1. Introduction
Emergent endotracheal intubation is a lifesaving procedure frequently performed in emergency departments (ED), intensive care units (ICU), and pre-hospital settings. While often routine in operating rooms (OR), where patients are prepared, monitored, and managed by anesthesiologists, intubations outside the OR carry a significantly higher risk of complications. Among these, rapid-onset hypoxia is the most common and most dangerous, particularly in critically ill or chemically paralyzed patients with limited oxygen reserves [
1].
Despite the widespread use of techniques such as preoxygenation and apneic oxygenation, oxygen saturation can decline precipitously during rapid sequence intubation (RSI). Studies have shown that patients may reach critical hypoxia within 45 to 60 seconds of apnea [
2], with severe desaturation (SpO₂ <75%) reported in over 20 percent of ICU and ED intubations [
3,
4]. Multiple intubation attempts, delays in securing an airway, and inadequate preoxygenation further exacerbate the risk. Existing adjuncts such as nasal cannulas or video laryngoscopy may improve visualization or slightly extend safe apnea time, but they do not reliably prevent hypoxia in patients who are not breathing [
5].
The Turbo® O₂ Cap was specifically designed to be the first device capable of mitigating hypoxia during difficult intubations by delivering high-flow oxygen during the apneic phase, even in paralyzed, non-breathing patients. Human studies will be necessary to confirm the device’s clinical effectiveness, and this animal study represents a critical first step in that validation process. The device is a low-complexity, high-flow oxygenation cap that attaches to standard adult endotracheal tubes and delivers 15 liters per minute of 100 percent oxygen. It incorporates dual pressure-relief valves to reduce the risk of barotrauma and allows for simultaneous passage of a stylet [
6,
7]. Although this study presents the preclinical performance of the device in a porcine model, the Turbo® O₂ Cap has since received FDA Class I designation and is now commercially available in the United States [
6].
This study evaluates the Turbo® O₂ Cap in a controlled porcine model designed to simulate difficult intubations in paralyzed, non-breathing animals under RSI conditions. The objective was to determine whether the device could prevent or delay the onset of hypoxia compared to standard practice without oxygen delivery during intubation.
2. Materials and Methods
2.1. Study Design and Ethics
The study was conducted at Absorption Systems California, LLC (ASC), a Pharmaron Company, using a porcine model to evaluate the Turbo® O₂ Cap during simulated difficult emergent intubation. The protocol was approved by ASC’s Institutional Animal Care and Use Committee (IACUC) on March 22, 2022.
2.2. Device Description
Designed to mitigate hypoxia during intubation, the Turbo® O₂ Cap is a disposable, FDA Class I device that delivers high-flow oxygen through a standard adult endotracheal tube (ETT). The cap connects to a universal 15 mm ETT connector, making it compatible with any commercially available adult endotracheal tube. It delivers 15 liters per minute of 100% oxygen via a side-port tubing inlet. The device includes a stylet passthrough port to allow standard intubation techniques, and is designed for use with ETTs sized 5.5 mm to 9.0 mm internal diameter. The entire unit is constructed from rigid medical-grade polycarbonate and medical-grade silicone components [
6].
The Turbo® O₂ Cap is intended to be removed immediately after successful intubation and inflation of the ETT cuff, at which point the operator transitions to conventional ventilation methods, such as manual ventilation using a bag-valve-mask (BVM) or connection to a mechanical ventilator. If the cap is inadvertently left in place after the ETT cuff is inflated, the now closed respiratory circuit could begin to build pressure. To prevent over pressurization in this unlikely scenario, the device includes two redundant silicone pressure-relief valves, which automatically activate at approximately 20 cm H₂O. These valves are purely a safety mechanism and are not used during routine operation of the device. The engineering development and benchtop validation of the device have been previously described in detail [
7].
2.3. Animal Model
Three Yorkshire pigs (Sus scrofa domesticus), each weighing approximately 65–85 kg, were used in the study. The animals were obtained from an ASC-approved vendor and acclimated for at least five days prior to testing. All animals were kept under deep sedation throughout the entire procedure. During designated segments of the study, the animals were also chemically paralyzed to simulate apneic, non-breathing conditions. Sedation was maintained at all times to ensure that the animals did not experience pain or awareness during any part of the protocol.
