1. Introduction
Heart failure (HF) remains a leading cause of morbidity and mortality worldwide [
1,
2]. Despite transformative advances in HF therapies, their implementation and up-titration in clinical practice lags alarmingly behind. Only 22% of HF patients receive some form of triple therapy, and a mere 1% reach the recommended target doses of all essential medications [
3].
Previously reported barriers of GDMT implementation include therapeutic inertia, misperceptions about a “clinically stable” status, potential biases against older, female, or co-morbid patients and concerns over therapy-related adverse events (AEs) as hypotension, impaired renal function or hyperkalemia [
3,
4,
5,
6]. Clinicians tend to most frequently underuse GDMT in advanced HF (AHF) patients, due to a lack of strong evidence in this patient population and concerns about drug-related AEs, while these patients potentially benefit most [
7].
This study aimed to i) explore whether AHF patients can be up-titrated safely with HF medications and ii) assess the development of drug (and disease)-related AEs during medical up-titration in a dedicated HF outpatient unit in a tertiary care center.
2. Materials and Methods
Patient Population
Since 2015 a HFrEF registry at the Vienna General Hospital includes chronic HFrEF outpatients defined by a history of documented left ventricular ejection fraction (LVEF) of ≤35% by echocardiography and a history of significantly elevated NT-proBNP levels >1500pg/ml. Clinical data, patient history, cardiac imaging results, medications and laboratory values are routinely documented at every visit. For this study, data of patients between January 2015 and December 2023 were analyzed, who completed a baseline, short-term (2±1 months, 2M visit) and long-term follow-up visit (12±6 months, 12M visit). The investigations were conducted in strict adherence to the principles outlined in the Declaration of Helsinki and received institutional ethics committee approval (EK1612/2015). All participants provided written informed consent.
Patient and Public Involvement
Patients were not involved in the design, conduct, reporting, or dissemination plans of this research. This study was based on a prospective heart failure registry capturing routine clinical care at a tertiary outpatient heart failure clinic. While patients did not directly contribute to the study design, the research question was shaped by a clear clinical need to address gaps in evidence around the tolerability and implementation of heart failure therapies, particularly in advanced disease stages. The findings are intended to inform future clinical practice and improve therapeutic outcomes for patients with heart failure.
Assessing GDMT in HF
Precise use and exact dosage of HF medications, i.e., BB, RASi, MRA and SGLT2i were documented at each visit. For comparability, HF medication dosages were expressed as a percentage of the recommended target dosages. Non-receipt of a specific drug was recorded as 0%. Additionally, triple-therapy intensity was assessed by calculating the mean percentage of the TD for BB, RASi, and MRA [
] [
8,
9]. SGLT2i data in this paper was only analyzed for patients enrolled after 2021, in alignment with the ESC recommendations for SGLT2i use.
Definition of Clinical Factors Limiting GDMT Up-Titration - Adverse Events
The presence of clinical factors potentially limiting GDMT up-titration / AEs related to HF treatment were assessed at each visit. AEs were defined according to the 2021 ESC HF-guidelines [
8]. The most common clinical AEs were defined as follows: bradycardia, indicated by a resting heart rate (RHR) of <50 beats per minute (bpm); asymptomatic hypotension, identified by an office systolic blood pressure (SBP) of <90mmHg without symptoms; symptomatic hypotension, identified by an office SBP of <90mmHg with symptoms; impaired renal function, indicated by an estimated glomerular filtration rate (eGFR) of <30ml/min/1.73m
2; and hyperkalemia (HK), indicated by a serum potassium (K) level of >5.0mmol/l or >5.5mmol/l. AEs which were not present as the initial visit but developed at FUP during up-titration were termed as new AEs. In case HF-drugs were not up-titrated beyond 50% TD, medical records were investigated to identify the reason for no up-titration.
Statistical Analysis
To describe the patient population, baseline characteristics are presented. Continuous data are expressed as median and interquartile range (IQR), categorial data as counts and percentages. To compare the BL characteristics according to up-titrational success, counts were analyzed by the 2-sided Fisher’s exact test, and continuous variables by the Kruskal-Wallis test.
To assess the success of GDMT up-titration, TDs were compared by the Friedman test across all timepoints (paired, non-parametric test). To identify specific differences post hoc analysis was performed using pairwise Wilcoxon Signed-Rank Tests (paired, non-parametric test). To correct for multiple comparisons the Bonferroni correction was applied. For visualization TDs were summarized in four clinically relevant groups, i.e zero (0%), low (>0%–<50%), medium (≥50%–<90%), and high (≥90%) TDs [
9].
To identify factors associated with up-titrational success i.e., TDs of ≥90% at 12-month follow-up, a logistic bootstrap regression model with stepwise forward selection (p ≤0.05 for inclusion) as an exploratory method was used. Potential influencing factors considered included: age, sex, BMI, NYHA class, SBP, HR, K, eGFR, blood urea nitrogen (BUN), sodium, butyrylcholinesterase (BChE), GOT, GPT, GGT, bilirubin, transferrin saturation (TSAT), hemoglobin, triglycerides, C-reactive protein (CRP), NT-proBNP, number of comorbidities, and baseline TDs. The selection process followed the method described by Harrell [
10]. It accounts for potential non-linear associations and initially excludes variables with weak bivariate correlations (p >0.2). Variables selected in fewer than 40% of 500 bootstrap samples using forward selection were then excluded step by step. A final logistic regression with forward selection was performed on the remaining predictors. Results are reported in units of standard deviation. Alternative selection methods were tested by using continuous TD values, case-by-case exclusion of missing data or imputation by the mean, and consideration of first-order intercorrelations. These alternatives produced qualitatively consistent results for the key variables (p ≤0.01).
