Submitted:
02 June 2025
Posted:
03 June 2025
You are already at the latest version
Abstract
Keywords:
Introduction
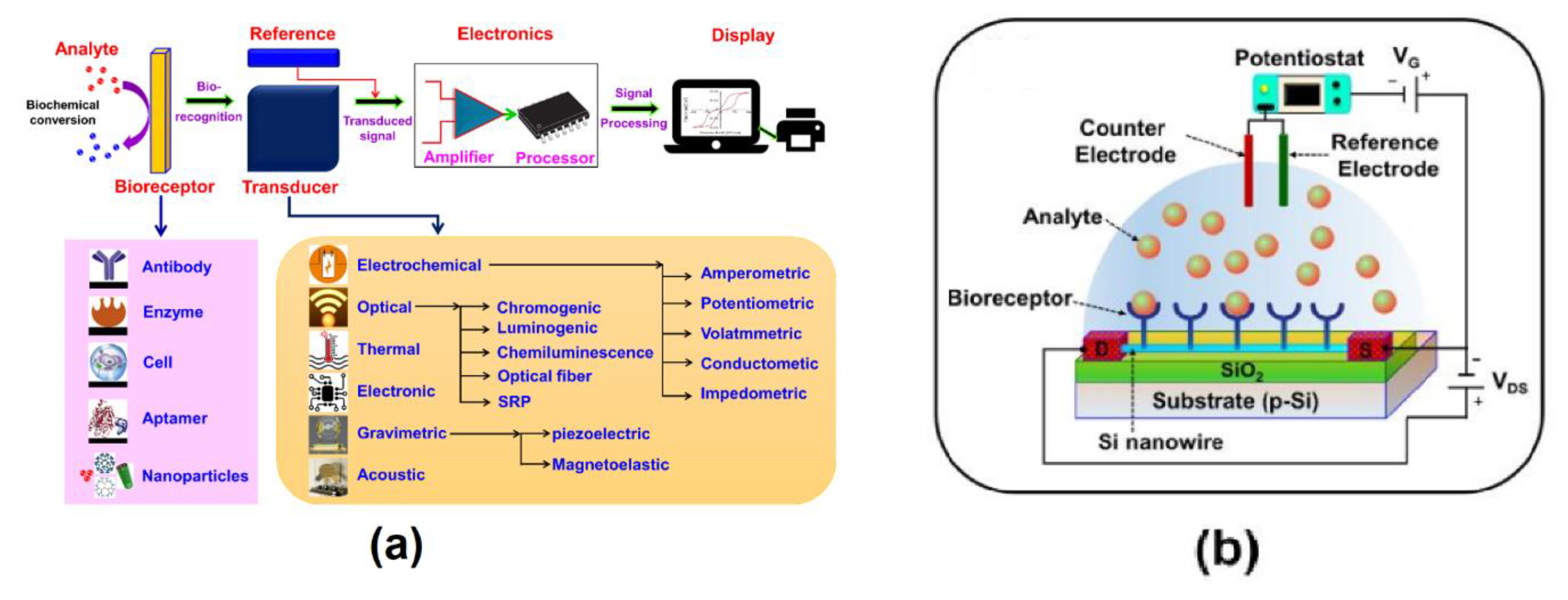
Advanced FET Biosensor Architectures and Fabrication
Junctionless and Gate-All-Around (GAA) Nanowire Based Fabrication
Dual-Material Gate and FinFET-Based Fabrication
Ion-Sensitive and Extended Gate Field Effect Transistor (ISFET and EGFET) Biosensors
GaN, AlGaN/GaN-Based, and Other III-V Material Fabrication
Advanced Fabrication Techniques and Specialized Applications
Nanomaterials for High-Performance FET Biosensors
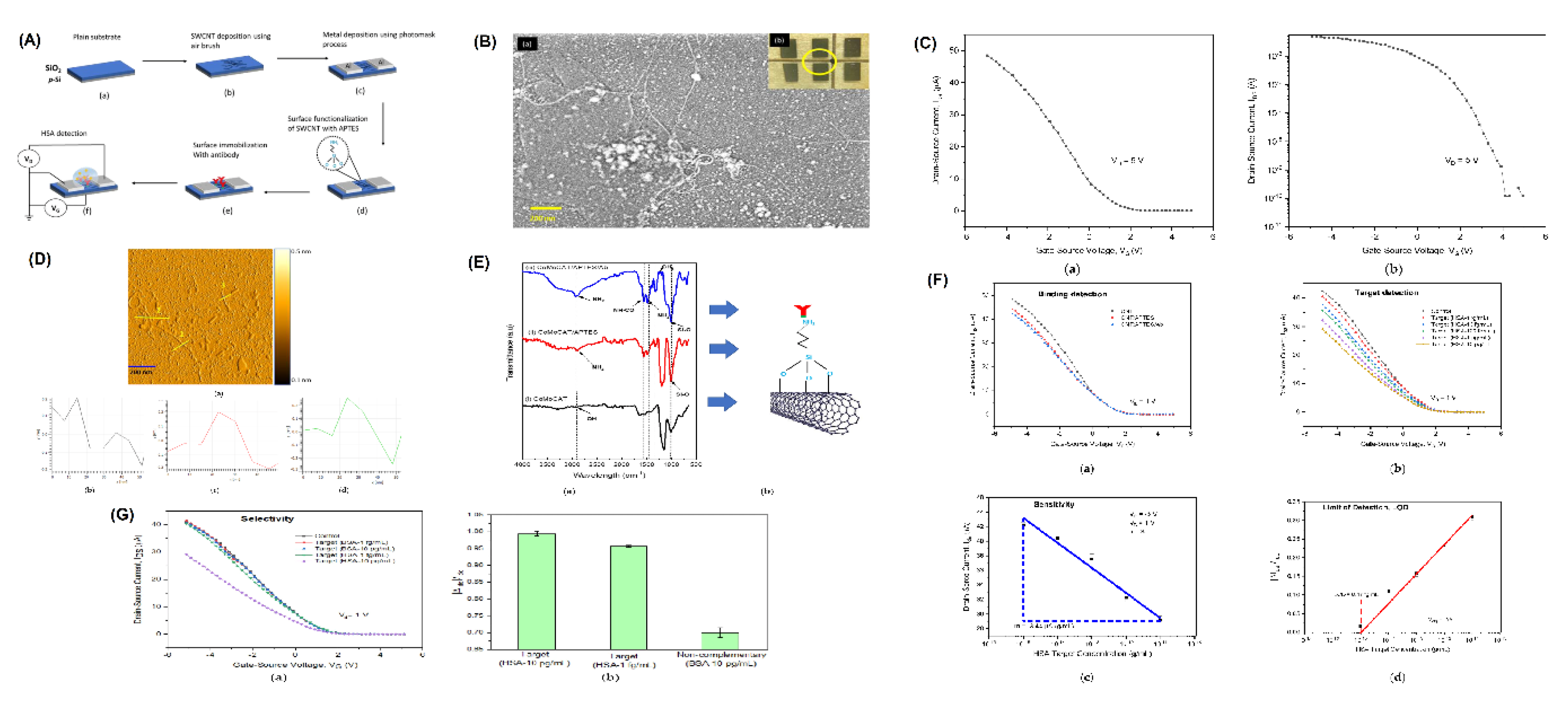
Carbon-Based Materials
Metal-Oxide Semiconductors
Gallium Nitride (GaN) and Related Materials
Silicon-Based Nanowires and Related Structures
FET-Based Biosensors for Breast Cancer Detection
Dielectric-Modulated TFETs for Breast Cancer Detection
Heterojunction FETs for Cancer Biomarkers
Nanomaterial-Based FETs
GaN- and CNT-Based FETs for Breast Cancer
Multi-Target FET Biosensor
Conclusion
Funding
Acknowledgments
Authors Contribution
Availability of data and material
Ethics approval and consent to participate
Consent for publication
Competing interests
References
- Singh, S., N. K. Singh, and S. Singh, Breast-cancer biomarker (C-erbB-2) detection in saliva/serum based on InGaAs/Si heterojunction dopingless TFET biosensor. IEEE Transactions on NanoBioscience, 2021. 22(1): p. 28-37.
- Shalini, V.; Kumar, P. Design and analysis of interconnected multichannel Schottky FinFET for the detection of breast cancer cells. Micro Nanostructures 2025, 199. [Google Scholar] [CrossRef]
- Camarca, A.; Varriale, A.; Capo, A.; Pennacchio, A.; Calabrese, A.; Giannattasio, C.; Almuzara, C.M.; D’auria, S.; Staiano, M. Emergent Biosensing Technologies Based on Fluorescence Spectroscopy and Surface Plasmon Resonance. Sensors 2021, 21, 906. [Google Scholar] [CrossRef]
