1. Introduction
Classical BCR-ABL-negative myeloproliferative neoplasms (MPNs) are a type of myeloid malignancy, resulting from the malignant clonal proliferation of myeloid cell lineages derived from hematopoietic stem cells (HSCs). This includes essential thrombocythemia (ET), polycythemia vera (PV), and primary myelofibrosis (PMF) [
1]. Clinically, MPN patients typically present with symptoms such as fatigue, fever, night sweats, weight loss, pruritus, satiety, and complications like bleeding or thrombosis. The severity of these symptoms is linked to the patient’s tumor burden and existing comorbidities, often leading to disease progression towards bone marrow failure or acute leukemia, which can ultimately result in death [
2,
3]. Driver mutations in HSCs are considered the primary factors in MPN development, including Janus kinase 2 (
JAK2), calreticulin (
CALR), and myeloproliferative leukemia virus (
MPL) gene mutations. These mutations lead to hyperactivation of the JAK2 signaling pathway, resulting in the abnormal proliferation of HSCs [
4]. JAK2V617F is the most common driver mutation, found in over 95% of PV patients and 50% of ET and PMF patients [
5]. In addition to driver mutations, non-driver mutations such as
ASXL1,
EZH2,
TET2, and
U2AF1 also contribute to disease progression [
6]. In approximately 10% of MPN patients, driver mutations cannot be detected, classifying them as “triple-negative” patients [
7].
MPNs are also regarded as an inflammatory tumor disease. Patients with MPN often exhibit elevated levels of pro-inflammatory cytokines, chemokines, and growth factors such as IL-1β, IL-6, IL-8, IL-10, TNF-α, and TGF-β in the bone marrow microenvironment and peripheral blood [
8,
9,
10]. This pro-inflammatory profile exacerbates genetic and epigenetic instability in MPNs, leading to cardiovascular events, fibrosis progression, and leukemic transformation in patients [
11]. Additionally, various immune cells are dysregulated in MPNs, including neutrophils, lymphocytes, regulatory T cells, dendritic cells (DC), monocyte and myeloid-derived suppressor cells (MDSC) [
12]. One recent study of bone marrow mononuclear cells in PMF patients with single cell sequence technology identified 15 subsets of T and NK cells and eight CD14+ monocyte subsets. Functional module analysis demonstrated an increased cytotoxic score of most T cells and a higher prevalence of LAG3 expressing effector T cells in overt-PMF compared to that of pre-PMF. An increased frequency of Mono 3 was also noticed in overt-PMF which expressed M-MDSC markers [
13]. Several studies focusing on monocytes demonstrated its abnormality both in number and quality, which correlates strongly with MPN prognosis, particularly PMF prognosis [
14,
15]. Monocytes are unique due to their high plasticity in differentiating into fibrocytes, osteoclasts, DC, or macrophages in MPNs [
16,
17]. Furthermore, they perform essential functions such as antigen presentation, migration, cytokine secretion, and interaction with T cells [
18,
19]. Several studies focusing on monocytes have revealed that abnormal monocytes may play a significant role in MPN progression through pro-angiogenesis by expressing Tie2, pro-fibrosis by expressing SLAMF7, pro-tumor activity via cytokine secretion such as TNF-α, and inhibiting T cell function through increased expression of PDL1, as well as modulating the tumor microenvironment by differentiating into tumor-associated macrophages, etc. Therefore, this review aims to summarize recent advances in the biology of monocytes and particularly their involvement in pathophysiology of MPNs. The plasticity and heterogeneity of monocytes contribute to their highly complex functions in different tissues under various stimuli. Initial studies have demonstrated the phenotypic and transcriptional disturbances of monocytes in MPNs; however, the origins of these disturbances and their intricate roles in the pathogenesis and progression of MPNs still need to be elucidated. A summary of the advancements in understanding the underlying mechanisms could help guide further studies aimed at unmasking these complex processes, offering new therapeutic targets and proposing innovative treatment approaches in the future.
2. Overview of Monocytes
2.1. Monocyte Development and Differentiation
Distinct developmental pathways of monocytes were proposed using mouse models with novel experimental approaches such as fate mapping and single cell analysis [
20]. Monocytes are derived in the bone marrow from HSCs, which differentiate into multipotent progenitor cells (MPPs) and then further into common myeloid progenitor cells (CMPs). These cells subsequently differentiate into granulocyte-macrophage progenitors (GMPs) or monocyte-dendritic cell progenitors (MDPs), MDPs could differentiate directly into Ly6cHi monocytes, However, GMPs eventually become monocyte progenitors (cMOPs) and ultimately mature monocytes [
20,
21]. Circulating monocytes can migrate into tissues in response to various signals, replenishing tissue macrophages or differentiating into monocyte-derived dendritic cells (mo-DCs) to transport antigens to lymph nodes or present them to T cells. Monocytes play a crucial role in the immune response by recognizing danger signals through pathogen pattern receptors. They can phagocytose, present antigens, secrete cytokines, and migrate into tissues [
22].
2.2. Monocyte Heterogeneity and Its Homeostasis
Currently, there are three main subsets of human monocytes: classical monocytes (CMs: CD14++CD16-), intermediate monocytes (ICMs: CD14+CD16+), and non-classical monocytes (NCMs: CD14+/-CD16++) [
22]. In healthy individuals, CMs typically account for approximately 85% of the monocyte population, while ICMs and NCMs comprise around 5% and 5-10%, respectively [
23]. CMs have a relatively short lifespan in the bloodstream, surviving for about 1.6 days, after which the majority exit circulation to migrate into tissues. In contrast, circulating ICMs and NCMs last approximately 4 days and 7 days, respectively [
24]. Notably, there is a linear relationship between these subsets, with CMs transitioning into ICMs, and subsequently into NCMs [
25].
The transition from CMs to ICMs and NCMs occurs primarily in the diaphysis, as evidenced by Bianchini et al., who evaluated the distribution of monocyte subsets. They found the proportions of CMs, ICMs, and NCMs in the diaphysis to be 14:1:3, compared to 2:1:2 in the epiphysis. Further research indicated that the number of Transitional Zone (TZ) vessels within the bone marrow correlates positively with the total amount of PD-L1+ cells, which include both ICMs and NCMs, with NCMs predominating. Circulating CMs preferentially localize to the diaphyseal marrow, remaining in this region for extended periods before transiting into ICMs and NCMs following interactions with TZ vessels [
26].
Monocytes maintain a homeostatic state, which can be disrupted under various conditions, such as chronic inflammation or infection, a topic that has been extensively reviewed elsewhere [
27,
28].
2.3. Phenotypic Features of Monocytes
Human peripheral blood monocytes express a variety of surface markers, including cluster differentiation antigens, chemokine receptors, and cytokine receptors [
18]. Key markers include CD14, CD16, CD64 (Fc gamma RI), CD192 (also known as CCR2, a crucial mediator of monocyte migration), CX3CR1 (the fractalkine receptor), HLA-DR (which is expressed at the highest levels in the intermediate monocyte population), and CD195 (CCR5). Additionally, monocytes express TNFR1 (CD120a) and TNFR2 (CD120b) [
29]. Recent studies on the immunophenotype of peripheral blood monocytes have shown that different subsets exhibit distinct phenotypic characteristics [
30,
31]. CMs particularly exhibit high level expression of CD11b, CD15s, and SSEA-1, CD35 (complement receptor type 1), CD36, CD38, CD49e (integrin α-5), CD89 (immunoglobulin-α Fc receptor), CDw93, CD96, CD99, CD114, CD182 (CXCR2), CD166 (ALCAM), CD181 (CXCR1), and leukotriene B4 receptor 1 (BLTR1), which also significantly differentiated them from the intermediate subset. ICMs express relatively high levels of CD32, CD39 (NTPDase-1), CD54, CD72, CD74, CD91 (α2-macroglobulin receptor), CD105 (endoglin), CD163, CD195 (CCR5), CD275, CD305 (LAIR1), CDw328 (Siglec-7), and HLA-DR/DQ. NCMs present the high expression levels of CD29 (integrinβ-1), CD31 (PECAM1), CD45RA, CD75, CD120b (TNFR2), CD132 (interleukin IL-2 receptor subunit gamma), CD244 (SLAMF4), CD294, and HPC (hematopoietic progenitor cell) [
30,
31].
2.4. Functional Features of Monocytes
2.4.1. Cytokine Secretion
Cytokine production in response to Toll-like receptor (TLR) agonists is a prominent feature of monocytes and macrophages. Under stimulation with TLR1-9 agonists, CMs secrete significantly higher levels of IL-1β, IL-6, and TNF-α compared to NCMs and ICMs, with NCMs exhibiting the weakest secretion ability [
21,
32]. Notably, when stimulated with TLR4 agonists, CMs can produce large amounts of TNF-α, IL-6, and IL-1β [
32]. Studies have shown that upon exposure to increasing concentrations of lipopolysaccharide (LPS), CMs generate the highest levels of G-CSF, IL-10, CCL2, and IL-6 compared to ICMs and NCMs. Conversely, NCMs are more adept at producing inflammatory cytokines like TNF-αand IL-1, while ICMs have the least capacity for cytokine secretion [
32]. Additionally, LPS-stimulated DCs derived from CD16– monocytes produce higher levels of IL-12 mRNA and lower levels of TGF-β1 mRNA compared to DCs derived from CD16+ monocytes, with corresponding protein levels confirmed [
33].
2.4.2. Differentiation Potential
Monocytes can differentiate into monocyte derived dendritic cells (moDCs) and macrophages under various culture conditions, but their differentiation potential varies among the three subsets. Comparative studies indicate that CMs fully differentiate into moDCs after one week, while only a limited number of NCMs and ICMs undergo this differentiation, with most cells undergoing apoptosis [
32]. Functional assays have demonstrated that these differentiated cells exhibit defects in inducing T-cell proliferation or IFN-γ production. None of the three monocyte subsets can differentiate into plasmacytoid dendritic cells (p-DCs) [
32]. Furthermore, moDCs derived from CD16+ monocyte express higher levels of CD86, CD11a, and CD11c, while showing lower expression of CD1a and CD32 compared to moDCs derived from CD16–monocytes. When co-cultured with T cells, both moDCs derived from CD16+ and CD16– monocytes induce T-cell proliferation without significant differences; however, moDCs derived from CD16+ monocytes promote higher secretion of IL-4 and IFN-γ from T cells [
33].