To visualize the main results of the logistic regression HF TDs groups were displayed for clinically relevant variables, i.e., NT-proBNP, eGFR, age, sex, BMI and comorbidity burden. In this analysis trends for GDMT up-titration were analyzed between the strata by the Jonckheere-Terpstra test (unpaired, non-parametric test, which calculates p for trend). Sex differences were compared via the Kruskal-Wallis H test (unpaired, non-parametric test).
The prevalence of AEs was displayed as percentages at different timepoints. AEs were compared by the McNemar-Bowker test (paired, categorical test) between timepoints. For an explorative analysis, the distribution of new AEs after 12 months according to subgroups (NT-proBNP, eGFR, age, BMI, sex and number of comorbidities) was compared by the 2-sided Fisher’s exact test (unpaired, categorial test).
IBM SPSS 25.0 and GraphPad Prism 9 were used to perform statistical analyses and create the figures. GChaos 31.3 statistical software written in C++ by one of the authors (GS) was used for the bootstrap selection process. A two-tailed p-value <0.05 was deemed statistically significant. In case of multiple testing, p-values were adjusted by Bonferroni correction.
3. Results
Study Population
Baseline characteristics for the total cohort (n=373) are displayed in
Table 1. The median age was 62years (IQR: 50-72), 23.3% of patients were women. 7.8%, 51.6% and 40.6% of patients were NYHA class I, II and III/IV, median NT-proBNP was 2363pg/ml (IQR: 1014-5009).
Up-Titration of HF Medication
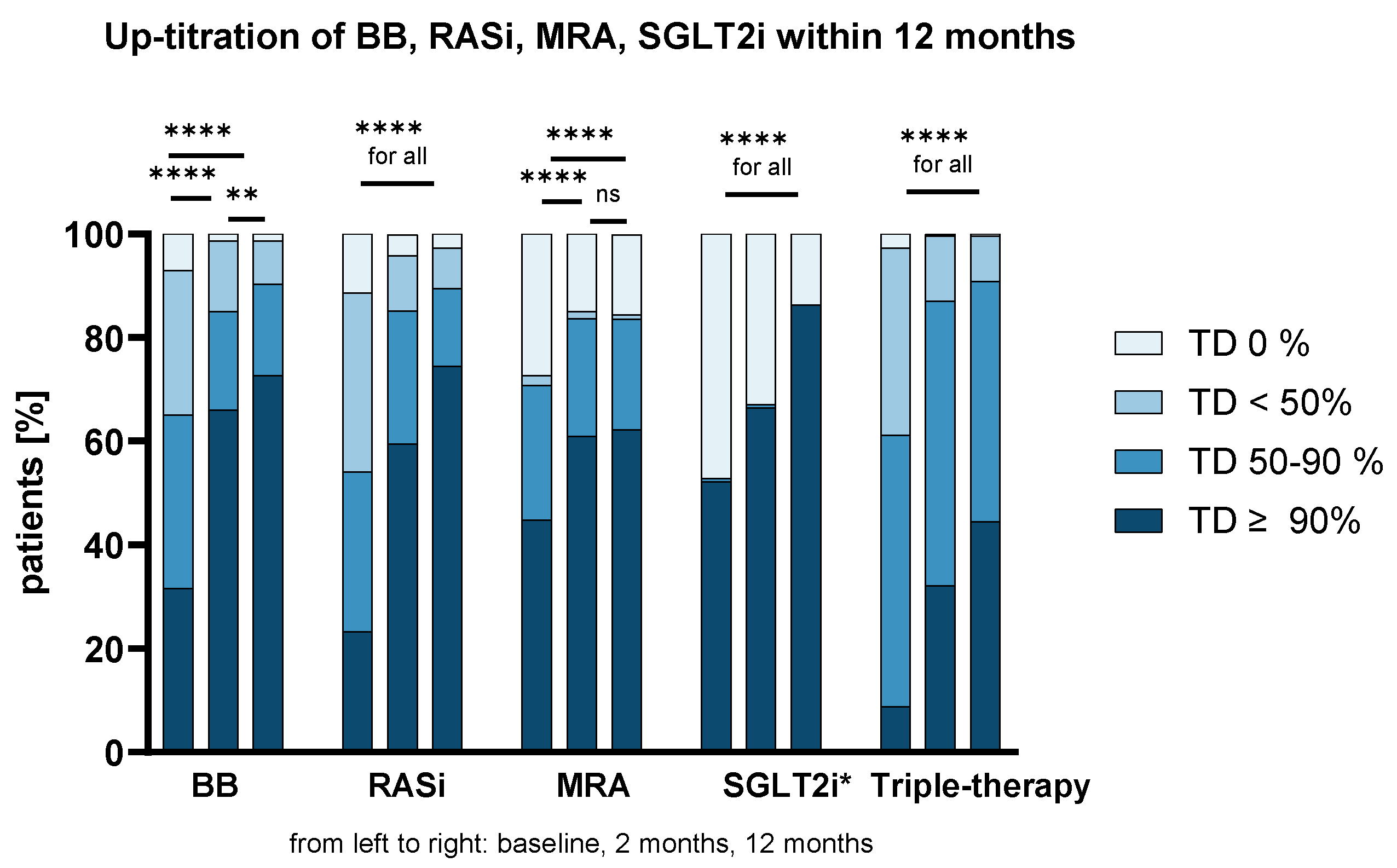
The achieved TDs for all HF drug classes at BL, 2M and 12M are shown in
Figure 1. A significant increase in TDs could be observed for all drug classes during FUP (p<0.001 for comparisons between BL vs 2M and BL vs 12M for the continuous variable of TDs). The majority of patients received ≥50% and ≥90% of the recommended TDs at 12M (for ≥50%: 90%, 90%, 84%, 86% and 91%; and for ≥90%: 73%, 75%, 62%, 86% and 45% for BB, RASi, MRA, SGLT2i and triple-therapy, respectively).
