Submitted:
30 May 2025
Posted:
30 May 2025
You are already at the latest version
Abstract
Keywords:
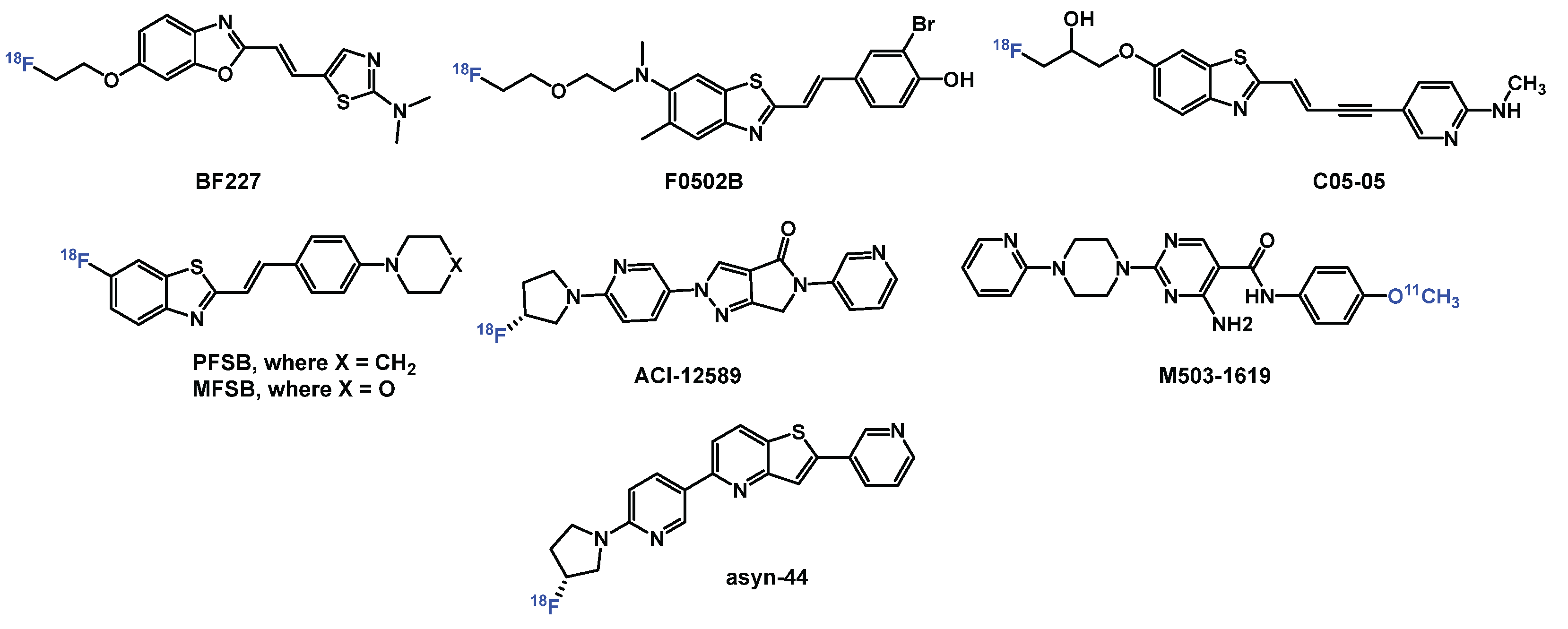
1. Introduction
2. Materials and Methods
General
Postmortem Tissues
In Vitro Competition (Ki) Assays
Two Point Screening Assays
Metabolic Stability in Human Liver Microsomes
In Vitro Transport Studies Using MDR1-Transfected MDCK Cells
- P_app is the apparent permeability,
- V_r is the volume of the receiver chamber,
- C₀ is the initial concentration in the donor chamber,
- S is the surface area of the cell monolayer (0.7 cm² for a 24-well insert),
- dC/dt is the rate of appearance of the compound in the receiver chamber.
