Submitted:
14 June 2025
Posted:
16 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Mechanism of Action of AREG
3. AREG and Fibrotic Diseases
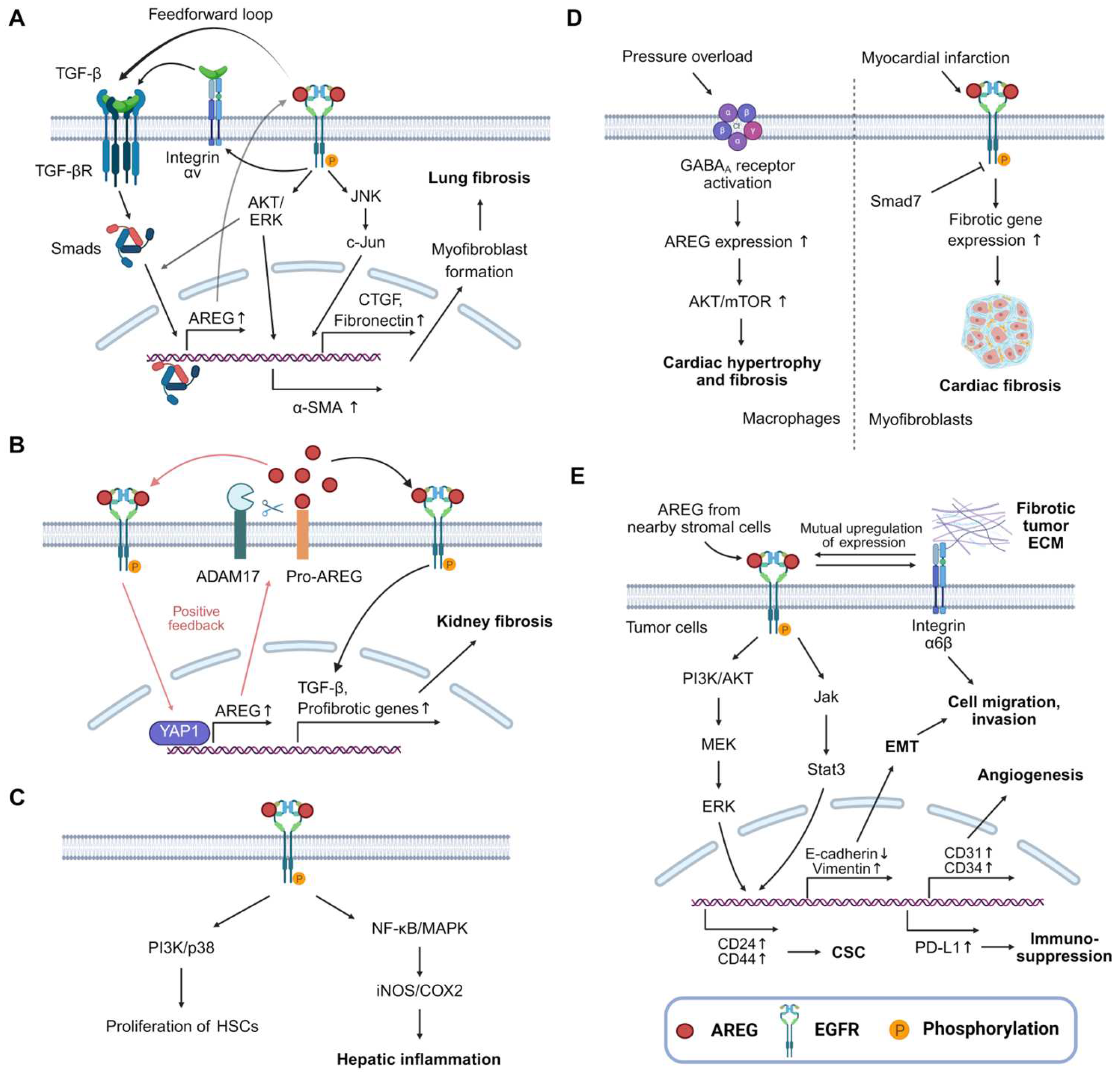
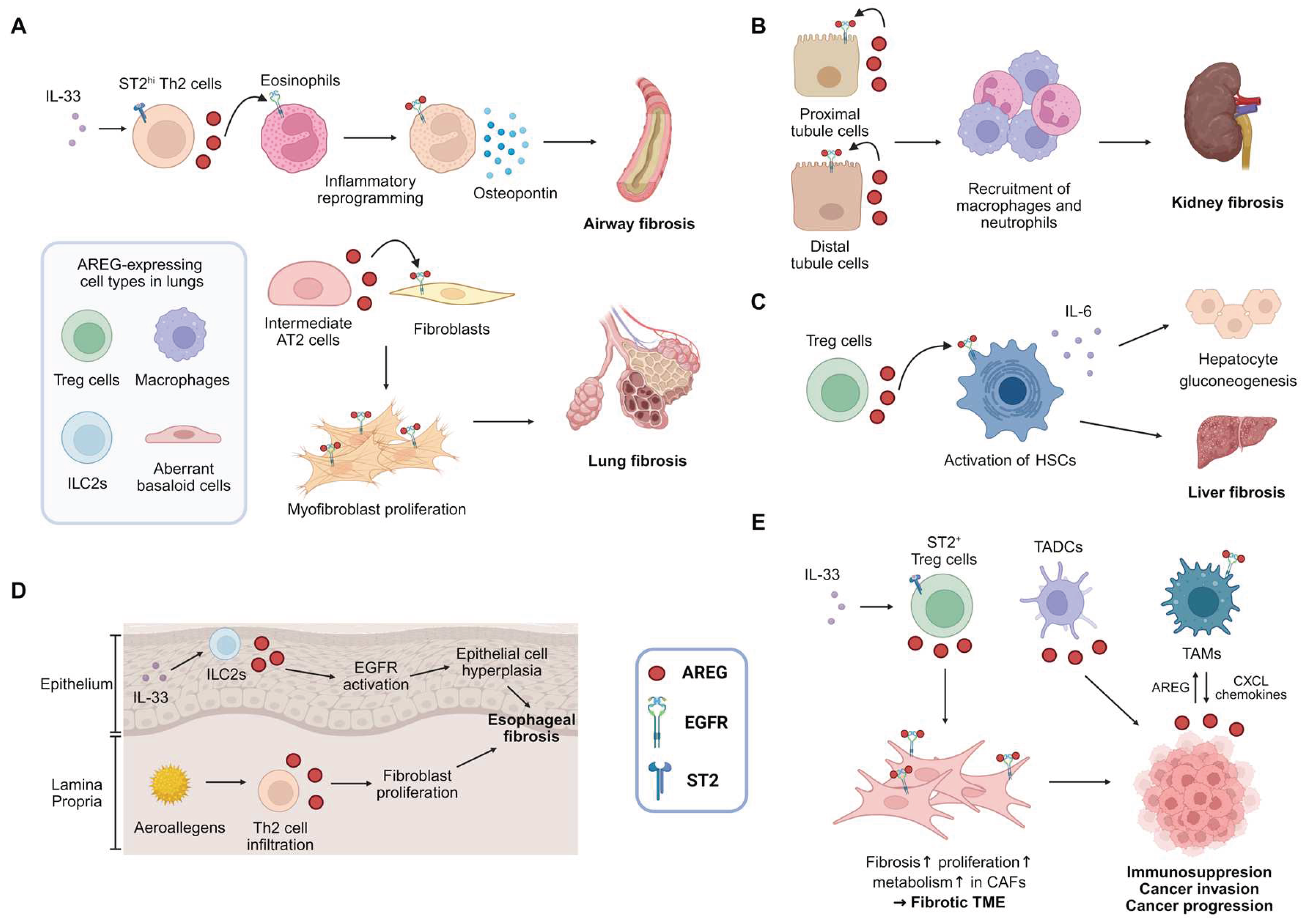
3.1. Lung Fibrosis
3.2. Kidney Fibrosis
3.3. Liver Fibrosis
3.4. Cardiac Fibrosis
3.5. Intestinal Fibrosis
3.6. Radiation-Induced Fibrosis
3.7. Other Types of Fibrosis
4. AREG and Cancer
5. Therapeutic Targeting of AREG: Preclinical and Clinical Trials for Human Application
6. Conclusion and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADAM | A disintegrin and metalloprotease |
| AKI | Acute kidney injury |
| AREG | Amphiregulin |
| α-SMA | α-smooth muscle actin |
| CCl4 | Carbon tetrachloride |
| CKD | Chronic kidney disease |
| DKD | Diabetic kidney disease |
| ECM | Extracellular matrix |
| EGF | Epidermal growth factor |
| EGFR | Epidermal growth factor receptor |
| EMT | Epithelial-mesenchymal transition |
| EoE | Eosinophilic esophagitis |
| ERK | Extracellular signal-regulated kinase |
| FPC | Fructose, palmitate, and cholesterol-rich |
| HB-EGF | Heparin-binding EGF-like growth factor |
| HSCs | Hepatic stellate cells |
| IBD | Inflammatory bowel disease |
| IIM | Inflammatory myopathy |
| IL | Interleukin |
| ILC2s | type 2 innate lymphoid cells |
| IPF | Idiopathic pulmonary fibrosis |
| MAIT | Mucosal-associated invariant T |
| MAPK | Mitogen-activated protein kinase |
| MASH | Metabolic dysfunction-associated steatohepatitis |
| PI3K | Phosphoinositide 3-kinase |
| PLCγ | Phospholipase C-γ |
| RNAi | RNA interference |
| SAMiRNA | Self-Assembled-Micelle inhibitory RNA |
| SSc | Systemic sclerosis |
| SSc-ILD | Systemic sclerosis-associated interstitial lung disease |
| TBI | Total-body irradiation |
| TGF | transforming growth factor |
| Th | T helper |
| TKI | Tyrosine kinase inhibitor |
| TME | Tumor microenvironment |
References
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Kisseleva, T.; Brenner, D.A. Fibrogenesis of parenchymal organs. Proc. Am. Thorac. Soc. 2008, 5, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Wu, X.Q.; Zhang, D.D.; Wang, Y.N.; Guo, Y.; Li, P.; Xiong, Q.; Zhao, Y.Y. Deciphering the cellular mechanisms underlying fibrosis-associated diseases and therapeutic avenues. Pharmacol. Res. 2021, 163, 105316. [Google Scholar] [CrossRef]
- Lurje, I.; Gaisa, N.T.; Weiskirchen, R.; Tacke, F. Mechanisms of organ fibrosis: Emerging concepts and implications for novel treatment strategies. Mol. Aspects Med. 2023, 92, 101191. [Google Scholar] [CrossRef]
- Kis, K.; Liu, X.; Hagood, J.S. Myofibroblast differentiation and survival in fibrotic disease. Expert. Rev. Mol. Med. 2011, 13, e27. [Google Scholar] [CrossRef]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef] [PubMed]
- Dees, C.; Chakraborty, D.; Distler, J.H.W. Cellular and molecular mechanisms in fibrosis. Exp. Dermatol. 2021, 30, 121–131. [Google Scholar] [CrossRef]
- Umbarkar, P.; Ejantkar, S.; Tousif, S.; Lal, H. Mechanisms of Fibroblast Activation and Myocardial Fibrosis: Lessons Learned from FB-Specific Conditional Mouse Models. Cells 2021, 10. [Google Scholar] [CrossRef]
- Friedman, S.L.; Sheppard, D.; Duffield, J.S.; Violette, S. Therapy for fibrotic diseases: nearing the starting line. Sci. Transl. Med. 2013, 5, 167sr161. [Google Scholar] [CrossRef]
- Distler, J.H.; Feghali-Bostwick, C.; Soare, A.; Asano, Y.; Distler, O.; Abraham, D.J. Review: Frontiers of Antifibrotic Therapy in Systemic Sclerosis. Arthritis Rheumatol. 2017, 69, 257–267. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, L.; Wang, M.; Zhou, S.; Lu, Y.; Cui, H.; Racanelli, A.C.; Zhang, L.; Ye, T.; Ding, B.; et al. Targeting fibrosis, mechanisms and cilinical trials. Signal Transduct. Target. Ther. 2022, 7, 206. [Google Scholar] [CrossRef] [PubMed]
- Zaiss, D.M.W.; Gause, W.C.; Osborne, L.C.; Artis, D. Emerging functions of amphiregulin in orchestrating immunity, inflammation, and tissue repair. Immunity 2015, 42, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Berasain, C.; Avila, M.A. Amphiregulin. Semin. Cell Dev. Biol. 2014, 28, 31–41. [Google Scholar] [CrossRef]
- Singh, S.S.; Chauhan, S.B.; Kumar, A.; Kumar, S.; Engwerda, C.R.; Sundar, S.; Kumar, R. Amphiregulin in cellular physiology, health, and disease: Potential use as a biomarker and therapeutic target. J. Cell Physiol. 2022, 237, 1143–1156. [Google Scholar] [CrossRef]
- Shoyab, M.; McDonald, V.L.; Bradley, J.G.; Todaro, G.J. Amphiregulin: a bifunctional growth-modulating glycoprotein produced by the phorbol 12-myristate 13-acetate-treated human breast adenocarcinoma cell line MCF-7. Proc. Natl. Acad. Sci. U S A 1988, 85, 6528–6532. [Google Scholar] [CrossRef] [PubMed]
- Busser, B.; Sancey, L.; Brambilla, E.; Coll, J.L.; Hurbin, A. The multiple roles of amphiregulin in human cancer. Biochim. Biophys. Acta 2011, 1816, 119–131. [Google Scholar] [CrossRef]
- Zaiss, D.M.; Minutti, C.M.; Knipper, J.A. Immune- and non-immune-mediated roles of regulatory T-cells during wound healing. Immunology 2019, 157, 190–197. [Google Scholar] [CrossRef]
- Minutti, C.M.; Modak, R.V.; Macdonald, F.; Li, F.; Smyth, D.J.; Dorward, D.A.; Blair, N.; Husovsky, C.; Muir, A.; Giampazolias, E.; et al. A Macrophage-Pericyte Axis Directs Tissue Restoration via Amphiregulin-Induced Transforming Growth Factor Beta Activation. Immunity 2019, 50, 645–654 e646. [Google Scholar] [CrossRef]
- Stoll, S.W.; Johnson, J.L.; Bhasin, A.; Johnston, A.; Gudjonsson, J.E.; Rittie, L.; Elder, J.T. Metalloproteinase-mediated, context-dependent function of amphiregulin and HB-EGF in human keratinocytes and skin. J. Invest. Dermatol. 2010, 130, 295–304. [Google Scholar] [CrossRef]
- Kefaloyianni, E.; Muthu, M.L.; Kaeppler, J.; Sun, X.; Sabbisetti, V.; Chalaris, A.; Rose-John, S.; Wong, E.; Sagi, I.; Waikar, S.S.; et al. ADAM17 substrate release in proximal tubule drives kidney fibrosis. JCI Insight 2016, 1. [Google Scholar] [CrossRef]
- Stoll, S.W.; Stuart, P.E.; Lambert, S.; Gandarillas, A.; Rittie, L.; Johnston, A.; Elder, J.T. Membrane-Tethered Intracellular Domain of Amphiregulin Promotes Keratinocyte Proliferation. J. Invest. Dermatol. 2016, 136, 444–452. [Google Scholar] [CrossRef]
- Berasain, C.; Garcia-Trevijano, E.R.; Castillo, J.; Erroba, E.; Lee, D.C.; Prieto, J.; Avila, M.A. Amphiregulin: an early trigger of liver regeneration in mice. Gastroenterology 2005, 128, 424–432. [Google Scholar] [CrossRef]
- Schelfhout, V.R.; Coene, E.D.; Delaey, B.; Waeytens, A.A.; De Rycke, L.; Deleu, M.; De Potter, C.R. The role of heregulin-alpha as a motility factor and amphiregulin as a growth factor in wound healing. J. Pathol. 2002, 198, 523–533. [Google Scholar] [CrossRef]
- Liu, T.; De Los Santos, F.G.; Ding, L.; Wu, Z.; Phan, S.H. Amphiregulin Promotes Fibroblast Activation in Pulmonary Fibrosis. The FASEB Journal 2016, 30, 50.56–50.56. [Google Scholar] [CrossRef]
