Submitted:
30 May 2025
Posted:
30 May 2025
Read the latest preprint version here
Abstract
Keywords:
Introduction
Food Polyphenols Rescue the Altered Biophysics and Biochemical Homeostasis
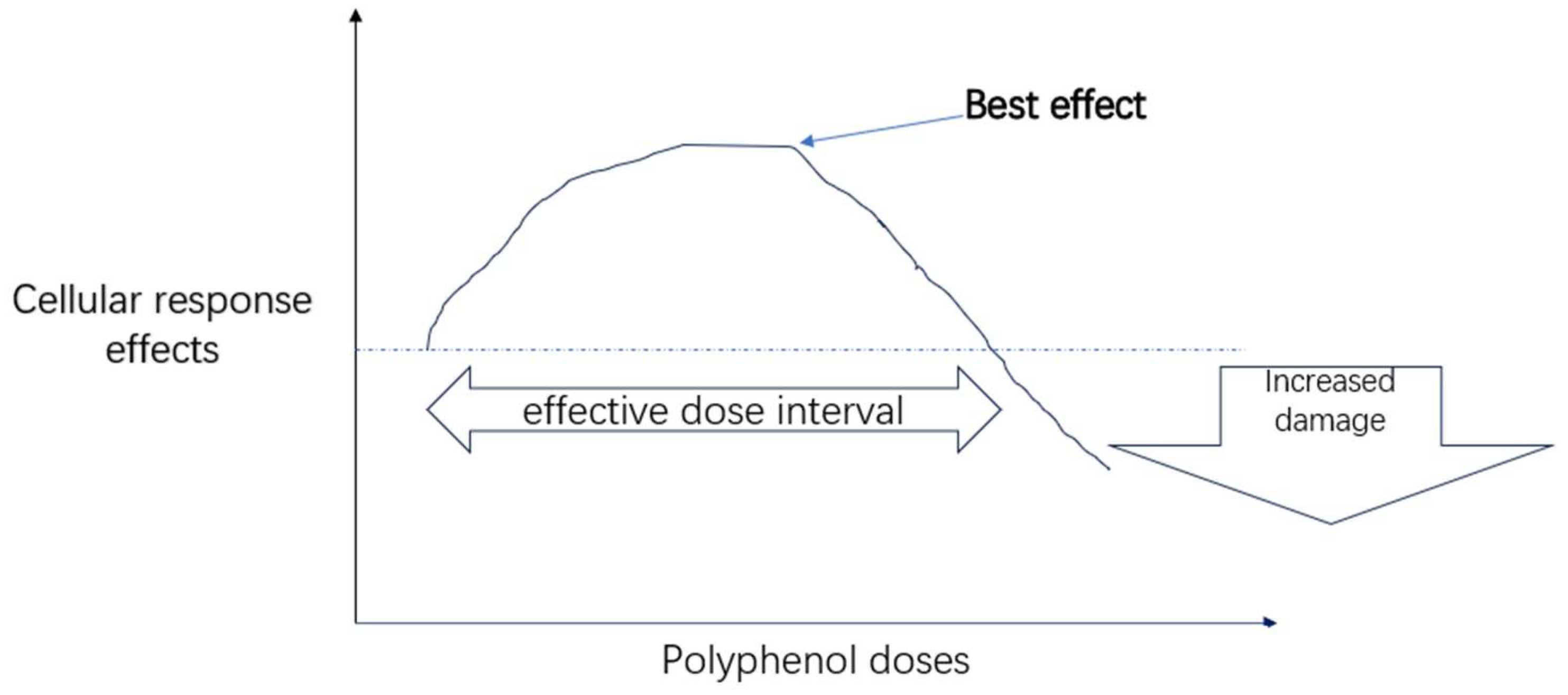
2.1. Polyphenols Hormesis Effects
2.2 Polyphenols Regulate Redox Homeostasis Status
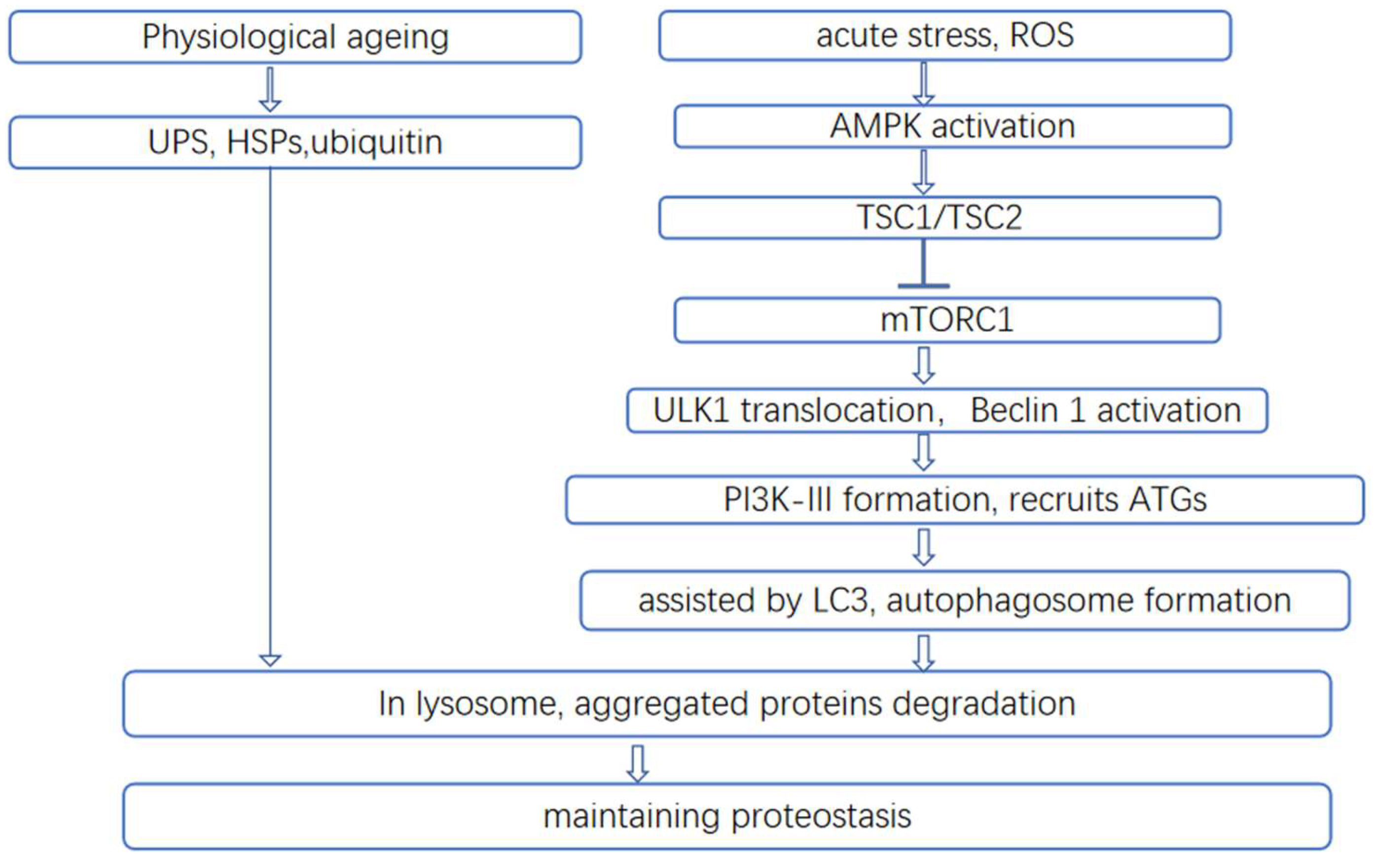
2.3. Polyphenols Modulate Protein Homeostasis
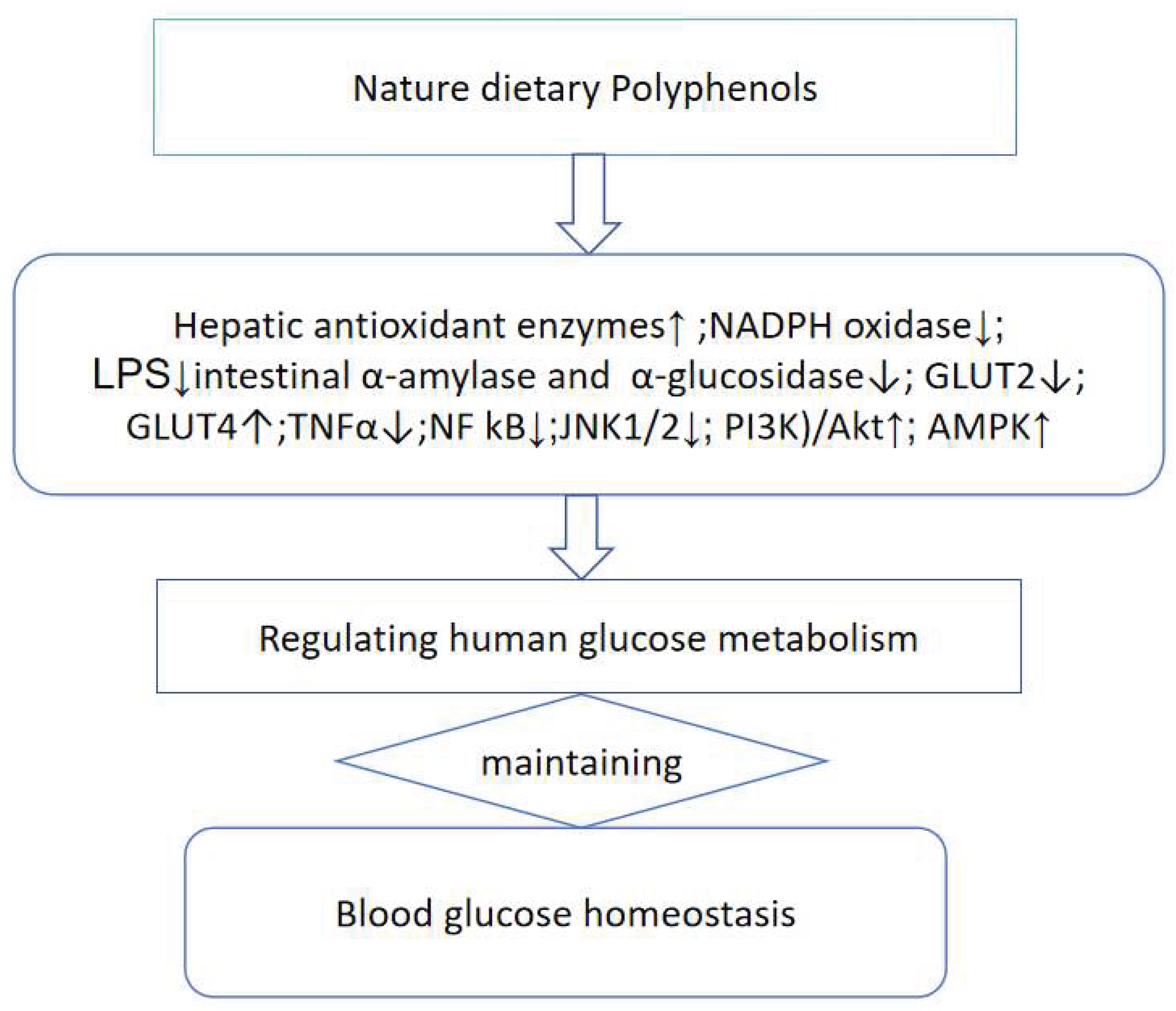
2.4. Polyphenols Regulate Glucose Metabolism Homeostasis
3. Polyphenols Regulate Gene Expression
3.1. Food Polyphenols Activate Antioxidant Protection Vitagene Signaling Pathways.
3.2. Food Polyphenols Modulate Genomic Stability
3.3. Food Polyphenols Improve Epigenetic Function
4. Perspectives
5. Conclusions
References
- Sara Zumerle , Miles Sarill, Miriam Saponaro, Manuel Colucci, Liliana Contu, Edoardo Lazzarini, Roberta Sartori1, Camilla Pezzini, Anna Rinaldi, Anna Scanu, Jacopo Sgrignani, Patrizia Locatelli, Marianna Sabbadin, Aurora Valdata, Daniela Brina, Isabella Giacomini, Beatrice Rizzo, Alessandra Pierantoni, Saman Sharifi, Silvia Bressan, Claudia Altomare, Yulia Goshovska, Chiara Giraudo, Roberto Luisetto, Luca Iaccarino, Cristina Torcasio, Simone Mosole, Emiliano Pasquini, Andrea Rinaldi, Laura Pellegrini, Gregorio Peron, Matteo Fassan, Stefano Masiero, Andrea Maria Giori, Stefano Dall’Acqua, Johan Auwerx, Pietro Cippà, Andrea Cavalli, Marco Bolis, Marco Sandri, Lucio Barile, Monica Montopoli & Andrea Alimonti. Targeting senescence induced by age or chemotherapy with a polyphenol rich natural extract improves longevity and healthspan in mice. Nature Aging 2024, 4(9):1231-1248. [CrossRef]