- Ma, X. , et al., Recent advances in ion-sensitive field-effect transistors for biosensing applications. Electrochemical Science Advances, 2023. 3(3): p. e2100163.
- Das, R.R.; Rajalekshmi, T.R.; James, A. FinFET to GAA MBCFET: A Review and Insights. IEEE Access 2024, 12, 50556–50577. [Google Scholar] [CrossRef]
- McCreary, A.; Kazakova, O.; Jariwala, D.; Al Balushi, Z.Y. An outlook into the flat land of 2D materials beyond graphene: synthesis, properties and device applications. 2D Mater. 2020, 8, 013001. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Sanjay; Kumar, V. ; Vohra, A. Sensitivity enhancement using triple metal gate work function engineering of junctionless cylindrical gate all around SiNW MOSFET based biosensor for neutral biomolecule species detection for upcoming sub 14 nm technology node. Mater. Sci. Eng. B 2024, 306. [Google Scholar] [CrossRef]
- Kumar, P.; Raj, B.; Wadhwa, G.; Singh, B.; Kumar, R. Design and Analysis of Junctionless-Based Gate All Around N+ Doped Layer Nanowire TFET Biosensor. ECS J. Solid State Sci. Technol. 2024, 13, 017002. [Google Scholar] [CrossRef]
- Kumar, S.; Chauhan, R.K. Tweaking the Performance of Dielectric Modulated Junctionless Double Gate Metal Oxide Field Effect Transistor-Based Label-Free Biosensor. J. Electrochem. Soc. 2024, 171, 017503. [Google Scholar] [CrossRef]
- Hussian, A.; Alkhammash, H.I.; Wani, M.S.; Loan, S.A. Metal Strip Implanted Tunneling Field-Effect Transistor Biosensor as a Label-Free Biosensor. ACS Appl. Bio Mater. 2024, 7, 4633–4641. [Google Scholar] [CrossRef]
- Kumar, A.; Kale, S. Spacer-Engineered Reconfigurable Silicon Nanowire Schottky Barrier Transistor as a Label-Free Biosensor. Silicon 2023, 16, 2023–2036. [Google Scholar] [CrossRef]
- Kumar, P. and K. Koley, Breast Cancer and Prostate Cancer Detection Considering Transconductance Generation Factor (g m/I DS) as a Sensing Metric for III-V Gate-all-around Tunnel FET Biosensor. IEEE Sensors Journal, 2023.
