1. Introduction
Highly pathogenic nematode parasites pose a significant threat to humans and animals, causing widespread morbidity and significant socio-economic losses at the global level. Chemotherapy remains the mainstay for controlling all helminthiases, but there are at list two main problems that compromise the use of antinematodal drugs: increasing resistance of parasitic nematodes and the toxicity of drugs if their doses are increased. The neuromuscular system of parasitic nematodes has proven to be an efficient pharmacological target for antihelmintics [
1]. Some of the most frequently used antiparasitic drugs are agonists of nicotinic acetylcholine receptors (nAChRs) (imidazothiazoles and tetrahydropyrimidines) or activators of both glutamate-gated chloride channels (GluCls) and GABA-receptors (macrocyclic lactones). Cholinergic agonists such as levamisole, pyrantel and oxantel selectively open ligand-gated acetylcholine ion channels expressed in nematode body wall muscles to induce spastic contraction of muscle cells leading to paralysis of the worms [
2]. Plants produce natural active organic compounds of secondary metabolism. Essential oils and their active ingredients, based on previous pharmacological studies, may be able to efficiently and securely replace (or act as adjuncts to) traditional antiparasitic drugs. The focuses of our research are terpenoid active ingredients (AIs) of plant essential oils (EOs). This is in line with the global need to reduce the use of synthetic veterinary medicines and transition to new plant-based drugs. On the other hand, considering the specific mechanism of antinematodal action of AIs of EOs, this can be an effective way to neutralize parasitic helminths. Our previous results on plant monoterpenoid evidenced that mechanism of antinematodal effects of carvacrol involved inhibition of parasite muscle contraction. Specifically, carvacrol inhibited acetylcholine (ACh) induced depolarizations of muscle cells indicating a direct interaction with nAChRs in Ascaris suum [
3,
4]. We observed that carvacrol enhanced the inhibitory effect of monepantel on A. suum contractions, which may have an effective clinical application. On the other hand, carveol potentiated the contractile effect of ACh in A. suum, indicating significant platform for potentiating the antinematodal action of nicotinic acetylcholine receptor (nAChR) agonists [
5]. It is obvious that AIs of plant EOs possess anthelmintic potential that could be applied in the pharmacotherapy of parasitic infections in humans and animals.
Here, we decided to examine the properties of geraniol, a cyclic monoterpene alcohol that is used as a repellent [
6,
7]. Geraniol is the main ingredient of rose oil, but is also present in many other EOs such as Palmarosa oil [
8]. Geraniol was found to be the most effective constituent of Pelargonium graveolens EO against the parasitic root-knot nematode Meloidogyne incognita [
9,
10]. Another investigation assessing the anthelmintic activity of the Cymbopogon martinii EO on Caenorhabditis elegans, resulted in geraniol as the anthelmintic component of palmarosa oil [
11]. Geraniol also exhibited larvicidal activity against the genus of roundworms Contracaecum [
12] and against marine nematodes Anisakis simplex [
13]. Also, geraniol disrupts the hatching of eggs of different strains of H. contortus in vitro, with an EC
50 value of 651.60 to 681.70μM, as well as the development of larvae with an EC
50 of 9.43 to 13.12mM [
14]. However, there are no data on the mechanism of antinematodal action of geraniol.
The aim of this study was to compare the antinematodal effects of geraniol and carvacrol, as well as their interaction in the model nematode Caenorhabditis elegans, as well as on the contractile model of the neuromuscular preparation A. suum and on the nicotinic acetylcholine receptor of A. suum ACR-16, expressed on Xenopus oocytes.
2. Results
2.1. Activity of Geraniol and Carvacrol on C. elegans
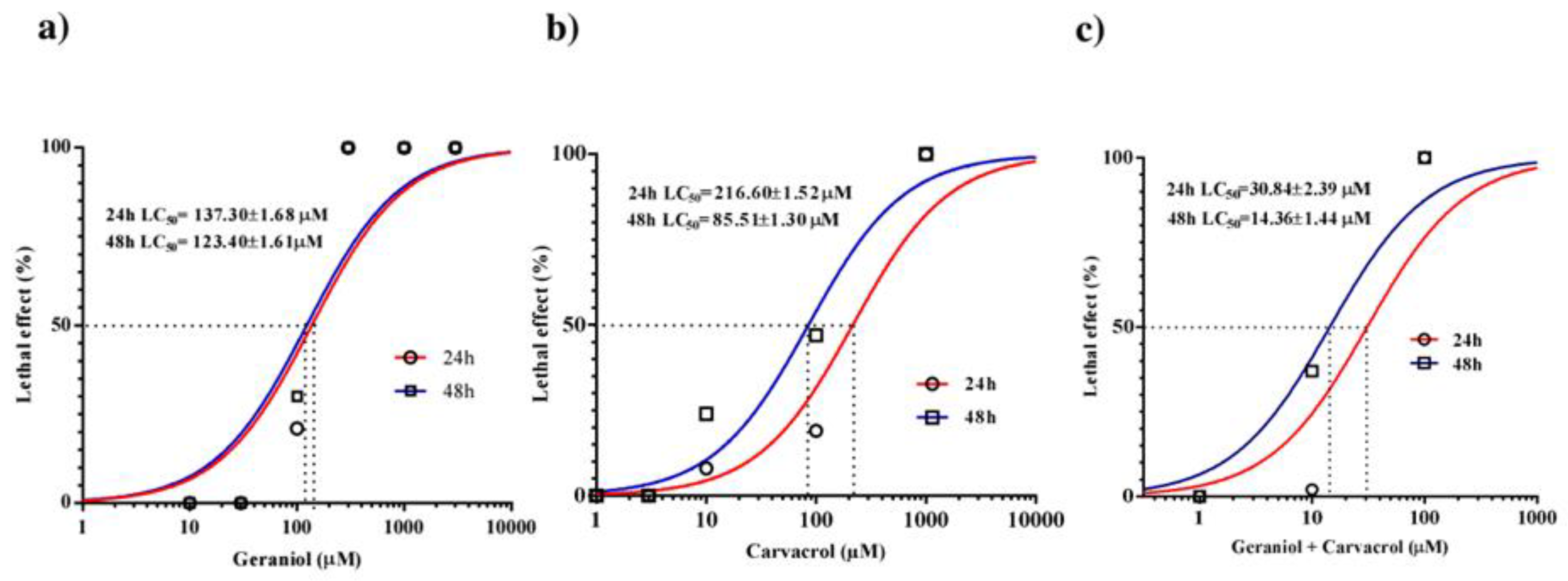
The median lethal concentration (LC
50) of geraniol for C. elegans after 24h of exposure was 137.30±1.68 μM and did not differ significantly after 48h, although it decreased to 123.40±1.61 μM (
Figure 1a). Geraniol caused atonic paralysis of the nematodes, which occurred before pharyngeal pumping ceased. The LC
50 value of carvacrol was 215.08±1.18 μM after 24 hours, and 84±1.13 μM after 48 hours (
Figure 1b). In the control experiments, no death of adult C. elegans individuals was recorded. Analyzing real-time motility recordings, it was observed that exposure to carvacrol leads to a slowing and cessation of pharyngeal pumping, which occurs before nematode movement ceases. When C. elegans was exposed to a combination of geraniol and carvacrol, the LC
50 value after 24 hours was 30.86±2.39 μM. After 48 hours of exposure, the LC
50 value decreased twice to 14.36±1.44μM (
Figure 1c).
