Printing and dyeing industry is a large industrial wastewater discharge. According to statistics, the printing and dyeing industry discharges more than 4 million tons of wastewater every day in China. Dyeing wastewater is diverse, characterized by high chromaticity, poor biochemistry, many toxic and hazardous substances, and high salt content. And if it cannot be properly treated, it will seriously pollute the environment and jeopardize human health [
1].
Printing and dyeing wastewater treatment technologies mainly include adsorption, coagulation, membrane separation, chemical oxidation, photocatalytic oxidation, electrochemical method and biological treatment. Traditional treatment methods, such as adsorption [
2,
3,
4], coagulation and precipitation [
5,
6], etc., are mainly used for low-concentration printing and dyeing wastewater or as a pre-treatment process for biological treatment to improve the biochemistry of the printing and dyeing wastewater. And it is difficult to make the high-concentration printing and dyeing wastewater meet the discharge standards. The use of chemical oxidation [
7,
8,
9] requires the constant addition of oxidants, membrane separation technology [
10,
11] has high energy consumption and high operating cost. The photocatalytic oxidation technology [
12] requires high-quality preparation of photocatalysts. Printing and dyeing wastewater has poor biochemistry, many toxic and hazardous substances, and high salinity will inhibit the growth of microorganisms, so the biological method is often combined with other methods for the treatment of printing and dyeing wastewater [
13]. Under the condition of high salinity, the wastewater has high electrical conductivity, this characteristic provides a good development space for the application of electrochemical method in the treatment of high salinity printing and dyeing wastewater.
Iron-carbon micro-electrolysis utilizes the principle of electrochemical corrosion of metals. In the filler, Fe with a low potential and C with a high potential generate a potential difference in wastewater with certain electrical conductivity, forming countless tiny primary batteries. The wastewater was treated through the electrode reaction as well as a series of synergistic effects, such as coagulation, sedimentation, and air flotation [
14], which was often used to treat dyeing and printing wastewater [
15,
16,
17].He Wei et al. [
18] pretreated methyl orange simulated printing and dyeing wastewater using micro-electrolytic materials with a wide range of pH adaptability. The optimal process parameters were determined, and the BOD/COD value of the effluent was increased by nearly 5 times after pretreatment. Jia Yanping et al. [
19] treated the rinsing workshop wastewater of a woolen mill in Jilin City by iron-carbon micro-electrolysis. The highest colorimetric removal rate was 75.34%. The iron-carbon micro-electrolysis process could efficiently decompose pollutants such as esters and alcohols, and convert them into small-molecule organic pollutants that were easy to be biochemically processed. The biochemistry of the wastewater was improved. She Shuaiqi et al. [
20] studied the mechanism of action of iron-carbon micro-electrolysis for the treatment of printing and dyeing wastewater. The combination of iron-carbon micro-electrolysis and coagulation sedimentation improves the efficiency of COD removal and reduces the treatment cost. Ren Qingqing [
21] used iron carbon micro-electrolysis to remove four kinds of dyes in wastewater, determined the optimal experimental conditions and the degree of influence of each factor. The iron carbon micro-electrolysis process, as a pretreatment technology, had achieved good treatment effects on the four types of wastewater. It can be seen that the former use of iron carbon micro-electrolysis process to treat printing and dyeing wastewater, mainly focused on the pretreatment of wastewater, after pretreatment to improve the biochemistry of wastewater.
The wastewater containing azo dyes has a high chemical oxygen demand (COD
Cr), a large number of refractory substances, and a high chroma, which poses a difficult problem for the decolorization and degradation of dye wastewater [
22,
23]. In this experiment, Direct Scarlet 4BE with a typical azo structure was selected for experimental research. The influences of factors such as the initial pH value, reaction time, mass ratio of iron to carbon, and the mode of solid-liquid separation on the treatment effect were investigated. The optimal treatment conditions for the wastewater were determined. Then iron and carbon micro-electrolysis with coagulation or Fenton’s reagent was used for the coupling of the wastewater, which achieved a better decolorization effect. After treatment, the wastewater met the chromaticity emission standard of 80 times as specified in the “Emission Standard for Water Pollutants in Textile Dyeing and Finishing Industry” (GB4287-2012).
1. Experimental Design and Sample Testing
1.1. Materials, Reagents and Instruments
The printing and dyeing wastewater was formulated with Direct Big Red 4BE dye, with a concentration of 1500 mg/L, a salt content of 20 g/L, an initial pH of 8.34, and a chromaticity of 80,000 times. The main experimental chemicals were: Direct Big Red 4BE dye, sodium chloride, iron powder, granular activated carbon, sodium hydroxide, concentrated sulfuric acid and so on. Main instruments: electronic analytical balance (model BSA223S); electric constant temperature blast drying oven (model DHG-9070); pH meter (model PHS-3C); rotary oscillator (model HZ-81B); desktop centrifuge (model 800); ultraviolet and visible spectrophotometer (model SP-1105).
