1. Introduction
As many as one in five children in the United States experience deficits in cognitive, adaptive, communicative, and psychomotor abilities [
1,
2]. While genetic factors appear to play a substantial role in these deficits, a growing literature has identified that environmental factors, e.g., chemical, biological, and nutritional exposures, may significantly contribute to their etiology [
3,
4,
5,
6,
7].
Insecticides, a diverse group of chemicals often designed to disrupt the neurological systems of pests, pose potential risks to human neurodevelopment. Household insecticide exposure, in particular, has emerged as a suspected contributor to neurodevelopmental toxicity during early life. Before the early 2000s, organophosphorus (OP) compounds were the predominant active ingredients in household insecticide sprays, after the banning of organochlorines in household insecticides in the United States during the 1970s and 1980s. Notably, OP insecticides such as chlorpyrifos and diazinon were extensively used in residential settings until they were banned by the United States Environmental Protection Agency (EPA) from household products, with phased eliminations occurring in 2001 for chlorpyrifos and in 2002 and 2004 for indoor and outdoor formulations of diazinon, respectively [
8,
9]. Following this phase-out, pyrethroids became the main active compounds in household insecticides [
10].
Epidemiological studies have reported associations between both OP and pyrethroid exposures and neurodevelopmental outcomes, including autism spectrum disorder (ASD) [
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21]. While most of these studies have focused on proximity to agricultural pesticide applications [
12,
13,
19] or short-lived biomarkers measured at specific time points during pregnancy or early childhood [
14,
22,
23,
24], our group recently found an association between caregiver reports of professionally and non-professionally applied household insecticides, especially repeated exposures during pregnancy and early life, and ASD in the CHARGE study [
21]. While the neurodevelopmental risks associated with OP exposure are well-documented, the evidence regarding pyrethroid exposure is less conclusive.
Pet flea and tick control products are widely used in the United States, where an estimated 70% of households own pets, with dogs and cats being the most common [
25]. To manage flea and tick infestations, a variety of chemical treatments are employed, including topical spot-on products, collars, oral medications, and soaps/shampoos, many of which contain neuroactive compounds such as pyrethroids, neonicotinoids, fipronil, and insect growth regulators [
26]. These products are designed to disrupt the nervous systems or developmental cycles of fleas and ticks, however, little is known about their neurodevelopmental effects in humans, particularly during critical windows of development.
This study leverages extensive caregiver-reported data on household insecticide use from the CHARGE (Childhood Autism Risk from Genetics and the Environment) study, detailing the timing and frequency of insecticide use from three months before conception through the first two years of a child’s life. We aimed to examine potential impacts of these exposures on cognitive and adaptive behaviors in children, utilizing standardized composite and subscale scores from the Mullen Scales for Early Learning (MSEL) [
27] and Vineland Adaptive Behavior Scales (VABS) [
28]. Analyses were conducted separately for children with ASD, developmental delay without ASD (DD), and typical development (TD) classifications.
2. Materials and Methods
2.1. Study Population
The study sample was drawn from the CHARGE study, a population-based case-control study initiated in 2003 to investigate environmental and genetic risk factors for autism and other neurodevelopmental disorders, along with gene-by-environment interactions [
29]. Eligible participants were recruited into CHARGE from within California and included children aged 24 to 60 months at the time of recruitment, born in California, and currently residing with at least one biologically related parent who was proficient in English or Spanish. Children who either had ASD or DD were identified through the California Department of Developmental Services’ (DDS), which coordinate services to children with developmental disabilities through their system of Regional Centers (RCs). Families of children with ASD were also recruited from other UC Davis MIND Institute studies, or from people who contacted the study team directly. General population (GP) controls were selected through the California State Vital Statistics birth files and frequency-matched to ASD cases based on age, sex assigned at birth, and RC catchment area distribution. Subjects were excluded from analyses if the questionnaire on insecticide exposures was not completed or the child had a known genetic cause for their DD diagnosis (e.g., Down syndrome, Fragile X syndrome, etc.).
The study was approved by institutional review boards of the University of California. Written informed consent was obtained from all parents or legal guardians prior to collection of any data.
2.2. Diagnostic and Outcome Measurements
Upon enrollment, most children were assessed at the UC Davis MIND Institute to confirm diagnoses, however, a small percentage were seen at the UCLA Neuropsychiatric Institute. Children with previous ASD diagnoses were assessed using the Autism Diagnostic Observation Schedules-2 (ADOS-2) [
30,
31] and the Autism Diagnostic Interview–Revised (ADI-R) [
32,
33,
34]. Children without a prior ASD diagnoses were screened for ASD symptoms using either the Social Communication Questionnaire (SCQ) [
35] or clinician judgement. If the total SCQ score was 15 or above [
36], or if the clinician suspected ASD, assessments with the ADI-R and ADOS-2 were conducted during a second visit. Cognitive function was evaluated in all children using the Mullen Scales of Early Learning (MSEL) and adaptive skills were assessed by administering the Vineland Adaptive Behavior Scales (VABS) to the primary caregiver. Children who did not meet the CHARGE criteria for ASD on both the ADI-R and ADOS-2 were further classified based solely on their MSEL and VABS composite scores. Those scoring below 70 on either measure were classified as having DD, while those scoring 70 or above on both were classified as TD.
The MSEL is a clinician-administered tool designed to evaluate cognitive functioning in young children across multiple domains. It generates an Early Learning Composite score based on performance in four subscales: receptive language, expressive language, visual reception, and fine motor skills. Standard scores for the MSEL subscales are normalized against age-based norms, with a mean ± SD score of 100 ± 15. To mitigate the potential for floor effects in children with significant developmental delays, developmental quotients (DQ) were calculated. DQs were derived by dividing the age-equivalent score obtained from each subscale by the child’s chronological age and multiplying by 100, with higher DQs indicating better cognitive performance.
Adaptive functioning was measured using the VABS, a semi-structured interview administered to caregivers, designed to assess daily adaptive behaviors necessary for personal and social sufficiency. The VABS provides an Adaptive Behavior Composite score, alongside subscale scores in the domains of communication, daily living skills, socialization, and motor skills. Similar to the MSEL, scores provided are standardized scores with a mean ± SD of 100 ± 15. DQs are used and calculated the same way as with MSEL. Higher DQs reflect greater adaptive functioning.
