Submitted:
23 June 2025
Posted:
25 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Fabrication of Polymeric 3D-Printed MNAs
2.1. Overview of 3D Printing Techniques
2.2. Polymeric Material Used for 3D Printing of MNAs
2.2.1. Photopolymer Resins
2.2.2. Biodegradable Polymers
2.2.3. Hydrogels
2.2.4. Composite Resins and Materials
2.2.5. Innovative Material
3. Non-Transdermal Applications of Polymeric 3D-Printed MNAs
3.1. Brain/Central Nervous System (CNS)
3.2. Oral Cavity
3.3. Ocular (Eye)
3.4. Gastrointestinal Tract
3.5. Cardiovascular System
3.6. Reproductive System
3.7. Other Emerging Areas
3.7.1. Inner Ear
3.7.2. Targeted Organ Delivery and Tumor Therapy
3.7.3. Point-of-Care 3D Printing of MNAs
4. Challenges and Future Directions
5. Conclusion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szabó A, De Decker I, Semey S, et al. Photo-crosslinkable polyester microneedles as sustained drug release systems toward hypertrophic scar treatment. Drug Delivery 2024, 31, 2305818. [Google Scholar] [CrossRef] [PubMed]
- Kim J, Kim MY, Han Y, et al. Development of an electrochemical biosensor for non-invasive cholesterol monitoring via microneedle-based interstitial fluid extraction. Talanta 2024, 280, 126771. [Google Scholar] [CrossRef] [PubMed]
- Hema, Jindal A, Bala R, singh A. A review on recent advances and challenges of microneedle technology for enhanced topical treatment of skin disorders. Arch Dermatol Res. 2025, 317, 706. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi M, Alexander Ninan J, Akbari M. Advancements in Materials for 3D-Printed Microneedle Arrays: Enhancing Performance and Biocompatibility. Micromachines 2024, 15, 1433. [Google Scholar] [CrossRef]
- Nguyen, HX. Beyond the Needle: Innovative Microneedle-Based Transdermal Vaccination. Medicines 2025, 12, 4. [Google Scholar] [CrossRef]
- Merzougui C, Yang X, Meng D, Huang Y, Zhao X. Microneedle Array-Based Dermal Interstitial Fluid Biopsy for Cancer Diagnosis: Advances and Challenges. Advanced Healthcare Materials 2025, 14, 2404420. [Google Scholar] [CrossRef]
- Aroche AF, Nissan HE, Daniele MA. Hydrogel-Forming Microneedles and Applications in Interstitial Fluid Diagnostic Devices. Advanced Healthcare Materials 2025, 14, 2401782. [Google Scholar] [CrossRef]
- Yang JH, Seong KY, Kang M, Jang S, Yang SY, Hahn YK. Turbulence-enhanced microneedle immunoassay platform (TMIP) for high-precision biomarker detection from skin interstitial fluid. Biosensors and Bioelectronics 2025, 282, 117480. [Google Scholar] [CrossRef]
- Razzaghi M, Ninan JA, Azimzadeh M, et al. Remote-Controlled Sensing and Drug Delivery via 3D-Printed Hollow Microneedles. Adv Healthcare Materials, 4 June 2024. [CrossRef]
- Razzaghi M, Seyfoori A, Pagan E, Askari E, Hassani Najafabadi A, Akbari M. 3D Printed Hydrogel Microneedle Arrays for Interstitial Fluid Biomarker Extraction and Colorimetric Detection. Polymers 2023, 15, 1389. [Google Scholar] [CrossRef]
- Lee K, Goudie MJ, Tebon P, et al. Non-transdermal microneedles for advanced drug delivery. Advanced Drug Delivery Reviews. [CrossRef]
- Uddin MJ, Economidou SN, Guiraud L, Kazi M, Alanazi FK, Douroumis D. Monoclonal Antibody Delivery Using 3D Printed Biobased Hollow μNe3dle Arrays for the Treatment of Osteoporosis. Mol Pharmaceutics 2024, 21, 4465–4475. [Google Scholar] [CrossRef]
- Liu Z, Xu X, Huang S, et al. Multichannel microneedle dry electrode patches for minimally invasive transdermal recording of electrophysiological signals. Microsyst Nanoeng 2024, 10, 1–18. [Google Scholar] [CrossRef]
- Cao X, and Chen G. Advances in microneedles for non-transdermal applications. Expert Opinion on Drug Delivery 2022, 19, 1081–1097. [Google Scholar] [CrossRef] [PubMed]
- Vanwersch P, Evens T, Van Bael A, Castagne S. Design, fabrication, and penetration assessment of polymeric hollow microneedles with different geometries. Int J Adv Manuf Technol. 2024, 132, 533–551. [Google Scholar] [CrossRef]
- Turner JG, Lay E, Jungwirth U, et al. 3D-Printed Hollow Microneedle-Lateral Flow Devices for Rapid Blood-Free Detection of C-Reactive Protein and Procalcitonin. Adv Materials Technologies 2023, 8, 2300259. [Google Scholar] [CrossRef]
- Sarker S, Colton A, Wen Z, et al. 3D-Printed Microinjection Needle Arrays via a Hybrid DLP-Direct Laser Writing Strategy. Adv Materials Technologies 2023, 8, 2201641. [Google Scholar] [CrossRef]
- Monou PK, Andriotis EG, Tsongas K, et al. Fabrication of 3D Printed Hollow Microneedles by Digital Light Processing for the Buccal Delivery of Actives. ACS Biomater Sci Eng. 2023, 9, 5072–5083. [Google Scholar] [CrossRef]
- Li R, Zhang L, Jiang X, et al. 3D-printed microneedle arrays for drug delivery. Journal of Controlled Release 2022, 350, 933–948. [Google Scholar] [CrossRef]
- Ghanbariamin D, Samandari M, Ghelich P, et al. Cleanroom-Free Fabrication of Microneedles for Multimodal Drug Delivery. Small 2023, 19, 2207131. [Google Scholar] [CrossRef]
- Yadav V, Sharma PK, Murty US, et al. 3D printed hollow microneedles array using stereolithography for efficient transdermal delivery of rifampicin. International Journal of Pharmaceutics 2021, 605, 120815. [Google Scholar] [CrossRef]
- Silva TEAR, Kohler S, Bartzsch N, Beuschlein F, Guentner AT. 3D printing by two-photon polymerization of hollow microneedles for interstitial fluid extraction. Published online , 2024. 15 October. [CrossRef]
- Kawre S, Suryavanshi P, Lalchandani DS, et al. Bioinspired labrum-shaped stereolithography (SLA) assisted 3D printed hollow microneedles (HMNs) for effectual delivery of ceftriaxone sodium. European Polymer Journal 2024, 204, 112702. [Google Scholar] [CrossRef]
- Loh JM, Lim YJL, Tay JT, Cheng HM, Tey HL, Liang K. Design and fabrication of customizable microneedles enabled by 3D printing for biomedical applications. Bioactive Materials 2024, 32, 222–241. [Google Scholar] [CrossRef] [PubMed]
- Li R, Liu X, Yuan X, et al. Fast Customization of Hollow Microneedle Patches for Insulin Delivery. International Journal of Bioprinting 2022, 8, 553. [Google Scholar] [CrossRef]
- Ghaznavi A, Xu J, Lee CU, Hara SA. 3D-Printed Hollow Microneedles Array with Luer Lock Connection for Facile and Painless Intradermal Injection: A Proof of Concept. Adv Materials Technologies, 14 June 2024. [CrossRef]
- Liu Y, He C, Qiao T, et al. Coral-Inspired Hollow Microneedle Patch with Smart Sensor Therapy for Wound Infection. Advanced Functional Materials 2024, 34, 2314071. [Google Scholar] [CrossRef]
