Introduction
Iron deficiency (ID) is a prevalent comorbidity of heart failure (HF) that significantly worsens clinical outcome(s) and prognosis [
1,
2]. Studies have demonstrated that ID leads to impaired mitochondrial function and decreased myocyte contractility, highlighting its detrimental effects independent of anaemia in patients diagnosed with HF [
3,
4]. While most studies investigating ID in HF have focused on stable patients with reduced ejection fraction (EF) [
2,
5] recent clinical observations have reported ID in up to 80% of patients diagnosed with acute decompensated HF (ADHF) [
6]. Moreover, ID was associated with short-term adverse outcomes in ADHF [
6,
7]. Although the negative impact of ID on left ventricular (LV) systolic function is well established, its effects on right ventricular (RV) function, particularly RV global longitudinal strain (RV-GLS), as an indicator of early subclinical dysfunction, remain insufficiently studied [
8,
9,
10].
Previous studies have demonstrated the adverse effects of ID on RV systolic function in patients diagnosed with ADHF [
11]. However, the specific impact of ID on RV-GLS, which reflects early subclinical impairment, has not yet been adequately explored. As such, the present study aimed to evaluate the relationship between ID and RV-GLS in patients diagnosed with ADHF.
Methods
Study Population
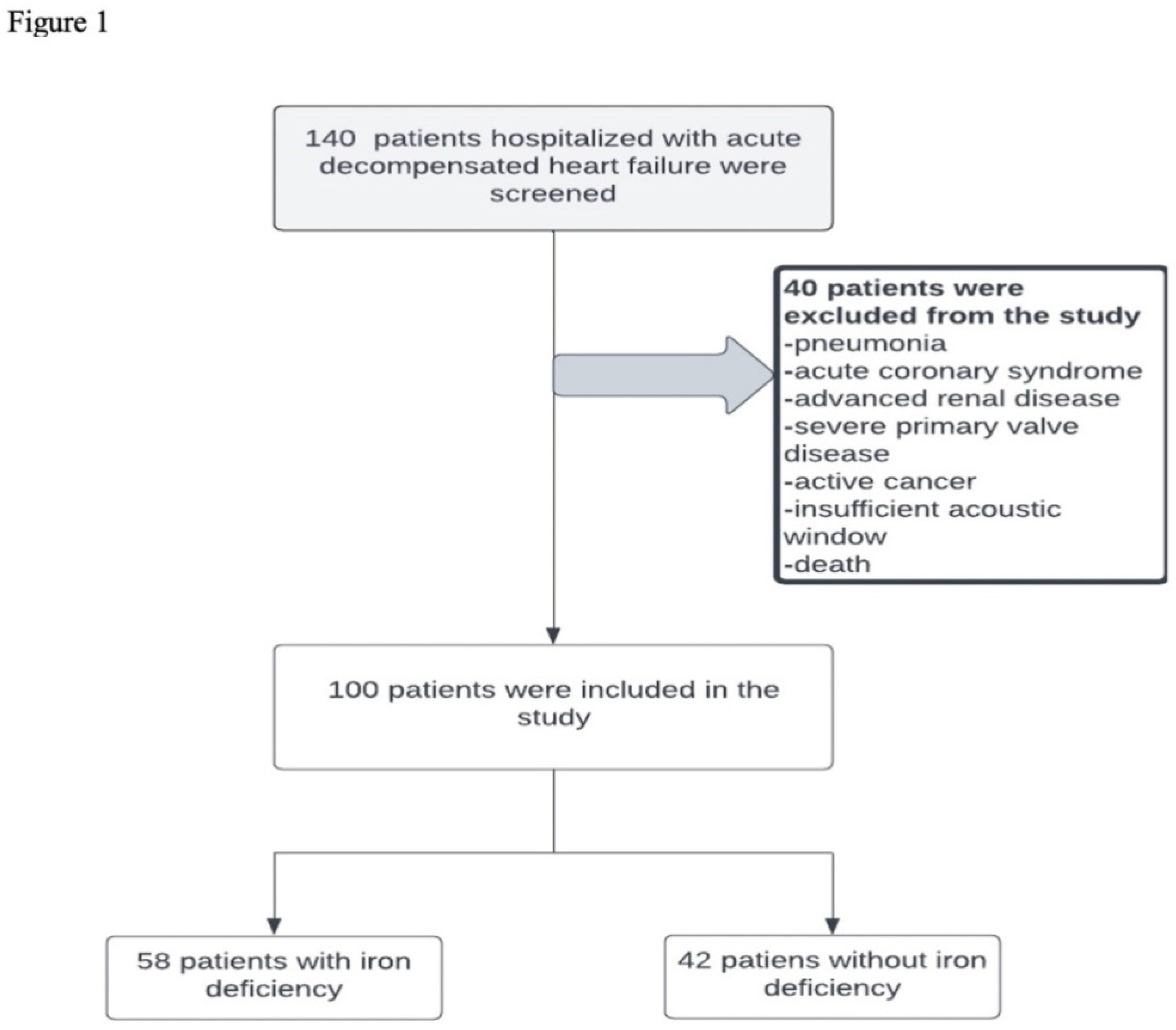
Data from 100 patients hospitalised with ADHF between January 2022 and September 2022 were included in this study. Acute heart failure was diagnosed based on the European Society of Cardiology (ESC) criteria and defined as rapid onset or worsening of HF signs and symptoms requiring intravenous (IV) diuretic therapy or hospitalisation [
12].
