Submitted:
03 June 2025
Posted:
03 June 2025
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Biosensing Technology
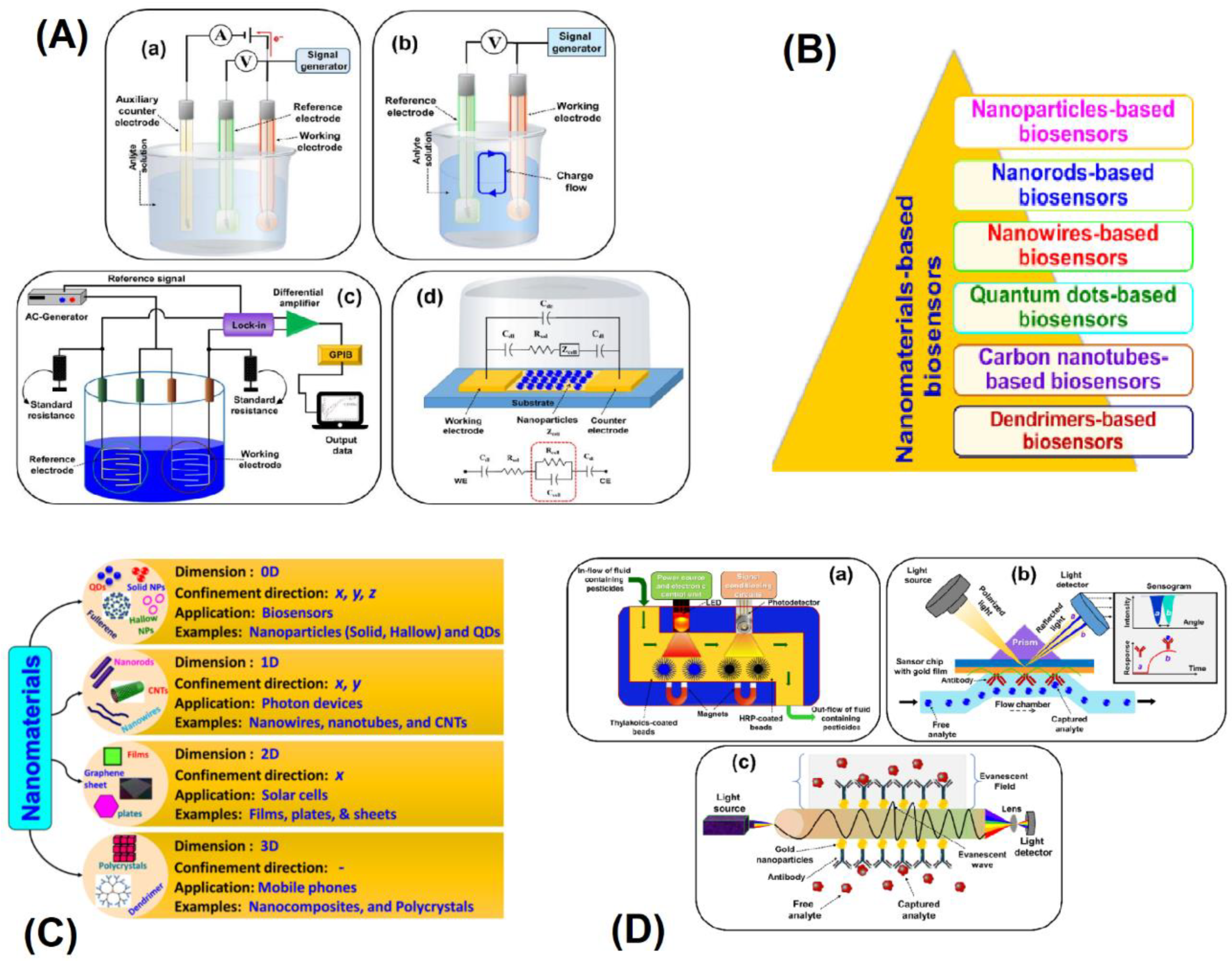
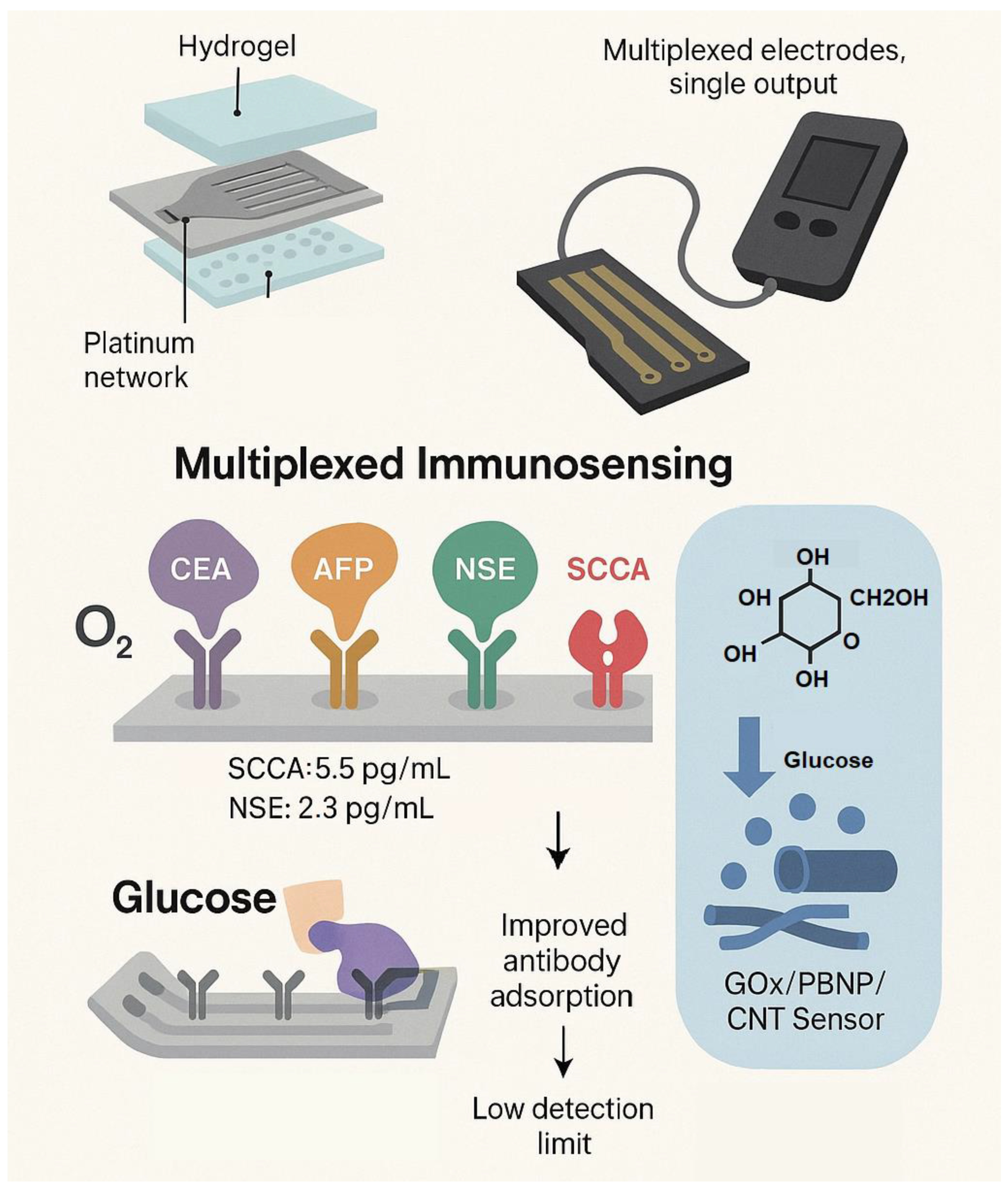
2.1. Lab On-Chip Devices
2.2. Biosensors
2.3. Microfluidic Devices
2.4. Assay
2.5. BioMEMS
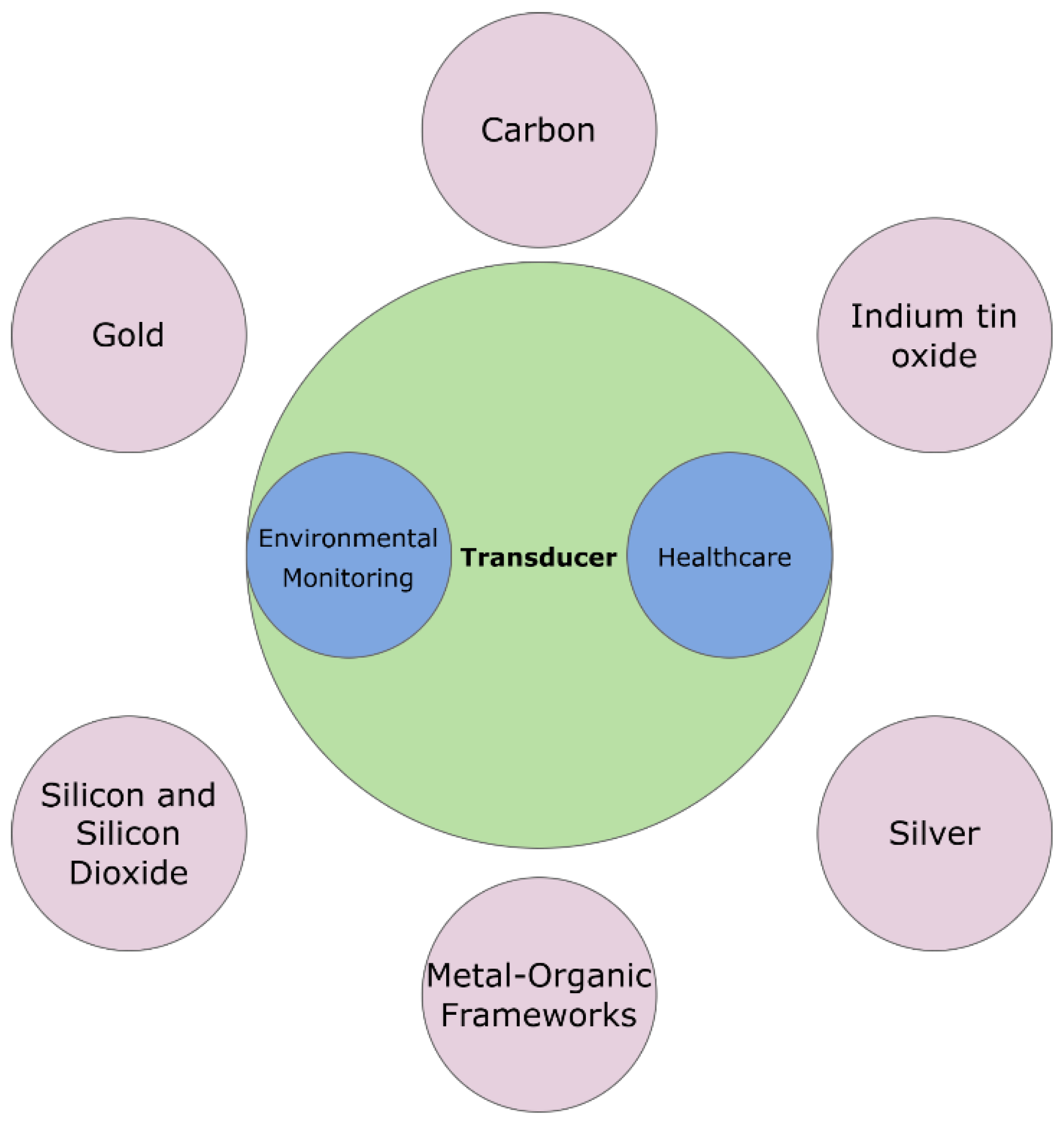
3. Material as Transducer
3.1. Gold (Au) Electrodes
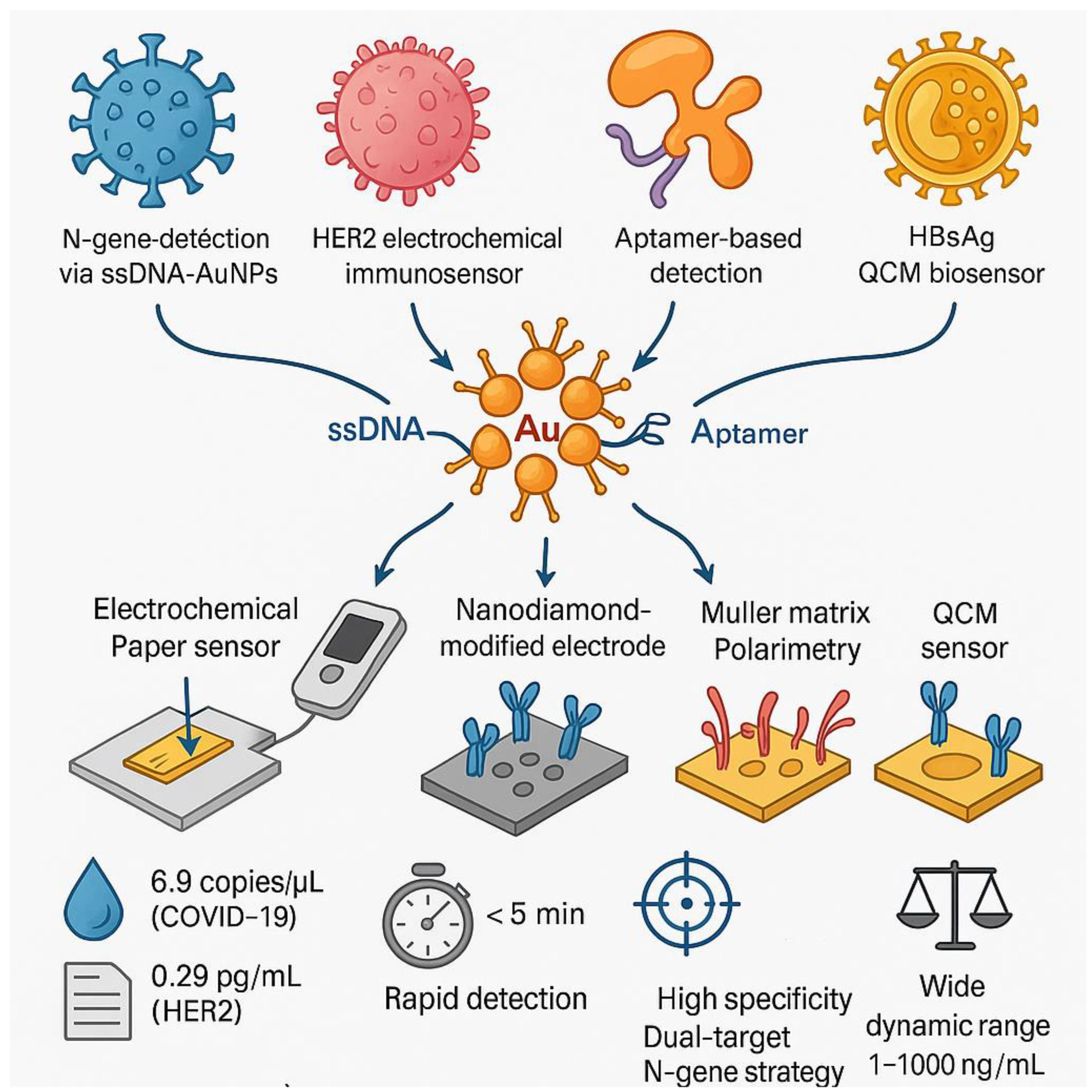
Gold Nanoparticles for Biomolecule Detection
Electrochemical Detection in Biological Monitoring
Advanced Nanostructures for Specific Detection
3.2. Carbon-Based Electrodes
Electrochemical Sensors for Biological Molecules
Electrochemical and Optical Sensors for Environmental and Pharmaceutical Applications
3.3. Indium Tin Oxide (ITO)
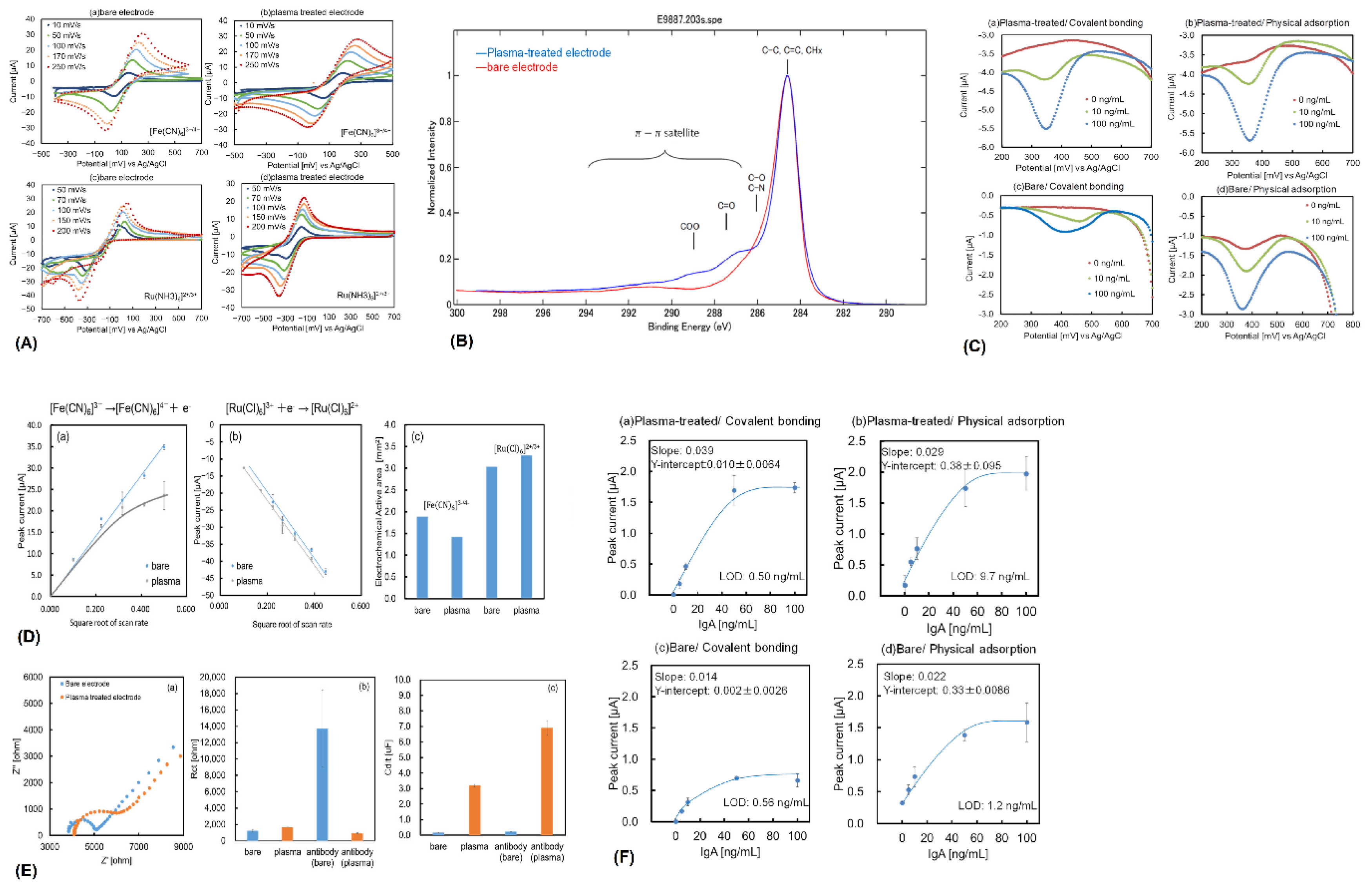
Electrochemical Biosensors Using ITO for Pathogen Detection
Biosensors Using ITO for Environmental and Health Applications
Advanced Nanomaterial-Based Sensors
3.4. Silver Nanoparticles
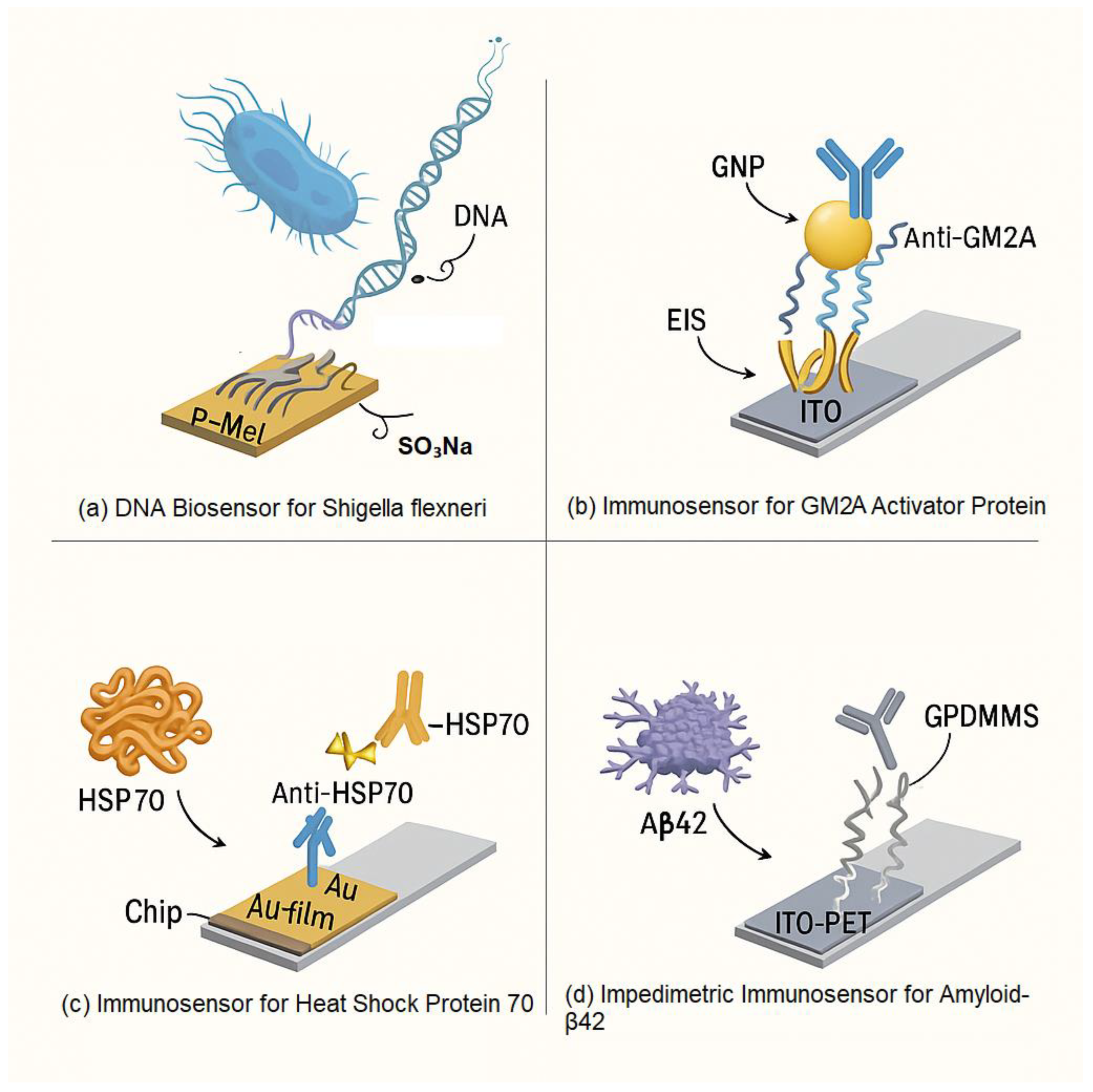
Glucose Detection Using Nanomaterials
Detection Using AgNPs and Other Nanoparticles
Biosensors Using Specific Electrode Materials
3.5. Metal‒Organic Frameworks
Electrochemical Detection with Aptamer/Enzyme Modification
Electrochemical Detection with Metal/Carbon Nanocomposites
3.6. Silicon and Silicon Dioxide
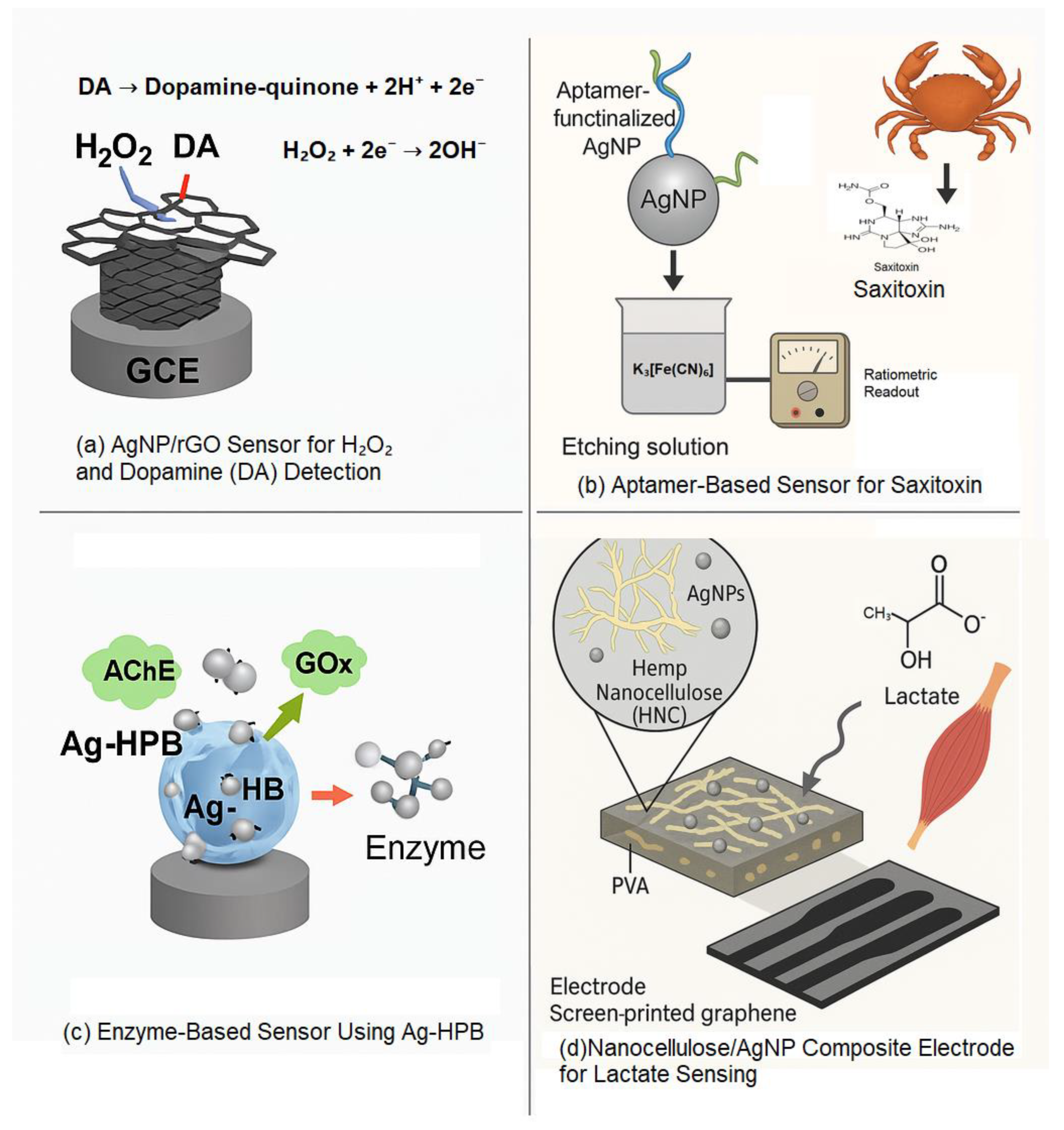
Electrochemical Etching and Functionalization
Lithography and Etching Techniques
Microcontact and Layer-by-Layer Techniques
Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naresh, V.; Lee, N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors, 2021, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, L.; Cui, Y. Transdermal amperometric biosensors for continuous glucose monitoring in diabetes. Talanta, 2023, 253, 124033. [Google Scholar] [CrossRef]
- Pesaran, S.; Rafatmah, E.; Hemmateenejad, B. An all-in-one solid state thin-layer potentiometric sensor and biosensor based on three-dimensional origami paper microfluidics. Biosensors, 2021, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Zaccariotto, G.C.; et al. A novel method for the detection of SARS-CoV-2 based on graphene-impedimetric immunosensor. Materials, 2021, 14, 4230. [Google Scholar] [CrossRef]
- Sciuto, E.L.; et al. Miniaturized electrochemical biosensor based on whole-cell for heavy metal ions detection in water. Biotechnology and Bioengineering, 2021, 118, 1456–1465. [Google Scholar] [CrossRef]
- Serebrennikova, K.V.; et al. Raman scattering-based biosensing: new prospects and opportunities. Biosensors, 2021, 11, 512. [Google Scholar] [CrossRef]
- Asghari, A.; et al. Fast, accurate, point-of-care COVID-19 pandemic diagnosis enabled through advanced lab-on-chip optical biosensors: Opportunities and challenges. Applied physics reviews 2021, 8. [Google Scholar] [CrossRef]
- Wu, J.; et al. Lab-on-a-Chip Platforms for Detection of Cardiovascular Disease and Cancer Biomarkers. Sensors, 2017, 17, 2934. [Google Scholar] [CrossRef]
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical glucose sensors in diabetes management: an updated review (2010–2020). Chemical Society Reviews, 2020, 49, 7671–7709. [Google Scholar] [CrossRef]
- Bradley, Z.; Bhalla, N. Point-of-care diagnostics for sepsis using clinical biomarkers and microfluidic technology. Biosensors and Bioelectronics, 2023, 227, 115181. [Google Scholar] [CrossRef]
- Vázquez, M.; et al. Use of some cost-effective technologies for a routine clinical pathology laboratory. Lab on a Chip, 2021, 21, 4330–4351. [Google Scholar] [CrossRef] [PubMed]
- Chircov, C.; Grumezescu, A.M. Microelectromechanical systems (MEMS) for biomedical applications. Micromachines, 2022, 13, 164. [Google Scholar] [CrossRef] [PubMed]
- Alafeef, M.; et al. Rapid, ultrasensitive, and quantitative detection of SARS-CoV-2 using antisense oligonucleotides directed electrochemical biosensor chip. ACS nano, 2020, 14, 17028–17045. [Google Scholar] [CrossRef]
- Olorundare, F.O.; et al. A nanoDiamond/gold nanoparticle-based electrochemical immunosensor for the detection of HER 2 cancer biomarker. Biosensors and Bioelectronics: X, 2024, 18, 100483. [Google Scholar] [CrossRef]
- Le, T.-H.; et al. Gold nanoparticle-based biosensor for Lysozyme-DNA detection utilizing decomposition Mueller matrix polarimetry. Optics & Laser Technology, 2024, 179, 111302. [Google Scholar]
