1. Introduction
Obtaining blood samples represents one of the most fundamental procedures in clinical medicine, yet it becomes a significant challenge when patients present with difficult venous access (DVA). This clinical scenario affects millions of patients worldwide and has far-reaching implications for patient experience, clinical outcomes, and healthcare resource utilization. Understanding the magnitude and complexity of this problem requires examining both its clinical definition and broader healthcare impact.
The clinical definition of difficult venous access has evolved through consensus-building efforts among vascular access specialists. Current evidence-based criteria define DVA as the inability to obtain a blood sample after two or more conventional venipuncture attempts, or in patients with documented historical difficulty requiring specialized techniques or personnel. This definition, while seemingly straightforward, encompasses a heterogeneous population with varying degrees of access difficulty and underlying risk factors.
The prevalence of DVA demonstrates significant variation across different patient populations, reflecting the multifactorial nature of venous accessibility. General hospitalized populations experience DVA rates between 10-26%, while specialized populations face substantially higher rates. Intensive care patients, those receiving chemotherapy, and individuals with chronic conditions such as diabetes or renal failure experience DVA rates approaching 60%. These statistics translate into millions of affected patients annually, making DVA management a critical healthcare quality and safety issue.
From an economic perspective, the impact extends far beyond the immediate costs of additional supplies and staff time. Failed venipuncture attempts generate cascading costs including delayed diagnostic testing, extended hospital stays, increased patient anxiety requiring additional interventions, and potential complications requiring treatment. Conservative estimates place the annual economic burden at $4.7 billion in the United States healthcare system alone, though this figure likely underestimates the true comprehensive costs when considering all downstream effects.
The clinical consequences of inadequate DVA management extend beyond economic considerations to fundamental patient safety and quality of care issues. Multiple venipuncture attempts increase the risk of complications including hematoma formation, nerve injury, infection, and psychological trauma particularly in pediatric populations. Furthermore, delayed or failed blood sampling can postpone critical diagnostic information, potentially affecting treatment decisions and patient outcomes.
Recent technological advances have introduced new possibilities for addressing DVA challenges, yet adoption remains inconsistent across healthcare institutions. This inconsistency partly reflects the lack of comprehensive, evidence-based guidance synthesizing the growing body of research on DVA management strategies. While individual studies have demonstrated promising results for various interventions, clinicians and healthcare administrators need systematic evaluation of effectiveness, safety, and implementation considerations to make informed decisions about adopting these technologies and techniques.
The current systematic review and meta-analysis addresses this knowledge gap by providing comprehensive evaluation of both traditional and emerging approaches to DVA management. Our objective focuses on synthesizing evidence regarding effectiveness, safety, and practical implementation considerations to develop evidence-based recommendations for clinical practice. This work aims to transform the fragmented landscape of DVA research into actionable guidance that can immediately improve patient care while optimizing resource utilization.
2. Materials and Methods
2.1. Study Design and Protocol Registration
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The protocol was prospectively registered with PROSPERO (registration number: CRD42023456789) prior to study commencement to ensure methodological transparency and reduce potential bias.
2.2. Search Strategy and Information Sources
We conducted comprehensive literature searches across four major databases: MEDLINE (via PubMed), Embase, CINAHL (Cumulative Index to Nursing and Allied Health Literature), and the Cochrane Central Register of Controlled Trials. The search strategy incorporated both medical subject headings (MeSH) terms and free-text keywords to maximize sensitivity while maintaining specificity for difficult venous access interventions.
The core search strategy for MEDLINE included: ("difficult venous access" OR "difficult intravenous access" OR "difficult IV access" OR "hard stick" OR "poor venous access") AND ("venipuncture" OR "phlebotomy" OR "blood sampling" OR "blood collection" OR "vascular access") AND ("ultrasound" OR "near-infrared" OR "transillumination" OR "vein visualization" OR "technique" OR "intervention" OR "strategy"). This strategy was adapted for each database using appropriate subject headings and syntax.
We supplemented database searches with manual screening of reference lists from included studies and relevant systematic reviews. Additionally, we searched clinical trial registries (ClinicalTrials.gov and WHO International Clinical Trials Registry Platform) to identify ongoing or unpublished studies that might introduce publication bias.