- Liu H, Zhou X, Nail A, et al. Multi-material 3D printed eutectogel microneedle patches integrated with fast customization and tunable drug delivery. Journal of Controlled Release 2024, 368, 115–130. [Google Scholar] [CrossRef]
- Liu H, Nail A, Meng D, et al. Recent progress in the 3D printing of microneedle patches for biomedical applications. International Journal of Pharmaceutics 2025, 668, 124995. [Google Scholar] [CrossRef]
- Dabbagh SR, Sarabi MR, Rahbarghazi R, Sokullu E, Yetisen AK, Tasoglu S. 3D-printed microneedles in biomedical applications. iScience 2021, 24, 102012. [Google Scholar] [CrossRef]
- Elahpour N, Pahlevanzadeh F, Kharaziha M, Bakhsheshi-Rad HR, Ramakrishna S, Berto F. 3D printed microneedles for transdermal drug delivery: A brief review of two decades. International Journal of Pharmaceutics 2021, 597, 120301. [Google Scholar] [CrossRef]
- Schmidleithner C, Kalaskar DM, Schmidleithner C, Kalaskar DM. Stereolithography. In: 3D Printing. IntechOpen; 2018. [CrossRef]
- Yeung C, Chen S, King B, et al. A 3D-printed microfluidic-enabled hollow microneedle architecture for transdermal drug delivery. Biomicrofluidics 2019, 13, 064125. [Google Scholar] [CrossRef]
- Parrilla M, Sena-Torralba A, Steijlen A, Morais S, Maquieira Á, De Wael K. A 3D-printed hollow microneedle-based electrochemical sensing device for in situ plant health monitoring. Biosensors and Bioelectronics 2024, 251, 116131. [Google Scholar] [CrossRef]
- Ogundele M, Okafor HK. Transdermal Drug Delivery: Microneedles, Their Fabrication and Current Trends in Delivery Methods. Journal of Pharmaceutical Research International, 19 September 2017. [CrossRef]
- Bhuskute H, Shende P, Prabhakar B. 3D Printed Personalized Medicine for Cancer: Applications for Betterment of Diagnosis, Prognosis and Treatment. AAPS PharmSciTech 2021, 23, 8. [Google Scholar] [CrossRef]
- Fitaihi R, Abukhamees S, Chung SH, Craig DQM. Optimization of stereolithography 3D printing of microneedle micro-molds for ocular drug delivery. International Journal of Pharmaceutics 2024, 658, 124195. [Google Scholar] [CrossRef] [PubMed]
- Economidou SN, Pere CPP, Reid A, et al. 3D printed microneedle patches using stereolithography (SLA) for intradermal insulin delivery. Materials Science and Engineering: C 2019, 102, 743–755. [Google Scholar] [CrossRef]
- Economidou SN, Uddin MdJ, Marques MJ, et al. A novel 3D printed hollow microneedle microelectromechanical system for controlled, personalized transdermal drug delivery. Additive Manufacturing 2021, 38, 101815. [Google Scholar] [CrossRef]
- Lu Y, Mantha SN, Crowder DC, et al. Microstereolithography and characterization of poly(propylene fumarate)-based drug-loaded microneedle arrays. Biofabrication 2015, 7, 045001. [Google Scholar] [CrossRef]
- Xenikakis I, Tzimtzimis M, Tsongas K, et al. Fabrication and finite element analysis of stereolithographic 3D printed microneedles for transdermal delivery of model dyes across human skin in vitro. European Journal of Pharmaceutical Sciences 2019, 137, 104976. [Google Scholar] [CrossRef]
- Johnson AR, Procopio AT. Low cost additive manufacturing of microneedle masters. 3D Print Med. 2019, 5, 2. [Google Scholar] [CrossRef]
- Kadian S, Sundar Sahoo S, Shukla S, J. Narayan R. Development of 3D-printed conducting microneedle-based electrochemical point-of-care device for transdermal sensing of chlorpromazine. Published online , 2025. 5 February. [CrossRef]
- Lijnse T, Mendes M, Shu W, O’Cearbhaill E. Development of Digital Light Processing 3D Printed Microneedles for Biomedical Applications. Published online , 2024. 27 June. [CrossRef]
- Lu Y, Mapili G, Suhali G, Chen S, Roy K. A digital micro-mirror device-based system for the microfabrication of complex, spatially patterned tissue engineering scaffolds. J Biomed Mater Res A 2006, 77, 396–405. [Google Scholar] [CrossRef]
- Mathew E, Pitzanti G, Larrañeta E, Lamprou DA. 3D Printing of Pharmaceuticals and Drug Delivery Devices. Pharmaceutics 2020, 12, 266. [Google Scholar] [CrossRef]
- Mathew E, Pitzanti G, Gomes dos Santos AL, Lamprou DA. Optimization of Printing Parameters for Digital Light Processing 3D Printing of Hollow Microneedle Arrays. Pharmaceutics 2021, 13, 1837. [Google Scholar] [CrossRef]
- Yao W, Li D, Zhao Y, et al. 3D Printed Multi-Functional Hydrogel Microneedles Based on High-Precision Digital Light Processing. Micromachines 2020, 11, 17. [Google Scholar] [CrossRef]
- Liu Y, Yu Q, Luo X, Yang L, Cui Y. Continuous monitoring of diabetes with an integrated microneedle biosensing device through 3D printing. Microsyst Nanoeng 2021, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Chen Z, Wu H, Zhao S, et al. 3D-Printed Integrated Ultrasonic Microneedle Array for Rapid Transdermal Drug Delivery. Mol Pharmaceutics 2022, 19, 3314–3322. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi M, Akbari M. The Effect of 3D Printing Tilt Angle on the Penetration of 3D-Printed Microneedle Arrays. Micromachines 2023, 14, 1157. [Google Scholar] [CrossRef] [PubMed]
- Ngo TD, Kashani A, Imbalzano G, Nguyen KTQ, Hui D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Composites Part B: Engineering 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Vanhooydonck A, Vleugels J, Parrilla M, Clerx P, Watts R. Optimizing high accuracy 8K LCD 3D-printed Hollow Microneedles: Methodology and ISO-7864:2016 Guided Evaluation for Enhanced Skin Penetration. In: Health Informatics and Biomedical Engineering Applications. Vol 142. AHFE Open Acces; 2024. [CrossRef]
- Xenikakis I, Tsongas K, Tzimtzimis EK, Tzetzis D. ADDITIVE MANUFACTURING OF HOLLOW MICRONEEDLES FOR INSULIN DELIVERY. IJMMT 2021, 13, 185–190. [Google Scholar] [CrossRef]
- Xenikakis I, Tsongas K, Tzimtzimis EK, et al. Transdermal delivery of insulin across human skin in vitro with 3D printed hollow microneedles. Journal of Drug Delivery Science and Technology 2022, 67, 102891. [Google Scholar] [CrossRef]
- Quan H, Zhang T, Xu H, Luo S, Nie J, Zhu X. Photo-curing 3D printing technique and its challenges. Bioactive Materials 2020, 5, 110–115. [Google Scholar] [CrossRef]
- Tsolakis IA, Papaioannou W, Papadopoulou E, Dalampira M, Tsolakis AI. Comparison in Terms of Accuracy between DLP and LCD Printing Technology for Dental Model Printing. Dentistry Journal 2022, 10, 181. [Google Scholar] [CrossRef]
- Prabhu A, Baliga V, Shenoy R, Dessai AD, Nayak UY. 3D printed microneedles: revamping transdermal drug delivery systems. Drug Deliv and Transl Res, 5 August 2024. [CrossRef]
