Submitted:
13 August 2025
Posted:
14 August 2025
You are already at the latest version
Abstract
Keywords:
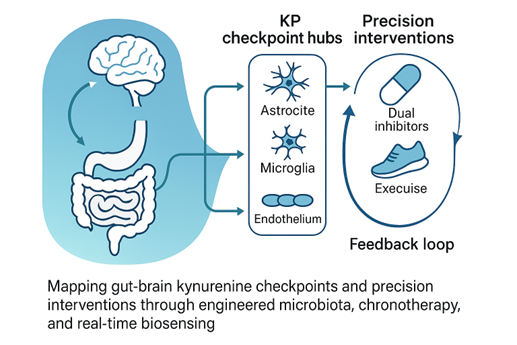
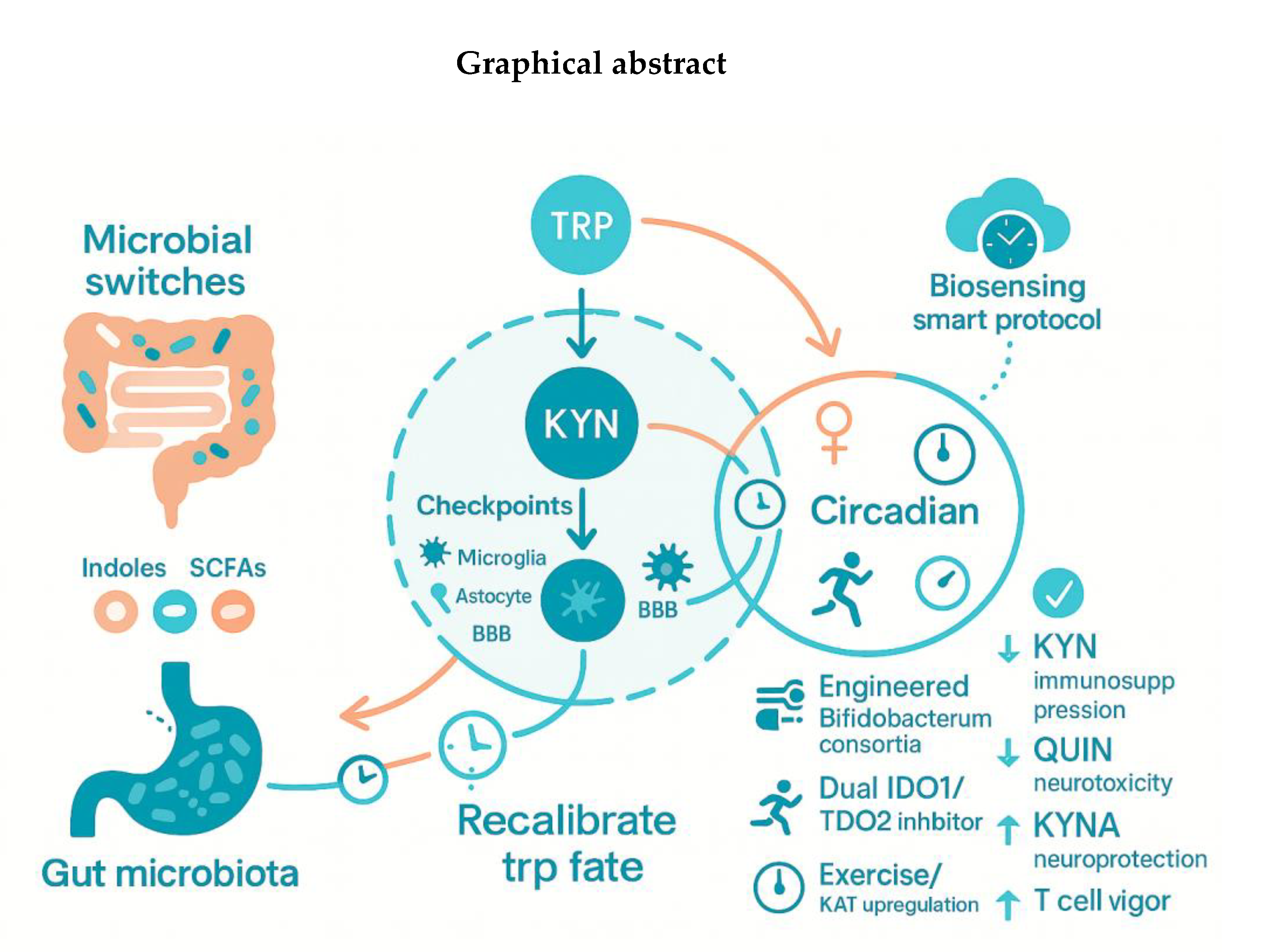
Graphical abstract
1. Introduction
2. Microbiota-Driven Modulation of Indoleamine-2,3-Dioxygenase-1 (IDO1) and Tryptophan-2,3-Dioxygenase (TDO2) Signaling
2.1. Literature Review: Microbial Metabolites as Modulators of Intestinal Integrity and Systemic Disease
2.2. Research gaps: Gaps in Dosing Strategies, Longitudinal Efficacy, and Mechanistic Insights
2.3. Time-Stamped Isotope-Tracing in Gnotobiotic Mice Can Tag Flux Through Indoleamine-2,3-Dioxygenase-1 (IDO1) Versus Tryptophan-2,3-Dioxygenase (TDO2)
2.4. Single-Cell Proteomics in Intestinal Organoids Could Reveal Which Epithelial or Immune Subsets Sense each “Metabokine”
2.5. Synthetic Consortia with Inducible Kyn Operons Would Let Us Dial Metabolite Output Like a Volume Knob
2.6. Molecular Mechanisms Linking Gut Microbiota to Kynurenine Pathway Enzymes
3. Kynurenine (KYN) Metabolic Pathway “Checkpoints” in the Brain’s Cellular Grid
3.1. Literature Review: Mapping Kynurenine (KYN) Dynamics Across Neurovascular and Immune Landscapes
3.2. Research Gaps: Mapping, Monitoring, and Modulating Kynurenine (KYN) Checkpoints Across Systems
3.3. CRISPRi “Zip-Codes” Delivered by Adeno-Associated Virus (AAV) Can Silence Kynurenine 3-Monooxygenase (KMO) or Kynureninase (KYNU) Only in Perivascular Endothelium and Watch Downstream Glutamatergic Sync Crash—Or Not
3.4. Light-Addressable Riboswitches Could Let Us Pulse KP Enzymes in Astrocytes and Read Real-Time Calcium Waves
4. Sex and the Circadian City: Hidden Modifiers
4.1. Literature Review: Circadian Misalignment, Mood Vulnerability, and Emerging Chronotherapeutics
4.2. Research Gaps: Timing, Sex, and Biomarker Integration for Precision Kynurenine (KYN) Intervention
4.3. Multi-Time-Point Plasma Kynurenine (KYN) Profiles Stratified by Sex and Hormonal Phase
4.4. Wearable Light-Exposure + Metabolite Logging to See if Circadian Misalignment Exaggerates the Quinolinic Spike
4.5. Adaptive Trial Designs that Randomize Dose-Timing Rather Than Just Dose Size
5. Microbiota Engineering as a Precision Switch
5.1. Literature Review: Microbiota-Targeted Strategies for Modulating Mood and Inflammation
5.2. Research Gaps: Live Biotherapeutic Products Against Multi-Drug Resistant (MDR) Enteric Pathogens: Research Gaps
5.3. Designer Strains with Kill-Switches and Inducible Kynurenine Aminotransferase (KAT) Expression
5.4. Encapsulated “Post-Biotics” (e.g., Stabilized KYNA) to Bypass Colonization
5.5. Cloud-Linked Stool Metabolomics Dashboards to Guide Weekly Probiotic Titration
6. Intervention 2.0: Dual Inhibitors, Exercise, and Real-Time Biosensing
6.1. Literature Review. Dual Inhibition and Kynurenines (KYNs) Modulation
6.2. Research gaps: Adaptive Dose-Timing and Real-Time Monitoring
6.3. Crosstalk Between Kynurenine Pathway Modulation and Broader Metabolic Networks
6.4. Phase-Ib “Smart Protocols”: Micro-Dosed Dual Inhibitors Guided by Saliva KYNA Sensors
6.5. Conceptual and Translational Limitations
6.6. AI-Driven Feedback Loops that Auto-Adjust Evening Treadmill Sessions or Probiotic Cocktails Based on Morning KYN/TRP slope.AI-Driven KYN/TRP Feedback Loops
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAV | adeno-associated virus |
| AD | Alzheimer’s disease |
| AhR | aryhydorcarbon receptor |
| AI | artificial intelligence |
| BBB | blood–brain barrier |
| CM | Circadian misalignment |
| COVID-19 | coronavirus disease 2019 |
| CRISPR | clustered regularly interspaced short palindromic repeats |
| CRISPRi | clustered regularly interspaced short palindromic repeats interference |
| IDO1 | indoleamine-2,3-dioxygenase 1 |
| KMO | kynurenine 3-monooxygenase |
| KYN | kynurenine |
| KYNU | kynureninase |
| KYNA | kynurenic acid |
| KAT | kynurenine aminotransferase |
| LBPs | biotherapeutic products |
| LC-MS | liquid chromatography–mass spectrometry |
| LD | Linear dichroism |
| NAD | nicotinamide adenine dinucleotide |
| QUIN | quinolinic acid |
| TDO2 | tryptophan-2,3-dioxygenase-2 |
| TLA | Three letter acronym |
| TLR | Toll-like receptor |
| Trp | tryptophan |
| ZIM3 | zinc finger protein 3 |
References
- Sun, M.; Ma, N.; He, T.; Johnston, L.J.; Ma, X. Tryptophan (Trp) modulates gut homeostasis via aryl hydrocarbon receptor (AhR). Critical reviews in food science and nutrition 2020, 60, 1760–1768. [Google Scholar] [CrossRef]
- Roth, W.; Zadeh, K.; Vekariya, R.; Ge, Y.; Mohamadzadeh, M. Tryptophan metabolism and gut-brain homeostasis. International journal of molecular sciences 2021, 22, 2973. [Google Scholar] [CrossRef]
- Su, X.; Gao, Y.; Yang, R. Gut microbiota-derived tryptophan metabolites maintain gut and systemic homeostasis. Cells 2022, 11, 2296. [Google Scholar] [CrossRef]
- Hou, Y.; Li, J.; Ying, S. Tryptophan Metabolism and Gut Microbiota: A Novel Regulatory Axis Integrating the Microbiome, Immunity, and Cancer. Metabolites 2023, 13. [Google Scholar] [CrossRef]
- Hyland, N.P.; Cavanaugh, C.R.; Hornby, P.J. Emerging effects of tryptophan pathway metabolites and intestinal microbiota on metabolism and intestinal function. Amino Acids 2022, 54, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo Godoy, A.C.; Frota, F.F.; Araújo, L.P.; Valenti, V.E.; Pereira, E.; Detregiachi, C.R.P.; Galhardi, C.M.; Caracio, F.C.; Haber, R.S.A.; Fornari Laurindo, L.; et al. Neuroinflammation and Natural Antidepressants: Balancing Fire with Flora. Biomedicines 2025, 13. [Google Scholar] [CrossRef]
- Chen, X.; Xu, D.; Yu, J.; Song, X.-J.; Li, X.; Cui, Y.-L. Tryptophan metabolism disorder-triggered diseases, mechanisms, and therapeutic strategies: a scientometric review. Nutrients 2024, 16, 3380. [Google Scholar] [CrossRef] [PubMed]
- Vécsei, L.; Szalárdy, L.; Fülöp, F.; Toldi, J. Kynurenines in the CNS: recent advances and new questions. Nat Rev Drug Discov 2013, 12, 64–82. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. A Decade of Dedication: Pioneering Perspectives on Neurological Diseases and Mental Illnesses. Biomedicines 2024, 12. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Battaglia, S.; Giménez-Llort, L.; Chen, C.; Hepsomali, P.; Avenanti, A.; Vécsei, L. Innovation at the Intersection: Emerging Translational Research in Neurology and Psychiatry. Cells 2024, 13. [Google Scholar] [CrossRef]
- Tanaka, M.; Battaglia, S.; Liloia, D. Navigating Neurodegeneration: Integrating Biomarkers, Neuroinflammation, and Imaging in Parkinson’s, Alzheimer’s, and Motor Neuron Disorders. Biomedicines 2025, 13. [Google Scholar] [CrossRef]
- de Lima, E.P.; Laurindo, L.F.; Catharin, V.C.S.; Direito, R.; Tanaka, M.; Jasmin Santos German, I.; Lamas, C.B.; Guiguer, E.L.; Araújo, A.C.; Fiorini, A.M.R.; et al. Polyphenols, Alkaloids, and Terpenoids Against Neurodegeneration: Evaluating the Neuroprotective Effects of Phytocompounds Through a Comprehensive Review of the Current Evidence. Metabolites 2025, 15. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Leme Boaro, B.; da Silva Camarinha Oliveira, J.; Patočka, J.; Barbalho Lamas, C.; Tanaka, M.; Laurindo, L.F. Molecular Mechanisms Underlying Neuroinflammation Intervention with Medicinal Plants: A Critical and Narrative Review of the Current Literature. Pharmaceuticals (Basel) 2025, 18. [Google Scholar] [CrossRef]
- Pagotto, G.L.O.; Santos, L.; Osman, N.; Lamas, C.B.; Laurindo, L.F.; Pomini, K.T.; Guissoni, L.M.; Lima, E.P.; Goulart, R.A.; Catharin, V.; et al. Ginkgo biloba: A Leaf of Hope in the Fight against Alzheimer’s Dementia: Clinical Trial Systematic Review. Antioxidants (Basel) 2024, 13. [Google Scholar] [CrossRef]
- Valotto Neto, L.J.; Reverete de Araujo, M.; Moretti Junior, R.C.; Mendes Machado, N.; Joshi, R.K.; Dos Santos Buglio, D.; Barbalho Lamas, C.; Direito, R.; Fornari Laurindo, L.; Tanaka, M.; et al. Investigating the Neuroprotective and Cognitive-Enhancing Effects of Bacopa monnieri: A Systematic Review Focused on Inflammation, Oxidative Stress, Mitochondrial Dysfunction, and Apoptosis. Antioxidants (Basel) 2024, 13. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Li, G.; Zheng, Q.; Gu, X.; Shi, Q.; Su, Y.; Chu, Q.; Yuan, X.; Bao, Z.; Lu, J. Tryptophan metabolism in health and disease. Cell Metabolism 2023, 35, 1304–1326. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Sujino, T.; Kanai, T. The tryptophan metabolic pathway of the microbiome and host cells in health and disease. International immunology 2024, 36, 601–616. [Google Scholar] [CrossRef]
- Cellini, B.; Zelante, T.; Dindo, M.; Bellet, M.M.; Renga, G.; Romani, L.; Costantini, C. Pyridoxal 5′-phosphate-dependent enzymes at the crossroads of host–microbe tryptophan metabolism. International Journal of Molecular Sciences 2020, 21, 5823. [Google Scholar] [CrossRef]
- Tanaka, M.; Szabó, Á.; Vécsei, L. Redefining Roles: A Paradigm Shift in Tryptophan-Kynurenine Metabolism for Innovative Clinical Applications. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef]
- Vandereyken, K.; Sifrim, A.; Thienpont, B.; Voet, T. Methods and applications for single-cell and spatial multi-omics. Nature Reviews Genetics 2023, 24, 494–515. [Google Scholar] [CrossRef] [PubMed]
- Bressan, D.; Battistoni, G.; Hannon, G.J. The dawn of spatial omics. Science 2023, 381, eabq4964. [Google Scholar] [CrossRef]
- Hrovatin, K.; Fischer, D.S.; Theis, F.J. Toward modeling metabolic state from single-cell transcriptomics. Molecular metabolism 2022, 57, 101396. [Google Scholar] [CrossRef]
- Guan, D.; Lazar, M.A. Interconnections between circadian clocks and metabolism. J Clin Invest 2021, 131. [Google Scholar] [CrossRef]
- Lok, R.; Qian, J.; Chellappa, S.L. Sex differences in sleep, circadian rhythms, and metabolism: implications for precision medicine. Sleep medicine reviews 2024, 101926. [Google Scholar] [CrossRef]
- Lévi, F.A.; Okyar, A.; Hadadi, E.; Innominato, P.F.; Ballesta, A. Circadian regulation of drug responses: toward sex-specific and personalized chronotherapy. Annual review of pharmacology and toxicology 2024, 64, 89–114. [Google Scholar] [CrossRef]
- Weger, M.; Weger, B.D.; Gachon, F. Understanding circadian dynamics: current progress and future directions for chronobiology in drug discovery. Expert Opinion on Drug Discovery 2023, 18, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Truong, T.; Saxton, A.J.; Boekweg, H.; Payne, S.H.; Van Ry, P.M.; Kelly, R.T. HyperSCP: combining isotopic and isobaric labeling for higher throughput single-cell proteomics. Analytical chemistry 2023, 95, 8020–8027. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, J.; Altea-Manzano, P.; Pranzini, E.; Fendt, S.-M. Stable isotopes for tracing mammalian-cell metabolism in vivo. Trends in biochemical sciences 2020, 45, 185–201. [Google Scholar] [CrossRef]
- Rottinghaus, A.G.; Ferreiro, A.; Fishbein, S.R.; Dantas, G.; Moon, T.S. Genetically stable CRISPR-based kill switches for engineered microbes. Nature communications 2022, 13, 672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, Y.; Jiang, N.; Yetisen, A.K. Wearable artificial intelligence biosensor networks. Biosensors and Bioelectronics 2023, 219, 114825. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, M. A Bayesian Dynamic Model-Based Adaptive Design for Oncology Dose Optimization in Phase I/II Clinical Trials. Pharm Stat 2025, 24, e2451. [Google Scholar] [CrossRef]
- Joisten, N.; Kummerhoff, F.; Koliamitra, C.; Schenk, A.; Walzik, D.; Hardt, L.; Knoop, A.; Thevis, M.; Kiesl, D.; Metcalfe, A.J.; et al. Exercise and the Kynurenine pathway: Current state of knowledge and results from a randomized cross-over study comparing acute effects of endurance and resistance training. Exerc Immunol Rev 2020, 26, 24–42. [Google Scholar]
- Jankovskaja, S.; Engblom, J.; Rezeli, M.; Marko-Varga, G.; Ruzgas, T.; Björklund, S. Non-invasive skin sampling of tryptophan/kynurenine ratio in vitro towards a skin cancer biomarker. Sci Rep 2021, 11, 678. [Google Scholar] [CrossRef] [PubMed]
- Cordaillat-Simmons, M.; Rouanet, A.; Pot, B. Live biotherapeutic products: the importance of a defined regulatory framework. Exp Mol Med 2020, 52, 1397–1406. [Google Scholar] [CrossRef]