- Naheed Akhtar, Amna Jabbar Siddiqui, Muhammad Ramzan, Jalal Uddin , Mufarreh Asmari.
- Hesham R. El-Seedi and Syed Ghulam Musharraf. Investigation of Pharmacologically Important Polyphenolic Secondary Metabolites in Plant-based Food Samples Using HPLC-DAD. Plants 2024, 13: 1311. [CrossRef]
- Barry Halliwell. Understanding mechanisms of antioxidant action in health and disease. Nature Reviews Molecular Cell Biology 2024(1), 25: 13–33. [CrossRef]
- Benjamin Fair, Carlos F Buen Abad Najar, Junxing Zhao, Stephanie Lozano, Austin Reilly, Gabriela Mossian, Jonathan P Staley, Jingxin Wang, Yang I Li. Global impact of unproductive splicing on human gene expression. Nature genetics 2024(9), 56: 1851-1861. [CrossRef]
- Coral Del Val, Elisa Díaz de la Guardia-Bolívar, Igor Zwir, Pashupati P Mishra, Alberto Mesa, Ramiro Salas, Guillermo F Poblete, Gabriel de Erausquin, Emma Raitoharju, Mika Kähönen, Olli Raitakari, Liisa Keltikangas-Järvinen, Terho Lehtimäki, Claude Robert Cloninger. Gene expression networks regulated by human personality. Molecular psychiatry 2024(7), 129: 2241-2260. [CrossRef]
- Akira Murakami.Impact of hormesis to deepen our understanding of the mechanisms underlying the bioactivities of polyphenols. Current Opinion in Biotechnology 2024, 86, 103074. [CrossRef]
- Akira Murakami. Novel mechanisms underlying bioactivities of polyphenols via hormesis.Current Opinion in Toxicology 2022, 30: 100337. [CrossRef]
