Introduction
Ultra-high contrast MRI is a term used to describe MR imaging that shows abnormalities with high contrast when little or no abnormality is seen using conventional state-of-the-art MRI sequences. It is achieved without the use of increased static or gradient magnetic fields. For soft tissues, Xray CT can be regarded as high contrast, conventional state-of-the-art MRI as very high contrast and MRI which shows abnormalities with obvious contrast when they are not seen with conventional MRI as ultra-high contrast (UHC) [
1].
A common mechanism for achieving UHC MRI is synergistic contrast in which changes in a single tissue property such as T
1 are used twice or more in the same sequence to increase contrast rather than just once which is the case with most conventional sequences [
2]. An example is the divided subtracted inversion recovery (dSIR) sequence in which the signals from two inversion recovery (IR) sequences with different inversion times (TIs) are subtracted to increase the contrast produced by small changes in T
1. This contrast is increased further by division of the subtraction by the sum of the signals from the two sequences [
3].
Another way of achieving UHC MRI is to use a high quality single tissue property map such as a T1 or T2 map and retrospectively apply a shaped filter to the map. The filter can increase contrast in one or more domains of tissue property values and maintain anatomical detail in the rest of the image. This is unlike narrow windowing of images which, when used to increase contrast, is accompanied by saturation of the display gray scale at the upper and lower signal thresholds. Narrow windowing leads to loss of anatomical detail and limits the degree to which contrast can be increased by windowing.
The principal clinical focus of UHC MRI to date has been on small changes in tissue properties from normal that are insufficient to generate useful contrast with conventional state-of-the-art sequences. When abnormalities are already seen with high contrast using conventional sequences, there is no particular clinical gain in demonstrating them with even greater contrast. As a result, attention has been directed at normal or near normal appearing tissues as seen with conventional state-of-the-art imaging.
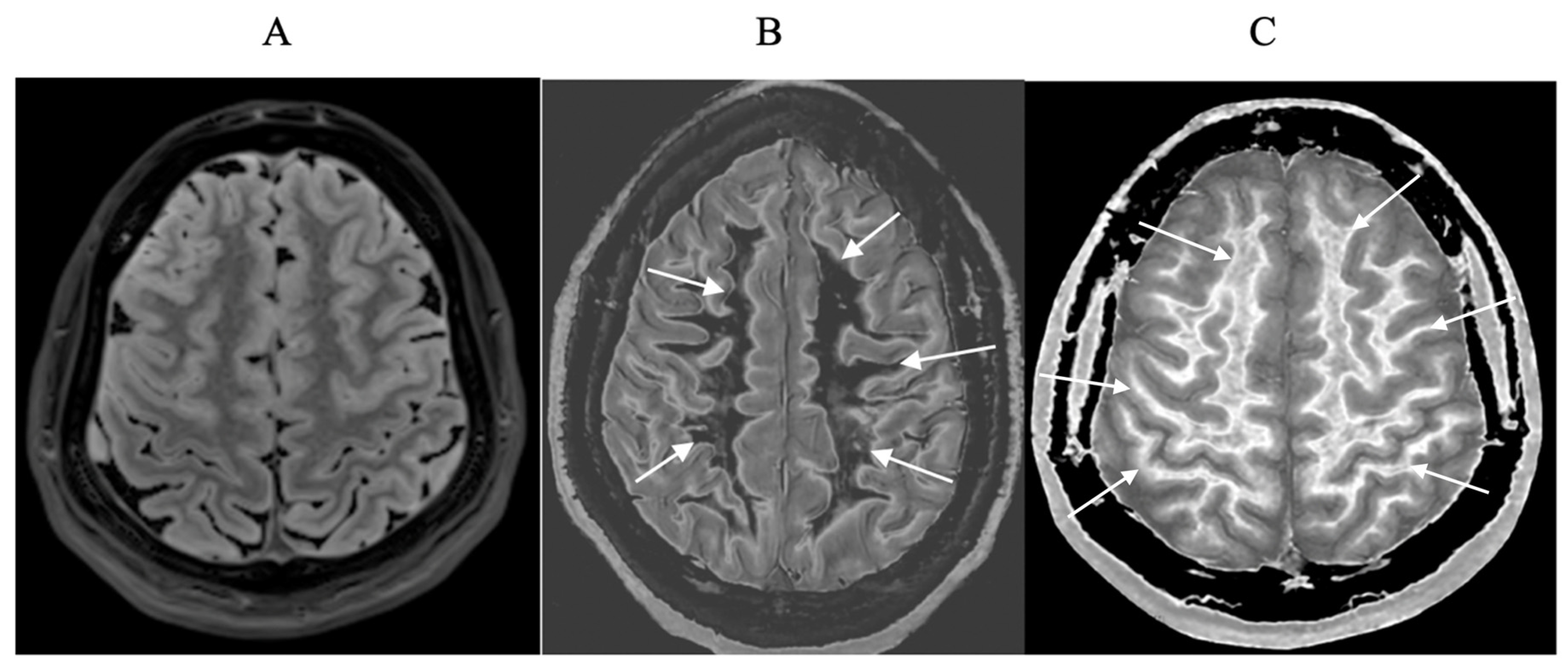
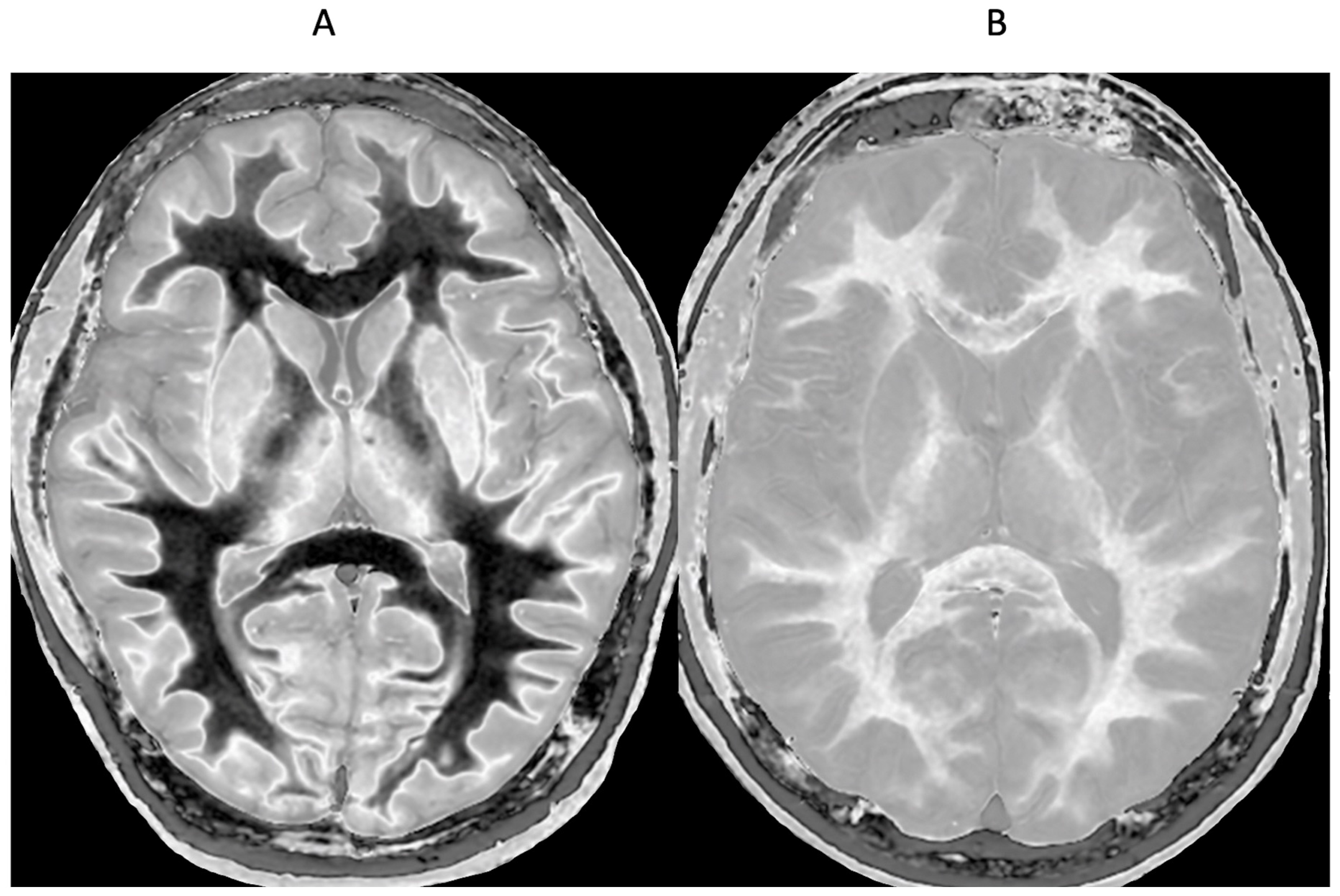
Small changes in T
1 may produce obvious abnormalities with the dSIR sequence as illustrated in a case of mild traumatic brain injury (mTBI) in an 18-year-old male patient shown in
Figure 1. No abnormality is seen on the T
2-FLAIR image in the patient (
Figure 1A). The dSIR sequence in a normal age matched control shows normal white matter in the cerebral hemispheres as low signal (dark) (arrows) in
Figure 1B. The same dSIR sequence in the patient shows abnormal high signal in his entire white matter (arrows) (
Figure 1C). High signal (light) well defined boundaries are seen between normal white and gray matter on the dSIR image in
Figure 1B. These are less obvious in
Figure 1C because of the high signal in the abnormal white matter.
High contrast abnormalities in white matter have been seen using dSIR T
1-bipolar filter (BLAIR) images of the type illustrated in
Figure 1 in areas where little or no abnormality has been seen with T
2-FLAIR or T
2-wSE sequences in cases of mTBI [
4], multiple sclerosis (MS) [
5], methamphetamine substance use disorder [
6] and Grinker's myelinopathy [
7].
The purpose of this educational review is to describe the basic physics underlying UHC MRI using BLAIR images and illustrate use of these images in patients in order to facilitate wider use of them in clinical practice.
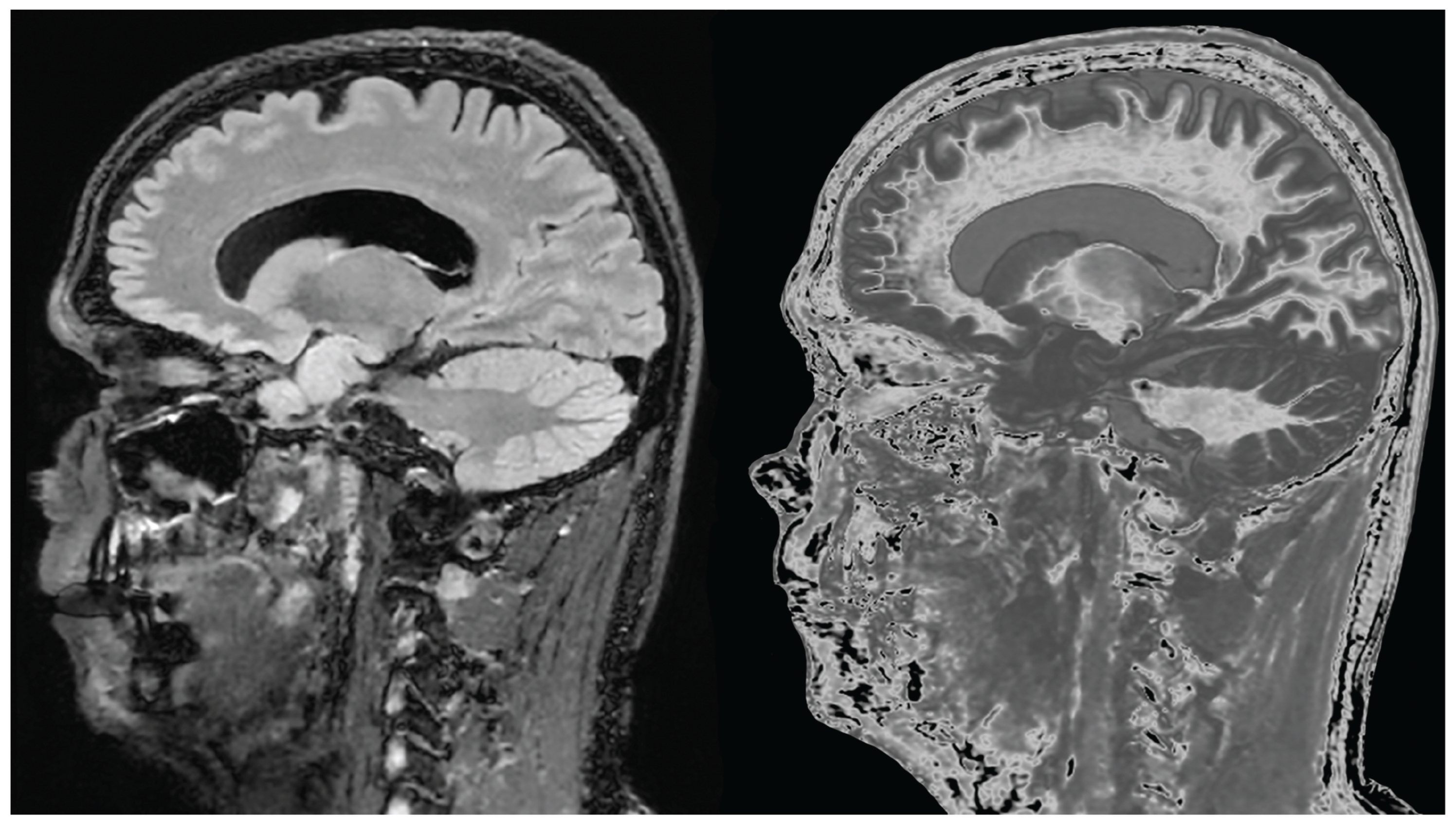
Figure 1.
Positionally matched images of the brain in a 24-year-old male patient with mTBI (A and C) and a normal control (B). (A) is a T2-FLAIR image in the patient which shows no abnormality. (B) is a narrow mD dSIR image of the brain in the normal control. The white matter in the central region of this image has a normal low signal (dark) appearance (arrows). (C) is a narrow mD dSIR image performed in the patient with the same sequence as in the control. This image shows all the patient's white matter with abnormal high signal (light) (arrows) rather than the normal low signal (dark) appearance in the control (arrows) (B). There is a night and day difference in signal between normal and abnormal white matter in (B) and (C). High signal boundaries are seen between normal white matter and normal gray matter in (B). These are less obvious in (C) because of the high signal in the abnormal white matter.
Figure 1.
Positionally matched images of the brain in a 24-year-old male patient with mTBI (A and C) and a normal control (B). (A) is a T2-FLAIR image in the patient which shows no abnormality. (B) is a narrow mD dSIR image of the brain in the normal control. The white matter in the central region of this image has a normal low signal (dark) appearance (arrows). (C) is a narrow mD dSIR image performed in the patient with the same sequence as in the control. This image shows all the patient's white matter with abnormal high signal (light) (arrows) rather than the normal low signal (dark) appearance in the control (arrows) (B). There is a night and day difference in signal between normal and abnormal white matter in (B) and (C). High signal boundaries are seen between normal white matter and normal gray matter in (B). These are less obvious in (C) because of the high signal in the abnormal white matter.
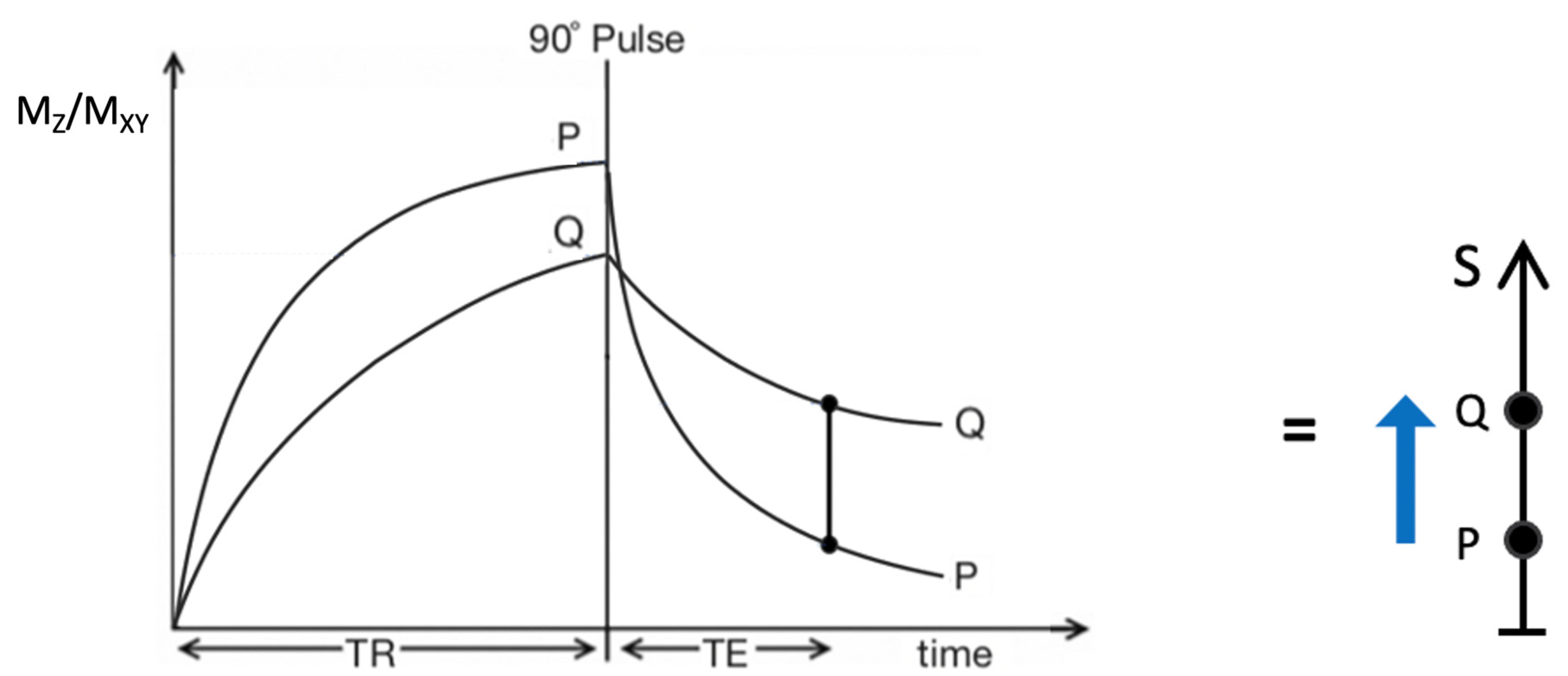
Figure 2.