3. Results and discussion
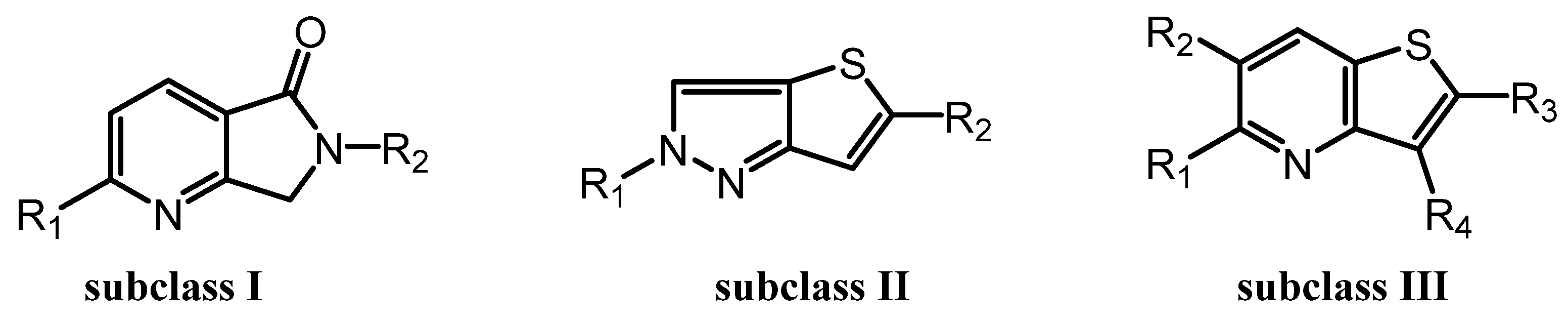
3.1. Chemical Synthesis
3.2. Binding Assays and SAR Studies
3.3. Metabolic Stability, Permeability, and Efflux Characteristics of selected LMD Compounds

| Compound ID | % remaining at 5 mins | % remaining at 30 mins | t1/2 (min) | Clint (µg/min/mg) | Clearance category2 | |
| LMD-032 | 53.3 | 5.02 | 7.05 | 197 | High | |
| LMD-046 | 3.43 | NC1 | NC | NC | NC | |
| LMD-051 | 75.8 | 30.3 | 17.0 | 81.5 | High | |
| LMD-052 | 39.7 | 5.61 | 7.60 | 182 | High | |
| LMD-044 | 45.7 | 3.71 | 6.37 | 218 | High | |
| LMD-045 | 65.3 | 13.5 | 10.5 | 132 | High | |
| LMD-006 | 39.6 | 4.32 | 6.81 | 203 | High | |
| LMD-022 | 92.4 | 75.4 | 75.3 | 18.4 | Moderate | |
| Verapamil | 43.6 | 4.64 | 6.89 | 201 | High |
| Compound ID |
A→B (×10⁻⁶ cm/s) |
B→A (×10⁻⁶ cm/s) |
Efflux Ratio (B→A / A→B) |
Permeability Category |
Efflux Category |
| LMD-022 | 40.6 | 37.6 | 0.93 | High | Not a substrate |
| Digoxin | 1.65 | 24 | 14.5 | Low | Substrate of efflux |
| Propranolol | 9.28 | 8.03 | 0.9 | Medium | Not a substrate |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PET | Positron Emission Tomography |
| PD | Parkinson’s disease |
| DLB | dementia with Lewy bodies |
| MSA | multiple system atrophy |
References
- Calabresi, P.; Mechelli, A.; Natale, G.; Volpicelli-Daley, L.; Di Lazzaro, G.; Ghiglieri, V., Alpha-synuclein in Parkinson’s disease and other synucleinopathies: from overt neurodegeneration back to early synaptic dysfunction. Cell Death & Disease 2023, 14, (3), 176.
- Koga, S.; Sekiya, H.; Kondru, N.; Ross, O. A.; Dickson, D. W., Neuropathology and molecular diagnosis of Synucleinopathies. Molecular Neurodegeneration 2021, 16, (1), 83.
- Kuo, G.; Kumbhar, R.; Blair, W.; Dawson, V. L.; Dawson, T. M.; Mao, X., Emerging targets of α-synuclein spreading in α-synucleinopathies: a review of mechanistic pathways and interventions. Molecular Neurodegeneration 2025, 20, (1), 10.
- Tarutani, A.; Hasegawa, M., Chapter Eighteen - Prion-like propagation of α-synuclein in neurodegenerative diseases. In Progress in Molecular Biology and Translational Science, Teplow, D. B., Ed. Academic Press: 2019; Vol. 168, pp 323-348.