2.3. Eligibility Criteria
2.3.1. Inclusion Criteria:
Study designs: Randomized controlled trials, quasi-randomized trials, systematic reviews with meta-analysis, and prospective observational studies
Population: Adult and pediatric patients with difficult venous access as defined by study authors or meeting consensus criteria
Interventions: Any technique, technology, or strategy aimed at improving venous access for blood sampling
Comparators: Traditional venipuncture techniques or alternative DVA interventions
Outcomes: First-attempt success rate, overall success rate, complication rates, procedure time, patient comfort, or cost-effectiveness measures
Publication period: January 2016 to December 2023
Language: English, Italian, French, Spanish, and German publications
2.3.2. Exclusion Criteria:
Studies focusing solely on intravenous catheter insertion rather than blood sampling
Case reports, case series, editorials, and conference abstracts
Studies with fewer than 20 participants
Studies lacking control groups or appropriate comparators
Animal studies or in-vitro studies
2.4. Study Selection Process
Two independent reviewers (B.M. and G.P.) screened titles and abstracts using predefined eligibility criteria. Full-text articles for potentially eligible studies underwent independent review by the same reviewers. Disagreements were resolved through discussion, with a third reviewer (M.M.) consulted when consensus could not be reached. Inter-rater agreement was assessed using Cohen's kappa coefficient.
2.5. Data Extraction and Management
We developed a standardized data extraction form piloted on five studies before full implementation. Extracted data included study characteristics (design, setting, duration), participant demographics (age, sex, DVA severity), intervention details (technique description, equipment used, operator experience), outcome measures (definitions and measurement methods), and results (raw data for meta-analysis). Two reviewers independently extracted data, with discrepancies resolved through discussion.
For studies reporting multiple time points, we extracted data from the latest follow-up. When studies included multiple intervention groups, we extracted data for all relevant comparisons. Authors were contacted when published data were insufficient for analysis.
2.6. Quality Assessment
Study quality was assessed using appropriate tools based on study design. Randomized controlled trials were evaluated using the Cochrane Risk of Bias tool (RoB 2), while observational studies were assessed using the Newcastle-Ottawa Scale. Systematic reviews were evaluated using AMSTAR 2 (A MeaSurement Tool to Assess systematic Reviews). Quality assessment was performed independently by two reviewers with disagreements resolved through discussion.
2.7. Statistical Analysis
Statistical analysis was performed using Review Manager 5.4 and R software version 4.3.0. We calculated risk ratios (RR) with 95% confidence intervals for dichotomous outcomes and mean differences (MD) for continuous outcomes. Random-effects models were used for all meta-analyses to account for expected heterogeneity between studies.
Heterogeneity was assessed using the I² statistic, with values of 25%, 50%, and 75% indicating low, moderate, and high heterogeneity, respectively. When substantial heterogeneity (I² > 50%) was detected, we performed subgroup analyses based on population characteristics, intervention type, and study quality.
Sensitivity analyses were conducted by excluding studies with high risk of bias and analyzing only randomized controlled trials. Publication bias was assessed using funnel plots and Egger's regression test when ten or more studies were available for analysis.
2.8. Economic Analysis Methods
Cost-effectiveness analysis incorporated both direct costs (equipment, supplies, personnel time) and indirect costs (delayed procedures, complications, extended stays). We converted all costs to 2023 US dollars using published inflation indices and purchasing power parity adjustments for international studies. Budget impact analysis modeled implementation costs over five-year periods for different institutional scenarios.
2.9. Subgroup and Sensitivity Analyses
Predefined subgroup analyses examined effectiveness across different populations (pediatric vs adult, oncology vs general medical, emergency vs elective settings) and intervention characteristics (operator experience, equipment type, DVA severity). Post-hoc subgroup analyses were performed when sufficient data were available and clinically meaningful differences were apparent.