Regarding differentiation into macrophages, all monocyte subsets adopt macrophage morphology, characterized by a rounded, firmly adhered appearance with actin-rich podosomes and extensions for substrate adhesion. Compared to M1 and M2 macrophages generated from ICMs and NCMs, M1 and M2 macrophages derived from CMs exhibit stronger phagocytic abilities and secrete higher levels of cytokines such as IL-10, IL-6, and PDGF-BB [
32]. Under homeostatic conditions, classical monocytes can maintain their monocyte-like state without differentiating into macrophages or DCs, acting as a local reservoir and participating in non-immune tissue surveillance by transferring antigen information to lymph nodes [
34].
2.4.3. Antigen Presentation
In vitro studies have evaluated the ability of moDCs derived from CD16– and CD16+ monocytes to prime autologous and allogeneic CD4 T cells, revealing that moDCs from CD16+ monocytes elicit a relatively higher autologous response and a similar allogeneic response compared to moDCs derived from CD16–monocytes. moDCs derived from CD16+ monocytes consistently induce higher secretion of IL-4 by T lymphocytes, while the levels of IFN-γ produced by both moDC types are comparable [
33]. Beyond their role as antigen-presenting cells, monocytes themselves exhibit significant antigen-presenting capabilities. Animal studies suggest that monocytes can capture and present antigens in vivo, functioning similarly to conventional DCs. Other researches indicate that monocytes primarily transport antigens to lymph nodes, where they present them to other antigen-presenting cells, such as conventional dendritic cells (cDCs) [
34,
35,
36]. However, whether human monocyte in vitro could present antigen to T cells is still unknown, which needs to be further investigated.
2.4.4. Migration and Extravasation
After being produced in the bone marrow, monocytes enter peripheral blood circulation and rapidly migrate to damaged tissues in response to inflammatory or infection signals, playing a crucial role in immune defense. Mouse model studies demonstrated that monocyte migration is primarily regulated by specific chemokines. For instance, when CCR2 on monocytes binds to MCP-1, it triggers the release of monocytes from the bone marrow [38]. Similarly, CCL19 binds to CCR7 on monocyte surfaces to guide them to lymph nodes, where they perform their functions. Upon reaching target sites, monocytes can differentiate according to the local microenvironment, such as becoming macrophages that phagocytose pathogens, clear cellular debris, and secrete anti-inflammatory cytokines [39]. The migration ability of human monocytes was evaluated using transwell and transendothelial migration assays. The results revealed that CD16- and CD16+ monocytes are guided by different chemokines. CD16- monocytes preferentially respond to MCP-1 and MIP-1, while CD16+ monocytes, which express high levels of CX3CR1, are guided by the CX3CR1-FKN axis [40]. Other chemokines, such as CCR5 and CCR6, also play roles in the migration and extravasation of monocytes [39]. Further studies focusing on the migration of human monocytes are needed.
2.5. Transcriptional Features of Monocytes
Numerous studies have demonstrated transcriptional differences among human monocyte subsets [41,42,43,44,45]. CMs primarily participate in inflammatory responses and tissue repair, exhibiting gene expression profiles associated with these functions. Notably, CMs highly express genes from the S100 protein family, such as S100A8, and S100A12, which encode proteins crucial for signal transduction and cell recruitment during inflammation [42,43,44]. Additionally, they express angiogenesis and tissue repair-related genes, including VEGF, and TGF-β, which facilitate the repair and reconstruction of damaged tissues [43]. CMs also display elevated expression of neuregulin 1(NRG1), phospholipase A2 group VII (PLA2G7), ADAM19, Low-density lipoprotein receptor (LDLR), scavenger receptor class B member 1 (SCARB1), and stabilin 1(STAB1), all of which mediate inflammatory responses and participate in immune regulation [42].
ICMs play a vital role in antigen presentation, characterized by their highest expression of MHC-II among monocyte subsets, which enables them to effectively present antigens to T cells [41,45]. Furthermore, ICMs present the highest expression of genes involved in cell differentiation and cell function such as IRF5, IRF8 and NFKB1 [45].
In contrast, NCMs exhibit high expression of cytoskeletal regulatory genes, including CDC42 effector protein-4 (CDC42EP4), creatine kinase B (CKB), EMAP-like protein 4 (EML4), Enah/vasodilator-stimulated phosphoprotein-like (EVL), formin-like 2 (FMNL2), metastasis suppressor-1 (MTSS1), and RHOC [43]. These genes are involved in the migration and adhesion of non-monocytes, enabling NCMs to function as efficient patrolling cells that can rapidly migrate to sites of infection. Additionally, NCMs express genes related to both inflammatory and anti-inflammatory states, such as heme oxygenase-1(HMOX1), KLF1, and FCGR3B, reflecting their capability to modulate immune responses according to the tissue environment [42,44].
3. Monocyte Characteristics in MPNs
A significant decrease in CMs and an increased frequency of ICMs and NCMs was noticed in MPN patients after analyzing of peripheral blood monocytes using flow cytometry. Further analysis of monocytes from PMF patients indicated an increased proportion of CCR2+ monocytes, including CMs, ICMs, and non-classical NCMs. Conversely, the proportion of TNFR1+ monocytes decreased, while TNFR2+ monocytes increased. Additionally, the percentage of CX3CR1+ NCMs cells declined, while CCR5+ monocytes, comprising both CMs/ICMs and NCMs, increased [46]. Another study demonstrated that monocytes from PMF patients displayed an inflammatory state, resulting in a diminished capacity to secrete IL-8 following LPS stimulation [47]. Moreover, PMF monocytes showed impaired differentiation into dendritic cells (DCs), primarily characterized by the presence of immature DCs that continued to express CD14, while CD80 expression was reduced. In mature DCs, the expression of both CD40 and CD80 was also decreased [48].
Transcriptomic sequencing of CD163+ monocytes isolated from the bone marrow of MPN patients revealed that In PV, the upregulated pathways were found to include cytokine-cytokine receptor interactions, cytokine receptor pathways, the Rap1 signaling pathway, the cGMP-PKG signaling pathway, platelet activation, and complement and coagulation cascades. The downregulated pathways were associated with ribosomal function, the cell cycle, oxidative phosphorylation, and pathways related to neurodegeneration. In ET, the upregulated pathways included platelet activation, cell adhesion molecules, ECM-receptor interactions, focal adhesion, and regulation of the actin cytoskeleton. The down regulated pathways involved viral protein interactions with cytokines and their receptors, oxidative phosphorylation, thermogenesis, as well as the NF-kappa B signaling pathway, TNF signaling pathway, and IL-17 signaling pathway. In PMF, the upregulated pathways related to cytokine-cytokine receptor interactions, platelet activation, the JAK-STAT signaling pathway, complement and coagulation cascades, and Fc gamma R-mediated phagocytosis. In contrast, the only down regulated pathways were associated with coronavirus disease (COVID-19) and ribosomal function [49].
In another study, CD14+ monocytes were isolated using magnetic bead sorting and compared with healthy monocytes. A total of 101 differentially expressed genes (DEGs) shared among ET, PV, and PMF were primarily enriched in pathways related to coagulation, complement activation, JAK-STAT signaling, and TNF/NF-kB signaling. In PMF patients, the upregulated genes in monocytes were mainly associated with platelet function, including PPBP, ITGB3, and PF4, while the downregulated genes primarily related to lymphoid and erythroid lineages, such as DNTT, VPREB, various immunoglobulin transcripts, and GYPA [50]. Additionally, a bioinformatics analysis of peripheral blood monocytes from MPN patients showed that the top 20% of differentially expressed genes underwent enrichment analysis revealing significant associations with KRAS signaling, immune activation, response to hypoxia, stimuli of degranulation, complement system regulation, heme metabolism, and regulation of cell death. Subgroup analyses demonstrated that PV patients exhibited greater enrichment in the IFN-γ pathway, ET patients were more associated with IL-2-STAT5 signaling and fatty acid metabolism, and PMF patients displayed enhanced KRAS signaling, heme metabolism, and stimulation of neutrophil degranulation [51].