- N. Sulaimani, M.J. Houghton, M.P. Bonham1 and G. Williamson. Effect of dietary polyphenols on chronobiology in mammalian cells in vitro. Proceedings of the Nutrition Society 2024, 83 (OCE1), E134. [CrossRef]
- Hui-Min Liu, Ming-Yan Cheng, Meng-Han Xun, Zhi-Wei Zhao, Yun Zhang, Wei Tang, Jun Cheng, Jia Ni, Wei Wang. Possible Mechanisms of Oxidative Stress-Induced Skin Cellular Senescence, Inflammation, and Cancer and the Therapeutic Potential of Plant Polyphenols. International Journal of Molecular Sciences 2023,24: 3755. [CrossRef]
- Tong Zheng, Donna F. Bielinski, Derek R. Fisher, Jianyi Zhang and Barbara Shukitt-Hale.
- Protective Effects of a Polyphenol-Rich Blueberry Extract on Adult Human Neural Progenitor Cells. Molecules 2022, 27: 6152.
- Ronny Lesmana, Cynthia Parameswari, Gabriela Fernanda Mandagi, Julianne Fay Wahyudi, Noah Jefferson Permana, Putri Teesa Radhiyanti, Julia Windi Gunadi. The Role of Exercise-Induced Reactive Oxygen Species (ROS) Hormesis in Aging: Friend or Foe. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology 2022(6),56: 692-706.
- Abbasi N., Akhavan M.M., Rohbar-Roshandel N., Shafiei M. The effects of low and high concentrations of luteolin on cultured human endothelial cells under normal and glucotoxic conditions: Involvement of integrin-linked kinase and cyclooxygenase-2. Phytotherapy research 2014, 28: 1301–1307. [CrossRef]
- Abbasi N., Khosravi A., Aidy A., Shafiei M. Biphasic response to luteolin in G-osteobalst-like cells under high glucose-induced oxidative stress. Iranian Journal of Medical Sciences 2016, 41: 118–125.