2.3. Household Insecticide Exposure
We determined prenatal and early life exposure to indoor, outdoor, and flea and tick control insecticides, as described elsewhere [
21]. In brief, the primary caregiver (usually the mother) was asked during a comprehensive interview about the use of insecticides in the home, who applied them (which parent, others in the household, or professional applicators), where they were applied (indoor, outdoor, on pets), during which month and/or year of the index period (beginning 3 months before pregnancy through the second year of life) they were applied, and how frequently they were applied during that period. The questionnaire was modified part way through the study, with changes that included categorizing professionally and non-professionally applied insecticide products into indoor vs outdoor use at the time of data collection, rather than collecting free text descriptions of application location and manually categorizing them after data entry. Additionally, in the later version of the questionnaire separate questions were included for each type of pet flea/tick product while in the earlier version one question grouped pet sprays, powders, and skin applied treatments together, and responses were manually categorized to distinguish between skin-applied treatments and other forms (e.g., sprays, powders). Product text fields were manually reviewed to exclude items that did not contain insecticides and brand names were cross referenced with the EPA’s Insecticide Product and Label System (
https://ordspub.epa.gov/ords/insecticides/f?p=PPLS:1) to confirm presence of an active insecticidal ingredient. Use of borax-containing poisoned bait containers was not included because they have a small surface area of insecticide, which would result in low volatilization and thus limited exposure.
We categorized exposure types into several groups: professionally applied indoor insecticides, non-professionally applied indoor insecticides, any indoor insecticide application, non-professionally applied outdoor insecticides, any outdoor insecticide application, and pet flea and tick treatments including collar use, skin application, and a combined category for soap, shampoo, and powder use. Exposure frequency was classified into three categories for the pre-pregnancy period and each trimester: no exposure, exposure for 1-2 months, and exposure for all 3 months. Similarly, for the entire pregnancy period, exposure frequency was grouped into no exposure, exposure for 1-5 months, and exposure for 6-9 months. Exposure frequency was not asked for the postnatal period.
2.4. Confounders
To visually represent the presumed causal relationships among the study variables, a Directed Acyclic Graph (DAG) was created in the Dagitty web application [
37] to identify minimally sufficient adjustment sets of variables to minimize bias in the association between household insecticides and cognitive and adaptive skills (
Figure S1). This led to a minimally sufficient adjustment set that included the following covariates: year of conception (modeled as a cubic polynomial), maternal race/ethnicity (non-Hispanic white (NHW) vs non-NHW), maternal education (bachelor’s degree and higher vs. some college or less), home ownership at time of enrollment (yes/no), urbanicity (mother’s address at time of delivery within an area classified as urban by the US Census closest to their birth year [2000, 2010, or 2020]; yes/no), season of conception, and maternal mental health problems during pregnancy (yes/no). Maternal mental health data was not collected prior to October 2005 and thus was missing for n = 382 subjects. To avoid dropping those children from analyses, we conducted multiple imputation of this variable, as described elsewhere [
21]. In brief, maternal mental health was imputed with 50 imputations, based on a Markov Chain Monte Carlo imputation method that assumes a multivariate normal joint distribution of variables. The addition of maternal mental health in the final models had no impact on the effect estimates, and thus, to increase power, was not included in the final models.
Covariate data obtained from parent interviews were validated with birth and medical records, as described previously [
21]. The child’s birth address was classified as urban vs/ non-urban using census definitions and based on parent-reported addresses at birth in conjunction with Census population data.
2.5. Statistical Analyses
Univariate analyses were used to assess exposure and covariate distributions. All variables presented in
Table 1 were either collected directly or derived from study interview responses. We estimated betas and 95% confidence intervals (CIs) for associations between each of the various application types of household insecticides for each time period, separately, and the composite DQs for MSEL or VABS fitting multiple linear regression models adjusted for confounders, among each of the diagnostic groups (ASD, DD, TD). Considering the differences in diagnosis rates by the child’s sex, we assessed effect modification by sex by including the product of each exposure by child’s sex. A p-value <0.10 for the multiplicative term was considered significant evidence for an interaction. In secondary analyses, we examined the associations between each of the application types of household insecticides and the different subscales for the MSEL and VABS using separate multiple linear regressions, adjusted for the same confounders.
To address the issue of multiple hypothesis testing, we utilized the false discovery rate (FDR) method in our primary analyses, which involved examining the relationship between each type of insecticide application (yes/no) and either MSEL or VABS DQ. The Benjamini-Hochberg procedure was employed to control the proportion of false positives among the significant findings, thereby enhancing the reliability of our results across independent comparisons. Associations with a Q-value after FDR correction (QFDR) of less than 0.05 were considered statistically significant, while those with a QFDR less than 0.10 were deemed borderline significant. Stata (SE version 18.0; StataCorp, College Station, TX) was used for all analyses.
3. Results
3.1. Participant Characteristics
At the time of this study, n = 1,772 participants had been enrolled and classified as ASD (n = 875), DD (n = 343) or TD (n = 554). These included n = 599 recruited from GP (final study classification of these were: n = 7 ASD, 38 DD, 554 TD), with the remaining n = 1,173 children recruited from DDS, from other MIND studies, or from people who contacted the study team directly. Of these, n = 239 subjects were excluded from analyses for having a known genetic cause for their DD diagnosis (n = 137) or not completing the questionnaire on insecticide exposures (n = 65 ASD, n = 14 DD, n = 23 TD). Of the n = 1,533 remaining, n = 810 were classified as ASD, n = 192 as DD, and n = 531 as TD. A further n = 20 ASD, n = 7 DD, n = 11 TD were missing covariates.
Enrolled children were conceived between 1997 and 2018, with TD children born in slightly later years because enrollment in the first few years for TD children was delayed due to a lengthy process for obtaining access to the State’s birth files (
Table 1). The children in our study were ethnically diverse with 47% non-Hispanic white (derived from parent-reported parental race/ethnicity), 32% Hispanic, and 21% other races. Due to the high male:female ratio among those with ASD and our frequency matching of GP recruitment with ASD, participating children were predominately male (81%).
3.2. Distribution of MSEL and VABS DQs
Distributions (mean ± SD) of developmental quotients of MSEL and VABS composite and subscale DQs are presented in
Table S1. All mean DQs were roughly similar for ASD and DD, except for MSEL Fine Motor scores and VABS Motor Skills which were higher for ASD than for DD, and as expected, VABS Socialization which was lower for ASD than DD. TD DQs were 45-55 points higher than either ASD or DD.
