1. Introduction
ENDOPHTHALMITIS is infection with a resulting inflammation of intraocular tissues and fluids with pus filling vitreal cavity and possible infiltration of inner coats of the eye wall. It may be postoperative, post-injection, post-traumatic or endogenous.(Kato et al. 2022; Soliman et al. 2019) Bacteria are the most common cause, but fungi or viruses may also be responsible. It is an acute and urgent ocular pathology that poses a risk of losing not only vision but also the entire eyeball. If the pathogen is highly virulent, sepsis may also pose a serious risk.
Acute postoperative endophthalmitis typically appears 1-2 weeks following surgery, usually within 1-4 days. This is why it is of utmost importance to intervene immediately and perform complete surgery - Complete and Early Vitrectomy for Endophthalmitis (CEVE). This protocol was introduced in 2008, based on the historical indication „Ubi pus, ibi evacua.” (Kuhn 2016; Morris & Kuhn 2021; Soliman et al. 2019)
Endophthalmitis requires immediate treatment. From both medical and legal perspectives, it should be initiated without delay, regardless of the chosen treatment. Pathologically, the presence of pus inside the eye necessitates its evacuation as quickly as possible, primarily because it can rapidly lead to irreversible vision loss (Kuhn 2016; Morris & Kuhn 2021; Soliman et al. 2019). Therefore, we believe that surgical treatment should be introduced immediately, along with antibiotics such as vancomycin, first or second-generation cephalosporins, or fluoroquinolones, administered systemically. (Pietras-Baczewska et al. 2021; Rejdak et al. 2016)
Complete and Early Vitrectomy is essential for retinal survival, especially since it is initially not known which organism has caused the endophthalmitis and whether a fulminant infection will develop. (Morris & Kuhn 2021)
Currently, the majority of ocular surgeries are performed on an outpatient basis, which is why we propose a protocol for managing endophthalmitis in one-day surgery centers, as it ensures timely intervention.
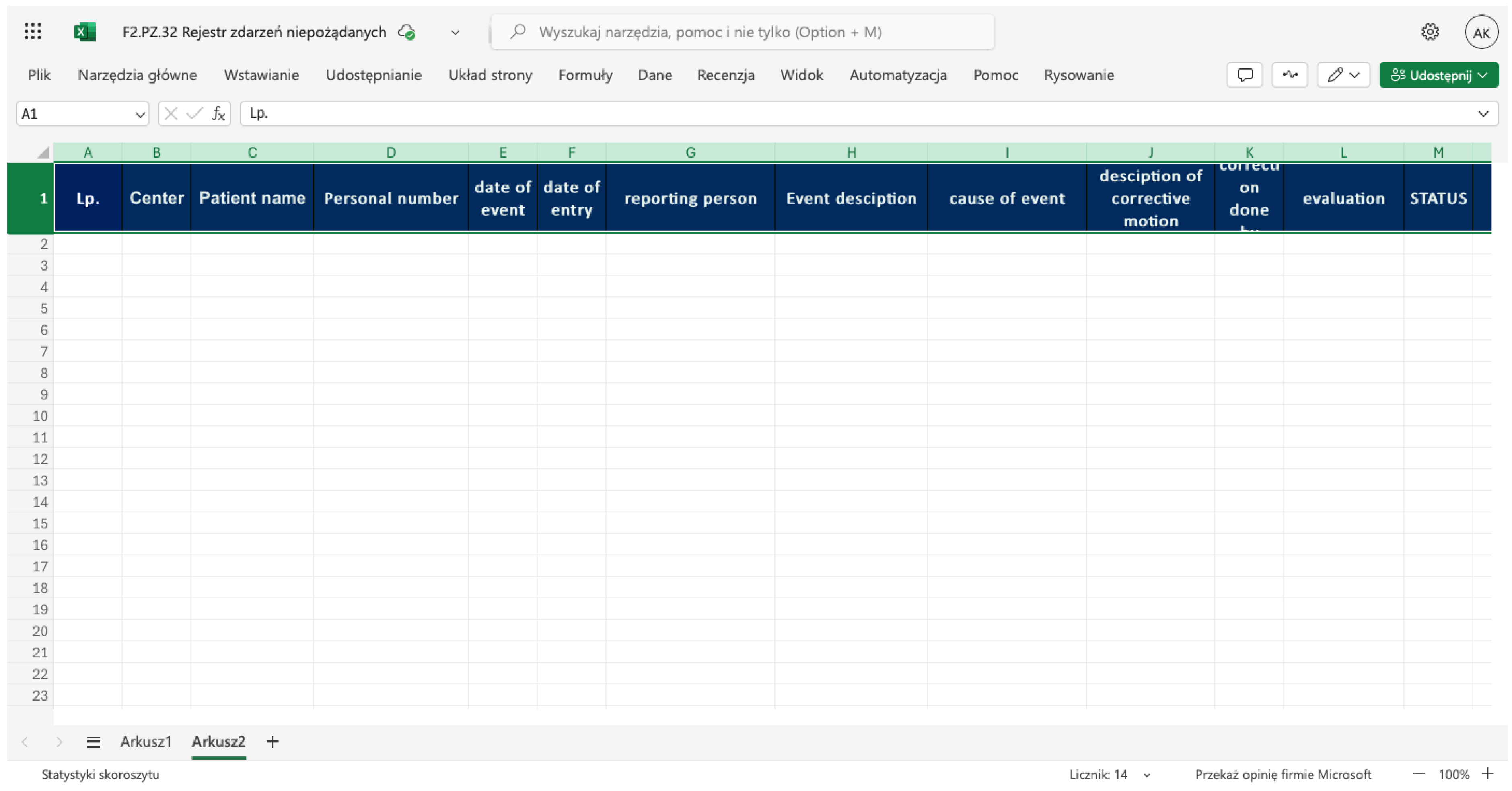
Endophthalmitis Management in Eye Surgery One-Day Clinic.
