Submitted:
06 June 2025
Posted:
06 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
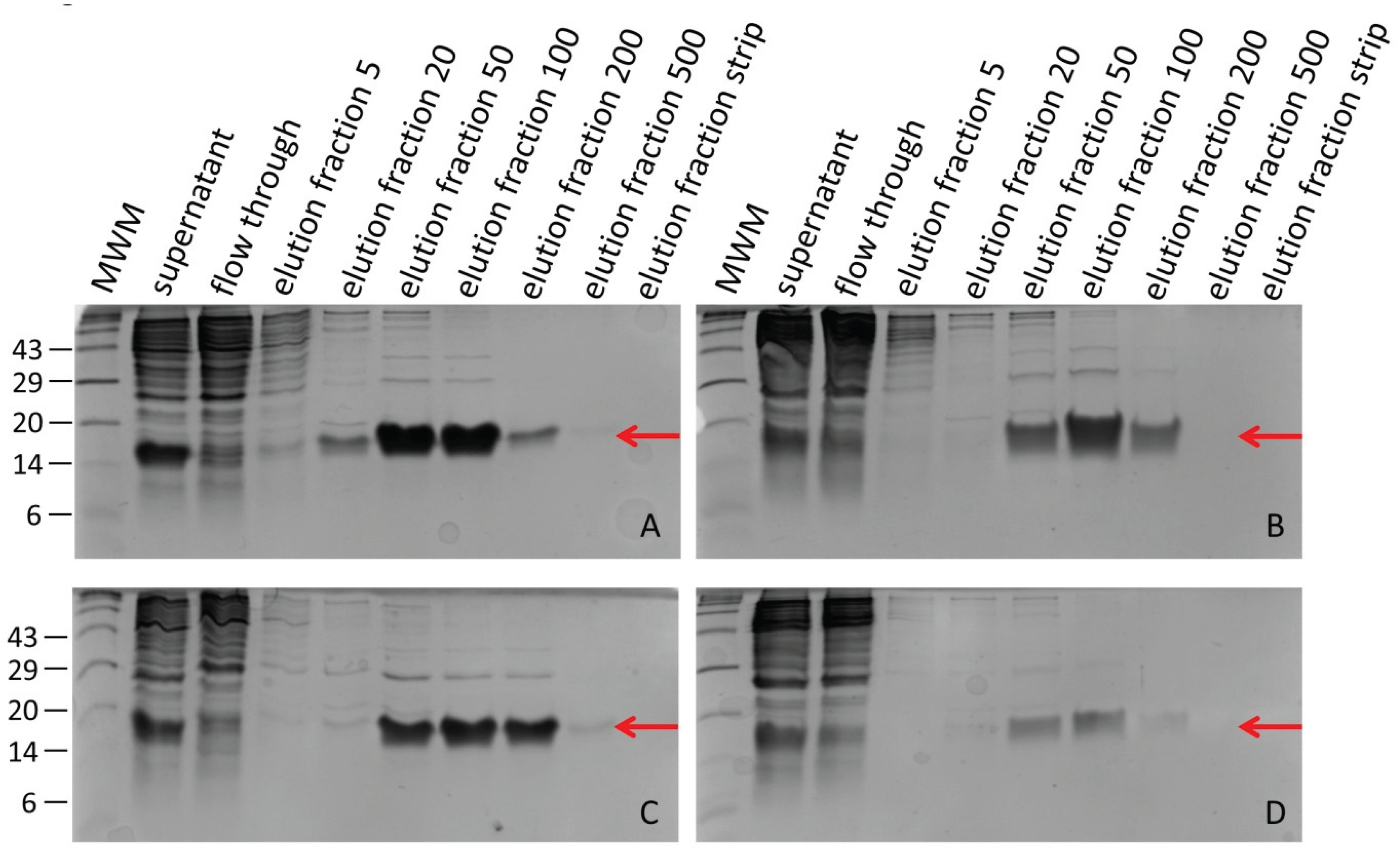
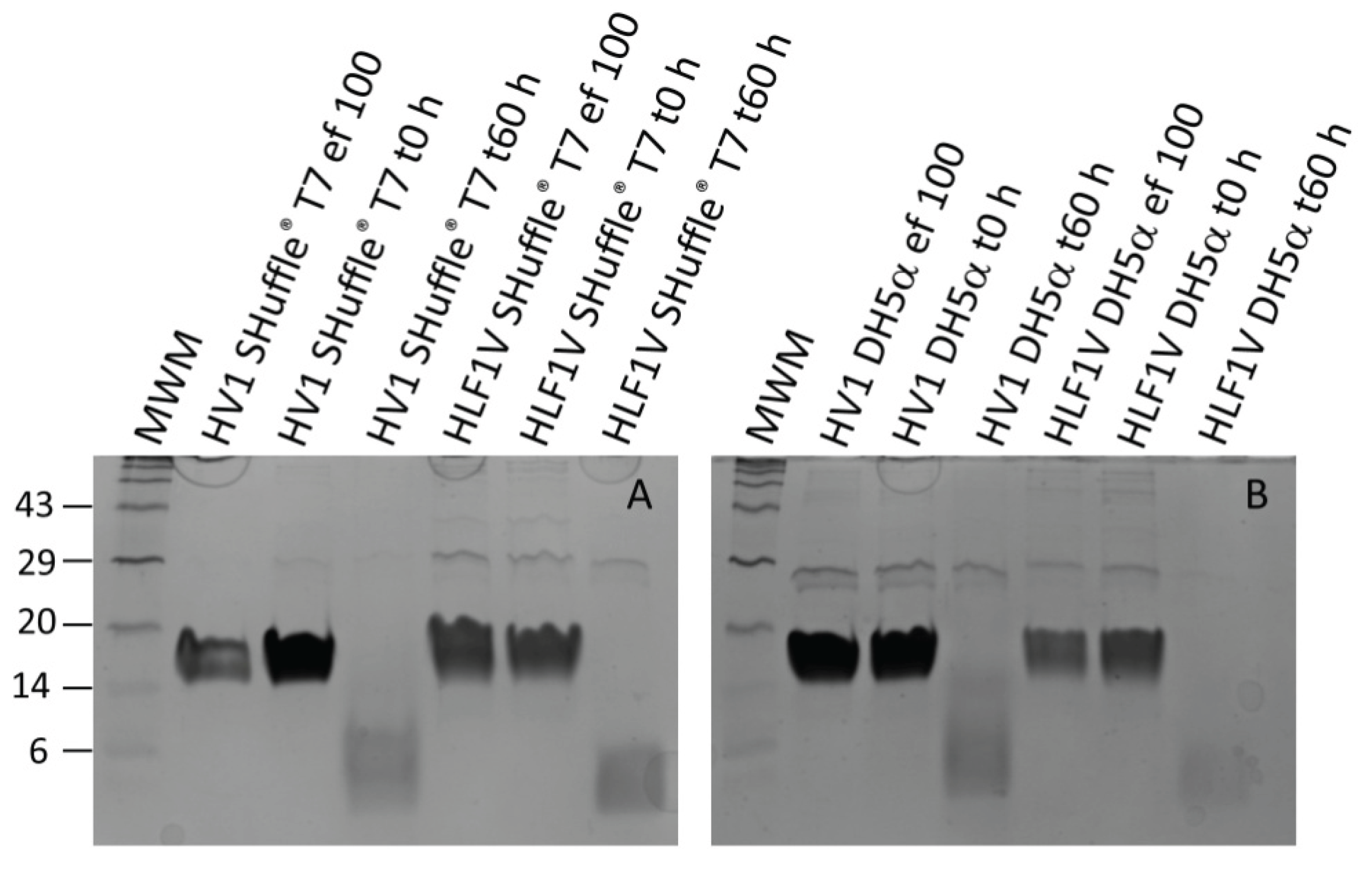
2.1. Expression of Recombinant Hirudin HV1 and HLF1V in E. coli