4. Mechanism of Monocyte Involvement in MPN Progression
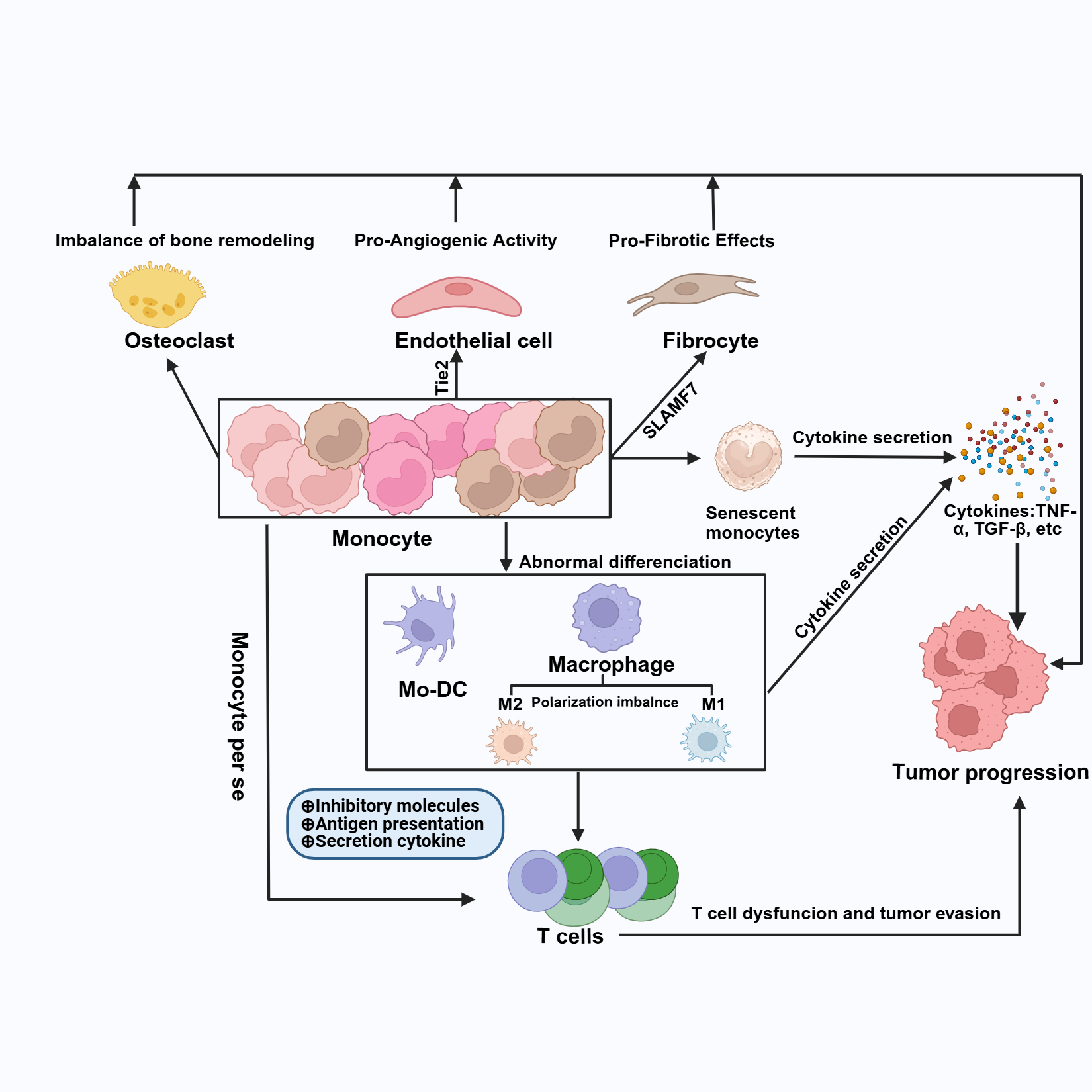
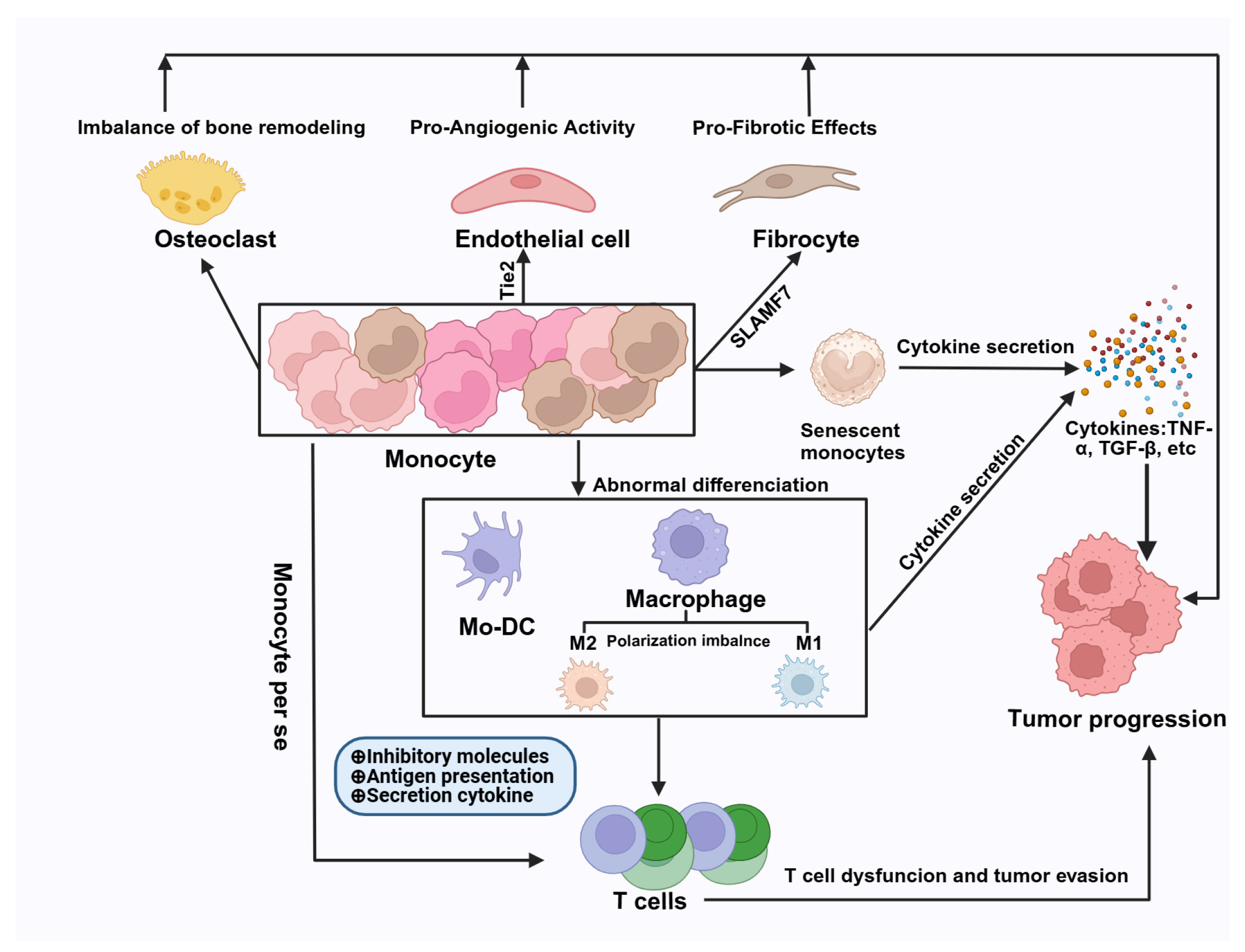
As described above, monocytes have been implicated in the prognosis of MPNs, particularly in PMF. In MPNs, monocytes demonstrate a redistribution of monocyte subsets, dysfunction of mo-DCs, and transcriptional dysregulation. This shift in monocyte populations is associated with aberrant functions that may contribute to MPN pathogenesis, although the underlying mechanisms remain to be fully elucidated. Several initial studies demonstrated that monocytes play a multifaceted role in the progression of MPNs through various mechanisms as described in
Table 1:
Pro-Aiogenic Activity: Monocytes may exhibit pro-angiogenic properties by expressing Tie2 or even differentiating into endothelial cells, thereby promoting vascularization and tumor progression [52,53].
Pro-Fibrotic Effects: Through the expression of SLAMF7 and differentiation into fibrocytes, monocytes may contribute to fibrotic processes within the bone marrow microenvironment [54].
Pro-Tumoral Effects: Monocytes can express programmed death-ligand 1 (PD-L1), which directly inhibits T cell function, thereby facilitating tumor progression. This immune evasion mechanism allows tumors to escape immune surveillance [55,56].
Cytokine Secretion: An imbalance in monocyte subsets can lead to abnormal cytokine secretion, particularly an excess of TNF-α. This cytokine is known to promote MPN progression. Additionally, intrinsic disturbances in monocyte function may result in abnormal interactions with T cells, further contributing to tumor growth [57].
Differentiation Potential: Monocytes present the ability to differentiate into various cell types, including DCs, macrophages. These differentiated cells can interact with T cells, potentially inhibiting their functions and facilitating tumor evasion [48].
Osteoclast Differentiation: Monocytes can also differentiate into osteoclasts, which may play a significant role in the progression of MPNs by affecting the bone marrow microenvironment [58].
Senescence and Dysfunction: Monocytes may enter a senescent state, leading to mitochondrial dysfunction. This impairment can result in abnormal migration, antigen presentation, and overall immune response, further complicating their role in MPNs [59,60].
These potential mechanisms underscore the complex contributions of monocytes to MPN progression and highlight the necessity for further research to fully understand their roles in disease pathology. A summary of these underlying mechanisms is illustrated in
Figure 1.
4.1. Pro-Angiogenesis via Expressing Tie2
The Tie2 receptor, a receptor tyrosine kinase, plays a crucial role in regulating physiological processes such as vascular growth and differentiation in recognizing their ligands such as angiopoietin-2. Its activation triggers a cascade of downstream signaling pathways essential for angiogenesis, the formation of new blood vessels, which is a critical rate-limiting step in tumor development. Tie2 is frequently expressed on vascular endothelial cells and some hematopoietic stem cells, particularly monocytes or macrophages [61]. These Tie2+ cells accounted for 35% to 75% of the CD14lowCD16+ monocytes, so this subset of monocytes expressing Tie2 known as Tie2-expressing monocytes (TEMs) [62]. Tie2+ cells accounted for 1.6% to 7.4% of the total PB mononuclear cells, further evaluation with PBMCs from cancer patients demonstrated the frequency of TEMs ranged from 1.8% to 10.1% of the total PBMCs. Further studies using tumor mouse models have demonstrated that the selective elimination of TEMs effectively reduces tumor angiogenesis, leading to tumor regression [63]. Initial clinical studies with Tie2 Kinase Inhibitor Rebastinib in treating myeloid malignancies and breast cancer demonstrated good tolerance, but its efficacy needs further evaluation with large sample size [64,65]. However, the existence and functional significance of Tie2+ macrophage in tumor environment was still in debate [66], it still needs further study.
As for its involvement in MPNs, there are only two clinical observations. An increased frequency of ICMs expressing Tie2 has been noted in the peripheral blood of patients with PMF [52]. Additionally, their frequency was found to be higher in spleen tissue-derived mononuclear cells (MNCs) from PMF patients compared to that of controls [53]. These preliminary findings suggest that Tie2+ monocytes may promote the progression of MPNs through their angiogenic properties, potentially contributing to complications such as thrombosis and splenomegaly.
