1. Introduction
Here I briefly review results functional imaging of language in neurotypical people that have revealed a reliable “language network” in the left frontal, temporal, and parietal cortex. These studies that have averaged data from healthy controls have yielded convergent, highly valuable insights into areas of the brain that work together to support language. Then I review longitudinal functional imaging studies of recovery of language after stroke that have reported average results across a group or groups. This review reveals contrasting results with respect to both changes in cortical activation and changes in connectivity associated with recovery across studies. I argue that such contrasting results are inevitable, as studies report average results across groups of people with different volumes and locations of strokes (as well as different demographics). I then review longitudinal functional imaging studies of individuals with post-stroke aphasia (PSA) that illustrate the many distinct ways brain networks can change with recovery of language, depending on not only time post-stroke, but also lesion characteristics, patient characteristics, and interventions.
2. Functional Imaging Studies of Language in People Without Brain Lesions
Functional imaging studies of heathy individuals have reliably revealed a network or cortical regions that are activated in nearly every language task. Although some designs have revealed distinct areas activated more during one language task versus another (e.g., naming actions versus objects) nearly all language tasks engage a network of regions of left hemisphere when contrasted with low level baselines that are primarily attentional or perceptual. Nodes of this “language network” nearly always include left (more than right) posterior inferior frontal gyrus (pIFG; often referred to as “Broca’s area”), posterior superior temporal cortex (pSTG, often referred to as “Wernicke’s area”), middle temporal gyrus (MTG), inferior temporal gyrus or fusiform gyrus (FuG), dorsolateral prefrontal cortex (DLPFC), supramarginal gyrus (SMG) and angular gyrus (AG). This language network has been revealed by functional MRI (fMRI) studies across tasks of word retrieval [
1], passive viewing and listening to discourse [
2], comprehension [
3], and reading [
4], as well as by PET studies (see[
5] for review). Unsurprisingly, tasks with visual stimuli also activate occipital areas not central to the language network unless compared to a baseline with similar visual demands.
These brain regions that comprise the “language network” are nodes of a network revealed by task-free (“resting state”) fMRI, as indicated by highly correlated Blood Oxygenation Level Dependent (BOLD) activation (referred to as “high connectivity”) [
6]. However, superior frontal cortex is also among the nodes with high connectivity with language network areas [
6].
Of note, essentially all functional imaging studies of language also show activation of at least some right hemisphere regions homologous to the language network, although typically lower than seen in the left hemisphere [
5,
7,
8]. These studies indicate that right hemisphere homologues to language network might have a supportive role in language processing.
Functional imaging studies of neurotypical controls that have formed our understanding of the brain network underlying language have largely been conducted in group studies, showing significant activation for the group. However, individual neurotypical controls reliably show the same areas of activation associated with language as do groups, although longer imaging studies (generating more data) are often required to obtain significant results.
3. Functional Imaging Studies of Recovery in Groups of People with PSA
Many clinical investigations are launched with the expressed aim to identify the areas of the brain activated by language when part of the language network is damaged by stroke. The goal of these studies is to use groups of patients averaged together to identify the neural basis of recovery from post-stroke aphasia. Do healthy regions in the left hemisphere or in the right hemisphere “take over” the functions of the damaged nodes of the network? Answers to such questions have important clinical as well as scientific impact. For example, should rehabilitation of aphasia focus on tasks or interventions (e.g., non-invasive brain stimulation) that recruit the right hemisphere?
Unfortunately, unlike broader characterization of healthy function, there are seemingly conflicting answers to these critical questions in groups of patients with lesions. A summary of results across studies are reported in
Table 1. An early study of a group of seven participants who had substantially recovered language after aphasia by the five months after stroke revealed significantly increased activation of right hemisphere areas, and non-significant increase in left hemisphere areas, compared to controls, associated with a lexical-semantic task [
9]. Participants with post-stroke aphasia who recovered best showed bilateral activation of language network regions and their homologues. In contrast, in a study of four participants with post-stroke aphasia and four controls, positive activation associated with picture identification was observed predominantly in peri-lesional areas (along with negative activation in the right hemisphere). Both PSA and control groups activated left frontal and temporal language areas, but in those with PSA, the frontal region activation was spread over a larger peri-lesional area. They concluded that people recovering from post-stroke aphasia use functional peri-lesional areas to perform language functions [
10]. However, a study of 82 people with PSA and 82 controls showed no evidence of peri-lesional activation associated with naming or semantic decision in post-stroke aphasia recovery [
11]. Instead, they proposed that aphasia recovery depends on normalization of language network and possibly recruitment of alternative areas of the cortex, independent of the distance from the lesion. Very recently, the same group reported a large study of 76 people with chronic PSA and 69 neurotypical controls engaged in a semantic decision task. They showed, on average, right hemisphere activation associated with the language task was higher in participants with PSA than in controls. Furthermore, greater right hemisphere activation was associated with higher education, younger age, and left-handedness, but (in contrast to previous studies) was not associated with lesion size or longer time since onset of stroke. Finally, only those with PSA activated right dorsal inferior frontal gyrus during this task [
12].
Saur and colleagues studied 14 people with PSA acutely (0-4 days), sub-acutely (2-3 weeks), and chronically (4-12 months) with a sentence comprehension task [
3]. They report minimal activation acutely, mostly increased right hemisphere activation sub-acutely, and finally normalization of left hemisphere language network chronically. But another group who studied 17 people with PSA at 2, 6, 17, 26, and 52 weeks post-stroke reported mostly increased left temporal and left cerebellar activation during a semantic fluency task over time [
13]. There was also a shift toward left frontal lateralization. They also observed temporary compensatory activation of the right hemisphere but concluded it was not important in recovery, as recovery was driven by re-activation of left fronto-temporal regions. However, a separate study of eight people with mild PSA and left IFG lesions showed increased activation of right frontal and left cerebellum associated with learning effects on a word-retrieval task [
14].
Some studies have divided PSA into separate groups to determine if there are distinct mechanisms of recovery or studied people with PSA longitudinally (as summarized in
Table 2). One study of nine individuals who had recovered well from PSA showed predominantly left hemisphere activation during language, while 18 people who had limited recovery showed mostly right hemisphere activation. They concluded that a right hemisphere “shift” is an ineffective mechanism in language recovery [
15]. Another evaluated 17 people with mostly frontal strokes and 17 with temporoparietal strokes as the cause of PSA. They found that both groups showed reduced activation during language, including in areas far from the lesion (diaschisis) followed by reactivation of the normal language network (i.e. resolution of diaschisis) by 2-3 weeks. By 6 months, both groups showed increased activity of perilesional cortex and reorganization of left temporal language areas in the chronic phase. They found activation of right hemisphere homologues in the group with frontal but not temporo-parietal lesions [
16]. An investigation of resting state connectivity before and after treatment in a trial of lexical therapy demonstrated differences between groups dichotomized by high improvement with therapy versus low improvement with therapy [
17]. Those who showed good improvement showed an increase in average connectivity between left fusiform gyrus (an area shown to be critical for picture naming) and other regions in the language network, as well as increased connectivity between left fusiform cortex and right hemisphere homologues after treatment. Those who showed no or poor improvement with treatment showed decreased connectivity between left fusiform gyrus and left language network and right hemisphere homologues.
A recent study that used functional near infrared spectroscopy (fNIRS) both to guide transcranial magnetic stimulation (TMS) and to evaluate changes in language activation before and after an intensive language therapy in eight people with chronic PSA. They found that average changes in activation depended on the treatment group [
18]. Participants who received low frequency stimulation to the right inferior frontal gyrus (IFG) showed reduced activation in both hemispheres, but stronger activation in left than right hemisphere with improvement in language after treatment. In contrast, those who received high frequency stimulation to right IFG showed increased right relative to left hemisphere activation with improved language after therapy. Hartwigsen and Saur recently provided a comprehensive review of fMRI and PET studies of aphasia recovery with various treatments, showing a variety of changes in average activation associated with treatment success across studies, including: (1) Upregulation of right hemisphere regions; (2) Downregulation of right hemisphere regions; (3) Upregulation of bilateral regions; (4) Upregulation of perilesional or spared left hemisphere regions; (5) Downregulation of perilesional or spared left hemisphere regions [
19].