2.4. Experimental Procedure
Three animals underwent four total cycles: two control cycles and two test cycles, for a total of 12 cycles across the study. Control cycles involved use of an ETT without oxygen; test cycles involved the ETT fitted with the Turbo® O₂ Cap and oxygen flowing at 15 L/min.
Each cycle included preparation, sedation, and administration of paralytics. The animals were placed in the prone position. After IV paralytic was given, a standard laryngoscope was used to elevate the jaw, and an endotracheal (ET) tube was placed in the back of the throat with the tip proximal to the vocal cords, in the area of the glottis but without advancing through it. The ET tube was positioned so that it did not obstruct visualization of the vocal cords, simulating a difficult intubation scenario.
In control cycles, the ET tube delivered no oxygen. In test cycles, the Turbo® O₂ Cap was attached to the ETT, with oxygen flowing at 15 L/min, directed near the entrance of the glottis.
Oxygen saturation was monitored and timed until it reached 75% or 10 minutes post paralytic injection, whichever came first. This marked the end of the cycle. The animal was then intubated and ventilated back to baseline or greater than 90% oxygen saturation.
Standard data collection included sex, age, weight, oxygen saturation per unit of time, and time to reach 75% saturation (if applicable).
2.5. Data Collection and Safety Monitoring
Oxygen saturation was continuously monitored by pulse oximetry. Vital signs, anesthetic depth, and post-procedure status were recorded by trained personnel. Animals were euthanized following completion of the acute procedures using potassium chloride (2 mEq/kg IV) under deep anesthesia, in accordance with AVMA 2020 euthanasia guidelines.
3. Results
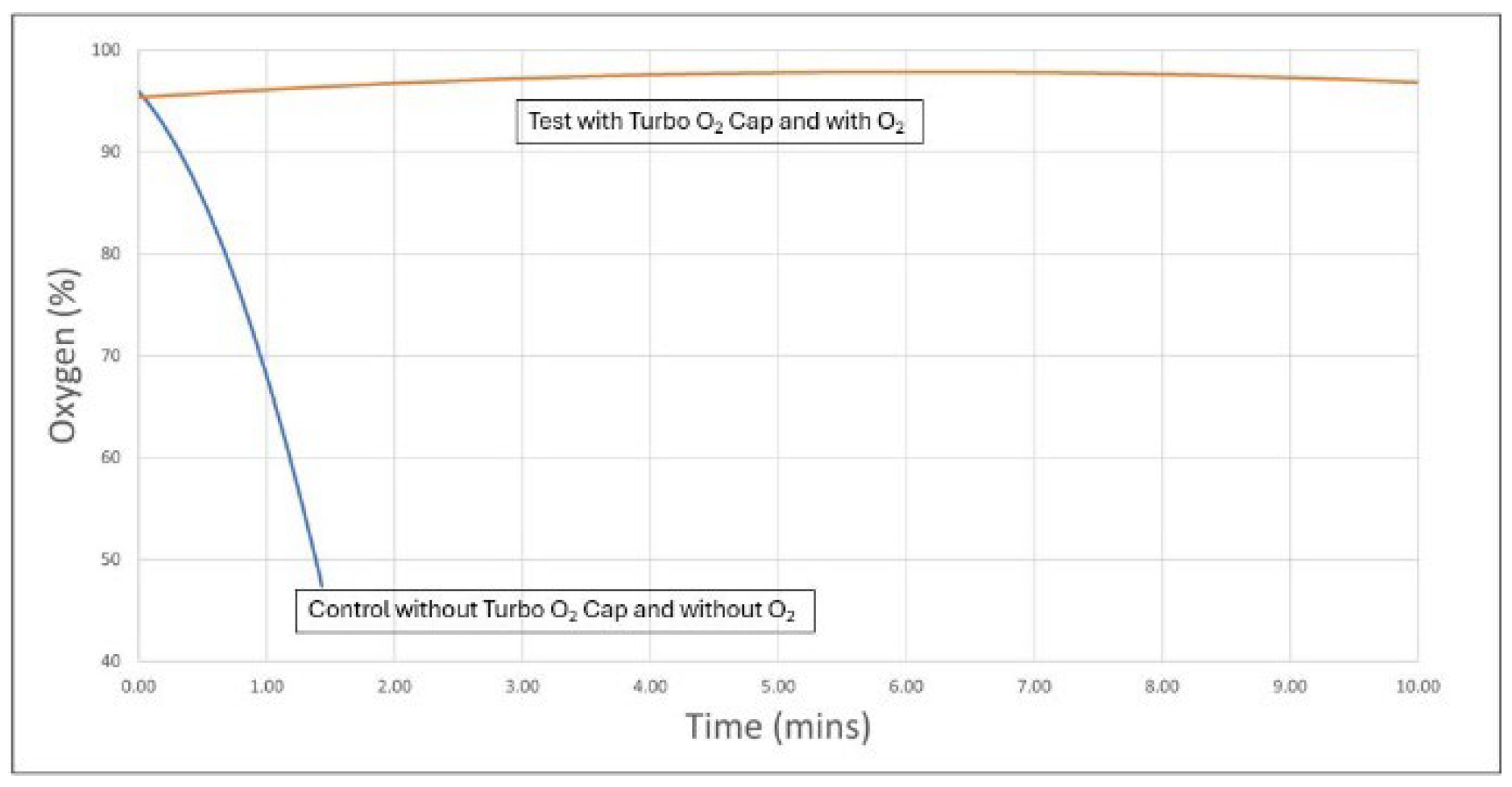