- Saffari, Z.; et al. A quartz crystal microbalance biosensor based on polyethylenimine-modified gold electrode to detect hepatitis B biomarker. Analytical Biochemistry, 2023, 661, 114981. [Google Scholar] [CrossRef]
- Yu, M.; et al. Gold nanostructure-programmed flexible electrochemical biosensor for detection of glucose and lactate in sweat. Journal of Electroanalytical Chemistry, 2021, 882, 115029. [Google Scholar] [CrossRef]
- Wang, R.; et al. Stretchable gold fiber-based wearable textile electrochemical biosensor for lactate monitoring in sweat. Talanta, 2021, 222, 121484. [Google Scholar] [CrossRef]
- Bi, R.; et al. Enzymatic biosensor based on dendritic gold nanostructure and enzyme precipitation coating for glucose sensing and detection. Enzyme and Microbial Technology, 2023, 162, 110132. [Google Scholar] [CrossRef]
- Singh, A.K.; et al. Electrochemical biosensors based on in situ grown carbon nanotubes on gold microelectrode array fabricated on glass substrate for glucose determination. Microchimica Acta, 2023, 190, 55. [Google Scholar] [CrossRef]
- Rachmawati, A.; et al. An acetylcholinesterase-based biosensor for isoprocarb using a gold nanoparticles-polyaniline modified graphite pencil electrode. Analytical Sciences, 2023, 39, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Batra, B.; et al. An electrochemical biosensor for the determination of folic acid in pregnant women based on DHFR/c-MWCNTs/TiO2NPs modified gold electrode. Sensors International, 2023, 4, 100235. [Google Scholar] [CrossRef]
- Zhao, L.; Han, H.; Ma, Z. Improved screen-printed carbon electrode for multiplexed label-free amperometric immuniosensor: Addressing its conductivity and reproducibility challenges. Biosensors and Bioelectronics, 2018, 101, 304–310. [Google Scholar] [CrossRef]
- Verma, R.; et al. Uricase conjugated nanostructured NiFe2O4/rGO modified flexible screen-printed carbon electrode based interface for long-range electrochemical determination of uric acid. Surfaces and Interfaces, 2024, 49, 104406. [Google Scholar] [CrossRef]
- Osaki, S.; et al. Surface Modification of Screen-Printed Carbon Electrode through Oxygen Plasma to Enhance Biosensor Sensitivity. Biosensors, 2024, 14, 165. [Google Scholar] [CrossRef]
- Xiao, Y.; et al. Noninvasive glucose monitoring using portable GOx-Based biosensing system. Analytica Chimica Acta, 2024, 1287, 342068. [Google Scholar] [CrossRef]
- Sharifi, J.; Fayazfar, H. Highly sensitive determination of doxorubicin hydrochloride antitumor agent via a carbon nanotube/gold nanoparticle based nanocomposite biosensor. Bioelectrochemistry, 2021, 139, 107741. [Google Scholar] [CrossRef]
- Shumeiko, V.; et al. A nanoscale optical biosensor based on peptide encapsulated SWCNTs for detection of acetic acid in the gaseous phase. Sensors and Actuators B: Chemical, 2021, 327, 128832. [Google Scholar] [CrossRef]
- Suwannachat, J.; et al. An electrochemical AChE-based biosensor for organophosphate pesticides using a modified CuNWs/rGO nanocomposite on a screen-printed carbon electrode. Food Chemistry, 2024, 434, 137431. [Google Scholar] [CrossRef]
- Ahmad, K.; et al. Fabrication of CaTiO3 modified glassy carbon electrode-based hydrogen peroxide sensor. Journal of the Iranian Chemical Society 2024, 1–11. [Google Scholar] [CrossRef]
- Yang, M.; et al. A 3D electrochemical biosensor based on Super-Aligned Carbon NanoTube array for point-of-care uric acid monitoring. Biosensors and Bioelectronics, 2021, 179, 113082. [Google Scholar] [CrossRef] [PubMed]
- Sangubotla, R.; Kim, J. Fiber-optic biosensor based on the laccase immobilization on silica-functionalized fluorescent carbon dots for the detection of dopamine and multi-color imaging applications in neuroblastoma cells. Materials Science and Engineering: C, 2021, 122, 111916. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; et al. Label free flexible electrochemical DNA biosensor for selective detection of Shigella flexneri in real food samples. Talanta, 2023, 253, 123909. [Google Scholar] [CrossRef] [PubMed]
- Aydın, E.B.; Aydın, M.; Sezgintürk, M.K. A label-free electrochemical biosensor for highly sensitive detection of GM2A based on gold nanoparticles/conducting amino-functionalized thiophene polymer layer. Sensors and Actuators B: Chemical, 2023, 392, 134025. [Google Scholar] [CrossRef]