- Cordeiro AS, Tekko IA, Jomaa MH, et al. Two-Photon Polymerisation 3D Printing of Microneedle Array Templates with Versatile Designs: Application in the Development of Polymeric Drug Delivery Systems. Pharm Res. 2020, 37, 174. [Google Scholar] [CrossRef]
- Faraji Rad Z, Prewett P, Davies G. High-resolution two-photon polymerization: the most versatile technique for the fabrication of microneedle arrays. Microsystems and Nanoengineering 2021, 7, 1–17. [Google Scholar] [CrossRef]
- Ovsianikov A, Chichkov B, Mente P, Monteiro-Riviere NA, Doraiswamy A, Narayan RJ. Two Photon Polymerization of Polymer–Ceramic Hybrid Materials for Transdermal Drug Delivery. International Journal of Applied Ceramic Technology 2007, 4, 22–29. [Google Scholar] [CrossRef]
- Szeto B, Aksit A, Valentini C, et al. Novel 3D-printed hollow microneedles facilitate safe, reliable, and informative sampling of perilymph from guinea pigs. Hearing Research 2021, 400, 108141. [Google Scholar] [CrossRef] [PubMed]
- Aksit A, Rastogi S, Nadal ML, et al. Drug delivery device for the inner ear: ultra-sharp fully metallic microneedles. Drug Deliv and Transl Res. 2021, 11, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Miller PR, Xiao X, Brener I, Burckel DB, Narayan R, Polsky R. Microneedle-Based Transdermal Sensor for On-Chip Potentiometric Determination of K+. Advanced Healthcare Materials 2014, 3, 876–881. [Google Scholar] [CrossRef]
- Olowe M, Parupelli SK, Desai S. A Review of 3D-Printing of Microneedles. Pharmaceutics 2022, 14, 2693. [Google Scholar] [CrossRef]
- Yang Q, Zhong W, Xu L, et al. Recent progress of 3D-printed microneedles for transdermal drug delivery. International Journal of Pharmaceutics 2021, 593, 120106. [Google Scholar] [CrossRef]
- Kouassi MC, Kallel A, Abdallah AB, et al. Assessment of fused deposition modeling (FDM) parameters for fabrication of solid and hollow microneedles using polylactic acid (PLA). Polymers for Advanced Technologies 2024, 35, e6548. [Google Scholar] [CrossRef]
- Luzuriaga MA, Berry DR, Reagan JC, Smaldone RA, Gassensmith JJ. Biodegradable 3D printed polymer microneedles for transdermal drug delivery. Lab Chip 2018, 18, 1223–1230. [Google Scholar] [CrossRef]
- Wang X, Jiang M, Zhou Z, Gou J, Hui D. 3D printing of polymer matrix composites: A review and prospective. Composites Part B: Engineering 2017, 110, 442–458. [Google Scholar] [CrossRef]
- Khosraviboroujeni A, Mirdamadian SZ, Minaiyan M, Taheri A. Preparation and characterization of 3D printed PLA microneedle arrays for prolonged transdermal drug delivery of estradiol valerate. Drug Deliv Transl Res. 2022, 12, 1195–1208. [Google Scholar] [CrossRef]
- Camović M, Biščević A, Brčić I, et al. Coated 3D Printed PLA Microneedles as Transdermal Drug Delivery Systems. In: Badnjevic A, Škrbić R, Gurbeta Pokvić L, eds. CMBEBIH 2019, 2020. [CrossRef]
- Rajan K, Samykano M, Kadirgama K, Harun WSW, Rahman MdM. Fused deposition modeling: process, materials, parameters, properties, and applications. Int J Adv Manuf Technol, 1: 120(3-4), 1531. [CrossRef]
- Chen YF, Wang YH, Tsai J che. Enhancement of surface reflectivity of fused deposition modeling parts by post-processing. Optics Communications 2019, 430, 479–485. [Google Scholar] [CrossRef]
- Jia B, Xia T, Xu Y, Li B. Morphology Design and Precision Control of Microneedles by PμSL 3D Printing. Polymers 2025, 17, 1351. [Google Scholar] [CrossRef]
- He Y, He D, Ren S, Fan L, Wang L, Sun J. 3D-printed microneedles loaded with madecassoside for periodontal soft tissue regeneration. International Journal of Pharmaceutics 2025, 676, 125569. [Google Scholar] [CrossRef]
- Sirbubalo M, Tucak A, Muhamedagić K, Rahić O, Čekić A, Vranić E. Photopolymerization-Based Technologies for Microneedle Arrays Production. In: Badnjevic A, Gurbeta Pokvić L, eds. CMBEBIH 2021, 2021. [CrossRef]
- Tabriz AG, Viegas B, Okereke M, et al. Evaluation of 3D Printability and Biocompatibility of Microfluidic Resin for Fabrication of Solid Microneedles. Micromachines 2022, 13, 1368. [Google Scholar] [CrossRef]
- Jia B, Xia T, Wang X, Xu Y, Li B. Investigation of biosensing properties in magnetron sputtered metallized UV-curable polymer microneedle electrodes. Journal of Biomaterials Science, Polymer Edition 2024, 35, 1008–1030. [Google Scholar] [CrossRef]
- Baykara D, Bedir T, Ilhan E, et al. Fabrication and optimization of 3D printed gelatin methacryloyl microneedle arrays based on vat photopolymerization. Front Bioeng Biotechnol 2023, 11, 1157541. [Google Scholar] [CrossRef]
- Xiang H, Wang X, Ou Z, et al. UV-curable, 3D printable and biocompatible silicone elastomers. Progress in Organic Coatings 2019, 137, 105372. [Google Scholar] [CrossRef]
- Zhou D, Ito Y. Visible light-curable polymers for biomedical applications. Sci China Chem 2014, 57, 510–521. [Google Scholar] [CrossRef]
- Wu L, Shrestha P, Iapichino M, Cai Y, Kim B, Stoeber B. Characterization method for calculating diffusion coefficient of drug from polylactic acid (PLA) microneedles into the skin. Journal of Drug Delivery Science and Technology 2021, 61, 102192. [Google Scholar] [CrossRef]
- Boehm RD, Daniels J, Stafslien S, Nasir A, Lefebvre J, Narayan RJ. Polyglycolic acid microneedles modified with inkjet-deposited antifungal coatings. Biointerphases 2015, 10, 011004. [Google Scholar] [CrossRef]
- Boehm RD, Jaipan P, Skoog SA, Stafslien S, VanderWal L, Narayan RJ. Inkjet deposition of itraconazole onto poly(glycolic acid) microneedle arrays. Biointerphases 2016, 11, 011008. [Google Scholar] [CrossRef]
- Panda A, Sharma PK, McCann T, Bloomekatz J, Repka MA, Murthy SN. Fabrication and development of controlled release PLGA microneedles for macromolecular delivery using FITC-Dextran as model molecule. Journal of Drug Delivery Science and Technology 2022, 68, 102712. [Google Scholar] [CrossRef]
- Braz Gomes K, D’Souza B, Vijayanand S, Menon I, D’Souza MJ. A dual-delivery platform for vaccination using antigen-loaded nanoparticles in dissolving microneedles. International Journal of Pharmaceutics 2022, 613, 121393. [Google Scholar] [CrossRef] [PubMed]
- Malek-Khatabi A, Sadat Razavi M, Abdollahi A, et al. Recent progress in PLGA-based microneedle-mediated transdermal drug and vaccine delivery. Biomater Sci. 2023, 11, 5390–5409. [Google Scholar] [CrossRef]
- Mao AS, Mooney DJ. Regenerative medicine: Current therapies and future directions. Proceedings of the National Academy of Sciences 2015, 112, 14452–14459. [Google Scholar] [CrossRef]
- Bahnick AJ, Dziewior CS, Li Y, et al. Controlled Transdermal Delivery of Dexamethasone for Pain Management via Photochemically 3D-Printed Bioresorbable Microneedle Arrays. Advanced Healthcare Materials 2024, 13, 2402113. [Google Scholar] [CrossRef]