2.2. Expression of Recombinant Hirudin HV1 in P. pastoris
2.3. Purification of Recombinant Hirudins HV1 and HLF1V
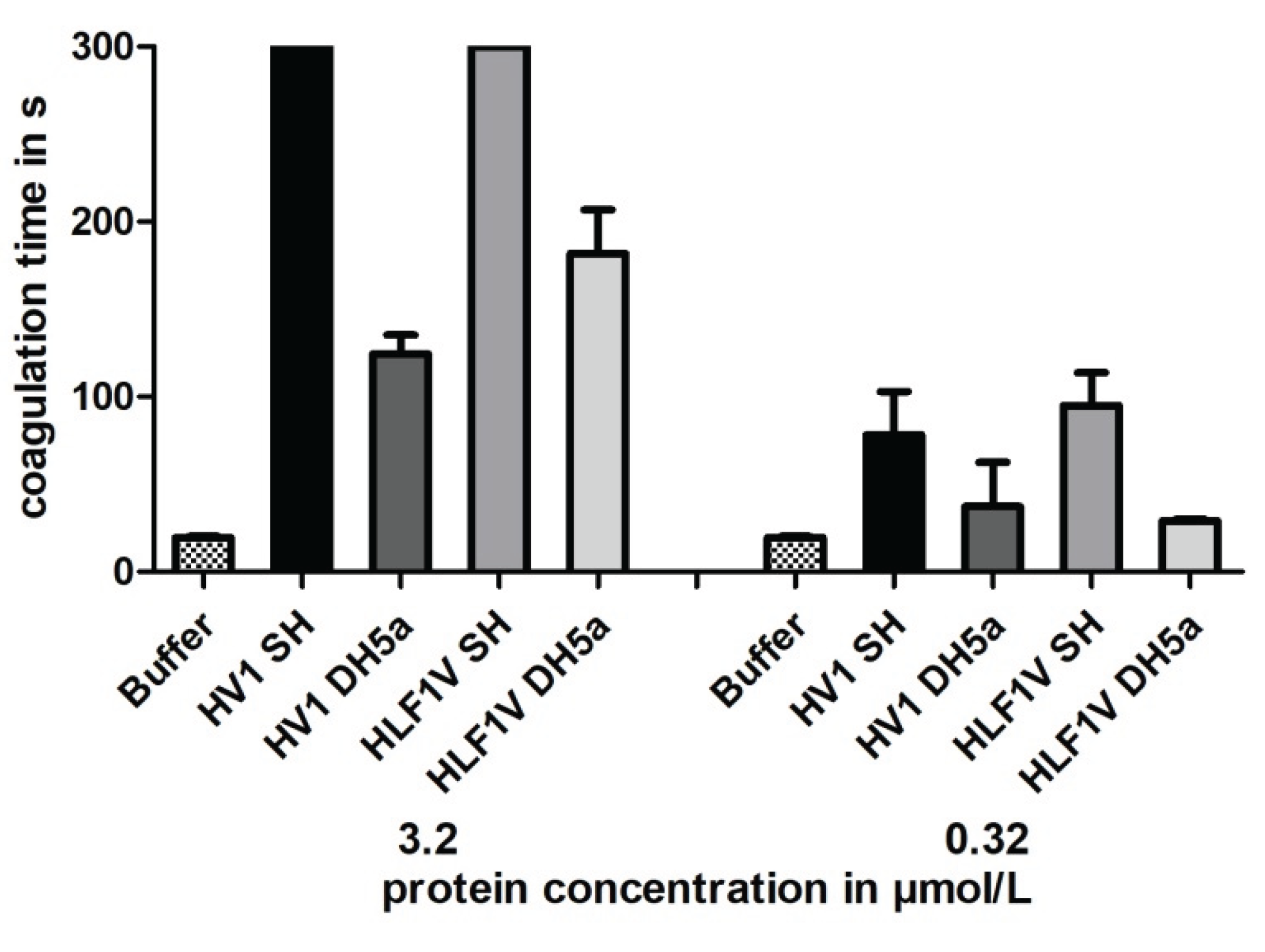
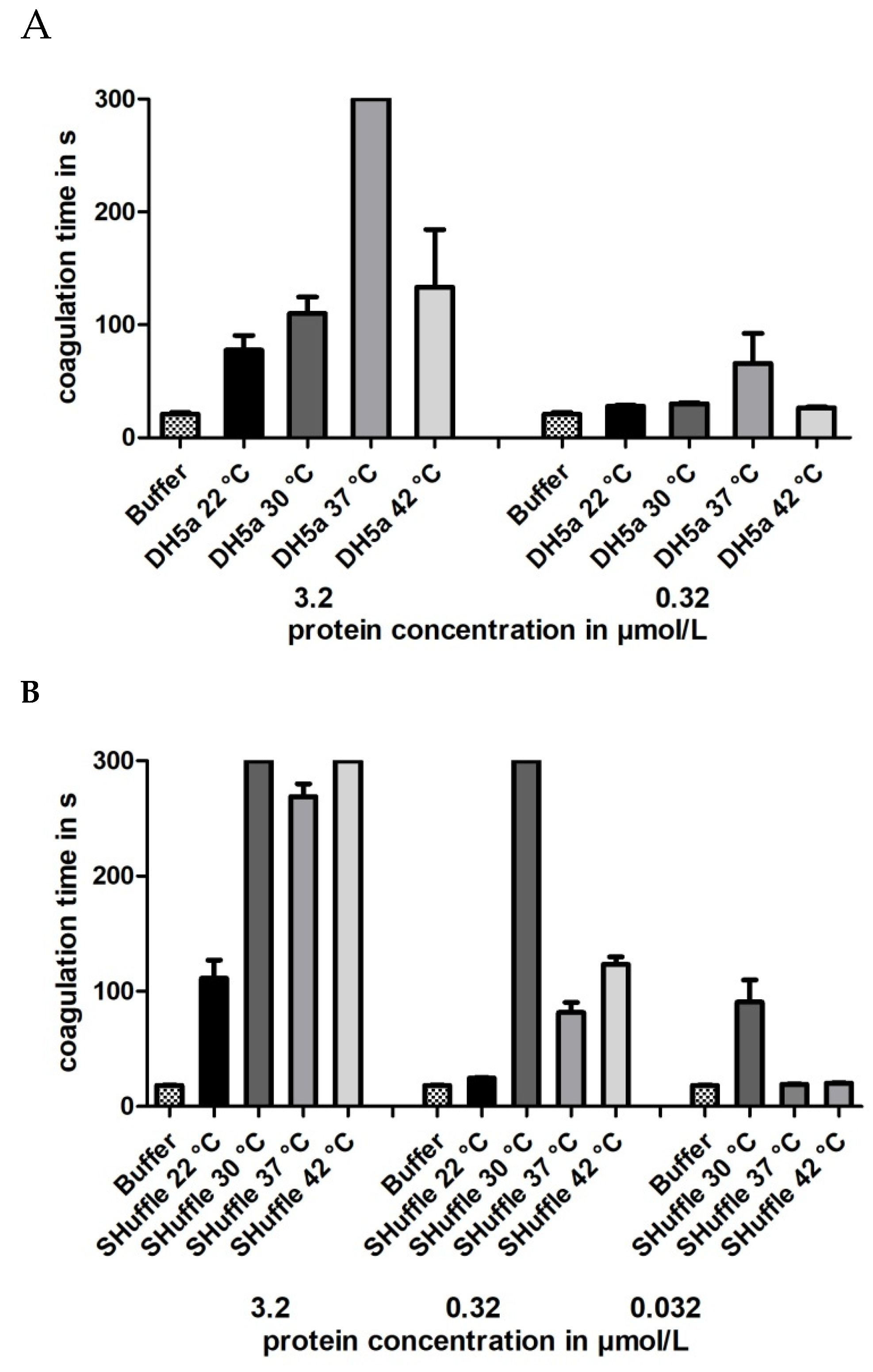