Patients with active infections, primary tricuspid valve disease, severe rheumatic mitral stenosis, acute coronary syndrome, pneumonia, advanced kidney or liver failure, pulmonary arterial hypertension, active cancer, or died during hospitalisation were excluded (
Figure 1).
The study protocol adhered to the ethical principles outlined in the Declaration of Helsinki, and informed consent was obtained from all participants before enrolment. Our study was initiated with the approval of the local ethics committee. (E-69291215-900-8625)
On admission, demographic data, comorbidities, vital signs, and standard treatments were recorded for each patient. Baseline assessments included 12-lead electrocardiogram and laboratory investigations for renal function and electrolyte, pro-B-type natriuretic peptide (pro-BNP), and troponin levels. Additional laboratory investigations, including complete blood count and ferritin, serum iron, cholesterol, and C-reactive protein levels, were performed approximately 120 h after admission.
Patients were categorised into two groups based on the presence or absence of ID, as defined by the ESC guidelines.
ID Parameters Assessment and Definitions
Blood parameters for diagnosing ID and assessing inflammation were obtained approximately 120 h after admission, following clinical stabilisation. Clinical stabilisation was defined as the absence of the need for IV diuretics, transition to oral diuretic therapy, and no requirement for mechanical support devices while maintaining haemodynamic stability.
The ESC criteria for ID diagnosis were used to define ID as ferritin level < 100 µg/L or ferritin levels between 100 and 299 µg/L with transferrin saturation (TSAT) < 20% [
12]. Anaemia is defined by the World Health Organization (WHO) as a haemoglobin level < 12 g/dL in women and < 13 g/dL in men. N-terminal proBNP levels were measured on admission. Ferritin, haemoglobin, and complete blood counts were analysed using a laboratory analyser (cobas 6000, Roche Diagnostics, Mannheim, Germany), and NT-proBNP levels were measured using the cobas E411 system (Roche, Germany). TSAT was calculated as serum iron divided by the total iron-binding capacity (TIBC).
Echocardiographic Evaluation
Standard two-dimensional (2D) echocardiography (2DE) evaluations were performed approximately 120 ± 24 h after stabilisation using a Epiq7 system (Philips Healthcare, Andover, MA, USA) equipped with an X5 transducer. Echocardiographic images were reviewed by two experienced cardiologists who were blinded to the clinical data.
The measurements followed the American Society of Echocardiography [
13] standards and included views from the parasternal long axis, parasternal short axis, apical four-chamber, three-chamber, and two-chamber. The key parameters assessed were as follows:
Left atrium (LA): diameter at end systole along the parasternal long axis.
Pulmonary valve: Assessed using continuous wave (CW) and pulsed wave (PW) Doppler on the parasternal short axis. Pulmonary acceleration time was measured using PW Doppler across the pulmonary valve.
LV systolic function: Calculated using the modified Simpson biplane method by manually tracing the apical four- and two-chamber views.
RV systolic function: Evaluated using the apical four-chamber view. The key parameters were as follows:
Tricuspid annular plane systolic excursion (TAPSE) measured via M-mode.
Peak systolic tricuspid annular velocity (RV TDI S′) was obtained using tissue Doppler imaging.
RV fractional area change (RV FAC) was calculated as the percentage of RV end-diastolic and end-systolic area changes.
Systolic pulmonary artery pressure was estimated using the peak velocity gradient of tricuspid regurgitation flow, right atrial pressure derived from the inferior vena cava dimensions, and respiratory variability.