1.2. Material Pre-Treatment
Pretreatment of iron powder and granular activated carbon was required before the start of the experiment. Iron powder (60 mesh) pretreatment, soaked in 10% sodium hydroxide for 6min (to remove oil and grease), and then rinsed under tap water to neutral, washed with 5% sulfuric acid for 5min (to remove iron oxides, and activate the iron powder), and rinsed in distilled water to neutral for drying and use. The pretreatment of activated carbon was as follows: rinsed it with water until clean (to wash away the carbon black), soaked it in the prepared wastewater for 48 hours, and then placed it in an oven to dry for use, so as to eliminate the interference of the adsorption effect of activated carbon on the experiment. In the experiment, iron powder should be prepared and used now to avoid re-oxidation.
1.3. Experimental Design
Iron-carbon micro-electrolysis experiments were carried out to investigate the effects of different reaction times, initial pH values, iron and carbon mass ratios on the degradation effects. Five 100mL water samples were poured into 250mL conical flasks respectively. Each conical flask was injected with a quantitative amount of iron powder and activated carbon. And after oscillating for a specified time, it was left to stand for 20min. The supernatant was filtered through a filter membrane (0.45μm) to measure the absorbance.
The reaction time ranged from 50 to 130 min. The initial pH of the wastewater was adjusted to 3.0, 4.0, 5.0, 6.0, and 7.0. The amount of activated carbon and iron powder was adjusted according to the Fe/C mass ratio of 1:2, 1:1, 2:1, and 3:1. The influence of different solid-liquid separation methods (centrifugal separation and microfiltration membrane filtration) on the degradation effect was investigated.
Under the optimal conditions of iron and carbon micro-electrolysis, the chromaticity of the treated wastewater was still high. It did not meet the emission standards for chromaticity in the Emission Standards for Water Pollutants in Textile Dyeing and Finishing Industry (GB4287-2012). Coagulation sedimentation and Fenton oxidation were respectively combined with the iron-carbon micro-electrolysis process for further treatment of wastewater.
1.4. Sample Testing and Data Processing Methods
The absorbance was measured by ultraviolet and visible spectrophotometer. According to the standard curve, the concentration value was obtained, and the decolorization rate was calculated to judge the treatment effect of iron-carbon micro-electrolysis process on direct red 4BE dye wastewater. The chromaticity was determined by the dilution multiplier method, and the pH value was determined by a pH meter.
