Submitted:
03 June 2025
Posted:
03 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Animals
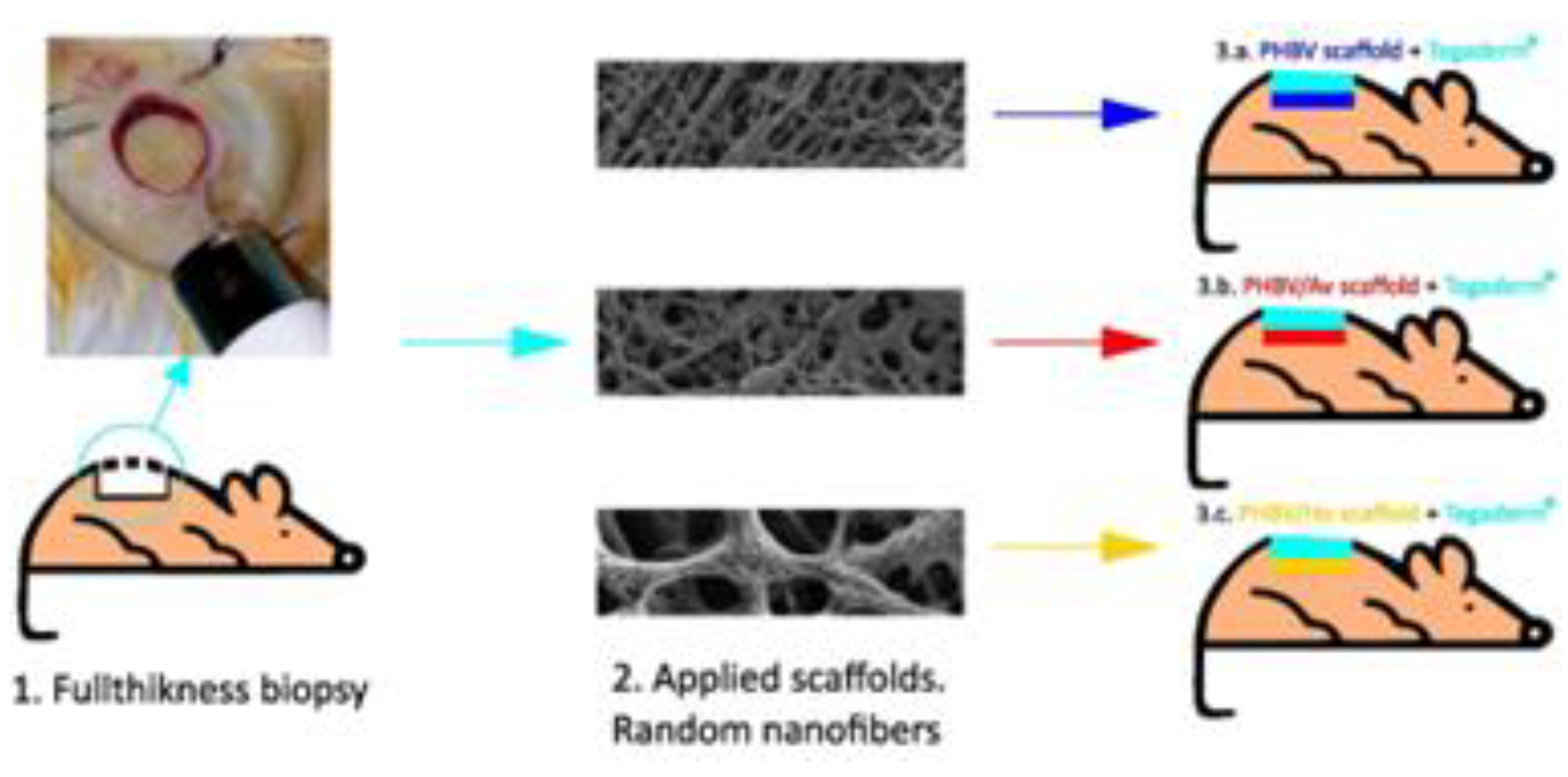
Surgical Procedure
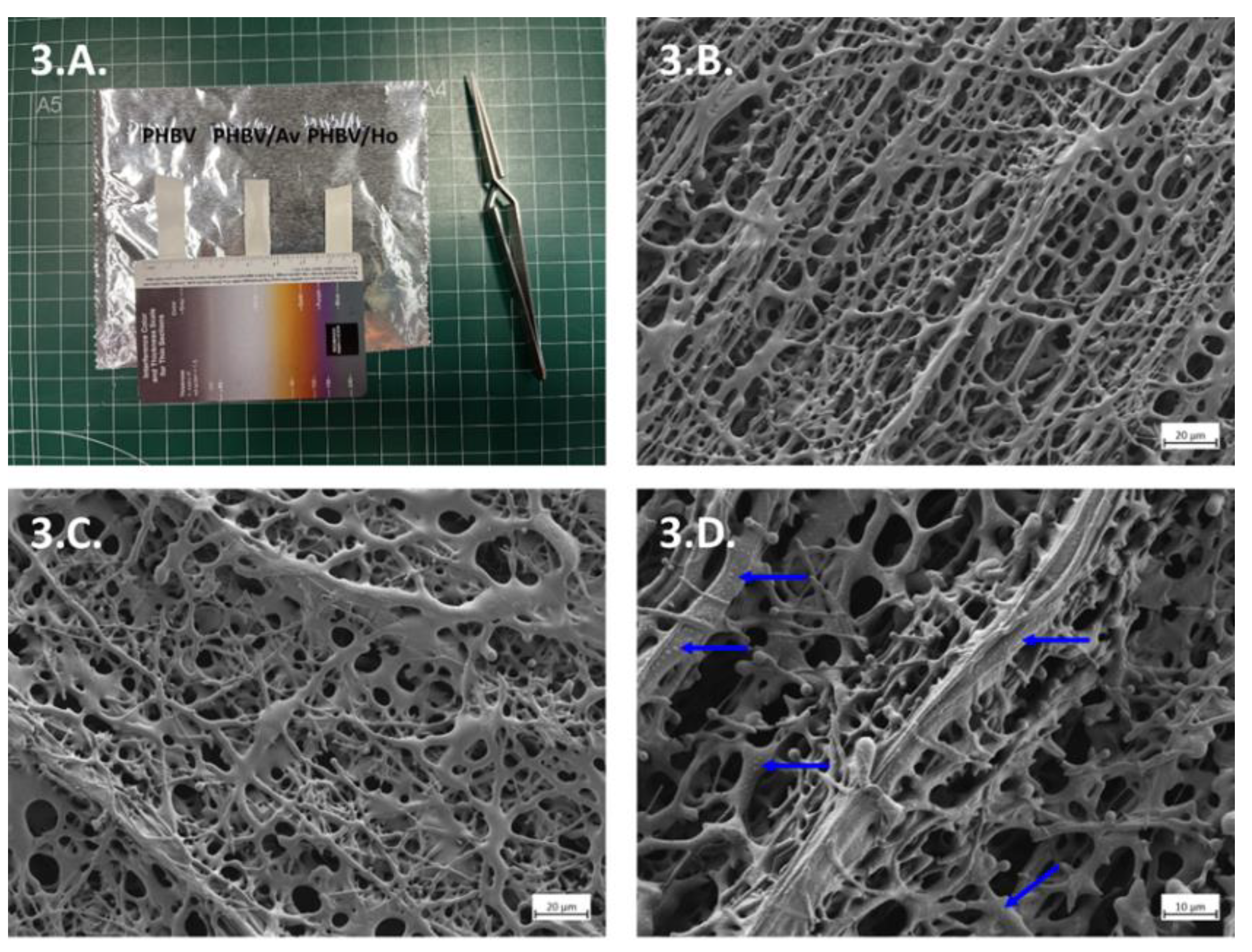
Scaffold Fabrication and Morphological Characterization
Control and Experimental Treatments
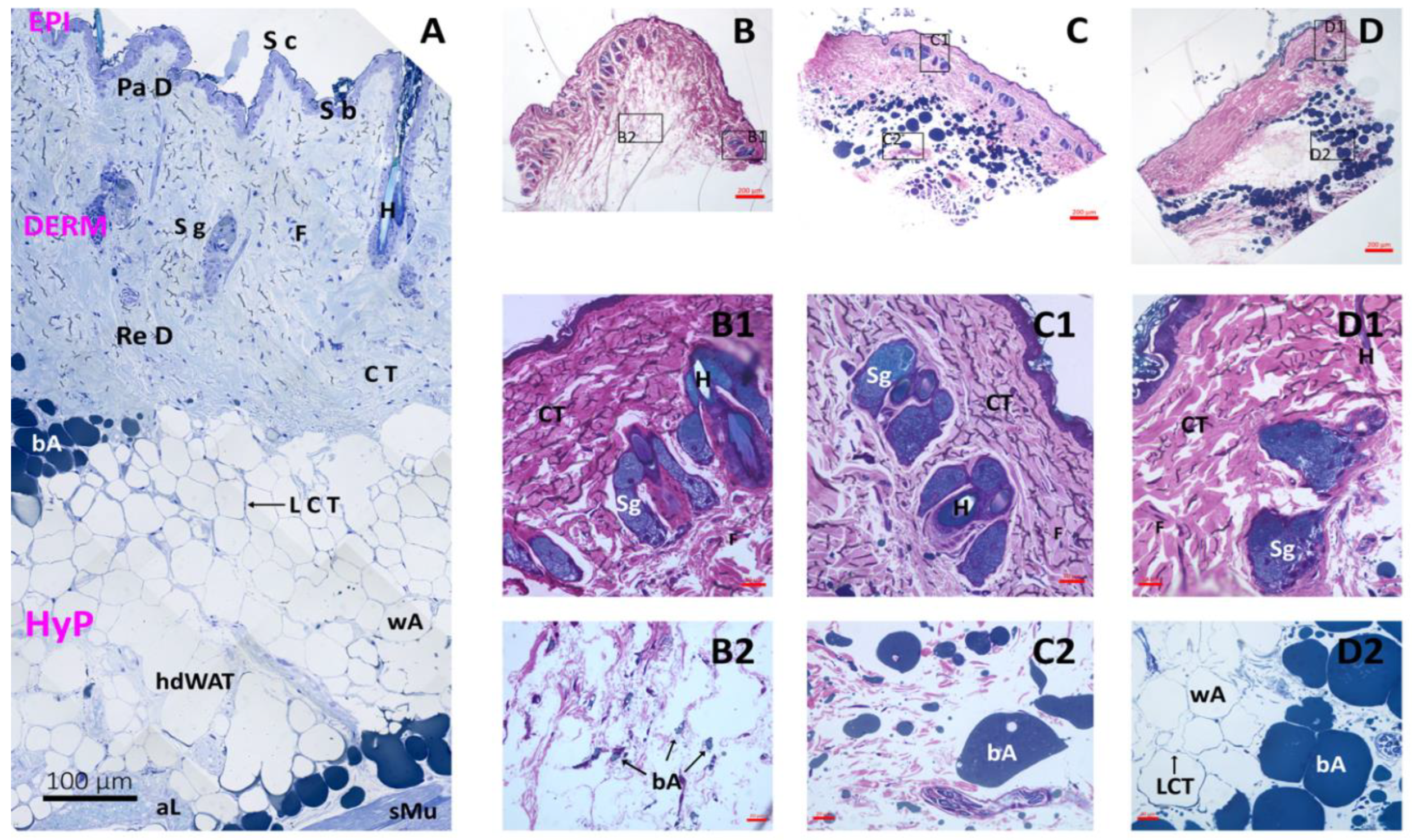
Histological Analysis
Statistical Analysis
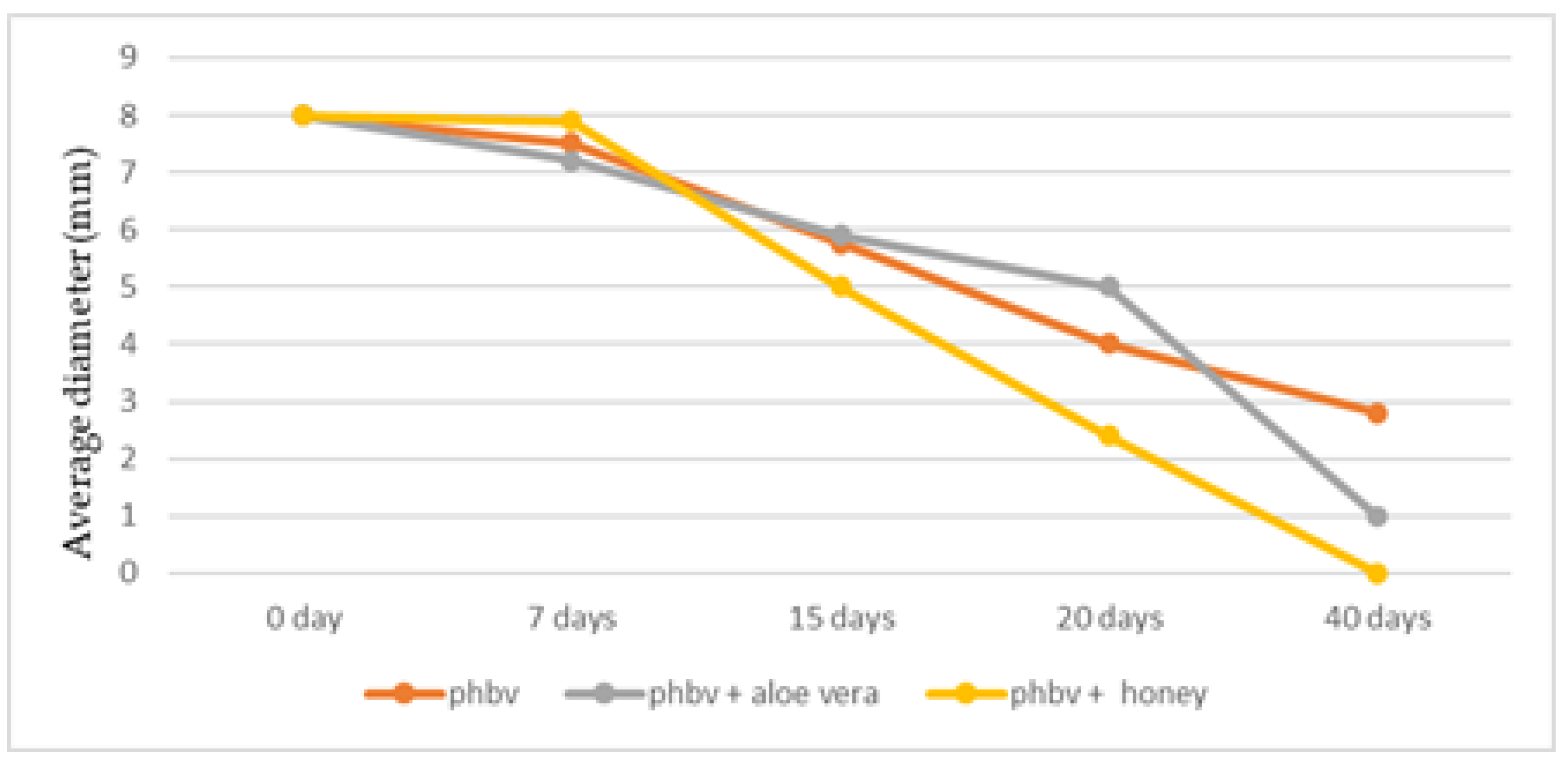
3. Results
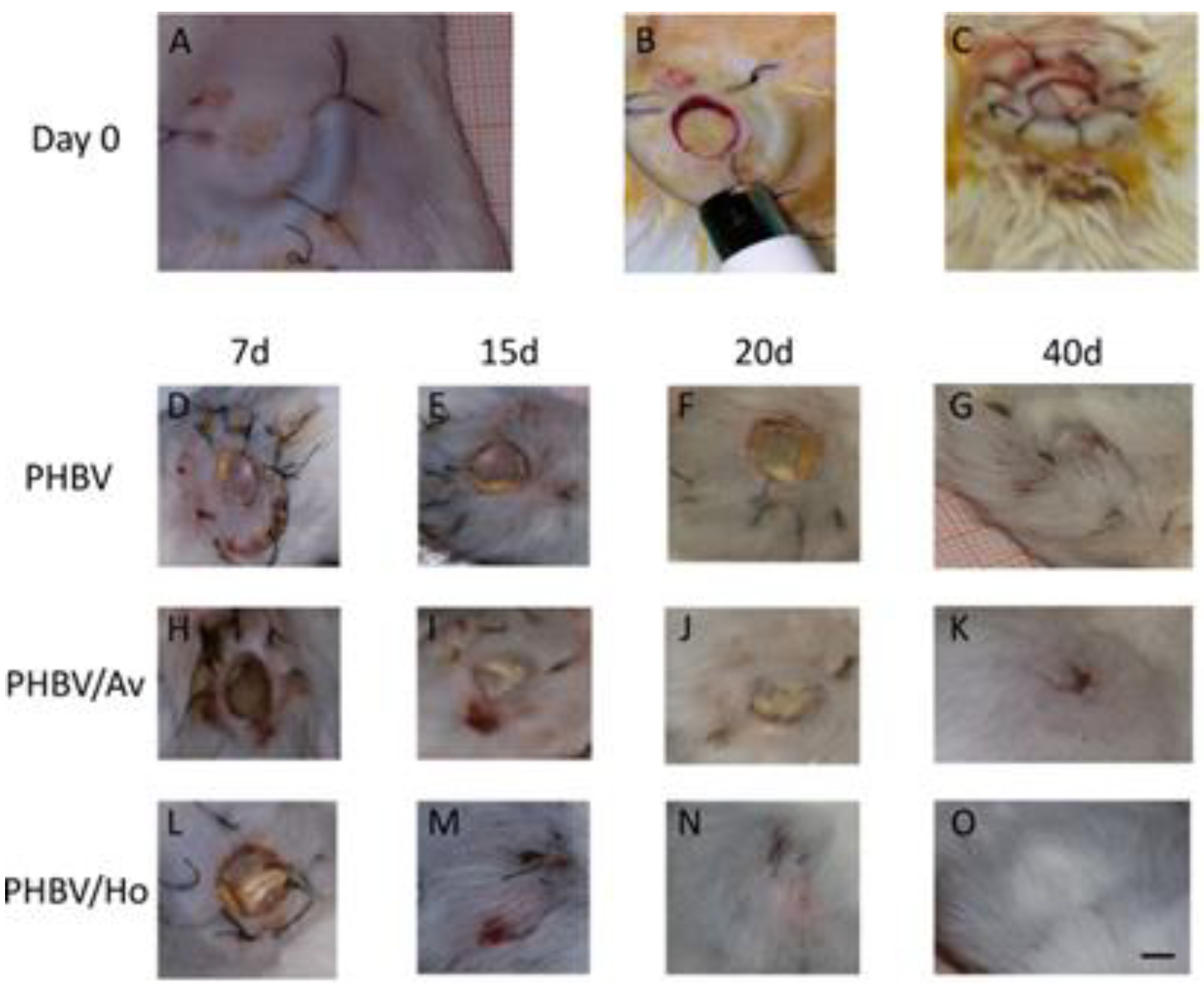
3.1. Animals
3.2. Scaffold Fabrication and Morphological Characterization
3.3. Histology
3.4. Statistical Analysis
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PHVB | Poly (3-hydroxybutyrate-co-3-hydroxy valerate) |
| PHBV/AV | Poly (3-hydroxybutyrate-co-3-hydroxy valerate) /Aloe Vera |
| PHBV/Ho | Poly (3-hydroxybutyrate-co-3-hydroxy valerate)/Honey |
| PBS | Phosphate Buffer Saline |
| ECM | Extracellular matrix |
| PVA | Polyvinyl alcohol |
| PCL | Polycaprolactone |
| ULPGC | Universidad de Las Palmas de Gran Canaria. |
| FESEM | Field Emission Scanning Electron Microscope |
| UV | Ultraviolet |
| AIC | Akaike Information Criterion |
| SE | Standard Errors |
| SIMACE | Advanced Confocal and Electron Microscopy Research Facility |
References
- Hernández-Fuentes, A. , Cháves-Borges D., Cenobio-Galindo A., Zepeda-Velázquez A., Figuera A., Jiménez-Alvarado R., Campos-Montiel R. Characterization of total phenol and flavonoids contents, color, functional properties form honey samples with different floral origins. Inter. J Food Stud. 2021, 10, 346–358. https://www.iseki-food-ejournal.com/ojs/index.php/e-journal/article/view/893/321. [CrossRef]