- Liu, R.; et al. Sensitive detection of HSP70 using a current-amplified biosensor based on antibody-loaded PS-AuNPs@ Cys/Au modified ITO chip. Microchimica Acta, 2024, 191, 1–10. [Google Scholar] [CrossRef]
- Altay, D.N.; Yagar, H.; Ozcan, H.M. A new ITO-based Aβ42 biosensor for early detection of Alzheimer's disease. Bioelectrochemistry, 2023, 153, 108501. [Google Scholar] [CrossRef]
- Wen, X.; et al. ZnO/Cu2O heterojunction integrated fiber-optic biosensor for remote detection of cysteine. Biosensors and Bioelectronics, 2023, 223, 115021. [Google Scholar] [CrossRef]
- Bhardwaj, H.; Sumana, G.; Marquette, C.A. Gold nanobipyramids integrated ultrasensitive optical and electrochemical biosensor for Aflatoxin B1 detection. Talanta, 2021, 222, 121578. [Google Scholar] [CrossRef]
- Ghrera, A.S. Ti3C2TxMXene modified indium tin oxide (ITO) electrode for electrochemical sensing of bilirubin based on a molecularly imprinted pyrrole polymer. Physica Scripta, 2024, 99, 055936. [Google Scholar]
- Viswanathan, P.; et al. Methionine-assisted electrodeposition of porous copper cobalt bi-metallic hetero-nanostructures on an indium tin oxide electrode: a disposable and stable electrode for non-enzymatic glucose sensing. Journal of Materials Chemistry C 2024. [Google Scholar] [CrossRef]
- Bharat, B.S.; Babu, A.R. Iodine-doped reduced graphene oxide and titanium dioxide nanocomposite as effective amperometric glucose biosensor. Materials Chemistry and Physics, 2024, 320, 129409. [Google Scholar] [CrossRef]
- Uhuo, O.; et al. Interferon gamma (IFN-γ)-sensitive TB aptasensor based on novel chitosan-indium nano-kesterite (χtCITS)-labeled DNA aptamer hairpin technology. Bioelectrochemistry, 2024, 158, 108693. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Krasnoslobodtsev, A.V. DNA-templated silver nanoclusters as dual-mode sensitive probes for self-powered biosensor fueled by glucose. Nanomaterials, 2023, 13, 1299. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; et al. Highly stable, stretchable, and transparent electrodes based on dual-headed Ag@ Au core-sheath nanomatchsticks for non-enzymatic glucose biosensor. Nano Research, 2023, 16, 1558–1567. [Google Scholar] [CrossRef]
- Pektaş, S.Ü.; et al. Green synthesis of silver nanoparticles and designing a new amperometric biosensor to determine glucose levels. Journal of Food Composition and Analysis, 2024, 129, 106133. [Google Scholar] [CrossRef]
- Zalke, J.B.; et al. Facile chemiresistive biosensor functionalized with PANI/GOx and novel green synthesized silver nanoparticles for glucose sensing. Microchemical Journal, 2024, 200, 110339. [Google Scholar] [CrossRef]
- Zhang, Y.; et al. Hydrogen Peroxide and Dopamine Sensors Based on Electrodeposition of Reduced Graphene Oxide/Silver Nanoparticles. Sensors, 2024, 24, 355. [Google Scholar] [CrossRef]
- Zeng, W.; et al. Development of a highly sensitive aptamer-based electrochemical sensor for detecting saxitoxin based on K3Fe (CN) 6 regulated silver nanoparticles. Analytica Chimica Acta, 2024, 1287, 342134. [Google Scholar] [CrossRef]
- Li, R.; et al. Hollow Prussian blue with ultrafine silver nanoparticle agents (Ag-HPB) integrated sensitive and flexible biosensing platform with highly enzyme loading capability. Talanta, 2024, 266, 125036. [Google Scholar] [CrossRef]
- Phumma, R.; et al. Fabrication of silver nanoparticle loaded into nanocellulose derived from hemp and poly (vinyl alcohol)-based composite as an electrode for electrochemical sensors for lactate determination. ACS omega, 2024, 9, 10371–10379. [Google Scholar] [CrossRef]
- Lansdorp, B.M.; Lamberg, P.; Hamid, R. Screen-Printed Silver/Silver Chloride Electrodes Inhibit Alcohol Oxidase Activity. ECS Sensors Plus, 2023, 2, 030602. [Google Scholar] [CrossRef] [PubMed]
- Tvorynska, S.; Barek, J.; Josypcuk, B. High-performance amperometric biosensor for flow injection analysis consisting of a replaceable lactate oxidase-based mini-reactor and a silver amalgam screen-printed electrode. Electrochimica Acta, 2023, 445, 142033. [Google Scholar] [CrossRef]
- Tian, J.; et al. An electrochemical dual-aptamer biosensor based on metal-organic frameworks MIL-53 decorated with Au@ Pt nanoparticles and enzymes for detection of COVID-19 nucleocapsid protein. Electrochimica Acta, 2021, 387, 138553. [Google Scholar] [CrossRef]