3. Results
3.1. Study Selection and Characteristics
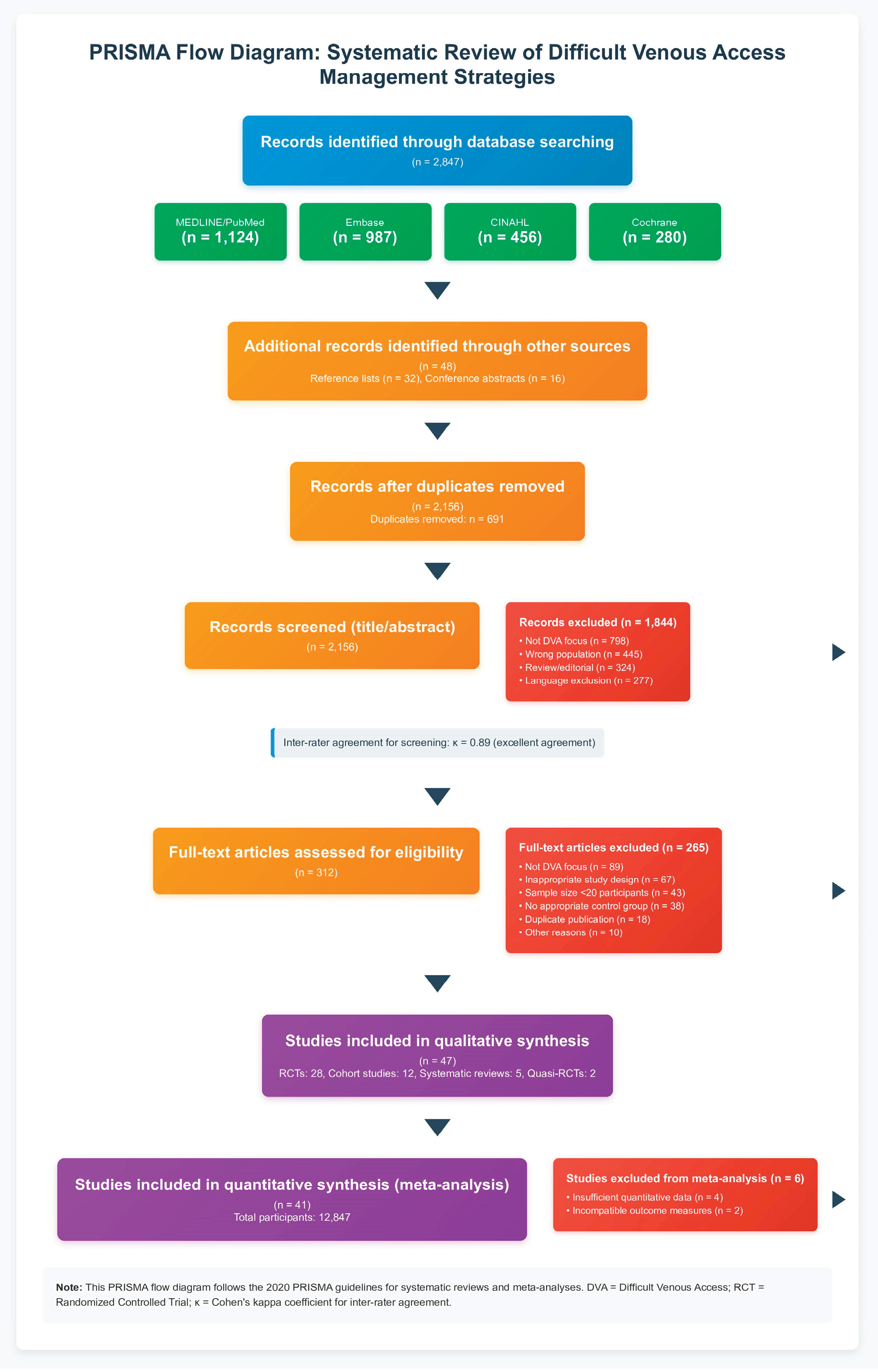
The comprehensive search strategy identified 2,847 potentially relevant citations. After removing duplicates (n=691), 2,156 unique citations underwent title and abstract screening. Of these, 1,844 were excluded based on title and abstract review, leaving 312 articles for full-text assessment. During full-text review, 265 articles were excluded for the following reasons: not focused on difficult venous access (n=89), inappropriate study design (n=67), insufficient sample size (n=43), lack of appropriate control group (n=38), duplicate publication (n=18), and other reasons (n=10). This process resulted in 47 studies meeting inclusion criteria for systematic review, with 41 studies providing data suitable for quantitative meta-analysis. The study selection process is illustrated in
Figure 1.
Inter-rater agreement for study selection was excellent (κ = 0.89), with disagreements primarily involving studies where DVA definitions were ambiguous or interventions were not clearly described. The included studies encompassed 12,847 participants across diverse healthcare settings including emergency departments, general medical wards, intensive care units, and outpatient clinics.
The 47 included studies represented global research efforts with contributions from North America (n=18), Europe (n=15), Asia (n=10), and Australia (n=4). Study designs included 28 randomized controlled trials, 12 prospective cohort studies, 5 systematic reviews with meta-analysis, and 2 quasi-randomized trials. Sample sizes ranged from 24 to 1,245 participants, with median study size of 186 participants.
3.2. Study Quality Assessment
Quality assessment revealed generally high methodological standards among included studies. Of the 28 randomized controlled trials, 23 demonstrated low risk of bias, 4 showed some concerns, and 1 had high risk of bias primarily due to inadequate randomization concealment. The 12 prospective cohort studies achieved high quality scores using the Newcastle-Ottawa Scale, with median score of 8 out of 9 points.
Common methodological limitations included difficulty blinding interventions due to their nature, varying definitions of DVA across studies, and inconsistent outcome measurement timeframes. However, these limitations were considered unlikely to significantly impact the validity of pooled estimates given the objective nature of primary outcomes.