2.2. Effect of Geraniol and Carvacrol on Ascaris suum Neuromuscular Contractions
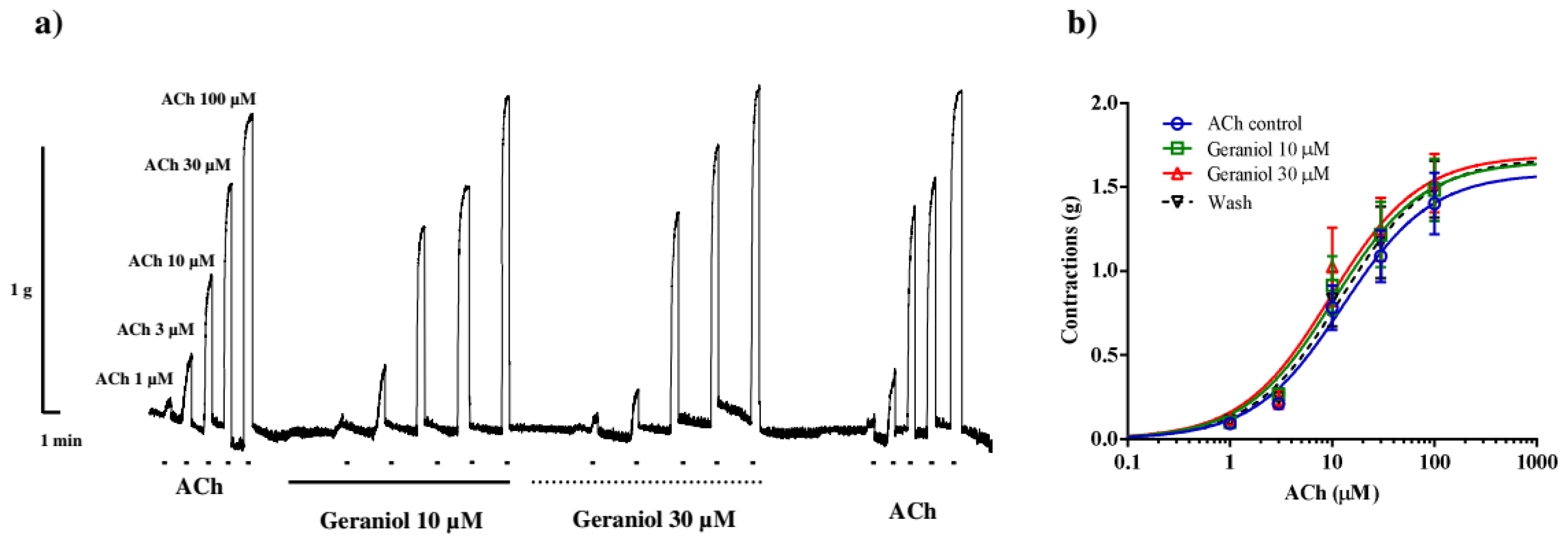
It was important for us to check whether geraniol affects the contractions of the neuromuscular preparation of A. suum induced by increasing concentrations of ACh (
Figure 2a). In the control series of contractions, the measured EC
50 value of ACh was 12.69±1.40 µM, while in the presence of 10 µM of geraniol, it was reduced to 10.32±1.43 µM, but the difference was not statistically significant (p=0.65). Geraniol at a concentration of 30 µM reduced the EC
50 of ACh to 9.60 ± 1.48 µM, but also without statistical significance (p=0.43). After washing, the EC
50 value was 12.11±1.44 µM (p=0.99). Incubation of the preparation with geraniol did not lead to a statistically significant change in the maximal contractile effect (E
max) of ACh. E
max in the control series of contractions was 1.58±0.16 g, and in the presence of 10 and 30µM of geraniol 1.65±0.0.17g and 1.69±0.19g, respectively (p=0.99 and 0.88). After washing and removing the geraniol from the bath solution, the E
max of contractions was 1.67±0.19g, which was not significantly different from the control value (p=0.98) (
Figure 2b).