4.2. Pro-Fibrosis via Expressing SLAMF7
SLAMF7, a member of the SLAM family, primarily induces B cell proliferation and autocrine signaling. Additionally, it plays a negative regulatory role in T-cell responses, inhibiting their activity and contributing to the modulation of pro-inflammatory responses in monocytes [67]. SLAMF7 is expressed on various immune cells, including plasma cells, NK cells, plasmacytoid dendritic cells (pDCs), T cells, B cells, monocytes, and Mo-DCs. It is also highly expressed in tumor cells, particularly in multiple myeloma, and on the surface of fibroblasts in both mice and humans [68]. In MPNs, monocytes have been found to express elevated levels of SLAMF7, an increased frequency of CD14+SLAMF7+ monocyte was noticed in MPN patients including PV and ET patients [54,69]. Specifically, SLAMF7highCD14+ monocytes are considered precursor cells for human fibrocytes, and their numbers significantly increase in MF patients, particularly in those with the JAK2V617F mutation [54]. Further research has demonstrated that the JAK2V617F mutation directly leads to increased SLAMF7 expression in monocytes [54]. The elevated presence of SLAMF7high monocytes with a higher JAK2V617F allelic burden, is associated with the development of PMF. This suggests that monocytes contribute to MPN pathogenesis through their differentiation into fibrocytes, promoting bone marrow fibrosis. Thus, SLAMF7 serves as a potential mechanism by which monocytes facilitate the fibrotic processes observed in MPNs. Ultimately, antibody targeting of SLAM7, such as Elotuzumab, could be a candidate treatment for PMF, as Elotuzumab has demonstrated its benefits in multiple myeloma [70]. Currently, there is one active clinical trial involving Elotuzumab for treating PMF, but it has not yet begun recruiting patients.
4.3. Protumoral Effect via Expressing PD-L1
PD-L1 plays a critical role in tumor immune evasion by binding to PD-1 on the surface of T cells, thereby inhibiting T cell-mediated immune responses against tumors [71]. Numerous studies have highlighted that PD-L1 is expressed on the surface of antigen-presenting cells, where it is pivotal in the efficacy of immune checkpoint inhibitor therapies [72,73]. Research by M. Bianchini et al. has shown that PD-L1 is predominantly expressed on non-classical monocytes, with minimal or absent expression on intermediate and classical monocytes, particularly in the context of cardiovascular diseases [26]. Under inflammatory conditions, monocytes can be activated by various cytokines, leading to the upregulation of PD-L1 expression, which subsequently suppresses T cell immune responses. Notably, the PD-L1 gene is located on chromosome 9, adjacent to the JAK2 gene [74]. Studies have demonstrated a correlation between JAK2 mutations and PD-L1 expression in both melanoma and MPNs [56,75]. For instance, a study on melanoma indicated that mutations in JAK1/JAK2 may contribute to resistance against PD-1 antibody treatments [75]. In the context of MPNs, research has shown that JAK2 mutations are associated with increased PD-1 expression on the surface of various cell types, including monocytes, MDSCs, and megakaryocytes [76]. For MPNs associated with CALR mutations, studies suggest that in vivo blockade of PD-1 can help restore or enhance T cell responses [56]. Two initial clinical trials conducted in 2021 indicated that PD-1 inhibitors could establish a new standard of care for patients with MPNs, potentially improving survival rates and enhancing quality of life. Additionally, a Phase II study investigating the combination of fedratinib and nivolumab in patients with myelofibrosis began in 2022 and is expected to conclude in December 2024 [77,78]. This study could provide more proof in using PD1 inhibitors to treat MPN. Continued investigation into the mechanisms of action and patient responses to PD-1 inhibition will be essential for optimizing treatment strategies and ensuring the best outcomes for individuals affected by this condition.
4.4. Tumorigenic with Abnormal Cytokine Secretion
Although various cytokine disturbances have been observed in MPN patients, particularly in TNF, IL-1β, IL-8, and IL-6, studies investigating the cytokine secretion capabilities of monocytes remain somewhat controversial. An initial study in 2017 using the ELISA method found that IL-8 levels in monocytes from MF patients were elevated compared to those in healthy monocytes, both in LPS-stimulated and non-stimulated groups. Additionally, TNF-α levels in MF monocytes increased after LPS stimulation compared to non-stimulated groups [47]. In 2018, Fisher DAC et al. demonstrated that 14 out of 15 cytokines were overproduced in MF patients upon stimulation, with monocytes identified as the principal cellular source for most of these cytokines, as determined by mass cytometry. The most prominent cytokines included TNF-α, IL-8, and IL-1RA. Moreover, TNF-α could induce a limited set of cytokines in monocytes, such as IL-8/CXCL8, IL-6, CCL4/MIP-1β, and IL-1RA [79]. The elevated concentrations of TNF-α in MPN patients are attributed to a defect in the negative regulation of Toll-like receptor (TLR) signaling in monocytes, leading to insensitivity to IL-10 and resulting in unchecked TNF-α production upon TLR activation [80]. In a subsequent study in 2020, Barone M et al. found that intracellular cytokine staining revealed a decrease in the proportions of IL-1β+, TNF+, and IL-6+ monocytes in MPN patients compared to healthy individuals, both in LPS-stimulated and non-stimulated groups. Following treatment with ruxolitinib, these monocyte proportions showed some recovery. ELISA measurements indicated that at baseline, the concentrations of IL-1β at 4 and 24 hours, TNF-α at 4 hours, IL-6 at 4 and 24 hours, and IL-10 at 24 hours were significantly reduced in the supernatants of MF monocytes compared to those of healthy controls. Notably, TNF-α levels at 24 hours demonstrated a slight increase in MF monocytes compared to healthy donors [46]. Collectively, MF monocytes are likely to secrete elevated levels of TNF-α and IL-8 following prolonged or persistent TLR stimulation, which may contribute to the progression of MPNs and could be considered as a treatment target for these diseases [81,82,83,84]. For instance, IL-8, via its receptor CXCR2, facilitates the infiltration of inflammatory cells into the bone marrow, promoting the expansion of mutated HSCsand exacerbating fibrosis [83,84]. Furthermore, blocking the CXCR1/2 axis using reparixin has shown promise in reducing bone marrow fibrosis in mouse models. An ongoing phase II clinical trial evaluating reparixin in patients with PMF is expected to be completed in 2026, which could provide further insights into its therapeutic potential.
4.5. Over-Differentiation Towards Osteoclasts
In MPNs, monocytes can abnormally differentiate into osteoclasts, leading to an imbalance between osteoclasts and osteoblasts, which contribute to the development of bone marrow fibrosis [85]. Research indicates that JAK2+ monocytes exhibit a greater propensity to differentiate into osteoclasts compared to normal monocytes. This increased differentiation results in an enriched osteoclast environment that promotes the proliferation and survival of mutated cells associated with MPNs [58,85]. Interestingly, studies have shown that osteoclasts in the context of MPNs are functionally impaired, primarily exhibiting a reduced capacity for bone resorption. This impairment can lead to a shift in the balance of bone remodeling towards bone regeneration, allowing osteoblasts to prevail and ultimately resulting in bone sclerosis [
17]. However, the proliferation of osteoblasts can further promote the development of fibrosis, as excessive osteoblast activity perpetuates the proliferation of clonal MPN cells [58]. The abnormal differentiation of monocytes into osteoclasts significantly impacts the progression of MPNs by disrupting the bone marrow structure, promoting fibrosis, exacerbating osteoporosis, and altering the microenvironment [86]. The cytokines and signaling pathways involved in this process present potential targets for future therapeutic strategies. Modulating osteoclast function may emerge as an important approach to inhibit the progression of MPNs, offering new avenues for treatment and management of the disease.
4.6. Abnormal Differentiation Towards Macrophages
Monocyte-derived macrophages play a critical role in chronic inflammation and are highly heterogeneous, capable of rapidly polarizing into either M1 (pro-inflammatory) or M2 (anti-inflammatory) macrophages in response to microenvironmental signals. Tumor-associated macrophages (TAMs) have been shown to be significant in tumor immunology, and dysregulation in their differentiation and function is crucial in the advancement of MPNs [
12]. Historical evidence from 1992 by Thiele et al. indicated that increased macrophage presence was observed in 25 out of 30 patients, suggesting their potential involvement in the generation of bone marrow fibrosis [87]. More recent studies by Molitor et al. reported a significant increase in CD68-positive macrophages in patients with PMF compared to those with chronic myeloid leukemia (CML) and healthy controls. Notably, the frequency of these macrophages in PMF was higher than in PV and ET, although CD68-positive macrophages were also elevated in PV compared to ET [88]. These macrophages can induce the proliferation of myofibroblasts via the vitamin D receptor, further contributing to fibrosis [89]. Transcriptomic analyses of isolated monocyte/macrophages from various MPN entities have highlighted their potential roles in MPN development [49]. In patients with ET, macrophages tend to polarize towards the M2 phenotype, which is generally associated with tissue repair, anti-inflammatory responses, and regulation of the tumor microenvironment. M2 macrophages inhibit excessive inflammatory responses by secreting anti-inflammatory cytokines such as IL-10 and TGF-β. Additionally, M2 macrophages are centrally involved in fibrosis through the secretion of pro-fibrotic cytokines in PMF, as well as in tissue regeneration and scar formation [49].
In experimental models, such as Asxl1-/-Jak2V617F mice, the proportions of macrophages, predominantly M1 macrophages, were markedly increased in the bone marrow and spleens compared to Jak2V617F mice. Further studies using a monocyte transfusion model demonstrated that the increased macrophages were neoplastic and derived from monocytes rather than primary tissue-resident macrophages [90]. These differentiated macrophages can further polarize into M1 macrophages under inflammatory stimulation, secreting cytokines that create a positive feedback loop, exacerbating the inflammatory response. However, further investigation is needed to determine whether there are differences in the differentiation capabilities of the three monocyte subpopulations in inflammatory environments and how the functions of the differentiated macrophages may change in the context of MPNs. Understanding these dynamics could provide insights into potential therapeutic targets for modulating macrophage activity in MPN patients.
4.7. Abnormal Differentiation Towards DCs
In vitro studies have shown that monocytes can differentiate into DCs with the assistance of granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4). The IL-4 signal transmits through the JAK1/3 signaling pathway. However, in the presence of JAK2 mutations, monocytes exhibit increased sensitivity to GM-CSF, which leads to a reduced ability to differentiate into Mo-DCs and a more pronounced macrophage-like phenotype [48]. In patients with MF, there is a notably decreased number of cDCs in peripheral blood compared to healthy donors (HD). Further phenotypic and functional assays of Mo-DCs in vitro have demonstrated that monocytes from patients with the JAK2V617F mutation show defects in differentiating towards immature Mo-DCs, characterized by a lack of upregulation of surface markers such as CD1a and CD80 [48]. Additionally, these monocytes exhibit decreased antigen presentation capabilities and increased endocytosis abilities, although their migratory capacity remains unaffected compared to HD. Interestingly, research indicates that ruxolitinib, the preferred treatment for MPNs, can adversely impact the differentiation and functionality of Mo-DCs [91]. Mouse model experiments have further revealed that defects in DCs can lead to the development of myeloproliferative diseases (MPDs) by disrupting the interactions between DCs and CD4+ T cells [92]. Given these findings, targeting the differentiation of monocytes into Mo-DCs and exploring the interactions between Mo-DCs and CD4+ T cells may provide new insights and therapeutic strategies for treating MPNs. This approach could help address the dysregulated immune responses and inflammation associated with MPNs, potentially leading to more effective treatments.