3.3. Distribution of Insecticide Exposure
The most common type of insecticide application reported in our study was any indoor insecticide (44.2%), followed by any outdoor insecticide (40.0%;
Table S2). Flea and tick collar use was the least commonly reported product type (6.3%), followed by indoor professionally applied insecticides (11.8%) and flea and tick soap/shampoo/powder (14.9%). Reported use of all insecticides, other than flea and tick collars, was lower during the pregnancy period than during the child’s first and second years of life: indoor and outdoor applications were generally about twice as common in the postnatal years. Apart from flea and tick applications, more participants reported use of insecticides during trimester 2 than during trimesters 1 or 3.
3.4. Insecticide Exposure and Neurodevelopmental Skills
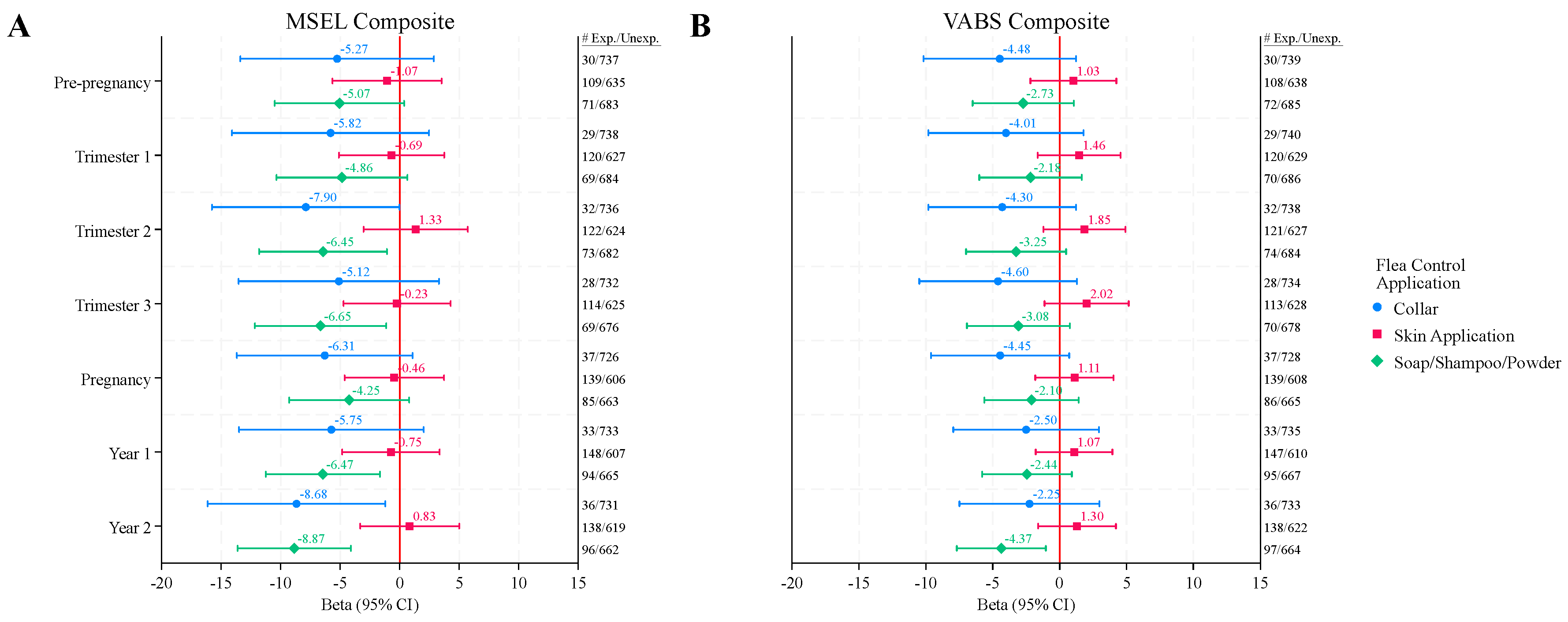
Of the flea and tick applications, reported use in the ASD group of soaps, shampoos, or powders or collars during any point in the index period was associated with MSEL Composite DQs 4 – 9 points lower than those that were not exposed (
Figure 1A,
Table S3). These associations reached statistical significance from mid-pregnancy (2nd trimester) through the 2nd year of life for soaps, shampoos, and powders, and in the second trimester and second year of life for collars, however, after FDR correction only soaps, shampoos, and powders exposure during the second year of life remained marginally significant (QFDR = 0.09).
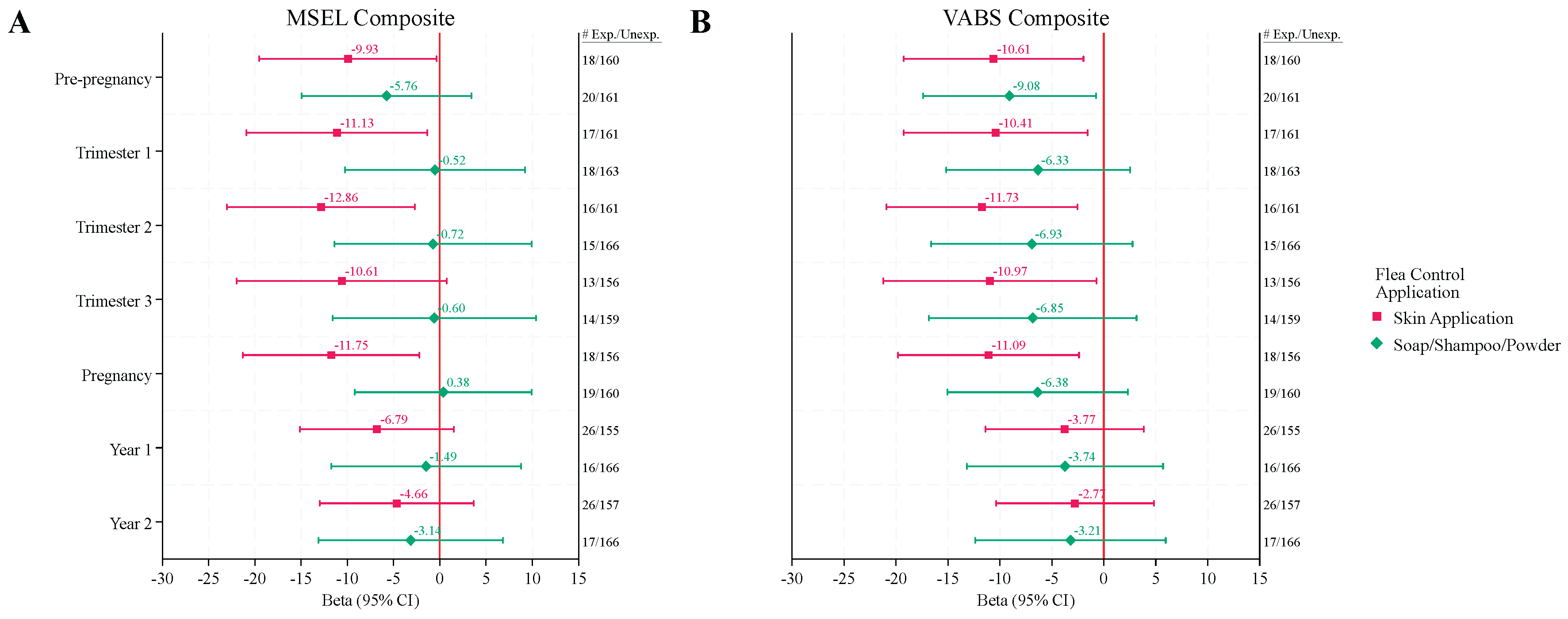
Reported use of skin applications of flea and tick control products in the DD group during any point in the pre-pregnancy or pregnancy periods were also associated with lower cognition with MSEL Composite DQs 10 – 13 points lower than those that were not exposed, which represents a loss of two thirds the SD or more for those exposed (
Figure 2A,
Table S3). While exposures during all periods except trimester 3 were statistically significant, the significance did not hold after FDR correction for multiple comparisons (QFDR = 0.51 – 0.72).
