1. Introduction to Targeted and Advanced Drug Delivery Systems
The evolution of drug delivery systems has changed from conventional dosage forms to more accurate and effective methods of drug delivery to increase therapeutic effectiveness while reducing side effects. Targeted drug delivery is an example of these efforts because targeted drug delivery concentrates its efforts on delivering therapeutic agents only to the site of action, with the goals of improving efficacy and reducing systemic exposure. The purpose of targeting drug delivery is to achieve the highest concentration of drug in the desired target area while interacting with the healthy tissues in a minimum way to optimize pharmacokinetics and pharmacodynamics [
1].
The groundwork for drug delivery was laid by Bangham et al. regarding the ability of phospholipid bilayers, which led to the idea of vesicular systems (liposomes). This finding was important because it provided a structural basis for developing carrier-mediated delivery systems [
2]. From this, controlled drug delivery took shape as a multidisciplinary effort to keep drugs within a therapeutic window for a prolonged period. Controlled drug delivery was an effort that combined elements of pharmacology, materials science, and biomedical engineering to maintain site-specific action and to provide controlled elution of therapeutic agents [
3].
2. Liposomal Drug Delivery Systems
Liposomal drug delivery systems are tiny (nanoscale), spherical vesicles containing phospholipid bilayers able to encapsulate both hydrophobic and hydrophilic drugs. They may be broadly classified based on their structural features: unilamellar vs multilamellar; and their preparation methods can greatly affect their size and drug loading. Drug release from liposomes can happen via diffusion, erosion, or pH sensitive activation depend upon the vesicle composition and design [
4]. Liposomal drug delivery systems are known to be advantageous because of their biocompatibility, their ability to solubilize poorly water-soluble drugs, and the decreased systemic toxicity they can cause [