- Pattnaik, A.; Mohapatra, S.K.; Dastidar, A.; Acharya, O.P.; AbdelAll, N.; A El-Badry, B.; Khouqeer, G.A.; Alodhayb, A.N. Design and Simulation of Dielectrically Modulated Dual Material Gate-Stack Double-Gate FinFET Biosensor. ECS J. Solid State Sci. Technol. 2024, 13, 057002. [Google Scholar] [CrossRef]
- Dharmender; Nigam, K. K.; Yadav, P.; Tikkiwal, V.A. Performance analysis of dual material control gate cavity on source electrically doped TFET biosensor for biomedical applications. Micro Nanostructures 2024, 191. [Google Scholar] [CrossRef]
- Das, S.; Kumar, B.B.; Bundela, P.; Singh, K. Performance assessment of Si based dual metal double gate vertical TFET biosensor. Micro Nanostructures 2024, 191. [Google Scholar] [CrossRef]
- Kumawat, M.; Gopal, G.; Varma, T. Design and analysis of hetero-dielectric Junctionless-TFET(JL-TFET) with N+ pocket as label free biosensors. Phys. Scr. 2024, 99, 045405. [Google Scholar] [CrossRef]
- Mishra, S.; Mohanty, S.S.; Mishra, G.P. Gate electrode stacked source/drain SON trench MOSFET for biosensing application. Phys. Scr. 2023, 98, 125027. [Google Scholar] [CrossRef]
- Gubanova, O.; Poletaev, A.; Komarova, N.; Grudtsov, V.; Ryazantsev, D.; Shustinskiy, M.; Shibalov, M.; Kuznetsov, A. A novel extended gate ISFET design for biosensing application compatible with standard CMOS. Mater. Sci. Semicond. Process. 2024, 177. [Google Scholar] [CrossRef]
- Chen, X.; Liang, Y.; Tang, N.; Li, C.; Zhang, Y.; Xu, F.; Shi, G.; Zhang, M. Ultrasensitive sensing urinary cystatin C via an interface-engineered graphene extended-gate field-effect transistor for non-invasive diagnosis of chronic kidney disease. Biosens. Bioelectron. 2024, 249, 116016. [Google Scholar] [CrossRef]
- Richardson, H.; Barahona, J.; Medwig, G.; Johns, A.; Pérez, L.M.A.; Sode, K.; Daniele, M.; Miller, F.J.; Lobaton, E.; Pavlidis, S. Towards monitoring of critical illness via the detection of histones with extended gate field-effect transistor sensors. Biosens. Bioelectron. X 2024, 19. [Google Scholar] [CrossRef]
- Kuznetsov, A.; Sheshil, A.; Smolin, E.; Grudtsov, V.; Ryazantsev, D.; Shustinskiy, M.; Tikhonova, T.; Kitiashvili, I.; Vechorko, V.; Komarova, N. Detection of α-Galactosidase A Reaction in Samples Extracted from Dried Blood Spots Using Ion-Sensitive Field Effect Transistors. Sensors 2024, 24, 3681. [Google Scholar] [CrossRef]
- Felix, A.T., M. Mulato, and E.M. Guerra, Evaluation of sensitivity of Extended Gate Field Effect Transistor-biosensor based on V2O5/GOx for glucose detection. Enzyme and Microbial Technology, 2024. 177: p. 110428.
- Mishra, G.S.; Mohankumar, N.; Singh, S.K. Sensitivity improvement in gate engineered technique dielectric modulated GaN MOSHEMT with InGaN notch for label-free biosensing. Eng. Res. Express 2024, 6, 025309. [Google Scholar] [CrossRef]
- Sriramani, P.; Mohankumar, N.; Prasamsha, Y. Drain current sensitivity analysis using a surface potential-based analytical model for AlGaN/GaN double gate MOS-HEMT. Micro Nanostructures 2023, 185. [Google Scholar] [CrossRef]
- Rufino, F.C.; de Almeida, C.R.; Sales, G.; César, R.; Vidal, M.; Delafiori, J.; de Oliveira, A.; Busanello, E.; Siciliano, R.; Nicolau, J.C.; et al. Non-Functionalized Graphene Ribbons FET Biosensor Platform: SARS-CoV-2 Detection on TiO2Gate Dielectric Windows. IEEE Sensors J. 2024, 24, 18791–18804. [Google Scholar] [CrossRef]
- Dixit, A. , et al., Biomolecule detection using GaAs1− xSbX FET based dielectric modulated label-free biosensor. Physica Scripta, 2024. 99(2): p. 025020.
- Bitra, J.; Komanapalli, G. An Improved Z-Shaped Dual-Material-Gate DM-SDZ-TFET Biosensor for Label-Free Detection. J. Electron. Mater. 2024, 53, 1445–1460. [Google Scholar] [CrossRef]
- Venkatesh, M.; Parthasarathy, P.; Kumar, U.A. Surface Potential Analysis of Dual Material Gate Silicon-Based Ferroelectric TFET for Biosensing Application. ECS J. Solid State Sci. Technol. 2024, 13, 017001. [Google Scholar] [CrossRef]
- Ahangari, Z. Design and simulation of a nano biosensor based on amorphous indium gallium zinc oxide (a-IGZO) thin film transistor. Semicond. Sci. Technol. 2024, 39, 035011. [Google Scholar] [CrossRef]
- Raj, A.; Sharma, S.K. Exploring the Potential of Dielectric Modulated SOI Junctionless FinFETs for Label-Free Biosensing. J. Electron. Mater. 2023, 53, 766–772. [Google Scholar] [CrossRef]
- Tomar, A. , et al. AlN/β-Ga₂O₃ MOSHEMT as Biosensor. in 2024 IEEE Applied Sensing Conference (APSCON). 2024. IEEE.
