Submitted:
11 June 2025
Posted:
13 June 2025
You are already at the latest version
Abstract
Keywords:
-
TOC (created with biorender.com)
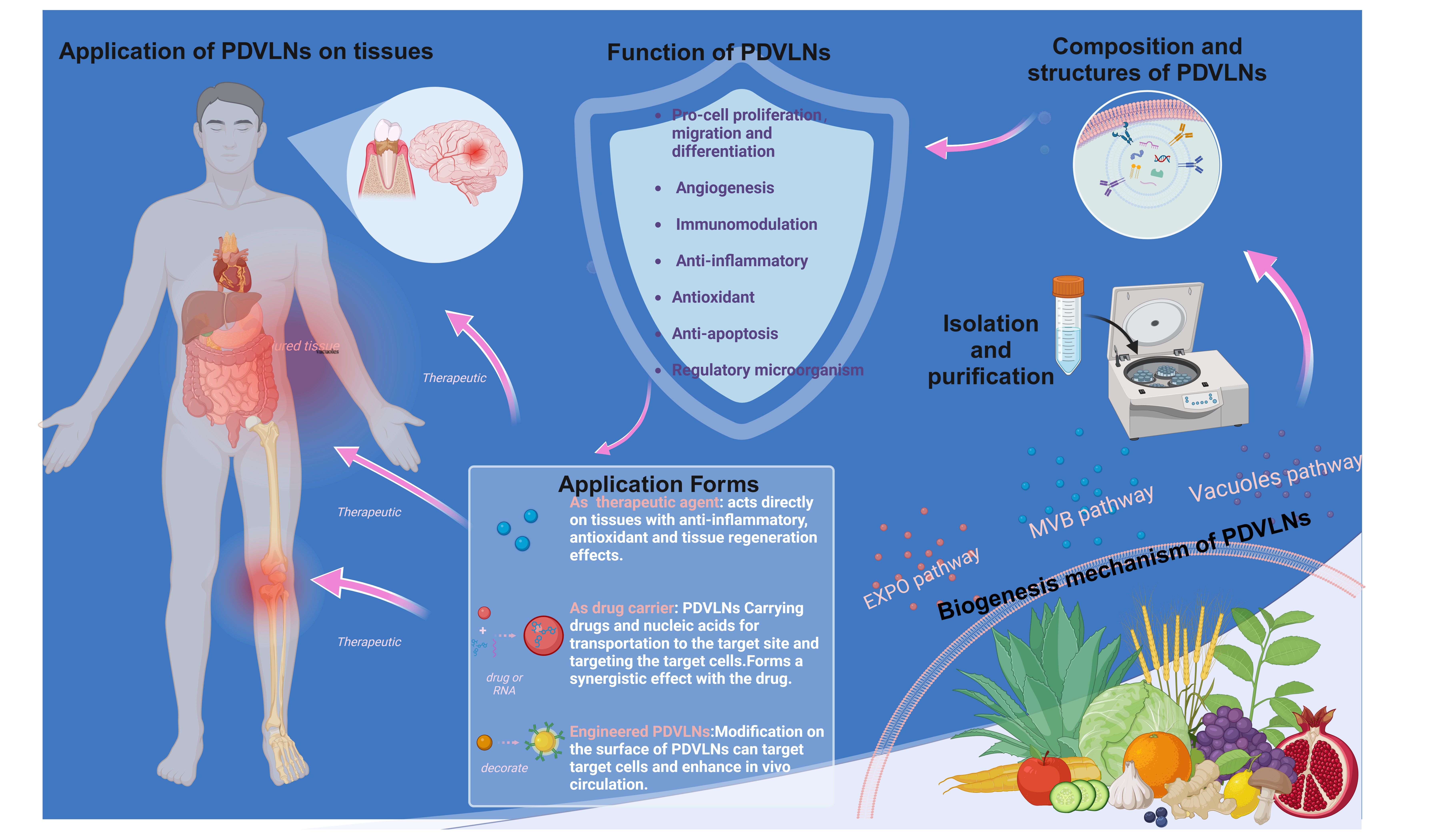
 Plant vesicle-like nanoparticles (PDVLNs) may become a new avenue for the treatment of tissue injury, promoting tissue repair and regeneration through their intrinsic bioactivity or as drug delivery carriers. In addition, PDVLNs can be engineered and modified to achieve better results.
Plant vesicle-like nanoparticles (PDVLNs) may become a new avenue for the treatment of tissue injury, promoting tissue repair and regeneration through their intrinsic bioactivity or as drug delivery carriers. In addition, PDVLNs can be engineered and modified to achieve better results.
1. Introduction
2. Overview of PDVLNs
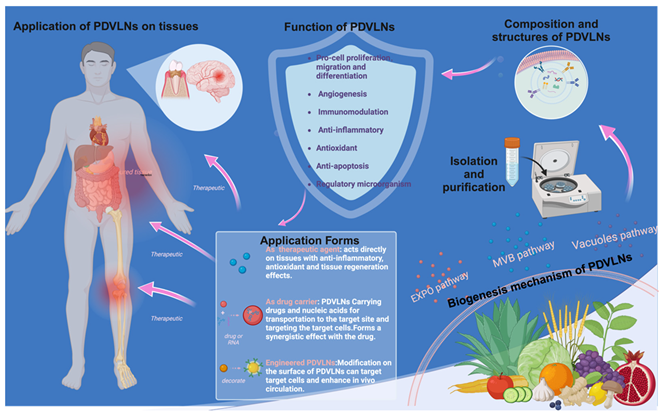
2.1. Biogenesis Mechanism of PDVLNs
2.2. Composition of PDVLNs
2.2.1. Lipids
2.2.2. Carbohydrates
2.2.3. Nucleic Acids
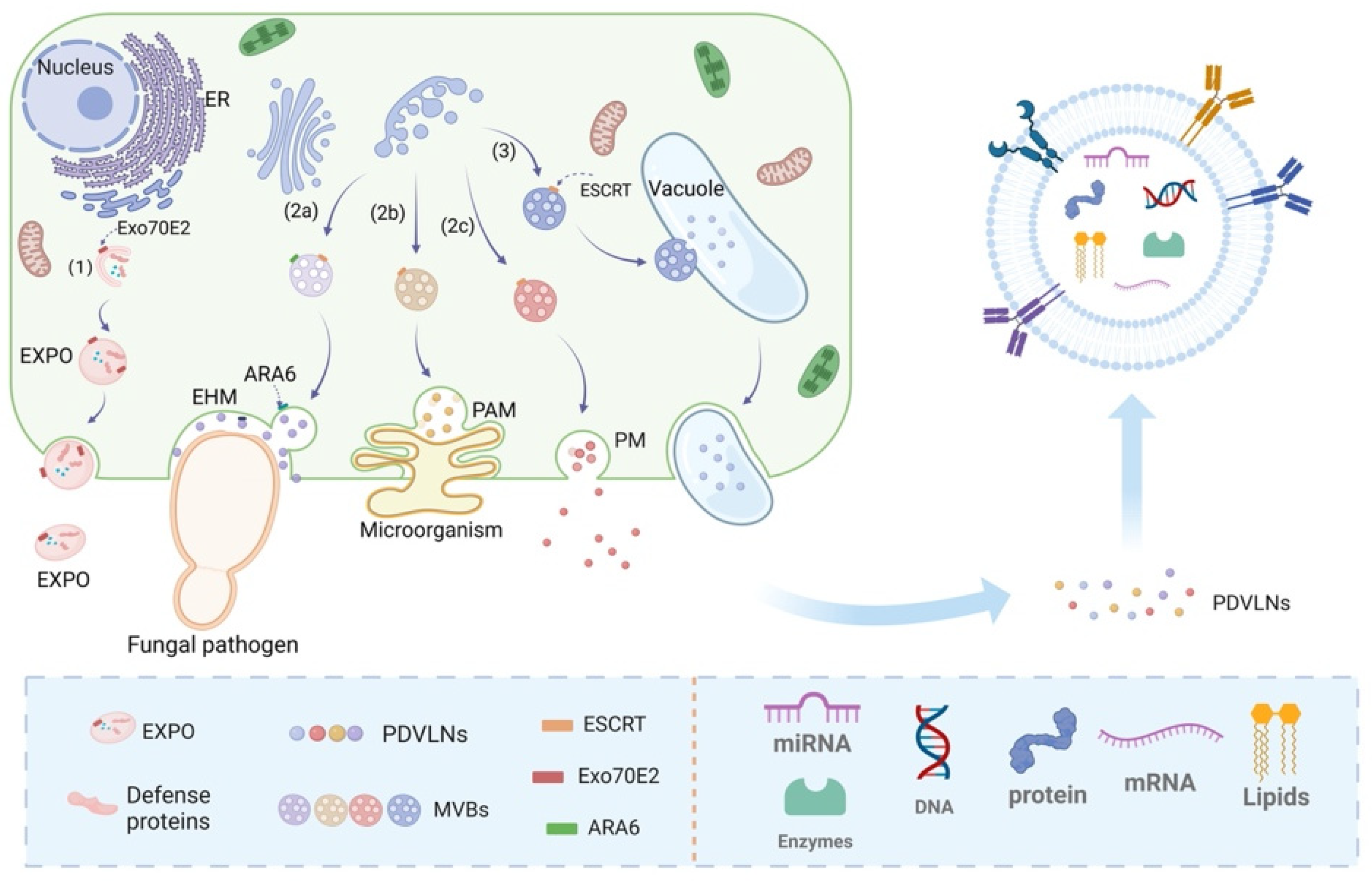
2.3. Preparation and Isolation Methods for PDVLNs
| Separation method |
Principle | Advantages | Drawbacks | Make superior | Reference |
|---|---|---|---|---|---|
| Ultracentrifugation | Separation of particles with large differences in size and density | Simple and economical | Formation of proteins, exosome aggregates; expensive equipment and expertise required | Combining density gradient centrifugation to obtain purer nanoparticles | [70,71,72,73,74,75] |
| Dense differential velocity gradient centrifugation | Centrifugal settling or sedimentation equilibrium in an inert gradient medium results in the formation of different separation zones. | Improved separation purity | Time-consuming, requires expensive equipment and specialized knowledge | Combine with differential ultracentrifugation | [69,73,74,75,76] |
| Ultrafiltration | Separation of large and small molecules by pressure membranes | Does not affect exosome activity, economical | Filter membranes prone to clogging, affecting recycling rates | Ultrafiltration pre-concentration of samples combined with size exclusion chromatography | [77,78,79,80,81,82] |
| Size exclusion chromatography (SEC) |
Based on particle size differences | High purity, high yield | Inability to selectively extract specific size exosomes | Combine with ultrafiltration pre-concentration | [83,84,85] |
| Asymmetric flow field flow separation | Field effects cause particles to move at different speeds in different channels | Wide range of separated nanoparticles | Inability to distinguish between differently shaped aggregates | Immunoaffinity capture after preliminary separation using asymmetric flow field flow separation method | [84,85,86] |
| Immunoaffinity capture method | Utilizes high affinity antigen-antibody binding | Highly selective | Requires specific antibodies or magnetic beads, may affect biological function | Combine with asymmetric flow field flow separation | [55,87,88,89,90,91,92] |
| PEG-based precipitation | Utilizes the intrinsic negative charge property on the surface of PDVLNs | Simultaneously removing impurities such as nucleic acids and proteins | Non-specificity, not suitable suitable for proteins that are sensitive to the presence of PEG | Combine with electrophoresis and dialysis | [93] |
2.4. Characterization Methods for PDVLNs
2.4.1. Particle Size Distribution of PDVLNs
2.4.2. Morphology of PDVLNs
2.4.3. Potentiation of PDVLNs
3. Mechanisms and Applications of PDVLNs in Tissue Repair and Regeneration
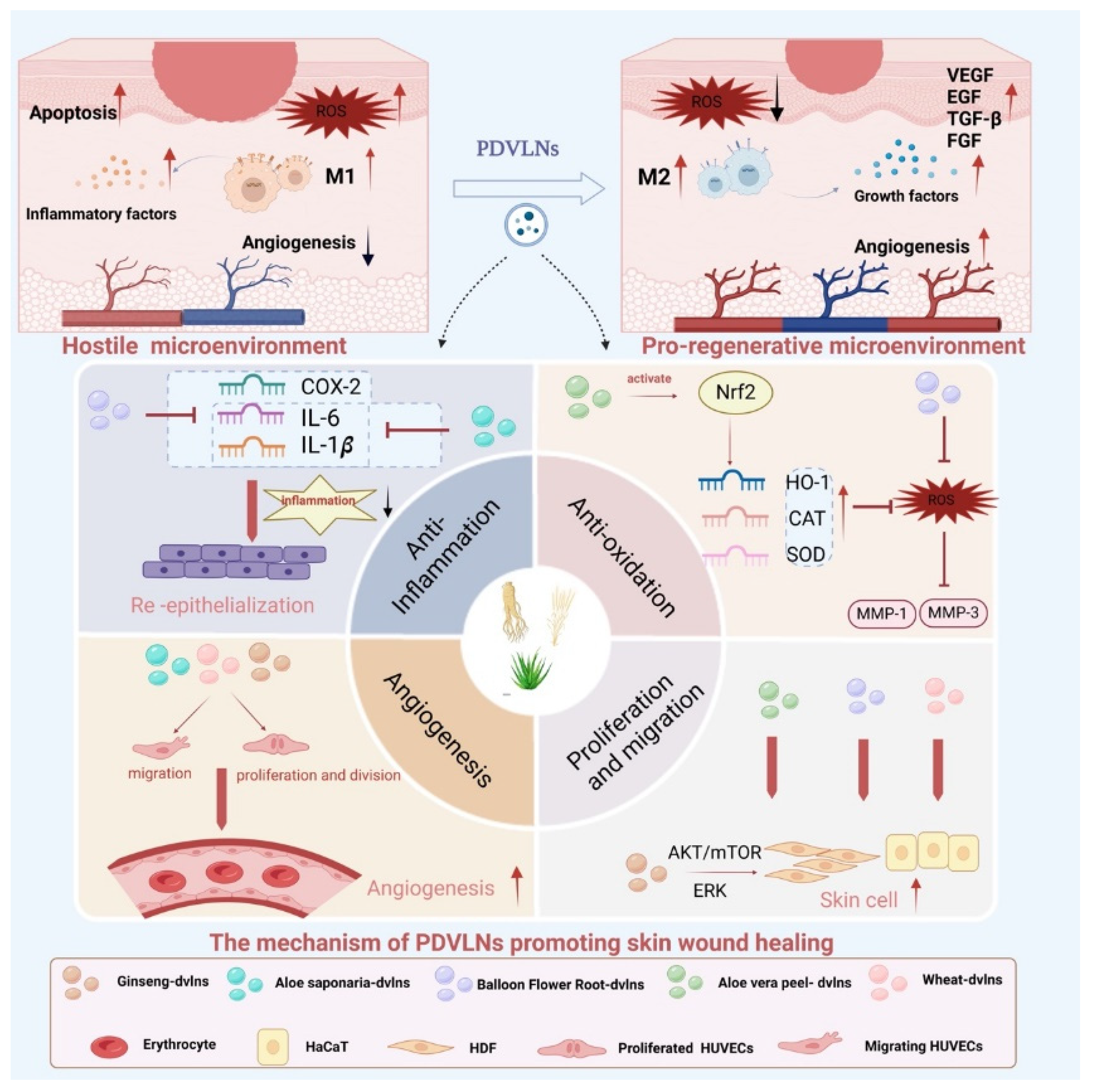
3.1. Skin: Wound Healing and Facial Rejuvenation
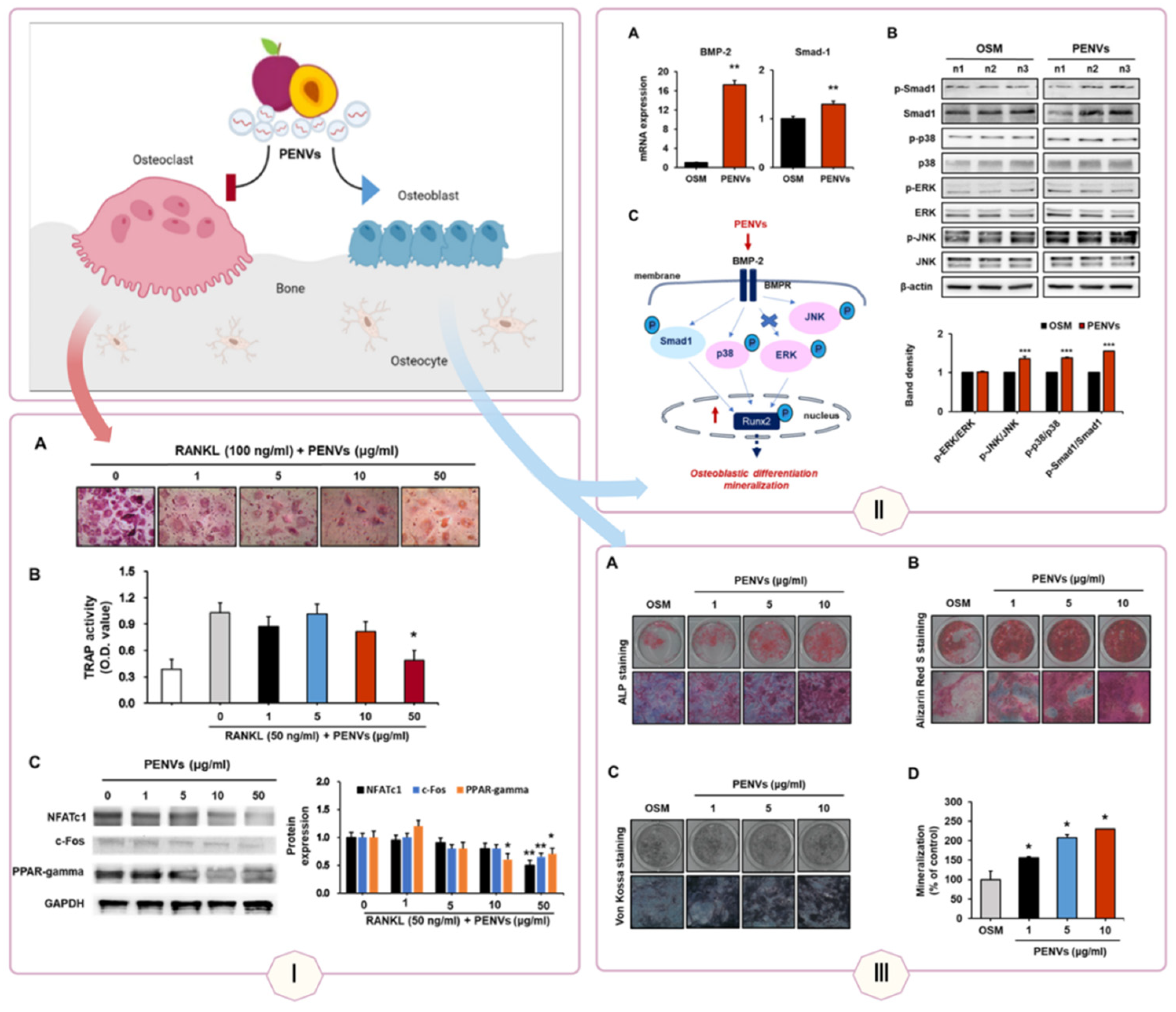
3.2. Bone: Osteogenesis and Osteoporosis Treatment
3.3. Nervous System: Neural Differentiation and Neuroprotection
3.4. Liver: Anti-Inflammatory and Antioxidant Effects
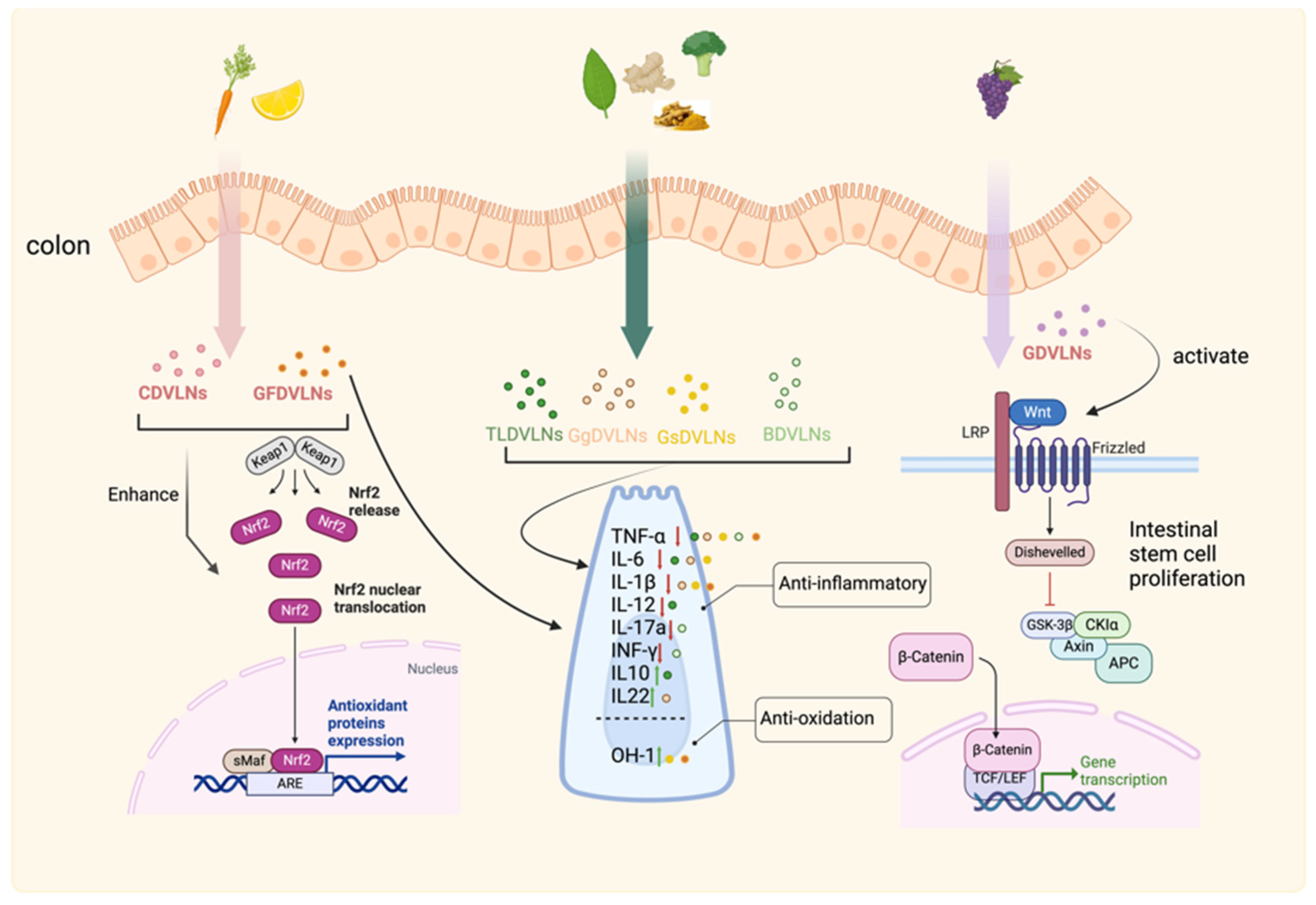
3.5. Gastrointestinal Tract: Inflammation Control and Mucosal Repair
3.6. Cardiovascular System: Antioxidant and Angiogenic Support
3.7. Dental Tissue: Periodontal Repair
| Tissue | Source | Method of administration | In vivo or in vitro models | Potential mechanism | Key findings | Main ingredients | Reference |
|---|---|---|---|---|---|---|---|
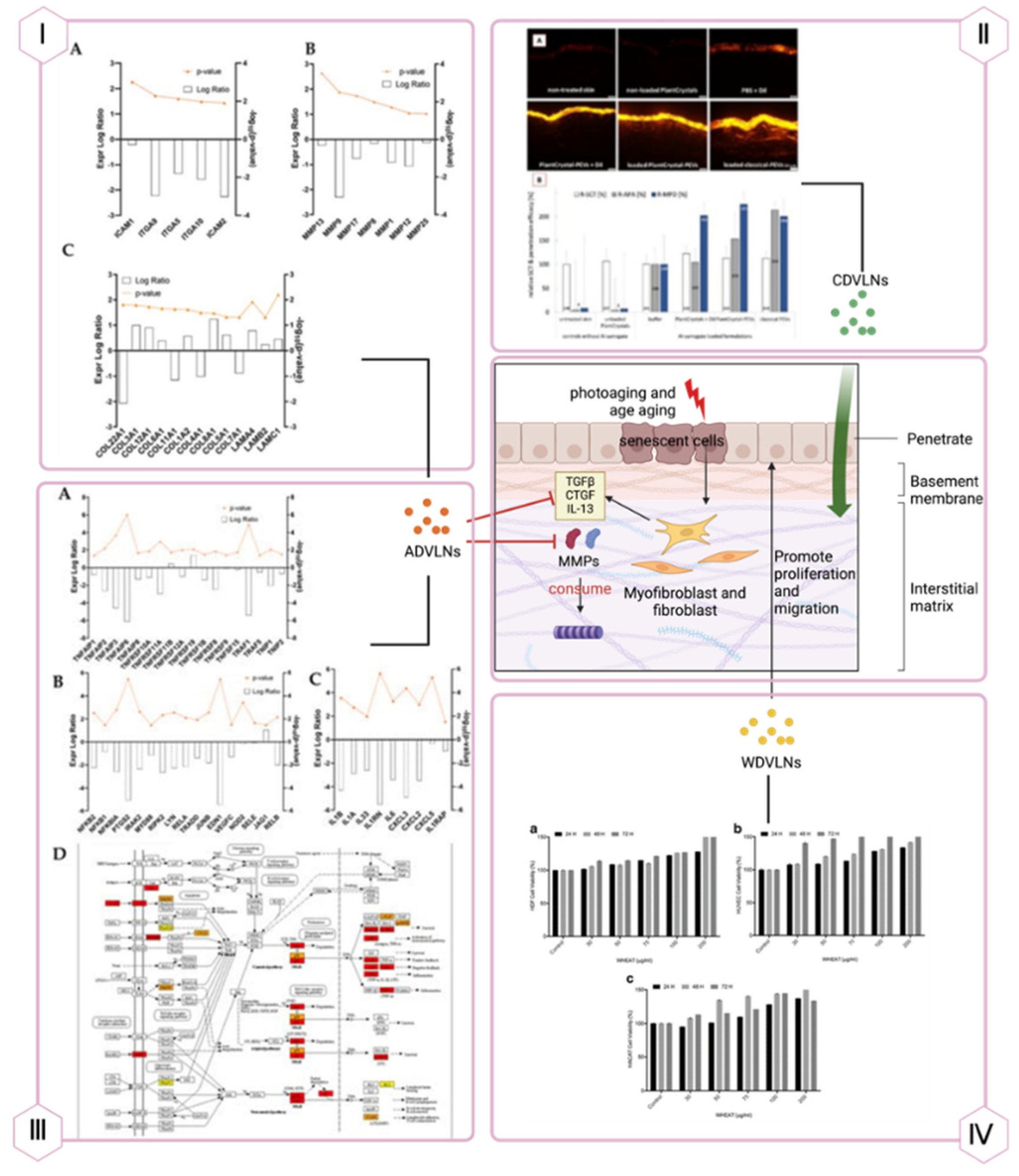
| Skin | Aloe-saponaria | co-incubation | In vitro: HDFs, HUVECs | Proliferation and migration of HDFs, Angiogenesis of HUVECs, Angiogenesis of HUVECs, Antioxidant | Promotes chronic wounds healing | unknown | [132] |
| Ginseng | co-incubation | In vitro: HaCaT, HUVECs, BJ | Enhanced cell migration and angiogenesis, Increased secretion of extracellular matrix proteins, Regulation of proliferation and inflammatory response via ERK and Akt/mTOR pathways | Promotes wound healing | ginsenoside | [109] | |
| Wheat | co-incubation | In vitro: HDFs, HUVECs, HaCaT | Enhancement of wound healing-related gene expression; activation of fibroblast function, coordination of vascularization process | Promotes wound healing | unknown | [108] | |
| BFR | co-incubation | In vitro: HDFs | Down-regulation of pro-inflammatory cytokines; Promotion of HDF proliferation and migration | Promotes wound healing | unknown | [133] | |