5].
Developments in liposome preparation aim to improve on encapsulation efficiency, stability and scalability. Certain innovations led to techniques such as using ethanol injection, reverse-phase evaporation and microfluidic mixing that allow researchers to formulate liposomes with more precision and reproducibility than previously known lipid preparation methods [
6]. New formulations of liposomes like proniosomes, dry formulations that turn into liposomes after hydration, offer use in a wider scope of lipid products and offer greater storage stability and transportation ease [
7].
When liposomal formulations are utilized, in terms of clinical translation, we see evidence it is most evident in relation the treatment of oncology and infectious disease. Approved products like Doxil have demonstrated the ability for liposomal formulations to enhance therapeutic indices to drugs through prolonged circulation, and reduced off target effects by minimizing systemic reactions [
8]. Continued refinement in liposomal design, including ligand-targeted and stimuli-responsive systems, has further expanded their role in personalized medicine and site-specific therapy [
9].
3. Nanoparticles and Lipid-Based Nanocarriers
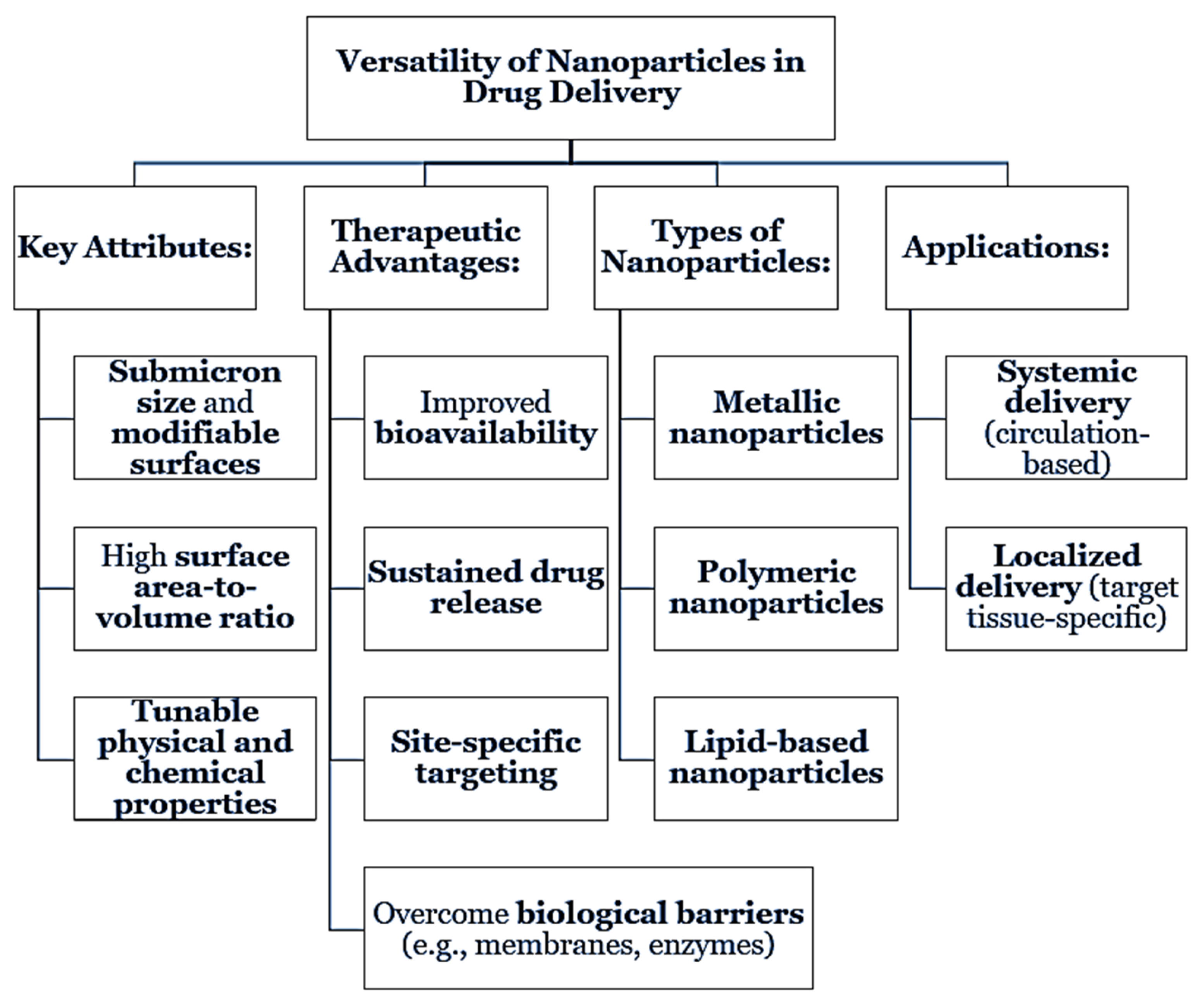
Nanoparticles are rapid and flexible delivery vehicles for drug delivery because of their submicron size and tunable surface properties. They may improve the bioavailability, allow for prolonged or sustained delivery, specific targeting or site-specific delivery, among a variety of other potential advantages. All types of nanoparticles including, metallic, polymeric, and lipid-based nanoparticles could allow to overcome biological barriers and support improved therapeutic indices [
10]. With high surface area to volume ratios, and tunability, nanoparticles are well suited for both systemic and localized delivery platforms [
11].