- Mishra, V.; Agarwal, L.; Tiwari, C.; Rathi, V. Dielectric Modulated Negative Capacitance Heterojunction TFET as Biosensor: Proposal and Analysis. Silicon 2024, 16, 3041–3053. [Google Scholar] [CrossRef]
- Krsihna, B.V.; Gangadhar, A.; Ravi, S.; Mohan, D.; Panigrahy, A.K.; Rajeswari, V.R.; Prakash, M.D. A Highly Sensitive Graphene-based Field Effect Transistor for the Detection of Myoglobin. Silicon 2022, 14, 11741–11748. [Google Scholar] [CrossRef]
- Krsihna, B.V.; Ahmadsaidulu, S.; Teja, S.S.T.; Jayanthi, D.; Navaneetha, A.; Reddy, P.R.; Prakash, M.D. Design and Development of Graphene FET Biosensor for the Detection of SARS-CoV-2. Silicon 2021, 14, 5913–5921. [Google Scholar] [CrossRef]
- Zamzami, M.A.; Rabbani, G.; Ahmad, A.; Basalah, A.A.; Al-Sabban, W.H.; Ahn, S.N.; Choudhry, H. Carbon nanotube field-effect transistor (CNT-FET)-based biosensor for rapid detection of SARS-CoV-2 (COVID-19) surface spike protein S1. Bioelectrochemistry 2021, 143, 107982–107982. [Google Scholar] [CrossRef]
- Yahya, I.; Hassan, M.A.; Maidin, N.N.M.; Mohamed, M.A. SWCNT Network-FET Device for Human Serum Albumin Detection. Sensors 2022, 22, 8212. [Google Scholar] [CrossRef]
- Wasfi, A.; Awwad, F.; Qamhieh, N.; Al Murshidi, B.; Palakkott, A.R.; Gelovani, J.G. Real-time COVID-19 detection via graphite oxide-based field-effect transistor biosensors decorated with Pt/Pd nanoparticles. Sci. Rep. 2022, 12, 1–15. [Google Scholar] [CrossRef]
- Ghosh, R., S. Sarkhel, and P. Saha, MoS 2 based dual gate MOSFET as ultra-sensitive SARs-CoV-2 biosensor for rapid screening of respiratory syndrome. IEEE Sensors Letters, 2023.
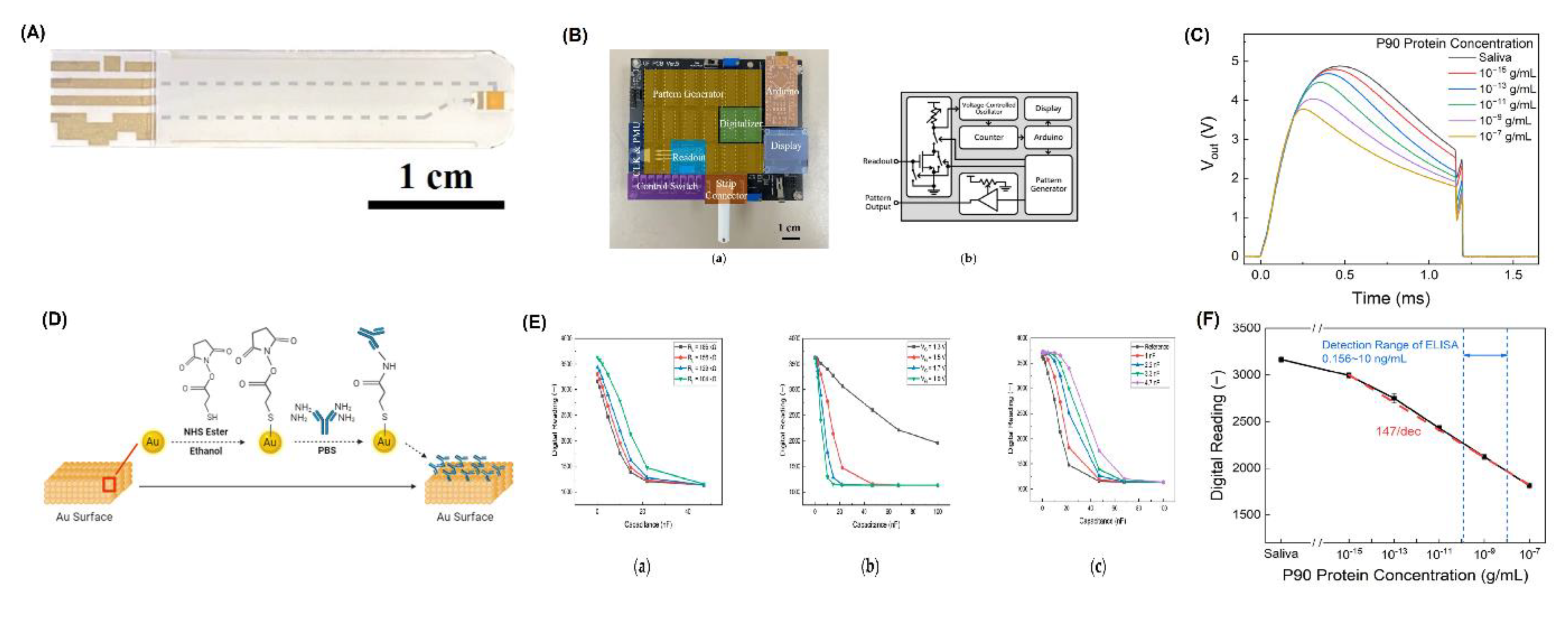
- Wan, H.-H.; Zhu, H.; Chiang, C.-C.; Li, J.-S.; Ren, F.; Tsai, C.-T.; Liao, Y.-T.; Neal, D.; Katz, J.; Esquivel-Upshaw, J.F. Sensitive Detection of Oral Leukoplakia: Analyzing P90 Biomarkers in Saliva and Tissue. Biosensors 2024, 14, 281. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.-H.; Zhu, H.; Chiang, C.-C.; Xia, X.; Li, J.-S.; Ren, F.; Tsai, C.-T.; Liao, Y.-T.; Chou, T.-C.; Neal, D.; et al. Point-of-Care Detection of HER2 and CA 15-3 in Breast Cancer Patients: Dual-Channel Biosensor Implementation. ECS J. Solid State Sci. Technol. 2024, 13, 057003. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Huang, W.; Wan, G.; Xia, M.; Chen, D.; Zhang, Y.; Wang, Y.; Guo, F.; Tan, J.; et al. Integrated Urinalysis Devices Based on Interface-Engineered Field-Effect Transistor Biosensors Incorporated With Electronic Circuits. Adv. Mater. 2022, 34, e2203224. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, Z.; Lan, K.; Wang, Z.; Qin, G.; Chen, R. Highly sensitive detection of multiple proteins from single cells by MoS2-FET biosensors. Talanta 2022, 236, 122839. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jeong, S.; Sarawut, S.; Kim, H.; Son, S.U.; Lee, S.; Rabbani, G.; Kwon, H.; Lim, E.-K.; Ahn, S.N.; et al. An immunosensor based on a high performance dual-gate oxide semiconductor thin-film transistor for rapid detection of SARS-CoV-2. Lab a Chip 2022, 22, 899–907. [Google Scholar] [CrossRef]
- Thakur, R.R.; Saini, A.K.; Jain, A.K.; Taliyan, R.; Chaturvedi, N. Label-free GaN HEMT-based biosensing platform for interferon-γ detection. Mater. Sci. Semicond. Process. 2024, 178. [Google Scholar] [CrossRef]