- Malla, P.; et al. Magnetic metal-organic frameworks as sensitive aptasensors for coronavirus spike protein. Analytica Chimica Acta, 2024, 1309, 342671. [Google Scholar] [CrossRef]
- Wang, M.; Wang, H.; Cheng, J. An Enzyme-Encapsulated Metal-Organic Frameworks Nanomesh Biosensor for Salivary Glucose Detection. Advanced Materials Technologies, 2024, 9, 2301678. [Google Scholar] [CrossRef]
- Rabiee, N.; et al. Bioactive hybrid metal-organic framework (MOF)-based nanosensors for optical detection of recombinant SARS-CoV-2 spike antigen. Science of The Total Environment, 2022, 825, 153902. [Google Scholar] [CrossRef]
- Li, X.; et al. Encapsulation of enzyme by metal-organic framework for single-enzymatic biofuel cell-based self-powered biosensor. Nano Energy, 2020, 68, 104308. [Google Scholar] [CrossRef]
- Kumari, D.; Prajapati, M.; Kant, C.R. Highly Efficient Non-Enzymatic Electrochemical Glucose Biosensor Based on Copper Metal Organic Framework Coated on Graphite Sheet. ECS Journal of Solid State Science and Technology, 2024, 13, 047007. [Google Scholar] [CrossRef]
- Su, M.; et al. Multi-walled carbon nanotubes-metal–organic framework nanocomposite based sensor for the monitoring of multiple monoamine neurotransmitters in living cells. Bioelectrochemistry 2024, 108776. [Google Scholar] [CrossRef]
- Ma, F.; et al. Nanocluster/metal-organic framework nanosheet-based confined ECL enhancement biosensor for the extracellular vesicle detection. Analytica Chimica Acta, 2024, 1301, 342488. [Google Scholar] [CrossRef]
- Xu, X.; et al. Cooperative Amplification of Prussian Blue as a Signal Indicator and Functionalized Metal-Organic Framework-Based Electrochemical Biosensor for an Ultrasensitive HE4 Assay. Biosensors and Bioelectronics 2024, 116541. [Google Scholar] [CrossRef] [PubMed]
- Maniya, N.H.; Srivastava, D.N. Fabrication of porous silicon based label-free optical biosensor for heat shock protein 70 detection. Materials Science in Semiconductor Processing, 2020, 115, 105126. [Google Scholar] [CrossRef]
- Sarkar, T.; Mukherjee, N.; Das, J. Glucose oxidase immobilized macro porous silicon based conductive glucose sensor. Applied Physics A, 2022, 128, 336. [Google Scholar] [CrossRef]
- Dervisevic, M.; et al. Electrochemical immunosensor for breast cancer biomarker detection using high-density silicon microneedle array. Biosensors and Bioelectronics, 2021, 192, 113496. [Google Scholar] [CrossRef]
- Naorem, M.; Singh, R.; Paily, R. Detection of hydrogen peroxide using rGO/PPy nanocomposites in silicon dioxide trench embedded field effect transistor. IEEE Sensors Journal, 2021, 21, 22426–22433. [Google Scholar] [CrossRef]
- Vankalkunti, S.; Singh, M. Plasmonic Biosensor for DNA Hybridization Using Integrated Graphene-Porous Silicon Waveguide. IEEE Sensors Journal, 2023, 23, 28797–28804. [Google Scholar] [CrossRef]
- Bahri, M.; et al. Capacitance electrochemical biosensor based on silicon nitride transducer for TNF-α cytokine detection in artificial human saliva: Heart failure (HF). Talanta, 2020, 209, 120501. [Google Scholar] [CrossRef]
- Sivakumar, M.; et al. Design and Fabrication of Biosensor for a Specific Microbe by Silicon-Based Interference Color System. Micromachines, 2024, 15, 741. [Google Scholar] [CrossRef]
- Prathap, P.; Prakruthi, H.; Saara, K. SiO2-Au-GO Based 2D Photonic Crystal Biosensor For Protein Analysis And iImmunological Evaluation. Journal of Optics 2024, 1–8. [Google Scholar] [CrossRef]
- Tabaru, T.E.; Karatutlu, A.; Ortaç, B. Phase-shifted bragg-grating consisting of silicon oxynitride doped silicon and silica alternating layers lab-on-fiber for biosensors with ultrahigh sensitivity and ultralow detection limit. Optics & Laser Technology, 2023, 167, 109693. [Google Scholar]
- Bai, L.; et al. Detection of pesticide residues based on a porous silicon optical biosensor with a quantum dot fluorescence label. IEEE Sensors Journal, 2021, 21, 21441–21449. [Google Scholar] [CrossRef]
- Ahmadi, A.; et al. Electrochemiluminescence paper-based screen-printed electrode for HbA1c detection using two-dimensional zirconium metal-organic framework/Fe3O4 nanosheet composites decorated with Au nanoclusters. Microchimica Acta, 2021, 188, 296. [Google Scholar] [CrossRef]