- Yasser Fakri Mustafa. Harmful Free Radicals in Aging: A Narrative Review of Their Detrimental Effects on Health. Indian journal of clinical biochemistry : IJCB. 2024(2),39:154-167. [CrossRef]
- Silvia Dossena and Angela Marin. Oxidative Stress and Antioxidants in Aging. Antioxidants 2024(11),13: 1288. [CrossRef]
- Luo Jing, Si Hongwei, Jia Zhenquan and LiuDongmin. Dietary Anti-Aging Polyphenols and Potential Mechanisms. Antioxidants. 2021, 10: 283. [CrossRef]
- Joy I Obeme-Nmom, Raliat O Abioye, Samanta S Reyes Flores, Chibuike C Udenigwe. Regulation of redox enzymes by nutraceuticals: a review of the roles of antioxidant polyphenols and peptides. Food & function. 2024(22), 15:10956-10980. [CrossRef]
- Srinivasaragavan Divyajanani, Kannan Harithpriya, Kumar Ganesan and Kunka Mohanram Ramkumar. Dietary Polyphenols Remodel DNA Methylation Patterns of NRF2 in Chronic Disease 2023,15: 3347. [CrossRef]
- Liang Zhang, Qingzheng Kang, Mengxiao Kang, Suwei Jiang, Feng Yang, Jun Gong, Gaozhi Ou, Song Wang. Regulation of main ncRNAs by Polyphenols: A Novel Anticancer Therapeutic Approach. Phytomedicine 2023,120: 155072. [CrossRef]
- Arthur J. Chu. Quarter-Century Explorations of Bioactive Polyphenols: Diverse Health Benefits. Frontiers in bioscience (Landmark edition) 2022,27(4): 134. [CrossRef]
- Vichitra Chandrasekaran, Tousif Ahmed Hediyal, Nikhilesh Anand, Pavan Heggadadevanakote Kendaganna, Vasavi Rakesh Gorantla, Arehally M Mahalakshmi, Ruchika Kaul Ghanekar, Jian Yang, Meena Kishore Sakharkar, Saravana Babu Chidambaram. Polyphenols, Autophagy and Neurodegenerative Diseases: A Review. Biomolecules 2023(8):1196. [CrossRef]
- Arunkumar Subramanian, T. Tamilanban, Vetriselvan Subramaniyan, Mahendran Sekar, Vipin Kumar, Ashok Kumar Janakiraman, Saminathan Kayarohanam. Establishing network pharmacology between natural polyphenols and Alzheimer’s disease using bioinformatic tools – An advancement in Alzheimer’s research. Toxicology Reports 2024,13: 101715. [CrossRef]
- Tharsius Raja William Raja, Veeramuthu Duraipandiyan, Savarimuthu Ignacimuthu, Udaiyappan Janakiraman, Soosaimanickam Maria Packiam. Role of Polyphenols in alleviating Alzheimer's disease: A Review. Current medicinal chemistry 2023,30:4032-4047. [CrossRef]
- Liu Chaoying, Wang Ye, Li Dahong, Li Tongbiao, Wang Mingcheng, Zhou Zhiwen, Jin Qianyi, Li Enzhong. Effects of resveratrol on hydrogen peroxide-pyroptosis, apoptosis, and autophagy. Journal of Biotech Research 2024,16: 334-344.
- Eun-Young Choi, Eun-Ji Han, Su-Ji Jeon, Sang-Woo Lee, Jun-Mo Moon, Soo-Hyun Jung, Young-Seok Park, Byung-Kwon Park, Byeong-Soo Kim, Sang-Ki Kim, Ji-Youn Jung. Kaempferol Inhibits Cervical Cancer Cells by Inducing Apoptosis and Autophagy via Inactivation of the PI3K/AKT/mTOR Signaling Pathway. Anticancer research 2024(7),44: 2961-2972.
- Jie Shao, Lin Yang, Zhichao Jin, Yanmin Bao, Jiawen He, Q. H. Le, Ponnurengam Malliappan Sivakumar, Mao Wang, Ruiping Wang. Antiproliferative Effect of Gallic Acid is Mediated via Mitochondrial- or ER-Stress-Induced Apoptosis and Canonical Autophagy in HT-29 Cells. Journal of Chemistry 2024(1): 7139556.
- Xiaoqin Li, Zhaojun Meng, Xiaolan He, Jiawei Wang, Lin Yang, Zongping Fang. Resveratrol attenuates neuroinflammation and alleviates emotional dysfunction in mice with sepsis-associated encephalopathy through promoting chaperone-mediated autophagy (CMA)]. Chinese journal of cellular and molecular immunology 2024(6),40: 481-487.
- Alaee Sanaz, Khodabandeh Zahra, Dara Mahintaj, Hosseini Elham, Sharma Mona. Curcumin mitigates acrylamide-induced ovarian antioxidant disruption and apoptosis in female Balb/c mice: A comprehensive study on gene and protein expressions. Food Science & Nutrition 2024(6),12: 2048-7177. [CrossRef]
- Joseph Ndacyayisenga, Festus M. Tolo, Fred Wamunyokoli, Esther N. Maina. Effects of tea catechin extracts from BB35 and purple (TRFK 306) tea clones on the gene expression of Egfr, App, Bcl2, Dnmt, Casp3, Hif1a, Gadd45b and Psmb5 genes involved in triple negative breast cancer diseases: In silico and in vitro study. Informatics in Medicine Unlocked. 2024,46: 101469. [CrossRef]
- Saleha Alqarni, Mashael Alsebai, Batool Adal Alsaigh, Abeer Sayer Alrashedy, Israa Talal Albahrani, Albandri Yousef Aljohar, Amjad Obaid Alazmi. Do polyphenols affect body fat and/or glucose metabolism? Frontiers in Nutrition. 2024, 11: 1376508. [CrossRef]
- Oleg Frumuzachi, Laura Ioana Gavrilaș, Dan Cristian Vodnar, Sascha Rohn, Andrei Mocan. Systemic Health Effects of Oleuropein and Hydroxytyrosol Supplementation: A Systematic Review of Randomized Controlled Trials. Antioxidants (Basel, Switzerland) 2024(9),13: 1040. [CrossRef]