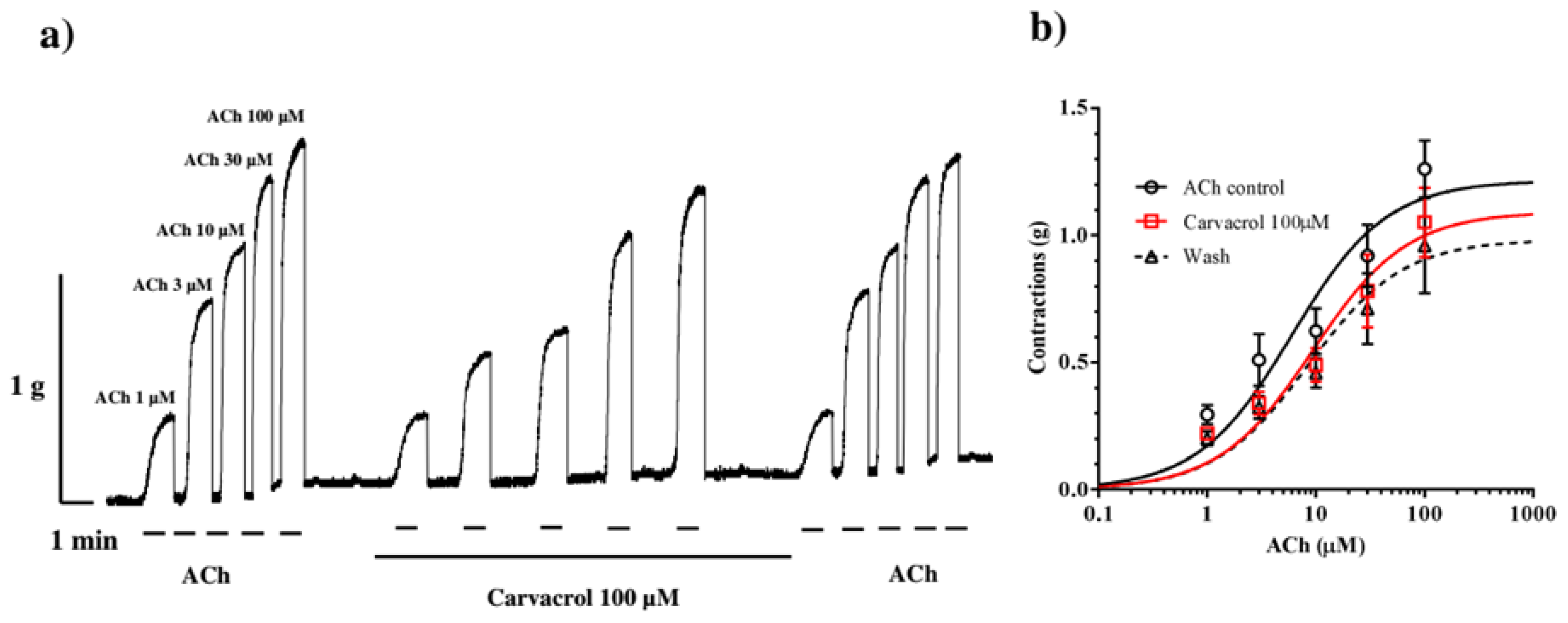
In a separate series of contractions, we examined the inhibitory effect of 100 μM of carvacrol on ACh-induced contractions. Increasing concentrations of ACh in the control series caused dose-dependent contractions of the Ascaris suum neuromuscular preparation with an EC
50 value of 6.03±1.40 μM (
Figure 3a). Carvacrol non-significantly increased the EC
50 value of ACh to 9.35±1.46 μM (p=0.64), and it did not change even after removing carvacrol from the experimental bath, being 8.57±0.20 μM (p=0.28). Also, carvacrol non-significantly reduced the E
max value of ACh. The control Emax was 1.22±0.11g, while in the presence of carvacrol 100 μM, it was 1.09±0.12g (p=0.73) and after washing 0.98±0.13 g (p=0.93) (
Figure 3b).
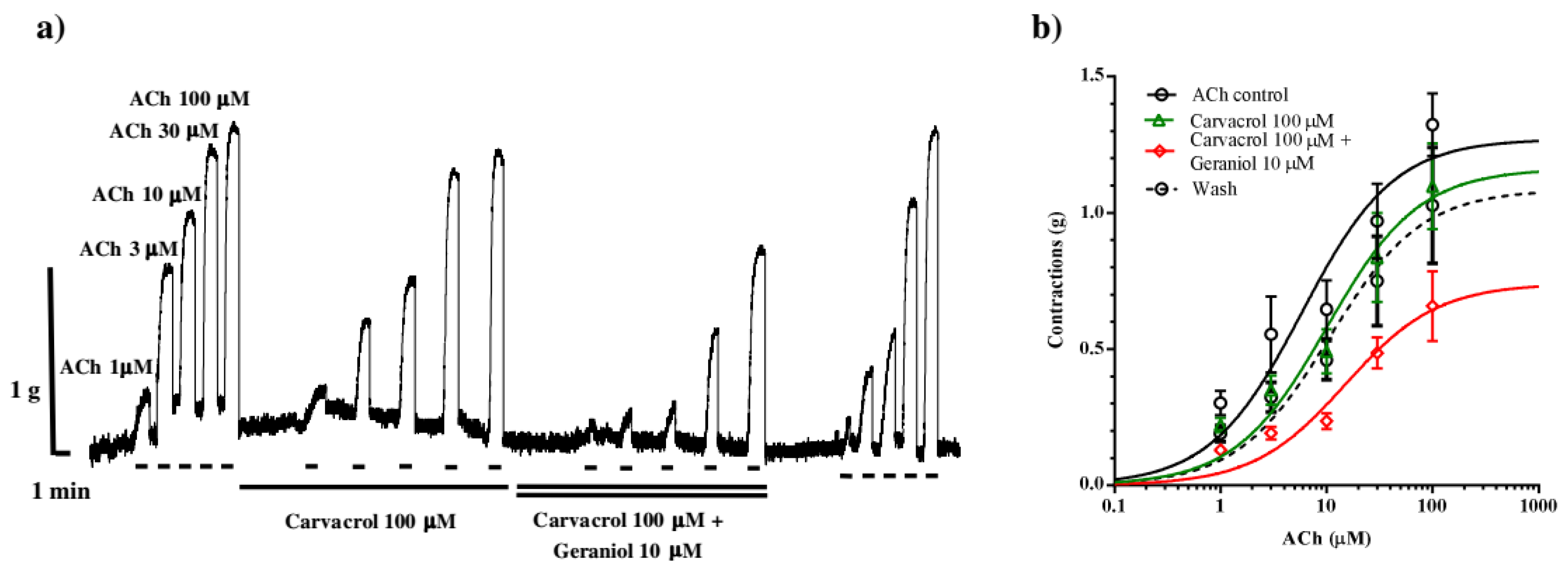
The interaction between carvacrol and geraniol were tested on the contractions of neuromuscular preparation of the parasitic nematode Ascaris suum in the same conditions as the previous examination of their individual effects (
Figure 4a). The obtained control EC
50 of ACh was 5.89±1.45 μM. Incubation of neuromuscular preparations with carvacrol 100 μM non-significantly increased the EC
50 to 10.10±1.51 μM (p=0.11). Furthermore, the addition of geraniol 10 μM in presence of carvacrol increased the EC
50 value of ACh significantly to 15.03±1.52 μM (p<0.0001). After removing of carvacrol and geraniol from the pharmacological bath, the EC
50 of acetylcholine was 10.64±1.64 μM (
Figure 4b). The maximal contractile effect of ACh in the control series was 1.27±0.12 g, and it did not significantly change after incubation with carvacrol (1.24±0.21 g). However, when the preparation was incubated with geraniol (10 μM) together with carvacrol, E
max decreased significantly (p=0.0272) to 0.77±0.14 g.