| Aloe vera Peels | co-incubation | In vitro: HaCaT, HDF | Decreased levels of ROS within HaCaT cells, Increased migration capacity of cells, Increased expression of mRNA for Nrf2, HO-1, CAT, and SOD genes | Promotes wound healing | unknown | [114] | |
| Grapefruit | co-incubation | In vitro: HaCaT, HUVEC | Reduction of ROS levels in HaCaT cells, Enhancement of proliferation- and migration-related gene expression in HaCaT, Enhancement of HUVEC cell tube formation | Promotes chronic wound healing | unknown | [134] | |
| Apple | co-incubation | In vitro: HDF | Inhibition of TLR4 activity, Down-regulation of NF-κB pro-inflammatory pathway, Enhancement of collagen synthesis, Inhibition of metalloproteinase production | Anti-aging of the skin | unknown | [115] | |
| Liver | Shiitake mushroom | none | In vivo: mouse model of acute liver injury (GaiN, LPS induced) | Inhibition of NLRP3 inflammatory vesicle activation, Decrease in IL-6 activity | Combating FHF; preventing GaIn/LPS-induced acute liver injury | unknown | [135] |
| Ginger | oral | In vivo: mouse model of alcoholic liver disease | Activation of Nrf2, Triggering of hepatic detoxification/antioxidant gene expression, Inhibition of ROS production | Protective effect against alcoholic liver injury in mice | shogaol | [136] | |
| Garlic | Oral or intravenous administration | In vivo: mice with acute liver injury | Inhibition of NLRP3 inflammatory vesicle activation pathway, Reduction in cysteinyl asparagin-1 autocleavage, inhibition of cytokine release and pyroptosis cell death in primary macrophages | Attenuating inflammation in chemically induced acute liver injury | DLPC | [123] | |
| Honey | none | In vivo: mice with acute liver injury | Inhibition of NLRP3 inflammatory vesicle activation, Reduction of IL-1β, IL-3, IL-6 and TNFα levels | Amelioration of inflammation and liver injury in acute liver injury | MiR-4057 | [65] | |
| Blueberry | intragastric administration | In vitro: HepG2 In vivo: male C57BL/6 mice (6-8 weeks) |
Acceleration of Nrf2 translocation, Decrease in AST and ALT levels, Improvement of insulin resistance, Inhibition of FAS and ACC1 expression | Treatment of NAFLD | unknown | [121] | |
| Lemon | co-incubation | In vitro: LGG | Inhibition of Msp1 and Msp3 production by RNase P-mediated degradation of specific tRNAs, Improvement of LGG tolerance to bile, Increase in LGG percentage | Regulates LGG to promote liver tissue repair | unknown | [125] | |
| Bone | Plum | co-incubation | In vitro: MC3T3-E1 cells, mouse bone marrow primary osteoblasts | Modulation of BMP-2/MAPK/Smad-1 pathway | Promotes osteoblast activation, Reduces osteoclast differentiation | unknown | [137] |
| Ginseng | co-incubation | In vitro: bone marrow-derived osteoclasts | Inhibits RANKL-induced signaling pathways, Regulates osteoclast maturation genes, Inhibits osteoclast differentiation | Anti-osteoporosis | ginsenoside | [138] | |
| Yam | oral | In vivo: mouse model of osteoporosis | Activates BMP-2/p-p38-dependent Runx2 pathway, Increases bone differentiation markers. | Promotes bone regeneration, Promotes osteoblast differentiation and mineralization | unknown | [116] | |
| Apple | none | In vitro: MC3T3-E1 | Regulates BMP3/Smad3 pathway, Activates ERK and JNK-related signaling, Promotes osteoblast growth and differentiation. | Anti-osteoporosis | unknown | [117] | |
| Pueraria lobata | co-incubation | In vitro: hBMSC In vivo: Ovariectomy (OVX)-induced osteoporosis in rats |
Promotes hBMSC differentiation and mineralization, Degradation of TMAO promotes autophagy. | Promotes bone formation and reduces bone resorption. | unknown | [118] | |
| Colon | Grapefruit | oral | In vivo: DSS-induced colitis model in mouse | Up-regulation of HO-1 expression, Inhibition of IL-1β and TNF-α production in intestinal macrophages | Anti-inflammatory, immune-modulatory, maintenance of intestinal macrophage homeostasis | unknown | [139] |
| Ginger | oral | In vivo: colitis model in mouse | Increased survival and proliferation of IECs, Decreased pro-inflammatory cytokines, Increased anti-inflammatory cytokines | Prevents and protects against IBD | 6-gingerol | [49] | |
| Ginger | oral | In vitro: Lactobacillus rhamnosus (LGG) In vivo: mouse model |
Activation of the AHR pathway, Induction of IL-22 expression | LGG-mediated inhibition of colitis in mice | unknown | [140] | |
| Mulberry bark | oral | In vitro: MC38 and human Caco2 colon cancer cell lines In vivo: mouse colitis model |
Promoting HSPA8-mediated activation of the AhR signaling pathway and anti-inflammatory via AhR-COPS8 | Prevention of colitis in mice | HSPA8 | [141] | |
| Grape | oral | In vivo: rat | Modulating stem cell microenvironment, Promotes proliferation of Lgr5+ intestinal stem cells |
Prevention of colitis in rats | unknown | [142] | |
| Grape | oral | In vivo: DSS-induced mouse colitis model | Induction of intestinal stem cell growth factor gene expression through the Wnt/β-catenin signaling pathway, Promotes proliferation of Lgr5+ intestinal stem cells | Promotes self-renewal of intestinal epithelium and accelerates the recovery of intestinal structure | unknown | [143] | |