Predictors for Successful Up-Titration– Impact of Disease Severity and Patient Characteristics
A multiple linear regression was conducted to determine the predictors of achieved TD of triple-therapy at 12 months (
Table 2). The overall model was statistically significant (R
2=0.150, Cox & Snell). Significant predictors were eGFR (ß=0.679, p<0.001), serum potassium (ß=-0.240, p=0.048) and triple-therapy at baseline (ß=0.523, p<0.001).
Successful up-titration was independent from heart failure severity reflected by NT-proBNP or NYHA class, but also age, comorbidity burden, sex and BMI.
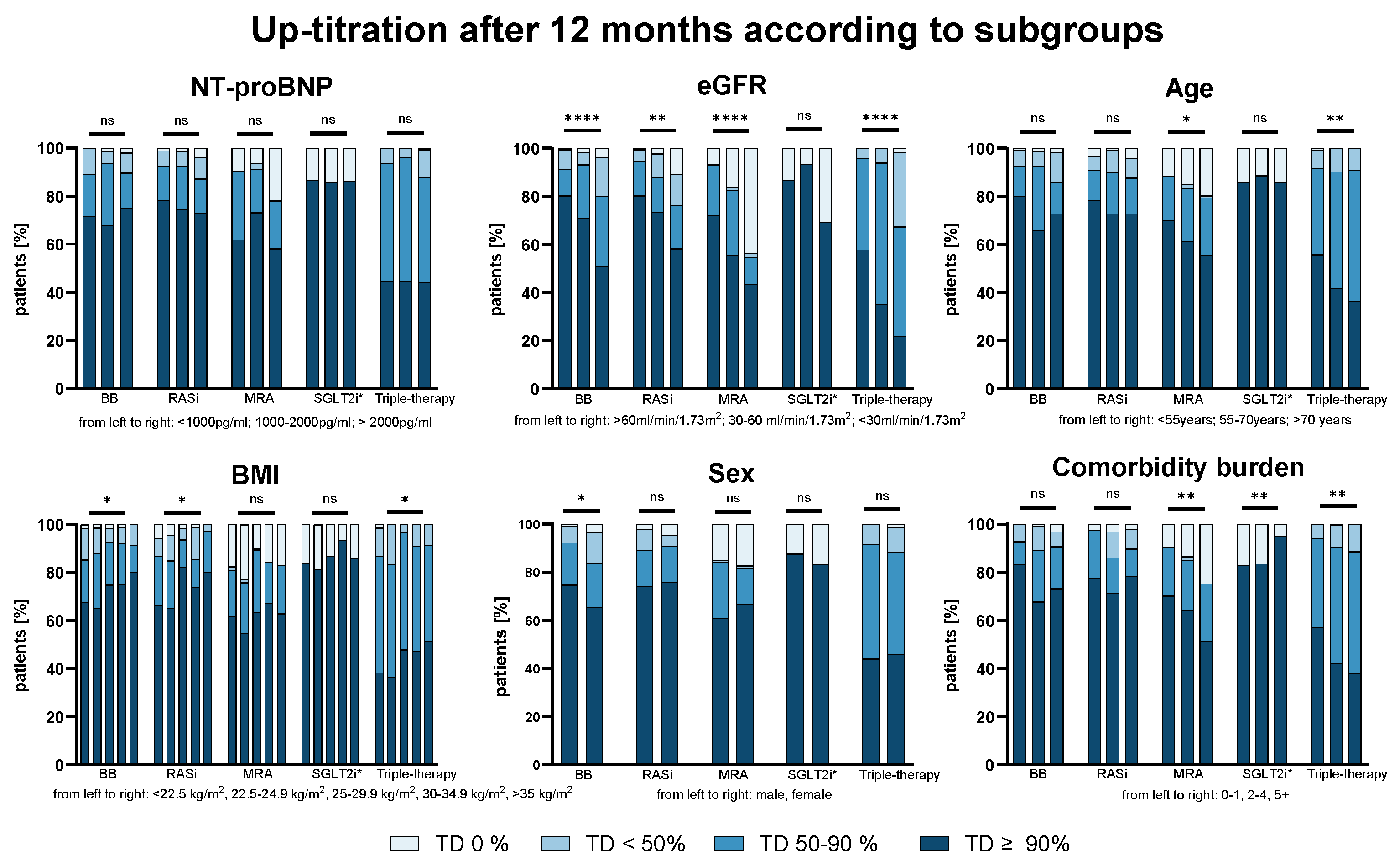
Figure 2 displays the main findings graphically. However, a consistent trend for lower GDMT was observable with increasingly impaired renal function (BB: p<0.001, RASi: p=0.001, MRA: p<0.001, SGLT2i: p=ns, triple-therapy: p<0.001).