- Kachhawa, P.; Mishra, S.; Jain, A.K.; Tripura, C.; Joseph, J.; Radha, V.; Chaturvedi, N. Antigen-Antibody Interaction-Based GaN HEMT Biosensor for C3G Detection. IEEE Sensors J. 2022, 22, 6256–6262. [Google Scholar] [CrossRef]
- Sriramani, P.; Mohankumar, N.; Prasamsha, Y.; Sarkar, A.; Chanda, M. Threshold and surface potential-based sensitivity analysis of symmetrical double gate AlGaN/GaN MOS-HEMT including capacitance effects for label-free biosensing. Phys. Scr. 2023, 98, 115036. [Google Scholar] [CrossRef]
- Chen, P.-H.; Huang, C.-C.; Wu, C.-C.; Tripathi, A.; Wang, Y.-L. Saliva-based COVID-19 detection: A rapid antigen test of SARS-CoV-2 nucleocapsid protein using an electrical-double-layer gated field-effect transistor-based biosensing system. Sensors Actuators B: Chem. 2022, 357, 131415–131415. [Google Scholar] [CrossRef] [PubMed]
- Wasfi, A.; Awwad, F.; Gelovani, J.G.; Qamhieh, N.; Ayesh, A.I. COVID-19 Detection via Silicon Nanowire Field-Effect Transistor: Setup and Modeling of Its Function. Nanomaterials 2022, 12, 2638. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Hu, J.; Liu, J.; Li, X.; Sun, S.; Luan, X.; Zhao, Y.; Wei, S.; Li, M.; Zhang, Q.; et al. Si nanowire Bio-FET for electrical and label-free detection of cancer cell-derived exosomes. Microsystems Nanoeng. 2022, 8, 1–12. [Google Scholar] [CrossRef]
- Prakash, M.D.; Krsihna, B.V.; Satyanarayana, B.V.V.; Vignesh, N.A.; Panigrahy, A.K.; Ahmadsaidulu, S. A Study of an Ultrasensitive Label Free Silicon Nanowire FET Biosensor for Cardiac Troponin I Detection. Silicon 2021, 14, 5683–5690. [Google Scholar] [CrossRef]
- Yang, C.-M.; Wei, C.-H.; Ughi, F.; Chang, J.-Y.; Pijanowska, D.G.; Lai, C.-S. High pH stability and detection of α-synuclein using an EGFET biosensor with an HfO2 gate deposited by high-power pulsed magnetron sputtering. Sensors Actuators B: Chem. 2024, 416. [Google Scholar] [CrossRef]
- Samanta, S.; Tiwari, V.S.; Sadhujan, S.; Harilal, S.; Eisenberg-Lerner, A.; Rotfogel, Z.; Pikhay, E.; Shima-Edelstein, R.; Greental, D.; Bashouti, M.Y.; et al. From sensing interactions to controlling the interactions: a novel approach to obtain biological transistors for specific and label-free immunosensing. Nanoscale 2024, 16, 6648–6661. [Google Scholar] [CrossRef]
- Dewan, B.; Chaudhary, S.; Singh, D.; Yadav, M. Label-free detection of breast cancer cell lines using dopingless heterojunction TFET considering non-ideal hybridization issue. Mater. Sci. Eng. B 2024, 302. [Google Scholar] [CrossRef]
- Bind, M.K.; Singh, S.V.; Nigam, K.K. Design and Investigation of the DM- PC-TFET-Based Biosensor for Breast Cancer Cell Detection. Trans. Electr. Electron. Mater. 2023, 24, 381–395. [Google Scholar] [CrossRef]
- Sravani, K.G.; Kumar, R.A.; Rao, K.S.; Edla, D.R.; Jannu, S.; Alkhayyat, A.; Mishra, A.K. Modeling and Evaluating the Performance of a Split-Gate T-Shape Channel DM DPDG-TFET Biosensor for Label-Free Detection. IEEE Trans. Consum. Electron. 2024, PP, 1–1. [Google Scholar] [CrossRef]
- Raut, P.; Panda, D.K.; Rashed, A.N.Z. Analysis of Dual Material Gate InSb/Si Heterojunction Silicon on Insulator Tunnel Field Effect Transistor (DMG-HJ-SOI-TFET) Biosensor for CREB-2 Protein Detection. Sens. Imaging 2025, 26, 1–21. [Google Scholar] [CrossRef]
- Kumar, P. and K. Koley, Breast Cancer and Prostate Cancer Detection Considering Transconductance Generation Factor (g m/I DS) as a Sensing Metric for III–V Gate-All-Around Tunnel FET Biosensor. IEEE Sensors Journal, 2023. 23(19): p. 22723-22730.