This review of the functional imaging group studies of PSA recovery clearly reveals conflicting results across studies. This conclusion is not surprising, given that the groups studied were variable in terms of stroke characteristics and given that different tasks were evaluated with fMRI. Moreover, each study averaged the results across people with aphasia who have different lesions, and often different times post-stroke, different degrees of recovery, different initial deficits. Some studies have shown that average site of activation does depend on the time post-stroke [
3,
16]; others have shown that average site of activation depends on lesion location [
16], or degree of recovery [
15]. Others found that average changes in activation depends on age, education, and left-handedness [
12]. Further, average changes differ depending on interventions for aphasia [
20].
Hence, while it is justifiable to average results of functional imaging of language in neurotypical controls to increase power, given that studies have shown a fairly high degree of homogeneity across neurotypical individuals engaged in the same task, it is not justifiable to average functional imaging across individuals after stroke. As we review below, individuals with PSA vary widely in what areas (and even what hemisphere) there is increased or decreased activation associated with any given language task. The average result for any given group may not be representative of any single participant in the study, and is certainly not generalizable to other people with PSA.
4. Functional Imaging Studies of Recovery in Individuals with PSA
4.a. Task-Related fMRI Studies of Recovery in Individuals with PSA
An early study evaluated fMRI in two patients with residual non-fluent PSA who received two different interventions. One person showed improvement on one treatment (focusing on “intention”) but not on the other treatment (focusing on “attention”). This individual showed a shift of activation to right presupplementary motor area and the right lateral frontal lobe after treatment. The second patient showed improvement on both treatments. Before treatment this individual showed right hemisphere activation during language that persisted after treatment, but also showed increased activation in the left posterior peri-sylvian cortex.
Another early fMRI study included seven people with left middle cerebral artery ischemic stroke infarction who had partially recovered comprehension after initial diagnosis of global aphasia. The most common areas of activation across individuals were