2.4. Blood Coagulation Assays
3. Results
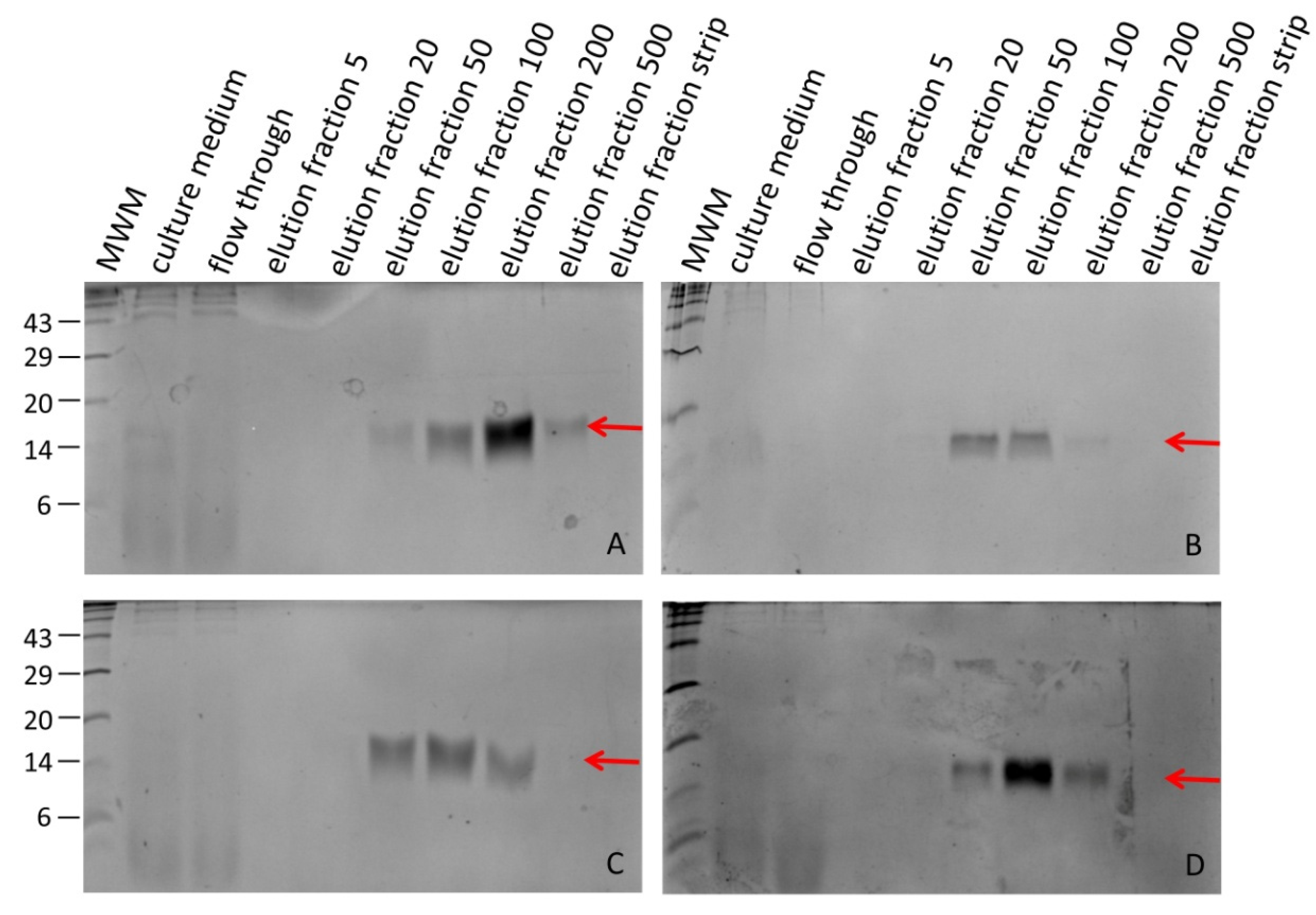
3.1. Expression of Recombinant Hirudin HV1 and HLF1V in Escherichia coli Strains
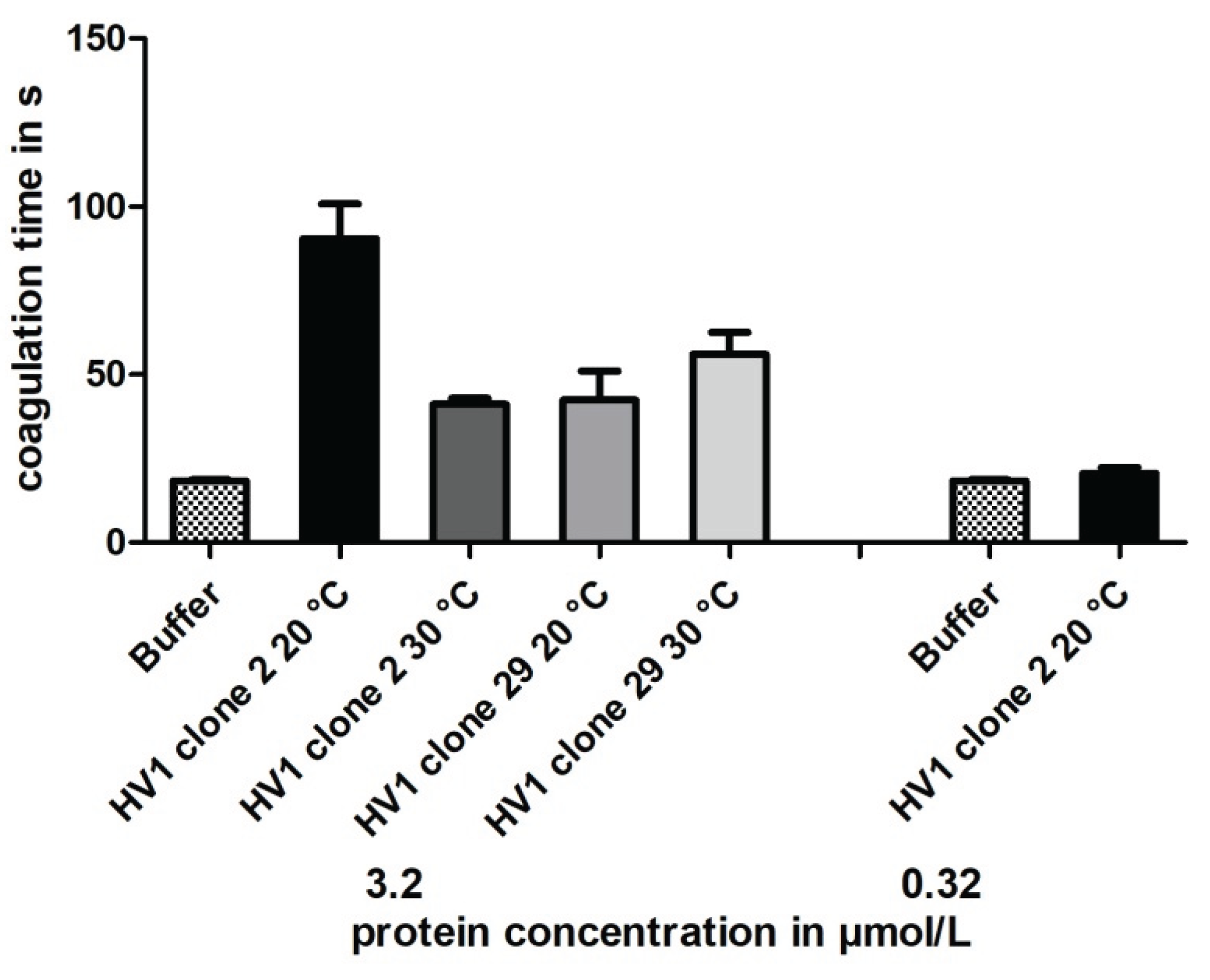
3.2. Functional Characterization of Hirudin HV1 and HLF1V Expressed in E. coli Strains
3.3. Expression of Recombinant Hirudin HV1 in P. pastoris GS115
3.4. Functional Characterization of Hirudin HV1 Expressed in P. pastoris GS115
4. Discussion
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
References
- Abdualkader AM, Ghawi AM, Alaama M, Awang M, Merzouk A (2013) Leech therapeutic applications. Indian J Pharm Sci 75(2):127–137.
