1. Introduction
Epidermal growth factor receptor (EGFR) mutated metastatic non-small cell lung cancer (mNSCLC) is the most common oncogene addicted lung cancer with a variable global prevalence based on ethnicity (1,2). The molecular landscape of EGFR mutations is diverse, with typical mutations like Exon 19 deletion and Exon 21 L858R substitution collectively accounting for nearly 90% of all mutations. Atypical alterations include mutations in EGFR like S768I, L861Q, and G719X and exon 20 insertions(3,4).
Following results from pivotal clinical trials, third generation EGFR tyrosine kinase inhibitors (TKIs) such as Osimertinib have become the standard of care treatment for patients with classical EGFR mutations as they have demonstrated superiority over 1st and 2nd generation EGFR inhibitors(5). However, median progression free survival (PFS) on Osimertinib is approximately 18 months. Furthermore, few of these patients have durable remissions and nearly one-third drop out after progression and thus do not receive second line therapy. (5). Therefore, it is imperative to strengthen the treatment paradigm in the first line.
Recent studies have looked at improving outcomes in 1st line treatment. This includes a combination of Osimertinib and chemotherapy in the FLAURA2 trial or alternatively a chemotherapy free approach in the MARIPOSA trial using a 3rd generation TKI Lazertinib and Amivantamab, which is a monoclonal bispecific antibody targeting EGFR and c-MET (6,7). Both these studies have shown promising outcomes with better PFS compared to Osimertinib alone. MARIPOSA also had a positive overall survival (OS) readout recently, with OS for FLAURA-2 showing a similar trend.(8)
Despite several new treatment approaches, there is uncertainty regarding the most favourable first line treatment. This is in part due to excellent tolerability of single agent Osimertinib, but also due to toxicity associated with the addition of chemotherapy and Amivantamab, and limited global access for newer drugs. Moreover, none of these strategies are curative. Therefore appropriate sequencing after progression on the first line therapy constitutes an important clinical challenge. This review will explore the complexities of managing EGFR-mutated mNSCLC in the first line treatment and beyond, in addition to focusing on novel agents and unmet needs in clinical practice. We will also highlight ongoing clinical trials in this space and the need for biomarker driven research.
2. Overview of First-Line Treatment (Table 1)
Osimertinib has been the standard of care for patients with mNSCLC with an EGFR mutation based on results of the FLAURA study (5). In this study, Osimertinib was compared to standard of care TKIs as first-line treatment in patients with EGFR-mutated mNSCLC (exon 19 deletions and exon 21 L858R substitutions) (n=556). Results demonstrated superior outcomes with Osimertinib, including significantly longer PFS (18.9 months vs. 10.2 months, hazard ratio [HR] 0.46, 95% confidence interval [CI] 0.37-0.57, p value < 0.001), median duration of response [mDOR] (17.2 months vs. 8.5 months, HR 0.39, 95% CI 0.29-0.53, p value < 0.001), and OS (38.6 months vs. 31.8 months, HR 0.80, 95% CI 0.64- 1.00, p value = 0.0046). Grade 3/4 toxicity occurred in 34% of participants in the Osimertinib group versus 45% in the comparator group.
This study also included a significant proportion of patients with central nervous system (CNS) metastases (n=116; 21%). Besides excellent systemic response, Osimertinib also showed a reduction in the incidence of CNS progression, with lower rates of severe toxicity, which made it the preferred first-line therapy option. PFS at 18 months among patients with CNS metastases was 58% (95% CI: 0.40 to 0.72) in the Osimertinib group in contrast to 40% (95% CI: 0.25 to 0.55) in the comparator group (HR, 0.48; 95% CI 0.26 to 0.86), however, long term CNS specific results were not reported. Interestingly, patients with Exon 19 deletion did better with Osimertinib [HR 0.68 (95% CI: .051- 0.90)] compared to Exon 21 L858R substitution [HR 1.00 (95% CI: 0.71- 1.40)].
Subsequent to the FLAURA study, the phase III FLAURA2 trial (n=557) evaluated Osimertinib combined with a platinum–pemetrexed doublet (4 cycles) followed by pemetrexed maintenance versus Osimertinib alone in previously untreated EGFR-mutated mNSCLC (exon 19 deletion or exon 21 L858R substitution) (7). Platinum exposure in the combination arm was typically 4 cycles (either cisplatin 75 mg/m² or carboplatin AUC 5), and most patients received a median of six additional pemetrexed cycles as maintenance. The trial recruited patients with stable or asymptomatic CNS metastases constituting approximately 40% of patients in each arm. Liver metastases were more frequent in the Osimertinib arm (23.7%) compared to Osimertinib + chemotherapy arm (15.4%). This combination resulted in an improved PFS for the combination of Osimertinib plus chemotherapy of 25.5 months versus 16.7 months [HR 0.62; 95% CI: 0.49 - 0.79; p < 0.001]. OS data remains immature but second interim OS analysis presented at the World Lung Cancer Conference 2024 (WLCC 2024) showed encouraging early results, with median OS not being reached in the Osimertinib plus chemotherapy arm compared to 36.7 months in Osimertinib arm (HR 0.75, 0.57-0.97, p-0.028) (9). However, this benefit came at the cost of increased toxicity, with 64% patients experiencing grade 3/4 adverse events (combination arm) compared to 27% (Osimertinib monotherapy arm). 38% of patients had a serious adverse event with 11% discontinued treatment in the combination arm compared to just 6% in the Osimertinib monotherapy arm(7). Subgroup analysis from this study revealed that combination therapy performed better in Asian Chinese and Non-Asians compared to Asian Non-Chinese, possibly due to heterogeneity in EGFR mutation subtypes. The differences in response amongst exon 19 deletion and exon 21 L858R substitution, which were noted in FLAURA study, were not seen in this study. This indicates that addition of chemotherapy to Osimertinib can offset the poor prognostic impact of Exon 21 L858R substitution. Patients with CNS metastasis had a better PFS with the combination therapy compared to Osimertinib monotherapy alone (24.9 months vs 13.8 months, HR.0.47). Similar benefits were also seen in other high-risk groups including younger patients (HR 0.59), those with liver metastasis (HR 0.63) and those harboring TP53 co-mutations (HR 0.57) (10)
A novel chemotherapy free approach using a newer third generation EGFR TKI (Lazertinib) and a bispecific EGFR-MET monoclonal antibody Amivantamab was then investigated in the phase III MARIPOSA trial(6). This study recruited 1074 treatment naïve patients with EGFR exon 19 deletion or exon 21 L858R substitution and randomized them in a 2:2:1 ratio into receiving Amivantamab-Lazertinib combination, Osimertinib monotherapy or Lazertinib monotherapy. The results mainly focused on Amivantamab-Lazertinib vs Osimertinib monotherapy arms. This study included patients with stable or asymptomatic brain metastases (41% in both arms), liver metastases (~15-17% in both arms) and TP53 co-mutation (54% in both arms).