- Jull, A.B. , Cullum N., Dumville J.C., Westby M.J., Deshpande S., Walker N. Honey as a topical wound treatment. Cochrane Database Syst Rev. 2015, 2015, CD005083. [Google Scholar] [CrossRef] [PubMed]
- Merckoll, P. , Jonassen T., Vad M., Jeansson S., Melby K. Bacteria, biofilm, and honey: A study of the effects of honey on ‘planktonic’ and biofilm-embedded chronic wound bacteria. Scand. J Infect. Dis. 2009, 41, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M. , Antunes M., Faleiro M. Honey as a complementary medicine. Integr. Med. Insights. 2017, 12, 1–15. [Google Scholar] [CrossRef]
- Pereira, F. , Bártolo, P. Traditional therapies for skin wound healing. Adv. Wound Care. 2016, 5, 208–229. [Google Scholar] [CrossRef] [PubMed]
- Ruttermann, M. , Maier-Hasselmann A., Nink-Grebe B., Burckhardt M. Local treatment of chronic wounds. Dtsch. Arztebl Int. 2013, 110, 25–31. [Google Scholar] [CrossRef]
- Lee, S.K. Cell growth-stimulating effect. In: Park, Y.I., Lee, S.K. (eds) New Perspectives on Aloe. 2006. Springer, Boston, MA. [CrossRef]
- Kumar, R. , Singh K., Gupta, A., Bishayee A., Pandey, A. Therapeutic potential of aloe vera-a miracle gift of nature. Phytomedicine. 2019, 60. [Google Scholar] [CrossRef]
- Lusby, P. , Coombes A., Wilkinson, J. Honey: A potent agent for wound healing? J Wound Ostomy Continence Nurs. Soc. 2002, 29, 295–300. [Google Scholar] [CrossRef]
- Mikołajczak, N. Potential health benefits of Aloe vera. J Educ. Health Sport. 2018, 8, 1420–1435. [Google Scholar] [CrossRef]
- Molan, P.C. The evidence supporting the use of honey as a wound dressing. Int. J Low. Extrem. Wounds. 2006, 5, 40–54. [Google Scholar] [CrossRef]
