1. Muscle Weakness: The Overlooked Pathological Finding
Musculoskeletal conditions affect approximately 1.7 billion people worldwide [
2], with far-reaching consequences. For individuals, pain and reduced function may lead to work, financial, and family hardships, along with diminished well-being and quality of life, and in some cases, opiate addiction [
3]. Employers in the US spend
$353 billion on musculoskeletal care [
4], with productivity losses averaging
$3,105 per affected employee annually [
5]. This contributes to an overall economic burden approaching
$1 trillion (5.6% of GDP) [
6]. The incidence is expected to increase as the population ages. Pain is a primary complaint in many musculoskeletal disorders.
1.1. Non-specific musculoskeletal disorders
An estimated 75-95% of patients with musculoskeletal complaints have non-specific musculoskeletal disorders (nsMSDs) — conditions lacking an “organic” lesion: a specific disease, pathology, or structural abnormality that could induce pain [
1,
2,
3,
4,
5,
6,
7,
8]. This establishes what O’Sullivan calls "a diagnostic and management vacuum," resulting in treatment of signs and symptoms "without consideration for the underlying basis or mechanism for the pain disorder" ([
9] p. 243).
Although ‘non-specific’ most often refers to idiopathic low back pain [
3,
6,
7,
10,
11,
12], the designation is also applied to other areas, including the temporomandibular joint, neck [
2], knees [
8], shoulders or arms [
5], but it can be applied to any condition in which no organic cause is found.
1.2. Motor Control, Muscle Weakness and Non-Specific Conditions
Over the last 30 years, aberrant motor control has been discussed as a factor in nsMSDs, whether active or in remission [
9,
11,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23]. Motor control refers to the nervous system’s coordination of muscle activation to produce purposeful movement and maintain posture. Clinically, muscle weakness, an indicator of aberrant motor control, is discussed as a factor in a wide range of non-specific conditions. These include non-specific chronic low back pain [
9,
18,
24], whiplash-associated disorders [
25], knee pain syndromes [
23,
26,
27], ankle instability [
28,
29], plantar fasciitis [
30], headaches [
31], and various "overuse" injuries [
32,
33]. Muscle weakness impairs rehabilitation outcomes [
34,
35,
36], but long-term restoration remains a challenge, as discussed in later sections.
Once organic causes of weakness — e.g., stroke, inflammatory myopathies, nerve impingement, or disuse atrophy — have been ruled out, a healthy muscle’s ability to contract depends on the activation level of its alpha motor neuron (αMN) pool. When contraction is insufficient, it reflects a diminished ‘central integrative state’ of the αMN pool; the net effect of its excitatory and inhibitory inputs [
37,
38,
39].
In physical therapy and orthopedic literature, the finding of weakness in otherwise healthy muscles is often called “arthrogenic muscle inhibition” (AMI). AMI theories posit that inhibition begins as a reflex mediated by spinal inhibitory interneurons. In response to excess mechanoreceptor activation in joints during or following injury, these interneurons presynaptically inhibit αMNs [
40,
41,
42] by moving the central integrative state of the αMNs further away from their firing threshold. AMI researchers have suggested that inhibition is a mechanism that protects the affected region from further injury. More recently, studies outlining supraspinal changes, particularly in the motor cortex, have described altered motor control associated with AMI. In seeming contradiction, studies have found that the motor cortex is more excitable when muscle inhibition is present. This suggests that the motor control system has to do more work to stabilize the region when muscles are inhibited [
27,
35,
43], but it is not evidence of supraspinal involvement in AMI [
44].
Most reporting on AMI has emerged from studies of knee pain and injury, particularly following anterior cruciate ligament (ACL) rupture and reconstruction, with quadriceps inhibition garnering the most attention [
45,
46,
47,
48,
49,
50,
51,
52,
53].
Several decades of experiments by Wolpaw and colleagues at the University at Albany have demonstrated that behavioral adaptations, the result of operant (instrumental) conditioning, can change the function of muscles [
54]. Using Hoffman (H)-reflex biofeedback, they found that a functional analog of AMI — downregulation of αMNs in humans and animals induced by operant conditioning — alters spinal and αMN morphology and function, but is ultimately sustained by cerebellar plasticity [
55,
56,
57,
58,
59]. The H-reflex measures motoneuron pool excitability and is analogous to the stretch reflex. H-reflex biofeedback electrically evokes this monosynaptic spinal reflex and provides visual or auditory feedback that allows subjects to monitor and potentially modulate reflex strength. Insufficient H-reflex amplitude is a common finding in muscles affected by AMI [
60].
Assuming that the participant is exerting full effort, muscle activation deficits, organic or not, can be caused by functional alterations occurring anywhere in a pathway from the sensorimotor cortices and cerebellum to the motor neuron or muscle itself. This includes the possibility of altered function of gamma motor neurons [
61,
62], which modulate the force of contraction in active muscles. Pain is known to trigger diverse alterations in movement patterns, including inhibition of muscles [
17,
24,
63,
64,
65].
1.3. Foundations of this Review
To the clinician discovering a weak muscle using manual muscle testing (MMT) or MMT with handheld dynamometry (HHD) on patients without organic conditions, the mechanisms that underlie the diminished contraction are a black box. ‘Idiopathic weakness’, would be an appropriate term for persistently weak muscles in these patients, but we will use the term “non-organic weakness” (NOw). Weakness that might be called AMI is a form of NOw. Unless otherwise specified, throughout this document, any mention of muscle weakness, its treatment, or MMT refers to weakness without a determinable organic cause.
In the relative absence of other theories accounting for the persistence of NOw, this may prove to be more than a designation of convenience. The hypotheses presented in this paper suggest that it represents a distinct pathological entity, characterized by maladaptive neuroplastic changes in motor control. A working definition of NOw is muscle weakness resulting from disturbances of motor control caused by maladaptive learning (neuroplasticity). As shown by AMI studies, affected muscles may exhibit deficits on tests including H-reflex and burst superimposition (described later); however, functional motor control deficits are concisely revealed through MMT. Specifically, these deficits are observed during the MMT “break test,” or through objective recordings of force and motion (F/M), alone or traced over time (FM/T) during MMT. Unique among all other muscle evaluations, the break test tracks a muscle’s response to increasing external force, which is dictated by motor control functions. Importantly, the designation also implies the potential for remediation, as these adaptations may be normalized by interventions that putatively alter or eliminate maladaptive motor learning. These corrections represent a potential method for remediation of motor control dysfunction.
Part I summarizes current knowledge of NOw, its relationship to motor control, and reviews existing treatments. Part II presents objective data that refine methods and interpretations of MMT. Part III expands on the “muscle-motor-movement post-traumatic stress disorder” (mPTSD) model, proposing a hypothetical mechanism for NOw. Part IV suggests clinical applications of these concepts, introducing additional theory grounded in clinical observation. Part V presents conclusions, limitations, and directions for future research.
1.4. The MMT “Break Test”
“Weakness”, applied to an individual muscle tested with MMT, is usually subclinical: patients complain of hamstring pain or tightness for example, not semimembranosus weakness. MMT, as we use the term here, is the “break test” performed without instrumentation. The following description of the break test, the most widely used form of MMT, essentially coincides with narrative descriptions from Kendall’s
Muscles, Testing, and Function. Now in its 6th edition, “Kendall” is one of two classic textbooks of MMT [
66]. The other, Daniels & Worthingham’s, emphasizes a different methodology not addressed here.
The MMT break test evaluates a muscle’s ability to maintain an isometric (unmoving) plateau under gradually increasing force. In this test, the examiner instructs the participant to hold the limb steady while resisting the examiner’s ramping force. As stated by several authors, once the muscle “breaks” — that is, when the limb begins to move — the test is considered complete and the muscle is classified as weak [
67,
68,
69,
70,
71].
Objective recordings of FM/T, however, reveal two distinct breaks. In our terminology, “Break 1” marks the onset of eccentric contraction, where the muscle lengthens while still resisting. “Break 2” represents complete failure, when resistance force falls to zero and the limb gives way entirely.
Several authors have argued that Break 2, which includes the force produced during eccentric contraction, should be considered part of the test [
67,
71]. HHD, which usually relies on peak force to estimate maximum voluntary (isometric) contraction (MVC), is also likely to capture eccentrically produced force, which exceeds isometric force [
72].
FM/T tracings, combined with current understanding of muscle physiology and mechanisms of weakness, suggest that MMT should generally be concluded at Break 1. With one exception, quality FM/T analyses have only been available since 2015, and their implications have not yet been incorporated into standard knowledge about muscle testing. This issue is examined further in Part II.
1.5. Expanding the Biopsychosocial Model; Mind-Body Treatment for Muscles
A “biopsychosocial model” for nsMSDs, holding that thoughts, emotions, beliefs, and social factors may contribute to non-specific conditions, particularly low back pain [
7,
19,
73,
74], has been gaining support. Similar constructs have been offered for potentially related conditions, including kinesiophobia — fear and avoidance of movement [
75] — and functional weakness (FW) — clinical paresis or paralysis that is considered a subset of functional motor or neurologic disorders [
76].
This review affirms and extends the biopsychosocial model by introducing a motor-specific mechanism through which psychological and sensorimotor experiences may directly alter movement patterns. While most biopsychosocial models focus on conscious beliefs, the mPTSD model highlights non-conscious influences on individual muscle activation and coordination. These include conditioned associations and sensorimotor predictions, responses guided by unconscious information processing [
77,
78,
79].
By integrating motor control and learning theory, this review outlines a new treatment paradigm that aligns with — but extends beyond — current mind-body approaches. Rather than focusing on broad functional activities, however, the mPTSD model focuses on muscle-by muscle testing and interventions.
1.6. Hypotheses and Methods
In the aftermath of musculoskeletal injury or in the presence of pain, the finding of treatment-resistant muscle weakness (NOw as we call it) has puzzled clinicians for centuries [
80,
81]. Guided by a desire to understand the etiology of unexplained weakness and its treatments, this review proposes three hypotheses linking its etiology, diagnosis, and treatment.
1.6.1. Hypotheses of Etiology, Diagnosis, and Treatment of mPTSD
Hodges and Moseley (2003) posited that anticipation of pain leads to delayed muscle activation, and that this finding represents a change in motor planning, a product of fear-avoidance learning [
82]. Neige et al. (2018) further connected this effect to conditioned learning, wherein learned associations between pain and particular movements would lead to adaptations in movement patterns. These adaptations may delay or avoid particular movements. Although protective movement strategies may initially reduce pain, they can have adverse long-term effects and contribute to the development of chronic pain [
83,
84,
85].
A mPTSD hypothesis was preliminarily supported in a 2021 proof-of-concept study, which showed that muscle weakness (NOw) could be immediately reversed by side-to-side eye movements — a key element in the trauma therapy Eye Movement Desensitization and Reprocessing (EMDR) [
86]. This review expands on the mPTSD Model, exploring maladaptive learning as a potential mechanism underlying NOw. We also examine how recent theoretical and phenomenological insights into MMT may improve the identification of affected muscles, and consider potential mechanisms by which certain therapies may resolve these maladaptive motor adaptations. These themes essentially combine models or theories from three sources:
Two-stage maladaptive learning, a novel hypothesis presented here for the first time. In stage 1, adaptive learning associates the use of specific muscles with prior trauma (e.g., pain, stress, or injury). These associations are encoded into internal models (mental representations of how the body moves and responds) used during predictive motor planning [
87,
88,
89]. In stage 2, compensatory strategies shift the load to unaffected muscles. These inefficient patterns persist, preventing re-integration of inhibited muscles. (See Part III.)
“Adaptive force” as an assessment of motor control, a pre-existing theory. At the level of individual muscles, the inability to sustain isometric holding — representing the failure of the muscle to adapt to changing pressure — is the key indicator of a motor control deficit, not reduced force. Failure to recognize this distinction may lead to false negatives with peak-force dynamometry. (
Section 3.4).
Maladaptive memories can be eliminated, a pre-existing theory. Building on memory reconsolidation theories, maladaptive motor memories — here, memories sustaining NOw — can be erased once reactivated. Therapies that reverse muscle inhibition may do so by engaging these reconsolidation mechanisms. (
Section 7.1.)
1.6.2. Methodology: Non-Systematic Review of Existing Literature
This non-systematic, integrative review draws from musculoskeletal medicine, neuroscience, psychology, physiology, motor control, and trauma research, synthesizing concepts rarely combined. It is, in part, an effort to understand the clinical outcomes presented in Appendix A — outcomes that would be considered improbable by conventional thinking — and to share long-observed phenomena in a form accessible to clinicians outside alternative practice spheres.
This format contains several limitations. First, the lack of formal inclusion and exclusion criteria introduces potential selection bias [
90]. Second, the transdisciplinary scope, while a strength in exploring novel models, makes it difficult to establish uniform standards of evidence across domains [
91]. Third, some proposed interpretations — particularly involving theories of adaptive force, predictive processing and reinforcement learning — rely on theoretical extrapolations rather than empirical validation. This necessitates further research. Finally, given the controversial nature of some source material, including case reports and observational data, conclusions are best viewed as provisional and hypothesis-generating rather than confirmatory.
Integrating data from diverse domains, some of which are themselves not empirically validated, this review blends established findings with hypothesis-driven interpretations. While every effort has been made to distinguish speculation from evidence, readers are encouraged to regard the proposed mechanisms as provisional models rather than definitive conclusions.
2. Current Knowledge and Practices
Before developing these concepts, we review the current baseline of knowledge and practice regarding the evaluation and treatment of aberrant motor control and muscle weakness.
Given the lack of clinical criteria and differential guidelines, distinguishing AMI from other potential causes of NOw is not currently possible, but alternative theories are few. Reports of resolution of weakness via treatment of trigger points [
92,
93], spinal manipulation [
94,
95,
96,
97,
98], reflex treatments [
39,
99,
100] and therapeutic eye movements and other dissimilar therapies imply a mechanism that has yet to be considered. Speculation on that mechanism is a central thrust of this review, covered in Part III.
2.1. Motor Priming, Learned Responses, MMT Outcomes, and Defensive Action
Two essentially opposing theories describe motor responses to pain and nociception. The “pain adaptation model” holds that inhibition is the primary response [
83,
101,
102], while the ‘vicious cycle theory’ holds that pain increases muscle activity [
2,
103,
104,
105]. Paul Hodges, a primary researcher of motor control in the context of musculoskeletal disorders, suggests instead that pain induces a redistribution of muscle activity, varying across tasks, individuals, and contexts [
106]. In
Section 7.6, we amend this view based on clinical observations.
A theory of muscle response supported by clinical observation and experiments is that there are at least three states of muscle facilitation or priming: 1) positively primed (just “primed” in most uses), 2) inhibited or negatively primed, and 3) normally facilitated or unprimed. In cognitive and motor neuroscience, both positive and negative motor priming (the unconscious preparation for motor responses) have been demonstrated in response to preceding stimuli, including subliminal or masked cues. For instance, motor responses can be facilitated (as shown by shorter reaction time and greater excitability) when congruent motor plans or positively valanced sensory inputs are unconsciously pre-activated [
107,
108,
109]. Motor responses are inhibited or negatively primed (showing slower, delayed or reduced excitability) when incongruent plans or negatively valenced sensory inputs are presented [
107,
109,
110].
Though it remains unstudied to our knowledge, delayed activation may be a common denominator in negative motor priming, motor control abnormalities found in musculoskeletal conditions, and MMT outcomes. Delayed activation has been observed in patients with musculoskeletal pain, even in remission [
22,
24,
82,
83,
111]. A related partially supported theory is that muscle weakness found with MMT represents muscles that are delayed in their activation [
112]. When specific MMT technique is used (see
Section 3.7), the failure of a muscle to respond is evident within the first milliseconds of force application [
113]. This generally supports the pain adaptation model.
Supporting the vicious cycle theory, pain and trauma are known to induce specific defensive and withdrawal movements as reactions to the details of the experience. These muscle responses may become habituated or learned as ongoing patterns [
114,
115,
116]. According to Levine (and others, in related explorations), one form of this response may be caused by the inherent impulse to complete unresolved defensive responses during trauma [
117,
118,
119,
120]. This persistent response, which may be equivalent to positive priming, contributes to symptoms of post-traumatic stress disorder (PTSD). If this is the case, antagonists of positively primed muscles may be negatively primed — weak, if tested — due to mechanisms of spinal reciprocal inhibition [
121] or supraspinal processes [
122,
123].
In this framing, negatively primed muscles test weak with MMT — unable to form a stable isometric plateau — while unprimed muscles test ‘strong’ or normal. Positive motor priming is more complex to detect. Clinicians identify a phasic state called
over-facilitation, evident only during voluntary contraction (e.g., MMT). Over-facilitated muscles may feel normal when tested but fail to weaken in response to stimuli that should transiently inhibit contraction, such as manual compression of muscle spindles, which in normal muscles produces temporary weakening on immediate retest [
124,
125]; ([
100] pp. 81–83). Clinically, these muscles may sometimes feel rigid from the outset of testing or even express as an aggressive, concentric push against the examiner’s hand, rather than isometric resistance. This can occur in single muscles or, in some cases, systemically, affecting all muscles. Later, we relate MMT outcomes to the “defense cascade,” in which weakness may reflect a freeze response and over-facilitation a second stage, priming for fight or flight [
126,
127,
128]. In
Section 7.5, we suggest a clinical strategy for remediating locally over-facilitated muscles.
2.1.1. Current Treatments for AMI
In the previous section, we noted that various therapies might be successful in reversing NOw. These will be explored in more detail shortly.
Although not a feature of all muscle inhibition ([
92] p. 7), atrophy (hypotrophy) is stated to be a common secondary effect of AMI. Muscles presumed to be affected by AMI often fail in hypertrophic development despite exercise and physiotherapy [
18,
36,
60,
129,
130,
131,
132,
133]. A scoping review of physiotherapy strategies for AMI concluded that “[p]ersistent impairments have been detected despite increases in [muscle] function.” … “Thus, it is probable that the present therapeutic approaches are failing.” ([
134] p. 2611) Exercising while in pain is discouraged by several authors, as it risks training (learning) of abnormal biomechanics which could make the altered function more indelible [
92,
135]. The validity of this assertion may be assessed by testing muscles in previously injured, now asymptomatic regions.
Therapies that block afferent output from joints, including transcutaneous neuromuscular stimulation (TENS), transcranial magnetic stimulation (TMS), and cryotherapy [
27,
35,
129,
134,
136,
137], can temporarily suspend weakness, enabling effective hypertrophic muscle training [
35,
48,
129,
137,
138]. Even with this therapy, AMI-related neural activation deficits often persist beyond both symptom resolution and functional recovery [
18,
134,
136,
139,
140,
141].
Electromyographic (EMG) biofeedback has shown some promise in the diagnosis and treatment of AMI [
142,
143]. Transforming physiological variables into visual or auditory signals, EMG biofeedback allows patients to learn to more precisely control individual muscles. Richaud et al. describe applying this method with AMI patients, showing how several weeks of therapy can improve or normalize function of both hypertonic and inhibited muscles [
144]. In
Section 6.5, we look at the possible neuroplastic mechanisms involved in this method.
2.2. Effects of Muscle Weakness on the Body
The term ‘muscle weakness’ is used here as may be used in the literature; often without clear etiology or standardized assessment criteria. Muscle weakness negatively affects coordination, stability, and overall function.
Key consequences include:
Altered movement patterns: To avoid inhibited muscles, patients adopt compensatory movements that reduce biomechanical and functional efficiency [
84,
145,
146,
147].
Joint instability: Weak stabilizers compromise joint function and may accelerate degeneration [
51,
148].
Reduced performance: Weakness impairs athletic performance and increases reinjury risk [
149,
150,
151].
Chronicity and recurrence: Unresolved weakness may promote the transition from acute to chronic conditions and increase the risk of recurrence, even in asymptomatic individuals [
85].
Kinetic chain dysfunction: Weakness in one region can disrupt proprioception and stability throughout the body, particularly when it affects the lower extremities [
152,
153].
Persistent pain: Muscle weakness and other motor control alterations are correlated with the presence of pain [
112,
154]. This may create a self-perpetuating loop, with pain and motor control abnormalities reinforcing one another [
11,
17,
82,
106].
Muscles are essential for joint mobility, stability, and shock absorption [
72]. Even in asymptomatic individuals, muscle weakness may contribute to functional impairment [
155,
156], ankle instability [
28,
157], gait disturbance [
49,
158], and osteoarthritis [
148,
159,
160]. Roos et al. (2010) noted that muscle function correlates more closely with joint pain than joint-space narrowing [
160]. Weakness, which may undermine the supportive role of muscles, might be a better predictor of disability than pain and structural findings [
161,
162]. Pain may not reflect actual tissue damage; it may act as a warning signal to impaired motor control or joint instability [
163,
164].
In occupational and athletic contexts, undetected (subclinical) weakness after apparent recovery poses a significant — but potentially correctable — risk for repetitive stress injuries, ongoing pain, and reinjury.
This underscores the utility of implementing comprehensive testing and correction methods that we will be discussing, practical tools that can help clinicians identify and resolve these hidden vulnerabilities
2.2.1. Muscle Weakness and Central Pain — A Vicious Cycle?
Nociception is the detection of potentially harmful stimuli — e.g., heat, cold, mechanical, or chemical inputs — by specialized peripheral receptors. Pain is the subjective experience that may accompany nociception. Either may occur without the other [
165].
The mechanisms for weakness proposed here are similar to those theorized for central pain sensitization or nociceptive pain. Cerritelli et al. describe how maladaptive predictive processing (which we cover later) can generate and maintain pain sensitization through persistent error signaling [
166]. This may create a vicious cycle: nociception and pain alter motor control [
17,
84], with muscle weakness as a key feature [
101,
102].
Biomechanical compromise resulting from weakness and other factors of altered motor control may increase nociceptive activity through altered joint loading patterns [
84] and abnormal tissue stress [
167]. This bidirectional relationship — where pain leads to weakness and weakness aggravates nociceptive inputs — highlights the need to address weakness to improve motor function and potentially interrupt persistent pain states.
2.3. Muscle Weakness, an Often-Unnoticed Gap in Treatment of nsMSDs
AMI literature focuses on objective tests for muscle inhibition, including EMG [
150], H-reflex testing [
145,
168,
169], and burst superimposition (interpolated twitch) testing, in which MVC is compared to electrically enhanced contraction [
28,
29,
46,
63,
170,
171]. High cost, time demands, and the limited number of muscles that can be tested mean these methods are rarely used clinically. Meanwhile, the most common clinical tools — MMT and MMT with HHD — receive little attention in AMI research.
Although EMG is considered the gold standard for the analysis of muscle activation [
172], it is not equivalent to MMT, particularly when MMT is applied according to the protocols reviewed here. The most easily used form in clinical settings, surface EMG (sEMG), has significant accuracy limitations, potentially suffering from artifact, cross-talk, and signal cancellation, particularly when sampling from deeper or nearby muscles. This limits its sensitivity and specificity [
173]. Intramuscular (needle) EMG offers greater precision but is invasive, painful, and logistically challenging for routine or large-scale testing [
174]. Leisman et al. (1995) showed that EMG amplitude can be elevated even in muscles that test weak on MMT, revealing possible dissociation between activation and functional muscle response [
175]. Nonetheless, as demonstrated earlier, sEMG has shown some promise as a diagnostic and therapeutic tool for AMI.
AMI is often overlooked by athletic trainers and physiotherapists, perhaps due to educational deficits [
137]. However, the subclinical nature of AMI, the scarcity of treatments that reverse it, and the lack of clinical diagnostic criteria may also discourage considering the role of muscle weakness in patients’ conditions. With most muscle testing texts listing tests for only ~200 of the 600 muscles of the body [
66,
176] even if MMT were accepted as a valid test for AMI, the extent of the condition would still be undetected. Despite the observation, discussed in
Section 2.5, that correction of muscle weakness can reduce musculoskeletal symptoms, clinical observation also suggests that the number of weak muscles does not necessarily correlate with the level of pain or disability. This means that MMT may not be a useful medico-legal tool for establishing levels of disability.
2.4. Motor Control, a Clinically Confounding Concept
Although the field has progressed in the last 20 years, Latash and Anson’s statement (2006, p. 1152) remains essentially true:
“In order to study a phenomenon, one has to be able to define it and to have tools that can identify the phenomenon or, even better, the tools to quantify it. In the area of the control and coordination of movements, including atypical movements that may be performed by patients seeking help from physical therapists, such tools and definitions are commonly absent.” [
177]
Latash reiterated the comment in 2021, noting the lack of diagnostic tools and the difficulty translating findings into clinical practice [
178].
Motor control deficits — including weakness of individual muscles — are consistently observed in nsMSDs, even during remission [
7,
9,
18,
21,
22,
23,
179]. In response to pain, muscles or subsets of motor units display altered force, timing, and coordination [
17,
27,
84,
102,
150,
180,
181]. Reflexive or supraspinal mechanisms may delay activation or disrupt normal muscle recruitment patterns [
24,
83,
111,
112,
182].
Abnormalities in individual muscles, detectable through sEMG or MMT, often accompany broader dysfunctional motor patterns in response to pain. These include co-contraction of antagonists [
183], reciprocal inhibition of opposing muscles [
121], abnormal variability in muscle function (excessive or overly limited) [
184], and compensatory hypertonicity, rigidity, or guarding when pain is anticipated or muscles are inhibited [
17,
20,
179,
185,
186].
Several clinical methods have been developed to assess motor control. These include sEMG [
187], balance and center-of-pressure measurements [
188,
189,
190], joint position sense evaluation, motion analysis, and functional movement control tests [
191,
192]. In clinical settings, however, efforts to treat symptoms linked to aberrant motor control face several challenges:
High individual variability in aberrant motor control presentation, and a lack of methods to identify and categorize these patterns [
187,
193].
A shortage of clinical studies and evidence-based treatments showing consistent improvement from motor control interventions [
187,
193].
A shortage of trained clinicians familiar with procedures (e.g., sEMG) and the interdisciplinary knowledge to integrate them [
187].
Limited effectiveness of motor control exercises and poor long-term compliance with prescribed routines [
160,
187,
194].
Uncertain clinical relevance of motor control changes: Ongoing debate regarding whether such changes are causal, compensatory, or merely associated with pain and disability [
177,
178,
195,
196,
197,
198,
199].
2.4.1. Persistent Motor Patterns as Learned Behavior
Motor control alterations acquired through pain or trauma often persist even after symptoms resolve. Learning theory and neuroplasticity studies suggest that new motor skills typically overlay, rather than erase, earlier patterns; new memories are built on the foundation of prior learning [
200,
201]. If muscle inhibition is a form of learned behavior, new movement patterns established during rehabilitation may still avoid using inhibited muscles [
202,
203]. In particular, motor inhibition learned through pain or trauma can persist and therefore shape subsequent motor learning [
204,
205,
206,
207].
Evidence shows that although motor control exercises may reduce symptoms, they often do not outperform other exercise therapies. Benefits are typically modest, and residual deficits may remain [
18,
21,
22,
179,
197,
208,
209]. Approaches to determine the personalized application of motor control interventions remain undeveloped [
184,
187,
197].
This review posits that MMT can serve as a valid clinical tool for pre- and post-treatment evaluation of motor control impairments, particularly when paired with the treatment principles introduced later. Although more studies on motor control metrics are needed, clinical observations of gait, posture, range of motion, muscle tone, and phasic over-facilitation suggest that correcting muscle weakness may improve key aspects of motor control [
39,
100,
210,
211,
212,
213,
214,
215].
2.5. Is NOw Reversible?
Some evidence cited here comes from Applied Kinesiology (AK), a field encompassing diverse methods that use novel applications of MMT. Over 60 years, clinicians practicing AK have produced thousands of case reports and clinical studies, including time-series outcomes covering >10,000 patients. Some reports contain detailed anatomical, physiological, and neurological analyses and innovative, though unproven, therapeutic approaches [
216,
217]. In addition, there is a growing body of peer-reviewed research (e.g., [
31,
113,
175,
218,
219,
220,
221,
222,
223]), along with several comprehensive textbooks [
39,
100] covering aspects of AK. Rigorous studies needed to establish evidence-based status have been lacking, however, adding to AK’s controversial history.
AK has been sharply attacked in non-peer-reviewed articles that did not address most of its existing literature [
224,
225,
226], though better founded criticisms also exist (including [
227,
228,
229]).
Part of the problem is that, over the years, the name “AK” has been colloquialized — applied to a wide variety of practices that use MMT in different ways and for different purposes, even by unlicensed practitioners [
69]. Against that backdrop, in its original context, AK holds that MMT can be used for functional neurologic assessment, particularly to gauge the integrity of neural circuits involved in motor execution [
37]. Today, an estimated 1 million practitioners worldwide use methods derived from the basic principles or findings of “AK”, centering around DCs, but including MDs, DOs, PTs, and DDSs [
230].
Among the many AK-based methods and procedures, however, this review relies on only one claim: the immediate reversibility of muscle weakness (which we have labeled NOw). Other claims of AK are unnecessary to the generation of our central hypotheses, and beyond our scope to address. (Nonetheless, certain evidence we examine, though not necessarily derived from AK, supports observations typically attributed to AK.)
2.5.1. The History and Current Uses of AK Inhibition Reversal Methods
The reversibility of NOw was discovered in 1964 by George Goodheart, founder of AK. Goodheart used Kendall’s testing methodology to determine whether the muscle was normal or weak — he did not attempt to estimate strength by grade. (
Section 3 shows that muscles capable of offering resistance yield a result best interpreted as binary.) He went on to discover that each muscle was associated with a unique set of reflexes thought to relate to the vascular, lymphatic, neurological, and acupuncture meridian supplies to the muscle. With 60 years of history and extensive individual experience, it is taken as fact by AK practitioners that manual treatment of these reflexes, along with manipulation of specific vertebrae and massaging the muscle’s origin and insertion, will immediately restore normal function to muscles in patients without organic causes for the weakness. [
39,
99,
100,
231] Although the reliability of these methods has not been rigorously studied, multiple case reports from the AK repository (e.g., [
86,
210,
211,
212,
213,
214,
215,
232,
233,
234,
235]) demonstrate functional restoration by various means.