Endophthalmitis
(Figure 1) is
a rare but
severe condition
that requires rapid
and efficient action to
preserve the patient’s vision,
eye, and,
in cases of highly virulent pathogens, even
the patient’s life.
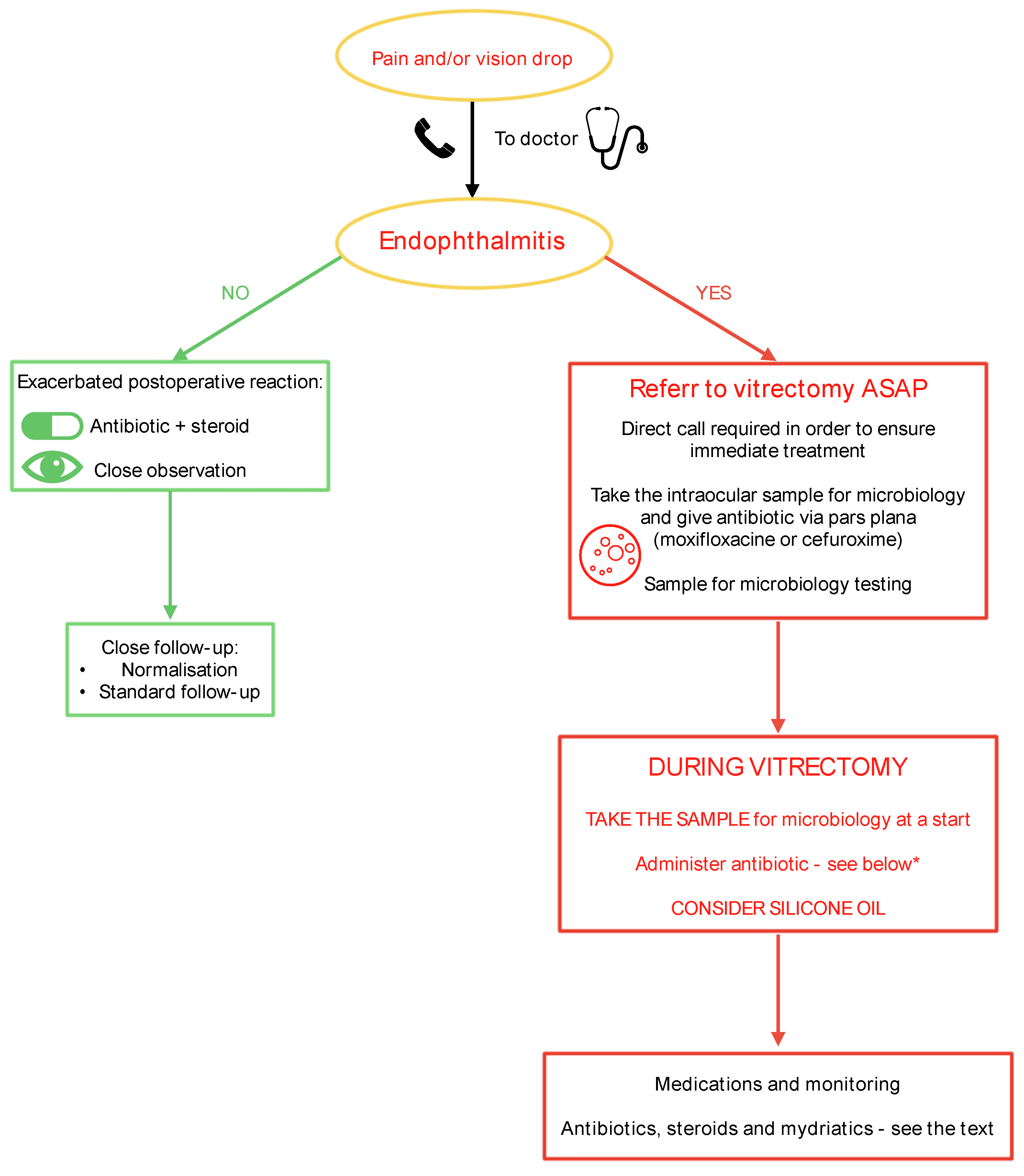
Every patient experiencing pain and/or vision deterioration after an operation or injection who contacts the registration office of the clinic should be examined as soon as possible, no later than on the same day. If the physician (CHANGE ALL IN THE MS) detects symptoms or suspects endophthalmitis, the patient should be urgently referred to a vitreoretinal (VR) surgeon. It is advisable to have direct contact with a VR surgeon affiliated with the clinic or to arrange an immediate referral to a hospital equipped with a VR surgery unit. A sample for microbiological testing should be obtained either through a pars plana tap before referring the patient to a VR surgeon or at the beginning of vitrectomy. (Althiabi et al. 2022; Dave et al. 2022; Gower et al. 2017)
If the patient must travel a significant distance to the VR surgery unit, it is advisable to take a vitreous tap before referral and administer an intravitreal antibiotic injection (e.g., moxifloxacin, levofloxacin, or cephalosporin) via pars plana. This may help control the infection until a proper vitrectomy can be performed. We recommend implementing the Complete and Early Vitrectomy for Endophthalmitis (CEVE) strategy, which has been proven to be the most effective approach for endophthalmitis management. (Kuhn 2016; Morris & Kuhn 2021).
Figure 2.
Endophthalmitis management in one-day clinic set-up.
Figure 2.
Endophthalmitis management in one-day clinic set-up.