- Hong X, Wu Z, Chen L, Wu F, Wei L, Yuan W. Hydrogel Microneedle Arrays for Transdermal Drug Delivery. Nano-Micro Lett. 2014, 6, 191–199. [Google Scholar] [CrossRef]
- Tiraton T, Suwantong O, Chuysinuan P, et al. Biodegradable microneedle fabricated from sodium alginate-gelatin for transdermal delivery of clindamycin. Materials Today Communications 2022, 32, 104158. [Google Scholar] [CrossRef]
- Yu W, Jiang G, Zhang Y, Liu D, Xu B, Zhou J. Polymer microneedles fabricated from alginate and hyaluronate for transdermal delivery of insulin. Materials Science and Engineering: C 2017, 80, 187–196. [Google Scholar] [CrossRef]
- Luo Z, Sun W, Fang J, et al. Biodegradable Gelatin Methacryloyl Microneedles for Transdermal Drug Delivery. Advanced Healthcare Materials 2019, 8, 1801054. [Google Scholar] [CrossRef]
- Zeng Z, Jiang G, Liu T, et al. Fabrication of gelatin methacryloyl hydrogel microneedles for transdermal delivery of metformin in diabetic rats. Bio-des Manuf. 2021, 4, 902–911. [Google Scholar] [CrossRef]
- Han D, Morde RS, Mariani S, et al. 4D Printing of a Bioinspired Microneedle Array with Backward-Facing Barbs for Enhanced Tissue Adhesion. Advanced Functional Materials 2020, 30, 1909197. [Google Scholar] [CrossRef]
- Barnum L, Quint J, Derakhshandeh H, et al. 3D-Printed Hydrogel-Filled Microneedle Arrays. Adv Healthcare Materials 2021, 10, 2001922. [Google Scholar] [CrossRef] [PubMed]
- Serris I, Serris P, Frey KM, Cho H. Development of 3D-Printed Layered PLGA Films for Drug Delivery and Evaluation of Drug Release Behaviors. AAPS PharmSciTech 2020, 21, 256. [Google Scholar] [CrossRef]
- Li X, Shan W, Yang Y, et al. Limpet Tooth-Inspired Painless Microneedles Fabricated by Magnetic Field-Assisted 3D Printing. Advanced Functional Materials 2021, 31, 2003725. [Google Scholar] [CrossRef]
- Zhou X, Liu H, Yu Z, et al. Direct 3D printing of triple-responsive nanocomposite hydrogel microneedles for controllable drug delivery. Journal of Colloid and Interface Science 2024, 670, 1–11. [Google Scholar] [CrossRef]
- Makvandi P, Jamaledin R, Chen G, et al. Stimuli-responsive transdermal microneedle patches. Materials Today 2021, 47, 206–222. [Google Scholar] [CrossRef]
- Nair SS, Mishra SK, Kumar D. Recent progress in conductive polymeric materials for biomedical applications. Polymers for Advanced Technologies 2019, 30, 2932–2953. [Google Scholar] [CrossRef]
- El-Sayed N, Vaut L, Schneider M. Customized fast-separable microneedles prepared with the aid of 3D printing for nanoparticle delivery. European Journal of Pharmaceutics and Biopharmaceutics 2020, 154, 166–174. [Google Scholar] [CrossRef]
- Pardridge, WM. Alzheimer’s disease drug development and the problem of the blood-brain barrier. Alzheimer’s & Dementia 2009, 5, 427–432. [Google Scholar] [CrossRef]
- Razzaghi M, Soleymani Eil Bakhtiari S, Charest G, Fortin D, Akbari M. Microneedle arrays for brain drug delivery: the potential of additive manufacturing. Trans Can Soc Mech Eng, 28 February 2025. [CrossRef]
- Prajapati BG, Alzaghari LF, Alam P, Fareed M, Kapoor DU. Revolutionizing neurological therapies: The role of 3D printed microneedles in precision brain targeted drug delivery. Journal of Drug Delivery Science and Technology 2025, 107, 106818. [Google Scholar] [CrossRef]
- Zhai X, Chen K, Wei X, et al. Microneedle/CD-MOF-mediated transdural controlled release of methylprednisolone sodium succinate after spinal cord injury. Journal of Controlled Release 2023, 360, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Wang Z, Yang Z, Jiang J, et al. Silk Microneedle Patch Capable of On-Demand Multidrug Delivery to the Brain for Glioblastoma Treatment. Advanced Materials 2022, 34, 2106606. [Google Scholar] [CrossRef]
- Banks, WA. From blood–brain barrier to blood–brain interface: new opportunities for CNS drug delivery. Nat Rev Drug Discov. 2016, 15, 275–292. [Google Scholar] [CrossRef]
- Jiang X, Xia W, Pan J, et al. Engineered microneedle systems for topical cancer therapy. Applied Materials Today 2023, 31, 101774. [Google Scholar] [CrossRef]
- Lee HJ, Son Y, Kim D, et al. A new thin silicon microneedle with an embedded microchannel for deep brain drug infusion. Sensors and Actuators B: Chemical 2015, 209, 413–422. [Google Scholar] [CrossRef]
- Lee Y, Kang T, Cho HR, et al. Localized Delivery of Theranostic Nanoparticles and High-Energy Photons using Microneedles-on-Bioelectronics. Advanced Materials 2021, 33, 2100425. [Google Scholar] [CrossRef]
- Wang Z, Han R, Shi Z, Mao Y, Tao TH, Qin N. Heterogeneous and Multifunctional Silk Microneedles for in Situ Treatment of Brain Glioma. In: 2020 IEEE 33rd International Conference on Micro Electro Mechanical Systems (MEMS). ; 2020, 369-371. [CrossRef]
- Wang Z, Liu K, Qin N, Tao TH. A Silk-Based Microneedle Patch for Controlled Multi-Drug Delivery in Glioma Treatment. In: 2021 21st International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers). ; 2021, 1416-1419. [CrossRef]
- Muresan P, McCrorie P, Smith F, et al. Development of nanoparticle loaded microneedles for drug delivery to a brain tumour resection site. European Journal of Pharmaceutics and Biopharmaceutics 2023, 182, 53–61. [Google Scholar] [CrossRef]
- Acharya JR, Kumar S, Girdhar GA, et al. 3D Bioprinting: Shaping the Future of Periodontal Tissue Regeneration and Disease Management. Cureus. [CrossRef]
- Zhang X, Hasani-Sadrabadi MM, Zarubova J, et al. Immunomodulatory microneedle patch for periodontal tissue regeneration. Matter 2022, 5, 666–682. [Google Scholar] [CrossRef]
- Ma Y, Tao W, Krebs SJ, Sutton WF, Haigwood NL, Gill HS. Vaccine Delivery to the Oral Cavity Using Coated Microneedles Induces Systemic and Mucosal Immunity. Pharm Res. 2014, 31, 2393–2403. [Google Scholar] [CrossRef]
- Gade S, Glover K, Mishra D, et al. Hollow microneedles for ocular drug delivery. Journal of Controlled Release 2024, 371, 43–66. [Google Scholar] [CrossRef] [PubMed]
- Levy JA, Straker MA, Stine JM, Beardslee LA, Ghodssi R. Magnetically triggered ingestible capsule for localized microneedle drug delivery. Device 2024, 2, 100438. [Google Scholar] [CrossRef]
- Abramson A, Caffarel-Salvador E, Soares V, et al. A luminal unfolding microneedle injector for oral delivery of macromolecules. Nat Med. 2019, 25, 1512–1518. [Google Scholar] [CrossRef]
- Engineers’ New Capsule Aims to Deliver Drugs—and Hope—to GI Patients. Maryland Today. , 2022. Accessed June 1, 2025. https://today.umd. 7 December.