4.8. Effect of Monocytes Per Se
Monocytes are innate immune cells that play several critical roles beyond their differentiation into other cell types, such as DCs or macrophages. They possess the ability to perform endocytosis and present antigens without undergoing differentiation. Additionally, monocytes can contribute to thrombosis formation by secreting tissue factor (TF) and interacting with platelets. Their intrinsic state, including factors like senescence and metabolic disturbances, particularly abnormal mitochondrial function, can significantly influence the progression of MPNs.
Monocytes serve as a primary vascular source of TF [93]. In the context of MPNs, which are chronic inflammatory disorders, monocytes are continuously exposed to inflammatory stimuli, leading to the release of significant amounts of TF. When these monocytes migrate to sites of vascular endothelial injury, they facilitate the binding of TF with factor VIIa, activating thrombin and initiating the coagulation cascade. MPN patients often present with elevated platelet counts, particularly in ET. Platelets expressing P-selectin interact with monocytes, enhancing platelet activation and aggregation, which can lead to thrombosis-a common complication in patients with MPNs. Simultaneously, platelets can stimulate monocyte activation and promote its secretion of pro-inflammatory cytokines [94].
The healthy state of monocytes is crucial; senescent monocytes tend to secrete excess cytokines, such as TNF-α [95]. Mitochondria are the primary source of cellular energy, and mitochondrial dysfunction can significantly impact monocyte function. In monocytes, the transition from an inflammatory phenotype to an immunosuppressive state involves mitochondrial metabolic reprogramming. During inflammation, damaged mitochondria accumulate, leading to increased production of reactive oxygen species (ROS) and activation of inflammasome signaling [96]. This metabolic shift results in a transition from oxidative phosphorylation to glycolysis and lactate production, with mitochondrial ATP production being replaced by succinate oxidation, further stimulating ROS and inflammatory cytokine production. Elevated oxidative stress and mitochondrial dysfunction can lead to increased apoptosis of monocytes [97]. Such abnormalities in mitochondrial function contribute to the progression of chronic inflammatory diseases, including atherosclerosis and chronic kidney disease [98]. Given that MPNs can affect individuals of all ages, with a majority of patients being 60 years or older [99], the presence of more senescent monocytes with dysfunctional mitochondria is particularly concerning. Therefore, studying the impact of abnormal mitochondrial function in monocytes on the development of MPNs holds significant clinical and research value.
4.9. Potential Treatments Targeting Monocytes for MPNs
As described above, dysregulated monocytes may be involved in the pathophysiology of MPNs through several mechanisms, which could potentially serve as targets. These can be summarized into three major strategies: ① Elimination or Decrease of Pathogenic Monocytes: This can be achieved using inhibitors such as Rebastinib, which targets Tie2, or monoclonal antibodies like Nivolumab, which targets the PD-1/PD-L1 axis, and Elotuzumab, which targets SLAMF7. ②Neutralization of Adverse Effects Induced by Cytokines: This strategy involves targeting cytokines secreted by monocytes by utilizing inhibitors that affect IL-1β, CXCL8/CXCR, and TNF/TNFR-mediated pathways. ③Reprogramming Dysregulated Monocytes: This strategy aims to convert dysregulated monocytes into a healthy state using small molecules, although this approach has not yet been extensively explored. Currently, there are only limited number of clinical studies involving Nivolumab, Elotuzumab, and Reparixin in the treatment of myeloproliferative neoplasms (MPNs), as summarized in
Table 2. There remain numerous potential targets to explore for managing MPNs, alongside a deeper understanding of the role of monocytes in the pathophysiology of this disease.
5. Conclusions
Abnormalities in both the quantity and quality of monocytes are closely associated with the prognosis of MPNs. Several studies have investigated the mechanisms by which monocytes contribute to MPN progression. Elevated monocyte levels are linked to disease advancement through both direct effects, such as pro-angiogenic activity via Tie2 expression, pro-fibrotic effects through SLAM7 expression, and tumorigenic processes driven by excessive cytokine production, as well as indirect effects, including PD-L1 expression that inhibits T cell function, and differentiation into osteoclasts, macrophages, and mo-DCs, all of which facilitate tumor evasion from immune surveillance. Moreover, the impaired functionality of monocytes, exacerbated by age-related abnormalities in mitochondrial quantity and quality, may significantly contribute to the initiation of MPNs. However, many questions remain to be elucidated. There may be differences between peripheral blood and bone marrow monocytes, and the presence of a small number of CD34+CD14+ cells within CD14+ monocytes complicates the analysis of true monocyte characteristics. Additionally, distinctions have not been made between genetically mutated clonal monocytes and relatively normal monocytes lacking gene mutations. The mechanisms underlying the production of abnormal monocytes in MPNs and their specific roles in the onset and progression of MPNs are still not fully understood, particularly concerning whether monocytes in the bone marrow microenvironment are recruited or resident after differentiation from myeloid stem cells in response to local tumor microenvironmental cues. Therefore, it is crucial to comprehensively investigate the characteristics and underlying mechanisms of peripheral blood and bone marrow monocytes, especially their roles in the pathogenesis of MPNs. Addressing these questions will not only enhance our understanding of monocyte involvement in MPN development but also provide valuable insights for the development of novel therapeutic strategies.
Author Contributions
YY.WANG planned and structured the manuscript. XD.LI and ML.XU wrote the first draft, generated the figure and prepared the references. YY.WANG critically reviewed and submitted the manuscript. All authors contributed to the manuscript revision and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (82000135).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka HM, Wang SA, Bagg A, Barbui T, Branford S, Bueso-Ramos CE, Cortes JE, Dal Cin P, DiNardo CD, Dombret H, Duncavage EJ, Ebert BL, Estey EH, Facchetti F, Foucar K, Gangat N, Gianelli U, Godley LA, Gökbuget N, Gotlib J, Hellström-Lindberg E, Hobbs GS, Hoffman R, Jabbour EJ, Kiladjian JJ, Larson RA, Le Beau MM, Loh ML, Löwenberg B, Macintyre E, Malcovati L, Mullighan CG, Niemeyer C, Odenike OM, Ogawa S, Orfao A, Papaemmanuil E, Passamonti F, Porkka K, Pui CH, Radich JP, Reiter A, Rozman M, Rudelius M, Savona MR, Schiffer CA, Schmitt-Graeff A, Shimamura A, Sierra J, Stock WA, Stone RM, Tallman MS, Thiele J, Tien HF, Tzankov A, Vannucchi AM, Vyas P, Wei AH, Weinberg OK, Wierzbowska A, Cazzola M, Döhner H, Tefferi A. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022 Sep 15;140(11):1200-1228. [CrossRef]
- Suttantapidok S, Owattanapanich W. Clinical Characteristics, Prognostic Factors, and Thrombotic and Bleeding Outcomes in Philadelphia Chromosome-Negative Myeloproliferative Neoplasms: A Single-Center Cohort Study in Thailand. Cureus. 2025 Apr 12;17(4):e82141. [CrossRef]
- Fisher DAC, Fowles JS, Zhou A, Oh ST. Inflammatory Pathophysiology as a Contributor to Myeloproliferative Neoplasms. Front Immunol. 2021 Jun 1;12:683401. [CrossRef]
- Hermouet, S. Mutations, inflammation and phenotype of myeloproliferative neoplasms. Front Oncol. 2023 May 22;13:1196817. [CrossRef]
- Luque Paz D, Kralovics R, Skoda RC. Genetic basis and molecular profiling in myeloproliferative neoplasms. Blood. 2023 Apr 20;141(16):1909-1921. [CrossRef]
- Li B, Gale RP, Xu Z, Qin T, Song Z, Zhang P, Bai J, Zhang L, Zhang Y, Liu J, Huang G, Xiao Z. Non-driver mutations in myeloproliferative neoplasm-associated myelofibrosis. J Hematol Oncol. 2017 May 2;10(1):99. [CrossRef]
