CrossRef]
- Post, E. H., Su, F., Hosokawa, K., Taccone, F. S., Herpain, A., Creteur, J., Vincent, J. L. and De Backer, D. (2017) Changes in kidney perfusion and renal cortex metabolism in septic shock: an experimental study. J Surg Res. 207, 145-154. [CrossRef]
- Schulz, J., Vollmer, C., Truse, R., Bauer, I., Beck, C., Picker, O. and Herminghaus, A. (2020) Effect of Pravastatin Pretreatment and Hypercapnia on Intestinal Microvascular Oxygenation and Blood Flow During Sepsis. Shock. 53, 88-94. [CrossRef]
- Stark, R. J. (2022) Endothelial-Dependent Responses Correlate with Pediatric SOFA Scores During Severe Sepsis and Septic Shock. J Cardiovasc Transl Res. 15, 903-905. [CrossRef]
- Sturm, T., Leiblein, J., Schneider-Lindner, V., Kirschning, T. and Thiel, M. (2018) Association of Microcirculation, Macrocirculation, and Severity of Illness in Septic Shock: A Prospective Observational Study to Identify Microcirculatory Targets Potentially Suitable for Guidance of Hemodynamic Therapy. J Intensive Care Med. 33, 256-266. [CrossRef]
- Tang, A. L., Shen, M. J. and Zhang, G. Q. (2022) Intestinal microcirculation dysfunction in sepsis: pathophysiology, clinical monitoring, and therapeutic interventions. World J Emerg Med. 13, 343-348. [CrossRef]
- Tannert, A., Ramoji, A., Neugebauer, U. and Popp, J. (2018) Photonic monitoring of treatment during infection and sepsis: development of new detection strategies and potential clinical applications. Anal Bioanal Chem. 410, 773-790. [CrossRef]
- Trzeciak, S. and Rivers, E. P. (2005) Clinical manifestations of disordered microcirculatory perfusion in severe sepsis. Crit Care. 9 Suppl 4, S20-26. [CrossRef]
- Connes, P., Möckesch, B., Tudor Ngo Sock, E., Hardy-Dessources, M. D., Reminy, K., Skinner, S., Billaud, M., Nader, E., Tressieres, B., Etienne-Julan, M., Guillot, N., Lemonne, N., Hue, O., Romana, M. and Antoine-Jonville, S. (2021) Oxidative stress, inflammation, blood rheology, and microcirculation in adults with sickle cell disease: Effects of hydroxyurea treatment and impact of sickle cell syndrome. Eur J Haematol. 106, 800-807. [CrossRef]
- Mohan, J. S., Vigilance, J. E., Marshall, J. M., Hambleton, I. R., Reid, H. L. and Serjeant, G. R. (2000) Abnormal venous function in patients with homozygous sickle cell (SS) disease and chronic leg ulcers. Clin Sci (Lond). 98, 667-672.
- Mohan, J. S., Lip, G. Y. H., Blann, A. D., Bareford, D. and Marshall, J. M. (2011) Endothelium-dependent and endothelium-independent vasodilatation of the cutaneous circulation in sickle cell disease. Eur J Clin Invest. 41, 546-551. [CrossRef]
- Cai, Q., Xu, G., Liu, J., Wang, L., Deng, G., Liu, J. and Chen, Z. (2016) A modification of intraluminal middle cerebral artery occlusion/reperfusion model for ischemic stroke with laser Doppler flowmetry guidance in mice. Neuropsychiatr Dis Treat. 12, 2851-2858. [CrossRef]
- Cuccione, E., Versace, A., Cho, T. H., Carone, D., Berner, L. P., Ong, E., Rousseau, D., Cai, R., Monza, L., Ferrarese, C., Sganzerla, E. P., Berthezène, Y., Nighoghossian, N., Wiart, M., Beretta, S. and Chauveau, F. (2017) Multi-site laser Doppler flowmetry for assessing collateral flow in experimental ischemic stroke: Validation of outcome prediction with acute MRI. J Cereb Blood Flow Metab. 37, 2159-2170. [CrossRef]