The absorbance value of the treated dye wastewater was measured at 500nm [
24]. The concentration value of the corresponding wastewater was converted through the standard curve. The decolorization rate η of the dye wastewater was calculated according to Equation (1):
C - concentration value of direct Big Red 4BE dye wastewater after treatment
C0- concentration value of direct Big Red 4BE dye effluent before treatment
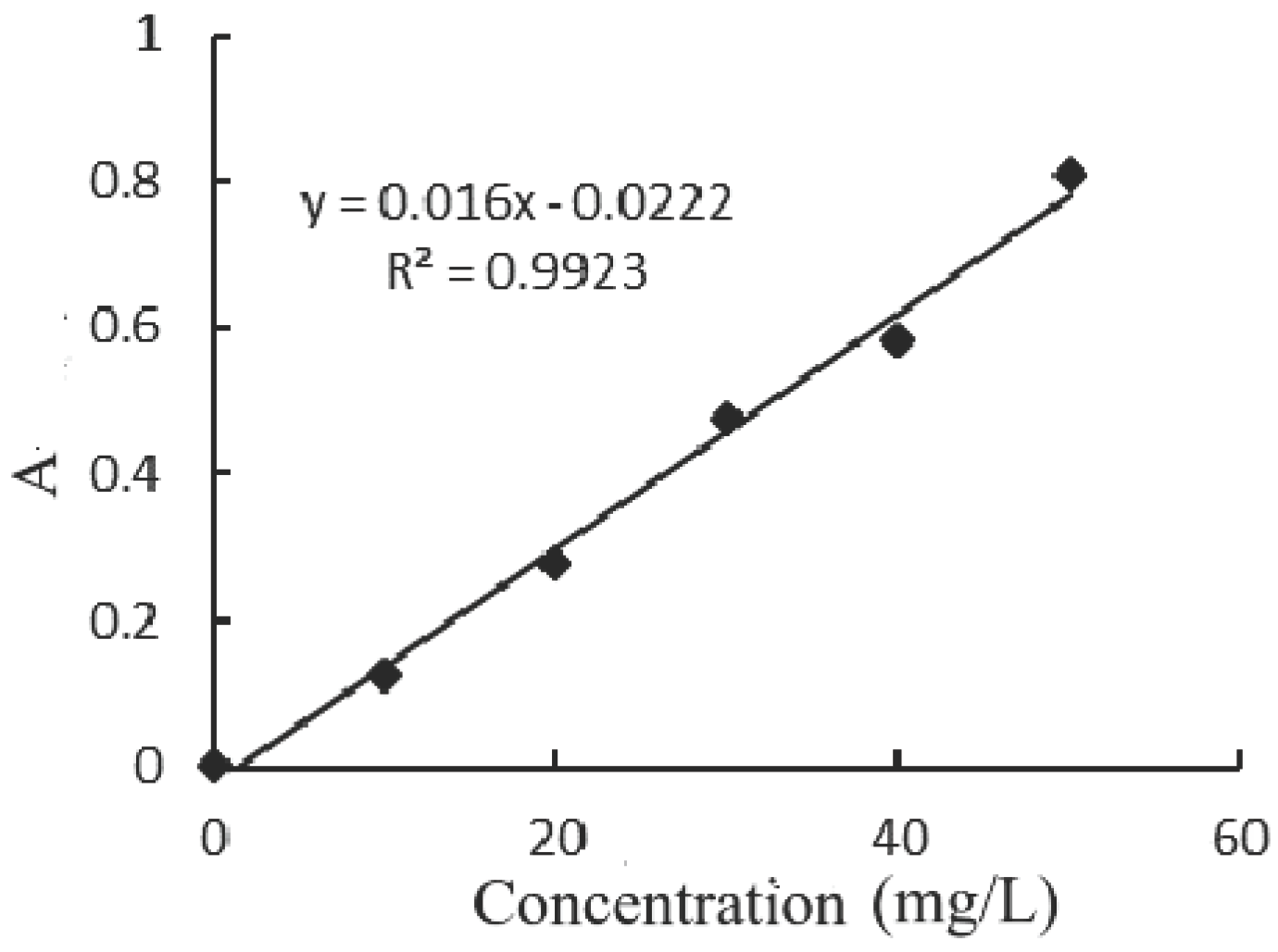
Plotting of the standard curve of direct red 4BE dye wastewater: The direct red 4BE solutions with dye concentrations of 0, 10, 20, 30, 40 and 50mg/L were prepared, the salt content is 20g/L. Distilled water as a reference, the absorbance of each water sample was determined by an ultraviolet-visible spectrophotometer. The standard curve was plotted with the concentration of direct scarlet 4BE as the abscissa and the absorbance value as the ordinate. It was shown in
Figure 1. It can be seen that the direct Big Red 4BE solution has a good linear relationship between the dye concentration and absorbance in the concentration range of 0-50 mg/L, with the correlation coefficient R
2 of 0.9923.
2. Results and Analysis
2.1. Single Factor Experiment of Iron-Carbon Micro-Electrolysis
2.1.1. Effect of Different Reaction Times
Five portions of 100 mL dye wastewater were poured into 250 mL conical flasks respectively. Quantitative iron powder and activated carbon (iron powder 20g/L, activated carbon 40g/L) were added to each water sample which was placed on the oscillator. After 50 min reaction, the water samples were taken every 20 min, and the absorbance value of each water sample was determined after standing and filtering. The absorbance values of the samples with reaction time of 50 min and 70 min were measured at 25 times of dilution, and the absorbance values of the samples with reaction time of 90 min were measured at 10 times of dilution. The concentration value and decolorization rate of the samples are shown in
Table 1.
It can be seen that the decolorization rate increased with the increase of reaction time. Within 50 ~ 130 min, the decolorization rate increased from 65.50 % to 99.14 %. After 110 min, the rate of color reduction slowed down, and the decolorization rate was above 96 %. In the early stage, the Fe of the primary battery anode is sufficient, the cathode dissolved oxygen is also very sufficient, and the reaction rate is fast. In the later stage, a large amount of Fe2+ was produced in the anode, and the micro-electrolysis reaction was weakened. The decolorization rate increased slowly under the enrichment of micro-electric field and the oxidation of dissolved oxygen. After comprehensive consideration, the optimal reaction time of dye wastewater is 110 min.