- Song, J.J. , Salcido R. Use of honey in wound care: an update. Adv. Skin Wound Care. 2010, 24, 40–44, quiz 45–46. [Google Scholar] [CrossRef] [PubMed]
- Ullah, K. , Naz S., Abudbos A. Towards a better understanding of the therapeutic applications and corresponding mechanisms of action of honey. Environ. Sci. Pollut. Res. 2017, 24, 27755–27766. [Google Scholar] [CrossRef]
- Yang, X. , Fan L., Ma L., Wang Y., Lin S., Yu F., Pan X., Luo G., Zhang D., Wang H. Green electrospun Manuka honey/silk fibroin fibrous matrices as a potential wound dressing. Mater. Des. 2017, 119, 76–84. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, J. , Martín-Barrasa J., Aragón-Sánchez J., Monzón-Mayor M., Pérez-Galván J., Saavedra-Santana P., Romero-Alemán M. The effect of honey, aloe vera, and hydrocolloid dressing on the healing process of murine excisional wounds. Int. J Low. Extrem. Wounds. 2023, 22, 1–9. [Google Scholar] [CrossRef]
- Almasaudi, S. The antibacterial activities of honey. Saudi J Biol. Sci. 2021, 28, 2188–2196. [Google Scholar] [CrossRef]
- Bucekova, M. , Buriova M., Pekarik L., Matjan V., Matjan J. Phytochemicals mediated hydrogen peroxide production is crucial for high antibacterial activity of honeydew honey. Sci. Rep. 2018, 8, 9061. [Google Scholar] [CrossRef]
- González-Gascón, R. , Del Dedo-Torre P. Actualización sobre el uso de miel en el tratamiento de úlceras y heridas. Caso clínico. Enferm. Glob. 2004, 4, 1–10. [Google Scholar] [CrossRef]
- Manjunatha, D. , Chua L. The anti-inflammatory and wound-healing properties of honey. Eur. Food Res. Technol. 2014, 239, 1003–1014. [Google Scholar] [CrossRef]