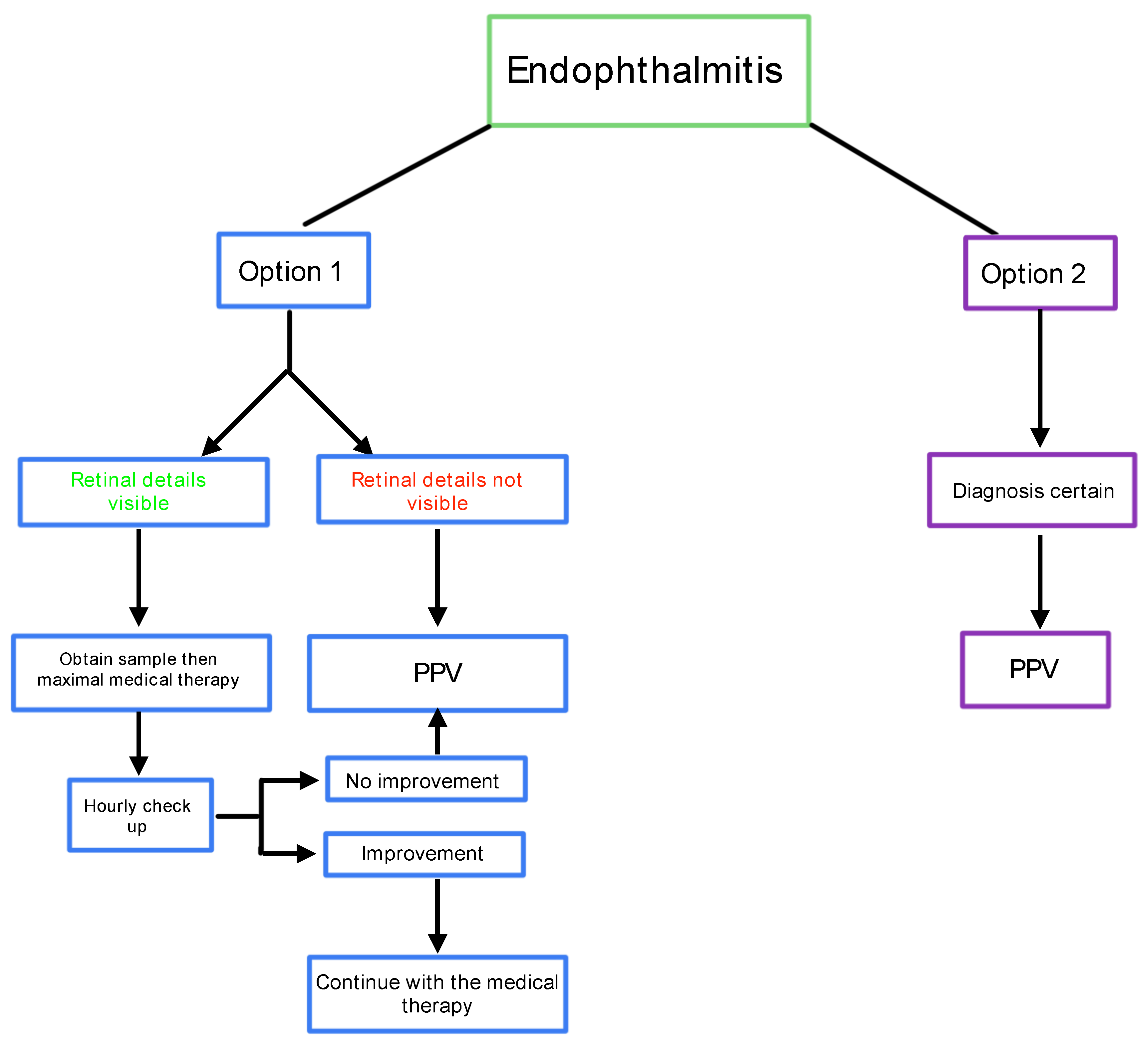
Management should follow a decision-making tree as illustrated on
Figure 3.
This algorithm focuses on the CEVE strategy and provides a straightforward guideline for eye surgeons: If there is any doubt, the condition should be treated as endophthalmitis, and vitrectomy should be performed as soon as possible. The presence of pus inside the eye requires its immediate evacuation and the use of all available treatment modalities against virulent pathogens. For this reason, pars plana vitrectomy is the default option. However, if the retina is visible, a sample may be taken via a vitreous tap, and the patient can be placed on maximum medical therapy with close follow-up. (Morris & Kuhn 2021)
Key Steps in Pars Plana Vitrectomy(Shao William B Yates I-Van Ho Andrew A Chang Matthew P Simunovic):
After trocar insertion, obtain samples from the vitreous cavity and the anterior chamber for microbiological testing.
During vitrectomy, administer a broad-spectrum antibiotic via infusion fluid (e.g., vancomycin 0.2 mg/ml in 500 ml Ringer’s solution) (Pietras-Baczewska et al. 2021; Rejdak et al. 2016)
Perform intraoperative anterior chamber lavage using a broad-spectrum antibiotic.
Remove pus, ensuring posterior vitreous detachment to clean the retinal surface as thoroughly as possible, and examine the ciliary body and vitreous base.
Address any pre-existing ocular pathologies, such as retinal detachment.
At the conclusion of the procedure, leave an appropriate antibiotic in the vitreous cavity (see
Table 1 for dosages).
Consider using a silicone oil tamponade, as silicone oil has bacteriostatic properties and may help prevent late complications such as retinal detachment or eyeball atrophy.(Sinisi et al. 2022; Weber et al. 2023)
The CEVE protocol means a vitrectomy that is performed as soon as possible once the diagnosis has been made. “Early” aims for a stage when the damage to intraocular tissues is still minimal, and a vitrectomy where safety is not compromised by either poor visibility or necrotic retina.
The CEVE protocol also means a vitrectomy where pus is not left on the posterior retina (“macular hypopyon”), which means the still-attached posterior hyaloid is surgically detached, consistent with safety.
Conversely, despite this vitrectomy being termed “complete”, it is limited anteriorly, at the vitreous base, to reduce the risk of iatrogenic retinal injury, which would threaten with postoperative retinal detachment.
Postoperatively, broad-spectrum antibiotics should be administered topically on an hourly basis. Topical steroids, such as dexamethasone (every 2-3 hours), and mydriatics (e.g., atropine or tropicamide, 2-3 times daily) should also be used. Once the antibiogram results are available, treatment should be adjusted accordingly.