- Cerqueira, F.P.; Jesus, A.M.C.; Cotrim, M.D. Adaptive Design: A Review of the Technical, Statistical, and Regulatory Aspects of Implementation in a Clinical Trial. Ther Innov Regul Sci 2020, 54, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Gao, Y.; Yang, R. Gut Microbiota-Derived Tryptophan Metabolites Maintain Gut and Systemic Homeostasis. Cells 2022, 11. [Google Scholar] [CrossRef]
- Colucci Cante, R.; Nigro, F.; Passannanti, F.; Lentini, G.; Gallo, M.; Nigro, R.; Budelli, A.L. Gut health benefits and associated systemic effects provided by functional components from the fermentation of natural matrices. Compr Rev Food Sci Food Saf 2024, 23, e13356. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front Cell Infect Microbiol 2018, 8, 13. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Mostafavi Abdolmaleky, H.; Zhou, J.R. Gut Microbiota Dysbiosis, Oxidative Stress, Inflammation, and Epigenetic Alterations in Metabolic Diseases. Antioxidants (Basel) 2024, 13. [Google Scholar] [CrossRef]
- Peña-Durán, E.; García-Galindo, J.J.; López-Murillo, L.D.; Huerta-Huerta, A.; Balleza-Alejandri, L.R.; Beltrán-Ramírez, A.; Anaya-Ambriz, E.J.; Suárez-Rico, D.O. Microbiota and Inflammatory Markers: A Review of Their Interplay, Clinical Implications, and Metabolic Disorders. Int J Mol Sci 2025, 26. [Google Scholar] [CrossRef] [PubMed]
- Hand, T.W.; Vujkovic-Cvijin, I.; Ridaura, V.K.; Belkaid, Y. Linking the Microbiota, Chronic Disease, and the Immune System. Trends Endocrinol Metab 2016, 27, 831–843. [Google Scholar] [CrossRef]
- Potrykus, M.; Czaja-Stolc, S.; Stankiewicz, M.; Kaska, Ł.; Małgorzewicz, S. Intestinal Microbiota as a Contributor to Chronic Inflammation and Its Potential Modifications. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Rizzetto, L.; Fava, F.; Tuohy, K.M.; Selmi, C. Connecting the immune system, systemic chronic inflammation and the gut microbiome: The role of sex. J Autoimmun 2018, 92, 12–34. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, D.; Song, P.; Zou, M.H. Tryptophan-kynurenine pathway is dysregulated in inflammation, and immune activation. Front Biosci (Landmark Ed) 2015, 20, 1116–1143. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.A.; Fu, J.; Chang, P.V. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A 2020, 117, 19376–19387. [Google Scholar] [CrossRef] [PubMed]
- Gasaly, N.; de Vos, P.; Hermoso, M.A. Impact of Bacterial Metabolites on Gut Barrier Function and Host Immunity: A Focus on Bacterial Metabolism and Its Relevance for Intestinal Inflammation. Front Immunol 2021, 12, 658354. [Google Scholar] [CrossRef]
- Mayengbam, S.; Chleilat, F.; Reimer, R.A. Dietary Vitamin B6 Deficiency Impairs Gut Microbiota and Host and Microbial Metabolites in Rats. Biomedicines 2020, 8. [Google Scholar] [CrossRef]
- Hou, C.; Shi, H.; Xiao, J.; Song, X.; Luo, Z.; Ma, X.; Shi, L.; Wei, H.; Li, J. Pomegranate Juice Supplemented with Inulin Modulates Gut Microbiota and Promotes the Production of Microbiota-Associated Metabolites in Overweight/Obese Individuals: A Randomized, Double-Blind, Placebo-Controlled Trial. J Agric Food Chem 2024, 72, 14663–14677. [Google Scholar] [CrossRef]
- Hiel, S.; Gianfrancesco, M.A.; Rodriguez, J.; Portheault, D.; Leyrolle, Q.; Bindels, L.B.; Gomes da Silveira Cauduro, C.; Mulders, M.; Zamariola, G.; Azzi, A.S.; et al. Link between gut microbiota and health outcomes in inulin -treated obese patients: Lessons from the Food4Gut multicenter randomized placebo-controlled trial. Clin Nutr 2020, 39, 3618–3628. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, S.; Ma, N.; Johnston, L.J.; Wu, C.; Ma, X. Metabolites of microbiota response to tryptophan and intestinal mucosal immunity: A therapeutic target to control intestinal inflammation. Med Res Rev 2021, 41, 1061–1088. [Google Scholar] [CrossRef]
- Wang, L.; Qin, N.; Shi, L.; Liu, R.; Zhu, T. Gut Microbiota and Tryptophan Metabolism in Pathogenesis of Ischemic Stroke: A Potential Role for Food Homologous Plants. Mol Nutr Food Res 2024, 68, e2400639. [Google Scholar] [CrossRef]
- Gupta, S.K.; Vyavahare, S.; Duchesne Blanes, I.L.; Berger, F.; Isales, C.; Fulzele, S. Microbiota-derived tryptophan metabolism: Impacts on health, aging, and disease. Exp Gerontol 2023, 183, 112319. [Google Scholar] [CrossRef]
- Meghani, S.; Frishkopf, M.; Park, T.; Montgomery, C.L.; Norris, C.; Papathanassoglou, E. Music-based interventions and theoretical mechanisms in post-ICU survivors: A critical narrative synthesis. Intensive Crit Care Nurs 2025, 86, 103777. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, T.; Della Porta, D.; Perschl, J.; Evers, A.W.M.; Magee, W.L.; Schaefer, R.S. Motivation and music interventions in adults: A systematic review. Neuropsychol Rehabil 2024, 34, 649–678. [Google Scholar] [CrossRef] [PubMed]
- Kuuse, A.K.; Paulander, A.S.; Eulau, L. Characteristics and impacts of live music interventions on health and wellbeing for children, families, and health care professionals in paediatric hospitals: a scoping review. Int J Qual Stud Health Well-being 2023, 18, 2180859. [Google Scholar] [CrossRef]
- Pakdeesatitwara, N.; Clark, I.; Tamplin, J. A mixed-studies systematic review of self-administered music interventions (SAMIs) for psychological wellbeing in people with chronic health conditions: Meta-analysis and narrative summary. Patient Educ Couns 2024, 118, 108006. [Google Scholar] [CrossRef]
- Chang, E.X.; Brooker, J.; Hiscock, R.; O’Callaghan, C. Music-based intervention impacts for people with eating disorders: A narrative synthesis systematic review. J Music Ther 2023, 60, 202–231. [Google Scholar] [CrossRef]
- Wang, G.; Fan, Y.; Zhang, G.; Cai, S.; Ma, Y.; Yang, L.; Wang, Y.; Yu, H.; Qiao, S.; Zeng, X. Microbiota-derived indoles alleviate intestinal inflammation and modulate microbiome by microbial cross-feeding. Microbiome 2024, 12, 59. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, N.; Su, X.; Gao, Y.; Yang, R. Gut-Microbiota-Derived Metabolites Maintain Gut and Systemic Immune Homeostasis. Cells 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.Q.; Wang, Y.N.; Feng, H.Y.; Guo, Z.Y.; Li, X.; Nie, X.L.; Zhao, Y.Y. Host/microbiota interactions-derived tryptophan metabolites modulate oxidative stress and inflammation via aryl hydrocarbon receptor signaling. Free Radic Biol Med 2022, 184, 30–41. [Google Scholar] [CrossRef]
- Zheng, D.; Ratiner, K.; Elinav, E. Circadian Influences of Diet on the Microbiome and Immunity. Trends Immunol 2020, 41, 512–530. [Google Scholar] [CrossRef]
- Frazier, K.; Frith, M.; Harris, D.; Leone, V.A. Mediators of Host-Microbe Circadian Rhythms in Immunity and Metabolism. Biology (Basel) 2020, 9. [Google Scholar] [CrossRef]
- Frazier, K.; Chang, E.B. Intersection of the Gut Microbiome and Circadian Rhythms in Metabolism. Trends Endocrinol Metab 2020, 31, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Krautkramer, K.A.; Fan, J.; Bäckhed, F. Gut microbial metabolites as multi-kingdom intermediates. Nat Rev Microbiol 2021, 19, 77–94. [Google Scholar] [CrossRef]
- Więdłocha, M.; Marcinowicz, P.; Janoska-Jaździk, M.; Szulc, A. Gut microbiota, kynurenine pathway and mental disorders - Review. Prog Neuropsychopharmacol Biol Psychiatry 2021, 106, 110145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tu, S.; Ji, X.; Wu, J.; Meng, J.; Gao, J.; Shao, X.; Shi, S.; Wang, G.; Qiu, J.; et al. Dubosiella newyorkensis modulates immune tolerance in colitis via the L-lysine-activated AhR-IDO1-Kyn pathway. Nat Commun 2024, 15, 1333. [Google Scholar] [CrossRef] [PubMed]
- Aslamkhan, A.G.; Xu, Q.; Loughlin, A.; Vu, H.; Pacchione, S.; Bhatt, B.; Garfinkel, I.; Styring, T.G.; LaFranco-Scheuch, L.; Pearson, K.; et al. Characterization of indoleamine-2,3-dioxygenase 1, tryptophan-2,3-dioxygenase, and Ido1/Tdo2 knockout mice. Toxicol Appl Pharmacol 2020, 406, 115216. [Google Scholar] [CrossRef]
- Peyraud, F.; Guegan, J.P.; Bodet, D.; Cousin, S.; Bessede, A.; Italiano, A. Targeting Tryptophan Catabolism in Cancer Immunotherapy Era: Challenges and Perspectives. Front Immunol 2022, 13, 807271. [Google Scholar] [CrossRef]
- Platten, M.; Friedrich, M.; Wainwright, D.A.; Panitz, V.; Opitz, C.A. Tryptophan metabolism in brain tumors - IDO and beyond. Curr Opin Immunol 2021, 70, 57–66. [Google Scholar] [CrossRef]
- Yu, L.; Lu, J.; Du, W. Tryptophan metabolism in digestive system tumors: unraveling the pathways and implications. Cell Commun Signal 2024, 22, 174. [Google Scholar] [CrossRef]
- Tijono, S.M.; Palmer, B.D.; Tomek, P.; Flanagan, J.U.; Henare, K.; Gamage, S.; Braun, L.; Ching, L.M. Evaluation of Novel Inhibitors of Tryptophan Dioxygenases for Enzyme and Species Selectivity Using Engineered Tumour Cell Lines Expressing Either Murine or Human IDO1 or TDO2. Pharmaceuticals (Basel) 2022, 15. [Google Scholar] [CrossRef] [PubMed]
- Naing, A.; Eder, J.P.; Piha-Paul, S.A.; Gimmi, C.; Hussey, E.; Zhang, S.; Hildebrand, V.; Hosagrahara, V.; Habermehl, C.; Moisan, J.; et al. Preclinical investigations and a first-in-human phase I trial of M4112, the first dual inhibitor of indoleamine 2,3-dioxygenase 1 and tryptophan 2,3-dioxygenase 2, in patients with advanced solid tumors. J Immunother Cancer 2020, 8. [Google Scholar] [CrossRef]
- Wu, C.; Spector, S.A.; Theodoropoulos, G.; Nguyen, D.J.M.; Kim, E.Y.; Garcia, A.; Savaraj, N.; Lim, D.C.; Paul, A.; Feun, L.G.; et al. Dual inhibition of IDO1/TDO2 enhances anti-tumor immunity in platinum-resistant non-small cell lung cancer. Cancer Metab 2023, 11, 7. [Google Scholar] [CrossRef]
- Juhász, L.; Spisák, K.; Szolnoki, B.Z.; Nászai, A.; Szabó, Á.; Rutai, A.; Tallósy, S.P.; Szabó, A.; Toldi, J.; Tanaka, M.; et al. The Power Struggle: Kynurenine Pathway Enzyme Knockouts and Brain Mitochondrial Respiration. J Neurochem 2025, 169, e70075. [Google Scholar] [CrossRef]
- Szabó, Á.; Galla, Z.; Spekker, E.; Szűcs, M.; Martos, D.; Takeda, K.; Ozaki, K.; Inoue, H.; Yamamoto, S.; Toldi, J.; et al. Oxidative and Excitatory Neurotoxic Stresses in CRISPR/Cas9-Induced Kynurenine Aminotransferase Knockout Mice: A Novel Model for Despair-Based Depression and Post-Traumatic Stress Disorder. Front Biosci (Landmark Ed) 2025, 30, 25706. [Google Scholar] [CrossRef]
- Tanaka, M.; Szatmári, I.; Vécsei, L. Quinoline Quest: Kynurenic Acid Strategies for Next-Generation Therapeutics via Rational Drug Design. Pharmaceuticals (Basel) 2025, 18. [Google Scholar] [CrossRef]
- Du, L.; Xing, Z.; Tao, B.; Li, T.; Yang, D.; Li, W.; Zheng, Y.; Kuang, C.; Yang, Q. Both IDO1 and TDO contribute to the malignancy of gliomas via the Kyn-AhR-AQP4 signaling pathway. Signal Transduct Target Ther 2020, 5, 10. [Google Scholar] [CrossRef]
- Capochiani de Iudicibus, R.; Tomek, P.; Palmer, B.D.; Tijono, S.M.; Flanagan, J.U.; Ching, L.M. Parallel discovery of selective and dual inhibitors of tryptophan dioxygenases IDO1 and TDO2 with a newly-modified enzymatic assay. Bioorg Med Chem 2021, 39, 116160. [Google Scholar] [CrossRef] [PubMed]
- MacCannell, A.D.; Roberts, L.D. Metabokines in the regulation of systemic energy metabolism. Curr Opin Pharmacol 2022, 67, 102286. [Google Scholar] [CrossRef] [PubMed]
- Mund, A.; Brunner, A.D.; Mann, M. Unbiased spatial proteomics with single-cell resolution in tissues. Mol Cell 2022, 82, 2335–2349. [Google Scholar] [CrossRef]
- Petelski, A.A.; Emmott, E.; Leduc, A.; Huffman, R.G.; Specht, H.; Perlman, D.H.; Slavov, N. Multiplexed single-cell proteomics using SCoPE2. Nat Protoc 2021, 16, 5398–5425. [Google Scholar] [CrossRef]
- Mansuri, M.S.; Williams, K.; Nairn, A.C. Uncovering biology by single-cell proteomics. Commun Biol 2023, 6, 381. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; Jung, S.B.; Chang, J.Y.; Shong, M. Cellular and Intercellular Homeostasis in Adipose Tissue with Mitochondria-Specific Stress. Endocrinol Metab (Seoul) 2021, 36, 1–11. [Google Scholar] [CrossRef]
- Wu, L.; Ai, Y.; Xie, R.; Xiong, J.; Wang, Y.; Liang, Q. Organoids/organs-on-a-chip: new frontiers of intestinal pathophysiological models. Lab Chip 2023, 23, 1192–1212. [Google Scholar] [CrossRef]