- Singh, N.K.; Kar, R.; Mandal, D. Simulation Study of Novel Charge-Plasma Based ArcTFET for Sensing the Breast Cancer Biomarker (C-erbB-2) in Serum. IEEE Trans. NanoBioscience 2022, 22, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Ming, P.; Li, J.; Yang, L.; Yu, Y.; Tang, L.; Zhou, H.; Zhang, Z.-Y.; Zhang, G.-J. A Drug Molecule-Modified Graphene Field-Effect Transistor Nanosensor for Rapid, Label-Free, and Ultrasensitive Detection of Estrogen Receptor α Protein. Anal. Chem. 2024, 96, 3454–3461. [Google Scholar] [CrossRef]
- Majd, S.M.; Mirzapour, F.; Shamsipur, M.; Manouchehri, I.; Babaee, E.; Pashabadi, A.; Moradian, R. Design of a novel aptamer/molecularly imprinted polymer hybrid modified Ag–Au@Insulin nanoclusters/Au-gate-based MoS2 nanosheet field-effect transistor for attomolar detection of BRCA1 gene. Talanta 2023, 257, 124394. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Tang, L.; Li, K.; Liu, B.; Xiao, M.-M.; Liu, N.; Ni, W.; Li, Y.; Zhang, Z.; Zhang, G.-J. Functionalized carbon nanotube field-effect transistor biosensor for highly sensitive detection of exosomal protein. Anal. Chim. Acta 2023, 1273, 341511. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Wang, Z.; Wang, S.; Wang, C.; Zhang, K.; Zhang, Y.; Han, L. Highly Stable InSe-FET Biosensor for Ultra-Sensitive Detection of Breast Cancer Biomarker CA125. Biosensors 2023, 13, 193. [Google Scholar] [CrossRef]
- Sehgal, H.D.; Pratap, Y.; Kabra, S. Detection of Breast Cancer Cell-MDA-MB-231 by Measuring Conductivity of Schottky Source/Drain GaN FinFET. IEEE Sensors J. 2022, 22, 6108–6115. [Google Scholar] [CrossRef]
- Chowdhury, R.; Mishra, S.; Singh, K.; Chaturvedi, N.; Chauhan, A.; Pande, S.; Sharma, N.; Parjapat, P.; Sharma, R.; Kothari, P.; et al. GaN HEMT based biosensor for the detection of breast cancer marker (C-erbB2). Semicond. Sci. Technol. 2021, 36, 045018. [Google Scholar] [CrossRef]
- Sharma, B.; Yadav, S.; Rewari, S.; Hasija, Y. DM-PA-CNTFET Biosensor for Breast Cancer Detection: Analytical Model. ECS J. Solid State Sci. Technol. 2024, 13, 087004. [Google Scholar] [CrossRef]
- Karmakar, P.; Sahu, P.; Mohapatra, S.; Alanazi, N.; Alodhayb, A.N. A modified gate oxide tunnel Field-Effect transistor (TFET) biosensor to identify receptor Tyrosine-Protein kinase 2 (C-erbB-2) in Serum/Saliva. Measurement 2024, 239. [Google Scholar] [CrossRef]
- Gao, J.; Wang, C.; Wang, C.; Chu, Y.; Wang, S.; Sun, M.Y.; Ji, H.; Gao, Y.; Wang, Y.; Han, Y.; et al. Poly-l-Lysine-Modified Graphene Field-Effect Transistor Biosensors for Ultrasensitive Breast Cancer miRNAs and SARS-CoV-2 RNA Detection. Anal. Chem. 2022, 94, 1626–1636. [Google Scholar] [CrossRef]
- Chakraborty, A.; Rafiq, M.; Zarkob, Y.H.; Chauhan, Y.S.; Sahay, S. Ferroelectric FET-Based Bayesian Inference Engine for Disease Diagnosis. IEEE Trans. Circuits Syst. I: Regul. Pap. 2025, 72, 1547–1559. [Google Scholar] [CrossRef]
- Kaushal, P.; Khanna, G. Breast Cancer Detection Using Si-Doped MoS2 Channel-Based Thickness Engineered TFET Biosensor. IEEE Sensors Lett. 2024, 8, 1–4. [Google Scholar] [CrossRef]