- Weston, R. The contribution of catalase and other natural products to the antibacterial activity of honey: A review. Food Chem. 2000, 71, 235–239. [Google Scholar] [CrossRef]
- Bucekova M, Jardekova L, Juricova V, et al. Antibacterial activity of different blossom honeys: new findings. Molecules. 2019, 24, 1573–1592. [CrossRef]
- Cianciosi, D. , Forbes-Hernández T.Y., Afrin S., et al. Phenolic compounds in honey and their associated health benefits: a review. Molecules. 2018, 23, 2322–2241. [Google Scholar] [CrossRef] [PubMed]
- Combarros-Fuertes, P. , Fresno J., Estevinho M., Sousa-Pimenta M., Tornadijo M., Estevinho, L. Honey: Another alternative in the fight against antibiotic resistant bacteria? Antibiotics. 2020, 9, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, P. , Gauche C., Gonzaga L., Costa A., Felt R. Honey: Chemical composition, stability, and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K. A current perspective on hydrogen peroxide production in honey. A review. Food Chem. 2020, 332, 127229. [Google Scholar] [CrossRef]
- Kwakman, P.H. , Te Velde A.A., de Boer L., Vandenbroucke-Grauls C.M., Zaat S.A. Two major medicinal honeys have different mechanisms of bactericidal activity. PLoS One. 2011, 6, e17709. [Google Scholar] [CrossRef]
- Maddocks, S. , Jenkins, R. (2013). Honey: a sweet solution to the growing problem of antimicrobial resistance? Future Microbiol. 2013, 8, 1419–1429. [Google Scholar] [CrossRef]
- Masoura, M. , Gkatzionis K. The antimicrobial mechanism of Greek thyme honeys against methicillin-resistant Staphylococcus aureus clinical isolates: a case study of comparison with manuka honey. Int. J of Food Sci. Tech. 2022, 57, 7076–7084. [Google Scholar] [CrossRef]