2.1.2. Effect of Initial pH Value
The initial pH values of the wastewater were adjusted to 3.0,4.0,5.0,6.0, and 7.0, respectively. Each water sample was added with a quantitative amount of iron powder and activated carbon (iron powder 20g/L, activated carbon 40g/L), and placed on an oscillator to oscillate for 110 min. After the reaction was finished, the samples were allowed to stand for 20 min, and the supernatant was filtered with a filter membrane to measure the absorbance value. The absorbance values of the two samples with initial pH values of 6 and 7 were measured by 10-fold dilution. The results are shown in
Table 2.
From
Table 2, it can be seen that when the initial pH value is in the range of 3~7, the decolorization rate of wastewater decreases with the increase of initial pH value. When the initial pH value was 3, the decolorization rate of dye wastewater was the highest (99.78%). When the initial pH value was 3 ~ 5, the decolorization rate was above 99%. When the initial pH was 7, the decolorization rate reached the lowest (84.20%). The reason for this phenomenon is that when the initial pH value is less than 5, the H
+ content in the water sample is more, and the anode iron powder will react violently. The stronger the acidity is, the greater the potential difference of the original battery in the solution is, and the stronger the reaction is. On the other hand, it may be that the lower the initial pH, the more Fe
2+ content in the water sample, the stronger the redox reaction, so as to remove the dye molecules in the wastewater. When the initial pH value is greater than 5, the acidity of the water sample is reduced, and the active performance of iron is also greatly reduced, thus inhibiting the effect of the iron-carbon microcell. Considering comprehensively, the optimal initial pH value of this experiment is 5.
2.1.3. Effect of Different Fe/C Mass Ratios
A total of 4 water samples, each water sample was added with a quantitative iron powder (20g/L). The amount of activated carbon was adjusted according to the Fe/C mass ratio of 2:1,1:1,1:2 and 1:3, that is, the amount of activated carbon was 10,20,40 and 60g/L, respectively. The oscillation reaction was 110min, and the absorbance value of each water sample was measured after standing and filtering. The samples with iron-carbon ratio of 2:1 and 1:1 were diluted 10 times and the absorbance was measured. The results are shown in
Table 3.
From
Table 3, it can be seen that when the mass ratio of iron to carbon is 2:1 and 1:1, the decolorization rate is low, the lowest is 90.20%. When the mass ratio of iron to carbon is 1:2 and 1:3, the decolorization rate of dye wastewater is higher, which is basically maintained at about 99%. The reason for this phenomenon may be that when the amount of iron powder is relatively large (iron-carbon ratio 2:1 and 1:1), the internal electrolysis and flocculation precipitation of iron powder play a major role. With the decrease of the mass ratio of iron to carbon, part of the iron powder and activated carbon form the primary battery, and the effect of the iron powder itself is weakened. When the mass ratio of iron to carbon is less than 1:1, the iron-carbon micro-cell plays a leading role, and the ability to remove organic matter is getting stronger and stronger. After comprehensive consideration, the best mass ratio of iron to carbon in this experiment is 1:2, the dosage of iron powder is 20g/L, and the dosage of activated carbon is 40g/L.
2.1.4. The Influence of Different Separation Methods
After the reaction, the solid-liquid separation was carried out in two ways: centrifugal separation and membrane filtration. In this experiment, membrane filtration was used for solid-liquid separation. Through a set of comparative experiments, the effects of different separation methods on the treatment of direct red 4BE dye wastewater by iron-carbon micro-electrolysis process were investigated.
Ten dye wastewater samples were divided into two groups, and the dosage of activated carbon in each water sample was 40g/L. The dosage of iron powder in each group was 20,30,40,50 and 60g/L, respectively. After 90min of oscillation reaction, the water samples were standing. The first group was separated by centrifugation, and the second group was filtered by 0.45μm microporous membrane. The absorbance of wastewater was measured, and the results were shown in
Table 4.