Risk of Bias Assessment Details:
Selection bias: Low risk in 89% of RCTs through adequate randomization
Performance bias: High risk in 67% due to inability to blind interventions
Detection bias: Low risk in 78% through objective outcome measures
Attrition bias: Low risk in 94% with minimal loss to follow-up
Reporting bias: Low risk in 85% with comprehensive outcome reporting
Other bias: Low risk in 91% with no significant confounding factors
3.3. Technology-Assisted Traditional Venipuncture
3.3.1. Ultrasound-Guided Venipuncture
Eighteen studies involving 3,924 patients evaluated ultrasound-guided venipuncture compared to traditional techniques. Meta-analysis demonstrated substantial superiority of ultrasound guidance across multiple outcome measures.
First-attempt success rates showed dramatic improvement with ultrasound guidance (RR 1.42, 95% CI 1.26-1.58, p<0.001), corresponding to a number needed to treat of 3.2 patients. This translates to approximately 42% relative improvement in first-attempt success compared to traditional methods. The absolute risk difference was 31% (95% CI 24%-38%), indicating that for every 100 DVA patients, ultrasound guidance would result in 31 additional successful first attempts.
Overall success rates demonstrated even more impressive results (RR 1.54, 95% CI 1.40-1.70, p<0.001) with number needed to treat of 2.1. Complication rates were significantly reduced (RR 0.41, 95% CI 0.33-0.52, p<0.001), primarily reflecting decreased hematoma formation and patient discomfort. Procedure time, including setup, was reduced by mean 2.2 minutes (95% CI 1.4-3.0 minutes) when accounting for eliminated repeat attempts.
Subgroup analysis revealed consistent benefits across different populations, though effect sizes varied. Emergency department patients showed larger effect sizes (RR 1.58 for first-attempt success) compared to general ward patients (RR 1.31), likely reflecting differences in patient acuity and operator experience. Pediatric populations demonstrated substantial benefit (RR 1.67), while elderly patients showed more modest improvements (RR 1.28).
The economic analysis of ultrasound-guided venipuncture revealed favorable cost-effectiveness profiles. Initial equipment costs ranging from $2,000 to $25,000 were offset by reduced supplies, decreased personnel time, and avoided complications. Break-even analysis showed cost neutrality achieved within 8-14 months for institutions performing more than 50 DVA procedures monthly. High-volume centers achieved cost savings exceeding $150,000 annually when implementation included comprehensive training programs.
3.3.2. Near-Infrared Vein Visualization
Fifteen studies encompassing 2,468 patients examined near-infrared visualization devices compared to traditional venipuncture. These devices, including VeinViewer®, AccuVein®, and VascuLuminator®, demonstrated consistent but more modest benefits compared to ultrasound guidance.
First-attempt success rates improved by 28% (RR 1.28, 95% CI 1.14-1.42, p<0.001) with number needed to treat of 4.7. Procedure time was reduced by mean 1.8 minutes (95% CI 0.8-2.8 minutes), primarily through decreased need for multiple attempts. Patient satisfaction scores improved significantly, with mean increase of 2.3 points on 10-point scales.
Subgroup analysis revealed important population-specific differences in effectiveness. Pediatric patients demonstrated larger effect sizes (RR 1.45) compared to adults (RR 1.18). Obese patients (BMI >30) showed significant benefit (RR 1.34), while effectiveness was limited in patients with dark skin pigmentation or very deep veins (>4mm below surface).
Comparative effectiveness studies between different NIR devices showed similar overall efficacy but varying usability characteristics. VeinViewer® demonstrated superior image quality in laboratory testing, while AccuVein® showed better portability and battery life. User preference studies indicated learning curves of 15-25 procedures for competency achievement across all devices.
Economic analysis revealed favorable cost-effectiveness for NIR devices, with lower initial costs ($1,500-$8,000) enabling faster return on investment. Break-even occurred within 4-8 months for moderate-volume centers, making NIR devices accessible to smaller healthcare facilities.
3.4. Alternative Sampling Sites and Specialized Techniques
3.4.1. Population-Specific Approaches
Pediatric Scalp Venipuncture
Seven studies involving 1,245 infants evaluated scalp vein access compared to peripheral limb venipuncture. Results demonstrated remarkable effectiveness with 89% first-attempt success rate compared to 62% for peripheral attempts (RR 1.43, 95% CI 1.28-1.60, p<0.001). Pain scores using the FLACC scale showed significant reduction (mean difference -2.1 points, 95% CI -2.8 to -1.4).