- Morais, A., Locascio, J. J., Sansing, L. H., Lamb, J., Nagarkatti, K., Imai, T., van Leyen, K., Aronowski, J., Koenig, J. I., Bosetti, F., Lyden, P., Ayata, C. and SPAN Investigators. (2023) Embracing Heterogeneity in The Multicenter Stroke Preclinical Assessment Network (SPAN) Trial. Stroke. 54, 620-631. [CrossRef]
- Dreier, J. P., Major, S., Manning, A., Woitzik, J., Drenckhahn, C., Steinbrink, J., Tolias, C., Oliveira-Ferreira, A. I., Fabricius, M., Hartings, J. A., Vajkoczy, P., Lauritzen, M., Dirnagl, U., Bohner, G., Strong, A. J. and group, C. s. (2009) Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain. 132, 1866-1881. [CrossRef]
- Mauritzon, S., Ginstman, F., Hillman, J. and Wardell, K. (2022) Analysis of laser Doppler flowmetry long-term recordings for investigation of cerebral microcirculation during neurointensive care. Front Neurosci. 16, 1030805. [CrossRef]
- Rejmstad, P., Akesson, G., Aneman, O. and Wardell, K. (2016) A laser Doppler system for monitoring cerebral microcirculation: implementation and evaluation during neurosurgery. Med Biol Eng Comput. 54, 123-131. [CrossRef]
- Schubert, G. A., Poli, S., Mendelowitsch, A., Schilling, L. and Thome, C. (2008) Hypothermia reduces early hypoperfusion and metabolic alterations during the acute phase of massive subarachnoid hemorrhage: a laser-Doppler-flowmetry and microdialysis study in rats. J Neurotrauma. 25, 539-548. [CrossRef]
- Tapper, S., Mauritzon, S., Martins, M. P., Ginstman, F., Tisell, A., Zsigmond, P. and Wårdell, K. (2025) Simultaneous MRI and laser Doppler Flowmetry: Assessing cerebral Macro- and microcirculation in neurointensive care. Neuroimage Clin. 47, 103821. [CrossRef]
- Herrick, A. L., Dinsdale, G. and Murray, A. (2020) New perspectives in the imaging of Raynaud's phenomenon. Eur J Rheumatol. 7, S212-S221. [CrossRef]
- Pellicano, C., Vaiarello, V., Colalillo, A., Gigante, A., Iannazzo, F. and Rosato, E. (2023) Role of kinurenic acid in the systemic sclerosis renal involvement. Clin Exp Med. 23, 1713-1719. [CrossRef]
- Rotondo, C., Nivuori, M., Chiala, A., Praino, E., Matucci Cerinic, M., Cutolo, M., Lapadula, G. and Iannone, F. (2018) Evidence for increase in finger blood flow, evaluated by laser Doppler flowmetry, following iloprost infusion in patients with systemic sclerosis: a week-long observational longitudinal study. Scand J Rheumatol. 47, 311-318. [CrossRef]
- Lin, B. S., Wang, C. C., Chang, M. H. and Chio, C. C. (2015) Evaluation of traumatic brain injury by optical technique. BMC Neurol. 15, 202. [CrossRef]
- Mendez, D. R., Cherian, L. and Robertson, C. S. (2004) Laser Doppler flow and brain tissue PO2 after cortical impact injury complicated by secondary ischemia in rats treated with arginine. J Trauma. 57, 244-250. [CrossRef]
- Marrakchi, S. and Maibach, H. I. (2006) Functional map and age-related differences in the human face: nonimmunologic contact urticaria induced by hexyl nicotinate. Contact Dermatitis. 55, 15-19. [CrossRef]
- Skrebova, N., Takiwaki, H., Miyaoka, Y. and Arase, S. (2001) Localized heat urticaria: a clinical study using laser Doppler flowmetry. J Dermatol Sci. 26, 112-118. [CrossRef]
- Secomb, T. W. (2008) Theoretical models for regulation of blood flow. Microcirculation. 15, 765-775. [CrossRef]
- Hofmeyr, J.-H. S. and Cornish-Bowden, A. (2000) Regulating the cellular economy of supply and demand. FEBS Lett. 476, 47-51.