As put forth by Suvvari (2024), case reports, though often discounted, “are indispensable in evidence-based medicine, offering crucial insights into rare cases and innovative treatments. While they are not as robust as randomized controlled trials or observational studies, case reports provide essential information that can guide clinical decision-making and stimulate further research. Embracing the significance of case reports can enrich medical education and improve patient outcomes [
236] (p. 5452).” Case reports can also be valuable for generating hypothesis [
236,
237], which is how they are used in this review.
Reversal of muscle weakness is further supported by the author’s clinical experience restoring function to thousands of muscles over decades (with short case reports in Appendix A), as well as textbooks and studies on AK practice [
31,
39,
100,
238]. Weissfeld (2021), described in detail in
Section 3.2.2, is a small study that confirmed the same finding using methodology not derived from AK.
The potential benefits of muscle weakness reversal were broadened in the late 1970s, when Alan Beardall, a student of Goodheart’s, enlarged upon Kendall’s catalog of documented muscle tests, bringing the number of testable muscles to nearly 600 bilaterally, while also presenting the set of reflexes to treat for each [
239]. In 2013 Buhler and Williams published
Muscles of the Neck, further expanding that number [
240]. To be considered definitive for each muscle, these extended test protocols need to be compared with other measures like EMG to confirm test specificity. Even without such confirmation however, they still comprise an enlarged survey of possible force vectors of the tested limb, assuming that a limb should be able to effectively resist incoming force from any vector.
Beginning in 1979, Buhler actualized this protocol as a team doctor for the National Basketball Association’s (NBA’s) Utah Jazz for more than two decades, eventually naming the method Advanced Muscle Integration Technique (AMIT
®). A retrospective analysis (1990–2010) showed Jazz players missed less than half as many games as the league average from 1990–2000, the period that overlapped Buhler’s tenure. [
241]. These findings, along with case reports, news articles, and interviews with players and team staff [
242,
243,
244,
245], suggest that this method may provide unexpectedly beneficial outcomes, though high quality evidence is lacking.
2.6. The Limitations of MMT as It Is Practiced
MMT is the most widely used clinical method for assessing muscle function [
219,
246,
247,
248], but reports on its reliability and validity vary. Reliability is supported in some studies [
221,
249,
250,
251], particularly when fixed or handheld dynamometers are used [
70,
247,
252,
253]. However, other studies report poor reliability [
247,
249,
254], and, particularly when performed without instrumentation, MMT’s subjectivity, insensitivity to small
-to
-moderate force changes, and uneven grading [
248,
250,
255,
256,
257,
258,
259], have led some to question whether MMT is adequate for making clinical judgments [
248,
254]. These concerns have likely limited MMT’s use in research, including AMI studies, hindering translation to clinical practice.
Not concerned with movement, most studies focused on force-related aspects of break tests, monitoring peak force to discover MVC. “Until a formal time-and-motion [FM/T] study of muscle testing has been performed for these methods,” Caruso and Leisman (2000 p. 683) argued, “claims of subjectivity or objectivity must be regarded as premature. [
113]” In Part II, we examine FM/T research that reveals a different model of MMT.
While the term “muscle weakness” is well used, researchers often state that the brain “thinks” in terms of movements, not individual muscles [
260]. Stimulation of specific motor cortex zones has been shown to trigger complex, naturalistic movements involving multiple muscles and joints [
261]. Clinicians performing MMT typically test “individual muscles,” but the number of possible distinct test vectors is vast; even slight changes in position or force direction can alter recruitment. Highlighting specific muscles is a practical convention for sampling representative movements. Joints with fewer degrees of freedom (e.g., knee or ankle) yield a more representative sample of all possible movements than highly mobile joints like the shoulder and hip.
The findings of this review argue that weakness is the most actionable of all abnormal motor expressions to assess and treat.
3. Motor Activation as a Binary Phenomenon
Conventional interpretations of MMT generally imply that its purpose is to measure or estimate voluntary strength (force resisted or produced) on a graded continuum. Kendall notes (p. 18): “Grading strength involves a subjective evaluation based on the amount of pressure applied. [
66]” Given the evidence in
Section 2.6 showing that MMT is insensitive to force increments, a better approach is to focus on movement — whether the tested muscle locks or unlocks.
Muscles can exert a range of force from a slight nudge to MVC — an analog capacity that is accurate but incomplete as a description. While voluntary activation is largely analog in execution (though not initiation), binary function plays a major role in muscle function and dysfunction. Here in Part II, we present an approach that frames muscle testing and function as fundamentally binary.
3.1. The Binary Nature of Muscle Function
The binary nature of muscle function can be illustrated by established principles of motor control. In Go/No-Go tasks, motor action is either initiated or inhibited in response to specific stimuli [
262]. Motor priming experiments show that stimuli — whether consciously perceived or subliminal — can instantly flip a binary switch affecting motor response accuracy and speed [
263,
264,
265]. Similarly, approach-avoidance and freeze responses are functionally binary, though their outward expressions can be complex and context-dependent [
126,
266]. Across these examples, immediate change in functional priming is a hallmark of binary responses.
The binary nature of muscle response is evident when comparing muscle performance before and after specific sensory inputs. Schaefer et al. (2022) (
Section 3.4,
Figure 1) showed that focusing on unpleasant imagery can trigger an immediate shift from isometric holding to eccentric yielding [
33] in normal muscles. Rosner et al. (2015) [
220] and Caruso and Leisman (2000, 2001) [
113,
267] found that muscles show similar binary changes when tested before and after exposure to certain cutaneous stimuli.
Binary facilitation extends to inputs from other sensory modalities, including: visceral [
39,
100,
268,
269,
270], olfactory [
271,
272], visual [
273], and various cutaneous inputs [
102,
168,
220,
270,
274,
275,
276], as well as emotional stressors such as making counterfactual statements (lying) [
218,
223]). These show that a muscle’s capacity to activate may be conditional, based on interpretations of the current internal and external environment. Although this review is focused on chronically, not transiently inhibited muscles, these findings regarding the conditionality of muscle activation support a key contention of AK [
37,
39,
100,
113,
220,
267].
NOw muscles may be understood to be chronically conditionally inhibited, as if there were a persistent interpretation that use of the muscle would lead to a noxious outcome. Additionally, as mentioned earlier, instead of being conditionally inhibited, a muscle can become conditionally over-facilitated in response to the presentation of a noxiously-interpreted stimulus; the muscle will not weaken to stimuli otherwise expected to induce weakness.
3.2. The Binary Nature of Muscle Dysfunction
Clinical evidence from treatments for NOw supports this model. Interventions addressing muscle weakness consistently produce a binary shift from dysfunction to normal function, not gradual improvement. This shift is seen in two categories of treatment: those that temporarily suspend AMI, and those that produce lasting normalization of muscle weakness (potentially including AMI).
3.2.1. Reversal of NOw Implies a Binary Functional Deficit
As noted in
Section 2.2.1, decreasing afferent output from associated joints can temporarily reverse muscle activation deficits. This supports a binary model of motor control pathology: muscles shift from inhibited to facilitated states, then return to inhibition when the effect wears off.
Treatments from AK, reviewed in
Section 2.5, also show this binary pattern, with muscle function restored immediately after treatment, but typically not reverting to weakness.
3.2.2. Experimental Evidence of Immediate Recovery
In addition to introducing the mPTSD concept, Weissfeld (2021) provides evidence for the binary nature of muscle dysfunction and suggests a role for maladaptive learning in muscle weakness. In the study, side-to-side eye movements, thought to work by eliminating maladaptive learning, are borrowed from EMDR [
277,
278].
Section 7.1 discusses potential mechanisms for this elimination.
EMDR has consistently demonstrated efficacy in treating PTSD and depression, with recent meta-analyses confirming significant reductions in symptom severity [
279,
280,
281]. It is generally considered safe when delivered by trained practitioners, though some clients may experience temporary emotional distress or resurgence of traumatic memories during or after sessions [
282]. Controversy persists regarding EMDR’s mechanism of action: Is bilateral stimulation is essential, or do benefits primarily derive from common therapeutic elements of exposure and processing. Also questioned are EMDR’s safety and efficacy across a wide variety of patients and conditions, when compared to other methods [
281,
283].
Encompassing both sexes, and ages ranging from their 20s to 60s, Weissfeld study recruited generally healthy individuals without major musculoskeletal complaints. Experienced MMT practitioners first identified and recorded a variety of muscles testing weak (by binary measure) in each participant. Each weak muscle was then retested, followed immediately by 15 seconds of EMDR-style eye movements. Muscles were tested again immediately afterward.
Immediately after eye movements, 91% of weak muscles tested normal. On retest about 15 days later, over 84% remained normal. In some cases, multiple muscles normalized after a single application, suggesting muscle weakness can resolve — in multi-muscle patterns. In a crossover control group evaluating 42 weak muscles, 88% remained weak without treatment over a similar period. When treated, these muscles showed similar improvement.
The study also included a case report of a patient who, 15 years earlier, had a skiing accident causing an ACL tear and other ligament damage. Although surgery was functionally successful, minor pain persisted. MMT revealed multiple subclinical weaknesses in the knee and ankle, consistent with typical AMI findings. After correction using the eye movement protocol, the muscles remained normal one month later, with pain resolved.
In each case, changes in muscle function were immediate and binary: muscles were either weak or normal, with no intermediate gradations.
Future studies could incorporate sham-control conditions (e.g., visual fixation without bilateral saccades) to rule out expectancy or examiner bias. Such controls would allow clearer attribution of immediate muscle normalization to the therapy’s effects. Other therapies — e.g., spinal manipulation, acupuncture, or other sham treatments — could be substituted for eye movements to assess the generalizability of these treatments.
3.3. Updating a Century of Criteria for MMT Interpretations
Originating in the 1910s to assess polio patients, MMT techniques and interpretations were designed for muscles with lower motor neuron compromise causing severe deficits [
66,
176]. MMT typically uses the Medical Research Council (MRC) scale, grading muscle strength from 0 (no contraction) to 5 (full isometric resistance against submaximal examiner force). Grades 0–2 indicate muscles unable to lift against gravity; Grade 3 can lift against gravity but not resist pressure; Grade 4 can resist pressure but cannot maintain isometric contraction [
66,
284]. Only Grades 4 and 5 produce measurable resistance to incoming force, as applied in the break test.
Beyond polio, MMT is used to assess total body involvement in Duchenne muscular dystrophy [
285] and is accepted as valid for other organic conditions, including myasthenia gravis, inflammatory myopathy [
71,
286], amyotrophic lateral sclerosis [
287,
288,
289], post-polio syndrome [
255,
289,
290], chronic inflammatory demyelinating polyneuropathy [
291], dermatomyositis, polymyositis, nerve impingements (disk, foraminal, or other), and atrophy from disuse or other causes [
176,
246,
253,
286,
288,
292,
293,
294,
295,
296].
In patients with nsMSDs, severe weakness (Grades 0-2) is not part of the usual symptom picture. Most muscles in these patients, when not directly affected by pain, test as either Grade 4 or 5, and occasionally Grade 3, though this observation has not been empirically validated. Because studies have shown that clinicians lack the capacity to accurately subdivide grades (e.g., 3+, 4-, 4, 4+) [
255,
257,
258,
297,
298], this leaves only two applicable grades in most cases, 4 or 5, effectively mandating a binary determination. Detailed analysis, however, suggests that binarity is more than just an artifact of the grading system; it may be built into the physiology and mechanics of the MMT break test and, our eventual hypothesis holds, the pathophysiology of NOw.
3.4. The Hidden Phenomenology of MMT
The break test is controlled by the examiner. After instructing the subject to resist their force, the examiner determines when to exert force, how quickly ramp that force, the maximal level of force to exert against a muscle that is not yielding to pressure, and how long to apply that pressure. Findings from FM/T analyses performed since 2000 suggest that rather than force estimation, muscles in patients without organic conditions can, and perhaps should be graded based solely on movement — their ability to maintain an isometric plateau.
Kendall’s
Muscles, Testing and Function is one of the two most respected MMT texts. The break test is Kendall’s recommended test. (The other, Daniels and Worthingham, emphasizes a method sometimes called the “make test” in which the subject is instructed to press into the hand of the examiner [
176]. We will not be deeply examining this form of testing.) Kendall (p.22) states that “normal” refers to a muscle that “can [isometrically] hold the test position against gravity and strong [though submaximal] pressure.” A normal muscle has “strength that is adequate for ordinary functional activities.” In performing the test, Kendall instructs a gradual initial force application that allows the subject to “get set and hold” ([
66] pp. 16, 18). This is referred to as “coupling” by Bittmann (2020). In Bittmann’s interpretation of MMT, coupling, and initial gradual increase in pressure during which the subject is allowed to adjust to the examiner’s pressure, can last several seconds [
299].
Figure 1, adopted from Schaefer et al. (2022) shows an overlay of three tests of the same muscle, each assessed while the subject is holding a differently valanced mental focus: unpleasant (red tracing), pleasant imagery (blue tracing), and a baseline test with no specific imagery (gray tracing). The top box shows force over time (F/T) and the bottom box movement over time (M/T).
Figure 1 has been slightly modified from the original. Some labels, unnecessary for the scope of this review, have been removed and others added to emphasize particular points. The key experimental finding in Schaefer — that normal muscle function can be disturbed by noxious neurological activation, mental focus in this case — is a theme that we will return to later. For now, we are interested in the phenomenological characteristics of the malfunction.
The first, and most striking difference between tracings is seen in the bottom box, which shows significant angular velocity (movement) in the red tracing beginning at just over 2.5 seconds. We are calling the moment of loss of isometric stability “Break 1.” The other two tracings, while significantly oscillating (the rapid small up and down movements of the tracings), do not show angular movement; they are isometrically stable or locked as indicated by the lack of vertical movement. The moment of complete loss of resistive capacity of the weak muscle we have labeled as “Break 2”. The blue and grey movement tracings do not break; they end when the examiner ends the test.
Second, in the top box all tracings reach similar peaks of force, between about 160 and 180 Newtons, but only the blue and grey can sustain the peak. Even as the muscle is giving way eccentrically following Break 1, the red force tracing is spiking, rising to a peak and then falling. Other FM/T studies show similar patterns of spiking force at normal levels [
220,
272]. A study showing F/T-only graphs of break tests taken to MVC also shows the same phenomenon [
71].
The observation, from these tracings, is that both normal and weak muscles are capable of producing equivalent force. This suggests that peak-force HHD, which adjudicates muscle function by its recording of the maximum force a muscle is capable of producing (MVC), may result in false negatives when testing weak muscles, though this proposition has never been directly studied. (This is seen in F/T-only tracings as well [
71]) presented Furthermore, since peak force is nearly equivalent in both weak and normal tests, the ability to attain an isometric lock is the primary finding that differentiates a weak muscle from a normal one.
This hypothesis of equivalent force in normal and weak muscles might be tested by comparing FM/T-based break test outcomes (binary lock vs unlock) with conventional peak-force dynamometry. If muscles that unlock under FM/T nevertheless generate normal peak force with HHD, this would confirm that the true deficit lies in adaptive force — the ability to sustain isometric stability — rather than in force generation. Such findings would indicate that dynamometry may miss clinically important motor control deficits.
3.5. Eccentric Action: Mechanical and Reflex Driven Force
The force produced or resisted during eccentric contractions is mechanically and neurologically distinct from force produced during isometric action in striated (voluntary) muscles. Eccentric contractions generate greater force with lower neural activation, recruit fewer motor units, and display a lower motor unit discharge rate compared to isometric or concentric actions. Like a stretching rubber band, during eccentric lengthening, elastic elements of the muscle and tendon convert kinetic energy into elastic energy, meaning incoming force is absorbed and resistance is greater. [
72,
300,
301,
302].
Continuing the test to full failure (Break 2) may be favored in research or athletic settings where maximal eccentric strength or endurance is of interest. Clinically, however, for treatment of nsMSD patients, our analysis will suggest that equal or greater diagnostic utility, efficiency, and accuracy will come from concluding the test at Break 1, with the conclusion that Beak 1, or its absence, is what establishes or rules out the presence of a motor control deficit affecting the muscle.
3.6. Adaptive Force, a New Way of Understanding MMT
Emerging perspectives regarding a capacity known as “adaptive force” are providing a basis for updating our views of MMT and its relationship to muscle function. As put forth by Schaefer et al. (2023 p. 1), “the adaptation of the neuromuscular system to external loads is usually not investigated in sports or movement sciences. Strength is commonly measured by pushing against resistance without considering the adaptive component. Adaptive force has been defined as "the neuromuscular capacity to adapt to external loads during holding [isometric] muscle actions similar to motions in real life and sports" ([
303]).
Clinically, adaptive force refers to the ability of a muscle to maintain isometric stability in response to changing force, as it is applied in the MMT break test [
33,
245,
246,
247]. Making the point that this is a major departure from conventional testing, Bittmann (2020 pp. 22-23) concludes that during MMT, "the aim is not to test the maximal strength of the patient or to ‘demonstrate’ that the patient is not able to resist the external force. The aim is to assess if the adaptation capacity of the neuromuscular system functions in a normal way. [
299]"
Reflecting the CNS’ ability to predict and adapt to changing force demands [
299], adaptive force arises from properties of ‘motor prediction, planning, and control’, a finer specification of what is usually referred to as motor control. This conceptual point is a bridge that will take us to the mPTSD model in Part III.
When adaptive force is intact, the tested muscle remains isometrically locked to the examiners full (though submaximal) pressure. When adaptive force is not effective, the muscle unlocks, yielding to eccentric motion even while force continues to rise as documented in
Figure 1. The dual possible outcome of the MMT break test, then, is locking vs. unlocking; a clear binary result.
Among currently available clinical tools for muscle assessment, only the MMT break test, which applies force that can measurably displace the limb causing eccentric contraction, directly challenges, and thereby evokes adaptive force. The H-reflex test, or any of the other aforementioned tests for AMI can induce and therefore measure the integrity of isometric holding. sEMG or EMG may differentiate between isometric and eccentric actions [
304], but they have not been studied in relation to adaptive force, and, as stated in
Section 2.3, their outcomes may not align with MMT outcomes.
Physiological oscillations observed during MMT offer further support for this interpretation. Oscillatory activity of about 10 Hz accompanies successful isometric contractions. Weak muscles typically have deficient or missing oscillations [
33,
305,
306].
This coincides with oscillations originating from the inferior olive, which are transmitted to cerebellar Purkinje cells via climbing fibers. Organizing movements and perhaps enabling the use of individual muscles [
307,
308,
309], these oscillations also coordinate timing between the cerebellum and motor cortex, supporting predictive and feedforward motor control, [
310,
311,
312], cortical motor learning, movement planning, and execution. [
313,
314]. Notably, individuals with PTSD exhibit reduced motor oscillations [
315], suggesting a common thread between PTSD and motor inhibition.
These findings challenge traditional interpretations of MMT and establish adaptive force as a binary, predictive control variable. When a muscle unlocks under load, the CNS has failed to maintain the necessary forward model of resistance. In short, this provides a basis for the hypothesis that NOw is a functional motor control disorder, and takes a step towards tying it to mechanisms of PTSD.
3.7. Another Way to Perform a Break Test?
In
Figure 1, we observe that the M/T tracing of the weak muscle (red tracing, bottom box) shows an isometric plateau during the first 2.5 seconds. This implies that weak muscles attain isometric locking for some period of time before beginning eccentric movement. These results are defined by examiners who are following a particular protocol for force application which generally coincides with Kendall’s narrative instructions. The initial seconds of the test are dedicated to slowly introducing the subject to the forces of the test. This can lead to tests taking 3-6 seconds to complete [
33,
299].
The initial isometric period may actually be an artifact of the examiner’s technique, however. For decades, AK practitioners have asserted that they determine the binary state of the muscle at the onset or “leading edge” of the test — the initial pressure applied to the limb — not after several seconds. The binary assessment, along with this style of testing has been called AK-MMT. Tracking FM/T, Caruso and Leisman (2000, 2001) set out to test this notion, producing an objective, verifiable visual and numerical record of these tests. The study focused on first milliseconds of the test, where the limb either remains steady or it gives way, a binary outcome.
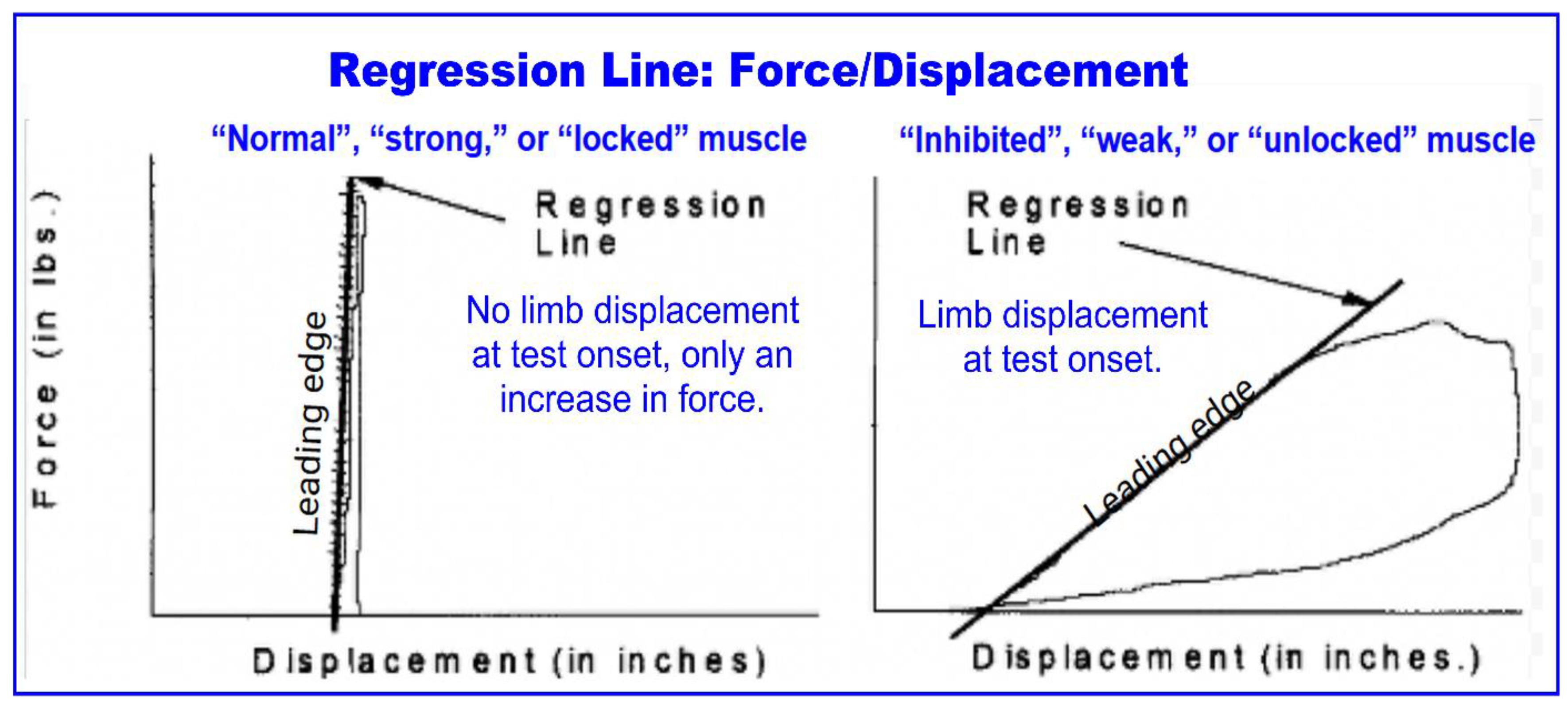
Figure 2 (from Caruso 2001) is a “force-displacement” graph which shows the leading edge or initial thrust of the test. The left tracing shows a normal muscle, which resists force without movement. The right tracing shows a weak muscle, which is immediately displaced with the onset of force.
A binary test might simply reinterpret Grades 5 and 4 as “strong” and “weak” respectively, but AK-MMT represent an alternative testing technique. Although it has not been explicitly delineated as such in the literature, this form of testing omits the “get set and hold” or “coupling” steps. The examiner increases force without the interplay that would allow the subject to catch up at the beginning of the test. The valid concern offered by Kendall and Caruso (2000) is that applying to much force too rapidly risks overpowering the subject. In fact, Caruso observed that experienced practitioners, utilizing this method, applied less force to weak muscles. Perhaps, they reasoned, these practitioners immediately sensed the muscle was giving way and therefore found it unnecessary to apply additional force.
This protocol might be called the “door handle” test. A quick press on a levered door handle reveals that it either gives way or it doesn’t. Extended pressure is unnecessary, making the test highly efficient in terms of both time and energy for both the examiner and the subject.
Comparing the subjective judgement of practitioners to objective outcomes, those with 5 or more years of experience had 98% agreement. That dropped to 64% for those with less than 5 years’ experience.
Several caveats exist regarding the door handle test, however. First, FM/T tracings of persistently weak (NOw) muscles have not been performed, to our knowledge. Like in
Figure 1, muscles depicted in
Figure 2 were weakened by stressful stimulus; in this case reflex points on the foot, so equivalence with NOw muscles is hypothetical. Further, a pilot study has shown that extending the time force is applied isometrically stable muscles may reveal weakness after several seconds, so the shorter test may miss hidden defects [
316]. More study is needed to pin down these variables. Finally, the door handle test works best for repeated tests of the same muscle as various stimuli are presented, as is done in AK. In Part IV, we show a hybrid technique that this author, at least has found beneficial for systematically testing many different muscles.
3.8. Clinical Utility of Binary MMT and the Case for Widespread Implementation
Despite the limitations of MMT noted in
Section 2.6, and the availability of other objective assessments, we argue that MMT — when applied using the methodology described here — offers unique advantages for evaluating motor control in clinical practice.
In addition to the aforementioned finding of 98% accuracy when used by experienced practitioners, Bohannan (2018) found near-perfect test-retest and inter-rater agreement was possible for most muscle actions when binary assessment was used [
250]. Applied as a binary test, MMT outperforms well-accepted clinical tools including deep tendon reflex testing [
317,
318], palpation [
319,
320,
321,
322,
323], and radiological evaluations [
324,
325,
326,
327]. Although to our knowledge FM/T devices are not yet commercially available, these findings suggest that such tools might improve training and accelerate proficiency.
In summary, these points argue for more widespread use of binary MMT for patients without organic conditions:
Only binary MMT directly evaluates the efficacy of adaptive force.
Focus on movement, not force, reduces limitations of sensitivity and subjectivity.
High concordance with objective FM/T tests, in experienced hands.
Highly scalable; >600 muscles can be assessed.
The binary state can be identified in tests taking well under 1 second, making it time-effective and less effortful.
These findings support the integration of binary MMT into standard musculoskeletal evaluation. As the following sections show, such weakness may represent not just a mechanical deficit but a learned, reversible motor inhibition — one that reflects the nervous system’s ongoing prediction and avoidance of negative outcomes.
4. Maladaptive Neuroplasticity, a Missing Link in Motor Dysfunction?
The diagnostic and therapeutic challenge of addressing nsMSDs requires understanding the top-down drivers of pain and motor dysfunction — i.e., maladaptive neuroplasticity or learning — and employing methods that target them.
Central sensitization, a well-documented condition often attributed to maladaptive neuroplasticity, involves structural, functional, and chemical changes that make the central nervous system (CNS) hypersensitive to sensory stimuli, particularly pain [
328]. Normally non-noxious stimuli become linked to pain pathways, leading to persistent pain states that extend beyond tissue healing [
329]. Past experiences of injury may intensify and prolong even unrelated pain responses [
330]. These altered neural patterns represent a form of implicit memory where the nervous system "remembers" and anticipates pain, even in its absence.
If maladaptive neuroplasticity is a central driver of pain, elimination of implicit pain memories may offer a solution to pain. According to Bonin and De Koninck (2014), spinal memories that might induce pain via this mechanism may be subject to erasure through processes related to memory reconsolidation [
331]. In
Section 7 and 7.1, we review similar possibilities for maladaptive motor memories.
Focusing on motor function, Pelletier, Higgins, and Bourbonnais (2015) argue that maladaptive neuroplasticity is a key factor in nsMSDs. This challenges the stated “structural-pathology paradigm,” which assumes symptoms originate locally at the pain or injury site. They propose that associative learning links movements with pain or trauma, altering sensory processing, motor control, and pain perception. They identify the failure to address these changes as a “missing link” in musculoskeletal care and call for a new paradigm [
332].
Similarly, Ablin et al. (2024) report that nociplastic pain — altered CNS pain modulation without clear tissue pathology — can involve functional and structural changes in motor networks [
333]. This suggests maladaptive motor learning may interact with central sensitization, compounding pain and motor dysfunction.
Changes in muscle function — including inhibition as part of maladaptive motor plasticity — may aggravate nociplastic pain or central sensitization, adding muscle and joint dysfunction to already altered CNS pain modulation.