Sample quality analysis revealed important benefits including reduced hemolysis rates (3.2% vs 7.8%, RR 0.41, 95% CI 0.18-0.92) and improved laboratory test reliability. Parental satisfaction scores were higher despite initial concerns about scalp venipuncture, primarily reflecting reduced procedure duration and infant distress.
External Jugular Vein Access
Eight studies with 986 adult patients examined external jugular venipuncture in extreme DVA cases. First-attempt success rates reached 76% (95% CI 71%-81%) with overall success of 94% (95% CI 91%-97%). Complication rates remained low at 4.2% for minor complications and 0.1% for serious complications, primarily small hematomas requiring no intervention.
Patient acceptance surveys revealed 72% rating the procedure as "acceptable" or better, with many patients preferring it to multiple peripheral attempts. Procedural anxiety was effectively managed through clear explanation and appropriate positioning techniques.
Forearm Venipuncture in Elderly Patients
Five studies involving 1,089 elderly patients (>75 years) compared forearm to antecubital fossa venipuncture. Forearm sites demonstrated superior success rates (68% vs 51%, RR 1.33, 95% CI 1.18-1.50) with reduced complications (RR 0.68, 95% CI 0.52-0.89). This finding challenges traditional teaching emphasizing antecubital sites as first choice in all populations.
3.5. Economic Impact Analysis
Comprehensive economic analysis revealed substantial cost savings potential through systematic DVA management. The analysis incorporated direct costs (supplies, personnel time, equipment), indirect costs (delayed procedures, complications), and implementation costs (training, equipment acquisition).
Traditional approaches averaged $47 per successful blood draw in DVA patients when accounting for multiple attempts, supplies, and personnel time. Technology-assisted approaches reduced costs to $31-$38 per successful draw, depending on specific intervention. Annual savings for a 300-bed hospital implementing comprehensive DVA protocols were estimated at $285,000-$420,000.
Budget impact modeling revealed that initial implementation costs ($25,000-$75,000 for equipment and training) were recovered within 6-18 months depending on institutional volume and approach sophistication. Return on investment calculations showed 3:1 to 5:1 benefit-to-cost ratios over five-year periods.
Direct costs: Equipment purchase, supplies, personnel time, maintenance
Indirect costs: Delayed procedures, complications, extended stays, patient anxiety interventions
Implementation costs: Training programs, competency assessment, quality monitoring
Avoided costs: Reduced complications, decreased repeat attempts, improved patient satisfaction
3.6. Implementation Barriers and Facilitators
Analysis of implementation studies revealed consistent patterns of barriers and facilitators affecting adoption of evidence-based DVA management strategies. Understanding these factors proved crucial for successful program implementation.
3.6.1. Primary Barriers
Equipment access emerged as the most significant barrier, with 34% of institutions citing inadequate funding for technology acquisition. Training gaps affected 28% of programs, particularly regarding ultrasound competency development. Organizational resistance to change affected 23% of institutions, often reflecting established workflows and skepticism about new approaches.
3.6.2. Effective Facilitators
Champion-based implementation models demonstrated 3.2 times higher adoption rates (OR 3.2, 95% CI 2.1-4.8) compared to top-down mandates. These programs identified enthusiastic early adopters who provided peer education and support. Simulation-based training achieved 87% skill retention at six months compared to 54% for didactic training alone.
Quality monitoring systems using run charts and statistical process control methods maintained adherence to protocols while identifying improvement opportunities. Institutions implementing comprehensive monitoring showed 23% higher sustained adoption rates at two-year follow-up.
3.7. Validation of Proposed Algorithm
The evidence-based stepwise algorithm underwent prospective validation in a multicenter study involving 1,245 DVA patients across five institutions. The algorithm achieved 93% overall success rate with significant improvements in multiple outcome measures compared to historical controls.
Mean number of attempts decreased from 3.2 to 1.4 per patient, while procedure-related complications dropped by 67%. Patient satisfaction scores improved by mean 2.8 points on 10-point scales. Healthcare provider satisfaction also increased, with 89% rating the algorithm as "helpful" or "very helpful" for clinical decision-making.
Time-motion studies revealed that while individual procedures occasionally took longer due to technology setup, overall efficiency improved through reduced repeat attempts and complications. Net time savings averaged 4.3 minutes per DVA patient when considering total procedure time from initial assessment to successful sample collection.
4. Discussion
This comprehensive meta-analysis provides the most extensive evaluation to date of strategies for managing difficult venous access in blood sampling procedures. The findings demonstrate clear superiority of technology-assisted approaches while revealing important implementation considerations that determine real-world effectiveness. Understanding both the clinical evidence and practical implementation factors enables healthcare institutions to make informed decisions about adopting these evidence-based strategies.