- Yaghoobi, R. , Kazerouni A.,Kazerouni O. Evidence for clinical use of honey in wound healing as an anti-bacterial, anti-inflammatory, anti-oxidant and anti-viral agent: a review. Jundishapur J. Nat. Pharm. Prod. 2013, 8, 100–104. [Google Scholar] [CrossRef]
- Cosmetic Ingredient Review Expert Panel. Final report on the safety assessment of AloeAndongensis Extract, Aloe Andongensis Leaf Juice, Aloe arborescens Leaf Extract, Aloe Arborescens Leaf Juice, Aloe Arborescens Leaf Protoplasts, Aloe Barbadensis Flower Extract, Aloe Barbadensis Leaf, Aloe Barbadensis Leaf Extract, Aloe Barbadensis Leaf Juice, aloe Barbadensis Leaf Polysaccharides, Aloe Barbadensis Leaf Water, Aloe Ferox Leaf Extract, Aloe Ferox Leaf Juice, and Aloe Ferox Leaf Juice Extract. Int. J. Toxicol. 2007, 26 (Suppl 2), 1–50. [CrossRef]
- Chung M., Choi S. Wound healing effect. In: Park, Y.I., Lee, S.K. (eds). New Perspectives on Aloe. 2006. Springer, Boston, MA. [CrossRef]
- Hernández-Martínez F.J., Jiménez-Díaz J.F., Rodríguez de Vera B., Quintana-Montesdeoca M.P., Chacón-Ferrera R., Estévez-García M.L. (2010). Therapeutic use of Aloe Vera in pressure ulcers (PU).[El uso terapéutico del Aloe Vera en las úlceras por presión (UPP)]. Revista CENIC Ciencias Biológicas. 2020, 41, 1–4. https://www.redalyc.org/articulo.oa?id=181220509066.
- Kim, K.W. Angiogenic effect. In: Park, Y.I., Lee, S.K. (eds). New Perspectives on Aloe. 2006. Springer, Boston, MA. [CrossRef]