Among children with ASD or TD, inverse or null associations were observed with all applications of reported indoor or outdoor insecticide exposure during the index period and MSEL Composite DQs (
Table S3). Among DD children, indoor applications during the pre-pregnancy and pregnancy periods were associated with decreased MSEL DQs, however, these associations did not reach statistical significance.
No associations were observed among TD children for any insecticide application in any period (
Table S3). Reported use of indoor professionally applied insecticides and flea/tick collars in the DD group could not be assessed due to small sample sizes. Similar, or slightly attenuated, results were observed for VABS composite DQs in all diagnostic groups (
Figure 1B and
Figure 2B,
Table S4).
Effect modification by child’s sex was observed in the ASD group for exposure to any outdoor insecticides in trimesters 2 and 3, and year 2, flea/tick soaps, shampoos, and powders in all time periods, and flea/tick skin applications in pre-pregnancy and trimester 1 (P for interaction < 0.10) on MSEL Composite DQs, with females having stronger negative effects of each insecticide product than males (
Table S5). While these associations did not reach statistical significance, the sample size for females was, by design, much lower than that of males. Among the TD children, effect modification by child’s sex was observed for exposure to indoor and outdoor insecticides (non-professionally applied and any application) in various time points and MSEL Composite DQs, with males generally having stronger negative effects of each insecticide than females. Effect modification by sex was not observed for the DD group, except with exposure to any outdoor insecticide in year 1, with stronger negatives effects observed in males. Similar results were observed with VABS Composite DQs (
Table S6). Effect modification by sex for flea/tick collars and indoor professionally applied insecticides among female children in all diagnostic groups, as well as outdoor non-professionally applied insecticide and flea and tick skin applications in the DD group, could not be evaluated due to small sample sizes of females.
Associations between each reported insecticide application and the subscales of MSEL were similar to the Composite scores for each diagnostic group (
Table S7). Associations with reported flea and tick collar use in year 2 among ASD children were stronger and reached statistical significance with Expressive Language and Visual Receptivity (Beta (95% CI): -10.60 (-18.90, -2.30) and -8.94 (-17.40, -0.48), respectively). Exposure during trimester 2 or the whole pregnancy period was associated with a statistically significant 8-point (nearly an SD) lower VABS Communication DQ, and an 8-point lower VABS Fine Motor Skills DQ in trimester 3 (
Table S8). Associations with reported flea/tick soap, shampoo, and powder use during any time period among ASD children were strongest with Receptive Language, with estimated mean scores 6 – 10.5 points lower among those exposed than those not exposed during the same time period, and in year 2, these associations reached statistical significance with every MSEL subscale as well as VABS Communication and Daily Living Skills subscales (
Tables S7 & S8).
DD children with reported exposure to flea and tick skin applications during pre-pregnancy, any pregnancy period, or the first year of life was statistically significantly associated with a decrease of 12 – 18 points (well over 1 SD difference) in MSEL Visual Receptivity DQs and 10 – 19 points (nearly 2 SD’s) in VABS Socialization DQs, compared to those that were not exposed (
Tables S7 & S8). MSEL Receptive Language DQs were also statistically significantly 10 – 14 points lower when DD children were exposed during pre-pregnancy or any pregnancy period compared to those that were not exposed.
High frequency of exposures (every month for trimesters or at least 6 months out of the entire pregnancy period) of non-professionally applied indoor insecticides in every time period in the DD group was associated with a statistically significant decrease in MSEL Composite DQs of 10 – 12.5 points (
Table S9). Associations were attenuated or null with less frequent use. While there was a similar trend towards decreasing VABS Composite DQs in these same time periods, none of these associations reached statistical significance (
Table S10). In the ASD and TD groups, associations were mostly null, or slightly inverse, with similar effect estimates observed in both frequency groups.
4. Discussion
This study builds upon the growing body of evidence that household insecticide exposure, including pet flea and tick control products and indoor spray insecticides, can adversely affect neurodevelopmental outcomes in children. By examining exposure across distinct time windows and application types, this analysis highlights nuanced associations between household insecticide use and cognitive and adaptive functioning, as measured by MSEL and VABS.
The association between pet flea and tick control products and lower cognitive and adaptive functioning scores, particularly in children with ASD, aligns with prior studies documenting the neurotoxic potential of insecticides.[
38] The consistent significance, in children with ASD, of exposures to flea and tick soaps, shampoos, and powders during the second year of life when a child interacts more with their environment and potentially with pets in the home, underscores the importance of examining chronic, low-level exposures during sensitive developmental periods. Similarly, the observed associations in DD children of exposure to flea and tick skin applications during pre-pregnancy and early pregnancy suggest that the timing of exposure is critical. Other possible reasons for the association in early childhood only occurring in the ASD group is that children with ASD are also known to bond more readily with their pets than with other humans.[
39,
40] and may have increased hand-to-mouth behaviors,[
41,
42] with both factors leading to a higher likelihood of ingesting flea/tick insecticides after touching their pets.