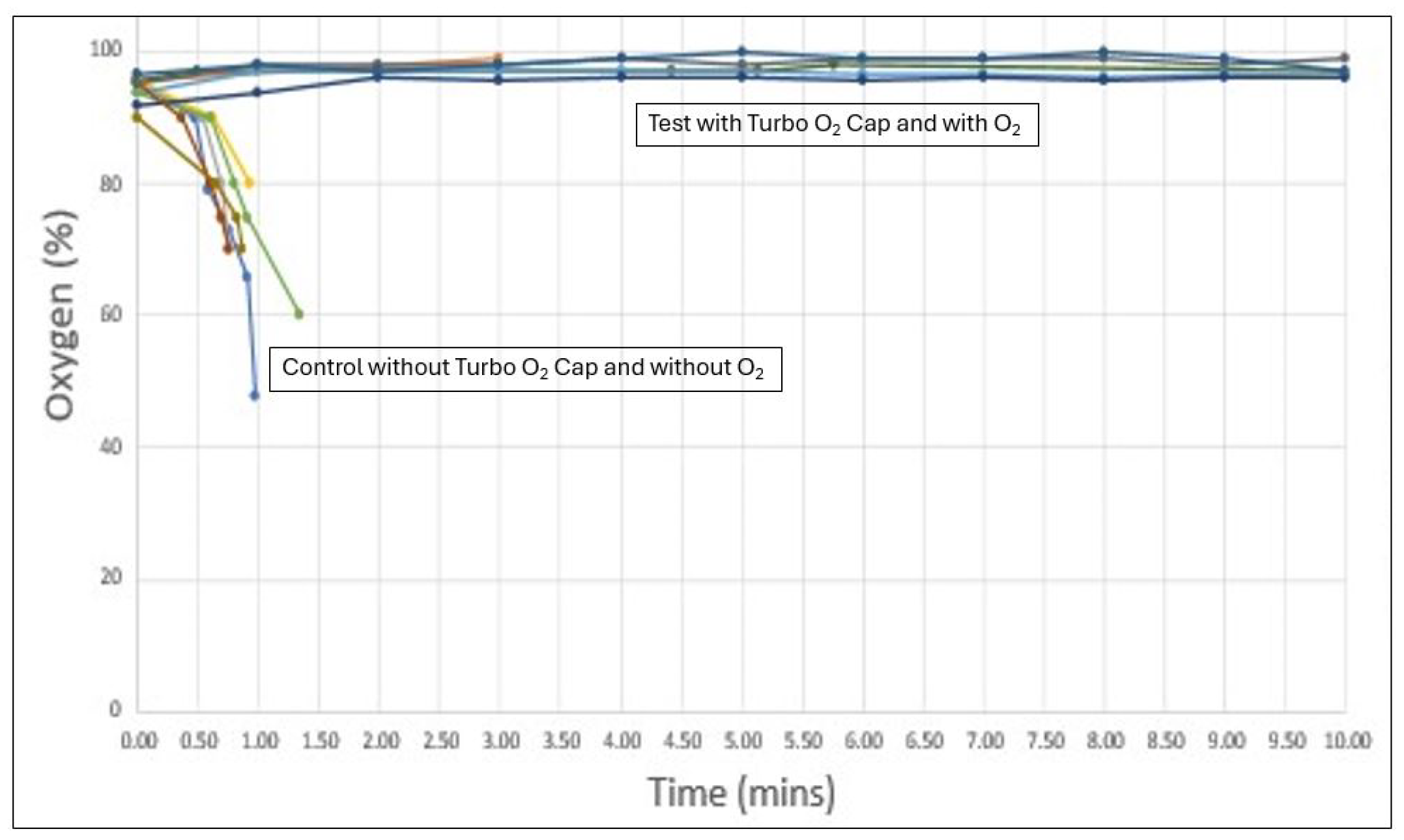
The results of the six test cycles (Turbo® O₂ Cap with oxygen) and six control cycles (no Turbo® O₂ Cap and no oxygen) are shown below in
Figure 1 and
Figure 2.
Figure 1 displays all 12 cycles individually. Key findings include:
In all six control cycles, oxygen saturation dropped from the mid-to-high 90s at the start of the procedure to below 75% within less than one minute, representing dangerous hypoxia.
In all six test cycles, oxygen saturation increased from the mid-to-high 90s at the start and remained in the high 90s for the full 10-minute observation period.
The test cycles showed highly consistent oxygen saturation curves with minimal variability across all six runs. A similar pattern of consistency was observed in the control cycles, where all animals demonstrated a rapid and uniform desaturation trend.
Figure 2 presents the same data in an aggregated format, summarizing the average results for the six test and six control cycles. The combined trendlines clearly show a steep decline in oxygen saturation to below 75% within one minute for the control group, while the test group showed a sustained increase to high 90s that was maintained throughout the entire 10-minute period [
6].
4. Discussion
This preclinical study demonstrates that the Turbo® O₂ Cap consistently prevented desaturation in a porcine model simulating difficult intubation under RSI conditions. In all control cycles, oxygen saturation dropped below 75% within one minute, a level considered to represent dangerous hypoxia. In contrast, all test cycles using the Turbo® O₂ Cap maintained oxygen saturation in the high 90s throughout the entire 10-minute observation period. This level of consistency across all animals and all cycles strongly suggests that the device may be effective in extending the safe apnea window during intubation.