| Tea leaves | oral | In vivo: mouse colitis model | Reduces ROS production, Inhibits pro-inflammatory cytokines, and Increases macrophage secretion of anti-inflammatory cytokines | Prevention of colitis-related colon cancer, antioxidant | galactose (CH2O6) | [144] | |
| Broccoli | oral | In vivo: mouse colitis model | Inhibition of pro-inflammatory cytokines and activation of AMPK are involved in inhibiting DC pro-inflammatory factor activation | Preventing DC activation and inducing tolerance to DC | unknown | [145] | |
| Turmeric | oral | In vitro: primitive 264.7 murine macrophages, NCM 460 and HT-29 colonic epithelial cells In vivo: ICR mice |
Inhibits pro-inflammatory cytokine expression, promotes antioxidant gene levels, regulates gut microbiota composition and abundance, and remodels the immune microenvironment | Anti-inflammatory, antioxidant, relieves symptoms of intestinal inflammation | unknown | [146] | |
| Carrot | oral | In vitro: intestinal macrophages and stem cells. In vivo: B6.Cg-Tg(BAT-lacZ)3Picc/J mice |
Promotion of Nrf2 nuclear translocation, induction of anti-inflammatory cytokines, antioxidant and activation of Wnt signaling gene expression | Anti-inflammatory, antioxidant, maintains intestinal homeostasis | unknown | [31] | |
| Pomegranate | co-incubation | In vitro: THP-1, Caco-2 cells | Improved Caco-2 cell survival at 5 ug/ml | Anti-inflammatory, antioxidant, promotes healing of intestinal epithelium | unknown | [147] | |
| Nervous system | Garlic | oral | In vivo: mouse high-fat diet model | Inhibition of cGAS/STING/IDO1/AHR inflammatory signaling cascade response | Alleviating brain inflammation and obesity in mice | PA(36:4) | [148] |
| Momordica charantia | none | In vivo: MCAO model in male SD rats | Up-regulation of AKT/GSK3β signaling pathway, inhibition of MMP-9, attenuation of BBB injury, and inhibition of neuronal apoptosis | Improving neurologic dysfunction in cerebral ischemia-reperfusion injury | Possibly related to miR5266 | [149] | |
| Cardiovascular system | M charantia | intraperitoneal injection | In vitro: H9C2 cardiomyocytes In vivo: thoracic-irradiated 5-6 week old BALB/c nude mice |
Promotes cell proliferation, inhibits apoptosis, attenuates DNA damage, scavenges mitochondrial ROS, and regulates p-AKT/AKT and p-ERK/ERK ratios | Reduces myocardial damage and fibrosis and is potentially protective against radiation-induced heart disease | unknown | [150] |
| Citrus limon (L.) Osbeck | sublingual administration | In vivo: healthy participants | Effect on waist circumference and LDL-C | Cardiometabolic risk factors for management of normolipidemic participants | unknown | [151] | |
| Carrot | co-incubation | In vitro: H2C5 cardiomyoblasts and SH-SY2Y cells | Inhibits decreased expression of antioxidant molecules, protects cells from oxidative stress, and inhibits ROS production and ROS-induced apoptosis | Novel drug candidates for treatment of myocardial infarction and Parkinson’s disease | unknown | [122] | |
| Oral cavity | Ginger | oral | In vitro: Porphyromonas gingivalis surface In vivo: mouse model of chronic periodontitis |
Acts with heme-binding protein 35 (HBP35) to inhibit the pathogenicity of Porphyromonas gingivalis, reduce the expression of anti-inflammatory factors in periodontal tissues, and reduce alveolar bone loss | Prevention/treatment of chronic periodontitis | PA(36:2) | [129] |
4. Therapeutic Advantages and Comparisons of PDVLNs in Regenerative Medicine
5. Engineering PDVLNs for Enhanced Tissue Repair and Regeneration
6. Challenges and Future Directions in PDVLN-Based Regenerative Medicine
Author Contributions
Funding
Ethics Approval
Consent to Participate
Consent for Publication
Data Availability Statement
Code Availability
Conflicts of Interest
References
- Mostafa, H. K. K. Different Cells of the Human Body: Categories and Morphological Characters. J. Microsc. Ultrastruct. 2022, 10(2), 40. [Google Scholar] [CrossRef] [PubMed]
- Şeker, Ş.; Elçin, A. E.; Elçin, Y. M. Advances in Regenerative Medicine and Biomaterials. In Gene, Drug, and Tissue Engineering; Pereira, G. C., Ed.; Methods in Molecular Biology; Springer US: New York, NY, 2023; Vol. 2575, pp. 127–152. [Google Scholar] [CrossRef]
- Farley, A.; McLafferty, E.; Hendry, C. Cells, Tissues, Organs and Systems. Nurs. Stand. R. Coll. Nurs. G. B. 1987 2012, 26(52), 40–45. [Google Scholar] [CrossRef]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C. T. Progress in 3D Bioprinting Technology for Tissue/Organ Regenerative Engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, X.-J.-N.; Fei, Y.-Y.; Han, H.-T.; Xu, J.; Cheng, L.; Li, X. Stem Cell Therapy in Liver Regeneration: Focus on Mesenchymal Stem Cells and Induced Pluripotent Stem Cells. Pharmacol. Ther. 2022, 232, 108004. [Google Scholar] [CrossRef]
- Zhang, D.; Wei, G.; Li, P.; Zhou, X.; Zhang, Y. Urine-Derived Stem Cells: A Novel and Versatile Progenitor Source for Cell-Based Therapy and Regenerative Medicine. Genes Dis. 2014, 1(1), 8–17. [Google Scholar] [CrossRef]
- Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies | Nature Medicine. Available online: https://www.nature.com/articles/nm.3267 (accessed on 17 March 2025).