Like PTSD and chronic anxiety, NOw may be a behavioral adaptation driven by maladaptive learning. It may be encoded through conditioned muscle- or movement–trauma associations and sustained by avoidance learning in a two-stage process [
334,
335,
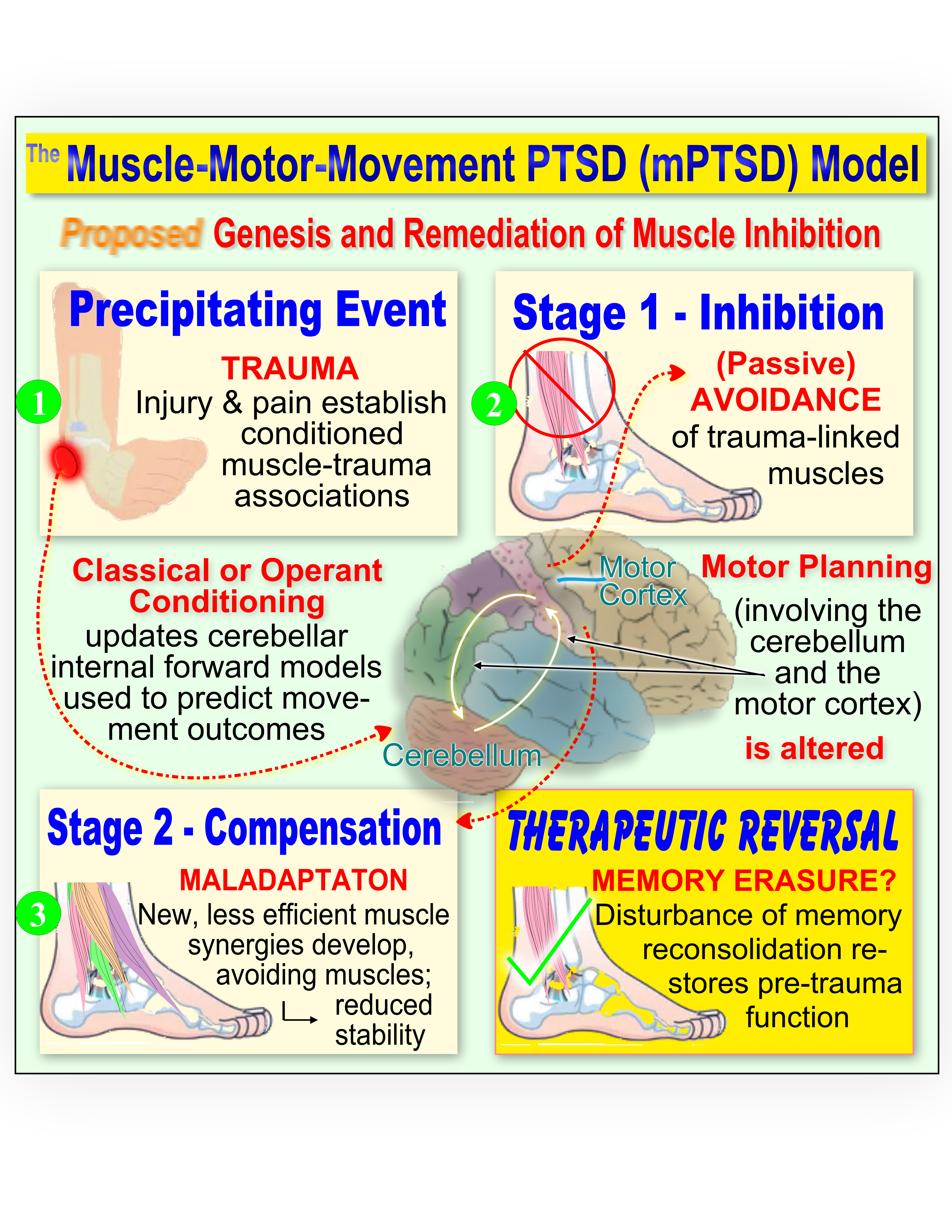
336]. Instead of focusing on “emotional” parameters, as in PTSD, these mechanisms may specifically affect motor function, detectable in individual muscles with MMT. The mPTSD model describes how muscle weakness may be acquired, sustained, and resolved, as shown in the graphical summary (of mPTSD) presented at the beginning of this paper of this paper.
Trauma (pain, stress or injury), occurring in temporal proximity to movements (contraction of muscles), associates the sensory and motor events. Persistent antalgic movements may add to or cause similar associations.
Th associations create ongoing predictions that using the muscle will (re)trigger the trauma, so it is avoided during movement planning — the first stage of the response.
In the second stage, new synergies are developed to avoid using affected muscles. These are likely to be less efficient and maladaptive, yet become the preferred choice for functional activities [
337]. (A muscle or motor synergy is a coordinated activation pattern in which muscles cooperate with specific timing and force to produce a segment or module of movement. Multiple synergies combine to achieve complex goals [
338,
339].)
As in two-stage avoidance learning models of PTSD or anxiety disorders, habitual avoidance prevents corrective experiences that could reintegrate the muscles once healing has occurred.
With avoidance encoded in cerebellar internal models via error-based learning, its interactions with the motor cortex establish new synergies that reformat future movements.
Successful treatments for mPTSD may work by eliminating muscle–pain/trauma associations through the disruption of memory reconsolidation.
These points will be elaborated upon in succeeding sections.
4.1. Psychoneurokinesiology: A Broad Framework for Motor Conditioning
We propose psychoneurokinesiology (PNK) as a framework for studying how psychological factors, mediated by the nervous system, influence movement and motor function. Like psychoneuroimmunology and psychoneuroendocrinology with the immune and endocrine systems, PNK considers how learning processes (behavioral conditioning) can shape motor behavior. In later sections, we return to PNK as a way to integrate potential causative factors of mPTSD with conditions like FW and kinesiophobia.
4.2. The Mechanism of mPTSD; Theoretical Background
Focused on prediction and learning mechanisms, the mPTSD model builds on the relationship between fear-avoidance and aberrant motor control. Hodges and Moseley (2003) proposed that changes in motor function can originate during movement planning, when the prediction (fear) of pain leads to predictive avoidance of activating certain muscles [
82]. This occurs upstream of the motor cortex’s dispersion of control signals to the spine. Neige et al. (2018) suggested that once such changes are acquired, they may persist, delaying muscle activation. While both center on potential changes in the motor cortex, evidence reviewed here suggests a larger role for the cerebellum, whose predictions modulate motor cortex activity [
340].
Focusing on the lumbopelvic area, Hodges and Moseley summarize a number of potential mechanisms by which pain and nociceptive signals might interfere with harmonious muscle function, potentially leading to further pain. Pain may evoke cortical inhibition, delayed central transmission, and motor neuron inhibition either directly or through reflex inhibition (AMI). Pain and nociception, which may alter motor output, also alter afferent (proprioceptive and somatic) signals, potentially leading to mismatches (prediction errors) with existing internal models of the body and its potential actions, managed by the cerebellum [
341]. Pain also evokes stress responses and fear, utilizing attentional resources. This may increase latencies and errors in movements.
Hodges and Moseley give particular focus to the importance of the “fear-avoidance model”, a behavioral theory with considerable support in the literature. They cite a number of examples in which motor control — the unconscious selection of motor patterns — is affected by fear. Fear-avoidance is also described in kinesiophobia, the outward behavioral response of avoiding movements or activities that have been associated with pain [
342,
343].
In one example that offers direct support for our thesis, they point to delayed contractions in the transverse abdominis frequently seen in patients with a history of low back pain. As suggested earlier, weak-testing muscles may exhibit delayed activation in MMT, and, as was shown in
Section 3.7 muscle weakness can be demonstrated in the immediate onset of MMT, implying the presence of delayed activation [
112,
113]. Although confirming evidence is needed, this logically connects fear-avoidance to MMT outcomes.
Hodges and Moseley further draw the association that (p. 365), “if fear of pain can disrupt the normal control of the trunk muscles, this may provide a link between psychosocial factors and physiological changes that lead to recurrence of pain. It could also be interpreted that these changes in motor control are an adaptation to limit loading and prevent recurrence.” Moreover, they state, “these adaptive strategies may provide a short-term solution with long-term [maladaptive] sequelae” that may result from reorganization of control through motor learning strategies.
Pointing to the relatively consistent finding of reduced activity of deep spinal muscles (and increased activity of superficial muscles) in patients — a pattern that has also been observed in the jaw and trunk — they note that this phenomenon supports the “pain adaptation model” introduced in
Section 2.1.
4.3. Predictive Processing and Reinforcement Learning, Intertwined Models
In the next two sections, we will be elaborating on two broad, intertwined paradigms of behavioral adaptation, predictive processing (PP) and reinforcement learning (RL), focusing on how they may be affecting movement to cause delayed activation or inhibition of muscles.
In psychology and behavioral sciences, RL is an umbrella term including theories of associative learning, classical (Pavlovian) conditioning, operant (instrumental) conditioning, extinction, counter-conditioning, and two-stage passive avoidance learning, all of which involve the cerebellum [
57,
344,
345,
346,
347,
348,
349]. In computational modeling, RL may specifically describe learning guided by reward prediction errors, with the basal ganglia playing a central role [
350]. Use of RL here is a reference to the behavioral science usage.
Arising in part as a reaction to Freud’s introspective psychology, early 20th century behavioral conditioning and modification theories from Pavlov, Thorndike, Watson, Skinner, and others were precursors to what is now called RL [
351]. Though the two paradigms certainly cooperate, RL is arguably more useful than PP for constructing an account of these two-stage learning processes.
PP frameworks have recently been gaining currency as a foundational principle of neurobiology. PP describes the brain as continuously generating and updating hierarchical internal models (or generative models) to predict the causes of incoming sensory input, and to generate responses to those predictions.
In terms of motor function, RL and PP paradigms jointly support cerebellar-dependent motor adaptations. The cerebellum implicitly learns from errors in prediction. With connections to sites of sensorimotor, executive, reward, and limbic function, the cerebellum is crucial to integrating responses to pain along with physical and psychological trauma [
352]. With further roles in anticipation and perception of pain, cerebellum also supports adaptation to pain by updating predictions about movement outcomes and their expected sensory consequences. In turn, these updates instruct new learning in the motor cortex [
87,
340,
353,
354].
In summary, predictive processing minimizes sensory prediction errors to update internal models, while reinforcement learning uses error-driven feedback to optimize motor behaviors. Pain and trauma-induced modifications in cerebellar function modulate this integrated learning system, influencing adaptive motor outcomes.
5. The Predictive Processing Framework of Motor Control
PP, including the free energy principle (FEP) and active inference (AInf) describe how the brain interprets sensory information and generates movement and other behaviors. Predictive processing frameworks are thought to offer explanatory power that traditional models lack.
When predictions differ from actual sensory input, the resulting prediction errors drive the updating of internal or generative models, helping the brain to improve future sensory predictions. Overall, PP refers to a cycle of prediction, error detection, and model updating as fundamental to perception, action (movement), and learning [
355,
356,
357]. Particularly relating to internal models of the body and the range of potential movements, this function is often attributed to the cerebellum [
358,
359].
5.1. Core Concepts of Predictive Processing
The brain continuously generates predictions based on prior experiences. These predictions form internal or generative models of the body and environment that guide perception and action. The FEP suggests that the brain actively seeks to minimize "free energy" — a proxy for surprise, uncertainty, or prediction error [
360]. Prediction error — the difference between the expected and actual states of the body (interoceptive) or the environment (exteroceptive) — can signal potential threats to homeostasis and therefore to survival.
AInf describes how organisms minimize surprise by adjusting either their internal predictions or their actions to align with incoming sensory input [
359].
The brain’s generative models constrain predictions to a limited set of plausible states of the body and the world based on prior experience. The brain’s goal is to keep operations within these bounded states, thereby minimizing surprise (free energy) [
361,
362].
5.2. Precision Weighting and Perceptual Bias
A crucial aspect of PP is precision weighting — the confidence assigned to either prior (top-down) beliefs or (bottom-up) sensory inputs. When prior beliefs are assigned high precision, perception is biased more toward expectations than toward actual sensory evidence. For example, placebo effects may reflect the weighted top-down prediction of a positive result from a treatment [
363]. Perceived capacities influence perception: infants who can crawl infants show fear when seeing a (fake) cliff, whereas non-crawling infants do not [
364].
Conversely, when sensory input is assigned high precision, perception is shaped more by that input than by expected outcomes [
365,
366]. For instance, individuals who are fatigued or carrying heavy packs estimate upcoming distances to be longer and hills to be steeper [
364].
5.3. Trauma, Pain, and Predictive Processing
In PTSD, the brain may develop hyperprecise priors — generative models biased toward predicting adverse outcomes — that override contradictory sensory input, potentially causing benign stimuli to trigger defensive responses [
367,
368]. For example, a door slamming may evoke predictions of threat in a combat veteran, despite cues indicating safety.
In response, the brain may invoke physiological defenses such as altered muscle activation, autonomic arousal, and postural adjustments, preparing to fight or flee [
127,
369]. Even unconscious interoceptive feedback can reinforce these maladaptive responses. Physiological arousal may be interpreted as confirmation of threat, providing evidence for the original prediction [
370,
371,
372]. This interoceptive feedback may be unconscious, though it may also be consciously interpreted as fear, anxiety, or other emotional states [
373].
Similar predictive processes may occur in chronic pain, where past experiences lead to fulfilled predictions of pain despite the absence of nociceptive stimuli [
372,
374,
375]. In other contexts, the occurrence of symptoms based on expectations is known as the nocebo effect [
186].
5.4. Motor Control and Active Inference
In the AInf framework, descriptions of movement challenge common notions. Rather than issuing commands to contract specific muscles, the motor system generates predictions of the desired sensory outcomes — for instance, particular joint positions or the feeling of grasping a cup. These predictions are compared with actual sensory input, and any mismatches (prediction errors) drive adjustments, made by spinal circuits, to align reality with expectation [
359].
Intentions and predictions are hierarchical, arising from top-down processes, while errors from sensory mismatches flow bottom-up. For example, when reaching for an object, the prefrontal cortex sets the goal, prompting the premotor cortex to generate predictions about trajectory and hand positioning. The primary motor cortex (M1) translates these into a motor plan and sends an efference copy (also called a corollary discharge) to the cerebellum, which simulates the sensory consequences and fine-tunes the plan. The updated plan is returned to M1 for implementation [
87,
340,
376].
Transmitted through the corticospinal tract, the predicted proprioceptive end state formulated by M1 acts as an attractor (target state) that the spinal cord seeks to fulfill. Like the cerebellum, the spinal cord itself may hold an internal model of limb dynamics, enabling movement adjustments independently of supraspinal control [
377]. In the spinal cord, these predictions are compared with proprioceptive and other input from sensory receptors. Mismatches generate prediction errors that activate spinal memories and reflexes to contract muscles, moving the body to minimize the error [
359,
360,
361,
378].
Prediction errors from the periphery that are not fully resolved by spinal control may be carried back up the hierarchical chain to the cerebellum via the spinocerebellar tract. Working with M1, the cerebellum mediates these errors and sends corrections back to the spinal cord for execution [
359,
379].
5.5. Arthrogenic Muscle Inhibition as Predictive Processing
AMI is often framed as an adaptive defense that protects injured tissues [
134]. When inhibition persists, the defense may become maladaptive. Bilateral weakness in unilateral conditions suggests CNS involvement. Evidence also indicates that AMI may begin locally (joint injury/pain) but can be sustained by central (and possibly peripheral) plasticity that maintains activation deficits in otherwise healthy muscles [
23,
35,
43,
134,
156,
380].
Viewed from the perspective of PP, injury constitutes a salient prediction error that updates generative models of movement (e.g., an ankle inversion violates the predicted smooth step) [
381,
382,
383,
384]. Pain or fear may accelerate and reinforce that learning [
343,
385,
386,
387].
After apparently successful recovery, the generative model may still encode failure for muscles implicated during the injury. The peroneus muscles, which may be rapidly stretched in ankle inversion, may acquire hyper-precise priors predicting failure; motor predictions (plans) then minimize reliance on those muscles. Clinically, peroneus weakness is frequently observed after inversion sprains, becoming a factor in ankle instability [
28,
29,
157,
388,
389].
Alternatively, or perhaps in addition, the sensory prediction error arising from the joint during the injury (accompanied by nociceptive signals and pain) may lead to an over-weighting of proprioceptive output from that joint, again tied to prediction of its failure. In research that led to the “arthrogenic” designation, the over activation of joint mechanoreceptors during injuries was found to trigger inhibitory spinal interneurons that presynaptically inhibit alpha motor neurons of muscles around the joint [
134,
150,
181,
390]. In PP terms, a bottom-up, hyper-precise prediction that joint afferents will inhibit αMNs can promote avoidance of muscles predicted to fail, yielding MMT weakness. This is supported by findings that blocking joint afferents temporarily abolishes AMI-type weakness, enabling exercise and potential reduction of atrophy [
35,
43,
48].
5.6. Kinesiophobia, Muscle Weakness, and Predictive Processing
Kinesiophobia — fear of movement due to anticipated pain or damage — can be understood as arising from maladaptive predictions within the brain’s generative models. These predictions may include consciously accessible beliefs, such as the idea that pain signifies ongoing or future damage to the body [
264,
378,
391]. Such beliefs can lead to avoidance of gross (e.g., whole joint or limb) movements or activities like walking, bending, or lifting [
392,
393,
394]. The capacity for conscious awareness of what is being avoided allows for cognitive therapies aimed at reframing these beliefs and encouraging re-engagement in feared activities [
328,
395,
396,
397].
In contrast, NOw, our hypothetical mechanisms contend, is characterized by unconscious, subclinical avoidance of movements or muscles. Patients, aware only of pain, stiffness, or limited range of motion, are frequently surprised at how many muscles test weak on MMT. While humans can consciously choose the goal of a movement and the trajectory of their limbs toward that goal, they cannot directly control the precise sequencing, timing, and relative force of each muscle involved (i.e., the muscle synergies).
We suggest that unresolved NOw, causing muscle and joint imbalance and increasing the potential for injury, may contribute to kinesiophobia by adding an actual biomechanical risk. For example, ankle instability — resulting from peroneal weakness and other causes [
28,
29,
131,
182,
398] — increases injury likelihood. This may create an unconscious, though essentially rational, distrust of relying on the affected ankle.
Because most literature does not address clinical methods for restoring inhibited muscle function, this potential link between weakness resolution and kinesiophobia reduction has been overlooked. In this author’s clinical experience, restoring muscle function often reduces or eliminates kinesiophobia, though some patients may still require encouragement to resume previously avoided activities. A case report describing resolution of kinesiophobia following restoration of abdominal muscle function appears in Appendix A.
5.7. Functional Weakness, Muscle Weakness and Predictive Processing
Functional weakness (FW) is a movement disorder often classified within the broader categories of functional motor disorder (FMD) or functional neurologic disorder (FND). FW denotes clinical weakness — observable paresis or, more rarely, complete paralysis in a limb or region of the body — presenting without an identifiable organic cause [
399]. Historically, FW has been referred to as a conversion disorder, reflecting its conceptualization as a somatic or visceral manifestation of unconscious psychogenic stress [
400].
Several authors have discussed FMD and FW in relation to “sense of agency” and “intentional binding” theories, which describe a diminished capacity to experience motor actions as self-initiated. Patients with FMD have shown decreased functional connectivity between the right temporo-parietal junction (TPJ) and bilateral sensorimotor regions. The TPJ is thought to compare actual external events with internal predictions of movement, enabling the sense of self-agency [
401,
402]. The cerebellum has a similar function — comparing predicted to actual outcomes [
403] — though its specific involvement in FW has not been directly investigated, to our knowledge.
A related observation is that decreased sense of limb ownership has been reported in complex regional pain syndrome, a condition of chronic pain, allodynia, and dysautonomia that may develop after trauma, with symptoms disproportionate to the triggering event [
404,
405].
Viewed through the PP framework, FW may involve hyperprecise predictions of weakness or failure of movement that override accurate sensory input of efficacy. This mismatch between intention and perception may create the sense that movements are not under voluntary control.
Kozlowska (2007) suggests that two innate defensive responses — the freeze response and appeasement behaviors — may contribute to FW. Freeze responses inhibit muscles, while appeasement can manifest as feigned injury or immobility. When these responses become predicted reactions to pain or trauma, they may persist beyond the original context. Musculoskeletal conditions, injuries and childhood trauma are common precursors to FW [
406].
Overly certain generative models predicting weakness may override contradictory proprioceptive or interoceptive sensory evidence of actual muscular efficacy. Bennett (2021) compares this to phantom limb syndrome, in which the brain predicts that an amputated limb is still present, leading to sensations attributed to the absent limb. In FW, the prediction may be opposite — anticipating that the limb is absent or ‘not my limb’ [
407].
Like kinesiophobia, the reduced sense of agency in FW could be caused or reinforced by NOw. If an individual unconsciously “knows” that certain muscles will not fully respond to voluntary commands, their sense of agency and control may diminish. We are not aware of definitive FW cases treated with muscle restoration methods.
6. Reinforcement Learning: the Second Theoretical Framework of Muscle Weakness
While PP accounts for the immediate suppression of maladaptive movements based on expected outcomes, reinforcement learning frameworks — including both classical and operant conditioning — provide a complementary and partially overlapping explanation for the long-term persistence of these patterns.
Associative learning, a form of RL, describes how temporally linked events become associated, forming persistent behavioral patterns [
201,
408,
409]. The neural remodeling underlying associative learning, Hebbian plasticity, is often stated as "neurons that fire together, wire together" [
410]. Thus, “neuroplasticity” is sometimes used as a non-specific synonym for associative learning. While predictive processing explains how sensory expectations shape motor suppression, RL may provide a better account for the persistence of these patterns through conditioning mechanisms.
6.1. Cerebellar–Motor Cortex Interactions
Cerebellar–motor cortex interactions are central to the development of motor or muscle synergies [
411,
412,
413,
414]. The cerebellum refines motor output through error-based supervised learning, continuously updating commands to minimize sensory prediction errors [
340,
415]. In the context of aberrant motor control (which includes NOw), such calibration may, we suggest, extend beyond ordinary adaptation, producing lasting avoidance of muscle use previously associated with pain or threat.
Concurrently, the M1 contributes unsupervised, experience-dependent plasticity that reshapes motor maps and synergy organization [
416]. Together, this cerebellar–M1 network may reorganize muscle synergies so that trauma-linked muscles are excluded or down-weighted, leading to the compensatory but less efficient strategies observed clinically with motor control evaluations or MMT, in the model we are suggesting. Evidence from pain studies supports this view: nociceptive input can alter M1 excitability and synergy recruitment [
417,
418], consistent with the hypothesis that cerebellar–cortical adaptations underlie both the persistence of NOw and the emergence of maladaptive synergies.
6.2. The Cerebellum and Suppressed Movement in the Face of Threat
Although much of the research on muscle inhibition focuses on the motor cortices, the cerebellum is integral to many of the responses examined here. Taking up just 10% of the brain’s volume, it holds more neurons than the rest of the brain, though its operation is outside of conscious awareness [
419]. Correlational evidence suggests that cerebellar activity may participate in the avoidance of movements using particular muscles. Although here we focus on the cerebellum’s role in RL, these functions can be reframed to model cerebellar activity in terms of PP [
358].
The motor cortex has been shown to be more, not less, active when engaging muscles affected by AMI. Experimental joint effusion increases corticospinal excitability, and chronic joint pathology likewise shows elevated motor cortex activity. As stated earlier this may reflect increased central drive when fewer muscles are engaged, but, as Rice (2015, p. 1) noted in studies of quadriceps inhibition, this provides “no evidence for a supraspinal contribution to quadriceps AMI” [
27,
44,
420]. Similar testing has not been completed with the cerebellum, to our knowledge.
The cerebellum participates in multiple levels of sensorimotor processing and conditioning, including the development and expression of classical and operant conditioning [
344,
345], passive avoidance conditioning [
421], and the adaptation of movements based on prediction [
345]. With reciprocal connections to prefrontal and limbic regions, including amygdala, hippocampus, hypothalamus, periaqueductal gray (PAG), and raphe nuclei, it plays a role in error-based learning, episodic and working memory, and cognitive-emotional function. With or without PTSD, cerebellar activity is associated with depression, fear, anxiety, and dissociation [
422,
423]. These properties have been implicated in the acquisition of and response to memories underlying PTSD [
424,
425,
426], and, based on correlation, to mPTSD, we propose. Despite all this, the cerebellum remains relatively underexplored [
422,
423].
As outlined in
Section 5.4, the cerebellum receives an efference copy of motor plans from the motor cortex, and uses its internal models of the body and its movements to predict the sensory consequences of the proposed movement. As the movement is underway, it also compares the expected sensory outcome with actual sensory outcomes (reafference), using mismatch (prediction error) to correct ongoing movements and update future plans. When the predicted sensory feedback matches the reafferent feedback, sensitivity to the self-generated sensation can be reduced [
427,
428,
429,
430]; for example, we cannot tickle ourselves [
431].
If part of a proposed motor plan has previously resulted in noxious prediction errors (i.e., tissue damage or pain occurred when a similar motor plan was enacted), the cerebellum may flag it as potentially threatening and suppress or modify it prior to execution. This can serve both immediate protective and long-term learning needs via associative mechanisms [
387,
432] which update internal models [
433,
434,
435]. Revised plans are returned via cerebello-thalamocortical circuits, updating motor cortex memories [
87,
376,
436,
437,
438].
While most forms of learning require repetition, passive avoidance learning — suspension of motor activity in response to a perceived threat — can be established in a single trial [
206,
439], a process involving the cerebellum [
421]. For example, a child burned when touching a hot stove may avoid repeating the action without further experiences. Avoidance — not doing — is simpler than active defensive responses, which require coordinated execution. Relatedly perhaps, brief nociceptive stimuli can trigger immediate and longer-term motor cortex inhibition, sometimes lasting hours after stimulus removal [
102].
The freeze or immobility response, which inhibits muscular action, is typically the first reaction to threats that do not immediately trigger active defensive or withdrawal movements. This represents a transient state of increased vigilance, “freezing for action”, as stated by Roelofs (2017) [
114,
115,
116,
127,
128]. This is distinct from tonic immobility (also called “playing dead” or “possum”), which may occur when fight or flight have failed [
127,
440]. Although freeze reactions are typically understood to affect all muscles, freeze responses initiated during preparation for a specific action may selectively inhibit the muscles required for that action [
127,
128].
Threat recognition and the freeze response can begin in the amygdala, which is closely connected to the PAG, which mediates behavioral responses to threat (e.g., fight-flight-freeze). In conjunction with the PAG, the cerebellum plays a role in the coordination of physiological responses to threat including changes in blood pressure and heart rate [
441]. Both conditioned and innate fear-evoked freeze behaviors are mediated in part by cerebellar-spinal connections [
442]. We suggest that transient freeze responses when faced with noxious sensory inputs — potential threats — may be observable with MMT.
6.3. Classical Conditioning in Cerebellar Motor Suppression
Classical conditioning models provide a framework for understanding how specific sensory inputs can become triggers for motor inhibition. A neutral stimulus (NS, e.g., a bell) that repeatedly coincides with an unconditioned stimulus (US, e.g., a puff of air to the eye) capable of eliciting a reflexive response (e.g., an eyeblink) can become a conditioned stimulus (CS) that evokes a similar response on its own [
443,
444]. In other words, the bell alone causes the blink.
In muscle inhibition, the US may be any neural activation capable of producing an unconditioned response of motor inhibition — the freeze response. There is strong support for the hypothesis that associative conditioning occurs after painful or traumatic events, and that a wide range of internal and external cues — including somatic, proprioceptive, interoceptive, biochemical, and cognitive-emotional signals, including muscle spindle activity from the inhibited muscle itself — can serve as CS eliciting passive or active avoidance as a CR. [
445,
446,
447,
448,
449,
450].
Because motor planning is feedforward, merely intending a movement that incorporates the CS may be enough to alter the plan, as the efference copy from the motor cortex is processed for potential errors by the cerebellum. Likewise, an attempt to contract a muscle associated with the CS — as during MMT — may, upon reafference, be interpreted as an error, reinforcing future inhibition [
451,
452].
Cerebellar involvement in conditioning thus aligns with its role in predictive processing and reinforcement learning: it does not simply react to sensory events, but encodes and recalls learned associations, enabling rapid, often unconscious, avoidance of movements predicted to cause harm — even when such movements are harmless in the present context. This mechanism may contribute to the persistence of maladaptive motor patterns in mPTSD.
The inhibition of peroneus muscles following ankle inversion injuries, a factor in chronic ankle instability, as mentioned earlier, may provide a direct example of conditioned (passive avoidance) learning of muscle inhibition. Through single-trial passive avoidance learning, interoceptive sensory activations taking place immediately prior to an accidental inversion [
445,
446] — spindle stretch, joint proprioception or other features of placing weight on the involved foot — may serve as the conditioned CS that triggers the conditioned response CR, the arthrogenic inhibitory reflex.
Indeed, the cerebellum has been shown to be involved in chronic ankle instability [
453]. Manda et al. (2025), also documented increased cerebellar activity in individuals with migraines, chronic low back pain, and irritable bowel syndrome. Confronting “traditional and simplistic”, ideas of the cerebellum and its motor functions, the article emphasizes (pp. 803-804) “the need for further investigation into cerebellar mechanisms and their clinical applications, potentially transforming pain treatment paradigms. [
454]” While this current review does not engage in direct investigation, it suggests plausible mechanisms for cerebellar involvement in muscle weakness, a ubiquitous finding in musculoskeletal pain conditions. If the treatments for muscle inhibition we cover are ultimately found to alter cerebellar memories, they may represent one way of addressing Manda’s call for transformation of pain treatment paradigms based on cerebellar mechanisms.
6.4. Implications of Maladaptive Plasticity for Rehabilitation
As stated earlier, in theories of central pain sensitization, movements that are predicted to cause pain based on prior experience may generate pain signals even without nociceptive input. Similarly, maladaptive motor learning can suppress harmless movements or the muscles that enable them, rendering certain actions unavailable and forcing reliance on alternative motor strategies that take time to acquire.
In the presence of muscle weakness, rehabilitation may be understood as the development of new compensatory patterns that allow avoidance of pain- or trauma-associated muscles. Dedicated rehabilitative practices may speed functional gains, and perhaps allow the replacements to be more effective, but even the best compensations are likely to be less stable and efficient than fully integrated motor patterns.