- Kip, A.M.; Soons, Z.; Mohren, R.; Duivenvoorden, A.A.M.; Röth, A.A.J.; Cillero-Pastor, B.; Neumann, U.P.; Dejong, C.H.C.; Heeren, R.M.A.; Olde Damink, S.W.M.; et al. Proteomics analysis of human intestinal organoids during hypoxia and reoxygenation as a model to study ischemia-reperfusion injury. Cell Death Dis 2021, 12, 95. [Google Scholar] [CrossRef]
- Whitehead, A.; Krause, F.N.; Moran, A.; MacCannell, A.D.V.; Scragg, J.L.; McNally, B.D.; Boateng, E.; Murfitt, S.A.; Virtue, S.; Wright, J.; et al. Brown and beige adipose tissue regulate systemic metabolism through a metabolite interorgan signaling axis. Nat Commun 2021, 12, 1905. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Lu, V.B.; Bany Bakar, R.; Miedzybrodzka, E.; Davison, A.; Goldspink, D.; Reimann, F.; Gribble, F.M. Single-cell transcriptomics of human organoid-derived enteroendocrine cell populations from the small intestine. J Physiol 2024. [Google Scholar] [CrossRef] [PubMed]
- James, K.R.; Elmentaite, R.; Teichmann, S.A.; Hold, G.L. Redefining intestinal immunity with single-cell transcriptomics. Mucosal Immunol 2022, 15, 531–541. [Google Scholar] [CrossRef]
- Burclaff, J.; Bliton, R.J.; Breau, K.A.; Ok, M.T.; Gomez-Martinez, I.; Ranek, J.S.; Bhatt, A.P.; Purvis, J.E.; Woosley, J.T.; Magness, S.T. A Proximal-to-Distal Survey of Healthy Adult Human Small Intestine and Colon Epithelium by Single-Cell Transcriptomics. Cell Mol Gastroenterol Hepatol 2022, 13, 1554–1589. [Google Scholar] [CrossRef]
- Labib, M.; Kelley, S.O. Single-cell analysis targeting the proteome. Nat Rev Chem 2020, 4, 143–158. [Google Scholar] [CrossRef]
- Schoof, E.M.; Furtwängler, B.; Üresin, N.; Rapin, N.; Savickas, S.; Gentil, C.; Lechman, E.; Keller, U.A.D.; Dick, J.E.; Porse, B.T. Quantitative single-cell proteomics as a tool to characterize cellular hierarchies. Nat Commun 2021, 12, 3341. [Google Scholar] [CrossRef]
- Petrosius, V.; Aragon-Fernandez, P.; Üresin, N.; Kovacs, G.; Phlairaharn, T.; Furtwängler, B.; Op De Beeck, J.; Skovbakke, S.L.; Goletz, S.; Thomsen, S.F.; et al. Exploration of cell state heterogeneity using single-cell proteomics through sensitivity-tailored data-independent acquisition. Nat Commun 2023, 14, 5910. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.J.; Lukowski, J.K.; Anderton, C.R. Spatially Resolved Mass Spectrometry at the Single Cell: Recent Innovations in Proteomics and Metabolomics. J Am Soc Mass Spectrom 2021, 32, 872–894. [Google Scholar] [CrossRef] [PubMed]
- Gebreyesus, S.T.; Siyal, A.A.; Kitata, R.B.; Chen, E.S.; Enkhbayar, B.; Angata, T.; Lin, K.I.; Chen, Y.J.; Tu, H.L. Streamlined single-cell proteomics by an integrated microfluidic chip and data-independent acquisition mass spectrometry. Nat Commun 2022, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Chen, S.; Bai, Y.; Chen, H.; Liao, G.; Mukherjee, N.; Vazquez, G.; McIlwain, D.R.; Tzankov, A.; Lee, I.T.; et al. Robust single-cell matching and multimodal analysis using shared and distinct features. Nat Methods 2023, 20, 304–315. [Google Scholar] [CrossRef]
- Hao, Y.; Stuart, T.; Kowalski, M.H.; Choudhary, S.; Hoffman, P.; Hartman, A.; Srivastava, A.; Molla, G.; Madad, S.; Fernandez-Granda, C. Dictionary learning for integrative, multimodal and scalable single-cell analysis. Nature biotechnology 2024, 42, 293–304. [Google Scholar] [CrossRef]
- Kelly, R.T. Single-cell Proteomics: Progress and Prospects. Mol Cell Proteomics 2020, 19, 1739–1748. [Google Scholar] [CrossRef]
- Truong, T.; Kelly, R.T. What’s new in single-cell proteomics. Curr Opin Biotechnol 2024, 86, 103077. [Google Scholar] [CrossRef]
- Qian, X.; Chen, L.; Sui, Y.; Chen, C.; Zhang, W.; Zhou, J.; Dong, W.; Jiang, M.; Xin, F.; Ochsenreither, K. Biotechnological potential and applications of microbial consortia. Biotechnol Adv 2020, 40, 107500. [Google Scholar] [CrossRef]
- Hampton, T. From the Literature. Circulation 2020, 142, 1491–1493. [Google Scholar] [CrossRef]
- Benninghaus, L.; Schwardmann, L.S.; Jilg, T.; Wendisch, V.F. Establishment of synthetic microbial consortia with Corynebacterium glutamicum and Pseudomonas putida: Design, construction, and application to production of γ-glutamylisopropylamide and l-theanine. Microb Biotechnol 2024, 17, e14400. [Google Scholar] [CrossRef]
- Peng, H.; Darlington, A.P.S.; South, E.J.; Chen, H.H.; Jiang, W.; Ledesma-Amaro, R. A molecular toolkit of cross-feeding strains for engineering synthetic yeast communities. Nat Microbiol 2024, 9, 848–863. [Google Scholar] [CrossRef]
- Gasparek, M.; Steel, H.; Papachristodoulou, A. Deciphering mechanisms of production of natural compounds using inducer-producer microbial consortia. Biotechnol Adv 2023, 64, 108117. [Google Scholar] [CrossRef]
- Alnahhas, R.N.; Sadeghpour, M.; Chen, Y.; Frey, A.A.; Ott, W.; Josić, K.; Bennett, M.R. Majority sensing in synthetic microbial consortia. Nat Commun 2020, 11, 3659. [Google Scholar] [CrossRef]
- Bodapati, S.; Daley, T.P.; Lin, X.; Zou, J.; Qi, L.S. A benchmark of algorithms for the analysis of pooled CRISPR screens. Genome Biol 2020, 21, 62. [Google Scholar] [CrossRef]
- Sun, P.; Wang, M.; Liu, Y.X.; Li, L.; Chai, X.; Zheng, W.; Chen, S.; Zhu, X.; Zhao, S. High-fat diet-disturbed gut microbiota-colonocyte interactions contribute to dysregulating peripheral tryptophan-kynurenine metabolism. Microbiome 2023, 11, 154. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol 2021, 19, 55–71. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhou, M.; Wang, J.; Yao, J.; Yu, J.; Liu, W.; Wu, L.; Wang, J.; Gao, R. Involvement of the microbiota-gut-brain axis in chronic restraint stress: disturbances of the kynurenine metabolic pathway in both the gut and brain. Gut Microbes 2021, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Clément, K.; Sokol, H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 2021, 70, 1174–1182. [Google Scholar] [CrossRef]
- Vernocchi, P.; Del Chierico, F.; Putignani, L. Gut Microbiota Metabolism and Interaction with Food Components. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef]
- Prukpitikul, P.; Sirivarasai, J.; Sutjarit, N. The molecular mechanisms underlying gut microbiota-miRNA interaction in metabolic disorders. Benef Microbes 2024, 15, 83–96. [Google Scholar] [CrossRef]
- Huang, Z.; Yao, Q.; Ma, S.; Zhou, J.; Wang, X.; Meng, Q.; Liu, Y.; Yu, Z.; Chen, X. The synergistic role of gut microbiota and RNA in metabolic diseases: mechanisms and therapeutic insights. Front Microbiol 2025, 16, 1504395. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, Y.; Yang, S.; Lu, J.; Jin, X.; Wu, M. Diet-gut microbiota-epigenetics in metabolic diseases: From mechanisms to therapeutics. Biomed Pharmacother 2022, 153, 113290. [Google Scholar] [CrossRef]
- Lim, R.; Cabatbat, J.J.T.; Martin, T.L.P.; Kim, H.; Kim, S.; Sung, J.; Ghim, C.M.; Kim, P.J. Large-scale metabolic interaction network of the mouse and human gut microbiota. Sci Data 2020, 7, 204. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tang, J.; Jiang, J.; Chen, Z. Metabolic checkpoints and novel approaches for immunotherapy against cancer. International Journal of Cancer 2022, 150, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yuan, Z.; Zhu, Y.; Liang, C.; Chen, Z.; Zhang, J.; Leng, L. Multi-omics analysis reveals GAPDH posttranscriptional regulation of IFN-γ and PHGDH as a metabolic checkpoint of microglia polarization. Brain, behavior, and immunity 2024, 117, 155–166. [Google Scholar] [CrossRef]
- Fang, J.; Lu, Y.; Zheng, J.; Jiang, X.; Shen, H.; Shang, X.; Lu, Y.; Fu, P. Exploring the crosstalk between endothelial cells, immune cells, and immune checkpoints in the tumor microenvironment: new insights and therapeutic implications. Cell death & disease 2023, 14, 586. [Google Scholar]
- Liu, M.; Hong, L.; Sridhar, S.; Jaynes, P.; Tipgomut, C.; Poon, L.; De Mel, S.; Lee, J.S.X.; Ng, S.-B.; Tan, C.L. Spatial-Resolved Transcriptomics Reveals Immune Landscape Variations in Primary Central Nervous System Lymphoma (PCNSL) and Diffuse Large B-Cell Lymphoma (DLBCL). Blood 2024, 144, 3004. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. Revolutionizing our understanding of Parkinson’s disease: Dr. Heinz Reichmann’s pioneering research and future research direction. J Neural Transm (Vienna) 2024, 131, 1367–1387. [Google Scholar] [CrossRef]
- Abdel-Rahman, S.A.; Gabr, M. Small molecule immunomodulators as next-generation therapeutics for glioblastoma. Cancers 2024, 16, 435. [Google Scholar] [CrossRef]
- de Lima, E.P.; Tanaka, M.; Lamas, C.B.; Quesada, K.; Detregiachi, C.R.P.; Araújo, A.C.; Guiguer, E.L.; Catharin, V.; de Castro, M.V.M.; Junior, E.B.; et al. Vascular Impairment, Muscle Atrophy, and Cognitive Decline: Critical Age-Related Conditions. Biomedicines 2024, 12. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Laurindo, L.F.; de Oliveira Zanuso, B.; da Silva, R.M.S.; Gallerani Caglioni, L.; Nunes Junqueira de Moraes, V.B.F.; Fornari Laurindo, L.; Dogani Rodrigues, V.; da Silva Camarinha Oliveira, J.; Beluce, M.E.; et al. AdipoRon’s Impact on Alzheimer’s Disease-A Systematic Review and Meta-Analysis. Int J Mol Sci 2025, 26. [Google Scholar] [CrossRef]
- Kim, J.E.; Patel, K.; Jackson, C.M. The potential for immune checkpoint modulators in cerebrovascular injury and inflammation. Expert opinion on therapeutic targets 2021, 25, 101–113. [Google Scholar] [CrossRef]
- Battaglia, S.; Fazio, C.D.; Borgomaneri, S.; Avenanti, A. Cortisol Imbalance and Fear Learning in PTSD: Therapeutic Approaches to Control Abnormal Fear Responses. Curr Neuropharmacol 2025, 23, 835–846. [Google Scholar] [CrossRef]
- Battaglia, S.; Di Fazio, C.; Mazzà, M.; Tamietto, M.; Avenanti, A. Targeting Human Glucocorticoid Receptors in Fear Learning: A Multiscale Integrated Approach to Study Functional Connectivity. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef] [PubMed]
- Tortora, F.; Hadipour, A.L.; Battaglia, S.; Falzone, A.; Avenanti, A.; Vicario, C.M. The Role of Serotonin in Fear Learning and Memory: A Systematic Review of Human Studies. Brain Sci 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune influencers in action: metabolites and enzymes of the tryptophan-kynurenine metabolic pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef] [PubMed]
- Fujigaki, H.; Yamamoto, Y.; Saito, K. L-Tryptophan-kynurenine pathway enzymes are therapeutic target for neuropsychiatric diseases: Focus on cell type differences. Neuropharmacology 2017, 112, 264–274. [Google Scholar] [CrossRef]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef] [PubMed]
- Klaessens, S.; Stroobant, V.; De Plaen, E.; Van den Eynde, B.J. Systemic tryptophan homeostasis. Frontiers in molecular biosciences 2022, 9, 897929. [Google Scholar] [CrossRef]
- Cheong, J.E.; Sun, L. Targeting the IDO1/TDO2–KYN–AhR pathway for cancer immunotherapy–challenges and opportunities. Trends in pharmacological sciences 2018, 39, 307–325. [Google Scholar] [CrossRef]
- Stone, T.W.; Williams, R.O. Tryptophan metabolism as a ‘reflex’feature of neuroimmune communication: sensor and effector functions for the indoleamine-2, 3-dioxygenase kynurenine pathway. Journal of Neurochemistry 2024, 168, 3333–3357. [Google Scholar] [CrossRef] [PubMed]
- Labadie, B.W.; Bao, R.; Luke, J.J. Reimagining IDO pathway inhibition in cancer immunotherapy via downstream focus on the tryptophan–kynurenine–aryl hydrocarbon axis. Clinical Cancer Research 2019, 25, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Vécsei, L. From Lab to Life: Exploring Cutting-Edge Models for Neurological and Psychiatric Disorders. Biomedicines 2024, 12. [Google Scholar] [CrossRef]
- Platten, M.; von Knebel Doeberitz, N.; Oezen, I.; Wick, W.; Ochs, K. Cancer immunotherapy by targeting IDO1/TDO and their downstream effectors. Frontiers in immunology 2015, 5, 673. [Google Scholar] [CrossRef] [PubMed]
- Triplett, T.A.; Garrison, K.C.; Marshall, N.; Donkor, M.; Blazeck, J.; Lamb, C.; Qerqez, A.; Dekker, J.D.; Tanno, Y.; Lu, W.-C. Reversal of indoleamine 2, 3-dioxygenase–mediated cancer immune suppression by systemic kynurenine depletion with a therapeutic enzyme. Nature biotechnology 2018, 36, 758–764. [Google Scholar] [CrossRef]
