Submitted:
13 June 2025
Posted:
16 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
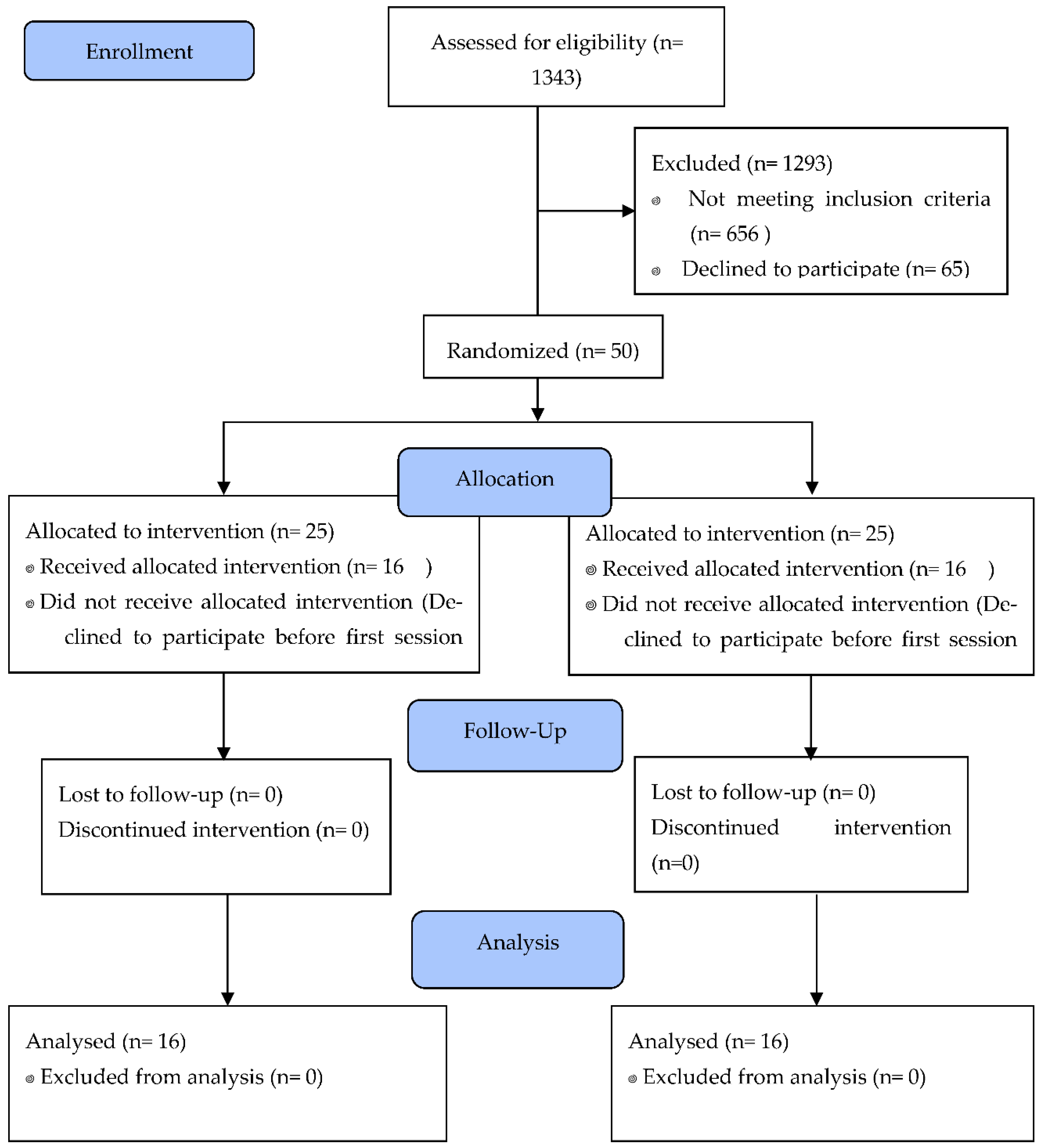
Study Design
Participants
Assessment Procedures
Intervention
Outcome Measures
Primary Outcome Measures
Secondary Outcome Measures
Background Measures
Sample Size Determination
Randomization and Blinding
Statistical Analysis
Bayesian Analyisis
3. Results
Baseline Characteristics
Mood and Cognitive Screening
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feigin VL, Brainin M, Norrving B, Martins SO, Pandian J, Lindsay P, et al. World Stroke Organization: Global Stroke Fact Sheet 2025. International Journal of Stroke [Internet]. 2025 Feb 3;20(2):132–44. Available online: https://journals.sagepub.com/doi/10.1177/17474930241308142.