- Hanaphy, P. Purdue University researchers 3D print bacteria grabbing colonoscopy capsules. 3D Printing Industry. , 2020. Accessed June 1, 2025. https://3dprintingindustry. 13 August 1745. [Google Scholar]
- Chen W, Wainer J, Ryoo SW, et al. Dynamic omnidirectional adhesive microneedle system for oral macromolecular drug delivery. Science Advances 2022, 8, eabk1792. [Google Scholar] [CrossRef]
- Xu J, Liao X, Chen D, Jia X, Niu X. Microneedles for non-transdermal drug delivery: design strategies and current applications. Bio-des Manuf. 2025, 8, 243–274. [Google Scholar] [CrossRef]
- Kaur SD, Choudhary S, Sen S, Pemmaraju DB, Singh SK, Kapoor DN. Microneedle patches: the next frontier in cardiovascular care. Drug Deliv and Transl Res, 29 January 2025. [CrossRef]
- Zhang X, Chen G, Wang Y, Fan L, Zhao Y. Arrowhead Composite Microneedle Patches with Anisotropic Surface Adhesion for Preventing Intrauterine Adhesions. Advanced Science 2022, 9, 2104883. [Google Scholar] [CrossRef]
- Wang N, Zhen Y, Jin Y, et al. Combining different types of multifunctional liposomes loaded with ammonium bicarbonate to fabricate microneedle arrays as a vaginal mucosal vaccine adjuvant-dual delivery system (VADDS). Journal of Controlled Release 2017, 246, 12–29. [Google Scholar] [CrossRef]
- 3D-Printed Microneedles Open Ears to New Treatments. Columbia University Irving Medical Center. , 2019. Accessed June 1, 2025. https://www.cuimc.columbia. 3 December.
- Chiang H, Yu M, Aksit A, et al. 3D-Printed Microneedles Create Precise Perforations in Human Round Window Membrane in Situ. Otology & Neurotology 2020, 41, 277. [Google Scholar] [CrossRef]
- 瑞思3D 列印專門店. The First 3D-Printed Microneedle for Hearing Loss Treatment. RAS3D. , 2025. Accessed June 1, 2025. https://www.ras.com. 5 January.
- vander Straeten A, Sarmadi M, Daristotle JL, et al. A microneedle vaccine printer for thermostable COVID-19 mRNA vaccines. Nat Biotechnol. 2024, 42, 510–517. [Google Scholar] [CrossRef]
- Listek, V. The Future of Vaccination? MIT’s 3D Printed Microneedles Show Promise. 3DPrint.com | The Voice of 3D Printing / Additive Manufacturing. , 2023. Accessed June 1, 2025. https://3dprint. 3 May 2998. [Google Scholar]
- Che QT, Seo JW, Charoensri K, Nguyen MH, Park HJ, Bae H. 4D-printed microneedles from dual-sensitive chitosan for non-transdermal drug delivery. International Journal of Biological Macromolecules 2024, 261, 129638. [Google Scholar] [CrossRef]
- Biswas AA, Dhondale MR, Agrawal AK, Serrano DR, Mishra B, Kumar D. Advancements in microneedle fabrication techniques: artificial intelligence assisted 3D-printing technology. Drug Deliv and Transl Res. 2024, 14, 1458–1479. [Google Scholar] [CrossRef] [PubMed]
- Defelippi KM, Kwong AYS, Appleget JR, et al. An Integrated Approach to Control the Penetration Depth of 3D-Printed Hollow Microneedles. Applied Mechanics 2024, 5, 233–260. [Google Scholar] [CrossRef]
- Donnelly RF, Singh TRR, Larrañeta E, McCrudden MTC. Microneedles for Drug and Vaccine Delivery and Patient Monitoring, 2018.