Frequency of AEs – Impact of Disease Severity and Patient Characteristics
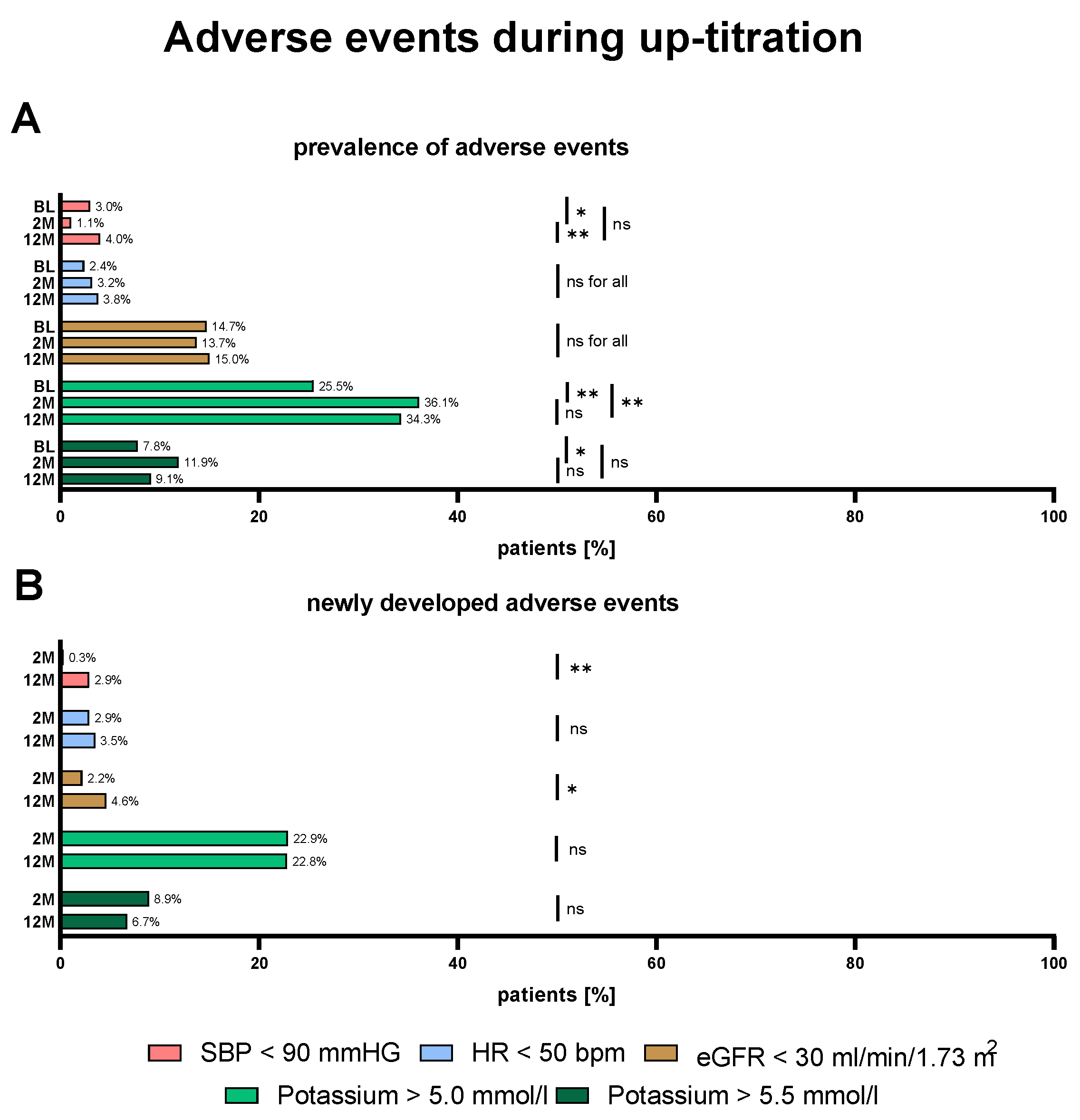
Figure 3 shows the prevalence and development of AEs at BL, 2M and 12M. At baseline 3% of patients exhibited hypotension, 2.4% bradycardia, 14.7% impaired renal function and 25.5% mild (K >5.0mmol/l) and 7.8% significant (K >5.5mmol/l) hyperkalemia. The development of new AEs was generally infrequent, with less than 5% of cases experiencing hypotension, bradycardia, and renal impairment at 2M and 12M. The prevalence of hypotension, bradycardia and impaired renal function remained similar during medical up-titration. New cases of hyperkalemia were the most frequent new AE and developed in 22.9% and 22.8% (K >5.0mmol/l) and 8.9% and 6.7% (K >5.5mmol/l) of cases after 2 and 12 months.
