Main Context
Human sensation commences when sensory receptors, located in the eyes, ears, nose, tongue, and skin, detect external stimuli and transform these physical signals into electrical impulses. These impulses are then transmitted through sensory neurons to specific regions of the brain referred to as primary sensory areas, where they are integrated by higher order processing areas, forming conscious perception.
The prevalent view currently holds that the brain cannot create “new images” from nothing; instead, it must interpret sensory information received from the eyes. When brain cells are not functioning correctly, the signals will not be processed into conscious images, even when provided with healthy signal transmission from healthy eyes. The central processing is reflected at the cellular level, when external light signals reach the central system, the resulted alteration in neuronal activities and spurred electrical signals are primarily driven by changes in synaptic connections and these changes rely on the distinctive feature of our neuronal cells - neuronal plasticity, which must be generated through biomolecular activations internally in the cells. The fact is visual perception as one of the most rapid human sensory responses, its central processing actually does not involve the synthesis of new molecular structures; instead, it primarily relies on the excitation of pre-existing molecules contrasting with other processes like learning and memory. We emphasize the critical role of these built-in biomolecular machineries.
To highlight, when external light signals are converted into electrical signals and reach central neurons, they do not trigger neuronal activity as swiftly as commonly assumed, instead, an intermediate step exists, wherein these signals serve as an energy source that empowers the neurons' pre-existing molecular machinery. This then, generates highly plastic activities, followed by refined synaptic connections, allowing visualization to be perceived and consciously understood. We emphasize a critical step that precedes the observable central neuronal activity- the activation of cellular molecular machinery, fueled by externally delivered energy, prior to the emergence of conscious visual perception - shifting the current focus emphasizing external signals as directly guiding central neuronal activity.
Different colors, varying intensity, and brightness correspond to distinct light wavelengths, which elicit specific electrical impulses. These impulses, in turn, fuel the corresponding scale and parts of the molecular machinery. Examples such as blue light with wavelengths at ~400–500 nm and green light at ~500–570 nm are well-known to excite cone cells, however it is often unnoticed the resulting electronic impulses from cone cells when reaching central neuronal cells continue serving as energy power to turn on their essential molecular engines. This is followed by refined plastic activity, synaptic connections, ultimately leading to the final perceptual experience.
As well-known, key molecules, such as AMPA receptors and PSD-95, are essential for synaptic formation and plasticity, shaping how objects are represented in visual perception. Neurotransmitters like glutamate further enhance accuracy and clarity. Visual defects, for example, in Alzheimer’s disease, is known resulted from cortical cell deterioration that disrupts central processing - a consequence we call from the collapse of molecular machinery during cells’ degeneration. In such cases, disrupted neural activity cannot be revived due the non-functional cellular machinery, even when provided with normal signals from eyes - the energy sources for cellular machinery, leading to the observed visual deficits. We regard the pre-existing molecular machineries as determinative elements of visual perception, fueled by the electronic energy provide by light signals, generating outputs that are received and understood.
We further highlight vision perception in central neurons does not in fact, rely on external light stimuli - given none of the critical molecules involved in this process require light stimuli for their synthesis. It is the energy sources that is the pivotal for corresponding molecular activities leading to vision perception, while light typically serves this role, regarding energy source, light shouldn’t be the only option.
When energy is provided by alternative sources, the essential molecular machinery may be activated in the same way as fueled by external light. The idea is reflected in the phenomena called sound-induced flash illusion and visual motions when central neurons in middle temporal visual area are enforced into activities under auditory stimuli - we describe this as central molecular machineries becoming activated to generate vision perception when electrical energy propagated from the auditory center, without the need of light.
There are reports that completely blind individuals including those born blind with no light received ever, may also experience called ‘visual imagery’ or ‘illusion’ known as the example of self-activation and vision perception. The energy source of ‘visual imagery’ in these completely blind individuals may be identified, but more importantly, it suggests when provided with therapeutic artificial energies, vision could be reconstructed artificially even for these blind individuals. The control switch is on the central biomolecular engine parts for therapeutic purpose, rather than the external lights and its sensing organ, or merely the external energy stimuli - a consideration shifting the current treatment focus for patients with defective vision, warranting more investigation.
We further suggest a more severe form of these visual imagery phenomena, referred to as vision hallucinational cases may fall under the same consideration.