Patients should remain under close observation, with daily follow-up visits if necessary.
Every
endophthalmitis case should be reported to the
Infection Control Group within
the clinic or hospital
(if applicable) and documented in
the Electronic Adverse Events Registry.
An example of such a registry is depicted in Figure 4.
2. Prevent Before It Happens
Endophthalmitis prophylaxis during ophthalmic surgical procedures, such as cataract extraction, vitreoretinal (VR) surgery, or any other surgery, should include preoperative, intraoperative, and postoperative management. Preoperatively, eyelid margin evaluation should be performed to assess for conditions such as rosacea, excessive biofilm on eyelashes or eyelid margins, and exacerbated dry eye. These conditions must be treated before surgery to maintain ocular surface balance and ensure a safer surgical outcome. Many infections arise from the patient’s own flora, introduced from the ocular surface into the eye during surgery. Staphylococcus epidermidis and even Staphylococcus aureus are part of the natural ocular surface flora (Kudasiewicz-Kardaszewska et al. 2023b). However, when introduced into the vitreous cavity, these bacteria may become virulent and cause severe infections.
While complete sterilization of the ocular surface is impossible, it is essential to restore its balance before surgery and disinfect it properly before the procedure (Grzybowski & Brona 2017).
2.1. Preoperative Measures
For patients with chronic blepharitis, standard treatment should include eyelid thermotherapy, hygiene with special wipes, and artificial tears as a minimum requirement (Kudasiewicz-Kardaszewska et al. 2023b).
Additionally, in cases of severe blepharitis, applying mupirocin ointment to the nose and/or face (e.g., Mupine, Mupirox) may be considered to reduce Staphylococcus populations on the eyelids and facial skin. This is particularly important for minimizing methicillin-resistant Staphylococcus aureus (MRSA) strains. The ointment should be applied to the nostrils 2-3 times daily for five days before surgery (Trautmann et al. 2007).
2.2. Prevention in the Operating Room (OR)
All personnel present in the operating room should wear proper OR attire, including gowns, caps, and boots specifically designated for the OR environment. Face masks should cover both the mouth and nose, particularly overthe sterile operating field. If a mask becomes moist, contaminated, or comes into contact with bodily fluids, it must be replaced immediately.
The laminar airflow system and/or air conditioning should be operational at least one hour before surgery begins and remain active throughout all procedures. Turning off the laminar flow system due to personal discomfort (e.g., feeling cold air on the neck) is discouraged. Instead, surgeons may wear an additional layer underneath their sterile gown for comfort.
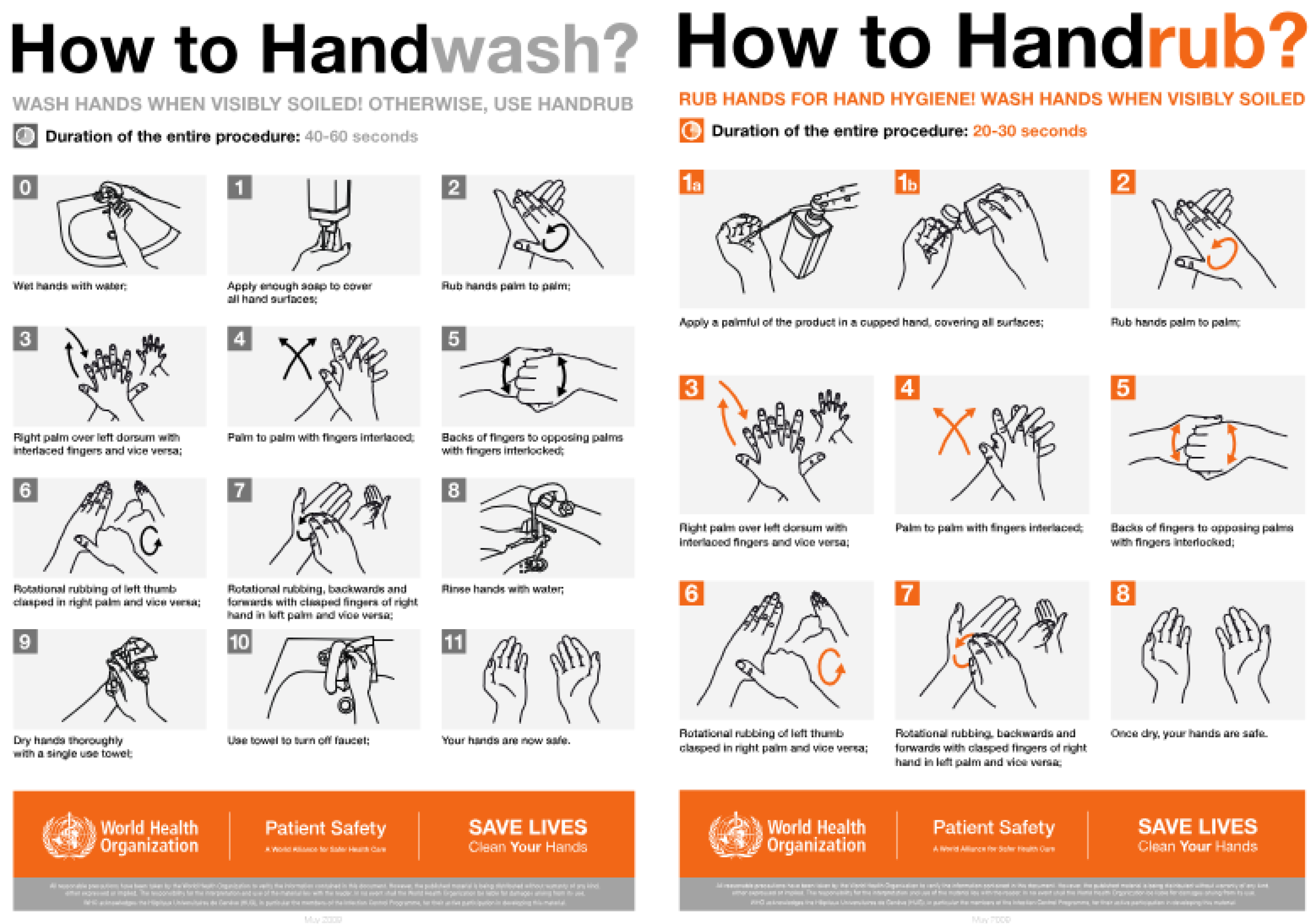
The
surgeon must carefully scrub their hands using
sterile soap and
warm water, paying special attention to nail hygiene.