- Harimalala Ranaivo, Zhengxiao Zhang, Maud Alligier, Laurie Van Den Berghe, Monique Sothier, Stéphanie Lambert-Porcheron, Nathalie Feugier, Charlotte Cuerq, Christelle Machon, Audrey Neyrinck, Benjamin Seethaler, Julie Rodriguez, Martin Roumain, Gullio Muccioli, Véronique Maquet, Martine Laville, Stephan Bischoff, Jens Walter, Nathalie Delzenne, Julie-Anne Nazare. Chitin-Glucan Supplementation Altered Gut Microbiota and Improved Postprandial Metabolism in Subjects at Cardiometabolic Risk. Current Developments in Nutrition 2022(Suppl 1),6: 331. [CrossRef]
- Xueni Zhang, Lei Jiang, Cankun Xie, Yidi Mo, Zihao Zhang, Shengxia Xu, Xiaoping Guo, Ke Xing, Yina Wang, Zhijian Su.The Recombinant Lactobacillus Strains with the Surface-Displayed Expression of Amuc_1100 Ameliorate Obesity in High-Fat Diet-Fed Adult Mice. Bioengineering (Basel, Switzerland)2024(6),11: 574. [CrossRef]
- Garcia-Serrano S, Ho-Plagaro A, Santiago-Fernandez C, Rodríguez-Díaz C, Martín-Reyes F, Valdes S, Moreno-Ruiz FJ, Lopez-Gómez C, García-Fuentes E, Rodríguez-Pacheco F. An Isolated Dose of Extra-Virgin Olive Oil Produces a Better Postprandial Gut Hormone Response, Lipidic, and Anti-Inflammatory Profile that Sunflower Oil: Effect of Morbid besity. Molecular nutrition & food research 2021(22),65: e2100071. [CrossRef]
- Yajuan Qin, Xiaoai Chen, Fei Xu, Kexue Zhu, Ping Wang, Yutong Zhang, Yanjun Zhang. Pectin enhances the inhibition of α-amylase via the mixture of rutin and quercetin. International Journal of Biological Macromolecules. 2025, 285: 138251. [CrossRef]
- Luzhe Zheng, Zhanzhan Wang, Bo Zhang, Lulu Yan, Pengfei Wang, Chao Zhao, Jun Wang, Yun Wang, Heizhao Lin, Lihua Qiu, Chuanpeng Zhou. Rhodiola rosea L. improved intestinal digestive enzyme activities, inflammatory response, barrier and microbiota dysbiosis in Lateolabrax maculatus juveniles fed with high-carbohydrate diets. Fish & Shellfish Immunology 2024,146: 109362. [CrossRef]
- Manan Kothari, Karthika Kannan, Revathy Sahadevan, Sruthi Vijaya Retnakumar, Camille Chauvin, Jagadeesh Bayry, Sushabhan Sadhukhan. Lipophilic derivatives of EGCG as potent α-amylase and α-glucosidase inhibitors ameliorating oxidative stress and inflammation. Bioorganic chemistry 2024,153: 107786. [CrossRef]
- Rizliya Visvanathan, Dang Truong Le, Sushil Dhital, Topul Rali, Rohan A Davis, Gary Williamson.Inhibition of Human Salivary and Pancreatic α-Amylase by Resveratrol Oligomers. Journal of medicinal chemistry 2024(21),67: 18753-18763. [CrossRef]
- Ji Wang, Jianyu Qu, Sha Liu, Qiurong Xu, Xiaowen Li, Yuanyuan Zhu, Xiangyan Liu, Jine Yi, Zhihang Yuan, Peng Huang, Yulong Yin, Lixin Wen, Jing Wu. Tannic Acid Ameliorates Systemic Glucose and Lipid Metabolic Impairment Induced by Low-Dose T-2 Toxin Exposure. Journal of agricultural and food chemistry 2023(33),71: 12574-12586. [CrossRef]
- Aysegül Tura, Viktoria Herfs, Tjorge Maaßen, Huaxin Zuo, Siranush Vardanyan, Michelle Prasuhn, Mahdy Ranjbar, Vinodh Kakkassery, Salvatore Grisanti.Quercetin Impairs the Growth of Uveal Melanoma Cells by Interfering with Glucose Uptake and Metabolism. International journal of molecular sciences 2024(8),25: 4292. [CrossRef]
- Mihai Babotă, Oleg Frumuzachi, Corneliu Tanase, Andrei Mocan. Efficacy of Myricetin Supplementation on Glucose and Lipid Metabolism: A Systematic Review and Meta-Analysis of In Vivo Mice Studies.Nutrients 2024(21),16: 3730. [CrossRef]
- Aoi Ito, Yuji Matsui, Masao Takeshita, Mitsuhiro Katashima, Chiho Goto, Kiyonori Kuriki. Gut microbiota-mediated associations of green tea and catechin intakes with glucose metabolism in individuals without type 2 diabetes mellitus: a four-season observational study with mediation analysis. Archives of microbiology 2023(5),205: 191. [CrossRef]
- Rohit Dutt, Pooja Mathur, Sweta Kamboj, Rohit Kamboj, Kumar Guarve, Shikha Kamboj, Rudrakash, Tanu Devi, Prerna Sharma. Anthocyanins as Nutraceuticals. Anthocyanins: Pharmacology and Nutraceutical Importance 2024: 48-67. [CrossRef]
- Abhinav Kakuturu, Heeyun Choi, Leah G Noe, Brianna N Scherer, Bikram Sharma, Bilon Khambu, Bhupal P Bhetwal.Bitter melon extract suppresses metastatic breast cancer cells (MCF-7 cells) growth possibly by hindering glucose uptake. microPublication biology 2023, 2023: 2578-9430. [CrossRef]
- Mahmoud Gamal, Mohamed A Awad, Azizeh Shadidizaji, Marwa A Ibrahim, Magdy A Ghoneim, Mohamad Warda. In vivo and In silico Insights into the Anti-Diabetic Efficacy of EVOO and Hydroxytyrosol in a Rat Model.The Journal of Nutritional Biochemistry 2024,135: 109775. [CrossRef]
- Luis Mojica1, Andrea Susana Ramos-Lopez, Oscar Abel Sánchez-Velázquez, Armando Gómez-Ojeda, Claudia Luevano-Contreras. Black bean (Phaseolus vulgaris L.) protein hydrolysates reduce acute postprandial glucose levels in adults with prediabetes and normal glucose tolerance. Journal of Functional Foods 2024,112: 105927. [CrossRef]