This debilitating symptom is observed across a wide range of health conditions, commonly in mental disorders, frequently in cases of direct or indirect brain damage, prolonged damaging environment, and in nearly all advanced stages of deteriorating health. We emphasize the concept of an aberrant central biomolecular engines underlying hallucination, challenging the commonly highlighted notion of abnormal neuronal hyperactivity, termed 'misfiring'.
Neuronal cells by themselves should be able to activate their own self-enforced metabolism to fuel essential molecular activities similar to cells in other parts of the system. The issue is that when the existing cellular machinery becomes excessively activated, to an extent surpassing the routine activities levels for normal external light signaling, it may lead to changes in synaptic connections resembling the process supported by external light-derived energy, resulting in a form of internally generated 'vision,' often termed as 'visual hallucination’.
The fact is any aberrant cellular activities are expected to stem from the aberrant activation of a normally tightly regulated molecular machinery, implying an ongoing internal disruptions. This, in turn, points to existing cellular internal stresses that could be threatening. Enhanced activities when exaggerated by unleashed neuronal plasticity, should further escalate molecular disruptions, cells are compelled to respond and unlikely to cease their response when the enhanced activities increasingly continue. However, paradoxically, enhanced neuronal plasticity is widely regarded as an advantageous evolutionary adaptation, underscoring the complexity of the underlying context.
We propose, under increasing disruptions from heightened activities caused by the unleashed plasticity itself, cells retrospectively exploit this unleashed plasticity actively in response to ongoing damaging situation. The rapidly increased neuronal activities as subsequently supported, most efficiently bolster metabolism to supply energy for cellular essentials, thereby securing survival and preparing for further adaptive actions. The enhanced neuronal plasticity acts more as a securing action; though not specifically termed a survival action, it safeguards essential cellular functions, challenging the view of merely serving as an evolved advantage to enhance survival. While more damages may occur under enhanced activities, cells must continue the response becoming regardless the cost and losing these activities could be detrimental.
Our notion is bolstered by the observation that some patients experience unresponsive symptoms preceding active visual hallucinations is marked by significant neuronal inactivity, often referred as dormancy [
4] - an obvious indicator of cellular response to survival threats, where cells adopt their survival strategy, dormancy.
Patients may remain unresponsive or gradually develop visual hallucinations, and some are recognized to exhibit fluctuations between unresponsive states and hallucinatory episodes. It was observed that the more extensive the neural damage from the underlying illness, the more likelihood of these fluctuation patterns. We describe it as a process seeking for survival, when dormancy can no longer sustain the cells, cellular response triggers to reenforce activities thus aggregating energy to secure cellular essentials, preparing for further adaptive actions. For example, deeper unresponsiveness was observed in subsequent episodes, which is usually perceived as aggregated neuronal inactivity- we call a deeper dormancy when cells get a chance to continue their further adaptation once their basic essentials are secured.
Suppose the observed enhanced neuronal plasticity posits a random beneficial as in current view, it just seems unlikely that cells with an already non-secured survival by losing the previous dormancy, could now be supported by random beneficial as this enhanced cellular plasticity, to transition into a more secured survival, e.g. deeper dormancy in subsequent cycles - clinically noted as deeper sedation. The observation underscores the secured cellular actions unleased by enhanced plasticity leading to heightened cellular activities, which subsequently, activates metabolism that rapidly fuels the energy needed to secure basic cellular essentials when previous dormancy mechanisms have failed, challenging the current view that serving merely as beneficial advantage contributing to enhance survival.
Fluctuating symptoms are more frequently observed when there is underlying damaging conditions such as Alzheimer’s disease. We propose that in these cases, damage to epigenetic machinery impairs the full unleashing of neuronal plasticity, subtly favoring a transition into protective shutdown or, otherwise cellular demise. In contrast, conditions that permit fully unleashed plasticity may lead to continued disruption, propelling cells into continued aggressive activity as the retrospective response while subtly reducing the possibility of entering a protective shutdown. Although cellular vitality may temporarily persist, the more prominent feature is often neuronal ‘misfiring’ within an already compromised system.
Enhanced plasticity is often recognized as evolutionarily beneficial for processes such as learning and memory, however, usually more aggressively enhanced neuronal plasticity is observed in ‘misfiring’ - we suggest it is when an inherited advantage must be aggressively executed and repurposed while evolving into crucial actions actively taken to secure cellular essentials, becoming regardless of more disruptions it may cause in the face of imminent threat.