- Martin SS, Aday AW, Allen NB, Almarzooq ZI, Anderson CAM, Arora P, et al. 2025 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation [Internet]. 2025 Feb 25;151(8). Available online: https://www.ahajournals.org/doi/10.1161/CIR.0000000000001303.
- El Husseini N, Katzan IL, Rost NS, Blake ML, Byun E, Pendlebury ST, et al. Cognitive Impairment After Ischemic and Hemorrhagic Stroke: A Scientific Statement From the American Heart Association/American Stroke Association. Stroke [Internet]. 2023 Jun 1;54(6):E272–91. Available online: https://www.ahajournals.org/doi/10.1161/STR.0000000000000430.
- Elendu C, Amaechi DC, Elendu TC, Ibhiedu JO, Egbunu EO, Ndam AR, et al. Stroke and cognitive impairment: understanding the connection and managing symptoms. Annals of Medicine & Surgery [Internet]. 2023 Dec;85(12):6057–66. Available online: https://journals.lww.com/10.1097/MS9.0000000000001441.
- Blomgren C, Samuelsson H, Blomstrand C, Jern C, Jood K, Claesson L. Long-term performance of instrumental activities of daily living in young and middle-aged stroke survivors—Impact of cognitive dysfunction, emotional problems and fatigue. Abete P, editor. PLoS One [Internet]. 2019 May 16;14(5):e0216822. Available online: https://dx.plos.org/10.1371/journal.pone.0216822.
- Stolwyk RJ, Mihaljcic T, Wong DK, Chapman JE, Rogers JM. Poststroke Cognitive Impairment Negatively Impacts Activity and Participation Outcomes. Stroke [Internet]. 2021 Feb 1;52(2):748–60. Available online: https://www.ahajournals.org/doi/10.1161/STROKEAHA.120.032215.
- de Menezes KKP, Scianni AA, Avelino PR, Faria-Fortini I, Bastos VS, Faria CDC de M. Contextual and clinical factors as explainers of stroke severity, residual motor impairments, and functional independence during hospitalization. Journal of Stroke and Cerebrovascular Diseases [Internet]. 2025 Jan 1;34(1):108154. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1052305724005974.
- Heldner MR, Chalfine C, Houot M, Umarova RM, Rosner J, Lippert J, et al. Cognitive Status Predicts Return to Functional Independence After Minor Stroke: A Decision Tree Analysis. Front Neurol [Internet]. 2022 Feb 17;13. Available online: https://www.frontiersin.org/articles/10.3389/fneur.2022.833020/full.
- MANCUSO M, IOSA M, ABBRUZZESE L, MATANO A, COCCIA M, BAUDO S, et al. The impact of cognitive function deficits and their recovery on functional outcome in subjects affected by ischemic subacute stroke: results from the Italian multicenter longitudinal study CogniReMo. Eur J Phys Rehabil Med [Internet]. 2023 Jun 1;59(3):284–93. Available online: https://www.minervamedica.it/index2.php?show=R33Y2023N03A0284.
- Lugtmeijer S, Lammers NA, de Haan EHF, de Leeuw FE, Kessels RPC. Post-Stroke Working Memory Dysfunction: A Meta-Analysis and Systematic Review. Neuropsychol Rev [Internet]. 2021 Mar 24;31(1):202–19. Available online: http://link.springer.com/10.1007/s11065-020-09462-4.