- Ryusei Uchio, Chinatsu Okuda-Hanafusa, Haruka Sakaguchi, Ryosuke Saji, Koutarou Muroyama, Shinji Murosaki, Yoshihiro Yamamoto, Yoshitaka Hirose. Curcuma longa extract reduces serum inflammatory markers and postprandial hyperglycemia in healthy but borderline participants with overweight and glycemia in the normal/prediabetes range: a randomized, double-blind, and placebo-controlled trial. Frontiers in nutrition 2024,11: 1324196. [CrossRef]
- Soto-Covasich J, Reyes-Farias M, Torres RF, Vásquez K, Duarte L, Quezada J, Jimenez P, Pino MT, Garcia-Nannig L, Mercado L, Garcia-Diaz DF. A polyphenol-rich Calafate (Berberis microphylla) extract rescues glucose tolerance in mice fed with cafeteria diet. J Funct Foods. 2020, 67: 103856. [CrossRef]
- Xia T, Duan W, Zhang Z, Fang B, Zhang B, Xu B, de la Cruz CBV, El-Seedi H, Simal-Gandara J, Wang S, Wang M. Polyphenol-rich extract of Zhenjiang aromatic vinegar ameliorates high glucose-induced insulin resistance by regulating JNKIRS-1 and PI3K/Akt signaling pathways. Food Chemistry 2021, 335: 127513. [CrossRef]
- Huaying Huang, Heye Chen, Yu Yao& …Xueyong Lou. Branched-chain amino acids supplementation induces insulin resistance and pro-inflammatory macrophage polarization via INFGR1/JAK1/STAT1 signal pathway. Molecular Medicine 2024(1), 30: 1528-3658.Ahmed Abdelbaset-Ismail, Katarzyna Brzezniakiewicz-Janus, Arjun Thapa, Janina Ratajczak, Magda Kucia, Mariusz Z Ratajczak. Pineal Gland Hormone Melatonin Inhibits Migration of Hematopoietic Stem/Progenitor Cells (HSPCs) by Downregulating Nlrp3 Inflammasome and Upregulating Heme Oxygenase-1 (HO-1) Activity. Stem cell reviews and reports 2024(1),20: 237-246. [CrossRef]
- Lokanath Mishra, Monalisa Mishra. Ribose-induced advanced glycation end products reduce the lifespan in Drosophila melanogaster by changing the redox state and down-regulating the Sirtuin genes. Biogerontology 2024(1),26: 28. [CrossRef]
- Arghadip Das, Sanchari Bhattacharya, Junaid Jibran Jawed. Enzymatically Digested Garlic Waste Conserved Most of Its Polyphenols and Contributed to Nrf2 Activation: Bio-accessibility. [CrossRef]
- Bioactivity, Gene Expression, and Genotoxicity Analysis. Waste and Biomass Valorization. 2024, 15: 4671–4698.
- Scuto M, Trovato Salinaro A, Caligiuri, Ontario ML, Greco V, Sciuto N, Crea R, Calabrese EJ, Rizzolio F, Canzonieri V, Calabrese V. Redox modulation of vitagenes via plant polyphenols and vitamin D: Novel insights for chemoprevention and therapeutic interventions based on organoid technology. Mechanisms of ageing and development 2021,199: 111551. [CrossRef]
- Maria Scuto, Maria Laura Ontario, Angela Trovato Salinaro, Isabella Caligiuri, Francesco Rampulla, Vincenzo Zimbone, Sergio Modafferi, Flavio Rizzolio, Vincenzo Canzonieri, Edward J Calabrese, Vittorio Calabrese. Redox modulation by plant polyphenols targeting vitagenes for chemoprevention and therapy: Relevance to novel anti-cancer interventions and mini-brain organoid technology. Free radical biology & medicine 2022,179: 59-75. [CrossRef]
- Lucía López-Gil, Amparo Pascual-Ahuir, Markus Proft. Genomic Instability and Epigenetic Changes during Aging. International journal of molecular sciences 2023(18),24: 14279. [CrossRef]
- Zeming Wu, Jing Qu, Guang-Hui Liu. Roles of chromatin and genome instability in cellular senescence and their relevance to ageing and related diseases. Nature Reviews Molecular Cell Biology 2024(12),125: 979-1000. [CrossRef]
- Cao Yu, Lu Jianying, Wang Han, Wang Xu, Ni Juan. Effects of curcumin and soy isoflavones on genomic instability of human colon cells NCM460 and SW620. Cellular and Molecular Biology, 2023, 69(1): 36-43. [CrossRef]
- Proshkina E., Shaposhnikov M., Moskalev A. Genome-Protecting Compounds as Potential Geroprotectors. International journal of molecular sciences. 2020, 21: 4484. [CrossRef]
- Rossiello F., Jurk D. Telomere dysfunction in ageing and age-related diseases. Nature 2022, 24: 135–147. [CrossRef]
- Stefania D'Angelo. Diet and Aging: The Role of Polyphenol-Rich Diets in Slow Down the Shortening of Telomeres: A Review. Antioxidants (Basel, Switzerland) 2023(12),12: 2086. [CrossRef]
- Stella Baliou, Petros Ioannou, Miruna-Maria Apetroaei, Elena Vakonaki, Persefoni Fragkiadaki, Evangelos Kirithras, Manolis N Tzatzarakis, Andreea Letitia Arsene, Anca Oana Docea, Aristides Tsatsakis. The Impact of the Mediterranean Diet on Telomere Biology: Implications for Disease Management-A Narrative Review. Nutrients 2024(15),16: 2525. [CrossRef]
- Gomez-Delgado F., Delgado-Lista J., Lopez-Moreno J., Rangel-Zuñiga O.A., Alcala-Diaz J.F., Leon-Acuña A., Corina A., Yubero-Serrano E., Torres-Peña J.D., Camargo A., et al. Telomerase RNA component genetic variants interact with the mediterranean diet modifying the inflammatory status and its relationship with aging: CORDIOPREV Study. JOURNALS OF GERONTOLOGY SERIES A-BIOLOGICAL SCIENCES AND MEDICAL SCIENCES. 2018, 73: 327–332.