- Jung JH, Jin SG. Microneedle for transdermal drug delivery: current trends and fabrication. J Pharm Investig 2021, 51, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Ren Y, Li J, Chen Y, et al. Customized flexible hollow microneedles for psoriasis treatment with reduced-dose drug. Bioengineering & Translational Medicine 2023, 8, e10530. [Google Scholar] [CrossRef]
- Bedir T, Kadian S, Shukla S, Gunduz O, Narayan R. Additive manufacturing of microneedles for sensing and drug delivery. Expert Opinion on Drug Delivery 2024, 21, 1053–1068. [Google Scholar] [CrossRef]
- Islam H, Poly TS, Tisha ZT, et al. 3D Printed Hollow Microneedles for Treating Skin Wrinkles Using Different Anti-Wrinkle Agents: A Possible Futuristic Approach. Cosmetics 2023, 10, 41. [Google Scholar] [CrossRef]
- Ingrole RSJ, Azizoglu E, Dul M, Birchall JC, Gill HS, Prausnitz MR. Trends of microneedle technology in the scientific literature, patents, clinical trials and internet activity. Biomaterials 2021, 267, 120491. [Google Scholar] [CrossRef]
- DeSimone JM, Robbins GR, Johnson AR. Polymeric microneedles and rapid additive manufacturing of the same. Published online , 2020. Accessed February 24, 2025. https://patents.google.com/patent/US10792857B2/en? 6 October.
- Nguyen TD, Tran K. Method of manufacturing a microneedle assembly. Published online , 2024. Accessed February 24, 2025. https://patents.google.com/patent/EP3761864B1/en? 1 May.
- CHANG, C. Microneedle Array Device, Methods Of Manufacturing And Use Thereof. Published online , 2024. Accessed February 24, 2025. https://patents.google.com/patent/US20240075267A1/en? 7 March.
- Creelman B, Frivold C, Jessup S, Saxon G, Jarrahian C. Manufacturing readiness assessment for evaluation of the microneedle array patch industry: an exploration of barriers to full-scale manufacturing. Drug Deliv and Transl Res. 2022, 12, 368–375. [Google Scholar] [CrossRef]
- Cárcamo-Martínez Á, Mallon B, Domínguez-Robles J, Vora LK, Anjani QK, Donnelly RF. Hollow microneedles: A perspective in biomedical applications. International Journal of Pharmaceutics 2021, 599, 120455. [Google Scholar] [CrossRef]
- Attaran, M. 3D Printing: Enabling a New Era of Opportunities and Challenges for Manufacturing. International Journal of Research in Engineering and Science 2016, 4, 2320–9364. [Google Scholar]
- Mu Q, Wang L, Dunn CK, et al. Digital light processing 3D printing of conductive complex structures. Additive Manufacturing 2017, 18, 74–83. [Google Scholar] [CrossRef]
- Dongre A, Nale T, Ramavajhala A, et al. The evolution of transdermal drug delivery: from patches to smart microneedle-biosensor systems. Journal of Knowledge Learning and Science Technology ISSN: 2959-6386 (online) 2024, 3, 160–168. [Google Scholar] [CrossRef]
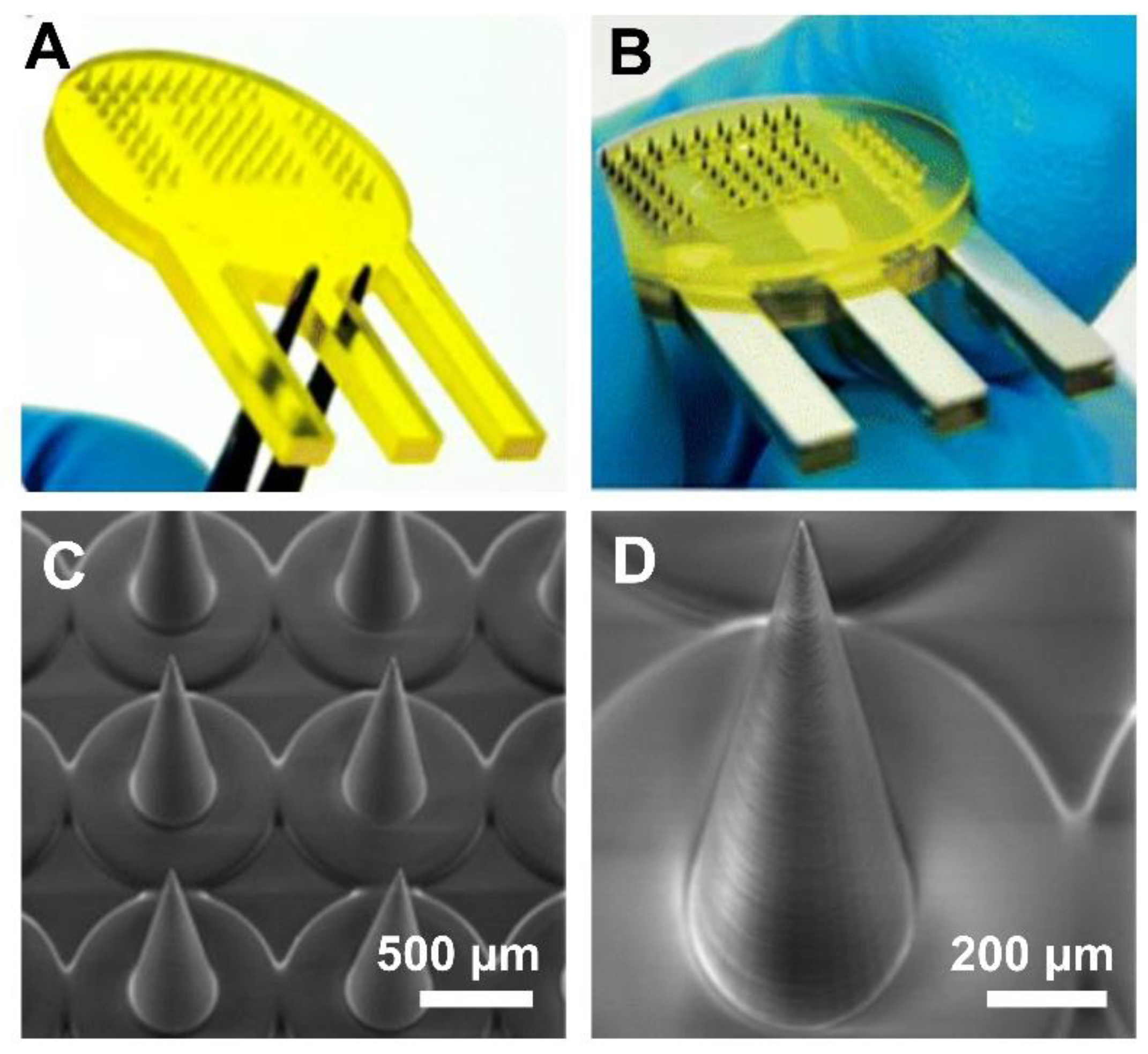
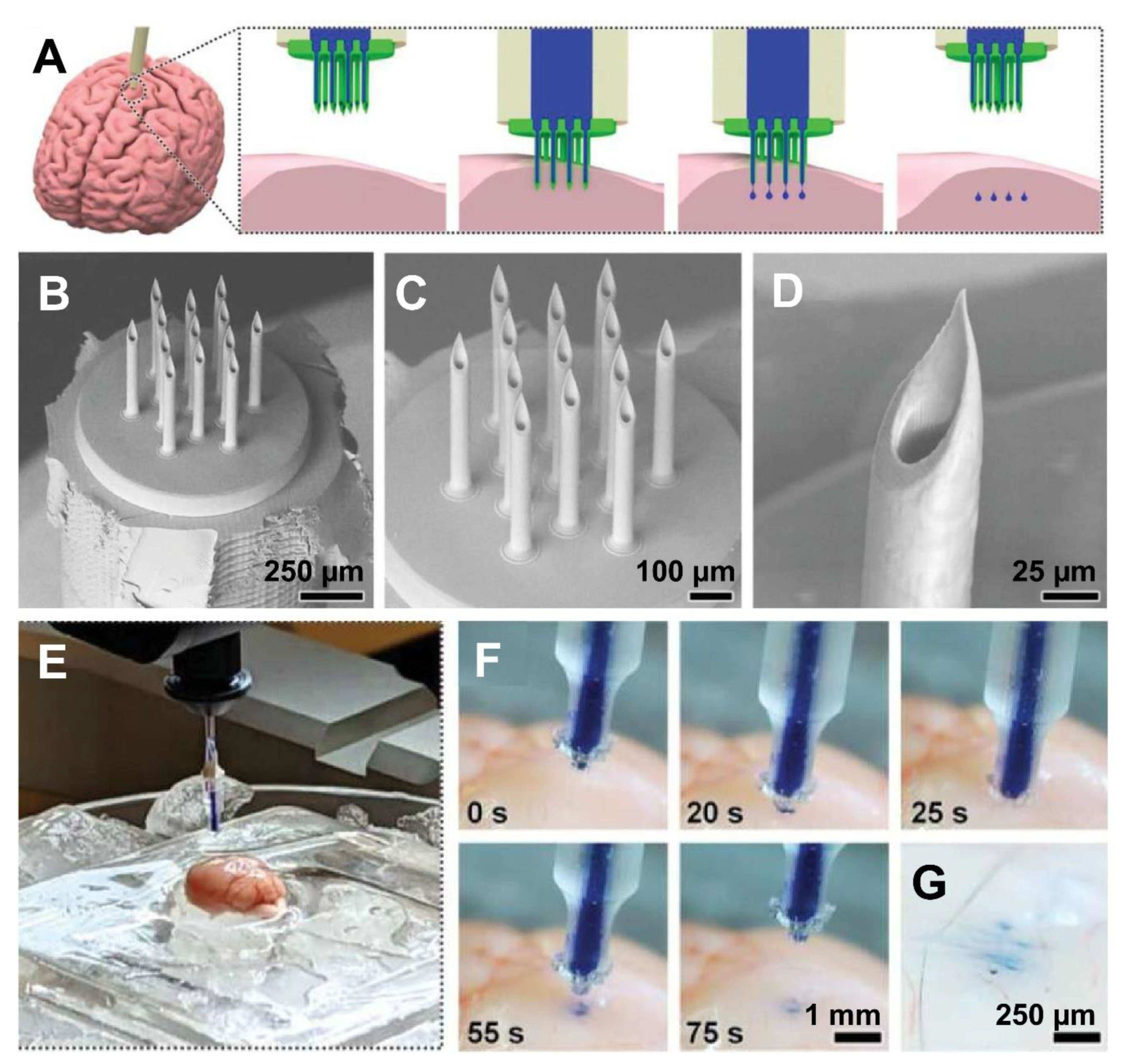
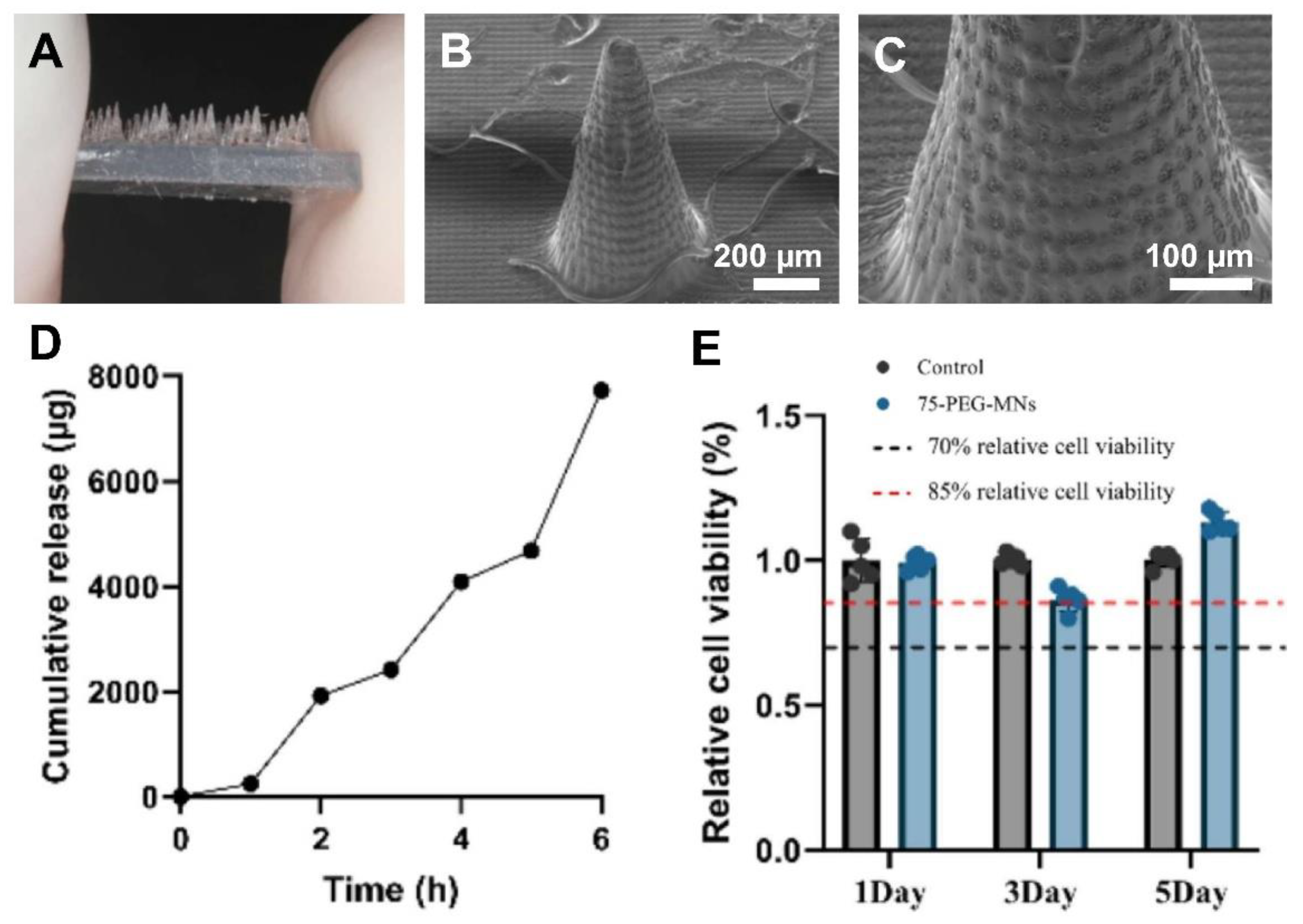
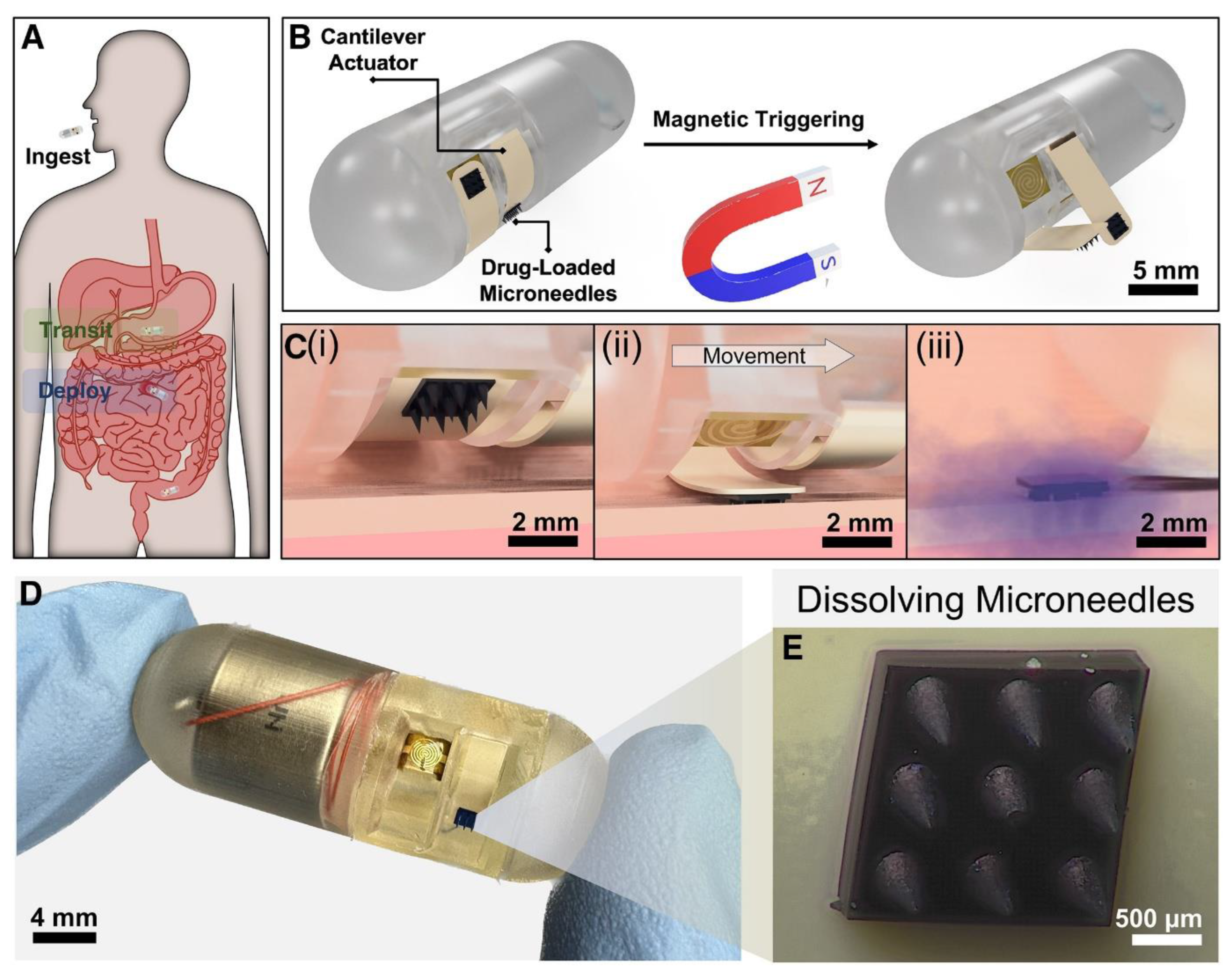
| Fabrication Technique | Advantages | Limitations | References |
|---|---|---|---|