left extra-sylvian posterior temporal and the
right posterior parietal cortex. Those with the best recovery of language comprehension showed activations in regions that were also activated in several neurotypical controls [
21].
The same group reported results of fMRI of word processing from two individuals with PSA who had partially recovered. One showed left perilesional inferior and middle frontal activation extending to parietal and left superior temporal gyrus as well as left thalamus, precuneus, and precentral gyrus. The second person showed a single highly significant cluster of activation in left posterior superior, middle and inferior temporal gyrus. Activation in parts of Wernicke’s area (posterior superior temporal gyrus) was the only overlap in activation associated with conceptual-semantic word processing. No right hemisphere regions were significantly activated [
22].
On the other hand, an fMRI study of word generation in three people with PSA who had partially recovered language, compared to six neurotypical controls, revealed no activation in left inferior frontal gyrus (whether or not there was a lesion in the left frontal lobe), but some right hemisphere activation in two people with PSA, in areas not activated by controls. The neurotypical controls, as expected, showed activations in left frontal, temporal, parietal and occipital regions.[
23]
One study obtained both diffusion weighted imaging to reveal the infarct and perfusion weighted imaging to reveal the area of dysfunctional but salvageable tissue beyond the infarct in two people with acute PSA. They who showed that successful restoration of blood flow resulted in task-specific activation in the perilesional tissue within the previous area of salvageable tissue on perfusion weighted imaging[
24]. These results may not reflect any “reorganization”, but recovery through reperfusion (see also [
25,
26])
A later study of individuals with multimodal imaging acutely and at subsequent time points also demonstrated distinct mechanisms of recovery, including: (a) reperfusion; (b) recovery from diaschisis; (c) "reorganization", whereby undamaged regions show increased activation during recovery [
27]. “Reorganization” may actually be a process of relying more heavily on previously present but “supportive” or underused networks (e.g., regions homologous to the left hemisphere language network) [
28]. While a previous group study indicated that, on average, diaschisis occurs acutely in PSA [