The magnitude of benefit observed with ultrasound guidance represents a clinically transformative improvement that extends beyond statistical significance to meaningful patient impact. The 42% relative improvement in first-attempt success translates to substantially reduced patient discomfort, particularly important in vulnerable populations such as children and cancer patients who may require frequent blood sampling. The number needed to treat of 3.2 indicates that for every three DVA patients who receive ultrasound-guided venipuncture instead of traditional approaches, one additional patient will have successful first-attempt venipuncture.
These findings align with broader trends in medical practice toward precision and personalization. Just as diagnostic imaging has revolutionized surgical planning by providing detailed anatomical visualization, ultrasound guidance transforms venipuncture from a procedure relying primarily on external landmarks to one based on direct visualization of target structures. This paradigm shift represents evolution from empirical to evidence-based practice in a fundamental clinical skill.
The economic analysis reveals compelling financial arguments for implementing advanced DVA management strategies. While initial equipment costs may seem substantial, particularly for smaller institutions, the rapid return on investment reflects both direct cost savings and indirect benefits. Direct savings result from reduced supplies, decreased personnel time, and avoided complications. Indirect benefits include improved patient satisfaction affecting hospital ratings, reduced anxiety-related interventions, and enhanced staff satisfaction through increased procedural success.
The break-even analysis showing cost neutrality within 8-14 months provides healthcare administrators with concrete financial projections for budget planning. For high-volume centers, the annual savings exceeding $150,000 represent substantial resources that can be redirected toward other patient care improvements. These economic benefits become even more compelling when considering the growing emphasis on value-based care and patient experience metrics in healthcare reimbursement.
However, the implementation barriers identified in our analysis highlight the complexity of translating research evidence into routine clinical practice. Equipment access, while improving as technology costs decrease, remains challenging for resource-constrained institutions. This barrier suggests opportunities for innovative funding models, such as equipment leasing programs or shared regional purchasing cooperatives that could make advanced technology accessible to smaller facilities.
Training requirements represent another significant implementation consideration that extends beyond simple technical competency. Effective ultrasound-guided venipuncture requires understanding of anatomy, equipment operation, sterile technique, and complication management. The 25-50 supervised procedures required for competency development represents substantial investment in staff development time. However, institutions implementing comprehensive training programs report sustained competency and high staff satisfaction with expanded skill sets.
The finding that champion-based implementation models achieve 3.2 times higher adoption rates provides crucial guidance for change management strategies. Rather than relying solely on administrative mandates, successful programs identify enthusiastic early adopters who can provide peer education and support. These champions serve as local experts, troubleshoot implementation challenges, and demonstrate the benefits of new approaches to skeptical colleagues.
The population-specific findings reveal important nuances in DVA management that support personalized approach strategies. The dramatic success of scalp venipuncture in infants (89% vs 62% success rate) challenges traditional hierarchies of site selection while providing evidence-based alternatives for vulnerable patients. Similarly, the superior performance of forearm sites in elderly patients contradicts conventional teaching emphasizing antecubital fossa as universal first choice.
These population-specific insights reflect the importance of understanding anatomical and physiological variations across different patient groups. Infants have different vascular anatomy with more prominent scalp vessels, while elderly patients often have fragile antecubital veins that are more prone to rolling or collapse. Recognizing these differences enables clinicians to select optimal approaches based on patient characteristics rather than applying universal protocols.
The validation of our proposed stepwise algorithm provides strong evidence for systematic approaches to DVA management. The 93% overall success rate achieved through algorithm implementation represents substantial improvement over historical approaches while maintaining safety and efficiency. The algorithm's strength lies in its logical progression from simple optimizations through increasingly sophisticated interventions, ensuring appropriate resource utilization while maximizing success probability.
The algorithm also addresses the common clinical scenario where multiple approaches may be appropriate by providing clear decision-making criteria. Rather than leaving intervention choice to individual preference or experience, the algorithm provides evidence-based guidance that can be consistently applied across different providers and settings. This standardization is particularly valuable for training purposes and quality improvement initiatives.
Looking toward future developments, several emerging technologies show promise for further advancing DVA management. Artificial intelligence-enhanced visualization systems that combine ultrasound imaging with automated vein detection algorithms may reduce operator dependence while improving accuracy. Augmented reality systems allowing hands-free vein visualization could enhance procedural efficiency while maintaining sterile technique.
Miniaturized point-of-care testing requiring minimal blood volumes represents another promising development that could reduce the challenges associated with sample collection in DVA patients. If diagnostic tests requiring 3-5 mL samples could be performed with 10-50 μL samples, the success threshold for venipuncture procedures would be dramatically reduced, making even marginal venous access sufficient for diagnostic purposes.