- Hsiu, H., Huang, S. M., Chao, P. T., Hsu, W. C., Hsu, C. L., Jan, M. Y., Wang, W. K. and Wang, Y. Y. (2007) Study on the microcirculatory blood velocity of acupoint monitored by laser Doppler signal. Annu Int Conf IEEE Eng Med Biol Soc. 2007, 959-962. [CrossRef]
- Hsiu, H., Huang, S. M., Chao, P. T., Jan, M. Y., Hsu, T. L., Wang, W. K. and Wang, Y. Y. (2007) Microcirculatory characteristics of acupuncture points obtained by laser Doppler flowmetry. Physiol Meas. 28, N77-86. [CrossRef]
- Hsiu, H., Hsu, W. C., Hsu, C. L., Huang, S. M. and Lin, Y. Y. (2009) Microcirculatory changes by laser Doppler after infrared heating over acupuncture points--relevance to moxibustion. Photomed Laser Surg. 27, 855-861. [CrossRef]
- Nielsen, A., Knoblauch, N. T., Dobos, G. J., Michalsen, A. and Kaptchuk, T. J. (2007) The effect of Gua Sha treatment on the microcirculation of surface tissue: a pilot study in healthy subjects. Explore (NY). 3, 456-466. [CrossRef]
- Guigui, A., Loader, J., Bellier, A. and Roustit, M. (2022) Commentary: Multiple laser doppler flowmetry probes increase the reproducibility of skin blood flow measurements. Front Physiol. 13, 1025905. [CrossRef]
- Guven, G., Dijkstra, A., Kuijper, T. M., Trommel, N., van Baar, M. E., Topeli, A., Ince, C. and van der Vlies, C. H. (2023) Comparison of laser speckle contrast imaging with laser Doppler perfusion imaging for tissue perfusion measurement. Microcirculation. 30, e12795. [CrossRef]
- Mahé, G., Durand, S., Humeau-Heurtier, A., Leftheriotis, G. and Abraham, P. (2012) Impact of experimental conditions on noncontact laser recordings in microvascular studies. Microcirculation. 19, 669-675. [CrossRef]
- Millet, C., Roustit, M., Blaise, S. and Cracowski, J. L. (2011) Comparison between laser speckle contrast imaging and laser Doppler imaging to assess skin blood flow in humans. Microvasc Res. 82, 147-151. [CrossRef]
- Roustit, M., Millet, C., Blaise, S., Dufournet, B. and Cracowski, J. L. (2010) Excellent reproducibility of laser speckle contrast imaging to assess skin microvascular reactivity. Microvasc Res. 80, 505-511. [CrossRef]
- Sun, S., Hayes-Gill, B. R., He, D., Zhu, Y., Huynh, N. T. and Morgan, S. P. (2016) Comparison of laser Doppler and laser speckle contrast imaging using a concurrent processing system. Optic Lasers Eng. 83, 1-9. [CrossRef]
- Hodges, G. J., Klentrou, P., Cheung, S. S. and Falk, B. (2020) Comparison of laser speckle contrast imaging and laser-Doppler fluxmetry in boys and men. Microvasc Res. 128, 103927. [CrossRef]
- Fredriksson, I., Larsson, M. and Stromberg, T. (2009) Measurement depth and volume in laser Doppler flowmetry. Microvasc Res. 78, 4-13. [CrossRef]
- Jakobsson, A. and Nilsson, G. E. (1993) Prediction of sampling depth and photon pathlength in laser Doppler flowmetry. Med Biol Eng Comput. 31, 301-307. [CrossRef]
- O'Doherty, J., McNamara, P., Clancy, N. T., Enfield, J. G. and Leahy, M. J. (2009) Comparison of instruments for investigation of microcirculatory blood flow and red blood cell concentration. J Biomed Opt. 14, 034025. [CrossRef]