The conventional therapeutic approach, which often involves inhibiting cellular activities like using haloperidol to block dopamine D2 receptors, while effective in retrieving some symptoms, often runs the risk of causing more aggressive issues in the long term. Our interpretation is such therapy approaches suppress excitability and neuronal plasticity – a necessary secure action under stress, cells are compelled to bounce back the activities when they still can, which may explain the clinical observation that patients can experience more aggressive hallucinations during and after treatment. Additionally, cell death and reductions in brain volume are commonly observed. We describe the eventual failure of all attempts, worsened by forced suppression of dopamine D2 receptors activities, thereafter the accumulated cell decline and death ultimately lead to impairment in a series of higher-level cognitive functions such as intelligence and memory through extensive disruption of the brain's highly interconnected neuronal networks.
This type of neuronal cells over activities by the nature should originate in our intrinsic biology. Human sleep for example, is essential for the system’s recovery from daytime activities particularly for the activities of visual cortex - which is the most distinctive feature of human sleeping. Remarkably, similar visual perceptions often occur in human dreaming and clinical visual hallucinations, both involving heightened neuronal activities, despite one being considered a normal process and the other classified as a disease condition. Our consideration is in both scenarios, an increase in neuronal activity through enhanced plasticity bolsters metabolism and survival mechanisms as secure actions, representing our neurons’ attempts to initiate recovery - in response to cellular disturbances.
While human dreaming appears to facilitate recovery successfully, vision hallucination reflects more persistent and exaggerated efforts to cope with substantial cellular disruptions. This revived vitality inevitably comes at the cost of more pronounced excitotoxicity, which in turn further triggers the activation process, striving more strenuous efforts to sustain. The intrinsic drive to secure essential functions, when executed overly aggressively, is often overshadowed by the disease condition, rather than being recognized as a sacrificial effort to preserve viability.
The nuanced aspect is the purpose of such neuronal activities is akin to the proliferative activities as already widely recognized in biology, which, although are specifically termed survival actions, typically enhance metabolic activity to secure essential cellular functions by providing increased energy and prepare for further actions, such as repair mechanisms and regeneration. Most tissues and cells in our system generally depend on inherited critical genes when epigenetic strategies become quickly strained in proliferative activity and other adaptations. In the contrast, neuronal plasticity supported by its epigenetic features stands out, which secures cellular essentials, prominently enabling further adaptive reactions and protecting cells. As widely recognized neuronal cells usually exhibit less mutations, known as more tolerant comparing to other tissues. It is the distinct plastic feature more active than other parts in the system that enables neuronal cells to quickly revitalize cellular machinery, granting our neuronal system heightened resilience in resisting damage, mutations and cancer.