- Cheng, W.H.; Kao, S.Y.; Chen, C.L.; Yuliani, F.S.; Lin, L.Y.; Lin, C.H.; Chen, B.C. Amphiregulin induces CCN2 and fibronectin expression by TGF-beta through EGFR-dependent pathway in lung epithelial cells. Respir. Res. 2022, 23, 381. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, Y.; Li, Y.; Zhang, Q.; Zhong, L.; Pan, W.; Ji, K.; Zhang, S.; Chen, Z.; Liu, Y.; et al. Cancer-associated fibroblasts derived amphiregulin promotes HNSCC progression and drug resistance of EGFR inhibitor. Cancer Lett. 2025, 622, 217710. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Wang, Z.; Wang, G.; Geng, J.; Wu, H.; Liu, X.; Bin, E.; Sui, J.; Dai, H.; Tang, N. Sustained amphiregulin expression in intermediate alveolar stem cells drives progressive fibrosis. Cell Stem Cell 2024, 31, 1344–1358 e1346. [Google Scholar] [CrossRef] [PubMed]
- Son, S.S.; Hwang, S.; Park, J.H.; Ko, Y.; Yun, S.I.; Lee, J.H.; Son, B.; Kim, T.R.; Park, H.O.; Lee, E.Y. In vivo silencing of amphiregulin by a novel effective Self-Assembled-Micelle inhibitory RNA ameliorates renal fibrosis via inhibition of EGFR signals. Sci. Rep. 2021, 11, 2191. [Google Scholar] [CrossRef] [PubMed]
- Son, B.; Kim, T.R.; Park, J.H.; Yun, S.I.; Choi, H.; Choi, J.W.; Jeon, C.; Park, H.O. SAMiRNA Targeting Amphiregulin Alleviate Total-Body-Irradiation-Induced Renal Fibrosis. Radiat. Res. 2022, 197, 471–479. [Google Scholar] [CrossRef]
- Yoon, P.O.; Park, J.W.; Lee, C.M.; Kim, S.H.; Kim, H.N.; Ko, Y.; Bae, S.J.; Yun, S.; Park, J.H.; Kwon, T.; et al. Self-assembled Micelle Interfering RNA for Effective and Safe Targeting of Dysregulated Genes in Pulmonary Fibrosis. J. Biol. Chem. 2016, 291, 6433–6446. [Google Scholar] [CrossRef]
- Gilmore, J.L.; Scott, J.A.; Bouizar, Z.; Robling, A.; Pitfield, S.E.; Riese, D.J., 2nd; Foley, J. Amphiregulin-EGFR signaling regulates PTHrP gene expression in breast cancer cells. Breast Cancer Res. Treat. 2008, 110, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Baldys, A.; Gooz, M.; Morinelli, T.A.; Lee, M.H.; Raymond, J.R., Jr.; Luttrell, L.M.; Raymond, J.R., Sr. Essential role of c-Cbl in amphiregulin-induced recycling and signaling of the endogenous epidermal growth factor receptor. Biochemistry 2009, 48, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Krall, J.A.; Beyer, E.M.; MacBeath, G. High- and low-affinity epidermal growth factor receptor-ligand interactions activate distinct signaling pathways. PLoS One 2011, 6, e15945. [Google Scholar] [CrossRef] [PubMed]
- Macdonald-Obermann, J.L.; Pike, L.J. Different epidermal growth factor (EGF) receptor ligands show distinct kinetics and biased or partial agonism for homodimer and heterodimer formation. J. Biol. Chem. 2014, 289, 26178–26188. [Google Scholar] [CrossRef]
- Deguchi, E.; Lin, S.; Hirayama, D.; Matsuda, K.; Tanave, A.; Sumiyama, K.; Tsukiji, S.; Otani, T.; Furuse, M.; Sorkin, A.; et al. Low-affinity ligands of the epidermal growth factor receptor are long-range signal transmitters in collective cell migration of epithelial cells. Cell Rep. 2024, 43, 114986. [Google Scholar] [CrossRef]
- Zaiss, D.M.W. Amphiregulin as a driver of tissue fibrosis. Am. J. Transplant. 2020, 20, 631–632. [Google Scholar] [CrossRef]
- Schramm, F.; Schaefer, L.; Wygrecka, M. EGFR Signaling in Lung Fibrosis. Cells 2022, 11. [Google Scholar] [CrossRef]
- Singh, B.; Carpenter, G.; Coffey, R.J. EGF receptor ligands: recent advances. F1000Res 2016, 5. [Google Scholar] [CrossRef]
- Xu, Q.; Chiao, P.; Sun, Y. Amphiregulin in Cancer: New Insights for Translational Medicine. Trends Cancer 2016, 2, 111–113. [Google Scholar] [CrossRef]
- Kefaloyianni, E.; Keerthi Raja, M.R.; Schumacher, J.; Muthu, M.L.; Krishnadoss, V.; Waikar, S.S.; Herrlich, A. Proximal Tubule-Derived Amphiregulin Amplifies and Integrates Profibrotic EGF Receptor Signals in Kidney Fibrosis. J. Am. Soc. Nephrol. 2019, 30, 2370–2383. [Google Scholar] [CrossRef]
- Zhou, Y.; Lee, J.Y.; Lee, C.M.; Cho, W.K.; Kang, M.J.; Koff, J.L.; Yoon, P.O.; Chae, J.; Park, H.O.; Elias, J.A.; et al. Amphiregulin, an epidermal growth factor receptor ligand, plays an essential role in the pathogenesis of transforming growth factor-beta-induced pulmonary fibrosis. J. Biol. Chem. 2012, 287, 41991–42000. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Marshall, J.F. The role of integrins in TGFbeta activation in the tumour stroma. Cell Tissue Res. 2016, 365, 657–673. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Baker, D.; Ten Dijke, P. TGF-beta-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Cruz da Silva, E.; Dontenwill, M.; Choulier, L.; Lehmann, M. Role of Integrins in Resistance to Therapies Targeting Growth Factor Receptors in Cancer. Cancers (Basel) 2019, 11. [Google Scholar] [CrossRef]
- Busser, B.; Sancey, L.; Josserand, V.; Niang, C.; Favrot, M.C.; Coll, J.L.; Hurbin, A. Amphiregulin promotes BAX inhibition and resistance to gefitinib in non-small-cell lung cancers. Mol. Ther. 2010, 18, 528–535. [Google Scholar] [CrossRef]
- Busser, B.; Sancey, L.; Josserand, V.; Niang, C.; Khochbin, S.; Favrot, M.C.; Coll, J.L.; Hurbin, A. Amphiregulin promotes resistance to gefitinib in nonsmall cell lung cancer cells by regulating Ku70 acetylation. Mol. Ther. 2010, 18, 536–543. [Google Scholar] [CrossRef]
- Rexer, B.N.; Engelman, J.A.; Arteaga, C.L. Overcoming resistance to tyrosine kinase inhibitors: lessons learned from cancer cells treated with EGFR antagonists. Cell Cycle 2009, 8, 18–22. [Google Scholar] [CrossRef]
- Wheeler, D.L.; Dunn, E.F.; Harari, P.M. Understanding resistance to EGFR inhibitors-impact on future treatment strategies. Nat. Rev. Clin. Oncol. 2010, 7, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Kindermann, M.; Knipfer, L.; Atreya, I.; Wirtz, S. ILC2s in infectious diseases and organ-specific fibrosis. Semin. Immunopathol. 2018, 40, 379–392. [Google Scholar] [CrossRef]
- Hirahara, K.; Aoki, A.; Morimoto, Y.; Kiuchi, M.; Okano, M.; Nakayama, T. The immunopathology of lung fibrosis: amphiregulin-producing pathogenic memory T helper-2 cells control the airway fibrotic responses by inducing eosinophils to secrete osteopontin. Semin. Immunopathol. 2019, 41, 339–348. [Google Scholar] [CrossRef]
- Adams, T.S.; Schupp, J.C.; Poli, S.; Ayaub, E.A.; Neumark, N.; Ahangari, F.; Chu, S.G.; Raby, B.A.; DeIuliis, G.; Januszyk, M.; et al. Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci. Adv. 2020, 6, eaba1983. [Google Scholar] [CrossRef] [PubMed]
- Neumark, N.; Cosme, C., Jr.; Rose, K.A.; Kaminski, N. The Idiopathic Pulmonary Fibrosis Cell Atlas. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 319, L887–L893. [Google Scholar] [CrossRef] [PubMed]
- Kathiriya, J.J.; Wang, C.; Zhou, M.; Brumwell, A.; Cassandras, M.; Le Saux, C.J.; Cohen, M.; Alysandratos, K.D.; Wang, B.; Wolters, P.; et al. Human alveolar type 2 epithelium transdifferentiates into metaplastic KRT5(+) basal cells. Nat. Cell Biol. 2022, 24, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Koma, Y.I.; Miyako, S.; Torigoe, R.; Yokoo, H.; Omori, M.; Yamanaka, K.; Ishihara, N.; Tsukamoto, S.; Kodama, T.; et al. AREG Upregulation in Cancer Cells via Direct Interaction with Cancer-Associated Fibroblasts Promotes Esophageal Squamous Cell Carcinoma Progression Through EGFR-Erk/p38 MAPK Signaling. Cells 2024, 13. [Google Scholar] [CrossRef]