- Thompson, O. B., Hirst, E. R. and Andrews, M. K. (2011) Is there a difference between laser speckle and laser Doppler in depth sensitivity? Proc SPIE 7898, 7898OE. [CrossRef]
- Bamps, D., Macours, L., Buntinx, L. and de Hoon, J. (2020) Laser speckle contrast imaging, the future DBF imaging technique for TRP target engagement biomarker assays. Microvasc Res. 129, 103965. [CrossRef]
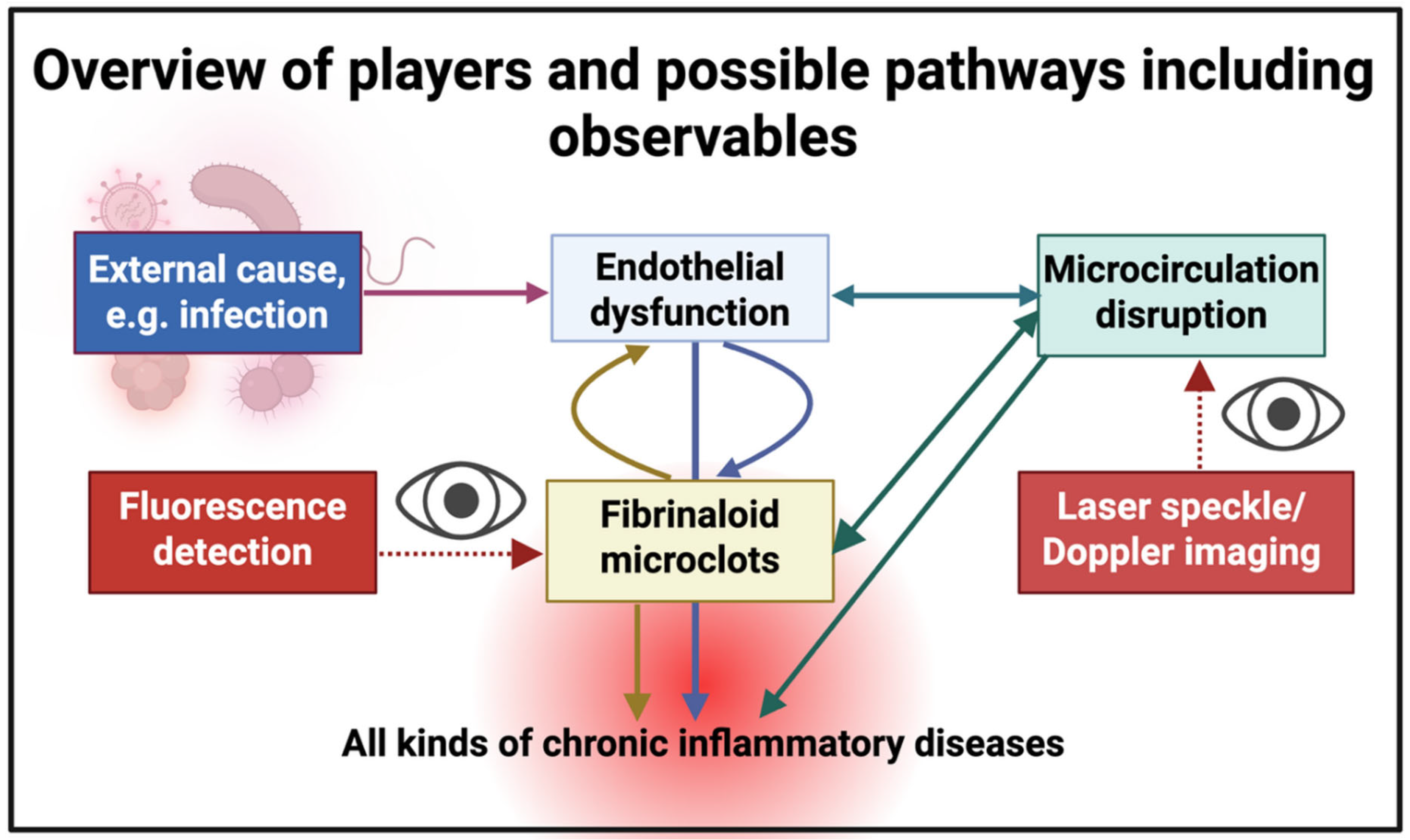


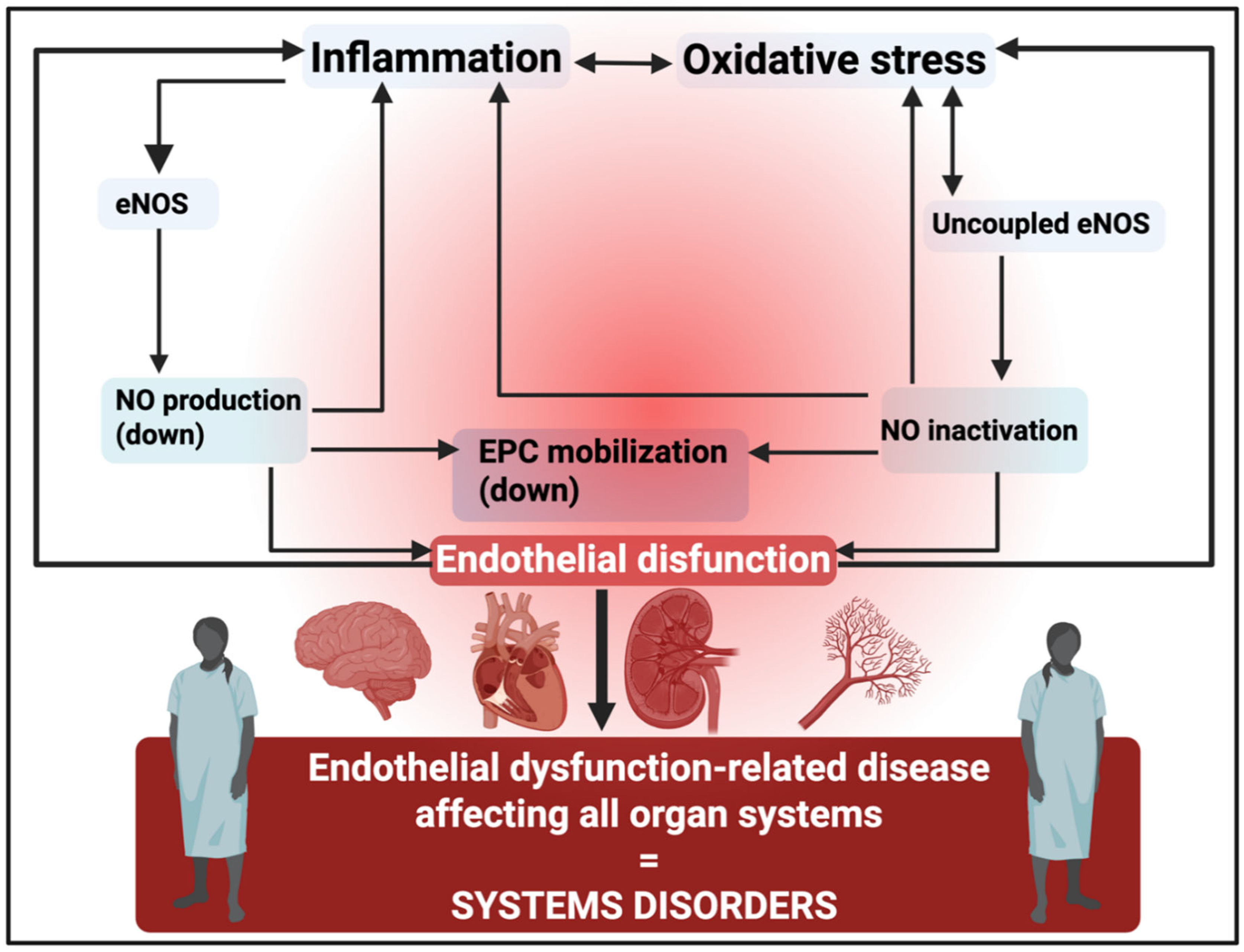

| Disease or syndrome | Comments | Selected references |
| Age-related macular degeneration (AMD) | Also related to cardiovascular issues. Note that the proteins in drusen, that is the insoluble material often associated with AMD, include amyloid A, amyloid-b, amyloid P, a1-antitrypsin, fibrinogen, etc. [12,13,14], importantly including abundant amyloid structures [15,16,17] that stain with the amyloid stain thioflavin T [18,19] | [20,21,22,23,24,25] |
| Cancers | Many vascular changes involved in all aspects of tumorigenesis, etc | [26,27,28,29,30,31,32] |
| Cardiovascular diseases | Strong relationship with microcirculation disruption | [33,34,35,36,37,38,39,40,41,42,43,44,45,46,47] |
| Choroid thickness after haemodialysis | [48] | |
| Chronic fatigue syndrome | Bears some similarities to Long COVID | [49,50,51,52,53,54] |
| Chronic venous insufficiency | [55] | |
| COVID and post-COVID | The key to recovery | [56,57,58,59,60,61,62,63,64,65,66,67,68] |
| Diabetes, type 2 | Recognised as a vascular disease | [69,70,71,72,73,74,75,76,77,78,79] |
| Diabetic complications | [80,81,82] | |
| Fibromyalgia | Clear likelihood of fibrinaloid microclot deposition | [83,84,85,86,87,88,89,90,91,92,93,94] |
| General reviews of microcirculation disruption | [95,96,97,98,99,100,101,102] | |
| Glaucoma | Relates to intraocular blood pressure | [103,104,105,106,107,108,109,110,111,112,113] |
| Hypertension | Capillary rarefaction seen as a major driver, at least in later stages. Increased blood pressure but lowered flux strongly implies that the latter causes the former. Put another way, there is an increased resistance to flow. This is entirely consistent with the known role of angiogenesis inhibitors in raising blood pressure [114,115]. | [41,74,114,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136] |
| Inflammatory bowel disease | [137,138,139,140,141,142] | |
| Metabolic syndrome | A comorbidity of many cardiovascular disease | [46,47,79,143,144,145,146,147,148,149,150,151] |
| Obstructive sleep apnoea | A common co-morbidity of many of these diseases, which implies the potential for a common aetiology and a common cure | [150,152,153,154,155] |
| Parkinson’s disease | [156] | |
| Pre-eclampsia | Clear hypertensive disorder, albeit involving cellular senescence [157] and likely an infectious origin [158,159] | [160,161,162,163] |
| Raynaud’s phenomenon | Strongly related to systemic scleroderma | [137,164,165,166,167,168,169,170] |
| Sepsis and septic shock | One of the most significant examples, with a high level of mortality. Strong evidence that lowered microcirculatory flux relates closely to mortality (and might hence offer protective treatments). | [171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200] |