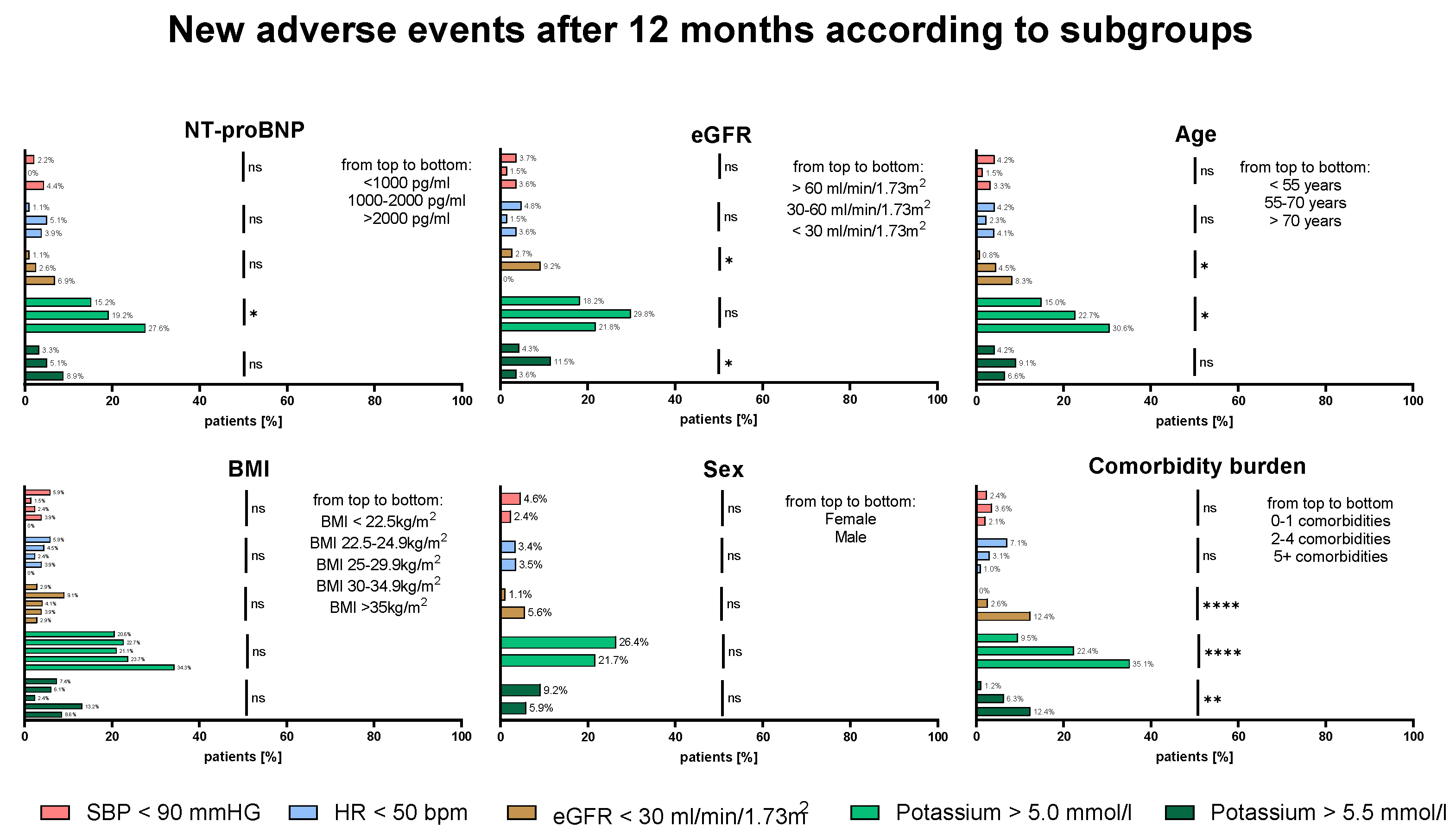
Figure 4 shows an explorative analysis for the development of HF drug related AEs at 12M for clinically relevant patient subgroups. New AEs as hypotension, bradycardia or impaired renal function were equally distributed across NT-proBNP strata, sex or BMI (p=ns for all). Only the development of hyperkalemia (K >5.0mmol/l at 12M) increased with higher NT-proBNP (15.2% vs 19.2% vs 27.6%, p=0.045). Generally, the development of hyperkalemia (K >5.0mmol/l at 12M) and impaired renal function (eGFR <30ml/min/1.73m
2) was associated with higher age and increased comorbidity burden. Both higher age and increased comorbidity burden predispose for worse baseline renal function.
Reasons for no Up-Titration (TD ≤ 50%) at 12M
Table 3 shows the reason for maintaining suboptimal TDs. After 12 months, 23%, 22%, and 38% of patients could not be up-titrated beyond 50% of the recommended TD. Likely causes could be identified for 92%, 91% and 75% of BB, RASi, and MRA cases. The most common causes were bradycardia and hypotension for BBs (in total accounting for 66%), hypotension and hyperkalemia for RASi (in total accounting for 61%), and hyperkalemia with or without impaired renal function for MRAs (in total accounting for 58%). Among all HFrEF medications, MRAs were most likely to remain at suboptimal dosages and showed the highest number of cases where no reason could be identified. Hyperkalemia accounted for a total of 31% and 43% of cases for suboptimal dosages of RASi and MRA.