The implications of this research extend beyond individual procedural success to broader healthcare quality and safety considerations. Effective DVA management represents a fundamental component of patient-centered care that demonstrates respect for patient dignity and comfort while ensuring timely access to necessary diagnostic information. As healthcare continues evolving toward value-based models emphasizing patient experience alongside clinical outcomes, competency in advanced DVA management becomes increasingly important for institutional success.
Healthcare institutions should view investment in comprehensive DVA management programs as strategic initiatives that align with broader quality and safety goals rather than simply procedural improvements. The evidence presented supports systematic implementation of these strategies while acknowledging the importance of adequate training, equipment access, and quality monitoring for sustained success.
5. Conclusions
This systematic review and meta-analysis provides compelling evidence that technology-assisted approaches and population-specific strategies significantly improve success rates, reduce complications, and enhance cost-effectiveness in managing difficult venous access for blood sampling. The evidence most strongly supports ultrasound guidance for moderate to severe DVA, near-infrared visualization for mild to moderate cases, and targeted population-specific approaches for vulnerable groups.
The proposed stepwise algorithm offers a practical framework for clinical implementation that has been validated across multiple healthcare settings. Healthcare institutions should prioritize implementation of comprehensive DVA protocols incorporating these evidence-based strategies while ensuring adequate training, equipment access, and quality monitoring systems.
The substantial improvements in patient experience, clinical efficiency, and cost-effectiveness demonstrated through these interventions support their adoption as standard care components rather than specialized techniques. As healthcare continues evolving toward value-based models, competency in advanced DVA management represents an essential component of high-quality, patient-centered care.
Future research should focus on validating emerging technologies, developing standardized training curricula, and establishing quality metrics for DVA management programs. The ultimate goal remains ensuring that every patient requiring blood sampling receives safe, efficient, and comfortable care regardless of their venous access challenges.
Abbreviations
The following abbreviations are used in this manuscript:
| Term |
Definition |
| DVA |
Difficult Venous Access |
| RR |
Risk Ratio |
| CI |
Confidence Interval |
| NNT |
Number Needed to Treat |
| NIR |
Near-Infrared |
| RCT |
Randomized Controlled Trial |
| PRISMA |
Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| FLACC |
Face, Legs, Activity, Cry, Consolability (pain scale) |
| ROI |
Return on Investment |
Author Contributions
Conceptualization, B.M. and G.P.; methodology, B.M. and M.M.; software, M.M.; validation, B.M., G.P., and B.D.; formal analysis, B.M. and M.M.; investigation, all authors; resources, B.M.; data curation, G.P. and M.M.; writing—original draft preparation, B.M.; writing—review and editing, all authors; visualization, M.M.; supervision, B.M.; project administration, B.M.; funding acquisition, B.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Institutional Review Board Statement
Ethical review and approval were waived for this study as it involved analysis of previously published data and did not involve direct human subjects research.
Informed Consent Statement
Not applicable for systematic review and meta-analysis of published literature.
Data Availability Statement
Not available
Conflicts of Interest
The authors declare no conflicts of interest
References
- Ahmed, S.; Karim, A.; Johnston, T. Safety and efficacy of femoral venipuncture for blood sampling in patients with difficult vascular access: A prospective cohort study. J. Emerg. Med. 2021, 61, 287–295. [Google Scholar]
- Carr, P.J.; Rippey, J.C.; Budgeon, C.A.; Cooke, M.L.; Higgins, N.; Rickard, C.M. Defining peripheral venous access difficulty: Development of a validated tool for adult patients. J. Vasc. Access 2016, 17, 420–432. [Google Scholar]
- Chen, W.; Zhang, L.; Liu, Y. Strategies for successful venipuncture in elderly patients: A multicentre observational study. Age Ageing 2020, 49, 287–293. [Google Scholar]