- Irfani Fitri F, Fithrie A, Rambe AS. Association between working memory impairment and activities of daily living in post-stroke patients. Med Glas [Internet]. 2020 Jul 12;17(2):433–8. Available online: https://medicinskiglasnik.ba/article/246.
- Malouin F, Belleville S, Richards CL, Desrosiers J, Doyon J. Working memory and mental practice outcomes after stroke. Arch Phys Med Rehabil [Internet]. 2004 Feb;85(2):177–83. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0003999303007718.
- Loetscher T, Potter KJ, Wong D, das Nair R. Cognitive rehabilitation for attention deficits following stroke. Cochrane Database of Systematic Reviews [Internet]. 2019 Nov 10;2019(11). Available online: https://doi.wiley.com/10.1002/14651858.CD002842.pub3.
- Loetscher T, Lincoln NB. Cognitive rehabilitation for attention deficits following stroke. Cochrane Database of Systematic Reviews [Internet]. 2013 May 31;2013(5). Available online: https://doi.wiley.com/10.1002/14651858.CD002842.pub2.
- Westerberg H, Jacobaeus H, Hirvikoski T, Clevberger P, Östensson ML, Bartfai A, et al. Computerized working memory training after stroke–A pilot study. Brain Inj [Internet]. 2007 Jan 3;21(1):21–9. Available online: http://www.tandfonline.com/doi/full/10.1080/02699050601148726.
- van de Ven RM, Murre JMJ, Veltman DJ, Schmand BA. Computer-Based Cognitive Training for Executive Functions after Stroke: A Systematic Review. Front Hum Neurosci [Internet]. 2016 Apr 20;10(APR2016):1–27. Available online: http://journal.frontiersin.org/Article/10.3389/fnhum.2016.00150/abstract.
- das Nair R, Cogger H, Worthington E, Lincoln NB. Cognitive rehabilitation for memory deficits after stroke. Cochrane Database of Systematic Reviews [Internet]. 2016 Sep 1;2016(9). Available online: http://doi.wiley.com/10.1002/14651858.CD002293.pub3.
- Kleim JA, Jones TA. Principles of Experience-Dependent Neural Plasticity: Implications for Rehabilitation After Brain Damage. Journal of Speech, Language, and Hearing Research [Internet]. 2008 Feb;51(1). Available online: http://pubs.asha.org/doi/10.1044/1092-4388%282008/018%29.
- Karbach J, Verhaeghen P. Making Working Memory Work: A Meta-Analysis of Executive-Control and Working Memory Training in Older Adults. Psychol Sci [Internet]. 2014 Nov 8;25(11):2027–37. Available online: https://journals.sagepub.com/doi/10.1177/0956797614548725.
- von Bastian CC, Eschen A. Does working memory training have to be adaptive? Psychol Res [Internet]. 2016 Mar 26;80(2):181–94. Available online: http://link.springer.com/10.1007/s00426-015-0655-z.
- Cahn-Weiner DA, Boyle PA, Malloy PF. Tests of Executive Function Predict Instrumental Activities of Daily Living in Community-Dwelling Older Individuals. Appl Neuropsychol [Internet]. 2002 Sep;9(3):187–91. Available online: http://www.tandfonline.com/doi/abs/10.1207/S15324826AN0903_8.
- M. Tucker A, Stern Y. Cognitive Reserve in Aging. Curr Alzheimer Res [Internet]. 2011 Jun 1;8(4):354–60. Available online: http://www.eurekaselect.com/openurl/content.php?genre=article&issn=1567-2050&volume=8&issue=4&spage=354.
- Simons DJ, Boot WR, Charness N, Gathercole SE, Chabris CF, Hambrick DZ, et al. Do “Brain-Training” Programs Work? Psychological Science in the Public Interest [Internet]. 2016 Oct 2;17(3):103–86. Available online: https://journals.sagepub.com/doi/10.1177/1529100616661983.
- Lövdén M, Bäckman L, Lindenberger U, Schaefer S, Schmiedek F. A theoretical framework for the study of adult cognitive plasticity. Psychol Bull [Internet]. 2010 Jul;136(4):659–76. Available online: https://doi.apa.org/doi/10.1037/a0020080.