4. Discussion
This is the first study to investigate differences in GDMT implementation according to the severity of heart failure and to deliver data on the development of AEs during up-titration of GDMT in AHF in a tertiary care outpatient HF center. GDMT therapy implementation was good, whereby 91% of patients achieved ≥50% of the TDs in all recommended drug classes at 1-year (Central Illustration). GDMT up-titration could be successfully achieved regardless of NT-proBNP strata or sex. Despite successful up-titration, new AEs remained rare, with only 4%, 4%, 5% and 7% of patients developing bradycardia, hypotension, severe renal impairment (eGFR <30ml/min/1.73m2) and severe hyperkalemia (K >5.5mmol/l). A trend for increasing number of AEs and lower average dosages in GDMT was observed in patients with older age, worse baseline renal function and higher comorbidity burden.
GDMT Evidence in AHF
In a recent Universal Definition of heart failure from multiple HF societies AHF failure is defined by several key characteristics, such as severe symptoms or symptoms at rest, recurrent HFH despite GDMT, and requirement of advanced therapies such as mechanical circulatory support [
11]. Most importantly, also intolerance to up-titration of GDMT is part of this definition.
Other definitions as the updated HFA-ESC definition, refer to symptoms, NT-proBNP, cardiac dysfunction, but do not mention the inability of up-titration [
12].
There is no established consensus on when to halt GDMT up-titration or how to accurately define true medication intolerance. Moreover, evidence on the efficacy or futility of HF medications in patients with AHF is scarce, leaving clinicians with limited guidance for optimizing treatment in this high-risk population.
Trials in high-risk, AHF patients, such as CONSENSUS, COPERNICUS, and RALES, demonstrated the efficacy of ACEi, BBs, and MRAs in this population, with significant reductions in mortality and the combined risk of death or HF hospitalization [
13,
14,
15]. Similarly, in PARADIGM-HF, ARNI showed consistent efficacy regardless of NT-proBNP levels, including those above and below the median of 1631pg/ml, although patients in general were lower risk [
16].
GDMT Implementation and Clinical Patient Profiles in AHF
Global registries reveal suboptimal use of GDMT in HFrEF, while the STRONG-HF study demonstrated better results after acute heart failure [
3,
6,
9,
18,
19].
Up-titration of all four pillars of GDMT was successfully achieved across all heart failure risk groups. TDs were consistent in the low-risk group with median NT-proBNP 508pg/ml [378-707], the intermediate-risk group with median NT-proBNP 1430pg/ml [1216-1719] corresponding to a PARADIGM-HF cohort, and the high-risk group with median NT-proBNP 4740pg/ml [3199-8397], corresponding to a typical cohort of patients treated with a ventricular assist device [
20].
Patient Characteristics Predisposing for Suboptimal GDMT
Patients with more severe heart failure, greater symptoms, or comorbidities often receive lower doses of GDMT. Studies consistently show that factors like older age, higher NYHA-class, greater NT-proBNP levels, low blood pressure, female sex, poor kidney function, and the presence of other comorbidities hold the physicians off up-titration of GDMT [
3,
4,
6,
18,
21,
22].
In the present dataset after structured up-titration no bias regarding GDMT implementation based on sex could be observed. Most importantly, GDMT implementation was comparably successful between NT-proBNP strata, indicating that up-titration is feasible and worth pursuing in most severe disease.
In line with previous reports besides eGFR, older age, lower BMI and high comorbidity burden were indeed associated with somewhat worse implementation of GDMT, in univariate analysis. In multivariate analysis kidney function and potassium remained significant, indicating that the influence of age, BMI and comorbidities is largely mediated through eGFR.
Notably, in a logistic regression model NT-proBNP did not predict up-titration at follow-up.
Development of Adverse Events During Up-Titration
The presumably largest limiting factor for GDMT implementation is the expectation of development of HF drug-related AEs. However, data on AEs during up-titration in AHF remains scarce, and factors limiting GDMT are often underreported or poorly documented in studies and registries. Notably, the achievement of high TDs in registries is far less common than observed in this study. In BIOSTAT-CHF drug intolerance was cited as a reason for not achieving target doses in 22% of BB patients and 26% of ACEi/ARB, though in most cases, the reasons were unknown or unrecorded [
6]. A secondary analysis of GUIDE-IT found that common reasons for avoiding up-titration included the perception of “clinically stable” status or being “already at maximally tolerated therapy” [
4]. GUIDE-IT reported an overall low rate of symptomatic hypotension (2%), symptomatic bradycardia (0%), hyperkalemia (2.5%), and worsening renal function (3.6%), but did not differentiate between disease severity [
17].
A recent meta-analysis highlighted the misperception of the development of AEs related to HF therapy. The study included landmark cardiovascular outcome trials with forced up-titration in HFrEF and investigated the occurrence of AEs between different HF drugs and placebo [
23]. Almost all clinically relevant AEs were rare and occurred at similar rates in both treatment and control groups. This indicates that events such as drops in blood pressure, worsening renal function, or hyperkalemia are not primarily attributable to specific therapies but probably rather reflect the underlying risks associated with HF itself. The overall frequency of drug-related AEs in these trials was low [
23]. It can be presumed that these underlying risks are a function of disease stages.