- L. , P. K.; Kandoi, S.; Misra, R.; S., V.; K., R.; Verma, R. S. The Mesenchymal Stem Cell Secretome: A New Paradigm towards Cell-Free Therapeutic Mode in Regenerative Medicine. Cytokine Growth Factor Rev. 2019, 46, 1–9. [Google Scholar] [CrossRef]
- Welsh, J. A.; Goberdhan, D. C. I.; O’Driscoll, L.; Buzas, E. I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T. A. P.; Erdbrügger, U.; Falcon-Perez, J. M.; Fu, Q.-L.; Hill, A. F.; Lenassi, M.; Lim, S. K.; Mahoney, M. G.; Mohanty, S.; Möller, A.; Nieuwland, R.; Ochiya, T.; Sahoo, S.; Torrecilhas, A. C.; Zheng, L.; Zijlstra, A.; Abuelreich, S.; Bagabas, R.; Bergese, P.; Bridges, E. M.; Brucale, M.; Burger, D.; Carney, R. P.; Cocucci, E.; Crescitelli, R.; Hanser, E.; Harris, A. L.; Haughey, N. J.; Hendrix, A.; Ivanov, A. R.; Jovanovic-Talisman, T.; Kruh-Garcia, N. A.; Ku’ulei-Lyn Faustino, V.; Kyburz, D.; Lässer, C.; Lennon, K. M.; Lötvall, J.; Maddox, A. L.; Martens-Uzunova, E. S.; Mizenko, R. R.; Newman, L. A.; Ridolfi, A.; Rohde, E.; Rojalin, T.; Rowland, A.; Saftics, A.; Sandau, U. S.; Saugstad, J. A.; Shekari, F.; Swift, S.; Ter-Ovanesyan, D.; Tosar, J. P.; Useckaite, Z.; Valle, F.; Varga, Z.; van der Pol, E.; van Herwijnen, M. J. C.; Wauben, M. H. M.; Wehman, A. M.; Williams, S.; Zendrini, A.; Zimmerman, A. J.; MISEV Consortium; Théry, C.; Witwer, K. W. Minimal Information for Studies of Extracellular Vesicles (MISEV2023): From Basic to Advanced Approaches. J. Extracell. Vesicles 2024, 13(2), e12404. [Google Scholar] [CrossRef]
- Nam, G.-H.; Choi, Y.; Kim, G. B.; Kim, S.; Kim, S. A.; Kim, I.-S. Emerging Prospects of Exosomes for Cancer Treatment: From Conventional Therapy to Immunotherapy. Adv. Mater. Deerfield Beach Fla 2020, 32(51), e2002440. [Google Scholar] [CrossRef]
- Vizoso, F. J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18(9), 1852. [Google Scholar] [CrossRef]
- Roomi, S.; Masi, A.; Conselvan, G. B.; Trevisan, S.; Quaggiotti, S.; Pivato, M.; Arrigoni, G.; Yasmin, T.; Carletti, P. Protein Profiling of Arabidopsis Roots Treated With Humic Substances: Insights Into the Metabolic and Interactome Networks. Front. Plant Sci. 2018, 9, 1812. [Google Scholar] [CrossRef]
- Qiao, S. A.; Roth, R. Messenger and Message: Uncovering the Roles, Rhythm and Regulation of Extracellular Vesicles in Plant Biotic Interactions. Curr. Opin. Plant Biol. 2025, 83, 102672. [Google Scholar] [CrossRef] [PubMed]
- Al-Masawa, M. E.; Alshawsh, M. A.; Ng, C. Y.; Ng, A. M. H.; Foo, J. B.; Vijakumaran, U.; Subramaniam, R.; Ghani, N. A. A.; Witwer, K. W.; Law, J. X. Efficacy and Safety of Small Extracellular Vesicle Interventions in Wound Healing and Skin Regeneration: A Systematic Review and Meta-Analysis of Animal Studies. Theranostics 2022, 12(15), 6455–6508. [Google Scholar] [CrossRef] [PubMed]
- Cong, M.; Tan, S.; Li, S.; Gao, L.; Huang, L.; Zhang, H.-G.; Qiao, H. Technology Insight: Plant-Derived Vesicles-How Far from the Clinical Biotherapeutics and Therapeutic Drug Carriers? Adv. Drug Deliv. Rev. 2022, 182, 114108. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Liu, K. Plant-Derived Extracellular Vesicles as Oral Drug Delivery Carriers. J. Control. Release Off. J. Control. Release Soc. 2022, 350, 389–400. [Google Scholar] [CrossRef]
- Samadi, P.; Sheykhhasan, M.; Mokhtari, K.; Yang, P.; Maghool, F.; Kalhor, N. Chapter 13 - Extracellular Vesicles: Unlocking Therapeutic Potential in Regenerative Medicine. In Extracellular Vesicles for Therapeutic and Diagnostic Applications; Anand, K., Vadivalagan, C., Gangadaran, P., Muthu, S., Peacock, B., Eds.; Nanotechnology in Biomedicine; Elsevier, 2025; pp 397–435. [CrossRef]
- Halperin, W.; Jensen, W. A. Ultrastructural Changes during Growth and Embryogenesis in Carrot Cell Cultures. J. Ultrastruct. Res. 1967, 18(3), 428–443. [Google Scholar] [CrossRef]
- Kalra, H.; Drummen, G. P. C.; Mathivanan, S. Focus on Extracellular Vesicles: Introducing the Next Small Big Thing. Int. J. Mol. Sci. 2016, 17(2), 170. [Google Scholar] [CrossRef]
- Scheuring, D.; Viotti, C.; Krüger, F.; Künzl, F.; Sturm, S.; Bubeck, J.; Hillmer, S.; Frigerio, L.; Robinson, D. G.; Pimpl, P.; Schumacher, K. Multivesicular Bodies Mature from the Trans-Golgi Network/Early Endosome in Arabidopsis. Plant Cell 2011, 23(9), 3463–3481. [Google Scholar] [CrossRef]
- Ung, T. H.; Madsen, H. J.; Hellwinkel, J. E.; Lencioni, A. M.; Graner, M. W. Exosome Proteomics Reveals Transcriptional Regulator Proteins with Potential to Mediate Downstream Pathways. Cancer Sci. 2014, 105(11), 1384–1392. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, Q.; Hu, S.; Jiang, L. Vacuole Biogenesis in Plants: How Many Vacuoles, How Many Models? Trends Plant Sci. 2020, 25(6), 538–548. [Google Scholar] [CrossRef]
- Ho, P. T. B.; Clark, I. M.; Le, L. T. T. MicroRNA-Based Diagnosis and Therapy. Int. J. Mol. Sci. 2022, 23(13), 7167. [Google Scholar] [CrossRef]
- Poulsen, C. P.; Dilokpimol, A.; Mouille, G.; Burow, M.; Geshi, N. Arabinogalactan Glycosyltransferases Target to a Unique Subcellular Compartment That May Function in Unconventional Secretion in Plants. Traffic Cph. Den. 2014, 15(11), 1219–1234. [Google Scholar] [CrossRef] [PubMed]
- Gayral, M.; Arias Gaguancela, O.; Vasquez, E.; Herath, V.; Flores, F. J.; Dickman, M. B.; Verchot, J. Multiple ER-to-Nucleus Stress Signaling Pathways Are Activated during Plantago Asiatica Mosaic Virus and Turnip Mosaic Virus Infection in Arabidopsis Thaliana. Plant J. Cell Mol. Biol. 2020, 103(3), 1233–1245. [Google Scholar] [CrossRef] [PubMed]
- Movahed, N.; Cabanillas, D. G.; Wan, J.; Vali, H.; Laliberté, J.-F.; Zheng, H. Turnip Mosaic Virus Components Are Released into the Extracellular Space by Vesicles in Infected Leaves. Plant Physiol. 2019, 180(3), 1375–1388. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Qiao, L.; Wang, M.; He, B.; Lin, F.-M.; Palmquist, J.; Huang, S.-D.; Jin, H. Plants Send Small RNAs in Extracellular Vesicles to Fungal Pathogen to Silence Virulence Genes. Science 2018, 360(6393), 1126–1129. [Google Scholar] [CrossRef]
- Wang, F.; Shang, Y.; Fan, B.; Yu, J.-Q.; Chen, Z. Arabidopsis LIP5, a Positive Regulator of Multivesicular Body Biogenesis, Is a Critical Target of Pathogen-Responsive MAPK Cascade in Plant Basal Defense. PLoS Pathog. 2014, 10(7), e1004243. [Google Scholar] [CrossRef]
- Cui, Y.; Shen, J.; Gao, C.; Zhuang, X.; Wang, J.; Jiang, L. Biogenesis of Plant Prevacuolar Multivesicular Bodies. Mol. Plant 2016, 9(6), 774–786. [Google Scholar] [CrossRef]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.-B.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; Zhang, H.-G. Interspecies Communication between Plant and Mouse Gut Host Cells through Edible Plant Derived Exosome-like Nanoparticles. Mol. Nutr. Food Res. 2014, 58(7), 1561–1573. [Google Scholar] [CrossRef]
- Maricchiolo, E.; Panfili, E.; Pompa, A.; De Marchis, F.; Bellucci, M.; Pallotta, M. T. Unconventional Pathways of Protein Secretion: Mammals vs. Plants. Front. Cell Dev. Biol. 2022, 10, 895853. [Google Scholar] [CrossRef]
- Bruns, C.; McCaffery, J. M.; Curwin, A. J.; Duran, J. M.; Malhotra, V. Biogenesis of a Novel Compartment for Autophagosome-Mediated Unconventional Protein Secretion. J. Cell Biol. 2011, 195(6), 979–992. [Google Scholar] [CrossRef]
- Gu, Y.; Innes, R. W. The KEEP ON GOING Protein of Arabidopsis Regulates Intracellular Protein Trafficking and Is Degraded during Fungal Infection. Plant Cell 2012, 24(11), 4717–4730. [Google Scholar] [CrossRef]
- Ito, Y.; Taniguchi, K.; Kuranaga, Y.; Eid, N.; Inomata, Y.; Lee, S.-W.; Uchiyama, K. Uptake of MicroRNAs from Exosome-Like Nanovesicles of Edible Plant Juice by Rat Enterocytes. Int. J. Mol. Sci. 2021, 22(7), 3749. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ding, Y.; Wang, J.; Hillmer, S.; Miao, Y.; Lo, S. W.; Wang, X.; Robinson, D. G.; Jiang, L. EXPO, an Exocyst-Positive Organelle Distinct from Multivesicular Endosomes and Autophagosomes, Mediates Cytosol to Cell Wall Exocytosis in Arabidopsis and Tobacco Cells. Plant Cell 2010, 22(12), 4009–4030. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D. G.; Ding, Y.; Jiang, L. Unconventional Protein Secretion in Plants: A Critical Assessment. Protoplasma 2016, 253(1), 31–43. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bermúdez, P.; Blesa, J.; Soriano, J. M.; Marcilla, A. Extracellular Vesicles in Food: Experimental Evidence of Their Secretion in Grape Fruits. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2017, 98, 40–50. [Google Scholar] [CrossRef]
- Cai, Q.; He, B.; Wang, S.; Fletcher, S.; Niu, D.; Mitter, N.; Birch, P. R. J.; Jin, H. Message in a Bubble: Shuttling Small RNAs and Proteins Between Cells and Interacting Organisms Using Extracellular Vesicles. Annu. Rev. Plant Biol. 2021, 72, 497–524. [Google Scholar] [CrossRef]
- Jeppesen, D. K.; Fenix, A. M.; Franklin, J. L.; Higginbotham, J. N.; Zhang, Q.; Zimmerman, L. J.; Liebler, D. C.; Ping, J.; Liu, Q.; Evans, R.; Fissell, W. H.; Patton, J. G.; Rome, L. H.; Burnette, D. T.; Coffey, R. J. Reassessment of Exosome Composition. Cell 2019, 177(2), 428–445.e18. [Google Scholar] [CrossRef]
- Alfieri, M.; Leone, A.; Ambrosone, A. Plant-Derived Nano and Microvesicles for Human Health and Therapeutic Potential in Nanomedicine. Pharmaceutics 2021, 13(4), 498. [Google Scholar] [CrossRef]
- Zhou, S.; Cao, Y.; Shan, F.; Huang, P.; Yang, Y.; Liu, S. Analyses of Chemical Components and Their Functions in Single Species Plant-Derived Exosome like Vesicle. TrAC Trends Anal. Chem. 2023, 167, 117274. [Google Scholar] [CrossRef]