| SLA | High-resolution, smooth surface finishes, ideal for intricate designs. | Slower speed com pared to DLP, limited build volume. |
[16,32,33,34,35,36,37,38,39,40,41,42,43,74] |
| DLP | Faster printing speed, high resolution, suitable for intricate designs. | Potential pixelation effects. | [9,18,42,47,48,49,50,75] |
| LCD | Affordable, large build volume, avoids pixel distortion. | Slightly lower resolution and accuracy compared to DLP. | [41,53,54,55] |
| SOPL | High precision, efficient for specific patterns, suitable for intricate designs. | Limited flexibility in pattern changes during printing. | [25] |
| 2PP | Extremely high resolution, suitable for nanoscale features. | Expensive, slow printing speed, limited material options. | [22,59,60,61,62,63,64] |
| FDM | Cost-effective, wide range of materials, user-friendly. | Low resolution. Often requires post-fabrication processes | [68,69,70,71] |
| Organ/Tissue applied | Results | Reference number |
|---|---|---|
| Brain (glioblastoma) | The developed MNA achieved programmable release of chemotherapeutics and resulted in prolonged survival in mice | [107] |
| Brain (glioma) | The developed MNA achieved effective, long-term drug release, with programmable degradation profiles. In vivo studies demonstrated significant suppression of tumor cell activity, confirming its potential for localized glioma therapy. The device maintained structural integrity on brain surfaces and enabled precise, multi-drug delivery without systemic toxicity. | [112] |
| Brain (glioma) | The developed MNA enabled coordinated, sequential delivery of thrombin, temozolomide, and bevacizumab—achieving hemostasis, anti-angiogenesis, and tumor apoptosis. In glioma mouse models, it reduced tumor volume, extended survival, and proved biocompatible and biodegradable, demonstrating on-demand multidrug delivery as an effective localized therapy | [113] |
| Brain | The developed MNA successfully delivered cannabidiol and olaparib nanoparticles into brain simulant and ex vivo brain tissue. It demonstrated strong potential for localized, sustained chemotherapy at brain tumor resection sites.imaging. | [114] |
| Brain (fluid injection) | The hybrid-fabricated MNAs demonstrated mechanical robustness, effective penetration into brain tissue, and precise, uniform delivery of fluids and nanoparticles. Microfluidic testing showed strong sealing with no leakage under high pressure. Compared to conventional needles, MNAs provided superior distribution of payloads, highlighting their potential for advanced biomedical microinjections. | [17] |
| Oral cavity/Gingival tissue | MNAs loaded with madecassoside significantly improved gingival height and thickness, increased collagen fiber area, and elevated type I collagen levels in rabbits. The treatment demonstrated strong regenerative potential for periodontal soft tissues, validating the approach as a promising, minimally invasive alternative to conventional surgical interventions. | [75] |