| Title | Summary | Target Analyte | Material | Technique | Detection Limit (LOD) | Sensitivity | Measurement Method | Key Features | Applications | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| Gold (Au) Electrodes for SARS-CoV-2 Detection | Biosensor using AuNPs capped with antisense ssDNA targeting the viral N-gene. | SARS-CoV-2 RNA | AuNPs, ssDNA | Paper-based electrochemical platform | 6.9 copies/μL | 231 (copies μL–1)−1 | Hand-held reader | Dual-target approach, no amplification needed, rapid detection (< 5 mins) | SARS-CoV-2 detection | [13] |
| Electrochemical Immunosensor for HER 2 Detection | Highly sensitive detection of HER 2 using a NanoDiamond and AuNP nanohybrid platform. | HER 2 | NanoDiamonds, AuNPs | GCE, DPV | 0.29 pg mL−1 | Not specified | Differential pulse voltammetry (DPV) | High specificity, low cross-reactivity, optimal at 35 °C, pH 7.2 | Breast cancer biomarker detection | [14] |
| Gold Nanoparticle-Based Biosensor for Lysozyme Detection | Detection of Lysozyme using gold nanoparticles and decomposition Muller matrix polarimetry. | Lysozyme | Gold nanoparticles, DNA aptamer | Muller matrix polarimetry | 1.24 fM | Not specified | Muller matrix calculation | High specificity, broad dynamic range (0.01 to 500 pM) | Lysozyme detection | [15] |
| Flexible Sensor for Glucose and Lactate Monitoring | Real-time monitoring of glucose and lactate in sweat using a flexible chip with AuNNs. | Glucose, Lactate | AuNNs | Electrochemical deposition, PEGDE | 7 μmol L−1 (glucose), 54 μmol L−1 (lactate) | Not specified | Electrochemical detection | High selectivity, stability, reproducibility | Glucose and lactate monitoring in sweat | [17] |
| Stretchable Gold Fiber-Based Lactate Biosensors | Fabrication of stretchable, strain-insensitive gold fibers for lactate sensing in wearable applications. | Lactate | Gold fibers | Dry-spinning, three-electrode system | Not specified | 19.13 μA/mM cm² (PBS), 14.6 μA/mM cm² (artificial sweat) | Electrochemical detection | High sensitivity, maintains performance under 100% strain | Wearable lactate monitoring | [18] |
| Flexible Enzymatic Glucose Biosensor | High-performance glucose biosensor using dendritic gold nanostructures on carbon cloth. | Glucose | Dendritic gold nanostructures, carbon cloth | Electrochemical deposition, enzyme precipitation coating | 6.7 μM | 72.45 μA mM−1 cm−2 | SEM, CV, chronoamperometry, EIS | High sensitivity, low detection limit, wide linear range, good selectivity, reproducibility, stability | Biomedical and health care | [19] |
| QCM-Biosensor for HBsAg Detection | QCM-biosensor for label-free detection of HBsAg. | HBsAg | Anti-HBsAg antibodies, gold electrode | PEI and thiolated-PEI surface modifications, RSM optimization | 3.14 ng/mL | Not specified | FESEM, AFM, ATR-FTIR, CA measurement | Broad dynamic range, high accuracy, good selectivity, stability, regenerability | Non-invasive monitoring of HBV-biomarker | [16] |
| Isoprocarb Detection Biosensor | Biosensor system based on AChE inhibition by isoprocarb using AuNPs-PANI-GPE. | Isoprocarb | Gold nanoparticles, polyaniline, graphite pencil electrode | Electro-polymerization, electro-deposition | 0.1615 nM | 1.7771 μA/μM.mm² | SEM-EDX, CV | High sensitivity, excellent stability | Real detection of isoprocarb | [21] |
| Electrochemical Sensor for Glucose Detection | Sensor using CNTs grown on Au MEA for glucose detection. | Glucose | CNTs, gold microelectrode arrays | Immobilizing GOx enzyme, poly (p-PDA) matrix | 0.2 μM | 168.03 kΩ−1 M−1 | CV, EIS | Good reproducibility, anti-interference, validated through HPLC | Multiple electroactive biomolecules detection | [20] |
| Folic Acid Biosensor | Biosensor for folic acid detection using DHFR immobilized on c-MWCNT/TiO2NPs modified Au electrode. | Folic Acid | c-MWCNT, TiO2 nanoparticles, gold electrode | TEM, XRD, FTIR, SEM, EIS, CV | 11.48 nM | 0.42 μA/nM/cm² | SEM, EIS, FTIR, CV | Wide linear range, good storage stability | Folic acid quantification in serum samples | [22] |