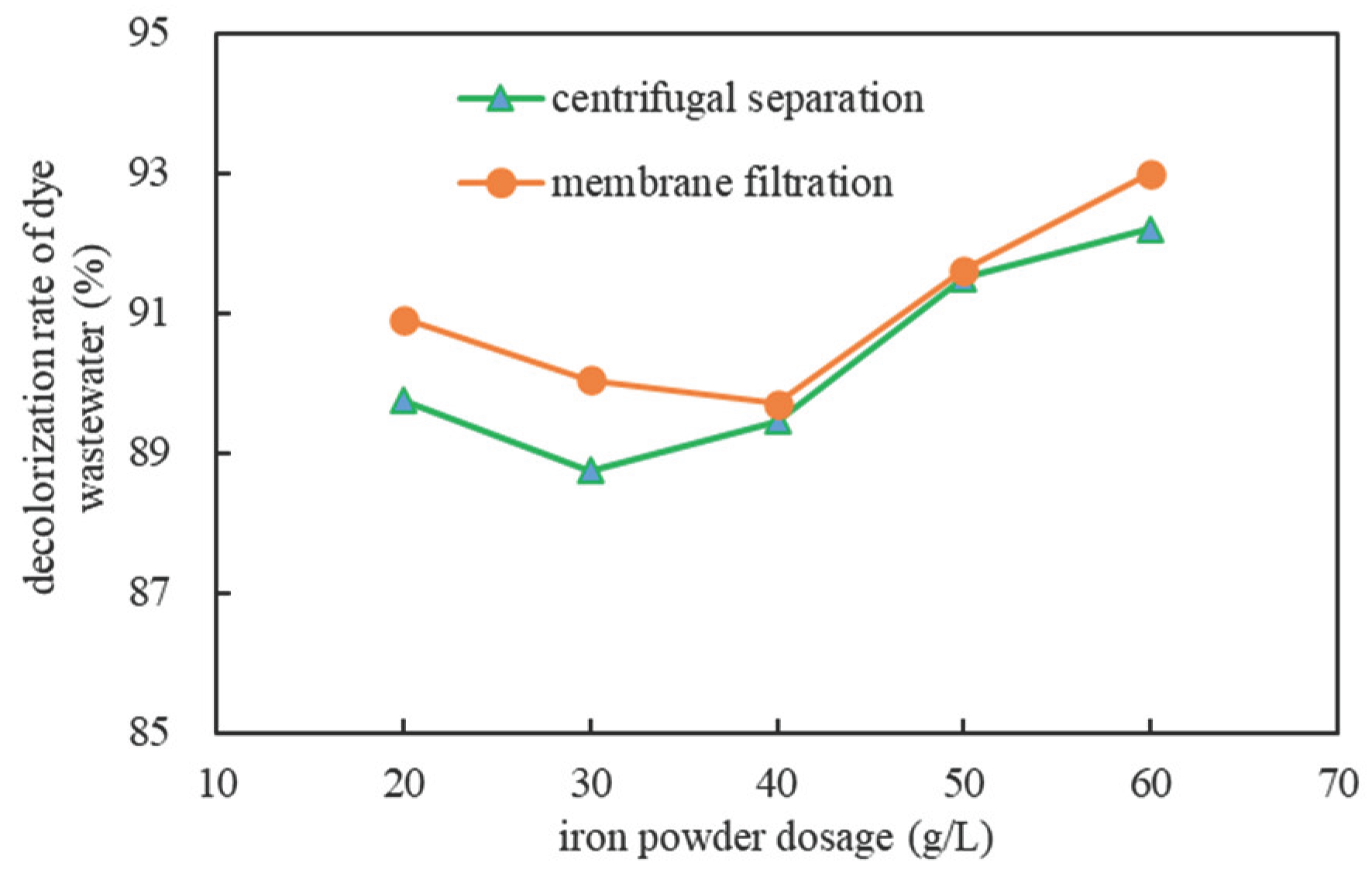
Figure 2 shows the variation curve of decolorization rate with iron powder dosage under two separation methods. It can be seen from
Figure 2 that the decolorization rates of the two samples were very close under the same iron powder dosage. Centrifugal separation is a little smaller than the overall decolorization rate of membrane filtration. This may be due to that the supernatant was first centrifuged in the experiment, and then the supernatant was taken for membrane filtration. The intermediate time difference leads to some deviation in the decolorization rate of the two separation methods. After comprehensive consideration, it is concluded that there is no difference between the two solid-liquid separation methods of centrifugal separation and filter membrane filtration in the iron-carbon micro-electrolysis experiment. In this experiment, the convenient and quick filter membrane filtration was used for solid-liquid separation.
2.1.5. Iron-Carbon Micro-Electrolysis Experiments Under Optimal Conditions
The optimal conditions for iron-carbon micro-electrolysis treatment of wastewater were determined through one-factor experiments: reaction time of 110 min, initial pH value of 5, iron-carbon mass ratio of 1:2 (iron powder 20g/L and activated carbon 40g/L). The experiment was carried out under the optimal conditions. The absorbance and chroma of the supernatant were measured after filtration. The experimental results are shown in
Table 5.
From
Table 5, it can be seen that after treatment with iron-carbon micro-electrolysis, the concentration of wastewater was reduced to 14.51 mg/L, the chromaticity was 3,000 times, and the decolorization rate reached 99.03%. It shows that the iron-carbon micro-electrolysis process has a good decolorization effect on the treatment of direct red 4BE dye wastewater.