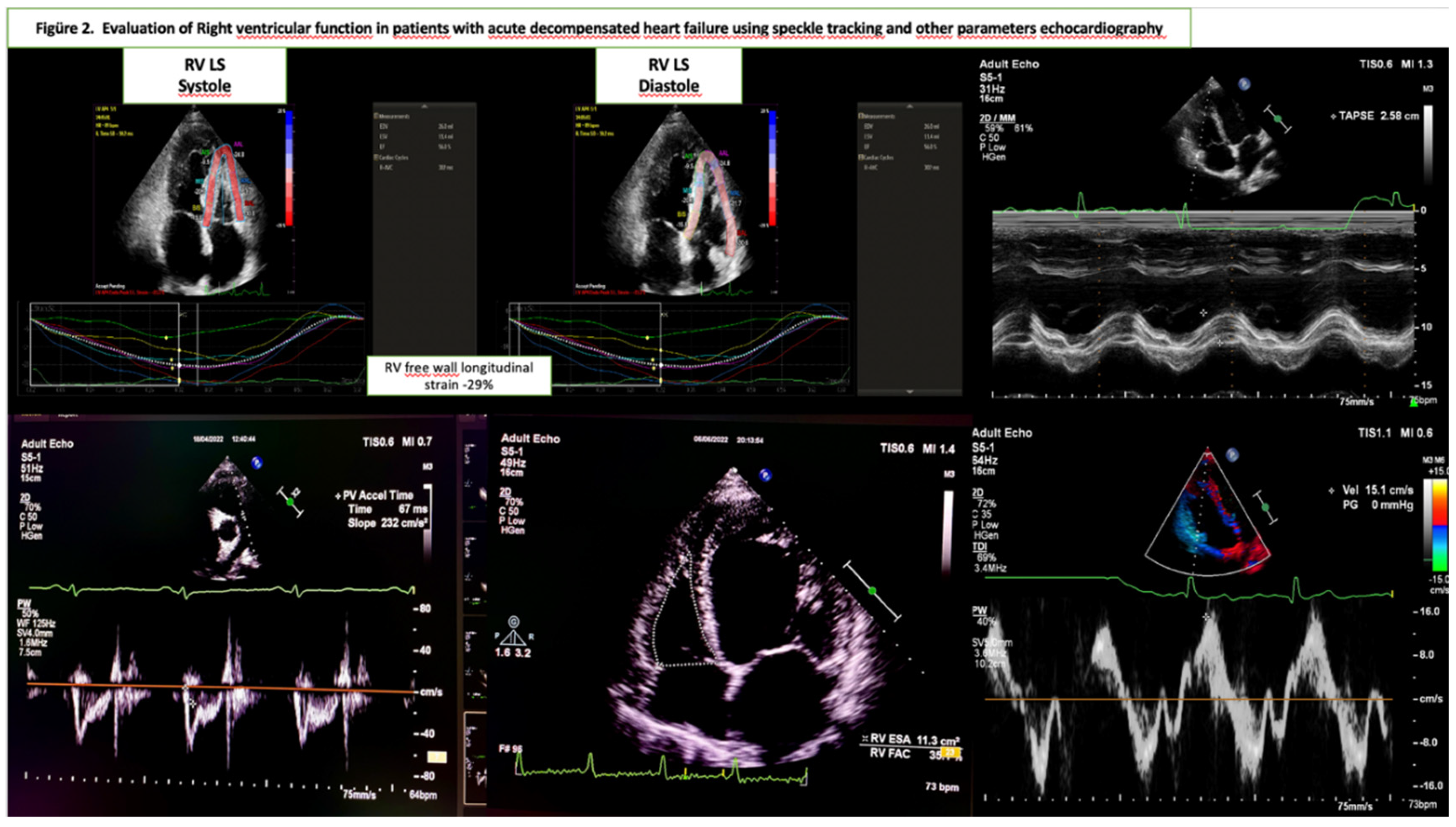
Two-dimensional strain imaging was used to assess myocardial deformation as an early marker of RV systolic dysfunction. The time of aortic valve closure was considered end-systole, whereas the R-wave peak on the electrocardiogram was considered end-diastole. For strain measurements, an apical four-chamber view of the RV was captured, ensuring that all RV segments and the LV apex were within the imaging frame [
14,
15].
Boundaries of the endocardium, myocardium, and epicardium were automatically defined and manually adjusted to avoid errors. Analysis was performed using QLAB-CMQ software on the Philips Epiq 7C system. The RV free wall was divided into three segments (basal, mid, and apical) and the mean longitudinal strain was calculated across these segments. Patients with incomplete imaging windows or unmeasurable strain values were excluded.
The parameters used for RV function evaluation in this study are reported in
Figure 2. [
14]
Statistical Analysis
Statistical analyses were performed using SPSS version 21 (IBM Corp., Armonk, NY, USA). Continuous variables are expressed as mean ± standard deviation (SD), while categorical variables are expressed as percentage. The normality of data distribution was assessed using the Kolmogorov–Smirnov test.