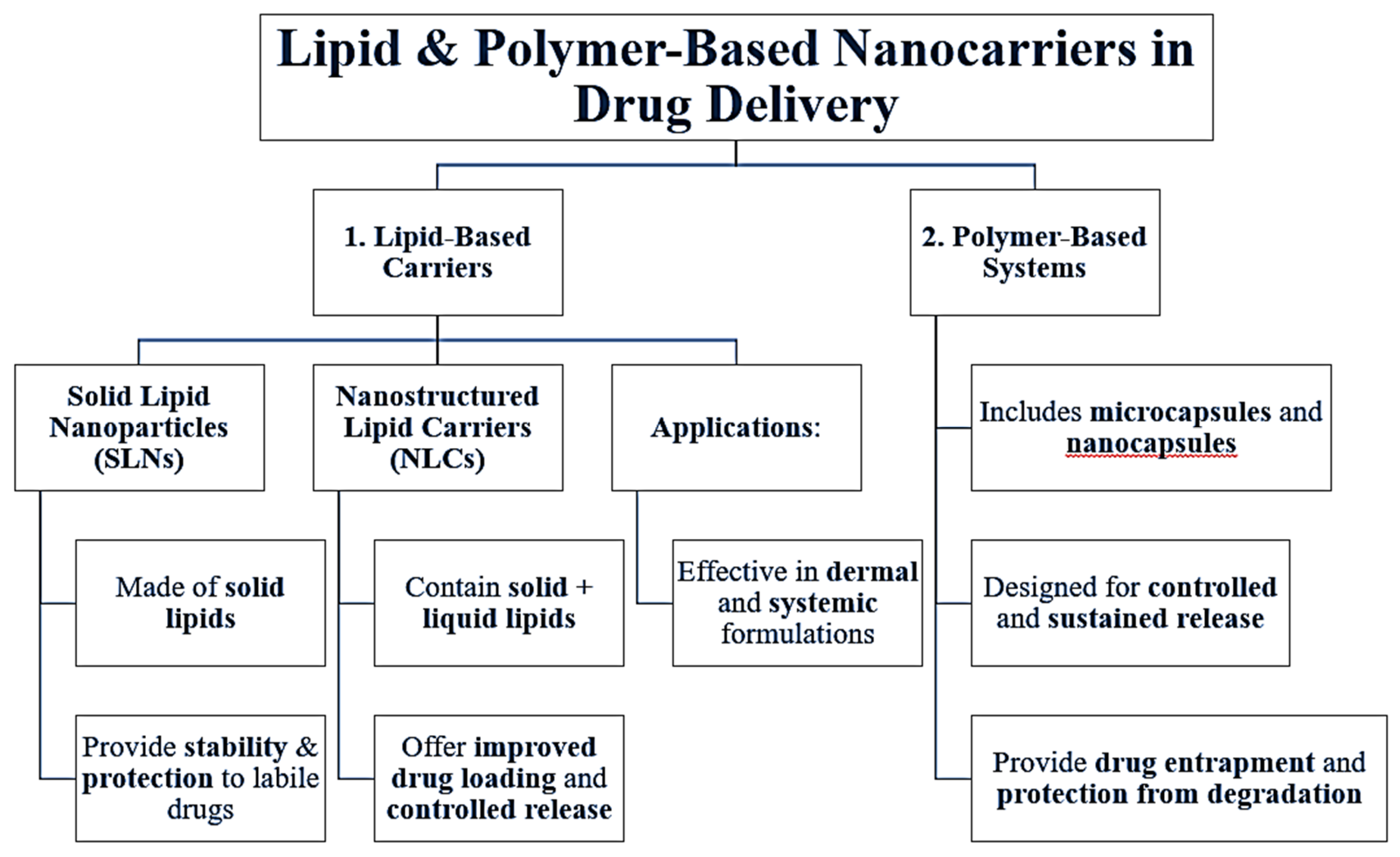
The lipid-based carriers, solid lipid nanoparticles (SLNs), and nanostructured lipid carriers (NLCs), are the most widely used. SLNs consist of solid lipids which stabilize labile drugs and protect against degradation, while NLCs are produced using solid lipids in combination with liquid lipids to allow for greater drug loading and controlled released. Various studies have demonstrated their efficacy when applied as a dermal or as a systemic formulations [
12]. Besides lipid-based carriers, polymer-based systems, including microcapsules and nanocapsules, have been tested as a way to provide controlled, sustained delivery as a suitable platform to entrap the more sensitive drug molecules and protect against degradation [
13].
These nanosystems can be applied to the cosmetic and pharmaceutical fields. For example, nanoparticle systems for nasal and pulmonary formulations may be viable alternatives for targeting respiratory diseases based on non-invasive routes of administration with fast systemic absorption—for drugs with poor oral bioavailability [
14].
Figure 1.
Versatility of Nanoparticles in Drug Delivery.
Figure 1.
Versatility of Nanoparticles in Drug Delivery.
Figure 2.
Lipid & Polymer-Based Nanocarriers in Drug Delivery.
Figure 2.