Plot of MZ/MXY against time for a T2-weighted version of the SE sequence for two tissues P (with a shorter T1 and T2) and Q (with a longer T1 and T2). Overall T1 and T2 dependent contrast between P and Q is shown with the positive blue arrow on the right.
Figure 2.
Plot of MZ/MXY against time for a T2-weighted version of the SE sequence for two tissues P (with a shorter T1 and T2) and Q (with a longer T1 and T2). Overall T1 and T2 dependent contrast between P and Q is shown with the positive blue arrow on the right.
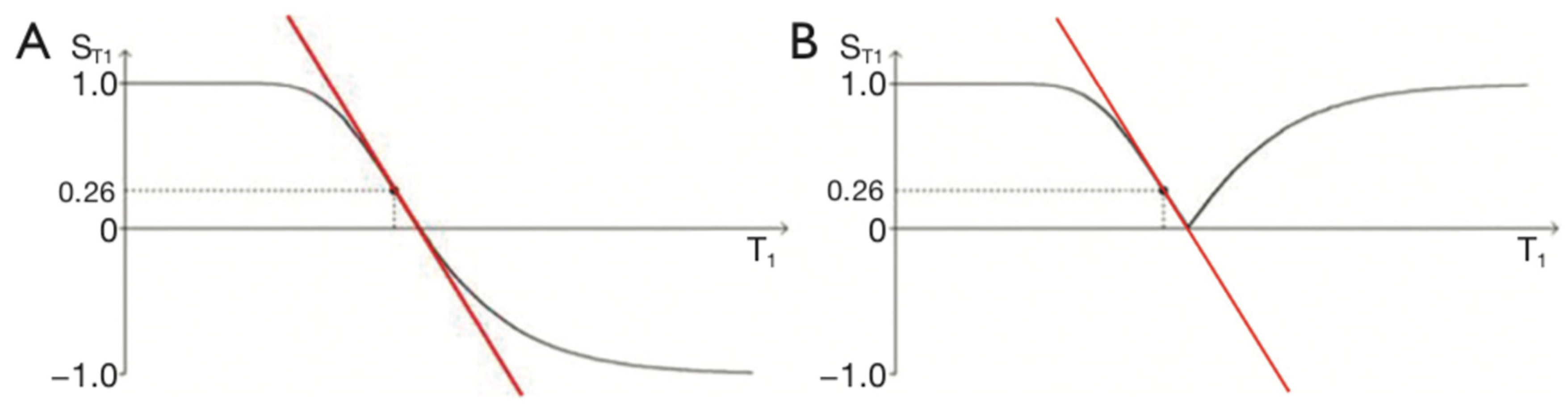
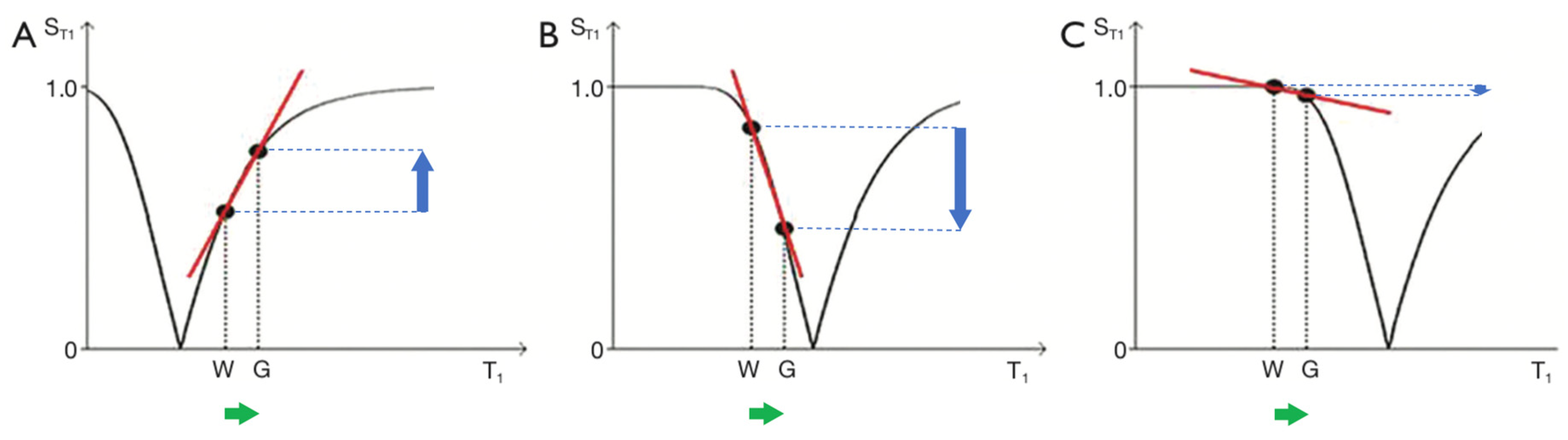
Figure 3.
IR T1-filters with plots of signal ST1 against T1 in phase-sensitive (A) and magnitude forms (B). (A) is a low pass filter and (B) is a negative unipolar filter. (A) shows both positive and negative values for ST1 whereas (B) shows negative values "reflected" across the X axis so they become positive. The maximum size of the slopes of the two T1-filters which are tangential to the slope are shown as red lines. The slopes are negative in both cases. .
Figure 3.
IR T1-filters with plots of signal ST1 against T1 in phase-sensitive (A) and magnitude forms (B). (A) is a low pass filter and (B) is a negative unipolar filter. (A) shows both positive and negative values for ST1 whereas (B) shows negative values "reflected" across the X axis so they become positive. The maximum size of the slopes of the two T1-filters which are tangential to the slope are shown as red lines. The slopes are negative in both cases. .
Figure 4.
The long TR IR sequence. Negative T1-unipolar filters for short TIs (A, left), intermediate TIi (B, centre) and long TIl (C, right) including white (W) and gray (G) matter. Signal ST1 is plotted against T1 in each case (black curves). The positions of W and G are the same at each of the three TIs. TI is increased from TIs (left) to TIi (centre) and increased further to TIl (right). In (A), (B) and (C), the increase in T1 from W to G (horizontal positive green arrows) is multiplied by the relevant slopes of the filters (red lines) to produce contrast. This is moderately positive, strongly negative, and mildly negative contrast in (A), (B) and (C) respectively (vertical blue arrows).
Figure 4.
The long TR IR sequence. Negative T1-unipolar filters for short TIs (A, left), intermediate TIi (B, centre) and long TIl (C, right) including white (W) and gray (G) matter. Signal ST1 is plotted against T1 in each case (black curves). The positions of W and G are the same at each of the three TIs. TI is increased from TIs (left) to TIi (centre) and increased further to TIl (right). In (A), (B) and (C), the increase in T1 from W to G (horizontal positive green arrows) is multiplied by the relevant slopes of the filters (red lines) to produce contrast. This is moderately positive, strongly negative, and mildly negative contrast in (A), (B) and (C) respectively (vertical blue arrows).
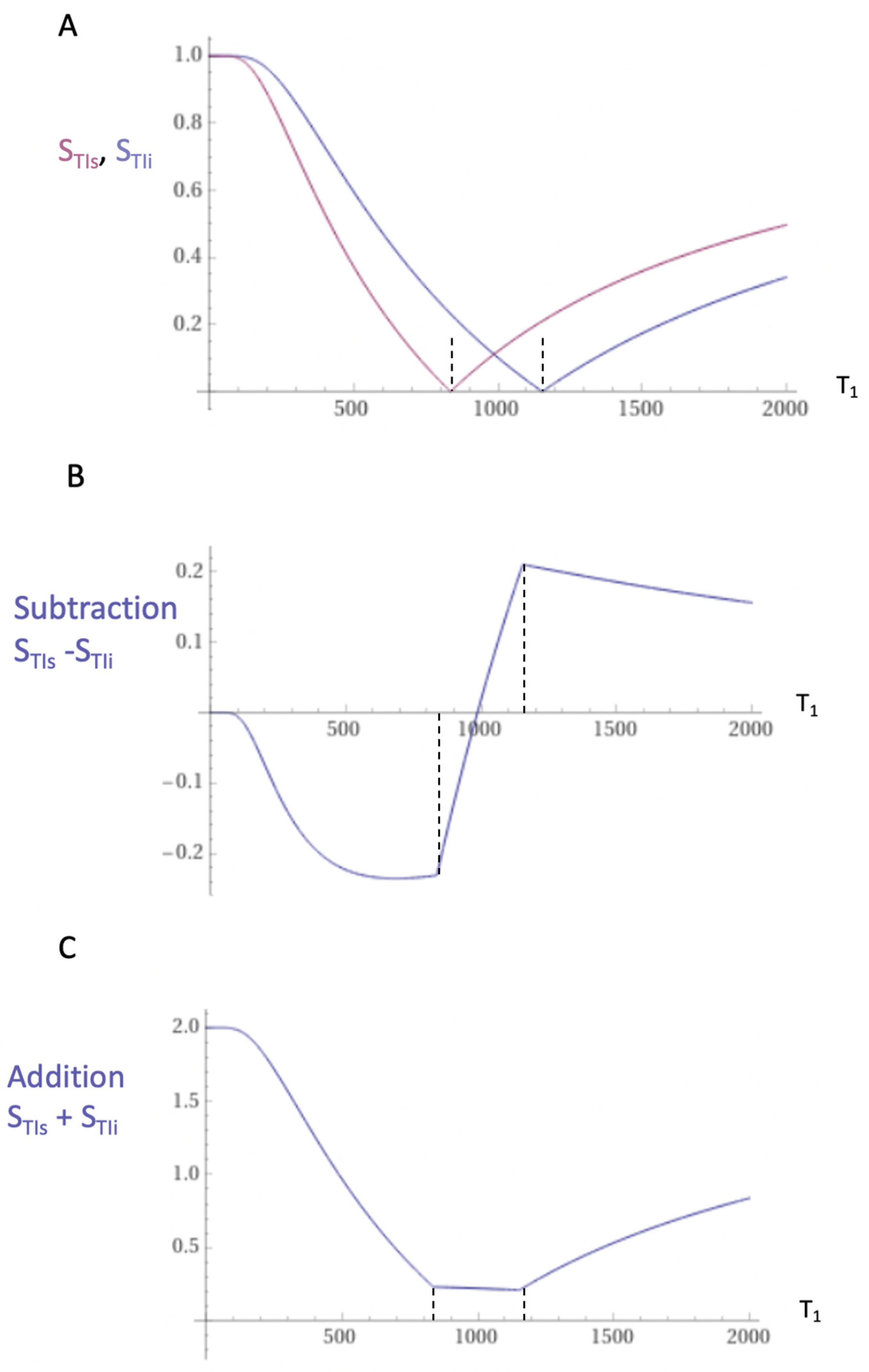
Figure 5.
Unipolar IR (A), subtracted IR (SIR) (B) and Added IR (AIR) (C) T1-filters. T1 is shown along the X axes in ms. (A) shows a TIs T1-unipolar filter (pink) and a TIi T1-unipolar filter (blue), (B) shows the subtraction (STIs - STIi) IR or SIR T1-bipolar filter, and (C) shows the addition (STIs + STIi) IR or AIR T1- filter. The vertical lines divide the X axes into lowest, middle and highest Domains (lD, mD and hD). The mD contains T1 values between the vertical dashed lines. In (B) the slope of the curve of the SIR T1-filter in the mD is about double that of the STIs T1-filter (pink in [A]). In (C) the signal at T1=0 is doubled to 2.0, and the signal in the mD is reduced to about 0.20 in the nearly linear, slightly downward sloping central part of the AIR T1-filter (i.e., in the mD).
Figure 5.
Unipolar IR (A), subtracted IR (SIR) (B) and Added IR (AIR) (C) T1-filters. T1 is shown along the X axes in ms. (A) shows a TIs T1-unipolar filter (pink) and a TIi T1-unipolar filter (blue), (B) shows the subtraction (STIs - STIi) IR or SIR T1-bipolar filter, and (C) shows the addition (STIs + STIi) IR or AIR T1- filter. The vertical lines divide the X axes into lowest, middle and highest Domains (lD, mD and hD). The mD contains T1 values between the vertical dashed lines. In (B) the slope of the curve of the SIR T1-filter in the mD is about double that of the STIs T1-filter (pink in [A]). In (C) the signal at T1=0 is doubled to 2.0, and the signal in the mD is reduced to about 0.20 in the nearly linear, slightly downward sloping central part of the AIR T1-filter (i.e., in the mD).
Figure 6.
Figure 6A shows division of the SIR T
1-bipolar filter in
Figure 5B by the addition T
1-filter AIR in
Figure 5C to give the dSIR T
1-bipolar filter. T
1 is shown along the X axis in ms.
Figure 6B shows comparison of the conventional IR S
TIs T
1-unipolar filter (pink) with the SIR T
1-bipolar filter (blue) for a small increase in T
1 (horizontal positive green arrow, ΔT
1).
Figure 6C is a comparison of the S
TIs T
1-unipolar filter (pink) with the dSIR T
1-bipolar filter (blue) for the same small increase in T
1. In 6B the contrast produced by the SIR T
1-bipolar filter is about twice that produced by the IR T
1-unipolar filter (blue and pink arrows). In 6C the contrast produced by the dSIR T
1-bipolar filter is about ten times greater than that produced by the IR T
1-unipolar filter (blue and pink arrows). The display gray scale has no negative values so white matter appears on it as zero signal (black) at the lowest part of the display scale when using both the T
1-filters shown in each of
Figure 6B and
Figure 6C.
Figure 6.
Figure 6A shows division of the SIR T
1-bipolar filter in
Figure 5B by the addition T
1-filter AIR in
Figure 5C to give the dSIR T
1-bipolar filter. T
1 is shown along the X axis in ms.
Figure 6B shows comparison of the conventional IR S
TIs T
1-unipolar filter (pink) with the SIR T
1-bipolar filter (blue) for a small increase in T
1 (horizontal positive green arrow, ΔT
1).
Figure 6C is a comparison of the S
TIs T
1-unipolar filter (pink) with the dSIR T
1-bipolar filter (blue) for the same small increase in T
1. In 6B the contrast produced by the SIR T
1-bipolar filter is about twice that produced by the IR T
1-unipolar filter (blue and pink arrows). In 6C the contrast produced by the dSIR T
1-bipolar filter is about ten times greater than that produced by the IR T
1-unipolar filter (blue and pink arrows). The display gray scale has no negative values so white matter appears on it as zero signal (black) at the lowest part of the display scale when using both the T
1-filters shown in each of
Figure 6B and
Figure 6C.