- Stone, T.W.; Williams, R.O. Modulation of T cells by tryptophan metabolites in the kynurenine pathway. Trends in Pharmacological Sciences 2023, 44, 442–456. [Google Scholar] [CrossRef]
- Williams, H.L.; Frei, A.L.; Koessler, T.; Berger, M.D.; Dawson, H.; Michielin, O.; Zlobec, I. The current landscape of spatial biomarkers for prediction of response to immune checkpoint inhibition. NPJ precision oncology 2024, 8, 178. [Google Scholar] [CrossRef]
- Kim, M.; Tomek, P. Tryptophan: A Rheostat of Cancer Immune Escape Mediated by Immunosuppressive Enzymes IDO1 and TDO. Front Immunol 2021, 12, 636081. [Google Scholar] [CrossRef]
- Campesato, L.F.; Budhu, S.; Tchaicha, J.; Weng, C.H.; Gigoux, M.; Cohen, I.J.; Redmond, D.; Mangarin, L.; Pourpe, S.; Liu, C.; et al. Blockade of the AHR restricts a Treg-macrophage suppressive axis induced by L-Kynurenine. Nat Commun 2020, 11, 4011. [Google Scholar] [CrossRef]
- Ala, M. The footprint of kynurenine pathway in every cancer: a new target for chemotherapy. European Journal of Pharmacology 2021, 896, 173921. [Google Scholar] [CrossRef] [PubMed]
- Zeitler, L.; Murray, P.J. IL4i1 and IDO1: Oxidases that control a tryptophan metabolic nexus in cancer. J Biol Chem 2023, 299, 104827. [Google Scholar] [CrossRef] [PubMed]
- Sadik, A.; Somarribas Patterson, L.F.; Öztürk, S.; Mohapatra, S.R.; Panitz, V.; Secker, P.F.; Pfänder, P.; Loth, S.; Salem, H.; Prentzell, M.T.; et al. IL4I1 Is a Metabolic Immune Checkpoint that Activates the AHR and Promotes Tumor Progression. Cell 2020, 182, 1252–1270.e1234. [Google Scholar] [CrossRef]
- Liu, M.; Wang, X.; Wang, L.; Ma, X.; Gong, Z.; Zhang, S.; Li, Y. Targeting the IDO1 pathway in cancer: from bench to bedside. J Hematol Oncol 2018, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Nazzi, C.; Di Fazio, C.; Borgomaneri, S. The role of pre-supplementary motor cortex in action control with emotional stimuli: A repetitive transcranial magnetic stimulation study. Ann N Y Acad Sci 2024, 1536, 151–166. [Google Scholar] [CrossRef]
- Battaglia, S.; Nazzi, C.; Fullana, M.A.; di Pellegrino, G.; Borgomaneri, S. ‘Nip it in the bud’: Low-frequency rTMS of the prefrontal cortex disrupts threat memory consolidation in humans. Behav Res Ther 2024, 178, 104548. [Google Scholar] [CrossRef]
- Tanaka, M.; He, Z.; Han, S.; Battaglia, S. Editorial: Noninvasive brain stimulation: a promising approach to study and improve emotion regulation. Front Behav Neurosci 2025, 19, 1633936. [Google Scholar] [CrossRef]
- Krolak, T.; Chan, K.Y.; Kaplan, L.; Huang, Q.; Wu, J.; Zheng, Q.; Kozareva, V.; Beddow, T.; Tobey, I.G.; Pacouret, S.; et al. A High-Efficiency AAV for Endothelial Cell Transduction Throughout the Central Nervous System. Nat Cardiovasc Res 2022, 1, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Gleichman, A.J.; Kawaguchi, R.; Sofroniew, M.V.; Carmichael, S.T. A toolbox of astrocyte-specific, serotype-independent adeno-associated viral vectors using microRNA targeting sequences. Nature Communications 2023, 14, 7426. [Google Scholar] [CrossRef]
- Alerasool, N.; Segal, D.; Lee, H.; Taipale, M. An efficient KRAB domain for CRISPRi applications in human cells. Nat Methods 2020, 17, 1093–1096. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, F.; Gao, G. CRISPR-Based Therapeutic Genome Editing: Strategies and In Vivo Delivery by AAV Vectors. Cell 2020, 181, 136–150. [Google Scholar] [CrossRef]
- Chen, X.; Wolfe, D.A.; Bindu, D.S.; Zhang, M.; Taskin, N.; Goertsen, D.; Shay, T.F.; Sullivan, E.E.; Huang, S.F.; Ravindra Kumar, S.; et al. Functional gene delivery to and across brain vasculature of systemic AAVs with endothelial-specific tropism in rodents and broad tropism in primates. Nat Commun 2023, 14, 3345. [Google Scholar] [CrossRef]
- Bolanos-Palmieri, P.; Kotb, A.; Schenk, H.; Bähre, H.; Schroder, P.; Schiffer, M. MO006 CHANGES IN THE KYNURENINE PATHWAY LEAD TO ALTERATIONS IN NAD BALANCE AND BIOENERGETICS PARAMETERS IN GLOMERULAR CELLS IN VITRO AND CONTRIBUTE TO PROTEINURIA IN A ZEBRAFISH MODEL. Nephrology Dialysis Transplantation 2021, 36, gfab079–002. [Google Scholar] [CrossRef]
- Kampmann, M. CRISPRi and CRISPRa Screens in Mammalian Cells for Precision Biology and Medicine. ACS Chem Biol 2018, 13, 406–416. [Google Scholar] [CrossRef]
- Battaglia, S.; Avenanti, A.; Vécsei, L.; Tanaka, M. Neurodegeneration in Cognitive Impairment and Mood Disorders for Experimental, Clinical and Translational Neuropsychiatry. Biomedicines 2024, 12. [Google Scholar] [CrossRef]
- Späth, M.R.; Hoyer-Allo, K.J.R.; Seufert, L.; Höhne, M.; Lucas, C.; Bock, T.; Isermann, L.; Brodesser, S.; Lackmann, J.W.; Kiefer, K.; et al. Organ Protection by Caloric Restriction Depends on Activation of the De Novo NAD+ Synthesis Pathway. J Am Soc Nephrol 2023, 34, 772–792. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.; Altermatt, M.; Dobreva, T.; Chen, S.; Wang, A.; Thomson, M.; Gradinaru, V. Deep Parallel Characterization of AAV Tropism and AAV-Mediated Transcriptional Changes via Single-Cell RNA Sequencing. Front Immunol 2021, 12, 730825. [Google Scholar] [CrossRef] [PubMed]
- Börner, K.; Kienle, E.; Huang, L.Y.; Weinmann, J.; Sacher, A.; Bayer, P.; Stüllein, C.; Fakhiri, J.; Zimmermann, L.; Westhaus, A.; et al. Pre-arrayed Pan-AAV Peptide Display Libraries for Rapid Single-Round Screening. Mol Ther 2020, 28, 1016–1032. [Google Scholar] [CrossRef]
- Flickinger, K.M.; Cantor, J.R. Uncovering the Conditionally Essential Roles of NAD Kinases in Human Cells. The FASEB Journal 2022, 36. [Google Scholar] [CrossRef]
- Walsh, S.; Gardner, L.; Deiters, A.; Williams, G.J. Intracellular light-activation of riboswitch activity. Chembiochem 2014, 15, 1346–1351. [Google Scholar] [CrossRef]
- Borrachero-Conejo, A.I.; Adams, W.R.; Saracino, E.; Mola, M.G.; Wang, M.; Posati, T.; Formaggio, F.; De Bellis, M.; Frigeri, A.; Caprini, M.; et al. Stimulation of water and calcium dynamics in astrocytes with pulsed infrared light. Faseb j 2020, 34, 6539–6553. [Google Scholar] [CrossRef]
- Spennato, D.; Leone, J.; Gundhardt, C.; Varnavski, O.; Fabbri, R.; Caprini, M.; Zamboni, R.; Benfenati, V.; Goodson, T. , 3rd. Investigations of Astrocyte Calcium Signaling and Imaging with Classical and Nonclassical Light. J Phys Chem B 2024, 128, 7966–7977. [Google Scholar] [CrossRef]
- Zhang, Y.; Rózsa, M.; Liang, Y.; Bushey, D.; Wei, Z.; Zheng, J.; Reep, D.; Broussard, G.J.; Tsang, A.; Tsegaye, G.; et al. Fast and sensitive GCaMP calcium indicators for imaging neural populations. Nature 2023, 615, 884–891. [Google Scholar] [CrossRef]
- Qiao, L.; Niu, L.; Wang, M.; Wang, Z.; Kong, D.; Yu, G.; Ye, H. A sensitive red/far-red photoswitch for controllable gene therapy in mouse models of metabolic diseases. Nature Communications 2024, 15, 10310. [Google Scholar] [CrossRef] [PubMed]
- Shemetov, A.A.; Monakhov, M.V.; Zhang, Q.; Canton-Josh, J.E.; Kumar, M.; Chen, M.; Matlashov, M.E.; Li, X.; Yang, W.; Nie, L.; et al. A near-infrared genetically encoded calcium indicator for in vivo imaging. Nat Biotechnol 2021, 39, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Lohr, C.; Beiersdorfer, A.; Fischer, T.; Hirnet, D.; Rotermund, N.; Sauer, J.; Schulz, K.; Gee, C.E. Using Genetically Encoded Calcium Indicators to Study Astrocyte Physiology: A Field Guide. Front Cell Neurosci 2021, 15, 690147. [Google Scholar] [CrossRef] [PubMed]
- Gorzo, K.A.; Gordon, G.R. Photonics tools begin to clarify astrocyte calcium transients. Neurophotonics 2022, 9, 021907. [Google Scholar] [CrossRef]
- Walton, J.C.; Bumgarner, J.R.; Nelson, R.J. Sex Differences in Circadian Rhythms. Cold Spring Harb Perspect Biol 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Tuka, B.; Vécsei, L. Navigating the Neurobiology of Migraine: From Pathways to Potential Therapies. Cells 2024, 13. [Google Scholar] [CrossRef]
- Minbay, M.; Khan, A.; Ghasemi, A.R.; Ingram, K.K.; Ay, A.A. Sex-specific associations between circadian-related genes and depression in UK Biobank participants highlight links to glucose metabolism, inflammation and neuroplasticity pathways. Psychiatry Res 2024, 337, 115948. [Google Scholar] [CrossRef]
- Abo, S.M.; Layton, A.T. Modeling the circadian regulation of the immune system: Sexually dimorphic effects of shift work. PLoS computational biology 2021, 17, e1008514. [Google Scholar] [CrossRef] [PubMed]
- Logan, R.W.; Xue, X.; Ketchesin, K.D.; Hoffman, G.; Roussos, P.; Tseng, G.; McClung, C.A.; Seney, M.L. Sex Differences in Molecular Rhythms in the Human Cortex. Biol Psychiatry 2022, 91, 152–162. [Google Scholar] [CrossRef]
- Tanaka, M.; Battaglia, S. Dualistic Dynamics in Neuropsychiatry: From Monoaminergic Modulators to Multiscale Biomarker Maps. Biomedicines 2025, 13. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.J.; Christofides, K.; Weissleder, C.; Huang, X.F.; Shannon Weickert, C.; Lim, C.K.; Newell, K.A. Sex- and suicide-specific alterations in the kynurenine pathway in the anterior cingulate cortex in major depression. Neuropsychopharmacology 2024, 49, 584–592. [Google Scholar] [CrossRef]
- Liloia, D.; Zamfira, D.A.; Tanaka, M.; Manuello, J.; Crocetta, A.; Keller, R.; Cozzolino, M.; Duca, S.; Cauda, F.; Costa, T. Disentangling the role of gray matter volume and concentration in autism spectrum disorder: A meta-analytic investigation of 25 years of voxel-based morphometry research. Neurosci Biobehav Rev 2024, 164, 105791. [Google Scholar] [CrossRef]
- Bailey, M.; Silver, R. Sex differences in circadian timing systems: implications for disease. Front Neuroendocrinol 2014, 35, 111–139. [Google Scholar] [CrossRef]
- Tanaka, M. Beyond the boundaries: Transitioning from categorical to dimensional paradigms in mental health diagnostics. Adv Clin Exp Med 2024, 33, 1295–1301. [Google Scholar] [CrossRef]
- Wu, F.; Langer, P.; Shim, J.; Fleisch, E.; Barata, F. Comparative Efficacy of Commercial Wearables for Circadian Rhythm Home Monitoring From Activity, Heart Rate, and Core Body Temperature. IEEE J Biomed Health Inform 2025, 29, 900–908. [Google Scholar] [CrossRef]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br J Pharmacol 2018, 175, 3190–3199. [Google Scholar] [CrossRef]
- Lightman, S.L.; Conway-Campbell, B.L. Circadian and ultradian rhythms: Clinical implications. J Intern Med 2024, 296, 121–138. [Google Scholar] [CrossRef]
- Steele, T.A.; St Louis, E.K.; Videnovic, A.; Auger, R.R. Circadian Rhythm Sleep-Wake Disorders: a Contemporary Review of Neurobiology, Treatment, and Dysregulation in Neurodegenerative Disease. Neurotherapeutics 2021, 18, 53–74. [Google Scholar] [CrossRef]
- Walker, W.H., 2nd; Walton, J.C.; DeVries, A.C.; Nelson, R.J. Circadian rhythm disruption and mental health. Transl Psychiatry 2020, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr Neuropharmacol 2017, 15, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Lévi, F.A.; Okyar, A.; Hadadi, E.; Innominato, P.F.; Ballesta, A. Circadian Regulation of Drug Responses: Toward Sex-Specific and Personalized Chronotherapy. Annu Rev Pharmacol Toxicol 2024, 64, 89–114. [Google Scholar] [CrossRef] [PubMed]
- Karaboué, A.; Innominato, P.F.; Wreglesworth, N.I.; Duchemann, B.; Adam, R.; Lévi, F.A. Why does circadian timing of administration matter for immune checkpoint inhibitors’ efficacy? Br J Cancer 2024, 131, 783–796. [Google Scholar] [CrossRef]
- Ohdo, S.; Koyanagi, S.; Matsunaga, N. Chronopharmacological strategies focused on chrono-drug discovery. Pharmacol Ther 2019, 202, 72–90. [Google Scholar] [CrossRef]
- Wang, C.; Zeng, Q.; Gül, Z.M.; Wang, S.; Pick, R.; Cheng, P.; Bill, R.; Wu, Y.; Naulaerts, S.; Barnoud, C.; et al. Circadian tumor infiltration and function of CD8(+) T cells dictate immunotherapy efficacy. Cell 2024, 187, 2690–2702.e2617. [Google Scholar] [CrossRef]
- Ye, Y.; Xiang, Y.; Ozguc, F.M.; Kim, Y.; Liu, C.J.; Park, P.K.; Hu, Q.; Diao, L.; Lou, Y.; Lin, C.; et al. The Genomic Landscape and Pharmacogenomic Interactions of Clock Genes in Cancer Chronotherapy. Cell Syst 2018, 6, 314–328.e312. [Google Scholar] [CrossRef]
- Ephraim, A.; Leatheng, C.; Lu, Z.E.; Xia, X.; Pirruccello, J.P.; Marotti, J.D.; MacKenzie, T.; Chamberlin, M.D. Association of plasma kynurenine (KYN) with plasma osteopontin (OPN) in patients with locally invasive breast cancer. 2024.