| Ocular (Eye) | The fabricated MNA achieved precise intrascleral delivery of rhodamine B via hollow MNAs. The adapters controlled injection angle and volume, enabling accurate depot formation in the sclera. |
[118] |
| Ocular (Eye) | The optimized cone-shaped MNs (1:2 aspect ratio), printed at a 67.5° orientation and 25 μm layer thickness, exhibited high fidelity, sharp tips, and robust mechanical strength. These MNs successfully penetrated ex vivo ocular tissues with minimal force, suggesting their potential for self-administered, minimally invasive ocular drug delivery. | [37] |
| GI Tract | The developed device safely delivered insulin-loaded MNAs into the small intestine of swine, achieving rapid systemic absorption with over 10% bioavailability compared to subcutaneous injection. Blood glucose levels dropped significantly, confirming effective drug delivery. The device caused no tissue perforation or retention, supporting its feasibility for oral biologic delivery. | [120] |
| GI Tract | The developed capsule successfully deployed MNAs into ex vivo intestinal tissue within 2.91 ± 0.48 seconds using a handheld magnet. It achieved precise localized drug delivery with significant diffusion and retention, demonstrating improved efficacy and targeting compared to conventional oral delivery, while minimizing systemic exposure and potential side effects. | [119] |
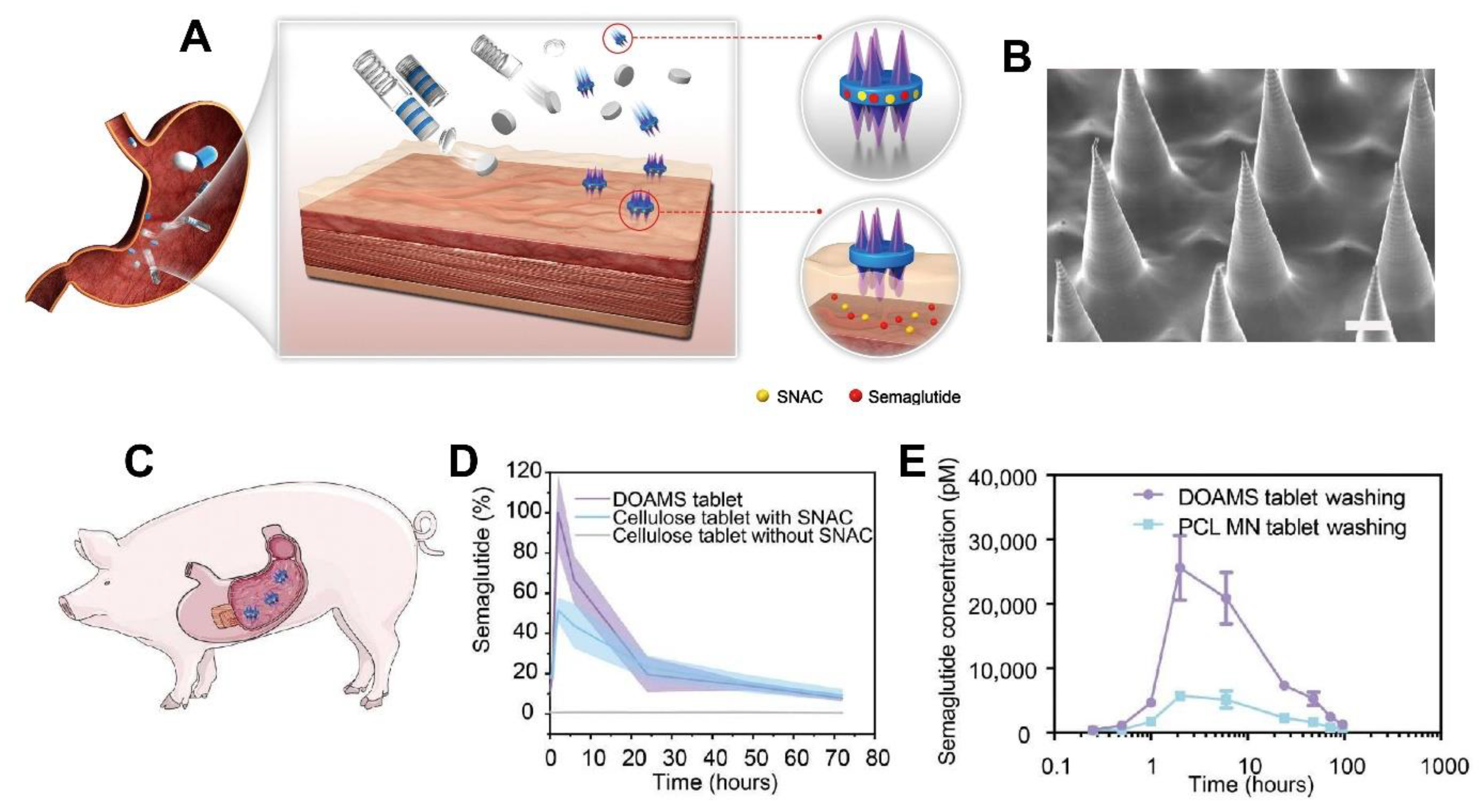
| GI Tract | The developed platform significantly enhanced oral delivery of semaglutide in swine, achieving twice the peak plasma concentration of standard tablets and sixfold higher levels with water. The system demonstrated strong mucoadhesion, prolonged gastric retention, and safe deployment, suggesting high potential for translating injectable biologics into effective oral formulations. | [123] |
| Inner ear | The MNAs successfully created consistent, slit-shaped perforations in the HRWM with minimal deformation or damage. Peak forces and displacements remained within safe limits, and the MNAs retained structural integrity post-use. These results support the feasibility of precise, safe HRWM perforation for future diagnostic and therapeutic applications in the inner ear. | [129] |
| Point-of-care 3D printing | The MNAs produced using the MVP effectively delivered mRNA vaccines, generated strong immune responses in mice, and remained stable for at least six months at room temperature. These patches offer a viable alternative to conventional vaccines by enabling decentralized, cold-chain-free immunization, particularly in resource-limited settings. | [131] |
| Point-of-care 3D printing | The HBCMA-based 4D-printed MNAs demonstrated successful soft tissue penetration and sustained drug release, with geometry and mechanical properties modulated by temperature. Drug delivery followed diffusion-dominated kinetics, and needle performance was enhanced through precise DLP printing and material responsiveness. These MNAs show strong potential for non-dermal therapeutic applications. | [133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