Comparisons between groups:
Continuous variables were analysed using one-way analysis of variance (ANOVA) for normally distributed data or the Kruskal–Wallis test for non-normally distributed data. Categorical variables were compared using the chi-squared (χ²) test.
The Spearman’s correlation test was used to investigate the relationships between continuous variables. Independent predictors of reductions in RV global longitudinal strain (GLS) reduction were identified using multiple linear regression analysis. The regression model included parameters such as haemoglobin, LVEF, heart rate, deceleration time (DKB), and troponin and ferritin levels.
Differences with p < 0.05 were considered to be statistically significant.
Results
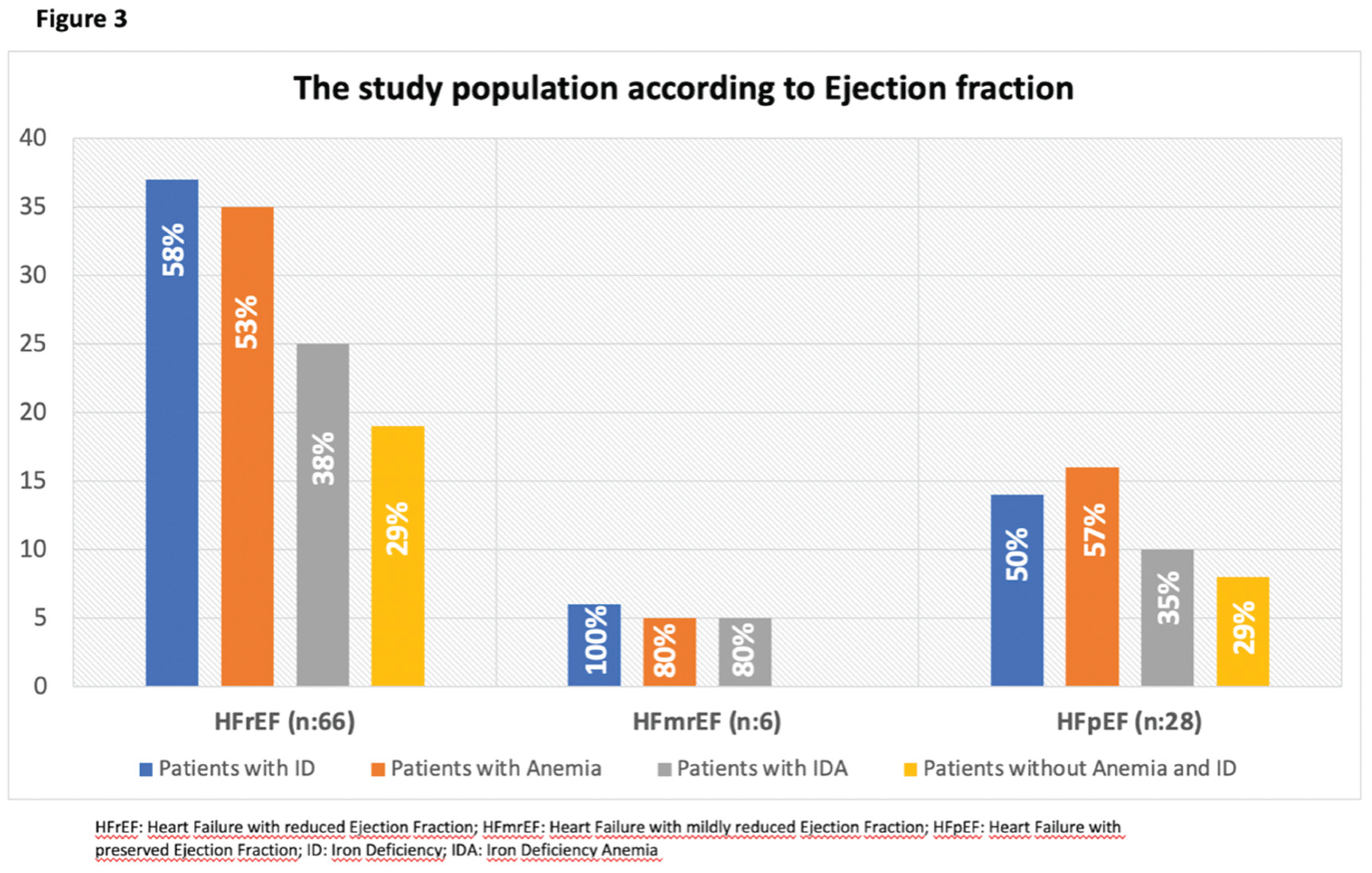
The distribution of patients according to HF classification is illustrated in
Figure 3. The majority (66%) of the population consisted of patients with HF with reduced ejection fraction (HFrEF). Additionally, 41% of patients fulfilled the criteria for both anaemia and ID.