- Chang, K.H.; Cheng, M.L.; Tang, H.Y.; Huang, C.Y.; Wu, Y.R.; Chen, C.M. Alternations of Metabolic Profile and Kynurenine Metabolism in the Plasma of Parkinson’s Disease. Mol Neurobiol 2018, 55, 6319–6328. [Google Scholar] [CrossRef]
- Chantrapanichkul, P.; Stevenson, M.O.; Suppakitjanusant, P.; Goodman, M.; Tangpricha, V. SERUM HORMONE CONCENTRATIONS IN TRANSGENDER INDIVIDUALS RECEIVING GENDER-AFFIRMING HORMONE THERAPY: A LONGITUDINAL RETROSPECTIVE COHORT STUDY. Endocr Pract 2021, 27, 27–33. [Google Scholar] [CrossRef]
- Kervezee, L.; Cermakian, N.; Boivin, D.B. Individual metabolomic signatures of circadian misalignment during simulated night shifts in humans. PLoS Biol 2019, 17, e3000303. [Google Scholar] [CrossRef] [PubMed]
- Skene, D.J.; Skornyakov, E.; Chowdhury, N.R.; Gajula, R.P.; Middleton, B.; Satterfield, B.C.; Porter, K.I.; Van Dongen, H.P.A.; Gaddameedhi, S. Separation of circadian- and behavior-driven metabolite rhythms in humans provides a window on peripheral oscillators and metabolism. Proc Natl Acad Sci U S A 2018, 115, 7825–7830. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, D.S.; Akhmetova, L.; Carlin, D.; Romero, H.; Welsh, D.K.; Colwell, C.S.; Desplats, P. Circadian modulation by time-restricted feeding rescues brain pathology and improves memory in mouse models of Alzheimer’s disease. Cell Metab 2023, 35, 1704–1721.e1706. [Google Scholar] [CrossRef]
- Cheng, P.; Walch, O.; Huang, Y.; Mayer, C.; Sagong, C.; Cuamatzi Castelan, A.; Burgess, H.J.; Roth, T.; Forger, D.B.; Drake, C.L. Predicting circadian misalignment with wearable technology: validation of wrist-worn actigraphy and photometry in night shift workers. Sleep 2021, 44. [Google Scholar] [CrossRef]
- Emens, J.S.; Burgess, H.J. Effect of Light and Melatonin and Other Melatonin Receptor Agonists on Human Circadian Physiology. Sleep Med Clin 2015, 10, 435–453. [Google Scholar] [CrossRef] [PubMed]
- Boivin, D.B.; Boudreau, P.; Kosmadopoulos, A. Disturbance of the Circadian System in Shift Work and Its Health Impact. J Biol Rhythms 2022, 37, 3–28. [Google Scholar] [CrossRef]
- Chen, X.; He, R.; Chen, X.; Jiang, L.; Wang, F. Optimizing dose-schedule regimens with bayesian adaptive designs: opportunities and challenges. Front Pharmacol 2023, 14, 1261312. [Google Scholar] [CrossRef]
- Lu, M.; Yuan, Y.; Liu, S. A Bayesian pharmacokinetics integrated phase I-II design to optimize dose-schedule regimes. Biostatistics 2024, 26. [Google Scholar] [CrossRef]
- Ballesta, A.; Innominato, P.F.; Dallmann, R.; Rand, D.A.; Lévi, F.A. Systems Chronotherapeutics. Pharmacol Rev 2017, 69, 161–199. [Google Scholar] [CrossRef]
- Dong, D.; Yang, D.; Lin, L.; Wang, S.; Wu, B. Circadian rhythm in pharmacokinetics and its relevance to chronotherapy. Biochem Pharmacol 2020, 178, 114045. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, R.; Yan, F.; Yap, T.A.; Yuan, Y. Shotgun: A Bayesian seamless phase I-II design to accelerate the development of targeted therapies and immunotherapy. Contemp Clin Trials 2021, 104, 106338. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Diekman, C.O. Dosing Time of Day Impacts the Safety of Antiarrhythmic Drugs in a Computational Model of Cardiac Electrophysiology. J Biol Rhythms 2025, 40, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Huang, Z.; Duraj-Thatte, A.M.; Ebert, M.P.; Zhang, F.; Burgermeister, E.; Liu, X.; Scott, B.M.; Li, G.; Zuo, T. Engineering the gut microbiome. Nature Reviews Bioengineering 2023, 1, 665–679. [Google Scholar] [CrossRef]
- Sorbara, M.T.; Pamer, E.G. Microbiome-based therapeutics. Nat Rev Microbiol 2022, 20, 365–380. [Google Scholar] [CrossRef]
- Mousa, W.K.; Al Ali, A. The Gut Microbiome Advances Precision Medicine and Diagnostics for Inflammatory Bowel Diseases. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef]
- Dodd, D.; Spitzer, M.H.; Van Treuren, W.; Merrill, B.D.; Hryckowian, A.J.; Higginbottom, S.K.; Le, A.; Cowan, T.M.; Nolan, G.P.; Fischbach, M.A.; et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 2017, 551, 648–652. [Google Scholar] [CrossRef]
- Han, S.; Van Treuren, W.; Fischer, C.R.; Merrill, B.D.; DeFelice, B.C.; Sanchez, J.M.; Higginbottom, S.K.; Guthrie, L.; Fall, L.A.; Dodd, D.; et al. A metabolomics pipeline for the mechanistic interrogation of the gut microbiome. Nature 2021, 595, 415–420. [Google Scholar] [CrossRef]
- Nunes, Y.C.; Mendes, N.M.; Pereira de Lima, E.; Chehadi, A.C.; Lamas, C.B.; Haber, J.F.S.; Dos Santos Bueno, M.; Araújo, A.C.; Catharin, V.C.S.; Detregiachi, C.R.P.; et al. Curcumin: A Golden Approach to Healthy Aging: A Systematic Review of the Evidence. Nutrients 2024, 16. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, S.; Sen, P.; Dickens, A.M.; Orešič, M.; Bertram, H.C. Gut metabolome meets microbiome: A methodological perspective to understand the relationship between host and microbe. Methods 2018, 149, 3–12. [Google Scholar] [CrossRef]
- Guo, H.; Liu, X.; Chen, T.; Wang, X.; Zhang, X. Akkermansia muciniphila Improves Depressive-Like Symptoms by Modulating the Level of 5-HT Neurotransmitters in the Gut and Brain of Mice. Mol Neurobiol 2024, 61, 821–834. [Google Scholar] [CrossRef]
- Tian, P.; O’Riordan, K.J.; Lee, Y.K.; Wang, G.; Zhao, J.; Zhang, H.; Cryan, J.F.; Chen, W. Towards a psychobiotic therapy for depression: Bifidobacterium breve CCFM1025 reverses chronic stress-induced depressive symptoms and gut microbial abnormalities in mice. Neurobiol Stress 2020, 12, 100216. [Google Scholar] [CrossRef]
- Ding, Y.; Bu, F.; Chen, T.; Shi, G.; Yuan, X.; Feng, Z.; Duan, Z.; Wang, R.; Zhang, S.; Wang, Q.; et al. A next-generation probiotic: Akkermansia muciniphila ameliorates chronic stress-induced depressive-like behavior in mice by regulating gut microbiota and metabolites. Appl Microbiol Biotechnol 2021, 105, 8411–8426. [Google Scholar] [CrossRef]
- Tian, P.; Chen, Y.; Zhu, H.; Wang, L.; Qian, X.; Zou, R.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; et al. Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: A randomized clinical trial. Brain Behav Immun 2022, 100, 233–241. [Google Scholar] [CrossRef]
- Yamamura, R.; Okubo, R.; Katsumata, N.; Odamaki, T.; Hashimoto, N.; Kusumi, I.; Xiao, J.; Matsuoka, Y.J. Lipid and Energy Metabolism of the Gut Microbiota Is Associated with the Response to Probiotic Bifidobacterium breve Strain for Anxiety and Depressive Symptoms in Schizophrenia. J Pers Med 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Su, Z.; Chen, Z.; Cao, J.; Liu, X.; Xu, F. Lactobacillus reuteri strain 8008 attenuated the aggravation of depressive-like behavior induced by CUMS in high-fat diet-fed mice through regulating the gut microbiota. Front Pharmacol 2023, 14, 1149185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, H.; Wu, P.; Yang, S.; Xue, W.; Xu, B.; Zhang, S.; Tang, B.; Xu, D. Akkermansia muciniphila: A promising probiotic against inflammation and metabolic disorders. Virulence 2024, 15, 2375555. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Zhao, J.; Li, L.; Wang, Y.; Chen, F.; Li, Y.; Cheng, R.; He, F.; Ze, X.; et al. Effects of Bifidobacterium breve 207-1 on regulating lifestyle behaviors and mental wellness in healthy adults based on the microbiome-gut-brain axis: a randomized, double-blind, placebo-controlled trial. Eur J Nutr 2024, 63, 2567–2585. [Google Scholar] [CrossRef]
- Liu, J.Y.; Lin, T.L.; Chiu, C.Y.; Hsieh, P.F.; Lin, Y.T.; Lai, L.Y.; Wang, J.T. Decolonization of carbapenem-resistant Klebsiella pneumoniae from the intestinal microbiota of model mice by phages targeting two surface structures. Front Microbiol 2022, 13, 877074. [Google Scholar] [CrossRef] [PubMed]
- Medlock, G.; Felix, C.; Alsharif, W.; Cornacchione, L.; Schinn, M.; Watson, A.; Bedard-Shurtleff, S.; Norman, J.; Faith, J.; Kuijper, E.J. 2521. VE707, a defined live biotherapeutic product for prevention of infection by multidrug-resistant gram-negative bacteria. In Proceedings of the Open Forum Infectious Diseases, 2023; p. ofad500. 2139.
- Tavoukjian, V. Faecal microbiota transplantation for the decolonization of antibiotic-resistant bacteria in the gut: a systematic review and meta-analysis. J Hosp Infect 2019, 102, 174–188. [Google Scholar] [CrossRef]
- Macareño-Castro, J.; Solano-Salazar, A.; Dong, L.T.; Mohiuddin, M.; Espinoza, J.L. Fecal microbiota transplantation for Carbapenem-Resistant Enterobacteriaceae: A systematic review. J Infect 2022, 84, 749–759. [Google Scholar] [CrossRef]
- Mortzfeld, B.M.; Palmer, J.D.; Bhattarai, S.K.; Dupre, H.L.; Mercado-Lubio, R.; Silby, M.W.; Bang, C.; McCormick, B.A.; Bucci, V. Microcin MccI47 selectively inhibits enteric bacteria and reduces carbapenem-resistant Klebsiella pneumoniae colonization in vivo when administered via an engineered live biotherapeutic. Gut Microbes 2022, 14, 2127633. [Google Scholar] [CrossRef]
- Osbelt, L.; Wende, M.; Almási, É.; Derksen, E.; Muthukumarasamy, U.; Lesker, T.R.; Galvez, E.J.C.; Pils, M.C.; Schalk, E.; Chhatwal, P.; et al. Klebsiella oxytoca causes colonization resistance against multidrug-resistant K. pneumoniae in the gut via cooperative carbohydrate competition. Cell Host Microbe 2021, 29, 1663–1679.e1667. [Google Scholar] [CrossRef] [PubMed]
- Heavey, M.K.; Durmusoglu, D.; Crook, N.; Anselmo, A.C. Discovery and delivery strategies for engineered live biotherapeutic products. Trends Biotechnol 2022, 40, 354–369. [Google Scholar] [CrossRef] [PubMed]
- Rottinghaus, A.G.; Ferreiro, A.; Fishbein, S.R.S.; Dantas, G.; Moon, T.S. Genetically stable CRISPR-based kill switches for engineered microbes. Nat Commun 2022, 13, 672. [Google Scholar] [CrossRef]
- Hartig, A.M.; Dai, W.; Zhang, K.; Kapoor, K.; Rottinghaus, A.G.; Moon, T.S.; Parker, K.M. Influence of Environmental Conditions on the Escape Rates of Biocontained Genetically Engineered Microbes. Environ Sci Technol 2024, 58, 22657–22667. [Google Scholar] [CrossRef]
- Gencay, Y.E.; Jasinskytė, D.; Robert, C.; Semsey, S.; Martínez, V.; Petersen, A.; Brunner, K.; de Santiago Torio, A.; Salazar, A.; Turcu, I.C.; et al. Engineered phage with antibacterial CRISPR-Cas selectively reduce E. coli burden in mice. Nat Biotechnol 2024, 42, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Tiwari, P.; Deep, A.; Kidwai, S.; Gupta, S.; Thakur, K.G.; Singh, R. System-Wide Analysis Unravels the Differential Regulation and In Vivo Essentiality of Virulence-Associated Proteins B and C Toxin-Antitoxin Systems of Mycobacterium tuberculosis. J Infect Dis 2018, 217, 1809–1820. [Google Scholar] [CrossRef]