Nails should be short and free of polish or debris. Special sterile spatulas can be used to clean under the nails. Hand washing should last
at least 40-60 seconds (Farhoudi et al. 2016). The
sequence of hand washing and disinfection is shown in
Figure 5.
According to WHO (2008), if antimicrobial soap and clean water are available, additional disinfection is not necessary. However, in practical settings, surgeons should perform surgical handwashing to remove any visible contaminants before entering the OR. Subsequently, a disinfectant should be applied to the hands and forearms for at least 1-3 minutes (up to 5 minutes if necessary). Disinfection targets the surgeon’s and nurses’ own flora and should be repeated between surgeries. Hand washing should also be performed after using the restroom, eating, coming into contact with blood or body fluids, or returning to the OR from another area. (Pittet 2009).
After hand scrubbing and disinfection, the surgical team (including the surgeon and scrub nurse) should wear a sterile gown and gloves. The scrub nurse should prepare a table with sterilized and disposable sterile equipment. Either the scrub nurse or the surgeon should disinfect the operative field. For eye surgery, this involves applying 7.5% or 10% povidone-iodine to the eyelids, orbital area, forehead, and cheek (twice for 30 seconds each time) and 5% povidone-iodine to the ocular surface before surgery and after speculum insertion (Grzybowski & Brona 2017). Povidone-iodine has been proven to be the most effective antiseptic for preventing postoperative endophthalmitis.(Grzybowski et al. 2022).
Povidone-iodine is a broad-spectrum antimicrobial agent that works by destroying microbial DNA and proteins(Singh et al. 2021). It is effective against bacteria, viruses, chlamydia, and fungi. Evidence-based data show that povidone-iodine reduces the bacterial population on the ocular surface (both cornea and conjunctiva) by a factor of 100.(Ciulla et al. 2002)
Figure 6.
Skin and ocular surface ready for operation: Skin disinfected with 10% povidone-iodine, ocular surface with 5% povidone-iodine, sterile drape applied, eyelids held open with a speculum, eyelashes separated from the surgical field using a surgical foil.
Figure 6.
Skin and ocular surface ready for operation: Skin disinfected with 10% povidone-iodine, ocular surface with 5% povidone-iodine, sterile drape applied, eyelids held open with a speculum, eyelashes separated from the surgical field using a surgical foil.
2.3. Controversies Regarding Preoperative Topical Antibiotics
There is ongoing debate regarding the use of topical antibiotics (e.g., levofloxacin or tobramycin) before surgery. Based on our experience, it may be beneficial, especially for patients with stage III or IV blepharitis (even in remission) or monocular patients. However, antibiotics should be instilled at least 30 minutes before surgery. Some surgeons advocate for administering antibiotic prophylaxis 3-5 days preoperatively; however, this should be based on microbiological analysis to avoid unnecessary antibiotic use and reduce the risk of bacterial resistance.
2.4. Proper Draping and Ocular Surface Preparation
After applying povidone-iodine, a sterile drape with plastic foil should be placed over the surgical field. The first application of 5% povidone-iodine drops should be instilled onto the ocular surface before draping. A second application (2 ml) should be performed after the drape and speculum are in place as a lavage over the entire ocular surface. If irrigation with normal saline or Ringer’s solution is performed, povidone-iodine should remain in contact with the ocular surface for at least three minutes to be effective(Borgia et al. 2023).
Table 2.
Preparation of 5% Povidone-Iodine Solution:.
Table 2.
Preparation of 5% Povidone-Iodine Solution:.
| Initial Povidone-Iodine Concentration |
Dilution Ratio |
Preparation Method (10 ml syringe) |
| 10% Povidone-Iodine |
1:1 |
5 ml of 10% PI + 5 ml Ringer’s solution or BSS |
| 7.5% Povidone-Iodine |
2:1 |
6 ml of 7.5% PI + 3 ml Ringer’s solution or BSS |
By following these standardized antiseptic and hygiene protocols, the risk of postoperative endophthalmitis can be significantly reduced.
If a monocular patient wears a prosthesis, it must be removed 3-5 days preoperatively and the conjunctival sac sterilized for 3-5 days using povidone-iodine 10%.
2.4.1. Intraoperative Endophthalmitis Prophylaxis
The use of intracameral antibiotics, such as cefuroxime (Aprokam, 0.1 mg/0.1 ml) or moxifloxacin (off-label in Europe but approved in India as Auromox 0.5%), is essential for reducing the risk of postoperative endophthalmitis. Studies have demonstrated that intracameral cefuroxime reduces the endophthalmitis rate from 0.59% to 0.039%, while moxifloxacin decreases the risk fourfold among cataract patients(Rękas et al. 2020). Research conducted outside Europe has also shown that intracameral moxifloxacin significantly lowers postoperative infection rates while ensuring safety.(Arshinoff & Modabber 2016; Haripriya et al. 2016; Leung et al. 2018).
Moxifloxacin is also used in the vitreous cavity at the conclusion of cataract surgery in so-called ‘dropless cataract surgery.’ Special formulations, such as Tri-Moxi (triamcinolone and moxifloxacin), have been administered via pars plana or transzonular routes to eliminate the need for postoperative antibiotic-steroid drops(Assil et al. 2021). However, Tri-Moxi-Vanc (triamcinolone, moxifloxacin, and vancomycin) has been associated with an increased incidence of hemorrhagic occlusive vasculitis, and its use is therefore discouraged. (American Society of Cataract and Refractive Surgery. Clinical alert: HORV association with intraocular vancomycin. Ascrs.org/clinical-education/clinical-reports/2016-cr-clinical-alert-horv-association-with-intraocular-vancomycin . Last accessed June 3, 2020. 2020)
The application of 5% povidone-iodine at the end of surgery, before any other drops or ointments, is another crucial antiseptic step in infection prevention(Grzybowski et al. 2022).