- Buechler, M.B.; Fu, W.; Turley, S.J. Fibroblast-macrophage reciprocal interactions in health, fibrosis, and cancer. Immunity 2021, 54, 903–915. [Google Scholar] [CrossRef]
- Ding, L.; Liu, T.; Wu, Z.; Hu, B.; Nakashima, T.; Ullenbruch, M.; Gonzalez De Los Santos, F.; Phan, S.H. Bone Marrow CD11c+ Cell-Derived Amphiregulin Promotes Pulmonary Fibrosis. J. Immunol. 2016, 197, 303–312. [Google Scholar] [CrossRef]
- Kurche, J.S.; Stancil, I.T.; Michalski, J.E.; Yang, I.V.; Schwartz, D.A. Dysregulated Cell-Cell Communication Characterizes Pulmonary Fibrosis. Cells 2022, 11. [Google Scholar] [CrossRef]
- Miyamoto, S.; Fukami, T.; Yagi, H.; Kuroki, M.; Yotsumoto, F. Potential for molecularly targeted therapy against epidermal growth factor receptor ligands. Anticancer. Res. 2009, 29, 823–830. [Google Scholar]
- Lofgren, K.A.; Sreekumar, S.; Jenkins, E.C., Jr.; Ernzen, K.J.; Kenny, P.A. Anti-tumor efficacy of an MMAE-conjugated antibody targeting cell surface TACE/ADAM17-cleaved Amphiregulin in breast cancer. Antib. Ther. 2021, 4, 252–261. [Google Scholar] [CrossRef]
- Hosur, V.; Farley, M.L.; Burzenski, L.M.; Shultz, L.D.; Wiles, M.V. ADAM17 is essential for ectodomain shedding of the EGF-receptor ligand amphiregulin. FEBS Open Bio 2018, 8, 702–710. [Google Scholar] [CrossRef]
- Lee, C.M.; Park, J.W.; Cho, W.K.; Zhou, Y.; Han, B.; Yoon, P.O.; Chae, J.; Elias, J.A.; Lee, C.G. Modifiers of TGF-beta1 effector function as novel therapeutic targets of pulmonary fibrosis. Korean J. Intern. Med. 2014, 29, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Fu, M.; Wang, M.; Wei, Y.; Wei, X. Targeting TGF-beta signal transduction for fibrosis and cancer therapy. Mol. Cancer 2022, 21, 104. [Google Scholar] [CrossRef]
- Trachalaki, A.; Sultana, N.; Wells, A.U. An update on current and emerging drug treatments for idiopathic pulmonary fibrosis. Expert. Opin. Pharmacother. 2023, 24, 1125–1142. [Google Scholar] [CrossRef]
- Suri, G.S.; Kaur, G.; Jha, C.K.; Tiwari, M. Understanding idiopathic pulmonary fibrosis—Clinical features, molecular mechanism and therapies. Exp. Gerontol. 2021, 153, 111473. [Google Scholar] [CrossRef] [PubMed]
- Zaman, T.; Lee, J.S. Risk factors for the development of idiopathic pulmonary fibrosis: A review. Curr. Pulmonol. Rep. 2018, 7, 118–125. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J. Cellular and Molecular Mechanisms in Idiopathic Pulmonary Fibrosis. Adv. Respir. Med. 2023, 91, 26–48. [Google Scholar] [CrossRef]
- Bonella, F.; Spagnolo, P.; Ryerson, C. Current and Future Treatment Landscape for Idiopathic Pulmonary Fibrosis. Drugs 2023, 83, 1581–1593. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.; Athar, Z.M.; Hiba, T. Current and Novel Treatment Modalities of Idiopathic Pulmonary Fibrosis. Cureus 2024, 16, e56140. [Google Scholar] [CrossRef]
- Cameli, P.; Refini, R.M.; Bergantini, L.; d’Alessandro, M.; Alonzi, V.; Magnoni, C.; Rottoli, P.; Sestini, P.; Bargagli, E. Long-Term Follow-Up of Patients With Idiopathic Pulmonary Fibrosis Treated With Pirfenidone or Nintedanib: A Real-Life Comparison Study. Front. Mol. Biosci. 2020, 7, 581828. [Google Scholar] [CrossRef]
- Marijic, P.; Schwarzkopf, L.; Schwettmann, L.; Ruhnke, T.; Trudzinski, F.; Kreuter, M. Pirfenidone vs. nintedanib in patients with idiopathic pulmonary fibrosis: a retrospective cohort study. Respir. Res. 2021, 22, 268. [Google Scholar] [CrossRef]
- Kalluri, M. Palliative care in advanced pulmonary fibrosis. Curr. Opin. Pulm. Med. 2024, 30, 530–539. [Google Scholar] [CrossRef]
- Hanata, N.; Nagafuchi, Y.; Sugimori, Y.; Kobayashi, S.; Tsuchida, Y.; Iwasaki, Y.; Shoda, H.; Fujio, K. Serum Amphiregulin and Heparin-Binding Epidermal Growth Factor as Biomarkers in Patients with Idiopathic Inflammatory Myopathy. J. Clin. Med. 2021, 10. [Google Scholar] [CrossRef]
- Shen, C.; Fan, X.; Mao, Y.; Jiang, J. Amphiregulin in lung diseases: A review. Medicine (Baltimore) 2024, 103, e37292. [Google Scholar] [CrossRef]
- Shen, M.; Luo, Z.; Zhou, Y. Regeneration-Associated Transitional State Cells in Pulmonary Fibrosis. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Guan, X.; Carraro, G.; Parimon, T.; Liu, X.; Huang, G.; Mulay, A.; Soukiasian, H.J.; David, G.; Weigt, S.S.; et al. Senescence of Alveolar Type 2 Cells Drives Progressive Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2021, 203, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ding, L.; Wu, Z.; Gonzalez De Los Santos, F.; Phan, S. Role of dendritic cell-derived amphiregulin in pulmonary fibrosis (CCR5P.203). The Journal of Immunology 2015, 194, 186.185–186.185. [Google Scholar] [CrossRef]
- Yao, H.C.; Zhu, Y.; Lu, H.Y.; Ju, H.M.; Xu, S.Q.; Qiao, Y.; Wei, S.J. Type 2 innate lymphoid cell-derived amphiregulin regulates type II alveolar epithelial cell transdifferentiation in a mouse model of bronchopulmonary dysplasia. Int. Immunopharmacol. 2023, 122, 110672. [Google Scholar] [CrossRef]
- Morimoto, Y.; Hirahara, K.; Kiuchi, M.; Wada, T.; Ichikawa, T.; Kanno, T.; Okano, M.; Kokubo, K.; Onodera, A.; Sakurai, D.; et al. Amphiregulin-Producing Pathogenic Memory T Helper 2 Cells Instruct Eosinophils to Secrete Osteopontin and Facilitate Airway Fibrosis. Immunity 2018, 49, 134–150 e136. [Google Scholar] [CrossRef]
- Conroy, K.P.; Kitto, L.J.; Henderson, N.C. alphav integrins: key regulators of tissue fibrosis. Cell Tissue Res. 2016, 365, 511–519. [Google Scholar] [CrossRef]
- Margadant, C.; Sonnenberg, A. Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 2010, 11, 97–105. [Google Scholar] [CrossRef]
- Sakao, S.; Tatsumi, K. Molecular mechanisms of lung-specific toxicity induced by epidermal growth factor receptor tyrosine kinase inhibitors. Oncol. Lett. 2012, 4, 865–867. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, I.E.; Eickelberg, O. The impact of TGF-beta on lung fibrosis: from targeting to biomarkers. Proc. Am. Thorac. Soc. 2012, 9, 111–116. [Google Scholar] [CrossRef]
- Suzuki, H.; Aoshiba, K.; Yokohori, N.; Nagai, A. Epidermal growth factor receptor tyrosine kinase inhibition augments a murine model of pulmonary fibrosis. Cancer Res. 2003, 63, 5054–5059. [Google Scholar] [PubMed]
- Ma, H.; Wu, X.; Li, Y.; Xia, Y. Research Progress in the Molecular Mechanisms, Therapeutic Targets, and Drug Development of Idiopathic Pulmonary Fibrosis. Front. Pharmacol. 2022, 13, 963054. [Google Scholar] [CrossRef] [PubMed]
- Koya, D. Diabetic kidney disease: Its current trends and future therapeutic perspectives. J. Diabetes Investig. 2019, 10, 1174–1176. [Google Scholar] [CrossRef]
- Humphreys, B.D. Mechanisms of Renal Fibrosis. Annu. Rev. Physiol. 2018, 80, 309–326. [Google Scholar] [CrossRef]