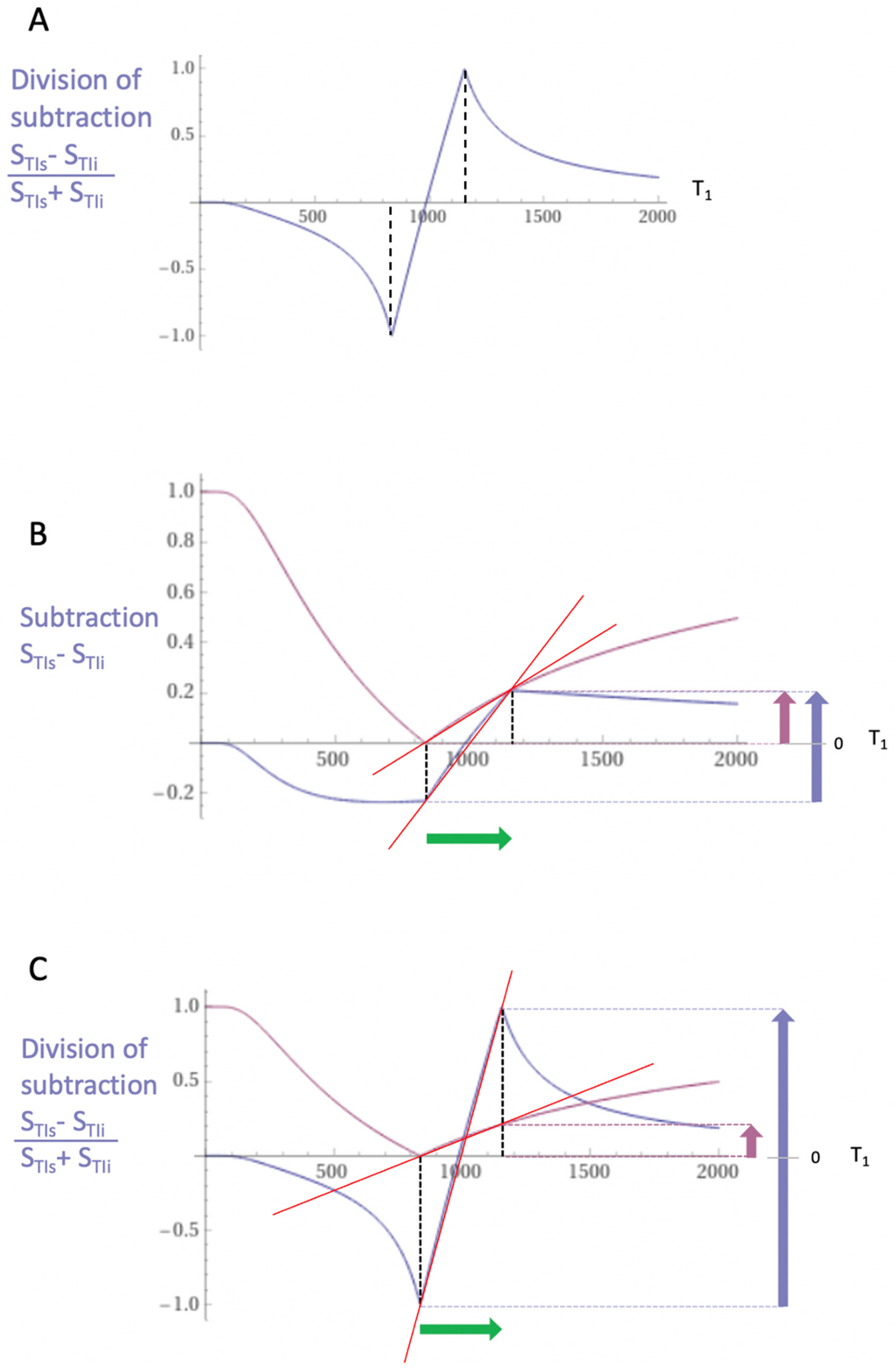
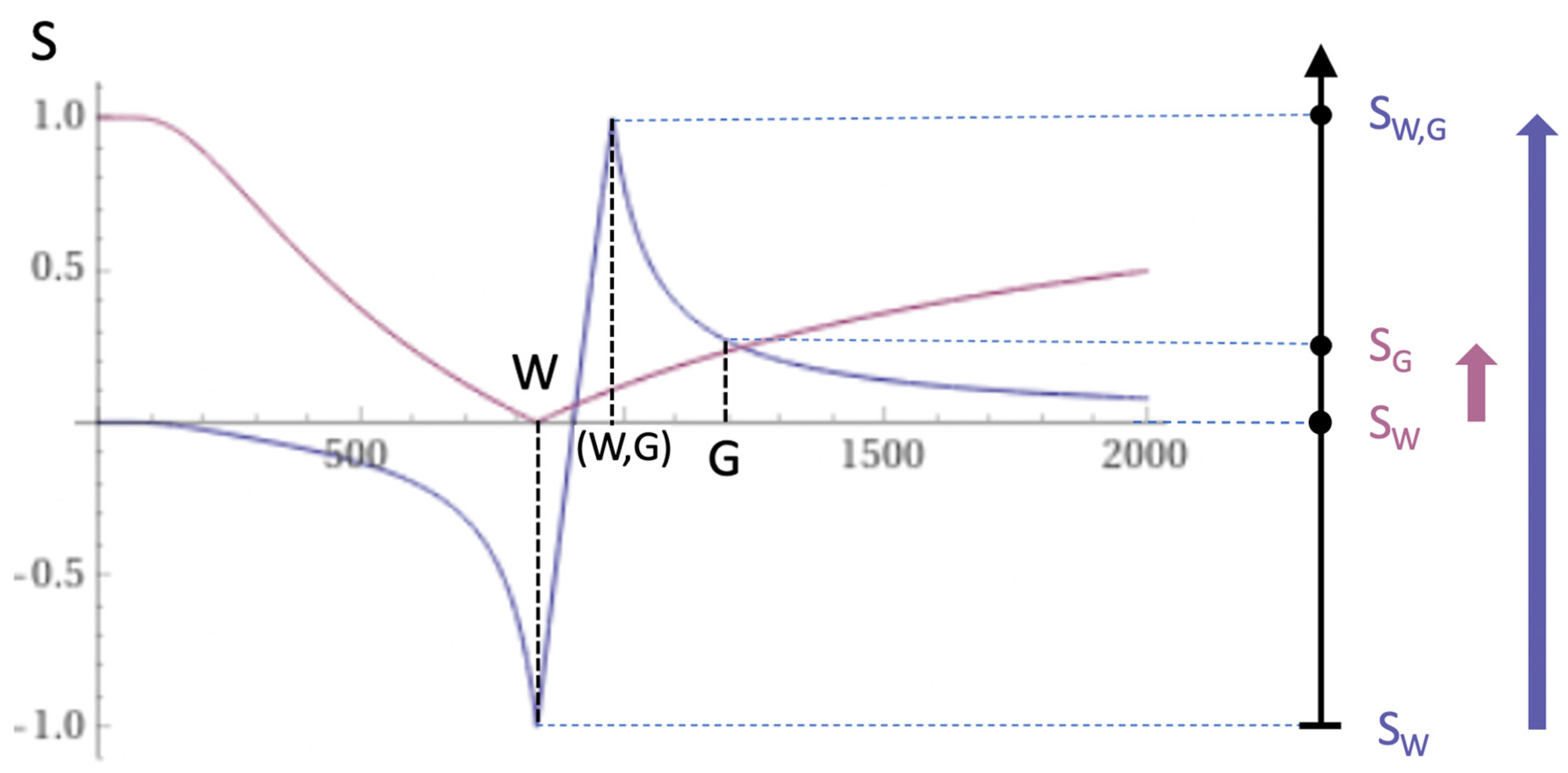
Figure 7.
Boundaries. This image shows a narrow mD dSIR T1-bipolar filter with a mD extending from white matter (W) to a T1W,G between the TIs of W and gray matter (G) (blue curve) as well as a white matter nulled conventional IR T1-unipolar filter e.g., MP-RAGE (pink curve). The X axis is shown in ms. The contrast produced from the difference in signal between W and G by the T1-unipolar filter is (SG minus SW, pink curve) and is shown by the vertical pink arrow. With the T1-bipolar filter (blue curve) there is a partial volume effect between W and G producing a T1W,G between the T1s of W and G which results in the high signal, SW,G shown on the T1-bipolar filter (blue curve). The contrast between this high signal and white matter is the difference (SW,G minus SW, in blue) and is shown by the vertical blue arrow. The contrast produced by the T1-bipolar filter at the boundary between W and G is far greater than that produced at the boundary between W and G by the T1-unipolar filter.
Figure 7.
Boundaries. This image shows a narrow mD dSIR T1-bipolar filter with a mD extending from white matter (W) to a T1W,G between the TIs of W and gray matter (G) (blue curve) as well as a white matter nulled conventional IR T1-unipolar filter e.g., MP-RAGE (pink curve). The X axis is shown in ms. The contrast produced from the difference in signal between W and G by the T1-unipolar filter is (SG minus SW, pink curve) and is shown by the vertical pink arrow. With the T1-bipolar filter (blue curve) there is a partial volume effect between W and G producing a T1W,G between the T1s of W and G which results in the high signal, SW,G shown on the T1-bipolar filter (blue curve). The contrast between this high signal and white matter is the difference (SW,G minus SW, in blue) and is shown by the vertical blue arrow. The contrast produced by the T1-bipolar filter at the boundary between W and G is far greater than that produced at the boundary between W and G by the T1-unipolar filter.
Figure 8.
Plots of ln S
TIs (TI
s = 350 ms) vs T
1 (pink), and ln S
TIi (TI
i = 500 ms) vs T
1 (blue). The plots have a negative unipolar form with steeply sloping signal gradients around their negative poles. The pink and blue ln S
TIs and ln S
TIi curves are steeper than the corresponding magnitude curves shown in
Figure 5A. The lSIR filter S
lSIR = ½(ln S
TIs - ln S
TIi) reverses the sign of the S
TIi filter so it becomes positive as seen in the following figure (
Figure 9) (orange curve).
Figure 8.
Plots of ln S
TIs (TI
s = 350 ms) vs T
1 (pink), and ln S
TIi (TI
i = 500 ms) vs T
1 (blue). The plots have a negative unipolar form with steeply sloping signal gradients around their negative poles. The pink and blue ln S
TIs and ln S
TIi curves are steeper than the corresponding magnitude curves shown in
Figure 5A. The lSIR filter S
lSIR = ½(ln S
TIs - ln S
TIi) reverses the sign of the S
TIi filter so it becomes positive as seen in the following figure (
Figure 9) (orange curve).
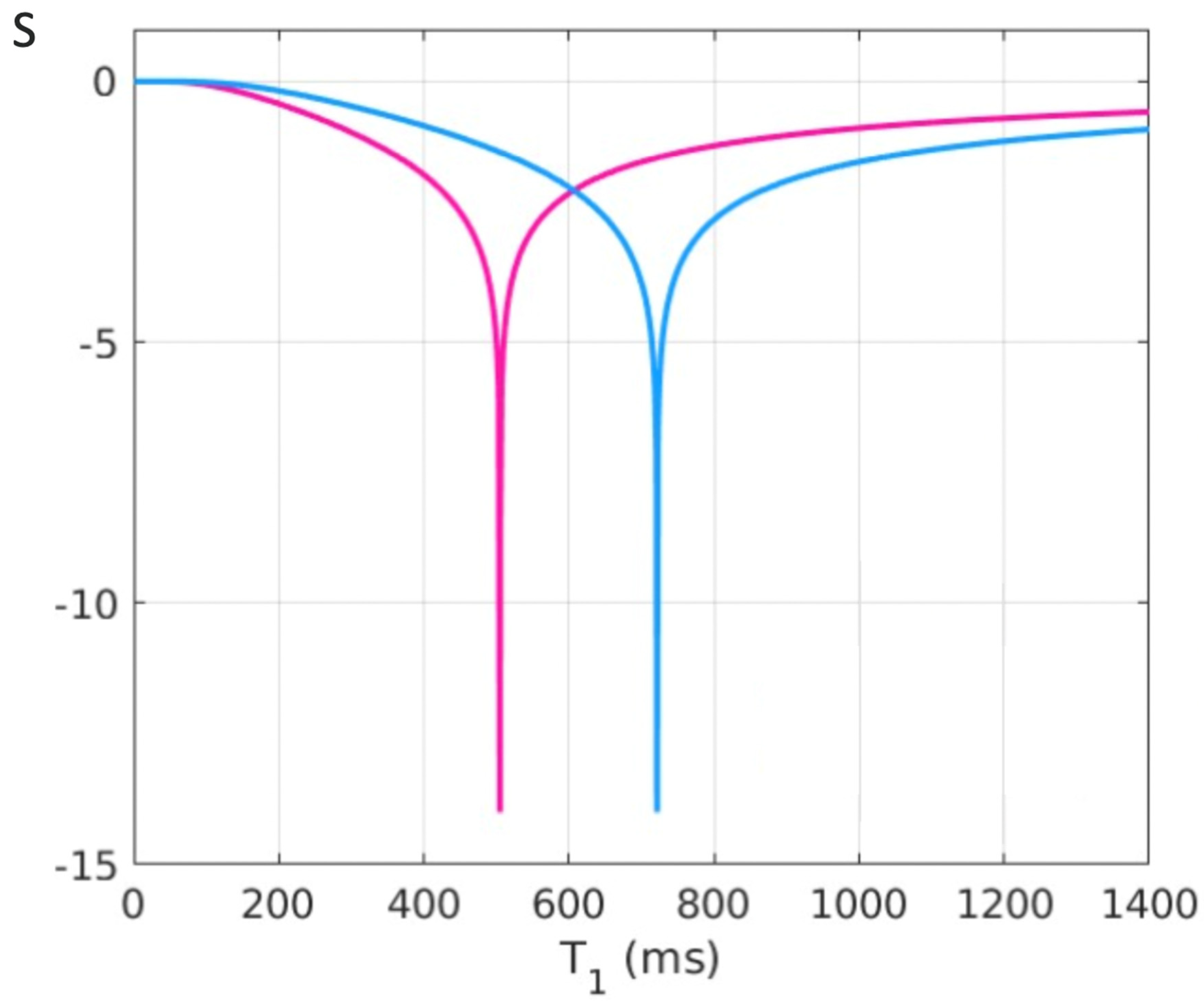
Figure 9.
Plot of signal vs T1 in ms for a dSIR T1-bipolar filter (blue curve) and the corresponding lSIR T1-bipolar filter with the same null points (orange curve) (TIs = 350 ms and TIi = 500 ms, ΔTI = 150 ms). The dSIR filter shows the usual pattern with maximum values of ±1. It has an essentially constant slope in the mD. The lSIR filter has similar values to the dSIR filter in the regions of lowest and highest values of T1 as well as in the centre of the mD. However around the lower and upper T1 nulling values it has much steeper slopes and proceeds asymptotically to minus and plus infinity respectively (values of S along the Y axis in the range of ± 2 are shown). From a practical point of view, the contrast for very small differences or changes in T1 is 2-3 times greater with the lSIR filter compared with the corresponding dSIR filter. For the same difference in T1 in the regions close to the null points the lSIR filter is steeper than the dSIR filter. This results in greater contrast and spatial resolution.
Figure 9.
Plot of signal vs T1 in ms for a dSIR T1-bipolar filter (blue curve) and the corresponding lSIR T1-bipolar filter with the same null points (orange curve) (TIs = 350 ms and TIi = 500 ms, ΔTI = 150 ms). The dSIR filter shows the usual pattern with maximum values of ±1. It has an essentially constant slope in the mD. The lSIR filter has similar values to the dSIR filter in the regions of lowest and highest values of T1 as well as in the centre of the mD. However around the lower and upper T1 nulling values it has much steeper slopes and proceeds asymptotically to minus and plus infinity respectively (values of S along the Y axis in the range of ± 2 are shown). From a practical point of view, the contrast for very small differences or changes in T1 is 2-3 times greater with the lSIR filter compared with the corresponding dSIR filter. For the same difference in T1 in the regions close to the null points the lSIR filter is steeper than the dSIR filter. This results in greater contrast and spatial resolution.