This approach demonstrated an improved PFS of 23.7 months for the combination therapy in contrast to 16.6 months for Osimertinib monotherapy (HR 0.70, 0.58-0.85, p<0.001). Overall response rate (ORR) was similar in both the subgroups at 86% and 85%, respectively. Final OS analysis presented at the European Lung Cancer Conference 2025 (ELCC 2025) demonstrated a statistically significant survival benefit with median OS not being reached for the combination arm compared to 36.7 months in the Osimertinib monotherapy (HR 0.75, 0.61-0.92, p<0.005), 3 year OS 56% vs 44%)(11). Notably, cross-over was not permitted in this study, and thus most of these patients in the Osimertinib monotherapy arm did not receive Amivantamab in later lines of therapy.
Toxicity was significant with 75% of patients having a grade 3 or higher adverse event, 49% having a serious adverse event and 35% discontinuing the combination therapy. These side effects were related to EGFR inhibition (paronychia 69%, rash 64%, diarrhea 32%, stomatitis 30%), MET inhibition (hypoalbuminemia 51% and edema 38%) and other common infusion reactions seen with Amivantamab (65%). Venous thromboembolism was noted in about 40% of patients, necessitating prophylactic anticoagulation. Subgroup analysis revealed that median PFS in patients with exon 19 deletion was 27.9 months (95%CI: 25.1–NE), HR 0.65 (0.51-0.85), which was significantly longer in comparison to patients with an exon 21 L858R substitution (24.7 months, 95%CI: 19.5–27.4), HR 0.78, 0.59-1.02). This observation was attributed to Ex19del mutations being more sensitive to TKIs. Addition of Amivantamab was hypothesized to block resistance pathways like MET amplification which frequently emerge in patients receiving EGFR TKIs. For patients with baseline brain metastasis, the combination showed superiority in median PFS (18.3 months with combination therapy vs 13 months with Osimertinib monotherapy, HR 0.69, p=0.01). Similarly, benefits were seen consistently in other high risk groups including patients with liver metastasis (median PFS 18.2 months vs 11 months, HR 0.58, p=0.017), those harboring TP53 co-mutations (median PFS 18.2 vs 12.9 months, HR 0.65, p=0.003), age less than 65 years (HR 0.50, 0.39-0.65) and those with detectable circulating tumour DNA (ctDNA) at baseline (20.3 months vs 14.8 months, HR 0.68, p=0.002)(12).
Table 1.
Overview of 1st line trials in EGFR mutated mNSCLC.
Table 1.
Overview of 1st line trials in EGFR mutated mNSCLC.
| Trial (Arm) |
Median Age (Range) |
Liver Metastases |
CNS Metastases |
TP53 Mutation |
Baseline ctDNA |
ORR |
PFS (median) |
CNS PFS (median) |
OS (median) |
|
FLAURA – Osimertinib |
64 years
(26-85) |
Not reported |
19.0% |
Not reported |
Not reported |
80%
(75-85%) |
18.9 months (95% CI 15.2–21.4) |
CNS PFS (18 months) 58% (95% CI 40-72) |
38.6 months (95% CI 34.5–41.8) |
|
FLAURA – Gefitinib/Erlotinib |
64 years
(35-93) |
Not reported |
23.0% |
Not reported |
Not reported |
76%
(70-81%) |
10.2 months (95% CI 9.6–11.1) |
CNS PFS (18 months) 40% (95% CI 25-55) |
31.8 months (95% CI 26.6–36.0) |
|
FLAURA2 – Osimertinib + Chemo |
61 years
(26-83) |
15.4% |
41.6% |
Not reported |
Not reported |
83%
(78-87%) |
25.5 months (95% CI ~) (HR 0.62) |
24.9 months
(patients with baseline CNS mets) |
NR (95% CI 38.0- NR), interim HR 0.75, p: 0.028 |
|
FLAURA2 – Osimertinib (monotherapy) |
62 years
(30-85) |
23.7% |
39.6% |
Not reported |
Not reported |
76%
(70-80%) |
16.7 months (95% CI ~) |
13.8 months
(patients with baseline CNS mets) |
36.7 months (95% CI 33.2- NR) |
|
MARIPOSA – Amivantamab + Lazertinib |
64 years
(25-88) |
15% |
41.4% |
56% |
69.2% |
86% (83-89%) |
23.7 months (95% CI 19.1–27.7) |
25.4 months (95% CI 20.1-29.5), HR 0.79, p: 0.07 |
NR (95% CI 42.9- NR) (interim HR 0.75, p<0.005) |
|
MARIPOSA – Osimertinib |
63 years
(28-88) |
17% |
40% |
52.5% |
71.4% |
85% (81-88%) |
16.6 months (95% CI 14.8–18.5) |
22.2 months (95%CI, 18.4-26.9) |
36.7 months
(95% CI 33.4–41.0) |
3. Choosing First Line Therapy
The first line treatment of EGFR mutated mNSCLC has become complex with the arrival of two new treatment options- Osimertinib plus chemotherapy and a chemotherapy free approach with Amivantamab and Lazertinib. These regimens have shown an improved PFS in large phase III trials and recent data suggests that they also improve OS. However, we believe that there are finer nuances to consider while selecting front line therapy and all patients may not require a combination approach.
Although most patients on Osimertinib experience chronic grade 1-2 toxicities, the incidence of grade 3-4 toxicities remains low with Osimertinib monotherapy(<5%). Clinical experience suggests that most patients on Osimertinib monotherapy remain functionally adept, enjoy a good quality of life and require few visits to clinic (minimal time toxicity)(5).