Associations between non-professionally applied indoor insecticides and lower neurodevelopmental scores, particularly cognitive measures, in DD children were only observed when the exposure occurred every month of a trimester or at least 6 months of the full pregnancy period, indicating higher and/or more consistent exposures. The lack of associations for fewer months of exposure may reflect the small sample size of DD children. Given the magnitude of the observed associations with frequent exposures, further investigation is warranted. The stronger associations observed with indoor applications is potentially driven by higher insecticide concentrations, given that indoor insecticides linger longer than outdoor ones, the latter being degraded by sunlight and dispersed more readily with wind.[
43,
44] Very few DD children were exposed to professionally-applied insecticides – too few for analysis (see
Table S2).
The findings align with established biological pathways for insecticide-induced neurotoxicity. Neonicotinoids, including imidacloprid and dinotefuran, are a class of insecticide commonly used in pet flea and tick control products, which act as an agonist of nicotinic acetylcholine receptors (nAChRs) in the central nervous system.[
45] Exposure to low concentrations of these compounds has been shown to stimulate the extracellular signal-regulated kinase (ERK/MAPK) signaling pathway through activation of nAChRs and subsequent intracellular calcium release.[
46] During critical periods of neurodevelopment, such as the second year of life, this over-stimulation can disrupt the balance of excitatory and inhibitory signaling, impairing synaptic plasticity and leading to long-term deficits in cognitive and behavioral function.[
47,
48] Additionally, imidacloprid exposure has been shown to induce oxidative stress and mitochondrial dysfunction,[
49,
50] both of which are linked to neuroinflammation and neuronal injury. Emerging evidence suggests that these processes may contribute to alterations in synaptic connectivity, a hallmark feature of neurodevelopmental disorders.[
51]
Fipronil, another common active ingredient in pet flea and tick control products, primarily acts as a gamma-aminobutyric acid (GABA) receptor antagonist, impairing inhibitory neurotransmission.[
52] This disruption can lead to hyperexcitation in neural networks, potentially affecting the maturation of neural circuits critical for cognitive and behavioral development. Fipronil exposure has also been associated with oxidative stress [
53,
54] and endocrine disruption,[
54,
55] both of which are relevant to neurodevelopmental outcomes.[
51,
56] Importantly, its lipophilic nature allows it to accumulate in fatty tissues, including the brain,[
54] potentially prolonging its effects during critical developmental windows.
4.1. Strengths and Limitations
While this study provides valuable insights into the neurotoxic effects of household insecticides, several limitations must be acknowledged. As with any self-reported exposure data, the responses of the primary caregivers to questions on insecticide use may have been subject to recall bias, if caregivers of children with developmental delays or ASD report differentially as compared with those caring for TD children. This could lead to overreporting of exposures perceived as harmful in those with affected children or under-reporting by those with unaffected children – in both cases, leading to differential misclassification that would tend to inflate associations. However, one study,[
57] aimed at ascertaining if self-report of multiple exposures during pregnancy was random or systematic, found that reporting errors of most exposures (75%) were random, with the authors concluding that recall bias is exposure-specific. If these recall errors were systematic, the potential exists that they are exaggerated away from the null. The reliance on self-reported exposure histories also raises concerns about the accuracy of timing and frequency of insecticide applications, especially over extended recall periods. However, these types of errors tend to be non-differential, biasing the results toward the null.
We were also unable to ascertain specific insecticide formulations or active ingredients, thereby limiting the capacity to determine the relative toxicity of a given ingredient. While we did ask participants for specific brand names of insecticides used, there were substantial recall issues with the exact name of each insecticide. For example, many participants would simply report “Raid” instead of “Raid Max Ant and Roach Killer” or “Raid Wasp & Hornet Killer.” Also, we did not ask for the year the insecticide was purchased, and therefore did not know the product formulation. This made it difficult to identify the specific insecticide(s) each participant was exposed to.
Additionally, insecticide residues in food and exposures outside the home environment, such as in workplaces or childcare settings, were not assessed. Many such locations routinely employ insecticide applications as part of their maintenance routines, likely exposing some mothers and children to additional sources of these chemicals beyond the home environment. This likely resulted in an underestimation of the total insecticide exposure. We also had very few people in the DD group that were exposed to certain insecticides, resulting in models that were underpowered, or unable to be assessed.
Lastly, residual confounding is another concern, particularly with respect to socioeconomic status (SES), which may influence both insecticide use patterns and neurodevelopmental outcomes. Despite efforts to adjust for SES-related variables, such as maternal education and homeownership, more nuanced SES factors may not have been fully accounted for. Families using pesticides may experience higher pest burdens due to environmental factors, such as older housing or poor maintenance, which could independently contribute to stressors or health risks that affect neurodevelopment. Conversely, those with higher SES might be more likely to get their homes professionally sprayed on a regular basis. This dynamic of structural factors may result in residual confounding that biases the results in unpredictable directions and complicates the interpretation of causality.
This study has several notable strengths. The use of detailed, self-reported data on the timing, type, and frequency of pesticide exposures provides valuable granularity, allowing for the examination of specific exposure windows critical for neurodevelopment. The large sample size, which includes children with and without ASD, enhances the statistical power and generalizability of findings. Additionally, the rigorous diagnostic classification of ASD using gold-standard tools, such as the ADOS-2 and ADI-R by trained clinicians who have attained reliability on the instruments they administer, ensures reliable identification of cases and minimizes the potential for outcome misclassification bias. The study’s diverse population, encompassing a broad range of socioeconomic, demographic, racial, ethnic and cultural backgrounds, further supports the relevance of findings to varied community settings.