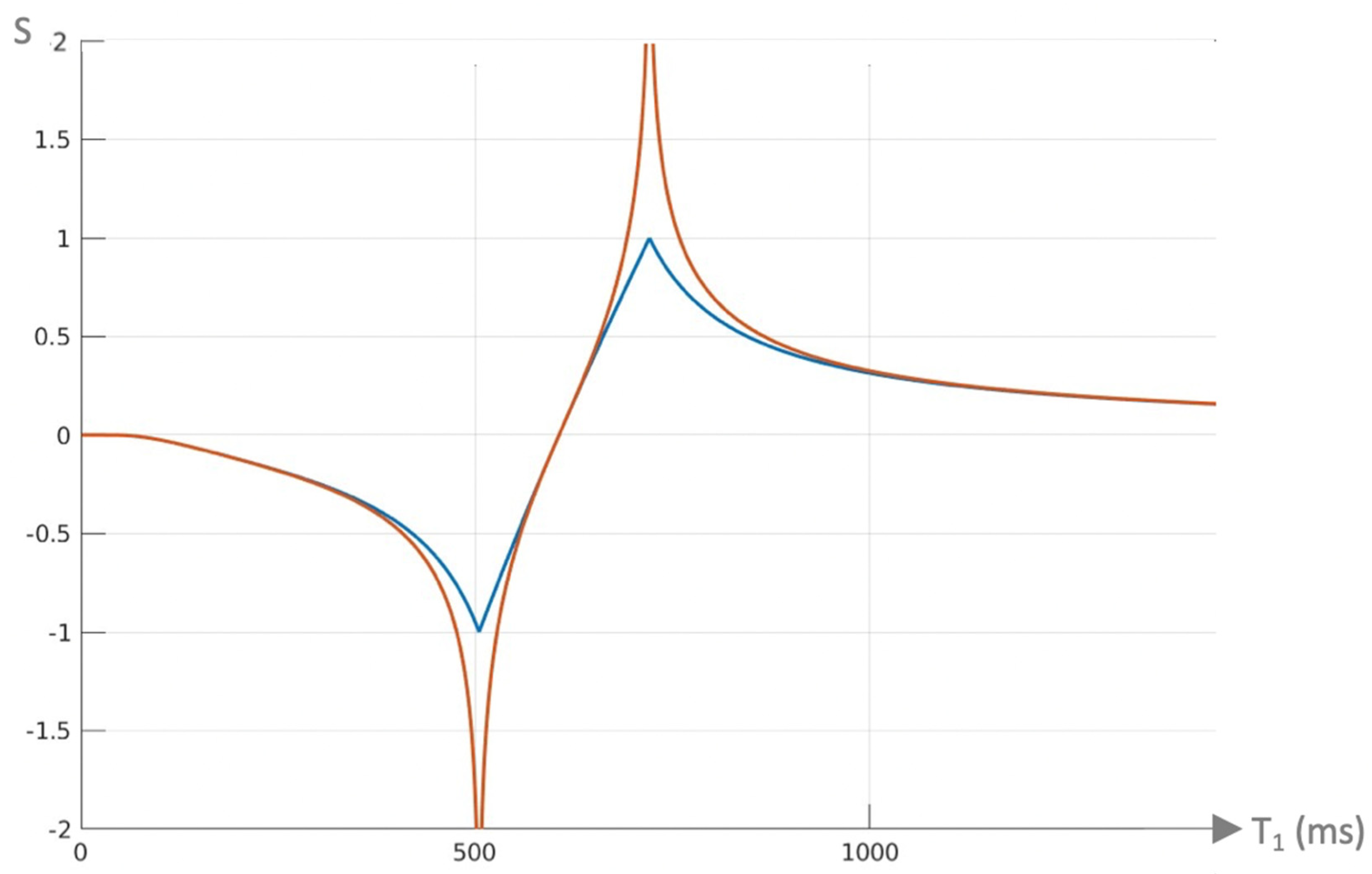
Figure 10.
Composite dSIR filters (cdSIRs with TIs = 350 ms and TIi = 500 ms). Attenuated first IR T1-filter (TIs) in (A) and attenuated second IR T1-filter (TIi) in (B). (A) shows the conventional T1-bipolar filter (blue) with SOF = 1, an attenuated first TIs filter (SOF = 0.5) (red) and a further attenuated first TIs filter (SOF = 0.25) (yellow). In (A), as SOF is decreased to 0.5 and 0.25 the filters around the first negative pole become wider and are positioned outside the blue filter. The attenuated filters around the second positive pole show higher contrast and become narrower. They are located inside the blue filter. (B) shows the conventional T1-bipolar filter (blue) with SOF = 1, and attenuated second TIi filter SOF = 0.5 (red) and a more attenuated second TIs filter (SOF = 0.25) (yellow). As SOF is decreased, the filters around the first negative pole become narrower and show higher contrast. They are positioned within the blue filter. The attenuated filters around the second positive pole become wider and show lower contrast. They are positioned outside of the blue filter. For T2, a short T2 increases attenuation relative to a longer T2 and this corresponds to the narrower and sharper peak with greater negative contrast and spatial resolution of the contrast (A).
Figure 10.
Composite dSIR filters (cdSIRs with TIs = 350 ms and TIi = 500 ms). Attenuated first IR T1-filter (TIs) in (A) and attenuated second IR T1-filter (TIi) in (B). (A) shows the conventional T1-bipolar filter (blue) with SOF = 1, an attenuated first TIs filter (SOF = 0.5) (red) and a further attenuated first TIs filter (SOF = 0.25) (yellow). In (A), as SOF is decreased to 0.5 and 0.25 the filters around the first negative pole become wider and are positioned outside the blue filter. The attenuated filters around the second positive pole show higher contrast and become narrower. They are located inside the blue filter. (B) shows the conventional T1-bipolar filter (blue) with SOF = 1, and attenuated second TIi filter SOF = 0.5 (red) and a more attenuated second TIs filter (SOF = 0.25) (yellow). As SOF is decreased, the filters around the first negative pole become narrower and show higher contrast. They are positioned within the blue filter. The attenuated filters around the second positive pole become wider and show lower contrast. They are positioned outside of the blue filter. For T2, a short T2 increases attenuation relative to a longer T2 and this corresponds to the narrower and sharper peak with greater negative contrast and spatial resolution of the contrast (A).
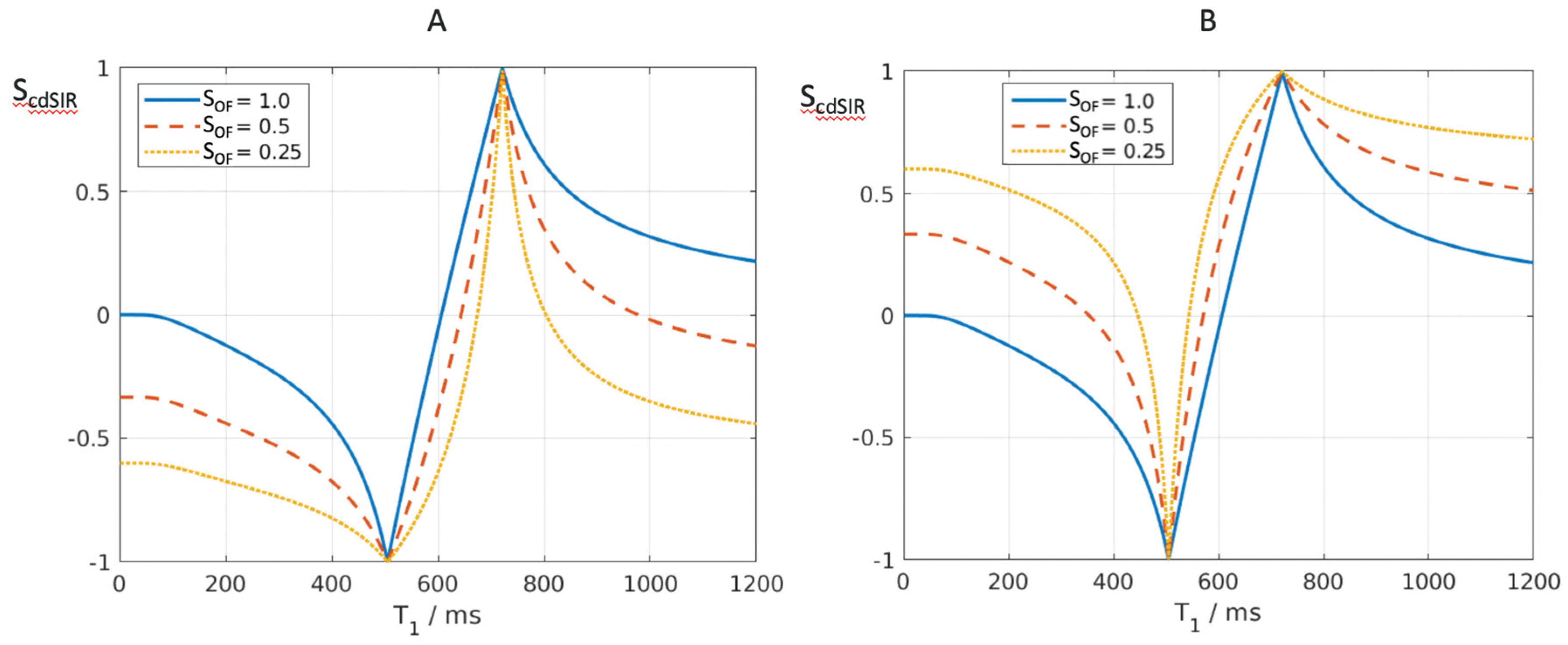
Figure 11.
Comparison of conventional windowing (A and B) and a dSIR T1-bipolar filter (C). (A) plots the display signal SD against image signal S for two tissues P and Q over the full image gray scale range shown along the Y axis. There is a linear relationship between SD and S over the full gray scale range. In (B) the image is narrowly windowed to place the signals from P and Q at the lower and upper ends of the SD range shown on the Y axis. The contrast between P and Q (difference in signal level shown on the Y axis) is increased in (B) compared with (A). In (B) signal values less than P on the X axis are all shown at the lowest signal level on the Y axis, and signal values along the X axis greater than Q are all shown at the highest signal level on the Y axis (horizontal lines). In these regions, voxels have the same signals and show no contrast between them. They form isointense blocks and anatomical detail is lost within the blocks. (C) plots signal against T1 using a dSIR T1-bipolar filter. Contrast between P and Q is high in the mD as in (B). Values of T1 less than P and greater than Q do not form plateaux as in (B) but follow the T1-bipolar filter in the lD and hD. There are therefore differences in signal between voxels so contrast is maintained and anatomical detail is preserved outside of the mD bounded by P and Q.
Figure 11.
Comparison of conventional windowing (A and B) and a dSIR T1-bipolar filter (C). (A) plots the display signal SD against image signal S for two tissues P and Q over the full image gray scale range shown along the Y axis. There is a linear relationship between SD and S over the full gray scale range. In (B) the image is narrowly windowed to place the signals from P and Q at the lower and upper ends of the SD range shown on the Y axis. The contrast between P and Q (difference in signal level shown on the Y axis) is increased in (B) compared with (A). In (B) signal values less than P on the X axis are all shown at the lowest signal level on the Y axis, and signal values along the X axis greater than Q are all shown at the highest signal level on the Y axis (horizontal lines). In these regions, voxels have the same signals and show no contrast between them. They form isointense blocks and anatomical detail is lost within the blocks. (C) plots signal against T1 using a dSIR T1-bipolar filter. Contrast between P and Q is high in the mD as in (B). Values of T1 less than P and greater than Q do not form plateaux as in (B) but follow the T1-bipolar filter in the lD and hD. There are therefore differences in signal between voxels so contrast is maintained and anatomical detail is preserved outside of the mD bounded by P and Q.
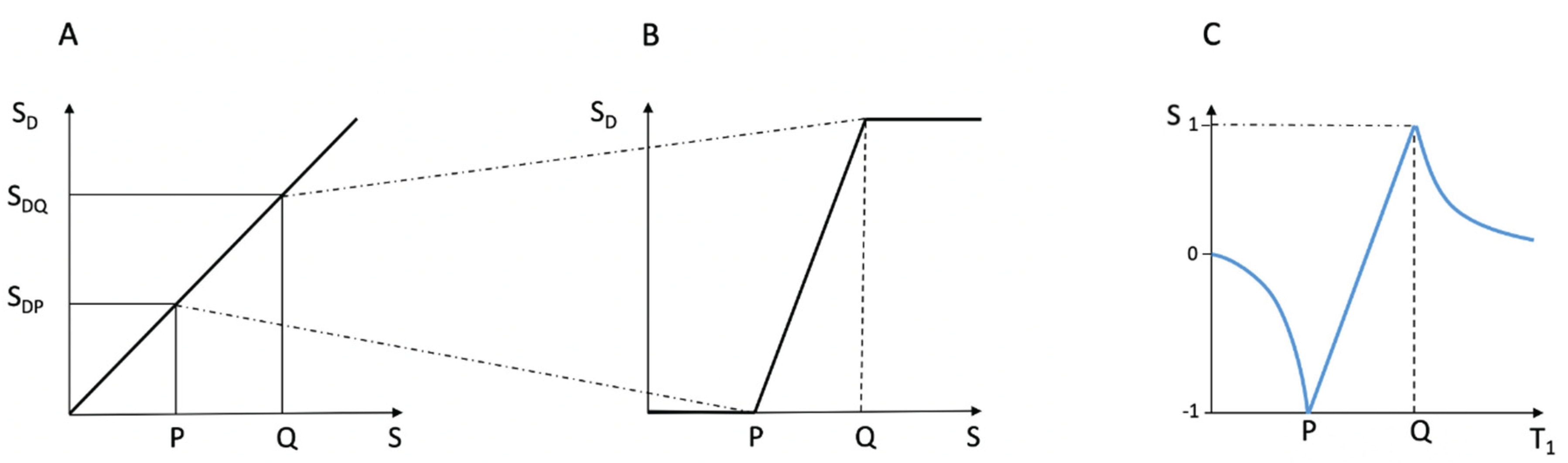
Figure 12.
18-year-old patient with mTBI 21h (A) and 64h (B) post injury imaged with the same narrow mD dSIR sequence. In (A) the patient shows a whiteout sign (grade 4 out of 5) with high signal in most of the white matter in the cerebral hemispheres except for the anterior and posterior central corpus callosum. The posterior limb of the internal capsule is high signal. The thalami show low internal contrast from medial to lateral (arrows on lateral margins of the thalami). There is also low external contrast between the lateral margins of the thalami and the adjacent posterior limbs of the internal capsule. On the follow up examination at 64h (B) the whiteout sign has resolved and there is low signal in the white matter including the posterior limbs of the internal capsule (except for the corticospinal tracts). The thalami now show high internal contrast from medial to lateral (arrows on lateral margins of the thalamus) which is the normal appearance at this age. There is now also very high external contrast between the lateral margin of the thalamus and the adjacent posterior limb of the internal capsule. Image (A) shows the grayout sign which is a reduction in the high contrast between the medial and lateral gray matter of the thalamus. The high contrast is restored in (B). No abnormality was seen on the corresponding T2-FLAIR images obtained at both time points.
Figure 12.
18-year-old patient with mTBI 21h (A) and 64h (B) post injury imaged with the same narrow mD dSIR sequence. In (A) the patient shows a whiteout sign (grade 4 out of 5) with high signal in most of the white matter in the cerebral hemispheres except for the anterior and posterior central corpus callosum. The posterior limb of the internal capsule is high signal. The thalami show low internal contrast from medial to lateral (arrows on lateral margins of the thalami). There is also low external contrast between the lateral margins of the thalami and the adjacent posterior limbs of the internal capsule. On the follow up examination at 64h (B) the whiteout sign has resolved and there is low signal in the white matter including the posterior limbs of the internal capsule (except for the corticospinal tracts). The thalami now show high internal contrast from medial to lateral (arrows on lateral margins of the thalamus) which is the normal appearance at this age. There is now also very high external contrast between the lateral margin of the thalamus and the adjacent posterior limb of the internal capsule. Image (A) shows the grayout sign which is a reduction in the high contrast between the medial and lateral gray matter of the thalamus. The high contrast is restored in (B). No abnormality was seen on the corresponding T2-FLAIR images obtained at both time points.
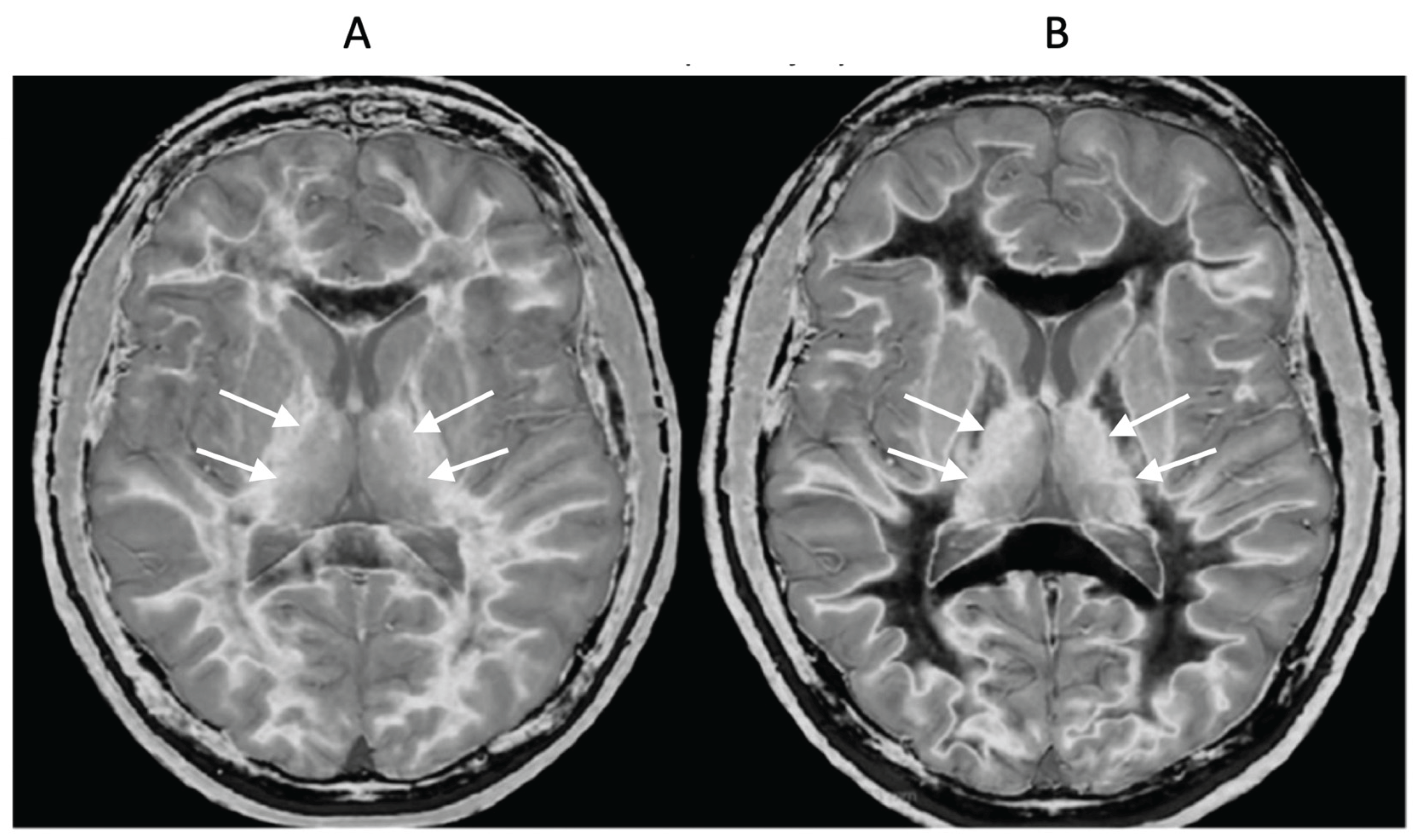
Figure 13.