- Radad, K.; Moldzio, R.; Krewenka, C.; Kranner, B.; Rausch, W.-D., Pathophysiology of non-motor signs in Parkinson’s disease: some recent updating with brief presentation. Exploration of Neuroprotective Therapy 2023, 3, (1), 24-46.
- Kotzbauer, P. T.; Tu, Z.; Mach, R. H., Current status of the development of PET radiotracers for imaging alpha synuclein aggregates in Lewy bodies and Lewy neurites. Clin Transl Imaging 2017, 5, (1), 3-14.
- Korat, Š.; Bidesi, N. S. R.; Bonanno, F.; Di Nanni, A.; Hoàng, A. N. N.; Herfert, K.; Maurer, A.; Battisti, U. M.; Bowden, G. D.; Thonon, D.; Vugts, D.; Windhorst, A. D.; Herth, M. M., Alpha-Synuclein PET Tracer Development—An Overview about Current Efforts. Pharmaceuticals 2021, 14, (9), 847.
- Alam, M. M.; Lee, S. H.; Wasim, S.; Lee, S.-Y., PET tracer development for imaging α-synucleinopathies. Archives of Pharmacal Research 2025, 48, (4), 333-350.
- Mekala, S.; Wu, Y.; Li, Y.-M., Strategies of positron emission tomography (PET) tracer development for imaging of tau and α-synuclein in neurodegenerative disorders. RSC Medicinal Chemistry 2025, 16, (2), 605-639.
- Park, H.; Kam, T.-I.; Dawson, V. L.; Dawson, T. M., α-Synuclein pathology as a target in neurodegenerative diseases. Nature Reviews Neurology 2025, 21, (1), 32-47.
- Fernandes Gomes, B.; Farris, C. M.; Ma, Y.; Concha-Marambio, L.; Lebovitz, R.; Nellgård, B.; Dalla, K.; Constantinescu, J.; Constantinescu, R.; Gobom, J.; Andreasson, U.; Zetterberg, H.; Blennow, K., α-Synuclein seed amplification assay as a diagnostic tool for parkinsonian disorders. Parkinsonism Relat Disord 2023, 117, 105807.
- Kim, H. Y.; Chia, W. K.; Hsieh, C.-J.; Saturnino Guarino, D.; Graham, T. J. A.; Lengyel-Zhand, Z.; Schneider, M.; Tomita, C.; Lougee, M. G.; Kim, H. J.; Pagar, V. V.; Lee, H.; Hou, C.; Garcia, B. A.; Petersson, E. J.; O’Shea, J.; Kotzbauer, P. T.; Mathis, C. A.; Lee, V. M. Y.; Luk, K. C.; Mach, R. H., A Novel Brain PET Radiotracer for Imaging Alpha Synuclein Fibrils in Multiple System Atrophy. Journal of Medicinal Chemistry 2023, 66, (17), 12185-12202.
- Di Nanni, A.; Saw, R. S.; Battisti, U. M.; Bowden, G. D.; Boeckermann, A.; Bjerregaard-Andersen, K.; Pichler, B. J.; Herfert, K.; Herth, M. M.; Maurer, A., A Fluorescent Probe as a Lead Compound for a Selective α-Synuclein PET Tracer: Development of a Library of 2-Styrylbenzothiazoles and Biological Evaluation of [18F]PFSB and [18F]MFSB. ACS Omega 2023, 8, (34), 31450-31467.
- Smith, R.; Capotosti, F.; Schain, M.; Ohlsson, T.; Vokali, E.; Molette, J.; Touilloux, T.; Hliva, V.; Dimitrakopoulos, I. K.; Puschmann, A.; Jögi, J.; Svenningsson, P.; Andréasson, M.; Sandiego, C.; Russell, D. S.; Miranda-Azpiazu, P.; Halldin, C.; Stomrud, E.; Hall, S.; Bratteby, K.; Tampio L’Estrade, E.; Luthi-Carter, R.; Pfeifer, A.; Kosco-Vilbois, M.; Streffer, J.; Hansson, O., The α-synuclein PET tracer [18F] ACI-12589 distinguishes multiple system atrophy from other neurodegenerative diseases. Nature Communications 2023, 14, (1), 6750.
- Kuebler, L.; Buss, S.; Leonov, A.; Ryazanov, S.; Schmidt, F.; Maurer, A.; Weckbecker, D.; Landau, A. M.; Lillethorup, T. P.; Bleher, D.; Saw, R. S.; Pichler, B. J.; Griesinger, C.; Giese, A.; Herfert, K., [11C]MODAG-001—towards a PET tracer targeting α-synuclein aggregates. European Journal of Nuclear Medicine and Molecular Imaging 2021, 48, (6), 1759-1772.