- Bitra, J.; Komanapalli, G. A Dielectric Modulated Step-Channel Junction-Less TFET (DM-SC-JLTFET) for Label-Free Detection of Breast Cancer Cells: Design and Sensitivity Analysis. Sens. Imaging 2023, 24, 1–22. [Google Scholar] [CrossRef]

| Title/Device | Detection Target | Technology Used | Simulation Tool | Key Features | Sensitivity Metrics | Reference |
|---|---|---|---|---|---|---|
| DM-DL-DMG-HJTFET | Breast cancer cell lines (healthy vs cancerous) | Dielectric modulated dual metal gate TFET | SILVACO ATLAS TCAD | - Nanogap beneath gate- Detects via dielectric property differences | Sensitivity in drain current, Vth, transconductance, ION/IOFF (exact values not given) | [54] |
| DM-PC-TFET | Breast Cancer Cells (BCCs), esp. T47D line | Dielectric modulated polarity control TFET | 2D TCAD Tool | - Works in microwave frequency band- Examines temp., geometry, charge density effects | Drain current: 7.82×10¹⁰ION/IOFF: 2.01×10⁹Transconductance: 2.32×10¹2 | [55] |
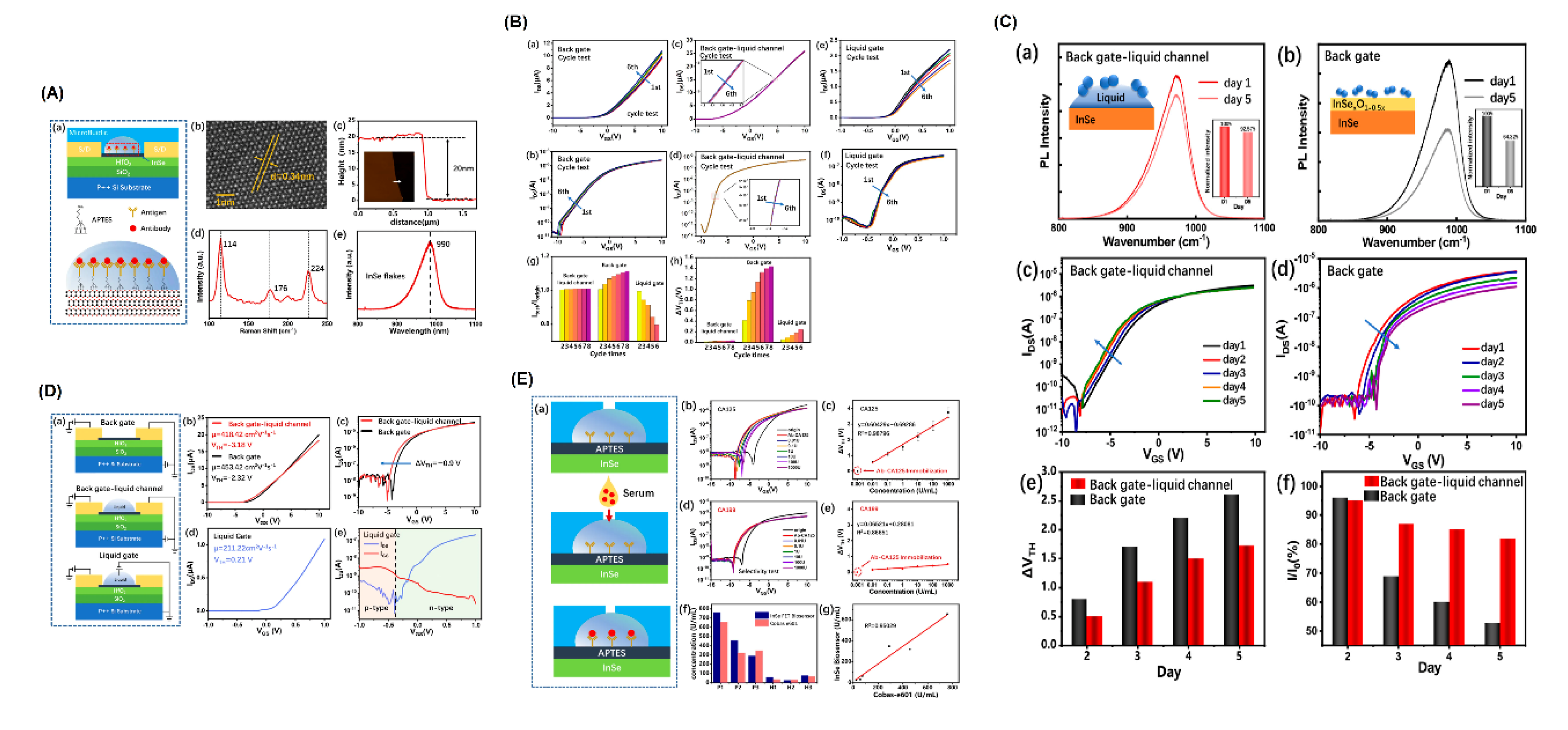
| InSe-FET | CA125 biomarker (clinical samples) | InSe-based FET with microfluidic integration | Not explicitly mentioned | - High electrical stability in liquids- Label-free detection | Detection range: 0.01–1000 U/mLTime: 20 min | [63] |
| GaN FinFET with Schottky Source/Drain | Breast cancer cells (MCF-10A, MDA-MB-231, T-47D, MCF-7, HS578t) | GaN FinFET, conductivity variation at microwave frequencies | Not specified | Studies dielectric property variation in cells; Optimized fin height, channel material, temp.; Comparative study with HEMT | 17% higher ΔIds compared to HEMT; Analysis at 900MHz and 10GHz | [64] |
| Si-doped MoS₂ TE-TFET | Breast cancer cells (Hs578T, MDA-MB-231, MCF-7, T47D) | Si-doped MoS₂ tunnel FET biosensor | Not specified | Dielectric charge modulation; Analyzes geometry variations, cavity occupancy | SVth: 0.38 (healthy), 0.77 (T47D); SION: 9.64894 (healthy), 110.37 (T47D) | [70] |
| III–V GAA TFET | Breast and prostate cancer (c-erbB-2, PSA serum) | Gate-all-around III–V TFET biosensor | Not specified | Subthreshold sensing via gm/IDS; Varies cavity lengths and BAs charge | gm/IDS: 45–61 V⁻¹; Sensitivity: 10–35; τBio: 103–419μs (varies by health condition) | [58] |
| DM-SC-JLTFET | Breast cancer cell T47D (K=32) | Dielectric modulated step channel Junctionless TFET | Not specified | Uses charge plasma; RDF reduction; Step-channel with low substrate thickness | Detection sensitivity: 2.683×10⁶; SS: 32 mV/dec | [71] |
| CP-GS-ArcTFET | C-erbB-2 protein | Charge-Plasma and Gate-Stack ArcTFET | Silvaco ATLAS TCAD | Central angle (θ) tuning; analysis of gm, gm2, gm3, fT, GBP, τ, TFP; ION/IOFF: 4.5×10⁹; SS: 35.97 mV/dec | Sensitivity to ON-current (SON), OFF-current (SOFF), and current-ratio; Impact of θ on sensitivity at 210μg/ml | [59] |
| DS-GAAE-CNTFET | MDA-MB-231 and HS578t breast cancer cells | Dual metal Gate-All-Around CNTFET with SiO2 and HfO2 stack | Not mentioned | CNT channel; dual nanocavity; early cancer detection | HS578t: 236.9 nA (Id), 285.58 mV (VT); MDA-MB-231: 343.35 nA (Id), 293.23 mV (VT) | [66] |