2.3. Effect of Geraniol and Carvacrol on Ascaris suum nAChR Expressed in Xenopus oocytes
Considering the recorded interaction of caracrol and geraniol on the contractions of the A. suum neuromuscular preparation, we examined their individual and joint effects on the homomeric A. suum nAChR (Asu-ACR-16) expressed in X. leavis oocytes. Perfusion of increasing concentrations of acetylcholine (ACh) caused a concentration-dependent increase in current, with an control EC50 value of 7.89±1.02 μM and large currents with maximum amplitude in the μA range (
Figure 5a).
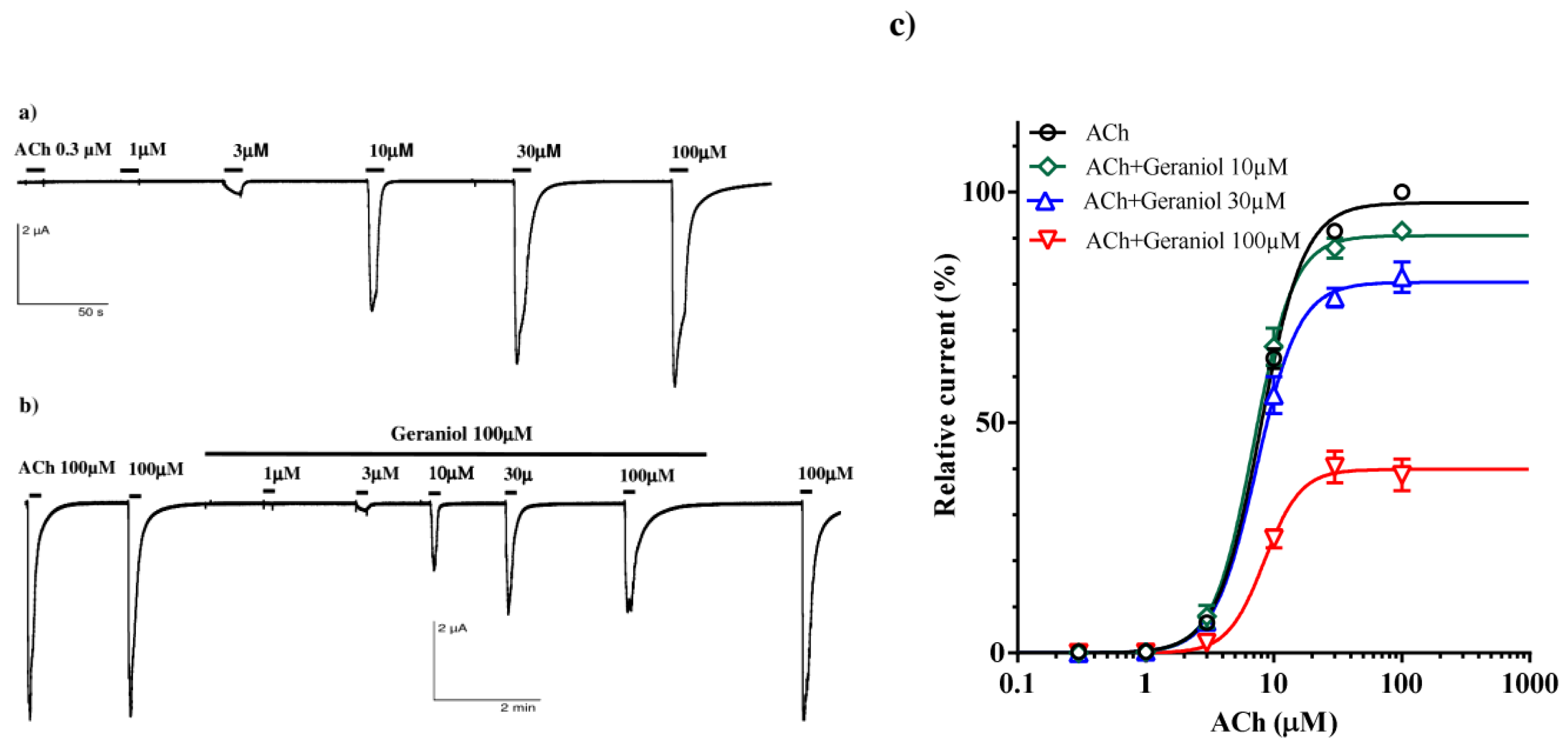
We tested the effect of 10, 30 and 100 μM (
Figure 5b) of geraniol on the current induced by increasing concentrations of ACh. We found that the ACh EC
50 values were 6.95±1.02 μM, 7.33±1.02 μM and 8.44±1.08 μM, in the presence of 10, 30 and 100 μM of geraniol, respectively. These values were not significantly different compared to the control. The ACh-evoked maximal response amplitude of current (E
max) in the control series was 97.63±0.92% and 10 μM of geraniol did not change this value significantly (90.53±1.46%, p=0.0803). However, higher concentrations, 30 and 100 μM of geraniol significantly decreased the E
max to 80.41±1.62% and 39.83±1.49% (p<0.0001; p<0.0001), respectively (
Figure 5c) indicating a non-competitive inhibition of ACh-elicited currents by geraniol.