| Title | Summary | Target Analyte | Material | Technique | Detection Limit (LOD) | Sensitivity | Measurement Method | Key Features | Applications | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| Impact of Ag/AgCl Electrodes on Alcohol Oxidase Stability | Investigates the interaction between Ag/AgCl electrodes and Alcohol Oxidase enzyme stability. | Alcohol Oxidase enzyme | Ag/AgCl electrodes | Screen-printed Ag/AgCl ink, optical absorbance assay | N/A | N/A | Optical absorbance assay | Significant enzyme activity degradation in presence of Ag/AgCl; enzyme activity halftime reduced from ~1 week to 10 hours | Enzymatic biosensors | [51] |
| Lactate Oxidase-Based Biosensor with Silver Amalgam Electrode | Design of a biosensor with LOx-based mini-reactor and silver amalgam electrode. | Lactate | Silver amalgam electrode, lactate oxidase (LOx) | LOx mini-reactor, mesoporous silica powder, amperometric monitoring | N/A | N/A | Amperometric monitoring | High operational stability (93.8% after 350 measurements, 96.9% after 7 months); Easy replacement of mini-reactor | Clinical diagnostics, food and beverage quality control | [52] |
| Dual-Mode Biosensor Using DNA-Templated Silver Nanoclusters | Fluorescent/electrochemical dual-mode biosensor for glucose detection. | Glucose | DNA-templated silver nanoclusters (AgNCs@DNA), glucose oxidase (GOx) | Fluorescence detection, electrochemical pathway | 23 μM (optical), 29 μM (electrochemical) | N/A | Fluorescence and electrochemical | Dual-mode detection, high sensitivity, small size | Glucose monitoring in body fluids | [43] |
| Core-Sheath Ag@Au Nanowires for Stretchable Transparent Electrodes | Development of Ag@Au NWs for improved STEs with high oxidation resistance. | Glucose | Ag@Au nanowires (NWs) | Capillary-force-induced welding | 125 µM | 967 µA·mM−1·cm−2 | Electrochemical | High optical transmittance (78.7%), high tensile strain (240%), excellent robustness | Flexible electronics, glucose biosensors | [44] |
| Green Synthesis of Silver Nanoparticles for Glucose Biosensors | Amperometric glucose biosensor using green synthesized WT-AgNPs. | Glucose | Green synthesized waste tea-based silver nanoparticles (WT-AgNPs), glucose oxidase enzyme | CPE modified with WT-AgNPs, enzyme immobilization | N/A | N/A | Amperometric | Low detection limit, high reproducibility, good selectivity | Glucose detection in food, environmental applications | [45] |
| HNC/AgNPs-PVA for Lactate Detection | Uses hemp-derived nanocellulose (HNC) with silver nanoparticles (AgNPs) for lactate detection. | Lactate | HNC, AgNPs, PVA | Alkali treatment, acid hydrolysis, self-reduction | Not specified | Not specified | Cyclic voltammetry (CV), Amperometry | High current response at 10 wt% HNC/AgNPs-PVA, Linear range: 0–25 mM | Wearable lactate sensor | [50] |
| Ag-HPB Nanocomposites for Biosensing | Hollow Prussian blue with ultra-small AgNPs for glucose and trichlorfon detection. | Glucose, Trichlorfon | AgNPs, Prussian blue (PB) | Coating-etching method | 2.28 pg/mL (trichlorfon) | 24.37 μA mM−1 cm−2 (glucose) | Amperometry | High sensitivity and enzyme loading, Flexible biosensing platform | Trichlorfon monitoring in apples | [49] |
| GPP-Based SPCIDE for Glucose Sensing | Glossy Photo-Paper based sensor for glucose detection. | Glucose | Graphene, Polyaniline (PANI), GS-AgNPs, Glucose oxidase (GOx) | Screen-printing, drop-casting | 198 nM | 291.19 µA mM−1 cm−2 | Amperometry | Low-cost, eco-friendly, Linear range: 198 nM to 30 mM | Point-of-Care glucose testing | [46] |
| Aptamer-Based Electrochemical Sensor for STX | Uses aptamer and K3Fe(CN)6 regulated AgNPs for STX detection. | Saxitoxin (STX) | AgNPs, Aptamer, K3Fe(CN)6 | Aptamer immobilization, K3Fe(CN)6 etching | 1 nM | Not specified | Differential pulse voltammetry (DPV) | High sensitivity, stability, and reproducibility | STX detection in seafood | [48] |
| AgNPs/rGO Nanocomposites for H2O2 and DA Detection | Electrochemical sensors using AgNPs/rGO nanocomposites. | Hydrogen peroxide (H2O2), Dopamine (DA) | AgNPs, Reduced graphene oxide (rGO) | Hydrothermal synthesis, electrodeposition | 3.19 μA (H2O2), 0.18 μM (DA) | 49 μA mM−1cm−2 (H2O2), 7.86 μA mM−1cm−2 (DA) | Amperometry, CV | Simultaneous detection of H2O2 and DA, Good stability | Detection of H2O2 and dopamine | [47] |