Standard treatment practices (e.g., RICE — rest, ice, compression and elevation) suggest that swelling after injuries such as ankle sprains may exceed what is necessary for healing [
455,
456]. Reports from Buhler, Utah Jazz players, and other observers have noted that when regional muscle function was promptly restored after injuries with minimal tissue damage, swelling and pain were often diminished or absent [
245,
457]. This suggests that persistent muscle inhibition after injury may be maladaptive and contribute to swelling, rather than being protective.
We speculate that neuroplastic recalibration of cerebellar associations could underlie this phenomenon. Given cerebellar roles in pain and nociception, its somatotopic connections to individual body areas [
353,
354,
434], its links to immune function, inflammation [
458], and hypothalamic connectivity [
459], memory-affecting treatments could remove muscle-movement-trauma associations from cerebellar internal models that could sustain the impression that the region has suffered serious injury. This impression may continue to ramp up the physiological responses appropriate to greater levels of tissue damage.
Nonetheless, inhibition could still serve a protective role at the moment of injury. For example, during ankle an inversion injury, rapid stretch may trigger excessive spindle activation in the peroneus muscles — the primary evertors of the foot — producing reflex contraction that could itself increase the risk of damage to those muscles. The arthrogenic inhibitory reflex from the deformation of joint mechanoreceptors may counteract this spindle response, protecting the muscle. Unfortunately, affected muscles may remain [353,354,434] inhibited indefinitely.
6.5. Learning Theory: Extinction, Counter-Conditioning, and Muscle Therapies
In RL theory, when a CS is repeatedly presented without reinforcement by the US, the CR (the behavioral response) weakens and may eventually disappear (become extinct). For instance, from our earlier example, if the CS (bell) is repeatedly rung without the US (air puff), the CR (blink) will eventually cease as a response. Neurologically, “extinction” does not erase the original associative memory. Rather, it creates a new memory that essentially holds the belief that the bell does not predict an air puff is coming. Extinction learning inhibits the behavioral expression of an existing memory by adding an additional memory that counteracts the first. [
460,
461,
462].
Counter-conditioning is similar to extinction. It differs in that the CS is not simply presented without reinforcement, but is paired with a new, alternative response. Whereas extinction suppresses expression of the original association, counter-conditioning actively builds a competing response that can replace the conditioned reaction [
201,
463]. An example is when an individual chooses to chew gum after a meal instead of smoking a cigarette. With repetition, the craving will shift to the new option.
If RL is the mechanism of muscle inhibition, extinction, allowing inhibited muscles to be re-integrated into movements, might be expected to occur as muscle use becomes pain-free, but several factors may prevent this from occurring.
The first is the phenomenon of two-stage learning.
After an injury, pain is experienced when using joints or muscles that are associated with the injury, potentially reinforcing trauma associations formed during the original event. In a second stage of learning achieved through operant (trial-and-error) conditioning, new motor synergies that avoid trauma-associated muscles may be developed [
145]. The experience of pain, whether it arises from actual tissue damage or other causes, leads to the development of new motor synergies [
2,
85,
132,
464,
465,
466,
467,
468,
469,
470,
471]. This learning is reinforced by the reward of avoiding pain. (Like classical conditioning, operant conditioning is dependent on the cerebellum [
145,
464,
472,
473].)
Two-stage learning is well documented in avoidance-based disorders such as agoraphobia and anxiety disorders. In these conditions, avoidance produces an immediate reward — reduced fear or anxiety — but prevents the corrective experiences needed for extinction. Agoraphobia, the fear of leaving one’s house or being in open or crowded places, is sometimes treated with exposure therapy — titrated experiences of the feared condition. Exposure provides repeated evidence that avoided experiences are safe while also building confidence in one’s ability to cope [
474,
475,
476,
477]. These new safety and efficacy beliefs do not erase the original association of open spaces with threat, but instead overlay it. Until the new learning — open spaces ≠ threat — is deeply ingrained through repetition, the older, less healthy or pathological responses can resurface, particularly under stress or in novel contexts [
478].
The mPTSD model proposes that in a similar two-stage process, new synergies are developed that omit or avoid reliance on trauma-associated muscles. In a seeming paradox, attempted exercise of affected muscles, which can be seen as analogous to exposure may prevent those muscles from being reintegrated into functional movements. This tendency may be furthered by the feedforward nature of muscle activity represented in pre-movement motor planning. Motor planning processes in the motor cortex and supplementary motor area choose which synergies will be used during a movement [
479,
480]. Aspects of motor synergies are also encoded in the brain stem and spinal cord [
481]. Very limited conscious modulation of synergies may be possible, but in most circumstances, this does not translate to control of individual muscles [
482,
483].
In directing movements, the brain also prefers movements that are habitual, rather than optimal [
337]. In sum, once new synergies that avoid reliance on particular muscles have been developed, their use becomes automatic. Voluntary attempts to contract these muscles — during MMT for instance — may emerge from motor planning with a delay and/or diminished force. The intended contraction is overridden by the habitual default.
Similarly, we might expect that exposure therapy would allow for the renewed use of avoided muscles. Assuming pain-free status after recovery, repeatedly contracting inhibited muscles should re-establish normal function. As stated earlier however, exercise therapies alone will not foster hypertrophy of inhibited muscles or reverse AMI; i.e., inhibited muscles apparently do not respond to exposure therapies.
However, using sEMG biofeedback to directly sense the function of individual muscles, what appears to be extinction (based on its properties) may be accomplished. Earlier we mentioned H-reflex and sEMG biofeedback, each of which makes individual muscle activation consciously accessible, allowing research subjects or patients, respectively, to alter the function of individual muscles. Using H-reflex biofeedback, both animals and humans have learned to up- or downregulate individual muscles, a process shown to depend on the cerebellum for both acquisition and maintenance [
56,
59,
145].
In AMI research, sEMG biofeedback has shown some promise [
142,
144]. With repeated reward-based learning, patients directly monitoring the amplitude of electrical signals produced by both inhibited and hypertonic muscles were able to normalize the output of those muscles [
484].
Assuming that learned inhibition is the cause of individual muscle weakness, and an understanding of the learning (neuroplasticity) principles applied, a number of limitations may exist with biofeedback treatments of muscle inhibition. First, the corrections, if established via extinction and counter-conditioning, are layered on top avoidance memories, making them unstable, especially under stress [
478]. Second, as a diagnostic method, sEMG is far less efficient than MMT. In the time it takes to set up the equipment and do bilateral comparison tests, an experienced practitioner of MMT could have tested dozens of muscles. Furthermore, sEMG is limited in its precision. Where MMT can test over 300 unilateral muscles [
239,
240], sEMG contains electrode placements for only 30 [
485]. Third, in contrast to the aforementioned immediate effect of corrections achieved by methods which have been and will be outlined here, the correction of a single muscle may take several weeks of practice [
144].
The broader question of how correction of inhibition alters multi-muscle synergies is taken up in
Section 7.5.
7. Can Memory-Altering Treatments Restore Normal Function?
As discussed in
Section 2.1, theoretical models of pain-related muscle adaptation — including the pain adaptation model, the vicious cycle theory, and Hodges’ redistribution framework — provide differing but overlapping accounts of how muscles respond to pain. Each emphasizes variability and complexity in adaptation, and Hodges in particular highlights the upstream influence of motor planning, consistent with the mPTSD model, though lacking in details regarding PP and RL frameworks [
106].
Although this review aligns with Hodges’ emphasis on variability and complexity, clinical observations suggest that inhibition, revealed by MMT, is the simplest, most accessible — and often reversible — marker of motor dysfunction.
The preceding hypothetical mPTSD model suggests that persistent NOw is a behavioral outcome resulting from maladaptive learning and its underlying neuroplasticity. If this is indeed its mechanism, therapies produce immediate normalization of weakness (AK-based methods and eye movements of EMDR) may be altering or eliminating the underlying maladaptive memories.
Memory reconsolidation theories describe mechanisms that may underlie rapid memory alteration. In this section, we present the theoretical and clinical aspects of interacting with maladaptive motor learning. We begin by outlining how reconsolidation allows reactivated memories to be updated or erased, providing a potential mechanism for the rapid reversal of NOw.
7.1. Learning Theory: Therapeutic Implications of Memory Storage
Memory reconsolidation refers to the process by which previously consolidated memories return to a labile and unstable state when reactivated (recalled, consciously or not). Reactivated memories must undergo reconsolidation — a process biochemically and neurologically similar to initial consolidation — to be restabilized. Known as the reconsolidation window, this labile period may last up to 6 hours [
382,
486,
487]. During the reconsolidation window, which is triggered by novel information or conditions that contradict some portion of the original memory, reactivated memories can be modified by new information. If the novel information is sufficiently contradictory to the existing memory, or the consolidation process itself is disrupted, the reactivated memory may fail to reconsolidate and be effectively erased [
381,
382].
EMDR has been suggested to operate based on the principles of reconsolidation, perhaps in conjunction with several potential mechanisms. Bilateral eye movements may tax working memory resources, potentially interfering with reconsolidation processes [
488]. Alternating bilateral stimulation may enhance communication between cerebral hemispheres, facilitating integration of emotional and sensorimotor information [
489]. Alternatively, the eye movements may trigger an investigatory reflex [
490] that contradicts the freeze response that may be associated with muscle inhibition.
Within PP models, successful interventions may create prediction errors — mismatches between expected and actual sensory input during movement [
491,
492]. As stated by Cerritelli and Esteves (2022 p. 5), interventions may “cause positive surprise and a high prediction error that will violate existing predictions and update the brain’s internal model.” These challenge the brain’s maladaptive predictions, potentially disrupting the reconsolidation of traumatic motor memories. This may effectively erase the associative memories that hold predictions of pain and trauma [
166,
365,
381,
382,
493], allowing pre-trauma functional motor patterns to resume.
The mPTSD model posits that the testing of a weak muscle reactivates the associative pain or trauma memory, or the motor learning associated with inhibition. Treatments that have been shown to successfully reverse NOw — e.g., AK-based interventions, spinal manipulation [
94,
95,
96,
97,
98], and trigger point therapy [
92] — each assumedly test weak muscles prior to administering treatments, potentially activating underlying maladaptive memories. Their therapeutic effect may then be founded on the ability to change the state of the CNS, interrupting the reconsolidation of the activated memory rather than their local effects. If this is the case, these therapies may be functionally interchangeable.
Overall, this represents what might be understood as “a common principle of change [
494]” which may be invoked by a variety therapeutic interventions, including spinal manipulation [
166,
365], acupuncture point therapies [
495,
496,
497,
498], and others [
499,
500]. This also coincides with AK’s broad model, which first focuses the nervous system on a particular imbalance, and then offers treatments that may, in the language of PP, invoke positive surprise [
166,
365,
491,
499,
501].
As noted in
Section 3.2.2, sham-controlled studies comparing eye-movement or other procedures with functionally inert controls would be useful in determining the veracity of this argument.
7.2. Clinical Replication of These Results
Although the muscle-by-muscle restoration of function is quite reliable, there are details and trends that emerge only with experience across many patients. The following clinical guidelines illustrate a few of these details. Reflecting techniques for reversing muscle weakness that differ from the broad methods of AK or its successor AMIT, the following details of clinical practice reflect the experience of this author alone.
Weissfeld (2021) demonstrated that many unknowns exist regarding the cause and treatment of NOw. That study provided the initial presentation and validation of the mPTSD hypothesis, describing a 4-step protocol for restoring function to weak muscles — a framework that may guide researchers attempting to replicate the study or clinicians seeking to improve patient outcomes:
Before beginning, record baseline symptoms, positive orthopedic tests, range of motion, pain-provoking or relieving movements, and any other criteria that can be reassessed after treatment.
Step 1: Scan for weak muscles using MMT according to the criteria in
Section 3.5–3.8, and list each one. In clinical practice, muscles surrounding the area of complaint are often tested as well (e.g., for knee pain, relevant muscles of the foot, ankle, and hip are also tested).
Step 2: Beginning at the top of the list, retest and immediately treat each muscle using the following procedure: Within 7 seconds of (re)testing the muscle, have the subject follow your finger from right to left and back with their eyes at ~1 cycle/second for ~15 seconds.
Step 3: Test the muscle again and record results; it either remains weak or it has normalized. You may find that some weak muscles have strengthened by the time they are reached on the list; this “spontaneous” recovery should be noted as well.
Step 4: When you have done this with all weak muscles, retest every muscle that was originally tested, not just those found to be weak on the first pass. A new strength-weakness pattern may be discovered, with some muscles that were normal becoming weak, and vice versa. If this occurs, repeat Steps 1-4 for as many rounds as needed to restore all muscles in the affected area(s).
When all, or most, muscles test normal, reassess baseline measures. Signs and symptoms often change following successful restoration of muscle function.
For research replication, subjects should be reassessed at least 24 hours later — or at intervals of weeks or months — to evaluate persistence of muscle and symptom changes.
For experimental purposes, other treatments, particularly those that arguably unwind stress in the nervous system, including spinal manipulation, acupuncture, or mental foci like positive visualizations may be substituted for the eye movements.
Testing multiple weak muscles before an intervention may destabilize a broad network of maladaptive memories, which could explain why a single therapy can sometimes normalize many muscles simultaneously. From the perspective of principles of memory reconsolidation outlined in the previous section, these therapies may be establishing broad neurological states that are contradictory (mismatched) to the states induced by testing muscles associated with pain or trauma [
381,
382,
493].
7.3. Layered Adaptations Revealed by Muscle Correction
Following correction of weakness as directed in 7.2, a second pass through all muscles in a region may reveal a new pattern of weakness. Muscles that tested normal the first time may now test weak. Such findings indicate layered compensatory patterns, with muscles shifting roles depending on the state of their counterparts. It suggests a cascade of attempted adaptations, each likely less efficient than the last. Regardless of the number of layers, progressive correction of weak muscles, in this author’s experience, moves the system toward a more integrated, non-adapted state.
In addition, when weak muscles are systematically corrected, the state of over-facilitated muscles often changes as well. They may normalize, or, if they test weak on a subsequent pass, they can simply be corrected as part of the next layer of adaptation.
7.4. “High Resolution” Muscle Testing; an Index of Motor Control Dysfunction?
As described in
Section 2.5, expanded protocols have been developed which allow for MMT assessment of ~600 muscles — typically 20–25 per major joint or region (e.g., ankle, wrist, knee), with roughly twice that in the shoulder complex. This can be called “high resolution” (HR) MMT.
In regions with a history of pain or injury, HR testing, this author finds, often reveals that more than 30% of muscles test weak, sometimes considerably more. This percentage does not always track with symptoms — asymptomatic individuals can sometimes show widespread weakness — but it provides a quantifiable metric nonetheless. For example, if 25 muscles are tested and 5 are weak, a 20% deficit is indicated.
We propose that this percentage could serve as an index of regional and potentially systemic motor control dysfunction, a tool that may assist both clinical assessment and future research, though it remains to be systematically validated. Because we assume that effective treatment will reverse existing muscle weakness, this measure of motor control deficit marks a starting point, rather than a long-term definition of deficit. Though verification on individual patients is required, continued symptoms after correction of weak muscles in painful regions may rule out aberrant motor control as a cause of those symptoms.
7.5. Neuropsychological Implications of MMT
Viewed in the ways that have been introduced in this review, MMT might be considered to be an assessment of aspects of neuropsychological function, since its outcomes reflect how predictive and associative processes shape motor output in real time. The influence of psychological and sensory cues on muscle function parallels the logic of neuropsychological testing, where structured tasks reveal higher-order cognitive or emotional influences on behavior. This characterization is provisional, but it underscores the possibility that MMT provides not only a measure of motor performance but also a window into the interaction of psychological states and motor control.
7.6. Does Disruption of Compensatory Adaptations Reveal Pre-Trauma Synergies?
Learning theory identifies extinction and counterconditioning as mechanisms by which conditioned responses are modified; a stimulus that once triggered one pattern of behavior can come to elicit a different one [
201,
502,
503].
According to the mPTSD model, muscles active during pain or trauma may become implicitly avoided, forcing reliance on compensatory synergies. Each layer of adaptation represents a novel solution, constructed from a reduced pool of available muscles. As layers accumulate, efficiency and resilience are likely to decline, leaving the system vulnerable to overload or failure when engaging in repetitive or demanding activities.
Correcting weak muscles in the currently dominant synergy may dissolve its poorly adaptive pattern, exposing the next layer beneath. This process may continue until latent pre-trauma synergies, drawing on a wider range of muscles, re-emerge, restoring more integrated and efficient motor control.
Clinical observations are consistent with this hypothesis. When most or all regional muscles are normalized, patients often report immediate ease of movement. The ease is often accompanied by observable improvements in posture, gait, and range of motion — without additional rehabilitation. This might be empirically tested using pre- and post-treatment measures of functional parameters. Appendix A reports some of these outcomes.
While sham-control designs suggested in
Section 3.2.2 address the first stage of the model (reversibility of trauma-linked inhibition), multi-muscle observations are needed to understand how motor patterns reorganize once inhibition is corrected. EMG or kinematic methods could document changes in synergies before and after correction, showing if, and how normalized muscles are incorporated into ongoing movement patterns. In addition, functional outcomes can be assessed directly: if resolving NOw is accompanied by immediate improvements in pain, range of motion, stability, or biomechanical efficiency, this would provide evidence that correction of inhibition has meaningful effects at the systems level. Observing changes in synergies indirectly supports the existence of a second stage of learning in the development of mPTSD.
8. Summary of Primary Hypotheses
This paper proposes psychoneurokinesiology (PNK) as a unifying framework for movement disorders that are not due to organic pathology. This covers motor control issues seen in non-specific musculoskeletal disorders (nsMSDs), functional weakness, kinesiophobia, and other conditions. We hypothesize that these conditions share a common mechanism: maladaptive motor learning (neuroplasticity).
8.1. The Genesis of NOw
As part of PNK, the “muscle-motor-movement post-traumatic stress disorder (mPTSD) model suggests a hypothesis in which experiences of trauma (pain, stress, or injury) become associated with contemporaneously contracting muscles, an instance of classical (Pavlovian) conditioning. Ongoing antalgic movements or postures may also cause or reinforce avoidance of muscles, an example of operant (instrumental) conditioning (i.e., trial-and-error learning). This leads to persistent passive avoidance — amounting to inhibition — of those muscles, implemented during the planning stages of movements by the cerebellum and motor cortex.
In a second stage of learning, new muscle synergies are developed via operant conditioning. These substitute other combinations of muscles for those that are inhibited. The ongoing preference for these new synergies, enforced during motor planning, is self-reinforcing: “healed” muscles do not get the opportunity to fully contract, preventing corrective experiences that might allow them to be reintegrated into future movements.
Persistent non-organic muscle weakness (NOw), established by these mechanisms, is proposed as a key clinical indicator of maladaptive motor learning. The mPTSD model builds on several existing constructs or theories:
Predictive processing (PP) and reinforcement learning (RL; behavioral conditioning), provide well-developed models of perception, action, and adaptive behavior.
RL mechanisms may include passive avoidance learning, which can develop after a single event, which incorporate freeze responses. From a PP perspective, this learning facilitates hyperprecise priors (prior beliefs) holding that particular sensory or motor activity will result in negative outcomes. Either way, this represents defensive adaptations to threat that suppress movement.
The “adaptive force” construct reframes MMT (using the break test) as a measure of the capacity of a muscle to maintain isometric stability when challenged by changing incoming force or pressure, a binary functional assessment of movement, not force.
Many of the features of mPTSD rely on the cerebellum for acquisition, maintenance, or expression. Its involvement in both implicit learning and error-driven updating of learned motor patterns makes it a plausible locus for maladaptive associations and a potential target of therapeutic interventions.
PNK, describing maladaptive motor learning, may extend to conditions such as functional weakness and kinesiophobia. These conditions may be consequences of inaccurate predictions or learning (beliefs) about movement outcomes. The addition of NOw provides a biomechanical correlate: muscle inhibition adds genuine motor insufficiency to the cognitive-emotional phenomena usually addressed as primary, potentially amplifying or underlying those conditions.
Applying this novel understanding of muscle weakness, several conclusions are demonstrated or suggested by available evidence:
NOw, a product of aberrant motor control, is ubiquitous across many musculoskeletal conditions.
NOw is the most easily evaluated form of aberrant motor control.
NOw can only be diagnosed with the MMT break test, which applies incoming force that might displace a limb.
With methodologies capable systematic testing a wider range muscles in a region, MMT may offer a comprehensive quantitative index of motor control dysfunction, presented as the percentage of muscles that are weak in that region.
Assuming the above, treatments that reverse NOw are a reliable and quantifiable method of addressing motor control deficits.
8.2. New Diagnostic and Therapeutic Directions; Clinical Psychoneurokinesiology
Clinical reports, peer-reviewed literature, and textbooks have been documenting methods that reverse muscle weakness (NOw) for over six decades, though definitive studies sufficient for evidence-based use have yet to occur. In these reports, and countless clinical observations, reversal is immediate following the application of an appropriate intervention, or it fails completely; treatment results are binary.
A 2021 study showed that over 90% of NOw muscles could be reversed from a single 15 second application of side-to-side eye movements, as used in Eye Movement Desensitization and Reprocessing (EMDR), with over 80% sustaining the correction for at least 2 weeks. It was hypothesized that this effect was due to the disruption of memory reconsolidation, which could eliminate the trauma-muscle association for those muscles.
Systematic treatments to all weak muscles in a region has been observed to immediately, without additional therapies or rehabilitation, restore integrated function at times, as indicated by ease of pain free action and improvement of clinical signs. This suggests that restoring function to individual muscles may restore pre-trauma muscle synergies, which used a wider selection of muscles.
9. Limitations and Future Directions
The framework developed here is largely theoretical, combining established models (predictive processing, memory reconsolidation, reinforcement learning) with emerging constructs (adaptive force showing the binarity of muscle function, newer therapies that may alter memories) to establish a novel hypothetical paradigm, mPTSD. While these syntheses are internally consistent and supported by clinical observations, they have not yet been validated through controlled experimental designs. The mPTSD model, in particular, should be regarded as the basis for developing new hypotheses rather than a proven mechanism. Further, although binary treatment outcomes align with both clinical reports and the logic of adaptive force testing, these require systematic replication to exclude bias, placebo effects, or examiner influence.
9.1. Methodological Limitations
Manual muscle testing (MMT), particularly when novelly reconceptualized as a measure of adaptive force, remains controversial in academic neuroscience. Criticisms stem from inconsistent methodology, examiner dependence, and limited inter-rater reliability. The objective adjuncts of force–motion-time (FM/T) tracings and high-resolution MMT protocols may address some of these concerns, but training standards need to be updated. Further, validation studies with structured protocols, blinded raters, quantitative endpoints, and independent replication are essential.
9.2. Clinical and Translational Directions
Clinical PNK, represents a translational framework for diagnosing and treating maladaptive motor learning. Future research might explore:
whether binary treatment outcomes generalize across therapeutic modalities (e.g., EMDR, spinal manipulation, acupuncture point stimulation, AK-derived procedures). Interchangeability reinforces the likelihood that there is a single underlying cause for NOw.
how training protocols can best prepare clinicians to reliably identify and address NOw. FM/T feedback may help trainees master the principles of MMT by objectively demonstrating test dynamics. This may help trainees to improve control, allowing them to establish techniques appropriate for different clinical contexts and various patient responses.
the role of the cerebellum and related neural networks in establishing, maintaining, and eliminating trauma-linked motor memories.
whether resolving biomechanical compromise associated with muscle inhibition influences conditions like kinesiophobia and functional weakness.
Until such studies are conducted, claims regarding causation or therapeutic efficacy should remain provisional. The priority of this paper is to offer a coherent conceptual model, generate testable hypotheses, and invite systematic investigation rather than to provide definitive clinical guidance or scientific certainty. As a baseline, however, interventions reported to normalize muscle function are already well established — though not widely established — in clinical practice.
At the very least, we hope this paper informs the broader medical community about the serious risks to musculoskeletal health posed by persistent NOw, its ubiquity across diverse conditions, and the potential of approaches that may restore normal function. Most importantly, we hope that patients suffering from musculoskeletal complaints gain greater access to treatments capable of restoring muscle function, thereby improving their chances of meaningful recovery.
Funding
This research received no external funding
Conflicts of Interest
The author has, for over 40 years, studied and applied some of the techniques described in this paper in private clinical practice.
Abbreviations
The following abbreviations are used in this manuscript:
| AInf |
Active inference |
| AK |
Applied Kinesiology |
| AMI |
Arthrogenic muscle inhibition |
CK
CNS |
Clinical Kinesiology
Central nervous system |
| CR |
Conditioned response |
| CS |
Conditioned stimulus |
| EMDR |
Eye Movement Desensitization and Reprocessing |
| EMG |
Electromyography |
| FEP |
Free energy principle |
| FM/T |
Force and Motion over Time |
| FMD |
Functional motor disorder |
| FND |
Functional neurologic disorder |
| FW |
Functional weakness |
| HHD |
Handheld dynamometry |
| HR |
High resolution |
| MCE |
Motor control exercise |
| MMT |
Manual muscle testing |
| motorPPC |
Motor prediction, planning, and control |
| mPTSD |
Muscle-motor-movement post-traumatic stress disorder |
| MVC/MVIC |
Maximum voluntary (isometric) contraction |
| nsMSD |
Non-specific musculoskeletal disorder |
| PAG |
Periaqueductal grey |
| PNK |
Psychoneurokinesiology |
| PP |
Predictive processing |
| PTSD |
Post-traumatic stress disorder |
| RL |
Reinforcement learning |
| TENS |
Transcutaneous electrical nerve stimulation |
| TMS |
Transcranial magnetic stimulation |
| TPJ |
Temporo-parietal junction |
| US |
Unconditioned stimulus |
References
- Balagué, F.; Mannion, A.F.; Pellisé, F.; Cedraschi, C. Non-specific low back pain. The Lancet 2012, 379, 482–491. [Google Scholar] [CrossRef]
- Fougeront, N.; Fleiter, B. Temporomandibular disorder and comorbid neck pain: Facts and hypotheses regarding pain-induced and rehabilitation-induced motor activity changes. Can. J. Physiol. Pharmacol. 2018, 96. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, J.R. Can testing of six individual muscles represent a screening approach to upper limb neuropathic conditions? BMC Neurol. 2014, 14, 90. [Google Scholar] [CrossRef] [PubMed]
- Maher, C.; Underwood, M.; Buchbinder, R. Non-specific low back pain. The Lancet 2017, 389, 736–747. [Google Scholar] [CrossRef]
- Moradi, A.; Ebrahimzadeh, M.H.; Ring, D. Nonspecific Arm Pain. Arch. Bone Jt. Surg. 2013, 1, 53–58. [Google Scholar]
- Oliveira, C.B.; Maher, C.G.; Pinto, R.Z.; Traeger, A.C.; Lin, C.-W.C.; Chenot, J.-F.; van Tulder, M.; Koes, B.W. Clinical practice guidelines for the management of non-specific low back pain in primary care: an updated overview. Eur. Spine J. 2018, 27, 2791–2803. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, P. It’s time for change with the management of non-specific chronic low back pain. Br. J. Sports Med. 2012, 46, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.R.; Villalobos, A. Evaluation and management of knee pain in young athletes: overuse injuries of the knee. Transl. Pediatr. 2017, 6, 190–198. [Google Scholar] [CrossRef]
- O’Sullivan, P. Diagnosis and classification of chronic low back pain disorders: maladaptive movement and motor control impairments as underlying mechanism. Man. Ther. 2005, 10, 242–255. [Google Scholar] [CrossRef]
- Casser, H.-R.; Seddigh, S.; Rauschmann, M. Acute Lumbar Back Pain. Dtsch. Ärztebl. Int. 2016, 113, 223–234. [Google Scholar] [CrossRef]
- Meier, M.L.; Vrana, A.; Schweinhardt, P. Low Back Pain: The Potential Contribution of Supraspinal Motor Control and Proprioception. The Neuroscientist 2019, 25, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Schmid, S.; Bangerter, C.; Schweinhardt, P.; Meier, M.L. Identifying Motor Control Strategies and Their Role in Low Back Pain: A Cross-Disciplinary Approach Bridging Neurosciences With Movement Biomechanics. Front. Pain Res. 2021, 2. [Google Scholar] [CrossRef]
- Szeto, G.P.Y.; Straker, L.M.; O’Sullivan, P.B. A comparison of symptomatic and asymptomatic office workers performing monotonous keyboard work—1: Neck and shoulder muscle recruitment patterns. Man. Ther. 2005, 10, 270–280. [Google Scholar] [CrossRef]
- OSHwiki Pathophysiological mechanisms of musculoskeletal disorders; European Agency for Safety and Health at Work, 2020.