5. Conclusions
These findings from the CHARGE Study contribute to the growing body of evidence suggesting that household insecticide use, particularly indoor insecticides and products used for flea and tick control, may negatively affect development of critical domains, namely cognitive/intellectual and adaptive/functional living skills, especially in children with ASD, but also for those with other neurodevelopmental impairments. Future research should aim to replicate these timing-specific associations, evaluate exposure frequency and intensity, and assess cumulative insecticide exposures from diverse sources, including dietary intake, occupational and childcare environments, and residential proximity to treated areas, to more fully elucidate their potential impact on neurodevelopment.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, Amanda J Goodrich, Rebecca Schmidt, Irva Hertz-Picciotto and Deborah Bennett; Data curation, Yunin Ludeña and Ekaterina Roudneva; Formal analysis, Amanda J Goodrich; Funding acquisition, Rebecca Schmidt, Irva Hertz-Picciotto and Deborah Bennett; Methodology, Amanda J Goodrich, Daniel Tancredi, Rebecca Schmidt, Irva Hertz-Picciotto and Deborah Bennett; Project administration, Rebecca Schmidt, Irva Hertz-Picciotto and Deborah Bennett; Software, Amanda J Goodrich, Yunin Ludeña and Ekaterina Roudneva; Supervision, Daniel Tancredi, Rebecca Schmidt and Deborah Bennett; Visualization, Amanda J Goodrich; Writing – original draft, Amanda J Goodrich; Writing – review & editing, Amanda J Goodrich, Daniel Tancredi, Yunin Ludeña, Ekaterina Roudneva, Rebecca Schmidt, Irva Hertz-Picciotto and Deborah Bennett. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by The National Institutes of Health (NIH) (Grant numbers: R21-ES021330, R01-ES031701, R01-ES015359, P01-ES11269) and the UC Davis MIND Institute.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Boards University of California (IRB number 226028, approval date: 04/09/2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets presented in this article are not readily available because the data are part of an ongoing study.
Acknowledgments
The authors would like to thank the University of California Davis MIND (Medical Investigations of Neurodevelopmental Disorders) Institute for their role in diagnosing participants. The authors would also like to thank the CHARGE staff and the participants for their valuable contributions.
Conflicts of Interest
Daniel Tancredi reports a relationship with Deerland Probiotics and Enzymes that includes: consulting or advisory. Daniel Tancredi reports a relationship with Synbiotic Health that includes: consulting or advisory. Deborah Bennett reports a relationship with LinusBio that includes: consulting or advisory. Rebecca Schmidt reports a relationship with LinusBio that includes: consulting or advisory. Rebecca Schmidt reports a relationship with Bia-Echo Foundation that includes: funding grants. Rebecca Schmidt reports a relationship with HEALthy Brain and Child Development (HBCD) Study that includes: travel reimbursement. Rebecca Schmidt reports a relationship with Organization of Teratology Information Specialists (OTIS) that includes: travel reimbursement. Rebecca Schmidt reports a relationship with Simons Foundation that includes: funding grants. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| ADI-R |
Autism Diagnostic Interview–Revised |
| ADOS-2 |
Autism Diagnostic Observation Schedules-2 |
| ASD |
Autism spectrum disorder |
| CHARGE |
Childhood Autism Risk from Genetics and the Environment |
| CI |
Confidence interval |
| DAG |
Directed Acyclic Graph |
| DD |
Developmental delay without autism spectrum disorder |
| DDS |
Department of Developmental Services |
| DQ |
Developmental quotients |
| EPA |
Environmental Protection Agency |
| FDR |
False discovery rate |
| GABA |
gamma-aminobutyric acid |
| GP |
General population |
| MSEL |
Mullen Scales of Early Learning |
| nAChRs |
nicotinic acetylcholine receptors |
| NHW |
Non-Hispanic white |
| OP |
Organophosphorus |
| QFDR |
Q-value after FDR correction |
| RC |
Regional Centers |
| SCQ |
Social Communication Questionnaire |
| SES |
Socioeconomic status |
| TD |
Typical development |
| VABS |
Vineland Adaptive Behavior Scales |
References
- Zablotsky, B.; Black, L.I.; Maenner, M.J.; Schieve, L.A.; Danielson, M.L.; Bitsko, R.H.; Blumberg, S.J.; Kogan, M.D.; Boyle, C.A. Prevalence and trends of developmental disabilities among children in the United States: 2009–2017. Pediatrics 2019, 144. [Google Scholar] [CrossRef] [PubMed]
- Gidziela, A.; Ahmadzadeh, Y.I.; Michelini, G.; Allegrini, A.G.; Agnew-Blais, J.; Lau, L.Y.; Duret, M.; Procopio, F.; Daly, E.; Ronald, A. A meta-analysis of genetic effects associated with neurodevelopmental disorders and co-occurring conditions. Nature human behaviour 2023, 7, 642–656. [Google Scholar] [CrossRef] [PubMed]
- Bourgeron, T. From the genetic architecture to synaptic plasticity in autism spectrum disorder. Nat Rev Neurosci 2015, 16, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Hallmayer, J.; Cleveland, S.; Torres, A.; Phillips, J.; Cohen, B.; Torigoe, T.; Miller, J.; Fedele, A.; Collins, J.; Smith, K.; et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry 2011, 68, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Lyall, K.; Schweitzer, J.B.; Schmidt, R.J.; Hertz-Picciotto, I.; Solomon, M. Inattention and hyperactivity in association with autism spectrum disorders in the CHARGE study. Research in Autism Spectrum Disorders 2017, 35, 1–12. [Google Scholar] [CrossRef]
- Mazina, V.; Gerdts, J.; Trinh, S.; Ankenman, K.; Ward, T.; Dennis, M.Y.; Girirajan, S.; Eichler, E.E.; Bernier, R. Epigenetics of autism-related impairment: copy number variation and maternal infection. J Dev Behav Pediatr 2015, 36, 61–67. [Google Scholar] [CrossRef]
- Sandin, S.; Lichtenstein, P.; Kuja-Halkola, R.; Hultman, C.; Larsson, H.; Reichenberg, A. The Heritability of Autism Spectrum Disorder. JAMA 2017, 318, 1182–1184. [Google Scholar] [CrossRef]