3], this case series found that acute diaschisis was far from universal [
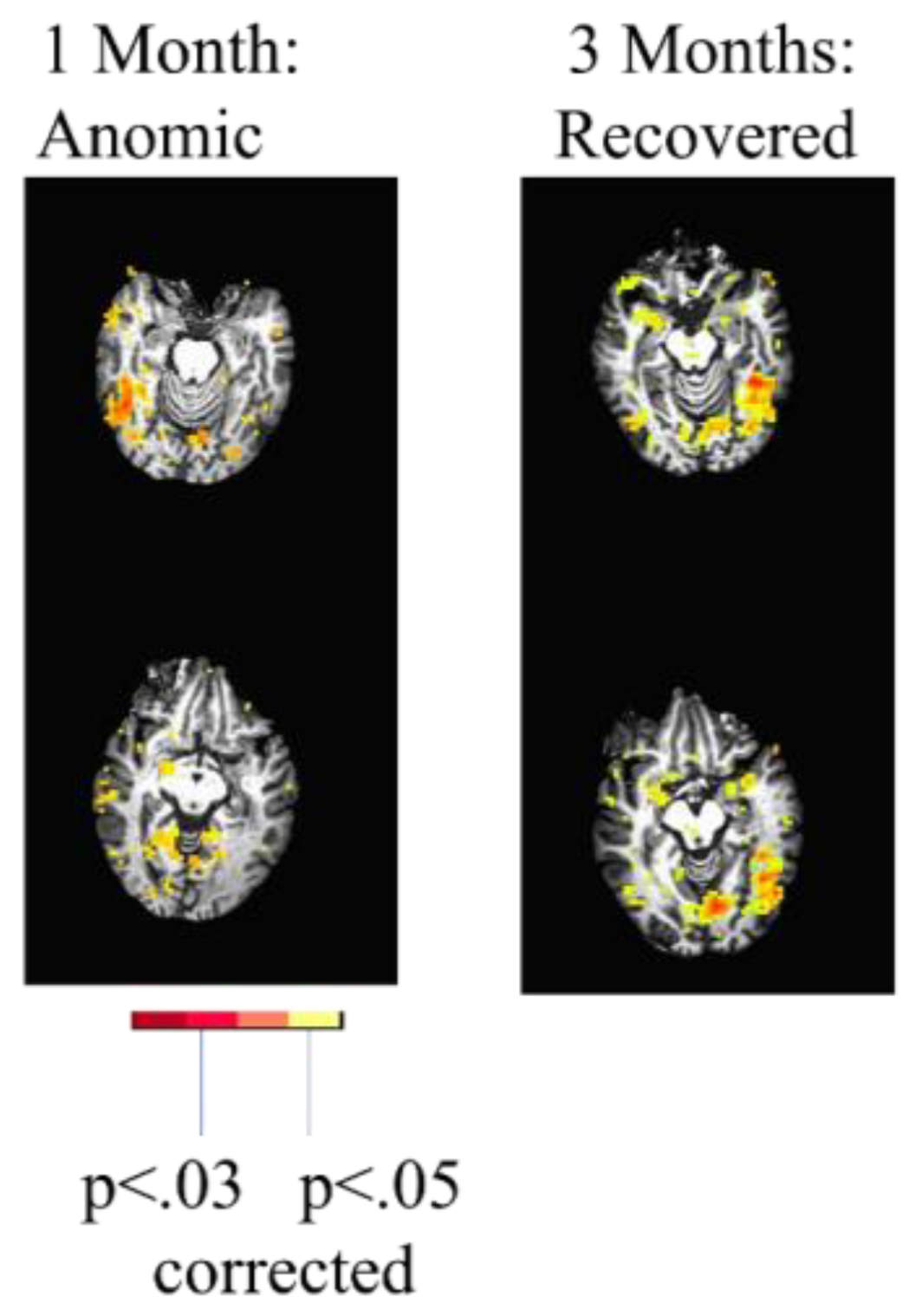
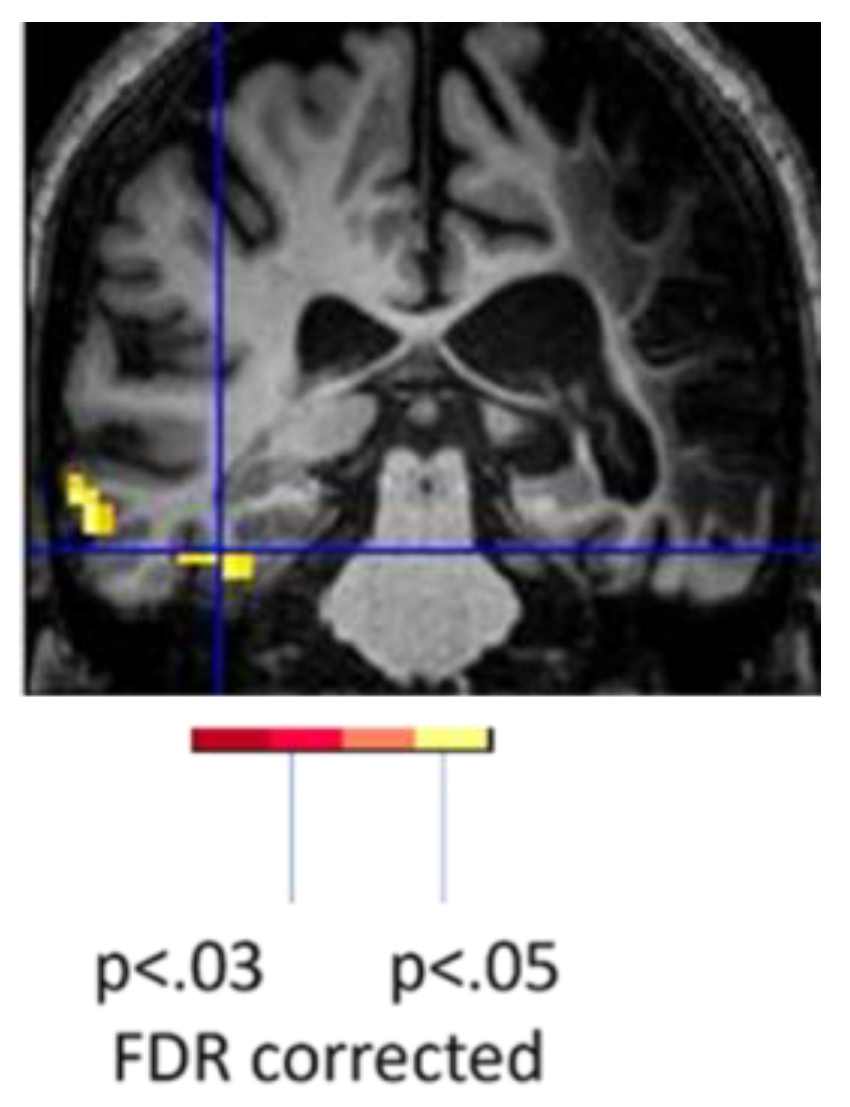
27]. One individual with PSA due to a left thalamic lesion showed clear evidence of recovery from diaschisis. This individual showed reduced activation in the left hemisphere language cortex but activation of the right hemisphere homologous network at one month post-stroke (subacutely) associated with picture naming (vs. saying “scrambled” in response to a scrambled picture), followed by re-activation of the left language network at 3 months post-stroke (
Figure 1).
However, other individuals showed activation of perilesional tissue in the language network even acutely as shown below. In this series of individual task-related fMRI studies at several points in the first year of stroke, the degree to which language activated normal left hemisphere language network depended not only on time from stroke onset, but also on the language task, accuracy of performance on the language task, the volume and location of the stroke.
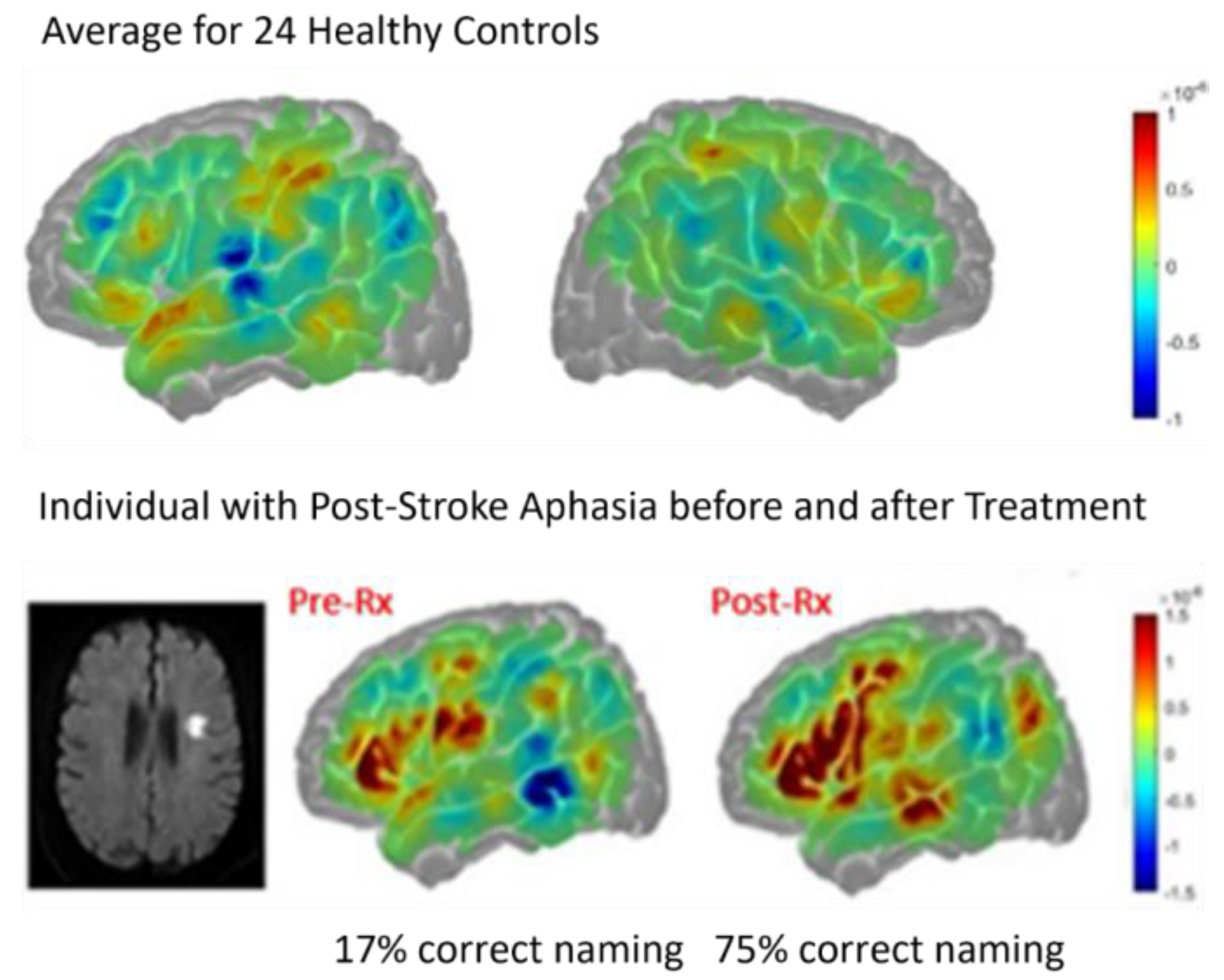
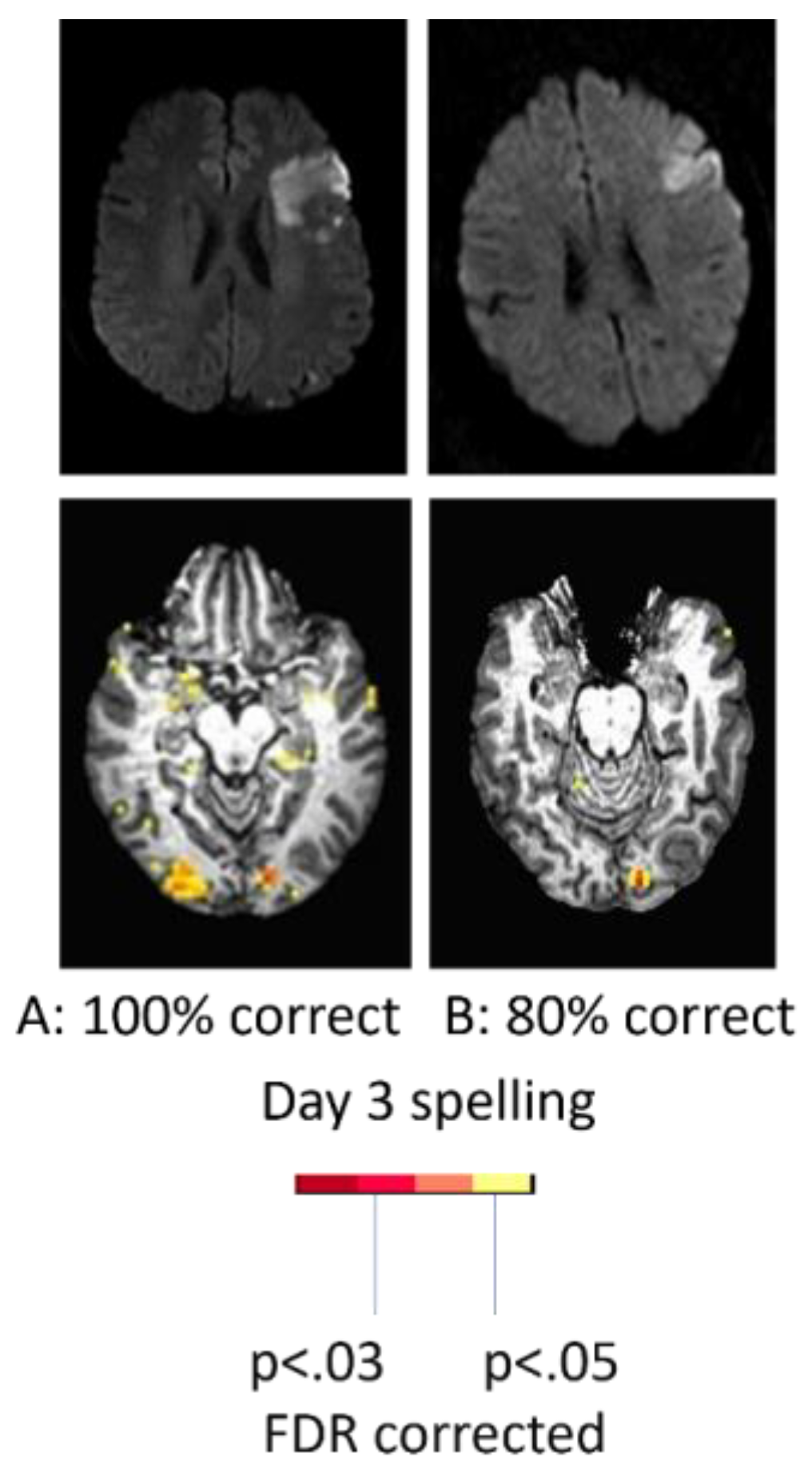
With regard to the effect of accuracy of performance, two women of similar ages with very similar infarcts involving left posterior inferior frontal cortex (“Broca’s area”), showed quite different patterns of activation during a spelling task (identifying the missing letter in a word vs identifying the letter with a different case in a word) at Day 3. While the participant with 100% accuracy showed bilateral (right more than left) activation in areas similar to controls, the participant with 80% accuracy showed only left hemisphere activation as shown in
Figure 2. These data raise doubts that right hemisphere homologous activation is detrimental, since it was associated with flawless performance [
27].
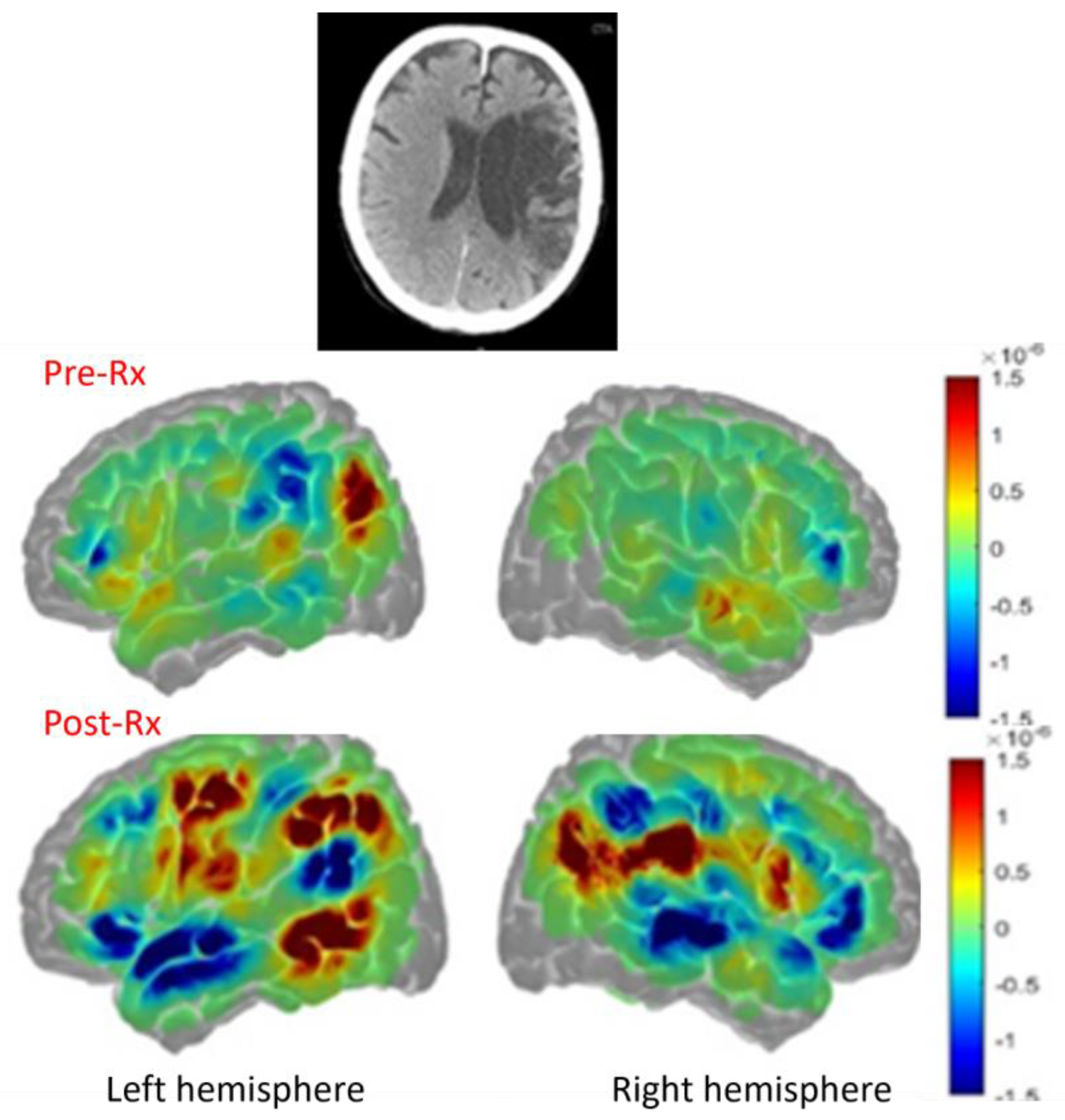
Likewise, another task related fMRI study from our group involved a man who recovered well from post-stroke global aphasia after a massive left middle cerebral artery stroke. Three years post-stroke he scored in the normal range on the Western Aphasia Battery [
29]. His reading was also 100% accurate and fluent. During a reading task (reading aloud words vs saying “skip” to letter strings), he showed only right hemisphere activation of fusiform cortex (
Figure 3), also confirming that right homologous activation can be positive, and associated with flawless performance, even at the chronic stage of stroke [
30].