| Sickle cell disease | Significant impacts on the microcirculation | [201,202,203,204,205,206,207,208] |
| Stroke (ischaemic) | Very clear evidence for a relation between microcirculation and multiple factors before and after an ischaemic stroke | [209,210,211,212,213,214,215,216,217,218,219,220,221] |
| Subarachnoid haemorrhage | Erythrocyte sedimentation rate (ESR) was the only measure predictive of a subsequent stroke in a detailed study [222] | [223,224,225,226,227,228,229,230,231,232,233] |
| Systemic sclerosis (scleroderma) | A major focus in the nailfold capillaroscopy field | [234,235,236,237,238,239,240,241,242,243] |
| Traumatic brain injury and other traumas | [244,245,246,247,248] |
| Disease or syndrome | Comments | Selected laser speckle imaging references |
Selected fibrinaloid microclot references (where tested) |
| Acute COVID-19 | Significant evidence of microvascular dysfunction | [56,436,437] | [317,318,319,320,321,322] |
| Acute respiratory distress syndrome | Severity correlates with lowered microcirculation | [56,438] | |
| Age-related macular degeneration | Also related to glaucoma | [439,440] | |
|
Alzheimer’s dementia (including mild cognitive impairment) |
Significantly lowered cerebral blood flow in Alzheimer’s dementia | [441,442,443,444,445,446,447,448,449] | [309,323,324,325,326] |
| Antineutrophil Cytoplasmic Antibody-Associated (ANCA) Vasculitis | Impaired microvascular function and blunted reactivity after occlusion | [450] | |
| Atopic dermatitis | Review showing marked differences, and treatment | [451] | |
| Behçet’s disease | Higher baseline flux | [450] | |
| Biliary cirrhosis | Significant microcirculation lesions |
[452] | |
| Burns | Lesions can occur at places distal to the burn site. Faster though less common than LDI. Useful in burn depth diagnosis. | [453,454,455,456,457,458,459,460,461,462,463,464,465,466,467] | |
| Cancers | There is a large literature, indicating issues with the microcirculation. A very small number of reviews at right. | [396,468] | |
| Chronic smokers | Led to Buerger’s disease, successful diagnosed (and cured) | [469] | |
| Cold urticaria | Attenuated response to cold challenge in patients with cold urticaria | [470] | |
| Connective tissue disorders | Includes Ehlers-Danlos syndrome | [471,472,473,474,475] | |
| Coronary heart disease | [476,477,478,479,480] | ||
| Dermatomyositis | [474,481] | ||
| Diabetes mellitus, type 1 | Decreased microcirculation flux. Can be ameliorated by a Chinese herbal formula. | [482,483,484,485] | |
|
Diabetes mellitus, type 2 |
Impaired microcirculation. Correlates with glycosylated haemoglobin A1c levels | [75,486,487,488,489,490] | [322,323,327,328,329] |
| Diabetic complications | Review | [491] | |
| Diabetic foot | [492,493,494] | ||
| Diabetic nephropathy | Decreased blood flow despite no lowering of vessel diameter (consistent with microclots) | [71] | |
| Diabetic neuropathy | [489,490,495,496] | ||