- Stone, D.L.; Sudakin, D.L.; Jenkins, J.J. Longitudinal trends in organophosphate incidents reported to the National Pesticide Information Center, 1995–2007. Environmental Health 2009, 8, 1–8. [Google Scholar] [CrossRef]
- Williams, M.K.; Rundle, A.; Holmes, D.; Reyes, M.; Hoepner, L.A.; Barr, D.B.; Camann, D.E.; Perera, F.P.; Whyatt, R.M. Changes in pest infestation levels, self-reported pesticide use, and permethrin exposure during pregnancy after the 2000–2001 US Environmental Protection Agency restriction of organophosphates. Environmental Health Perspectives 2008, 116, 1681–1688. [Google Scholar] [CrossRef]
- Saillenfait, A.-M.; Ndiaye, D.; Sabaté, J.-P. Pyrethroids: exposure and health effects–an update. International journal of hygiene and environmental health 2015, 218, 281–292. [Google Scholar] [CrossRef]
- Eskenazi, B.; Marks, A.R.; Bradman, A.; Harley, K.; Barr, D.B.; Johnson, C.; Morga, N.; Jewell, N.P. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environmental health perspectives 2007, 115, 792–798. [Google Scholar] [CrossRef]
- Roberts, E.M.; English, P.B.; Grether, J.K.; Windham, G.C.; Somberg, L.; Wolff, C. Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California Central Valley. Environmental health perspectives 2007, 115, 1482–1489. [Google Scholar] [CrossRef]
- Shelton, J.F.; Geraghty, E.M.; Tancredi, D.J.; Delwiche, L.D.; Schmidt, R.J.; Ritz, B.; Hansen, R.L.; Hertz-Picciotto, I. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: the CHARGE study. Environmental health perspectives 2014, 122, 1103–1109. [Google Scholar] [CrossRef]
- Furlong, M.A.; Barr, D.B.; Wolff, M.S.; Engel, S.M. Prenatal exposure to pyrethroid pesticides and childhood behavior and executive functioning. Neurotoxicology 2017, 62, 231–238. [Google Scholar] [CrossRef]
- Gunier, R.B.; Bradman, A.; Harley, K.G.; Kogut, K.; Eskenazi, B. Prenatal residential proximity to agricultural pesticide use and IQ in 7-year-old children. Environmental health perspectives 2017, 125, 057002. [Google Scholar] [CrossRef] [PubMed]
- Hicks, S.D.; Wang, M.; Fry, K.; Doraiswamy, V.; Wohlford, E.M. Neurodevelopmental delay diagnosis rates are increased in a region with aerial pesticide application. Frontiers in pediatrics 2017, 5, 116. [Google Scholar] [CrossRef] [PubMed]
- Lyall, K.; Croen, L.A.; Sjödin, A.; Yoshida, C.K.; Zerbo, O.; Kharrazi, M.; Windham, G.C. Polychlorinated biphenyl and organochlorine pesticide concentrations in maternal mid-pregnancy serum samples: association with autism spectrum disorder and intellectual disability. Environmental health perspectives 2017, 125, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.S.; Cheslack-Postava, K.; Rantakokko, P.; Kiviranta, H.; Hinkka-Yli-Salomäki, S.; McKeague, I.W.; Surcel, H.-M.; Sourander, A. Association of maternal insecticide levels with autism in offspring from a national birth cohort. American Journal of Psychiatry 2018, 175, 1094–1101. [Google Scholar] [CrossRef]
- von Ehrenstein, O.S.; Ling, C.; Cui, X.; Cockburn, M.; Park, A.S.; Yu, F.; Wu, J.; Ritz, B. Prenatal and infant exposure to ambient pesticides and autism spectrum disorder in children: population based case-control study. BMJ 2019, 364, l962. [Google Scholar] [CrossRef]
- Bennett, D.H.; Busgang, S.A.; Kannan, K.; Parsons, P.J.; Takazawa, M.; Palmer, C.D.; Schmidt, R.J.; Doucette, J.T.; Schweitzer, J.B.; Gennings, C. Environmental exposures to pesticides, phthalates, phenols and trace elements are associated with neurodevelopment in the CHARGE study. Environment international 2022, 161, 107075. [Google Scholar] [CrossRef]
- Goodrich, A.J.; Tancredi, D.J.; Ludeña, Y.J.; Bennett, D.H.; Hertz-Picciotto, I.; Schmidt, R.J. Professionally and non-professionally applied household insecticides during pregnancy and early life and their associations with autism spectrum disorder and developmental delay in the CHARGE case-control study. Environmental Research 2025, 267, 120651. [Google Scholar] [CrossRef] [PubMed]
- Domingues, V.F.; Nasuti, C.; Piangerelli, M.; Correia-Sá, L.; Ghezzo, A.; Marini, M.; Abruzzo, P.M.; Visconti, P.; Giustozzi, M.; Rossi, G. Pyrethroid pesticide metabolite in urine and microelements in hair of children affected by autism spectrum disorders: A preliminary investigation. International journal of environmental research and public health 2016, 13, 388. [Google Scholar] [CrossRef] [PubMed]
- Barkoski, J.M.; Philippat, C.; Tancredi, D.; Schmidt, R.J.; Ozonoff, S.; Barr, D.B.; Elms, W.; Bennett, D.H.; Hertz-Picciotto, I. In utero pyrethroid pesticide exposure in relation to autism spectrum disorder (ASD) and other neurodevelopmental outcomes at 3 years in the MARBLES longitudinal cohort. Environmental research 2021, 194, 110495. [Google Scholar] [CrossRef]
- Lee, K.-S.; Lim, Y.-H.; Lee, Y.A.; Shin, C.H.; Kim, B.-N.; Hong, Y.-C.; Kim, J.I. The association of prenatal and childhood pyrethroid pesticide exposure with school-age ADHD traits. Environment International 2022, 161, 107124. [Google Scholar] [CrossRef]
- National pet owners survey. Business/Finance Fact Sheet. Available online: americanpetproducts.org/Uploads/NPOS/21-22BusinessandFinance.pdf (accessed on.
- Rust, M.K. Recent advancements in the control of cat fleas. Insects 2020, 11, 668. [Google Scholar] [CrossRef] [PubMed]
- Mullen, E.M. Mullen scales of early learning manual; American Guidance Service: 1995.
- Sparrow, S.S.; Balla, D.A.; Cicchetti, D.V. Vineland Adaptive Behavior Scales Interview Edition Expanded Form Manual. 1984.
- Hertz-Picciotto, I.; Croen, L.A.; Hansen, R.; Jones, C.R.; van de Water, J.; Pessah, I.N. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environ Health Perspect 2006, 114, 1119–1125. [Google Scholar] [CrossRef]
- Lord, C.; Risi, S.; Lambrecht, L.; Cook, E.H.; Leventhal, B.L.; DiLavore, P.C.; Pickles, A.; Rutter, M. The Autism Diagnostic Observation Schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of autism and developmental disorders 2000, 30, 205–223. [Google Scholar] [CrossRef]
- Lord C, R.M., DiLavore PC, Risi S. Autism Diagnostic Observation Schedule Manual. 2003. 2003.