- Liu, N.-J.; Wang, N.; Bao, J.-J.; Zhu, H.-X.; Wang, L.-J.; Chen, X.-Y. Lipidomic Analysis Reveals the Importance of GIPCs in Arabidopsis Leaf Extracellular Vesicles. Mol. Plant 2020, 13(10), 1523–1532. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, M.; Merlin, D. Advances in Plant-Derived Edible Nanoparticle-Based Lipid Nano-Drug Delivery Systems as Therapeutic Nanomedicines. J. Mater. Chem. B 2018, 6(9), 1312–1321. [Google Scholar] [CrossRef]
- Laulagnier, K.; Motta, C.; Hamdi, S.; Roy, S.; Fauvelle, F.; Pageaux, J.-F.; Kobayashi, T.; Salles, J.-P.; Perret, B.; Bonnerot, C.; Record, M. Mast Cell- and Dendritic Cell-Derived Exosomes Display a Specific Lipid Composition and an Unusual Membrane Organization. Biochem. J. 2004, 380 Pt 1, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Record, M. Exosome-like Nanoparticles from Food: Protective Nanoshuttles for Bioactive Cargo. Mol. Ther. J. Am. Soc. Gene Ther. 2013, 21(7), 1294–1296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M. K.; Xiao, B.; Xu, C.; Srinivasan, S.; Merlin, D. Edible Ginger-Derived Nanoparticles: A Novel Therapeutic Approach for the Prevention and Treatment of Inflammatory Bowel Disease and Colitis-Associated Cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef] [PubMed]
- Szatanek, R.; Baj-Krzyworzeka, M.; Zimoch, J.; Lekka, M.; Siedlar, M.; Baran, J. The Methods of Choice for Extracellular Vesicles (EVs) Characterization. Int. J. Mol. Sci. 2017, 18(6), 1153. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Yu, J. Exosome-like Nanoparticles from Ginger Rhizomes Inhibited NLRP3 Inflammasome Activation. Mol. Pharm. 2019, 16(6), 2690–2699. [Google Scholar] [CrossRef]
- Goul, C.; Peruzzo, R.; Zoncu, R. The Molecular Basis of Nutrient Sensing and Signalling by mTORC1 in Metabolism Regulation and Disease. Nat. Rev. Mol. Cell Biol. 2023, 24(12), 857–875. [Google Scholar] [CrossRef]
- Kumar, A.; Sundaram, K.; Teng, Y.; Mu, J.; Sriwastva, M. K.; Zhang, L.; Hood, J. L.; Yan, J.; Zhang, X.; Park, J. W.; Merchant, M. L.; Zhang, H.-G. Ginger Nanoparticles Mediated Induction of Foxa2 Prevents High-Fat Diet-Induced Insulin Resistance. Theranostics 2022, 12(3), 1388–1403. [Google Scholar] [CrossRef]
- Xu, F.; Mu, J.; Teng, Y.; Zhang, X.; Sundaram, K.; Sriwastva, M. K.; Kumar, A.; Lei, C.; Zhang, L.; Liu, Q. M.; Yan, J.; McClain, C. J.; Merchant, M. L.; Zhang, H.-G. Restoring Oat Nanoparticles Mediated Brain Memory Function of Mice Fed Alcohol by Sorting Inflammatory Dectin-1 Complex Into Microglial Exosomes. Small Weinh. Bergstr. Ger. 2022, 18(6), e2105385. [Google Scholar] [CrossRef]
- Hu, X.-L.; Lu, H.; Hassan, M. M.; Zhang, J.; Yuan, G.; Abraham, P. E.; Shrestha, H. K.; Villalobos Solis, M. I.; Chen, J.-G.; Tschaplinski, T. J.; Doktycz, M. J.; Tuskan, G. A.; Cheng, Z.-M. M.; Yang, X. Advances and Perspectives in Discovery and Functional Analysis of Small Secreted Proteins in Plants. Hortic. Res. 2021, 8(1), 130. [Google Scholar] [CrossRef]
- Wang, X.; Chung, K. P.; Lin, W.; Jiang, L. Protein Secretion in Plants: Conventional and Unconventional Pathways and New Techniques. J. Exp. Bot. 2017, 69(1), 21–37. [Google Scholar] [CrossRef]
- Tang, Y.-T.; Huang, Y.-Y.; Zheng, L.; Qin, S.-H.; Xu, X.-P.; An, T.-X.; Xu, Y.; Wu, Y.-S.; Hu, X.-M.; Ping, B.-H.; Wang, Q. Comparison of Isolation Methods of Exosomes and Exosomal RNA from Cell Culture Medium and Serum. Int. J. Mol. Med. 2017, 40(3), 834–844. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, S.; Cai, Q.; Jin, H. Effective Methods for Isolation and Purification of Extracellular Vesicles from Plants. J. Integr. Plant Biol. 2021, 63(12), 2020–2030. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Lin, J.; Ye, J.; Wang, R.; Yang, C.; Gong, J.; Liu, Y.; Deng, C.; Liu, P.; Chen, C.; Cheng, Y.; Deng, X.; Zeng, Y. A Comprehensive Proteomic Analysis of Elaioplasts from Citrus Fruits Reveals Insights into Elaioplast Biogenesis and Function. Hortic. Res. 2018, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Kim, S. Q.; Kim, K.-H. Emergence of Edible Plant-Derived Nanovesicles as Functional Food Components and Nanocarriers for Therapeutics Delivery: Potentials in Human Health and Disease. Cells 2022, 11(14), 2232. [Google Scholar] [CrossRef]
- Ryu, H.; Fuwad, A.; Yoon, S.; Jang, H.; Lee, J. C.; Kim, S. M.; Jeon, T.-J. Biomimetic Membranes with Transmembrane Proteins: State-of-the-Art in Transmembrane Protein Applications. Int. J. Mol. Sci. 2019, 20(6), 1437. [Google Scholar] [CrossRef]
- Baldrich, P.; Rutter, B. D.; Karimi, H. Z.; Podicheti, R.; Meyers, B. C.; Innes, R. W. Plant Extracellular Vesicles Contain Diverse Small RNA Species and Are Enriched in 10- to 17-Nucleotide “Tiny” RNAs. Plant Cell 2019, 31(2), 315–324. [Google Scholar] [CrossRef]
- Perut, F.; Roncuzzi, L.; Avnet, S.; Massa, A.; Zini, N.; Sabbadini, S.; Giampieri, F.; Mezzetti, B.; Baldini, N. Strawberry-Derived Exosome-Like Nanoparticles Prevent Oxidative Stress in Human Mesenchymal Stromal Cells. Biomolecules 2021, 11(1), 87. [Google Scholar] [CrossRef]
- Yin, L.; Yan, L.; Yu, Q.; Wang, J.; Liu, C.; Wang, L.; Zheng, L. Characterization of the MicroRNA Profile of Ginger Exosome-like Nanoparticles and Their Anti-Inflammatory Effects in Intestinal Caco-2 Cells. J. Agric. Food Chem. 2022, 70(15), 4725–4734. [Google Scholar] [CrossRef]
- Wu, S.; Yang, J.; Xu, H.; Wang, X.; Zhang, R.; Lu, W.; Yang, J.; Li, X.; Chen, S.; Zou, Y.; Nan, A. Circular RNA circGLIS3 Promotes Bladder Cancer Proliferation via the miR-1273f/SKP1/Cyclin D1 Axis. Cell Biol. Toxicol. 2022, 38(1), 129–146. [Google Scholar] [CrossRef]
- Veerapandian, M.; Ramasundaram, S.; Jerome, P.; Chellasamy, G.; Govindaraju, S.; Yun, K.; Oh, T. H. Drug Delivery Application of Functional Nanomaterials Synthesized Using Natural Sources. J. Funct. Biomater. 2023, 14(8), 426. [Google Scholar] [CrossRef]
- Chen, X.; Liu, B.; Li, X.; An, T. T.; Zhou, Y.; Li, G.; Wu-Smart, J.; Alvarez, S.; Naldrett, M. J.; Eudy, J.; Kubik, G.; Wilson, R. A.; Kachman, S. D.; Cui, J.; Yu, J. Identification of Anti-Inflammatory Vesicle-like Nanoparticles in Honey. J. Extracell. Vesicles 2021, 10(4), e12069. [Google Scholar] [CrossRef] [PubMed]
- Saiyed, A. N.; Vasavada, A. R.; Johar, S. R. K. Recent Trends in miRNA Therapeutics and the Application of Plant miRNA for Prevention and Treatment of Human Diseases. Future J. Pharm. Sci. 2022, 8(1), 24. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Liang, Z.; Xu, J.; Zhao, Y.; Li, X.; Zhang, Y.; Zhao, D.; Chen, R.; Liu, Y.; Joshi, T.; Chang, J.; Wang, Z.; Zhang, Y.; Zhu, J.; Liu, Q.; Xu, D.; Jiang, C. Plant-Derived Phosphocholine Facilitates Cellular Uptake of Anti-Pulmonary Fibrotic HJT-sRNA-M7. Sci. China Life Sci. 2019, 62(3), 309–320. [Google Scholar] [CrossRef]
- Li, X.; Liang, Z.; Du, J.; Wang, Z.; Mei, S.; Li, Z.; Zhao, Y.; Zhao, D.; Ma, Y.; Ye, J.; Xu, J.; Zhao, Y.; Chang, J.; Qin, Y.; Yu, L.; Wang, C.; Jiang, C. Herbal Decoctosome Is a Novel Form of Medicine. Sci. China Life Sci. 2019, 62(3), 333–348. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K. W.; Aikawa, E.; Alcaraz, M. J.; Anderson, J. D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G. K.; Ayre, D. C.; Bach, J.-M.; Bachurski, D.; Baharvand, H.; Balaj, L.; Baldacchino, S.; Bauer, N. N.; Baxter, A. A.; Bebawy, M.; Beckham, C.; Bedina Zavec, A.; Benmoussa, A.; Berardi, A. C.; Bergese, P.; Bielska, E.; Blenkiron, C.; Bobis-Wozowicz, S.; Boilard, E.; Boireau, W.; Bongiovanni, A.; Borràs, F. E.; Bosch, S.; Boulanger, C. M.; Breakefield, X.; Breglio, A. M.; Brennan, M. Á.; Brigstock, D. R.; Brisson, A.; Broekman, M. L.; Bromberg, J. F.; Bryl-Górecka, P.; Buch, S.; Buck, A. H.; Burger, D.; Busatto, S.; Buschmann, D.; Bussolati, B.; Buzás, E. I.; Byrd, J. B.; Camussi, G.; Carter, D. R.; Caruso, S.; Chamley, L. W.; Chang, Y.-T.; Chen, C.; Chen, S.; Cheng, L.; Chin, A. R.; Clayton, A.; Clerici, S. P.; Cocks, A.; Cocucci, E.; Coffey, R. J.; Cordeiro-da-Silva, A.; Couch, Y.; Coumans, F. A.; Coyle, B.; Crescitelli, R.; Criado, M. F.; D’Souza-Schorey, C.; Das, S.; Datta Chaudhuri, A.; de Candia, P.; De Santana Junior, E. F.; De Wever, O.; del Portillo, H. A.; Demaret, T.; Deville, S.; Devitt, A.; Dhondt, B.; Di Vizio, D.; Dieterich, L. C.; Dolo, V.; Dominguez Rubio, A. P.; Dominici, M.; Dourado, M. R.; Driedonks, T. A.; Duarte, F. V.; Duncan, H. M.; Eichenberger, R. M.; Ekström, K.; EL Andaloussi, S.; Elie-Caille, C.; Erdbrügger, U.; Falcón-Pérez, J. M.; Fatima, F.; Fish, J. E.; Flores-Bellver, M.; Försönits, A.; Frelet-Barrand, A.; Fricke, F.; Fuhrmann, G.; Gabrielsson, S.; Gámez-Valero, A.; Gardiner, C.; Gärtner, K.; Gaudin, R.; Gho, Y. S.; Giebel, B.; Gilbert, C.; Gimona, M.; Giusti, I.; Goberdhan, D. C.; Görgens, A.; Gorski, S. M.; Greening, D. W.; Gross, J. C.; Gualerzi, A.; Gupta, G. N.; Gustafson, D.; Handberg, A.; Haraszti, R. A.; Harrison, P.; Hegyesi, H.; Hendrix, A.; Hill, A. F.; Hochberg, F. H.; Hoffmann, K. F.; Holder, B.; Holthofer, H.; Hosseinkhani, B.; Hu, G.; Huang, Y.; Huber, V.; Hunt, S.; Ibrahim, A. G.-E.; Ikezu, T.; Inal, J. M.; Isin, M.; Ivanova, A.; Jackson, H. K.; Jacobsen, S.; Jay, S. M.; Jayachandran, M.; Jenster, G.; Jiang, L.; Johnson, S. M.; Jones, J. C.; Jong, A.; Jovanovic-Talisman, T.; Jung, S.; Kalluri, R.; Kano, S.; Kaur, S.; Kawamura, Y.; Keller, E. T.; Khamari, D.; Khomyakova, E.; Khvorova, A.; Kierulf, P.; Kim, K. P.; Kislinger, T.; Klingeborn, M.; Klinke II, D. J.; Kornek, M.; Kosanović, M. M.; Kovács, Á. F.; Krämer-Albers, E.-M.; Krasemann, S.; Krause, M.; Kurochkin, I. V.; Kusuma, G. D.; Kuypers, S.; Laitinen, S.; Langevin, S. M.; Languino, L. R.; Lannigan, J.; Lässer, C.; Laurent, L. C.; Lavieu, G.; Lázaro-Ibáñez, E.; Le Lay, S.; Lee, M.