- Liu, B.C.; Tang, T.T.; Lv, L.L.; Lan, H.Y. Renal tubule injury: a driving force toward chronic kidney disease. Kidney Int. 2018, 93, 568–579. [Google Scholar] [CrossRef]
- Klinkhammer, B.M.; Boor, P. Kidney fibrosis: Emerging diagnostic and therapeutic strategies. Mol. Aspects Med. 2023, 93, 101206. [Google Scholar] [CrossRef]
- Huang, R.; Fu, P.; Ma, L. Kidney fibrosis: from mechanisms to therapeutic medicines. Signal Transduct. Target. Ther. 2023, 8, 129. [Google Scholar] [CrossRef]
- Melderis, S.; Hagenstein, J.; Warkotsch, M.T.; Dang, J.; Herrnstadt, G.R.; Niehus, C.B.; Neumann, K.; Panzer, U.; Berasain, C.; Avila, M.A.; et al. Amphiregulin Aggravates Glomerulonephritis via Recruitment and Activation of Myeloid Cells. J. Am. Soc. Nephrol. 2020, 31, 1996–2012. [Google Scholar] [CrossRef]
- Rayego-Mateos, S.; Rodrigues-Diez, R.; Morgado-Pascual, J.L.; Valentijn, F.; Valdivielso, J.M.; Goldschmeding, R.; Ruiz-Ortega, M. Role of Epidermal Growth Factor Receptor (EGFR) and Its Ligands in Kidney Inflammation and Damage. Mediators Inflamm. 2018, 2018, 8739473. [Google Scholar] [CrossRef]
- Osakabe, Y.; Taniguchi, Y.; Hamada Ode, K.; Shimamura, Y.; Inotani, S.; Nishikawa, H.; Matsumoto, T.; Horino, T.; Fujimoto, S.; Terada, Y. Clinical significance of amphiregulin in patients with chronic kidney disease. Clin. Exp. Nephrol. 2024, 28, 421–430. [Google Scholar] [CrossRef]
- Schmidt, I.M.; Kefalogianni, E.; Zhao, R.; Verma, A.; Sabbisetti, V.; Rahman, M.; Pradhan, N.; Srivastava, A.; He, J.; Chen, J.; et al. Associations of Serum Amphiregulin Levels With Kidney Failure and Mortality: The Chronic Renal Insufficiency Cohort (CRIC). Kidney Med. 2025, 7, 100958. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Xu, J.; Xie, J.; Harris, D.C.H.; Zheng, G. The Role of Macrophages in Kidney Fibrosis. Front. Physiol. 2021, 12, 705838. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Harris, D.C.; Wang, Y. Macrophages in kidney injury, inflammation, and fibrosis. Physiology (Bethesda) 2015, 30, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Black, L.M.; Lever, J.M.; Agarwal, A. Renal Inflammation and Fibrosis: A Double-edged Sword. J. Histochem. Cytochem. 2019, 67, 663–681. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Palau, V.; Pascual, J.; Soler, M.J.; Riera, M. Role of ADAM17 in kidney disease. Am. J. Physiol. Renal Physiol. 2019, 317, F333–F342. [Google Scholar] [CrossRef]
- Gu, Y.Y.; Liu, X.S.; Huang, X.R.; Yu, X.Q.; Lan, H.Y. Diverse Role of TGF-beta in Kidney Disease. Front. Cell Dev. Biol. 2020, 8, 123. [Google Scholar] [CrossRef]
- Higgins, S.P.; Tang, Y.; Higgins, C.E.; Mian, B.; Zhang, W.; Czekay, R.P.; Samarakoon, R.; Conti, D.J.; Higgins, P.J. TGF-beta1/p53 signaling in renal fibrogenesis. Cell Signal 2018, 43, 1–10. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, X.M.; Huang, X.R.; Lan, H.Y. The preventive and therapeutic implication for renal fibrosis by targetting TGF-beta/Smad3 signaling. Clin Sci (Lond) 2018, 132, 1403–1415. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Liu, N. EGFR signaling in renal fibrosis. Kidney Int Suppl (2011) 2014, 4, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Melderis, S.; Warkotsch, M.T.; Dang, J.; Hagenstein, J.; Ehnold, L.I.; Herrnstadt, G.R.; Niehus, C.B.; Feindt, F.C.; Kylies, D.; Puelles, V.G.; et al. The Amphiregulin/EGFR axis protects from lupus nephritis via downregulation of pathogenic CD4(+) T helper cell responses. J. Autoimmun. 2022, 129, 102829. [Google Scholar] [CrossRef]
- Buvall, L.; Menzies, R.I.; Williams, J.; Woollard, K.J.; Kumar, C.; Granqvist, A.B.; Fritsch, M.; Feliers, D.; Reznichenko, A.; Gianni, D.; et al. Selecting the right therapeutic target for kidney disease. Front. Pharmacol. 2022, 13, 971065. [Google Scholar] [CrossRef]
- Tawengi, M.; Al-Dali, Y.; Tawengi, A.; Benter, I.F.; Akhtar, S. Targeting the epidermal growth factor receptor (EGFR/ErbB) for the potential treatment of renal pathologies. Front. Pharmacol. 2024, 15, 1394997. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kim, S.I.; Choi, M.E. Therapeutic targets for treating fibrotic kidney diseases. Transl. Res. 2015, 165, 512–530. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, F.; Li, X.; Ma, Z.; Jiang, D. Quercetin inhibits the amphiregulin/EGFR signaling-mediated renal tubular epithelial-mesenchymal transition and renal fibrosis in obstructive nephropathy. Phytother. Res. 2023, 37, 111–123. [Google Scholar] [CrossRef]
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Unalp-Arida, A.; Ruhl, C.E. Liver fibrosis scores predict liver disease mortality in the United States population. Hepatology 2017, 66, 84–95. [Google Scholar] [CrossRef]
- Ng, C.H.; Lim, W.H.; Hui Lim, G.E.; Hao Tan, D.J.; Syn, N.; Muthiah, M.D.; Huang, D.Q.; Loomba, R. Mortality Outcomes by Fibrosis Stage in Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2023, 21, 931–939 e935. [Google Scholar] [CrossRef]
- Savage, T.M.; Fortson, K.T.; de Los Santos-Alexis, K.; Oliveras-Alsina, A.; Rouanne, M.; Rae, S.S.; Gamarra, J.R.; Shayya, H.; Kornberg, A.; Cavero, R.; et al. Amphiregulin from regulatory T cells promotes liver fibrosis and insulin resistance in non-alcoholic steatohepatitis. Immunity 2024, 57, 303–318 e306. [Google Scholar] [CrossRef] [PubMed]
- McKee, C.; Sigala, B.; Soeda, J.; Mouralidarane, A.; Morgan, M.; Mazzoccoli, G.; Rappa, F.; Cappello, F.; Cabibi, D.; Pazienza, V.; et al. Amphiregulin activates human hepatic stellate cells and is upregulated in non alcoholic steatohepatitis. Sci. Rep. 2015, 5, 8812. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, A.; Takemura, K.; Tanaka, A.; Matsumoto, M.; Katsuyama, M.; Okanoue, T.; Yamaguchi, K.; Itoh, Y.; Iwata, K.; Amagase, K.; et al. Carfilzomib shows therapeutic potential for reduction of liver fibrosis by targeting hepatic stellate cell activation. Sci. Rep. 2024, 14, 19288. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, W.M.; Sun, H.Y.; Peng, Y.; Huang, R.J.; Chen, C.Y.; Zhang, H.D.; Zhou, S.A.; Wu, H.P.; Tang, D.; et al. Hepatocyte-derived liver progenitor-like cells attenuate liver cirrhosis via induction of apoptosis in hepatic stellate cells. Hepatol. Commun. 2025, 9. [Google Scholar] [CrossRef]
- Keam, S.J. Resmetirom: First Approval. Drugs 2024, 84, 729–735. [Google Scholar] [CrossRef]
- Heo, Y.J.; Lee, N.; Choi, S.E.; Jeon, J.Y.; Han, S.J.; Kim, D.J.; Kang, Y.; Lee, K.W.; Kim, H.J. Amphiregulin Induces iNOS and COX-2 Expression through NF-kappaB and MAPK Signaling in Hepatic Inflammation. Mediators Inflamm. 2023, 2023, 2364121. [Google Scholar] [CrossRef]
- Hori, M.; Kita, M.; Torihashi, S.; Miyamoto, S.; Won, K.J.; Sato, K.; Ozaki, H.; Karaki, H. Upregulation of iNOS by COX-2 in muscularis resident macrophage of rat intestine stimulated with LPS. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G930–G938. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, M.K.; Lim, S.Y.; Sung, S.H.; Kim, Y.C. Inhibition of inducible NO synthase, cyclooxygenase-2 and interleukin-1beta by torilin is mediated by mitogen-activated protein kinases in microglial BV2 cells. Br. J. Pharmacol. 2009, 156, 933–940. [Google Scholar] [CrossRef]