- Lin, M.; Kussell, E. Inferring bacterial recombination rates from large-scale sequencing datasets. Nat Methods 2019, 16, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.; Kolida, S.; Marchesi, J.R.; Want, E.; Sidaway, J.E.; Swann, J.R. In Vitro Modeling of Bile Acid Processing by the Human Fecal Microbiota. Front Microbiol 2018, 9, 1153. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Wu, A.; Liu, C.; Cao, X.; Wang, R.; Shu, X.; Wang, L.; Zhang, Y.; Xiang, H.; Li, M. The toxin-antitoxin RNA guards of CRISPR-Cas evolved high specificity through repeat degeneration. Nucleic Acids Res 2022, 50, 9442–9452. [Google Scholar] [CrossRef]
- Wiechert, J.; Gätgens, C.; Wirtz, A.; Frunzke, J. Inducible Expression Systems Based on Xenogeneic Silencing and Counter-Silencing and Design of a Metabolic Toggle Switch. ACS Synth Biol 2020, 9, 2023–2038. [Google Scholar] [CrossRef]
- Schwarz, S.; Gildemeister, D.; Hein, A.; Schröder, P.; Bachmann, J. Environmental fate and effects assessment of human pharmaceuticals: lessons learnt from regulatory data. Environmental Sciences Europe 2021, 33, 68. [Google Scholar] [CrossRef]
- Richard, E.; Darracq, B.; Littner, E.; Vit, C.; Whiteway, C.; Bos, J.; Fournes, F.; Garriss, G.; Conte, V.; Lapaillerie, D.; et al. Cassette recombination dynamics within chromosomal integrons are regulated by toxin-antitoxin systems. Sci Adv 2024, 10, eadj3498. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Sun, Q.; Hui, Y.; Seth, A.; Petrovsky, N.; Zhao, C.X. Microfluidic formation of core-shell alginate microparticles for protein encapsulation and controlled release. J Colloid Interface Sci 2019, 539, 497–503. [Google Scholar] [CrossRef]
- Omer, A.M.; Ahmed, M.S.; El-Subruiti, G.M.; Khalifa, R.E.; Eltaweil, A.S. pH-Sensitive Alginate/Carboxymethyl Chitosan/Aminated Chitosan Microcapsules for Efficient Encapsulation and Delivery of Diclofenac Sodium. Pharmaceutics 2021, 13. [Google Scholar] [CrossRef]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan--a review. J Control Release 2006, 114, 1–14. [Google Scholar] [CrossRef]
- Qu, Q.; Yang, A.; Wang, J.; Xie, M.; Zhang, X.; Huang, D.; Xiong, R.; Pei, D.; Huang, C. Responsive and biocompatible chitosan-phytate microparticles with various morphology for antibacterial activity based on gas-shearing microfluidics. J Colloid Interface Sci 2023, 649, 68–75. [Google Scholar] [CrossRef]
- Feng, R.; Wang, L.; Zhou, P.; Luo, Z.; Li, X.; Gao, L. Development of the pH responsive chitosan-alginate based microgel for encapsulation of Jughans regia L. polyphenols under simulated gastrointestinal digestion in vitro. Carbohydr Polym 2020, 250, 116917. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.; Dai, S.; Lian, Z.; Tong, X.; Yang, S.; Chen, Y.; Qi, W.; Peng, X.; Wang, H.; Jiang, L. The Layered Encapsulation of Vitamin B(2) and β-Carotene in Multilayer Alginate/Chitosan Gel Microspheres: Improving the Bioaccessibility of Vitamin B(2) and β-Carotene. Foods 2021, 11. [Google Scholar] [CrossRef]
- Liu, X.; Liu, L.; Huang, F.; Meng, Y.; Chen, Y.; Wang, J.; Wang, S.; Luo, Y.; Li, J.; Liang, Y. pH-sensitive chitosan/sodium alginate/calcium chloride hydrogel beads for potential oral delivery of rice bran bioactive peptides. Food Chem 2025, 470, 142618. [Google Scholar] [CrossRef]
- Tian, Y.; Ran, H.; Wen, X.; Fu, G.; Zhou, X.; Liu, R.; Pan, T. Probiotics improve symptoms of patients with COVID-19 through gut-lung axis: a systematic review and meta-analysis. Front Nutr 2023, 10, 1179432. [Google Scholar] [CrossRef]
- Gutiérrez-Castrellón, P.; Gandara-Martí, T.; Abreu, Y.A.A.T.; Nieto-Rufino, C.D.; López-Orduña, E.; Jiménez-Escobar, I.; Jiménez-Gutiérrez, C.; López-Velazquez, G.; Espadaler-Mazo, J. Probiotic improves symptomatic and viral clearance in Covid19 outpatients: a randomized, quadruple-blinded, placebo-controlled trial. Gut Microbes 2022, 14, 2018899. [Google Scholar] [CrossRef] [PubMed]
- Xavier-Santos, D.; Padilha, M.; Fabiano, G.A.; Vinderola, G.; Gomes Cruz, A.; Sivieri, K.; Costa Antunes, A.E. Evidences and perspectives of the use of probiotics, prebiotics, synbiotics, and postbiotics as adjuvants for prevention and treatment of COVID-19: A bibliometric analysis and systematic review. Trends Food Sci Technol 2022, 120, 174–192. [Google Scholar] [CrossRef] [PubMed]
- Petrariu, O.A.; Barbu, I.C.; Niculescu, A.G.; Constantin, M.; Grigore, G.A.; Cristian, R.E.; Mihaescu, G.; Vrancianu, C.O. Role of probiotics in managing various human diseases, from oral pathology to cancer and gastrointestinal diseases. Front Microbiol 2023, 14, 1296447. [Google Scholar] [CrossRef]
- Schaub, A.C.; Schneider, E.; Vazquez-Castellanos, J.F.; Schweinfurth, N.; Kettelhack, C.; Doll, J.P.K.; Yamanbaeva, G.; Mählmann, L.; Brand, S.; Beglinger, C.; et al. Clinical, gut microbial and neural effects of a probiotic add-on therapy in depressed patients: a randomized controlled trial. Transl Psychiatry 2022, 12, 227. [Google Scholar] [CrossRef]
- Horvath, A.; Habisch, H.; Prietl, B.; Pfeifer, V.; Balazs, I.; Kovacs, G.; Foris, V.; John, N.; Kleinschek, D.; Feldbacher, N.; et al. Alteration of the Gut-Lung Axis After Severe COVID-19 Infection and Modulation Through Probiotics: A Randomized, Controlled Pilot Study. Nutrients 2024, 16. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, J.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Meta-analysis of randomized controlled trials of the effects of probiotics on functional constipation in adults. Clin Nutr 2020, 39, 2960–2969. [Google Scholar] [CrossRef]
- Xu, L.; Yang, C.S.; Liu, Y.; Zhang, X. Effective Regulation of Gut Microbiota With Probiotics and Prebiotics May Prevent or Alleviate COVID-19 Through the Gut-Lung Axis. Front Pharmacol 2022, 13, 895193. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Eskandari, M.H.; Djafarian, K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin Nutr 2019, 38, 522–528. [Google Scholar] [CrossRef]
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Małus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 2019, 100, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Platten, M.; Nollen, E.A.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov 2019, 18, 379–401. [Google Scholar] [CrossRef]
- Liang, H.; Li, T.; Fang, X.; Xing, Z.; Zhang, S.; Shi, L.; Li, W.; Guo, L.; Kuang, C.; Liu, H.; et al. IDO1/TDO dual inhibitor RY103 targets Kyn-AhR pathway and exhibits preclinical efficacy on pancreatic cancer. Cancer Lett 2021, 522, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.Z.; Zhou, J.; Zhu, Y.B.; He, L.J.; Miao, D.D.; Zhang, S.P.; Liu, X.P.; Zhang, C. Design, synthesis, and biological evaluation of a novel indoleamine 2,3-dioxigenase 1 (IDO1) and thioredoxin reductase (TrxR) dual inhibitor. Bioorg Chem 2020, 105, 104401. [Google Scholar] [CrossRef]
- Xing, Z.; Li, X.; He, Z.N.T.; Fang, X.; Liang, H.; Kuang, C.; Li, A.; Yang, Q. IDO1 Inhibitor RY103 Suppresses Trp-GCN2-Mediated Angiogenesis and Counters Immunosuppression in Glioblastoma. Pharmaceutics 2024, 16. [Google Scholar] [CrossRef]
- Lotz-Jenne, C.; Cren, S.; Joesch, C.; Ackerknecht, S.; Brandes, J.; Moebs, C.; Hartl, D.; Hartrampf, F.; Guerry, P.; Pothier, J. Superiority of dual IDO1/TDO2 inhibition versus IDO1 selective inhibition in reducing immunosuppressive KYN levels in tumors co-expressing IDO1 and TDO2. Cancer Research 2023, 83, 1849–1849. [Google Scholar] [CrossRef]
- Agudelo, L.Z.; Ferreira, D.M.S.; Dadvar, S.; Cervenka, I.; Ketscher, L.; Izadi, M.; Zhengye, L.; Furrer, R.; Handschin, C.; Venckunas, T.; et al. Skeletal muscle PGC-1α1 reroutes kynurenine metabolism to increase energy efficiency and fatigue-resistance. Nat Commun 2019, 10, 2767. [Google Scholar] [CrossRef]
- Spector, S.; Wu, C.; Nguyen, D.; Theodore, G.; Garcia, A.; Feun, L.; Savaraj, N.; Wangpaichitr, M. Targeting kynurenine pathway using novel IDO/TDO dual inhibitor (AT0174) to modulate tumor microenvironment in platinum resistant non-small cell lung cancer cancer: An immunometabolism compliment markers. Cancer Research 2022, 82, 2325–2325. [Google Scholar] [CrossRef]
- Martos, D.; Lőrinczi, B.; Szatmári, I.; Vécsei, L.; Tanaka, M. The Impact of C-3 Side Chain Modifications on Kynurenic Acid: A Behavioral Analysis of Its Analogs in the Motor Domain. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef]
- Martos, D.; Lőrinczi, B.; Szatmári, I.; Vécsei, L.; Tanaka, M. Decoupling Behavioral Domains via Kynurenic Acid Analog Optimization: Implications for Schizophrenia and Parkinson’s Disease Therapeutics. Cells 2025, 14. [Google Scholar] [CrossRef] [PubMed]
- He, X.; He, G.; Chu, Z.; Wu, H.; Wang, J.; Ge, Y.; Shen, H.; Zhang, S.; Shan, J.; Peng, K.; et al. Discovery of the First Potent IDO1/IDO2 Dual Inhibitors: A Promising Strategy for Cancer Immunotherapy. J Med Chem 2021, 64, 17950–17968. [Google Scholar] [CrossRef]
- Di Gregorio, F.; Steinhauser, M.; Maier, M.E.; Thayer, J.F.; Battaglia, S. Error-related cardiac deceleration: Functional interplay between error-related brain activity and autonomic nervous system in performance monitoring. Neurosci Biobehav Rev 2024, 157, 105542. [Google Scholar] [CrossRef]
- Nazzi, C.; Avenanti, A.; Battaglia, S. The Involvement of Antioxidants in Cognitive Decline and Neurodegeneration: Mens Sana in Corpore Sano. Antioxidants (Basel) 2024, 13. [Google Scholar] [CrossRef]
- Battaglia, S.; Nazzi, C.; Lonsdorf, T.B.; Thayer, J.F. Neuropsychobiology of fear-induced bradycardia in humans: progress and pitfalls. Mol Psychiatry 2024, 29, 3826–3840. [Google Scholar] [CrossRef]
- Heyes, M.P. Metabolism and neuropathologic significance of quinolinic acid and kynurenic acid. Biochemical Society Transactions 1993, 21, 83–89. [Google Scholar] [CrossRef]
- Damerell, V.; Klaassen-Dekker, N.; Brezina, S.; Ose, J.; Ulvik, A.; van Roekel, E.H.; Holowatyj, A.N.; Baierl, A.; Böhm, J.; Bours, M.J.L.; et al. Circulating tryptophan-kynurenine pathway metabolites are associated with all-cause mortality among patients with stage I-III colorectal cancer. Int J Cancer 2025, 156, 552–565. [Google Scholar] [CrossRef]