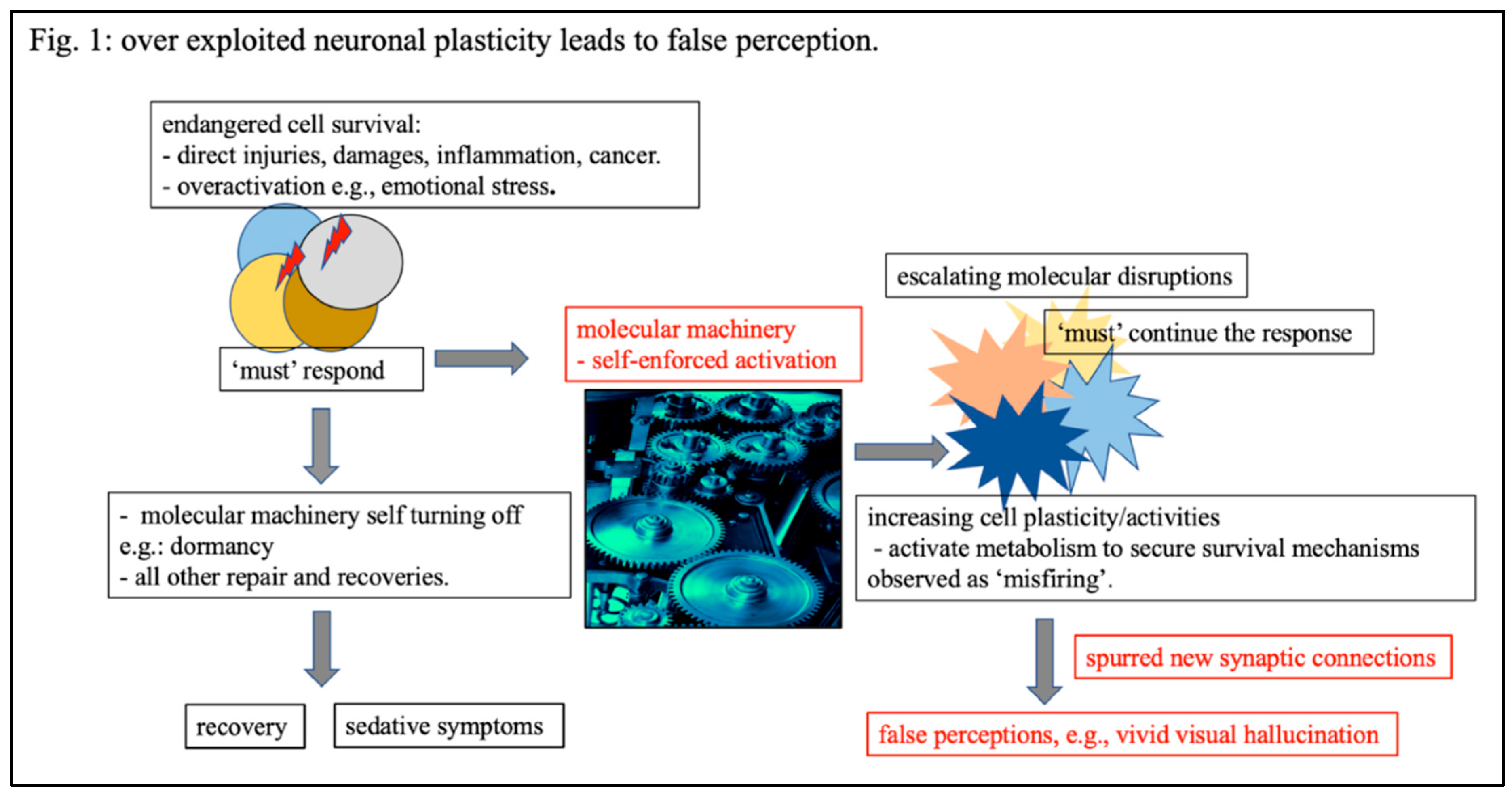
Figure 1.
Increasing cellular plasticity as an overexploited securing effort taken in response to endangered vitality. When neuronal cells’ survival is endangered under various stresses, they are provoked to respond and take actions. Among all various survival responses that otherwise lead to recovery, we highlight two processes: 1) self-turn-off characterized by a dormancy state and clinically observed as sedation, and 2), self-reinforced cellular plasticity and activities thereby revitalizing metabolism/energies to secure cellular essentials preparing for further adaptive actions. This self-reinforcement of cellular activity, often over exploited, leading to unstopped continuously strategies and aberrantly increasing in cellular activities, termed as ‘misfiring’, resulting in false perceptions, such as visual hallucinations.
Figure 1.
Increasing cellular plasticity as an overexploited securing effort taken in response to endangered vitality. When neuronal cells’ survival is endangered under various stresses, they are provoked to respond and take actions. Among all various survival responses that otherwise lead to recovery, we highlight two processes: 1) self-turn-off characterized by a dormancy state and clinically observed as sedation, and 2), self-reinforced cellular plasticity and activities thereby revitalizing metabolism/energies to secure cellular essentials preparing for further adaptive actions. This self-reinforcement of cellular activity, often over exploited, leading to unstopped continuously strategies and aberrantly increasing in cellular activities, termed as ‘misfiring’, resulting in false perceptions, such as visual hallucinations.
Several critical mutations, such as
COMT,
DISC1, and
NRG1, increase susceptibility to mental illness [
5,
6,
7] and are directly linked to neuronal cell survival. For instance,
COMT regulates dopamine levels to prevent oxidative stress, which can damage neurons.
Disc1 directly affects neuronal survival by inhibiting
GSK3β activity, thereby averting apoptosis and promoting cellular growth and differentiation. We propose that such mutations lead to cellular vulnerability, which increase susceptibility to trigger a neuronal response under survival stress. The response manifests as demanded neuronal activity, unleashed by heightened plasticity observed as ‘misfiring”, forcing the cells into a condition resembling genuine perception of light signals.
It is important to note that hallucinational symptoms often appear after periods of emotional stress, similar to how vivid dreams typically occur following more active days. We suggest that the overactivity in cells taxes the system’s timely response, a circumstance further compounded by the presence of the aforementioned vulnerability genes, rendering the system’s susceptibility in response to survival stress. When normal processes, such as vivid dreaming activities, no longer suffice for recovery, cells transcend these standard recovery protocols and must more strenuously counteract the perceived threat by exaggerating their activities supported by aggressively unleashed neuronal plasticity.