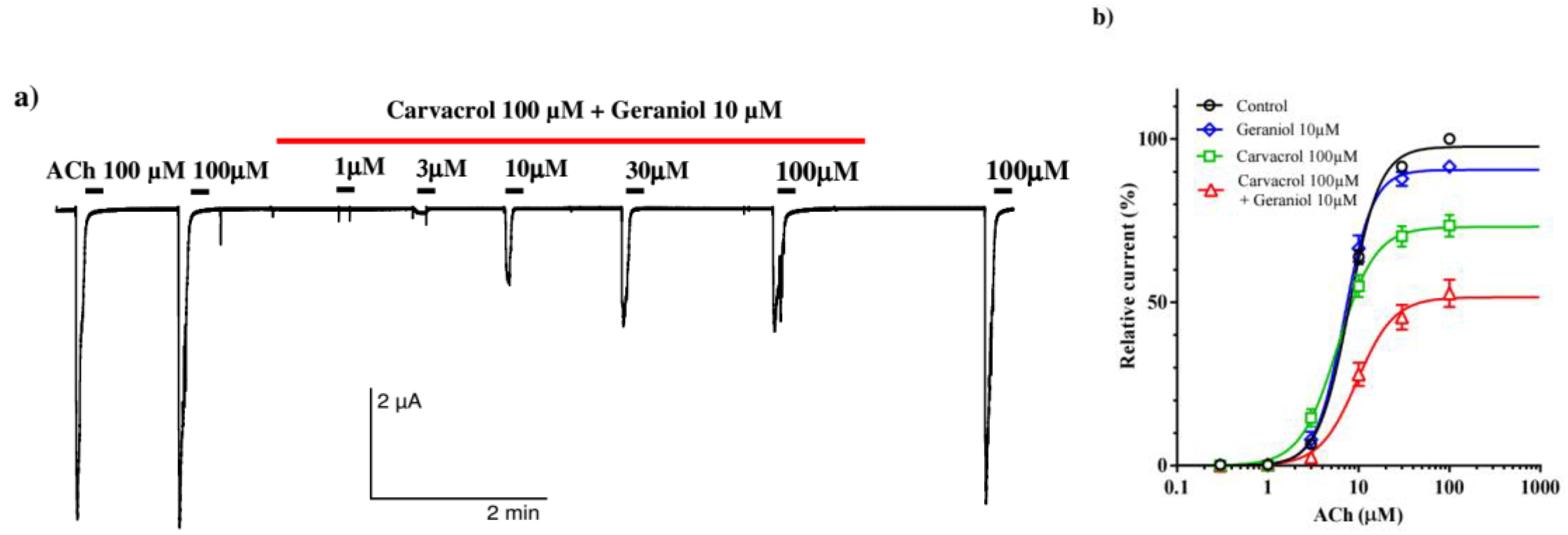
In the next series of experiments, we tested the effect of carvacrol and combination of geraniol and carvacrol on the ACh-evoked currents. Individually and in combination, carvacrol 100 μM and geraniol 10 μM did not significantly affect the value of EC
50 of ACh. The control value was 7.89±1.02 μM, in the presence of carvacrol 5.89±1.07 μM and geraniol 6.95±1.04 μM. Furthermore, the combination of geraniol and carvacrol insignificantly increased the EC
50 value to 9.57±1.11 μM (
Figure 6a). However, the effect of the combination of carvacrol and geraniol on E
max was different. Carvacrol 100 μM significantly reduced E
max to 73.09±1.87%, as we previously showed, geraniol 10 μM by itself did not significantly affect E
max, but in combination with carvacrol it reduced E
max by half, i.e., to 51.54±2.46% (
Figure 6b).
3. Discussion
Molecular docking analysis in our previously published research was indicated potential differences in the binding of carvacrol and geraniol to ACR-16, a homomeric nAChR widely distributed in Ascaris tissues [
5]. Carvacrol shows affinity for an allosteric binding site in the beta domain (a possible allosteric site composed of two sub-sites located close to each other), while geraniol potentially binds to the receptor at two sites in the alpha domain with lower affinity than carvacrol. The presence of geraniol or the presence of carvacrol, enhances their binding to the receptor. We previously also published that carvacrol dominantly exhibited characteristics of a non-competitive antagonist of nAChR in A. suum [
15,
16], so we considered it important to analyze the influence of geraniol on the effect of carvacrol. The prediction of molecular interaction is verified by examining the effects of carvacrol and geraniol on the motility and survival of adult C. elegans. In our study, geraniol showed better efficacy, but did not show a distinct time-dependent effect, on the other hand, carvacrol showed a slightly weaker efficacy but a clear time-dependent effect. However, when adult C. elegans was exposed to the combination of geraniol and carvacrol, the LC
50 value after 24h was reduced almost 10 times, and the effect was time-dependent. The prediction from the docking that the presence of one ligand increases the binding of the other ligand was confirmed. It is also interesting that geraniol caused atonic paralysis, but before paralysis it causes the cessation of pharyngeal pumping. Further investigation of this potential new target site for AI is of undoubted importance. These results are in agreement with the data that geraniol exhibits a nematocidal effects and at a concentration of 2% reduces the motility of L3s of H. contortus, T. axei and T. circumcincincta by 82, 90 and 94% [
17].