- Ben Ahmed R, Abilov A, Müller C (2024) Diversity of hirudin and hirudin-like factor genes in the North-African medicinal leech, Hirudo troctina. Parasitol Res 14;123(11):382. [CrossRef]
- Berkmen M (2012) Production of disulfide-bonded proteins in Escherichia coli. Protein Expr Purif 82(1):240-251. [CrossRef]
- Castillo-Corujo A, Uchida Y, Saaranen MJ, Ruddock LW (2024) Escherichia coli cytoplasmic expression of disulfide-bonded proteins: Side-by-side comparison between two competing strategies. J Microbiol Biotechnol 34(5):1126-1134. [CrossRef]
- Combet C, Blanchet C, Geourjon C, Deléage G (2000) NPS@: network protein sequence analysis. Trends Biochem Sci 25(3):147-150. [CrossRef]
- de Marco A (2009) Strategies for successful recombinant expression of disulfide bond-dependent proteins in Escherichia coli. Microb Cell Fact 8:26. [CrossRef]
- di Leandro L, Colasante M, Pitari G, Ippoliti R (2023) Hosts and heterologous expression strategies of recombinant toxins for therapeutic purposes. Toxins (Basel) 15(12):699. [CrossRef]
- Dodt J, Müller H-P, Seemüller U, Chang J-Y (1984) The complete amino acid sequence of hirudin, a thrombin specific inhibitor: Application of colour carboxymethylation. FEBS Lett 165 2(9):180-184 . [CrossRef]
- Dodt J, Seemüller U, Maschler R, Fritz H (1985) The complete covalent structure of hirudin. Localization of the disulfide bonds. Biol Chem Hoppe Seyler 366(4):379-385. [CrossRef]
- Dodt J, Machleidt W, Seemüller U, Maschler R, Fritz H (1986) Isolation and characterization of hirudin isoinhibitors and sequence analysis of hirudin PA. Biol Chem Hoppe Seyler 367(8):803–811. [CrossRef]
- Fernández FJ, Vega MC (2016). Choose a suitable expression host: A survey of available protein production platforms. In: Vega, M. (eds) Advanced technologies for protein complex production and characterization. Advances in experimental medicine and biology, vol 896. Springer, Cham. [CrossRef]
- Ferrer-Miralles N, Garcia-Fruitós E (2024) Heterologous expression of difficult to produce proteins in bacterial systems. Int J Mol Sci 25(2):822. [CrossRef]
- Francis DM, Page R (2010) Strategies to optimize protein expression in E. coli. Curr Protoc Protein Sci 61(1):5.24.1–5.24.29. doi: 10.1002/0471140864.ps0524s61.
- Gąciarz A, Veijola J, Uchida Y, Saaranen MJ, Wang C, Hörkkö S, Ruddock LW (2016) Systematic screening of soluble expression of antibody fragments in the cytoplasm of E. coli. Microb Cell Fact 15:22. https://doi. org/10.1186/s12934-016-0419-5.
- Gill SC, von Hippel PH (1989) Calculation of protein extinction coefficients from amino acid sequence data. Anal Biochem 182:319–326. https://doi. org/10. 1016/ 0003- 2697(89) 90602-7.
- Gomes AR, Byregowda SM, Veeregowda BM, Balamurugan V (2016) An overview of heterologous expression host systems for the production of recombinant proteins. Adv Anim Vet Sci 4:346-356. [CrossRef]
- Green M, Sambrook J (2012) Molecular Cloning: A Laboratory Manual. 4th Edition, Vol. II, Cold Spring Harbor Laboratory Press, New York.
- Greinacher A, Völpel H, Janssens U, Hach-Wunderle V, Kemkes-Matthes B, Eichler P, Mueller-Velten HG, Pötzsch B (1999) Recombinant hirudin (lepirudin) provides safe and effective anticoagulation in patients with heparin-induced thrombocytopenia: a prospective study. Circulation 99(1):73-80. [CrossRef]
- Greinacher A, Warkentin TE (2008) The direct thrombin inhibitor hirudin. Thromb Haemost 99(05): 819-829. [CrossRef]
- Hanahan D (1985) Techniques for transformation of E. coli. In: Glover D M, editor. DNA cloning: a practical approach. Vol. 1. Oxford, United Kingdom: IRL Press; 1985.