- Lord, C.; Pickles, A.; McLennan, J.; Rutter, M.; Bregman, J.; Folstein, S.; Fombonne, E.; Leboyer, M.; Minshew, N. Diagnosing autism: analyses of data from the Autism Diagnostic Interview. Journal of autism and developmental disorders 1997, 27, 501–517. [Google Scholar] [CrossRef]
- Lord, C.; Rutter, M.; Le Couteur, A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of autism and developmental disorders 1994, 24, 659–685. [Google Scholar] [CrossRef]
- Rutter, M.; Le Couteur, A.; Lord, C. Autism diagnostic interview-revised. Los Angeles, CA: Western Psychological Services 2003, 29, 30. [Google Scholar]
- Eaves, L.C.; Wingert, H.D.; Ho, H.H.; Mickelson, E.C. Screening for autism spectrum disorders with the social communication questionnaire. Journal of Developmental & Behavioral Pediatrics 2006, 27, S95–S103. [Google Scholar]
- Chandler, S.; Charman, T.; Baird, G.; Simonoff, E.; Loucas, T.; Meldrum, D.; Scott, M.; Pickles, A. Validation of the social communication questionnaire in a population cohort of children with autism spectrum disorders. Journal of the American Academy of Child & Adolescent Psychiatry 2007, 46, 1324–1332. [Google Scholar]
- Textor, J.; van der Zander, B.; Gilthorpe, M.S.; Liskiewicz, M.; Ellison, G.T. Robust causal inference using directed acyclic graphs: the R package ‘dagitty’. Int J Epidemiol 2016, 45, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Tu, Y.; Song, Y.; Yang, G.; You, M. The relationship between pesticide exposure during critical neurodevelopment and autism spectrum disorder: A narrative review. Environmental Research 2022, 203, 111902. [Google Scholar] [CrossRef]
- Carlisle, G.K. The social skills and attachment to dogs of children with autism spectrum disorder. Journal of Autism and Developmental Disorders 2015, 45, 1137–1145. [Google Scholar] [CrossRef]
- Wright, H.; Hall, S.; Hames, A.; Hardiman, J.; Mills, R.; Team, P.P.; Mills, D. Pet dogs improve family functioning and reduce anxiety in children with autism spectrum disorder. Anthrozoös 2015, 28, 611–624. [Google Scholar] [CrossRef]
- Fields, V.L.; Soke, G.N.; Reynolds, A.; Tian, L.H.; Wiggins, L.; Maenner, M.; DiGuiseppi, C.; Kral, T.V.; Hightshoe, K.; Schieve, L.A. Pica, autism, and other disabilities. Pediatrics 2021, 147. [Google Scholar] [CrossRef]
- Mayes, S.D.; Zickgraf, H. Atypical eating behaviors in children and adolescents with autism, ADHD, other disorders, and typical development. Research in Autism Spectrum Disorders 2019, 64, 76–83. [Google Scholar] [CrossRef]
- Miramontes Gonzalez, P.; Li, L. Evaluating the environmental persistence of liquid crystal monomers indoors and outdoors. Environmental Science & Technology Letters 2023, 11, 216–222. [Google Scholar]
- Shin, H.-M.; McKone, T.E.; Tulve, N.S.; Clifton, M.S.; Bennett, D.H. Indoor residence times of semivolatile organic compounds: model estimation and field evaluation. Environmental science & technology 2013, 47, 859–867. [Google Scholar]
- Bass, C.; Field, L.M. Neonicotinoids. Current biology 2018, 28, R772–R773. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, M.; Casida, J.E. Neonicotinoid insecticide toxicology: mechanisms of selective action. Annu Rev Pharmacol Toxicol 2005, 45, 247–268. [Google Scholar] [CrossRef] [PubMed]
- Bradford, B.R.; Whidden, E.; Gervasio, E.D.; Checchi, P.M.; Raley-Susman, K.M. Neonicotinoid-containing insecticide disruption of growth, locomotion, and fertility in Caenorhabditis elegans. PLoS One 2020, 15, e0238637. [Google Scholar] [CrossRef] [PubMed]
- Kara, M.; Yumrutas, O.; Demir, C.F.; Ozdemir, H.H.; Bozgeyik, I.; Coskun, S.; Eraslan, E.; Bal, R. Insecticide imidacloprid influences cognitive functions and alters learning performance and related gene expression in a rat model. International journal of experimental pathology 2015, 96, 332–337. [Google Scholar] [CrossRef]
- Duzguner, V.; Erdogan, S. Chronic exposure to imidacloprid induces inflammation and oxidative stress in the liver & central nervous system of rats. Pesticide biochemistry and physiology 2012, 104, 58–64. [Google Scholar]
- Wei, F.; Cheng, F.; Li, H.; You, J. Imidacloprid affects human cells through mitochondrial dysfunction and oxidative stress. Science of The Total Environment 2024, 951, 175422. [Google Scholar] [CrossRef]
- Zhao, T.; Alder, N.N.; Starkweather, A.R.; Chen, M.-H.; Matson, A.P.; Xu, W.; Balsbaugh, J.L.; Cong, X. Associations of mitochondrial function, stress, and neurodevelopmental outcomes in early life: A systematic review. Developmental neuroscience 2022, 44, 438–454. [Google Scholar] [CrossRef]
- Rosa, M.E.; Oliveira, R.S.; de Faria Barbosa, R.; Hyslop, S.; Dal Belo, C.A. Recent advances on the influence of fipronil on insect behavior. Current Opinion in Insect Science 2024, 101251. [Google Scholar] [CrossRef]
- Wang, X.; Martínez, M.A.; Wu, Q.; Ares, I.; Martínez-Larrañaga, M.R.; Anadón, A.; Yuan, Z. Fipronil insecticide toxicology: oxidative stress and metabolism. Critical reviews in toxicology 2016, 46, 876–899. [Google Scholar] [CrossRef]
- Suzuki, T.; Hirai, A.; Khidkhan, K.; Nimako, C.; Ichise, T.; Takeda, K.; Mizukawa, H.; Nakayama, S.M.; Nomiyama, K.; Hoshi, N. The effects of fipronil on emotional and cognitive behaviors in mammals. Pesticide Biochemistry and Physiology 2021, 175, 104847. [Google Scholar] [CrossRef]
- Lu, M.; Du, J.; Zhou, P.; Chen, H.; Lu, C.; Zhang, Q. Endocrine disrupting potential of fipronil and its metabolite in reporter gene assays. Chemosphere 2015, 120, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, V.; Gálvez-Ontiveros, Y.; González-Domenech, P.J.; Baca, M.Á.; Rodrigo, L.; Rivas, A. Role of endocrine disrupting chemicals in children’s neurodevelopment. Environmental Research 2022, 203, 111890. [Google Scholar] [CrossRef] [PubMed]
- WERLER, M.M.; POBER, B.R.; NELSON, K.; HOLMES, L.B. Reporting accuracy among mothers of malformed and nonmalformied infants. American Journal of Epidemiology 1989, 129, 415–421. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).