- Farzadinia, P. , Jofreh N., Khatamsaz S., Movahed A., Akbarzadeh S., Mohammadi M., Bargahi A. Anti-inflammatory and wound healing activities of aloe vera, honey, and milk ointment on second-degree burns in rats. Int. J. Lower Extrem. Wounds. 2016, 15, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Habeeb, F. , Satables G., Bradbury F., Nong S., Cameron P., Plevin R., Ferro V. (2007). The inner gel component of aloe vera suppresses bacterial-induced pro-inflammatory cytokines from human immune cells. Methods. 2007, 42, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Takzaree, N. , Hadjiakhndi A., Hassanzadeh G., Reza M., Manyi A., Majidi A. Transforming growth factor-β (TFG-β) activation in cutaneous wounds after topical application of aloe vera gel. Can. J Physiol. Pharmacol. 2016, 94, 1285–1290. [Google Scholar] [CrossRef]
- Choi, S. , Son B., Son Y., Park Y., Lee S., Chung M. (2001). The wound-healing effect of a glycoprotein fraction isolated from aloe vera. Br J Dermato. 2001, 145, 535–545. [Google Scholar] [CrossRef]
- Reynolds, T. , Dweck, A. Aloe vera leaf gel: a review update. J Ethnopharmacol. 1999, 68, 3–37. [Google Scholar] [CrossRef]
- Lee, I. , Kwon H., Meng W., Kang K. Nanofabrication of microbial polyester by electrospinning promotes cell attachment. Macromol. Res. 2004, 12, 374–378. [Google Scholar] [CrossRef]
- Chen Q., Wu. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials. 2005, 26, 6565–6578. [Google Scholar] [CrossRef]
- Romero-Alemán MM., Hernández-Rodríguez JE.,Pérez-Galván JM.,Monzón-Mayor,M. Hybrid electrospun PHBV/Aloe vera and PHBV/Honey nanofibers are scaffolds for rat dorsal root ganglion neurite outgrowth and guidance as well as for the regeneration of mouse skin after wounding. Glia. 2019, 67 (sup.S1), E654–E655,AbstractT16-0048:124-125.