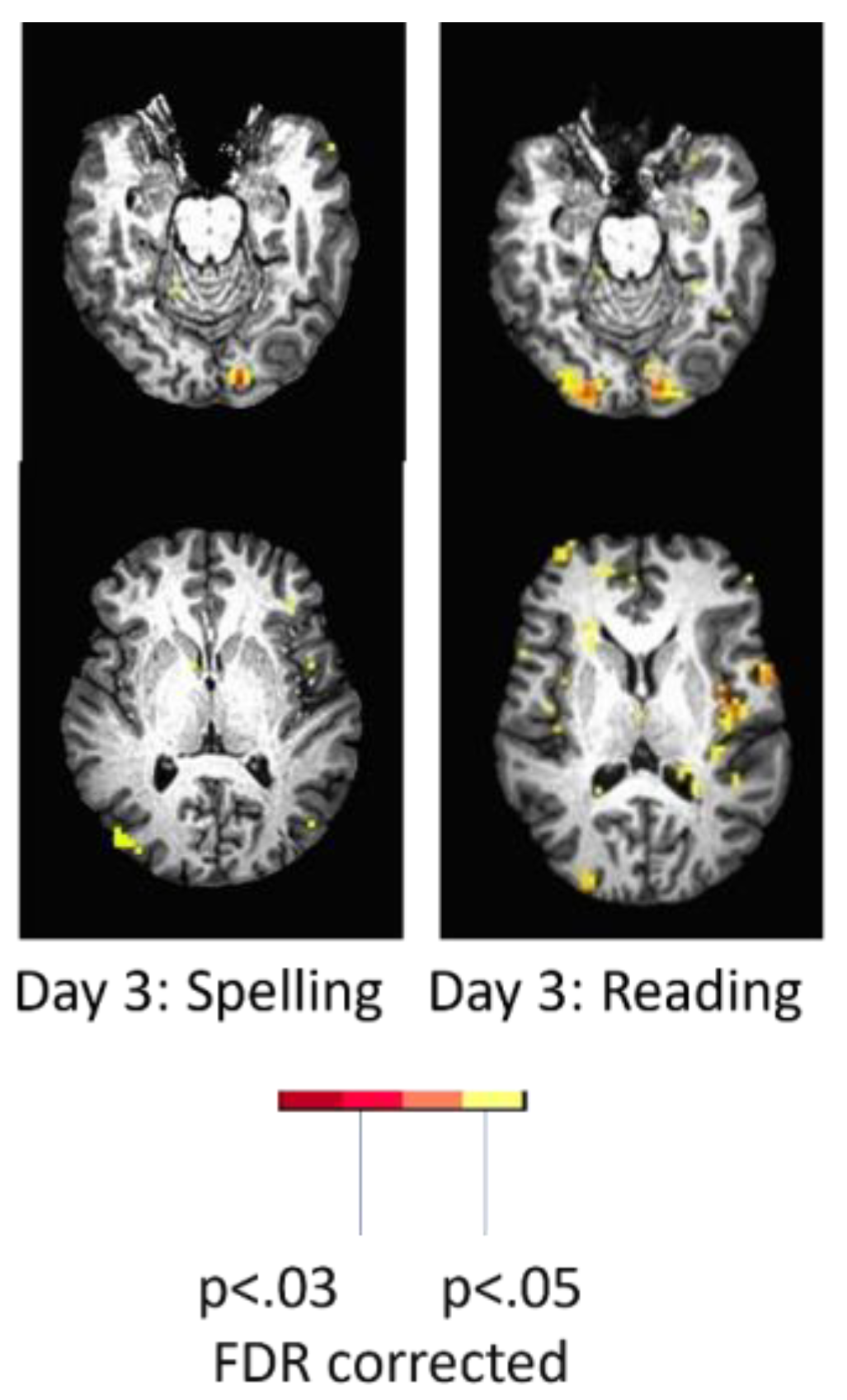
To illustrate the effect of language task, the woman described earlier who showed only left occipital activation with spelling showed bilateral activation and more left perilesional activation during reading (silent reading of words versus viewing of scrambled letter strings). (
Figure 4) She read the words with 100% accuracy outside of the scanner that day. Her activation with reading was essentially the normal pattern seen in neurotypical controls [31.] While it might be tempting to conclude that the minimal activation of perilesional tissue during
spelling was due to diaschisis, there was no evidence of diaschisis during the
reading task the same day [
27].
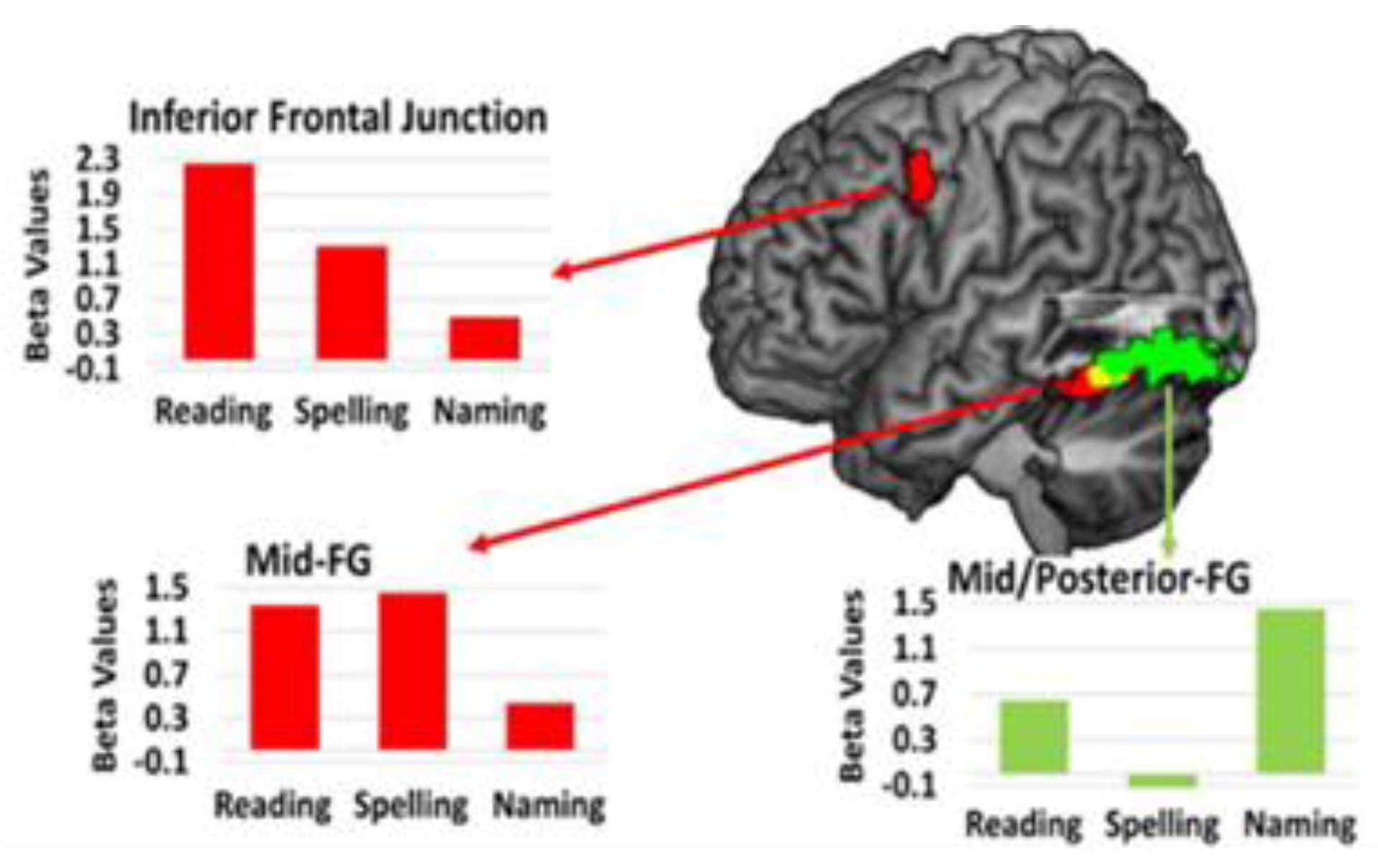
Another longitudinal fMRI study from our group, involving a third woman with PSA (with left occipital and splenial lesion) also showed distinct but overlapping areas of activation for reading, spelling, and naming at 4 time points from acute to 1 year after stroke. The areas where changes in activation were associated with improvement in each task (Time 4-Time 1), were also distinct, but adjacent, as shown in
Figure 5. Red bars show up-regulation of activation in the left inferior frontal gyrus and mid-fusiform gyrus (FG) reading and spelling. The green bars show up-regulation of activation in left posterior fusiform with recovery during reading and naming [
32].