We checked the prediction about the synergistic interaction of geraniol and carvacrol on the model of contractions of the neuromuscular preparation of A. suum. The tested concentrations of geraniol (10 and 30 μM) caused decrease in the EC
50 value of ACh (for 18.68%) as well as an increase in contractile E
max (for 4.43%), but without statistical significance. On the other hand, carvacrol at a concentration of 100 μM insignificantly increases the EC
50 of ACh and decreases the Emax of contractions, which is in agreement with our previously published results [
3]. However, when we incubated neuromuscular preparations of A. suum with the combination of carvacrol 100 μM and geraniol 10 μM, there was a significant increase in the value of EC
50 of ACh and a significant decrease in the value for contractile E
max. This corresponds to our results obtained with C. elegans, which indicate a synergistic interaction between carvacrol and geraniol against ACh-induced contractions.
To examine whether the interaction occurs at the receptor level, we tested the effects of carvacrol and geraniol on the homomeric Asu-ACR-16 expressed on X. leavis oocytes. In the presence of geraniol (30 and 100 μM) the ACh EC
50 value remained unchanged while the E
max was significantly reduced. This effect indicates a non-competitive antagonism or allosteric modulation caused by geraniol at Asu-ACR-16. Furthermore, we compared the effects of carvacrol (100 μM) and geraniol (10 μM), and their combination. As expected, carvacrol acted as a non-competitive antagonist on the A. suum N-AChR as described previously [
15,
18] while the addition of geraniol reduced E
max significantly on almost 50% of control value. This interaction is somewhat different from the interaction observed in the contraction tests. In both cases, the combined inhibitory effect in relation to ACh is greater than individual, but in contraction assays, in addition to the decrease in E
max, the EC
50 value of ACh increased. In tests on Asu-ACR-16, we obtained a synergistic inhibitory interaction of carvacrol and geraniol only in the reduction of E
max, without changes in EC
50. An explanation can be found in the fact that both antagonists bind to the allosteric site in Asu-ACR-16, resulting in non-competitive antagonism.
Asu-ACR-16 is a homopentameric nAChR with widespread distribution in the somatic muscle, pharynx, ovijector and head which indicates various tissue-related functions. The A. suum channel is most sensitive to nicotine, insensitive to levamisole and pyrantel when compared with same channel in C. elegans [
19]. The difference in the interaction between contractile tests and electrophysiology experiments on expressed Asu-ACR-16 can be explained by the fact that carvacrol and geraniol in contractions assay can act on all types of nAChRs in the neuromuscular preparation of Ascaris suum. Here, we evidenced the effect on Asu-ACR-16, but we can not rule out the possibility that carvacrol and geraniol could also act on other nAChR subtypes including either UNC-29/UNC-38 channels [
20], or ACR-26 channels [
21], as well as additional nAChRs from A. suum that have not been characterized so far [
22]. This hypothesis is supported by the significant effect of carvacrol previously reported on the morantel-sensitive nAChRs made of the ACR-26/ACR-27 subunits from Parascaris sp. [
15]. On the other hand, it is interesting to comment on the greater efficacy of geraniol on C. elegans. This can be explained by differences in the antagonist pharmacology between the two ACR-16 homologues. The A. suum channel is indeed most sensitive to nicotine, insensitive to levamisole and pyrantel, as also was observed with the C. elegans ACR-16 nAChR. Morantel behaved as a non-competitive antagonist of the A. suum nAChR but less potent in comparison to its effect on C. elegans receptor [
23,
24]. We do not comment more specifically though it is tempting to speculate that C. elegans ACR-16 is more sensitive to geraniol as well.
4. Materials and Methods
4.1. C. elegans Testing
C. elegans, N2 wild-type was obtained from the Caenorhabditis Genetics Center [
25]. Worms were cultivated and adults were separated for testing as we previously explained in Stojković et al. [
5]. Suspensions of adult nematodes (20μL) were inoculated on the Petri dish (diameter 3cm) with 2.5 ml of NGM substrate and increasing concentrations of carvacrol or geraniol (1, 3, 10, 30, 100, 300 ili 1000 µM) and a combination of geraniol and carvacrol 1:1 (1, 3, 10, 30, 100, 300 ili 1000 µM). The titer of adult worms was 20-37/20 μL and each concentration was tested on three Petri dishes. The three Petri dishes without the added test substances were untreated controls.
The plates inoculated with C. elegans were placed in a thermostat (Memmert IN30, Germany) at 20° C for 24 and 48 h. After incubation, the plates were observed under an inverted microscope (Motic AE 31, PRC) and the movement and pharyngeal pumping of C. elegans were recorded with a camera (Motic 5 MP, NRK) on the hard disk of a PC, for later analysis. The survival rate of the adult C. elegans was determined in the medium with carvacrol and geraniol, as well as in the untreated control medium. The lethality was determined by the cessation of movement and pharyngeal pumping. C. elegans was considered dead when it did not move and did not respond to repeated touching with a probe. Mortality was calculated for each treatment after 24 and 48 hours and expressed in percentages.
4.2. Ascaris suum Contractions
Ascaris muscle preparation for contraction studies were prepared as we previously described in Stojković et al. [
5]. The preparations were allowed to equilibrate for 15 min under the initial tension of 0.5 g. Contractions were monitored after increasing concentrations of acetylcholine (ACh) (1, 3, 10, 30 and 100 μM) and then in the presence of geraniol, carvacrol or carvacrol plus geraniol. The maximum contractions were observed prior to washing and subsequent application of ACh, with or without carvacrol and geraniol. The interval between the application of increasing doses of ACh was 1 min and 2 min when the preparation was incubated with carvacrol and geraniol. The responses for each concentration were expressed in grams (g), produced by each individual flap preparation. Contractions were monitored and recorded in real time on a PC computer, using a BioSmart interface, and eLAB 44 software (ElUnit, Belgrade). Sigmoidal concentration-response curves for ACh effects in the absence or presence of geraniol/carvacrol were described by the Hill equation.