- Chiu, L.C.; Tang, H.Y.; Fan, C.M.; Lo, C.J.; Hu, H.C.; Kao, K.C.; Cheng, M.L. Kynurenine Pathway of Tryptophan Metabolism Is Associated with Hospital Mortality in Patients with Acute Respiratory Distress Syndrome: A Prospective Cohort Study. Antioxidants (Basel) 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Hoong, C.W.S.; Chua, M.W.J. SGLT2 Inhibitors as Calorie Restriction Mimetics: Insights on Longevity Pathways and Age-Related Diseases. Endocrinology 2021, 162. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.F.; Clegg, D.J. Euglycemic Ketoacidosis as a Complication of SGLT2 Inhibitor Therapy. Clin J Am Soc Nephrol 2021, 16, 1284–1291. [Google Scholar] [CrossRef]
- Chen, C.; Rubin, E.H. Adaptive phase 2/3 designs for oncology drug development - Time to hedge. Contemp Clin Trials 2023, 125, 107047. [Google Scholar] [CrossRef] [PubMed]
- Hoeflich, K.P.; Merchant, M.; Orr, C.; Chan, J.; Den Otter, D.; Berry, L.; Kasman, I.; Koeppen, H.; Rice, K.; Yang, N.Y.; et al. Intermittent administration of MEK inhibitor GDC-0973 plus PI3K inhibitor GDC-0941 triggers robust apoptosis and tumor growth inhibition. Cancer Res 2012, 72, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Chen, J.; Xu, Z.; Yang, B.; He, Q.; Luo, P.; Yan, H.; Yang, X. Development and safety of PI3K inhibitors in cancer. Arch Toxicol 2023, 97, 635–650. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, C.; Lv, R.; Qin, Q.; Liu, N.; Yin, W.; Wang, R.; Sun, Y.; Wang, X.; Sun, Y.; et al. Design, synthesis, biological evaluation and pharmacophore model analysis of novel tetrahydropyrrolo [3,4-c]pyrazol derivatives as potential TRKs inhibitors. Eur J Med Chem 2021, 223, 113627. [Google Scholar] [CrossRef]
- Yan, W.; Lakkaniga, N.R.; Carlomagno, F.; Santoro, M.; McDonald, N.Q.; Lv, F.; Gunaganti, N.; Frett, B.; Li, H.Y. Insights into Current Tropomyosin Receptor Kinase (TRK) Inhibitors: Development and Clinical Application. J Med Chem 2019, 62, 1731–1760. [Google Scholar] [CrossRef] [PubMed]
- Altzerinakou, M.A.; Paoletti, X. An adaptive design for the identification of the optimal dose using joint modeling of continuous repeated biomarker measurements and time-to-toxicity in phase I/II clinical trials in oncology. Stat Methods Med Res 2020, 29, 508–521. [Google Scholar] [CrossRef]
- Pinsker, J.E.; Dassau, E.; Deshpande, S.; Raghinaru, D.; Buckingham, B.A.; Kudva, Y.C.; Laffel, L.M.; Levy, C.J.; Church, M.M.; Desrochers, H.; et al. Outpatient Randomized Crossover Comparison of Zone Model Predictive Control Automated Insulin Delivery with Weekly Data Driven Adaptation Versus Sensor-Augmented Pump: Results from the International Diabetes Closed-Loop Trial 4. Diabetes Technol Ther 2022, 24, 635–642. [Google Scholar] [CrossRef]
- Iasonos, A.; O’Quigley, J. Adaptive dose-finding studies: a review of model-guided phase I clinical trials. J Clin Oncol 2014, 32, 2505–2511. [Google Scholar] [CrossRef]
- Visser, M.M.; Charleer, S.; Fieuws, S.; De Block, C.; Hilbrands, R.; Van Huffel, L.; Maes, T.; Vanhaverbeke, G.; Dirinck, E.; Myngheer, N.; et al. Comparing real-time and intermittently scanned continuous glucose monitoring in adults with type 1 diabetes (ALERTT1): a 6-month, prospective, multicentre, randomised controlled trial. Lancet 2021, 397, 2275–2283. [Google Scholar] [CrossRef]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9. [Google Scholar] [CrossRef]
- Marszalek-Grabska, M.; Walczak, K.; Gawel, K.; Wicha-Komsta, K.; Wnorowska, S.; Wnorowski, A.; Turski, W.A. Kynurenine emerges from the shadows - Current knowledge on its fate and function. Pharmacol Ther 2021, 225, 107845. [Google Scholar] [CrossRef]
- Fiore, A.; Zeitler, L.; Russier, M.; Groß, A.; Hiller, M.K.; Parker, J.L.; Stier, L.; Köcher, T.; Newstead, S.; Murray, P.J. Kynurenine importation by SLC7A11 propagates anti-ferroptotic signaling. Mol Cell 2022, 82, 920–932.e927. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A. Role of indoleamine 2,3-dioxygenase 1 (IDO1) and kynurenine pathway in the regulation of the aging process. Ageing Res Rev 2022, 75, 101573. [Google Scholar] [CrossRef] [PubMed]
- Perry, M.W.D.; Abdulai, R.; Mogemark, M.; Petersen, J.; Thomas, M.J.; Valastro, B.; Westin Eriksson, A. Evolution of PI3Kγ and δ Inhibitors for Inflammatory and Autoimmune Diseases. J Med Chem 2019, 62, 4783–4814. [Google Scholar] [CrossRef]
- Bornaei, M.; Khajehsharifi, H.; Shahrokhian, S.; Sheydaei, O.; Zarnegarian, A. Differential pulse voltammetric quantitation of kynurenic acid in human plasma using carbon-paste electrode modified with metal-organic frameworks. Materials Chemistry and Physics 2023, 295, 127016. [Google Scholar] [CrossRef]
- Beatty, G.L.; O’Dwyer, P.J.; Clark, J.; Shi, J.G.; Bowman, K.J.; Scherle, P.A.; Newton, R.C.; Schaub, R.; Maleski, J.; Leopold, L.; et al. First-in-Human Phase I Study of the Oral Inhibitor of Indoleamine 2,3-Dioxygenase-1 Epacadostat (INCB024360) in Patients with Advanced Solid Malignancies. Clin Cancer Res 2017, 23, 3269–3276. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, H.; Wu, D.; Wang, X. Temperature and pH dual-stimuli-responsive phase-change microcapsules for multipurpose applications in smart drug delivery. J Colloid Interface Sci 2021, 583, 470–486. [Google Scholar] [CrossRef]
- Lin, R.; Zhou, Y.; Yan, F.; Li, D.; Yuan, Y. BOIN12: Bayesian Optimal Interval Phase I/II Trial Design for Utility-Based Dose Finding in Immunotherapy and Targeted Therapies. JCO Precis Oncol 2020, 4. [Google Scholar] [CrossRef]
- Liu, D.; Xin, Z.; Guo, S.; Li, S.; Cheng, J.; Jiang, H. Blood and Salivary MicroRNAs for Diagnosis of Oral Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. J Oral Maxillofac Surg 2021, 79, e1081–e1082. [Google Scholar] [CrossRef]
- Zahran, F.; Ghalwash, D.; Shaker, O.; Al-Johani, K.; Scully, C. Salivary microRNAs in oral cancer. Oral Dis 2015, 21, 739–747. [Google Scholar] [CrossRef]
- Hare, S.M.; Adhikari, B.M.; Mo, C.; Chen, S.; Wijtenburg, S.A.; Seneviratne, C.; Kane-Gerard, S.; Sathyasaikumar, K.V.; Notarangelo, F.M.; Schwarcz, R.; et al. Tryptophan challenge in individuals with schizophrenia and healthy controls: acute effects on circulating kynurenine and kynurenic acid, cognition and cerebral blood flow. Neuropsychopharmacology 2023, 48, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Tong, J.; Zhang, P.; Zhou, Y.; Li, Y.; Tan, S.; Wang, Z.; Yang, F.; Kochunov, P.; Chiappelli, J.; et al. Elevated salivary kynurenic acid levels related to enlarged choroid plexus and severity of clinical phenotypes in treatment-resistant schizophrenia. Brain Behav Immun 2022, 106, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Boßlau, T.K.; Wasserfurth, P.; Reichel, T.; Weyh, C.; Palmowski, J.; Nebl, J.; Joisten, N.; Belen, S.; Schenk, A.; Hahn, A.; et al. 12-week combined strength and endurance exercise attenuates CD8(+) T-cell differentiation and affects the kynurenine pathway in the elderly: a randomized controlled trial. Immun Ageing 2023, 20, 19. [Google Scholar] [CrossRef]
- Wnorowski, A.; Wnorowska, S.; Kurzepa, J.; Parada-Turska, J. Alterations in Kynurenine and NAD(+) Salvage Pathways during the Successful Treatment of Inflammatory Bowel Disease Suggest HCAR3 and NNMT as Potential Drug Targets. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Mor, A.; Tankiewicz-Kwedlo, A.; Krupa, A.; Pawlak, D. Role of Kynurenine Pathway in Oxidative Stress during Neurodegenerative Disorders. Cells 2021, 10. [Google Scholar] [CrossRef]
- Badawy, A.A. Tryptophan availability for kynurenine pathway metabolism across the life span: Control mechanisms and focus on aging, exercise, diet and nutritional supplements. Neuropharmacology 2017, 112, 248–263. [Google Scholar] [CrossRef]
- Jamshed, L.; Debnath, A.; Jamshed, S.; Wish, J.V.; Raine, J.C.; Tomy, G.T.; Thomas, P.J.; Holloway, A.C. An Emerging Cross-Species Marker for Organismal Health: Tryptophan-Kynurenine Pathway. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Cussotto, S.; Delgado, I.; Anesi, A.; Dexpert, S.; Aubert, A.; Beau, C.; Forestier, D.; Ledaguenel, P.; Magne, E.; Mattivi, F.; et al. Tryptophan Metabolic Pathways Are Altered in Obesity and Are Associated With Systemic Inflammation. Front Immunol 2020, 11, 557. [Google Scholar] [CrossRef]
- Cheng, C.K.; Ye, L.; Wang, Y.; Wang, Y.L.; Xia, Y.; Wong, S.H.; Chen, S.; Huang, Y. Exercised gut microbiota improves vascular and metabolic abnormalities in sedentary diabetic mice through gut‒vascular connection. J Sport Health Sci 2025, 14, 101026. [Google Scholar] [CrossRef]
- Tanaka, M. From Serendipity to Precision: Integrating AI, Multi-Omics, and Human-Specific Models for Personalized Neuropsychiatric Care. Biomedicines 2025, 13. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M. Parkinson’s Disease: Bridging Gaps, Building Biomarkers, and Reimagining Clinical Translation. Cells 2025. [Google Scholar] [CrossRef] [PubMed]
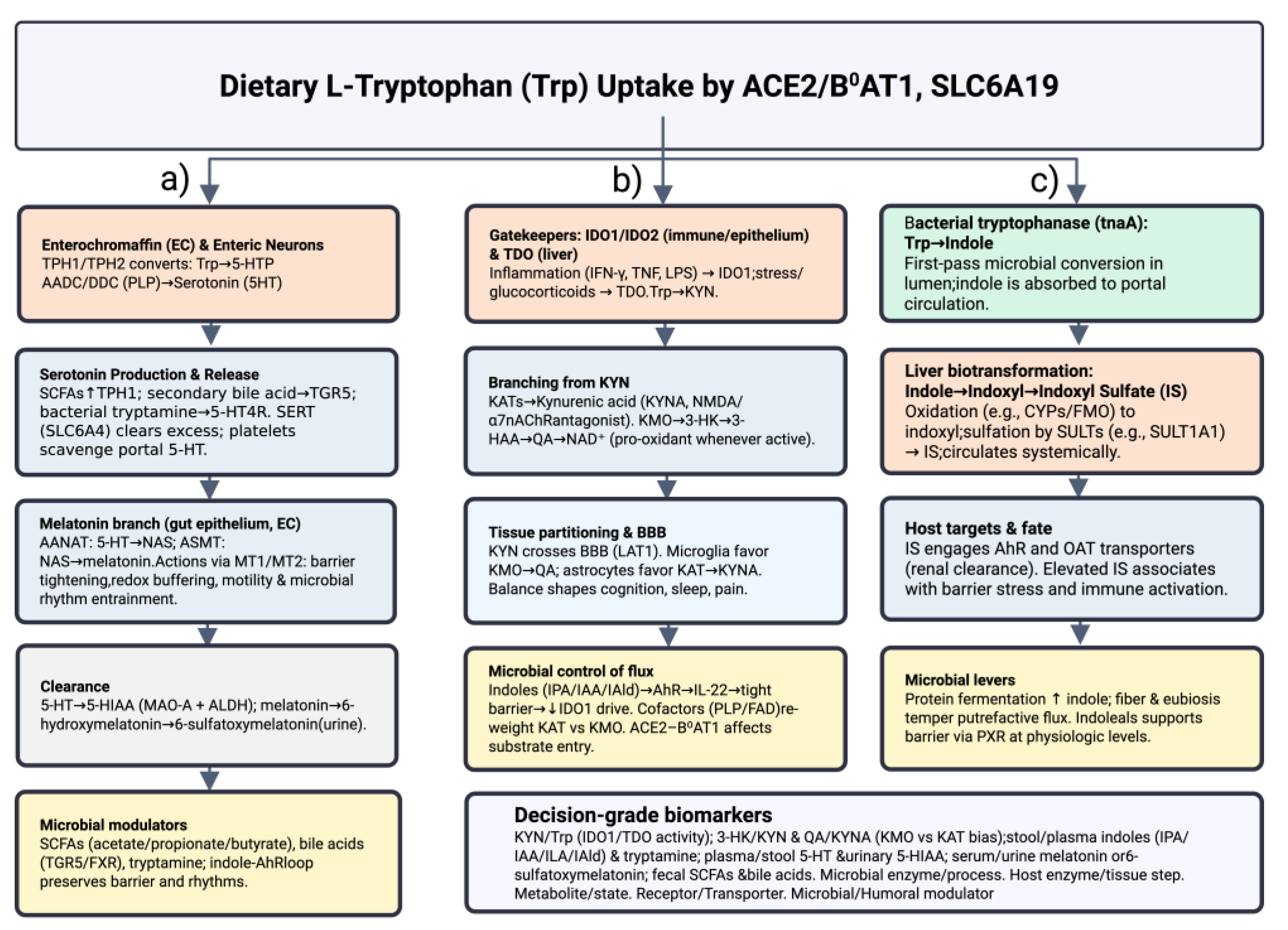
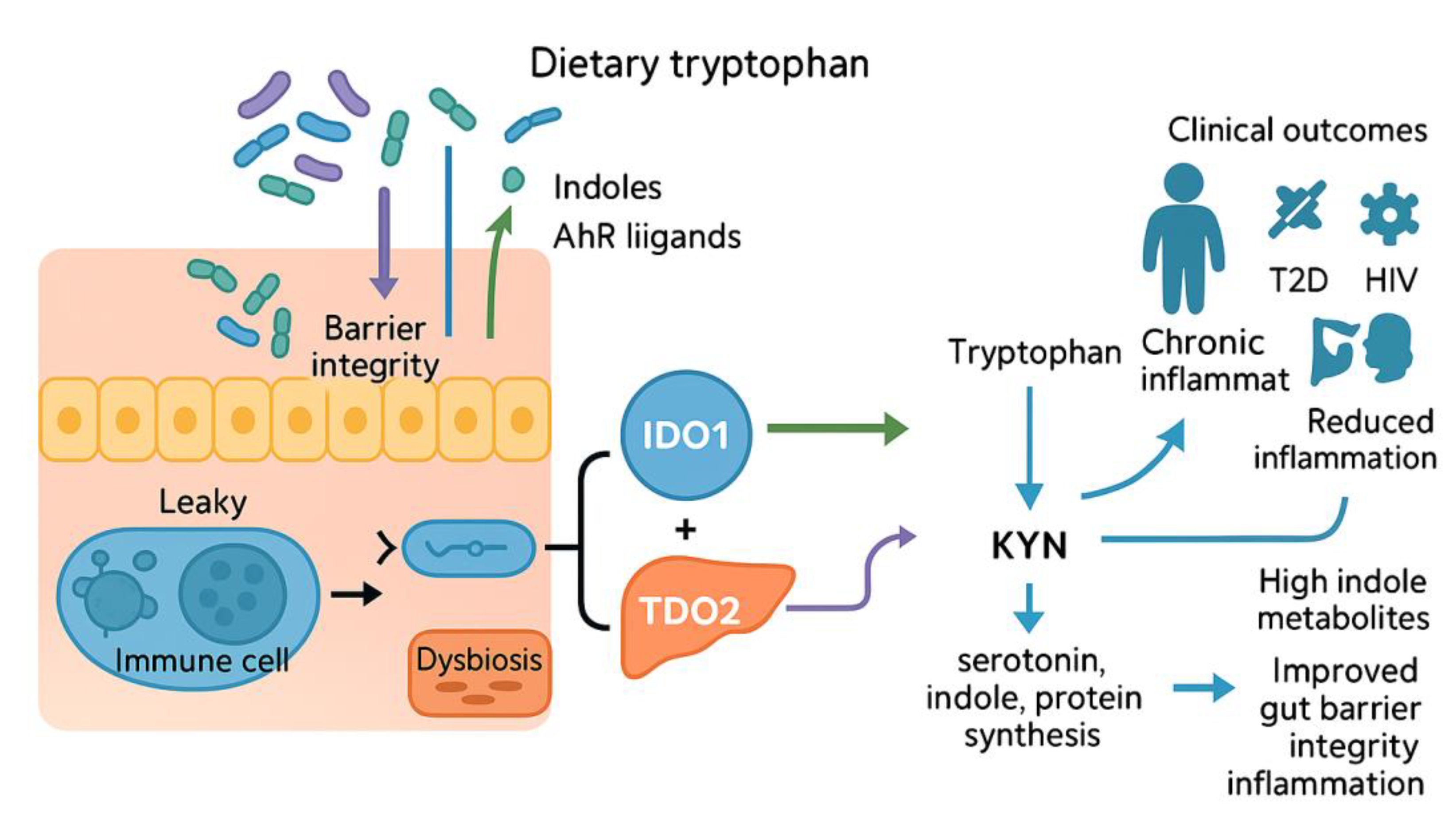
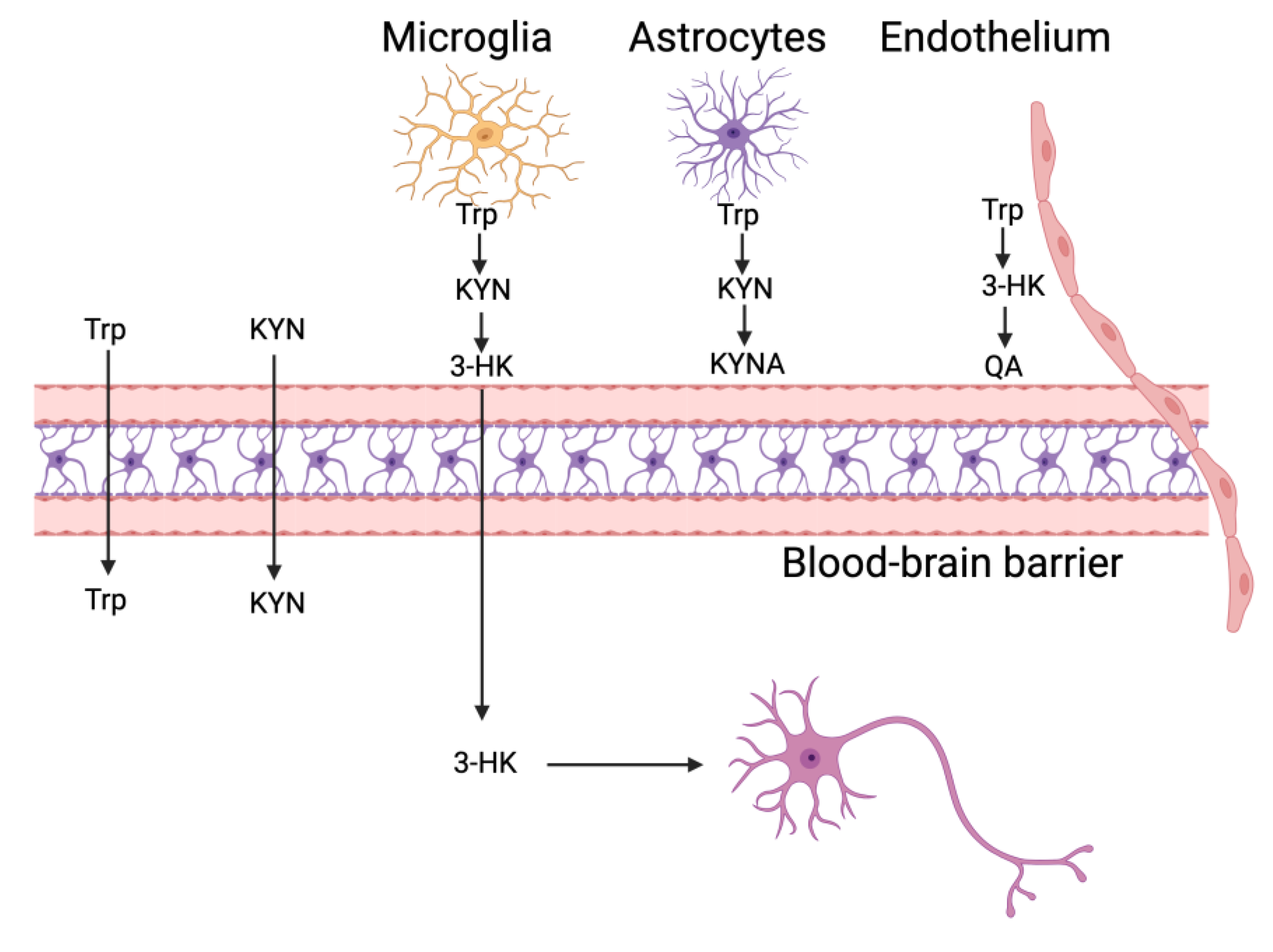
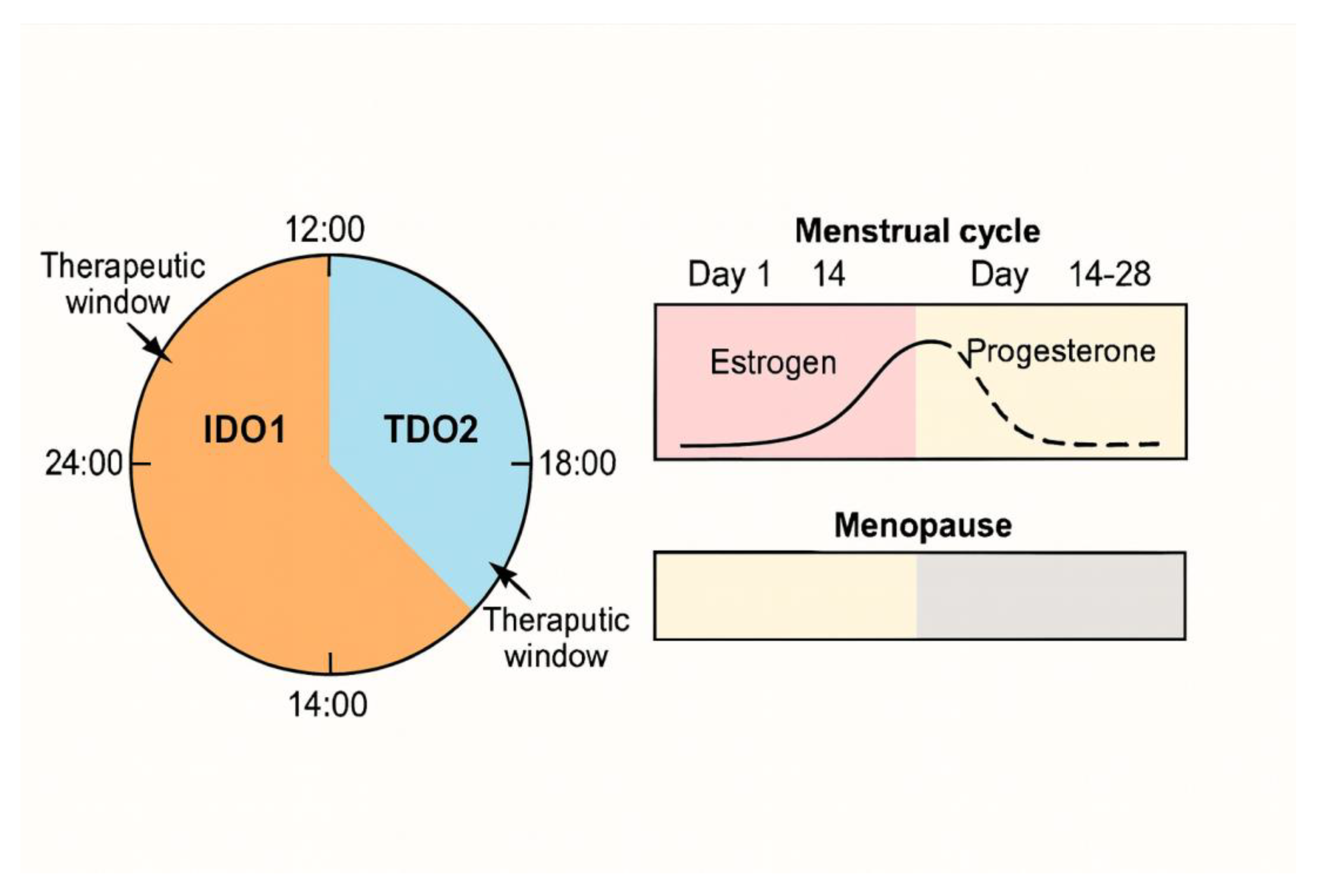
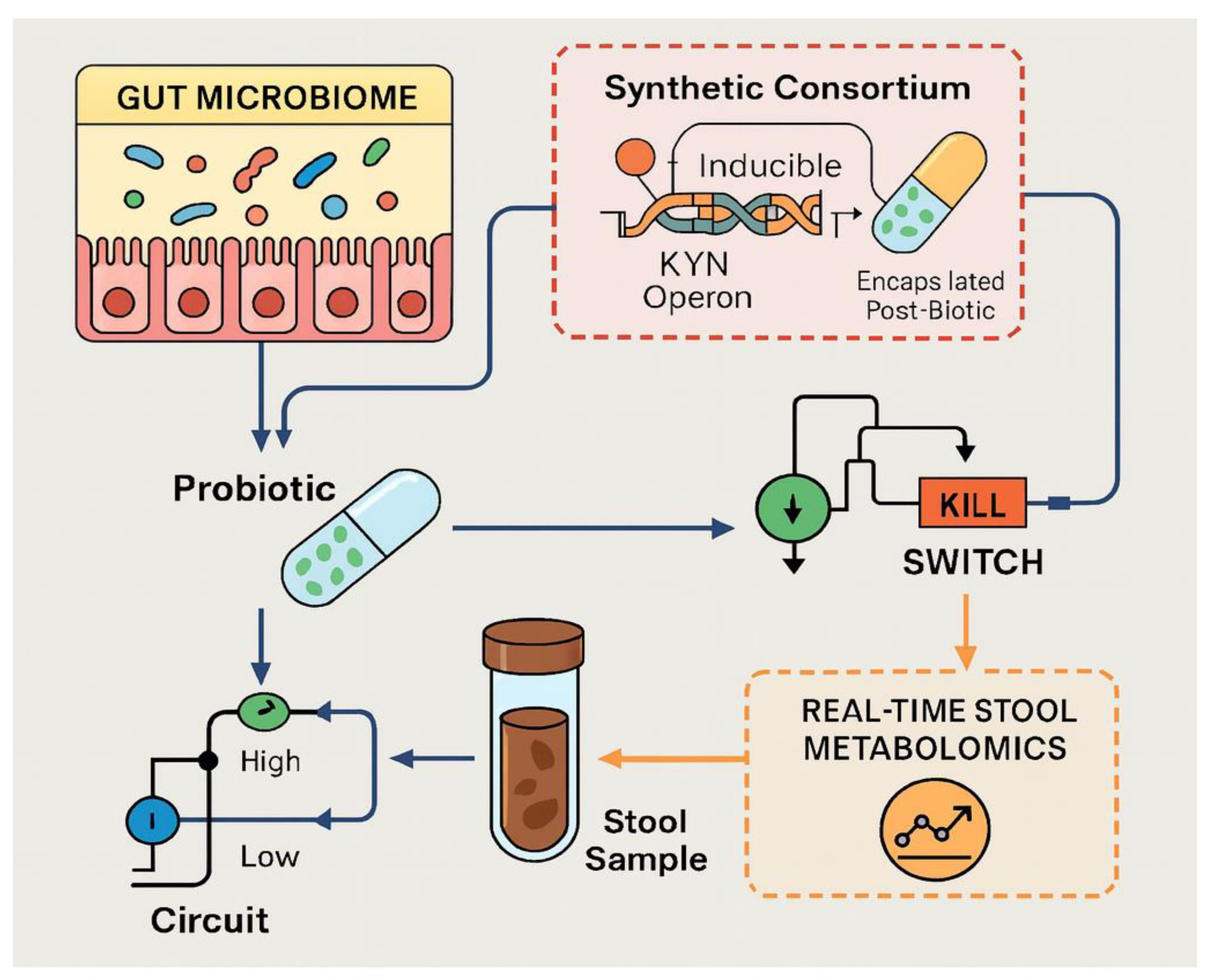
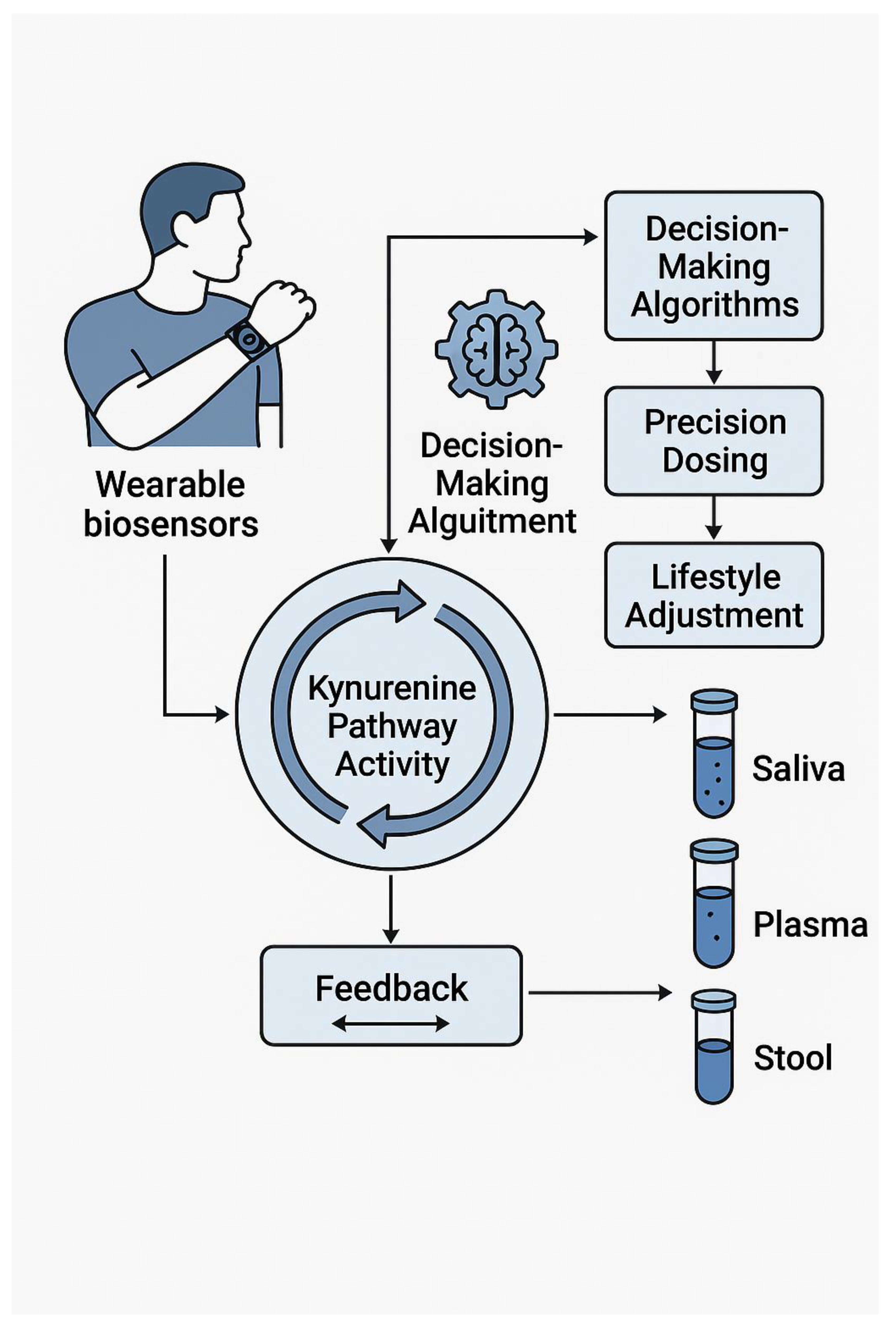
| Category | Description / Core Issue | Implication / Goal | |
|---|---|---|---|
| Translational Challenge | |||
| 1. | Causal Mapping | Thousands of disease associations but no causal framework linking microbiota, host enzymes (IDO1, TDO2) and downstream metabolites (KYNA, QUIN) to physiology | Limits precision design of probiotics, enzyme inhibitors, lifestyle prescriptions |
| 2. | Spatial Resolution | Bulk assays mask cell- and tissue-specific “checkpoints” (astrocytes, microglia, BBB endothelium) | Demands targeted modulation of localized hotspots rather than pathway-wide blockade |
| 3. | Temporal Dynamics | Trp–KYN flux oscillates with circadian rhythms and sex hormones; chronotherapeutic windows under-studied | Missing optimal timing may blunt efficacy or raise toxicity of interventions |
| Key Objective | |||
| 1. | Map Spatial Checkpoints | Chart localized KYN metabolism niches in brain and periphery | Inform cell-type-specific therapeutic targeting |
| 2. | Characterize Sex & Circadian Modifiers | Define how hormones and clocks tilt Trp metabolism toward neurotoxicity or resilience | Enable time- and sex-specific dosing strategies |
| 3. | Develop Microbiota-Based Precision Switches | Engineer probiotic consortia and post-biotics that reroute Trp flux | Provide modular, patient-tailored metabolic control |