Lipid & Polymer-Based Nanocarriers in Drug Delivery.
4. Proniosomes and Microencapsulation in Drug Delivery
Proniosomes are solid, free-flowing formulations that convert into niosomes upon hydration and are built from non-ionic surfactants and cholesterol. Advantages of proniosomes that generally lead to improved therapeutic efficacy include a more stable structure, ease of storage, storage period, and better control over vesicle size and entrapment efficiency, either orally and/or topically [
15]. Proniosomes provide better protection and permeation of the drug molecule, thus contributing to improved therapeutic efficacy.
Multiparticulate drug delivery systems, which include low density microspheres specifically designed for pulsatile drug release, have been developed to respond to biological rhythms or distinct physiological triggers. Multiparticular drug delivery systems allow for the timed or site-specific release of their payload; this may be especially useful in treating an illness with circadian variation or minimizing side effects [
16].
Microencapsulation refers to enwrapping active agents within microscopic carriers from polymers. Microencapsulation may be used to achieve controlled and sustained drug release, which helps to stabilize the drug and may contribute to decreasing dosing frequency. Microencapsulation is a basic method used for prolonging the therapeutic profile of hydrophilic and hydrophobic drugs [
17].
Table 1.
Proniosomes and Microencapsulation in Drug Delivery.
Table 1.
Proniosomes and Microencapsulation in Drug Delivery.
| Subsection |
Description |
| Proniosomes |
Dry, free-flowing formulations that convert to niosomes upon hydration. Composed of non-ionic surfactants and cholesterol, they enhance stability, storage, vesicle control, and drug entrapment [15]. |
| Therapeutic Advantages of Proniosomes |
Their vesicular structure improves drug permeation and offers protection, supporting enhanced therapeutic outcomes in both oral and topical delivery applications [15]. |
| Multiparticulate Systems |
Include low-density microspheres designed for pulsatile release. They respond to biological triggers or circadian rhythms, ensuring timed or site-specific drug administration [16]. |
| Microencapsulation Technology |
Involves enclosing drugs in microscopic polymer-based carriers. Offers controlled, sustained release, improves drug stability, and decreases dosing frequency [17]. |
| Applications of Microencapsulation |