As a result, such patients are frequently observed experiencing sleep deprivation, which ultimately leads to more vivid hallucinations. Despite the self-enforced aggressive activities potentially supporting vitality, they are often undertaken at the expense of an already vulnerable system observed as the aforementioned excitotoxicity. Neuronal cells are forced to continue responding and acting. If left without treatment and cells more aggressive reenforce the plasticity and activities, it eventually can lead to cell death - often clinically observed as vision impairment.
Survival stress can also arise as well-known from various conditions such as direct injuries, inflammation, infection, and cancer. As noted, direct brain cell injuries and emotional stress may not directly impact the visual cortex but can still pose risks to vision-related areas due to the highly interconnected neuronal networks within the brain.
Visual hallucinations in these conditions reflect our understanding of molecular disruptions stemming from the forced operation of cellular machinery when cells striving to enhance their featured plasticity. People with other medical conditions, even in the absence of direct brain injuries, can also develop visual delusions. Amid the neuronal cells self strenuously striving for survival under overall systemic deterioration, we interpret this occurrence also as critical neuronal centers such as those regulating respiratory and cardiovascular functions, are compelled to respond to impending decline of these vital organ systems, through unleashing neuronal plasticity to aggressively elevate cells activity thereby the central neuronal control for these vital organs. The process eventually unlocks the tightly controlled vision perception through internally connected neuronal networks, leading to similar clinical observations as in mental disorders.
Genes connected with neuronal activity, such as
BDNF,
NMDAR-1,
GFAP, and
IL-6 [
8,
9,
10,
11], are observed upregulated at the onset of mental disorders. However, they tend to become downregulated at the beginning of direct injury or other damaging medical conditions. We propose two separate processes, each arising from distinctive factors: one driven by overactivation that eventually exhausts cellular machinery resulting compromised vitality, and the other struck by direct damage. In both situations, cells must respond by rejuvenating their internal machinery, mitigating molecular constraints, and increasing activity as means to ensure survival.
Recent findings indicate that an enriched nutrient supply – for example, antioxidants and other essential compounds – confers protective effects and can mitigate hallucinational symptoms, including in schizophrenia or conditions associated with direct brain injuries. Our interpretation is that these nutrients reduce survival pressures by improving overall cellular conditions, thereby lessening the drive to excessively upregulate plasticity, and in turn, alleviating hallucinational symptoms. However, nutrients serving an external energy can also increase the susceptibility an already fragile system already fragile, the cellular machinery may be offered a chance to strive back when supplied with fuels. This explains why a significant proportion of rebound patients often present with increasingly aggressive hallucinational symptoms.
To support the health state of neuronal cells, critical genes in neuronal cell survival e.g., PI3K/AKT pathways, FOXO3, p53, and SOD1, have been considered in clinical practice contributing to genomic stability. However the effect usually not obvious, and still in the exploring process. Some emerging discussions highlight this gene therapy method may be less applicable to neurology disorders including mental illness. Our perspectives may add more insight.
We emphasize neuronal cells rely heavily on their featured plasticity to handle stress, and upon the loss of this main mechanism, they typically exhibit limited capacity for further adaptation. Cells exhibiting 'misfiring' typically either persist in heightened activity or shift into inactivity, may fluctuate between these states per our earlier discussion. This phenomena suggests epigenetic resources supporting neuronal plasticity are likely maintained throughout the cycle. However, once this mechanism becomes exhausted, cellular demise follows thereafter leaving no apparent opportunity for cells to take further actions such as accessing beneficial genes - differing other cells in the system.
There are two key aspects in this process, first, the renowned resilience supported by featured plasticity per our earlier discussion in securing essential cellular functions thereby reducing damaging processes, and second, the rapid onset of cell demise when this primary adaptive mechanism fails, leaving no opportunity for further damaging events. Both factors attribute to the observed infrequency of genome destabilization/mutation accumulation in neuronal cells. Thus in this regard, the gene therapies to stabilize the genome just seem less relevant. Moreover, once these cells are compromised, therapeutic genetic modulation, should carry risk of further exacerbating degeneration by limiting the remaining resources of new beneficial genes if could emerge from ongoing genome alterations. The dismissal of these last-resort resources, taken together, aggregates cell loss observed as serious impairments in higher-order cognitive functions as often reported in these studies.