Patients were divided into two groups based on the presence or absence of ID. The clinical, laboratory, demographic, and echocardiographic characteristics of the study groups are summarised in
Table 1. No significant differences were observed between the groups in terms of age, sex, or comorbidities including diabetes, hypertension, and asthma/chronic obstructive pulmonary disease. Laboratory investigations revealed similar levels of creatinine, sodium, potassium, creatine kinase, cholesterol, C-reactive protein, pro-BNP, and troponin. However, haemoglobin, haematocrit, ferritin, and TSAT levels were significantly lower in the group with ID (p < 0.01).
In the echocardiographic assessments, LVEF, LA diameter, FAC, pulmonary artery systolic blood pressure, and tissue Doppler imaging values for the RV were similar between groups. However, TAPSE, pulmonary acceleration time, and RV-GLS were significantly lower in the ID group (p = 0.05, p = 0.05, and p = 0.005, respectively).
In the subgroup analysis, patients with both ID and anaemia were compared to those with ID alone. The ECHO parameters of the two groups are summarized in
Table 2. Although TAPSE and RV-GLS values were numerically lower in the group with both ID and anaemia, the differences were not statistically significant (p = 0.08 and p = 0.66, respectively). No significant differences were observed in the other echocardiographic parameters.
Another subgroup analysis compared patients with ID only to those without ID or anaemia. The ECHO parameters of the two groups are summarised in
Table 2. While TAPSE exhibited no significant difference, RV-GLS was significantly lower in the ID group, reflecting subclinical systolic dysfunction (p = 0.05).
Table 2.
Echocardiographic findings in patients with iron deficiency and iron deficiency anemia and in patients with iron deficiency alone and without iron deficiency and anemia.
Table 2.
Echocardiographic findings in patients with iron deficiency and iron deficiency anemia and in patients with iron deficiency alone and without iron deficiency and anemia.
| |
ANEMIA (-)
ID (+)
n:17
|
ANEMIA (+)
ID (+)
n:41
|
P value |
ANEMIA (-)
ID(-)
N:27
|
ANEMIA (-)
ID(+)
N:17
|
P value |
| LVEF, (%) * |
35 (18-60) |
38 (18-60) |
0.41 |
30 (20-60) |
35 (18-60) |
0.42 |
| LAD (mm) * |
49 (44-60) |
46 (42-61) |
0.12 |
48 (40-61) |
49 (44-60) |
0.36 |
| TAPSE (mm) * |
18 (12-22) |
15.2 (12-22.9) |
0.08 |
17.3 (14.1-22.8) |
18 (12-22) |
0.90 |
| FAC (%) |
38.6 (20-50) |
36 (20-53) |
0.39 |
38 (18.5-50) |
38.6 (20.7-50) |
0.09 |
| RV-LS (%) * |
-16.2 (6.3-26.8) |
-14.7 (6.5-25.8) |
0.66 |
-20.4 (-9.9 27) |
-16.2((-6.3) - (-26.8)) |
0.05 |
| Pulmonaryacceleration time, (msn) |
92 (50-116) |
88 (56-132) |
0.91 |
96.9±22.1 |
86.9±21.1 |
0.20 |
| PASB, (mmHg) * |
50 (30-80) |
50 (28-70) |
0.72 |
45 (30-100) |
50 (30-80) |
0.68 |
| RV TDI S’ (CM/S) * |
10.3 (5.3-16.3) |
9.1 (6.4-17) |
0.78 |
012.2 (4.2-15.4) |
10.3 (5.3-16.3) |
0.21 |
Table 3.
Factors associated with right ventricular longitudinal strain in multiple linear regression analysis.
Table 3.
Factors associated with right ventricular longitudinal strain in multiple linear regression analysis.