The mechanism behind this effect is likely passive diffusion of high-concentration oxygen delivered directly to the glottic area during apnea. A simple theoretical explanation is that 100% oxygen is delivered in front of the vocal cords, while the lungs—just a few centimeters behind—contain lower concentrations of oxygen due to apnea. Because oxygen is a relatively light gas, this concentration difference enables passive diffusion. The Turbo® O₂ Cap positions the oxygen flow directly near the entrance of the glottis, reducing the distance oxygen molecules must travel to reach the lungs. In contrast, apneic oxygenation with a nasal cannula places the oxygen source at the external nares—several centimeters from the glottic inlet—where much of the oxygen is lost to ambient air, and only a fraction is available to diffuse across the upper airway. Conceptually and mechanically, the Turbo® O₂ Cap represents a fundamentally different approach by delivering oxygen at the anatomical root of the airway, just proximal to the vocal cords.
No currently available method has demonstrated this level of effectiveness in preventing hypoxia during difficult intubation, particularly under conditions of paralysis and apnea. Nasal cannula-based apneic oxygenation remains the only widely used adjunct, but its performance is inconsistent and often inadequate. The Turbo® O₂ Cap may be the first device capable of reliably mitigating hypoxia in this setting. While no formal statistical analysis was conducted due to the small sample size, the uniformity of the observed results—including the graphical trends across all 12 cycles—was striking. The clear separation between control and test groups, and the complete avoidance of desaturation in the test cycles, provides strong early support for this novel approach.