- Harvey RP, Degryse E, Stefani L, Schamber F, Cazenave JP, Courtney M, Tolstoshev P, Lecocq JP (1986) Cloning and expression of a cDNA coding for the anticoagulant hirudin from the bloodsucking leech, Hirudo medicinalis. Proc Nat Acad Sci USA 83(4):1084–1088. [CrossRef]
- Hatahet F, Nguyen VD, Salo KEH, Ruddock LW (2010) Disruption of reducing pathways is not essential for efficient disulfide bond formation in the cytoplasm of E. coli. Microb Cell Fact 9:67. [CrossRef]
- Hsieh YSY, Wijeyewickrema LC, Wilkinson BL, Pike RN, Payne RJ (2014) Total synthesis of homogeneous variants of hirudin P6: a post-translationally modified anti-thrombotic leech-derived protein. Angew Chem Int Ed Engl 53(15):3947-3951. [CrossRef]
- Huang Y, Zhang Y, Wu Y, Wang J, Liu X, Dai L, Wang L, Yu M, Mo W (2012) Expression, purification, and mass spectrometric analysis of 15N, 13C-labeled RGD-hirudin, expressed in Pichia pastoris, for NMR studies. PLoS One 7(8):e42207. [CrossRef]
- Jayakrishnan A, Rosli WRW; Tahir ARM, Razak FSA, Kee PE, Ng HS, Chew Y-L, Lee S-K, Ramasamy M, Tan CS, Liew KB (2024) Evolving paradigms of recombinant protein production in pharmaceutical industry: A rigorous review. Sci 6, 9. [CrossRef]
- Jayaraj R, Smooker PM (2009) So you need a protein—A guide to the production of recombinant proteins. Open Vet Sci J 3:28-34. [CrossRef]
- Johnson PH (1994) Hirudin: clinical potential of a thrombin inhibitor. Annu Rev Med 45:165-177. [CrossRef]
- Kim MD, Rhee SK, Seo JH (2001) Enhanced production of anticoagulant hirudin in recombinant Saccharomyces cerevisiae by chromosomal delta-integration. J Biotechnol 85(1):41-48. [CrossRef]
- Kochanowski R, Kotłowski R, Szweda P (2006) Novel method of expression and purification of hirudin based on pBAD TOPO, pTYB12 vectors and gene synthesis. Protein Expr Purif 50(1):25-30. [CrossRef]
- Lobstein J, Emrich CA, Jeans C, Faulkner M, Riggs R, Berkmen M (2012) SHuffle, a novel Escherichia coli protein expression strain capable of correctly folding disulfide bonded proteins in its cytoplasm. Microb Cell Fact 11:56. [CrossRef]
- Markwardt F (1970) Hirudin as an inhibitor of thrombin. Method Enzym 19: 924-932. [CrossRef]
- Markwardt F (1989) Development of hirudin as an antithrombotic agent. Semin Thromb Hemost 15(3):269-282. [CrossRef]
- Möller C, Rimkus N, Skala FFO, Merouze M, Böttcher D, Dörr M, Bornscheuer UT (2024) Improved recombinant expression of soluble cathepsin B and L in Escherichia coli. Appl Microbiol Biotechnol 108(1):536. [CrossRef]
- Müller C, Mescke K, Liebig S, Mahfoud H, Lemke S, Hildebrandt J-P (2016) More than just one: multiplicity of Hirudins and Hirudin-like Factors in the Medicinal Leech, Hirudo medicinalis. Mol Genet Genomics 291(1):227-240. [CrossRef]
- Müller C, Haase M, Lemke S, Hildebrandt J-P (2017) Hirudins and hirudin-like factors in Hirudinidae: implications for function and phylogenetic relationships. Parasitol Res 116(1):313-325. [CrossRef]
- Müller C, Lukas P, Böhmert M, Hildebrandt J-P (2020a) Hirudin or hirudin-like factor—that is the question: insights from the analyses of natural and synthetic HLF variants. FEBS Lett 594(5):841-850. [CrossRef]
- Müller C, Lukas P, Sponholz D, Hildebrandt J-P (2020b) The hirudin-like factors HLF3 and HLF4-hidden hirudins of European medicinal leeches. Parasitol Res 119(6):1767-1775. [CrossRef]
- Müller C, Wang Z, Hamann M, Sponholz D, Hildebrandt J-P (2022) Life without blood: Molecular and functional analysis of hirudins and hirudin-like factors of the Asian non-hematophagous leech Whitmania pigra. J Thromb Haemost 20(8):1808-1817. [CrossRef]
- Nguyen VD, Hatahet F, Salo KEH, Enlund E, Zhang C, Ruddock LW (2011) Pre-expression of a sulfhydryl oxidase significantly increases the yields of eukaryotic disulfide bond containing proteins expressed in the cytoplasm of E.coli. Microb Cell Fact 10:1. [CrossRef]
- Nowak G (2002) Pharmacology of recombinant hirudin. Semin Thromb Hemost 28(5):415-424. [CrossRef]