Model
|
Unstandardized coefficients |
Standardized coefficients |
P value |
95% CI |
| B |
Std. Error |
Beta |
| Constant* |
-0.961 |
7.507 |
|
0.89 |
-15.872- 13.950 |
| HGB |
0.652 |
0.248 |
0.252 |
0.01 |
0.160-1.145 |
| LV EF |
0.158 |
0.035 |
0.429 |
<0.01 |
0.088 – 0.228 |
| Model |
B |
Partial correlations |
Collinearity statistics
Tolerance
|
P value |
| eGFR |
-0.010 |
-0.047 |
0.924 |
0.66 |
| Heart Rate |
-0.046 |
-0.164 |
0.881 |
0.12 |
| DBP |
0.008 |
0.013 |
0.882 |
0.90 |
| Troponin |
5.444 |
0.017 |
0.915 |
0.87 |
| Ferritin |
0.007 |
0.150 |
0.843 |
0.15 |
| LAD |
0.126 |
0.138 |
0.936 |
0.19 |
Independent predictors of reduced RV-GLS were evaluated using multiple regression analysis. The model included haemoglobin, LVEF, glomerular filtration rate, heart rate, DKB, troponin, ferritin, and LA diameter. The adjusted R2 value for the model was 0.23. Haemoglobin levels (B = 0.652, p = 0.010) and LVEF (B = 0.158, p < 0.0001) were identified as independent predictors of RV-GLS deterioration.
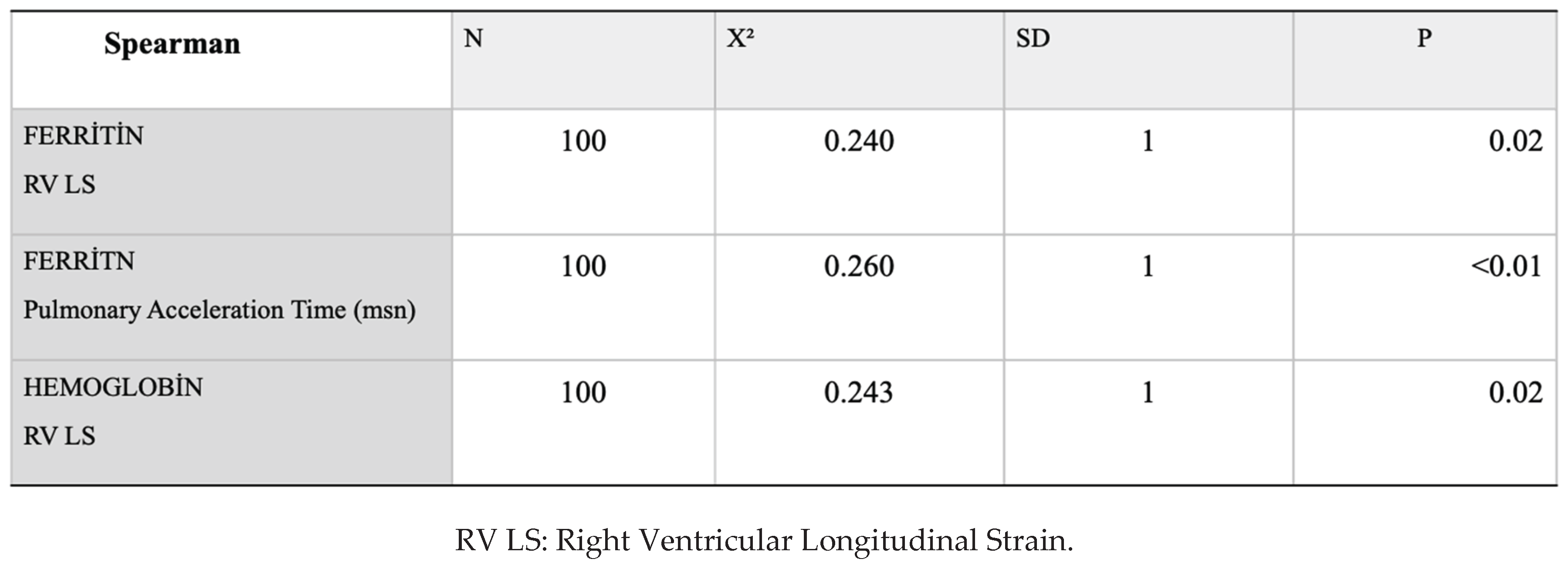
Spearman correlation analysis revealed a weak―but statistically significant―relationship between ferritin and RV-GLS, haemoglobin and RV-GLS and pulmonary acceleration time (
Table 4).
Discussion
The present study investigated the effect of ID on RV systolic function in patients hospitalised for ADHF who underwent transthoracic echocardiography. The main findings are as follows. First, in the ID group, TAPSE (15.6 mm vs. 17.2 mm; p=0.05) and RV-GLS (-14.7% vs. -18.2%; p=0.005) were significantly lower compared with the non-ID group. Second, in patients with ID but no anaemia, the RV-GLS (-20.4% vs. -16.2%, p=0.05) was significantly lower than that in non-ID patients. Third, multiple linear regression analysis revealed that haemoglobin (B = 0.652, p=0.010) and LVEF (B = 0.158, p<0.0001) independently predicted impairment in RV-GLS.