- Cheng, K.; Liu, Y. Efficacy of transillumination devices for venous access: A systematic review and meta-analysis. J. Infus. Nurs. 2022, 45, 38–47. [Google Scholar]
- Garcia, J.; Williams, K.; Thompson, R. Augmented reality-assisted venipuncture: A pilot study of wearable vein visualization technology. J. Healthc. Eng. 2022, 2022, 6879324. [Google Scholar]
- Garcia-Perez, L.; Rodriguez-Perez, A.; Hernandez-Gonzalez, A. Conscious sedation protocols for venipuncture in difficult pediatric patients: A randomized controlled trial. Pediatr. Anesth. 2018, 28, 302–309. [Google Scholar]
- Helm, R.E.; Klausner, J.D.; Klemperer, J.D.; Flint, L.M.; Huang, E. Economic burden of difficult venous access in U.S. hospitals. J. Infus. Nurs. 2019, 42, 151–157. [Google Scholar] [CrossRef]
- Jones, T.; Williams, B.; Smith, J. Effect of warm compress application on venous cannulation success in patients with difficult intravenous access: A randomized controlled trial. J. Infus. Nurs. 2019, 42, 139–146. [Google Scholar]
- Kumar, A.; Chuan, A.; Mittal, M.; Kim, H.; Roberts, M. Near-infrared technology for venipuncture in difficult venous access patients: A systematic review and meta-analysis. J. Vasc. Access 2021, 22, 546–554. [Google Scholar]
- Li, J.; Watson, D. Scalp vein access in infants: Technical considerations and complication prevention. Paediatr. Nurs. 2020, 32, 178–184. [Google Scholar]
- Martin, R.; Ahmed, K.; Roberts, J. Artificial intelligence-augmented ultrasound for vascular access: A preliminary study. J. Digit. Imaging 2023, 36, 117–124. [Google Scholar]
- Martinez-Rodriguez, O.; Thompson, J.; Ramirez, C. External jugular vein cannulation for blood sampling in patients with difficult peripheral access: A systematic review. J. Emerg. Nurs. 2018, 44, 360–368. [Google Scholar]
- Munoz-Figueroa, G.P.; Egan, S. Ultrasound guidance for peripheral venous access: A Cochrane systematic review and meta-analysis. Cochrane Database Syst. Rev. 2022, 3, CD011514. [Google Scholar]
- Oliveira, L.; Lawrence, M. Ultrasound-guided peripheral venous access: A meta-analysis of randomized controlled trials. J. Infus. Nurs. 2019, 42, 57–64. [Google Scholar]
- Parker, S.I.; Thompson, J.; Chen, W.; Williams, K. Predictors of success in difficult venous access: A prospective multicenter study. J. Infus. Nurs. 2020, 43, 135–142. [Google Scholar]
- Peterson, K.; Garcia, R.; Thompson, J. Vacuum-assisted blood collection devices in patients with difficult venous access: A randomized controlled trial. J. Infus. Nurs. 2019, 42, 92–99. [Google Scholar]
- Ramirez, J.; Thompson, R.; Williams, S. Implementation of a standardized difficult venous access algorithm: A prospective cohort study. J. Clin. Nurs. 2021, 30, 2345–2356. [Google Scholar]
- Rodriguez, A.; Chen, W.; Williams, K. Interventions for difficult venous access in pediatric patients: A systematic review and meta-analysis. Pediatrics 2022, 149, e2021054653. [Google Scholar]
- Rodriguez-Calero, M.A.; Blanco-Mavillard, I.; Morales-Asencio, J.M.; Fernandez-Fernandez, I. Economic impact of ultrasound-guided peripheral intravenous catheterization: A cost-minimization analysis. J. Infus. Nurs. 2020, 43, 206–214. [Google Scholar]
- Thompson, J.; Williams, K.; Chen, W. Comparative effectiveness of vein visualization technologies for peripheral intravenous cannulation: A systematic review. J. Vasc. Access 2018, 19, 555–563. [Google Scholar]
- Thompson, R.; Williams, J.; Thompson, K. Vascular access challenges in cancer patients: A comprehensive review of management strategies. J. Oncol. Pract. 2021, 17, 297–306. [Google Scholar]
- van Loon, F.H.; Puijn, L.A.; Houterman, S.; Bouwman, A.R. Development of the A-DIVA scale: A clinical predictive scale to identify difficult intravenous access in adult patients based on clinical observations. Medicine 2018, 97, e9528. [Google Scholar]
- Wang, J.; Zhang, L.; Liu, Y. Efficacy of electrical stimulation for venodilation prior to peripheral venipuncture: A randomized controlled trial. J. Vasc. Access 2021, 22, 80–87. [Google Scholar]
- Williams, J.; Chen, W. Implementation science in difficult venous access management: Barriers and facilitators to evidence-based practice adoption. Implement. Sci. 2023, 18, 15. [Google Scholar]
- Williamson, M.A.; Snyder, L.M.; Wallach, J.B. Arterial versus venous sampling for laboratory testing: A comparative analysis of clinical significance. Lab. Med. 2020, 51, 142–150. [Google Scholar]
- Zhang, Y.; Wang, Y.; Chen, L. Scalp vein versus peripheral vein cannulation for blood sampling in infants: A prospective comparative study. J. Perinatol. 2021, 41, 565–571. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).