2.2. Analysis of the Treatment Effect Of Iron-Carbon Micro-Electrolysis Combined Process
Under the optimal operating conditions, the chromaticity of the wastewater after iron-carbon micro-electrolysis treatment is 3000 times. However, it does not meet the emission standard of 80 times the chromaticity in the “Discharge Standard of Water Pollutants for Textile Dyeing and Finishing Industry” (GB4287-2012). In the field of refractory wastewater treatment, coagulation sedimentation [
25,
26,
27], Fenton reagent oxidation [
28,
29,
30,
31] and other processes are often used, or combined with other technologies to enhance the treatment effect. Therefore, in order to further improve the treatment effect of printing and dyeing wastewater, iron-carbon micro-electrolysis was combined with coagulation sedimentation or Fenton oxidation to deeply treat direct red 4BE dye wastewater.
2.2.1. Iron-Carbon Micro-Electrolysis and Coagulation Sedimentation Combined Process
Iron-carbon micro-electrolysis and coagulation sedimentation [
32,
33,
34] were combined to form a combined process to treat printing and dyeing wastewater. The supernatant of iron-carbon micro-electrolysis under the optimal treatment conditions was taken for coagulation experiments. The coagulant was FeSO4·7H2O.The effects of factors such as coagulant dosage, pH value, and coagulation standing time on the treatment effect were mainly considered. The optimum treatment conditions of coagulation experiment were determined by single factor experiment: coagulant dosage 100 mg/L, pH value 9.5, and coagulation standing time 50 min.
After the coagulation experiment, the supernatant was filtered to measure its absorbance and chromaticity. The experimental results are shown in
Table 6.
The comparison before and after the treatment of dye wastewater by iron-carbon microelectrolysis + coagulation sedimentation combined process is shown in
Figure 3.
It can be seen from
Table 6 that the concentration of wastewater decreased from 1500mg/L to 3.51mg/L and the chromaticity decreased from 80,000 times to 60 times after the combined process of iron-carbon micro-electrolysis and coagulation sedimentation. The decolorization rate reached 99.77 %. In
Figure 3, the color of the dye wastewater becomes lighter and lighter with the progress of the experiment, and the chromaticity reaches the emission standard of 80 times chromaticity in the “Discharge Standard of Water Pollutants for Textile Dyeing and Finishing Industry” (GB4287-2012).
2.2.2. Iron Carbon Micro-Electrolysis and Fenton Oxidation Combined Process
The combination of iron-carbon micro-electrolysis and Fenton oxidation [
35,
36,
37,
38] was used to further treat the direct red 4BE dye wastewater and improve the decolorization effect of wastewater. The supernatant of dye wastewater after iron-carbon micro-electrolysis treatment was taken out. The effects of factors such as FeSO
4 and H
2O
2 dosage, pH value, reaction time and other factors on the degradation effect were considered separately. The optimal treatment conditions were determined through one-factor experiment: the dosages of FeSO4 and H
2O
2 were 0.6g/L and 20mL/L respectively, the pH was 2, the reaction time was 40min. Experiments were carried out under the optimal treatment conditions for Fenton oxidation. The supernatant was taken and filtered to measure its absorbance and chromaticity, the experimental results are shown in
Table 7.
The comparison before and after the treatment of dye wastewater by iron-carbon microelectrolysis + Fenton oxidation combined process is shown in
Figure 4.
From
Table 7, It can be seen that the concentration of the wastewater treated by the combined process of iron-carbon micro-electrolysis and Fenton method was reduced to 3.14 mg/L, the chromaticity was reduced to 40 times, and the decolorization rate reached 99.79 %. In
Figure 4, with the progress of the reaction, the chromaticity of the dye wastewater is getting lower and lower, which meets the emission standard of 80 times chromaticity in the “Discharge Standard of Water Pollutants for Textile Dyeing and Finishing Industry” (GB4287-2012).
It can be seen that the above two combined processes have good treatment effects on printing and dyeing wastewater. However, the Fenton oxidation method needs to add hydrogen peroxide, the dosage of ferrous sulfate (0.6g/L) is higher than that of coagulation precipitation method (0.1g/L), and more sludge is produced. Coagulation treatment of printing and dyeing wastewater has the advantages of simple process, convenient operation and management, and low equipment investment. Therefore, the combination of iron-carbon micro-electrolysis and coagulation sedimentation process has a higher cost performance for the treatment of direct red 4BE dye wastewater.