- Qixia Xu, Qiang Fu, Zi Li, Hanxin Liu, Ying Wang , Xu Lin, Ruikun He, Xuguang Zhang, Zhenyu Ju, Judith Campisi, James L. Kirkland and Yu Sun . The flavonoid procyanidin C1 has senotherapeutic activity and increases lifespan in mice. Nature Metabolism 2021, 3(11): 1706–1726. [CrossRef]
- Jia Y, Mao Q, Yang J, Du N, Zhu Y, Min W. Epigallocatechin-3-Gallate Protects Human Skin Fibroblasts from Ultraviolet a Induced Photoaging. Clinical, Cosmetic and Investigational Dermatology. 2023,16: 149-159. [CrossRef]
- Aikaterini E Mantadaki, Stella Baliou, Manolis Linardakis, Elena Vakonaki, Manolis N Tzatzarakis, Aristides Tsatsakis, Emmanouil K Symvoulakis. Quercetin Intake and Absolute Telomere Length in Patients with Type 2 Diabetes Mellitus: Novel Findings from a Randomized Controlled Before-and-After Study. Pharmaceuticals (Basel, Switzerland) 2024(9),17: 1136. [CrossRef]
- Inhae Sohn, Chol Shin, Inkyung Baik. Associations of green tea, coffee, and soft drink consumption with longitudinal changes in leukocyte telomere length. Scientific Reports 2023(1),13: 1-7. [CrossRef]
- Dwivedi Lalitaa, Jaiswal Aishwaryab, Punia Reenub, Kumar R Sureshd, Yim Dongsoole, Rajamani Paulraja, Singh Rana P. Decursin Inhibits Expression and Nuclear Localization of DNMT and HDAC, and Suppresses the Growth and Survival of Prostate Cancer Cells. Asia Pacific Journal of Cancer Biology 2024(3),9: 271-281. [CrossRef]
- Xing Lu, Ziwei Wang, Yu Zhang, Ti Meng, Xuehua Chen, Rongmiao Yuan, Bing Liu, Huan He, Xin Ding, Silong Zhang. A curcumin-based HDACs inhibitor for targeted sonodynamic therapy of breast cancer. International journal of biological macromolecules. 2024,138616. [CrossRef]
- Beatriz Silva Urias, Aline Renata Pavan, Gabriela Ribeiro Albuquerque, Igor Muccilo Prokopczyk, Tânia Mara Ferreira Alves, Thais Regina Ferreira de Melo, Geraldo Rodrigues Sartori, João Hermínio Martins da Silva, Chung Man Chin, Jean Leandro Dos Santos. Optimization of Resveratrol Used as a Scaffold to Design Histone Deacetylase (HDAC-1 and HDAC-2) Inhibitors. Pharmaceuticals (Basel, Switzerland) 2022(10),15: 1260. [CrossRef]
- Manvi Sharma, Trygve O Tollefsbol. Combinatorial epigenetic mechanisms of sulforaphane, genistein and sodium butyrate in breast cancer inhibition. Experimental cell research 2022(1),416: 113160. [CrossRef]
- Peramaiyan Rajendran, Salaheldin Abdelraouf Abdelsalam, Kaviyarasi Renu, Vishnupriya Veeraraghavan, Rebai Ben Ammar, Emad A Ahmed. Polyphenols as Potent Epigenetics Agents for Cancer. International journal of molecular sciences 2022(19), 123: 11712. [CrossRef]
- Wojciech Pawłowski, Miłosz Caban, Urszula Lewandowska. Cancer Prevention and Treatment with Polyphenols: Type IV Collagenase-Mediated Mechanisms. Cancers 2024(18), 16: 3193. [CrossRef]
- Sajjad Tezerji, Hamid abdolazimi, Azadeh Fallah, Behrouz Talaei. The effect of resveratrol and quercetin intervention on azoxymethane-induced colon cancer in Rats model. Clinical Nutrition Open Science 2022,45: 91-102. [CrossRef]
- Nadir Mustafa Qadir Nanakali, Parisa Maleki Dana, Fatemeh Sadoughi, Zatollah Asemi, Mehran Sharifi, Reza Asemi, Bahman Yousefi. The role of dietary polyphenols in alternating DNA methylation in cancer. Critical Reviews in Food Science and Nutrition 2023(33),63: 12256-12269. [CrossRef]
- Anshu Agarwal, Vikash Kansal, Humaira Farooqi, Ram Prasad, Vijay Kumar Singh. Epigallocatechin Gallate (EGCG), an Active Phenolic Compound of Green Tea, Inhibits Tumor Growth of Head and Neck Cancer Cells by Targeting DNA Hypermethylation. Biomedicines 2023(3),11: 789. [CrossRef]
- Maria Vrânceanu, Damiano Galimberti, Roxana Banc, Ovidiu Dragoş, Anamaria Cozma-Petruţ, Simona-Codruţa Hegheş, Oliviu Voştinaru, Magdalena Cuciureanu, Carmina Mariana Stroia, Doina Miere, Lorena Filip. The Anticancer Potential of Plant-Derived Nutraceuticals via the Modulation of Gene Expression. Plants (Basel, Switzerland) 2022(19),11: 2524. [CrossRef]
- Kaavya Gunasekaran, Bala Murali Krishna Vasamsetti, Priyadharshini Thangavelu, Karthi Natesan, Bonaventure Mujyambere, Viswanathan Sundaram, Rama Jayaraj, Yeon-Jun Kim, Suja Samiappan, Jae-Won Choi. Cytotoxic Effects of Nanoliposomal Cisplatin and Diallyl Disulfide on Breast Cancer and Lung Cancer Cell Lines. Biomedicines 2023,11: 1021. [CrossRef]
- Vassallo V., Stellavato A., Finamore R., Giuliano M. T., Toro G., Iolascon G., Schiraldi C. Polyphenols from Mediterranean herbs as bioactive molecules for improving human health. FEBS OPEN BIO. 2024,14: 170.