Addition of a platinum doublet adds to hematological (anemia, neutropenia, thrombocytopenia) and non-hematological toxicity (fatigue, nausea) with nearly twice the number of patients discontinuing treatment compared to Osimertinib monotherapy. It also increases the number of hospital visits (three weekly infusions) and lab visits thereby increasing “time toxicity” (7). Duration of chemotherapy required to achieve benefit also remains uncertain and whether time limited chemotherapy can achieve similar benefit as continuous chemotherapy is a relevant future research question given chronic cumulative toxicities of chemotherapy. It would be interesting to see quality of life (QOL) data from the FLAURA2 study which would shed light on patient reported outcomes.
Surprisingly, the chemotherapy free regimen tested in MARIPOSA trial has even higher rates of adverse events with substantial burden of dermatological and hematological side effects, risk of venous thromboembolism (VTE) and infusion reactions. Rate of discontinuation is nearly three times that of Osimertinib and chemotherapy, thereby highlighting the challenges in administering this regimen. It is also associated with a significant burden on the health care system and an increased amount of time toxicity for patients, as Amivantamab is required to be infused at a weekly interval for five weeks followed by two weekly intervals thereafter(13). Various strategies have been tried to reduce the associated toxicities including enhanced dermatological prophylaxis (14,15), premedication with dexamethasone for infusion reactions(16,17) and prophylactic anticoagulation which may need to be incorporated routinely in clinical practice.
Despite these toxicities, there are certain patients who have a high risk of early progression on single agent Osimertinib. These include younger patients (age <65 years) with brain metastasis at the time of diagnosis, exon 21 L858R substitution, presence of liver metastasis and those harboring TP53 co-mutation. The presence of detectable ctDNA and lack of early clearance have also recently been associated with a shorter survival (18). In such patients, the benefits of using a combination therapy may outweigh the risks. For patients without these high-risk features, treatment with single agent Osimertinib remains a very reasonable choice.
Among the different combination regimens available, either the regimen of Osimertinib plus chemotherapy or Amivantamab plus Lazertinib is reasonable given lack of comparative data between these two. Positive OS benefit seen for Amivantamab and Lazertinib is a step in the right direction however lack of data on patient reported outcomes from both FLAURA-2 and MARIPOSA makes these discussions with patients challenging. High risk features including TP53 co-mutation and evaluation of CNS PFS seem more robust in the MARIPOSA trial and this regimen may be preferred in patients with brain metastasis or those with a TP53 co-mutation. However, given the vastly different toxicity profiles of these regimens, a nuanced discussion with patients regarding their -goals and preferences is essential. Shared decision making is vital. Data suggest that despite survival benefit, patients may prefer Osimertinib monotherapy over a combination, even at the cost of reduced survival (19). More studies looking at patient preferences and biomarkers to limit treatment duration and reduce toxicity while benefitting patients at high risk is imperative.
4. Mechanisms of Resistance and Second Line Treatment
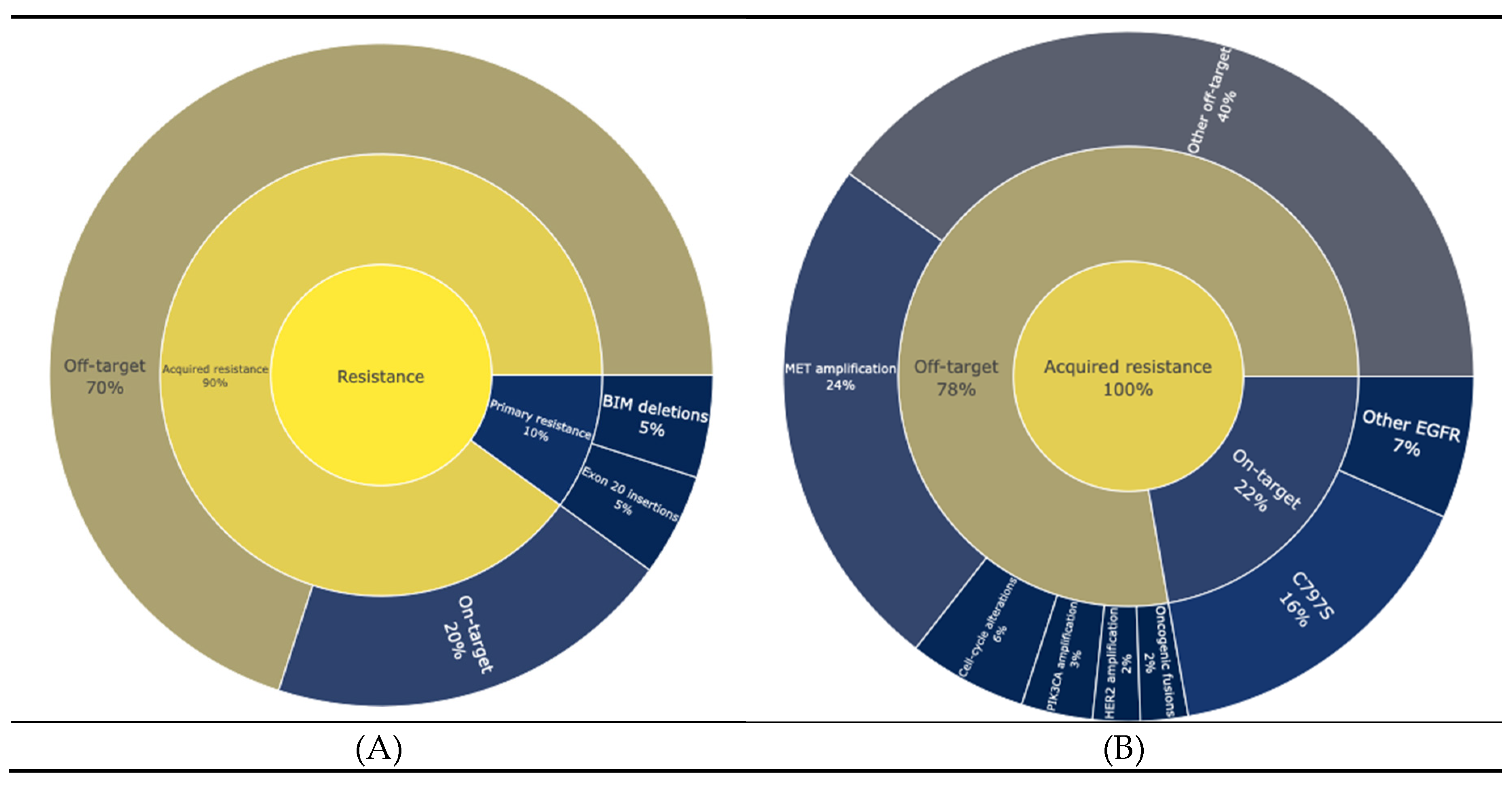
Resistance to EGFR targeted therapies can be broadly categorized into primary and acquired resistance (
Figure 1A). Primary resistance refers to instances where tumours fail to respond to treatment from the outset. For instance, NSCLCs with specific activating mutations in EGFR, such as exon 20 insertions, are largely unresponsive to most EGFR-TKIs(20). Additionally, concurrent genetic abnormalities such as BIM (BCL2L11) deletions, have been associated with reduced responsiveness to TKIs, even in patients with EGFR sensitizing mutations (21,22). In contrast, acquired resistance develops after an initial period of therapeutic efficacy and is classified as either on-target or off-target. On target resistance mechanisms include mutations in the C797S domain occurring in 14% of cases, while less frequent mutations include C797G, L792H/F, G796S and L718Q. Off-target resistance stems from mechanisms that bypass EGFR signaling, such as MET Amplification (22%), HER2 amplification, PIK3CA amplification, alterations in cell cycle genes, and oncogenic fusions such as FGFR3-TACC3, NTRK1-TPM3, RET-ERC1, and RET-CCDC6(23,24) (
Figure 1B)
In the context of newer combination strategies discussed above, For patients receiving combination therapy with Osimertinib and chemotherapy in the FLAURA-2 trial, mechanisms of resistance identified were similar compared to Osimertinib monotherapy, however fewer patients developed a resistance pathway mutation in the combination arm compared to monotherapy (40% vs 46%)(25).