- Robertson IH, Murre JMJ. Rehabilitation of brain damage: Brain plasticity and principles of guided recovery. Psychol Bull [Internet]. 1999;125(5):544–75. Available online: https://doi.apa.org/doi/10.1037/0033-2909.125.5.544.
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3 Pt 1):179–186. [CrossRef]
- Vallat-Azouvi C, Pradat-Diehl P, Azouvi P. The Working Memory Questionnaire: a scale to assess everyday life problems related to deficits of working memory in brain injured patients. Neuropsychol Rehabil. 2012;22(4):634–649. [CrossRef]
- Wechsler D. Wechsler Adult Intelligence Scale–Fourth Edition (WAIS–IV) manual. San Antonio (TX): Pearson Assessment; 2008.
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. [CrossRef]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. [CrossRef]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. [CrossRef]
- Jeffreys H. Theory of probability. 3rd ed. Oxford: Oxford University Press; 1961.
- Maier M, Ballester BR, Verschure PFMJ. Principles of neurorehabilitation after stroke based on motor learning and brain plasticity mechanisms. Front Syst Neurosci. 2019;13:74. [CrossRef]
- Simons DJ, Boot WR, Charness N, Gathercole SE, Chabris CF, Hambrick DZ, et al. Do “brain-training” programs work? Psychol Sci Public Interest. 2016;17(3):103–186. [CrossRef]
- Melby-Lervåg M, Redick TS, Hulme C. Working memory training does not improve performance on measures of intelligence or other measures of “far transfer”: Evidence from a meta-analytic review. Perspect Psychol Sci. 2016;11(4):512–534. [CrossRef]
- Mulhern M. Cognitive rehabilitation interventions for post-stroke populations. Delaware J Public Health. 2023;9(3):70–74. [CrossRef]
- Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51(1 Suppl):S225–S239. [CrossRef]
- von Bastian CC, Eschen A. Does working memory training have to be adaptive? Psychol Res. 2016;80(2):181–194. [CrossRef]
- Soni AK, Kumar M, Kothari S. Efficacy of home-based computerized adaptive cognitive training in patients with post-stroke cognitive impairment: a randomized controlled trial. Sci Rep. 2025;15:1072. [CrossRef]
| Domain | Task | Outcome measure |
|---|---|---|
| Primary outcome measure | ||
| IADL | Lawton Instrumental activities of daily living: (Cronbach’s α = 0.94). | Number of activities performed:
|
| e.g. Using the telephone |
|
|
| Working memory Questionnaire: (Cronbach’s α = 0.89). e.g. When you shop, do you often spend more than the budget you set for yourself? |
30 questions Each question was rated on a five-point Likert-type scale, ranging from 0 (“no problem at all”) to 4 (“very severe problem in everyday life”). Three sub- scores were computed, for each of the three domains (maximal score 40 for each), as well as a total score (out of 120). Higher scores corresponded to more difficulties/complaints. |