- Pace CN, Vajdos F, Fee L, Grimsley G, Gray T (1995) How to measure and predict the molar absorption coefficient of a protein. Protein Sci 4:2411–2423. [CrossRef]
- Pouresmaeil M, Azizi-Dargahlou S (2023) Factors involved in heterologous expression of proteins in E. coli host. Arch Microbiol 205(5):212. [CrossRef]
- Qiu J, Lingna W, Jinghong H, Yongqing Z (2019) Oral administration of leeches (Shuizhi): A review of the mechanisms of action on antiplatelet aggregation. J Ethnopharmacol 232:103-109. [CrossRef]
- Riehl-Bellon N, Carvallo D, Acker M, Van Dorsselaer A, Marquet M, Loison G, Lemoine Y, Brown SW, Courtney M, Roitsch C (1989) Purification and biochemical characterization of recombinant hirudin produced by Saccharomyces cerevisiae. Biochemistry 28(7):2941-2949. [CrossRef]
- Rydel TJ, Ravichandran KG, Tulinsky A, Bode W, Huber R, Roitsch C, Fenton 2nd JW (1990) The structure of a complex of recombinant hirudin and human alpha-thrombin. Science 249(4966):277-280. [CrossRef]
- Rydel TJ, Tulinsky A, Bode W, Huber R (1991) Refined structure of the hirudin-thrombin complex. J Mol Biol 221(2):583-601. [CrossRef]
- Saaranen MJ, Ruddock LW (2019) Applications of catalyzed cytoplasmic disulfide bond formation. Biochem Soc Trans 47(5):1223-1231. [CrossRef]
- San-Miguel T, Pérez-Bermúdez P, Gavidia I (2013) Production of soluble eukaryotic recombinant proteins in E. coli is favoured in early log-phase cultures induced at low temperature. Springerplus 2:89. doi: 10.1186/2193-1801-2-89.
- Schein C and Noteborn M (1988) Formation of soluble recombinant proteins in Escherichia coli is favored by lower growth temperature. Nat Biotechnol 6:291–294. doi: 10.1038/nbt0388-291.
- Schütz A, Bernhard F, Berrow N, Buyel JF, Ferreira-da-Silva F, Haustraete J, van den Heuvel J, Hoffmann J-E, de Marco A, Peleg Y, Suppmann S, Unger T, Vanhoucke M, Witt S, Remans K (2023) A concise guide to choosing suitable gene expression systems for recombinant protein production. STAR Protoc 4(4):102572. [CrossRef]
- Sig AK, Guney M, Guclu AU, Ozmen E (2017) Medicinal leech therapy—an overall perspective. Integr Med Res 6(4):337–343. [CrossRef]
- So K-K, Le NMT, Nguyen N-L, Kim D-H (2023) Improving expression and assembly of difficult-to-express heterologous proteins in Saccharomyces cerevisiae by culturing at a sub-physiological temperature. Microb Cell Fact 22(1):55. [CrossRef]
- Sohn J-H, Lee S-K, Choi E-S, Rhee S-K (1991) Gene expression and secretion of the anticoagulant hirudin in Saccharomyces cerevisiae. J Microbiol Biotechnol 1(4):266-273.
- Steiner V, Knecht R, Gruetter M, Raschdorf F, Gassmann E, Maschler R (1990) Isolation and purification of novel hirudins from the leech Hirudinaria manillensis by high-performance liquid chromatography. J Chromatogr 530(2):273-282. [CrossRef]
- Steiner V, Knecht R, Börnsen KO, Gassmann E, Stone SR, Raschdorf F, Schlaeppi JM, Maschler R (1992) Primary structure and function of novel O-glycosylated hirudins from the leech Hirudinaria manillensis. Biochemistry 31(8):2294-2298. [CrossRef]
- Tuttle AR, Trahan ND, Son MS (2021) Growth and maintenance of Escherichia coli laboratory strains. Curr Protoc 1, e20. [CrossRef]
- Warkentin TE (2004) Bivalent direct thrombin inhibitors: hirudin and bivalirudin. Best Pract Res Clin Haematol 17(1):105-125. [CrossRef]
- Wüstenhagen DA, Lukas P, Müller C, Aubele SA, Hildebrandt J-P, Kubick S (2020) Cell-free synthesis of the hirudin variant 1 of the blood-sucking leech Hirudo medicinalis. Sci Rep 10(1):19818. [CrossRef]
| Expression system | Hirudin HV1 | HLF1V |
| SHuffle® T7 | 132.4 µmol/L 931 µg/mL |
154.9 µmol/L 1011 µg/mL |
| DH5α | 190.9 µmol/L 1341 µg/mL |
128.2 µmol/L 842 µg/mL |
| Cultivation temperature | Clone 2 | Clone 29 |
| 20 °C | 59.3 µmol/L 416 µg/mL |
50.7 µmol/L 356 µg/mL |
| 30 °C | 11.3 µmol/L 79 µg/mL |
34.3 µmol/L 241 µg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