- Dashek, R.J.; Cunningham, R.P.; Taylor, C.L.; Alessi, I.; Diaz, C.; Meers, G.M.; Wheeler, A.A.; Ibdah, J.A.; Parks, E.J.; Yoshida, T.; et al. Hepatocellular RECK as a Critical Regulator of Metabolic Dysfunction-associated Steatohepatitis Development. Cell Mol. Gastroenterol. Hepatol. 2024, 18, 101365. [Google Scholar] [CrossRef]
- Cuevas, M.J.; Tieppo, J.; Marroni, N.P.; Tunon, M.J.; Gonzalez-Gallego, J. Suppression of amphiregulin/epidermal growth factor receptor signals contributes to the protective effects of quercetin in cirrhotic rats. J. Nutr. 2011, 141, 1299–1305. [Google Scholar] [CrossRef]
- Berasain, C.; Garcia-Trevijano, E.R.; Castillo, J.; Erroba, E.; Santamaria, M.; Lee, D.C.; Prieto, J.; Avila, M.A. Novel role for amphiregulin in protection from liver injury. J. Biol. Chem. 2005, 280, 19012–19020. [Google Scholar] [CrossRef]
- Liu, Q.; Rehman, H.; Krishnasamy, Y.; Haque, K.; Schnellmann, R.G.; Lemasters, J.J.; Zhong, Z. Amphiregulin stimulates liver regeneration after small-for-size mouse liver transplantation. Am. J. Transplant. 2012, 12, 2052–2061. [Google Scholar] [CrossRef] [PubMed]
- Perugorria, M.J.; Latasa, M.U.; Nicou, A.; Cartagena-Lirola, H.; Castillo, J.; Goni, S.; Vespasiani-Gentilucci, U.; Zagami, M.G.; Lotersztajn, S.; Prieto, J.; et al. The epidermal growth factor receptor ligand amphiregulin participates in the development of mouse liver fibrosis. Hepatology 2008, 48, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Fuchs, B.C.; Yamada, S.; Lauwers, G.Y.; Kulu, Y.; Goodwin, J.M.; Lanuti, M.; Tanabe, K.K. Mouse model of carbon tetrachloride induced liver fibrosis: Histopathological changes and expression of CD133 and epidermal growth factor. BMC Gastroenterol. 2010, 10, 79. [Google Scholar] [CrossRef]
- Crespo, I.; San-Miguel, B.; Fernandez, A.; Ortiz de Urbina, J.; Gonzalez-Gallego, J.; Tunon, M.J. Melatonin limits the expression of profibrogenic genes and ameliorates the progression of hepatic fibrosis in mice. Transl. Res. 2015, 165, 346–357. [Google Scholar] [CrossRef]
- Ikeno, Y.; Ohara, D.; Takeuchi, Y.; Watanabe, H.; Kondoh, G.; Taura, K.; Uemoto, S.; Hirota, K. Foxp3+ Regulatory T Cells Inhibit CCl(4)-Induced Liver Inflammation and Fibrosis by Regulating Tissue Cellular Immunity. Front. Immunol. 2020, 11, 584048. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Fan, J.; Zhou, H. Bile acid-mediated signaling in cholestatic liver diseases. Cell Biosci. 2023, 13, 77. [Google Scholar] [CrossRef]
- Santamaria, E.; Rodriguez-Ortigosa, C.M.; Uriarte, I.; Latasa, M.U.; Urtasun, R.; Alvarez-Sola, G.; Barcena-Varela, M.; Colyn, L.; Arcelus, S.; Jimenez, M.; et al. The Epidermal Growth Factor Receptor Ligand Amphiregulin Protects From Cholestatic Liver Injury and Regulates Bile Acids Synthesis. Hepatology 2019, 69, 1632–1647. [Google Scholar] [CrossRef]
- Xiao, M.H.; Wu, S.; Liang, P.; Ma, D.; Zhang, J.; Chen, H.; Zhong, Z.; Liu, J.; Jiang, H.; Feng, X.; et al. Mucosal-associated invariant T cells promote ductular reaction through amphiregulin in biliary atresia. EBioMedicine 2024, 103, 105138. [Google Scholar] [CrossRef]
- Mohagheghi, S.; Geramizadeh, B.; Nikeghbalian, S.; Khodadadi, I.; Karimi, J.; Khajehahmadi, Z.; Gharekhanloo, F.; Tavilani, H. Intricate role of yes-associated protein1 in human liver cirrhosis: TGF-beta1 still is a giant player. IUBMB Life 2019, 71, 1453–1464. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Cardiac fibrosis. Cardiovasc. Res. 2021, 117, 1450–1488. [Google Scholar] [CrossRef] [PubMed]
- Hinderer, S.; Schenke-Layland, K. Cardiac fibrosis—A short review of causes and therapeutic strategies. Adv. Drug Deliv. Rev. 2019, 146, 77–82. [Google Scholar] [CrossRef]
- Yin, X.; Yin, X.; Pan, X.; Zhang, J.; Fan, X.; Li, J.; Zhai, X.; Jiang, L.; Hao, P.; Wang, J.; et al. Post-myocardial infarction fibrosis: Pathophysiology, examination, and intervention. Front. Pharmacol. 2023, 14, 1070973. [Google Scholar] [CrossRef] [PubMed]
- Talman, V.; Ruskoaho, H. Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res. 2016, 365, 563–581. [Google Scholar] [CrossRef]
- Schlittler, M.; Pramstaller, P.P.; Rossini, A.; De Bortoli, M. Myocardial Fibrosis in Hypertrophic Cardiomyopathy: A Perspective from Fibroblasts. Int. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.Y.; Lopez, B.; Coelho-Filho, O.R.; Lakdawala, N.K.; Cirino, A.L.; Jarolim, P.; Kwong, R.; Gonzalez, A.; Colan, S.D.; Seidman, J.G.; et al. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N. Engl. J. Med. 2010, 363, 552–563. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Qu, Z.; Weiss, J.N. Cardiac fibrosis and arrhythmogenesis: the road to repair is paved with perils. J. Mol. Cell Cardiol. 2014, 70, 83–91. [Google Scholar] [CrossRef]
- Fujiu, K.; Shibata, M.; Nakayama, Y.; Ogata, F.; Matsumoto, S.; Noshita, K.; Iwami, S.; Nakae, S.; Komuro, I.; Nagai, R.; et al. A heart-brain-kidney network controls adaptation to cardiac stress through tissue macrophage activation. Nat. Med. 2017, 23, 611–622. [Google Scholar] [CrossRef]
- Sugita, J.; Fujiu, K.; Nakayama, Y.; Matsubara, T.; Matsuda, J.; Oshima, T.; Liu, Y.; Maru, Y.; Hasumi, E.; Kojima, T.; et al. Cardiac macrophages prevent sudden death during heart stress. Nat. Commun. 2021, 12, 1910. [Google Scholar] [CrossRef]
- Koeppen, M.; Lee, J.W.; Seo, S.W.; Brodsky, K.S.; Kreth, S.; Yang, I.V.; Buttrick, P.M.; Eckle, T.; Eltzschig, H.K. Hypoxia-inducible factor 2-alpha-dependent induction of amphiregulin dampens myocardial ischemia-reperfusion injury. Nat. Commun. 2018, 9, 816. [Google Scholar] [CrossRef]
- Bu, J.; Huang, S.; Wang, J.; Xia, T.; Liu, H.; You, Y.; Wang, Z.; Liu, K. The GABA(A) Receptor Influences Pressure Overload-Induced Heart Failure by Modulating Macrophages in Mice. Front. Immunol. 2021, 12, 670153. [Google Scholar] [CrossRef]
- Zuo, C.; Li, X.; Huang, J.; Chen, D.; Ji, K.; Yang, Y.; Xu, T.; Zhu, D.; Yan, C.; Gao, P. Osteoglycin attenuates cardiac fibrosis by suppressing cardiac myofibroblast proliferation and migration through antagonizing lysophosphatidic acid 3/matrix metalloproteinase 2/epidermal growth factor receptor signalling. Cardiovasc. Res. 2018, 114, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Humeres, C.; Shinde, A.V.; Hanna, A.; Alex, L.; Hernandez, S.C.; Li, R.; Chen, B.; Conway, S.J.; Frangogiannis, N.G. Smad7 effects on TGF-beta and ErbB2 restrain myofibroblast activation and protect from postinfarction heart failure. J. Clin. Invest. 2022, 132. [Google Scholar] [CrossRef] [PubMed]
- Grzebyk, E.; Pazgan-Simon, M.; Jagas, J.; Zuwala-Jagiello, J.; Gorka-Dynysiewicz, J. Left ventricular function is related with amphiregulin and fibrosis markers in cirrhotic cardiomyopathy. J. Physiol. Pharmacol. 2022, 73. [Google Scholar] [CrossRef]