| 4. | Outline Integrated “Intervention 2.0” Platform | Combine dual-enzyme inhibitors, exercise, and AI-driven biosensing | Create closed-loop, adaptive therapeutics |
| Experimental Strategy | Mechanistic Lever / Tools | Mechanistic Lever / Tools | Expected Outcome / Advantage |
|---|---|---|---|
| Endothelial CRISPRi “zip-code” AAV-targeted knock-down of KMO or KYNU in perivascular endothelium | • ZIM3-KRAB CRISPRi cassette• Endothelial-specific promoters + miRNA “clearing tags” • Bar-coded AAV libraries |
• Build bar-coded AAV panels to sharpen endothelial specificity • Validate knock-down and KYN-metabolite flux in brain-slice co-cultures • Monitor glutamate dynamics in vivo with optogenetic reporters • Test impact on tumor infiltration & behavior (KMO-high metastasis models) • Run parallel safety screens for NAD pools & mitochondrial stress |
Precise, vessel-restricted suppression of 3-HK/ QUIN → dampened excitotoxicity and immune escape with minimal systemic off-target effects |
| Light-addressable riboswitch control in astrocytesMillisecond, reversible tuning of KMO / KYNU translation | • Photocleavable or Z-lock riboswitch fused to target mRNA • Pulsed IR/visible light for on-off gating • Simultaneous GCaMP or glutamate sensor read-outs |
• Package riboswitch construct in astrocyte-specific AAV • Benchmark translation kinetics vs. Ca2⁺ rise in organotypic slices • Map spatial spread of KYN pulses and gliotransmitter waves • Deploy fiber-coupled two-photon uncaging in vivo to test network excitability during sleep, seizure, learning |
Real-time, non-invasive “dimmer switch” for KP activity with built-in metabolic read-outs; ideal for dissecting causal links between KYN flux and neural circuitry |
| Circadian / Sex-Specific Gap | Why It Matters | Biomarker-Guided Next Step | Anticipated Pay-Off |
|---|---|---|---|
| Absence of chrono-pharmacology trials for IDO1/TDO2, KMO or KAT inhibitors | Optimal dosing windows are unknown; schedules may blunt efficacy or raise toxicity | Launch Bayesian adaptive trials that co-randomize dose and clock time, using real-time KYN / QUIN read-outs as decision boundaries | Evidence-based chrono-dosing algorithms, reduced off-target effects |
| Inadequate stratification by circadian phase and sex | Female-specific PK/PD and toxicity signals vanish when averaged | Embed wearable-derived chronotype + hormonal phase into inclusion criteria; pre-specify sex-by-time interaction models | Sex-aware precision medicine; higher treatment tolerability |
| Undefined mechanistic links between clock genes, hormones & KYN enzyme activity | Surrogate biomarkers risk misinterpretation without pathway context | Overlay 24 h cortisol / melatonin rhythms onto multi-time-point KYN, QUIN, KYNA panels; apply mixed-effects chronobiology models | Mechanistic targets for combination therapy; validated biomarkers |
| Wearable metrics (light, sleep) not integrated into study design | Zeitgebers that modulate KYN flux are ignored | Trigger capillary micro-sampling when lux-derived phase-angle deviation crosses threshold (“biomarker-in-the-loop”) | Personalised sampling & dosing windows; lower noise in endpoints |
| Sex- and light-cycle biases in pre-clinical models | Male-only, fixed-light studies limit translation | Use sex-balanced rodents under rotating light cycles; validate with humanised microbiome models | Higher translational validity of pre-clinical findings |
| Lack of validated rapid biomarkers to couple KYN swings to outcomes | Real-time dose adjustment impossible | Develop saliva / finger-stick electrochemical strips for KYN / TRP / QUIN; calibrate against plasma & microdialysate | Closed-loop dose titration; faster early-phase trials |
| Circadian-Misalignment Focus: QUIN spikes during night-shift work | Neurotoxic burden may rise, especially in vulnerable chronotypes | Pilot cross-over study: shift-workers + hourly capillary sampling + light & activity trackers Model QUIN vs lux-derived phase angle (mixed-effects) Overlay cortisol & melatonin to disentangle stress vs circadian drivers Test timed blue-light blockers, melatonin, or time-restricted feeding |
Identifies high-risk chronotypes and intervention windows; informs occupational health policies |
| Adaptive Gap / Focus Area | Actionable Strategy & Tool Kit | Key Operational Step(s) | Intended Pay-Off |
|---|---|---|---|
| Dose-Time Randomization | Bayesian hierarchical designs that co-randomize dose level + clock-time | • Simulate designs borrowing strength across adjacent time bins • Integrate wearable-derived chronotype into priors • Embed rolling interim analyses that drop unfavorable time windows rather than doses | Evidence-based chrono-dosing rules; smaller, faster trials |
| Sensor–Data Pipeline | Validated software bridges from CGM / lactate / KYN sensors → electronic TMF | • Build real-time API between biosensors and trial master file • Version-control data streams for audit compliance | Seamless biomarker ingestion; regulatory-ready data fidelity |
| Biomarker Validation | Rapid KYN / TRP / QUIN saliva or finger-stick electrochemical strips | • Cross-validate saliva, plasma, tumor microdialysate after micro-dosed dual-inhibitor (e.g., RY103) crossover PK study | Closed-loop dosing feasible at point-of-care |
| Safety Governance | Rules for rapid dose-time shifts in outpatient settings | • Use melatonin / cortisol point-of-care assays as safety triggers • Pre-define algorithmic “pause” thresholds | Protects patients while enabling flexible chrono-titration |
| Patient-Centric Metrics | PROMs tuned to circadian toxicity (fatigue, cognition) | • Embed in ePRO platform; couple to Bayesian controller as soft constraints | Holistic tolerability; improves adherence |
| Pilot Implementation | First-in-human chrono-trials for drugs with known chronotoxicities | • Pilot crossover study: micro-dosed dual IDO1/TDO2 inhibitor + real-time KYNA pacing • Roll out in oncology & mood-disorder cohorts | Proof-of-concept that algorithm-guided timing beats fixed BID regimens |
| Development Challenge / Key Insight | Why It Matters | Precision Strategy or Next Step |
|---|---|---|
| 1 ▪ Unpredictable engraftment & variable response • Engineered consortia (e.g., VE303) often need antibiotic conditioning. • Multispecies probiotics cut transit time only in diet-matched responders. | Long-term decolonization or mood relief can fade. | — Screen baseline diet & Lactobacillus/Bifidobacterium colonization predictors before enrollment.— Tailor consortia composition to individual fermentable-fiber intake. |
| 2 ▪ Scarce durability & mechanistic data • Limited longitudinal sequencing of phage–bacteria–host dynamics. | Regulatory roadblock; patient safety. | — Pair shotgun metagenomics with phageomics in 6–12-month follow-up.— Validate causal metabolites via gnotobiotic “plug-and-play” models. |
| 3 ▪ Safety & horizontal-gene-transfer (HGT) risks • CRISPR-edited E. coli microcin strains could acquire resistance cassettes. | Batch-to-batch variability in metabolite output undermines reproducibility. | — Stack orthogonal kill-switches (CRISPRi + toxin–antitoxin + auxotrophy).— Deploy long-read sequencing of shed strains to monitor HGT. |
| 4 ▪ Manufacturing & QC deficits | Missing optimal dosing windows weakens efficacy. | — Adopt GMP-aligned metabolite “fingerprinting” for every lot.— Introduce in-line mass-spec release tests. |
| 5 ▪ Timing factors ignored • Circadian rhythms gate colonization resistance; probiotics modulate clock-gene expression. | Jurisdictional differences delay global rollout. | — Run adaptive trials that modulate dosing relative to light exposure, meals, & antibiotics.— Schedule capsule release (enteric or pH-triggered) to late-day circadian troughs in QUIN. |
| 6 ▪ Fragmented regulatory pathways | One-size-fits-all blends underperform. | — Establish harmonized guidance for genetically modified live biotherapeutic products (LBPs) via international consortia. |
| 7 ▪ Personalized strain selection • Foodborne lactic-acid-bacteria genomes map “starter” strains for bespoke consortia. | Long-term decolonization or mood relief can fade. | — Build a strain library ranked by individual dietary patterns & metabolite deficits; auto-compose patient-specific mixes. |
| Innovation Track | Key Development Step | Implementation Path / Intended Pay-Off |
|---|---|---|
| Encapsulated Post-biotics (e.g., KYNA) | 1 ▸ Screen GRAS-grade polymers & hydrogels for KYNA stability under accelerated aging.2 ▸ Map release profiles in simulated GI fluids & pig colonic explants.3 ▸ Test near-infrared–triggered nanocapsules for on-demand bursts during inflammation.4 ▸ Quantify systemic vs. luminal KYNA via LC-MS in gnotobiotic mice, benchmark against Bifidobacterium output. | • Locks in metabolite potency until it reaches the colon.• Enables flare-responsive “burst” therapy.• Provides pharmacokinetic data to support dosing equivalence to live-microbe production. |
| Adaptive Probiotic Titration | 1 ▸ Build a reference library of weekly stool metabolomes from diverse cohorts on fixed probiotic regimens.2 ▸ Train adaptive Bayesian models that recommend dose or strain tweaks when SCFA / indole scores drift.3 ▸ Integrate wearable-captured feeding rhythms to optimize capsule timing.4 ▸ Run N-of-1 cross-over trials comparing dashboard-guided vs. static dosing. | • Converts one-size-fits-all probiotics into dynamic, biomarker-steered therapies.• Aligns delivery with each person’s meal schedule and gut motility.• Generates individualized responder fingerprints for precision formulation. |
| AI-Driven Gut–Brain Feedback Loops | 1 ▸ Leverage ClinAIOps frameworks for continuous therapeutic monitoring (glucose, lactate, KYN sensors).2 ▸ Fuse real-time metabolite slopes with sensor-driven exercise platforms (e.g., auto-pacing treadmills).3 ▸ Embed adaptive probiotic dashboards into the same loop for daily strain/dose updates. | • Closed-loop system that tweaks movement and microbes to keep KYN/TRP in a protective range.• Minimizes clinician workload—algorithm adjusts interventions overnight.• Sets the stage for fully autonomous “gut-brain wearables” in neuropsychiatric care. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