| Title | Summary | Target Analyte | Material | Technique | Detection Limit (LOD) | Sensitivity | Measurement Method | Key Features | Applications | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| MOF-based Aptasensor for COVID-19 Detection | Biosensor using MIL-53(Al) with Au@Pt nanoparticles and enzymes for detecting 2019-nCoV-NP. | 2019-nCoV-NP | MIL-53(Al), Au@Pt nanoparticles, HRP, hemin, G-quadruplex DNAzyme | Thiol-modified aptamers, Au@Pt/MIL-53(Al), HRP, hemin/G-quadruplex DNAzyme | 8.33 pg/mL | Not specified | Aptamer-protein-nanoprobe sandwich electrochemical detection | Wide linear range (0.025 to 50 ng/mL), high sensitivity, selectivity, reliability | Early COVID-19 diagnosis | [53] |
| MOF-5/CoNi2S4 Nanobiosensor for SARS-CoV-2 Detection | Enhanced sensitivity for detecting SARS-CoV-2 spike antigen using MOF-5/CoNi2S4 with porphyrin. | SARS-CoV-2 spike antigen | MOF-5, CoNi2S4, H2TMP | Surface roughness measurement, cell viability assay | 5 nM | Not specified | Atomic force microscopy, MTT assay | High biocompatibility, low cytotoxicity, tunability | SARS-CoV-2 detection | [56] |
| ZIF-8 Encapsulated Laccase Electrode for BPA Detection | Encapsulation of laccase in ZIF-8 combined with BC and c-MWCNTs for detecting BPA. | Bisphenol A (BPA) | ZIF-8, BC, c-MWCNTs, laccase | Enzymatic biofuel cell | 1.95 x 10⁻³ mM | Not specified | Biofuel cell-driven sensing platform | High flexibility, excellent mechanical properties, conductivity | BPA detection | [57] |
| ECL Biosensor for HbA1c Detection | Paper-based ECL biosensor using nanocomposite tracing tag for HbA1c detection. | HbA1c | Zr-MOF, Fe3O4, TMC, AuNCs, APBA-functionalized GO | Electrochemiluminescence, cyclic voltammetry | 0.07% | Not specified | ECL and cyclic voltammetry measurements | Wide response range (2% to 18%), high sensitivity | HbA1c detection | [72] |
| Cu MOF-Based Glucose Biosensor | Non-enzymatic glucose sensor using Cu MOF for electrochemical detection. | Glucose | Cu MOF, BTC, Copper nitrate trihydrate | Electrochemical detection, cyclic voltammetry | 0.019 mM | 229.4 μAmM⁻¹ cm⁻² | Cyclic voltammetry, chronoamperometry | Exceptional stability, short response time, good repeatability | Glucose detection | [58] |
| Electrochemical Immunosensor for HE4 | A sandwich-type electrochemical immunosensor for HE4 detection using Prussian blue (PB) and functionalized MOF nanocomposites. | HE4 | PB, TiMOF-KB@AuNPs | Electrochemical immunosensor | 0.02 ng/mL | N/A | Electrochemical | PB as signal indicator, TiMOF-KB@AuNPs for signal amplification | Clinical ovarian cancer diagnosis | [61] |
| Non-Invasive Glucose Detection | An enzymatic biosensing platform using MOF nanomesh for sensitive and stable glucose detection. | Glucose | MOF nanomesh, enzyme encapsulation | Enzymatic biosensor | 16.57 µm | 86.86 µA mm−1 cm−2 | Electrochemical | High sensitivity and stability | Home-based glucose testing | [55] |
| ECL Biosensor for GC Detection | An electrochemiluminescence (ECL) biosensor for detecting microRNA in GC extracellular vesicles. | microRNA (miRNA-421) | Cu NCs, Zn MOF nanosheet, Au NPs/MXene, phospholipid layer | ECL biosensor | 0.5 fM | N/A | Electrochemiluminescence | Cu NCs/Zn MOF nanosheet for high quantum yield and stability | GC peritoneal metastasis diagnosis | [60] |
| MNT Detection with Electrochemical Sensor | A platform using MWCNTs and UIO-66-NH2 nanocomposite for detecting multiple monoamine neurotransmitters (MNTs). | Dopamine (DA), Adrenaline (Adr), Norepinephrine (NE), 5-Hydroxytryptamine (5-HT) | MWCNTs, UIO-66-NH2 | Electrochemical sensor | Low detection limit for DA, Adr, NE, 5-HT | N/A | Electrochemical | Synergistic effect between MWCNTs and UIO-66-NH2 | Clinical diagnosis of MNT-related disorders | [59] |
| MMOF-Based Electrochemical Aptasensor for SARS-CoV-2 | An MMOF-based aptasensor for detecting SARS-CoV-2 spike proteins using electrochemical methods. | SARS-CoV-2 spike proteins | MMOF, aptamer-biotin, streptavidin-HRP | Electrochemical aptasensor | 6 pM (voltammetry), 5.12 pM (impedance spectroscopy) | N/A | Electrochemical | MMOF for easy washing and deposition, high sensitivity | Point-of-care testing for SARS-CoV-2 | [54] |
| Title | Summary | Target Analyte | Material | Technique | Detection Limit (LOD) | Sensitivity | Measurement Method | Key Features | Applications | Ref | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Porous Silicon Optical Biosensor | High sensitivity for detecting HSP70 | Heat Shock Protein 70 (HSP70) | Porous Silicon (PSi) | Electrochemical etching, thermal oxidation, antibody functionalization with APTES and glutaraldehyde | 1290 ± 160 ng/mL | N/A | Fiber Optic Spectrometer, SEM, FTIR, Contact Angle Measurement | High selectivity, -10 dB wide bandwidth, porosity: 68% ± 3%, thickness: 2400 ± 30 nm | Label-free optical biosensing | [62] | ||||||||||