- Romero-Alemán MD, Pérez-Galván JM, Hernández-Rodríguez JE, Monzón-Mayor M. The Potential of Aloe Vera in Solution and in Blended Nanofibers Containing Poly (3-Hydroxybutyrate-Co-3-Hydroxyvalerate) as Substrates for Neurite Outgrowth. J Biomed Mater Res A. 2025, 113, e37825. [CrossRef]
- Masaeli, E. , Morshed M., Nasr-Esfahani M.H., et al. Fabrication, characterization, and cellular compatibility of poly(hydroxy alkanoate) composite nanofibrous scaffolds for nerve tissue engineering. PLoS ONE. 2013, 8, e57157. [Google Scholar] [CrossRef]
- Satalkar, P. , Elger B., Shaw D. Defining nano, nanotechnology, and nanomedicine: Why should it matter? Sci. Eng. Ethics. 2016, 22, 1255–1276. [Google Scholar] [CrossRef] [PubMed]
- Chen, W. , Tong Y. PHBV microspheres as neural tissue engineering scaffolds support neuronal cell growth and axon–dendrite polarization. Acta Biomaterialia. 2012, 8, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, M. , Vatankhah E., Ramakrishna S. Electrospun aligned PHBV/collagen nanofibers as substrates for nerve tissue engineering. Biotechnol. Bioeng. 2013, 110, 2775–2784. [Google Scholar] [CrossRef] [PubMed]
- Dumontel B, Conejo-Rodríguez V, Vallet-Regí M, Manzano M. Natural Biopolymers as Smart Coating Materials of Mesoporous Silica Nanoparticles for Drug Delivery. Pharmaceutics. 2023, 15, 447. Published 2023. [CrossRef]
- Jaldin-Crespo, L. , Silva N., Martínez J. Nanomaterials based on honey and propolis for wound healing—a mini-review. Nanomaterials. 2022, 12, 4409. [Google Scholar] [CrossRef]
- Yupanqui, M. , Vyas C., Aslan E., Humphreys G., Diver C., Bartolo P. Honey: an advanced antimicrobial and wound healing biomaterial for tissue engineering applications. Pharmaceutics. 2022, 14, 1663. [Google Scholar] [CrossRef]
- Rahman, S. , Carter P., Bhattarai N. Aloe vera for tissue engineering applications. J Funct. Biomater. 2017, 8, 6–22. [Google Scholar] [CrossRef]
- Thompson, Z. , Rahman S,. Yarmolenko S., Sankar J., Kumar D., Bhattarai N. Fabrication and characterization of magnesium ferrite-based PCL/Aloe vera nanofibers. Materials (Basel). 2017, 10, 937–948. [Google Scholar] [CrossRef]
- Shishatskaya, E. , Volova T., Puzyr A., Mogilnaya O., Efremov S. Tissue response to the implantation of biodegradable polyhydroxyalkanoate sutures. J Mat. Sci. Mater. Med. 2004, 15, 719–728. [Google Scholar] [CrossRef]
- Rubiano-Navarrete, A.F. , Rosas C. R. A., Torres P.Y., Gómez-Pachón E. Y. From fibers electrospun with honey to the healing of wounds: a review. Ingeniería y competitividad. 2024, 26, e–30112811. [Google Scholar] [CrossRef]
- Davidson, J. , Yu F., Opalenik S. Splinting strategies to overcome confounding wound contraction in experimental animal models. Adv Wound Care. 2013, 2, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Ren, L. , Zhou B., Chen L. Silicone ring implantation in an excisional murine wound model. Wound. 2012, 24, 36–42. [Google Scholar] [PubMed]
- Ansell, D. , Campbell L., Thomason H., Brass A., Hardman M. A statistical analysis of murine incisional and excisional acute wound models. Wound Rep. Reg. 2014, 22, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Directive 2010/63/EU of the European Parliament and the Council. Official Journal of the European Union ( 2010.
- Mukai, K. , Koike M., Nakamura S., et al. Evaluation of the effects of a combination of Japanese honey and hydrocolloid dressing on cutaneous wound healing in male mice. Evid Based Complement Alternat Med. 2015, 2015, 910605. [Google Scholar] [CrossRef]
- Martins, S. , Torres O., Santos O., Limeira-Júnior A., Sauaia-Filho, E., Melo S., Santos, R., Silva V. Analysis of the healing process of the wounds occurring in rats using laser therapy associated with hydrocolloid. Acta Ci.r Bras. 2015, 30, 681–685. [Google Scholar] [CrossRef]
- Takeuchi, T. , Ito M., Yamaguchi S., Watanabe S., Honda M., Imahashi T., Yamada T., Kokubo T. Hydrocolloid dressing improves wound healing by increasing M2 macrophage polarization in diabetic mice. Nagoya J Med. Sci. 2020, 82, 487–498. [Google Scholar] [CrossRef]
- Rasband W.S. ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA. https://imagej.nih.gov/ij/, 1997-2018.
- Molina A., Moyano M., Peña F., Lora A., Moreno S., Serrano, J. Central Nervous System depressants and anaesthesia in experimental rodents [Depresores del Sistema Nervioso Central y anestesia en roedores de experimentación]. RECVET. 2008, 3. https://www.researchgate.net/publication/353849333_Depresores_del_Sistema_Nervioso_Central_y_anestesia_en_roedores_de_experimentacion_Central_Nervous_System_depressant_and_anaesthesia_of_Laboratory_rodents.
- Scepankova H., Combarros-Fuertes P., Fresno J.M., et al. Role of honey in advanced wound care. Molecules. 2021, 26, 4784. [CrossRef]
- Giusto G, Vercelli C, Comino F, Caramello V, Tursi M, Gandini M. A new, easy-to-make pectin-honey hydrogel enhances wound healing in rats. BMC Complement Altern. Med. 2017, 17, 266. [CrossRef]
- Monzón, M., Romero, M., Hernández,JE., Pérez,JM. Hybrid Aloe vera nanofibers. European Patent Office. EP 3461788, 12.10, 2022. Munich.