Suitable for both hydrophilic and hydrophobic drugs, this method extends the therapeutic profile and supports long-term and patient-compliant drug delivery strategies [17]. |
5. Challenges and Strategies in Oral Delivery of Proteins and Peptides
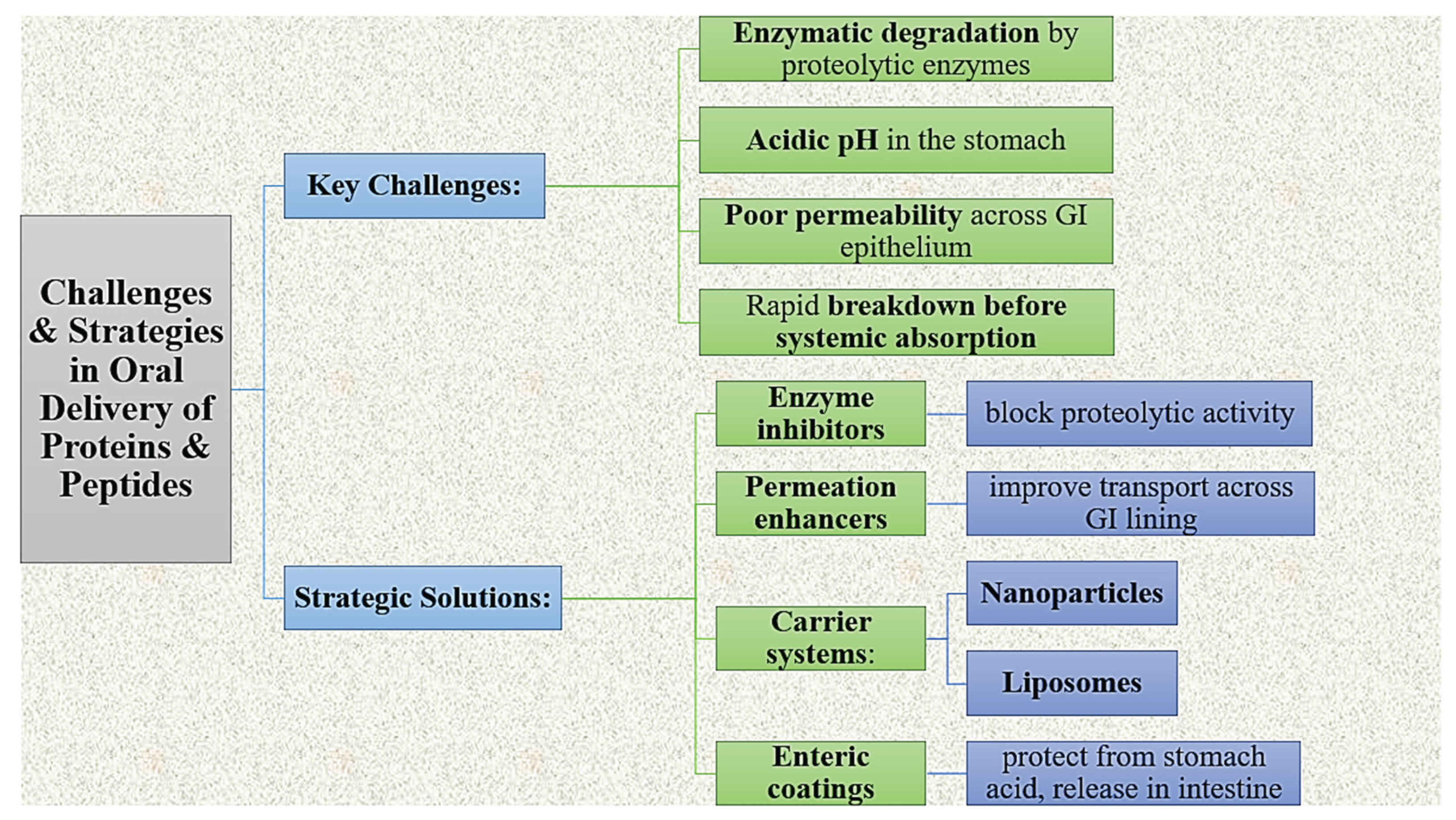
The oral administration of proteins and peptides can present difficulty because of enzymatic degradation and low permeability across the gastrointestinal (GI) epithelium. Since macromolecules are broken down by proteolytic enzymes under acidic pH conditions, they can be eliminated quickly before achieving access to systemic circulation. In addressing these factors, solutions involving enzyme inhibitors, permeation enhancers, carrier systems (e.g., lipid based delivery systems, nanoparticles, liposomes) and enteric coated preparations have been developed to increase absorption while also protecting the bioactivity of proteins and peptides [
18].
Figure 3.
Challenges & Strategies in Oral Delivery of Proteins & Peptides.
Figure 3.
Challenges & Strategies in Oral Delivery of Proteins & Peptides.
6. Fast Dissolving and Patient-Centric Oral Dosage Forms
Fast-dissolving dosage forms are purposefully designed with rapid disintegration or dissolution in the oral cavity, which can enhance compliance among patients, especially pediatric, geriatric, and dysphagic populations. Oral dissolving films, such as those formulated for propranolol hydrochloride, are an examples of a product that has quick onset, and allows drug administration without using water [
19]. Tablets formulated with superdisintegrants will rapidly break the dosage form apart and release the drug in the oral cavity, improving bioavailability, and, potentially, patient adherence [
20,
21]. Effervescent and chewable tablets can make patient-focused design a reality by offering patients a palatable dosage form that is easy and convenient to swallow. Effervescent systems, with e.g., paracetamol, will solubilize the drug and mask unpleasant tastes [
22]. Chewable tablets have a dosage form that may be chewed or broken mechanically, which is also amenable to rapid disintegration and absorption [
23]. New effervescent tablet studies began to demonstrate formulation techniques that could control stability, effervescence and taste for the best user experience [
24]. Current formulation trends focus on fast dissociation or disintegration, taste-masking, and facilitating ease of administration, and now are more frequently supported by the development of new excipients and research on drug-polymer science to innovate for modern patients [
25].