- Falla, D.; Bilenkij, G.; Jull, G. Patients With Chronic Neck Pain Demonstrate Altered Patterns of Muscle Activation During Performance of a Functional Upper Limb Task. Spine 2004, 29, 1436. [Google Scholar] [CrossRef]
- Szeto, G.P.Y.; Lin, J.K.M. A study of forearm muscle activity and wrist kinematics in symptomatic office workers performing mouse-clicking tasks with different precision and speed demands. J. Electromyogr. Kinesiol. 2011, 21, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Hodges, P.W. Motor control and pain. In Mechanisms and Management of Pain for the Physical Therapist; Sluka, K., Ed.; Philadelphia, PA: Wolters Kluwer Health, 2016. [Google Scholar]
- Russo, M.; Deckers, K.; Eldabe, S.; Kiesel, K.; Gilligan, C.; Vieceli, J.; Crosby, P. Muscle Control and Non-specific Chronic Low Back Pain. Neuromodulation J. Int. Neuromodulation Soc. 2018, 21, 1–9. [Google Scholar] [CrossRef]
- Sullivan, M.J. Toward a biopsychomotor conceptualization of pain: implications for research and intervention. Clin. J. Pain 2008, 24, 281–290. [Google Scholar] [CrossRef]
- Dankaerts, W.; O’Sullivan, P.; Burnett, A.; Straker, L. Altered Patterns of Superficial Trunk Muscle Activation During Sitting in Nonspecific Chronic Low Back Pain Patients: Importance of Subclassification. Spine 2006, 31, 2017–2023. [Google Scholar] [CrossRef]
- Hides, J.A.; Richardson, C.A.; Jull, G.A. Multifidus Muscle Recovery Is Not Automatic After Resolution of Acute, First-Episode Low Back Pain. Spine 1996, 21, 2763–2769. [Google Scholar] [CrossRef]
- MacDonald, D.; Moseley, G.L.; Hodges, P.W. Why do some patients keep hurting their back? Evidence of ongoing back muscle dysfunction during remission from recurrent back pain. PAIN® 2009, 142, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Werner, S. Anterior knee pain: an update of physical therapy. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2014, 22, 2286–2294. [Google Scholar] [CrossRef] [PubMed]
- Hodges, P.W.; Richardson, C.A. Delayed postural contraction of transversus abdominis in low back pain associated with movement of the lower limb. J. Spinal Disord. 1998, 11, 46–56. [Google Scholar] [CrossRef]
- Prushansky, T.; Gepstein, R.; Gordon, C.; Dvir, Z. Cervical muscles weakness in chronic whiplash patients. Clin. Biomech. Bristol Avon 2005, 20, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Barton, C.J.; Lack, S.; Malliaras, P.; Morrissey, D. Gluteal muscle activity and patellofemoral pain syndrome: a systematic review. Br. J. Sports Med. 2013, 47, 207–214. [Google Scholar] [CrossRef]
- Sherman, D.A.; Rush, J.; Glaviano, N.R.; Norte, G.E. Knee joint pathology and efferent pathway dysfunction: Mapping muscle inhibition from motor cortex to muscle force. Musculoskelet. Sci. Pract. 2024, 74, 103204. [Google Scholar] [CrossRef]
- Palmieri-Smith, R.M.; Hopkins, J.T.; Brown, T.N. Peroneal activation deficits in persons with functional ankle instability. Am. J. Sports Med. 2009, 37, 982–988. [Google Scholar] [CrossRef]
- Dong, S.; Liu, Y.; Liu, Z.; Shen, P.; Sun, H.; Zhang, P.; Fong, D.T.; Song, Q. Can Arthrogenic Muscle Inhibition Exist in Peroneal Muscles Among People with Chronic Ankle Instability? A Cross-sectional Study. Sports Med. - Open 2024, 10, 35. [Google Scholar] [CrossRef]
- Lee, J.H.; Jung, H.W.; Jang, W.Y. A prospective study of the muscle strength and reaction time of the quadriceps, hamstring, and gastrocnemius muscles in patients with plantar fasciitis. BMC Musculoskelet. Disord. 2020, 21, 722. [Google Scholar] [CrossRef]
- Cuthbert, S.; Blum, C.; Rosner, A. The Association of Manual Muscle Tests and Treatment Outcomes With Headache and Cranial Dysfunctions: A Retrospective Case Series Report. Altern. Ther. Health Med. 2018, 24, 8–21. [Google Scholar]
- Wilder, R.P.; Sethi, S. Overuse injuries: tendinopathies, stress fractures, compartment syndrome, and shin splints. Clin. Sports Med. 2004, 23, 55–81. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L.V.; Dech, S.; Wolff, L.L.; Bittmann, F.N. Emotional Imagery Influences the Adaptive Force in Young Women: Unpleasant Imagery Reduces Instantaneously the Muscular Holding Capacity. Brain Sci. 2022, 12, 1318. [Google Scholar] [CrossRef]
- Hopkins, J.T.; Ingersoll, C.D. Arthrogenic muscle inhibition: a limiting factor in joint rehabilitation. J. Sport Rehabil. 2000, 9, 135–159. [Google Scholar] [CrossRef]
- Norte, G.; Rush, J.; Sherman, D. Arthrogenic Muscle Inhibition: Best Evidence, Mechanisms, and Theory for Treating the Unseen in Clinical Rehabilitation. J. Sport Rehabil. 2021, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hurley, M.V.; Jones, D.W.; Newham, D.J. Arthrogenic quadriceps inhibition and rehabilitation of patients with extensive traumatic knee injuries. Clin. Sci. 1994, 86, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, W.H.; Yanuck, S.F. Expanding the neurological examination using functional neurologic assessment: part II neurologic basis of applied kinesiology. Int. J. Neurosci. 1999, 97, 77–108. [Google Scholar] [CrossRef]
- Belli, R. Applied Kinesiology and the Motor Neuron | Dynamic Chiropractic. Available online: https://dynamicchiropractic.com/article/9140-applied-kinesiology-and-the-motor-neuron (accessed on July 13, 2025).
- Walther, D.S. Applied Kinesiology Synopsis 2nd Edition; Systems DC Pueblo, CO, 2000.
- Stokes, M.; Young, A. The contribution of reflex inhibition to arthrogenous muscle weakness. Clin. Sci. 1984, 67, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.; Dixon, A.S.J. Joint distension and reflex muscle inhibition in the knee. JBJS 1965, 47, 313–322. [Google Scholar]
- Fahrer, H.; Rentsch, H.U.; Gerber, N.J.; Beyeler, C.; Hess, C.W.; Grunig, B. Knee effusion and reflex inhibition of the quadriceps. A bar to effective retraining. J. Bone Joint Surg. Br.
- Lepley, A.S.; Lepley, L.K. Mechanisms of Arthrogenic Muscle Inhibition. J. Sport Rehabil. 2021, 1, 1–10. [Google Scholar] [CrossRef]
- Rice, D.A.; Graven-Nielsen, T.; Lewis, G.N.; McNair, P.J.; Dalbeth, N. The effects of experimental knee pain on lower limb corticospinal and motor cortex excitability. Arthritis Res. Ther. 2015, 17, 204. [Google Scholar] [CrossRef]
- Criss, C.R.; Lepley, A.S.; Onate, J.A.; Clark, B.C.; Simon, J.E.; France, C.R.; Grooms, D.R. Brain activity associated with quadriceps strength deficits after anterior cruciate ligament reconstruction. Sci. Rep. 2023, 13, 8043. [Google Scholar] [CrossRef]
- Frissora, K.N. Reliability of Measuring Voluntary Quadriceps Activation Using the Burst Superimposition and Interpolated Twitch Techniques. 2014.
- Hart, J.M.; Pietrosimone, B.; Hertel, J.; Ingersoll, C.D. Quadriceps activation following knee injuries: a systematic review. J. Athl. Train. 2010, 45, 87–97. [Google Scholar] [CrossRef]
- Hart, J.M.; Kuenze, C.M.; Pietrosimone, B.G.; Ingersoll, C.D. Quadriceps function in anterior cruciate ligament-deficient knees exercising with transcutaneous electrical nerve stimulation and cryotherapy: a randomized controlled study. Clin. Rehabil. 2012, 26, 974–981. [Google Scholar] [CrossRef]
- Kim, K.-M.; Kim, J.-S.; Needle, A.R. Soleus arthrogenic muscle inhibition following acute lateral ankle sprain correlates with symptoms and ankle disability but not with postural control. J. Sport Health Sci. 2024. [Google Scholar] [CrossRef]
- Patel, H.H.; Berlinberg, E.J.; Nwachukwu, B.; Williams, R.J.; Mandelbaum, B.; Sonkin, K.; Forsythe, B. Quadriceps Weakness is Associated with Neuroplastic Changes Within Specific Corticospinal Pathways and Brain Areas After Anterior Cruciate Ligament Reconstruction: Theoretical Utility of Motor Imagery-Based Brain-Computer Interface Technology for Rehabilitation. Arthrosc. Sports Med. Rehabil. 2022, 5, e207–e216. [Google Scholar] [CrossRef]
- Pietrosimone, B.G.; Hertel, J.; Ingersoll, C.D.; Hart, J.M.; Saliba, S.A. Voluntary quadriceps activation deficits in patients with tibiofemoral osteoarthritis: a meta-analysis. PM R 2011, 3, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Sonnery-Cottet, B.; Saithna, A.; Quelard, B.; Daggett, M.; Borade, A.; Ouanezar, H.; Thaunat, M.; Blakeney, W.G. Arthrogenic muscle inhibition after ACL reconstruction: a scoping review of the efficacy of interventions. Br J Sports Med, 2017. [Google Scholar] [CrossRef]
- Sonnery-Cottet, B.; Hopper, G.P.; Gousopoulos, L.; Vieira, T.D.; Thaunat, M.; Fayard, J.-M.; Freychet, B.; Ouanezar, H.; Cavaignac, E.; Saithna, A. Arthrogenic Muscle Inhibition Following Knee Injury or Surgery: Pathophysiology, Classification, and Treatment. Video J. Sports Med. 2022, 2, 26350254221086295. [Google Scholar] [CrossRef]
- University at Albany Jonathan Wolpaw. Available online: https://www.albany.edu/sph/faculty/jonathan-wolpaw (accessed on Apr 9, 2024).
- Carp, J.S.; Wolpaw, J.R. Motoneuron plasticity underlying operantly conditioned decrease in primate H-reflex. J. Neurophysiol. 1994, 72, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.K.; Wolpaw, J.R. The Simplest Motor Skill: Mechanisms and Applications of Reflex Operant Conditioning. Exerc. Sport Sci. Rev. 2014, 42, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Wolpaw, J.R.; Chen, X.Y. The cerebellum in maintenance of a motor skill: A hierarchy of brain and spinal cord plasticity underlies H-reflex conditioning. Learn. Mem. 2006, 13, 208–215. [Google Scholar] [CrossRef]
- Wolpaw, Jonathan. R. Spinal cord plasticity in acquisition and maintenance of motor skills. Acta Physiol. 2007, 189, 155–169. [Google Scholar] [CrossRef]
- Chen, X.Y.; Wolpaw, J.R. Ablation of cerebellar nuclei prevents H-reflex down-conditioning in rats. Learn. Mem. 2005, 12, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, J.T.; Ingersoll, C.D.; Cordova, M.L.; Edwards, J.E. Intrasession and intersession reliability of the soleus H-reflex in supine and standing positions. Electromyogr. Clin. Neurophysiol. 2000, 40, 89–94. [Google Scholar] [PubMed]
- Ingram, T. Is bilateral quadriceps inhibition in unilateral anterior knee pain attributable to gamma loop dysfunction? masters, Memorial University of Newfoundland, 2014.
- Konishi, Y.; Yoshii, R.; Ingersoll, C.D. Gamma Loop Dysfunction as a Possible Neurophysiological Mechanism of Arthrogenic Muscle Inhibition: A Narrative Review of the Literature. J. Sport Rehabil. 2022, 31, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Verbunt, J.A.; Seelen, H.A.; Vlaeyen, J.W.; Bousema, E.J.; van der Heijden, G.J.; Heuts, P.H.; Knottnerus, J.A. Pain-related factors contributing to muscle inhibition in patients with chronic low back pain: an experimental investigation based on superimposed electrical stimulation. Clin. J. Pain 2005, 21, 232–240. [Google Scholar] [CrossRef]
- Graven-Nielsen, T.; Lund, H.; Arendt-Nielsen, L.; Danneskiold-Samsøe, B.; Bliddal, H. Inhibition of maximal voluntary contraction force by experimental muscle pain: a centrally mediated mechanism. Muscle Nerve 2002, 26, 708–712. [Google Scholar] [CrossRef]
- Le Pera, D.; Graven-Nielsen, T.; Valeriani, M.; Oliviero, A.; Di Lazzaro, V.; Tonali, P.A.; Arendt-Nielsen, L. Inhibition of motor system excitability at cortical and spinal level by tonic muscle pain. Clin. Neurophysiol. 2001, 112, 1633–1641. [Google Scholar] [CrossRef]
- Conroy, V.M.; Murray, J.B.N.; Alexopulos, Q.T.; McCreary, J. Kendall’s Muscles: Testing and Function with Posture and Pain; 6th edition.; Wolters Kluwer Health, 2022.
- Bohannon, R.W. Make tests and break tests of elbow flexor muscle strength. Phys. Ther. 1988, 68, 193–194. [Google Scholar] [CrossRef]
- Schaefer, L.V.; Dech, S.; Bittmann, F.N. Adaptive Force and emotionally related imaginations – preliminary results suggest a reduction of the maximal holding capacity as reaction to disgusting food imagination. Heliyon 2021, 7, e07827. [Google Scholar] [CrossRef]
- Schmitt, W.; Cuthbert, S. Common errors and clinical guidelines for manual muscle testing: Chiropr. Man. Ther. 2008, 16, 16. [Google Scholar] [CrossRef]
- Stratford, P.W.; Balsor, B.E. A comparison of make and break tests using a hand-held dynamometer and the Kin-Com. J. Orthop. Sports Phys. Ther. 1994, 19, 28–32. [Google Scholar] [CrossRef]
- Van der Ploeg, R.J.; Oosterhuis, H.J. The" make/break test" as a diagnostic tool in functional weakness. J. Neurol. Neurosurg. Psychiatry 1991, 54, 248–251. [Google Scholar] [CrossRef]
- Roberts, T.J.; Azizi, E. The series-elastic shock absorber: tendons attenuate muscle power during eccentric actions. J. Appl. Physiol. 2010, 109, 396–404. [Google Scholar] [CrossRef]
- Darnall, B.D.; Carr, D.B.; Schatman, M.E. Pain Psychology and the Biopsychosocial Model of Pain Treatment: Ethical Imperatives and Social Responsibility. Pain Med. 2017, 18, 1413–1415. [Google Scholar] [CrossRef]
- Sarno, J.E. The Divided Mind: The Epidemic of Mindbody Disorders; 1 edition.; Harper Perennial: New York, 2007; ISBN 978-0-06-117430-8. [Google Scholar]
- Karartı, C.; Basat, H.Ç.; Özsoy, İ.; Özyurt, F.; Özsoy, G.; Kodak, M.İ.; Özüdoğru, A.; Uçar, İ. Biopsychosocial Approach in Identifying Risk Factors of Kinesiophobia in Persons with Subacromial Pain Syndrome and Developing a Clinical Prediction Tool. Indian J. Orthop. 2022, 57, 124–136. [Google Scholar] [CrossRef]
- Saxena, A.; Godena, E.; Maggio, J.; Perez, D.L. Towards an Outpatient Model of Care for Motor Functional Neurological Disorders: A Neuropsychiatric Perspective. Neuropsychiatr. Dis. Treat. 2020, 16, 2119–2134. [Google Scholar] [CrossRef] [PubMed]
- Torres-Oviedo, G.; Ting, L.H. Subject-Specific Muscle Synergies in Human Balance Control Are Consistent Across Different Biomechanical Contexts. J. Neurophysiol. 2010, 103, 3084–3098. [Google Scholar] [CrossRef] [PubMed]
- Scheer, C.; Kubowitsch, S.; Dendorfer, S.; Jansen, P. Happy Enough to Relax? How Positive and Negative Emotions Activate Different Muscular Regions in the Back - an Explorative Study. Front. Psychol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Huis in ‘t Veld, E.M.J.; Van Boxtel, G.J.M.; de Gelder, B. The Body Action Coding System I: Muscle activations during the perception and expression of emotion. Soc. Neurosci. 2014, 9, 249–264. [Google Scholar] [CrossRef]
- Gabel, C.P.; Guy, B.; Mokhtarinia, H.R.; Melloh, M. Slacklining: A narrative review on the origins, neuromechanical models and therapeutic use. World J. Orthop. 2021, 12, 360–375. [Google Scholar] [CrossRef] [PubMed]
- Dehne, E. The Spinal Adaptation Syndrome (A Theory Based on the Study of Sprains). Clin. Orthop. Relat. Res. 1955, 5, 211–220. [Google Scholar]
- Hodges, P.W.; Moseley, G.L. Pain and motor control of the lumbopelvic region: effect and possible mechanisms. J. Electromyogr. Kinesiol. 2003, 13, 361–370. [Google Scholar] [CrossRef]
- Neige, C.; Mavromatis, N.; Gagné, M.; Bouyer, L.J.; Mercier, C. Effect of movement-related pain on behaviour and corticospinal excitability changes associated with arm movement preparation. J. Physiol. 2018, 596, 2917–2929. [Google Scholar] [CrossRef]
- Hodges, P.W.; Tucker, K. Moving differently in pain: a new theory to explain the adaptation to pain. Pain 2011, 152, S90–S98. [Google Scholar] [CrossRef] [PubMed]
- Summers, S.J.; Chipchase, L.S.; Hirata, R.; Graven-Nielsen, T.; Cavaleri, R.; Schabrun, S.M. Motor adaptation varies between individuals in the transition to sustained pain. PAIN 2019, 160, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Weissfeld, R.K. Mind, trauma & muscle inhibition Part I: Experiment and case history yield novel theory of muscular PTSD [Hypothesis]. 2021, 2. 2.
- Ito, M. Bases and implications of learning in the cerebellum—adaptive control and internal model mechanism. Prog. Brain Res. 2005, 148, 95–109. [Google Scholar] [PubMed]
- Butz, M.V.; Sigaud, O.; Gerard, P. Internal models and anticipations in adaptive learning systems. In Anticipatory behavior in adaptive learning systems; Springer, 2003; pp. 86–109.
- Kawato, M.; Wolpert, D. Internal models for motor control. Novartis Found. Symp. 1998, 218, 291–304. [Google Scholar] [CrossRef]
- Grant, M.J.; Booth, A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Inf. Libr. J. 2009, 26, 91–108. [Google Scholar] [CrossRef]
- Whittemore, R.; Knafl, K. The integrative review: updated methodology. J. Adv. Nurs. 2005, 52, 546–553. [Google Scholar] [CrossRef]
- Mense, S.; Gerwin, R.D. (Eds.) Muscle Pain: Diagnosis and Treatment. 2010 edition. Heidelberg; New York: Springer; 2010.
- Simons, D.G.; Travell, J.G.; Simons, L.S. Myofascial Pain and Dysfunction 1: The Trigger Point Manual. Upper Half of Body; Lippincott Williams & Wilkins, 1999. ISBN 978-0-683-08363-7.
- Dunning, J.; Rushton, A. The effects of cervical high-velocity low-amplitude thrust manipulation on resting electromyographic activity of the biceps brachii muscle. Man. Ther. 2009, 14, 508–513. [Google Scholar] [CrossRef]
- Hillermann, B.; Gomes, A.N.; Korporaal, C.; Jackson, D. A pilot study comparing the effects of spinal manipulative therapy with those of extra-spinal manipulative therapy on quadriceps muscle strength. J. Manipulative Physiol. Ther. 2006, 29, 145–149. [Google Scholar] [CrossRef]
- Morgan, B. The effects of sacroiliac manipulation on arthrogenic muscle inhibition in the hip musculature in patients with sacroiliac syndrome, 2005.
- Shambaugh, P. Changes in electrical activity in muscles resulting from chiropractic adjustment: a pilot study. J. Manipulative Physiol. Ther. 1987, 10, 300. [Google Scholar] [PubMed]
- Suter, E.; McMorland, G.; Herzog, W.; Bray, R. Conservative lower back treatment reduces inhibition in knee-extensor muscles: a randomized controlled trial. J. Manipulative Physiol. Ther. 2000, 23, 76–80. [Google Scholar] [CrossRef]
- Gin, R.; Green, B. George Goodheart, Jr., D.C., and a history of applied kinesiology. J. Manipulative Physiol. Ther. 1997, 20, 331–337. [Google Scholar]
- Frost, R. Applied kinesiology: a training manual and reference book of basic principles and practices; North Atlantic Books: Berkeley, Calif, 2013; ISBN 978-1-58394-612-1. [Google Scholar]
- Lund, J.P.; Donga, R.; Widmer, C.G.; Stohler, C.S. The pain-adaptation model: a discussion of the relationship between chronic musculoskeletal pain and motor activity. Can. J. Physiol. Pharmacol. 1991, 69, 683–694. [Google Scholar] [CrossRef]
- Nijs, J.; Daenen, L.; Cras, P.; Struyf, F.; Roussel, N.; Oostendorp, R.A.B. Nociception affects motor output: a review on sensory-motor interaction with focus on clinical implications. Clin. J. Pain 2012, 28, 175–181. [Google Scholar] [CrossRef]
- Merkle, S.L.; Sluka, K.A.; Frey-Law, L.A. The interaction between pain and movement. J. Hand Ther. Off. J. Am. Soc. Hand Ther. 2020, 33, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Peck, C.; Murray, G.; Gerzina, T. How does pain affect jaw muscle activity? The Integrated Pain Adaptation Model. Aust. Dent. J. 2008, 53, 201–207. [Google Scholar] [CrossRef]
- Sutter, B.A.; Radke, J. Vicious Cycle Theory vs Pain Adaptation Model: A Structured Critical Analysis with 2 Case Presentations. Adv. Dent. Technol. Tech. 2022, 29–46. [Google Scholar]
- Hodges, P.W. Pain and motor control: from the laboratory to rehabilitation. J. Electromyogr. Kinesiol. 2011, 21, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Neumann, O.; Klotz, W. Motor responses to nonreportable, masked stimuli: Where is the limit of direct parameter specification. In Attention and performance 15: Conscious and nonconscious information processing; Attention and performance series; The MIT Press: Cambridge, MA, US, 1994; pp. 123–150. ISBN 978-0-262-21012-6. [Google Scholar]
- Dehaene, S.; Naccache, L.; Le Clec’H, G.; Koechlin, E.; Mueller, M.; Dehaene-Lambertz, G.; van de Moortele, P.F.; Le Bihan, D. Imaging unconscious semantic priming. Nature 1998, 395, 597–600. [Google Scholar] [CrossRef]
- Eimer, M.; Schlaghecken, F. Response facilitation and inhibition in subliminal priming. Biol. Psychol. 2003, 64, 7–26. [Google Scholar] [CrossRef]
- Sumner, P. Negative and positive masked-priming - implications for motor inhibition. Adv. Cogn. Psychol. 2008, 3, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Cowan, S.M.; Schache, A.G.; Brukner, P.; Bennell, K.L.; Hodges, P.W.; Coburn, P.; Crossley, K.M. Delayed onset of transversus abdominus in long-standing groin pain. Med. Sci. Sports Exerc. 2004, 36, 2040–2045. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.G.; Kirkwood, R.N.; Oliveira, G.M. Clinimetric Properties of the Applied Kinesiology Manual Muscle Test in Adults With and Without Pain: A Methodological Study. J. Chiropr. Med. 2022. [Google Scholar] [CrossRef]
- Caruso, W.; Leisman, G. A Force/Displacement Analysis of Muscle Testing. Percept. Mot. Skills 2000, 91, 683–692. [Google Scholar] [CrossRef]
- Andersen, O.K.; Sonnenborg, F.A.; Arendt-Nielsen, L. Reflex receptive fields for human withdrawal reflexes elicited by non-painful and painful electrical stimulation of the foot sole. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2001, 112, 641–649. [Google Scholar] [CrossRef]
- Dimitrova, A.; Kolb, F.P.; Elles, H.G.; Maschke, M.; Forsting, M.; Diener, H.C.; Timmann, D. Cerebellar Responses Evoked by Nociceptive Leg Withdrawal Reflex as Revealed by Event-Related fMRI. J. Neurophysiol. 2003, 90, 1877–1886. [Google Scholar] [CrossRef]
- Jarrell, F.; Jacks, A.; Faubert, J. An introduction to Spinal Reflex Analysis. J. Aust. Assoc. Massage Ther. 2009, 7, 6–11. [Google Scholar]
- Ogden, P.; Minton, K.; Pain, C.; Siegel, D.J.; van der Kolk, B.A. Trauma and the Body: A Sensorimotor Approach to Psychotherapy; 1 edition.; W. W. Norton & Company: New York, 2006; ISBN 978-0-393-70457-0. [Google Scholar]
- Levine, P.A. Trauma and memory: Brain and body in a search for the living past: A practical guide for understanding and working with traumatic memory; North Atlantic Books, 2015.
- Payne, P.; Levine, P.A.; Crane-Godreau, M.A. Somatic experiencing: using interoception and proprioception as core elements of trauma therapy. Front. Psychol. 2015, 6, 93. [Google Scholar] [CrossRef]
- van der Kolk, B.A. The Body Keeps the Score: Brain, Mind, and Body in the Healing of Trauma; Reprint edition.; Penguin Books: New York, NY, 2015; ISBN 978-0-14-312774-1. [Google Scholar]
- Binder MD, Hirokawa N, Windhorst U, editors. Sherrington’s Law of Reciprocal Innervation. In: Encyclopedia of Neuroscience. Springer Berlin Heidelberg; 2009. p. 3687–3687.
- Capaday, C. The special nature of human walking and its neural control. Trends Neurosci. 2002, 25, 370–376. [Google Scholar] [CrossRef]
- Geminiani, A.; Mockevičius, A.; D’Angelo, E.; Casellato, C. Cerebellum Involvement in Dystonia During Associative Motor Learning: Insights From a Data-Driven Spiking Network Model. Front. Syst. Neurosci. 2022, 16. [Google Scholar] [CrossRef]
- Proske, U.; Gandevia, S.C. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol. Rev. 2012, 92, 1651–1697. [Google Scholar] [CrossRef]
- Bittmann, F.N.; Dech, S.; Schaefer, L.V. Another Way to Confuse Motor Control: Manual Technique Supposed to Shorten Muscle Spindles Reduces the Muscular Holding Stability in the Sense of Adaptive Force in Male Soccer Players. Brain Sci. 2023, 13, 1105. [Google Scholar] [CrossRef]
- Klaassen, F.H.; Held, L.; Figner, B.; O’Reilly, J.X.; Klumpers, F.; de Voogd, L.D.; Roelofs, K. Defensive freezing and its relation to approach–avoidance decision-making under threat. Sci. Rep. 2021, 11, 12030. [Google Scholar] [CrossRef]
- Kozlowska, K.; Walker, P.; McLean, L.; Carrive, P. Fear and the Defense Cascade: Clinical Implications and Management. Harv. Rev. Psychiatry 2015, 23, 263–287. [Google Scholar] [CrossRef]
- Roelofs, K. Freeze for action: neurobiological mechanisms in animal and human freezing. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.M.; Kuenze, C.M.; Diduch, D.R.; Ingersoll, C.D. Quadriceps Muscle Function After Rehabilitation With Cryotherapy in Patients With Anterior Cruciate Ligament Reconstruction. J. Athl. Train. 2014, 49, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Hurley, M.V. The effects of joint damage on muscle function, proprioception and rehabilitation. Man. Ther. 1997, 2, 11–17. [Google Scholar] [CrossRef]
- McVey, E.D.; Palmieri, R.M.; Docherty, C.L.; Zinder, S.M.; Ingersoll, C.D. Arthrogenic muscle inhibition in the leg muscles of subjects exhibiting functional ankle instability. Foot Ankle Int. 2005, 26, 1055. [Google Scholar] [CrossRef] [PubMed]
- Needle, A.R.; Lepley, A.S.; Grooms, D.R. Central nervous system adaptation after ligamentous injury: a summary of theories, evidence, and clinical interpretation. Sports Med. 2017, 47, 1271–1288. [Google Scholar] [CrossRef]
- Park, J.; Grindstaff, T.L.; Hart, J.M.; Hertel, J.N.; Ingersoll, C.D. Knee-Extension Exercise’s Lack of Immediate Effect on Maximal Voluntary Quadriceps Torque and Activation in Individuals with Anterior Knee Pain. J. Sport Rehabil. 2012, 21, 119–126. [Google Scholar] [CrossRef]
- Makkuva, A.; Surve, P.; Makkuva, A.; Surve, P. Exploring physiotherapy strategies in arthrogenic muscle inhibition: A scoping review. Int. J. Sci. Res. Arch. 2024, 12, 2609–2620. [Google Scholar] [CrossRef]
- Wyss, J. Therapeutic Programs for Musculoskeletal Disorders; Demos Medical Publishing, 2012. ISBN 978-1-61705-079-4.