HF is a global health problem that requires frequent hospitalisation and is associated with high mortality and morbidity rates. ID is a common condition in patients with HF, affecting 30%–50% of those with chronic HF and up to 70–80% of those with acute HF. Previous studies have demonstrated that ID impairs cardiomyocyte contractile function, and that iron replacement therapy improves this function [
16,
17]. Studies have also reported significant symptom improvement and better clinical outcomes in patients with HF treated with iron replacement therapy [
18,
19].
Although the effect of ID on LV systolic and diastolic functions has been well studied, its impact on RV systolic function remains unclear. Previous research has shown that RV systolic dysfunction in patients with HF is associated with poorer outcomes, regardless of LVEF [
20,
21,
22]. RV function is particularly vulnerable to conditions that increase workload and comorbidities, such as ID, which negatively affect systolic and diastolic function [
23,
24]. In a study involving 903 patients with acute HF, Minana et al. [
11] reported that ID was associated with a reduction in TAPSE. However, this study did not find a relationship between ID and LVEF. Similarly, our findings confirmed a significant reduction in TAPSE in the ID group, while no difference in the LVEF was observed. Additionally, our study revealed that RV-GLS was significantly lower in patients with ID, suggesting subclinical myocardial dysfunction.
In our subgroup analysis, patients with ID were compared to those without ID. RV-GLS was lower in the ID group, indicating that ID may cause subclinical impairment of RV systolic function due to myocardial deformation, even when TAPSE is preserved in the early stages of ID. Previous studies have compared RV-GLS in patients with and without anaemia, but have not specifically focused on those with ID or ID anaemia. For example, Burns et al. [
25] investigated echocardiographic parameters in patients with HF, preserved ejection fraction, and anaemia. Their study found reduced FAC and increased systolic pulmonary artery pressure but no differences in LVEF or strain parameters between the groups. Similarly, studies by Opeyemi et al. [
26] and Barbosa et al. [
27] assessed RV function in patients with sickle cell anaemia and found that, while the FAC was similar between the groups, TAPSE and tissue Doppler imaging values were altered. Our findings are consistent with these studies, further emphasising the unique effect of ID on RV function.
Mechanistically, several pathways may explain the observed myocardial dysfunction caused by ID [
3,
28,
29]. Iron plays a critical role in oxygen transport and storage, cellular energy metabolism via the electron transport chain, contractile protein synthesis, and antioxidant defense mechanisms. ID can lead to oxidative stress, impaired contractile function, and myocyte apoptosis. These mechanisms may explain the reduction in RV-GLS observed in this study, even in the absence of significant changes in traditional echocardiographic parameters.
Limitations
The present study was observational in design; as such, causality between ID and RV dysfunction could not be established. In addition, the relatively small sample size may limit the generalisability of our findings. Further research, including interventional studies, is required to explore the effects of iron replacement on RV function and clinical outcomes.
Conclusions
ID was associated with the subclinical impairment of RV systolic function in patients diagnosed with ADHF. This dysfunction was evident even in the absence of anaemia, highlighting the need for routine assessment of iron status and the potential benefits of iron replacement therapy in improving myocardial function and clinical outcomes.
References
- Magrì D, De Martino F, Moscucci F, Agostoni P, Sciomer S. Anemia and iron deficiency in heart failure: clinical and prognostic role. Heart Failure Clinics. 2019;15(3):359-69.
- Anand IS, Gupta P. Anemia and iron deficiency in heart failure: current concepts and emerging therapies. Circulation. 2018;138(1):80-98.
- Alnuwaysir RI, Hoes MF, van Veldhuisen DJ, van der Meer P, Grote Beverborg N. Iron deficiency in heart failure: mechanisms and pathophysiology. Journal of clinical medicine. 2021;11(1):125.
- Zhang H, Jamieson KL, Grenier J, Nikhanj A, Tang Z, Wang F, et al. Myocardial iron deficiency and mitochondrial dysfunction in advanced heart failure in humans. Journal of the American Heart Association. 2022;11(11):e022853.
- Loncar G, Obradovic D, Thiele H, von Haehling S, Lainscak M. Iron deficiency in heart failure. ESC heart failure. 2021;8(4):2368-79.
- Beale A, Carballo D, Stirnemann J, Garin N, Agoritsas T, Serratrice J, et al. Iron deficiency in acute decompensated heart failure. Journal of clinical medicine. 2019;8(10):1569.
- Palau P, Llàcer P, Domínguez E, Tormo JP, Zakarne R, Mollar A, et al. Iron deficiency and short-term adverse events in patients with decompensated heart failure. Clinical Research in Cardiology. 2021;110:1292-8.