- Aguirre LE, Jain A, Ball S, Ali NA, Volpe VO, Tinsley-Vance S, Sallman D, Sweet K, Lancet J, Padron E, Yun S, Kuykendall A, Komrokji R. Triple-Negative Myelofibrosis: Disease Features, Response to Treatment and Outcomes. Clin Lymphoma Myeloma Leuk. 2024 Jul;24(7):459-467. [CrossRef]
- Gleitz HFE, Benabid A, Schneider RK. Still a burning question: the interplay between inflammation and fibrosis in myeloproliferative neoplasms. Curr Opin Hematol. 2021 Sep 1;28(5):364-371. [CrossRef]
- Cominal JG, Cacemiro MDC, Berzoti-Coelho MG, Pereira IEG, Frantz FG, Souto EX, Covas DT, de Figueiredo-Pontes LL, Oliveira MC, Malmegrim KCR, de Castro FA. Bone Marrow Soluble Mediator Signatures of Patients With Philadelphia Chromosome-Negative Myeloproliferative Neoplasms. Front Oncol. 2021 May 18;11:665037. [CrossRef]
- Cacemiro MDC, Cominal JG, Tognon R, Nunes NS, Simões BP, Lôbo de Figueiredo-Pontes L, Bazzo Catto LF, Traina F, Xisto Souto E, Albani Zambuzi F, Frantz FG, de Castro FA. Philadelphia-negative myeloproliferative neoplasms as disorders marked by cytokine modulation. Hematol Transfus Cell Ther. 2018 Apr-Jun;40(2):120-131. [CrossRef]
- Koschmieder S, Chatain N. Role of inflammation in the biology of myeloproliferative neoplasms. Blood Rev. 2020 Jul;42:100711. [CrossRef]
- Strickland M, Quek L, Psaila B. The immune landscape in BCR-ABL negative myeloproliferative neoplasms: inflammation, infections and opportunities for immunotherapy. Br J Haematol. 2022 Mar;196(5):1149-1158. [CrossRef]
- Jung SH, Lee SE, Yun S, Min DE, Shin Y, Chung YJ, Lee SH. Different inflammatory, fibrotic, and immunologic signatures between pre-fibrotic and overt primary myelofibrosis. Haematologica. 2025 Apr 1;110(4):938-951. [CrossRef]
- Morsia E, Gangat N. Myeloproliferative Neoplasms with Monocytosis. Curr Hematol Malig Rep. 2022 Feb;17(1):46-51. [CrossRef]
- Bassan VL, Barretto GD, de Almeida FC, Palma PVB, Binelli LS, da Silva JPL, Fontanari C, Castro RC, de Figueiredo Pontes LL, Frantz FG, de Castro FA. Philadelphia-negative myeloproliferative neoplasms display alterations in monocyte subpopulations frequency and immunophenotype. Med Oncol. 2022 Sep 29;39(12):223. [CrossRef]
- CE, Newberry KJ, Prijic S, Knez L, Bozinovic K, Harris DM, Spaeth EL, Post SM, Multani AS, Rampal RK, Ahn J, Levine RL, Creighton CJ, Kantarjian HM, Estrov Z. Role of neoplastic monocyte-derived fibrocytes in primary myelofibrosis. J Exp Med. 2016 Aug 22;213(9):1723-40. [CrossRef]
- Spanoudakis E, Papoutselis M, Bazdiara I, Lamprianidi E, Kordella X, Tilkeridis C, Tsatalas C, Kotsianidis I. The JAK2V617F Point Mutation Increases the Osteoclast Forming Ability of Monocytes in Patients with Chronic Myeloproliferative Neoplasms and Makes their Osteoclasts more Susceptible to JAK2 Inhibition. Mediterr J Hematol Infect Dis. 2018 Nov 1;10(1):e2018058. [CrossRef]
- Williams H, Mack C, Baraz R, Marimuthu R, Naralashetty S, Li S, Medbury H. Monocyte Differentiation and Heterogeneity: Inter-Subset and Interindividual Differences. Int J Mol Sci. 2023 May 15;24(10):8757. [CrossRef]
- Kiss M, Caro AA, Raes G, Laoui D. Systemic Reprogramming of Monocytes in Cancer. Front Oncol. 2020 Sep 17;10:1399. [CrossRef]
- Ng LG, Liu Z, Kwok I, Ginhoux F. Origin and Heterogeneity of Tissue Myeloid Cells: A Focus on GMP-Derived Monocytes and Neutrophils. Annu Rev Immunol. 2023 Apr 26;41:375-404. [CrossRef]
- Guilliams M, Mildner A, Yona S. Developmental and Functional Heterogeneity of Monocytes. Immunity. 2018 Oct 16;49(4):595-613. [CrossRef]
- Ginhoux F, Mildner A, Gautier EL, Schlitzer A, Jakubzick C, Varol C, Bain C, Guermonprez P. Editorial: Monocyte Heterogeneity and Function. Front Immunol. 2021 Jan 7;11:626725. [CrossRef]
- Canè S, Ugel S, Trovato R, Marigo I, De Sanctis F, Sartoris S, Bronte V. The Endless Saga of Monocyte Diversity. Front Immunol. 2019 Aug 6;10:1786. [CrossRef]
- Patel AA, Zhang Y, Fullerton JN, Boelen L, Rongvaux A, Maini AA, Bigley V, Flavell RA, Gilroy DW, Asquith B, Macallan D, Yona S. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J Exp Med. 2017 Jul 3;214(7):1913-1923. [CrossRef]
- Tak T, Drylewicz J, Conemans L, de Boer RJ, Koenderman L, Borghans JAM, Tesselaar K. Circulatory and maturation kinetics of human monocyte subsets in vivo. Blood. 2017 Sep 21;130(12):1474-1477. [CrossRef]
- Bianchini M, Duchêne J, Santovito D, Schloss MJ, Evrard M, Winkels H, Aslani M, Mohanta SK, Horckmans M, Blanchet X, Lacy M, von Hundelshausen P, Atzler D, Habenicht A, Gerdes N, Pelisek J, Ng LG, Steffens S, Weber C, Megens RTA. PD-L1 expression on nonclassical monocytes reveals their origin and immunoregulatory function. Sci Immunol. 2019 Jun 21;4(36):eaar3054. [CrossRef]
- Kapellos TS, Bonaguro L, Gemünd I, Reusch N, Saglam A, Hinkley ER, Schultze JL. Human Monocyte Subsets and Phenotypes in Major Chronic Inflammatory Diseases. Front Immunol. 2019 Aug 30;10:2035. [CrossRef]
- Robinson A, Han CZ, Glass CK, Pollard JW. Monocyte Regulation in Homeostasis and Malignancy. Trends Immunol. 2021 Feb;42(2):104-119. [CrossRef]
- Guglietta S, Krieg C. Phenotypic and functional heterogeneity of monocytes in health and cancer in the era of high dimensional technologies. Blood Rev. 2023 Mar;58:101012. [CrossRef]
- Li C, Xiao M, Geng S, Wang Y, Zeng L, Lai P, Gong Y, Chen X. Comprehensive analysis of human monocyte subsets using full-spectrum flow cytometry and hierarchical marker clustering. Front Immunol. 2024 Apr 29;15:1405249.
- Hoffmann J, Fišer K, Liebetrau C, Staubach N, Kost D, Voss S, Heiden AZ, Dörr O, Lipps C, Nef HM, Möllmann H, Hamm CW, Keller T, Troidl C. High-Content Immunophenotyping and Hierarchical Clustering Reveal Sources of Heterogeneity and New Surface Markers of Human Blood Monocyte Subsets. Thromb Haemost. 2020 Jan;120(1):141-155. [CrossRef]
- Boyette LB, Macedo C, Hadi K, Elinoff BD, Walters JT, Ramaswami B, Chalasani G, Taboas JM, Lakkis FG, Metes DM. Phenotype, function, and differentiation potential of human monocyte subsets. PLoS One. 2017 Apr 26;12(4):e0176460. [CrossRef]
- Sánchez-Torres C, García-Romo GS, Cornejo-Cortés MA, Rivas-Carvalho A, Sánchez-Schmitz G. CD16+ and CD16- human blood monocyte subsets differentiate in vitro to dendritic cells with different abilities to stimulate CD4+ T cells. Int Immunol. 2001 Dec;13(12):1571-81. [CrossRef]
- Huang MN, Nicholson LT, Batich KA, Swartz AM, Kopin D, Wellford S, Prabhakar VK, Woroniecka K, Nair SK, Fecci PE, Sampson JH, Gunn MD. Antigen-loaded monocyte administration induces potent therapeutic antitumor T cell responses. J Clin Invest. 2020 Feb 3;130(2):774-788. [CrossRef]
- Tacke F, Ginhoux F, Jakubzick C, van Rooijen N, Merad M, Randolph GJ. Immature monocytes acquire antigens from other cells in the bone marrow and present them to T cells after maturing in the periphery. J Exp Med. 2006 Mar 20;203(3):583-97. [CrossRef]
- Jakubzick C, Gautier EL, Gibbings SL, Sojka DK, Schlitzer A, Johnson TE, Ivanov S, Duan Q, Bala S, Condon T, van Rooijen N, Grainger JR, Belkaid Y, Ma’ayan A, Riches DW, Yokoyama WM, Ginhoux F, Henson PM, Randolph GJ. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013 Sep 19;39(3):599-610. [CrossRef]
- Jakubzick CV, Randolph GJ, Henson PM. Monocyte differentiation and antigen-presenting functions. Nat Rev Immunol. 2017 Jun;17(6):349-362. [CrossRef]
- Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011 Oct 10;11(11):762-74. [CrossRef]
- Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007 Jan;117(1):185-94. [CrossRef]
- Ancuta P, Rao R, Moses A, Mehle A, Shaw SK, Luscinskas FW, Gabuzda D. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J Exp Med. 2003 Jun 16;197(12):1701-7. [CrossRef]
- Cormican S, Griffin MD. Human Monocyte Subset Distinctions and Function: Insights From Gene Expression Analysis. Front Immunol. 2020 Jun 4;11:1070. [CrossRef]
- Anbazhagan K, Duroux-Richard I, Jorgensen C, Apparailly F. Transcriptomic network support distinct roles of classical and non-classical monocytes in human. Int Rev Immunol. 2014 Nov-Dec;33(6):470-89. [CrossRef]
- Schmidl C, Renner K, Peter K, Eder R, Lassmann T, Balwierz PJ, Itoh M, Nagao-Sato S, Kawaji H, Carninci P, Suzuki H, Hayashizaki Y, Andreesen R, Hume DA, Hoffmann P, Forrest AR, Kreutz MP, Edinger M, Rehli M; FANTOM consortium. Transcription and enhancer profiling in human monocyte subsets. Blood. 2014 Apr 24;123(17):e90-9. [CrossRef]