This study reinforces these findings showing that the development of HF drug-attributable AEs during medical up-titration is low and that up-titration can be achieved in a dedicated setting. Moreover, it contributes new insights into the tolerability of GDMT and the occurrence of AEs across different risk groups, especially AHF and other HF subpopulations. Our analysis identified two key factors as particularly relevant for GDMT up-titration: hyperkalemia and impaired kidney function, both of which were more common in high-risk patients. Notably, higher baseline eGFR and lower potassium levels emerged as strong predictors of successful up-titration. Importantly, modern evidence suggests that neither impaired kidney function nor HK should be considered insurmountable barriers to GDMT optimization, as both conditions can often be effectively managed.
HK is a common issue in heart failure, linked to use of RASi and MRA, as well as severe HF, renal impairment, diabetes, and older age [
24]. However, HK is now manageable with potassium-binding agents, enabling higher doses of RASi and MRAs [
25,
26]. Poor kidney function should not preclude GDMT, as studies like STOP-ACEi and the Swedish HF Registry show continuing RASi and MRAs is safe, even in severe renal dysfunction [
27,
28]. Moreover, therapies like SGLT2i and ARNI stabilize renal function long-term [
29,
30]. Additionally, a recent study demonstrated benefits of ARNI treatment even in patients with end stage renal disease requiring dialysis [
31]. These findings highlight the importance of maintaining GDMT despite HK or renal concerns to optimize outcomes in high-risk HF patients.
5. Conclusions
This study demonstrates that GDMT can be successfully implemented at much higher rates than reported in previous registries, especially among AHF. Importantly, AEs are generally infrequent and mainly not directly attributable to AHF. These findings emphasize that AEs, though more common in high-risk patients, should not deter GDMT up-titration. Further research is needed to establish stronger evidence on the safety, tolerability, and especially benefits of GDMT up-titration in AHF.
Limitations
This study has several limitations. The specialized HF outpatient setting may not reflect non-specialized care, limiting applicability to primary care or community hospitals. Potential confounders, such as patient adherence, patient requests, and multidisciplinary management, could influence treatment consistency and up-titration decisions. While the study emphasized GDMT up-titration and AEs, other critical factors like quality of life, functional improvement, and risk reduction warrant consideration. Additionally, evolving therapies, such as SGLT2i, Vericiguat, and potassium-binders, were gradually implemented in our patient cohort, which may have impacted GDMT implementation and related factors during the study period (2015–2023).
Author Contributions
Conceptualization, N.G.P., N.P. and M.H.; methodology, N.G.P., G.S., N.P. and M.H.; software, M.S., G.S.; validation, G.S.; formal analysis, N.G.P, A.W., S.P., M.S., G.S.; investigation, G.H., H.A., G.S., G.G., P.E.B. and C.H.; resources, C.H.; data curation, N.G.P, M.S, G.S.; writing—original draft preparation, N.G.P.; writing—review and editing, N.G.P., A.W., S.P., M.S., G.S., G.S., G.G., H.A., P.E.B. G.H., C.H., N.P., M.H.; visualization, N.G.P.; supervision, N.P., M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of the Medical University of Vienna (EK1612/2015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data underlying this article cannot be shared publicly due to ethical and privacy concerns regarding the individuals who participated in the study. The data will be shared on reasonable request to the corresponding author.
Acknowledgments
The central illustration was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACEi |
Angiotensin-Converting Enzyme Inhibitor |
| AHF |
Advanced Heart Failure |
| ARB |
Angiotensin Receptor Blocker |
| ARNI |
Angiotensin Receptor-Neprilysin Inhibitor |
| BB |
Beta Blocker |
| eGFR |
Estimated Glomerular Filtration Rate |
| GDMT |
Guideline Directed Medical Therapy |
| HF |
Heart Failure |
| HFA |
Heart Failure Association |
| HFrEF |
Heart Failure with reduced Ejection Fraction |
| HK |
Hyperkalemia |
| IQR |
Interquartile Range |
| LVEF |
Left Ventricular Ejection Fraction |
| MRA |
Mineralocorticoid Receptor Antagonist |
| NYHA |
New York Heart Association |
| RASi |
Renin Angiotensin System Inhibitor |
| RHR |
Resting Heart Rate |
| SGLT2i |
Sodium-Glucose Transporter 2 Inhibitor |
References
- Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats AJS. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. 2023;118(17):3272-3287. [CrossRef]
- Mamas MA, Sperrin M, Watson MC, et al. Do patients have worse outcomes in heart failure than in cancer? A primary care-based cohort study with 10-year follow-up in Scotland. Eur J Heart Fail. 2017;19(9):1095-1104. [CrossRef]
- Greene SJ, Butler J, Albert NM, et al. Medical Therapy for Heart Failure With Reduced Ejection Fraction. J Am Coll Cardiol. 2018;72(4):351-366. [CrossRef]