3. Conclusion
In this thesis, the experiment of iron and carbon micro-electrolysis treatment of direct Big Red 4BE dye wastewater was carried out to study the influence law of different reaction time, different initial pH value and different iron and carbon mass ratio on the treatment effect, so as to determine the optimal treatment conditions. Data analysis showed that the optimal reaction time of 110 min, initial pH value of 5, iron and carbon mass ratio of 1:2 (iron powder dosage of 20g/L, activated carbon dosage of 40g/L), the decolorization rate of dye wastewater could reach 99.03 %, the concentration decreased from 1500mg/L to 14.51mg/L, and the chromaticity decreased from 80000 times to 3000 times. However, it does not meet the emission standard of dye wastewater. The dye wastewater treated under the optimal conditions of iron-carbon micro-electrolysis was treated by coagulation precipitation and Fenton oxidation process, respectively. The total decolorization rate of the wastewater could be improved up to 99.79%, and the chromaticity reached the 80 times chromaticity in the “Discharge Standard of Water Pollutants for Textile Dyeing and Finishing Industry” (GB4287-2012).
References
- LEME D M, PRIMO F L, GOBO G G, et al. Genotoxicity assessment of reactive and disperse textile dyes using human dermal equivalent (3D cell culture system) [J]. J Toxicol Environ Heal A, 2015,78(7): 466-480. [CrossRef]
- Sun Xiaoxu, Xu Jin, Chu Zhiqiang. Experimental study of absorption of methylene blue with activated carbon prepared by modification pomelo peel in different methods [J]. Journal of Hohai University(Natural Sciences), 2021,49(06):536-542. [CrossRef]
- Li Zhuobing. Study on the preparation and adsorption characteristics of auricula chaff charcoal [D]. Northeast Electric Power University,2024.
- Zhang Changfeng. Study on preparation of almond shell-based activated carbon and its adsorption of methylene blue [J]. Industrial Microbiology, 2023, 53(03):59 -62.
- Luo Xinhao. Study on O3-coagulation enhanced pretreatment and O3-SBBR advanced treatment of wastewater from a printing and dyeing industrial park in Foshan [D]. South China University of Technology, 2022.
- Ding jing, Zhang jianliang, Ren ye, et al. Treatment of dyeing wastewater by a combined process of coagulation,A/O,flotation,ozone, and magnetic coagulation [J]. China water & wastewater, 2021, 37(20):112-115.
- Li Zhonghua, Chang Na, Xu Yinong et al. Fenton process treating small pore-sized ultrafiltration membrane concentrate of printing and dyeing wastewater [J]. Chinese Journal of Environmental Engineering,2024,18(01):41-50. [CrossRef]
- Feng Yabing, Sun Rong, Zhao Jing,et al. Engineering design of Fenton oxidation process in advanced treatment of printing and dyeing wastewater [J]. Water and wastewater Engineering, 2023,59(07):58 -63.
- He Yujie, Chen Wei, Zheng Xiaoying, et al. Pretreatment experiment of high-concentration aromatic tobacco flavor wastewater with Fenton reagent and PAM coagulation [J]. Journal of Hohai University(Natural Sciences),2012,40(05):525-529.
- Jing Xinjun, Cai Daniu, Li Bin, et al. Progress in the deep treatment technology of printing and dyeing wastewater [J]. Technology of Water Treatment, 2022,48(6):13 -19.
- Jing Xinjun. Study on the advanced treatment process of high concentration printing and dyeing wastewater [D]. Hebei University of Technology, 2023.
- Zhang Lianke, Wang Chang, Li Yumei et al. Mechanism of complex superior oxidation system Vis/LaFeO3 /PDS for methylene blue degradation [J]. Water Resources Protection,2021,37(06):142-149.
- Zhang Zhen. Study on the efficiency and mechanism of iron carbon microelectrolysis treatment of dyeing wastewater [D]. Northeast Electric Power University,2020.
- Zhen-Zhu Sun, Zhong-Hai Liu, Le Han, et al. Study on the treatment of simulated azo dye wastewater by a novel micro-electrolysis filler [J]. Water Science & Technology,2019,79(12): 2279 -2288. [CrossRef]
- LIU W W, TU X Y, WANG X P,et al. Pretreatment of coking wastewater by acid out micro-electrolysis process with in situ electrochemical peroxidation reaction [J]. Chem Eng J, 2012,200: 720 -728.
- ZHU Q S, GUO S H,GUO C M,et al. Stability of Fe-C micro-electrolysis and biological process in treating ultra-high concentration organic wastewater [ J].Chem Eng J,2014,255:535 -540.
- Chen Kun, Gong Minghui, Li Rui, et al. Comprehensive Experimental Design of Fe-C Micro-electrolysis for Treating Dye Wastewate [J]. Research and Exploration in Laboratory,2023,42(04):183 -187+193.