Normal control (A) and patient with mTBI (B) showing a whiteout sign and grayout signs (narrow mD dSIR images). The normal control shows the heads of the caudate nuclei with higher signal than the adjacent CSF. Contrast is also seen between the cortex and CSF. In (B), the patient shows a whiteout sign. There are grayout signs in the thalami and putamina. In addition, contrast between the heads of the caudate nuclei and CSF is lost and there is little or no contrast between cortex and CSF which are isointense. These are also grayout signs. No abnormality was seen on the T2-FLAIR images in the normal control or patient.
Figure 13.
Normal control (A) and patient with mTBI (B) showing a whiteout sign and grayout signs (narrow mD dSIR images). The normal control shows the heads of the caudate nuclei with higher signal than the adjacent CSF. Contrast is also seen between the cortex and CSF. In (B), the patient shows a whiteout sign. There are grayout signs in the thalami and putamina. In addition, contrast between the heads of the caudate nuclei and CSF is lost and there is little or no contrast between cortex and CSF which are isointense. These are also grayout signs. No abnormality was seen on the T2-FLAIR images in the normal control or patient.
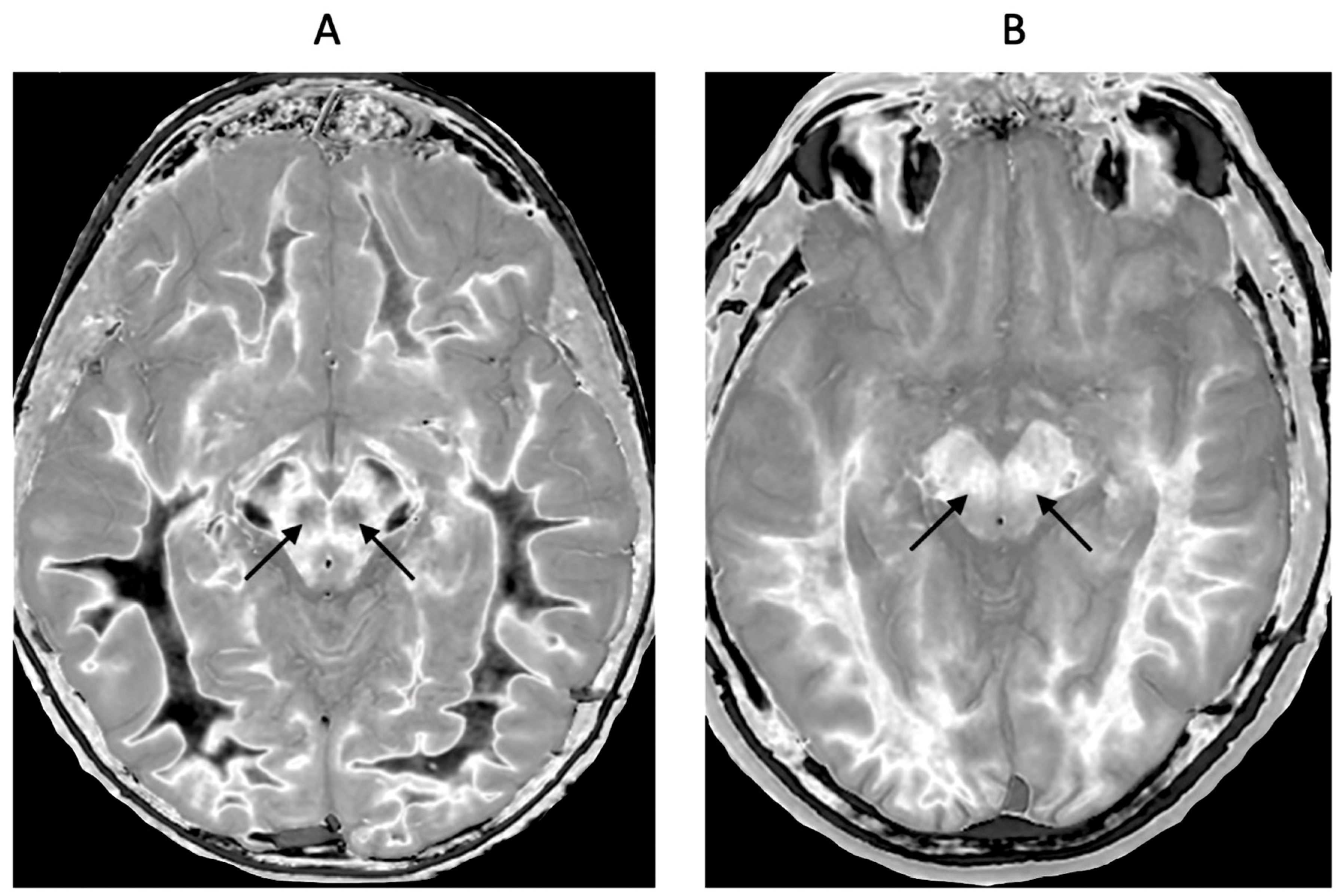
Figure 14.
Red nuclei in an 18-year-old normal control (A) and an 18-year-old male patient with mTBI (B). 2D narrow mD dSIR images. In (A) the normal control shows low signal in the white matter of the cerebral hemisphere, cortical spinal tracts and the ascending sensory tracts. The red nuclei (arrows) have an intermediate mid-gray signal. In (B) the patient shows high signal in the cerebral white matter, the corticospinal tracts and the ascending sensory tracts (whiteout sign, grade 4 out of 5). In addition, the red nuclei are higher signal than in (A) (arrows). No abnormality was seen on the T2-FLAIR images in the normal control or patient.
Figure 14.
Red nuclei in an 18-year-old normal control (A) and an 18-year-old male patient with mTBI (B). 2D narrow mD dSIR images. In (A) the normal control shows low signal in the white matter of the cerebral hemisphere, cortical spinal tracts and the ascending sensory tracts. The red nuclei (arrows) have an intermediate mid-gray signal. In (B) the patient shows high signal in the cerebral white matter, the corticospinal tracts and the ascending sensory tracts (whiteout sign, grade 4 out of 5). In addition, the red nuclei are higher signal than in (A) (arrows). No abnormality was seen on the T2-FLAIR images in the normal control or patient.
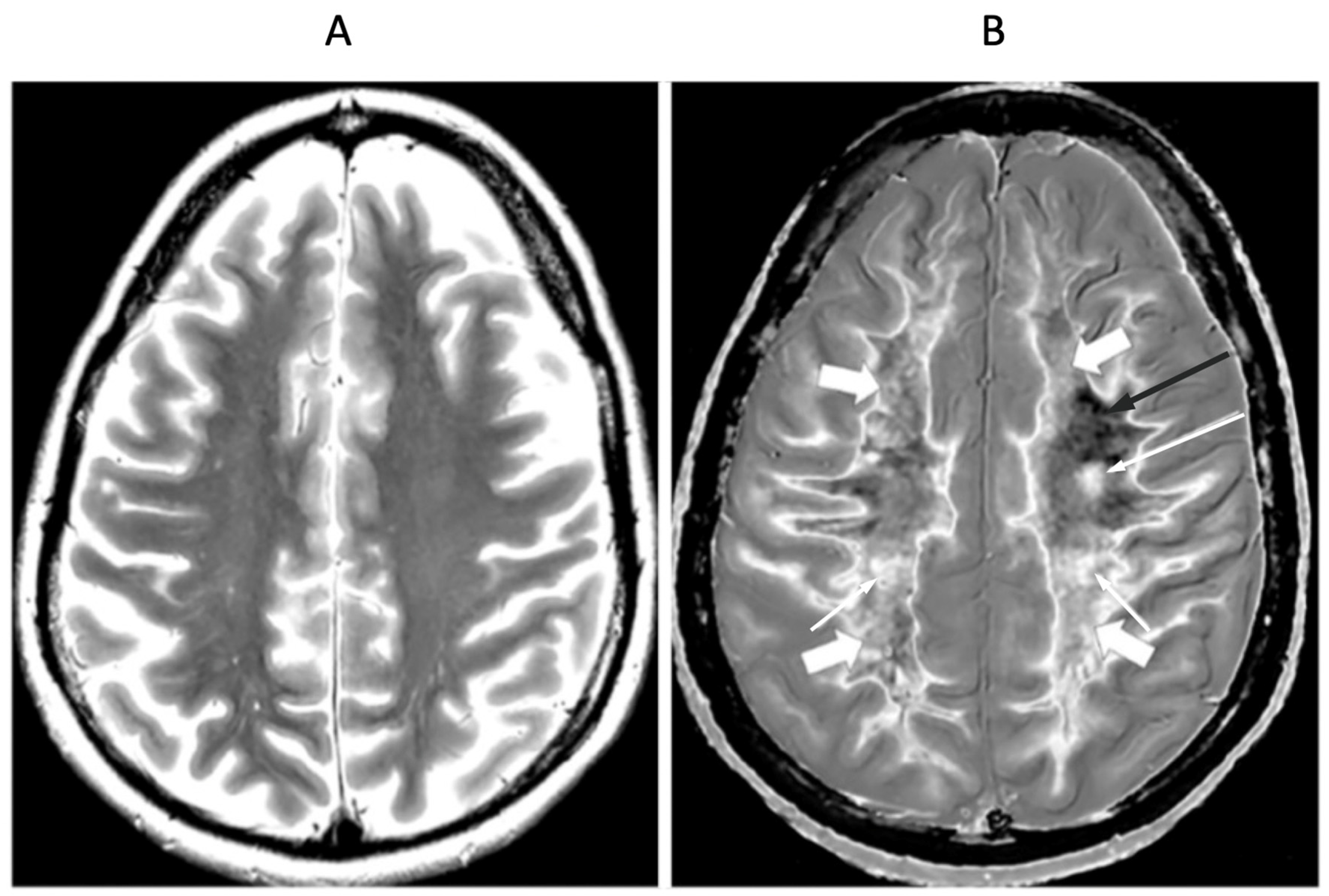
Figure 15.
41-year-old female patient with MS in remission. 2D T2-wSE (A) and narrow mD dSIR (B) images at the same level. No abnormality is seen in (A). A focal lesion is seen in (B) (long narrow white arrow) and the corticospinal tracts show a high signal (short narrow white arrows). In addition there is patchy increased signal in white matter (short thick white arrows) with only a small region showing a normal or near normal low signal appearance (long black arrow). High contrast and high spatial resolution contrast are seen at the boundaries between normal white matter and normal gray matter in (B). These features are less obvious in areas where the white matter has abnormal high signal.
Figure 15.
41-year-old female patient with MS in remission. 2D T2-wSE (A) and narrow mD dSIR (B) images at the same level. No abnormality is seen in (A). A focal lesion is seen in (B) (long narrow white arrow) and the corticospinal tracts show a high signal (short narrow white arrows). In addition there is patchy increased signal in white matter (short thick white arrows) with only a small region showing a normal or near normal low signal appearance (long black arrow). High contrast and high spatial resolution contrast are seen at the boundaries between normal white matter and normal gray matter in (B). These features are less obvious in areas where the white matter has abnormal high signal.
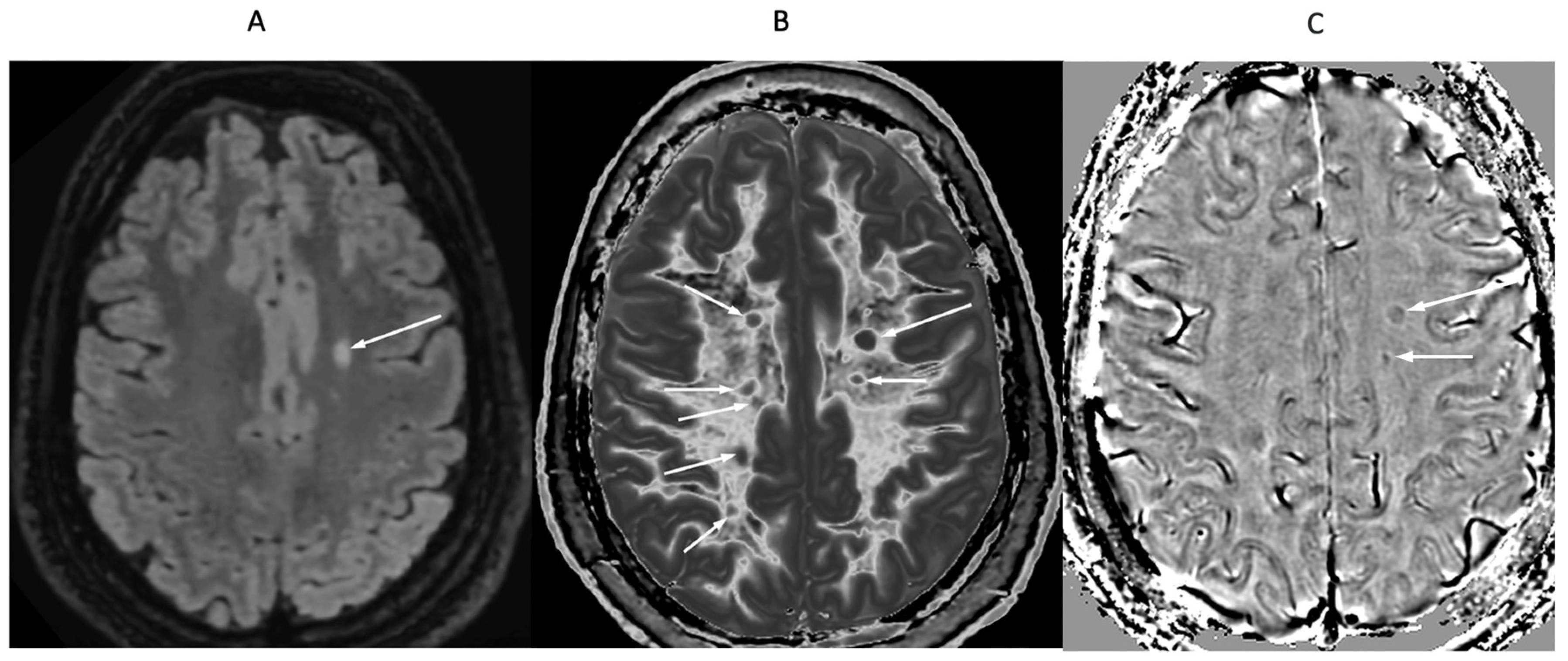
Figure 16.
32-year-old female with MS during a relapse. T
2-FLAIR (A), synthetic narrow mD dSIR (B) and filtered gradient echo (C) images. On the T
2-FLAIR image (A), one lesion is seen (long arrow). The surrounding white matter appears normal. On the dSIR image (B), the lesion shown on the T
2-FLAIR image is seen (long arrow) as well as six other lesions (short arrows). High signal boundaries around lesions are also seen in (B). Some of these lesions show paramagnetic rims on the filtered gradient echo image (arrows) in (C). In addition, most of the white matter in (B) is high signal corresponding to a high grade 4/5 whiteout sign [
1]. The whiteout sign is not seen on the T
2-FLAIR image (A).
Figure 16.
32-year-old female with MS during a relapse. T
2-FLAIR (A), synthetic narrow mD dSIR (B) and filtered gradient echo (C) images. On the T
2-FLAIR image (A), one lesion is seen (long arrow). The surrounding white matter appears normal. On the dSIR image (B), the lesion shown on the T
2-FLAIR image is seen (long arrow) as well as six other lesions (short arrows). High signal boundaries around lesions are also seen in (B). Some of these lesions show paramagnetic rims on the filtered gradient echo image (arrows) in (C). In addition, most of the white matter in (B) is high signal corresponding to a high grade 4/5 whiteout sign [
1]. The whiteout sign is not seen on the T
2-FLAIR image (A).
Figure 17.
24-year-old female patient with MS in remission (TI
s = 350 ms, TI
i = 500 ms). Narrow mD dSIR (A) and composite (T
1 and T
2) cdSIR (TI
s/TE
s = 350/7 and 80 ms; TI
i/TE
i = 500/7 ms) (B) images. A leukocortical lesion is seen on the dSIR image (A) and with higher contrast on the cdSIR image in (B) (arrows). White matter and gray matter boundaries are higher contrast and narrower on the composite filter cdSIR image (B). Also white matter is more uniformly low signal in (B). These features are consistent with the attenuation of the first IR TI
s filter signal increasing contrast and narrowing boundaries at the positive pole and decreasing these at the negative pole as shown in
Figure 10A.