| Diabetic retinopathy | Decreased blood flow despite no lowering of vessel diameter (consistent with microclots). Microcirculation decrease precedes retinopathy. | [71,497,498,499] | |
| Digital ulcers | [500,501,502,503,504,505,506] | ||
| Endothelial (dys)function | [507,508,509,510] | [338] | |
| Erythromelalgia | [511] | ||
| Fibromyalgia | Seemingly no studies done | See [354], and for amyloid deposition in skeletal muscle [512] | |
| Gaucher disease | Seemingly no studies done | ||
| General reviews | [36,242,396,401,426,472,513,514,515,516] | ||
| Glaucoma | Evidence for vasculopathies | [104,105,106,517,518,519,520] | |
| Heart failure | [476,521,522,523,524,525] | ||
| Hepatitis, viral | Seemingly no studies done | ||
| Hypertension and hypertensives | As expected, raised blood pressure correlates with lower flow rates (implying that the latter is a cause of the former) | [526,527,528,529,530,531,532,533] | |
| Long COVID | Observable effects on the microcirculation well after the acute phase. Surprisingly few studies. | [436,534] | [313,314,315,330,331,332,333,334,335,336,337,338] |
| Lupus (systemic lupus erythematosus, SLE) | Functional and morphological microvascular impairments in patients with SLE |
[472,535,536,537,538] | |
| Migraine | Significant microcirculation changes relative to controls | [539,540,541,542,543,544] | [340] |
| Myalgic encephalopathy/ chronic fatigue syndrome | Despite the fact that is is clearly an endotheliopathy associated with a deranged microcirculation, and with similarities to Long COVID [52,341,545] we have found no relevant studies | [275,342,343,344,355] | |
| Obstructive sleep apnoea | [150,155,489,546] | ||
| Parkinson’s disease | Allowed analysis of function of vasomotor small fibers | [547] | [323,345,346] |
| Polycythemia vera |
[548] | ||
| Polymyositis | [474,481] | ||
| Port wine stain | Convenient non-invasive measurement / diagnostic | [549] | |
| Pre-eclampsia | Microcirculation impaired | [550,551,552] | |
| Psoriasis | Perilesional increased perfusion and perfusion inhomogeneity predictive of lesion expansion after two weeks |
[553,554,555,556] | |
| Pulmonary arterial hypertension |
[501,502,503,557] | ||
| Raynaud’s disease or Raynaud’s phenomenon (a transient digital ischaemia, often related to systemic sclerosis) | Laser speckle analysis is little known relative to nailfold capillaroscopy [169]. | [241,538,558,559,560,561,562,563] | |
| Rheumatoid arthritis | [564,565,566,567] | [347,348,349] | |
| Sarcopenia | [568,569,570] | ||
| Sepsis and septic shock | Can discriminate sepsis from septic shock and lowered blood flow is a high predictor of mortality. The odds ratio of predicting survival based on the presence of fibrinaloid microclots was more than 5 [296]. | [571,572,573,574,575] | [296] and see [290] |
| Sickle cell disease | Microcirculation significantly impaired | [201,576,577,578] | |
| Stroke (ischaemic) | Very useful technique for monitoring and prediction | [570,576,579,580,581,582,583,584,585,586,587,588,589,590] | |
| Subarachnoid haemorrhage |