- Rice, D.A.; McNair, P.J.; Lewis, G.N. Mechanisms of quadriceps muscle weakness in knee joint osteoarthritis: the effects of prolonged vibration on torque and muscle activation in osteoarthritic and healthy control subjects. Arthritis Res. Ther. 2011, 13, R151. [Google Scholar] [CrossRef]
- Rush, J.L.; Sherman, D.A.; Bazett-Jones, D.M.; Ingersoll, C.D.; Norte, G.E. Understanding Athletic Trainers’ Knowledge, Intervention, and Barriers Toward Arthrogenic Muscle Inhibition. J. Sport Rehabil. 2021, 1, 1–9. [Google Scholar] [CrossRef]
- Hopkins, J.T.; Ingersoll, C.D.; Edwards, J.; Klootwyk, T.E. Cryotherapy and Transcutaneous Electric Neuromuscular Stimulation Decrease Arthrogenic Muscle Inhibition of the Vastus Medialis After Knee Joint Effusion. J. Athl. Train. 2002, 37, 25–31. [Google Scholar]
- Tayfur, B.; Charuphongsa, C.; Morrissey, D.; Miller, S.C. Neuromuscular Function of the Knee Joint Following Knee Injuries: Does It Ever Get Back to Normal? A Systematic Review with Meta-Analyses. Sports Med. Auckl. Nz 2020, 51, 321. [Google Scholar] [CrossRef]
- Capin, J.J.; Bade, M.J.; Jennings, J.M.; Snyder-Mackler, L.; Stevens-Lapsley, J.E. Total Knee Arthroplasty Assessments Should Include Strength and Performance-Based Functional Tests to Complement Range-of-Motion and Patient-Reported Outcome Measures. Phys. Ther. 2022, 102, pzac033. [Google Scholar] [CrossRef]
- Lepley, A.S.; Ericksen, H.M.; Sohn, D.H.; Pietrosimone, B.G. Contributions of neural excitability and voluntary activation to quadriceps muscle strength following anterior cruciate ligament reconstruction. The Knee 2014, 21, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Gabler, C.; Kitzman, P.H.; Mattacola, C.G. Targeting quadriceps inhibition with electromyographic biofeedback: a neuroplastic approach. Crit. Rev. Biomed. Eng. 2013, 41, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Pietrosimone, B.; McLeod, M.M.; Florea, D.; Gribble, P.A.; Tevald, M.A. Immediate increases in quadriceps corticomotor excitability during an electromyography biofeedback intervention. J. Electromyogr. Kinesiol. Off. J. Int. Soc. Electrophysiol. Kinesiol. 2015, 25, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Richaud, T.; Lacaze, K.; Fassio, A.; Nicolas, P.; Ourliac, M.; Hennart, B.; Ina, J.G.; Sonnery-Cottet, B.; Cavaignac, E. How Biofeedback With Surface EMG Can Contribute to the Diagnosis and Treatment of AMI in the Knee. Video J. Sports Med. 2024, 4, 26350254241241084. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.Y.; Jakeman, L.B.; Schalk, G.; Stokes, B.T.; Wolpaw, J.R. The Interaction of a New Motor Skill and an Old One: H-Reflex Conditioning and Locomotion in Rats. J. Neurosci. 2005, 25, 6898–6906. [Google Scholar] [CrossRef]
- Alizadehkhaiyat, O.; Fisher, A.C.; Kemp, G.J.; Vishwanathan, K.; Frostick, S.P. Assessment of functional recovery in tennis elbow. J. Electromyogr. Kinesiol. 2009, 19, 631–638. [Google Scholar] [CrossRef]
- Izadi, M.; Franklin, S.; Bellafiore, M.; Franklin, D.W. Motor Learning in Response to Different Experimental Pain Models Among Healthy Individuals: A Systematic Review. Front. Hum. Neurosci. 2022, 16, 863741. [Google Scholar] [CrossRef] [PubMed]
- Hurley, M.V. The role of muscle weakness in the pathogenesis of osteoarthritis. Rheum. Dis. Clin. N. Am. 1999, 25, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Pietrosimone, B.; Blackburn, J.T.; Harkey, M.S.; Luc, B.A.; Pamukoff, D.N.; Hart, J.M. Clinical Strategies for Addressing Muscle Weakness Following Knee Injury. Clin. Sports Med. 2015, 34, 285–300. [Google Scholar] [CrossRef]
- McPherson, A.L.; Schilaty, N.D.; Anderson, S.; Nagai, T.; Bates, N.A. Arthrogenic muscle inhibition after anterior cruciate ligament injury: Injured and uninjured limb recovery over time. Front. Sports Act. Living 2023, 5. [Google Scholar] [CrossRef]
- Palermi, S.; Vittadini, F.; Vecchiato, M.; Corsini, A.; Demeco, A.; Massa, B.; Pedret, C.; Dorigo, A.; Gallo, M.; Pasta, G.; et al. Managing Lower Limb Muscle Reinjuries in Athletes: From Risk Factors to Return-to-Play Strategies. J. Funct. Morphol. Kinesiol. 2023, 8, 155. [Google Scholar] [CrossRef]
- de Sousa, C.S.; de Jesus, F.L.A.; Machado, M.B.; Ferreira, G.; Ayres, I.G.T.; de Aquino, L.M.; Fukuda, T.Y.; Gomes-Neto, M. Lower limb muscle strength in patients with low back pain: a systematic review and meta-analysis. J. Musculoskelet. Neuronal Interact. 2019, 19, 69–78. [Google Scholar]
- Reed-Jones, R.J.; Vallis, L.A. Proprioceptive deficits of the lower limb following anterior cruciate ligament deficiency affect whole body steering control. Exp. Brain Res. Exp. Hirnforsch. Expérimentation Cérébrale 2007, 182, 249–260. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, S.; Falla, D.; Elliott, J.M.; Jull, G. Muscle Dysfunction in Cervical Spine Pain: Implications for Assessment and Management. J. Orthop. Sports Phys. Ther. 2009, 39, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.K.; Cochrane, T. Mobility impairment, muscle imbalance, muscle weakness, scapular asymmetry and shoulder injury in elite volleyball athletes. J. Sports Med. Phys. Fitness 2001, 41, 403. [Google Scholar] [PubMed]
- Schmitt, L.C.; Paterno, M.V.; Hewett, T.E. The Impact of Quadriceps Femoris Strength Asymmetry on Functional Performance at Return to Sport Following Anterior Cruciate Ligament Reconstruction. J. Orthop. Sports Phys. Ther. 2012, 42, 750–759. [Google Scholar] [CrossRef]
- Munn, J.; Sullivan, S.J.; Schneiders, A.G. Evidence of sensorimotor deficits in functional ankle instability: A systematic review with meta-analysis. J. Sci. Med. Sport 2010, 13, 2–12. [Google Scholar] [CrossRef]
- Lewek, M.; Rudolph, K.; Axe, M.; Snyder-Mackler, L. The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Clin. Biomech. 2002, 17, 56–63. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, S.; Jones, A.; Doherty, M. Muscle weakness in osteoarthritis. Curr. Opin. Rheumatol. 1997, 9, 259–262. [Google Scholar] [CrossRef]
- Roos, E.M.; Herzog, W.; Block, J.A.; Bennell, K.L. Muscle weakness, afferent sensory dysfunction and exercise in knee osteoarthritis. Nat. Rev. Rheumatol. 2010, 7, 57–63. [Google Scholar] [CrossRef]
- Dekker, J.; Tola, P.; Aufdemkampe, G.; Winckers, M. Negative affect, pain and disability in osteoarthritis patients: the mediating role of muscle weakness. Behav. Res. Ther. 1993, 31, 203–206. [Google Scholar] [CrossRef]
- Dubois, J.-D.; Abboud, J.; St-Pierre, C.; Piché, M.; Descarreaux, M. Neuromuscular adaptations predict functional disability independently of clinical pain and psychological factors in patients with chronic non-specific low back pain. J. Electromyogr. Kinesiol. 2014, 24, 550–557. [Google Scholar] [CrossRef]
- Roth, R. A Warning Signal to the Body. Dtsch. Ärztebl. Int. 2016, 113, 562–563. [Google Scholar] [CrossRef]
- Pontén, M.; Fust, J.; D’Onofrio, P.; Dorp, R. van; Sunnergård, L.; Ingre, M.; Axelsson, J.; Jensen, K. The pain alarm response - an example of how conscious awareness shapes pain perception. Sci. Rep. 2019, 9, 12478. [Google Scholar] [CrossRef]
- Mischkowski, D.; Palacios-Barrios, E.E.; Banker, L.; Dildine, T.C.; Atlas, L.Y. Pain or nociception? Subjective experience mediates the effects of acute noxious heat on autonomic responses. PAIN 2018, 159, 699–711. [Google Scholar] [CrossRef]
- Cerritelli, F. 1; Esteves, J.E. 2 1 C. -B.H.R.D.; osteojorge@gmail. com 2 Clinical-Based Human Research Department, F.C.C.; osteojorge@gmail.com; Malta ICOM Educational, G. 1071 G. An Enactive–Ecological Model to Guide Patient-Centered Osteopathic Care. 2022, 1092. [Google Scholar] [CrossRef]
- Staud, R. Evidence for shared pain mechanisms in osteoarthritis, low back pain, and fibromyalgia. Curr. Rheumatol. Rep. 2011, 13, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Sakamoto, M.; Endoh, T.; Komiyama, T. Location-specific and task-dependent modulation of cutaneous reflexes in intrinsic human hand muscles. Clin. Neurophysiol. 2006, 117, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Oishi, K.; Kimura, M.; Yasukawa, M.; Yoneda, T.; Maeshima, T. Amplitude reduction of H-reflex during mental movement simulation in elite athletes. Behav. Brain Res. 1994, 62, 55–61. [Google Scholar] [CrossRef]
- Behm, D.; Power, K.; Drinkwater, E. Comparison of interpolation and central activation ratios as measures of muscle inactivation. Muscle Nerve 2001, 24, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, T.; Johnson, C.D.; Nicholas, S.J.; McHugh, M.P. Muscle hypotrophy, not inhibition, is responsible for quadriceps weakness during rehabilitation after anterior cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2019, 27, 573–579. [Google Scholar] [CrossRef]
- Elamvazuthi, I.; Zulkifli, Z.; Ali, Z.; Khan, M.K.A.A.; Parasuraman, S.; Balaji, M.; Chandrasekaran, M. Development of Electromyography Signal Signature for Forearm Muscle. Procedia Comput. Sci. 2015, 76, 229–234. [Google Scholar] [CrossRef]
- Karacan, I.; Türker, K.S. A comparison of electromyography techniques: surface versus intramuscular recording. Eur. J. Appl. Physiol. 2025, 125, 7–23. [Google Scholar] [CrossRef]
- Al-Ayyad, M.; Owida, H.A.; De Fazio, R.; Al-Naami, B.; Visconti, P. Electromyography Monitoring Systems in Rehabilitation: A Review of Clinical Applications, Wearable Devices and Signal Acquisition Methodologies. Electronics 2023, 12, 1520. [Google Scholar] [CrossRef]
- Leisman, G.; Zenhausern, R.; Ferentz, A.; Tefera, T.; Zemcov, A. Electromyographic effects of fatigue and task repetition on the validity of estimates of strong and weak muscles in applied kinesiological muscle-testing procedures. Percept. Mot. Skills 1995, 80, 963–977. [Google Scholar] [CrossRef] [PubMed]
- Avers, D.; Lott, D.J.; Brown, M. Daniels and Worthingham’s Muscle Testing; 11th edition.; Saunders, 2024. ISBN 978-0-323-82420-0.
- Latash, M.L.; Anson, J.G. Synergies in Health and Disease: Relations to Adaptive Changes in Motor Coordination. Phys. Ther. 2006, 86, 1151–1160. [Google Scholar] [CrossRef]
- Latash, M.L. One more time about motor (and non-motor) synergies. Exp. Brain Res. 2021, 239, 2951–2967. [Google Scholar] [CrossRef]
- Falla, D.L.; Jull, G.A.; Hodges, P.W. Patients With Neck Pain Demonstrate Reduced Electromyographic Activity of the Deep Cervical Flexor Muscles During Performance of the Craniocervical Flexion Test. Spine 2004, 29, 2108. [Google Scholar] [CrossRef]
- Hodges, P.W.; Butler, J.; Tucker, K.; MacDonell, C.W.; Poortvliet, P.; Schabrun, S.; Hug, F.; Garland, S.J. Non-uniform Effects of Nociceptive Stimulation to Motoneurones during Experimental Muscle Pain. Neuroscience 2021, 463, 45–56. [Google Scholar] [CrossRef]
- Schilaty, N.D.; McPherson, A.L.; Nagai, T.; Bates, N.A. Arthrogenic muscle inhibition manifests in thigh musculature motor unit characteristics after anterior cruciate ligament injury. Eur. J. Sport Sci. 2023, 23, 840–850. [Google Scholar] [CrossRef]
- Löfvenberg, R.; Kärrholm, J.; Sundelin, G.; Ahlgren, O. Prolonged Reaction Time in Patients with Chronic Lateral Instability of the Ankle. Am. J. Sports Med. 1995, 23, 414–417. [Google Scholar] [CrossRef]
- van Dieën, J.H.; Selen, L.P.J.; Cholewicki, J. Trunk muscle activation in low-back pain patients, an analysis of the literature. J. Electromyogr. Kinesiol. Off. J. Int. Soc. Electrophysiol. Kinesiol. 2003, 13, 333–351. [Google Scholar] [CrossRef]
- Stergiou, N.; Decker, L.M. Human Movement Variability, Nonlinear Dynamics, and Pathology: Is There A Connection? Hum. Mov. Sci. 2011, 30, 869–888. [Google Scholar] [CrossRef] [PubMed]
- Moseley, G.L.; Hodges, P.W. Are the changes in postural control associated with low back pain caused by pain interference? Clin. J. Pain 2005, 21, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Rossettini, G.; Colombi, A.; Carlino, E.; Manoni, M.; Mirandola, M.; Polli, A.; Camerone, E.M.; Testa, M. Unraveling Negative Expectations and Nocebo-Related Effects in Musculoskeletal Pain. Front. Psychol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Merletti, R.; Temporiti, F.; Gatti, R.; Gupta, S.; Sandrini, G.; Serrao, M. Translation of surface electromyography to clinical and motor rehabilitation applications: The need for new clinical figures. Transl. Neurosci. 2023, 14, 20220279. [Google Scholar] [CrossRef] [PubMed]
- Brumagne, S.; Janssens, L.; Janssens, E.; Goddyn, L. Altered postural control in anticipation of postural instability in persons with recurrent low back pain. Gait Posture 2008, 28, 657–662. [Google Scholar] [CrossRef]
- Mientjes, M.I.; Frank, J.S. Balance in chronic low back pain patients compared to healthy people under various conditions in upright standing. Clin. Biomech. Bristol Avon 1999, 14, 710–716. [Google Scholar] [CrossRef]
- Mok, N.W.; Brauer, S.G.; Hodges, P.W. Failure to use movement in postural strategies leads to increased spinal displacement in low back pain. Spine 2007, 32, E537–543. [Google Scholar] [CrossRef]
- Luomajoki, H.; Kool, J.; de Bruin, E.D.; Airaksinen, O. Movement control tests of the low back; evaluation of the difference between patients with low back pain and healthy controls. BMC Musculoskelet. Disord. 2008, 9, 170. [Google Scholar] [CrossRef]
- Revel, M.; Andre-Deshays, C.; Minguet, M. Cervicocephalic kinesthetic sensibility in patients with cervical pain. Arch. Phys. Med. Rehabil. 1991, 72, 288–291. [Google Scholar]
- van Dieën, J.H.; Reeves, N.P.; Kawchuk, G.N.; Van Dillen, L.R.; Hodges, P.W. Analysis of Motor Control in Patients With Low Back Pain: A Key to Personalized Care? J. Orthop. Sports Phys. Ther. 2019, 49, 380–388. [Google Scholar] [CrossRef]
- Mapinduzi, J.; Ndacayisaba, G.; Mahaudens, P.; Hidalgo, B. Effectiveness of motor control exercises versus other musculoskeletal therapies in patients with pelvic girdle pain of sacroiliac joint origin: A systematic review with meta-analysis of randomized controlled trials. J. Back Musculoskelet. Rehabil. 2022, 35, 713–728. [Google Scholar] [CrossRef]
- van Vliet, P.M.; Heneghan, N.R. Motor control and the management of musculoskeletal dysfunction. Man. Ther. 2006, 11, 208–213. [Google Scholar] [CrossRef]
- Sterling, M.; Jull, G.; Wright, A. The effect of musculoskeletal pain on motor activity and control. J. Pain 2001, 2, 135–145. [Google Scholar] [CrossRef]
- van Dieën, J.H.; Reeves, N.P.; Kawchuck, G.; Van Dillen, L.R.; Hodges, P.W. Motor Control Changes in Low Back Pain: Divergence in Presentations and Mechanisms. J. Orthop. Sports Phys. Ther. 2019, 49, 370–379. [Google Scholar] [CrossRef]
- van Dieën, J.H.; Flor, H.; Hodges, P.W. Low-Back Pain Patients Learn to Adapt Motor Behavior With Adverse Secondary Consequences. Exerc. Sport Sci. Rev. 2017, 45, 223–229. [Google Scholar] [CrossRef]
- van Dieën, J.H.; Moseley, G.L.; Hodges, P.W. Chapter 18 - Motor control changes and low back pain: cause or effect? In Spinal Control; Hodges, P.W., Cholewicki, J., van Dieën, J.H., Eds.; Churchill Livingstone, 2013; pp. 207–217. ISBN 978-0-7020-4356-7.
- Chang, J.C.; Perich, M.G.; Miller, L.E.; Gallego, J.A.; Clopath, C. De novo motor learning creates structure in neural activity that shapes adaptation. Nat. Commun. 2024, 15, 4084. [Google Scholar] [CrossRef] [PubMed]
- Bouton, M.E. Learning and Behavior: A Contemporary Synthesis; 1st edition.; Sinauer Associates: Sunderland, Mass, 2006; ISBN 978-0-87893-063-0. [Google Scholar]
- Schabrun, S.M.; Palsson, T.S.; Thapa, T.; Graven-Nielsen, T. Movement Does Not Promote Recovery of Motor Output Following Acute Experimental Muscle Pain. Pain Med. 2018, 19, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Kantak, S.S.; Johnson, T.; Zarzycki, R. Linking Pain and Motor Control: Conceptualization of Movement Deficits in Patients With Painful Conditions. Phys. Ther. 2022, 102, pzab289. [Google Scholar] [CrossRef] [PubMed]
- Solomon, R.L.; Wynne, L.C. Traumatic avoidance learning: Acquisition in normal dogs. Psychol. Monogr. Gen. Appl. 1953, 67, 1–19. [Google Scholar] [CrossRef]
- Glogan, E.; Liu, P.; Meulders, A. Generalization of Costly Pain-Related Avoidance Based on Real-Life Categorical Knowledge. Psychol. Sci. 2023, 09567976231170878. [Google Scholar] [CrossRef]
- Cornwell, B.R.; Overstreet, C.; Krimsky, M.; Grillon, C. Passive avoidance is linked to impaired fear extinction in humans. Learn. Mem. 2013, 20, 164–169. [Google Scholar] [CrossRef]
- Encyclopedia.com Passive (Inhibitory) Avoidance, Fear Learning. Learn. Mem.
- Saragiotto, B.T.; Maher, C.G.; Yamato, T.P.; Costa, L.O.P.; Menezes Costa, L.C.; Ostelo, R.W.J.G.; Macedo, L.G. Motor control exercise for chronic non-specific low-back pain. Cochrane Database Syst. Rev. 2016, CD012004. [Google Scholar] [CrossRef]
- Hug, F.; Hodges, P.W.; Carroll, T.J.; Martino, E.D.; Magnard, J.; Tucker, K. Motor Adaptations to Pain during a Bilateral Plantarflexion Task: Does the Cost of Using the Non-Painful Limb Matter? PLOS ONE 2016, 11, e0154524. [Google Scholar] [CrossRef]
- McCulloch, R.H. Sternalis – an anatomical review and its AK clinical importance. In Experimental Observations of Members of the ICAK; International College of Applied Kinesiology-U.S.A., 2014; pp. 97–102.
- Alikhan, Z.H. Serratus Posterior: Incorporating the Superior and Inferior Division into Professional Applied Kinesiology. In Experimental Observations of Members of the ICAK; International College of Applied Kinesiology-U.S.A., 2024; Vol. V, pp. 449–460.
- Palomar, J. P-DTR Approach to the PiLUS Pattern Dysfunction. In: Proceedings of the Annual Meeting of the International College of Applied Kinesiology-U.S.A. International College of Applied Kinesiology-U.S.A.; 2013. p. 111–6.
- Genest, D.C. , Judith A Sacroiliac Joint Dysfunction: An Applied Kinesiology Approach Treatment. In Proceedings of the Experimental Observations of Members of the ICAK; 2017; Vol. 1; pp. 39–44. [Google Scholar]
- Tolhurst, W.H. Soft Tissue Manipulation of the Appendix Region for Correction of Persistent Bilateral Quadratus Lumborum Muscle Facilitation Error (Weakness). Proc. ICAK - USA.
- Blumenthal, J.P. The Pectineus Muscle as an Indicator of TMJ and Gait Imbalances. Proc. ICAK - USA.
- Cuthbert, S. Applied Kinesiology Past, Present, and Future | The American Chiropractor | JULY 2015. Available online: https://theamericanchiropractor.com/article/2015/7/1/applied-kinesiology-past-present-and-future (accessed on 30 June 2025).
- Collected Proceedings/Template/Guidelines – ICAK-USA. https://icakusa.com/collected-proceedings/. Accessed 29 June 2025.
- Jensen, A.M.; Stevens, R.J.; Burls, A.J. Estimating the accuracy of muscle response testing: two randomised-order blinded studies. BMC Complement. Altern. Med. 2016, 16, 492. [Google Scholar] [CrossRef] [PubMed]
- Conable, K.M.; Rosner, A.L. A narrative review of manual muscle testing and implications for muscle testing research. J. Chiropr. Med. 2011, 10, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Rosner, A.L.; Leisman, G.; Gilchriest, J.; Charles, E.; Keschner, M.G.; Minond, M. Reliability and validity of therapy localization as determined from multiple examiners and instrumentation. Funct. Neurol. Rehabil. Ergon. 2015, 5, 365. [Google Scholar]
- Cuthbert, S.; Goodheart, G.J. On the reliability and validity of manual muscle testing: a literature review. Chiropr. Man. Ther. 2007, 15, 4. [Google Scholar] [CrossRef]
- Leisman, G.; Shambaugh, P.; Ferentz, A.H. Somatosensory evoked potential changes during muscle testing. Int. J. Neurosci. 1989, 45, 143–151. [Google Scholar] [CrossRef]
- Monti, D.A.; Sinnott, J.; Marchese, M.; Kunkel, E.J.; Greeson, J.M. Muscle test comparisons of congruent and incongruent self-referential statements. Percept. Mot. Skills 1999, 88, 1019–1028. [Google Scholar] [CrossRef]
- Hall, H. Applied Kinesiology and Other Chiropractic Delusions | Skeptical Inquirer. 2020. https://skepticalinquirer.org/2020/05/applied-kinesiology-and-other-chiropractic-delusions/. Accessed 29 June 2025.
- Hyman, R. How People Are Fooled by Ideomotor Action. 2003. https://quackwatch.org/related/ideomotor/. Accessed 11 Nov 2020.
- Ingraham, P. Applied Kinesiology is Extreme Chiropractic Quackery. www.PainScience.com. 2015. Available online: https://www.painscience.com/articles/applied-kinesiology.php (accessed on 12 July 2025).
- Haas, M.; Cooperstein, R.; Peterson, D. Disentangling manual muscle testing and Applied Kinesiology: critique and reinterpretation of a literature review. Chiropr. Osteopat. 2007, 15, 11. [Google Scholar] [CrossRef]
- Schwartz, S.A.; Utts, J.; Spottiswoode, S.J.P.; Shade, C.W.; Tully, L.; Morris, W.F.; Nachman, G. A Double-Blind, Randomized Study to Assess the Validity of Applied Kinesiology (AK) as a Diagnostic Tool and as a Nonlocal Proximity Effect. Explore J. Sci. Heal. 2014, 10, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Brennan, S.; Murano, M.; McDonald, S.; McKenzie, J.; Turner, S. Kinesiology protocol (Systematic review of evidence on the clinical effectiveness of kinesiology: a protocol). 2024. [Google Scholar] [CrossRef]
- Jensen, A.M. Estimating the prevalence of use of kinesiology-style manual muscle testing: A survey of educators. Adv. Integr. Med. 2015, 2, 96–102. [Google Scholar] [CrossRef]
- Goodheart, G.J. You’ll Be Better: The Story of Applied Kinesiology 2005.
- Obersteadt ACU H Louis, DC, DIBAK, DCBCN. Migraines – A Case Study, Lessons Learned. In: Experimental Observations of Members of the ICAK. 2014. p. 7–10.
- McBride, D.C. , Adam S. ,.B.S. Resolution of Epigastric Pain & Chronic Hiccups Following Chiropractic Care, Applied Kinesiology (AK) and Related Techniques: A Case Study. In Proceedings of the Experimental Observations of Members of the ICAK; 2023; Vol. 1; pp. 91–107. [Google Scholar]
- Mincey, T. The Real “Great Pretender”- The Iliocecal Valve and Digestive Stasis Secondary to Hypochlohydria - A Case Study. Proc. ICAK - USA.
- Cuthbert, S. What Are You Doing About Muscle Weakness? (Part I). Dynamic Chiropractic 2009.
- Suvvari, T.K. Are case reports valuable? Exploring their role in evidence based medicine and patient care. World J. Clin. Cases 2024, 12, 5452–5455. [Google Scholar] [CrossRef] [PubMed]
- Burns, P.B.; Rohrich, R.J.; Chung, K.C. The Levels of Evidence and their role in Evidence-Based Medicine. Plast. Reconstr. Surg. 2011, 128, 305–310. [Google Scholar] [CrossRef]
- Force, M. The Scientific Foundations of Applied Kinesiology.
- Beardall, A.G. Clinical Kinesiology, Vols. I, II, III, IV, V.; Beardall, DC. Inc., Lake Oswego, OR, 1980.
- Buhler, C.F.; Williams, S. Muscles of the neck; 2013.
- Wilczynski, M.; 20-Year History of Missed Games in the NBA. Weak Side Awareness. 2011. Available online: https://weaksideawareness.wordpress.com/2011/11/28/20-year-history-of-missed-games-in-the-nba/ (accessed on 14 February 2020).
- Weissfeld, R.K. Mind, trauma & muscle inhibition Part II: When muscle inhibition is not ignored. Evidence of effectiveness from the NBA. Asia-Pac. Chiropr. J. 2021, 2. [Google Scholar]
- Buckley, T. Battle on Wounded Knee Doctors are in awe, teammates are amazed, yet Hornacek insists he’s just doing his job. Available online: https://www.deseret.com/2000/4/21/19557822/jeff-hornacek-battle-on-wounded-knee (accessed on Feb 12, 2020).
- Southern Utah University Technology continues to help enhance the health of athletes. Available online: https://www.deseret.com/2010/4/15/20108829/technology-continues-to-help-enhance-the-health-of-athletes (accessed on Dec 26, 2021).
- Jarvis, K.B. Chiropractic and all that Jazz. Dynamic Chiropractic. 19 October 1998.
- Durfee, W.K.; Iaizzo, P.A. Rehabilitation and muscle testing. Encycl. Med. Devices Instrum. 2006. [Google Scholar]
- Nadler, S.F.; DePrince, M.L.; Hauesien, N.; Malanga, G.A.; Stitik, T.P.; Price, E. Portable dynamometer anchoring station for measuring strength of the hip extensors and abductors. Arch. Phys. Med. Rehabil. 2000, 81, 1072–1076. [Google Scholar] [CrossRef]
- Fisher, M.I.; Harrington, S.E. Research Round-up: Manual Muscle Testing. Rehabil. Oncol. 2015, 33, 51. [Google Scholar] [CrossRef]
- Wadsworth, C.T.; Krishnan, R.; Sear, M.; Harrold, J.; Nielsen, D.H. Intrarater Reliability of Manual Muscle Testing and Hand-held Dynametric Muscle Testing. Phys. Ther. 1987, 67, 1342–1347. [Google Scholar] [CrossRef]
- Bohannon, R.W. Reliability of manual muscle testing: A systematic review. Isokinet. Exerc. Sci. 2018, 26, 245–252. [Google Scholar] [CrossRef]
- Paternostro-Sluga, T.; Grim-Stieger, M.; Posch, M.; Schuhfried, O.; Vacariu, G.; Mittermaier, C.; Bittner, C.; Fialka-Moser, V. Reliability and validity of the Medical Research Council (MRC) scale and a modified scale for testing muscle strength in patients with radial palsy. J. Rehabil. Med. 2008, 40, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W. Intertester Reliability Of Hand-Held Dynamometry: A Concise Summary Of Published Research. Percept. Mot. Skills 1999, 88, 899–902. [Google Scholar] [CrossRef]
- Baschung Pfister, P.; de Bruin, E.D.; Sterkele, I.; Maurer, B.; de Bie, R.A.; Knols, R.H. Manual muscle testing and hand-held dynamometry in people with inflammatory myopathy: An intra- and interrater reliability and validity study. PLoS ONE 2018, 13, e0194531. [Google Scholar] [CrossRef]
- Frese, E.; Brown, M.; Norton, B.J. Clinical reliability of manual muscle testing middle trapezius and gluteus medius muscles. Phys. Ther. 1987, 67, 1072–1076. [Google Scholar] [CrossRef] [PubMed]
- Beasley, W.C. Influence of method on estimates of normal knee extensor force among normal and postpolio children. Phys. Ther. Rev. 1956, 36, 21–41. [Google Scholar] [CrossRef]
- Bohannon, R.W. Considerations and Practical Options for Measuring Muscle Strength: A Narrative Review. BioMed Research International 2019, 2019, e8194537. [Google Scholar] [CrossRef] [PubMed]
- Dvir, Z. Grade 4 in manual muscle testing: the problem with submaximal strength assessment. Clin. Rehabil. 1997, 11, 36–41. [Google Scholar] [CrossRef]
- Knepler, C.; Bohannon, R.W. Subjectivity of Forces Associated with Manual-Muscle Test Grades of 3+, 4-, and 4. Percept. Mot. Skills 1998, 87, 1123–1128. [Google Scholar] [CrossRef]
- Bohannon, R.W. Manual muscle testing: does it meet the standards of an adequate screening test? Clin. Rehabil. 2005, 19, 662–667. [Google Scholar] [CrossRef]
- Ward, Z.B. Muscles or Movements? Representation in the Nascent Brain Sciences. J. Hist. Biol. 2023, 56, 5–34. [Google Scholar] [CrossRef]
- Graziano, M.S. Ethological action maps: a paradigm shift for the motor cortex. Trends Cogn. Sci. 2016, 20, 121–132. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Hsu, C.-Y.; Jaiswal, S.; Muggleton, N.G.; Liang, W.-K.; Juan, C.-H. To Go or Not to Go: Degrees of Dynamic Inhibitory Control Revealed by the Function of Grip Force and Early Electrophysiological Indices. Front. Hum. Neurosci. 2021, 15. [Google Scholar] [CrossRef] [PubMed]
- Mayr, S.; Buchner, A.; Dentale, S. Prime retrieval of motor responses in negative priming. J. Exp. Psychol. Hum. Percept. Perform. 2009, 35, 408. [Google Scholar] [CrossRef] [PubMed]
- Ocampo, B.; Painter, D.R.; Kritikos, A. Event coding and motor priming: how attentional modulation may influence binding across action properties. Exp. Brain Res. 2012, 219, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Praamstra, P.; Seiss, E. The neurophysiology of response competition: motor cortex activation and inhibition following subliminal response priming. J. Cogn. Neurosci. 2005, 17, 483–493. [Google Scholar] [CrossRef]
- Riskind, J.H.; Sagliano, L.; Trojano, L.; Conson, M. Dysfunctional Freezing Responses to Approaching Stimuli in Persons with a Looming Cognitive Style for Physical Threats. Front. Psychol. 2016, 7. [Google Scholar] [CrossRef]
- Caruso, W.; Leisman, G. The clinical utility of force/displacement analysis of muscle testing in applied kinesiology. Int. J. Neurosci. 2001, 106, 147–157. [Google Scholar] [CrossRef]
- Gangemi, S.C. A newly discovered muscle-organ relationship: the pectoralis minor and the parotid gland. Collect. Pap. Int. Coll. Appl. Kinesiol. 2006, 2005–2006, 75–77. [Google Scholar]
- Hoffman, J.; Mendel, R. ; Carpenter Evaluation of Muscle-Organ Association, Part II. J Clin Chiro 1977, III, 42–60. [Google Scholar]
- Pollard, H.P.; Bablis, P.; Bonello, R. ; others Commentary: The Ileocecal valve point and muscle testing: A possible mechanism of action. Chiropr. J. Aust. 2006, 36, 122. [Google Scholar]
- Iravani, B.; Schaefer, M.; Wilson, D.A.; Arshamian, A.; Lundström, J.N. The human olfactory bulb processes odor valence representation and cues motor avoidance behavior. Proc. Natl. Acad. Sci. U. S. A. 2021, 118, e2101209118. [Google Scholar] [CrossRef]
- Schaefer, L.V.; Dech, S.; Aehle, M.; Bittmann, F.N. Disgusting odours affect the characteristics of the Adaptive Force in contrast to neutral and pleasant odours. Sci. Rep. 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Price, R.S.; Maples, W.C. Physiological Effects of Low-Plus Lenses: Manual Muscle Testing and Nearpoint Lens Prescription. Available online: https://cdn.ymaws.com/www.covd.org/resource/resmgr/ovd36/93_98_PriceMaples.pdf (accessed on Jan 21, 2025).