-S.; Lee, Y. X. F.; Lemos, D. S.; Lenassi, M.; Leszczynska, A.; Li, I. T.; Liao, K.; Libregts, S. F.; Ligeti, E.; Lim, R.; Lim, S. K.; Linē, A.; Linnemannstöns, K.; Llorente, A.; Lombard, C. A.; Lorenowicz, M. J.; Lörincz, Á. M.; Lötvall, J.; Lovett, J.; Lowry, M. C.; Loyer, X.; Lu, Q.; Lukomska, B.; Lunavat, T. R.; Maas, S. L.; Malhi, H.; Marcilla, A.; Mariani, J.; Mariscal, J.; Martens-Uzunova, E. S.; Martin-Jaular, L.; Martinez, M. C.; Martins, V. R.; Mathieu, M.; Mathivanan, S.; Maugeri, M.; McGinnis, L. K.; McVey, M. J.; Meckes Jr, D. G.; Meehan, K. L.; Mertens, I.; Minciacchi, V. R.; Möller, A.; Møller Jørgensen, M.; Morales-Kastresana, A.; Morhayim, J.; Mullier, F.; Muraca, M.; Musante, L.; Mussack, V.; Muth, D. C.; Myburgh, K. H.; Najrana, T.; Nawaz, M.; Nazarenko, I.; Nejsum, P.; Neri, C.; Neri, T.; Nieuwland, R.; Nimrichter, L.; Nolan, J. P.; Nolte-’t Hoen, E. N.; Noren Hooten, N.; O’Driscoll, L.; O’Grady, T.; O’Loghlen, A.; Ochiya, T.; Olivier, M.; Ortiz, A.; Ortiz, L. A.; Osteikoetxea, X.; Østergaard, O.; Ostrowski, M.; Park, J.; Pegtel, D. M.; Peinado, H.; Perut, F.; Pfaffl, M. W.; Phinney, D. G.; Pieters, B. C.; Pink, R. C.; Pisetsky, D. S.; Pogge von Strandmann, E.; Polakovicova, I.; Poon, I. K.; Powell, B. H.; Prada, I.; Pulliam, L.; Quesenberry, P.; Radeghieri, A.; Raffai, R. L.; Raimondo, S.; Rak, J.; Ramirez, M. I.; Raposo, G.; Rayyan, M. S.; Regev-Rudzki, N.; Ricklefs, F. L.; Robbins, P. D.; Roberts, D. D.; Rodrigues, S. C.; Rohde, E.; Rome, S.; Rouschop, K. M.; Rughetti, A.; Russell, A. E.; Saá, P.; Sahoo, S.; Salas-Huenuleo, E.; Sánchez, C.; Saugstad, J. A.; Saul, M. J.; Schiffelers, R. M.; Schneider, R.; Schøyen, T. H.; Scott, A.; Shahaj, E.; Sharma, S.; Shatnyeva, O.; Shekari, F.; Shelke, G. V.; Shetty, A. K.; Shiba, K.; Siljander, P. R.-M.; Silva, A. M.; Skowronek, A.; Snyder II, O. L.; Soares, R. P.; Sódar, B. W.; Soekmadji, C.; Sotillo, J.; Stahl, P. D.; Stoorvogel, W.; Stott, S. L.; Strasser, E. F.; Swift, S.; Tahara, H.; Tewari, M.; Timms, K.; Tiwari, S.; Tixeira, R.; Tkach, M.; Toh, W. S.; Tomasini, R.; Torrecilhas, A. C.; Tosar, J. P.; Toxavidis, V.; Urbanelli, L.; Vader, P.; van Balkom, B. W.; van der Grein, S. G.; Van Deun, J.; van Herwijnen, M. J.; Van Keuren-Jensen, K.; van Niel, G.; van Royen, M. E.; van Wijnen, A. J.; Vasconcelos, M. H.; Vechetti Jr, I. J.; Veit, T. D.; Vella, L. J.; Velot, É.; Verweij, F. J.; Vestad, B.; Viñas, J. L.; Visnovitz, T.; Vukman, K. V.; Wahlgren, J.; Watson, D. C.; Wauben, M. H.; Weaver, A.; Webber, J. P.; Weber, V.; Wehman, A. M.; Weiss, D. J.; Welsh, J. A.; Wendt, S.; Wheelock, A. M.; Wiener, Z.; Witte, L.; Wolfram, J.; Xagorari, A.; Xander, P.; Xu, J.; Yan, X.; Yáñez-Mó, M.; Yin, H.; Yuana, Y.; Zappulli, V.; Zarubova, J.; Žėkas, V.; Zhang, J.; Zhao, Z.; Zheng, L.; Zheutlin, A. R.; Zickler, A. M.; Zimmermann, P.; Zivkovic, A. M.; Zocco, D.; Zuba-Surma, E. K. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7(1), 1535750. [Google Scholar] [CrossRef]
- Jia, Y.; Yu, L.; Ma, T.; Xu, W.; Qian, H.; Sun, Y.; Shi, H. Small Extracellular Vesicles Isolation and Separation: Current Techniques, Pending Questions and Clinical Applications. Theranostics 2022, 12(15), 6548–6575. [Google Scholar] [CrossRef]
- Bobrie, A.; Colombo, M.; Krumeich, S.; Raposo, G.; Théry, C. Diverse Subpopulations of Vesicles Secreted by Different Intracellular Mechanisms Are Present in Exosome Preparations Obtained by Differential Ultracentrifugation. J. Extracell. Vesicles 2012, 1. [Google Scholar] [CrossRef]
- Mathivanan, S.; Fahner, C. J.; Reid, G. E.; Simpson, R. J. ExoCarta 2012: Database of Exosomal Proteins, RNA and Lipids. Nucleic Acids Res. 2012, 40(Database issue), D1241–1244. [Google Scholar] [CrossRef]
- Ali, N. B.; Abdull Razis, A. F.; Ooi, D. J.; Chan, K. W.; Ismail, N.; Foo, J. B. Theragnostic Applications of Mammal and Plant-Derived Extracellular Vesicles: Latest Findings, Current Technologies, and Prospects. Mol. Basel Switz. 2022, 27(12), 3941. [Google Scholar] [CrossRef]
- Zou, Z.; Li, H.; Xu, G.; Hu, Y.; Zhang, W.; Tian, K. Current Knowledge and Future Perspectives of Exosomes as Nanocarriers in Diagnosis and Treatment of Diseases. Int. J. Nanomedicine 2023, 18, 4751–4778. [Google Scholar] [CrossRef]
- Greening, D. W.; Xu, R.; Ji, H.; Tauro, B. J.; Simpson, R. J. A Protocol for Exosome Isolation and Characterization: Evaluation of Ultracentrifugation, Density-Gradient Separation, and Immunoaffinity Capture Methods. Methods Mol. Biol. Clifton NJ 2015, 1295, 179–209. [Google Scholar] [CrossRef]
- Pertoft, H. Fractionation of Cells and Subcellular Particles with Percoll. J. Biochem. Biophys. Methods 2000, 44(1–2), 1–30. [Google Scholar] [CrossRef] [PubMed]
- Brakke, M. K. Density Gradient Centrifugation: A New Separation Technique1. ACS Publications. [CrossRef]
- Buschmann, D.; Mussack, V.; Byrd, J. B. Separation, Characterization, and Standardization of Extracellular Vesicles for Drug Delivery Applications. Adv. Drug Deliv. Rev. 2021, 174, 348–368. [Google Scholar] [CrossRef]
- Lobb, R. J.; Becker, M.; Wen, S. W.; Wong, C. S. F.; Wiegmans, A. P.; Leimgruber, A.; Möller, A. Optimized Exosome Isolation Protocol for Cell Culture Supernatant and Human Plasma. J. Extracell. Vesicles 2015, 4, 27031. [Google Scholar] [CrossRef]
- Xu, D.; Di, K.; Fan, B.; Wu, J.; Gu, X.; Sun, Y.; Khan, A.; Li, P.; Li, Z. MicroRNAs in Extracellular Vesicles: Sorting Mechanisms, Diagnostic Value, Isolation, and Detection Technology. Front. Bioeng. Biotechnol. 2022, 10, 948959. [Google Scholar] [CrossRef]
- Alzhrani, G. N.; Alanazi, S. T.; Alsharif, S. Y.; Albalawi, A. M.; Alsharif, A. A.; Abdel-Maksoud, M. S.; Elsherbiny, N. Exosomes: Isolation, Characterization, and Biomedical Applications. Cell Biol. Int. 2021, 45(9), 1807–1831. [Google Scholar] [CrossRef]
- Contreras-Naranjo, J. C.; Wu, H.-J.; Ugaz, V. M. Microfluidics for Exosome Isolation and Analysis: Enabling Liquid Biopsy for Personalized Medicine. Lab. Chip 2017, 17(21), 3558–3577. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, X.; Zhao, X.; Shen, J.; Wu, X.; Gao, P.; Yang, P.; Chen, J.; An, W. Multifunctional Milk-Derived Small Extracellular Vesicles and Their Biomedical Applications. Pharmaceutics 2023, 15(5), 1418. [Google Scholar] [CrossRef]
- Garzo, E.; Sánchez-López, C. M.; Fereres, A.; Soler, C.; Marcilla, A.; Pérez-Bermúdez, P. Isolation of Extracellular Vesicles from Phloem Sap by Size Exclusion Chromatography. Curr. Protoc. 2023, 3(10), e903. [Google Scholar] [CrossRef]
- Monguió-Tortajada, M.; Gálvez-Montón, C.; Bayes-Genis, A.; Roura, S.; Borràs, F. E. Extracellular Vesicle Isolation Methods: Rising Impact of Size-Exclusion Chromatography. Cell. Mol. Life Sci. CMLS 2019, 76(12), 2369–2382. [Google Scholar] [CrossRef]
- Martínez-Greene, J. A.; Hernández-Ortega, K.; Quiroz-Baez, R.; Resendis-Antonio, O.; Pichardo-Casas, I.; Sinclair, D. A.; Budnik, B.; Hidalgo-Miranda, A.; Uribe-Querol, E.; Ramos-Godínez, M. D. P.; Martínez-Martínez, E. Quantitative Proteomic Analysis of Extracellular Vesicle Subgroups Isolated by an Optimized Method Combining Polymer-Based Precipitation and Size Exclusion Chromatography. J. Extracell. Vesicles 2021, 10(6), e12087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lyden, D. Asymmetric-Flow Field-Flow Fractionation Technology for Exomere and Small Extracellular Vesicle Separation and Characterization. Nat. Protoc. 2019, 14(4), 1027–1053. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Cai, Q.; Qiao, L.; Huang, C.-Y.; Wang, S.; Miao, W.; Ha, T.; Wang, Y.; Jin, H. RNA-Binding Proteins Contribute to Small RNA Loading in Plant Extracellular Vesicles. Nat. Plants 2021, 7(3), 342–352. [Google Scholar] [CrossRef]
- Liangsupree, T.; Multia, E.; Riekkola, M.-L. Modern Isolation and Separation Techniques for Extracellular Vesicles. J. Chromatogr. A 2021, 1636, 461773. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; He, B.; Jin, H. Isolation of Extracellular Vesicles from Arabidopsis. Curr. Protoc. 2022, 2(1), e352. [Google Scholar] [CrossRef]
- Ackermann, B. L. Understanding the Role of Immunoaffinity-Based Mass Spectrometry Methods for Clinical Applications. Clin. Chem. 2012, 58(12), 1620–1622. [Google Scholar] [CrossRef]
- Mondal, S. K.; Whiteside, T. L. Immunoaffinity-Based Isolation of Melanoma Cell-Derived and T Cell-Derived Exosomes from Plasma of Melanoma Patients. Methods Mol. Biol. Clifton NJ 2021, 2265, 305–321. [Google Scholar] [CrossRef]
- Liu, W.-Z.; Ma, Z.-J.; Kang, X.-W. Current Status and Outlook of Advances in Exosome Isolation. Anal. Bioanal. Chem. 2022, 414(24), 7123–7141. [Google Scholar] [CrossRef]
- He, B.; Cai, Q.; Qiao, L.; Huang, C.-Y.; Wang, S.; Miao, W.; Ha, T.; Wang, Y.; Jin, H. RNA-Binding Proteins Contribute to Small RNA Loading in Plant Extracellular Vesicles. Nat. Plants 2021, 7(3), 342–352. [Google Scholar] [CrossRef]
- Staubach, S.; Bauer, F. N.; Tertel, T.; Börger, V.; Stambouli, O.; Salzig, D.; Giebel, B. Scaled Preparation of Extracellular Vesicles from Conditioned Media. Adv. Drug Deliv. Rev. 2021, 177, 113940. [Google Scholar] [CrossRef]
- Suresh, A. P.; Kalarikkal, S. P.; Pullareddy, B.; Sundaram, G. M. Low pH-Based Method to Increase the Yield of Plant-Derived Nanoparticles from Fresh Ginger Rhizomes. ACS Omega 2021, 6(27), 17635–17641. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Jeong, H.; Jang, E.; Kim, E.; Yoon, Y.; Jang, S.; Jeong, H.-S.; Jang, G. Isolation of High-Purity and High-Stability Exosomes from Ginseng. Front. Plant Sci. 2022, 13, 1064412. [Google Scholar] [CrossRef] [PubMed]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical Evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the Measurement of Nanoparticles and Protein Aggregates. Pharm. Res. 2010, 27(5), 796–810. [Google Scholar] [CrossRef]