- Fiuzat M, Ezekowitz J, Alemayehu W, et al. Assessment of Limitations to Optimization of Guideline-Directed Medical Therapy in Heart Failure From the GUIDE-IT Trial: A Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol. 2020;5(7):1. [CrossRef]
- Savarese G, Lindberg F, Christodorescu RM, et al. Physician perceptions, attitudes, and strategies towards implementing guideline-directed medical therapy in heart failure with reduced ejection fraction. A survey of the Heart Failure Association of the ESC and the ESC Council for Cardiology Practice. Eur J Heart Fail. 2024;26(6):1408-1418. [CrossRef]
- Ouwerkerk W, Voors AA, Anker SD, et al. Determinants and clinical outcome of uptitration of ACE-inhibitors and beta-blockers in patients with heart failure: a prospective European study. Eur Heart J. 2017;38(24):1883-1890. [CrossRef]
- Peterson PN, Rumsfeld JS, Liang L, et al. Treatment and risk in heart failure: gaps in evidence or quality? Circ Cardiovasc Qual Outcomes. 2010;3(3):309-315. [CrossRef]
- McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2022;24(1):4-131. [CrossRef]
- Cotter G, Deniau B, Davison B, et al. Optimization of Evidence-Based Heart Failure Medications After an Acute Heart Failure Admission: A Secondary Analysis of the STRONG-HF Randomized Clinical Trial. JAMA Cardiol. 2024;9(2):114-124. [CrossRef]
- Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361-387. [CrossRef]
- Bozkurt B, Coats AJ, Tsutsui H, et al. Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail. 2021;27(4):387-413. [CrossRef]
- Crespo-Leiro MG, Metra M, Lund LH, et al. Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20(11):1505-1535. [CrossRef]
- The Consensus Trial Study Group. Effects of Enalapril on Mortality in Severe Congestive Heart Failure. N Engl J Med. 1987;316(23):1429-1435. [CrossRef]
- Packer M, Fowler MB, Roecker EB, et al. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002;106(17):2194-2199. [CrossRef]
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709-717. [CrossRef]
- McMurray JJV, Packer M, Desai AS, et al. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. N Engl J Med. 2014;371(11):993-1004. [CrossRef]
- Felker GM, Anstrom KJ, Adams KF, et al. Effect of Natriuretic Peptide–Guided Therapy on Hospitalization or Cardiovascular Mortality in High-Risk Patients With Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA. 2017;318(8):713-720. [CrossRef]
- Brunner-La Rocca HP, Linssen GC, Smeele FJ, et al. Contemporary Drug Treatment of Chronic Heart Failure With Reduced Ejection Fraction: The CHECK-HF Registry. JACC Hear Fail. 2019;7(1):13-21. [CrossRef]
- Mebazaa A, Davison B, Chioncel O, et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): a multinational, open-label, randomised, trial. Lancet. 2022;400(10367):1938-1952. [CrossRef]
- Adlbrecht C, Hülsmann M, Wurm R, et al. Outcome of conservative management vs. assist device implantation in patients with advanced refractory heart failure. Eur J Clin Invest. 2016;46(1):34-41. [CrossRef]
- Cowie MR, Schöpe J, Wagenpfeil S, et al. Patient factors associated with titration of medical therapy in patients with heart failure with reduced ejection fraction: data from the QUALIFY international registry. ESC Hear Fail. 2021;8(2):861. [CrossRef]
- Greene SJ, Fonarow GC, DeVore AD, et al. Titration of Medical Therapy for Heart Failure With Reduced Ejection Fraction. J Am Coll Cardiol. 2019;73(19):2365-2383. [CrossRef]
- Harrington J, Fonarow GC, Khan MS, et al. Medication-Attributable Adverse Events in Heart Failure Trials. JACC Hear Fail. 2023;11(4):425-436. [CrossRef]
- Grobbee DE, Filippatos G, Desai NR, et al. Epidemiology and risk factors for hyperkalaemia in heart failure. ESC Hear Fail. 2024;11(4):1821. [CrossRef]
- Butler J, Anker SD, Lund LH, et al. Patiromer for the management of hyperkalemia in heart failure with reducedejection fraction: the DIAMOND trial. Eur Heart J. 2022;43(41):4362. [CrossRef]
- Weir MR, Rossignol P, Pitt B, et al. Patiromer-Facilitated Renin-Angiotensin-Aldosterone System Inhibitor Utilization in Patients with Heart Failure with or without Comorbid Chronic Kidney Disease: Subgroup Analysis of DIAMOND Randomized Trial. Am J Nephrol. 2024;55(6):1-18. [CrossRef]
- Bhandari S, Mehta S, Khwaja A, et al. Renin–Angiotensin System Inhibition in Advanced Chronic Kidney Disease. N Engl J Med. 2022;387(22):2021-2032. [CrossRef]
- Guidetti F, Lund LH, Benson L, et al. Safety of continuing mineralocorticoid receptor antagonist treatment in patients with heart failure with reduced ejection fraction and severe kidney disease: Data from Swedish Heart Failure Registry. Eur J Heart Fail. 2023;25(12):2164-2173. [CrossRef]
- The EMPA-KIDNEY Collaborative Group, Herrington WG, Staplin N, et al. Empagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2023;388(2):117-127. [CrossRef]
- Damman K, Gori M, Claggett B, et al. Renal Effects and Associated Outcomes During Angiotensin-Neprilysin Inhibition in Heart Failure. JACC Hear Fail. 2018;6(6):489-498. [CrossRef]
- Le D, Grams ME, Coresh J, Shin JI. Sacubitril-Valsartan in Patients Requiring Hemodialysis. JAMA Netw Open. 2024;7(8):e2429237-e2429237. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).