- Inés Domínguez-López, Camila Arancibia-Riveros, María Marhuenda-Muñoz, Anna Tresserra-Rimbau, Estefanía Toledo, Montserrat Fitó, Emilio Ros, Ramon Estruch, Rosa M Lamuela-Raventós. Association of microbiota polyphenols with cardiovascular health in the context of a Mediterranean diet. Food research international (Ottawa, Ont.). 2023,165: 112499. [CrossRef]
- Lucia Potenza, Roberta Saltarelli, Francesco Palma, Laura Di Patria, Giosuè Annibalini, Sabrina Burattini, Pietro Gobbi, Laura Valentini, Giovanni Caprioli, Agnese Santanatoglia, Sauro Vittori, Elena Barbieri. Morphological Characterization, Polyphenolic Profile, and Bioactive Properties of Limoncella, an Ancient Mediterranean Variety of Sweet Citrus. Biomolecules 2024(10),14: 1275. [CrossRef]
- Sabrina Berger, Ian Oesterle, Kolawole I Ayeni, Chibundu N Ezekiel, Annette ompel, Benedikt Warth. Polyphenol exposure of mothers and infants assessed by LC-MS/MS based biomonitoring in breast milk. Analytical and bioanalytical chemistry 2024(7),416: 1759-1774. [CrossRef]
- Sonia Zhan-Dai, Blanca Grases-Pintó, Rosa M Lamuela-Raventós, Margarida astell, Francisco J Pérez-Cano, Anna Vallverdú-Queralt, Maria José Rodríguez-Lagunas. Exploring the Impact of Extra Virgin Olive Oil on Maternal Immune System and Breast Milk Composition in Rats. Nutrients 2024(11),16: 1785. [CrossRef]
- Jimena Ríos, Viviana Valero-Jara, Samanta Thomas-Valdés. Phytochemicals in breast milk and their benefits for infants. Critical reviews in food science and nutrition 2022(25),62: 6821-6836. [CrossRef]
- Ruonan Yan, Chi-Tang Ho, Yanan Liu, Shengnan Zhan, Zufang Wu, Xin Zhang. The modulatory effect of oolong tea polyphenols on intestinal flora and hypothalamus gene expression in a circadian rhythm disturbance mouse model. Food Science and Human Wellness 2024, 13: 748-764. [CrossRef]
- Tobias Goris and Annett Braune. Genomics and physiology of Catenibacillus, human gut bacteria capable of polyphenol C-deglycosylation and flavonoid degradation. Microbial Genomics 2024, 10: 001245. [CrossRef]
| Number | Mechanisms |
| 1 | Polyphenols directly scavenge ROS, because of the presence of phenolic hydroxyl groups on polyphenols molecules[14,16,17]. |
| 2 | Polyphenols exert antioxidant activity by regulating endogenous antioxidant enzymes[16,17]. |
| 3 | Polyphenols directly or indirectly protect mitochondrial dysfunction through antioxidant roles[16,17]. |
| 4 | polyphenols enhance cellular antioxidant activity via regulating Nrf2-mediated pathway[18]. |
| 5 | Polyphenols counteract ROS via regulating mircoRNAs[19]. |
| 6 | Polyphenols reduce the formation of metal dependent 锦hydroxyl radicals by chelation action[14,16,17]. |
| Polyphenols | Function mechanism |
| Hydroxytyrosol, olivine glycoside | AMPK↑; inhibiting mTOR1↓[20,21,23,24]. |
| Resveratrol, kaempferol, and gallic acid | PI3K/AKT/mTORe↓[24,25,26]. |
| Resveratrol | Hsp70↑[27]. |
| Resveratrol | Aβ↓[22,25,26,27]. |
| Curcumins ,green tea | Hsp↑[28,29]. |
| Polyphenols | Enhancing blood glucose absorption | Reducing postprandial hyperglycemia. |
| oleuropein aglycone (OLE), hydroxytyrosol (HT) | NADH oxidase↓[31] | + |
| extra virgin olive oil(EVOO) | NADH oxidase↓,derived bacterial lipopolysaccharide↓[32,33,34] | + |
| quercetin, myricetin, luteolin, epigallocatechin-3-gallate (EGCG), resveratrol | α-amylase and α-glucosidase↓[35,36,37,38] | + |
| tannic acid, quercetin, , myricetin | GLUT2↓[39,40,41] | + |
| catechins of green tea, anthocyanins of grape seeds, bitter melon, extra virgin olive oil, black beans | glucose transporters(GLUT4)↑[42,43,44,45] | + |
| Polyphenols | Models | Main effects |
| resveratrol | mouse embryonic fibroblasts | ARF/p53↑ |
| proanthocyanidins and procyanidins | human lymphocytes | antioxidant and anti-inflammatory ↑; apoptosis↓ |
| EGCG and quercetin | myocardial cell | telomere shortening ↓ |
| Polyphenols | Function mechanism, target of action. |
| curcumin, resveratrol, and catechins. | HDACs and DNMTs[68,69,70] |
| genistein | DNMTs↓[71]. |
| Resveratrol | PGC1α/SIRT1/AMPK, DNMT [72,73,74,75] |
| EGCG | DNMTs↓, HDAC, HATs[76]. |
| Quercetin | HAT and HDAC↓[77]. |
| DADS | HDACs[78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