[591] | ||
| Systemic sclerosis | Also the commonest area for nailfold capillaroscopy | [237,500,501,502,503,504,505,592,593,594,595,596,597,598,599] | |
| Traumatic brain injury | Clear effects in decreasing microcirculation | [600,601,602,603] |
| Disease or syndrome | Comments | Selected laser Doppler imaging references |
Selected fibrinaloid microclot references (where tested) |
| Acute COVID-19 | Significant evidence of microvascular dysfunction | [57,59,63,605,606,607,608] | [317,318,319,320,321,322] |
| Acute respiratory distress syndrome | Few studies but low microcirculation clearly observable | [609,610] | |
|
Alzheimer’s dementia (including mild cognitive impairment) |
Significantly lowered cerebral blood flow in Alzheimer’s dementia. Many more studies than with LSI. Care needed with age matching though [604]. Vascular impairment clearly related to Ab deposition. | [444,611,612,613,614,615,616,617,618,619,620,621,622,623] | [309,323,324,325,326] |
| Antineutrophil Cytoplasmic Antibody-Associated (ANCA) Vasculitis | Impaired microvascular function | [624] | |
| Atopic dermatitis | Evidence of impaired microvascular function, but surpisingly little recent literature | [625,626] | |
| Biliary cirrhosis | Significant microcirculation lesions |
[627,628] | |
| Burns | Utility in burn depth assessment for assisting clinical judgement. Microcirculation problems also occur at distal sites. Seemingly more frequently used here than LSI. | [456,466,629,630,631,632,633,634,635,636,637,638,639,640,641] | |
| Cancer | Somewhat lesser literature than for LSI (given the importance to tumours of vascularisation), but there are issues with the microcirculation in cancer and its treatment. A very small number of articles at right. | [642,643,644] | |
| Chronic smokers | Impaired microcirculation (many more papers than for LSI) | [645,646,647,648,649,650] | |
| Connective tissue disorders | [640,651,652,653,654,655] | ||
| Coronary heart disease | Surprisingly little directly | [656,657,658,659] | |
| Dermatomyositis | Lowered flow rate correlates with disease severity | [170,236,660,661] | |
| Diabetes mellitus, type 1 | A large literature implicating microcirculation defects | [73,662,663,664] | |
|
Diabetes mellitus, type 2 |
Impaired microcirculation. Correlates with glycosylated haemoglobin A1c levels | [72,307,646,647,662,664,665,666,667,668,669] | [322,323,327,328,329] |
| Diabetic complications | Reviews (note that most complications follow from impaired microcirculation | [491,664] | |
| Diabetic foot (ulcers) | [397,670,671,672,673] | ||
| Diabetic nephropathy | Decreased blood flow despite no lowering of vessel diameter (consistent with microclots) | [674] | |
| Diabetic neuropathy | [675,676] | ||
| Diabetic retinopathy | Decreased blood flow despite no lowering of vessel diameter (consistent with microclots). Microcirculation decrease precedes retinopathy. | [677,678] | |
| Digital ulcers | Often coupled to systemic sclerosis | [234,505,649,679,680,681] | |
| Endothelial (dys)function generally | [608,682,683,684,685,686,687,688] | [338] | |