- Wong KL, Tai JJ, Wong WC, Han H, Sem X, Yeap WH, Kourilsky P, Wong SC. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011 Aug 4;118(5):e16-31. [CrossRef]
- Gren ST, Rasmussen TB, Janciauskiene S, Håkansson K, Gerwien JG, Grip O. A Single-Cell Gene-Expression Profile Reveals Inter-Cellular Heterogeneity within Human Monocyte Subsets. PLoS One. 2015 Dec 9;10(12):e0144351. [CrossRef]
- Barone M, Catani L, Ricci F, Romano M, Forte D, Auteri G, Bartoletti D, Ottaviani E, Tazzari PL, Vianelli N, Cavo M, Palandri F. The role of circulating monocytes and JAK inhibition in the infectious-driven inflammatory response of myelofibrosis. Oncoimmunology. 2020 Jun 23;9(1):1782575. [CrossRef]
- de la Guardia RD, Correa JG, López-Millán B, Juan M, Bueno C, Cervantes F, Menéndez P. Detection of inflammatory monocytes but not mesenchymal stem/stromal cells in peripheral blood of patients with myelofibrosis. Br J Haematol. 2018 Apr;181(1):133-137. [CrossRef]
- Romano M, Sollazzo D, Trabanelli S, Barone M, Polverelli N, Perricone M, Forte D, Luatti S, Cavo M, Vianelli N, Jandus C, Palandri F, Catani L. Mutations in JAK2 and Calreticulin genes are associated with specific alterations of the immune system in myelofibrosis. Oncoimmunology. 2017 Jul 5;6(10):e1345402. [CrossRef]
- Fan W, Cao W, Shi J, Gao F, Wang M, Xu L, Wang F, Li Y, Guo R, Bian Z, Li W, Jiang Z, Ma W. Contributions of bone marrow monocytes/macrophages in myeloproliferative neoplasms with JAK2V617F mutation. Ann Hematol. 2023 Jul;102(7):1745-1759. [CrossRef]
- Kong T, Laranjeira ABA, Letson CT, Yu L, Lin S, Fowles JS, Fisher DAC, Ng S, Yang W, He F, Youn M, Mark K, Jose AS, Liu J, Kim AB, Cox MJ, Fulbright MC, Jayanthan A, Los G, Rentschler SL, Ding L, Sakamoto KM, Dunn SE, Challen GA, Oh ST. RSK1 is an exploitable dependency in myeloproliferative neoplasms and secondary acute myeloid leukemia. Nat Commun. 2025 Jan 16;16(1):492. [CrossRef]
- Bassan VL, de Freitas Martins Felício R, Ribeiro Malmegrim KC, Attié de Castro F. Myeloproliferative Neoplasms Transcriptome Reveals Pro-Inflammatory Signature and Enrichment in Peripheral Blood Monocyte-Related Genes. Cancer Invest. 2024 Aug;42(7):605-618. [CrossRef]
- Campanelli R, Rosti V, Fois G, Bonetti E, Barosi G, Massa M. CD14(bright)CD16(low) intermediate monocytes expressing Tie2 are increased in the peripheral blood of patients with primary myelofibrosis. Exp Hematol. 2014 Apr;42(4):244-6. [CrossRef]
- Campanelli R, Fois G, Catarsi P, Poletto V, Villani L, Erba BG, Maddaluno L, Jemos B, Salmoiraghi S, Guglielmelli P, Abbonante V, Di Buduo CA, Balduini A, Iurlo A, Barosi G, Rosti V, Massa M; AGIMM Investigators. Tie2 Expressing Monocytes in the Spleen of Patients with Primary Myelofibrosis. PLoS One. 2016 Jun 9;11(6):e0156990. [CrossRef]
- Maekawa T, Kato S, Kawamura T, Takada K, Sone T, Ogata H, Saito K, Izumi T, Nagao S, Takano K, Okada Y, Tachi N, Teramoto M, Horiuchi T, Hikota-Saga R, Endo-Umeda K, Uno S, Osawa Y, Kobayashi A, Kobayashi S, Sato K, Hashimoto M, Suzu S, Usuki K, Morishita S, Araki M, Makishima M, Komatsu N, Kimura F. Increased SLAMF7high monocytes in myelofibrosis patients harboring JAK2V617F provide a therapeutic target of elotuzumab. Blood. 2019 Sep 5;134(10):814-825. [CrossRef]
- Cimen Bozkus C, Roudko V, Finnigan JP, Mascarenhas J, Hoffman R, Iancu-Rubin C, Bhardwaj N. Immune Checkpoint Blockade Enhances Shared Neoantigen-Induced T-cell Immunity Directed against Mutated Calreticulin in Myeloproliferative Neoplasms. Cancer Discov. 2019 Sep;9(9):1192-1207. [CrossRef]
- Shin DS, Zaretsky JM, Escuin-Ordinas H, Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, Grasso CS, Hugo W, Sandoval S, Torrejon DY, Palaskas N, Rodriguez GA, Parisi G, Azhdam A, Chmielowski B, Cherry G, Seja E, Berent-Maoz B, Shintaku IP, Le DT, Pardoll DM, Diaz LA Jr, Tumeh PC, Graeber TG, Lo RS, Comin-Anduix B, Ribas A. Primary Resistance to PD-1 Blockade Mediated by JAK1/2 Mutations. Cancer Discov. 2017 Feb;7(2):188-201. [CrossRef]
- Fleischman AG, Aichberger KJ, Luty SB, Bumm TG, Petersen CL, Doratotaj S, Vasudevan KB, LaTocha DH, Yang F, Press RD, Loriaux MM, Pahl HL, Silver RT, Agarwal A, O’Hare T, Druker BJ, Bagby GC, Deininger MW. TNFα facilitates clonal expansion of JAK2V617F positive cells in myeloproliferative neoplasms. Blood. 2011 Dec 8;118(24):6392-8. [CrossRef]
- Veletic I, Manshouri T, Multani AS, Yin CC, Chen L, Verstovsek S, Estrov Z. Myelofibrosis osteoclasts are clonal and functionally impaired. Blood. 2019 May 23;133(21):2320-2324. [CrossRef]
- De Maeyer RPH, Chambers ES. The impact of ageing on monocytes and macrophages. Immunol Lett. 2021 Feb;230:1-10. [CrossRef]
- Gibellini L, De Biasi S, Paolini A, Borella R, Boraldi F, Mattioli M, Lo Tartaro D, Fidanza L, Caro-Maldonado A, Meschiari M, Iadisernia V, Bacca E, Riva G, Cicchetti L, Quaglino D, Guaraldi G, Busani S, Girardis M, Mussini C, Cossarizza A. Altered bioenergetics and mitochondrial dysfunction of monocytes in patients with COVID-19 pneumonia. EMBO Mol Med. 2020 Dec 7;12(12):e13001. [CrossRef]
- Jones N, Iljin K, Dumont DJ, Alitalo K. Tie receptors: new modulators of angiogenic and lymphangiogenic responses. Nat Rev Mol Cell Biol. 2001 Apr;2(4):257-67. [CrossRef]
- Venneri MA, De Palma M, Ponzoni M, Pucci F, Scielzo C, Zonari E, Mazzieri R, Doglioni C, Naldini L. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood. 2007 Jun 15;109(12):5276-85. [CrossRef]
- De Palma M, Murdoch C, Venneri MA, Naldini L, Lewis CE. Tie2-expressing monocytes: regulation of tumor angiogenesis and therapeutic implications. Trends Immunol. 2007 Dec;28(12):519-24. [CrossRef]
- Cortes J, Talpaz M, Smith HP, Snyder DS, Khoury J, Bhalla KN, Pinilla-Ibarz J, Larson R, Mitchell D, Wise SC, Rutkoski TJ, Smith BD, Flynn DL, Kantarjian HM, Rosen O, Van Etten RA. Phase 1 dose-finding study of rebastinib (DCC-2036) in patients with relapsed chronic myeloid leukemia and acute myeloid leukemia. Haematologica. 2017 Mar;102(3):519-528. [CrossRef]
- Anampa JD, Flynn DL, Leary C, Oh S, Xue X, Oktay MH, Condeelis JS, Sparano JA. Phase Ib Clinical and Pharmacodynamic Study of the TIE2 Kinase Inhibitor Rebastinib with Paclitaxel or Eribulin in HER2-Negative Metastatic Breast Cancer. Clin Cancer Res. 2025 Jan 17;31(2):266-277. [CrossRef]
- Zhang Y, Brekken RA. Are TEMs Canceled? Questioning the Functional Relevance of Tie2-Expressing Macrophages. Cancer Res. 2022 Apr 1;82(7):1172-1173. [CrossRef]
- Altalbawy FMA, Babamuradova Z, Baldaniya L, Singh A, Singh KU, Ballal S, Sabarivani A, Sead FF, Alam R, Alshahrani MY. The multifaceted role of CS1 (SLAMF7) in immunoregulation: Implications for cancer therapy and autoimmune disorders. Exp Cell Res. 2025 Apr 1;447(1):114516. [CrossRef]
- Zhang Z, Zhang Y, Chen Z, Xia L. Emerging roles of SLAMF7 in immune cells and related diseases. Innate Immun. 2025 Jan-Dec;31:17534259251326700. [CrossRef]
- Van Egeren D, Kamaz B, Liu S, Nguyen M, Reilly CR, Kalyva M, DeAngelo DJ, Galinsky I, Wadleigh M, Winer ES, Luskin MR, Stone RM, Garcia JS, Hobbs GS, Michor F, Cortes-Ciriano I, Mullally A, Hormoz S. Transcriptional differences between JAK2-V617F and wild-type bone marrow cells in patients with myeloproliferative neoplasms. Exp Hematol. 2022 Mar;107:14-19. [CrossRef]
- Martino EA, Palmieri S, Galli M, Derudas D, Mina R, Della Pepa R, Zambello R, Vigna E, Bruzzese A, Mangiacavalli S, Zamagni E, Califano C, Musso M, Conticello C, Cerchione C, Mele G, Di Renzo N, Offidani M, Tarantini G, Casaluci GM, Rago A, Ria R, Uccello G, Barilà G, Palumbo G, Pettine L, De Magistris C, Vincelli ID, Brunori M, Accardi F, Amico V, Amendola A, Fontana R, Bongarzoni V, Rossini B, Cotzia E, Gozzetti A, Rizzi R, Sgherza N, Curci P, Mancuso K, Reddiconto G, Maroccia A, Franceschini L, Bertuglia G, Nappi D, Barbieri E, Quaresima M, Petrucci MT, Di Raimondo F, Neri A, Tripepi G, Musto P, Morabito F, Gentile M. Outcomes and prognostic indicators in daratumumab-refractory multiple myeloma: a multicenter real-world study of elotuzumab, pomalidomide, and dexamethasone in 247 patients. ESMO Open. 2025 Jan 7;10(2):104084. [CrossRef]