- HE Wei, LI Guiju, SONG Jie et al. Research on a new type of micro-electrolysis material for the degradation of printing and dyeing wastewater [J]. Industrial Water Treatment,2018,38(10):71-74.
- JIA Yanping, ZHANG Zhen, TONG Zewei et al. Study on efficiency and mechanism of iron-carbon microelectrolysis treatment of dyeing wastewater [J]. CIESC Journal, 2020, 71(4): 1791-1801.
- SHE Shuaiqi, CHEN Hong, XUE Gang, et al. Mechanism for treatment of dyeing wastewater by Fe-C micro-electrolysis [J]. Environmental Protection of Chemical Industry,2021,41(6):699-704.
- Ren Qingqing. Removal of direct dyes and reactive dyes from printing and dyeing wastewater by microelectrolysis and the mechanism of decolorization [D]. Lanzhou Jiaotong University,2022.
- Chen G. Study on biological decolorization of azo dye wastewater and aerobic degradation performance of typical decolorization products [D].Donghua University,2012.
- Liu Xiaobo. Study on the Effect and Decolorization Mechanism of Azo Dye Wastewater Treatment by Micro Electrolysis [D]. Lanzhou Jiaotong University,2021.
- BAI Bo, CHEN Zhihong, WANG Liping. Treatment of direct scarlet 4BE dye wastewater by the hybrid method of Fe/C microelectrolysis and ultrasound radiation [J]. Applied Chemical Industry,2007, 36(02): 130-133.
- FAN Jinhui, ZHOU Weidong, YANG Xiedong, et al. Effect of Fe2+ and PMS synchronous coagulation and oxidation pretreatment on purification of sludge water by ultrafiltration [J].Water Resources Protection, 2021, 37(02):148-152.
- LI Chenlu, GUO Yani, ZHENG Libing et al. DOM removal characteristics and coagulation mechanism of silica sand loading coagulation process [J]. Water Resources Protection,2021,37(4):148-155.
- WEI Yuansong, ZHENG Libing, ZHANG Chun et al. Progress of application and research of advanced treatment technologies for reclaimed water reuse in thermal power generation plant in China [J]. Water Resources Protection,2018,34(6):1-11+16.
- Feng Yabing, Sun Rong, Zhao Jing et al. Engineering design of Fenton oxidation process in advanced treatment of printing and dyeing wastewater [J]. Water &Wastewater Engineering,2023,49(7): 58-63.
- Zhang Kunpu. Study on treatment of aromatic compounds in printing and dyeing wastewater by fenton oxidation [D]. Beijing University of Chemical Technology,2023.
- ZHAO Hazy, LI Mei, BAI Maomao et al. Research progress of advanced oxidation technology in dyeing wastewater treatment [J]. Applied Chemical Industry,2023,52(6):1884-1890.
- Qingwei, Zhao Tingyue, Liu Jian et al. Pretreatment of phosphoric ester flame-retardant wastewater with Fenton oxidation technique [J]. Water Resources Protection,2016,32(6):90-92.
- Yang Xingxia. Study on the performance of PAM-PAFC in degrading printing and dyeing wastewater [D]. Guizhou University,2022.
- Chen Xi, Wu Linfeng, Zhang Li et al. Advanced treatment of practical dyeing wastewater by electron beam coupled with coagulation technology [J]. Industrial Water Treatment, 2025,45(05):143-150.
- SUN Shaozhe, SHEN Yaxue, LI Jun et al. Analysis on Flocculation Decolorization and COD Removal of Dye Wastewater by Aluminum Salt Coagulant [J]. Contemporary Chemical Industry, 2025,54(04):972-977.
- PANG Yue, LOU Honghai, GAO Rui et al. Study on Advanced Treatment of Dyeing Wastewater by Fenton Oxidation [J]. Technology of Water Treatment,2023,49(05):40-44.
- QIAO Tianyu, YANG Kailin, FENG Mingming et al. Research Progress on Application of Micro Electrolysis-Fenton Oxidation Combined Technology in Industrial Wastewater Treatment [J]. China Resources Comprehensive Utilization,2023,41(7):89-92.
- Kong Zhangliang. Treatment of Azo Dye Wastewater by Iron-Carbon Microelectrolysis Combined with Fenton Reagent [D]. Jiangxi University of Science and Technology,2019.
- Yan Wang, Xianwei Wu, Ju Yi, et al. Pretreatment of printing and dyeing wastewater by Fe/C micro-electrolysis combined with H2O2 process [J]. Water Science & Technology,2018,244:1-11. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).