| Fibromyalgia | Much more frequent use of LDI than of LSI | [88,91,689,690,691] (and for Complex Regional Pain Syndrome [692]) | See [354], and for amyloid deposition in skeletal muscle [512] |
| General reviews | [397,516,655,693,694,695,696] | ||
| ] | |||
| Glaucoma | [697,698,699] | ||
| Heart failure | Decreased microcirculation seen as a risk factor (causative) for worse outcomes | [700,701,702,703,704,705,706,707,708,709,710] | |
| Hepatitis, viral | [711,712] | ||
| Hypertension and hypertensives | As also seen with LSI, raised blood pressure correlates with lower flow rates (implying that the latter is a cause of the former) | [130,679,713,714,715,716] | |
| Inflammatory bowel disease | Measured as rectal blood flow | [717,718] | |
| Long COVID | Observable effects on the microcirculation well after the acute phase. Surprisingly few studies. | [52,67] | [313,314,315,330,331,332,333,334,335,336,337,338] |
| Lupus (systemic lupus erythematosus, SLE) | Functional and morphological microvascular impairments in patients with SLE |
[719,720,721] | |
| Migraine | [722] | [340] | |
| Myalgic encephalopathy/ chronic fatigue syndrome | Despite the fact that is is clearly an endotheliopathy associated with a deranged microcirculation, and with similarities to Long COVID [52,341,545] we have found only one relevant study | [52] | [275,342,343,344,355] |
| Obstructive sleep apnoea | Both improved by treatment | [152,723,724,725] | |
| Parkinson’s disease | Very few studies | [726] | [323,345,346] |
| Peripheral artery disease | Often related to diabetes | [649,679,727,728,729,730,731] | |
| Polymyositis | [170,661] | ||
| Port wine stain | Convenient non-invasive measurement / diagnostic | [549] | |
| Pre-eclampsia | Microcirculation impaired | [732,733,734,735,736] | |
| Psoriasis | [737,738,739,740,741,742] | ||
| Raynaud’s disease or Raynaud’s phenomenon (a transient digital ischaemia, often related to systemic sclerosis) | [169,170,562,743,744,745,746,747,748] | ||
| Rheumatoid arthritis | [749,750,751,752,753,754,755,756] | [347,348,349] | |
| Sarcopenia | [72] | ||
| Sepsis and septic shock | Microcirculation very important in sepsis. As with LSI, can discriminate sepsis from septic shock and lowered blood flow is a high predictor of mortality. | [757,758,759,760,761,762,763,764,765] | [296] and see [290] |
| Sjögren’s syndrome | [170] | ||
| Sickle cell disease | Microcirculation significantly impaired | [202,206,766,767,768] | |
| Stroke (ischaemic) | Very useful technique for monitoring and prediction | [769,770,771] | |
| Subarachnoid haemorrhage |
Note that impaired blood flow (measured by ESR) was the only predictor of a subsequent stroke [222] | [772,773,774,775,776] | |
| Systemic sclerosis | [169,234,235,236,241,243,366,504,505,514,557,592,594,595,679,680,681,744,777,778,779] | ||
| Traumatic brain injury | Clear effects in decreasing microcirculation as a result of damage following the trauma | [244,245,780,781] | |
| Urticaria | [782,783] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