- Fortunato, D.; Mladenović, D.; Criscuoli, M.; Loria, F.; Veiman, K.-L.; Zocco, D.; Koort, K.; Zarovni, N. Opportunities and Pitfalls of Fluorescent Labeling Methodologies for Extracellular Vesicle Profiling on High-Resolution Single-Particle Platforms. Int. J. Mol. Sci. 2021, 22(19), 10510. [Google Scholar] [CrossRef]
- Sharma, S.; Gillespie, B. M.; Palanisamy, V.; Gimzewski, J. K. Quantitative Nanostructural and Single-Molecule Force Spectroscopy Biomolecular Analysis of Human-Saliva-Derived Exosomes. Langmuir ACS J. Surf. Colloids 2011, 27(23), 14394–14400. [Google Scholar] [CrossRef]
- Koliqi, R.; Breznica, P.; Daka, A.; Koshi, B. Application of Design of Expert Software for Evaluating the Influence of Formulation Variables on the Encapsulation Efficacy, Drug Content and Particle Size of PEO-PPO-PEO/Poly (DL-Lactide-Co-Caprolactone) Nanoparticles as Carriers for SN-38. Med. Pharm. Rep. 2021, 94(4), 483–497. [Google Scholar] [CrossRef]
- Bommakanti, V.; Banerjee, M.; Shah, D.; Manisha, K.; Sri, K.; Banerjee, S. An Overview of Synthesis, Characterization, Applications and Associated Adverse Effects of Bioactive Nanoparticles. Environ. Res. 2022, 214 Pt 2, 113919. [Google Scholar] [CrossRef]
- Nagelkerke, A.; Ojansivu, M.; van der Koog, L.; Whittaker, T. E.; Cunnane, E. M.; Silva, A. M.; Dekker, N.; Stevens, M. M. Extracellular Vesicles for Tissue Repair and Regeneration: Evidence, Challenges and Opportunities. Adv. Drug Deliv. Rev. 2021, 175, 113775. [Google Scholar] [CrossRef]
- Chen, F.-M.; Wu, L.-A.; Zhang, M.; Zhang, R.; Sun, H.-H. Homing of Endogenous Stem/Progenitor Cells for in Situ Tissue Regeneration: Promises, Strategies, and Translational Perspectives. Biomaterials 2011, 32(12), 3189–3209. [Google Scholar] [CrossRef]
- Jahani, M.; Rezazadeh, D.; Mohammadi, P.; Abdolmaleki, A.; Norooznezhad, A.; Mansouri, K. Regenerative Medicine and Angiogenesis; Challenges and Opportunities. Adv. Pharm. Bull. 2020, 10(4), 490–501. [Google Scholar] [CrossRef]
- Ding, J.-Y.; Chen, M.-J.; Wu, L.-F.; Shu, G.-F.; Fang, S.-J.; Li, Z.-Y.; Chu, X.-R.; Li, X.-K.; Wang, Z.-G.; Ji, J.-S. Mesenchymal Stem Cell-Derived Extracellular Vesicles in Skin Wound Healing: Roles, Opportunities and Challenges. Mil. Med. Res. 2023, 10(1), 36. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Liang, J.; Liu, H.; Zhang, K.; Li, J. Nanomaterials for Angiogenesis in Skin Tissue Engineering. Tissue Eng. Part B Rev. 2020, 26(3), 203–216. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Tao, R.; Chen, L.; Xiong, Y.; Xue, H.; Hu, L.; Yan, C.; Xie, X.; Lin, Z.; Panayi, A. C.; Mi, B.; Liu, G. Exosomes Derived from Pioglitazone-Pretreated MSCs Accelerate Diabetic Wound Healing through Enhancing Angiogenesis. J. Nanobiotechnology 2021, 19(1), 150. [Google Scholar] [CrossRef]
- Şahin, F.; Koçak, P.; Güneş, M. Y.; Özkan, İ.; Yıldırım, E.; Kala, E. Y. In Vitro Wound Healing Activity of Wheat-Derived Nanovesicles. Appl. Biochem. Biotechnol. 2019, 188(2), 381–394. [Google Scholar] [CrossRef]
- Yang, S.; Lu, S.; Ren, L.; Bian, S.; Zhao, D.; Liu, M.; Wang, J. Ginseng-Derived Nanoparticles Induce Skin Cell Proliferation and Promote Wound Healing. J. Ginseng Res. 2023, 47(1), 133–143. [Google Scholar] [CrossRef]
- Hu, Y.; Tao, R.; Chen, L.; Xiong, Y.; Xue, H.; Hu, L.; Yan, C.; Xie, X.; Lin, Z.; Panayi, A. C.; Mi, B.; Liu, G. Exosomes Derived from Pioglitazone-Pretreated MSCs Accelerate Diabetic Wound Healing through Enhancing Angiogenesis. J. Nanobiotechnology 2021, 19(1), 150. [Google Scholar] [CrossRef]
- Yang, S.; Lu, S.; Ren, L.; Bian, S.; Zhao, D.; Liu, M.; Wang, J. Ginseng-Derived Nanoparticles Induce Skin Cell Proliferation and Promote Wound Healing. J. Ginseng Res. 2023, 47(1), 133–143. [Google Scholar] [CrossRef]
- Slavkovsky, R.; Kohlerova, R.; Tkacova, V.; Jiroutova, A.; Tahmazoglu, B.; Velebny, V.; Rezačová, M.; Sobotka, L.; Kanta, J. Zucker Diabetic Fatty Rat: A New Model of Impaired Cutaneous Wound Repair with Type II Diabetes Mellitus and Obesity. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2011, 19(4), 515–525. [Google Scholar] [CrossRef]
- Lobmann, R.; Ambrosch, A.; Schultz, G.; Waldmann, K.; Schiweck, S.; Lehnert, H. Expression of Matrix-Metalloproteinases and Their Inhibitors in the Wounds of Diabetic and Non-Diabetic Patients. Diabetologia 2002, 45(7), 1011–1016. [Google Scholar] [CrossRef]
- Kim, M. K.; Choi, Y. C.; Cho, S. H.; Choi, J. S.; Cho, Y. W. The Antioxidant Effect of Small Extracellular Vesicles Derived from Aloe Vera Peels for Wound Healing. Tissue Eng. Regen. Med. 2021, 18(4), 561–571. [Google Scholar] [CrossRef]
- Trentini, M.; Zanolla, I.; Zanotti, F.; Tiengo, E.; Licastro, D.; Dal Monego, S.; Lovatti, L.; Zavan, B. Apple Derived Exosomes Improve Collagen Type I Production and Decrease MMPs during Aging of the Skin through Downregulation of the NF-κB Pathway as Mode of Action. Cells 2022, 11(24), 3950. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-H.; Park, Y.-S.; Kim, H.-S.; Kim, D.; Lee, S.-H.; Lee, C.-H.; Lee, S.-H.; Kim, J.-E.; Lee, S.; Kim, H. M.; Kim, H.-W.; Kim, J.; Seo, W.; Kwon, H.-J.; Song, B.-J.; Kim, D.-K.; Baek, M.-C.; Cho, Y.-E. Yam-Derived Exosome-like Nanovesicles Stimulate Osteoblast Formation and Prevent Osteoporosis in Mice. J. Controlled Release 2023, 355, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Sim, Y.; Seo, H.-J.; Kim, D.-H.; Lee, S.-H.; Kwon, J.; Kwun, I.-S.; Jung, C.; Kim, J.-I.; Lim, J.-H.; Kim, D.-K.; Baek, M.-C.; Cho, Y.-E. The Effect of Apple-Derived Nanovesicles on the Osteoblastogenesis of Osteoblastic MC3T3-E1 Cells. J. Med. Food 2023, 26(1), 49–58. [Google Scholar] [CrossRef] [PubMed]
- Zhan, W.; Deng, M.; Huang, X.; Xie, D.; Gao, X.; Chen, J.; Shi, Z.; Lu, J.; Lin, H.; Li, P. Pueraria Lobata-Derived Exosome-like Nanovesicles Alleviate Osteoporosis by Enhacning Autophagy. J. Control. Release Off. J. Control. Release Soc. 2023, 364, 644–653. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, Z.; Xu, L.; Lu, D.; Tang, Y.; Mai, H. Plant Extracellular Vesicles as Emerging Neuroprotective Agents for Central Nervous System Disorders. J. Adv. Res. 2025. [Google Scholar] [CrossRef]
- Xu, X.-H.; Yuan, T.-J.; Dad, H. A.; Shi, M.-Y.; Huang, Y.-Y.; Jiang, Z.-H.; Peng, L.-H. Plant Exosomes As Novel Nanoplatforms for MicroRNA Transfer Stimulate Neural Differentiation of Stem Cells In Vitro and In Vivo. Nano Lett. 2021, 21(19), 8151–8159. [Google Scholar] [CrossRef]
- Zhao, W.-J.; Bian, Y.-P.; Wang, Q.-H.; Yin, F.; Yin, L.; Zhang, Y.-L.; Liu, J.-H. Blueberry-Derived Exosomes-like Nanoparticles Ameliorate Nonalcoholic Fatty Liver Disease by Attenuating Mitochondrial Oxidative Stress. Acta Pharmacol. Sin. 2022, 43(3), 645–658. [Google Scholar] [CrossRef]
- Kim, D. K.; Rhee, W. J. Antioxidative Effects of Carrot-Derived Nanovesicles in Cardiomyoblast and Neuroblastoma Cells. Pharmaceutics 2021, 13(8), 1203. [Google Scholar] [CrossRef]
- Liu, B.; Li, X.; Yu, H.; Shi, X.; Zhou, Y.; Alvarez, S.; Naldrett, M. J.; Kachman, S. D.; Ro, S.-H.; Sun, X.; Chung, S.; Jing, L.; Yu, J. Therapeutic Potential of Garlic Chive-Derived Vesicle-like Nanoparticles in NLRP3 Inflammasome-Mediated Inflammatory Diseases. Theranostics 2021, 11(19), 9311–9330. [Google Scholar] [CrossRef]
- He, X.-Y.; Gao, Z.-Y.; Liang, W.; Sun, Y.-C. Ameliorative Effect of Ginsenoside Rg1 on Dextran Sulfate Sodium-Induced Colitis: Involvement of Intestinal Barrier Remodeling in Mice. Ann. Transl. Med. 2022, 10(24), 1328. [Google Scholar] [CrossRef]
- Lei, C.; Teng, Y.; He, L.; Sayed, M.; Mu, J.; Xu, F.; Zhang, X.; Kumar, A.; Sundaram, K.; Sriwastva, M. K.; Zhang, L.; Chen, S.-Y.; Feng, W.; Zhang, S.; Yan, J.; Park, J. W.; Merchant, M. L.; Zhang, X.; Zhang, H.-G. Lemon Exosome-like Nanoparticles Enhance Stress Survival of Gut Bacteria by RNase P-Mediated Specific tRNA Decay. iScience 2021, 24(6), 102511. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Luo, T.; Wang, D.; Zhao, Y.; Jin, Y.; Yang, G.; Zhang, X. Therapeutic Application and Potential Mechanism of Plant-Derived Extracellular Vesicles in Inflammatory Bowel Disease. J. Adv. Res. 2025, 68, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Yang, L.; Zhu, H.; Li, D.; Pan, S.; Yuan, F. Stability of Blueberry Extracellular Vesicles and Their Gene Regulation Effects in Intestinal Caco-2 Cells. Biomolecules 2023, 13(9), 1412. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, X.; Luo, Q.; Liu, X.; Wang, X.; Cui, Z.; He, A.; He, S.; Jiang, Z.; Wu, N.; Chen, P.; Yu, K.; Zhuang, J. Retinoblastoma Cell-Derived Exosomes Promote Angiogenesis of Human Vesicle Endothelial Cells through microRNA-92a-3p. Cell Death Dis. 2021, 12(7), 695. [Google Scholar] [CrossRef]
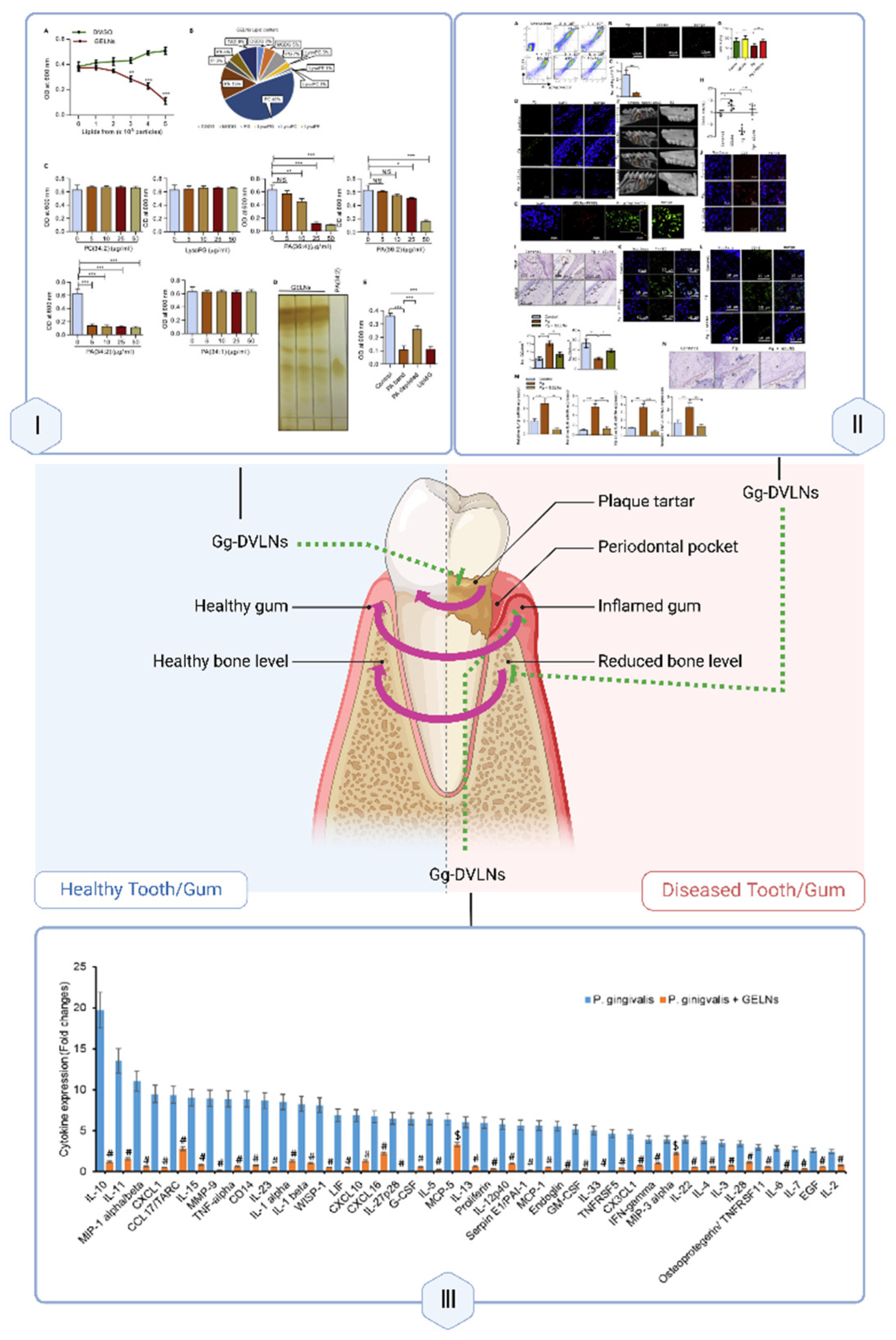
- Sundaram, K.; Miller, D. P.; Kumar, A.; Teng, Y.; Sayed, M.; Mu, J.; Lei, C.; Sriwastva, M. K.; Zhang, L.; Yan, J.; Merchant, M. L.; He, L.; Fang, Y.; Zhang, S.; Zhang, X.; Park, J. W.; Lamont, R. J.; Zhang, H.-G. Plant-Derived Exosomal Nanoparticles Inhibit Pathogenicity of Porphyromonas Gingivalis. iScience 2019, 21, 308–327. [Google Scholar] [CrossRef]
- Bui, F. Q.; Almeida-da-Silva, C. L. C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D. M. Association between Periodontal Pathogens and Systemic Disease. Biomed. J. 2019, 42(1), 27–35. [Google Scholar] [CrossRef]