4.3. Electrophysiological Recordings
The functional reconstitution of the A. suum nicotine-sensitive acetylcholine receptors (nAChRs) was carried out in Xenopus laevis oocytes as described previously [
24]. Briefly, capped cRNAs encoding the A. suum ACR-16 subunit were synthesized in vitro using the mMessage mMachine T7 transcription kit (Thermofisher). Defolliculated Xenopus laevis oocytes (Ecocyte Bioscience) were micro-injected with 36 nL of A. suum ACR-16 cRNA at 50 ng/µL using the Nanoject II microinjector (Drummond) and incubated 3 days at 19 °C to allow nAChR expression. Two micro-electrode voltage-clamp experiments were performed using an Oocyte Clamp OC-725D amplifier (Warner Instruments) under voltage clamp at − 60 mV as previously described [
5,
15]. Data were collected and analyzed using the pCLAMP 10.4 package (Molecular Devices).
4.4. Drugs
Acetylcholine, geraniol and carvacrol were obtained from Sigma-Aldrich Co. (St Louis, MO, United States). Acetylcholine was dissolved in the APF-Ringer and Tyrode solution. Geraniol and carvacrol were dissolved in ethanol, with a final concentration of ethanol in the APF-Ringer and Tyrode Solution of 0.1 %v/v.
4.5. Statistical Analyses
The results of the study of the lethal effect of carvacrol and geraniol is presented in in percentage (%) and the determination of the Median Lethal Concentration (LC50) were processed by non-linear regression. The results of muscle contraction assay are expressed as means ± S.E. in grams (g) of contractions. The dose-response relationship was analyzed by non-linear regression and the values of the Median Effective Concentration (EC50) of the agonist (ACh), without and in the presence of geraniol and carvacrol was determined. Whole cell current electrophysiology responses were analyzed using the pCLAMP 10.4 package (Molecular Devices). EC50 values were determined using non-linear regression on normalized data (100 µM ACh as maximal response) using GraphPad Prism® software. One-way analysis of variance (ANOVA) was applied for the comparison of the differences between the EC50 value and the maximal effect (Rmax). Differences were considered significant when the p value was < 0.05. The statistical analysis was conducted using GraphPad Prism® software (San Diego, CA, USA), while all values are expressed as mean ± standard error (S.E.).
5. Conclusions
The presented research confirms the significant anthelmintic potential of the active ingredients of essential plant oils and the synergistic effect of their combinations. On the other hand, it is obvious that one of the important sites of synergistic anthelmintic interaction is the nematode nACh receptor. The possibility of application of active ingredients of essential plant oils that exhibit a synergistic anthelmintic effect, considering the specific mechanism of action, can be an important platform for the development of new drugs and new therapeutic procedures.
Author Contributions
Conceptualization, M.S. and S.M.T.; Methodology, M.S., S.M.T. and C.L.C.; Software, D.S.M. and S.M.T.; Formal analysis, M.S., D.S.M., D.M., C.L.C. and S.M.T.; Investigation, M.S., D.S.M., D.M., C.L.C. and S.M.T.; Resources, C.L.C. and S.M.T.; Data curation, M.S., D.S.M., D.M., C.L.C. and S.M.T.; Writing—original draft, M.S. and S.M.T.; Writing-review & editing, M.S., D.S.M., D.M., C.L.C. and S.M.T.; Visualization, D.S.M. and D.M.; Supervision, C.L.C. and S.M.T.; Project administration, D.M.; Funding acquisition, S.M.T. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia (Contract number 451-03-136/2025-03/200143), Science Fund of the Republic of Serbia, #GRANT No, 7355 Project title – FARMASCA (
https://farmasca.vet.bg.ac.rs), and by INRAE (
http://www.inrae.fr/).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are incorporated into the article.
Acknowledgments
Not applicable.
Conflicts of Interest
Dr. Claude L. Charvet is an employee of MSD Animal Health Innovation GmbH, other authors declare no conflicts of interest.
References
- Martin, R.J.; Murray, I.; Robertson, A.P.; Bjorn, H.; Sangster, N. Anthelmintics and ion-channels: After a puncture, use a patch. Int. J. Parasitol. 1998, 28, 849–862. [Google Scholar] [CrossRef]
- Colquhoun, L.; Holden-Dye, L.; Walker, R.J. The pharmacology of cholinoceptors on the somatic muscle cells of the parasitic nematode Ascaris suum. J Exp Biol. 1991, 158, 509–530. [Google Scholar] [CrossRef]
- Trailović, S.M.; Marjanović, D.S.; Nedeljković Trailović, J.; Robertson, A.P.; Martin, R.J. Interaction of carvacrol with the Ascaris suum nicotinic acetylcholine receptors and gamma-aminobutyric acid receptors, potential mechanism of antinematodal action. Parasitol. Res. 2015, 114, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- Marjanović, D.S.; Zdravković, N.; Milovanović, M.; Trailović, J.N.; Robertson, A.P.; Todorović, Z.; Trailović, S.M. Carvacrol acts as a potent selective antagonist of different types of nicotinic acetylcholine receptors and enhances the effect of monepantel in the parasitic nematode Ascaris suum. Vet. Parasitol. 2020, 278, 109031. [Google Scholar] [CrossRef] [PubMed]
- Stojković, M.; Todorović, Z.; Protic, D.; Stevanovic, S.; Medić, D.; Charvet, L.C.; Courtot, E.; Marjanović, D.S.; Nedeljković Trailović, J.; Trailović, S.M. Corrigendum: Pharmacological effects of monoterpene carveol on the neuromuscular system of nematodes and mammals. Front. Pharmacol. 2024, 15, 1466575. [Google Scholar] [CrossRef]