- Monzón, M., Romero, M., Hernández,JE., Pérez,JM. Hybrid honey nanofibers. European Patent Office. EP 3428117, 27.07, 2022. Munich.
- Yixiang, D. , Yong, T., Liao, S., Chan, C. K., Ramakrishna, S2008. Degradation of electrospun nanofiber scaffold by short wave length ultraviolet radiation treatment and its potential applications in tissue engineering. Tissue Eng. Part A. 2008, 14, 1321–1329. [Google Scholar] [CrossRef]
- Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA. https://imagej.nih.gov/ij/, 1997-2018.
- Laird, N.M. , Ware J.H. Random-effects models for longitudinal data. Biometrics. 1982, 38, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh J., Neath A. The Akaike information criterion: Background, derivation, properties, application, interpretation, and refinements. WIREs Comput Stat. 2019, 11, e1460. [CrossRef]
- R Development Core Team. R: a language and environment for statistical computing (Version 3.6.1). R Foundation for Statistical Computing, Vienna, Austria. 2019. https://www.R-project.org/.
- Sosiati, H., Nur Fatihah, W., Yusmaniar, Nur Rahman, M.B., 2018. Characterization of the Properties of Electrospun Blended Hybrid Poly(Vinyl Alcohol)_Aloe Vera/Chitosan Nano-Emulsion Nanofibrous Membranes. KEM. [CrossRef]
- Van Zutphen L., Baumans V, Beynen A. Principles of Laboratory Animal Science, second edition, Elsevier Science, Amsterdam, 2001: https://www.humane-endpoints.info/es/rata/parametros-fisiologicos.
- Rossiter, K. , Cooper A.J., Voegeli D., Lwaleed B.A. Honey promotes angiogenic activity in the rat aortic ring assay. J Wound Care. 2010, 19, 440–446. [Google Scholar] [CrossRef]
- Sarkar S., Chaudhary A., Kumar S.T., Kumar D.A., Chatterjee J. Modulation of collagen population under honey-assisted wound healing in a diabetic rat model. Wound Medicine. 2018, 20, 7–17. [CrossRef]
- Andreu V., Mendoza G., Arruebo M., Irusta S. Smart dressings based on nanostructured fibers containing natural origin antimicrobial, anti-Inflammatory, and regenerative compounds. Materials (Basel). 2015, 8, 5154–5193. Published 2015 Aug 11. [CrossRef]
- Maleki, H. , Gharehaghaji A, Dijkstra P. A novel honey-based nanofibrous scaffold for wound dressing application. J Appl. Polym. Sci. 2013, 127, 4086–4092. [Google Scholar] [CrossRef]
- Abrigo, M. , MacArthur S., Kingshott. Electrospun nanofibers as dressings for chronic wound care: advances, challenges, and prospects. Macromol Biosci. 2014, 14, 772–792. [Google Scholar] [CrossRef]
- Pilehvar-Soltanahmadi, Y. , Dadashpour M., Mohajeri A., Fattahi A., Sheervalilou R., Zarghami N. An overview on application of natural substances incorporated with electrospun nanofibrous scaffolds to development of innovative wound dressings. Mini Rev. Med. Chem. 2018, 18, 414–427. [Google Scholar] [CrossRef]
- Suwantong, O. , Waleetorncheepsawat S., Sanchavanakit N., et al. In vitro biocompatibility of electrospun poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) fiber mats. Int. J Biol. Macromol. 2007, 40, 217–223. [Google Scholar] [CrossRef]
- Sendil, D. , Gürsel I., Wise D.L., Hasirci V. Antibiotic release from biodegradable PHBV microparticles. J Control Release. 1999, 59, 207–217. [Google Scholar] [CrossRef]
- Rahimnejad, M. , Derakhshanfar S., Zhong W. Biomaterials and tissue engineering for scar management in wound care. Burns Trauma. 2017, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Gavillero-Martin A., Juliá-Roca M.,Serra-Guillén I., Rodríguez-Hernández A.,Manrique-Silva E.,López-Sundh A.E.,Nagore E. Secondary Intention healing time of postoperative surgical cancer skin wounds with a biosynthetic porcine type I collagen dressing: A 306-patient retrospective, observational study. Actas Dermo-Sifiliográficas.[In press]; 2024. [CrossRef]
- Molina G., E. , Sherry Y.H., Neel V. A. Observations Regarding Infection Risk in Lower-Extremity Wound Healing by Second Intention. Dermatologic Surgery. 2020, 46, 1342–1344. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound Healing: A Cellular Perspective. Physiol Rev. 2019, 99, 665–706. [CrossRef]
| Treatment | Day 0(first) | Day 40(last) | Percent reduction* |
| PHBV | |||
| 8(8,8) | 2,5(2.5,3.2) | 68.8(60,68.8)a | |
| PHBV + Honey | |||
| 8(8,8) | 0(0,0) | 100(100,100)b | |
| PHBV + Aloe Vera | |||
| 8(8,8) | 0(0,1) | 100(87.2,100)a,b | |
| Coefficient (SE) | p-value | |
| Intercept Treatment |
||
| 2.334(0.085) | <0.001 | |
| PHBV(reference) | ||
| 0 | - | |
| PHBV + Honey | ||
| -0.382(0.107) | <0.001 | |
| PHBV +Aloe Vera | ||
| -0.136(0.109) | 0,211 | |
| Time, per day | -0.054(0.004) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