Table 2.
Fast Dissolving and Patient-Centric Oral Dosage Forms.
Table 2.
Fast Dissolving and Patient-Centric Oral Dosage Forms.
| Subsection |
Description |
| Fast-Dissolving Dosage Forms |
Designed to disintegrate quickly in the oral cavity, improving compliance in pediatric, geriatric, and dysphagic patients. Mouth-dissolving films (e.g., propranolol hydrochloride) enable rapid, water-free dosing [19]. |
| Superdisintegrant-Based Tablets |
Utilize agents that promote rapid tablet disintegration and drug release in the mouth, improving bioavailability and patient adherence [20,21]. |
| Effervescent Tablets |
Enhance drug solubilization and taste masking, offering improved palatability (e.g., effervescent paracetamol formulations) [22]. |
| Chewable Tablets |
Provide a mechanically breakable oral dosage that promotes rapid disintegration and absorption, suitable for populations with swallowing difficulties [23]. |
| Formulation Innovations |
Modern trends focus on taste masking, disintegration speed, and user-friendly formats. Recent developments explore excipients and polymer interactions to improve patient experience and compliance [24,25]. |
5. Conclusions
The changes in dosage forms used for drug delivery has fundamentally improved the efficacy of therapeutic products by addressing physiological and pharmacokinetic challenges. In this article, we reviewed a comprehensive suite of advanced and targeted drug delivery technology. These included liposomal formulations, nanoparticles, proniosomes, microencapsulation and patient-centric oral dosage forms. The original rationale for targeting drugs has been discovered over the past few decades via ongoing scientific developments. This underlying science can support the transport of drugs in a safe and accurate manner. Liposomes have significant advantages in drug transport and versatility. Nanoparticle-based systems have comparable benefits however with better control, stability and targeting.
The advances in proniosomes and microencapsulation provide clear advantages in controlling and site being administered with respect to deliver drugs while protecting labile molecules and enhancing bioavailability. For the outstanding barrier related to oral delivery of peptides and proteins on stability and absorption, there are a suite of innovative strategies currently being utilized. The advances in fast-dissolve, effervescent and chewable dosage forms can have a significant value for improving patient adherence to medications in vulnerable populations.
In sum, the potential applications of nanotechnology integrated with both established and new pharmaceutical routines promise to certainly revolutionize the future of therapeutic delivery. These research developments will approach any medication purported therapeutic efficacy through an infinite potential to improve healthcare while complementing patient-centric aspects of care while supporting the continual connection we know exists between drug formulation sciences and clinical efficacy.
References
-
Sengar, A. (2023). Targeting methods: A short review including rationale, goal, causes, strategies for targeting. International Journal of Research Publication and Reviews, 4(8), 1379–1384.
-
Bangham, A. D., Standish, M. M., & Watkins, J. C. (1965). Diffusion of univalent ions across the lamellae of swollen phospholipids. Journal of Molecular Biology, 13(1), 238–252.
-
Vyas, S. P., & Khar, R. K. (2002). Controlled drug delivery: concepts and advances. Vallabh Prakashan.
-
Akbarzadeh, A., Rezaei-Sadabady, R., Davaran, S., Joo, S. W., Zarghami, N., Hanifehpour, Y., ... & Nejati-Koshki, K. (2013). Liposome: classification, preparation, and applications. Nanoscale Research Letters, 8(1), 1–9.
-
Jagrati, K. M., & Sengar, A. (2024). Liposomal vesicular delivery system: An innovative nano carrier. World Journal of Pharmaceutical Research, 13(13), 1155–1169.
-
Has, C., & Sunthar, P. (2020). A comprehensive review on recent preparation techniques of liposomes. Journal of Liposome Research, 30(4), 336–365. [CrossRef]
-
Sengar, A., Saha, S., Sharma, L., Hemlata, Saindane, P. S., & Sagar, S. D. (2024). Fundamentals of proniosomes: Structure & composition, and core principles. World Journal of Pharmaceutical Research, 13(21), 1063–1071.