Figure 17.
24-year-old female patient with MS in remission (TI
s = 350 ms, TI
i = 500 ms). Narrow mD dSIR (A) and composite (T
1 and T
2) cdSIR (TI
s/TE
s = 350/7 and 80 ms; TI
i/TE
i = 500/7 ms) (B) images. A leukocortical lesion is seen on the dSIR image (A) and with higher contrast on the cdSIR image in (B) (arrows). White matter and gray matter boundaries are higher contrast and narrower on the composite filter cdSIR image (B). Also white matter is more uniformly low signal in (B). These features are consistent with the attenuation of the first IR TI
s filter signal increasing contrast and narrowing boundaries at the positive pole and decreasing these at the negative pole as shown in
Figure 10A.
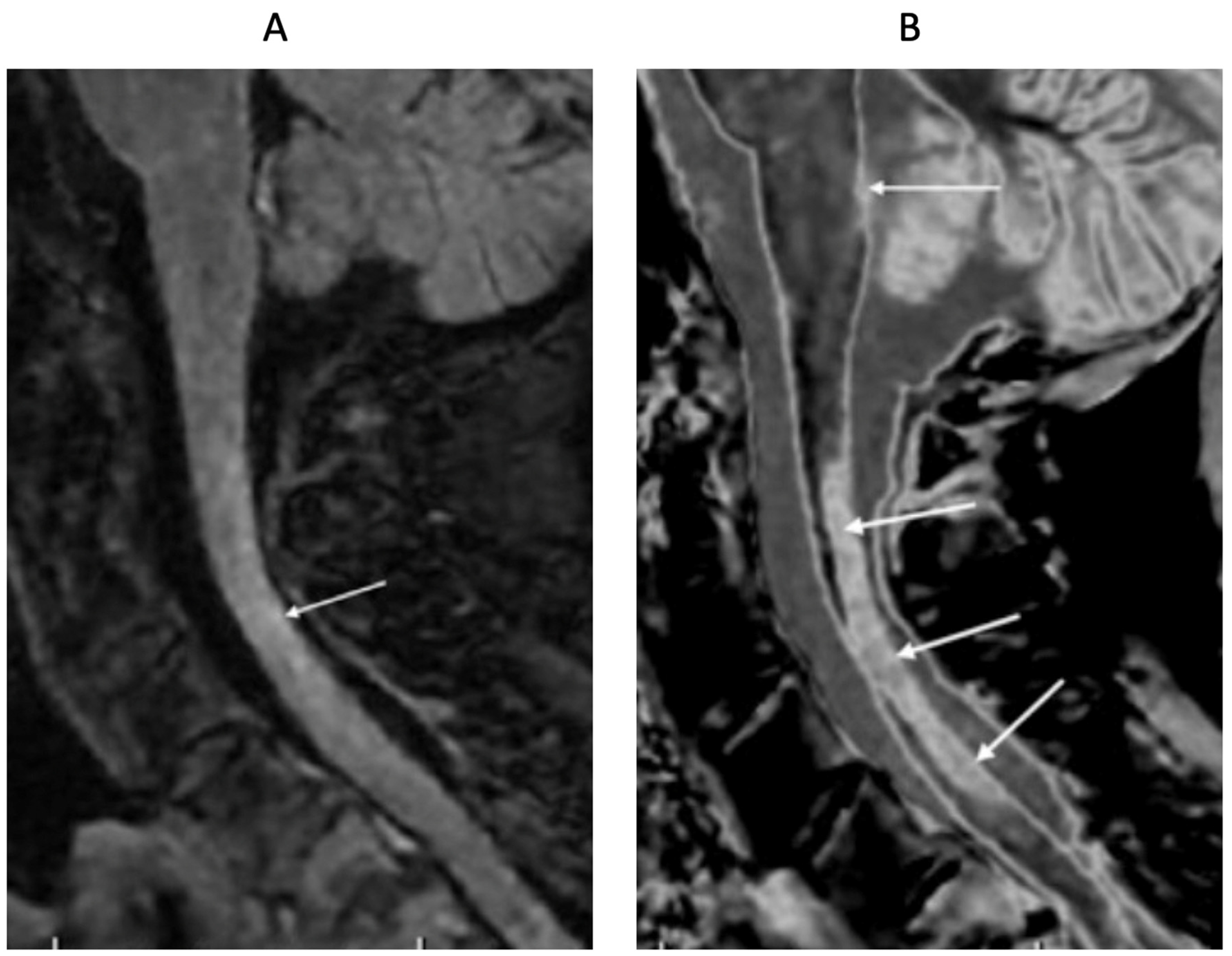
Figure 18.
76-year-old female patient in remission with a diagnosis of MS. Sagittal 3D T2-FLAIR (A) and 3D wide mD dSIR (B) images. The T2-FLAIR image shows a poorly defined area of increased signal in the cervical cord (arrow). The dSIR image shows a high contrast lesion with sharply defined ("punched out") boundaries in the cervical cord (lower three arrows). This is much more extensive than in (A). An additional lesion is seen in the medulla on the dSIR image in the region of the area postrema (highest arrow) (B) but not on the T2-FLAIR image (A). The extended lesion in the cervical cord and the lesion in the medulla raise the possibility of neuromyelitis spectrum disorder. Other conventional sequences such as MP-RAGE, T2-wSE or STIR may perform better than T2-FLAIR in the cervical cord.
Figure 18.
76-year-old female patient in remission with a diagnosis of MS. Sagittal 3D T2-FLAIR (A) and 3D wide mD dSIR (B) images. The T2-FLAIR image shows a poorly defined area of increased signal in the cervical cord (arrow). The dSIR image shows a high contrast lesion with sharply defined ("punched out") boundaries in the cervical cord (lower three arrows). This is much more extensive than in (A). An additional lesion is seen in the medulla on the dSIR image in the region of the area postrema (highest arrow) (B) but not on the T2-FLAIR image (A). The extended lesion in the cervical cord and the lesion in the medulla raise the possibility of neuromyelitis spectrum disorder. Other conventional sequences such as MP-RAGE, T2-wSE or STIR may perform better than T2-FLAIR in the cervical cord.
Figure 19.
51-year-old male patient with methamphetamine substance use disorder after one month's abstinence (A) and after nine months' abstinence (B). Matched narrow mD dSIR images. In (A) there is extensive high signal in the white matter of the cerebral hemispheres with only small areas of normal or near normal white matter (dark) at the periphery of the white matter (whiteout sign, grade 4 out of 5). After nine months’ abstinence (B), the high signal areas in (A) have markedly regressed. There is some intermediate signal in the more central white matter and lower signal in the peripheral white matter (whiteout sign grade 2 out of 5, where grade 1 is normal). No abnormality was seen in either examination on the corresponding T2-FLAIR images. A previous mTBI in the patient may have been a confounder.
Figure 19.
51-year-old male patient with methamphetamine substance use disorder after one month's abstinence (A) and after nine months' abstinence (B). Matched narrow mD dSIR images. In (A) there is extensive high signal in the white matter of the cerebral hemispheres with only small areas of normal or near normal white matter (dark) at the periphery of the white matter (whiteout sign, grade 4 out of 5). After nine months’ abstinence (B), the high signal areas in (A) have markedly regressed. There is some intermediate signal in the more central white matter and lower signal in the peripheral white matter (whiteout sign grade 2 out of 5, where grade 1 is normal). No abnormality was seen in either examination on the corresponding T2-FLAIR images. A previous mTBI in the patient may have been a confounder.
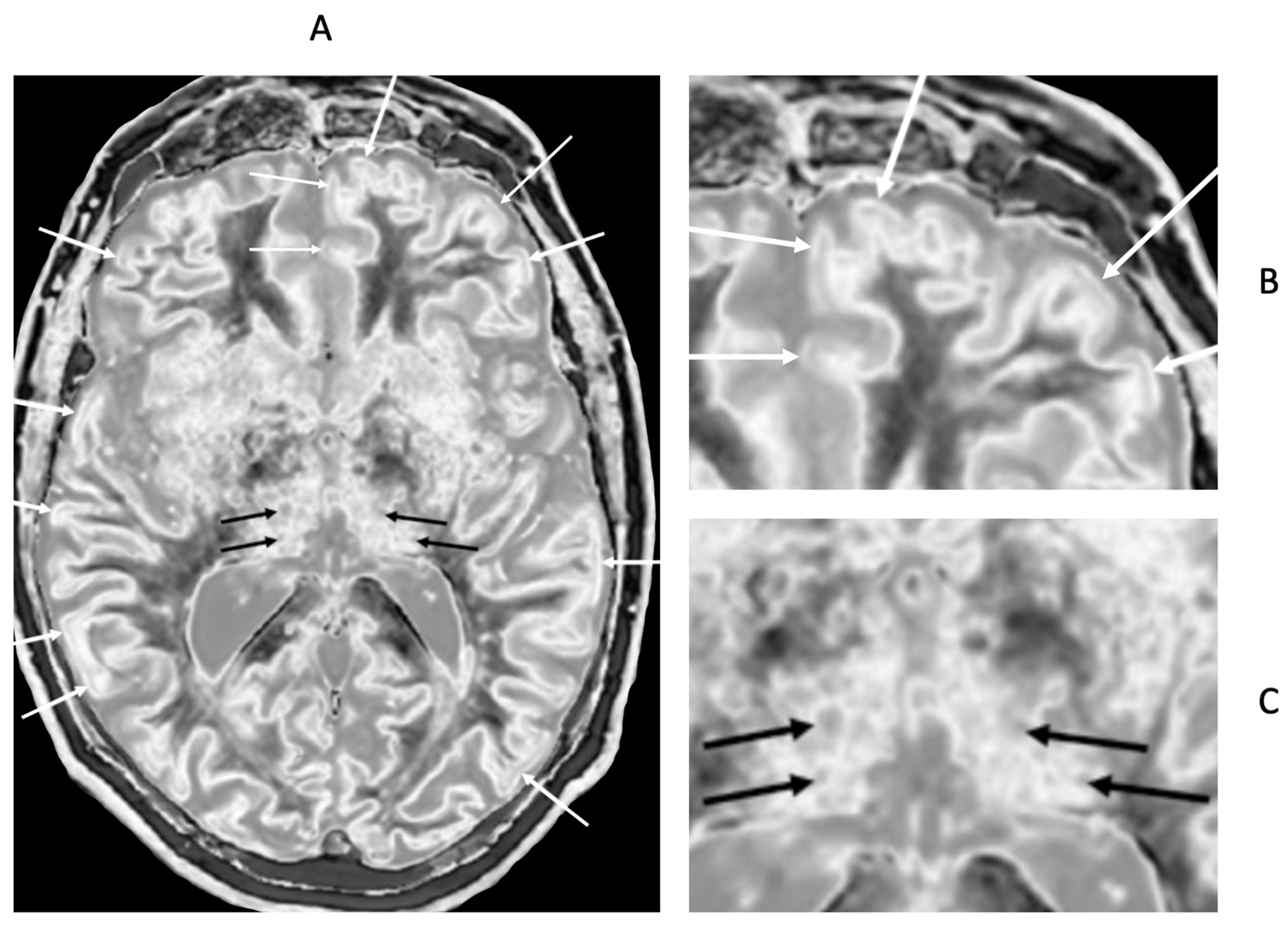
Figure 20.
72-year-old male patient with Parkinson's disease. 2D narrow mD dSIR image (A) with insets of anterior left cortex (B) and thalami (C). There is high signal in the superficial layer of the cortex at multiple sites (bilaminar cortex signs) which are more obvious on the inset (white arrows) (B). There are circular appearances in the thalami, putamen and heads of the caudate nuclei (bubble signs) which are more obvious on the inset (e.g., black arrows) (C). The bubble sign is due to focal reductions in T
1 probably from the presence of free iron. The thalamic signal is slightly higher medially compared with laterally unlike the appearance of the normal thalamus in the 18-year-old patient shown in
Figure 12B.
Figure 20.
72-year-old male patient with Parkinson's disease. 2D narrow mD dSIR image (A) with insets of anterior left cortex (B) and thalami (C). There is high signal in the superficial layer of the cortex at multiple sites (bilaminar cortex signs) which are more obvious on the inset (white arrows) (B). There are circular appearances in the thalami, putamen and heads of the caudate nuclei (bubble signs) which are more obvious on the inset (e.g., black arrows) (C). The bubble sign is due to focal reductions in T
1 probably from the presence of free iron. The thalamic signal is slightly higher medially compared with laterally unlike the appearance of the normal thalamus in the 18-year-old patient shown in
Figure 12B.
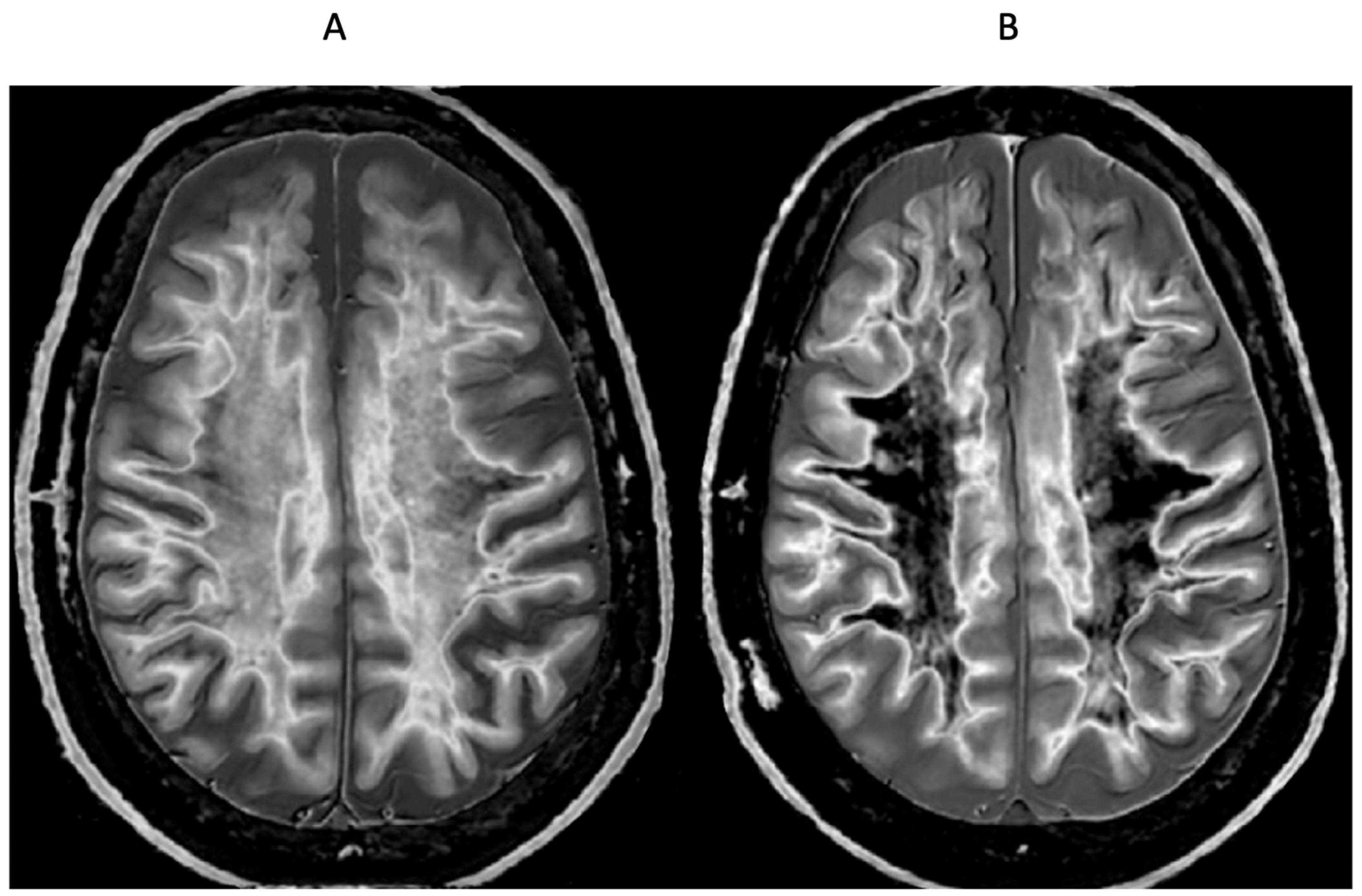
Figure 21.
65-year-old female with a glioma (not shown) post chemotherapy. Parasagittal T2-FLAIR (A) and synthetic narrow mD dSIR (B) images. On the T2-FLAIR image no abnormality is seen. On the dSIR image (B), the white matter has a high signal corresponding to a high grade whiteout sign. This includes the cerebellar white matter which is similar in intensity to the cerebral white matter. There is some sparing of the peripheral white matter in (B).
Figure 21.