2.4.2. Postoperative Management
It is recommended to prescribe
a high-concentration antibiotic for a short duration. In practice, a combination antibiotic-steroid drop should be used
four to five times daily for seven days, followed by a steroid-only drop for an additional seven days. Non-steroidal anti-inflammatory drugs (NSAIDs) are advisable
twice to four times daily for 2-4 weeks postoperatively. There is no need for gradual steroid tapering. The proposed postoperative treatment regimens are detailed in
Table 3.
2.4.3. Special Considerations for Intravitreal Injections
Millions of intravitreal injections are administered worldwide daily for macular and retinal diseases. While the procedure is simple, a lack of caution can lead to devastating complications, such as isolated or clustered cases of endophthalmitis (e.g., 2-6 cases in a row). (Einan-Lifshitz et al. 2021; Pietras-Baczewska et al. 2021).
t is recommended that intravitreal injections be performed either in the OR or in a dedicated treatment room. All staff should wear appropriate attire, including OR gowns, boots, caps, and surgical masks covering the nose and mouth. Strict adherence to face mask policies is crucial, as studies have demonstrated that properly worn masks are more effective in preventing oral flora-associated infections than a ‘no talking’ policy(Patel et al. 2021). The scrub nurse should prepare medications for injections in sterile manner(Grzybowski et al. 2018).
Patients should also be encouraged to practice home eyelid hygiene using sterile ocular wipes particularly in monocular patients or those receiving multiple injections over extended periods.(Baudin et al. 2022)
2.4.4. Povidone-Iodine Use in Intravitreal Injections
The following povidone-iodine concentrations should be used before intravitreal injections (Einan-Lifshitz et al. 2021; Reibaldi et al. 2019; Safar & Dellimore 2007):
7.5-10% for skin disinfection before draping and speculum insertion (applied twice).
5% for ocular surface disinfection before skin preparation, after draping, immediately before injection, and again post-injection before removing the drape.
Povidone-iodine should be free from soap components such as glycerol to avoid disruption of the mucin layer in the tear film. Since patients often return regularly for injections, protecting the ocular surface is crucial. The use of an operating microscope or magnifying lamp for injections is recommended to improve precision and visualization(Teberik et al. 2019).
2.4.5. Managing Patients with Povidone-Iodine Sensitivity
A true allergy to povidone-iodine (i.e., a Type I hypersensitivity reaction) is rare (Hinkle et al.; Nair et al. 2023) Most adverse reactions involve mild irritation, such as itching, excessive tearing, or conjunctival redness. If a patient has a history of iodine allergy, povidone-iodine can still be used at its standard concentration but should be rinsed out with balanced salt solution (BSS) immediately afterward. The patient should remain under observation for 30-60 minutes post-procedure. If excessive itching, swelling, or redness occurs, systemic antihistamines (e.g., cetirizine or bilastine) and mild topical steroids can be prescribed for 5-7 days.
If povidone-iodine must be avoided, chlorhexidine can be used instead for skin and ocular surface disinfection.(Kanclerz & Myers 2022)
2.4.6. Antibiotics Following Intravitreal Injections: To Prescribe or Not?
Evidence-based medicine (EBM) does not support routine antibiotic prescription after anti-VEGF injections. Studies have shown that topical antibiotics do not reduce the rate of endophthalmitis but do contribute to bacterial resistance(Baudin et al. 2022; Morioka et al. 2020).
Doctors may consider antiseptic drops (e.g., containing chlorhexidine or polyhexanide) for high-risk patients, such as monocular or immunocompromised individuals, or those with severe ocular surface disease(Kanclerz & Myers 2021).
An exception to these recommendations is dexamethasone implant injection, where broad-spectrum antibiotics should be prescribed
for 5-7 days post-injection. This is due to the
larger (19 g) needle used for drug delivery, increasing the risk of bacterial contamination.However, since dexamethasone implants are administered
only every 4-6 months, rather than as frequently as anti-VEGF agents, antibiotic prophylaxis remains justified. (
https://ec.europa.eu/health/documents/community-register/2017/20171115139165/anx_139165_pl.pdf)
By implementing these comprehensive intraoperative and postoperative protocols, the incidence of endophthalmitis can be substantially reduced, improving patient safety and surgical outcomes.
3. Conclusions
The Complete and Early Vitrectomy for Endophthalmitis (CEVE) strategy is the most effective treatment for endophthalmitis, regardless of its underlying cause.
Perioperative povidone-iodine application remains the most effective measure for preventing endophthalmitis in ocular surgery. The broad-spectrum antimicrobial properties of povidone-iodine help significantly reduce bacterial load, lowering the risk of postoperative infection.
The use of antibiotics for endophthalmitis prevention should be limited to a high concentration for a short duration when administered as eye drops. However, intracameral antibiotics are strongly recommended at the conclusion of eye surgery, particularly following cataract extraction, phaco-vitrectomy, or MIGS procedures involving cataract removal.
Despite ongoing controversy, we strongly recommend against the routine use of prophylactic antibiotics following intravitreal injections, as they do not reduce the risk of endophthalmitis but contribute to bacterial resistance. Instead, proper antiseptic measures, including povidone-iodine application, remain the most reliable approach to infection prevention.
By adhering to comprehensive infection control measures, including rapid surgical intervention, optimized prophylactic regimens, and strict perioperative protocols, ophthalmic surgeons can substantially reduce the risk of endophthalmitis and improve patient outcomes.