| Graphene FET | Estrogen receptor α (ERα) | Liquid-gated graphene FET with drug molecule as capture probe | Not mentioned | Label-free, fast detection; synthesized drug molecule as probe | LOD: 2.62 fM; Kd: 7.35 ± 0.06 pM; Response time: 30 min | [60] |
| MoS2 FET with Apta-MIP | BRCA1 ssDNA | Electrolyte-gated MoS2 FET with aptamer-MIP hybrid receptor | Not mentioned | Ag–Au@InsNCs; electropolymerized hybrid receptor | Sensitivity: 0.4851 μA/decade; LOD: 3.0 aM (buffer), 6.4 aM (serum) | [61] |
| GaN HEMT Biosensor | C-erbB2 protein | Compact GaN High Electron Mobility Transistor (HEMT) | Not mentioned | Two-finger gate (125 µm width, 5 µm length); Au–S complex; Functionalized with thioglycolic acid; High-resolution biosensing | 31% change in drain current after 6 h incubation | [65] |
| Poc-MGOTFET | C-erbB-2 in serum and saliva | Modified Gate Oxide TFET with Pocket and Dual Cavity | TCAD Sentaurus (2D simulation) | Interface charge modulation; Enhanced tunneling rate; Extended gate structure; Dual cavity under gate | Sensitivity increased by 10⁶; Improved ION/IOFF ratio | [67] |
| CNT FET Biosensor | Exosomal MUC1 protein | Polymer-sorted CNT film-based FET with AuNP-aptamer | Not mentioned | PLL-modified CNT; Aptamer-functionalized AuNPs; High-purity CNTs; Label-free detection | LOD: 0.34 fg/mL | [62] |
| IC–S-FinFET Biosensor | Various breast cancer cell lines (MCF-10A, Hs578T, MDA-MB-231, MCF-7, T47D) | Dielectrically Controlled Interconnected Multichannel Schottky FinFET | Not mentioned | Dielectric variation; Fill factor analysis; Linear and noise characterization | Improved Sn with fill factor; Evaluated drain current, SS, and ION/IOFF for different charges | [2] |
| PGFET Biosensor | Breast cancer miRNAs, SARS-CoV-2 RNA | PLL-functionalized Graphene FET | Not mentioned | PLL immobilizes DNA probes; detection from 2 μL serum/swab; 113% sensitivity enhancement over GFET | Detection limit: 1 fM; Detection time: 20 min | [68] |
| FeFET-based Muller C-element | Cancer classification via Bayesian inference | Ferroelectric FET (FeFET) | Experimentally calibrated compact model | Energy-efficient probabilistic computing; Bayesian inference; Compact (0.07 μm2); Energy: 4.1 fJ | High accuracy on Wisconsin dataset; low energy and area footprint | [69] |
| DM DPDG TFET Biosensor | SARS-CoV-2, Breast cancer cells, MCF-10A | Drain Pocket Dual Gate TFET with Nanocavity | Silvaco ATLAS TCAD | Dielectric modulation; K=22 for MDA-MB-231; optimized oxide/nanocavity thickness | Ion=0.183 mA/μm, Vth=1.712V, gm=0.581 mA/V-μm, SS=25.86 mV/dec | [56] |
| DMG-HJ-SOI-TFET | Breast Cancer Cells (BCC, e.g., T47D) | InSb/Si Heterojunction SOI TFET with Dual Material Gate | Not mentioned | Label-free detection; dielectric modulation; low power; K=32 for T47D | SS=39 mV/dec; SIon=104 | [57] |
| HJ-DL-TFET | C-erbB-2 in saliva/serum | In1-xGaxAs/Si Heterojunction Dopingless Tunnel FET | SILVACO ATLAS TCAD | Dual cavity; interface charge modulation; extended gate; x=0.2 | High ION/IOFF ratio; sensitivity ~10⁶ | [1] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