- Rosa, C. D. D. R. D.; Gomes, J. M. de L.; Moraes, S. L. D. de; Lemos, C. A. A.; da Fonte, T. P.; Limirio, J. P. J. de O.; Pellizzer, E. P. Use of Chlorhexidine Chip after Scaling and Root Planning on Periodontal Disease: A Systematic Review and Meta-Analysis. Saudi Dent. J. 2021, 33(1), 1–10. [Google Scholar] [CrossRef]
- Kim, M.; Park, J. H. Isolation of Aloe Saponaria-Derived Extracellular Vesicles and Investigation of Their Potential for Chronic Wound Healing. Pharmaceutics 2022, 14(9), 1905. [Google Scholar] [CrossRef]
- Kim, M.; Jang, H.; Park, J. H. Balloon Flower Root-Derived Extracellular Vesicles: In Vitro Assessment of Anti-Inflammatory, Proliferative, and Antioxidant Effects for Chronic Wound Healing. Antioxidants 2023, 12(6), 1146. [Google Scholar] [CrossRef]
- Savcı, Y.; Kırbaş, O. K.; Bozkurt, B. T.; Abdik, E. A.; Taşlı, P. N.; Şahin, F.; Abdik, H. Grapefruit-Derived Extracellular Vesicles as a Promising Cell-Free Therapeutic Tool for Wound Healing. Food Funct. 2021, 12(11), 5144–5156. [Google Scholar] [CrossRef]
- Liu, B.; Lu, Y.; Chen, X.; Muthuraj, P. G.; Li, X.; Pattabiraman, M.; Zempleni, J.; Kachman, S. D.; Natarajan, S. K.; Yu, J. Protective Role of Shiitake Mushroom-Derived Exosome-Like Nanoparticles in D-Galactosamine and Lipopolysaccharide-Induced Acute Liver Injury in Mice. Nutrients 2020, 12(2), 477. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Deng, Z.-B.; Mu, J.; Zhang, L.; Yan, J.; Miller, D.; Feng, W.; McClain, C. J.; Zhang, H.-G. Ginger-Derived Nanoparticles Protect against Alcohol-Induced Liver Damage. J. Extracell. Vesicles 2015, 4, 28713. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-S.; Kim, H.-W.; Hwang, J.-H.; Eom, J.-Y.; Kim, D.-H.; Park, J.; Tae, H.-J.; Lee, S.; Yoo, J.-G.; Kim, J.-I.; Lim, J.-H.; Kwun, I.-S.; Baek, M.-C.; Cho, Y.-E.; Kim, D.-K. Plum-Derived Exosome-like Nanovesicles Induce Differentiation of Osteoblasts and Reduction of Osteoclast Activation. Nutrients 2023, 15(9), 2107. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.; Yoo, J. H.; Kim, J.; Min, S. J.; Heo, D. N.; Kwon, I. K.; Moon, H.-J. Ginseng-Derived Exosome-like Nanovesicles Extracted by Sucrose Gradient Ultracentrifugation to Inhibit Osteoclast Differentiation. Nanoscale 2023, 15(12), 5798–5808. [Google Scholar] [CrossRef]
- Wang, B.; Zhuang, X.; Deng, Z.-B.; Jiang, H.; Mu, J.; Wang, Q.; Xiang, X.; Guo, H.; Zhang, L.; Dryden, G.; Yan, J.; Miller, D.; Zhang, H.-G. Targeted Drug Delivery to Intestinal Macrophages by Bioactive Nanovesicles Released from Grapefruit. Mol. Ther. J. Am. Soc. Gene Ther. 2014, 22(3), 522–534. [Google Scholar] [CrossRef]
- Teng, Y.; Ren, Y.; Sayed, M.; Hu, X.; Lei, C.; Kumar, A.; Hutchins, E.; Mu, J.; Deng, Z.; Luo, C.; Sundaram, K.; Sriwastva, M. K.; Zhang, L.; Hsieh, M.; Reiman, R.; Haribabu, B.; Yan, J.; Jala, V. R.; Miller, D. M.; Van Keuren-Jensen, K.; Merchant, M. L.; McClain, C. J.; Park, J. W.; Egilmez, N. K.; Zhang, H.-G. Plant-Derived Exosomal MicroRNAs Shape the Gut Microbiota. Cell Host Microbe 2018, 24(5), 637–652.e8. [Google Scholar] [CrossRef]
- Sriwastva, M. K.; Deng, Z.-B.; Wang, B.; Teng, Y.; Kumar, A.; Sundaram, K.; Mu, J.; Lei, C.; Dryden, G. W.; Xu, F.; Zhang, L.; Yan, J.; Zhang, X.; Park, J. W.; Merchant, M. L.; Egilmez, N. K.; Zhang, H.-G. Exosome-like Nanoparticles from Mulberry Bark Prevent DSS-Induced Colitis via the AhR/COPS8 Pathway. EMBO Rep. 2022, 23(3), e53365. [Google Scholar] [CrossRef]
- Rahimi Ghiasi, M.; Rahimi, E.; Amirkhani, Z.; Salehi, R. Leucine-Rich Repeat-Containing G-Protein Coupled Receptor 5 Gene Overexpression of the Rat Small Intestinal Progenitor Cells in Response to Orally Administered Grape Exosome-like Nanovesicles. Adv. Biomed. Res. 2018, 7, 125. [Google Scholar] [CrossRef]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.-B.; Wang, B.; Zhang, L.; Roth, M.; Welti, R.; Mobley, J.; Jun, Y.; Miller, D.; Zhang, H.-G. Grape Exosome-like Nanoparticles Induce Intestinal Stem Cells and Protect Mice from DSS-Induced Colitis. Mol. Ther. J. Am. Soc. Gene Ther. 2013, 21(7), 1345–1357. [Google Scholar] [CrossRef]
- Zu, M.; Xie, D.; Canup, B. S. B.; Chen, N.; Wang, Y.; Sun, R.; Zhang, Z.; Fu, Y.; Dai, F.; Xiao, B. “Green” Nanotherapeutics from Tea Leaves for Orally Targeted Prevention and Alleviation of Colon Diseases. Biomaterials 2021, 279, 121178. [Google Scholar] [CrossRef]
- Deng, Z.; Rong, Y.; Teng, Y.; Mu, J.; Zhuang, X.; Tseng, M.; Samykutty, A.; Zhang, L.; Yan, J.; Miller, D.; Suttles, J.; Zhang, H.-G. Broccoli-Derived Nanoparticle Inhibits Mouse Colitis by Activating Dendritic Cell AMP-Activated Protein Kinase. Mol. Ther. J. Am. Soc. Gene Ther. 2017, 25(7), 1641–1654. [Google Scholar] [CrossRef]
- Gao, C.; Zhou, Y.; Chen, Z.; Li, H.; Xiao, Y.; Hao, W.; Zhu, Y.; Vong, C. T.; Farag, M. A.; Wang, Y.; Wang, S. Turmeric-Derived Nanovesicles as Novel Nanobiologics for Targeted Therapy of Ulcerative Colitis. Theranostics 2022, 12(12), 5596–5614. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, C. M.; Manzaneque-López, M. C.; Pérez-Bermúdez, P.; Soler, C.; Marcilla, A. Characterization and Bioactivity of Extracellular Vesicles Isolated from Pomegranate. Food Funct. 2022, 13(24), 12870–12882. [Google Scholar] [CrossRef]
- Garlic exosome-like nanoparticles reverse high-fat diet induced obesity via the gut/brain axis - PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/35154484/ (accessed on 27 March 2025).
- Cai, H.; Huang, L.-Y.; Hong, R.; Song, J.-X.; Guo, X.-J.; Zhou, W.; Hu, Z.-L.; Wang, W.; Wang, Y.-L.; Shen, J.-G.; Qi, S.-H. Momordica Charantia Exosome-Like Nanoparticles Exert Neuroprotective Effects Against Ischemic Brain Injury via Inhibiting Matrix Metalloproteinase 9 and Activating the AKT/GSK3β Signaling Pathway. Front. Pharmacol. 2022, 13, 908830. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.-W.; Ye, C.; Wang, K.-X.; Yang, X.; Zhu, P.-Y.; Hu, K.; Lan, T.; Huang, L.-Y.; Wang, W.; Gu, B.; Yan, C.; Ma, P.; Qi, S.-H.; Luo, L. Momordica. Charantia-Derived Extracellular Vesicles-Like Nanovesicles Protect Cardiomyocytes Against Radiation Injury via Attenuating DNA Damage and Mitochondria Dysfunction. Front. Cardiovasc. Med. 2022, 9, 864188. [Google Scholar] [CrossRef]
- Raimondo, S.; Nikolic, D.; Conigliaro, A.; Giavaresi, G.; Lo Sasso, B.; Giglio, R. V.; Chianetta, R.; Manno, M.; Raccosta, S.; Corleone, V.; Ferrante, G.; Citarrella, R.; Rizzo, M.; De Leo, G.; Ciaccio, M.; Montalto, G.; Alessandro, R. Preliminary Results of CitraVesTM Effects on Low Density Lipoprotein Cholesterol and Waist Circumference in Healthy Subjects after 12 Weeks: A Pilot Open-Label Study. Metabolites 2021, 11(5), 276. [Google Scholar] [CrossRef]
- Li, L.; Zhong, D.; Wang, S.; Zhou, M. Plant-Derived Materials for Biomedical Applications. Nanoscale 2025, 17(2), 722–739. [Google Scholar] [CrossRef]
- Wang, Q.; Zhuang, X.; Mu, J.; Deng, Z.-B.; Jiang, H.; Zhang, L.; Xiang, X.; Wang, B.; Yan, J.; Miller, D.; Zhang, H.-G. Delivery of Therapeutic Agents by Nanoparticles Made of Grapefruit-Derived Lipids. Nat. Commun. 2013, 4, 1867. [Google Scholar] [CrossRef]
- Doyle, L. M.; Wang, M. Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8(7), 727. [Google Scholar] [CrossRef]
- Umezu, T.; Takanashi, M.; Murakami, Y.; Ohno, S.-I.; Kanekura, K.; Sudo, K.; Nagamine, K.; Takeuchi, S.; Ochiya, T.; Kuroda, M. Acerola Exosome-like Nanovesicles to Systemically Deliver Nucleic Acid Medicine via Oral Administration. Mol. Ther. Methods Clin. Dev. 2021, 21, 199–208. [Google Scholar] [CrossRef]
- Seow, S. L. S.; Hong, S. L.; Lee, G. S.; Malek, S. N. A.; Sabaratnam, V. 6-Shogaol, a Neuroactive Compound of Ginger (Jahe Gajah) Induced Neuritogenic Activity via NGF Responsive Pathways in PC-12 Cells. BMC Complement. Altern. Med. 2017, 17(1), 334. [Google Scholar] [CrossRef] [PubMed]
- Lozano, O.; Lázaro-Alfaro, A.; Silva-Platas, C.; Oropeza-Almazán, Y.; Torres-Quintanilla, A.; Bernal-Ramírez, J.; Alves-Figueiredo, H.; García-Rivas, G. Nanoencapsulated Quercetin Improves Cardioprotection during Hypoxia-Reoxygenation Injury through Preservation of Mitochondrial Function. Oxid. Med. Cell. Longev. 2019, 2019, 7683051. [Google Scholar] [CrossRef] [PubMed]
- Dad, H. A.; Gu, T.-W.; Zhu, A.-Q.; Huang, L.-Q.; Peng, L.-H. Plant Exosome-like Nanovesicles: Emerging Therapeutics and Drug Delivery Nanoplatforms. Mol. Ther. J. Am. Soc. Gene Ther. 2021, 29(1), 13–31. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Lin, H.; Jiang, X.; Li, W.; Gao, Y.; Li, M.; Yu, Y.; Chen, N.; Gao, J. Exosome-like Nanoparticles Derived from Fruits, Vegetables, and Herbs: Innovative Strategies of Therapeutic and Drug Delivery. Theranostics 2024, 14(12), 4598–4621. [Google Scholar] [CrossRef]
- Yi, C.; Lu, L.; Li, Z.; Guo, Q.; Ou, L.; Wang, R.; Tian, X. Plant-Derived Exosome-like Nanoparticles for microRNA Delivery in Cancer Treatment. Drug Deliv. Transl. Res. 2025, 15(1), 84–101. [Google Scholar] [CrossRef]
- Lozano, O.; Lázaro-Alfaro, A.; Silva-Platas, C.; Oropeza-Almazán, Y.; Torres-Quintanilla, A.; Bernal-Ramírez, J.; Alves-Figueiredo, H.; García-Rivas, G. Nanoencapsulated Quercetin Improves Cardioprotection during Hypoxia-Reoxygenation Injury through Preservation of Mitochondrial Function. Oxid. Med. Cell. Longev. 2019, 2019, 7683051. [Google Scholar] [CrossRef]
- Tian, J.; Han, Z.; Song, D.; Peng, Y.; Xiong, M.; Chen, Z.; Duan, S.; Zhang, L. Engineered Exosome for Drug Delivery: Recent Development and Clinical Applications. Int. J. Nanomedicine 2023, 18, 7923–7940. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