Author Contributions
Conceptualization: Agnieszka Kudasiewicz-Kardaszewska, Małgorzata Ozimek; Methodology: Agnieszka Kudasiewicz-Kardaszewska, Małgorzata Ozimek, Karolina Bonińska; Data Curation: Małgorzata Ozimek, Aleksandra Kardaszewska; Investigation: Aleksandra Kardaszewska, Małgorzata Ozimek, Karolina Bonińska; Software and resources: Aleksandra Kardaszewska, Karolina Bonińska; Formal Analysis: Ferenc Kuhn, Sławomir Cisiecki; Validation: Ferenc Kuhn, Sławomir Cisiecki; Writing – Original Draft Preparation: Agnieszka Kudasiewicz-Kardaszewska, Aleksandra Kardaszewska; Writing – Review & Editing: Małgorzata Ozimek, Karolina Bonińska, Ferenc Kuhn, Sławomir Cisiecki; Visualization and literature gathering: Aleksandra Kardaszewska; Supervision: Sławomir Cisiecki.
Funding
This research has no external funding.
Conflicts of Interest
The Authors declare no conflict of interest.
References
- Ali FS, Jenkins TL, Boparai RS, Obeid A, Ryan ME, et al. 2021. Aqueous Chlorhexidine Compared with Povidone-Iodine as Ocular Antisepsis before Intravitreal Injection: A Randomized Clinical Trial. Ophthalmol Retina. 5(8):788–96. [CrossRef]
- Althiabi S, Aljbreen AJ, Alshutily A, Althwiny FA. 2022. Postoperative Endophthalmitis After Cataract Surgery: An Update. [CrossRef]
- American Society of Cataract and Refractive Surgery. Clinical alert: HORV association with intraocular vancomycin. Ascrs.org/clinical-education/clinical-reports/2016-cr-clinical-alert-horv-association-with-intraocular-vancomycin . Last accessed June 3, 2020. 2020.
- Arshinoff SA, Modabber M. 2016. Dose and administration of intracameral moxifloxacin for prophylaxis of postoperative endophthalmitis. J Cataract Refract Surg. 42(12):1730–41. [CrossRef]
- Assil KK, Greenwood MD, Gibson A, Vantipalli S, Metzinger JL, Goldstein MH. 2021. Dropless cataract surgery: modernizing perioperative medical therapy to improve outcomes and patient satisfaction. [CrossRef]
- Barry P, Gettinby G, Lees F, Peterson M, Revie C, et al. 2007. Prophylaxis of postoperative endophthalmitis following cataract surgery: Results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 33(6). [CrossRef]
- Baudin F, Benzenine E, Mariet AS, Ghezala I Ben, Bron AM, et al. 2022. Topical Antibiotic Prophylaxis and Intravitreal Injections: Impact on the Incidence of Acute Endophthalmitis—A Nationwide Study in France from 2009 to 2018. Pharmaceutics. 14(10). [CrossRef]
- Borgia A, Mazzuca D, Della Corte M, Gratteri N, Fossati G, et al. 2023. Prophylaxis of Ocular Infection in the Setting of Intraocular Surgery: Implications for Clinical Practice and Risk Management. [CrossRef]
- Brockhaus L, Goldblum D, Eggenschwiler L, Zimmerli S, Marzolini C. 2019. Revisiting systemic treatment of bacterial endophthalmitis: a review of intravitreal penetration of systemic antibiotics. [CrossRef]
- Ciulla TA, Starr MB, Masket S. 2002. Bacterial endophthalmitis prophylaxis for cataract surgery: An evidence-based update. Ophthalmology. 109(1). [CrossRef]
- Dave V, Singh V, Reddy J, Sharma S, Joseph J, Das T. 2022. Clinical features and microbiology of post-cataract surgery endophthalmitis with and without intracameral moxifloxacin prophylaxis: Endophthalmitis prophylaxis study report 3. Indian J Ophthalmol. 70(1):158. [CrossRef]
- Einan-Lifshitz A, Sorkin N, Smollan G, Barequet I. 2021. The effect of recurrent povidone-iodine usage on conjunctival flora in diabetic patients undergoing intravitreal injections. Eur J Ophthalmol. 31(2). [CrossRef]
- Farhoudi F, Dashti AS, Davani MH, Ghalebi N, Sajadi G, Taghizadeh R. 2016. Impact of WHO Hand Hygiene Improvement Program Implementation: A Quasi-Experimental Trial. Biomed Res Int. 2016. [CrossRef]
- Gower EW, Lindsley K, Tulenko SE, Nanji AA, Leyngold I, Mcdonnell PJ. 2017. Perioperative antibiotics for prevention of acute endophthalmitis after cataract surgery. Cochrane Database of Systematic Reviews. 2017(2). [CrossRef]
- Grzybowski A, Brona P. 2017. Povidone-iodine is still a premium antiseptic measure in ocular surgery. [CrossRef]
- Grzybowski A, Shimada H, Nakashizuka H, Koerner J. 2022. Low-concentration povidone-iodine for the prevention of intraocular infections in ophthalmic surgery. [CrossRef]
- Grzybowski A, Told R, Sacu S, Bandello F, Moisseiev E, et al. 2018. 2018 Update on Intravitreal Injections: Euretina Expert Consensus Recommendations. Ophthalmologica. 239(4):181–93. [CrossRef]
- Haripriya A, Chang DF, Namburar S, Smita A, Ravindran RD. 2016. Efficacy of Intracameral Moxifloxacin Endophthalmitis Prophylaxis at Aravind Eye Hospital. Ophthalmology. 123(2):302–8. [CrossRef]
- Hinkle JW, Wykoff CC, Lim JI, Hahn P, Kim SJ, et al. “Iodine Allergy” and the Use of Povidone Iodine for Endophthalmitis Prophylaxis. J Vitreoretin Dis. 2020(1):65–68. [CrossRef]
- https://ec.europa.eu/health/documents/community-register/2017/20171115139165/anx_139165_pl.pdf.