Conversely, in the MARIPOSA study, combination therapy with Amivantamab and Lazertinib reduced emergence of MET amplification (4.4% vs 13.6% for monotherapy) and EGFR dependent resistance mechanisms (0.9% vs 7.9%). There was also reduction of TP53/RB1 loss (0.9% vs 2.9%) which has traditionally been associated with transformation to small cell phenotype. Bypass pathway alterations were higher in patients treated with Amivantamab and Lazertinib, including HER2 amplification (7.1%), RAS/RAF kinase mutations (9.7%), PI3K pathway activation(8%) and cell cycle pathway alterations (13.3%). In addition, there was also a higher incidence of complex resistance mechanisms in patients treated with Amivantamab and Lazertinib (26).
5. 2nd Line Treatment Options and Ongoing Trials:
Based on available evidence, treatment options for EGFR mutated mNSCLC in the first line include Osimertinib monotherapy, combination therapy with Osimertinib plus chemotherapy and a chemotherapy free combination of Amivantamab and Lazertinib. Post progression therapy poses a challenge and various strategies targeting different resistance mechanisms are being currently explored in clinical trials (
Table 2):
6. Targeting On-Target EGFR Resistance
Fourth-generation EGFR tyrosine-kinase inhibitors (TKIs) are being developed to counter C797S substitution—the prototypical on-target mechanism of resistance emerging after Osimertinib therapy. Early phase I findings with BLU-945, evaluated in the SYMPHONY study, demonstrate favourable tolerability both as monotherapy and in combination with Osimertinib(27). Additional fourth-generation agents like BDTX-1535 and BBT-176 have been designed to suppress triple-mutant EGFR variants. BDTX-1535, notable for its central-nervous-system penetration and broad mutant coverage, has produced preliminary tumour regressions in relapsed or refractory EGFR-mutant NSCLC(28). When T790M and C797S occur in trans, dual inhibition with Osirmertinib plus a first-generation TKI restores drug sensitivity. This approach is currently being formally tested in the adaptive ORCHARD platform trial, which includes a cohort receiving Osirmertinib + Gefitinib for patients who acquire EGFR C797X after frontline Osirmertinib treatment(29).
7. Off Target Inhibition:
Simultaneous angiogenesis and immune checkpoint blockers have been hypothesized to offset resistance to EGFR-directed therapy based on the ABCP arm of the IMpower150 study. This generated an early signal of efficacy in tumours harbouring EGFR or ALK driver alterations(30). The bispecific antibody Ivonescimab, which co-targets vascular endothelial growth factor (VEGF) and programmed cell death-1 (PD-1), produced encouraging results in a phase II cohort of EGFR-mutant metastatic NSCLC(31). This has laid the foundation for HARMONI-A trial, a randomised multicentre study conducted across 55 sites in China with patients who have progressed on prior EGFR-TKI therapy. Ivonescimab combined with platinum-based chemotherapy has prolonged median PFS to 7.1 months versus 4.8 months with chemotherapy alone (hazard ratio 0.46; 95% CI 0.34–0.62). The overall-survival results are immature for formal analysis(32).
The MARIPOSA-2 trial tested Amivantamab chemothetapy and Amivantamab-lazertinib-chemotherapy -versus chemotherapy alone in patients who had disease progression on Osimertinib. While this study was not powered to detect differences between arms containing Amivantamab, it showed that either regimen improved PFS compared to chemotherapy alone (median improvement of 2 months for Amivantamab plus chemotherapy and 4 months for Amivantamab- Lazertinib- chemotherapy)(33). Toxicities were consistent with what those described for Amivantamab and Lazertinib in the MARIPOSA trial, however, OS benefit has not been demonstrated and QOL data is not yet available.
8. Combined On-Target and Off Target Inhibition
Multiple strategies have been explored to target MET amplification in conjunction with EGFR inhibition. The MARIPOSA-2 trial included a treatment arm combining Amivantamab, Lazertinib and chemotherapy, however the specific contribution of Lazertinib remained unclear as the trial was not statistically powered for this direct comparison(33). Level 1 evidence supporting the efficacy of subcutaneous (SC) Amivantamab combined with Lazertinib in patients experiencing progression on Osimertinib and chemotherapy was demonstrated in the PALOMA-3 trial. This study highlighted superior tolerability with the SC formulation, reporting reduced infusion-related reactions (13% vs 66%) and fewer venous thromboembolic events (9% vs 14%). This study further demonstrated improved clinical outcomes, including a prolonged median PFS with subcutaneous Amivantamab of 6.1 months compared to 4.3 months, and an OS benefit (HR 0.62; 95% CI: 0.42–0.92)(34).