|
| Secondary outcome measure | ||
| Working memory | Working memory Index: Arithmetic task, forward, backward and sequencing digit span. (Cronbach’s α = 0.94). e.g. There are 25 gum tablets in each package. How many tablets are there in 8 packages? |
Age-corrected z-scores of total number of correct items |
| Variables | Group | Mean | SD | SE | C.V | U | S.S | BF₁₀ | ||
|---|---|---|---|---|---|---|---|---|---|---|
| IADL- I | Control | 10.188 | 3.619 | 0.905 | 0.355 | 144.500 | 0.543 | 0.370 | ||
| Experimental | 9.688 | 3.516 | 0.879 | 0.363 | ||||||
| IADL-AR | Control | 2.688 | 2.938 | 0.734 | 1.093 | 123.500 | 0.878 | 0.358 | ||
| Experimental | 3.000 | 3.651 | 0.913 | 1.217 | ||||||
| IADL-D | Control | 1.125 | 1.893 | 0.473 | 1.683 | 111.000 | 0.504 | 0.408 | ||
| Experimental | 1.313 | 1.537 | 0.384 | 1.171 | ||||||
| WMQ (Storing) | Control | 14.188 | 5.764 | 1.441 | 0.406 | 114.000 | 0.610 | 0.371 | ||
| Experimental | 15.375 | 7.464 | 1.866 | 0.485 | ||||||
| WMQ (Attention) | Control | 13.063 | 6.005 | 1.501 | 0.460 | 132.500 | 0.880 | 0.345 | ||
| Experimental | 13.188 | 6.493 | 1.623 | 0.492 | ||||||
| WMQ (Executive) | Control | 9.063 | 5.709 | 1.427 | 0.630 | 120.500 | 0.791 | 0.362 | ||
| Experimental | 10.188 | 6.969 | 1.742 | 0.684 | ||||||
| WMQ (Full Scale) | Control | 36.313 | 14.988 | 3.747 | 0.413 | 123.500 | 0.880 | 0.337 | ||
| Experimental | 38.750 | 18.746 | 4.686 | 0.484 | ||||||
| WMI | Control | 89.938 | 7.380 | 1.845 | 0.082 | 91.000 | 0.165 | 0.601 | ||
| Experimental | 92.625 | 8.539 | 2.135 | 0.092 |
| Variables | Group | Mean | SD | SE | C.V | U | S.S | BF₁₀ |
|---|---|---|---|---|---|---|---|---|
| IADL- I | Control | 12.563 | 2.476 | 0.619 | 0.197 | 141.500 | 0.598 | 0.375 |
| Experimental | 12.563 | 1.931 | 0.483 | 0.154 | ||||
| IADL-AR | Control | 0.900 | 1.197 | 0.379 | 1.330 | 24.500 | 0.088 | 0.992 |
| Experimental | 1.889 | 1.453 | 0.484 | 0.769 | ||||
| IADL-D | Control | 1.750 | 2.315 | 0.818 | 1.323 | 19.000 | 0.939 | 0.483 |
| Experimental | 1.200 | 1.095 | 0.490 | 0.913 | ||||
| WMQ (Storing) | Control | 12.438 | 6.470 | 1618 | 0.520 | 128.000 | 0.999 | 0.337 |
| Experimental | 12.438 | 7.711 | 1928 | 0.620 | ||||
| WMQ (Attention) | Control | 9.250 | 6.191 | 1548 | 0.669 | 109.000 | 0.485 | 0.412 |
| Experimental | 11.125 | 6.292 | 1573 | 0.566 | ||||
| WMQ (Executive) | Control | 8.125 | 5.353 | 1338 | 0.659 | 137.500 | 0.734 | 0.353 |
| Experimental | 7.938 | 5.285 | 1321 | 0.666 | ||||
| WMQ (Full Scale) | Control | 29.813 | 15.510 | 3878 | 0.520 | 126.500 | 0.970 | 0.341 |
| Experimental | 31.500 | 18.221 | 4555 | 0.578 | ||||
| WMI | Control | 93.688 | 7.040 | 1760 | 0.075 | 63.500 | 0.015 | 2.412 |
| Experimental | 103.938 | 11.509 | 2877 | 0.111 |
| Variables | Mean | S.D | C.V. | W | z | p | BF10 |
|---|---|---|---|---|---|---|---|