4.b. Functional Connectivity MRI Studies of Individuals with PSA
Both task-related and resting state (or task-free) fMRI studies of PSA have also frequently reported averaged results across participants with different lesions, as described in
Section 3 above. However, studies that report results of changes in connectivity show distinct changes in connectivity over time, even in individuals with similar lesions.
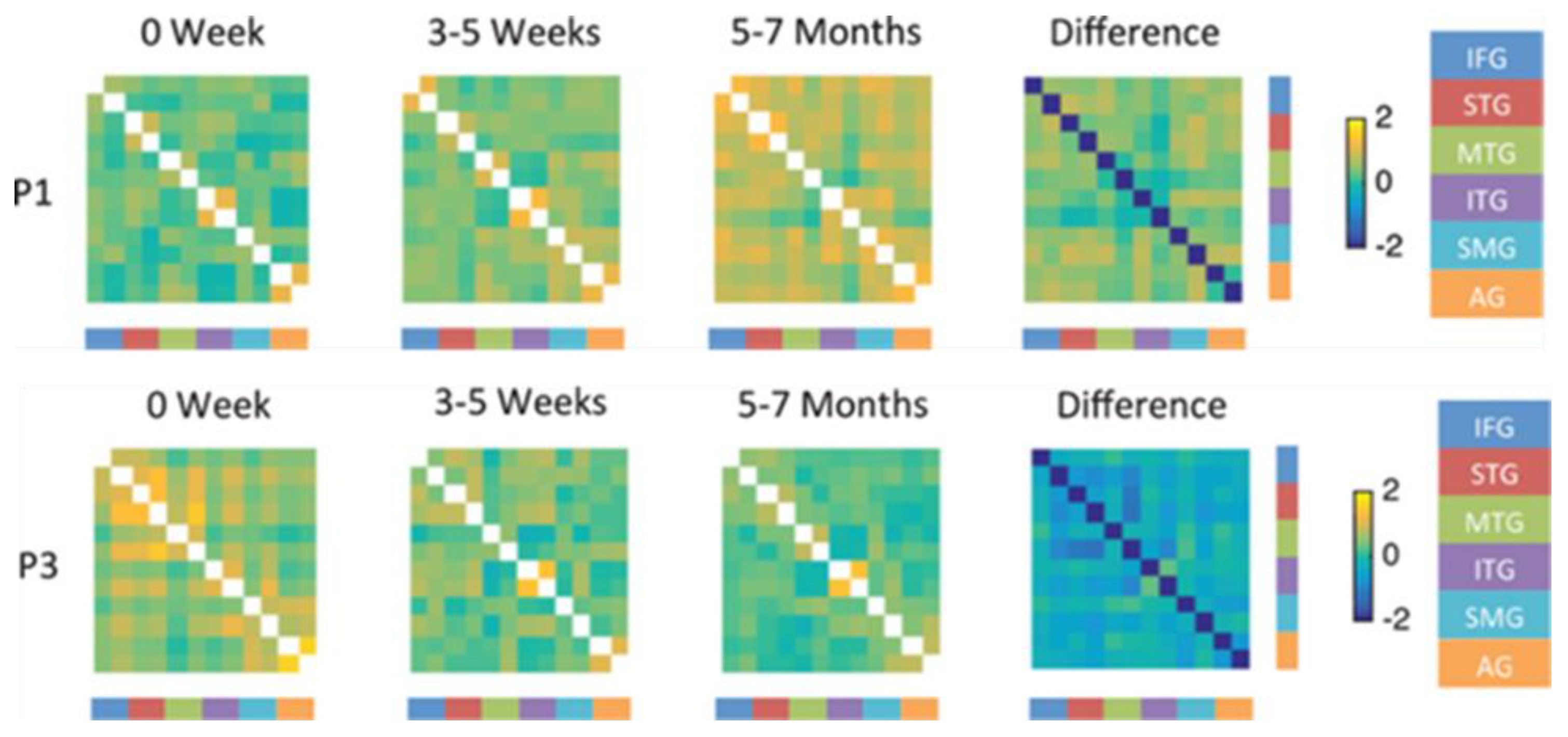
For example, one longitudinal study of four individuals, all with aphasia due stroke in the left posterior cerebral artery territory reported different patterns of activation associated with an overt naming task at each of four times in the first year after stroke [
33]. All of the individuals showed activation in left language network regions and right hemisphere homologues, but with different degrees of activation at each time point. The best recovery of naming was associated with increased balance in activation across nodes of the left hemisphere language network (including left inferior frontal gyrus, posterior temporal gyrus, angular gyrus, supramarginal gyrus and fusiform gyrus) and their right hemisphere homologues, again raising doubts that homologous activation is generally detrimental. This study also included connectivity analysis of the longitudinal fMRI in the four individuals. Three individuals showed improvement in naming accuracy from the acute to the chronic stage, which was associated in each case with increased connectivity within and between left hemisphere language regions, and their right hemisphere homologues (e.g., P1,
Figure 6). In contrast, the individual who showed a worsening naming deficit (P3) showed weak and decreasing connectivity within and between left and right hemisphere language network areas, as shown in
Figure 6.
One study that that reported both group and individual results from longitudinal resting state connectivity fMRI emphasized the role of network modularity in language and other networks in functional recovery [
34]. They studied 107 people with left or right hemisphere stroke and found that the degree of within-network integration (within and across hemispheres) and across-network segregation between networks (visual, default-mode, somato-motor, auditory, salience, dorsal attention, ventral attention, cingulo-opercular, fronto-parietal) was significantly reduced at 2 weeks (n = 107), but increased at 3 months (n = 85), and 12 months (n = 67) post-stroke. Within-network connectivity (and across network segregation (“modularity”) correlated with recovery of language, spatial memory, and attention, but not with motor or visual function. Detailed analysis of an individual with severe aphasia due to a left temporo-parietal stroke showed loss of connectivity between frontal and temporo-parietal regions within and across hemispheres at 2 weeks and substantial recovery of both language performance and modularity in multiple networks by three months post-stroke.
4.d. Transcranial Magnetic Stimulation (TMS) with Functional Imaging of Recovery in Individuals with PSA
Studies combining functional imaging, behavioral, and transcranial magnetic stimulation in individuals with aphasia have also provided enlightening results. One woman showed improvement in naming after inhibitory TMS to the right IFG pars triangularis, maintained two months later. fMRI showed reduced activation at the site of TMS target (without increased activation of the left hemisphere homologue). Three months after her treatment with TMS, she sustained a right hemisphere stroke that caused worsened aphasia, indicating that while right pars triangularis activation was detrimental (such that inhibition improved naming), other right hemisphere areas had been supporting language recovery (such that damage caused worse language) [
36].