| Capacitance Electrochemical Biosensor for TNF-α Detection | High sensitivity and selectivity for TNF-α cytokines | Tumor Necrosis Factor Alpha (TNF-α) | Silicon Nitride (Si3N4/SiO2/Si[P]/Al) | Micro-contact printing, fluorescence microscopy, contact angle measurement | 0.38 pg/mL (PBS), 1 pg/mL (AS) | 4 mV·pM−1 (PBS), 4.4 mV·pM−1 (AS) | Mott-Schottky analysis, Fluorescence Microscopy, Contact Angle Measurement | High linearity, selectivity against Cortisol and Interleukin-10 | Early detection of inflammatory responses | [67] | ||||||||||
| RNA Aptamer-Based Color Sensor for Pathogen Detection | Detection of specific bacteria with visual color changes | Sphingobium yanoikuyae | Silicon | Layer-by-layer deposition, RNA aptamers | 2 × 106 CFU/mL | N/A | Visual color change, UV-Vis reflectance spectrophotometry, AFM, XPS | Visual detection, iridescent color changes, UV-Vis confirmation | Pathogen detection in non-beverage alcohols | [68] | ||||||||||
| Photonic Crystal-Based Biosensor for Protein Analysis | Responsive biosensor for protein analysis and immunological evaluation | Proteins, antibodies, antigens | SiO2, Gold (Au), Graphene Oxide (GO) | Gold plating, graphene oxide coating, FDTD method | N/A | 100 nm/RIU | FDTD method | High Quality factor: 597, increased plasmonic attraction | Clinical decision systems | [69] | ||||||||||
| Fabry-Perot Optical Fiber Sensor for Glucose Detection | Detecting ultralow glucose concentrations with high sensitivity | Glucose | Siliconoxynitrite (SiON) doped silicon (Si) | Plasma enhanced chemical vapor deposition (PECVD), Phase Shifted Bragg-Grating (PSBG) | 1.98 × 10−6 RIU | 14904 nm/RIU | Lab-on-Fiber (LOF) | Temperature insensitive (25°C-45°C), high sensitivity | In-vivo biosensing applications | [70] | ||||||||||
| 3-D Integrated Graphene-Porous Silicon Plasmonic Waveguide for DNA Hybridization | Studies a 3-D integrated graphene-p-Si plasmonic waveguide-based nanostructure for DNA hybridization. | DNA | Graphene, porous silicon, silicon dioxide | Full-vectorial finite element method, Maxwell Garnett model | N/A | 318.5 nm/RIU | COMSOL multiphysics software | High sensitivity, tunability, reduced ohmic losses | Lab-on-a-chip biological applications | [66] | ||||||||||
| High-Performance Hydrogen Peroxide Sensing FET | Demonstrates a high-performance H2O2 sensing FET using rGO/PPy nanocomposites. | Hydrogen peroxide (H2O2) | Reduced graphene oxide, polypyrrole, SiO2 | Lithography, etching techniques | 10:00 PM | N/A | Electrical signal measurement | Fast response, high stability, high selectivity | Liquid sensing applications | [65] | ||||||||||
| Conductive Glucose Sensor Using Macro Porous Silicon | Studies glucose sensor using glucose oxidase immobilized on macro porous silicon. | Glucose | Glucose oxidase, macro porous silicon, silver electrodes | Electrochemical etching, physisorption | N/A | N/A | Current–voltage characteristics | Good response, high sensitivity, excellent repetitive behavior | Glucose sensing | [63] | ||||||||||
| Gold-Coated Silicon Microneedle Arrays for Breast Cancer Biomarker Detection | Uses Au-Si-MNA for biomarker extraction and electrochemical transduction for breast cancer diagnosis. | Epidermal growth factor receptor 2 (ErbB2) | Gold, silicon, artificial interstitial fluid | Gold coating, electrochemical transducer | 4.8 ng/mL | N/A | Electrochemical measurement | High selectivity, simultaneous extraction and quantification | Wearable point-of-care devices | [64] | ||||||||||
| Fluorescence Image-Based Pesticide Detection Using PSi Bragg Mirrors | Proposes a grayscale variation of fluorescence image using PSi Bragg mirrors for pesticide detection. | Acetamiprid (pesticide) | CdSe/ZnS quantum dots, PSi Bragg mirrors, aptamers | Digital imaging method | 2.8 nM | N/A | Fluorescence intensity measurement | Low-cost, rapid, simple detection | Rapid and simple pesticide detection | [71] | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