- Müller, G.C.; Junnila, A.; Butler, J.; Kravchenko, V.D.; Revay, E.E.; Weiss, R.W.; Schlein, Y. Efficacy of the botanical repellents geraniol, linalool, and citronella against mosquitoes. J. Vector Ecol. 2023, 34, 2–8. [Google Scholar] [CrossRef]
- Popescu, I.E.; Gostin, I.N.; Blidar, C.F. An Overview of the Mechanisms of Action and Administration Technologies of the Essential Oils Used as Green Insecticides. AgriEngineering. 2024, 6, 1195–1217. [Google Scholar] [CrossRef]
- Clark, G.S. Geraniol, Perfumer & Flavorist; Cameron & Stuart Inc.: Easton, MD, USA, 1998; Volume 23, pp. 19–25. [Google Scholar]
- Faria, J.M.S.; Rusinque, L.; Vicente, C.S.L.; Inácio, M.L. Bioactivity of Monoterpene Alcohols as an Indicator of Biopesticidal Essential Oils against the Root Knot Nematode Meloidogyne ethiopica. Biol. Life Sci. Forum. 2022, 16, 15. [Google Scholar]
- Leela, N.K.; Khan, R.M.; Reddy, P.P.; Nidiry, E.S.J. Nematicidal activity of essential oil of pelargonium graveolens against the –Knot nematode Meloidogyne incognita. Nematol. Mediterr. 1992, 20, 57–58. [Google Scholar]
- Kumaran, A.M.; D’Souza, P.; Agarwal, A.; Bokkolla, R.M.; Balasubramaniam, M. Geraniol, the putative anthelmintic principle of Cymbopogon martinii. Phytother. Res. 2003, 17, 957. [Google Scholar] [CrossRef]
- Barros, L.A.; Yamanaka, A.R.; Silva, L.E.; Vanzeler, M.L.; Braum, D.T.; Bonaldo, J. In vitro larvicidal activity of geraniol and citronellal against Contracaecum sp (Nematoda: Anisakidae). Braz. J. Med. Biol. Res. 2009, 42, 918–920. [Google Scholar] [CrossRef]
- Hierro, I.; Valero, A.; Pérez, P.; González, P.; Cabo, M.M.; Montilla, M.P.; Navarro, M.C. Action of different monoterpenic compounds against Anisakis simplex s.l. L3 larvae. Phytomedicine. 2004, 11, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Frota, G.A.; Santos, V.O.D.; Rodrigues, J.F.V.; Oliveira, B.R.; Albuquerque, L.B.; Vasconcelos, F.R.C.; Silva, A.C.; Teixeira, M.; Brito, E.S.; Santos, J.M.L.D.; Vieira, L.D.S.; Monteiro, J.P. Biological activity of cinnamaldehyde, citronellal, geraniol and anacardic acid on Haemonchus contortus isolates susceptible and resistant to synthetic anthelmintics. Rev. Bras. Parasitol. Vet. 2023, 32, e006023. [Google Scholar] [CrossRef]
- Trailovic, S.M.; Rajkovic, M.; Marjanovic, D.S.; Neveu, C.; Charvet, C.L. Action of Carvacrol on Parascaris sp. and Antagonistic Effect on Nicotinic Acetylcholine Receptors. Pharmaceuticals (Basel). 2021, 14, 505. [Google Scholar] [CrossRef] [PubMed]
- Marjanović, D.S.; Trailović, S.M.; Milovanović, M. Interaction of agonists of a different subtype of the nAChR and carvacrol with GABA in Ascaris suum somatic muscle contractions. J. Nematol. 2021, 53, e2021-22. [Google Scholar] [CrossRef]
- Helal, M.A.; Abdel-Gawad, A.M.; Kandil, O.M.; Khalifa, M.M.E.; Cave, G.W.V.; Morrison, A.A.; Bartley, D.J.; Elsheikha, H.M. Nematocidal Effects of a Coriander Essential Oil and Five Pure Principles on the Infective Larvae of Major Ovine Gastrointestinal Nematodes In Vitro. Pathogens. 2020, 9, 740. [Google Scholar] [CrossRef]
- Choudhary, S.; Marjianović, D.S.; Wong, C.R.; Zhang, X.; Abongwa, M.; Coats, J.R.; Trailović, S.M.; Martin, R.J.; Robertson, A.P. Menthol acts as a positive allosteric modulator on nematode levamisole sensitive nicotinic acetylcholine receptors. Int. J. Parasitol. Drugs Drug Resist. 2019, 9, 44–53. [Google Scholar] [CrossRef]
- Abongwa, M.; Martin, R.J.; Robertson, A.P. A brief review on the mode of action of antinematodal drugs. Acta Vet (Beogr). 2017, 67, 137–152. [Google Scholar] [CrossRef]
- Williamson, S.M.; Robertson, A.P.; Brown, L.; Williams, T.; Woods, D.J.; Martin, R.J.; Sattelle, D.B.; Wolstenholme, A.J. The nicotinic acetylcholine receptors of the parasitic nematode Ascaris suum: Formation of two distinct drug targets by varying the relative expression levels of two subunits. PLoS Pathog. 2009, 5, e1000517. [Google Scholar] [CrossRef]
- Bennett, H.M.; Williamson, S.M.; Walsh, T.K.; Woods, D.J.; Wolstenholme, A.J. ACR-26: A novel nicotinic receptor subunit of parasitic nematodes. Mol. Biochem. Parasitol. 2012, 183, 151–157. [Google Scholar] [CrossRef]
- Holden-Dye, L.; Joyner, M.; O’Connor, V.; Walker, R.J. Nicotinic acetylcholine receptors: A comparison of the nAChRs of Caenorhabditis elegans and parasitic nematodes. Parasitol. Int. 2013, 62, 606–615. [Google Scholar] [CrossRef]
- Raymond, V.; Mongan, N.P.; Sattelle, D.B. Anthelmintic actions on homomer-forming nicotinic acetylcholine receptor subunits: Chicken alpha7 and ACR-16 from the nematode Caenorhabditis elegans. Neuroscience. 2000, 101, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Abongwa, M.; Baber, K.E.; Martin, R.J.; Robertson, A.P. The cholinomimetic morantel as an open channel blocker of the Ascaris suum ACR-16 nAChR. Invert. Neurosci. 2016, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Charvet, C.L.; Guégnard, F.; Courtot, E.; Cortet, J.; Neveu, C. Nicotine-sensitive acetylcholine receptors are relevant pharmacological targets for the control of multidrug resistant parasitic nematodes. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 540–549. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).