- Enjuanes C, Klip IT, Bruguera J, Cladellas M, Ponikowski P, Banasiak W, et al. Iron deficiency and health-related quality of life in chronic heart failure: results from a multicenter European study. International journal of cardiology. 2014;174(2):268-75.
- Santas E, Miñana G, Cardells I, Palau P, Llàcer P, Fácila L, et al. Short-term changes in left and right systolic function following ferric carboxymaltose: a substudy of the Myocardial-IRON trial. ESC Heart Failure. 2020;7(6):4222-30.
- Martens, P. The effect of iron deficiency on cardiac function and structure in heart failure with reduced ejection fraction. Cardiac Failure Review. 2022;8.
- Miñana G, Santas E, de la Espriella R, Núñez E, Lorenzo M, Núñez G, et al. Right ventricular function and iron deficiency in acute heart failure. European Heart Journal Acute Cardiovascular Care. 2021;10(4):406-14.
- McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. European heart journal. 2021;42(36):3599-726.
- Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography: endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. Journal of the American society of echocardiography. 2010;23(7):685-713.
- Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. European Heart Journal-Cardiovascular Imaging. 2018;19(6):591-600.
- Lee J-H, Park J-H. Strain analysis of the right ventricle using two-dimensional echocardiography. Journal of cardiovascular imaging. 2018;26(3):111-24.
- Kobak KA, Radwańska M, Dzięgała M, Kasztura M, Josiak K, Banasiak W, et al. Structural and functional abnormalities in iron-depleted heart. Heart failure reviews. 2019;24:269-77.
- Chung YJ, Luo A, Park KC, Loonat AA, Lakhal-Littleton S, Robbins PA, et al. Iron-deficiency anemia reduces cardiac contraction by downregulating RyR2 channels and suppressing SERCA pump activity. JCI insight. 2019;4(7).
- Beck-da-Silva L, Piardi D, Soder S, Rohde LE, Pereira-Barretto AC, de Albuquerque D, et al. IRON-HF study: a randomized trial to assess the effects of iron in heart failure patients with anemia. International journal of cardiology. 2013;168(4):3439-42.
- Avni T, Leibovici L, Gafter-Gvili A. Iron supplementation for the treatment of chronic heart failure and iron deficiency: systematic review and meta-analysis. European journal of heart failure. 2012;14(4):423-9.
- Meyer P, Filippatos GS, Ahmed MI, Iskandrian AE, Bittner V, Perry GJ, et al. Effects of right ventricular ejection fraction on outcomes in chronic systolic heart failure. Circulation. 2010;121(2):252-8.
- Bosch L, Lam CS, Gong L, Chan SP, Sim D, Yeo D, et al. Right ventricular dysfunction in left-sided heart failure with preserved versus reduced ejection fraction. European journal of heart failure. 2017;19(12):1664-71.
- Raina A, Meeran T. Right ventricular dysfunction and its contribution to morbidity and mortality in left ventricular heart failure. Current Heart Failure Reports. 2018;15:94-105.
- Das P, Thandavarayan RA, Watanabe K, Velayutham R, Arumugam S. Right ventricular failure: a comorbidity or a clinical emergency? Heart failure reviews. 2021:1-15.
- Melenovsky V, Hwang S-J, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. European heart journal. 2014;35(48):3452-62.
- Burns JA, Sanchez C, Beussink L, Daruwalla V, Freed BH, Selvaraj S, et al. Lack of association between anemia and intrinsic left ventricular diastolic function or cardiac mechanics in heart failure with preserved ejection fraction. The American journal of cardiology. 2018;122(8):1359-65.
- Oni O, Adebiyi A, Aje A, Akingbola T. Right ventricular function assessment in sickle cell anaemia patients using echocardiography. Haematol Int J. 2019;1:136.
- Barbosa MM, Vasconcelos MCM, Ferrari TCA, Fernandes BM, Passaglia LG, Silva CM, et al. Assessment of ventricular function in adults with sickle cell disease: role of two-dimensional speckle-tracking strain. Journal of the American Society of Echocardiography. 2014;27(11):1216-22.
- Maeder MT, Khammy O, Dos Remedios C, Kaye DM. Myocardial and systemic iron depletion in heart failure: implications for anemia accompanying heart failure. Journal of the American College of Cardiology. 2011;58(5):474-80.
- Zhang H, Zhabyeyev P, Wang S, Oudit GY. Role of iron metabolism in heart failure: From iron deficiency to iron overload. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2019;1865(7):1925-37.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).