- Ji, M.; Liu, Y.; Zuo, Z.; Xu, C.; Lin, L.; Li, Y. Downregulation of amphiregulin improves cardiac hypertrophy via attenuating oxidative stress and apoptosis. Biol. Direct 2022, 17, 21. [Google Scholar] [CrossRef]
- Warunek, J.J.; Fan, L.; Zhang, X.; Wang, S.; Sanders, S.M.; Li, T.; Mathews, L.R.; Dwyer, G.K.; Wood-Trageser, M.A.; Traczek, S.; et al. Dysregulated Treg repair responses lead to chronic rejection after heart transplantation. J. Clin. Invest. 2024, 134. [Google Scholar] [CrossRef]
- Suzuki, Y.; Emoto, T.; Sato, S.; Yoshida, T.; Shoda, M.; Endoh, H.; Nagao, M.; Hamana, T.; Inoue, T.; Hayashi, T.; et al. Left atrial single-cell transcriptomics reveals amphiregulin as a surrogate marker for atrial fibrillation. Commun. Biol. 2024, 7, 1601. [Google Scholar] [CrossRef]
- Porvari, K.; Horioka, K.; Kaija, H.; Pakanen, L. Amphiregulin is overexpressed in human cardiac tissue in hypothermia deaths; associations between the transcript and stress hormone levels in cardiac deaths. Ann. Med. 2024, 56, 2420862. [Google Scholar] [CrossRef]
- Monticelli, L.A.; Osborne, L.C.; Noti, M.; Tran, S.V.; Zaiss, D.M.; Artis, D. IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin-EGFR interactions. Proc. Natl. Acad. Sci. U S A 2015, 112, 10762–10767. [Google Scholar] [CrossRef]
- Irie, E.; Ishihara, R.; Mizushima, I.; Hatai, S.; Hagihara, Y.; Takada, Y.; Tsunoda, J.; Iwata, K.; Matsubara, Y.; Yoshimatsu, Y.; et al. Enrichment of type I interferon signaling in colonic group 2 innate lymphoid cells in experimental colitis. Front. Immunol. 2022, 13, 982827. [Google Scholar] [CrossRef]
- Shao, J.; Sheng, H. Amphiregulin promotes intestinal epithelial regeneration: roles of intestinal subepithelial myofibroblasts. Endocrinology 2010, 151, 3728–3737. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhou, B.; Pang, X.; Song, X.; Gu, Y.; Xie, R.; Liu, T.; Xu, X.; Wang, B.; Cao, H. Clostridium butyricum, a butyrate-producing potential probiotic, alleviates experimental colitis through epidermal growth factor receptor activation. Food Funct. 2022, 13, 7046–7061. [Google Scholar] [CrossRef] [PubMed]
- Xiu, W.; Chen, Q.; Wang, Z.; Wang, J.; Zhou, Z. Microbiota-derived short chain fatty acid promotion of Amphiregulin expression by dendritic cells is regulated by GPR43 and Blimp-1. Biochem. Biophys. Res. Commun. 2020, 533, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Zeka, F.; Angori, S.; Rutishauser, D.; Moch, H.; Posovszky, C.; Amin, K.; Holtan, S.; Gungor, T.; Drozdov, D. High Amphiregulin Expression in Intestinal Biopsies of Pediatric Patients with Severe Acute Graft-Versus-Host Disease. Transplant. Cell Ther. 2025. [Google Scholar] [CrossRef]
- D’Alessio, S.; Ungaro, F.; Noviello, D.; Lovisa, S.; Peyrin-Biroulet, L.; Danese, S. Revisiting fibrosis in inflammatory bowel disease: the gut thickens. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 169–184. [Google Scholar] [CrossRef]
- Alfredsson, J.; Wick, M.J. Mechanism of fibrosis and stricture formation in Crohn’s disease. Scand. J. Immunol. 2020, 92, e12990. [Google Scholar] [CrossRef]
- Santacroce, G.; Lenti, M.V.; Di Sabatino, A. Therapeutic Targeting of Intestinal Fibrosis in Crohn’s Disease. Cells 2022, 11. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, B.; Jin, T.; Ocansey, D.K.W.; Jiang, J.; Mao, F. Intestinal Fibrosis in Inflammatory Bowel Disease and the Prospects of Mesenchymal Stem Cell Therapy. Front. Immunol. 2022, 13, 835005. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, W.; Yu, T.; Yu, Y.; Cui, X.; Zhou, Z.; Yang, H.; Yu, Y.; Bilotta, A.J.; Yao, S.; et al. Th17 Cell-Derived Amphiregulin Promotes Colitis-Associated Intestinal Fibrosis Through Activation of mTOR and MEK in Intestinal Myofibroblasts. Gastroenterology 2023, 164, 89–102. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.; Giammarile, F.; Carrara, M.; Paez, D.; Hricak, H.; Ayati, N.; Li, J.J.; Mueller, M.; Aggarwal, A.; Al-Ibraheem, A.; et al. Radiotherapy and theranostics: a Lancet Oncology Commission. Lancet Oncol. 2024, 25, e545–e580. [Google Scholar] [CrossRef]
- Fijardo, M.; Kwan, J.Y.Y.; Bissey, P.A.; Citrin, D.E.; Yip, K.W.; Liu, F.F. The clinical manifestations and molecular pathogenesis of radiation fibrosis. EBioMedicine 2024, 103, 105089. [Google Scholar] [CrossRef] [PubMed]
- Meulenbroeks, C.; van Weelden, H.; Schwartz, C.; Voehringer, D.; Redegeld, F.A.M.; Rutten, V.; Willemse, T.; Sijts, A.; Zaiss, D.M.W. Basophil-derived amphiregulin is essential for UVB irradiation-induced immune suppression. J. Invest. Dermatol. 2015, 135, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Allanore, Y.; Simms, R.; Distler, O.; Trojanowska, M.; Pope, J.; Denton, C.P.; Varga, J. Systemic sclerosis. Nat. Rev. Dis. Primers 2015, 1, 15002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Fang, S.; Gao, H.; Zhang, X.; Gu, D.; Liu, Y.; Wan, J.; Xie, J. A critical role of AREG for bleomycin-induced skin fibrosis. Cell Biosci. 2021, 11, 40. [Google Scholar] [CrossRef]
- Padilla, C.M.; Valenzi, E.; Tabib, T.; Nazari, B.; Sembrat, J.; Rojas, M.; Fuschiotti, P.; Lafyatis, R. Increased CD8+ tissue resident memory T cells, regulatory T cells and activated natural killer cells in systemic sclerosis lungs. Rheumatology (Oxford) 2024, 63, 837–845. [Google Scholar] [CrossRef]
- Morina, G.; Sambataro, D.; Libra, A.; Palmucci, S.; Colaci, M.; La Rocca, G.; Ferro, F.; Carli, L.; Baldini, C.; Liuzzo, S.V.; et al. Recognition of Idiopathic Inflammatory Myopathies Underlying Interstitial Lung Diseases. Diagnostics (Basel) 2025, 15. [Google Scholar] [CrossRef]
- Ceribelli, A.; Tonutti, A.; Isailovic, N.; De Santis, M.; Selmi, C. Interstitial lung disease associated with inflammatory myositis: Autoantibodies, clinical phenotypes, and progressive fibrosis. Front Med (Lausanne) 2023, 10, 1068402. [Google Scholar] [CrossRef]
- Sehgal, S.; Patel, A.; Chatterjee, S.; Fernandez, A.P.; Farver, C.; Yadav, R.; Li, Y.; Danoff, S.K.; Saygin, D.; Huapaya, J.A.; et al. Idiopathic inflammatory myopathies related lung disease in adults. Lancet Respir. Med. 2025, 13, 272–288. [Google Scholar] [CrossRef]
- Layoun, H.; Hajal, J.; Saliba, Y.; Smayra, V.; Habr, B.; Fares, N. Pirfenidone mitigates TGF-beta1-mediated fibrosis in an idiopathic inflammatory myositis-associated interstitial lung disease model. Cytokine 2022, 154, 155899. [Google Scholar] [CrossRef]
- Lim, M.; Kim, T.; Kim, H.; Jang, B.G.; Myung, J.K.; Kim, H.Y. Esophageal ILC2s mediate abnormal epithelial remodeling in eosinophilic esophagitis via Areg-EGFR signaling. Cell Mol. Immunol. 2025, 22, 97–110. [Google Scholar] [CrossRef]