- Nicholas, J.A.; Melvin, M.; Saraniti, A.J. Neurophysiologic inhibition of strength following tactile stimulation of the skin. Am. J. Sports Med. 1980, 8, 181–186. [Google Scholar] [CrossRef]
- Levinsson, A.; Holmberg, H.; Broman, J.; Zhang, M.; Schouenborg, J. Spinal Sensorimotor Transformation: Relation between Cutaneous Somatotopy and a Reflex Network. J. Neurosci. 2002, 22, 8170–8182. [Google Scholar] [CrossRef]
- Fallon, J.B.; Bent, L.R.; McNulty, P.A.; Macefield, V.G. Evidence for strong synaptic coupling between single tactile afferents from the sole of the foot and motoneurons supplying leg muscles. J. Neurophysiol. 2005, 94, 3795–3804. [Google Scholar] [CrossRef]
- Novo Navarro, P.; Landin-Romero, R.; Guardiola-Wanden-Berghe, R.; Moreno-Alcázar, A.; Valiente-Gómez, A.; Lupo, W.; García, F.; Fernández, I.; Pérez, V.; Amann, B.L. 25 years of Eye Movement Desensitization and Reprocessing (EMDR): The EMDR therapy protocol, hypotheses of its mechanism of action and a systematic review of its efficacy in the treatment of post-traumatic stress disorder. Rev. Psiquiatr. Salud Ment. 2018, 11, 101–114. [Google Scholar] [CrossRef]
- Schubert, S.J.; Lee, C.W.; Drummond, P.D. The efficacy and psychophysiological correlates of dual-attention tasks in eye movement desensitization and reprocessing (EMDR). J. Anxiety Disord. 2011, 25, 1–11. [Google Scholar] [CrossRef]
- Simpson, E.; Carroll, C.; Sutton, A.; Forsyth, J.; Rayner, A.; Ren, S.; Franklin, M.; Wood, E. Clinical and cost-effectiveness of eye movement desensitization and reprocessing for treatment and prevention of post-traumatic stress disorder in adults: A systematic review and meta-analysis. Br. J. Psychol. n/a. [CrossRef]
- Seok, J.-W.; Kim, J.I. The Efficacy of Eye Movement Desensitization and Reprocessing Treatment for Depression: A Meta-Analysis and Meta-Regression of Randomized Controlled Trials. J. Clin. Med. 2024, 13, 5633. [Google Scholar] [CrossRef] [PubMed]
- Gainer, D.; Alam, S.; Alam, H.; Redding, H. A FLASH OF HOPE: Eye Movement Desensitization and Reprocessing (EMDR) Therapy. Innov. Clin. Neurosci. 2020, 17, 12–20. [Google Scholar] [PubMed]
- Guy-Evans, O. Dangers of EMDR Therapy: Side Effects & Misconceptions 2025. Available online: https://www.simplypsychology.org/dangers-of-emdr-therapy.html (accessed on 26 August 2025).
- Lipscomb, A.; Ashley, W. A Critical Analysis of the Utilization of Eye Movement Desensitization and Reprocessing (EMDR) Psychotherapy with African American Clients. 7.
- Medical Research Council Aids to the examination of the peripheral nervous system. Available online: https://www.ukri.org/publications/aids-to-the-examination-of-the-peripheral-nervous-system/ (accessed on Mar 17, 2023).
- Aitkens, S.; Lord, J.; Bernauer, E.; Fowler, W.M.; Lieberman, J.S.; Berck, P. Relationship of manual muscle testing to objective strength measurements. Muscle Nerve 1989, 12, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.B.; Tucker-Lipscomb, B.; Massey, J.M. A Simple Manual Muscle Test for Myasthenia Gravis. Ann. N. Y. Acad. Sci. 2003, 998, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Andres, P.L.; Thibodeau, L.M.; Finison, L.J.; Munsat, T.L. Quantitative assessment of neuromuscular deficit in ALS. Neurol. Clin. 1987, 5, 125–141. [Google Scholar] [CrossRef]
- Colombo, R.; Mazzini, L.; Mora, G.; Parenzan, R.; Creola, G.; Pirali, I.; Minuco, G. Measurement of isometric muscle strength: a reproducibility study of maximal voluntary contraction in normal subjects and amyotrophic lateral sclerosis patients. Med. Eng. Phys. 2000, 22, 167–174. [Google Scholar] [CrossRef]
- Meldrum, D.; Cahalane, E.; Conroy, R.; Fitzgerald, D.; Hardiman, O. Maximum voluntary isometric contraction: reference values and clinical application. Amyotroph. Lateral Scler. 2007, 8, 47–55. [Google Scholar] [CrossRef]
- Milner-Brown, H.S. Muscle strengthening in a post-polio subject through a high-resistance weight-training program. Arch. Phys. Med. Rehabil. 1993, 74, 1165–1167. [Google Scholar] [CrossRef]
- Fillyaw, M.J.; Tandan, R.; Bradley, W.G. Serial evaluation of neuromuscular function in management of chronic inflammatory demyelinating polyneuropathy. A case report. Phys. Ther. 1987, 67, 1708–1711. [Google Scholar] [CrossRef]
- Paternostro-Sluga, T.; Grim-Stieger, M.; Posch, M.; Schuhfried, O.; Vacariu, G.; Mittermaier, C.; Bittner, C.; Fialka-Moser, V. Reliability and Validity of the Medical Research Council (MRC) Scale and a Modified Scale for Testing Muscle Strength in Patients with Radial Palsy. J. Rehabil. Med. Off. J. UEMS Eur. Board Phys. Rehabil. Med. 2008, 40, 665–671. [Google Scholar] [CrossRef]
- Florence Peterson, K.; McCreary, E.K.; Provance, P.G.; Rodgers, M.M.; Romani, W.A. Muscles: Testing and Function, with Posture and Pain; 5 edition.; LWW: Baltimore, MD, 2005; ISBN 978-0-7817-4780-6. [Google Scholar]
- Rider, L.G.; Koziol, D.; Giannini, E.H.; Jain, M.S.; Smith, M.R.; Whitney-Mahoney, K.; Feldman, B.M.; Wright, S.J.; Lindsley, C.B.; Pachman, L.M.; et al. Validation of Manual Muscle Testing and a Subset of Eight Muscles (MMT8) for Adult and Juvenile Idiopathic Inflammatory Myopathies. Arthritis Care Res. 2010, 62, 465–472. [Google Scholar] [CrossRef]
- Sharrard, W.J. Muscle recovery in poliomyelitis. J. Bone Joint Surg. Br. 79. [CrossRef]
- Shefner, J.M. Strength Testing in Motor Neuron Diseases. Neurotherapeutics 2017, 14, 154–160. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.; Jaszczak, S.L.T.; Steffensen, A.K.S.; Debrabant, B. Using 4+ to grade near-normal muscle strength does not improve agreement. Chiropr. Man. Ther. 2017, 25, 28. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Corrigan, D. A Broad Range of Forces is Encompassed by the Maximum Manual Muscle Test Grade of Five. Percept. Mot. Skills 2000, 90, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Bittmann, F.N.; Dech, S.; Aehle, M.; Schaefer, L.V. Manual Muscle Testing—Force Profiles and Their Reproducibility. Diagnostics 2020, 10. [Google Scholar] [CrossRef]
- Franchi, M.V.; Reeves, N.D.; Narici, M.V. Skeletal Muscle Remodeling in Response to Eccentric vs. Concentric Loading: Morphological, Molecular, and Metabolic Adaptations. Front. Physiol. 2017, 8, 447. [Google Scholar] [CrossRef]
- Linnamo, V.; Moritani, T.; Nicol, C.; Komi, P.V. Motor unit activation patterns during isometric, concentric and eccentric actions at different force levels. J. Electromyogr. Kinesiol. 2003, 13, 93–101. [Google Scholar] [CrossRef]
- Radák, Z. Chapter 2 - Skeletal Muscle, Function, and Muscle Fiber Types. In The Physiology of Physical Training; Radák, Z., Ed.; Academic Press, 2018; pp. 15–31. [CrossRef]
- Schaefer, L.V.; Carnarius, F.; Dech, S.; Bittmann, F.N. Repeated measurements of Adaptive Force: Maximal holding capacity differs from other maximal strength parameters and preliminary characteristics for non-professional strength vs. endurance athletes. Front. Physiol. 2023, 14, 1020954. [Google Scholar] [CrossRef]
- Kay, D.; St Clair Gibson, A.; Mitchell, M.J.; Lambert, M.I.; Noakes, T.D. Different neuromuscular recruitment patterns during eccentric, concentric and isometric contractions. J. Electromyogr. Kinesiol. 2000, 10, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L.V.; Torick, A.H.; Matuschek, H.; Holschneider, M.; Bittmann, F.N. Synchronization of Muscular Oscillations Between Two Subjects During Isometric Interaction. Eur. J. Transl. Myol. 2014, 24, 2237. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L.V.; Bittmann, F.N. Two forms of isometric muscle function: Interpersonal motor task supports a distinction between a holding and a pushing isometric muscle action 2020, 2020. 08.17.25 3633. [CrossRef]
- Welsh, J.P.; Llinás, R. Chapter 26 Some organizing principles for the control of movement based on olivocerebellar physiology. In Progress in Brain Research; C.I. De Zeeuw, P.S. and J.V., Ed.; Elsevier, 1997; Vol. Volume 114, pp. 449–461. ISBN 0079-6123.
- Welsh, J.P.; Lang, E.J.; Suglhara, I.; Llinás, R. Dynamic organization of motor control within the olivocerebellar system. Nature 1995, 374, 453–457. [Google Scholar] [CrossRef]
- Lefler, Y.; Torben-Nielsen, B.; Yarom, Y. Oscillatory activity, phase differences, and phase resetting in the inferior olivary nucleus. Front. Syst. Neurosci. 2013, 7. [Google Scholar] [CrossRef]
- Courtemanche, R.; Robinson, J.C.; Aponte, D.I. Linking oscillations in cerebellar circuits. Front. Neural Circuits 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- Llinás, R.; Yarom, Y. Oscillatory properties of guinea-pig inferior olivary neurones and their pharmacological modulation: an in vitro study. J. Physiol. 1986, 376, 163. [Google Scholar] [CrossRef] [PubMed]
- Loyola, S.; Hoogland, T.M.; Hoedemaker, H.; Romano, V.; Negrello, M.; De Zeeuw, C.I. How inhibitory and excitatory inputs gate output of the inferior olive. eLife 2023, 12, e83239. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, D.A.; Casula, E.P.; Koch, G. The Cerebellum and the Motor Cortex: Multiple Networks Controlling Multiple Aspects of Behavior. The Neuroscientist 2024, 30, 723–743. [Google Scholar] [CrossRef]
- Schubert, C.; Dabbagh, A.; Classen, J.; Krämer, U.M.; Tzvi, E. Alpha oscillations modulate premotor-cerebellar connectivity in motor learning: Insights from transcranial alternating current stimulation. NeuroImage 2021, 241, 118410. [Google Scholar] [CrossRef]
- Lobo, I.; Campagnoli, R.R.; Figueira, J.S.; Andrade, I.; Figueira, I.; Gama, C.; Gonçalves, R.M.; Keil, A.; Pereira, M.G.; Volchan, E.; et al. Hidden wounds of violence: Abnormal motor oscillatory brain activity is related to posttraumatic stress symptoms. NeuroImage 2021, 224, 117404. [Google Scholar] [CrossRef]
- Conable, K.M. Intraexaminer comparison of applied kinesiology manual muscle testing of varying durations: a pilot study. J. Chiropr. Med. 2010, 9, 3–10. [Google Scholar] [CrossRef]
- Caso, M. AK Manual Muscle Testing: As Reliable as the Deep Tendon Reflex? | Dynamic Chiropractic. Available online: https://dynamicchiropractic.com/article/9243-ak-manual-muscle-testing-as-reliable-as-the-deep-tendon-reflex (accessed on July 13, 2025).
- Marshall, G.L.; Little, J.W. Deep Tendon Reflexes: A Study Of Quantitative Methods. J. Spinal Cord Med. 2002, 25, 94–99. [Google Scholar] [CrossRef]
- Türp, J.C.; Minagi, S. Palpation of the lateral pterygoid region in TMD--where is the evidence? J. Dent. 2001, 29, 475–483. [Google Scholar] [CrossRef]
- Rathbone, A.T.L.; Grosman-Rimon, L.; Kumbhare, D.A. Interrater Agreement of Manual Palpation for Identification of Myofascial Trigger Points: A Systematic Review and Meta-Analysis. Clin. J. Pain 2017, 33, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Konieczka, C.; Gibson, C.; Russett, L.; Dlot, L.; MacDermid, J.; Watson, L.; Sadi, J. What is the reliability of clinical measurement tests for humeral head position? A systematic review. J. Hand Ther. Off. J. Am. Soc. Hand Ther. 2017, 30, 420–431. [Google Scholar] [CrossRef]
- Cooperstein, R.; Hickey, M. The reliability of palpating the posterior superior iliac spine: a systematic review. J. Can. Chiropr. Assoc. 2016, 60, 36–46. [Google Scholar]
- Seffinger, M.A.; Najm, W.I.; Mishra, S.I.; Adams, A.; Dickerson, V.M.; Murphy, L.S.; Reinsch, S. Reliability of spinal palpation for diagnosis of back and neck pain: a systematic review of the literature. Spine 2004, 29, E413–E425. [Google Scholar] [CrossRef]
- Hancock, M.J.; Maher, C.G.; Jarvik, J.G.; Battié, M.C.; Elliott, J.M.; Jensen, T.S.; Panagopoulos, J.; Jenkins, H.; Pardey, M.C.; McIntosh, J.; et al. Reliability and validity of subjective radiologist reporting of temporal changes in lumbar spine MRI findings. PM&R 2022, 14, 1325–1332. [Google Scholar] [CrossRef]
- Loeb, M.B.; Carusone, S.B.C.; Marrie, T.J.; Brazil, K.; Krueger, P.; Lohfeld, L.; Simor, A.E.; Walter, S.D. Interobserver Reliability of Radiologists’ Interpretations of Mobile Chest Radiographs for Nursing Home–Acquired Pneumonia. J. Am. Med. Dir. Assoc. 2006, 7, 416–419. [Google Scholar] [CrossRef]
- Mucci, B.; Murray, H.; Downie, A.; Osborne, K. Interrater variation in scoring radiological discrepancies. Br. J. Radiol. 2013, 86. [Google Scholar] [CrossRef]
- Khan, L.; Mitera, G.; Probyn, L.; Ford, M.; Christakis, M.; Finkelstein, J.; Donovan, A.; Zhang, L.; Zeng, L.; Rubenstein, J.; et al. Inter-rater reliability between musculoskeletal radiologists and orthopedic surgeons on computed tomography imaging features of spinal metastases. Curr. Oncol. 2011, 18, e282–e287. [Google Scholar] [CrossRef]
- Nijs, J.; Paul van Wilgen, C.; Van Oosterwijck, J.; van Ittersum, M.; Meeus, M. How to explain central sensitization to patients with “unexplained” chronic musculoskeletal pain: practice guidelines. Man. Ther. 2011, 16, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011, 152, S2–15. [Google Scholar] [CrossRef]
- Baumbach, J.L.; Mui, C.Y.Y.; Leonetti, A.M.; Martin, L.J. A history of injury enhances affective and sensory responses to predator threat by sensitizing corticosterone release through TRPA1 receptor signaling. Curr. Biol. 2025, 0. [Google Scholar] [CrossRef]
- Bonin, R.P.; De Koninck, Y. A spinal analog of memory reconsolidation enables reversal of hyperalgesia. Nat. Neurosci. 2014, 17, 1043–1045. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, R.; Higgins, J.; Bourbonnais, D. Is neuroplasticity in the central nervous system the missing link to our understanding of chronic musculoskeletal disorders? BMC Musculoskelet. Disord. 2015, 16. [Google Scholar] [CrossRef] [PubMed]
- Ablin, J.N. Nociplastic Pain: A Critical Paradigm for Multidisciplinary Recognition and Management. J. Clin. Med. 2024, 13, 5741. [Google Scholar] [CrossRef] [PubMed]
- Aldao, A.; Nolen-Hoeksema, S.; Schweizer, S. Emotion-regulation strategies across psychopathology: A meta-analytic review. Clin. Psychol. Rev. 2010, 30, 217–237. [Google Scholar] [CrossRef]
- Nitta, Y.; Takahashi, T.; Haitani, T.; Sugimori, E.; Kumano, H. Avoidance Behavior Prevents Modification of Fear Memory During Reconsolidation. Psychol. Rep. 2020, 123, 224–238. [Google Scholar] [CrossRef]
- Lissek, S.; Meurs, B. van Learning Models of PTSD: Theoretical Accounts and Psychobiological Evidence. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 2014, 98, 594. [Google Scholar] [CrossRef]
- Rugy, A. de; Loeb, G.E.; Carroll, T.J. Muscle Coordination Is Habitual Rather than Optimal. J. Neurosci. 2012, 32, 7384–7391. [Google Scholar] [CrossRef]
- Bizzi, E.; Cheung, V.C.K. The neural origin of muscle synergies. Front. Comput. Neurosci. 2013, 7. [Google Scholar] [CrossRef]
- Cheung, V.C.K.; d’Avella, A.; Tresch, M.C.; Bizzi, E. Central and Sensory Contributions to the Activation and Organization of Muscle Synergies during Natural Motor Behaviors. J. Neurosci. 2005, 25, 6419–6434. [Google Scholar] [CrossRef]
- Galea, J.M.; Vazquez, A.; Pasricha, N.; Xivry, J.-J.O. de; Celnik, P. Dissociating the Roles of the Cerebellum and Motor Cortex during Adaptive Learning: The Motor Cortex Retains What the Cerebellum Learns. Cereb. Cortex 2011, 21, 1761–1770. [Google Scholar] [CrossRef] [PubMed]
- Hore, J.; Vilis, T. A Cerebellar-dependent Efference Copy Mechanism for Generating Appropriate Muscle Responses to Limb Perturbations. In Cerebellar Functions; Bloedel, P.D.J.R., Dichgans, P.D.J., Precht, P.D.W., Eds.; Proceedings in Life Sciences; Springer Berlin Heidelberg, 1985; pp. 24–35. ISBN 978-3-642-69982-5.
- Alaiti, R.K.; Reis, F.J.J.; Arruda-Sanchez, T.; Caneiro, J.; Meulders, A. Unraveling the role of fear and avoidance behavior in chronic musculoskeletal pain: from theory to physical therapy clinical practice. Braz. J. Phys. Ther. 2025, 29, 101197. [Google Scholar] [CrossRef] [PubMed]
- Meulders, A.; Vansteenwegen, D.; Vlaeyen, J.W.S. The acquisition of fear of movement-related pain and associative learning: A novel pain-relevant human fear conditioning paradigm. PAIN 2011, 152, 2460–2469. [Google Scholar] [CrossRef]
- Ernst, T.M.; Brol, A.E.; Gratz, M.; Ritter, C.; Bingel, U.; Schlamann, M.; Maderwald, S.; Quick, H.H.; Merz, C.J.; Timmann, D. The cerebellum is involved in processing of predictions and prediction errors in a fear conditioning paradigm. eLife 2019, 8, e46831. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.-D.; Kim, S.J.; Lee, Y.-S. Cerebellar Circuits for Classical Fear Conditioning. Front. Cell. Neurosci. 2022, 16. [Google Scholar] [CrossRef]
- Burguière, E.; Arabo, A.; Jarlier, F.; De Zeeuw, C.I.; Rondi-Reig, L. Role of the cerebellar cortex in conditioned goal-directed behavior. J. Neurosci. 2010, 30, 13265–13271. [Google Scholar] [CrossRef]
- Dahhaoui, M.; Caston, J.; Auvray, N.; Reber, A. Role of the cerebellum in an avoidance conditioning task in the rat. Physiol. Behav. 1990, 47, 1175–1180. [Google Scholar] [CrossRef]
- Harvey, J.A.; Welsh, J.P. Learning and performance: a critical review of the role of the cerebellum in instrumental and classical conditioning. In The Acquisition of Motor Behavior in Vertebrates; MIT Press, 1996; pp. 115–142. ISBN 978-0-262-02404-4.
- Lalonde, R. Cerebellar contributions to instrumental learning. Neurosci. Biobehav. Rev. 1994, 18, 161–170. [Google Scholar] [CrossRef]
- Therrien, A.S.; Wolpert, D.M.; Bastian, A.J. Effective reinforcement learning following cerebellar damage requires a balance between exploration and motor noise. Brain 2016, 139, 101–114. [Google Scholar] [CrossRef]
- Psychology Town The Evolution of Behaviour Modification: Key Figures and Developments 2024. Available online: https://psychology.town/psychotherapeutic-methods/evolution-behaviour-modification-key-figures-developments/ (accessed on 27 July 2025).
- Blithikioti, C.; Nuño, L.; Guell, X.; Pascual-Diaz, S.; Gual, A.; Balcells-Olivero, Μ.; Miquel, L. The cerebellum and psychological trauma: A systematic review of neuroimaging studies. Neurobiol. Stress 2022, 17, 100429. [Google Scholar] [CrossRef]
- Li, C.N.; Keay, K.A.; Henderson, L.A.; Mychasiuk, R. Re-examining the Mysterious Role of the Cerebellum in Pain. J. Neurosci. 2024, 44, e1538232024. [Google Scholar] [CrossRef]
- Manda, O.; Hadjivassiliou, M.; Varrassi, G.; Zavridis, P.; Zis, P. Exploring the Role of the Cerebellum in Pain Perception: A Narrative Review. Pain Ther. 2025. [Google Scholar] [CrossRef]
- Clark, A. Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behav. Brain Sci. 2013, 36, 181–204. [Google Scholar] [CrossRef]
- Constant, A.; Ramstead, M.J.; Veissière, S.P.; Friston, K. Regimes of Expectations: An Active Inference Model of Social Conformity and Decision Making. Front. Psychol. 2019, 10, 679. [Google Scholar] [CrossRef]
- Parr, T.; Pezzulo, G.; Friston, K.J. Active Inference: The Free Energy Principle in Mind, Brain, and Behavior; The MIT Press, 2022.
- Friston, K.; Herreros, I. Active Inference and Learning in the Cerebellum. Neural Comput. 2016, 28, 1812–1839. [Google Scholar] [CrossRef]
- Adams, R.A.; Shipp, S.; Friston, K.J. Predictions not commands: active inference in the motor system. Brain Struct. Funct. 2013, 218, 611–643. [Google Scholar] [CrossRef]
- Friston, K. The free-energy principle: a unified brain theory? Nat. Rev. Neurosci. 2010, 11, 127–138. [Google Scholar] [CrossRef]
- Parr, T.; Friston, K.; Pezzulo, G. Generative models for sequential dynamics in active inference. Cogn. Neurodyn. 2024, 18, 3259–3272. [Google Scholar] [CrossRef] [PubMed]
- Parr, T.; Friston, K.J. The Anatomy of Inference: Generative Models and Brain Structure. Front. Comput. Neurosci. 2018, 12, 90. [Google Scholar] [CrossRef] [PubMed]
- Ongaro, G.; Kaptchuk, T.J. Symptom perception, placebo effects, and the Bayesian brain. Pain 2019, 160, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Suttie, J. Eight Ways Your Perception of Reality Is Skewed. Available online: https://greatergood.berkeley.edu/article/item/eight_reasons_to_distrust_your_own_perceptions (accessed on 29 March 2025).
- Bohlen, L.; Shaw, R.; Cerritelli, F.; Esteves, J.E. Osteopathy and Mental Health: An Embodied, Predictive, and Interoceptive Framework. Front. Psychol. 2021, 12, 767005. [Google Scholar] [CrossRef]
- Kuperman, P.; Talmi, D.; Katz, N.; Treister, R. Certainty in ascending sensory signals – The unexplored driver of analgesic placebo response. Med. Hypotheses 2020, 143, 110113. [Google Scholar] [CrossRef] [PubMed]
- Linson, A.; Parr, T.; Friston, K.J. Active inference, stressors, and psychological trauma: A neuroethological model of (mal)adaptive explore-exploit dynamics in ecological context. Behav. Brain Res. 2020, 380, 112421. [Google Scholar] [CrossRef] [PubMed]
- Seriès, P. Post-traumatic stress disorder as a disorder of prediction. Nat. Neurosci. 2019, 22, 334–336. [Google Scholar] [CrossRef]
- Barrett, L.F.; Simmons, W.K. Interoceptive predictions in the brain. Nat. Rev. Neurosci. 2015, 16, 419. [Google Scholar] [CrossRef] [PubMed]
- Harricharan, S.; McKinnon, M.C.; Lanius, R.A. How Processing of Sensory Information From the Internal and External Worlds Shape the Perception and Engagement With the World in the Aftermath of Trauma: Implications for PTSD. Front. Neurosci. 2021, 15. [Google Scholar] [CrossRef]
- Kube, T.; Berg, M.; Kleim, B.; Herzog, P. Rethinking post-traumatic stress disorder - A predictive processing perspective. Neurosci. Biobehav. Rev. 2020, 113, 448–460. [Google Scholar] [CrossRef]
- Van den Bergh, O.; Witthöft, M.; Petersen, S.; Brown, R.J. Symptoms and the body: Taking the inferential leap. Neurosci. Biobehav. Rev. 2017, 74, 185–203. [Google Scholar] [CrossRef]
- Barrett, L.F. How Emotions Are Made; Pan Macmillan, 2017. ISBN 978-1-5098-3750-2.
- Castejón, J.; Chen, F.; Yasoda-Mohan, A.; Ó Sé, C.; Vanneste, S. Chronic pain – A maladaptive compensation to unbalanced hierarchical predictive processing. NeuroImage 2024, 297, 120711. [Google Scholar] [CrossRef]
- Edwards, M.J.; Adams, R.A.; Brown, H.; Pareés, I.; Friston, K.J. A Bayesian account of ‘hysteria. ’ Brain 2012, 135, 3495–3512. [Google Scholar] [CrossRef]
- Gao, Z.; Davis, C.; Thomas, A.M.; Economo, M.N.; Abrego, A.M.; Svoboda, K.; De Zeeuw, C.I.; Li, N. A cortico-cerebellar loop for motor planning. Nature 2018, 563, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Kurtzer, I.L.; Pruszynski, J.A.; Scott, S.H. Long-Latency Reflexes of the Human Arm Reflect an Internal Model of Limb Dynamics. Curr. Biol. 2008, 18, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Clark, A. Predictions, precision, and agentive attention. Conscious. Cogn. 2017, 56, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Pruszynski, J.A.; Scott, S.H. Optimal feedback control and the long-latency stretch response. Exp. Brain Res. 2012, 218, 341–359. [Google Scholar] [CrossRef]
- Javadian, Y.; Adabi, M.; Heidari, B.; Babaei, M.; Firouzjahi, A.; Ghahhari, B.Y.; Hajian-Tilaki, K. Quadriceps Muscle Strength Correlates With Serum Vitamin D and Knee Pain in Knee Osteoarthritis. Clin. J. Pain 2017, 33, 67–70. [Google Scholar] [CrossRef]
- Osan, R.; Tort, A.B.L.; Amaral, O.B. A Mismatch-Based Model for Memory Reconsolidation and Extinction in Attractor Networks. PLoS ONE 2011, 6, e23113. [Google Scholar] [CrossRef]
- Pedreira, M.E.; Pérez-Cuesta, L.M.; Maldonado, H. Mismatch Between What Is Expected and What Actually Occurs Triggers Memory Reconsolidation or Extinction. Learn. Mem. 2004, 11, 579–585. [Google Scholar] [CrossRef]
- Ploghaus, A.; Tracey, I.; Clare, S.; Gati, J.S.; Rawlins, J.N.P.; Matthews, P.M. Learning about pain: The neural substrate of the prediction error for aversive events. Proc. Natl. Acad. Sci. 2000, 97, 9281–9286. [Google Scholar] [CrossRef]
- Wacongne, C.; Changeux, J.-P.; Dehaene, S. A Neuronal Model of Predictive Coding Accounting for the Mismatch Negativity. J. Neurosci. 2012, 32, 3665–3678. [Google Scholar] [CrossRef]
- Dancey, E. The effect of experimental pain on neural function and motor learning, 2017.