65-year-old female with a glioma (not shown) post chemotherapy. Parasagittal T2-FLAIR (A) and synthetic narrow mD dSIR (B) images. On the T2-FLAIR image no abnormality is seen. On the dSIR image (B), the white matter has a high signal corresponding to a high grade whiteout sign. This includes the cerebellar white matter which is similar in intensity to the cerebral white matter. There is some sparing of the peripheral white matter in (B).
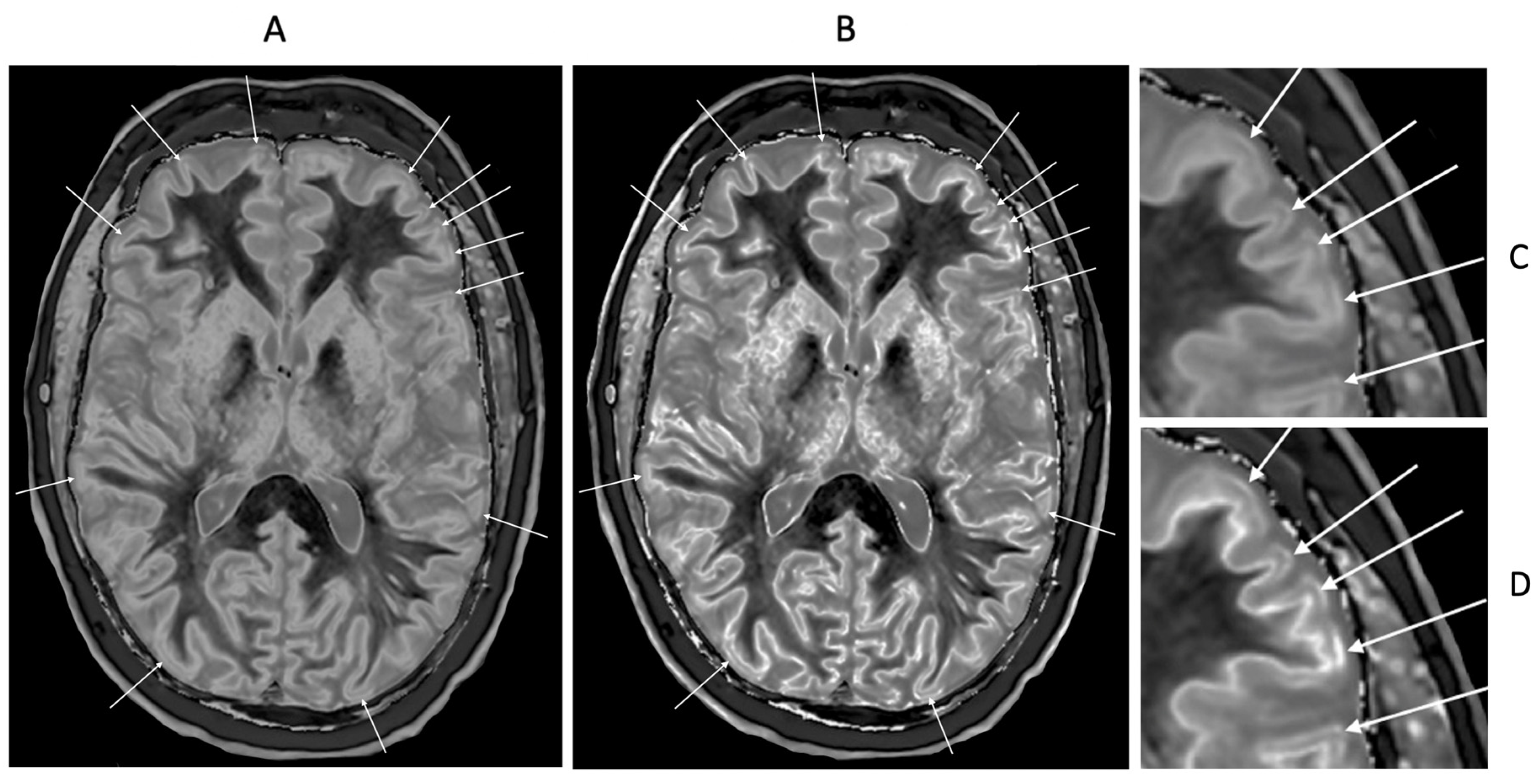
Figure 22.
46-year-old normal control. dSIR (A) and lSIR (B) images with insets of anterior left cortex dSIR in (C) and anterior left cortex lSIR in (D). The boundaries between white matter and gray matter are seen with higher contrast and the contrast is seen with higher spatial resolution in (B) and (D) compared with (A) and (C). Bilaminar cortex signs are also seen with higher contrast and spatial resolution in (B) and (D) (white arrows). There are also small bubble signs in the putamen and medial thalamus which are better seen on the lSIR image (B) compared with the dSIR image (A).
Figure 22.
46-year-old normal control. dSIR (A) and lSIR (B) images with insets of anterior left cortex dSIR in (C) and anterior left cortex lSIR in (D). The boundaries between white matter and gray matter are seen with higher contrast and the contrast is seen with higher spatial resolution in (B) and (D) compared with (A) and (C). Bilaminar cortex signs are also seen with higher contrast and spatial resolution in (B) and (D) (white arrows). There are also small bubble signs in the putamen and medial thalamus which are better seen on the lSIR image (B) compared with the dSIR image (A).
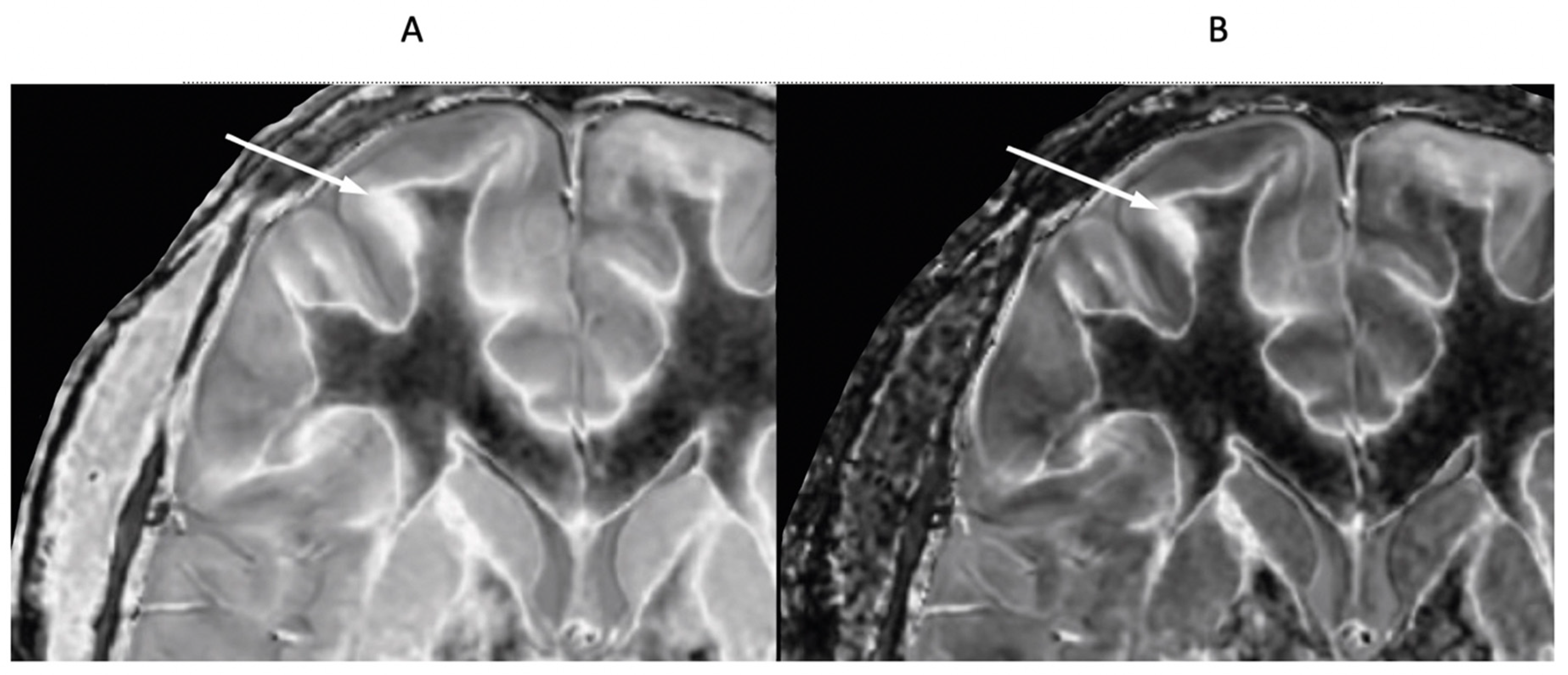
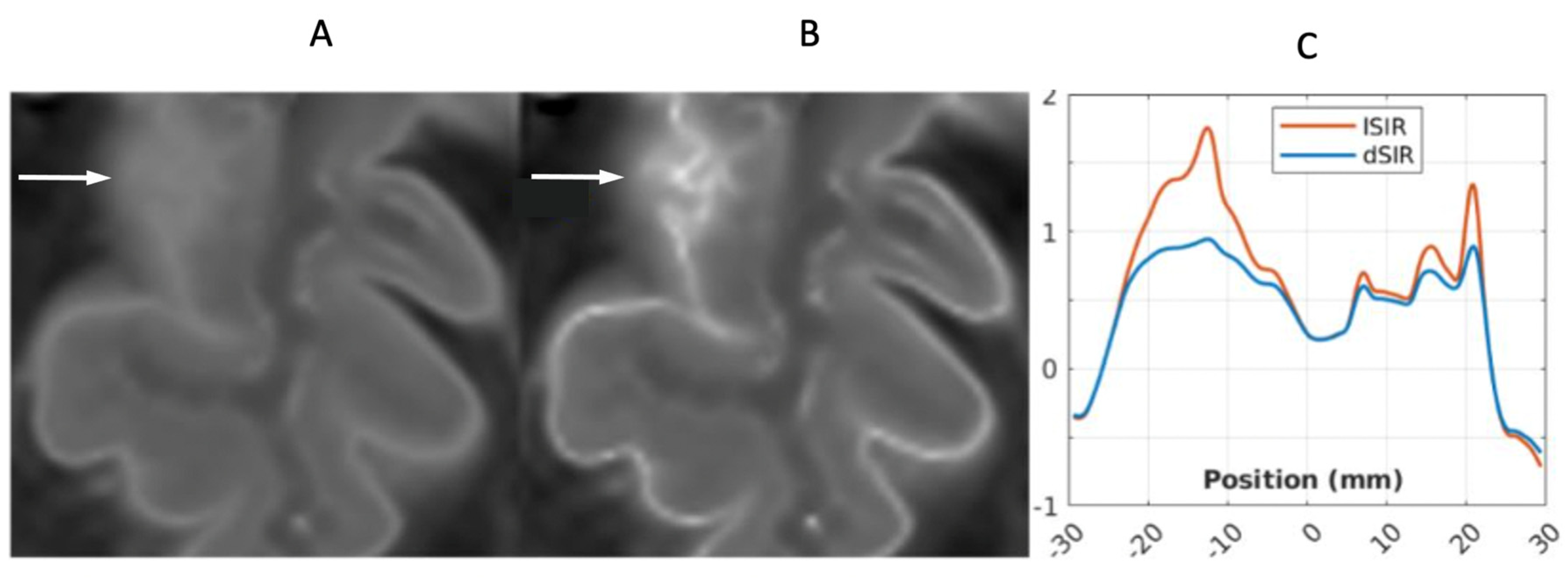
Figure 23.
41-year-old female patient with MS. A leukocortical lesion in the right medial frontal region is shown on the narrow mD dSIR image (A) and a matching lSIR image (B) (arrows). There are also left to right profiles with signal plotted against position (in mm) for the dSIR (blue) and lSIR (orange) images (C) at the level of the horizontal arrows shown in (A) and (B). No boundary between white matter and gray matter is seen within the lesion in (A). A disrupted high signal boundary between white matter and gray matter is seen in the lesion in (B). The lSIR profile (orange) has higher signal and steeper slopes than the dSIR profile (blue) in (C). The difference in signal (or contrast) achieved for the same change in position is generally greater with the lSIR filter i.e., the contrast shown on the lSIR image generally has a higher spatial resolution.
Figure 23.
41-year-old female patient with MS. A leukocortical lesion in the right medial frontal region is shown on the narrow mD dSIR image (A) and a matching lSIR image (B) (arrows). There are also left to right profiles with signal plotted against position (in mm) for the dSIR (blue) and lSIR (orange) images (C) at the level of the horizontal arrows shown in (A) and (B). No boundary between white matter and gray matter is seen within the lesion in (A). A disrupted high signal boundary between white matter and gray matter is seen in the lesion in (B). The lSIR profile (orange) has higher signal and steeper slopes than the dSIR profile (blue) in (C). The difference in signal (or contrast) achieved for the same change in position is generally greater with the lSIR filter i.e., the contrast shown on the lSIR image generally has a higher spatial resolution.
Figure 24.
Thalamus. dSIR narrow mD (TIs = 350 ms and TIi = 500 ms). T1-bipolar filter for normal appearance, abnormal increase in T1 in lateral thalamic gray matter within the hD with loss of contrast with medial thalamic gray matter (striped green arrow), and subsequent decrease in T1 to normal with restoration of contrast with medial thalamic gray matter (solid green arrow). The normal thalamus gray matter shows a decrease in T1 from medial (TGmed) to lateral (TGlat) (highest horizontal negative solid green arrow) and corresponding positive contrast (first vertical positive blue arrow). With disease there is an increase in T1 in the lateral thalamus (middle horizontal positive striped green arrow) with a loss of contrast with the medial thalamus (blue circle). With later regression of this and return of the T1 difference between the lateral and medial thalamus to normal (lowest negative solid green arrow), normal positive contrast is seen between the medial and lateral thalamus (second vertical positive blue arrow).
Figure 24.
Thalamus. dSIR narrow mD (TIs = 350 ms and TIi = 500 ms). T1-bipolar filter for normal appearance, abnormal increase in T1 in lateral thalamic gray matter within the hD with loss of contrast with medial thalamic gray matter (striped green arrow), and subsequent decrease in T1 to normal with restoration of contrast with medial thalamic gray matter (solid green arrow). The normal thalamus gray matter shows a decrease in T1 from medial (TGmed) to lateral (TGlat) (highest horizontal negative solid green arrow) and corresponding positive contrast (first vertical positive blue arrow). With disease there is an increase in T1 in the lateral thalamus (middle horizontal positive striped green arrow) with a loss of contrast with the medial thalamus (blue circle). With later regression of this and return of the T1 difference between the lateral and medial thalamus to normal (lowest negative solid green arrow), normal positive contrast is seen between the medial and lateral thalamus (second vertical positive blue arrow).