- Kanclerz P, Myers WG. 2022. Chlorhexidine and other alternatives for povidone-iodine in ophthalmic surgery: review of comparative studies. [CrossRef]
- Kato A, Horita N, Namkoong H, Nomura E, Masuhara N, et al. 2022. Prophylactic antibiotics for postcataract surgery endophthalmitis: a systematic review and network meta-analysis of 6.8 million eyes. Scientific Reports 2022 12:1. 12(1):1–10. [CrossRef]
- Kudasiewicz-Kardaszewska A, Grant-Kels JM, Grzybowski A. 2023a. Meibomian gland dysfunction and blepharitis: A common and still unsolved ophthalmic problem. Clin Dermatol. 41(4). [CrossRef]
- Kudasiewicz-Kardaszewska A, Grant-Kels JM, Grzybowski A. 2023b. Meibomian gland dysfunction and blepharitis: A common and still unsolved ophthalmic problem. Clin Dermatol. 41(4). [CrossRef]
- Kuhn F. 2016. Vitreoretinal Surgery: Strategies and Tactics. Cham: Springer International Publishing.
- Leung EH, Gibbons A, Stout JT, Koch DD. 2018. Intracameral moxifloxacin for endophthalmitis prophylaxis after cataract surgery: Cost-effectiveness analysis. J Cataract Refract Surg. 44(8):971–78. [CrossRef]
- Morioka M, Takamura Y, Nagai K, Yoshida S, Mori J, et al. 2020. Incidence of endophthalmitis after intravitreal injection of an anti-VEGF agent with or without topical antibiotics. Sci Rep. 10(1). [CrossRef]
- Morris RE, Kuhn F. 2021. Complete and early vitrectomy for endophthalmitis. Eur J Ophthalmol. 31(6):2794–95. [CrossRef]
- Nair S, Zhu A, Jaffry M, Choudhry H, Dastjerdi MH. 2023. Povidone-Iodine Adverse Effects and Alternatives for Ocular Procedures. https://home.liebertpub.com/jop. [CrossRef]
- Patel SN, Hsu J, Sivalingam MD, Chiang A, Kaiser RS, et al. 2021. The Impact of Physician Face Mask Use on Endophthalmitis After Intravitreal Anti–Vascular Endothelial Growth Factor Injections. Am J Ophthalmol. 222. [CrossRef]
- Pietras-Baczewska A, Jasińska E, Toro MD, Bonfiglio V, Reibaldi M, et al. 2021. Urgent Vitrectomy with Vancomycin Infusion, Silicone Oil Endotamponade, and General Antibiotic Treatment in Multiple Cases of Endophthalmitis from a Single Day of Intravitreal Injections—Case Series. Journal of Clinical Medicine 2021, Vol. 10, Page 1059. 10(5):1059. [CrossRef]
- Pittet D. 2009. WHO Guidelines on Hand Hygiene in Health Care : A Summary First Global Patient Safety Challenge Clean Care is Safer Care. World Health Organization. 30(1).
- Reibaldi M, Avitabile T, Bandello F, Longo A, Bonfiglio V, et al. 2019. The effectiveness of 0.6% povidone iodine eye drops in reducing the conjunctival bacterial load and needle contamination in patients undergoing anti-vegf intravitreal injection: A prospective, randomized study. J Clin Med. 8(7). [CrossRef]
- Rejdak R, Choragiewicz T, Kalinowska A, Koss MJ, Ksiazek P, et al. 2016. Vancomycin in infusion during vitrectomy in surgical treatment of acute postoperative and posttraumatic endophthalmitis. BMC Infect Dis. 16(1):1–9. [CrossRef]
- Rękas M, Młyńczak K, Dobrowolska I, Niewada M, Golicki D. 2020. Cefuroxime (Aprokam®) in the Prophylaxis of Postoperative Endophthalmitis After Cataract Surgery Versus Absence of Antibiotic Prophylaxis: A Cost-Effectiveness Analysis in Poland. Value Health Reg Issues. 22. [CrossRef]
- Safar A, Dellimore MC. 2007. The effect of povidone iodine flush versus drops on conjunctival colonization before intravitreal injections. Int Ophthalmol. 27(5). [CrossRef]
- Shao William B Yates I-Van Ho Andrew A Chang Matthew P Simunovic EH. Endophthalmitis: Changes in Presentation, Management and the Role of Early Vitrectomy.
- Singh S, Sawant OB, Mian SI, Kumar A. 2021. Povidone-iodine attenuates viral replication in ocular cells: Implications for ocular transmission of RNA viruses. Biomolecules. 11(5). [CrossRef]
- Sinisi F, Della Santina M, Loiudice P, Figus M, Casini G. 2022. The Role of Silicone Oil in the Surgical Management of Endophthalmitis: A Systematic Review. [CrossRef]
- Soliman MK, Gini G, Kuhn F, Iros M, Parolini B, et al. 2019. International Practice Patterns for the Management of Acute Postsurgical and Postintravitreal Injection Endophthalmitis: European Vitreo-Retinal Society Endophthalmitis Study Report 1. Ophthalmol Retina. 3(6):461–67. [CrossRef]
- Teberik K, Eski MT, Çalişkan E, Kilinçel Ö, Kaya M, Ankarali H. 2019. Effects of topical azithromycin, moxifloxacin, and povidone iodine on conjunctival bacterial flora in patients undergoing intravitreal injection. Arq Bras Oftalmol. 82(1). [CrossRef]
- Trautmann M, Stecher J, Hemmer W, Luz K, Panknin HT. 2007. Intranasal Mupirocin Prophylaxis in Elective SurgeryA Review of Published Studies. Chemotherapy. 54(1):9–16. [CrossRef]
- Weber C, Stasik I, Herrmann P, Schmitz-Valckenberg S, Holz FG, Liegl R. 2023. Early Vitrectomy with Silicone Oil Tamponade in the Management of Postoperative Endophthalmitis. J Clin Med. 12(15). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).