Limitations of this study include its small sample size (three animals), the use of a preclinical model, and the absence of arterial blood gas (ABG) data. Additionally, while the model mimics the apneic state typical of RSI, it does not account for variables such as airway obstruction, trauma, or anatomical variability that may be present in human patients. Nonetheless, these results offer compelling early evidence that the Turbo® O₂ Cap may address one of the most persistent and dangerous problems in airway management: severe hypoxia during intubation, which can cause patient harm, irreversible injury, or death. If confirmed in larger studies, this device could represent a paradigm shift in how we protect patients during high-risk airway procedures.
5. Conclusions
In this preclinical study, the Turbo® O₂ Cap completely prevented oxygen desaturation during simulated difficult intubation in paralyzed, non-breathing animals. All control cycles without oxygen experienced rapid hypoxia, similar to the desaturation patterns that may be observed in human patients undergoing rapid sequence intubation. In contrast, all test cycles using the Turbo® O₂ Cap maintained oxygen saturation in the high 90s for the full 10-minute observation period. The results support the hypothesis that delivering high-concentration oxygen directly to the glottic area can extend the safe apnea window during intubation.
The Turbo® O₂ Cap may be the first device shown to reliably mitigate hypoxia under rapid sequence intubation conditions. While larger and more comprehensive studies will be required to validate these findings in human subjects, this animal study establishes a strong technical foundation and offers early evidence of clinical potential.
6. Patents and Commercial Disclosure
The Turbo® O₂ Cap is protected by four issued U.S. patents and three additional pending applications, covering various design features including high-flow oxygen delivery, pressure relief mechanisms, and endotracheal tube compatibility [
7]. The device has received FDA Class I designation and is now commercially available in the United States.
This study was funded by SharpMed, LLC (Scottsdale, AZ, USA), the manufacturer of the Turbo® O₂ Cap. Author Chris Salvino, MD, is the CEO of SharpMed and the inventor of the Turbo® O₂ Cap. The other authors have no financial or proprietary relationship with SharpMed.
Funding
This research was funded by SharpMed, LLC (Scottsdale, AZ, USA), the manufacturer of the Turbo® O₂ Cap.
Conflict of Interest
Chris Salvino, MD, is the CEO of SharpMed, LLC and the inventor of the Turbo® O₂ Cap. Robert Goodwin, DO, and Brion Benninger, MD, declare no conflicts of interest.
References
- Driver, B.E. , et al. Effect of Use of a Bougie vs Endotracheal Tube With Stylet on Successful Intubation on the First Attempt Among Critically Ill Patients Undergoing Tracheal Intubation. JAMA 2021, 326(24), 2488–2497. [Google Scholar] [PubMed]
- Weingart, S.D. , Levitan, R.M. Preoxygenation and Prevention of Desaturation During Emergency Airway Management. Ann. Emerg. Med. 2011, 59(3), 165–175. [Google Scholar] [CrossRef]
- Bodily, J.B. , et al. Incidence and Duration of Continuously Measured Oxygen Desaturation During Emergency Department Intubation. Ann. Emerg. Med. 2016, 67(3), 389–395. [Google Scholar] [CrossRef]
- Simpson, G.D. , et al. Tracheal Intubation in the Critically Ill: A Multi-Centre National Study of Practice and Complications. Br. J. Anaesth. 2012, 108, 792–799. [Google Scholar] [CrossRef]
- McKown, A.C. , et al. Risk Factors for and Prediction of Hypoxemia During Tracheal Intubation of Critically Ill Adults. Ann. Am. Thorac. Soc. 2018, 15(11), 1325–1332. [Google Scholar]
- SharpMed, LLC. Turbo® O₂ Cap Device Overview and Technical Specification One-Pager. Internal publication, 2022.
- Salvino, C. , et al. Development of an Endotracheal Tube Cap for Oxygen Delivery During Intubation. J. Med. Devices 2023, 17(2), 024502. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).