Another study reported results from 11 individuals with post-stroke aphasia. Using PET, they found activation of inferior frontal gyrus (IFG) during language in the left hemisphere only in three participants and in both hemispheres in eight participants. Five of the eight individuals who showed right IFG (and none who had only left IFG activation) showed increased errors or latency in a semantic task with inhibitory TMS to the right IFG, indicating an essential role of right IFG in language function in five of 11 participants [
37].
5. Discussion
Here I have illustrated the divergent results of studies of language recovery in PSA that have used data averaged across groups, which have yielded conflicting conclusions. I have proposed an alternative approach to identifying the various ways the brain adapts to a sudden, focal lesion to the “language network” to recover language, using longitudinal single subject functional imaging. These single subject case series unveil distinct patterns of recovery across individuals, which may depend on the location and extent of stroke, time since stroke, performance accuracy, and perhaps various demographic factors. The conclusions drawn from averaged group results are necessarily limited to the group studied, and have minimal contributions to understanding the recovery of any individual in the group, much less to the understanding recovery of individuals with different lesions and abilities.
Group studies of aphasia recovery using functional imaging may provide insights into changes in activation or connectivity that correlate with recovery, however. For example, a longitudinal fMRI study of PSA showed that in the subacute stage of recovery, right supplementary motor area activation correlated with language improvement [
3]. A recent fNIRS study of 20 people with PSA showed that stronger resting state connectivity within right hemisphere and between hemispheres significantly correlated with higher aphasia quotient and better naming on standardized tests [
38]. A longitudinal study of fMRI in 17 people with aphasia due to frontal or temporoparietal stroke revealed that correlations between (1) language improvement and (2) task-related functional interactions between the language network and the multiple-demand network depended on lesion location and changed over time. [
39]. However, these results may not generalize to other groups of people with PSA.
There are also some common findings from group studies of aphasia recovery. For example, most show that the best recovery from aphasia is associated with the extent to which the individual can recruit the normal “language network” in the left hemisphere. But this conclusion seems relatively obvious. Unfortunately, many people with large left middle cerebral artery strokes are unable to recruit the normal left language network as it has been obliterated by the stroke. A recent review of fMRI studies of aphasia recovery reported that most often there is a global network breakdown acutely after stroke, followed by normalization of left hemisphere language networks with language recovery. But importantly, the authors noted that individual characteristics were associated with increased right hemisphere activation and sometimes activation of bilateral domain-general regions with recovery[
40]. (see also [
41]) It is important to understand how individuals with destruction of the left hemisphere language network (who cannot show normalization of this network) sometimes recover language. Combining their results with those with strokes that preserve the language network and reporting group averages will not help in this endeavor.
6. Conclusions
While group studies of aphasia recovery have yielded important insights into the dynamic changes in activation and connectivity between the language network regions and their right hemisphere homologues, longitudinal functional imaging studies of individuals are essential to discover the various ways the neural networks change with language recovery (or language decline) after stroke. To make progress in this area it is essential to abandon the common practice of averaging functional imaging data across participants with distinct lesions and divergence in other variables that affect recovery. Case series of functional imaging of recovery should systematically investigate the range of changes in the neural networks that support recovery of a variety of language tasks, as illustrated above for a small number of tasks [
21,
22,
23,
42].
Funding
This work was supported by NIH through NIDCD R01 DC05375 and P50 DC014664.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
Non-financial interests: AEH receives remuneration from the American Heart Association as Editor-in-Chief of Stroke. She receives grant funding from National Institute of Deafness and Communication Disorders and from the National Institute of Neurological Diseases and Stroke.
References
- Parker Jones, O.; Green, D.W.; Grogan, A.; Pliatsikas, C.; Filippopolitis, K.; Ali, N.; Lee, H.L.; Ramsden, S.; Gazarian, K.; Prejawa, S.; et al. Where, When and Why Brain Activation Differs for Bilinguals and Monolinguals during Picture Naming and Reading Aloud. Cereb Cortex 2012, 22, 892–902. [CrossRef]
- Bartels, A.; Zeki, S. Brain Dynamics during Natural Viewing Conditions--a New Guide for Mapping Connectivity in Vivo. Neuroimage 2005, 24, 339–349. [CrossRef]
- Saur, D.; Lange, R.; Baumgaertner, A.; Schraknepper, V.; Willmes, K.; Rijntjes, M.; Weiller, C. Dynamics of Language Reorganization after Stroke. Brain 2006, 129, 1371–1384. [CrossRef]
- Turkeltaub, P.E.; Messing, S.; Norise, C.; Hamilton, R.H. Are Networks for Residual Language Function and Recovery Consistent across Aphasic Patients? Neurology 2011, 76, 1726–1734. [CrossRef]
- Démonet, J.F.; Wise, R.; Frackowiak, R.S.J. Language Functions Explored in Normal Subjects by Positron Emission Tomography: A Critical Review. Hum Brain Mapp 1993, 1, 39–47. [CrossRef]
- Branco, P.; Seixas, D.; Castro, S.L. Mapping Language with Resting-state Functional Magnetic Resonance Imaging: A Study on the Functional Profile of the Language Network. Hum Brain Mapp 2020, 41, 545–560. [CrossRef]
- Frith, C.D.; Friston, K.J.; Liddle, P.F.; Frackowiak, R.S.J. A PET Study of Word Finding. Neuropsychologia 1991, 29, 1137–1148. [CrossRef]
- Patel, T.; Morales, M.; Pickering, M.J.; Hoffman, P. A Common Neural Code for Meaning in Discourse Production and Comprehension. Neuroimage 2023, 279, 120295. [CrossRef]
- Cao, Y.; Vikingstad, E.M.; George, K.P.; Johnson, A.F.; Welch, K.M. Cortical Language Activation in Stroke Patients Recovering from Aphasia with Functional MRI. Stroke 1999, 30, 2331–2340. [CrossRef]
- Szaflarski, J.P.; Eaton, K.; Ball, A.L.; Banks, C.; Vannest, J.; Allendorfer, J.B.; Page, S.; Holland, S.K. Poststroke Aphasia Recovery Assessed with Functional Magnetic Resonance Imaging and a Picture Identification Task. J Stroke Cerebrovasc Dis 2011, 20, 336–345. [CrossRef]
- DeMarco, A.T.; van der Stelt, C.; Paul, S.; Dvorak, E.; Lacey, E.; Snider, S.; Turkeltaub, P.E. Absence of Perilesional Neuroplastic Recruitment in Chronic Poststroke Aphasia. Neurology 2022, 99, e119–e128. [CrossRef]
- Turkeltaub, P.E.; Martin, K.C.; Laks, A.B.; DeMarco, A.T. Right Hemisphere Language Network Plasticity in Aphasia. medRxiv 2025. [CrossRef]
- Nenert, R.; Allendorfer, J.B.; Martin, A.M.; Banks, C.; Vannest, J.; Holland, S.K.; Hart, K.W.; Lindsell, C.J.; Szaflarski, J.P. Longitudinal FMRI Study of Language Recovery after a Left Hemispheric Ischemic Stroke. Restor Neurol Neurosci 2018, 36, 359–385. [CrossRef]
- Connor, L.T.; DeShazo Braby, T.; Snyder, A.Z.; Lewis, C.; Blasi, V.; Corbetta, M. Cerebellar Activity Switches Hemispheres with Cerebral Recovery in Aphasia. Neuropsychologia 2006, 44, 171–177. [CrossRef]
- Szaflarski, J.P.; Allendorfer, J.B.; Banks, C.; Vannest, J.; Holland, S.K. Recovered vs. Not-Recovered from Post-Stroke Aphasia: The Contributions from the Dominant and Non-Dominant Hemispheres. Restor Neurol Neurosci 2013, 31, 347–360. [CrossRef]
- Stockert, A.; Wawrzyniak, M.; Klingbeil, J.; Wrede, K.; Kümmerer, D.; Hartwigsen, G.; Kaller, C.P.; Weiller, C.; Saur, D. Dynamics of Language Reorganization after Left Temporo-Parietal and Frontal Stroke. Brain 2020, 143, 844–861. [CrossRef]
- Stockbridge, M.D.; Faria, A. V; Fridriksson, J.; Rorden, C.; Bonilha, L.; Hillis, A.E. Subacute Aphasia Recovery Is Associated with Resting-State Connectivity within and beyond the Language Network. Ann Clin Transl Neurol 2023, 10, 1525–1532. [CrossRef]
- Hara, T.; Abo, M.; Kakita, K.; Mori, Y.; Yoshida, M.; Sasaki, N. The Effect of Selective Transcranial Magnetic Stimulation with Functional Near-Infrared Spectroscopy and Intensive Speech Therapy on Individuals with Post-Stroke Aphasia. Eur Neurol 2017, 77, 186–194. [CrossRef]
- Hartwigsen, G.; Saur, D. Neuroimaging of Stroke Recovery from Aphasia - Insights into Plasticity of the Human Language Network. Neuroimage 2019, 190, 14–31. [CrossRef]
- Saur, D.; Hartwigsen, G. Neurobiology of Language Recovery after Stroke: Lessons from Neuroimaging Studies. Arch Phys Med Rehabil 2012, 93, S15-25. [CrossRef]
- Zahn, R.; Huber, W.; Drews, E.; Specht, K.; Kemeny, S.; Reith, W.; Willmes, K.; Schwarz, M. Recovery of Semantic Word Processing in Transcortical Sensory Aphasia: A Functional Magnetic Resonance Imaging Study. Neurocase 2002, 8, 376–386. [CrossRef]
- Zahn, R.; Drews, E.; Specht, K.; Kemeny, S.; Reith, W.; Willmes, K.; Schwarz, M.; Huber, W. Recovery of Semantic Word Processing in Global Aphasia: A Functional MRI Study. Brain Res Cogn Brain Res 2004, 18, 322–336. [CrossRef]
- Xu, X.; Zhang, M.; Shang, D.; Wang, Q.; Luo, B.; Weng, X. Cortical Language Activation in Aphasia: A Functional MRI Study. Chin Med J (Engl) 2004, 117, 1011–1016.