Additional therapeutic approaches have examined combining Osimertinib with selective MET inhibitors. The Phase Ib TATTON trial (NCT02143466) assessed Osimertinib in combination with Savolitinib, a potent MET inhibitor, in patients with MET-amplified NSCLC who had previously developed resistance following treatment with at least one prior EGFR TKI. This combination achieved an objective response rate (ORR) of 52%, with a median duration of response (mDOR) of 7.1 months(35). Similarly, the INSIGHT-2 study evaluated Tepotinib, another selective MET inhibitor, in combination with Osimertinib in the same clinical context. This regimen demonstrated an ORR of 50%, mDOR of 8.5 months, median PFS of 5.6 months, and median OS of 17.8 months(36). In another phase II SAVANNAH study of patients with EGFR mutated mNSCLC with MET overexpression (3+ by IHC) or amplification (>10 copies by FISH), combination of Savolitinib plus Osimertinib was superior to Savolitinib alone again highlighting the importance of continued EGFR inhibition in the presence of MET amplification (37)
9. Targeting Tumor Antigens- ADC’s
Antibody–drug conjugates (ADCs) have emerged as compelling later-line options as their cytotoxic payloads retain activity across diverse resistance pathways. Datopotamab deruxtecan (Dato-DXd) has received FDA Breakthrough Therapy designation for EGFR-mutated NSCLC that has progressed after Osimertinib and platinum chemotherapy based on the strength of data from the TROPION-Lung05 trial and the EGFR-mutant subset of TROPION-Lung01(38,39). In TROPION-Lung05, heavily pre-treated patients achieved an ORR of 43.8 % with a median duration of response of approximately seven months. Patritumab deruxtecan (HER3-DXd), an ADC directed against HER3, has likewise demonstrated modest PFS benefit in the phase III HERTHENA-Lung02 study among patients who relapsed after Osimertinib, OS data are still pending (40). Sacituzumab Tirumotecan (sac-TMT) is a Trop2 directed ADC which has also shown promising clinical activity with an ORR of 45% vs 16% with docetaxel, median PFS 6.9 vs 2.8 months, HR 0.30, 0.20-0.46, and 12 month OS 73% vs 54%, HR 0.36, 0.20-0.66 in 3rd line setting in patients who have had disease progression on an EGFR TKI and platinum based chemotherapy in a randomised trial done in China (41).
10. Choosing Second Line therapy (Figure 2):
The optimal post-progression strategy is dependent upon the frontline regimen and molecular alterations that subsequently emerge. In patients who relapse after single-agent Osimertinib, the combination of Amivantamab plus platinum–pemetrexed chemotherapy is now FDA-approved on the basis of the MARIPOSA-2 study. Whether the addition of Lazertinib meaningfully augments the antibody–chemotherapy doublet remains uncertain, and the triplet is associated with higher toxicity. Moreover, PFS benefit with either regimen is modest at best and neither regimen has yet demonstrated an OS advantage. Subcutaneous Amivantamab promises to be an important step to reduce infusion reactions and mitigate burden on the patients and the health care system and regulatory approval is eagerly awaited. At disease progression, repeat molecular profiling should be pursued to guide enrolment into trials of antibody–drug conjugates or agents matched to specific resistance pathways. Bispecific antibodies such as Ivonescimab are intriguing, but confirmation in larger ethnically diverse cohorts is required prior to widespread adoption. Outside a study context, systemic chemotherapy remains the cornerstone of management. While ablative radiotherapy to oligoprogressive sites can extend first-line benefit; participation in trials such as the CURB-2 is encouraged(43,44).
For tumours progressing on the FLAURA-2 regimen (osimertinib plus chemotherapy), Amivantamab combined with Lazertinib is supported by PALOMA-3 data, with the subcutaneous formulation of Amivantamab expected to reduce infusion-related toxicity and improve resource utilisation. Given that resistance patterns after Osimertinib + chemotherapy mirror those seen with Osimertinib alone, bispecific antibodies and ADCs are likely to play a central role in this setting as well.
Albeit an OS benefit has been demonstrated with using Amivantamab–Lazertinib in the first line in the MARIPOSA study, its delivery is operationally complex and no validated options exist once resistance develops. Currently, platinum-based chemotherapy constitutes the standard of care after progression on this doublet and enrolment on clinical trials for drugs matched to resistance mechanisms and ADC’s is highly encouraged.
Figure 2.
Choosing second line treatment.
Figure 2.
Choosing second line treatment.
11. Conclusions
Targeted therapy has transformed the treatment landscape of EGFR-mutated metastatic NSCLC, with Osimertinib firmly established as the standard first-line agent owing to its excellent systemic and intracranial efficacy, ease of administration, low risk of high grade toxicity and patient preferences for oral therapy. Yet, biologic heterogeneity like the divergent outcomes observed between exon 19 deletions and L858R substitutions demand an increasingly individualised approach. Phase III trials such as FLAURA2 and MARIPOSA which incorporate platinum–pemetrexed chemotherapy or pairing EGFR inhibition with MET blockade, demonstrate that intensification strategies can extend disease control for biologically high-risk cohorts. However, these gains are counterbalanced by greater toxicity, higher cost, and logistical complexity thereby underscoring the need for judicious patient selection.
Resistance to Osimertinib is frequently driven by MET amplification or secondary EGFR alterations. Combination regimens that add selective MET TKIs to Osimertinib, alongside fourth-generation EGFR inhibitors capable of suppressing C797S and other tertiary mutations, are emerging as promising salvage options. In the post-progression setting, cytotoxic chemotherapy, antibody drug conjugates and clinical-trial enrolment based on emerging resistance mutations continue to serve as essential pillars of care.
Moving forward, our aim should be to refine therapeutic sequencing, mitigate cumulative toxicity and expand access to novel agents. Therefore, advancements in biomarker-directed trial designs that pre-empt resistance, interventions that delay or forestall disease evolution, and globally inclusive studies to bridge disparities in drug availability are essential for optimal outcome.
Author Contributions
Conceptualization, PGR and AM.; Methodology, PGR, AM, SV and NP.; Resources, PGR, AM; Data Curation, PGR, AM, DR, NP, SV.; Writing – Original Draft Preparation, PGR, AM.; Writing – Review & Editing, PGR, NP, SV, DR, AM, AG, NM, CM, PSM; Supervision, AM, AG, NM, CM, PSM; Project Administration, AM, AG, NM, CM, PSM.