- Kaneko, T.; Iwamura, C.; Kiuchi, M.; Kurosugi, A.; Onoue, M.; Matsumura, T.; Chiba, T.; Nakayama, T.; Kato, N.; Hirahara, K. Amphiregulin-producing T(H)2 cells facilitate esophageal fibrosis of eosinophilic esophagitis. J. Allergy Clin. Immunol. Glob. 2024, 3, 100287. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Long, Q.; Zhu, D.; Fu, D.; Zhang, B.; Han, L.; Qian, M.; Guo, J.; Xu, J.; Cao, L.; et al. Targeting amphiregulin (AREG) derived from senescent stromal cells diminishes cancer resistance and averts programmed cell death 1 ligand (PD-L1)-mediated immunosuppression. Aging Cell 2019, 18, e13027. [Google Scholar] [CrossRef] [PubMed]
- Sanmamed, M.F.; Chen, L. Inducible expression of B7-H1 (PD-L1) and its selective role in tumor site immune modulation. Cancer J. 2014, 20, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, N.; Daigo, Y.; Takano, A.; Taniwaki, M.; Kato, T.; Hayama, S.; Murakami, H.; Takeshima, Y.; Inai, K.; Nishimura, H.; et al. Increases of amphiregulin and transforming growth factor-alpha in serum as predictors of poor response to gefitinib among patients with advanced non-small cell lung cancers. Cancer Res. 2005, 65, 9176–9184. [Google Scholar] [CrossRef]
- Masago, K.; Fujita, S.; Hatachi, Y.; Fukuhara, A.; Sakuma, K.; Ichikawa, M.; Kim, Y.H.; Mio, T.; Mishima, M. Clinical significance of pretreatment serum amphiregulin and transforming growth factor-alpha, and an epidermal growth factor receptor somatic mutation in patients with advanced non-squamous, non-small cell lung cancer. Cancer Sci. 2008, 99, 2295–2301. [Google Scholar] [CrossRef]
- Hobor, S.; Van Emburgh, B.O.; Crowley, E.; Misale, S.; Di Nicolantonio, F.; Bardelli, A. TGFalpha and amphiregulin paracrine network promotes resistance to EGFR blockade in colorectal cancer cells. Clin. Cancer Res. 2014, 20, 6429–6438. [Google Scholar] [CrossRef]
- Kappler, C.S.; Guest, S.T.; Irish, J.C.; Garrett-Mayer, E.; Kratche, Z.; Wilson, R.C.; Ethier, S.P. Oncogenic signaling in amphiregulin and EGFR-expressing PTEN-null human breast cancer. Mol. Oncol. 2015, 9, 527–543. [Google Scholar] [CrossRef]
- Higginbotham, J.N.; Demory Beckler, M.; Gephart, J.D.; Franklin, J.L.; Bogatcheva, G.; Kremers, G.J.; Piston, D.W.; Ayers, G.D.; McConnell, R.E.; Tyska, M.J.; et al. Amphiregulin exosomes increase cancer cell invasion. Curr. Biol. 2011, 21, 779–786. [Google Scholar] [CrossRef]
- Carvalho, S.; Lindzen, M.; Lauriola, M.; Shirazi, N.; Sinha, S.; Abdul-Hai, A.; Levanon, K.; Korach, J.; Barshack, I.; Cohen, Y.; et al. An antibody to amphiregulin, an abundant growth factor in patients’ fluids, inhibits ovarian tumors. Oncogene 2016, 35, 438–447. [Google Scholar] [CrossRef]
- Khambata-Ford, S.; Garrett, C.R.; Meropol, N.J.; Basik, M.; Harbison, C.T.; Wu, S.; Wong, T.W.; Huang, X.; Takimoto, C.H.; Godwin, A.K.; et al. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J. Clin. Oncol. 2007, 25, 3230–3237. [Google Scholar] [CrossRef]
- Kim, S.A.; Park, H.; Kim, K.J.; Kim, J.W.; Sung, J.H.; Nam, M.; Lee, J.H.; Jung, E.H.; Suh, K.J.; Lee, J.Y.; et al. Amphiregulin can predict treatment resistance to palliative first-line cetuximab plus FOLFIRI chemotherapy in patients with RAS wild-type metastatic colorectal cancer. Sci. Rep. 2021, 11, 23803. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, L.; Zhang, H.; Lu, J.; Zhang, Z.; Wu, H.; Liang, Z. AREG mediates the epithelial-mesenchymal transition in pancreatic cancer cells via the EGFR/ERK/NF-kappaB signalling pathway. Oncol. Rep. 2020, 43, 1558–1568. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Wang, S.; Pan, Y.; Zhi, W.; Gu, C.; Guo, T.; Zhai, J.; Li, C.; Chen, Y.Q.; Wang, R. Development, opportunities, and challenges of siRNA nucleic acid drugs. Mol. Ther. Nucleic Acids 2025, 36, 102437. [Google Scholar] [CrossRef]
- Wilson, K.J.; Gilmore, J.L.; Foley, J.; Lemmon, M.A.; Riese, D.J., 2nd. Functional selectivity of EGF family peptide growth factors: implications for cancer. Pharmacol. Ther. 2009, 122, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Piersma, B.; Hayward, M.K.; Weaver, V.M. Fibrosis and cancer: A strained relationship. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188356. [Google Scholar] [CrossRef]
- Landolt, L.; Spagnoli, G.C.; Hertig, A.; Brocheriou, I.; Marti, H.P. Fibrosis and cancer: shared features and mechanisms suggest common targeted therapeutic approaches. Nephrol. Dial. Transplant. 2022, 37, 1024–1032. [Google Scholar] [CrossRef]
- Henke, E.; Nandigama, R.; Ergun, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2019, 6, 160. [Google Scholar] [CrossRef]
- Benelli, R.; Vene, R.; Minghelli, S.; Carlone, S.; Gatteschi, B.; Ferrari, N. Celecoxib induces proliferation and Amphiregulin production in colon subepithelial myofibroblasts, activating erk1-2 signaling in synergy with EGFR. Cancer Lett. 2013, 328, 73–82. [Google Scholar] [CrossRef]
- Wang, C.; Long, Q.; Fu, Q.; Xu, Q.; Fu, D.; Li, Y.; Gao, L.; Guo, J.; Zhang, X.; Lam, E.W.; et al. Targeting epiregulin in the treatment-damaged tumor microenvironment restrains therapeutic resistance. Oncogene 2022, 41, 4941–4959. [Google Scholar] [CrossRef]
- Wu, F.; Yang, J.; Liu, J.; Wang, Y.; Mu, J.; Zeng, Q.; Deng, S.; Zhou, H. Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer. Signal Transduct. Target. Ther. 2021, 6, 218. [Google Scholar] [CrossRef]
- Sun, R.; Zhao, H.; Gao, D.S.; Ni, A.; Li, H.; Chen, L.; Lu, X.; Chen, K.; Lu, B. Amphiregulin couples IL1RL1(+) regulatory T cells and cancer-associated fibroblasts to impede antitumor immunity. Sci. Adv. 2023, 9, eadd7399. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.; Wang, Y.; Ye, P.; Li, J.; Li, H.; Ding, Q.; Xia, J. Amphiregulin Confers Regulatory T Cell Suppressive Function and Tumor Invasion via the EGFR/GSK-3beta/Foxp3 Axis. J. Biol. Chem. 2016, 291, 21085–21095. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Huang, M.S.; Cheng, D.E.; Hung, J.Y.; Yang, C.J.; Chou, S.H.; Kuo, P.L. Lung tumor-associated dendritic cell-derived amphiregulin increased cancer progression. J. Immunol. 2011, 187, 1733–1744. [Google Scholar] [CrossRef]
- Ballester, B.; Milara, J.; Cortijo, J. Idiopathic Pulmonary Fibrosis and Lung Cancer: Mechanisms and Molecular Targets. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, T.; Ohba, M.; Ohmori, T. Molecular-Targeted Therapies for Epidermal Growth Factor Receptor and Its Resistance Mechanisms. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Chandler, C.; Liu, T.; Buckanovich, R.; Coffman, L.G. The double edge sword of fibrosis in cancer. Transl. Res. 2019, 209, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Hegde, S.; DeNardo, D.G. Tumor-associated fibrosis as a regulator of tumor immunity and response to immunotherapy. Cancer Immunol. Immunother. 2017, 66, 1037–1048. [Google Scholar] [CrossRef]
- Akhurst, R.J.; Hata, A. Targeting the TGFbeta signalling pathway in disease. Nat. Rev. Drug Discov. 2012, 11, 790–811. [Google Scholar] [CrossRef]
- Colak, S.; Ten Dijke, P. Targeting TGF-beta Signaling in Cancer. Trends Cancer 2017, 3, 56–71. [Google Scholar] [CrossRef]
- Hawinkels, L.J.; Ten Dijke, P. Exploring anti-TGF-beta therapies in cancer and fibrosis. Growth Factors 2011, 29, 140–152. [Google Scholar] [CrossRef]