- Liu R, Li HF, Li S. PD-1-mediated inhibition of T cell activation: Mechanisms and strategies for cancer combination immunotherapy. Cell Insight. 2024 Feb 23;3(2):100146. [CrossRef]
- Oh SA, Wu DC, Cheung J, Navarro A, Xiong H, Cubas R, Totpal K, Chiu H, Wu Y, Comps-Agrar L, Leader AM, Merad M, Roose-Germa M, Warming S, Yan M, Kim JM, Rutz S, Mellman I. PD-L1 expression by dendritic cells is a key regulator of T-cell immunity in cancer. Nat Cancer. 2020 Jul;1(7):681-691. [CrossRef]
- Asai A, Yasuoka H, Matsui M, Tsuchimoto Y, Fukunishi S, Higuchi K. Programmed Death 1 Ligand Expression in the Monocytes of Patients with Hepatocellular Carcinoma Depends on Tumor Progression. Cancers (Basel). 2020 Aug 14;12(8):2286. [CrossRef]
- Prestipino A, Emhardt AJ, Aumann K, O’Sullivan D, Gorantla SP, Duquesne S, Melchinger W, Braun L, Vuckovic S, Boerries M, Busch H, Halbach S, Pennisi S, Poggio T, Apostolova P, Veratti P, Hettich M, Niedermann G, Bartholomä M, Shoumariyeh K, Jutzi JS, Wehrle J, Dierks C, Becker H, Schmitt-Graeff A, Follo M, Pfeifer D, Rohr J, Fuchs S, Ehl S, Hartl FA, Minguet S, Miething C, Heidel FH, Kröger N, Triviai I, Brummer T, Finke J, Illert AL, Ruggiero E, Bonini C, Duyster J, Pahl HL, Lane SW, Hill GR, Blazar BR, von Bubnoff N, Pearce EL, Zeiser R. Oncogenic JAK2V617F causes PD-L1 expression, mediating immune escape in myeloproliferative neoplasms. Sci Transl Med. 2018 Feb 21;10(429):eaam7729. [CrossRef]
- Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L, Saco J, Homet Moreno B, Mezzadra R, Chmielowski B, Ruchalski K, Shintaku IP, Sanchez PJ, Puig-Saus C, Cherry G, Seja E, Kong X, Pang J, Berent-Maoz B, Comin-Anduix B, Graeber TG, Tumeh PC, Schumacher TN, Lo RS, Ribas A. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med. 2016 Sep 1;375(9):819-29. [CrossRef]
- Wang JC, Chen C, Kundra A, Kodali S, Pandey A, Wong C, Cheung T, Gotlieb V, Joseph G, Tribie S. Programmed Cell Death Receptor (PD-1) Ligand (PD-L1) expression in Philadelphia chromosome-negative myeloproliferative neoplasms. Leuk Res. 2019 Apr;79:52-59. [CrossRef]
- Hobbs G, Cimen Bozkus C, Moshier E, Dougherty M, Bar-Natan M, Sandy L, Johnson K, Foster JE, Som T, Macrae M, Marble H, Salama M, El Jamal SM, Zubizarreta N, Wadleigh M, Stone R, Bhardwaj N, Iancu-Rubin C, Mascarenhas J. PD-1 inhibition in advanced myeloproliferative neoplasms. Blood Adv. 2021 Dec 14;5(23):5086-5097. [CrossRef]
- Isfort S, von Bubnoff N, Al-Ali HK, Becker H, Götze T, le Coutre P, Griesshammer M, Moskwa C, Wohn L, Riedel J, Palandri F, Manz K, Hochhaus A, Döhner K, Heidel FH. FRACTION: protocol of a phase II study of Fedratinib and Nivolumab combination in patients with myelofibrosis and resistance or suboptimal response to JAK-inhibitor treatment of the German MPN study group (GSG-MPN). Ann Hematol. 2024 Aug;103(8):2775-2785. [CrossRef]
- Fisher DAC, Miner CA, Engle EK, Hu H, Collins TB, Zhou A, Allen MJ, Malkova ON, Oh ST. Cytokine production in myelofibrosis exhibits differential responsiveness to JAK-STAT, MAP kinase, and NFκB signaling. Leukemia. 2019 Aug;33(8):1978-1995. [CrossRef]
- Lai HY, Brooks SA, Craver BM, Morse SJ, Nguyen TK, Haghighi N, Garbati MR, Fleischman AG. Defective negative regulation of Toll-like receptor signaling leads to excessive TNF-α in myeloproliferative neoplasm. Blood Adv. 2019 Jan 22;3(2):122-131. [CrossRef]
- Heaton WL, Senina AV, Pomicter AD, Salama ME, Clair PM, Yan D, Bell RN, Gililland JM, Prchal JT, O’Hare T, Deininger MW. Autocrine Tnf signaling favors malignant cells in myelofibrosis in a Tnfr2-dependent fashion. Leukemia. 2018 Nov;32(11):2399-2411. [CrossRef]
- Müller P, Baldauf CK, Haage TR, Waldleben AM, Richter F, Pfizenmaier K, Fischer T. Anti-inflammatory treatment in MPN: targeting TNFR1 and TNFR2 in JAK2-V617F-induced disease. Blood Adv. 2021 Dec 14;5(23):5349-5359. [CrossRef]
- Andrew Dunbar, Min Lu, Mirko Farina, et al. Increased Interleukin-8 (IL8)-CXCR2 Signaling Promotes Progression of Bone Marrow Fibrosis in Myeloproliferative Neoplasms. Blood 2020; 136 (Supplement 1): 6–7.
- Vermeersch G, Proost P, Struyf S, Gouwy M, Devos T. CXCL8 and its cognate receptors CXCR1/CXCR2 in primary myelofibrosis. Haematologica. 2024 Jul 1;109(7):2060-2072. [CrossRef]
- Yahara Y, Nguyen T, Ishikawa K, Kamei K, Alman BA. The origins and roles of osteoclasts in bone development, homeostasis and repair. Development. 2022 Apr 15;149(8):dev199908. [CrossRef]
- Curto-Garcia N, Harrison C, McLornan DP. Bone marrow niche dysregulation in myeloproliferative neoplasms. Haematologica. 2020 May;105(5):1189-1200. [CrossRef]
- Thiele J, Braeckel C, Wagner S, Falini B, Dienemann D, Stein H, Fischer R. Macrophages in normal human bone marrow and in chronic myeloproliferative disorders: an immunohistochemical and morphometric study by a new monoclonal antibody (PG-M1) on trephine biopsies. Virchows Arch A Pathol Anat Histopathol. 1992;421(1):33-9. [CrossRef]
- Molitor DCA, Boor P, Buness A, Schneider RK, Teichmann LL, Körber RM, Horvath GL, Koschmieder S, Gütgemann I. Macrophage frequency in the bone marrow correlates with morphologic subtype of myeloproliferative neoplasm. Ann Hematol. 2021 Jan;100(1):97-104. [CrossRef]
- Wakahashi K, Minagawa K, Kawano Y, Kawano H, Suzuki T, Ishii S, Sada A, Asada N, Sato M, Kato S, Shide K, Shimoda K, Matsui T, Katayama Y. Vitamin D receptor-mediated skewed differentiation of macrophages initiates myelofibrosis and subsequent osteosclerosis. Blood. 2019 Apr 11;133(15):1619-1629. [CrossRef]
- Shi Z, Liu J, Zhao Y, Yang L, Cai Y, Zhang P, Xu Z, Qin T, Qu S, Pan L, Wu J, Yan X, Li Z, Zhang W, Yan Y, Huang H, Huang G, Li B, Wu X, Xiao Z. ASXL1 mutations accelerate bone marrow fibrosis via EGR1-TNFA axis-mediated neoplastic fibrocyte generation in myeloproliferative neoplasms. Haematologica. 2023 May 1;108(5):1359-1373. [CrossRef]
- Heine A, Held SA, Daecke SN, Wallner S, Yajnanarayana SP, Kurts C, Wolf D, Brossart P. The JAK-inhibitor ruxolitinib impairs dendritic cell function in vitro and in vivo. Blood. 2013 Aug 15;122(7):1192-202. [CrossRef]
- Humblet-Baron S, Barber JS, Roca CP, Lenaerts A, Koni PA, Liston A. Murine myeloproliferative disorder as a consequence of impaired collaboration between dendritic cells and CD4 T cells. Blood. 2019 Jan 24;133(4):319-330. [CrossRef]
- Sachetto ATA, Mackman N. Monocyte Tissue Factor Expression: Lipopolysaccharide Induction and Roles in Pathological Activation of Coagulation. Thromb Haemost. 2023 Nov;123(11):1017-1033. [CrossRef]
- He F, Laranjeira AB, Kong T, Lin S, Ashworth KJ, Liu A, Lasky NM, Fisher DA, Cox MJ, Fulbright MC, Antunes-Heck L, Yu L, Brakhane M, Gao B, Sykes SM, D’Alessandro A, Di Paola J, Oh ST. Multiomic profiling reveals metabolic alterations mediating aberrant platelet activity and inflammation in myeloproliferative neoplasms. J Clin Invest. 2024 Feb 1;134(3):e172256. [CrossRef]
- Wang C, Cheng Y, Li B, Qiu X, Hu H, Zhang X, Lu Z, Zheng F. Transcriptional characteristics and functional validation of three monocyte subsets during aging. Immun Ageing. 2023 Sep 27;20(1):50. [CrossRef]
- Cortés M, Brischetto A, Martinez-Campanario MC, Ninfali C, Domínguez V, Fernández S, Celis R, Esteve-Codina A, Lozano JJ, Sidorova J, Garrabou G, Siegert AM, Enrich C, Pintado B, Morales-Ruiz M, Castro P, Cañete JD, Postigo A. Inflammatory macrophages reprogram to immunosuppression by reducing mitochondrial translation. Nat Commun. 2023 Nov 17;14(1):7471. [CrossRef]
- Sardenberg C, Suassuna P, Watanabe R, Cruz Andreoli MC, Aparecida Dalboni M, Faria Seabra V, Draibe SA, Cendoroglo Neto M, Jaber B. Balance between cytokine production by peripheral blood mononuclear cells and reactive oxygen species production by monocytes in patients with chronic kidney disease. Ren Fail. 2004 Nov;26(6):673-81. [CrossRef]
- Ravi S, Mitchell T, Kramer P, Chacko B, Darley-Usmar VM. Mitochondria in monocytes and macrophages-implications for translational and basic research. Int J Biochem Cell Biol. 2014 Aug;53:202-207. [CrossRef]
- Shallis RM, Zeidan AM, Wang R, Podoltsev NA. Epidemiology of the Philadelphia Chromosome-Negative Classical Myeloproliferative Neoplasms. Hematol Oncol Clin North Am. 2021 Apr;35(2):177-189. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).