| IADL-I-PRE | 9.688 | 3.516 | 0.363 | 9.500 | -2.699 | 0.007 | 34.076 |
| IADL-I-POS | 12.563 | 1.931 | 0.154 | ||||
| IADL-AR-PRE | 3.000 | 3.651 | 1.217 | 17.000 | -0.140 | 0.943 | 0.326 |
| IADL-AR-POS | 1.889 | 1.453 | 0.769 | ||||
| IADL-D-PRE | 1.313 | 1.537 | 1.171 | 6.000 | 1.604 | 0.174 | 1.439 |
| IADL-D-POS | 1.200 | 1.095 | 0.913 | ||||
| WMQ (Storing)PRE | 15.375 | 7.464 | 0.485 | 98.000 | 2.158 | 0.033 | 3.827 |
| WMQ (Storing)POS | 12.438 | 7.711 | 0.620 | ||||
| WMQ (Attention) PRE | 13.188 | 6.493 | 0.492 | 85.000 | 1.420 | 0.163 | 0.856 |
| WMQ (Attention)POS | 11.125 | 6.292 | 0.566 | ||||
| WMQ (Executive)PRE | 10.188 | 6.969 | 0.684 | 88.500 | 1.619 | 0.109 | 1.291 |
| WMQ (Executive)POS | 7.938 | 5.285 | 0.666 | ||||
| WMQ (Full Scale) PRE | 38.750 | 18.746 | 0.484 | 104.500 | 1.887 | 0.062 | 2.279 |
| WMQ (Full Scale)POS | 31.500 | 18.221 | 0.578 | ||||
| WMI-PRE | 92.625 | 8.539 | 0.092 | 3.000 | -3.361 | <0.001 | 180.797 |
| WMI- POS | 103.938 | 11.509 | 0.111 |
| Variables | Mean | S.D | C.V. | W | z | p | BF10 |
|---|---|---|---|---|---|---|---|
| IADL-I-PRE | 10.188 | 3.619 | 0.355 | 0.000 | -3.059 | 0.002 | 134.676 |
| IADL-I-POS | 12.563 | 2.476 | 0.197 | ||||
| IADL-AR-PRE | 2.688 | 2.938 | 1.093 | 21.000 | 2.201 | 0.035 | 6.687 |
| IADL-AR-POS | 0.900 | 1.197 | 1.330 | ||||
| IADL-D-PRE | 1.125 | 1.893 | 1.683 | 6.000 | 0.365 | 0.854 | 0.398 |
| IADL-D-POS | 1.750 | 2.315 | 1.323 | ||||
| WMQ (Storing)PRE | 14.188 | 5.764 | 0.406 | 78.000 | 1.601 | 0.116 | 1.147 |
| WMQ (Storing)POS | 12.438 | 6.470 | 0.520 | ||||
| WMQ (Attention) PRE | 13.063 | 6.005 | 0.460 | 111.500 | 2.925 | 0.004 | 77.418 |
| WMQ (Attention)POS | 9.250 | 6.191 | 0.669 | ||||
| WMQ (Executive)PRE | 9.063 | 5.709 | 0.630 | 88.000 | 1.034 | 0.312 | 0.383 |
| WMQ (Executive)POS | 8.125 | 5.353 | 0.659 | ||||
| WMQ (Full Scale) PRE | 36.313 | 14.988 | 0.413 | 102.000 | 2.385 | 0.018 | 3.357 |
| WMQ (Full Scale)POS | 29.813 | 15.510 | 0.520 | ||||
| WMI-PRE | 89.938 | 7.380 | 0.082 | 6.500 | -2.550 | 0.012 | 21.669 |
| WMI- POS | 93.688 | 7.040 | 0.075 |
| Group | Likely Model | P(M|data) | Time Effect (BF_incl) | DYT Effect (BF_incl) | Mean R² (95% CI) |
|---|---|---|---|---|---|
| Experimental | Time | 0.506 | 17.237 (Very Strong) | 0.860 (Weak) | 0.406 [0.197–0.578] |
| Control | Time + DYT | 0.656 | 14.531 (Very Strong) | 2.314 (Moderate) | 0.580 [0.319–0.754] |
| Grupo | Likely Model | P(M|data) | Time Effect (BF_incl) | MOCA TOT Effect (BF_incl) | Mean R² (95% CI) |
|---|---|---|---|---|---|
| Experimental | Time + MOCA TOT | 0.534 | 628.129 (very strong) | 1.149 (Anecdotical) | 0.692 [0.518–0.812] |
| Control | Time + MOCA TOT | 0.779 | 11.688 (strong) | 5.559 (Moderate to strong) | 0.717 [0.433–0.857] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