Conflicts of Interest
Prabhat Gautam Roy- None. Davida Reingold- None. Neha Pathak- None. Conso Molto-Honoraria from Merck and AstraZeneca. Nicholas Meti- Honoraria from AstraZeneca, Merck, Pfizer, Novartis, Gilead. Prabhat Singh Malik- None. Aarushi Gupta-None. Geordie Linford- Honoraria from: Merck, Bayer, BMS, Knight Pharmaceuticals. Consultancy: Pfizer. Abhenil Mittal- Honoraria from Merck, Astrazeneca, Gilead, Janssen, Novartis, Roche; Advisory Board for Gilead and Janssen.
References
- Graham, R.P.; Treece, A.L.; Lindeman, N.I.; Vasalos, P.; Shan, M.; Jennings, L.J.; Rimm, D.L. Worldwide Frequency of Commonly Detected EGFR Mutations. Arch. Pathol. Lab. Med. 2018, 142, 163–167. [CrossRef]
- Melosky, B.; Kambartel, K.; Häntschel, M.; Bennetts, M.; Nickens, D.J.; Brinkmann, J.; Kayser, A.; Moran, M.; Cappuzzo, F. Worldwide Prevalence of Epidermal Growth Factor Receptor Mutations in Non-Small Cell Lung Cancer: A Meta-Analysis. Mol. Diagn. Ther. 2021, 26, 7–18. [CrossRef]
- Shi, Y.; Au, J.S.-K.; Thongprasert, S.; Srinivasan, S.; Tsai, C.-M.; Khoa, M.T.; Heeroma, K.; Itoh, Y.; Cornelio, G.; Yang, P.-C. A Prospective, Molecular Epidemiology Study of EGFR Mutations in Asian Patients with Advanced Non–Small-Cell Lung Cancer of Adenocarcinoma Histology (PIONEER). J. Thorac. Oncol. 2014, 9, 154–162. [CrossRef]
- D’ANgelo, S.P.; Pietanza, M.C.; Johnson, M.L.; Riely, G.J.; Miller, V.A.; Sima, C.S.; Zakowski, M.F.; Rusch, V.W.; Ladanyi, M.; Kris, M.G. Incidence of EGFR Exon 19 Deletions and L858R in Tumor Specimens From Men and Cigarette Smokers With Lung Adenocarcinomas. J. Clin. Oncol. 2011, 29, 2066–2070. [CrossRef]
- Soria, J.-C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [CrossRef]
- Cho, B.C.; Lu, S.; Felip, E.; Spira, A.I.; Girard, N.; Lee, J.-S.; Lee, S.-H.; Ostapenko, Y.; Danchaivijitr, P.; Liu, B.; et al. Amivantamab plus Lazertinib in Previously Untreated EGFR -Mutated Advanced NSCLC. New Engl. J. Med. 2024, 391, 1486–1498. [CrossRef]
- Planchard, D.; Jänne, P.A.; Cheng, Y.; Yang, J.C.-H.; Yanagitani, N.; Kim, S.-W.; Sugawara, S.; Yu, Y.; Fan, Y.; Geater, S.L.; et al. Osimertinib with or without Chemotherapy in EGFR -Mutated Advanced NSCLC. New Engl. J. Med. 2023, 389, 1935–1948. [CrossRef]
- Yang, J.-H.; Kim, Y.; Lee, S.-H.; Liu, B.; Ostapenko, Y.; Lu, S.; Alip, A.; Korbenfeld, E.; Dias, J.; Danchaivijitr, P.; et al. 4O: Amivantamab plus lazertinib vs osimertinib in first-line (1L) EGFR-mutant (EGFRm) advanced NSCLC: Final overall survival (OS) from the phase III MARIPOSA study. J. Thorac. Oncol. 2025, 20, S6–S8. [CrossRef]
- PVI_slides_LakeTahoe24.pdf [Internet]. [cited 2025 Apr 22]. Available from: https://c.peerview.com/live/programs/150210399-1/downloads/PVI_slides_LakeTahoe24.pdf.
- Planchard, D.; Jänne, P.A.; Cheng, Y.; Yang, J.C.-H.; Yanagitani, N.; Kim, S.-W.; Sugawara, S.; Yu, Y.; Fan, Y.; Geater, S.L.; et al. Osimertinib with or without Chemotherapy in EGFR -Mutated Advanced NSCLC. New Engl. J. Med. 2023, 389, 1935–1948. [CrossRef]
- Yang, J.-H.; Kim, Y.; Lee, S.-H.; Liu, B.; Ostapenko, Y.; Lu, S.; Alip, A.; Korbenfeld, E.; Dias, J.; Danchaivijitr, P.; et al. 4O: Amivantamab plus lazertinib vs osimertinib in first-line (1L) EGFR-mutant (EGFRm) advanced NSCLC: Final overall survival (OS) from the phase III MARIPOSA study. J. Thorac. Oncol. 2025, 20, S6–S8. [CrossRef]
- Felip et al. - 2024 - Amivantamab plus lazertinib versus osimertinib in .pdf [Internet]. [cited 2025 Apr 22]. Available from: https://www.annalsofoncology.org/article/S0923-7534(24)00702-6/pdf.
- Felip, E.; Cho, B.; Gutiérrez, V.; Alip, A.; Besse, B.; Lu, S.; Spira, A.; Girard, N.; Califano, R.; Gadgeel, S.; et al. Amivantamab plus lazertinib versus osimertinib in first-line EGFR-mutant advanced non-small-cell lung cancer with biomarkers of high-risk disease: a secondary analysis from MARIPOSA. Ann. Oncol. 2024, 35, 805–816. [CrossRef]
- Study Details | Enhanced Dermatological Care to Reduce Rash and Paronychia in Epidermal Growth Factor Receptor (EGRF)-Mutated Non-Small Cell Lung Cancer (NSCLC) Treated First-line With Amivantamab Plus Lazertinib | ClinicalTrials.gov [Internet]. [cited 2025 Apr 22]. Available from: https://www.clinicaltrials.gov/study/NCT06120140#study-overview.