- McNally, G.P.; Westbrook, R.F. Predicting danger: The nature, consequences, and neural mechanisms of predictive fear learning. Learn. Mem. 2006, 13, 245–253. [Google Scholar] [CrossRef]
- Salomoni, S.E.; Marinovic, W.; Carroll, T.J.; Hodges, P.W. Motor Strategies Learned during Pain Are Sustained upon Pain-free Reexposure to Task. Med. Sci. Sports Exerc. 2019, 51, 2334–2343. [Google Scholar] [CrossRef]
- Mendez-Rebolledo, G.; Guzmán-Venegas, R.; Cruz-Montecinos, C.; Watanabe, K.; Calatayud, J.; Martinez-Valdes, E. Individuals with chronic ankle instability show altered regional activation of the peroneus longus muscle during ankle eversion. Scand. J. Med. Sci. Sports 2024, 34, e14535. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Suzuki, T. Relationship between chronic ankle sprain instability and ultrasonographic evaluation of the peroneus during a single-leg standing task. J. Phys. Ther. Sci. 2020, 32, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, R.M.; Weltman, A.; Edwards, J.E.; Tom, J.A.; Saliba, E.N.; Mistry, D.J.; Ingersoll, C.D. Pre-synaptic modulation of quadriceps arthrogenic muscle inhibition. Knee Surg. Sports Traumatol. Arthrosc. 2005, 13, 370–376. [Google Scholar] [CrossRef]
- Brown, H.; Friston, K.J.; Bestmann, S. Active Inference, Attention, and Motor Preparation. Front. Psychol. 2011, 2. [Google Scholar] [CrossRef]
- Hapidou, E.G.; O’Brien, M.A.; Pierrynowski, M.R.; de las Heras, E.; Patel, M.; Patla, T. Fear and Avoidance of Movement in People with Chronic Pain: Psychometric Properties of the 11-Item Tampa Scale for Kinesiophobia (TSK-11). Physiother. Can. 2012, 64, 235–241. [Google Scholar] [CrossRef]
- Núñez-Cortés, R.; Horment-Lara, G.; Tapia-Malebran, C.; Castro, M.; Barros, S.; Vera, N.; Pérez-Alenda, S.; Pablo Santelices, J.; Rivera-Lillo, G.; Cruz-Montecinos, C. Role of kinesiophobia in the selective motor control during gait in patients with low back-related leg pain. J. Electromyogr. Kinesiol. Off. J. Int. Soc. Electrophysiol. Kinesiol. 2023, 71, 102793. [Google Scholar] [CrossRef]
- Picavet, H.S.J.; Vlaeyen, J.W.S.; Schouten, J.S.A.G. Pain Catastrophizing and Kinesiophobia: Predictors of Chronic Low Back Pain. Am. J. Epidemiol. 2002, 156, 1028–1034. [Google Scholar] [CrossRef]
- Larsson, C.; Ekvall Hansson, E.; Sundquist, K.; Jakobsson, U. Kinesiophobia and its relation to pain characteristics and cognitive affective variables in older adults with chronic pain. BMC Geriatr. 2016, 16. [Google Scholar] [CrossRef]
- Nijs, J.; Lluch Girbés, E.; Lundberg, M.; Malfliet, A.; Sterling, M. Exercise therapy for chronic musculoskeletal pain: Innovation by altering pain memories. Man. Ther. 2015, 20, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Santi, M.; Diener, I.; Oostendorp, R. Assessment and treatment of patients with kinesiophobia: A Delphi consensus. J. Nov. Physiother. Rehabil. 2022, 6, 023–028. [Google Scholar] [CrossRef]
- Delahunt, E.; Monaghan, K.; Caulfield, B. Altered Neuromuscular Control and Ankle Joint Kinematics During Walking in Subjects With Functional Instability of the Ankle Joint. Am. J. Sports Med. 2006, 34, 1970–1976. [Google Scholar] [CrossRef]
- Stone, J.; Warlow, C.; Sharpe, M. The symptom of functional weakness: a controlled study of 107 patients. Brain 2010, 133, 1537–1551. [Google Scholar] [CrossRef]
- Mayo Clinic Functional neurologic disorder/conversion disorder - Symptoms and causes. Available online: https://www.mayoclinic.org/diseases-conditions/conversion-disorder/symptoms-causes/syc-20355197 (accessed on Apr 21, 2023).
- Bühler, J.; Weber, S.; Loukas, S.; Walther, S.; Aybek, S. Non-invasive neuromodulation of the right temporoparietal junction using theta-burst stimulation in functional neurological disorder. BMJ Neurol. Open 2024, 6. [Google Scholar] [CrossRef]
- Moccia, L.; di Luzio, M.; Conte, E.; Modica, M.; Ambrosecchia, M.; Ardizzi, M.; Lanzotti, P.; Kotzalidis, G.D.; Janiri, D.; Di Nicola, M.; et al. Sense of agency and its disturbances: A systematic review targeting the intentional binding effect in neuropsychiatric disorders. Psychiatry Clin. Neurosci. 2024, 78, 3–18. [Google Scholar] [CrossRef]
- Welniarz, Q.; Worbe, Y.; Gallea, C. The Forward Model: A Unifying Theory for the Role of the Cerebellum in Motor Control and Sense of Agency. Front. Syst. Neurosci. 2021, 15, 22. [Google Scholar] [CrossRef] [PubMed]
- Halicka, M.; Vittersø, A.D.; Proulx, M.J.; Bultitude, J.H. Neuropsychological Changes in Complex Regional Pain Syndrome (CRPS). Behav. Neurol. 2020, 2020, 4561831. [Google Scholar] [CrossRef] [PubMed]
- Filbrich, L.; Verfaille, C.; Vannuscorps, G.; Berquin, A.; Barbier, O.; Libouton, X.; Fraselle, V.; Mouraux, D.; Legrain, V. Atypical influence of biomechanical knowledge in Complex Regional Pain Syndrome-towards a different perspective on body representation. Sci. Rep. 2023, 13, 520. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, K. The Developmental Origins of Conversion Disorders. Clin. Child Psychol. Psychiatry 2007, 12, 487–510. [Google Scholar] [CrossRef]
- Bennett, K.; Diamond, C.; Hoeritzauer, I.; Gardiner, P.; McWhirter, L.; Carson, A.; Stone, J. A practical review of functional neurological disorder (FND) for the general physician. Clin. Med. 2021, 21, 28–36. [Google Scholar] [CrossRef]
- Balsam, P.D.; Gallistel, C.R. Temporal maps and informativeness in associative learning. Trends Neurosci. 2009, 32, 73–78. [Google Scholar] [CrossRef]
- Eliassen, J.C.; Souza, T.; Sanes, J.N. Experience-Dependent Activation Patterns in Human Brain during Visual-Motor Associative Learning. J. Neurosci. 2003, 23, 10540–10547. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.H.; Kairiss, E.W.; Keenan, C.L. Hebbian synapses: biophysical mechanisms and algorithms. Annu. Rev. Neurosci. 1990, 13, 475–511. [Google Scholar] [CrossRef] [PubMed]
- Rathelot, J.-A.; Dum, R.P.; Strick, P.L. Posterior parietal cortex contains a command apparatus for hand movements. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 4255–4260. [Google Scholar] [CrossRef]
- Rispal-Padel, L. [Contribution of cerebellar efferents to the organization of motor synergy]. Rev. Neurol. (Paris) 1993, 149, 716–727. [Google Scholar]
- Thach, W.T.; Perry, J.G.; Kane, S.A.; Goodkin, H.P. Cerebellar nuclei: rapid alternating movement, motor somatotopy, and a mechanism for the control of muscle synergy. Rev. Neurol. (Paris) 1993, 149, 607–628. [Google Scholar]
- Kelly, R.M.; Strick, P.L. Cerebellar Loops with Motor Cortex and Prefrontal Cortex of a Nonhuman Primate. J. Neurosci. 2003, 23, 8432–8444. [Google Scholar] [CrossRef]
- Ito, M. Cerebellar circuitry as a neuronal machine. Prog. Neurobiol. 2006, 78, 272–303. [Google Scholar] [CrossRef]
- Doya, K. What are the computations of the cerebellum, the basal ganglia and the cerebral cortex? Neural Netw. 1999, 12, 961–974. [Google Scholar] [CrossRef]
- Nurmikko, T.; MacIver, K.; Bresnahan, R.; Hird, E.; Nelson, A.; Sacco, P. Motor Cortex Reorganization and Repetitive Transcranial Magnetic Stimulation for Pain—A Methodological Study. Neuromodulation 2016, 19, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Mercier, C.; Léonard, G. Interactions between Pain and the Motor Cortex: Insights from Research on Phantom Limb Pain and Complex Regional Pain Syndrome. Physiother. Can. 2011, 63, 305–314. [Google Scholar] [CrossRef]
- Koch, C. What Is Consciousness? Nature 2018, 557, S8–S12. [Google Scholar] [CrossRef]
- Rice, D.A.; McNair, P.; Lewis, G.; Dalbeth, N. Quadriceps arthrogenic muscle inhibition: the effects of experimental knee joint effusion on motor cortex excitability. Arthritis Res. Ther. 2014, 16, 502. [Google Scholar] [CrossRef]
- Guillaumin, S.; Dahhaoui, M.; Caston, J. Cerebellum and memory: an experimental study in the rat using a passive avoidance conditioning test. Physiol. Behav. 1991, 49, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Gil-Paterna, P.; Furmark, T. Imaging the cerebellum in post-traumatic stress and anxiety disorders: a mini-review. Front. Syst. Neurosci. 2023, 17. [Google Scholar] [CrossRef] [PubMed]
- Blithikioti, C.; Nuño, L.; Guell, X.; Pascual-Diaz, S.; Gual, A.; Balcells-Olivero, Μ.; Miquel, L. The cerebellum and psychological trauma: A systematic review of neuroimaging studies. Neurobiol. Stress 2022, 17, 100429. [Google Scholar] [CrossRef]
- Blithikioti, C.; Nuño, L.; Guell, X.; Pascual-Diaz, S.; Gual, A.; Balcells-Olivero, Μ.; Miquel, L. The cerebellum and psychological trauma: A systematic review of neuroimaging studies. Neurobiol. Stress 2022, 17, 100429. [Google Scholar] [CrossRef]
- Blithikioti, C.; Duek, O.; Gordon, C.; Krystal, J.H.; Levy, I.; Harpaz-Rotem, I.; Schiller, D.; Perl, O. Cerebellar Contributions to Traumatic Autobiographical Memory in People with Post-Traumatic Stress Disorder. The Cerebellum 2024, 23, 2332–2340. [Google Scholar] [CrossRef]
- Terpou, B.A.; Densmore, M.; Thome, J.; Frewen, P.; McKinnon, M.C.; Lanius, R.A. The Innate Alarm System and Subliminal Threat Presentation in Posttraumatic Stress Disorder: Neuroimaging of the Midbrain and Cerebellum. Chronic Stress 2019, 3, 2470547018821496. [Google Scholar] [CrossRef] [PubMed]
- Jékely, G.; Godfrey-Smith, P.; Keijzer, F. Reafference and the origin of the self in early nervous system evolution. Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20190764. [Google Scholar] [CrossRef]
- Cullen, K.E.; Brooks, J.X.; Jamali, M.; Carriot, J.; Massot, C. Internal models of self-motion: computations that suppress vestibular reafference in early vestibular processing. Exp. Brain Res. 2011, 210, 377–388. [Google Scholar] [CrossRef]
- Person, A.L. Corollary discharge signals in the cerebellum. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2019, 4, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Niziolek, C.A.; Nagarajan, S.S.; Houde, J.F. What Does Motor Efference Copy Represent? Evidence from Speech Production. J. Neurosci. 2013, 33, 16110–16116. [Google Scholar] [CrossRef] [PubMed]
- Scientific American Why can’t a person tickle himself? Available online: https://www.scientificamerican.com/article/why-cant-a-person-tickle/ (accessed on 11 January 2018).
- Coombes, S.A.; Tandonnet, C.; Fujiyama, H.; Janelle, C.M.; Cauraugh, J.H.; Summers, J.J. Emotion and motor preparation: A transcranial magnetic stimulation study of corticospinal motor tract excitability. Cogn. Affect. Behav. Neurosci. 2009, 9, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Shadmehr, R.; Smith, M.A.; Krakauer, J.W. Error correction, sensory prediction, and adaptation in motor control. Annu. Rev. Neurosci. 2010, 33, 89–108. [Google Scholar] [CrossRef]
- Welman, M.; Smit, A.E.; Jongen, J.L.M.; Tibboel, D.; van der Geest, J.N.; Holstege, J.C. Pain Experience is Somatotopically Organized and Overlaps with Pain Anticipation in the Human Cerebellum. The Cerebellum 2018, 17, 447–460. [Google Scholar] [CrossRef]
- Boillat, Y.; Bazin, P.-L.; van der Zwaag, W. Whole-body somatotopic maps in the cerebellum revealed with 7T fMRI. NeuroImage 2020, 211, 116624. [Google Scholar] [CrossRef]
- Angel, R.W. Efference copy in the control of movement. Neurology 1976, 26, 1164–1164. [Google Scholar] [CrossRef]
- Chabrol, F.P.; Blot, A.; Mrsic-Flogel, T.D. Cerebellar Contribution to Preparatory Activity in Motor Neocortex. Neuron 2019, 103, 506–519.e4. [Google Scholar] [CrossRef]
- R.C. Miall The cerebellum, predictive control and motor coordination. In Sensory Guidance of Movement; John Wiley & Sons, 2008. ISBN 978-0-470-51557-0.
- Jarvik, M.E.; Kopp, R. An improved one-trial passive avoidance learning situation. Psychol. Rep. 1967, 21, 221–224. [Google Scholar] [CrossRef]
- Linson, A.; Friston, K. Reframing PTSD for computational psychiatry with the active inference framework. Cognit. Neuropsychiatry 2019, 24, 347–368. [Google Scholar] [CrossRef]
- da Silva, G.N.; Seiffert, N.; Tovote, P. Cerebellar contribution to the regulation of defensive states. Front. Syst. Neurosci. 2023, 17. [Google Scholar] [CrossRef] [PubMed]
- Koutsikou, S.; Crook, J.J.; Earl, E.V.; Leith, J.L.; Watson, T.C.; Lumb, B.M.; Apps, R. Neural substrates underlying fear-evoked freezing: the periaqueductal grey–cerebellar link. J. Physiol. 2014, 592, 2197–2213. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Thompson, R.F. Cerebellar circuits and synaptic mechanisms involved in classical eyeblink conditioning. Trends Neurosci. 1997, 20, 177–181. [Google Scholar] [CrossRef]
- Schreurs, B.G.; Burhans, L.B. Eyeblink Classical Conditioning and Post-Traumatic Stress Disorder – A Model Systems Approach. Front. Psychiatry 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- De Peuter, S.; Diest, I.; Vansteenwegen, D.; Bergh, O.; Vlaeyen, J.W. Understanding fear of pain in chronic pain: interoceptive fear conditioning as a novel approach. Eur. J. Pain 2011, 15, 889–894. [Google Scholar] [CrossRef]
- Zaman, J.; De Peuter, S.; Van Diest, I.; Van den Bergh, O.; Vlaeyen, J.W.S. Interoceptive cues predicting exteroceptive events. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 2016, 109, 100–106. [Google Scholar] [CrossRef]
- Farb, N.; Daubenmier, J.; Price, C.J.; Gard, T.; Kerr, C.; Dunn, B.D.; Klein, A.C.; Paulus, M.P.; Mehling, W.E. Interoception, contemplative practice, and health. Front. Psychol. 2015, 6. [Google Scholar] [CrossRef]
- Bevins, R.A.; Besheer, J. Interoception and Learning: Import to Understanding and Treating Diseases and Psychopathologies. ACS Chem. Neurosci. 2014, 5, 624–631. [Google Scholar] [CrossRef]
- Ursin, H. The Psychology In Psychoneuroendocrinology. Psychoneuroendocrinology 1998, 23, 555–570. [Google Scholar] [CrossRef]
- Ader, R.; Cohen, N. Psychoneuroimmunology: conditioning and stress. Annu. Rev. Psychol. 1993, 44, 53–85. [Google Scholar] [CrossRef]
- Ernst, T.M.; Brol, A.E.; Gratz, M.; Ritter, C.; Bingel, U.; Schlamann, M.; Maderwald, S.; Quick, H.H.; Merz, C.J.; Timmann, D. The cerebellum is involved in processing of predictions and prediction errors in a fear conditioning paradigm. eLife 2019, 8, e46831. [Google Scholar] [CrossRef]
- Dimitrova, A.; Kolb, F.P.; Elles, H.-G.; Maschke, M.; Gerwig, M.; Gizewski, E.; Timmann, D. Cerebellar activation during leg withdrawal reflex conditioning: an fMRI study. Clin. Neurophysiol. 2004, 115, 849–857. [Google Scholar] [CrossRef]
- Xue, X.; Rong Lu; Zang, D. ; LIi, H.; Zhang, H.; Xu, H.; LI, Q.; Ma, T.; Tang, W.; Chen, S.; et al. Low Regional Homogeneity of Intrinsic Cerebellar Activity in Ankle Instability: An Externally Validated rs-fMRI Study. Med. Sci. Sports Exerc. 2022, 54, 2037–2044. [Google Scholar] [CrossRef]
- Manda, O.; Hadjivassiliou, M.; Varrassi, G.; Zavridis, P.; Zis, P. Exploring the Role of the Cerebellum in Pain Perception: A Narrative Review. Pain Ther. 2025, 14, 803–816. [Google Scholar] [CrossRef] [PubMed]
- van den Bekerom, M.P.J.; Struijs, P.A.A.; Blankevoort, L.; Welling, L.; van Dijk, C.N.; Kerkhoffs, G.M.M.J. What Is the Evidence for Rest, Ice, Compression, and Elevation Therapy in the Treatment of Ankle Sprains in Adults? J. Athl. Train. 2012, 47, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Rucinski, T.J.; Hooker, D.N.; Prentice, W.E.; Shields, E.W.; Coté-Murray, D.J. The Effects of Intermittent Compression on Edema in Postacute Ankle sprains. J. Orthop. Sports Phys. Ther. 1991, 14, 65–69. [Google Scholar] [CrossRef] [PubMed]
- AMIT Method Effect on Sprained Ankle [Video]; 2016.
- Kharrazian, D.; Brock, B. Cerebellum and Immunity. In Proceedings of the Functional Neuroloty Seminars; 2016; 2016. [Google Scholar]
- Benagiano, V.; Rizzi, A. Autonomic functions of the cerebellum: Anatomical bases and clinical implications. Ital. J. Anat. Embryol. 2024, 128, 113–123. [Google Scholar] [CrossRef]
- Haubrich, J.; Machado, A.; Boos, F.Z.; Crestani, A.P.; Sierra, R.O.; Alvares, L. de O.; Quillfeldt, J.A. Enhancement of extinction memory by pharmacological and behavioral interventions targeted to its reactivation. Sci. Rep. 2017, 7, 10960. [Google Scholar] [CrossRef]
- Kalmbach, B.E.; Mauk, M.D. Multiple sites of extinction for a single learned response. J. Neurophysiol. 2012, 107, 226–238. [Google Scholar] [CrossRef]
- Robleto, K.; Poulos, A.M.; Thompson, R.F. Brain Mechanisms of Extinction of the Classically Conditioned Eyeblink Response. Learn. Mem. 2004, 11, 517–524. [Google Scholar] [CrossRef]
- Ferrara, N.C.; Kwapis, J.L.; Trask, S. Memory retrieval, reconsolidation, and extinction: Exploring the boundary conditions of post-conditioning cue exposure. Front. Synaptic Neurosci. 2023, 15. [Google Scholar] [CrossRef]
- Liew, B.X.W.; Vecchio, A.D.; Falla, D. The influence of musculoskeletal pain disorders on muscle synergies—A systematic review. PLOS ONE 2018, 13, e0206885. [Google Scholar] [CrossRef] [PubMed]
- Ervilha, U.F.; Farina, D.; Arendt-Nielsen, L.; Graven-Nielsen, T. Experimental muscle pain changes motor control strategies in dynamic contractions. Exp. Brain Res. 2005, 164, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Bank, P. j. m.; Peper, C. e.; Marinus, J.; Beek, P. j.; van Hilten, J. j. Motor consequences of experimentally induced limb pain: A systematic review. Eur. J. Pain 2013, 17, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Muceli, S.; Falla, D.; Farina, D. Reorganization of muscle synergies during multidirectional reaching in the horizontal plane with experimental muscle pain. J. Neurophysiol. 2014, 111, 1615–1630. [Google Scholar] [CrossRef]
- Tucker, K.; Butler, J.; Graven-Nielsen, T.; Riek, S.; Hodges, P. Motor Unit Recruitment Strategies Are Altered during Deep-Tissue Pain. J. Neurosci. 2009, 29, 10820–10826. [Google Scholar] [CrossRef]
- Manickaraj, N.; Bisset, L.M.; Devanaboyina, V.S.P.T.; Kavanagh, J.J. Chronic pain alters spatiotemporal activation patterns of forearm muscle synergies during the development of grip force. J. Neurophysiol. 2017, 118, 2132–2141. [Google Scholar] [CrossRef]
- Bouffard, J.; Salomoni, S.E.; Mercier, C.; Tucker, K.; Roy, J.-S.; van den Hoorn, W.; Hodges, P.W.; Bouyer, L.J. Effect of experimental muscle pain on the acquisition and retention of locomotor adaptation: different motor strategies for a similar performance. J. Neurophysiol. 2018, 119, 1647–1657. [Google Scholar] [CrossRef]
- van den Hoorn, W.; Hodges, P.W.; van Dieën, J.H.; Hug, F. Effect of acute noxious stimulation to the leg or back on muscle synergies during walking. J. Neurophysiol. 2015, 113, 244–254. [Google Scholar] [CrossRef]
- Doyon, J.; Penhune, V.; Ungerleider, L.G. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia 2003, 41, 252–262. [Google Scholar] [CrossRef]
- Hull, C. Prediction signals in the cerebellum: Beyond supervised motor learning. eLife 2020, 9, e54073. [Google Scholar] [CrossRef]
- de Silva, P.; Rachman, S. Does escape behaviour strengthen agoraphobic avoidance? A preliminary study. Behav. Res. Ther. 1984, 22, 87–91. [Google Scholar] [CrossRef]
- Hamm, A.O.; Richter, J.; Pané-Farré, C.; Westphal, D.; Wittchen, H.-U.; Vossbeck-Elsebusch, A.N.; Gerlach, A.L.; Gloster, A.T.; Ströhle, A.; Lang, T.; et al. Panic disorder with agoraphobia from a behavioral neuroscience perspective: Applying the research principles formulated by the Research Domain Criteria (RDoC) initiative: Panic disorder with agoraphobia. Psychophysiology 2016, 53, 312–322. [Google Scholar] [CrossRef]
- Krypotos, A.-M.; Effting, M.; Kindt, M.; Beckers, T. Avoidance learning: a review of theoretical models and recent developments. Front. Behav. Neurosci. 2015, 9. [Google Scholar] [CrossRef]
- Ito, L.M.; Araujo, L.A.D.; Tess, V.L.C.; Barros-Neto, T.P.D.; Asbahr, F.R.; Marks, I. Self-exposure therapy for panic disorder with agoraphobia: Randomised controlled study of external v. interoceptive self-exposure. Br. J. Psychiatry 2001, 178, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Bouton, M.E. Context and Behavioral Processes in Extinction. Learn. Mem. 2004, 11, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.; Yani, M.S.; Asavasopon, S.; Fisher, B.E.; Kutch, J.J. Brain Connectivity Associated with Muscle Synergies in Humans. J. Neurosci. 2015, 35, 14708–14716. [Google Scholar] [CrossRef] [PubMed]
- Leo, A.; Handjaras, G.; Bianchi, M.; Marino, H.; Gabiccini, M.; Guidi, A.; Scilingo, E.P.; Pietrini, P.; Bicchi, A.; Santello, M.; et al. A synergy-based hand control is encoded in human motor cortical areas. eLife 2016, 5, e13420. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.; Cheung, V.C.K.; Bizzi, E. Modules in the brain stem and spinal cord underlying motor behaviors. J. Neurophysiol. 2011, 106, 1363–1378. [Google Scholar] [CrossRef]
- Chvatal, S.A.; Ting, L.H. Voluntary and Reactive Recruitment of Locomotor Muscle Synergies during Perturbed Walking. J. Neurosci. 2012, 32, 12237–12250. [Google Scholar] [CrossRef]
- Togo, S.; Imamizu, H. Empirical Evaluation of Voluntarily Activatable Muscle Synergies. Front. Comput. Neurosci. 2017, 11. [Google Scholar] [CrossRef]
- Malik, K.; Dua, A. Advancing Patient Care With Biofeedback. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2025. [Google Scholar]
- seniam. Available online: http://seniam.org/ (accessed on Sept 9, 2025).
- Agren, T. Human reconsolidation: A reactivation and update. Brain Res. Bull. 2014, 105, 70–82. [Google Scholar] [CrossRef]
- LeDoux, J.E. Consolidation: challenging the traditional view. In Science of Memory: Concepts; Dudai, Y., Roediger, H.L., Fitzpatrick, S.M., Eds.; Oxford University Press: London ; New York, 2007; pp. 171–175. ISBN 978-0-19-531044-3.
- van den Hout, M.A.; Engelhard, I.M. How does EMDR work? J. Exp. Psychopathol. 2012, 3, 724–738. [Google Scholar] [CrossRef]
- Propper, R.E.; Christman, S.D. Interhemispheric interaction and saccadic horizontal eye movements: Implications for episodic memory, EMDR, and PTSD. J. EMDR Pract. Res. 2008, 2, 269–281. [Google Scholar] [CrossRef]
- Armstrong, M.S.; Vaughan, K. An orienting response model of eye movement desensitization. J. Behav. Ther. Exp. Psychiatry 1996, 27, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Chamberlin, D.E. The Predictive Processing Model of EMDR. Front. Psychol. 2019, 10, 2267. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, A.H.; Manalili, G.M.; Brunec, I.K.; Adcock, R.A.; Barense, M.D. Prediction errors disrupt hippocampal representations and update episodic memories. Proc. Natl. Acad. Sci. 2021, 118, e2117625118. [Google Scholar] [CrossRef]
- Sevenster, D.; Beckers, T.; Kindt, M. Prediction error demarcates the transition from retrieval, to reconsolidation, to new learning. Learn. Mem. 2014, 21, 580–584. [Google Scholar] [CrossRef]
- Welling, H. Transformative emotional sequence: Towards a common principle of change. J. Psychother. Integr. 2012, 22, 109. [Google Scholar] [CrossRef]
- Van Nuys, David; Orloff, Judith Energy Psychiatry and Emotional Freedom 2009.
- Church, D.; Geronilla, L.; Dinter, I. Psychological symptom change in veterans after six sessions of Emotional Freedom Techniques (EFT): An observational study. Electron. J. Artic. Int. J. Heal. Caring 2009, 9. [Google Scholar]
- Wells, S.; Polglase, K.; Andrews, H.B.; Carrington, P.; Baker, A.H. Evaluation of a meridian-based intervention, Emotional Freedom Techniques (EFT), for reducing specific phobias of small animals. J. Clin. Psychol. 2003, 59, 943–966. [Google Scholar] [CrossRef]
- Feinstein, D. Rapid treatment of PTSD: Why psychological exposure with acupoint tapping may be effective. Psychother. Theory Res. Pract. Train. 2010, 47, 385. [Google Scholar] [CrossRef]
- Chamberlin, D.E. The Active Inference Model of Coherence Therapy. Front. Hum. Neurosci. 2023, 16. [Google Scholar] [CrossRef]
- Coherence Psychology Institute Psychotherapy unification via memory reconsolidation. Available online: https://www.coherencetherapy.org/discover/TRP-documented-therapies.htm (accessed on Mar 16, 2024).
- Assouline, A.; Mendelsohn, A.; Reshef, A. Memory-directed acupuncture as a neuromodulatory treatment for PTSD: Theory, clinical model and case studies. Transl. Psychiatry 2022, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Keller, N.; Hennings, A.; Dunsmoor, J. Behavioral and neural processes in counterconditioning: past and future directions. Behav. Res. Ther. 2020, 125, 103532. [Google Scholar] [CrossRef] [PubMed]
- Meulders, A.; Karsdorp, P.A.; Claes, N.; Vlaeyen, J.W.S. Comparing Counterconditioning and Extinction as Methods to Reduce Fear of Movement-Related Pain. J. Pain 2015, 16, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).