-
Torchilin, V. P. (2005). Recent advances with liposomes as pharmaceutical carriers. Nature Reviews Drug Discovery, 4(2), 145–160. [CrossRef]
-
Allen, T. M., & Cullis, P. R. (2013). Liposomal drug delivery systems: from concept to clinical applications. Advanced Drug Delivery Reviews, 65(1), 36–48. [CrossRef]
-
Prajapati, R. N., Jagrati, K., Sengar, A., & Prajapati, S. K. (2024). Nanoparticles: Pioneering the future of drug delivery and beyond. World Journal of Pharmaceutical Research, 13(13), 1243–1262.
-
Kaur, G., & Mehta, S. K. (2017). Developments of nanoparticles for drug delivery: a review. Materials Science and Engineering: C, 76, 1276–1285.
-
Müller, R. H., Radtke, M., & Wissing, S. A. (2002). Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Advanced Drug Delivery Reviews, 54, S131–S155. [CrossRef]
-
Singh, M. N., Hemant, K. S. Y., Ram, M., & Shivakumar, H. G. (2010). Microencapsulation: a promising technique for controlled drug delivery. Research in Pharmaceutical Sciences, 5(2), 65–77.
-
Sengar, A., Jagrati, K., & Khatri, S. (2024). Enhancing therapeutics: A comprehensive review on naso-pulmonary drug delivery systems for respiratory health management. World Journal of Pharmaceutical Research, 13(13), 1112–1140.
-
Sengar, A., Saha, S., Sharma, L., Hemlata, Saindane, P. S., & Sagar, S. D. (2024). Fundamentals of proniosomes: Structure & composition, and core principles. World Journal of Pharmaceutical Research, 13(21), 1063–1071.
-
Sharma, S., & Pawar, A. (2006). Low-density multiparticulate system for pulsatile release of meloxicam. International Journal of Pharmaceutics, 313(1-2), 150–158. [CrossRef]
-
Singh, M. N., Hemant, K. S. Y., Ram, M., & Shivakumar, H. G. (2010). Microencapsulation: a promising technique for controlled drug delivery. Research in Pharmaceutical Sciences, 5(2), 65–77.
-
Zhu, Q., Chen, Z., Paul, P. K., & Wu, W. (2021). Oral delivery of proteins and peptides: challenges and strategies. Acta Pharmaceutica Sinica B, 11(8), 2396–2412.
-
Sengar, A., Yadav, S., & Niranjan, S. K. (2024). Formulation and evaluation of mouth-dissolving films of propranolol hydrochloride. World Journal of Pharmaceutical Research, 13(16), 850–861.
-
Patel, R. P., & Patel, M. M. (2007). Formulation and evaluation of mouth dissolving tablets of cinnarizine. Indian Journal of Pharmaceutical Sciences, 69(4), 568–570.
-
Kamboj, S., Saroha, K., & Goel, M. (2013). Formulation and evaluation of mouth dissolving tablets of amlodipine besylate using different superdisintegrants. International Journal of Pharmaceutical Sciences and Research, 4(2), 649–657.
-
Kumar, R., & Singh, S. (2012). Formulation and evaluation of effervescent tablets of paracetamol. International Journal of Pharmaceutical Sciences and Research, 3(1), 218–223.
-
Sengar, A., Vashisth, H., Chatekar, V. K., Gupta, B., Thange, A. R., & Jillella, M. S. R. S. N. (2024). From concept to consumption: A comprehensive review of chewable tablets. World Journal of Pharmaceutical Research, 13(16), 176–189.
-
Sengar, A., Tile, S. A., Sen, A., Malunjkar, S. P., Bhagat, D. T., & Thete, A. K. (2024). Effervescent tablets explored: Dosage form benefits, formulation strategies, and methodological insights. World Journal of Pharmaceutical Research, 13(18), 1424–1435.
-
Gupta, A., & Mishra, A. K. (2011). Recent trends of fast dissolving tablets: an overview of formulation technology. International Journal of Pharmaceutical & Biological Archives, 2(1), 1–10.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).