- Girard, N.; Li, W.; Spira, A.; Feldman, J.; Mak, M.; Sauder, M.; Bozorgmehr, F.; Voon, P.-J.; Yang, C.; Cundom, J.; et al. 10MO: Preventing moderate to severe dermatologic adverse events in first-line EGFR-mutant advanced NSCLC treated with amivantamab plus lazertinib: Early success of the COCOON trial. J. Thorac. Oncol. 2025, 20, S14–S16. [CrossRef]
- Study Details | Premedication to Reduce Amivantamab Associated Infusion Related Reactions | ClinicalTrials.gov [Internet]. [cited 2025 Apr 22]. Available from: https://www.clinicaltrials.gov/study/NCT05663866.
- Spira AI, Paz-Ares L, Han JY, Shih JY, Mascaux C, Roy UB, et al. Preventing Infusion-Related Reactions With Intravenous Amivantamab-Results From SKIPPirr, a Phase 2 Study: A Brief Report. J Thorac Oncol. 2025 Jun;20(6):809–16.
- ESMO. ctDNA-Guided Treatment Influence on Survival in Advanced NSCLC [Internet]. [cited 2025 Apr 22]. Available from: https://www.esmo.org/oncology-news/ctdna-guided-treatment-influence-on-survival-in-advanced-nsclc.
- Cancer Network [Internet]. 2023 [cited 2025 Apr 22]. Osimertinib/Chemo Elicits Meaningful PFS in EGFR+ NSCLC. Available from: https://www.cancernetwork.com/view/osimertinib-chemo-elicits-meaningful-pfs-in-egfr-nsclc.
- Pao, W.; Miller, V.A.; Politi, K.A.; Riely, G.J.; Somwar, R.; Zakowski, M.F.; Kris, M.G.; Varmus, H. Acquired Resistance of Lung Adenocarcinomas to Gefitinib or Erlotinib Is Associated with a Second Mutation in the EGFR Kinase Domain. PLoS Med. 2005, 2, e73. [CrossRef]
- Ramalingam SS, Cheng Y, Zhou C, Ohe Y, Imamura F, Cho BC, et al. Mechanisms of acquired resistance to first-line osimertinib: Preliminary data from the phase III FLAURA study. Annals of Oncology [Internet]. 2018 Oct 1 [cited 2024 Dec 13];29:viii740. Available from: https://www.annalsofoncology.org/article/S0923-7534(19)50454-9/fulltext.
- Chmielecki, J.; Mok, T.; Wu, Y.-L.; Han, J.-Y.; Ahn, M.-J.; Ramalingam, S.S.; John, T.; Okamoto, I.; Yang, J.C.-H.; Shepherd, F.A.; et al. Analysis of acquired resistance mechanisms to osimertinib in patients with EGFR-mutated advanced non-small cell lung cancer from the AURA3 trial. Nat. Commun. 2023, 14, 1–8. [CrossRef]
- Oxnard, G.R.; Hu, Y.; Mileham, K.F.; Husain, H.; Costa, D.B.; Tracy, P.; Feeney, N.; Sholl, L.M.; Dahlberg, S.E.; Redig, A.J.; et al. Assessment of Resistance Mechanisms and Clinical Implications in Patients WithEGFRT790M–Positive Lung Cancer and Acquired Resistance to Osimertinib. JAMA Oncol. 2018, 4, 1527–1534. [CrossRef]
- Yang Z, Yang N, Ou Q, Xiang Y, Jiang T, Wu X, et al. Investigating Novel Resistance Mechanisms to Third-Generation EGFR Tyrosine Kinase Inhibitor Osimertinib in Non-Small Cell Lung Cancer Patients. Clin Cancer Res. 2018 Jul 1;24(13):3097–107.
- Yang JC, Robichaux J, Planchard D, Kobayashi K, Lee CK, Sugawara S, et al. MA12.03 FLAURA2: Resistance, and Impact of Baseline TP53 Alterations in Patients Treated With 1L Osimertinib ± Platinum-Pemetrexed. Journal of Thoracic Oncology. 2024 Oct 1;19:S101–2.
- Besse, B.; Lee, S.-H.; Lu, S.; Stroyakovskiy, D.; Yazici, O.; Cid, J.R.; Hayashi, H.; Nguyen, D.; Yang, J.-H.; Gottfried, M.; et al. LBA55 Mechanisms of acquired resistance to first-line amivantamab plus lazertinib versus osimertinib in patients with EGFR-mutant advanced non-small cell lung cancer: An early analysis from the phase III MARIPOSA study. Ann. Oncol. 2024, 35, S1245–S1246. [CrossRef]
- Shum, E.; Elamin, Y.Y.; Piotrowska, Z.; Spigel, D.R.; Reckamp, K.L.; Rotow, J.K.; Tan, D.S.-W.; Lim, S.M.; Kim, T.M.; Lin, C.-C.; et al. A phase 1/2 study of BLU-945 in patients with common activating EGFR-mutant non–small cell lung cancer (NSCLC): SYMPHONY trial in progress.. J. Clin. Oncol. 2022, 40, TPS9156–TPS9156. [CrossRef]
- Black Diamond Therapeutics, Inc. A Phase 1/2 Study to Assess BDTX-1535, an Oral EGFR Inhibitor, in Patients With Glioblastoma or Non-Small Cell Lung Cancer [Internet]. clinicaltrials.gov; 2025 Mar [cited 2025 Apr 22]. Report No.: NCT05256290. Available from: https://clinicaltrials.gov/study/NCT05256290.
- AstraZeneca. A Biomarker-directed Phase 2 Platform Study in Patients With Advanced Non-Small Lung Cancer Whose Disease Has Progressed on First-Line Osimertinib Therapy. [Internet]. clinicaltrials.gov; 2025 Jan [cited 2025 Apr 22]. Report No.: NCT03944772. Available from: https://clinicaltrials.gov/study/NCT03944772.