- Pees, A.; Tong, J.; Birudaraju, S.; Munot, Y. S.; Liang, S. H.; Saturnino Guarino, D.; Mach, R. H.; Mathis, C. A.; Vasdev, N., Development of Pyridothiophene Compounds for PET Imaging of α-Synuclein. Chemistry 2024, 30, (23), e202303921.
- Xiang, J.; Zhang, Z.; Wu, S.; Ye, K., Positron emission tomography tracers for synucleinopathies. Molecular Neurodegeneration 2025, 20, (1), 1.
- Xiang, J.; Tao, Y.; Xia, Y.; Luo, S.; Zhao, Q.; Li, B.; Zhang, X.; Sun, Y.; Xia, W.; Zhang, M.; Kang, S. S.; Ahn, E.-H.; Liu, X.; Xie, F.; Guan, Y.; Yang, J. J.; Bu, L.; Wu, S.; Wang, X.; Cao, X.; Liu, C.; Zhang, Z.; Li, D.; Ye, K., Development of an α-synuclein positron emission tomography tracer for imaging synucleinopathies. Cell 2023, 186, (16), 3350-3367.e19.
- Endo, H.; Ono, M.; Takado, Y.; Matsuoka, K.; Takahashi, M.; Tagai, K.; Kataoka, Y.; Hirata, K.; Takahata, K.; Seki, C.; Kokubo, N.; Fujinaga, M.; Mori, W.; Nagai, Y.; Mimura, K.; Kumata, K.; Kikuchi, T.; Shimozawa, A.; Mishra, S. K.; Yamaguchi, Y.; Shimizu, H.; Kakita, A.; Takuwa, H.; Shinotoh, H.; Shimada, H.; Kimura, Y.; Ichise, M.; Suhara, T.; Minamimoto, T.; Sahara, N.; Kawamura, K.; Zhang, M.-R.; Hasegawa, M.; Higuchi, M., Imaging α-synuclein pathologies in animal models and patients with Parkinson’s and related diseases. Neuron 2024, 112, (15), 2540-2557.e8.
- Tian, G.-L.; Hsieh, C.-J.; Guarino, D. S.; Graham, T. J. A.; Lengyel-Zhand, Z.; Schmitz, A.; Chia, W. K.; Young, A. J.; Crosby, J.-G.; Plakas, K.; Huang, T.; Jiang, H.; Yu, Y.; Hou, C.; Lee, H.; Petersson, E. J.; Giannakoulias, S.; O’Shea, J.; Kotzbauer, P.; Tu, Z.; Mathis, C. A.; Mach, R. H., The development of a PET radiotracer for imaging alpha synuclein aggregates in Parkinson’s disease. RSC Medicinal Chemistry 2025.
- Graham, T. J. A.; Lindberg, A.; Tong, J.; Stehouwer, J. S.; Vasdev, N.; Mach, R. H.; Mathis, C. A., In Silico Discovery and Subsequent Characterization of Potent 4R-Tauopathy Positron Emission Tomography Radiotracers. J Med Chem 2023, 66, (15), 10628-10638.
- Come, J. H.; Collier, P. N.; Henderson, J. A.; Pierce, A. C.; Davies, R. J.; Le Tiran, A.; O’Dowd, H.; Bandarage, U. K.; Cao, J.; Deininger, D.; Grey, R.; Krueger, E. B.; Lowe, D. B.; Liang, J.; Liao, Y.; Messersmith, D.; Nanthakumar, S.; Sizensky, E.; Wang, J.; Xu, J.; Chin, E. Y.; Damagnez, V.; Doran, J. D.; Dworakowski, W.; Griffith, J. P.; Jacobs, M. D.; Khare-Pandit, S.; Mahajan, S.; Moody, C. S.; Aronov, A. M., Design and Synthesis of a Novel Series of Orally Bioavailable, CNS-Penetrant, Isoform Selective Phosphoinositide 3-Kinase γ (PI3Kγ) Inhibitors with Potential for the Treatment of Multiple Sclerosis (MS). Journal of Medicinal Chemistry 2018, 61, (12), 5245-5256.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