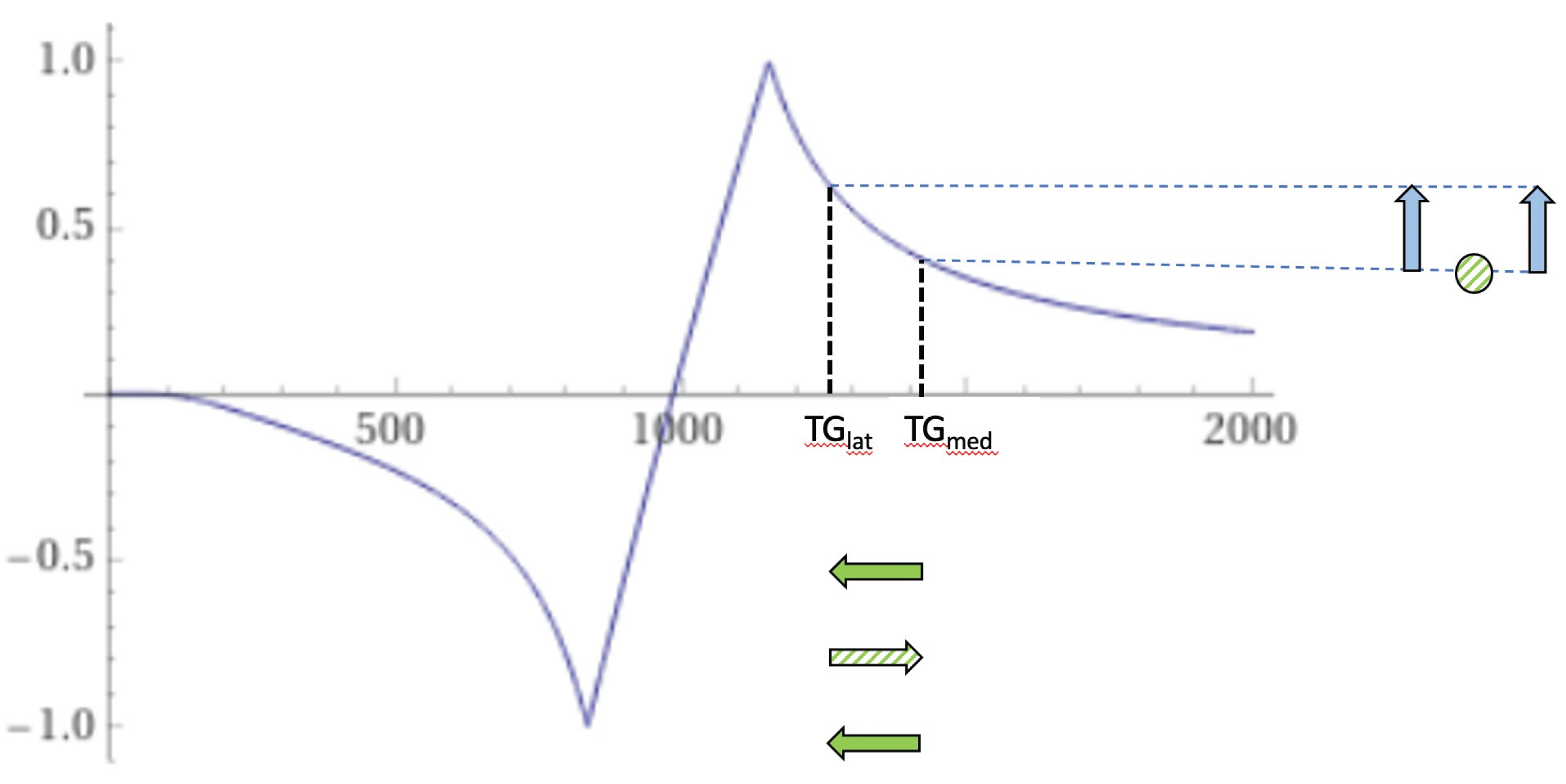
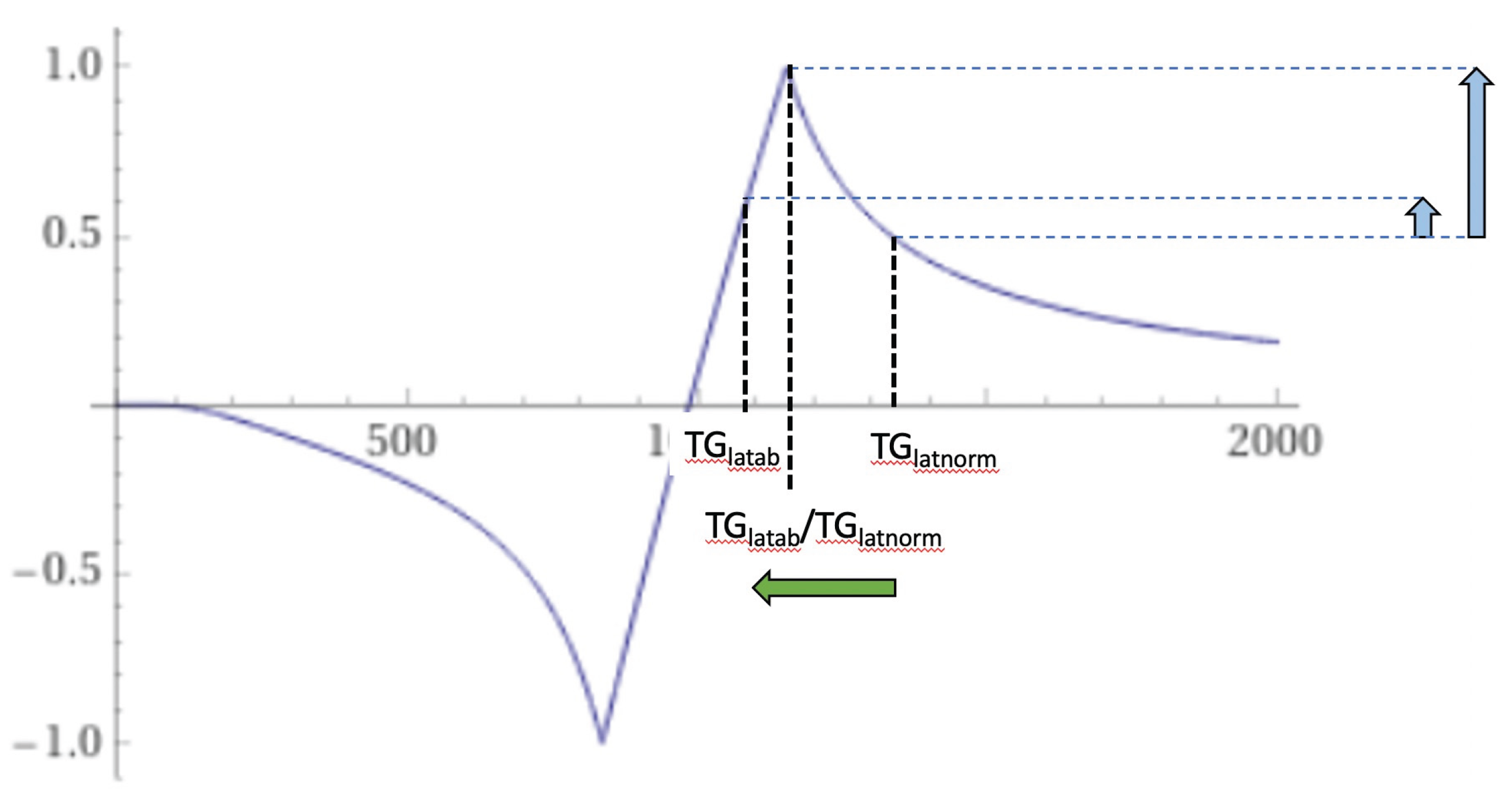
Figure 25.
Thalamus. Narrow mD (TIs = 350 ms and TIi = 500 ms). dSIR T1-bipolar filter for a larger abnormal decrease in T1 in lateral thalamic gray matter from normal (TGlatnorm) to abnormal (TGlatab) extending from the hD through the signal peak to the mD (horizontal negative green arrow). Signal in the lateral thalamus is highest where there is partial volume effect between abnormal and normal gray matter of the thalamus (TGlatab/TGlatnorm) (longer vertical blue arrow). Signal from abnormal lateral thalamus in the middle Domain (TGlatab) (shorter blue arrow) is less than the signal in the lateral thalamus at the boundary between normal and abnormal thalamus (longer blue arrow). The higher and lower signals (vertical blue arrows) result in a bubble sign with higher signal in the periphery where there are partial volume effects and lower signal centrally where there is only abnormal gray matter. The same argument applies to the medial thalamus.
Figure 25.
Thalamus. Narrow mD (TIs = 350 ms and TIi = 500 ms). dSIR T1-bipolar filter for a larger abnormal decrease in T1 in lateral thalamic gray matter from normal (TGlatnorm) to abnormal (TGlatab) extending from the hD through the signal peak to the mD (horizontal negative green arrow). Signal in the lateral thalamus is highest where there is partial volume effect between abnormal and normal gray matter of the thalamus (TGlatab/TGlatnorm) (longer vertical blue arrow). Signal from abnormal lateral thalamus in the middle Domain (TGlatab) (shorter blue arrow) is less than the signal in the lateral thalamus at the boundary between normal and abnormal thalamus (longer blue arrow). The higher and lower signals (vertical blue arrows) result in a bubble sign with higher signal in the periphery where there are partial volume effects and lower signal centrally where there is only abnormal gray matter. The same argument applies to the medial thalamus.
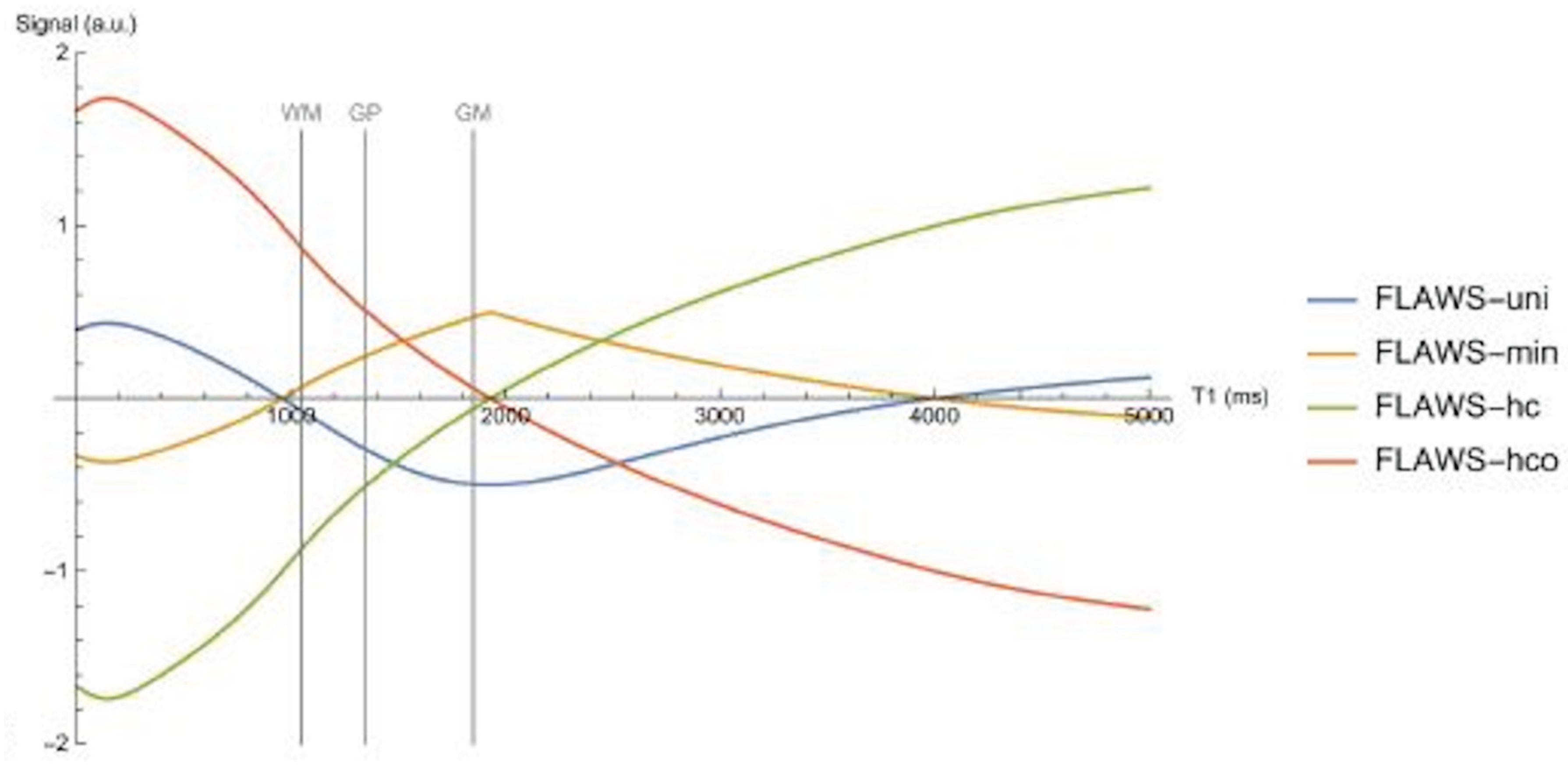
Table 1.
Pulse sequences and pulse sequence parameters used at 3T. Only 2D scans were performed on the Siemens Healthineers Skyra system.
Table 1.
Pulse sequences and pulse sequence parameters used at 3T. Only 2D scans were performed on the Siemens Healthineers Skyra system.
| # |
Sequence |
TR (ms) |
TI (ms) |
TE (ms) |
Matrix size Voxel size
(mm) |
Number of slices |
Slice thickness (mm) |
| 1* |
2D FSE IR (for white nulling matter) |
9,192 |
350 |
7, 80 |
256 x 224
0.9 x 0.1
Z512
0.4 x 0.4 |
26 |
4 |
| 2* |
2D FSE IR (used with #1 for narrow mD dSIR) |
5,796 |
500 |
7, 80 |
256 x 224
0.9 x 0.1
Z512
0.4 x 0.4 |
26 |
4 |
| 3* |
2D FSE IR (for longer T1 nulling) |
5,796 |
600 |
7 |
256 x 224
0.9 x 0.1
Z512
0.4 x 0.4 |
26 |
4 |
| 4* |
2D FSE IR (used with #1 for wide mD dSIR) |
5,796 |
800 |
7 |
256 x 224
0.9 x 0.1
Z512
0.4 x 0.4 |
26 |
4 |
| 5 |
3D BRAVO (for white matter nulling) |
2,000 |
400 |
6 |
256 x 256
0.8 x 0.8
Z512 |
220 |
0.8 |
| 6 |
3D BRA
VO (used with #5 for wide mD dSIR) |
2,000 |
800 |
6 |
256 x 256
0.8 x 0.8
Z512 |
220 |
0.8 |
| 7* |
2D T2-FLAIR |
6,300 |
1,851 |
102 |
320 x 240
0.7 x 0.7
Z512
0.4 x 0.4 |
26 |
4 |
| 8 |
3D T2-FLAIR |
6,300 |
1,850 |
102 |
256 x 256
0.8 x 0.8
Z512
0.6 x 0.6 |
252 |
0.8 |
| 9 |
3D susceptibility weighted |
40
|
- |
32 |
300 x 300
0.8 x 0.8
Z512 |
110 |
2 |
Table 2.
Directly acquired tissue property bipolar filters.
Table 2.
Directly acquired tissue property bipolar filters.
| Bipolar filter |
Reverse bipolar filter |
Tissue properties
single (upper)
composite (lower) |
| SIR |
rSIR |
T1
|
dSIR
cdSIR |
drSIR
cdrSIR |
T1
T1; T2, T2, D*, c and/or MT |
lSIR
clSIR |
lrSIR
clrSIR |
T1
T1; T2, T2*, D*, c and/or MT |
Table 3.
Signal equations for bipolar filters and other functions.
Table 3.
Signal equations for bipolar filters and other functions.
| # |
Filter, other functions |
Signal Equation |
Figure # |
| 1 |
IR, TIs
|
STIs = 1-2e-TIs/T1
|
3,4,5 |
| 2 |
IR, TIi
|
STIi = 1-2e-TIi/T1
|
3,4,5 |
| 3 |
SIR |
SSIR = STIs - STIi
|
5 |
| 4 |
dSIR
|
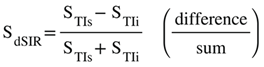 |
6,9 |
| 5 |
dSIR
|
 (in lD and hD) (in lD and hD)
ΔTI = TIi – TIs
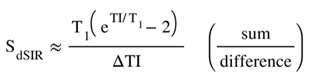 (in mD) (in mD) |
6,9 |
| 6 |
cdSIR
|
|
10 |
| 7 |
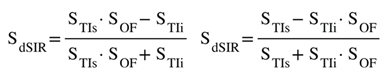
cdSIR, SOF
|
SOF = ±e-DTE/T2, ± e-DTE/T2*, ±e-DbD*, etc |
10 |
| 8 |
dSIR, SdSIR
|
(in mD) |
6 |
| 9 |
dSIR, T1
|
(in mD) |
6 |
| 10 |
lSIR |
SlSIR = ½(ln STIs - ln STIi) |
8,9 |
| 11 |
clSIR |
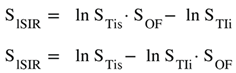 |
|
| 12 |
clSIR, lSIR |
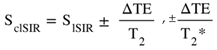 , ±DbD*, etc , ±DbD*, etc |
|
| 13 |
lSIR, dSIR† |
SlSIR = atanh SdSIR (in mD) |
9 |
| 14 |
dSIR, T1D†† |
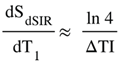 (in mD) (in mD) |
6,9 |
| 15 |
lSIR, T1D ††† |
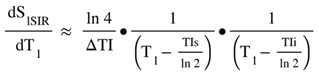 (in mD) (in mD) |
9 |
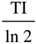 and ST1 = (2e-TI/T1 -1) for T1 >
and ST1 = (2e-TI/T1 -1) for T1 > 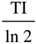 are used in the subsequent text unless otherwise specified.
are used in the subsequent text unless otherwise specified.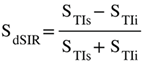






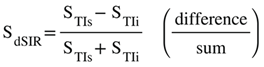
 (in lD and hD)
(in lD and hD)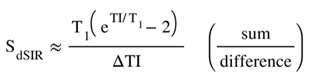 (in mD)
(in mD)
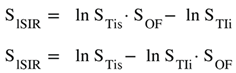
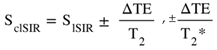 , ±DbD*, etc
, ±DbD*, etc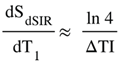 (in mD)
(in mD)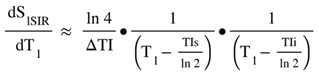 (in mD)
(in mD)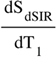 is the T1 derivative (T1D) of SdSIR and gives the slope of dSIR filter in the mD which is essentially constant and equal to
is the T1 derivative (T1D) of SdSIR and gives the slope of dSIR filter in the mD which is essentially constant and equal to 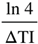 (Figure 9). †††
(Figure 9). ††† 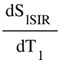 is the T1 derivative (T1D) of the SlSIR filter in the mD which varies. It has values of minus or plus infinity when
is the T1 derivative (T1D) of the SlSIR filter in the mD which varies. It has values of minus or plus infinity when 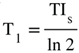 and
and 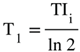 respectively (Figure 9).
respectively (Figure 9).