- Socinski, M.A.; Nishio, M.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; et al. IMpower150 Final Overall Survival Analyses for Atezolizumab Plus Bevacizumab and Chemotherapy in First-Line Metastatic Nonsquamous NSCLC. J. Thorac. Oncol. 2021, 16, 1909–1924. [CrossRef]
- Fang, W.; Zhao, Y.; Yang, Y.; Zhou, N.; Chen, L.; Huang, Y.; Chen, J.; Zhuang, L.; Du, Y.; Zhuang, W.; et al. Phase II results of ivonescimab (AK112/ SMT112), a novel PD-1/VEGF bispecific, in combination with chemotherapy for first line treatment of advanced or metastatic non-small cell lung cancer (NSCLC) without actionable genomic alterations (AGA) in EGFR/ALK.. J. Clin. Oncol. 2023, 41, 9087–9087. [CrossRef]
- HARMONi-A Study Investigators; Fang, W.; Zhao, Y.; Luo, Y.; Yang, R.; Huang, Y.; He, Z.; Zhao, H.; Li, M.; Li, K.; et al. Ivonescimab Plus Chemotherapy in Non–Small Cell Lung Cancer With EGFR Variant. JAMA 2024, 332, 561–570. [CrossRef]
- Passaro, A.; Wang, J.; Wang, Y.; Lee, S.-H.; Melosky, B.; Shih, J.-Y.; Azuma, K.; Juan-Vidal, O.; Cobo, M.; Felip, E.; et al. Amivantamab plus chemotherapy with and without lazertinib in EGFR-mutant advanced NSCLC after disease progression on osimertinib: primary results from the phase III MARIPOSA-2 study. Ann. Oncol. 2023, 35, 77–90. [CrossRef]
- Leighl, N.B.; Akamatsu, H.; Lim, S.M.; Cheng, Y.; Minchom, A.R.; Marmarelis, M.E.; Sanborn, R.E.; Yang, J.C.-H.; Liu, B.; John, T.; et al. Subcutaneous Versus Intravenous Amivantamab, Both in Combination With Lazertinib, in Refractory Epidermal Growth Factor Receptor–Mutated Non–Small Cell Lung Cancer: Primary Results From the Phase III PALOMA-3 Study. J. Clin. Oncol. 2024, 42, 3593–3605. [CrossRef]
- Oxnard, G.R.; Yang, J.C.-H.; Yu, H.; Kim, S.-W.; Saka, H.; Horn, L.; Goto, K.; Ohe, Y.; Mann, H.; Thress, K.S.; et al. TATTON: a multi-arm, phase Ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann. Oncol. 2020, 31, 507–516. [CrossRef]
- Wu, Y.-L.; Guarneri, V.; Voon, P.J.; Lim, B.K.; Yang, J.-J.; Wislez, M.; Huang, C.; Liam, C.K.; Mazieres, J.; Tho, L.M.; et al. Tepotinib plus osimertinib in patients with EGFR-mutated non-small-cell lung cancer with MET amplification following progression on first-line osimertinib (INSIGHT 2): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2024, 25, 989–1002. [CrossRef]
- Levy, B.P.; de Marinis, F.; Bonanno, L.; Sacher, A.G.; Chu, Q.S.; Baik, C.S.; Bironzo, P.; Bazhenova, L.; Tiseo, M.; Proto, C.; et al. Efficacy and CNS results from a randomized subset of the phase 2 SAVANNAH study comparing savolitinib (savo) + osimertinib (osi) combination with savo + placebo (PBO).. J. Clin. Oncol. 2025, 43, 8513–8513. [CrossRef]
- Ahn, M.-J.; Tanaka, K.; Paz-Ares, L.; Cornelissen, R.; Girard, N.; Pons-Tostivint, E.; Baz, D.V.; Sugawara, S.; Cobo, M.; Pérol, M.; et al. Datopotamab Deruxtecan Versus Docetaxel for Previously Treated Advanced or Metastatic Non–Small Cell Lung Cancer: The Randomized, Open-Label Phase III TROPION-Lung01 Study. J. Clin. Oncol. 2025, 43, 260–272. [CrossRef]
- Sands, J.; Ahn, M.-J.; Lisberg, A.; Cho, B.C.; Blumenschein, G.; Shum, E.; Tostivint, E.P.; Goto, Y.; Yoh, K.; Heist, R.; et al. Datopotamab Deruxtecan in Advanced or Metastatic Non–Small Cell Lung Cancer With Actionable Genomic Alterations: Results From the Phase II TROPION-Lung05 Study. J. Clin. Oncol. 2025, 43, 1254–1265. [CrossRef]
- Mok, T.; A Jänne, P.; Nishio, M.; Novello, S.; Reck, M.; Steuer, C.; Wu, Y.-L.; Fougeray, R.; Fan, P.-D.; Meng, J.; et al. HERTHENA-Lung02: phase III study of patritumab deruxtecan in advanced EGFR -mutated NSCLC after a third-generation EGFR TKI. Futur. Oncol. 2023, 20, 969–980. [CrossRef]
- Fang W, Li X, Wang Q, Meng X, Zheng W, Sun L, et al. Sacituzumab tirumotecan versus docetaxel for previously treated EGFR-mutated advanced non-small cell lung cancer: multicentre, open label, randomised controlled trial. BMJ. 2025 Jun 5;389:e085680.
- Oxnard, G.R.; Cantarini, M.; Frewer, P.; Hawkins, G.; Peters, J.; Howarth, P.; Ahmed, G.F.; Sahota, T.; Hartmaier, R.; Li-Sucholeiki, X.; et al. SAVANNAH: A Phase II trial of osimertinib plus savolitinib for patients (pts) with EGFR-mutant, MET-driven (MET+), locally advanced or metastatic non-small cell lung cancer (NSCLC), following disease progression on osimertinib. J. Clin. Oncol. 2019, 37 (Suppl. 15), TPS9119–TPS9119. [CrossRef]
- Tsai, C.J.; Yang, J.T.; Shaverdian, N.; Patel, J.; Shepherd, A.F.; Guttmann, D.; Yeh, R.; Gelblum, D.Y.; Namakydoust, A.; Preeshagul, I.; et al. Standard-of-care systemic therapy with or without stereotactic body radiotherapy in patients with oligoprogressive breast cancer or non-small-cell lung cancer (Consolidative Use of Radiotherapy to Block [CURB] oligoprogression): an open-label, randomised, controlled, phase 2 study. Lancet 2023, 403, 171–182. [CrossRef]
- Willmann, J.; Badra, E.V.; Adilovic, S.; Ahmadsei, M.; Christ, S.M.; Tanadini-Lang, S.; Mayinger, M.; Guckenberger, M.; Andratschke, N. Stereotactic body radiotherapy for oligoprogression with or without switch of systemic therapy. Clin. Transl. Radiat. Oncol. 2024, 45, 100748. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).