- Kleiser, R.; Wittsack, H.-J.; Bütefisch, C.M.; Jörgens, S.; Seitz, R.J. Functional Activation within the PI-DWI Mismatch Region in Recovery from Ischemic Stroke: Preliminary Observations. Neuroimage 2005, 24, 515–523. [CrossRef]
- Hillis, A.E.; Kleinman, J.T.; Newhart, M.; Heidler-Gary, J.; Gottesman, R.; Barker, P.B.; Aldrich, E.; Llinas, R.; Wityk, R.; Chaudhry, P. Restoring Cerebral Blood Flow Reveals Neural Regions Critical for Naming. Journal of Neuroscience 2006, 26, 8069–8073. [CrossRef]
- Hillis, A.E.; Rorden, C.; Fridriksson, J. Brain Regions Essential for Word Comprehension: Drawing Inferences from Patients. Ann Neurol 2017, 81, 759–768. [CrossRef]
- Jarso, S.; Li, M.; Faria, A.; Davis, C.; Leigh, R.; Sebastian, R.; Tsapkini, K.; Mori, S.; Hillis, A.E. Distinct Mechanisms and Timing of Language Recovery after Stroke. Cogn Neuropsychol 2013, 30. [CrossRef]
- Tilton-Bolowsky, V.; Stockbridge, M.D.; Hillis, A.E. Remapping and Reconnecting the Language Network after Stroke. Brain Sci 2024, 14. [CrossRef]
- Kertesz, A. Western Aphasia Battery–Revised; APA, 2006;
- Hillis, A.E. The Soriano Lecture: Language Recovery after Stroke: Evidence of Reorganization. In Proceedings of the 147th Meeting of the American Neurological Association. Annals of Neurology 92; October 2022; p. S131.
- Turkeltaub, P.E.; Eden, G.F.; Jones, K.M.; Zeffiro, T.A. Meta-Analysis of the Functional Neuroanatomy of Single-Word Reading: Method and Validation. Neuroimage 2002, 16, 765–780. [CrossRef]
- Purcell, J.; Sebastian, R.; Leigh, R.; Jarso, S.; Davis, C.; Posner, J.; Wright, A.; Hillis, A.E. Recovery of Orthographic Processing after Stroke: A Longitudinal FMRI Study. Cortex 2017, 92. [CrossRef]
- Sebastian, R.; Long, C.; Purcell, J.J.; Faria, A. V; Lindquist, M.; Jarso, S.; Race, D.; Davis, C.; Posner, J.; Wright, A.; et al. Imaging Network Level Language Recovery after Left PCA Stroke. Restor Neurol Neurosci 2016, 34, 473–489. [CrossRef]
- Siegel, J.S.; Seitzman, B.A.; Ramsey, L.E.; Ortega, M.; Gordon, E.M.; Dosenbach, N.U.F.; Petersen, S.E.; Shulman, G.L.; Corbetta, M. Re-Emergence of Modular Brain Networks in Stroke Recovery. Cortex 2018, 101, 44–59. [CrossRef]
- Bunker, L.B.; Meier, E.; Kim, H.; Durfee, A.; Hillis, A.E. Changes in Activation and Functional Connectivity in Subacute Aphasia Following Treatment: An FNIRS Cares Series Investigation. . In Proceedings of the Annual Meeting of the Academy of Aphasia; Reading, UK., 2023; pp. 10–11.
- Turkeltaub, P.E.; Coslett, H.B.; Thomas, A.L.; Faseyitan, O.; Benson, J.; Norise, C.; Hamilton, R.H. The Right Hemisphere Is Not Unitary in Its Role in Aphasia Recovery. Cortex 2012, 48, 1179–1186. [CrossRef]
- Winhuisen, L.; Thiel, A.; Schumacher, B.; Kessler, J.; Rudolf, J.; Haupt, W.F.; Heiss, W.D. Role of the Contralateral Inferior Frontal Gyrus in Recovery of Language Function in Poststroke Aphasia: A Combined Repetitive Transcranial Magnetic Stimulation and Positron Emission Tomography Study. Stroke 2005, 36, 1759–1763. [CrossRef]
- Meier, E.L.; Bunker, L.D.; Kim, H.; Hillis, A.E. Resting-State Connectivity in Acute and Subacute Poststroke Aphasia: A Functional Near-Infrared Spectroscopy Pilot Study. Brain Connect 2023, 13, 441–452. [CrossRef]
- Jiang, Z.; Kuhnke, P.; Stockert, A.; Wawrzyniak, M.; Halai, A.; Saur, D.; Hartwigsen, G. Dynamic Reorganization of Task-Related Network Interactions in Post-Stroke Aphasia Recovery. Brain 2025. [CrossRef]
- Li, R.; Mukadam, N.; Kiran, S. Functional MRI Evidence for Reorganization of Language Networks after Stroke. Handb Clin Neurol 2022, 185, 131–150. [CrossRef]
- Crosson, B.; McGregor, K.; Gopinath, K.S.; Conway, T.W.; Benjamin, M.; Chang, Y.-L.; Moore, A.B.; Raymer, A.M.; Briggs, R.W.; Sherod, M.G.; et al. Functional MRI of Language in Aphasia: A Review of the Literature and the Methodological Challenges. Neuropsychol Rev 2007, 17, 157–177. [CrossRef]
- Sebastian, R.; Long, C.; Purcell, J.J.; Faria, A. V; Lindquist, M.; Jarso, S.; Race, D.; Davis, C.; Posner, J.; Wright, A.; et al. Imaging Network Level Language Recovery after Left PCA Stroke. Restor Neurol Neurosci 2016, 34, 473–489. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).