- Huynh, L.K.; Hipolito, C.J.; Ten Dijke, P. A Perspective on the Development of TGF-beta Inhibitors for Cancer Treatment. Biomolecules 2019, 9. [Google Scholar] [CrossRef]
- Neuzillet, C.; Tijeras-Raballand, A.; Cohen, R.; Cros, J.; Faivre, S.; Raymond, E.; de Gramont, A. Targeting the TGFbeta pathway for cancer therapy. Pharmacol. Ther. 2015, 147, 22–31. [Google Scholar] [CrossRef]
- Ong, C.H.; Tham, C.L.; Harith, H.H.; Firdaus, N.; Israf, D.A. TGF-beta-induced fibrosis: A review on the underlying mechanism and potential therapeutic strategies. Eur. J. Pharmacol. 2021, 911, 174510. [Google Scholar] [CrossRef]
- Guernsey-Biddle, C.; High, P.; Carmon, K.S. Exploring the Potential of Epiregulin and Amphiregulin as Prognostic, Predictive, and Therapeutic Targets in Colorectal Cancer. Onco 2024, 4, 257–274. [Google Scholar] [CrossRef]
- Huang, W.S.; Wu, K.L.; Chen, C.N.; Chang, S.F.; Lee, D.Y.; Lee, K.C. Amphiregulin Upregulation in Visfatin-Stimulated Colorectal Cancer Cells Reduces Sensitivity to 5-Fluororacil Cytotoxicity. Biology (Basel) 2024, 13. [Google Scholar] [CrossRef] [PubMed]
- Nagathihalli, N.S.; Beesetty, Y.; Lee, W.; Washington, M.K.; Chen, X.; Lockhart, A.C.; Merchant, N.B. Novel mechanistic insights into ectodomain shedding of EGFR Ligands Amphiregulin and TGF-alpha: impact on gastrointestinal cancers driven by secondary bile acids. Cancer Res. 2014, 74, 2062–2072. [Google Scholar] [CrossRef]
- Li, H.; Fang, R.; Ma, R.; Long, Y.; He, R.; Lyu, H.; Chen, L.; Wen, Y. Amphiregulin promotes activated regulatory T cell-suppressive function via the AREG/EGFR pathway in laryngeal squamous cell carcinoma. Head. Face Med. 2024, 20, 62. [Google Scholar] [CrossRef] [PubMed]
- Ebott, J.; McAdams, J.; Kim, C.; Jansen, C.; Woodman, M.; De La Cruz, P.; Schrol, C.; Ribeiro, J.; James, N. Enhanced amphiregulin exposure promotes modulation of the high grade serous ovarian cancer tumor immune microenvironment. Front. Pharmacol. 2024, 15, 1375421. [Google Scholar] [CrossRef]
- Jiang, Y.J.; Ho, T.L.; Chao, C.C.; He, X.Y.; Chen, P.C.; Cheng, F.J.; Huang, W.C.; Huang, C.L.; Liu, P.I.; Tang, C.H. Particulate matter facilitates amphiregulin-dependent lung cancer proliferation through glutamine metabolism. Int. J. Biol. Sci. 2024, 20, 3126–3139. [Google Scholar] [CrossRef]
- Tu, C.Y.; Wang, B.W.; Cheng, F.J.; Chen, C.H.; Hsia, T.C.; Wei, Y.L.; Chen, C.Y.; Hsieh, I.S.; Yeh, Y.L.; Wang, L.Y.; et al. Incense burning smoke sensitizes lung cancer cells to EGFR TKI by inducing AREG expression. Am. J. Cancer Res. 2018, 8, 2575–2589. [Google Scholar]
- Thul, P.J.; Lindskog, C. The human protein atlas: A spatial map of the human proteome. Protein Sci. 2018, 27, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Luetteke, N.C.; Qiu, T.H.; Fenton, S.E.; Troyer, K.L.; Riedel, R.F.; Chang, A.; Lee, D.C. Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development 1999, 126, 2739–2750. [Google Scholar] [CrossRef] [PubMed]
- Jay, F.F.; Vaidya, M.; Porada, S.M.; Andrukhova, O.; Schneider, M.R.; Erben, R.G. Amphiregulin lacks an essential role for the bone anabolic action of parathyroid hormone. Mol. Cell Endocrinol. 2015, 417, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Ciarloni, L.; Mallepell, S.; Brisken, C. Amphiregulin is an essential mediator of estrogen receptor alpha function in mammary gland development. Proc. Natl. Acad. Sci. U S A 2007, 104, 5455–5460. [Google Scholar] [CrossRef]
- Kim, T.R.; Kim, H.Y.; Kim, I.H.; Kim, K.C.; Ko, Y.; Park, J.H.; Yun, S.; Lee, I.C.; Kim, S.H.; Park, H.O. Safety pharmacology of self-assembled-micelle inhibitory RNA-targeting amphiregulin (SAMiRNA-AREG), a novel siRNA nanoparticle platform. Toxicol. Rep. 2021, 8, 839–845. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, T.R.; Kim, S.H.; Kim, I.H.; Lim, J.O.; Park, J.H.; Yun, S.; Lee, I.C.; Park, H.O.; Kim, J.C. Four-Week Repeated Intravenous Dose Toxicity of Self-Assembled-Micelle Inhibitory RNA-Targeting Amphiregulin in Mice. Int. J. Toxicol. 2021, 40, 453–465. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, T.R.; Kim, S.H.; Kim, I.H.; Ko, Y.; Yun, S.; Lee, I.C.; Park, H.O.; Kim, J.C. Genotoxicity evaluation of self-assembled-micelle inhibitory RNA-targeting amphiregulin (SAMiRNA-AREG), a novel siRNA nanoparticle for the treatment of fibrotic disease. Drug Chem. Toxicol. 2022, 45, 2109–2115. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Kim, T.-R.; Kim, S.-H.; Kim, I.-H.; Kim, W.-I.; Park, J.-H.; Ko, Y.; Yun, S.; Park, H.-O.; Kim, J.-C. Systemic toxicity and toxicokinetics study of self-assembled-micelle inhibitory RNA-targeting amphiregulin in cynomolgus monkeys following intravenous injection. Toxicological Research 2025. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Addison, M.L.; Dear, J.W.; Webb, D.J. Small interfering RNA: Discovery, pharmacology and clinical development-An introductory review. Br. J. Pharmacol. 2023, 180, 2697–2720. [Google Scholar] [CrossRef]
- Gavrilov, K.; Saltzman, W.M. Therapeutic siRNA: principles, challenges, and strategies. Yale J. Biol. Med. 2012, 85, 187–200. [Google Scholar]
- Judge, A.; MacLachlan, I. Overcoming the innate immune response to small interfering RNA. Hum. Gene Ther. 2008, 19, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Robbins, M.; Judge, A.; MacLachlan, I. siRNA and innate immunity. Oligonucleotides 2009, 19, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Sioud, M. Does the understanding of immune activation by RNA predict the design of safe siRNAs? Front. Biosci. 2008, 13, 4379–4392. [Google Scholar] [CrossRef] [PubMed]
- Bora, R.S.; Gupta, D.; Mukkur, T.K.; Saini, K.S. RNA interference therapeutics for cancer: challenges and opportunities (review). Mol. Med. Rep. 2012, 6, 9–15. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, C.C.; Choy, K.W.; Du, Q.; Chen, J.; Wang, Q.; Li, L.; Chung, T.K.; Tang, T. Therapeutic potentials of gene silencing by RNA interference: principles, challenges, and new strategies. Gene 2014, 538, 217–227. [Google Scholar] [CrossRef]
- Sajid, M.I.; Moazzam, M.; Kato, S.; Yeseom Cho, K.; Tiwari, R.K. Overcoming Barriers for siRNA Therapeutics: From Bench to Bedside. Pharmaceuticals (Basel) 2020, 13. [Google Scholar] [CrossRef]
- Yun, S.I.; Lee, S.K.; Goh, E.A.; Kwon, O.S.; Choi, W.; Kim, J.; Lee, M.S.; Choi, S.J.; Lim, S.S.; Moon, T.K.; et al. Weekly treatment with SAMiRNA targeting the androgen receptor ameliorates androgenetic alopecia. Sci. Rep. 2022, 12, 1607. [Google Scholar] [CrossRef]
- Ali Zaidi, S.S.; Fatima, F.; Ali Zaidi, S.A.; Zhou, D.; Deng, W.; Liu, S. Engineering siRNA therapeutics: challenges and strategies. J. Nanobiotechnology 2023, 21, 381. [Google Scholar] [CrossRef]
- Kaushal, A. Innate immune regulations and various siRNA modalities. Drug Deliv. Transl. Res. 2023, 13, 2704–2718. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




